
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 23 September 2022
Sec. Viral Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1003094
This article is part of the Research TopicA year in review: Coronavirus infection in Humans and Viral ImmunologyView all 21 articles
It has been shown that SARS-CoV-2 shares homology and cross-reacts with vaccines, other viruses, common bacteria and many human tissues. We were inspired by these findings, firstly, to investigate the reaction of SARS-CoV-2 monoclonal antibody with different pathogens and vaccines, particularly DTaP. Additionally, since our earlier studies have shown immune reactivity by antibodies made against pathogens and autoantigens towards different food antigens, we also studied cross-reaction between SARS-CoV-2 and common foods. For this, we reacted monoclonal and polyclonal antibodies against SARS-CoV-2 spike protein and nucleoprotein with 15 different bacterial and viral antigens and 2 different vaccines, BCG and DTaP, as well as with 180 different food peptides and proteins. The strongest reaction by SARS-CoV-2 antibodies were with DTaP vaccine antigen, E. faecalis, roasted almond, broccoli, soy, cashew, α+β casein and milk, pork, rice endochitinase, pineapple bromelain, and lentil lectin. Because the immune system tends to form immune responses towards the original version of an antigen that it has encountered, this cross-reactivity may have its advantages with regards to immunity against SARS-CoV-2, where the SARS-CoV-2 virus may elicit a “remembered” immune response because of its structural similarity to a pathogen or food antigen to which the immune system was previously exposed. Our findings indicate that cross-reactivity elicited by DTaP vaccines in combination with common herpesviruses, bacteria that are part of our normal flora such as E. faecalis, and foods that we consume on a daily basis should be investigated for possible cross-protection against COVID-19. Additional experiments would be needed to clarify whether or not this cross-protection is due to cross-reactive antibodies or long-term memory T and B cells in the blood.
The SARS-CoV-2 virus that is responsible for the COVID-19 pandemic is part of the family of coronaviruses that normally cause from mild to moderate upper-respiratory tract illness very similar to that of the common cold. However, the difference between SARS-CoV-2 and other viruses is its induction of serious illness with the involvement of multiple tissue abnormalities that may result in death (1).
When the body is exposed to different pathogens, it will launch an immune response, and afterwards the body will retain some disease-fighting cells called memory T and memory B cells. Upon exposure to the same pathogen or cross-reactive antigens, these memory cells are ready to fight again with greater speed and more efficiency (2). In some people, pre-existing memory cells generated against, for example, common cold coronaviruses, can cross-recognize the SARS-CoV-2 virus because of cross-reactive antigen binding between SARS-CoV-1 and SARS-CoV-2 (3, 4). This was shown by the generation of memory T cell lines that recognized many fragments from spike and non-spike regions of SARS-CoV-2, which were then tested for epitope similarity against a peptide pool of other coronaviruses. It was found that memory T cells made against SARS-CoV-2 cross-reacted with 57% of “common cold” coronavirus fragments (5, 6).
Furthermore, after the performance of antigen-specific T-cell studies, it was reported that 20-50% of individuals unexposed to SARS-CoV-2 had significant T-cell reactivity to various SARS-CoV-2 peptide sequences (2, 3, 7–10). This may be an answer to why, after contracting COVID-19, some people present only mild or moderate symptoms, but others get severely ill (11–13). The memory T cells generated against common cold coronaviruses may be responsible for this extensive heterogeneity in the human immune response to SARS-CoV-2 and its contribution to herd immunity (6, 13, 14).
All of this indicates that, immunologically, humans are not naïve, and that when they encounter new infections, the host immune system will activate its memory B and T cells, allowing quicker immune responses to a multitude of antigens, resulting in the production of both protective and cross-reactive antibodies (1, 2). In this context, we refer to the potential cross-reactivity to SARS-CoV-2 from common human pathogens and vaccines (15). This is based on the hypothesis that children may already have some degree of protection against SARS-CoV-2 due to the presence of cross-reactive immunity induced through their vaccinations with different bacterial and viral antigens (16–18). Because the immunity elicited by vaccines declines with aging, the adult population becomes more susceptible to COVID-19 (15). Of course, this possible cross-protection resulting from cross-reactive immunity most likely would not give the same degree of protection as specific SARS-CoV-2 vaccines.
Based on the hypothesis that the elderly are more prone to SARS-CoV-2 infection and children are largely spared due to pediatric vaccinations, a systemic search for peptide matches in 18 viruses and 7 bacteria with SARS-CoV-2 was conducted to identify potential cross-reactive epitopes by Reche in 2020 (15). While other researchers found that common herpesviruses were poor sources of cross-reactivity, Reche found that the combination of diphtheria, tetanus and pertussis (DTaP) in the DTaP vaccine proved to be a significant cause of cross-reactive immunity to SARS-CoV-2 (15). Comparing the amino acid sequences of overlapping 15-mer peptides from some of these pathogens with 10 SARS-CoV-2 residues, he reported as low as 1 epitope (polio virus) to 3,807 epitopes (Bacillus Calmette-Guerin or BCG) that cross-reacted with SARS-CoV-2 spike protein. Measles had 8 cross-reactive epitopes, HSV 1&2 had 77, Epstein-Barr virus 94, cytomegalovirus 169, diphtheria 340, tetanus 601, and Bordetella pertussis 3,359 (15). Based on these and other findings, Reche concluded that cross-reactive immunity elicited by DT antigens in combination with DTaP vaccines is likely responsible for keeping children safe from worldwide infection with SARS-CoV-2 (15).
In search of possible cross-reactivity between SARS-CoV-2 and human autoantigens, in our own earlier studies we first reacted animal and then human monoclonal antibodies (19, 20) made against SARS-CoV-2 spike protein and nucleoproteins with more than 55 human tissue antigens, and found that the SARS-CoV-2 antibodies had moderate to strong reactions with more than 20 of the human tissue antigens. We concluded that a potential risk for autoimmunity may come from cross-reactivity between SARS-CoV-2 antigens and our own tissue antigens (19, 20).
Furthermore, in additional studies we investigated antigenic mimicry between dietary proteins and human autoantigens not only by epitope sharing but also through the interaction of food-specific antibodies with human tissue antigens and vice-versa (21–24). We found that an extensive number of food antigens reacted with tissue-specific antibodies, and many food antibodies such as lectins and agglutinins reacted with numerous tissue antigens (25, 26). We also showed significant immunological cross-reactivity between different viruses and other pathogens (27). Observing this interaction between polyclonal and monoclonal antibodies made against food antigens and pathogens with various tissue antigens led us to hypothesize the following:
1. SARS-CoV-2 antibody may cross-react with common viral and bacterial antigens, including some which were not examined in the 2020 study by Reche (15).
2. SARS-CoV-2 proteins may share cross-reactive epitopes with many food antigens that have not previously been studied.
3. The production of cross-reactive antibodies against viral, bacterial and food antibodies may be responsible for extensive heterogeneity in their response to SARS-CoV-2 in different countries in the world.
To identify possible cross-reactive immunity to SARS-CoV-2, we reacted SARS-CoV-2 monoclonal antibodies with 14 different viral and bacterial antigens, and 180 different food antigens and peptides. We then reacted human sera with very low levels of antibodies to selected pathogens and food antigens versus sera with very high levels of these same antigens, for comparative purposes, with SARS-CoV-2 antigens. Finally, we conducted systematic searches for sequences shared by SARS-CoV-2, and pathogens and food antigens with which SARS-CoV-2 antibodies had reacted.
E. coli and Salmonella lipopolysaccharides (LPS), and Enterococcus faecalis were purchased from Sigma Aldrich (St. Louis, MO USA).
ELISA microwell plates coated with B. burgdorferi, EBV-VCA, EBV-EAD, EBV-EBNA, CMV, VZV and measles antigens were purchased from Trinity Biotech (Jamestown, NY USA).
HHV-6 A and B were purchased from Bio-Synthesis (Lewisville, TX, USA).
ELISA well plates coated with HSV 1 + 2 antigens were obtained from Gold Standard Diagnostics (Davis, CA USA).
H. influenza, BCG and DTaP vaccines were purchased from the local pharmacy.
Human IgG1 monoclonal antibody made against SARS-CoV-2 spike protein S1 domain (CR3022) Catalog #NBP3-11813 was purchased from Novus Biologicals (Centennial, CO USA). This antibody binds specifically to amino acids 318-510 in the S (spike) domain of the SARS-CoV spike protein as well as the SARS-CoV-2 (COVID-19) spike protein
Human IgG1 monoclonal antibody made against SARS-CoV-2 nucleoprotein (CR3009) SKU: MAB12434 was obtained from The Native Antigen Company (Kidlington, Oxfordshire, UK). This antibody recognizes and binds the non-linear/conformational epitope of the N protein of SARS-CoV and SARS-CoV-2.
Recombinant SARS-CoV-2 spike protein S1 subunit and recombinant SARS-CoV-2 nucleocapsid protein were purchased from RayBiotech (Atlanta, GA USA).
For the preparation of the food antigens, food products were purchased from a Whole Foods supermarket. Food proteins undergo structural epitope transformation when the food is cooked, so, when necessary, foods underwent preparation so that the resulting food proteins would accurately represent the raw and cooked foods of typical human diets. Using a process similar to the one used in our earlier study (28), a total of 180 different foods representing different meats, seafoods, vegetables, fruits, grains, nuts, seeds, beans, spices, gums and more were prepared.
Lectins and agglutinins such as wheat germ agglutinin (WGA), soybean agglutinin, phytohemagglutinin, peanut agglutinin and concanavalin A were purchased from Sigma Aldrich (St. Louis, MO USA).
Gliadin peptides were synthesized by Bio-Synthesis (Lewisville, TX USA).
Food products were ground at 4°C in either 70% ethanol, or coco buffer containing 0.55 M of NaHCO3, 1% NaCl pH 8.5. Each food item was left on the stirrer at 25°C for 4 h. The food processor was decontaminated after each food was stirred. The mixture was centrifuged for 15 minutes at 2000 g, after which the top layer containing oil bodies was discarded. To ensure that all small molecules were removed, each solvent’s liquid phase was dialyzed against a buffer of 0.01 M phosphate buffered saline (PBS) using dialysis bags with a cutoff of 6000 DA for 72 h, with the buffer changed every 24 h. The protein concentration was subsequently measured using a kit obtained from Biorad (Hercules, CA USA). The complete list of the 180 foods can be found in Supplementary Material.
Rabbit anti-gluten was purchased from Sigma Aldrich (St. Louis, MO USA).
Rabbit anti-phytohemagglutinin was obtained from Abcam (Fremont, CA USA).
Rabbit anti-WGA, anti-soybean agglutinin, anti-wheat, anti-a-gliadin, anti-egg, anti-corn, anti-peanut agglutinin and others were prepared by Bio-Synthesis (Lewisville, TX USA).
Commercially available microwell plates coated with different bacterial and viral antigens including BCG vaccine, DTaP vaccine, SARS-CoV-2 spike protein and nucleoprotein were prepared at an optimal concentration of 1-3 mg/mL. After dilution at 1:100 in 0.01 M PBS, pH 7.4, 100 microliters containing 1-3 mg of these antigens were added to a series of 96-well microtiter plates.
Food antigens were prepared at a concentration of 1 mg/mL. In coating the ELISA plate, we determined the optimal concentration of each food antigen by examining the concentration of antigens that gave the most reproducible results in quadruplicate. Consequently, we diluted the stock solution from 1:25 to 1:100 in 0.1 M carbonate-bicarbonate buffer (pH 9.5). One hundred microliters were added to each well of the polystyrene flat-bottom ELISA plate. All plates were kept for 6 h at room temperature (RT) and 18 h at 4°C. Plates were washed 4 times using an ELISA washer, and 200 microliters of 2% BSA were added to each well and incubated for 24 h at 4°C in order to block the non-specific binding of the antibody to the antigen-coated wells. To examine the binding of SARS-CoV-2 antibodies to each one of these antigens, 100 microliters of human anti-spike protein and human anti-nucleoprotein at optimal dilutions of 1:100-1:200, and rabbit anti-envelope and rabbit anti-membrane proteins at a dilution of 1:100 were each added to quadruplicate wells of microtiter plates coated with various antigens. After 1 h of incubation and washing, an optimal dilution of alkaline-phosphatase-labeled anti-human or anti-rabbit IgG was added to the appropriate sets of plates, which were then incubated again for 1 h at RT. To remove the unbound antibodies, plates were washed 5 times, and 100 microliters of substrate para-nitrophenylphosphate were added. After 45 mins, color development was measured using an ELISA reader at 405 nm. We calculated the means of the respective quadruplicate wells and used them in the graphs.
We calculated the percentage of each antibody’s tissue reaction according to the following formula:
In order to determine the specificity of human monoclonal and rabbit polyclonal antibodies in binding to pathogens, vaccines and food antigens, these antibodies were replaced with the same dilution of human serum from a healthy subject or with non-immunized rabbit serum and added to quadruplicate wells. Furthermore, the antibodies and other reagents were added to 4 wells coated with 2% HSA and BSA alone and used as negative controls. After completion of all ELISA steps, the ODs of these control wells were measured.
For the demonstration of the specificity of SARS-CoV-2 antibody binding to SARS-CoV-2 antigens, bacterial, viral, vaccine and food antigens were prepared at a concentration of 1 mg/mL. Each antigen was then diluted 1:200, 1:400, and 1:800, after which 100 microliters of each antigen was added to different rows of microtiter plates. This way, each row was coated with no antigen (blank), or with final amounts of 500, 250 and 125 nanograms of each antigen: E. faecalis, HSV 1 + 2, EBV EAD, DTaP, α+β casein, gliadin peptide, and peanut proteins. These antigens were chosen because they showed from low to strong reactivity with either SARS-CoV-2 spike and nucleoprotein antibodies. After the completion of the antigen-coating steps, SARS-CoV-2 monoclonal antibodies at a dilution of 1:100 was added to the wells coated with different concentrations of antigens. After completion of the other ELISA steps, the ODs were recorded at 405 nM.
Different wells of ELISA plates were coated with 0.5 micrograms of either spike protein or nucleoprotein that had been dissolved in 100 ml of 0.01 M PBS pH 7.4 and were then kept for 8 h at RT followed by 16 h at 4°C. Plates were washed 4 times with 0.01 M PBS containing 0.05% Tween 20. After washing, 200 ml of 2% BSA was added and incubated again for 8 h at RT, and 16 h at 4°C to block the uncoated surfaces. Following removal of the BSA and washing, the plates were ready for antibody reactivity. 100 microliters of serum diluent were added to the first 4 wells of a microtiter plate coated with spike protein or nucleoprotein. 100 microliters of unimmunized rabbit serum diluted 1:100 was added to the second set of 4 wells, and 100 microliters of rabbit anti-spike or anti-nucleoprotein antibody was added to a third set of 4 wells. The second and third sets of wells, 4 in each set, 8 in total, served as negative and positive controls. 100 microliters of rabbit serum with very high titers of IgG antibody to wheat, α-gliadin, WGA, milk, α+β casein, soy, soy agglutinin, peanut agglutinin, phytohemagglutinin, egg, and corn antibodies diluted 1:100. After 60 mins of incubation and washing, 100 microliters of goat anti-rabbit IgG labeled with alkaline phosphatase at a dilution of 1:200 was added. Color development and optical densities were recorded at 405 nM after completion of ELISA steps.
Using ELISA methodology, we screened 200 sera for the presence or absence of IgG antibodies against EBV EAD, HSV 1 + 2, HHV-6, peanut proteins, wheat, gliadin peptide, gluteomorphin + dynorphin, milk, α + β casein, and pineapple bromelain. We then selected 24 sera with very low levels and 24 sera with very high levels of antibodies against each of these viral or food antigens-coated plates. After dilution of each serum 1:50, and the completion of ELISA steps, the ODs were compared.
Using SARS-CoV-2 Zeus ELISA, we screened many sera and selected 24 with non-detectable and 24 with high levels of IgG antibody to SARS-CoV-2 spike protein and nucleoprotein. We then applied the selected sera at a dilution of 1:20 to ELISA plates coated with EBV EA, CMV, HSV 1 + 2, HHV-6, DTaP and BCG vaccines, peanut butter, wheat, gliadin peptide, gluteomorphin + dynorphin, milk, α+β casein, and pineapple bromelain. The selection of these vaccines, viral and food antigens was based on their reactivity to SARS-CoV-2 monoclonal antibody. After completion of all the ELISA steps, the ODs were recorded.
We used the NIH/US National Library of Medicine’s BLAST (Basic Local Alignment Search Tool) sequence matching program to study the degrees of possible mimicry or amino acid (AA) sequence homology shared by SARS-CoV-2 spike protein and nucleoprotein with EBV EA, EBV VCA, EBV EBNA, CMV, HSV-1, HSV-2, HHV-6, peanut ARA H2 allergen, peanut agglutinin, wheat gliadin, glutenin, wheat germ agglutinin, casein, lentil lectin, pineapple bromelain and rice endochitinase.
Statistical analysis was performed by comparing the ODs obtained for the reactive tissue antigens with the mean OD of non-reactive tissue antigens + 3SD using STATA 14.2 software. Independent t-tests were performed to evaluate mean differences of optical densities between controls and antigens. A Bonferroni adjustment was conducted to account for type 1 errors with multiple comparisons and alpha was set to< 0.001.
We used human monoclonal antibodies made against SARS-CoV-2 spike proteins and nucleoproteins to measure the degree of immune reactivity of these antibodies with 15 different bacterial and viral antigens and 2 different vaccines, Bacillus Calmette-Guérin (BCG) and Diphtheria, Tetanus and Pertussis (DTaP). An earlier study showed that these 2 vaccines had a high degree of amino acid sequence similarity with SARS-CoV-2 (18). As expected, in comparison to the blank OD of 0.1 or less, the reaction of spike protein and nucleoprotein antibodies with recombinant spike proteins and nucleoproteins was greater than 3.5, which is very close to the maximum detection limit of the assay.
As shown in Figure 1, with spike protein reactivity as 100%, significant immune reactivity was observed between spike protein monoclonal antibody and DTaP vaccine (OD 1.4, or 36%), E. faecalis (OD 1.3 or 32%), and HSV 1 + 2 (OD 0.9, or 22%), but not with BCG vaccine. The immune reactivity was lower with CMV and most EBV antigens, ranging from 0.58 (13%) – 0.84 (21%) ODs. The ODs for VZV, measles and HHV-6 ranged from 0.38 (7%) – 0.43 (9%). Finally, for antigens such as LPS, E. coli+Salmonella CDT peptides, EBV VCA, and Borrelia burgdorferi, the ODs were very close to 3SD above the background OD of the controls, or OD< 0.27. The percentages of significant reactivity between SARS-CoV-2 spike antibodies and different bacterial and viral antigens are also shown in Figure 1. Overall, DTaP, E. faecalis, HSV 1 + 2, EBV-EAD, EBV-EBNA, CMV, HHV-6, measles, and VZV had significant p values (p< 0.001), while the other antigens were insignificant.
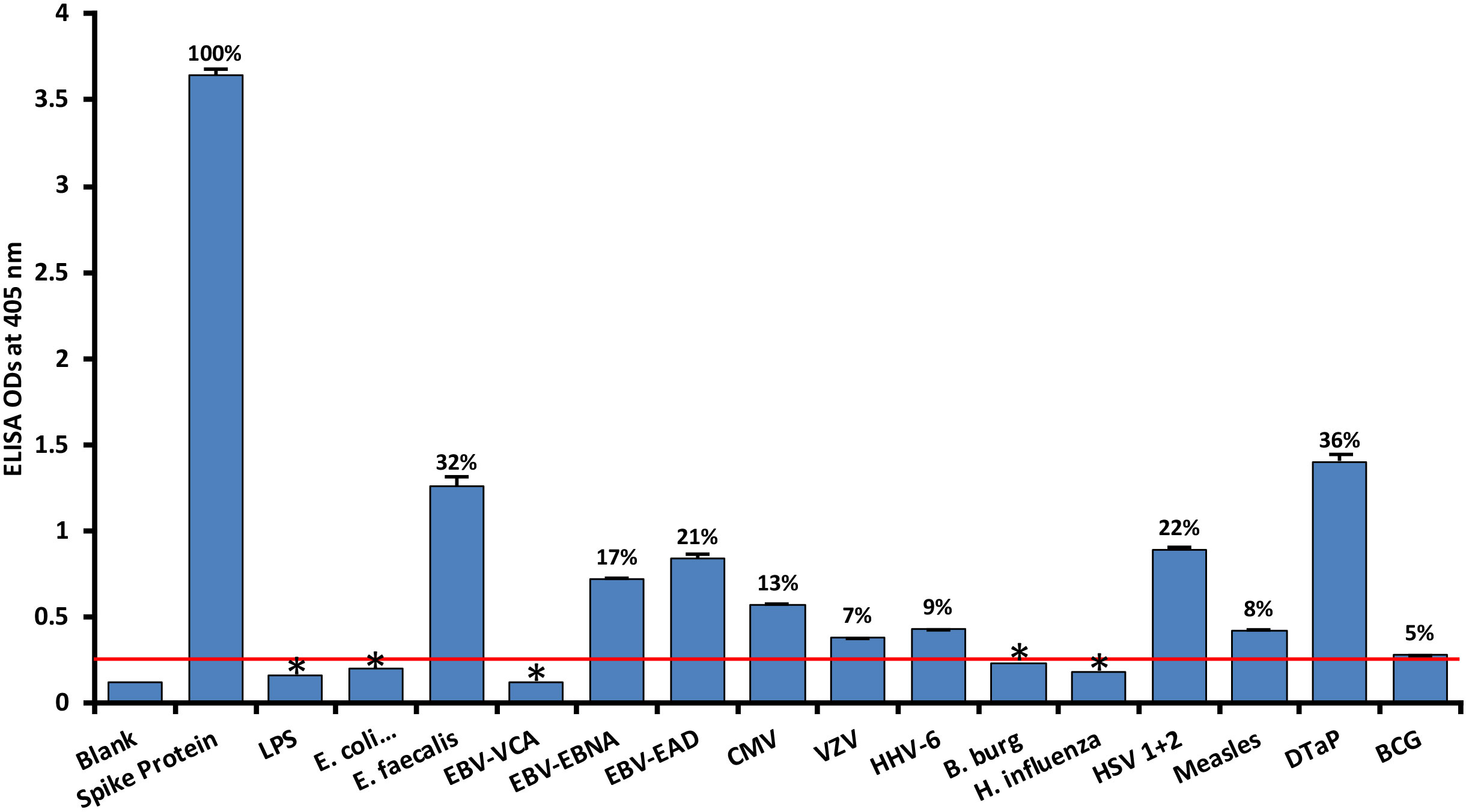
Figure 1 Reaction of human SARS-CoV-2 spike protein monoclonal antibody with spike protein and different bacterial and viral antigens, including DTaP and BCG vaccines. At 3SD above the OD of background or 0.27, significant reaction between spike protein antibody and bacterial and viral antigens was observed. Each determination of antigen-antibody reaction was performed in quadruplicate. The standard deviations (SDs) for all the reactions were less than 0.1, and are shown as error bars. In these experiments, when monoclonal anti-SARS-CoV-2 antibodies were replaced with sera from healthy subjects, non-significant reactions with a mean of 0.25 were observed from the sera with spike protein. Percentages of significant reactivity are shown. DTaP is shown to be the most reactive. * = Antigens whose reactions were under the cutoff and/or close to the blank.
At 3SD above the background OD of the controls (0.3), the human monoclonal antibody to nucleoprotein had weak to strong reactions with 10 out of the 14 bacterial and viral antigens. As shown in Figure 2, with nucleoprotein reactivity as 100%, the strongest immune reactivity was with DTaP (OD 1.58 or 39%), E. faecalis (OD 0.93 or 21%), EBV-VCA (OD 0.82 or 18%), CMV (OD 0.77 or 17%) and Borrelia burgdorferi (OD 0.63 or 13%), all of which had p values< 0.001. For E. coli + Salmonella CDT peptides, EBV-EBNA, EBV-EAD, VZV, HHV-6, H. influenza, HSV 1 + 2, measles, and BCG, the ODs were below the cutoff or very close to the blank, and their p values were insignificant. The percentages of significant reactivity between SARS-CoV-2 nucleoprotein antibodies and different bacterial and viral antigens are also shown in Figure 2.
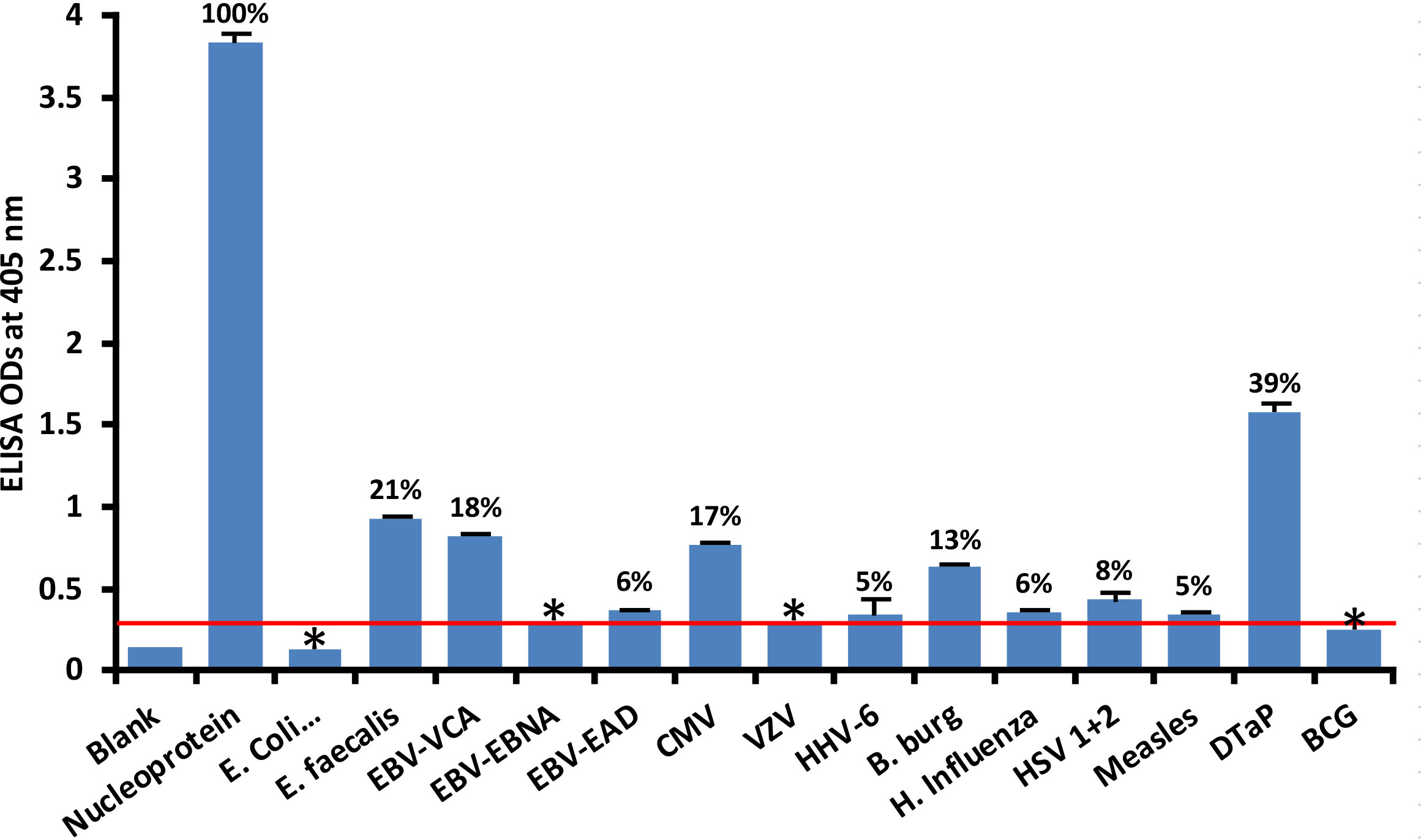
Figure 2 Reaction of human SARS-CoV-2 nucleoprotein monoclonal antibody with nucleoprotein and different bacterial and viral antigens, including DTaP and BCG vaccines. At 3SD above the OD of background or 0.3, significant reaction between spike protein antibody and bacterial and viral antigens was observed. Each determination of antigen-antibody reaction was performed in quadruplicate. The SDs for all the reactions were less than 0.12, and are shown as error bars. In these experiments, when monoclonal anti-SARS-CoV-2 antibodies were replaced with sera from healthy subjects, non-significant reactions with a mean of 0.29 were observed from the sera with nucleoprotein. The ODs of these reactions were lower than 0.3. Percentages of significant reactivity are also shown. DTaP is shown to be the most reactive. * = Antigens whose reactions were under the cutoff and/or close to the blank.
Similar to what we did with pathogen and vaccine antigens, we reacted monoclonal antibody made against SARS-CoV-2 spike proteins and nucleoproteins with 180 different commonly consumed food proteins and peptides.
Anti-SARS-CoV-2 spike protein antibody had a significant reaction with 28 out of 180 food antigens. Reactions with beef and corn were weaker. The reactivity between SARS-CoV-2 monoclonal antibody and different foods was considered positive only if the obtained ODs were higher than the reagent controls and the mean ODs of the other foods + 3SD. The cutoff OD for spike protein antibody reaction with various foods was determined to be 0.56. Figure 3 shows that the most significant reactions (p< 0.001) of spike protein antibody were with soy (35%), α+β casein (34%), roasted almond (32%), lentil lectin (31%), milk (30%), gliadin toxic peptide (28%), squid (28%), cooked chicken (27%), broccoli (27%), and pea protein (26%).
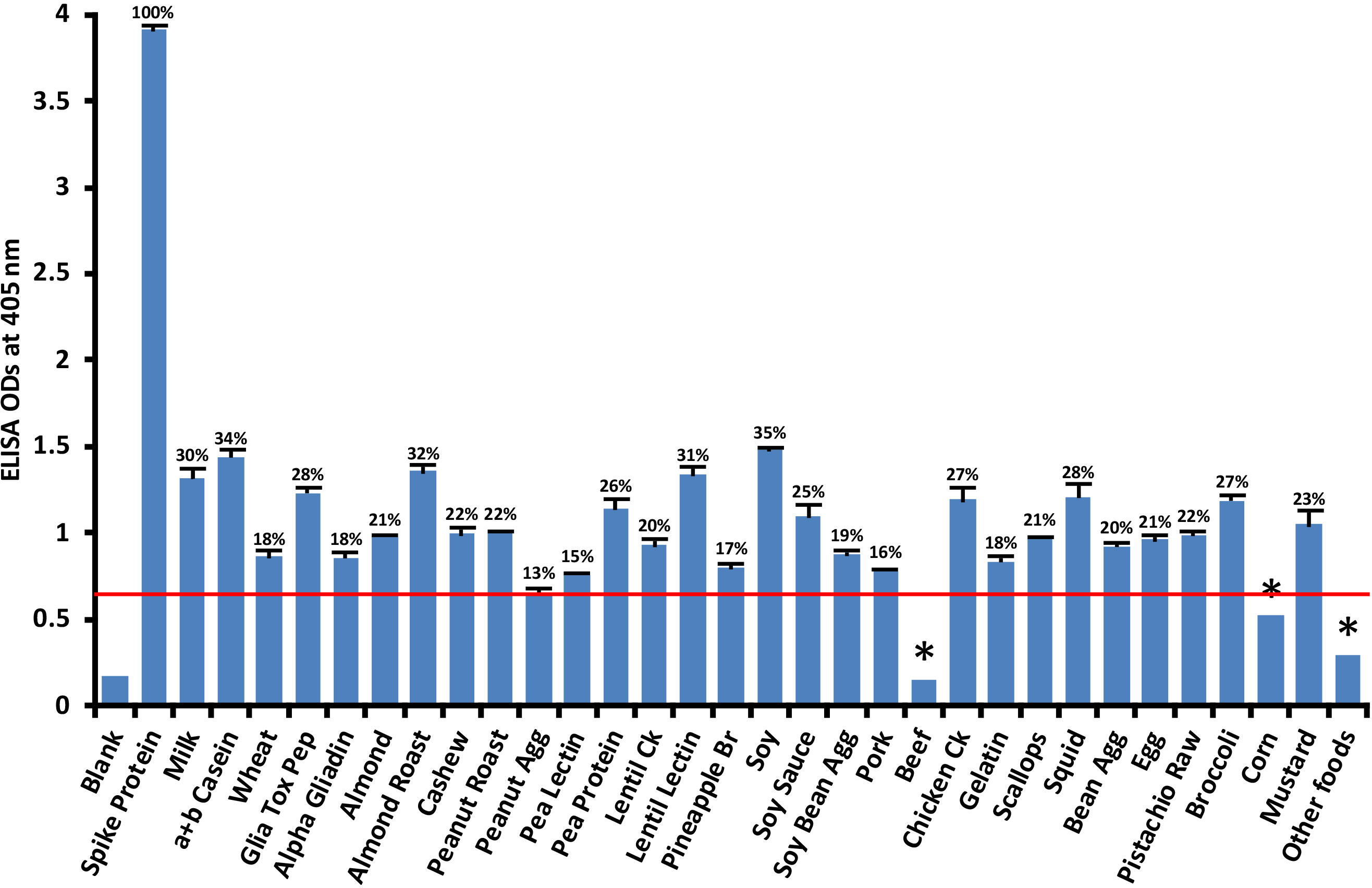
Figure 3 Reaction of human SARS-CoV-2 spike protein monoclonal antibody with various food antigens. At 3SD above the OD of antibody reaction with non-reactive foods or OD of 0.56, the spike protein antibody reacted significantly with 28 out of 180 tested food proteins and peptides. These reactions of SARS-CoV-2 spike protein antibody with different food antigens was obtained from quadruplicate testing. The SDs for all the reactions were less than 0.1, and are shown as error bars. * = Antigens whose reactions were under the cutoff and/or close to the blank. Glia Tox Pep, Gliadin Toxic Peptide; Peanut Agg, Peanut Agglutinin; Soy Bean Agg, Soy Bean Agglutinin; Bean Agg, Bean Agglutinin; Pineapple Br, Pineapple Bromelain; Ck, Cooked.
Compared to its reaction with SARS-CoV-2 nucleoprotein (100%), the application of SARS-CoV-2 nucleoprotein antibody to 180 food antigens resulted in the strongest reactions with the following foods: broccoli (39%), roasted almond (39%), cashew (34%), soy bean (32%), squid (32%), rice endochitinase (32%), pork (31%), pineapple bromelain (30%), and gliadin toxic peptide (30%). The cutoff OD for nucleoprotein was 0.64. The reactions with an additional 24 foods were not as strong; those still above the cutoff but weaker ranged from peanut agglutinin (14%) to egg (29%) while roasted peanut, beef and corn were below the cutoff (Figure 4). Overall, the difference in reactivity of SARS-CoV-2 monoclonal antibody with 22 of the reactive foods was very significant at p< 0.001, significant with 4 foods at p = 0.001, and insignificant for the rest.
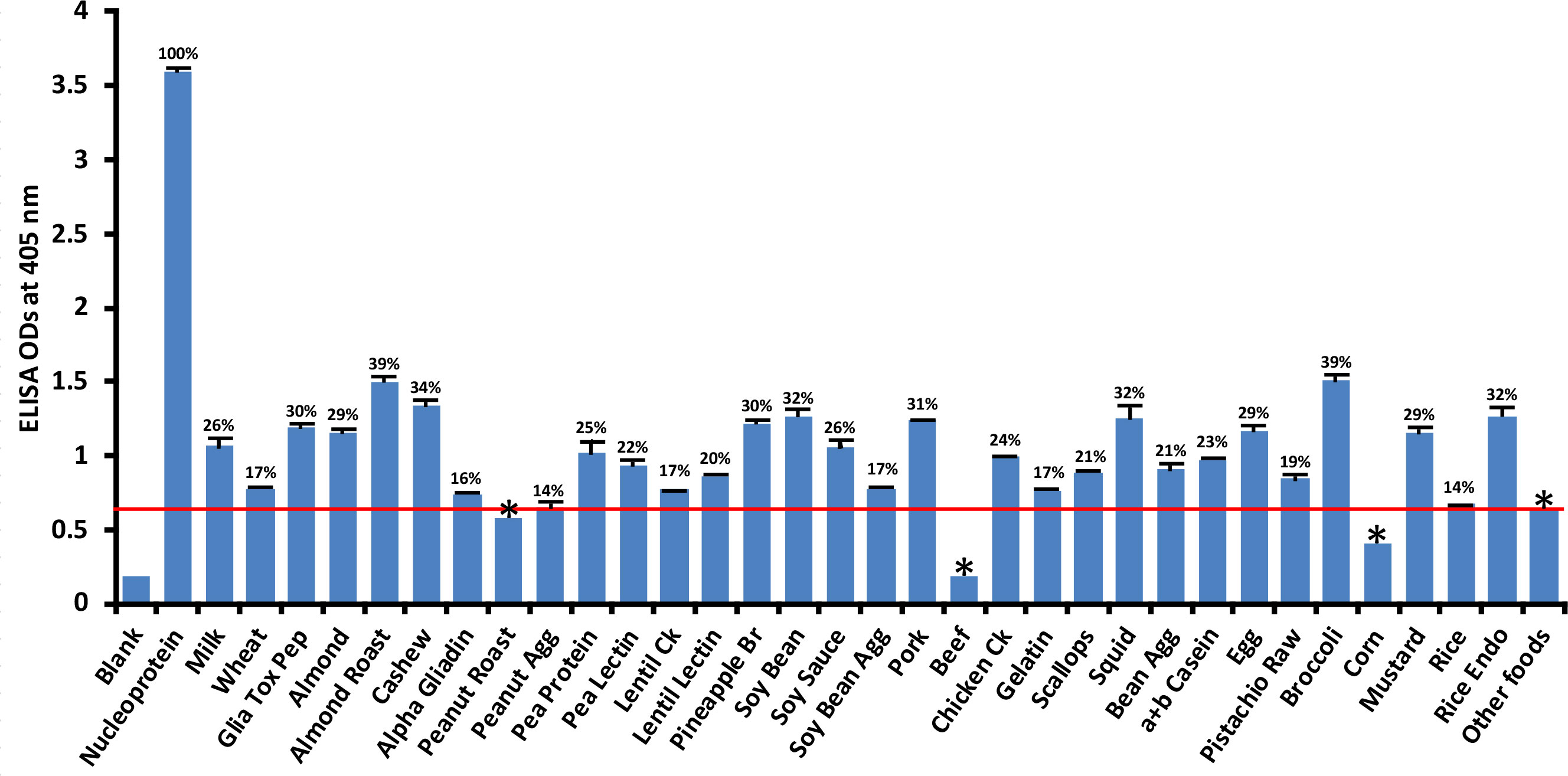
Figure 4 Reaction of human SARS-CoV-2 nucleoprotein monoclonal antibody with various food antigens. At 3SD above the OD of antibody reaction with non-reactive foods or OD of 0.64, the nucleoprotein antibody reacted significantly with 26 out of 180 tested food proteins and peptides. The data was obtained from quadruplicate testing. The SDs for all the reactions were less than 0.1, and are shown as error bars. * = Antigens whose reactions were under the cutoff and/or close to the blank. Glia Tox Pep, Gliadin Toxic Peptide; Peanut Agg, Peanut Agglutinin; Soy Bean Agg, Soy Bean Agglutinin; Bean Agg, Bean Agglutinin; Pineapple Br, Pineapple Bromelain; Rice Endo, Rice Endochitinase; Ck, Cooked.
Monoclonal antibodies made against both spike protein and nucleoprotein were applied to spike protein and nucleoprotein, as well as bacterial, viral and food antigens that had been serially diluted at dilutions of 1:200, 1:400 and 1:800. These antigens were selected because they had reacted significantly with these antibodies in prior experiments. As shown in Figure 5, the reaction of the anti-SARS-CoV-2 spike protein antibodies with E. faecalis, HSV 1 + 2, EBV-EAD, DTaP vaccine, α+β casein, gliadin toxic peptide and pea protein decreased in proportion to an increase in the dilution.
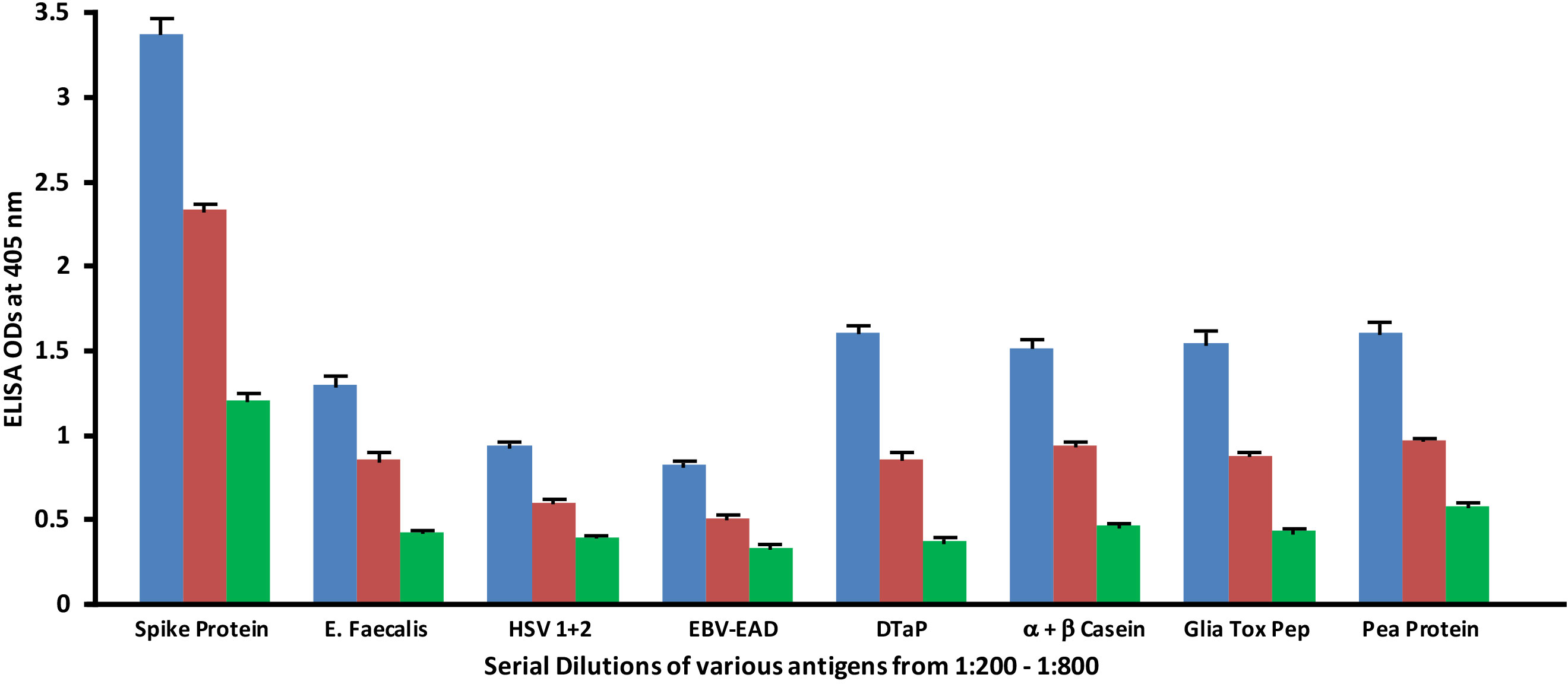
Figure 5 Reaction of human SARS-CoV-2 spike protein monoclonal antibody with different bacterial, viral and food antigens at dilutions of 1:200  , 1:400
, 1:400  , and 1:800
, and 1:800  . Note that in proportion to the dilution of the antigens, a significant decline in antibody-antigen reaction is observed. Each determination of antibody-antigen reaction was performed in triplicate. The SDs for all the reactions were less than 0.1, and are shown as error bars.
. Note that in proportion to the dilution of the antigens, a significant decline in antibody-antigen reaction is observed. Each determination of antibody-antigen reaction was performed in triplicate. The SDs for all the reactions were less than 0.1, and are shown as error bars.
Similar to spike protein antibody, the reaction of SARS-CoV-2 nucleoprotein antibody with bacterial, viral and food antigens decreased in proportion to an increase in the dilution (Figure 6). This corollary decline, however, is more pronounced and noticeable with antigens that reacted strongly with anti-nucleoprotein antibody, such as DTaP, gliadin toxic peptide, E. faecalis, and pea protein, while the decline with HSV 1 + 2, EBV-EAD and α+β casein, although present, are less obvious due to the closeness of their ODs to the background.
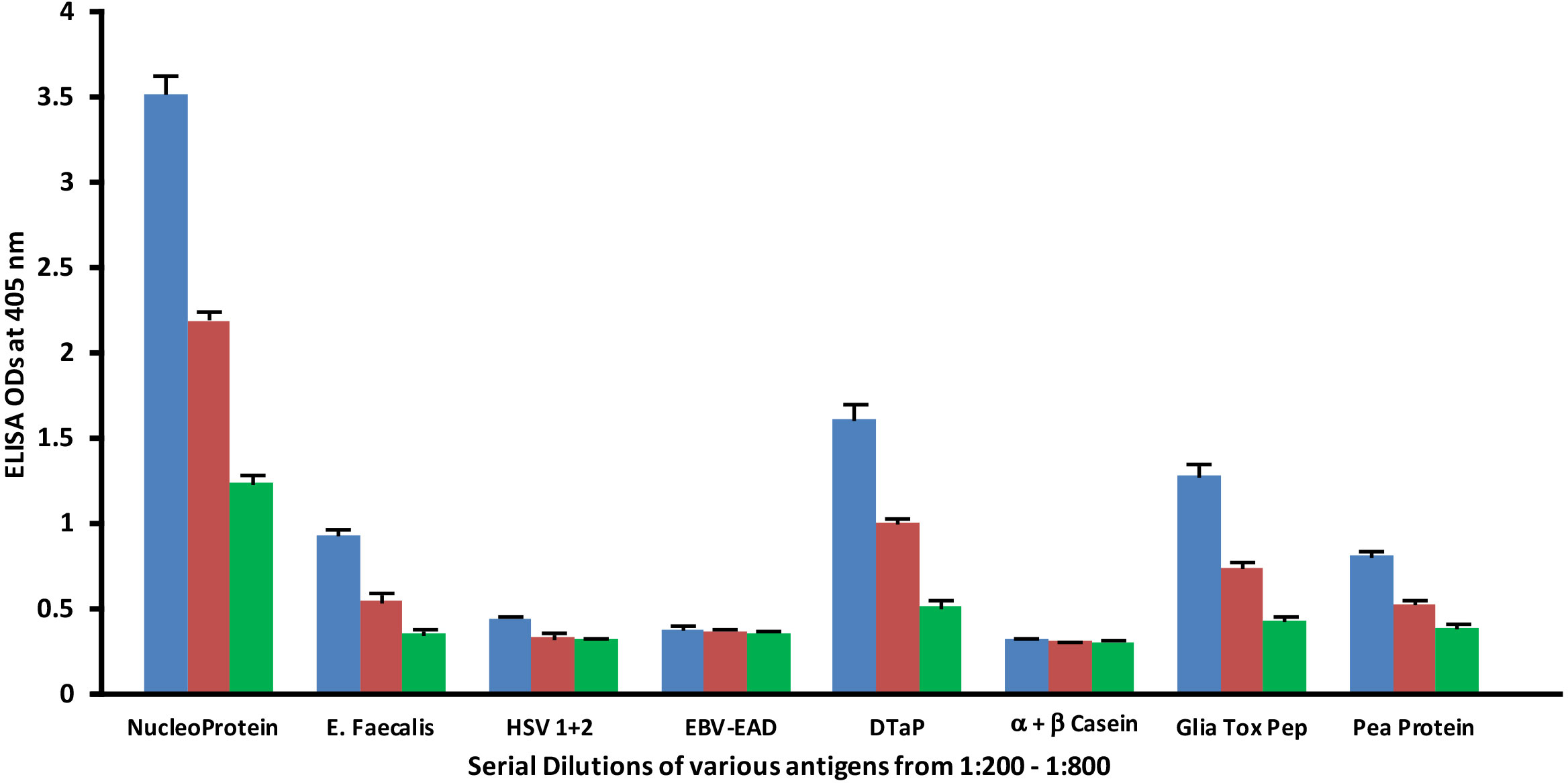
Figure 6 Reaction of human SARS-CoV-2 nucleoprotein monoclonal antibody with different bacterial, viral and food antigens at dilutions of 1:200  , 1:400
, 1:400  , and 1:800
, and 1:800  . Note that in proportion to the dilution of the antigens, a significant decline in antibody-antigen reaction is observed only in the antigens with ODs that are >0.5. Each determination of antibody-antigen reaction was performed in triplicate. The SDs for all the reactions were 0.1 or less, and are shown as error bars. .
. Note that in proportion to the dilution of the antigens, a significant decline in antibody-antigen reaction is observed only in the antigens with ODs that are >0.5. Each determination of antibody-antigen reaction was performed in triplicate. The SDs for all the reactions were 0.1 or less, and are shown as error bars. .
Using ELISA methodology, we reacted affinity-purified antibodies made against phytohemagglutinin (PHA), soy protein, soy agglutinin, peanut agglutinin, wheat, wheat germ agglutinin (WGA), α-gliadin-33 mer, milk, α+β casein, egg and corn by applying them to ELISA microwells coated with spike protein or nucleoprotein. We found that unimmunized rabbit serum diluted 1:100 did not react significantly with SARS-CoV-2 proteins. The ELISA indices for the unimmunized rabbit serum for all the reactions were within 3SD above the mean OD of control wells (0.36 – 0.39). The reactions and percentages of reactivity between SARS-CoV-2 antibodies and different food antigens are shown in Figure 7.
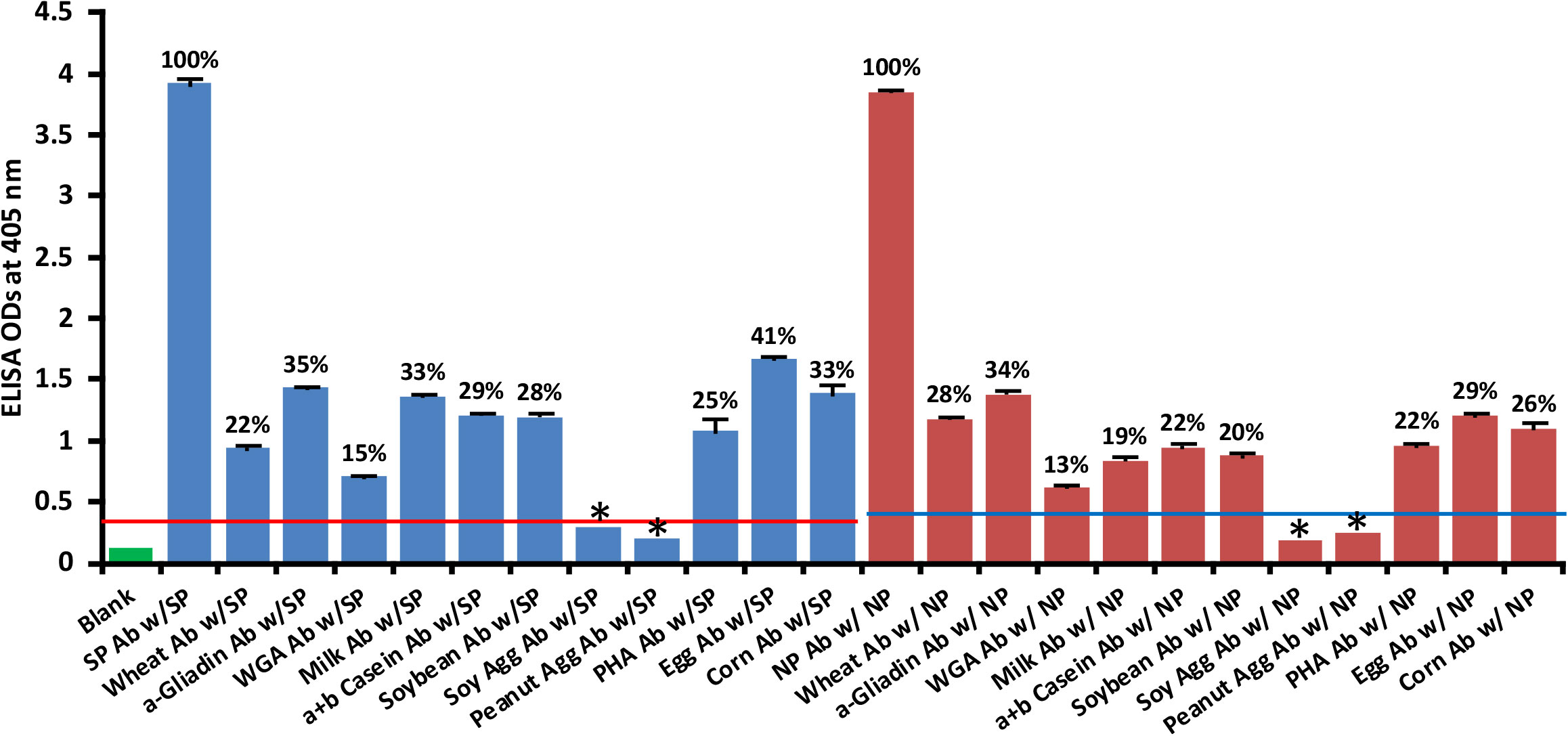
Figure 7 Reaction of affinity-purified rabbit polyclonal antibodies made against different food antigens with recombinant SARS-CoV-2 spike  or nucleoprotein
or nucleoprotein  . Each determination of antigen-antibody reaction was performed in quadruplicate. The SDs for all the reactions were 0.1 or less, and are shown as error bars. * = Antigens whose reactions were under the cutoff and/or close to the blank. WGA, Wheat Germ Agglutinin; Peanut Agg, Peanut Agglutinin; Soy Agg, Soy Agglutinin; PHA, Phytohemagglutinin. Percentages of significant reactivity are also shown.
. Each determination of antigen-antibody reaction was performed in quadruplicate. The SDs for all the reactions were 0.1 or less, and are shown as error bars. * = Antigens whose reactions were under the cutoff and/or close to the blank. WGA, Wheat Germ Agglutinin; Peanut Agg, Peanut Agglutinin; Soy Agg, Soy Agglutinin; PHA, Phytohemagglutinin. Percentages of significant reactivity are also shown.
The data presented in Figure 7 show that the following food antibodies had moderate to strong reactions with both SARS-CoV-2 spike proteins (SP) and nucleoproteins (NP): anti-wheat (SP 22%, NP 28%), anti-α-gliadin (SP 35%, NP 34%), anti-milk (SP 33%, NP 19%), anti-α+β casein (SP 29%, NP 22%), anti-soy (SP 28%, NP 20%), anti-PHA (SP 25%, NP 22%), anti-egg (SP 41%, NP 29%) and anti-corn (SP 33%, NP 26%). The reactions of WGA antibody with the SARS-CoV-2 proteins were low (SP 15%, NP 13%), and the reactions of anti-soy agglutinin and anti-peanut agglutinin with those proteins was comparable to reaction of unimmunized rabbit serum with SARS-CoV-2 spike and nucleoproteins. To facilitate the clarity of results, the percentages of reactivity are also shown in Figure 7.
Summary results of the reaction of sera containing low or high levels of IgG antibodies against EBV-EAD, HSV 1 + 2 and HHV-6 with SARS-CoV-2 spike protein are shown in Figure 8. Compared to the reaction of 24 sera with low levels (negative) of IgG antibody against EBV-EAD, HSV 1 + 2 and HHV-6, the reaction of 24 sera containing high levels of antibodies against these viral antigens with SARS-CoV-2 spike protein resulted in higher ELISA ODs with p values of 0.052, 0.028 and 0.006 respectively for EBV-EAD, HSV 1 + 2, and HHV-6.
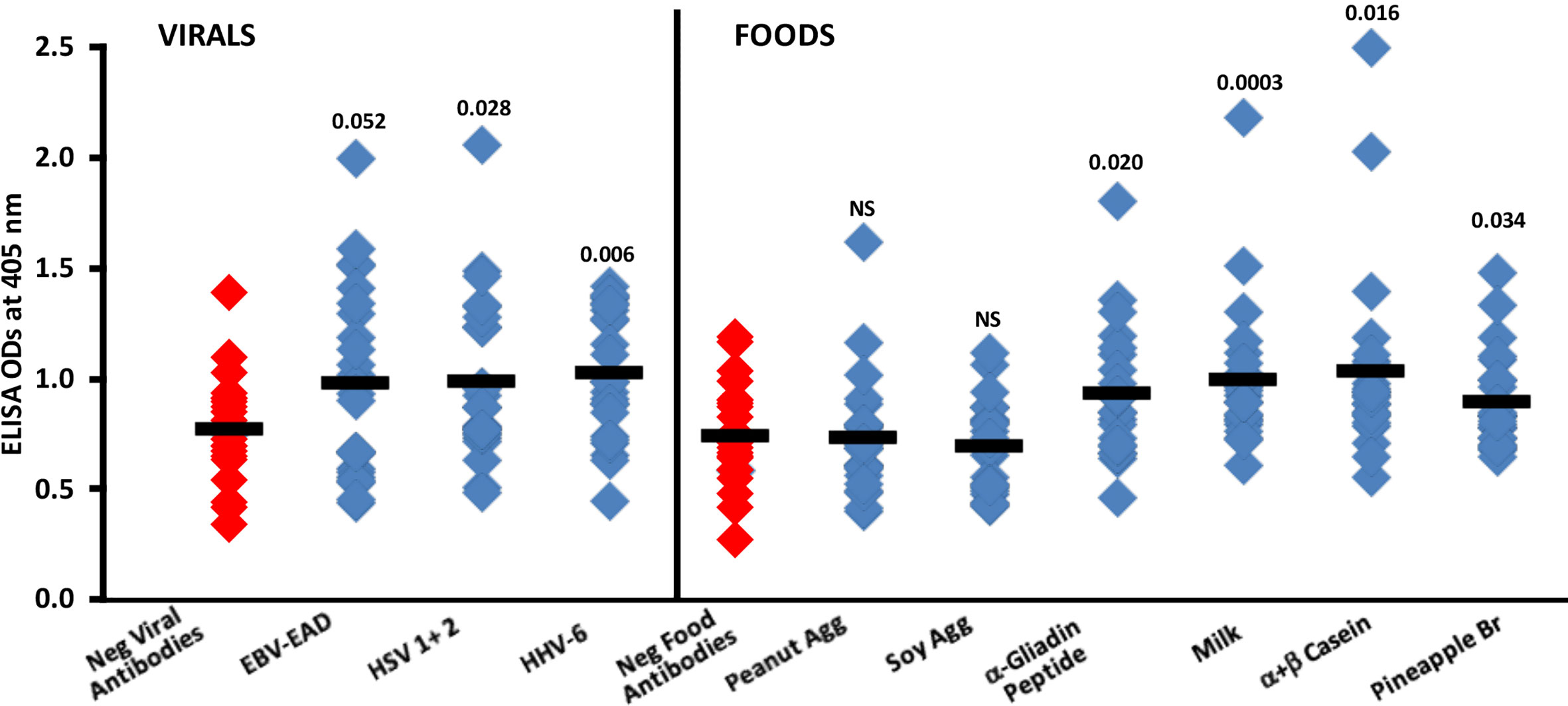
Figure 8 Reaction of human sera with low (Negative) or high levels of IgG antibody against EBV-EAD, HSV 1 + 2, HHV-6 and different food antigens with SARS-CoV-2 spike protein-coated plates. Black bars = means.
In Figure 8, the data shows that the reactions of human sera containing high levels of IgG antibody to peanut agglutinin and soy agglutinin with spike protein were comparable to those of sera containing low levels (negative) of antibody with non-significant p-values. However, most sera with high levels of IgG antibody against α-gliadin peptide, milk, α+β casein, and pineapple bromelain reacted strongly with SARS-CoV-2 spike protein, resulting respectively in p values of 0.020, 0.0003, 0.016, and 0.034.
We used BLAST to find the degree of identity between SARS-CoV-2 proteins and other viruses and pathogens, including HSV-1, HSV-2, EBV, CMV, HHV-6, measles, VZV and Borrelia burgdorferi. SARS-CoV-2 proteins shared a significant number of peptides with each of these pathogens, as can be seen in Table 1A and Table 1B. These SARS-CoV-2 sequences shared 50-100% identity with different viruses. An almost similar number of peptide sequences with identity percentages ranging from 30 to 49% were also observed but are not shown in these tables. Similar to SARS-CoV-2 homology with other viruses and pathogens, we used BLAST to find a significant number of peptides from different foods that are consumed on a daily basis which shared 50-73% identity with SARS-CoV-2 sequences. These foods were peanuts, almonds, wheat, milk, rice, lentil and pineapple (see Table 2A and Table 2B). In both cases these subject sequences actually made a match with more than one section of the SARS-CoV-2 sequences; the multiple matches are indicated by asterixes in Tables 1(A & B) and 2(A & B).
In our earlier investigation, we applied anti-SARS-CoV-2 monoclonal antibody to 55 different tissue antigens and showed that these specific antibodies reacted with 28 autoantigens (20). We also sought selective peptide matches shared by spike protein and nucleoproteins with mitochondria M2, F-actin, and thyroid peroxidase, and found extensive cross-reactivity between them (20).
In another article in the same journal (15), Reche et al. explored potential cross-reactive immunity to SARS-CoV-2 from common human pathogens and vaccines. Among the tested 25 human pathogen and vaccine antigens, they found that viruses such as mumps, measles and rubella used in pediatric vaccinations did not contain SARS-CoV-2 cross-reactive epitopes, and concluded that immunity against these viruses may not provide any general protection against SARS-CoV-2. In comparison, the authors found combination vaccines against diphtheria, tetanus and pertussis (DTaP vaccine) to be significant sources of possible cross-reactive immunity to SARS-CoV-2 spike protein, which included numerous CD4, CD8, and B cell epitopes (15).
We were inspired by these findings, firstly, to investigate the reaction of SARS-CoV-2 monoclonal antibody with different pathogens and vaccines, particularly DTaP. Secondly, since in additional studies we showed evidence of immune reactivity by antibodies made against pathogens and human autoantigens towards different food antigens (22, 23, 26), we extended this current research to cross-reaction between SARS-CoV-2 proteins and foods that we consume on a daily basis. This is because it has been shown that the entry of undigested food antigens into the circulation results in the production of food-specific antibodies not just in individuals with disturbed microbiota and enhanced intestinal permeability but also in healthy subjects (23–33). The cross-reactive immunity elicited by food antigens and peptides in combination with bacterial, viral, and vaccine antigens is highly important since it may protect the general population against SARS-CoV-2 and other cross-reactive viruses (15, 27).
However, because epitope sharing between two proteins by itself is not necessarily an indication of immune cross-reactivity (34), based on our earlier experience (19, 20), we applied monoclonal antibodies made against SARS-CoV-2 spike protein and nucleoprotein to DTaP and BCG vaccine antigens, as well as to several common viruses and bacteria to which our immune system has most likely been exposed during our lifetime, to determine if in fact the shared epitopes and homology actually resulted in cross-reactions (16, 17, 35–37).
We chose to use human IgG1 monoclonal antibody made against SARS-CoV-2 spike protein S1 domain and human IgG1 monoclonal antibody made against SARS-CoV-2 nucleoprotein. We used rabbit polyclonal antibodies to react with different food antigens.
Why did we choose to focus mainly on monoclonal antibodies to study reactivity by SARS-CoV-2 with pathogens and vaccines? It is true that polyclonal antibodies have the ability to detect multiple epitopes on an antigen, giving them a higher overall affinity for their antigen and, therefore, greater detection efficiency. However, the heterogenous nature of polyclonal antibodies also makes them more prone to batch-to-batch variability and cross-reactivity with other molecules, resulting in a higher background. Monoclonal antibodies, on the other hand, only detect one epitope per antigen, thus reducing cross-reactivity with other molecules. This also reduces the possibility of false positives, which is precisely the reason why we chose to focus mainly on monoclonal antibodies (38, 39).
We chose this specific antibody from Novus because it binds specifically to amino acids 318-510 in the S (spike) domain of the SARS-CoV spike protein as well as the SARS-CoV-2 (COVID-19) spike protein, giving us a broad range with which to work. More importantly, this antibody binds to the receptor binding domain (RBD), which is the most important component of the viral spike glycoprotein that is found on SARS-CoV-2. The virus uses this spike protein to anchor itself to the ACE2 receptor on human cells before infecting them. The RBD ise used as an antigen to generate neutralizing antibodies that are used in monoclonal antibody therapy against the progression of COVID-19 (40, 41).
Likewise, we used human SARS-Cov-2 nucleoprotein monoclonal antibody because it recognizes and binds the non-linear/conformational epitope of the N protein of SARS-CoV and SARS-CoV-2. This protein, as opposed to other viral components (S1, S2), can induce innate memory in human primary monocytes. This innate memory from viral nucleoproteins may contribute to the overall response to viral or bacterial infections or the response to vaccination (42).
In this present study, we found that SARS-CoV-2 spike protein specific antibody reacted most significantly with DTaP vaccine, to a somewhat lesser degree with E. faecalis bacteria which commonly resides in the human gut, to even lesser degrees with EBV-EAD, EBNA, HSV 1 + 2 CMV, and B. burgdorferi, but not significantly at all with BCG, measles, H. influenza, EBV-VCA, HHV-6, VZV, E. coli CdT and LPS (Figure 1). While the reaction of SARS-CoV-2 nucleoprotein monoclonal antibody with DTaP vaccine was the strongest, the overall reaction of anti-nucleoprotein antibody with the vaccines, viral and bacterial antigens was less strong (Figure 2). These results further confirm the findings of Reche (15), that the combination of DTaP vaccines are significant sources of T and B cell cross-reactivity to SARS-CoV-2, and cross-reactive immunity to DTaP vaccines can be protective against SARS-CoV-2.
BCG is a live attenuated strain of M. bovis used against tuberculosis. It has been shown that BCG can elicit protective heterologous immunity to SARS-CoV-2 (43–47). This protection of BCG and the induction of heterologous protective immunity against SARS-CoV-2 and other viruses is explained by the induction of trained immunity and functional reprogramming of innate immunity (15, 48–50). This conclusion was based on the observation that countries that implement BCG vaccination have fewer COVID-19 cases (43–46). Thus, we were consequently surprised when our purchased monoclonal antibodies made against both SARS-CoV-2 spike protein and nucleoprotein did not react with BCG vaccine antigens. In fact, the OD obtained from these reactions was comparable to the ELISA background, or less than 0.3. The lack of immune reactivity by SARS-CoV-2 spike protein and nucleoprotein antibodies with BCG may show that the presence of cross-reactive epitopes between two proteins does not necessarily result in cross-reactive immunity (34). However, our own results may be due to our use of human SARS-CoV-2 spike protein monoclonal antibody that binds specifically to amino acids 318-510 in the S domain of the SARS-CoV and SARS-CoV-2 spike proteins, and to our use of human SARS-Cov-2 nucleoprotein monoclonal antibody which recognizes and binds the non-linear/conformational epitope of the N protein of SARS-CoV and SARS-CoV-2. We admit that it is all too possible that BCG may be reacting with one or more of the many other SARS-CoV-2 epitopes different from the ones we used, such as the non-structural proteins also shown in our tables. Interestingly, while studying potential cross-reactivity between SARS-CoV-2 and BCG, Reche found 120, out of which 41 were for B-cell epitopes, 21 were for CD8 epitopes, and 11 were for CD4 epitopes (15).
Regarding common bacteria and viruses, including herpesviruses, we found significant reactivity by monoclonal antibody to spike protein with E. faecalis and moderate reactions with some of the herpes family of viruses (Figure 1). These reactions with this enterobacter, EBV and HSV 1 + 2 may be significant, because IgG antibody against these pathogens is found in various degrees in the blood of the general population (51). More research is needed on whether or not these common bacteria and viruses can be protective against SARS-CoV-2.
Because in our earlier studies we had shown that antibodies specific to both SARS-CoV-2 and food reacted with a variety of human tissue antigens (19–28, 30–32), in this study we hypothesized that many food proteins and peptides may share homology with SARS-CoV-2 proteins, and thus immune reaction against food proteins may be protective against SARS-CoV-2 infection. To test this hypothesis, we applied monoclonal antibody against SARS-CoV-2 spike protein to 180 different food proteins or peptides. This resulted in a significant reaction with 28 foods and weaker reactions with 2 foods, while immune reactivity with the other 150 food resulted in ODs of less than 0.56 or the mean ± 3SD of all the non-reactive foods, the ODs of which were very close to the background. Milk, α+β casein, gliadin toxic peptide, soy, pea protein, roasted almond, lentil lectin and other commonly consumed foods were among those that reacted with SARS-CoV-2 spike protein antibody (Figure 3). With SARS-CoV-2 nucleoprotein antibody the reaction was strongest with broccoli, roasted almond, cashew, soy bean, rice endochitinase, pork, pineapple bromelain, and gliadin toxic peptide. The reaction between SARS-CoV-2 nucleoprotein antibody and 20 other foods, although significant, was not as strong (Figure 4). Additional experiments performed in this research supports the hypothesis that this anti-SARS-CoV-2-specific antibody reaction with common vaccine antigens (DTaP), bacteria (E. faecalis), common viruses (EBV, HSV 1 + 2), and food proteins such as α+β casein, gliadin peptide, pea protein and others is specific:
1. Reaction of anti-SARS-CoV-2 protein antibodies resulted in a significant decline in antibody reaction with vaccine, viral and food antigens in proportion to their dilutions (Figures 5, 6)
2. Reaction of affinity-purified polyclonal antibodies made against different foods with SARS-CoV-2 spike protein and nucleoprotein resulted in a significant reaction only if the anti-food antibodies were made against the food items with which SARS-CoV-2 antibody had reacted in the earlier experiment (Figures 5–7). Rabbit polyclonal antibodies were used because monoclonal antibodies for these foods are not available.
3. Only human sera that had high levels of IgG antibodies to herpesviruses and food antigens such as gliadin, milk, α+β casein and pineapple bromelain, reacted significantly with SARS-CoV-2 spike protein (Figure 8)
4. We found significant homology between SARS-CoV-2 proteins and vaccine antigens as well as common viruses shown previously (15) and in this study (Tables 1, 2), and between SARS-CoV-2 proteins and different food items shown for the first time in this manuscript
Finally, we would like to admit that at this level we don’t know if these cross-reactive antibodies produced against a virus like EBV or foods like gliadin or α+β casein are protective or not against SARS-CoV-2. Considering the phenomenon that has been referred to as the “original antigenic sin” (17, 52), “whereby a history of a response to cross-reactive antigens can bias the response towards those antigens and inhibit the response to a new infection or vaccine” (17, 52), we should interpret our results with caution. Especially, since, very recently in different articles, it was shown that not only is EBV DNA increased in COVID-19 patients, but EBV reactivation and lytic replication induces ACE2 expression and enhances SARS-CoV-2 entry into the epithelial cell (53–55). Additionally, antibody cross-reactivity between casein and myelin associated glycoprotein (MAG) was shown to result in central nervous system demyelination (56). Thus, we do not definitively know if cross-reactive antibodies produced against viral and food epitopes that share similarity with SARS-CoV-2 proteins are helpful in controlling SARS-CoV-2 infections.
The findings presented in this manuscript indicate that cross-reactivity elicited by DTaP vaccines in combination with common herpesviruses to which we are exposed at an early age, bacteria that are part of our normal flora (E. faecalis, E. coli), and food that we consume on a daily basis may be keeping some individuals safe from COVID-19 in different parts of the world. This cross-reactivity between different pathogens and food antigens may explain why a significant percentage of the population who were repeatedly exposed to different variants of SARS-CoV-2 never became seriously ill. Additional in vivo and in vitro experiments are needed to clarify whether or not this cross-protection was due to the presence of cross-reactive antibodies or long-term memory T and B cells in the blood. Although cross-reactivity is mainly viewed as negative, this cross-reaction involving vaccine antigens, common viruses and food antigens may be protective.
● We admit that there are several limitations to our study.
● We applied human monoclonal antibodies made against SARS-CoV-2 spike protein and nucleoprotein in some experiments but used purified rabbit polyclonal antibodies in others due to lack of availability. Upon their availability, experiments should be performed comparing different clones of monoclonal antibodies and different preparations of polyclonal antibodies, testing their reactivity with different vaccines, pathogens and food proteins.
● We studied only a limited number of vaccines, bacteria and viruses in comparison to 180 different food antigens for the presence of cross-reactive immunity.
● Due to the high costs of SARS-CoV-2 recombinant antigens, for determining the specificity of antibody-antigen reaction we performed only serial dilutions and did not perform inhibition studies, which require high concentrations of antigens
● We used the BLAST sequence matching program to study the degrees of possible amino acid sequence homology shared by SARS-CoV-2 proteins with different viruses and food antigens, but not with T- and B-cell cross-reactive epitopes, as was done by Reche (15).
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
We purchased human monoclonal and rabbit polyclonal antibodies from certified, regulated commercial sources who use immunization protocols for the animals that conform to The Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, publication no 85-23, 1985. Human sera from healthy donors were obtained from commercial sources and were screened for presence or absence of antibodies against different viral and food antigens.
AV, EV, and JR designed the study. AV, EV, and AM performed the ELISA assays. AM and JR did the BLAST search. AV and EV wrote the manuscript, and the other authors read and approved it. All authors contributed to the article and approved the submitted version.
All funding was provided by Immunosciences Lab., Inc., of which the corresponding author, Aristo Vojdani, is co-owner, CEO, and technical director.
The authors wish to thank Joel Bautista for making the graphs, editing the manuscript for English corrections, and typing the manuscript for submission.
Corresponding author Aristo Vojdani is the co-owner, CEO and Technical Director of Immunosciences Lab., Inc. He is also the Chief Scientific Advisor of Cyrex Labs, LLC on a consultancy basis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1003094/full#supplementary-material
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
2. Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS and uninfected controls. Nature (2020) 584(7821):457–62. doi: 10.1038/s41586-020-2550-z
3. Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell (2020) 181(7):1489–501. doi: 10.1016/j.cell.2020.05.015
4. Huibin LV, Wu NC, Tsang OTY, Yuan M, Perera RAPM, Leung WS, et al. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. Cell Rep (2020) 31(9):107725. doi: 10.1016/j.celrep.2020.107725
5. Sette A, Crotty S. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat Rev Immunol (2020) 20:457–8. doi: 10.1038/s41577-020-0389-z
6. Mateus J, Grifoni A, Tarke A, Sidney J, Ramirez SI, Dan JM, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science (2020) 370(6512):89–94. doi: 10.1126/science.abd3871
7. Quiros-Fernandez I, Poorebrahim M, Fakhr E, Cid-Arregui A. Immunogenic T cell epitopes of SARS-CoV-2 are recognized by circulating memory and naive CD8 T cells of unexposed individuals. EBioMedicine (2021) 72:103610. doi: 10.1016/j.ebiom.2021.103610
8. Meckiff BJ, Ramirez-Suástegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H, et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4+ T cells in COVID-19. Cell (2020) 183(5):1340–53. doi: 10.1016/j.cell.2020.10.001
9. Weiskopf D, Schmitz KS, Raadsen MP, Grifoni A, Okba NMA, Endeman H, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol (2020) 5(48):eabd2071. doi: 10.1126/sciimmunol.abd2071
10. Braun J, Loyal L, Frentsch M, Wendisch D, Georg P, Kurth F, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature (2020) 587:270–4. doi: 10.1038/s41586-020-2598-9
11. Nickbakhsh S, Ho A, Marques DFP, McMenamin J, Gunson RN, Murcia PR. Epidemiology of seasonal coronaviruses: Establishing the context for the emergence of coronavirus disease 2019. J Infect Dis (2020) 222:17–25. doi: 10.1093/infdis/jiaa185
12. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Protecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science (2020) 368:860–8. doi: 10.1126/science.abb5793
13. Lipsitch M, Grad YH, Sette A, Crotty S. Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nat Rev Immunol (2020) 20(11):709–13. doi: 10.1038/s41577-020-00460-4
14. Grifoni A, Sidney J, Vita R, Peters B, Crotty S, Weiskopf D, et al. SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19. Cell Host Microbe (2021) 29(7):1076–92. doi: 10.1016/j.chom.2021.05.010
15. Reche PA. Potential cross-reactive immunity to SARS-CoV-2 from common human pathogens and vaccine. Front Immunol (2020) 11:586984. doi: 10.3389/fimmu.2020.586984
16. Agrawal B. Heterologous immunity: Role in natural and vaccine-induced resistance to infections. Front Immunol (2019) 10:2631. doi: 10.3389/fimmu.2019.02631
17. Welsh RM, Fujinami RS. Pathogenic epitopes, heterologous immunity and vaccine design. Nat Rev Microbiol (2007) 5(7):555–63. doi: 10.1038/nrmicro1709
18. Gu XX, Plotkin SA, Edwards KM, Sette A, Mills KHG, Levy O, et al. Waning immunity and microbial vaccines – workshop of the national institute of allergy and infectious diseases. Clin Vaccine Immunol (2017) 24(7):e00034–17. doi: 10.1128/CVI.00034-17
19. Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol (2020) 217:108480. doi: 10.1016/j.clim.2020.108480
20. Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: Implications for autoimmune diseases. Front Immunol (2021) 11:61708919. doi: 10.3389/fimmu.2020.617089
21. Vojdani A, Tarash I. Cross-reaction between gliadin and different food and tissue antigens. Food Nutr Sci (2013) 44:20–32. doi: 10.4236/fns.2013.41005
22. Vojdani A, Vojdani E. Immunoreactivity of anti-AβP-42 specific antibody with toxic chemicals and food antigens. J Alzheimers Dis Parkinsonism (2018) 8(3):441. doi: 10.4172/2161-0460.1000441
23. Vojdani A, Turnpaugh CC. Antibodies against group a streptococcus, dopamine receptors, and ganglioside GM1 cross-react with a variety of food antigens, potentially interfering with biomarkers for PANS and PANDAS. Biomarkers Neuropsychiatry (2020) 3:100023. doi: 10.1016/j.bionps.2020.100023
24. Vojdani A, Gushgari LR, Vojdani E. Interaction between food antigens and the immune system: Association with autoimmune disorders. Autoimmun Rev (2020) 19(3):102459. doi: 10.1016/j.autrev.2020.102459
25. Vojdani A. Reaction of food-specific antibodies with different tissue antigens. Int J Food Sci Tech (2020) 55(4):1800–15. doi: 10.1111/ijfs.14467
26. Vojdani A, Lerner A, Vojdani E. Cross-reactivity and sequence homology between alpha-synuclein and food products: A step further for parkinson's disease synucleinopathy. Cells (2021) 10(5):1111. doi: 10.3390/cells10051111
27. Vojdani A, Monro J, Lanzisera F, Sadeghi H. Serological cross-reactivity between viruses and their contribution to autoimmunity. Autoimmun Rev (2021) 20:102840. doi: 10.1016/j.autrev.2021.102840
28. Kharrazian D, Herbert M, Vojdani A. Detection of islet cell immune reactivity with low glycemic index foods – is this a concern for type 1 diabetes? J Diabetes Res (2017) 2017:4124967. doi: 10.1155/2017/4124967
29. Riccio P, Rossano R. Undigested food and gut microbiota may cooperate in the pathogenesis of neuroinflammatory diseases: A matter of barriers and a proposal on the origin of organ specificity. Nutrients (2019) 11:2714. doi: 10.3390/nu11112714
30. Hvatum M, Kanerud L, Hallgren R, Brandtzaeg P. The gut-joint axis: Cross-reactive food antibodies in rheumatoid arthritis. Gut (2006) 55:1240–7. doi: 10.1136/gut.2005.076901
31. Vojdani A, Mukherjee PS, Berookhim J, Kharrazian D. Detection of antibodies against human and plant aquaporins in patients with multiple sclerosis. Autoimmun Dis (2015) 2015:905208. doi: 10.1155/2015/905208
32. Vojdani A. Molecular mimicry as a mechanism for food immune reactivities and autoimmunity. ATHM (2015) 21:34–45.
33. Vojdani A, Vojdani E, Kharrazian D. Fluctuation of zonulin levels in blood versus stability of antibodies. World J Gastroenterol (2017) 23:5669–79. doi: 10.3748/wjg.v23.i31.5669
34. Lee CH, Salio M, Napolitani G, Ogg G, Simmons A, Koohy H. Predicting cross-reactivity and antigen-specificity of T cell receptors. Front Immunol (2020) 11:565096. doi: 10.3389/fimmu.2020.565096
35. Mosca A, LeClerc M, Hugot JP. Gut microbiota diversity and human diseases: Should we reintroduce key predators in our ecosystem? Front Microbiol (2016) 7:455. doi: 10.3389/fmicb.2016.00455
36. Carrasco Pro S, Lindestam Arlehamn CS, Dhanda SK, Carpenter C, Lindvall M, Faruqi AA, et al. Microbiota epitope similarity either dampens or enhances the immunogenicity of disease-associated antigenic epitopes. PLoSOne (2018) 13:e0196551. doi: 10.1371/journal.pone.0196551
37. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell (2014) 157:121–41. doi: 10.1016/j.cell.2014.03.011
38. Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR J (2005) 46:258–68. doi: 10.1093/ilar.46.3.258
39. Busby M, Xue C, Li C, Farjoun Y, Gienger E, Yofe I, et al. Systemic comparison of monoclonal versus polyclonal antibodies for mapping histone modifications by ChIP-seq. Epigenet Chromatin (2016) 9:49. doi: 10.1186/s13072-016-0100-6
40. Makdasi E, Levy Y, Alcalay R, Noy-Porat T, Zahavy E, Mechaly A, et al. Neutralizing monoclonal anti-SARS-CoV-2 antibodies isolated from immunized rabbits define novel vulnerable spike-protein epitope. Viruses (2021) 13:566. doi: 10.3390/v13040566
41. Min L, Sun Q. Antibodies and vaccines target RBD of SARS-CoV-2. Front Mol Biosci (2021) 8:671633. doi: 10.3389/fmolb.2021.671633
42. Urban P, Italiani P, Boraschi D, Gloria S. The SARS-CoC-2 nucleoprotein induces innate memory in human monocytes. Front Immunol (2022) 13:963627. doi: 10.3389/fimmu.2022.963627
43. Miller A, Reandelar MJ, Fasciglione K, Roumanova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv (2020). doi: 10.1101/2020.03.24.20042937
44. Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG vaccination to reduce the impact of COVID-19. Lancet (2020) 396:1546–6. doi: 10.1016/S0140-6736(20)31025-4
45. O’Neill LAJ, Netea MG. BCG-Induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol (2020) 20:335–7. doi: 10.1038/s41577-020-0337-y
46. Wickramasinghe D, Wickramasinghe N, Kamburugamuwa SA, Arambepola C, Samarasekera N. Correlation between immunity from BCG and the morbidity and mortality of COVID-19. Trop Diseases Travel Med Vaccines (2020) 6:17. doi: 10.1186/s40794-020-00117-z
47. Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis (2014) 58:470–80. doi: 10.1093/cid/cit790
48. Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep (2016) 17:2562–71. doi: 10.1016/j.celrep.2016.11.011
49. Moorlag S, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect (2019) 25:1473–8. doi: 10.1016/j.cmi.2019.04.020
50. Sanchez-Ramon S, Conejero L, Netea MG, Sancho D, Palomares I, Subiza JL. Trained immunity-based vaccines: A new paradigm for the development of broad-spectrum anti-infectious formulations. Front Immunol (2018) 9:2936. doi: 10.3389/fimmu.2018.02936
51. Smatti MK, Yassine HM, AbuOdeh R, Almarawani A, Taleb SA, Althani AA, et al. Prevalence and molecular profiling of Epstein Barr virus (EBV) among healthy blood donors from different nationalities in Qatar. PloS One (2017) 12(12):e0189033. doi: 10.1371/journal.pone.0189033
52. Zhang A, Stacey HD, Mullarkey CE, Miller MS. Original antigenic sin: How first exposure shapes lifelong anti-influenza virus immune responses. J Immunol (2019) 202(2):335–40. doi: 10.4049/jimmunol.1801149
53. Paoilucci S, Cassaniti I, Novazzi F, Fiorina L, Pirallas A, Comolli G, et al. San Matteo pavia COVID-19 task force. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int J Infect Dis (2021) 104:315–9. doi: 10.1016/j.ijid.2020.12.051
54. Gold JE, Okyay RA, Licht WE, Hurley DJ. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens (2021) 10(6):763. doi: 10.3390/pathogens10060763
55. Verma D, Church TM, Swaminathan S. Epstein-Barr Virus lytic replication induces ACE2 expression and enhances SARS-CoV-2 pseudotyped virus entry in epithelial cells. J Virol (2021) 95(13):e0019221. doi: 10.1128/JVI.00192-21
Keywords: SARS-CoV-2, pathogens, vaccines, food antigens, molecular mimicry, antibodies
Citation: Vojdani A, Vojdani E, Melgar AL and Redd J (2022) Reaction of SARS-CoV-2 antibodies with other pathogens, vaccines, and food antigens. Front. Immunol. 13:1003094. doi: 10.3389/fimmu.2022.1003094
Received: 25 July 2022; Accepted: 23 August 2022;
Published: 23 September 2022.
Edited by:
Richard Kennedy, Mayo Clinic, United StatesReviewed by:
Peter J. Eggenhuizen, Monash University, AustraliaCopyright © 2022 Vojdani, Vojdani, Melgar and Redd. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aristo Vojdani, ZHJhcmlAbXNuLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.