
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 05 October 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1002938
This article is part of the Research Topic Immune Drivers of Malignancy View all 7 articles
 Xiaorong Dong1†
Xiaorong Dong1† Yu Huang1†
Yu Huang1† Tienan Yi2
Tienan Yi2 Chunhong Hu3
Chunhong Hu3 Quanli Gao4
Quanli Gao4 Yuan Chen5
Yuan Chen5 Jing Zhang6
Jing Zhang6 Jianhua Chen7
Jianhua Chen7 Li Liu1
Li Liu1 Rui Meng1
Rui Meng1 Sheng Zhang1
Sheng Zhang1 Xiaofang Dai1
Xiaofang Dai1 Shihong Fei1
Shihong Fei1 Yang Jin8
Yang Jin8 Ping Yin9
Ping Yin9 Yanping Hu6*
Yanping Hu6* Gang Wu1*
Gang Wu1*Background: Preclincal studies showed the promising efficacy of tumor cell-derived microparticles packaging methotrexate (TMPs-MTX) to treat advanced non-squamous non-small cell lung cancer (NSCLC) with malignant pleural effusion (MPE).
Methods: This randomized, double-blind, placebo-controlled study was conducted at six hospitals in China from 20 July 2015 to 25 April 2019. Patients newly diagnosed with non-squamous NSCLC with MPE were randomly assigned to receive TMPs-MTX (group A) or saline (group B). Patients in both groups received pemetrexed (500 mg/m2 d1) and cisplatin (75 mg/m2 in total for d1-d2). Intrapleural infusion (50 mL saline containing 5 units of TMPs-MTX per perfusion, once every 48 hours, six total perfusions) was initiated on day 5 after pemetrexed-cisplatin chemotherapy. The primary outcome was the objective response rate (ORR) of MPE. Secondary outcomes included the ORR of target lesions, progression-free survival (PFS), overall survival (OS), toxicity, and pleural fluid properties.
Results: A total of 86 patients were enrolled in this study and randomly assigned to either group A or group B. Of these, 79 patients were evaluable for response. The ORR of MPE in group A was significantly higher than that in group B (82.50% vs. 58.97%, P = 0.0237). The ORR of target lesions was 25.64% in group A and 20.51% in group B (P = 0.5909), respectively. With a median follow-up time of 18.8 months, median PFS were 6.4 (95% CI, 4.5-12.3) months in group A and 7.3 (95% CI, 6.1-10.4) months in group B (P = 0.6893), and median OS were 19.9 (95% CI, 17.1-28.5) months and 17.5 (95% CI, 11.6-25.0) months (P = 0.4500), respectively. The incidence rates of adverse events were similar in the two groups. The most common treatment-related adverse events were chemotherapy-induced toxicities, including fever, gastrointestinal reactions, hepatic dysfunction, and leukopenia.
Conclusion: Intrapleural infusion of TMPs-MTX combined with pemetrexed-cisplatin chemotherapy is safe and effective against MPE in patients with advanced non-squamous NSCLC.
Clinical trial registration: http://www.chictr.org.cn (ChiCTR-ICR-15006304).
Malignant pleural effusion (MPE) is common among patients with lung cancer, who account for approximately 30%–40% of MPE cases (1). MPE symptoms include chest distress, shortness of breath, palpitation, pain, and inability to lie prostrate. These symptoms further impact the quality of life of patients with lung cancer (2–4). The prognosis of MPE is poor, with a median survival time of 3 to 12 months (5). MPE prognosis is even worse in patients with lung cancer (5). MPE treatment methods include systemic chemotherapy, molecular targeted therapy such as tyrosine kinase inhibitors, immunotherapy and locoregional therapies (6). Locoregional MPE treatment involves the local perfusion of talc, chemotherapeutic agents, biological agents, and anti-angiogenic drugs into the pleural cavity (7–10). As there are no standardized locoregional therapies for MPE, systemic therapies and drainage through the indwelling pleural catheter are the main treatment methods. New therapeutic approaches are urgently needed for the treatment of MPE.
Microparticles (MPs) are extracellular vesicles with a size ranging between 100 and 1,000 nm. These vesicles are shed by direct budding of the cell membrane under physiological or pathological conditions. MPs regulate the communication between cells by transferring signaling molecules (proteins, lipids, nucleic acids) from donor cells to recipient cells (11). Tumor cells are able to release extracellular vesicles labeled as tumor MPs. These MPs are promising natural carriers to deliver chemotherapeutic drugs or oncolytic viruses to tumor cells (12, 13). Tumor cell-derived MPs (TMPs) can act as a cell-free tumor vaccine and stimulate dendritic cells via cGAS/STING signaling (14, 15). Incorporating drugs such as methotrexate (MTX) into TMPs may yield chemo-immunotherapeutic, dual-functional MPs.
TMPs packaging methotrexate (TMPs-MTX) have been proved to be safe and effective in killing tumor cells and reversing drug resistance (12, 16, 17). The exploratory clinical study showed that TMPs-MTX alleviated MPE in patients with lung cancer by modulating the pleural immune microenvironment (17). Based on the potential benefit of TMPs-MTX, we conducted a multicenter, randomized clinical trial to investigate the efficacy and safety of TMPs-MTX combined with pemetrexed-cisplatin in patients with MPE and advanced non-squamous non-small cell lung cancer (NSCLC).
This multicenter, randomized, double-blind, placebo-controlled study was carried out in six hospitals from 20 July 2015 to 25 April 2019. A total of 86 patients with MPE were enrolled. Eligible patients were 18–70 years old and were newly diagnosed with advanced non-squamous NSCLC and MPE. All patients had malignant cells in the pleural fluid. All study subjects had Karnofsky performance status (KPS) scores ≥ 70. Exclusion criteria were as follows: prior treatment with chemotherapy or intrapleural infusion, pregnancy, lactation, history of drug allergies or allergic constitution, and severe underlying diseases (e.g., cardiac and pulmonary failure, hepatic dysfunction, and renal dysfunction). The study was conducted in compliance with the Good Clinical Practice principles and the Declaration of Helsinki. All patients provided informed consent. The study was approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology and was registered on the Chinese Clinical Trial Registry (ChiCTR-ICR-15006304) on April 20, 2015.
This was a randomized block study, with six patients per block and an allocation ratio of 1:1 in the microparticles group and the placebo group. The statisticians used SAS software to generate random number tables and the investigators were blinded to treatment allocation. Patients were randomly assigned (1:1) to receive TMPs-MTX or 0.9% saline. Patients in both groups received the same systemic chemotherapy regimen (pemetrexed 500 mg/m2 d1, cisplatin 75 mg/m2 in total for d1–d2). The indwelling pleural catheter for intrapleural infusion was inserted as per standard clinical practice. The first intrapleural infusion (50 mL per dose) was conducted at day 5 post-chemotherapy, once every other day, six times consecutively. Patients in group A were treated with 50 mL saline containing 5 units of TMPs-MTX (5 μg methotrexate/1 × 107 vesicles/unit). Patients in Group B were treated with 50 mL saline as a control.
TMPs-MTX (manufacturers specifications: 50 mL/bag) were dissolved in 0.9% sodium chloride injection solution (50 mL/bag; Baite company, Shanghai, China; approval number: GuoYaoZhunZi-H19994067) so that each dose contained 5 units of methotrexate vesicles (5 μg methotrexate/1 × 107 vesicles/unit). The solution was kept at 2–8°C and was returned to room temperature before use.
Objective response rate (ORR) for MPE was the primary study outcome. Treatment efficacy was evaluated using the World Health Organization (WHO) evaluation criteria (18). The volume of pleural effusion was measured on computed tomography (CT) layer by layer using volume rendering in the American general post-processing workstation (GE Advance Workstation 4.5), and was visualized using post-processing software. Changes in the pleural effusion before and after treatment were determined. Complete remission (CR) was defined as complete resolution of pleural effusion for at least four weeks. Partial remission (PR) was defined as > 50% reduction in pleural effusion for at least four weeks, and stable disease (SD) was defined as < 50% reduction in pleural effusion with partial remission of clinical symptoms. No change (NC) was defined as no significant decrease or increase in the volume of MPE. The ORR of pleural effusion was calculated as CR + PR.
Secondary outcomes included the ORR of target lesions, progression-free survival (PFS), overall survival (OS), KPS score, the variation in pleural fluid properties, and treatment toxicity. Tumor markers and other biomarkers in the blood and pleural effusions were assessed before and after treatment. Tumor response was evaluated by the response evaluation criteria in solid tumors (RECIST 1.1.) (19). The target lesions met the definition of measurability as described in RECIST 1.1, including primary lung lesions, lymph nodes and metastases. The ORR of target lesions was defined as the combined proportion of patients with CR or PR. PFS was calculated from the first day of chemotherapy to the date of disease progression. OS was defined from the first day of chemotherapy to death due to any cause. The severity of adverse events was scored using the Common Terminology Criteria for Adverse Events Version 5.0 (CTCAE v5.0).
Categorical variables were compared using either the chi-square test or the Wilcoxon rank-sum test. Numerical variables were compared using the independent sample t-test. A superiority trial was conducted to compare the efficacy of the treatment over the placebo. Treatment efficacy was compared using the Cochran-Mantel-Haensel method to adjust for the central effect. The chi-square test or the Fisher exact test was used to compare adverse events between the two groups and describe the changes in laboratory markers before and after treatment. Two-sided P-values < 0.05 were considered statistically significant. Statistical analyses were conducted using SAS version 9.4 (SAS Institute).
This study included 86 patients with advanced non-squamous NSCLC and MPE. Patients in group A (n = 43) were treated with TMPs-MTX, and patients in group B (n = 43) received saline (Figure 1). The full analysis set and per-protocol set consisted of 79 (91.86%) patients who completed their treatment and attended all follow-up visits. Six patients withdrew and one patient was lost to follow-up. Therefore, seven patients (three from group A and four from group B) were excluded from our analyses (Figure 1). No significant differences in the baseline characteristics (age, gender, smoking history, Eastern Cooperative Oncology Group (ECOG) Performance Status, histological type, baseline volume of pleural effusion, metastatic sites and gene mutations) were observed between the two groups (P > 0.05; Table 1).
Among the 40 patients in group A, there were 10 CR cases, 23 PR cases, 1 SD case, and 6 NC cases. Among the 39 patients in group B, there were 6 CR cases, 17 PR cases, 8 SD cases, and 8 NC cases. The ORR for MPE in group A was significantly higher than that in group B (82.50% vs. 58.97%; P = 0.0237; Table 2). These results suggest that TMPs-MTX alleviate MPE in patients with advanced non-squamous NSCLC.
Among the 39 evaluable patients of target lesions in group A, there were 0 CR cases, 10 PR cases, 28 SD cases, and one PD case. The ORR was 25.64%, and the disease control rate (DCR; CR+PR+SD) was 97.44%. Among the 39 patients in group B, the numbers of patients with CR, PR, SD, and PD were 0, 8, 28, and 3, respectively. The ORR and DCR were 20.51% and 92.31%, respectively (Table 2). Both ORR and DCR in group A were higher than those in group B, although their differences were not statistically significant (P = 0.5909 and P = 0.6077, respectively).
With a median follow-up time of 18.8 months, the median OS in group A and group B were 19.9 (95% CI, 17.1-28.5) and 17.5 (95% CI, 11.6-25.0) months, respectively (Figure 2); the difference in OS was not statistically significant (P = 0.4500). The half-year OS (100.00% vs. 89.74%) and one-year OS (77.50% vs. 58.97%) rates in group A were higher than those in group B, although their differences were not statistically significant. The median PFS were 6.4 (95% CI, 4.5-12.3) months in group A and 7.3 (95% CI, 6.1-10.4) months in group B (P = 0.6893; Figure 3).
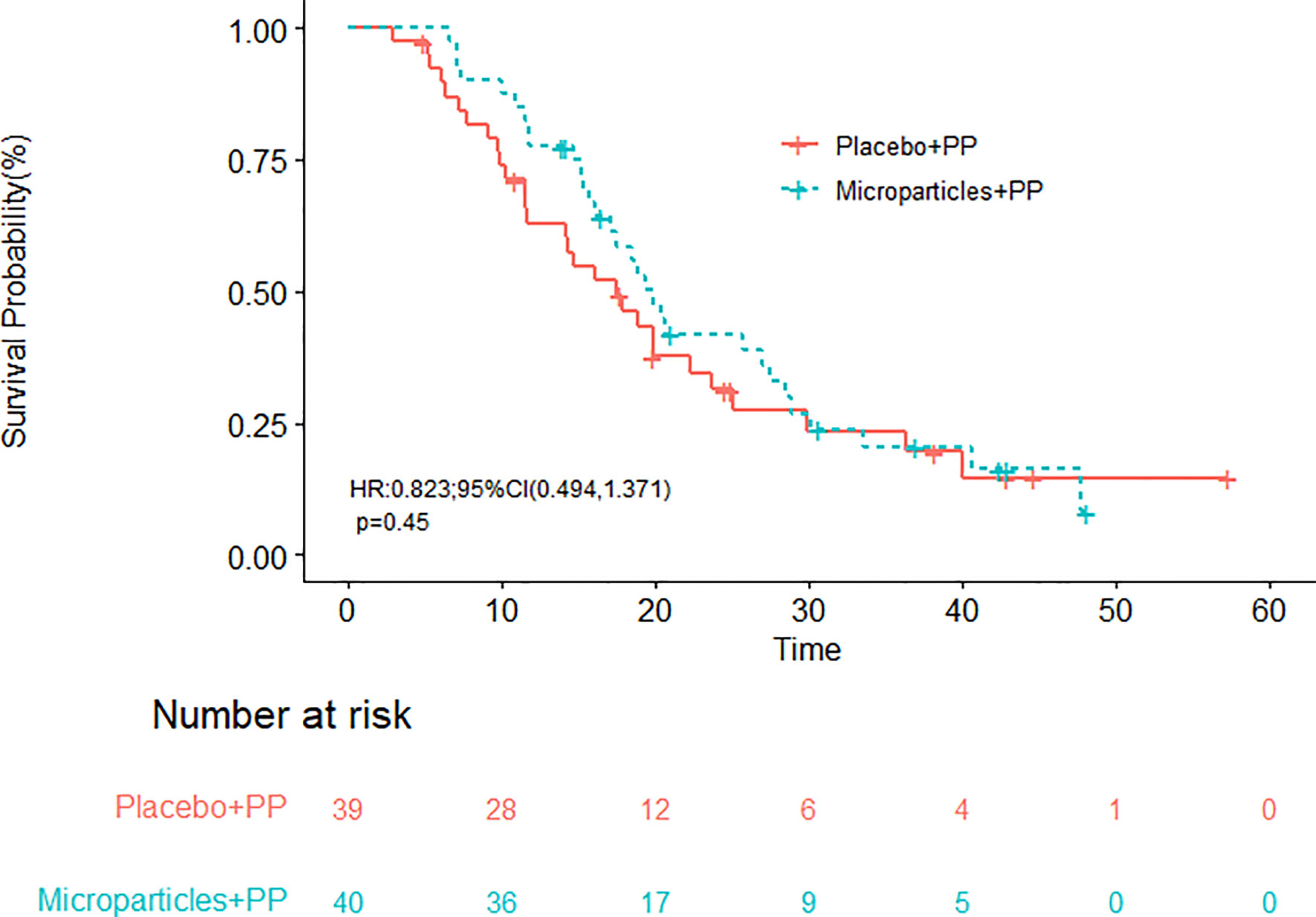
Figure 2 Overall survival of patients receiving the tumor cell-derived microparticles packaging methotrexate (TMPs-MTX) or saline combined with pemetrexed-cisplatin (PP) chemotherapy.
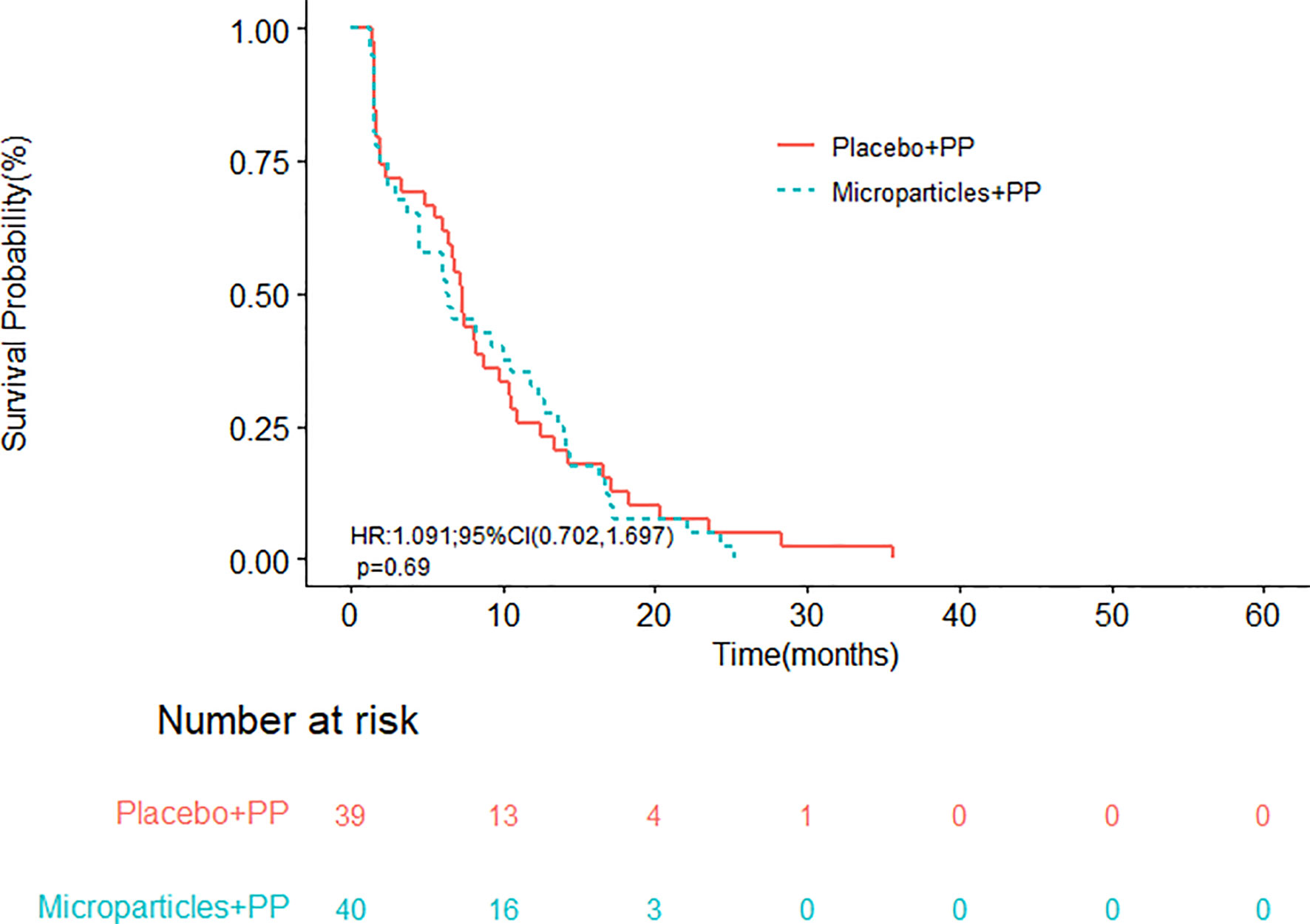
Figure 3 Progression-free survival of patients receiving the tumor cell-derived microparticles packaging methotrexate (TMPs-MTX) or saline combined with pemetrexed-cisplatin (PP) chemotherapy.
There was no significant difference in the KPS scores before and after treatment between the two groups (P >0.05). Moreover, we found no significant differences in the blood levels of the tumor markers CEA, CYFRA21-1, CA125, and CA19-9 between the two groups (P >0.05, Supplementary Table 1). Furthermore, there were no statistically significant differences in Rivalta test parameters (pleural fluid routine) between the two groups (Supplementary Table 2). Similarly, no significant differences in the levels of total protein, glucose, lactate dehydrogenase, and CEA in the pleural fluid were observed between the two groups (Supplementary Table 3).
A total of 56 adverse events (cumulative number: 194 cases) was reported, with 38 drug-related adverse events (cumulative number: 124 cases). 30 adverse events were reported in group A, and 26 adverse events were reported in group B. No statistically significant differences were observed in the incidence of adverse events between the two groups (P = 0.4647). There were 21 (50%) drug-related adverse events in group A and 17 (39.53%) drug-related adverse events in group B. The differences in the rates of drug-related adverse events between the two groups were also not statistically significant (P = 0.4891). 7 serious adverse events were reported: 4 in group A and 3 in group B. The incidence of serious adverse events did not differ significantly between the two groups. One drug-related serious adverse event was reported in group A; this adverse event was hepatic dysfunction (grade 3). The most common treatment-related adverse events were chemotherapy-induced toxicity, including fever, gastrointestinal reactions, hepatic dysfunction, leukopenia, asthenia, anemia, and hypoalbuminemia. The incidence rates of fever and hepatic dysfunction were slightly higher in group A than in group B (Table 3).
In this study, we evaluated the efficacy of the TMPs-MTX in the treatment of MPE in patients with advanced non-squamous NSCLC. We found that, compared with the control treatment, TMPs-MTX significantly alleviated MPE in patients with NSCLC and revealed good safety. Most adverse events were manageable. Although no significant improvements were observed in the secondary outcomes, including the ORR of target lesions, OS and PFS. Intrapleural infusion of TMPs-MTX still provides a new locoregional treatment option for patients with MPE.
Indwelling pleural catheters are often used to control MPE symptoms in patients with solid tumors (20–23). However, this method increases the risk of certain infections blockage, pneumothorax and catheter track metastasis (24, 25). Furthermore, no significant difference in the relief of breathlessness was observed between indwelling catheter alone and talc administration through an indwelling pleural catheter (26). In the previous studies of MPE, patients with different types of cancers were recruited, including lung cancer, breast cancer, mesothelioma, ovarian cancer and so on. As the systemic treatment regimens were non-uniform among these previous studies, the therapeutic effect of systemic treatment on MPE could not be comparable, and this may lead to research bias. In the management of MPE caused by NSCLC, bevacizumab intrapleural infusion showed higher response rate than intravenous infusion (27). Cytotoxic drugs such as nedaplatin or cisplatin were also infused intrapleurally for controlling MPE, but only 50%–60% patients responded to this treatment (28). These cytotoxic drugs might cause gastrointestinal side effects or other toxicities (28). Debulking surgery and hyperthermic intrathoracic chemotherapy achieved encouraging outcome in the treatment of selected patients with NSCLC and MPE (29). But the quality of evidence is still weak to confirm the effectiveness of this treatment (29). Lung cancer is one of the most common causes of MPE. The median survival time of lung cancer patients with pleural effusion was found to be only 74 days (5). Thus, novel therapeutic approaches for the treatment of MPE in patients with NSCLC are urgently required.
A recent study by Guo et al. (17) showed that TMPs-MTX were safe and effective in 11 patients with advanced lung cancer and MPE. The ORR was 90.91%, including 4 CRs and 6 PRs. The median time of pleurodesis was seven days. Long-term follow-up revealed that 9 of 11 patients did not need further therapeutic pleural drainage until death (17). The viability of malignant cells in the MPE was also tested in previous research. CD45- cells, which were confirmed to be tumor cells by HE staining, were efficiently removed from the MPE in TMPs-MTX treated patients. However, in the saline-treated patients, the proportions of CD45- cells were not altered (30). Our study is the first randomized controlled trial evaluating the efficacy of TMPs-MTX in the treatment of MPE. We found that the combination of TMPs-MTX with pemetrexed-cisplatin significantly alleviated MPE in patients with advanced non-squamous NSCLC. However, patients in both groups received first-line pemetrexed-cisplatin chemotherapy. The same systemic therapy contributed to similar ORR of target lesions and PFS between these two groups. In addition, some patients with epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) alterations might be treated with EGFR or ALK tyrosine kinase inhibitors (TKIs) after disease progression of chemotherapy. Therefore, the OS was also influenced by second-line treatment. Patient reported outcomes including symptoms and quality-of-life scores improvement for intrapleural infusion of TMPs-MTX might be worth exploring.
The biological mechanisms underlying the therapeutic effects of TMPs-MTX (Supplementary Figure 1) have been explored in recent studies (12, 16, 31). It has been reported that intrapleural infusion of TMPs-MTX significantly decreased the numbers of tumor cells and CD163+ macrophages in the pleural immune microenvironment. TMPs-MTX also stimulated IL-2 secretion in CD4+ T cells and IFN-γ secretion in CD8+ T cells (17). Upon methotrexate-loaded vesicle entry into the MPE, the vesicles were recognized and engulfed by tumor cells, leading to tumor cell death (17). Phagocytosis of TMPs-MTX by macrophages induced the release of CXCL1 and CXCL2, promoting neutrophil chemotaxis toward the MPE (30). These activated neutrophils enhanced the elimination of tumor cells. Moreover, activated neutrophils were reported to release web-like DNA-containing structures (i.e., neutrophil extracellular traps) to entrap pathogens and inhibit endothelial damage, thereby attenuating the inflammatory response in MPE (30).
Each unit of drug loaded microparticles contained about 5 ± 1ug of methotrexate. The earliest safe dose exploration test began with the perfusion of 3 units TMPs-MTX for each time, while 5 units were the second dose group. The previous study showed that intrapleural delivery of 5 units TMPs-MTX were safe and effective in lung cancer patients with MPE (17). The adverse reactions were only grade 1-2 (17). Therefore, the same dose of 5 units of TMPs-MTX was used in this clinical study and TMPs-MTX were well-tolerated. There were 38 drug-related adverse events in this study. Notably, we found no significant differences in the incidence of adverse events between the two groups, suggesting that TMPs-MTX were safe in patients with advanced non-squamous NSCLC.
Limitations of this study included the small sample size. And the immune related factors in pleural fluid or blood were not tested. Additionally, participant-reported health-related quality of life and symptoms in these two groups were not compared in this study. But many secondary outcomes were discussed, including KPS scores, levels of tumor markers in the blood, and pleural fluid properties. Despite these limitations, our study is one of the very few randomized controlled trials focusing on MPE in patients with advanced non-squamous NSCLC.
In conclusion, MPs might be an attractive drug delivery system and TMPs-MTX was reported to be chemo-immunotherapeutic, dual-functional in previous studies. Our findings suggest that intrapleural infusion of TMPs-MTX is an effective and safe approach to treat MPE in patients with advanced non-squamous NSCLC. MPE often require pleural intervention for symptom control. TMPs-MTX may provide a new locoregional strategy to treat malignancies with MPE.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
XDo (1st author) conceived and designed the study, reviewed and edited the manuscript. YuH (2nd author) interpreted the data and wrote the manuscript. TY, CH, QG, YC, JZ, LL, RM, SZ, XDa (12th author) and SF contributed to patient management. JC and YJ interpreted the data. PY conducted the statistical analysis. YaH (16th author) provided effective suggestion for treatment and modification, interpreted the data. GW was involved in the study concept and design, critical revision of the manuscript and study supervision. All authors contributed to the article and approved the final version of the manuscript.
This study was supported by the grants from the National Key R&D Program of China (Grant No. 2019YFC1316205) and grants from Key Research and Develop Program of Hunan Province, China (Grant No. 2017WK2061) and the Key Research and Development Program of Hubei (Grant No. 2020BCA068).
The authors thank all the patients, their families and the clinical study teams who participated in the study. We thank Yao Xiao (Department of Epidemiology and Biostatistics, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology) for the assistance of statistical data analysis. The study was also sponsored by Soundny (Sheng-Qi-An) Biotech.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SF declared a shared parent affiliation with the author CH to the handling editor at the time of the review
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1002938/full#supplementary-material
1. Antunes G, Neville E, Duffy J, Ali N. BTS guidelines for the management of malignant pleural effusions. Thorax (2003) 58:ii29–38. doi: 10.1136/thorax.58.suppl_2.ii29
2. Thomas R, Jenkins S, Eastwood PR, Lee YC, Singh B. Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med (2015) 21:338–45. doi: 10.1097/MCP.0000000000000174
3. Iyer NP, Reddy CB, Wahidi MM, Lewis SZ, Diekemper RL, Feller-Kopman D, et al. Indwelling pleural catheter versus pleurodesis for malignant pleural effusions. a systematic review and meta-analysis. Ann Am Thorac Soc (2019) 16:124–31. doi: 10.1513/AnnalsATS.201807-495OC
4. Rahman NM, Pepperell J, Rehal S, Saba T, Tang A, Ali N, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: The TIME1 randomized clinical trial. JAMA (2015) 314:2641–53. doi: 10.1001/jama.2015.16840
5. Clive AO, Kahan BC, Hooper CE, Bhatnagar R, Morley AJ, Zahan-Evans N, et al. Predicting survival in malignant pleural effusion: Development and validation of the LENT prognostic score. Thorax (2014) 69:1098–104. doi: 10.1136/thoraxjnl-2014-205285
6. Tissot C, Gay P, Brun C, Froudarakis ME. Novel insights into the systemic treatment of lung cancer malignant pleural effusion. Clin Respir J (2019) 13:131–8. doi: 10.1111/crj.13005
7. Bhatnagar R, Keenan EK, Morley AJ, Kahan BC, Stanton AE, Haris M, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med (2018) 378:1313–22. doi: 10.1056/NEJMoa1716883
8. Skok K, Hladnik G, Grm A, Crnjac A. Malignant pleural effusion and its current management: A review. Medicina (2019) 55:490. doi: 10.3390/medicina55080490
9. Nie K, Zhang Z, You Y, Zhuang X, Zhang C, Ji Y. A randomized clinical study to compare intrapleural infusion with intravenous infusion of bevacizumab in the management of malignant pleural effusion in patients with non-Small-Cell lung cancer. Thorac Cancer (2020) 11:8–14. doi: 10.1111/1759-7714.13238
10. Jones DR, Taylor MD, Petroni GR, Shu J, Burks SG, Daniel TM, et al. Phase I trial of intrapleural docetaxel administered through an implantable catheter in subjects with a malignant pleural effusion. J Thorac Oncol (2010) 5:75–81. doi: 10.1097/JTO.0b013e3181c07ddc
11. Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-Cell communication. Leukemia (2006) 20:1487–95. doi: 10.1038/sj.leu.2404296
12. Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun (2012) 3:1282. doi: 10.1038/ncomms2282
13. Ran L, Tan X, Li Y, Zhang H, Ma R, Ji T, et al. Delivery of oncolytic adenovirus into the nucleus of tumorigenic cells by tumor microparticles for virotherapy. Biomaterials (2016) 89:56–66. doi: 10.1016/j.biomaterials.2016.02.025
14. Zhang H, Tang K, Zhang Y, Ma R, Ma J, Li Y, et al. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer Immunol Res (2015) 3:196–205. doi: 10.1158/2326-6066.CIR-14-0177
15. Ma J, Wei K, Zhang H, Tang K, Li F, Zhang T, et al. Mechanisms by which dendritic cells present tumor microparticle antigens to CD8 + T cells. Cancer Immunol Res (2018) 6:1057–68. doi: 10.1158/2326-6066.CIR-17-0716
16. Ma J, Zhang Y, Tang K, Zhang H, Yin X, Li Y, et al. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res (2016) 26:713–27. doi: 10.1038/cr.2016.53
17. Guo M, Wu F, Hu G, Chen L, Xu J, Xu P, et al. Autologous tumor cell-derived microparticle-based targeted chemotherapy in lung cancer patients with malignant pleural effusion. Sci Transl Med (2019) 11:eaat5690. doi: 10.1126/scitranslmed.aat5690
18. Palmer MK. WHO handbook for reporting results of cancer treatment. Br J Cancer (1982) 45: 484–5. doi: 10.1038/bjc.1982.83
19. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (Version 1.1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
20. Fysh ETH, Waterer GW, Kendall PA, Bremner PR, Dina S, Geelhoed E, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest (2012) 142:394–400. doi: 10.1378/chest.11-2657
21. Thomas R, Fysh ETH, Smith NA, Lee P, Kwan BCH, Yap E, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA (2017) 318:1903–12. doi: 10.1001/jama.2017.17426
22. Muruganandan S, Azzopardi M, Fitzgerald DB, Shrestha R, Kwan BCH, Lam DCL, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med (2018) 6:671–80. doi: 10.1016/S2213-2600(18)30288-1
23. Wahidi MM, Reddy C, Yarmus L, Feller-Kopman D, Musani A, Shepherd RW, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. ASAP Trial. Am J Respir Crit Care Med (2017) 195:1050–7. doi: 10.1164/rccm.201607-1404OC
24. Fysh ETH, Tremblay A, Feller-Kopman D, Mishra EK, Slade M, Garske L, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest (2013) 144:1597–602. doi: 10.1378/chest.12-3103
25. Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med (2011) 26:70–6. doi: 10.1007/s11606-010-1472-0
26. Davies HE, Mishra EK, Kahan BC, Wrightson JM, Stanton AE, Guhan A, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA (2012) 307:2383–9. doi: 10.1001/jama.2012.5535
27. Nie K, Zhang Z, You Y, Zhuang X, Zhang C, Ji Y. A randomized clinical study to compare intrapleural infusion with intravenous infusion of bevacizumab in the management of malignant pleural effusion in patients with non-Small-Cell lung cancer. Thorac Cancer (2020) 11:8–14. doi: 10.1111/1759-7714.13238
28. Zhong LZ, Xu HY, Zhao ZM, Zhang GM, Lin FW. Comparison of efficacy and toxicity between nedaplatin and cisplatin in treating malignant pleural effusion. Onco Targets Ther (2018) 11:5509–12. doi: 10.2147/OTT.S168391
29. Migliore M, Nardini M. Does cytoreduction surgery and hyperthermic intrathoracic chemotherapy prolong survival in patients with N0-N1 nonsmall cell lung cancer and malignant pleural effusion? Eur Respir Rev (2019) 28:190018. doi: 10.1183/16000617.0018-2019
30. Xu P, Tang K, Ma J, Zhang H, Wang D, Zhu L, et al. Chemotherapeutic tumor microparticles elicit a neutrophil response targeting malignant pleural effusions. Cancer Immunol Res (2020) 8:1193–205. doi: 10.1158/2326-6066.CIR-19-0789
Keywords: intrapleural infusion, methotrexate, microparticles, malignant pleural effusion, non-squamous non-small cell lung cancer
Citation: Dong X, Huang Y, Yi T, Hu C, Gao Q, Chen Y, Zhang J, Chen J, Liu L, Meng R, Zhang S, Dai X, Fei S, Jin Y, Yin P, Hu Y and Wu G (2022) Intrapleural infusion of tumor cell-derived microparticles packaging methotrexate or saline combined with pemetrexed-cisplatin chemotherapy for the treatment of malignant pleural effusion in advanced non-squamous non-small cell lung cancer: A double-blind, randomized, placebo-controlled study. Front. Immunol. 13:1002938. doi: 10.3389/fimmu.2022.1002938
Received: 25 July 2022; Accepted: 20 September 2022;
Published: 05 October 2022.
Edited by:
Chengdi Wang, Independent researcher, Chengdu, ChinaReviewed by:
Marcello Migliore, University of Catania, ItalyCopyright © 2022 Dong, Huang, Yi, Hu, Gao, Chen, Zhang, Chen, Liu, Meng, Zhang, Dai, Fei, Jin, Yin, Hu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Wu, eGh6bHdnQDE2My5jb20=; Yanping Hu, aC15LXBAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.