
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 23 September 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1002742
This article is part of the Research Topic Delivering Nucleic Acids to Immune and Non-Immune Cells View all 16 articles
Osteosarcoma (OS) is a primary bone cancer that is highly prevalent among adolescents and adults below the age of 20 years. The prognostic outcome of metastatic OS or relapse is extremely poor; thus, developing new diagnostic and therapeutic strategies for treating OS is necessary. Extracellular vesicles (EVs) ranging from 30–150 nm in diameter are commonly produced in different cells and are found in various types of body fluids. EVs are rich in biologically active components like proteins, lipids, and nucleic acids. They also strongly affect pathophysiological processes by modulating the intercellular signaling pathways and the exchange of biomolecules. Many studies have found that EVs influence the occurrence, development, and metastasis of osteosarcoma. The regulation of inflammatory communication pathways by EVs affects OS and other bone-related pathological conditions, such as osteoarthritis and rheumatoid arthritis. In this study, we reviewed the latest findings related to diagnosis, prognosis prediction, and the development of treatment strategies for OS from the perspective of EVs.
Osteosarcoma (OS) predominantly occurs among individuals below 20 years and is a form of aggressive primary bone cancer (1, 2). The etiology of OS is mainly characterized by epidemiological, genetic, and environmental factors (3). Several risk factors are associated with tumorigenesis of OS, such as alkylating agents, hereditary retinoblastoma, Paget’s disease, ionizing radiation, and chromosomal abnormalities (4, 5). The diagnosis of OS relies mainly on clinical manifestations, medical imaging, tissue biopsy, and laboratory tests. The standard treatment regimens for OS include neoadjuvant chemotherapy, surgical resection, chemotherapy, and interventional therapy (6, 7). Recent developments related to the treatment of OS include extensive research on stem cell therapy, immunotherapy, and gene therapy (8–10). However, due to the complexity of therapeutic interventions and the genetic differences between laboratory animals and humans, these strategies are limited to preclinical studies. Additionally, patients with OS have a high incidence of early lung metastasis, except for other bone tissue metastasis. About 18% of OS patients show signs of micrometastasis at the time of diagnosis, and the five-year survival rate of patients with stage III OS or higher stages of OS is very low (11–13). Moreover, the treatment outcomes are suboptimal because of the difficulty in early diagnosis, the early onset of metastasis, and high malignancy (14, 15). The five-year survival of OS patients who do not receive chemotherapy is below 30%. Pulmonary metastasis is the main cause of OS-related mortality. Moreover, the chemotherapeutic intervention can partially control pulmonary metastasis of OS and increase the five-year survival to 50%. For OS cases with pulmonary metastasis, the two-year survival is less than 25%. Additionally, although there are several alternatives, the survival period during treatment might stabilize without any improvement. Therefore, implementing traditional treatment strategies might not yield the best results (16, 17). Hence, determining the mechanism of the occurrence and metastasis of OS might help to find new clinical diagnostic markers and efficient therapeutic targets.
Extracellular vesicles (EVs) are specialized membranous vesicles originating from endonuclear bodies with particles ranging from 30 to 100 nm in diameter (18, 19). EVs were first identified as a component of blood erythrocytes. They appeared as a lipid bilayer structure surrounded by cytoplasm and devoid of any organelles (20). These EVs were discovered approximately 40 years ago (20). The understanding of the role of EVs in human pathophysiological processes has improved significantly.
Several studies have shown that EVs are produced by various cancer and healthy cells (21–23). When EVs were discovered, their primary function was thought to be the excretion of metabolic wastes from cells (24). However, various studies highlighted the ability of EVs to perform cellular communication, which is essential during various biological processes and disease progression. This communication is possible due to the presence of various nucleic acids and proteins that are responsible for distinguishing the transmission of important biological information between cells (25–28). Thus, EVs can be used as nano-cargos for delivering nucleic acids (such as messenger RNA) (29) and therapeutic agents (such as paclitaxel) (30). Cells within the tumor microenvironment (TME) of OS can secrete EVs, which can deliver non-coding RNAs (ncRNAs) and proteins within the tumor matrix essential for cellular communication. Thus, EVs can effectively regulate the TME within OS and accelerate cell proliferation and metastasis. Additionally, EVs show high systemic stability and are not susceptible to cellular enzymes. They also have good therapeutic and diagnostic potential. In this article, we reviewed the different types of EVs and their biological properties, along with their potential in the diagnosis and treatment of OS.
Extracellular vesicles secreted by drug-resistant cells facilitate and transfer drug resistance to different types of tumors, including breast, prostate, colon, lung, and gastric cancer, as well as, osteosarcoma (31). Doxorubicin and cisplatin resistance are transferred from OS resistant cells to sensitive cells through EVs that carry P-glycoprotein, MDR-1 mRNA, or the circular RNA hsa_circ_103801 [178.179]. Bone marrow-derived mesenchymal stem cell-derived extracellular vesicles (BMSC-EVs) can promote the proliferation, invasion, and migration of osteosarcoma cells via the MALAT1/miR-143/NRSN2/Wnt/β-catenin axis (32). Additionally, EVs secreted by the osteosarcoma 143B cell line contain a pro-osteoclastogenic cargo, which includes MMPs (MMP-1 and MMP-13), RANK-L (Receptor Activator of Nuclear Factor κ B Ligand), CD-9, and TGF-β. These findings highlighted that EVs from different sources exhibit different biological activities.
Extracellular vesicles released from most cells contain various proteins, RNA, genomic DNA (gDNA), non-coding RNAs (ncRNAs), lipids, and metabolites (33, 34). EVs can be categorized into three types based on their size and release mechanisms and include EVs, microvesicles, and apoptotic vesicles, with vesicle sizes ranging from 30 to 150 nm, 100 to 1,000 nm, and 50 to 1,500 nm, respectively (35, 36). EVs are cultured from OS cells obtained in vivo and purified by differential centrifugation. The separated and purified EVs are assessed according to their purity and morphology, followed by protein profiling and sequencing of the components. The assessment of the morphology of EVs by electron microscopy remains a gold standard. Additionally, flow cytometry (FCM) might also be performed for assessing EVs. For particle size analysis of EVs, Nanoparticle Tracking Analysis Technology (NTA) is frequently used. The production of EVs involves the initiation of endocytosis, the formation of multivesicular bodies (MVBs), and the production of exosomes (37, 38). EVs start to develop with the initial formation of plasma membrane invaginations into a cup-like structure containing cell surface proteins, soluble proteins, and endoplasmic reticulum (ER). This cup-shaped structure, together with trans Golgi, promotes the formation of early endonucleosomes (39). Early intranucleosomes mature into late intranucleosomes, resulting in the formation of MVBs. These MVBs may fuse with the plasma membrane to release the intraluminal vesicles (ILVs) associated with EVs or may fuse with autophagosomes or lysosomes for degradation (40, 41). EVs are found in different types of body fluids, such as urine, plasma, breast milk, and ascites (42, 43), which makes EVs a significant tool with great diagnostic potential.
Extracellular vesicles are usually formed by endosomal endocytosis, in contrast to other conventional membrane outgrowth processes, which deform membranes from organelles into the cytoplasm. The endosomal limiting membrane undergoes multiple depressions with inward growth resulting in the formation ILVs. These ILVs are then converted into MVBs, which have a dynamic subcellular architecture. Interestingly, MVB formation can occur at the endosomal limiting membrane by the endosomal sorting complex required for the transport (ESCRT) mechanism (44, 45). The ESCRT machinery functions through a set of cytoplasmic protein complexes by recognizing the ubiquitinylated modified membrane proteins. The first ESCRT complex (ESCRT-0) can recognize ubiquitin markers, showing high levels of enrichment in the endosomal membrane during the transport of ubiquitinated complex into ESCRT I/II. Within ESCRT I, tumor susceptibility gene 101 protein (TSG101) can detect disulfide bonds and induce depression of the endosomal membrane. They function as shears in the bud neck under the influence of ESCRT III and lead to the formation of MVBs (46, 47). However, MVBs can still be formed in the absence of ESCRT. The process is initiated by an accessory protein ALG-2 interacting protein X (AIix). AIix directly binds to the intracellular bridging protein syntenin, which is further involved in EV formation (48, 49). Such ESCRT-independent MVBs are produced under the action of the abundant tetra-transmembrane protein CD63-α on MVBs and by ceramide-mediated cell membrane outgrowth (50, 51). These MVBs can fuse with lysosomes, degrade their contents, and recirculate them. The sorting of MVBs is significantly regulated by their cholesterol levels. For example, MVBs rich in cholesterol are targeted to cell membranes to be released as EVs, whereas, MVBs with low cholesterol levels are targeted for transport toward lysosomes (52).
Extracellular vesicles are generally responsible for inducing functional responses in receptor cells by delivering their contents, promoting phenotypic changes in receptor cells, and affecting their physiological state (25, 53). EV-mediated intercellular communication within plasma membrane relies on the activation of surface receptors on recipient cells and initiates cell signaling. The uptake of EVs by recipient cells is facilitated by cytokinesis (54, 55). The mechanisms of exosome cell membrane interaction and the transport of exosomes and endosomes are not fully understood. However, some studies have shown that these mechanisms are associated with the origin of EVs, receptor cells, and downstream processes involved in the same. Some studies have shown the activity of EVs derived from certain cells along with their application in the treatment of diseases (56, 57). The interaction between proteins significantly expressed on EVs and surface receptors of the recipient cell membrane can be used to assess the target cell specificity (58, 59). The known mediators of cell communication also include transmembrane tetraspanins, integrins, lipids, and extracellular matrix components (60, 61).
Extracellular vesicles influence the exclusion of redundant and nonfunctional cellular components (62, 63). They can also act as intercellular linkers for protein, nucleic acid, and lipid transport between host and recipient cells. They strongly affect different biological processes, such as antigen presentation, angiogenesis, inflammation, and apoptosis (64–67). These processes might be related to the metastasis of biomolecules and cell crosstalk that leads to cancer-related events (47, 68, 69). The constituent nucleic acids, proteins, and lipids captured by EVs during production might reflect their cellular origin and physiological state.
These biomolecules have high disease specificity and might act as potential biomarkers. Additionally, EVs function as carriers for these biomolecules and prevent their enzymatic degradation. Various tumor-associated events involve EVs for cell proliferation, apoptosis, metastasis, and angiogenesis, and thus, may be used as a noninvasive diagnostic biomarker in various types of cancer (70–72). For example, miR-21, miR-124–3p, and miR-222 in serum EVs might be used as molecular biomarkers for assessing early cancer development during postsurgical management of high-grade gliomas (HGG) (73). Shin et al. reported the expression of miR-21, miR-451, and miR-636 in urinary EVs in prostate cancer patients, which indicated a close resemblance with preoperative prostate-specific antigen (PSA) levels. Thus, urinary exosome-derived miRNAs might be used as noninvasive markers for predicting prostate cancer prognostic outcomes and metastasis (74). Wang et al. showed that plasma exosome-derived miR-363–5p was necessary for differentiating LN-positive breast cancer (BC) patients from LN-negative patients. Additionally, upregulation of miR-363–5p was strongly associated with overall survival (75). Exosome therapeutic research is focused on three main areas, which include biomedicine, drug delivery, and regenerative medicine. EVs are promising for treating disorders due to their nontumorigenic risk and bactericidal infiltration. Due to their small size, EVs can reach the site of injury through internal circulation and lower immunogenicity, which makes them an ideal candidate for developing treatment against various disorders (76, 77). EVs also facilitate gene delivery to recipient cells, thus alteringtheir biological activity. They are also capable of carrying therapeutic payloads such as proteins, RNAs, and chemotherapeutic agents and delivering them to the target site across different biological barriers (47, 78, 79). EVs can be engineered to target cell signaling pathways or specific recipient cells using a ligand-targeted approach (27, 80). Chemotherapeutic loaded EVs can target tumors with a significant reduction in dose-dependent side effects of chemotherapeutic agents and an increase in their efficacy in cancer treatment (55, 68, 81). Mesenchymal stem cell (MSC)-derived EVs can be used in the field of regeneration and repair. Additionally, some in vitro and in vivo studies have investigated its regenerative potential and therapeutic applications. In some studies, EVs were found to outperform MSCs in the treatment of various diseases (19, 82, 83).
Extracellular vesicles affect cellular communication between cells within the TME, thus influencing cell proliferation and metastasis in cancer. Bone marrow-derived mesenchymal stem cell-derived extracellular vesicles (BMSC-EVs) can promote proliferation, invasion, and migration of osteosarcoma cells via the MALAT1/miR-143/NRSN2/Wnt/β-catenin axis (32). This enhancement in cell proliferation and metastasis is facilitated by the epithelial-mesenchymal transition (EMT) in related cell types. Moreover, the TME significantly accelerates tumor neovascularization, immunosuppression through stromal cells, and the transformation of cancer-associated fibroblasts (84–87). In conclusion, EVs have a strong effect on OS cell proliferation, migration, invasion, and angiogenesis by participating in intercellular communication and controlling cellular signaling (Figure 1).
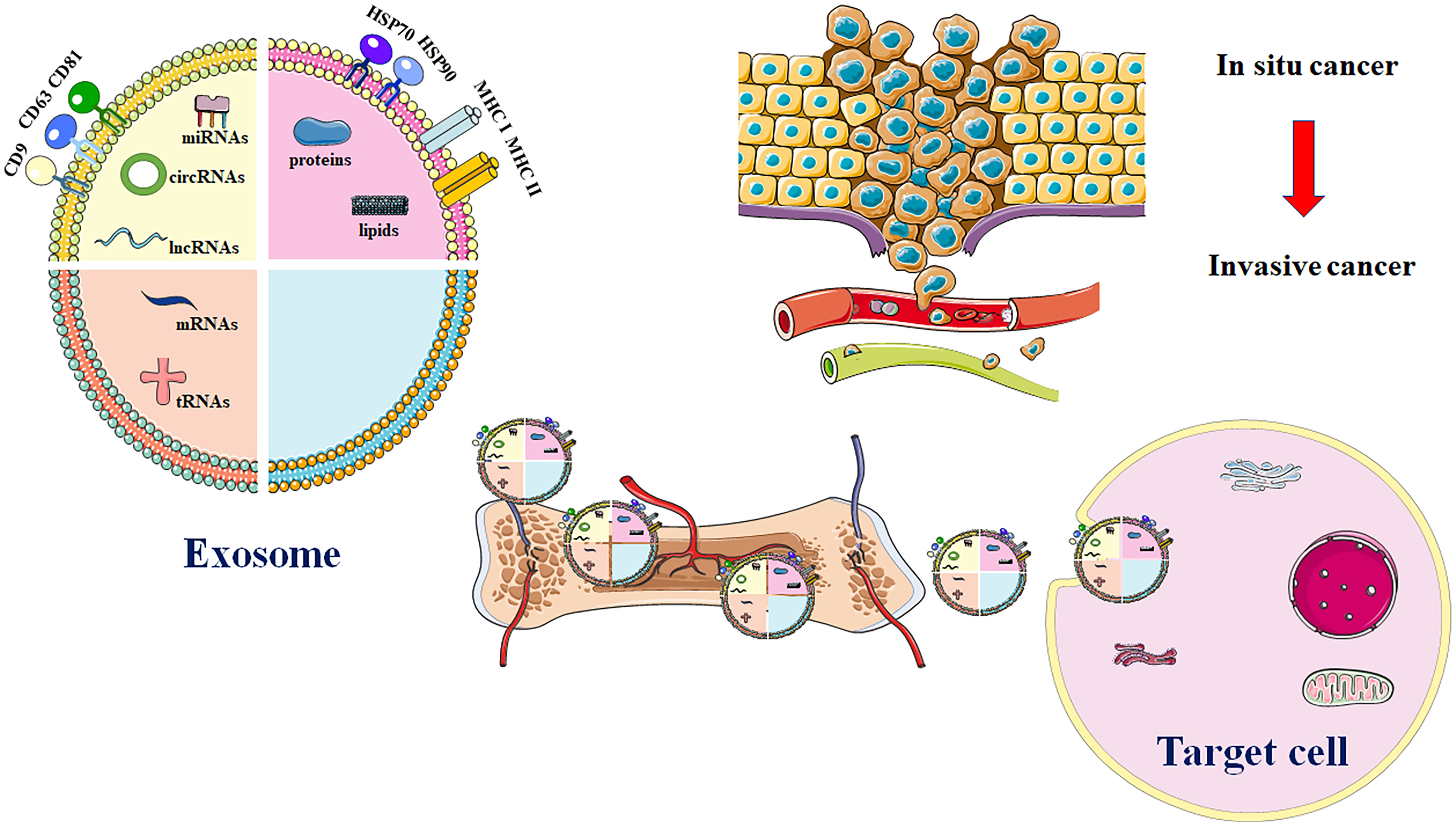
Figure 1 Major exosome release process in OS. EVs are comprised of various proteins and nucleic acids. These evolutionarily conserved proteins that can be used as biomarkers, like HSP70, CD9, CD63, and CD81. Additionally, exosomal cargos are also involved in transport of multiple biomolecules such as DNA or RNA. EVs that carry genetic materials are utilized in development of treatment for OS through enhancing drug resistance, immune evasion, migration, invasion, and angiogenesis. Source cell-derived exosomal cargos are also carried into recipient cells via blood circulation. Highly invasive OS cells enhance cell migration and invasion through production of exosomes.
Cancer cells undergo indefinite proliferation (88). In contrast, normal tissues have precise and controlled release of pro-growth signals, which cyclically initiate cell proliferation and differentiation up to a finite number of cell divisions. However, tumor cells can inherently produce growth factor receptors, thus escaping negative feedback regulation against proliferation (89, 90). EVs also have an important effect on proliferation in OS (Table 1). Zhang et al. reported the effect of exosomal miR-208a obtained from bone marrow-derived mesenchymal stem cells (BMSCs) on OS cell proliferation and apoptosis. They found that OS cell growth was enhanced and apoptosis was inhibited when PDCD4 expression was suppressed. This, in turn, activated the Hippo and ERK1/2 pathways. In contrast, the exosomal miR-206 obtained from BMSCs suppressed cell growth, invasion, and migration. It also promoted apoptosis by targeting TRA2B in OS cells (102). Additionally, BMSC-derived EVs could encapsulate and translocate PVT1 in OS cells, and PVT1 promoted cancer development and migration by binding to miR-183–5p and facilitating the expression of ERG (94). BMSC-EVs could enhance OS cell growth, migration, and invasion through MALAT1/miR-143/NRSN2/Wnt/β-catenin signaling (93). Huang et al. showed the effect of EVs obtained from hBMSCs on tumorigenesis and migration. The EVs showed enhanced tumorigenesis and migration by promoting oncogenic autophagy in OS (95). EVs derived from ADSC could enhance OS cell growth, invasion, and migration by delivering COLGALT2 to OS cells, leading to the malignant progression of OS (96). Li et al. found that OS cells that showed AXL upregulation promoted the secretion of EVs into cells with downregulated AXL, and this promoted cell growth, invasion, and migration via the linc00852/miR-7–5p/AXL regulatory axis (103). Ge et al. found that BMSC-derived EVs translocate into OS cells and promote OS growth and migration by LCP1/JAK2/STAT3 signaling and inhibit OS progression via miR-135a-5p/LCP1 signaling (98). The MG-63 cell-derived EVs, which were co-cultured using HOS and MG-63 cell lines, significantly enhanced OS cell growth and inhibited apoptosis. This effect might be related to the interaction of Hic-5 with smad4 and a decrease in the expression of TCF/LEF that regulates Wnt/β-catenin signaling (99). Han et al. found that exosomal miR-1307 obtained from OS cells can promote OS cell growth, invasion, and migration by inhibiting AGAP1 expression. This finding indicated that the miR-1307-AGAP1 axis might act as an anti-OS therapeutic target (100). Wu et al. found that exosomal miR-15a expression decreased in plasma EVs, and exosomal miR-15a was absorbed by OS cells, which suppressed GATA2/MDM2 signaling via the p53 pathway. This inhibited OS cell growth and migration in vitro (104).
In epithelial-mesenchymal transition (EMT), the epithelial properties of epithelial cells are lost, while the mesenchymal phenotype is acquired. This phenomenon is widely involved in physiological regulation and pathological changes and is closely related to embryogenesis, tissue regeneration, invasion, and metastasis of cancer tissue (105–107). When EMT occurs, the main features of epithelial cells are lost, resulting in a change from polygonal to spindle-shaped fibroblast-like morphology. Additionally, the cells also lose their polarity, show reduced adhesion, and gain the ability to invade and metastasize (108, 109). EVs have a strong effect on OS invasive metastasis (Table 2). When in-vitro synthesized miR-143 was transported into OS cells via EVs, they significantly inhibited the invasive ability of the cells (110). Gong et al. found that highly invasive OS cells secreted exosomal miR-675 into recipient cells and further suppressed CALN1 expression to enhance migration and invasion of OS cells. Additionally, serum exosomal miR-675 levels among OS cases are strongly associated with the prognosis of OS (111). Mazumdar et al. found that EVs derived from 143-B cells with high metastasis capacity and SAOS-2 cells with low metastasis capacity could induce the recruitment of BMCs into the lungs. The components of EVs might inhibit distant metastasis of OS (113). Zhong et al. showed that the Rab22a-NeoF1 fusion protein with PYK2 could be sorted into EVs in OS. The exosomal Rab22a-NeoF1 fusion protein promotes premetastatic lung niche generation by recruiting bone marrow-derived macrophages (BMDMs) (112). Han et al. showed that exosomal miR-1307 obtained from OS cells enhanced OS cell growth, invasion, and migration by inhibiting AGAP1 expression; thus, targeting miR-1307 might inhibit the malignant progression of OS (100).
Angiogenesis is the formation of new blood vessels in capillaries or venules behind capillaries (114, 115). This process is regulated by the interaction between proangiogenic and antiangiogenic factors. Although these factors are stable under normal physiological conditions, they can be activated or inactivated by external stimuli (12, 116). Different types of cells (cancer and healthy cells) require nutrients, which are supplied through blood capillaries. These capillaries can also excrete metabolic waste generated within cells (117, 118). Tumor-derived EVs are associated with an important mechanism that promotes angiogenesis. Moreover, EVs have a critical effect on angiogenesis in OS (Table 3). Yoshida et al. found that the expression of miR-25–3p increased in OS tissues, which promoted cancer development, drug resistance, and invasion by inhibiting the expression of DKK3. Embedding synthetic miR-25–3p into tumor-derived EVs significantly promoted the capillary formation and vascular endothelial cell (EC) invasion (119). Tao et al. showed that angiogenesis in OS could be promoted by EWSAT1. Therefore, including exosomes increases the sensitivity of vascular endothelial cells, which directly induces an increase in the secretion of angiogenic factors (120). Li et al. showed that osteosarcoma cells with high exosome abundance could modulate autophagy and angiogenesis in OS via ATG and miR-153 by secreting exosomal lnc-OIP5-AS1 into other OS cells (121).
The natural response of the body to any foreign material is expressed by immune system activation and production of EVs (22, 83, 122). EVs can also regulate and modulate immune cells and participate in the immune response (21, 123, 124). EVs obtained from cancer cells can deliver tumor-associated antigens (TAAs) to stimulate immune cells and generate antitumor immune responses. However, they can also interfere with immune recognition and inhibit tissue-associated cells, T cells, immune-related cells, and natural killer (NK) cells, thus accelerating tumor cell escape and metastasis (25, 125). Moreover, EVs are responsible for regulating cancer cell development via TME-derived immune cells (126, 127). Additionally, the immune microenvironment within OS cells is strongly affected by EVs (Table 4). Cancer-associated fibroblast (CAFs)-secreted exosomal miR-1228 can enhance OS migration and invasion via SCAI. This can be further used in the development of miR-1228-based anti-OS therapy (119). Raimondi et al. found that EVs can promote osteoclast bone resorption and differentiation. EVs can also enhance tube formation in ECs while increasing the expression of angiogenic markers. Specific miRNAs, including miR-21–5p and miR-148a, have important effects on the tumor microenvironment, as determined by second-generation sequencing (128). The EVs of metastatic OS cells secrete exosomal TGFβ2 into tumor-associated macrophages, which in turn promote the M2 phenotype and contribute to immunosuppression and tumorigenesis (129). Mazumdar et al. showed that EVs obtained from OS cells can promote the differentiation of myofibroblasts/CAF, the generation of fibronectin, and the expression of smooth muscle actin. They can also significantly promote the invasive ability of human lung fibroblasts (130). Cheng et al. showed that OS-obtained EVs can promote the polarization of M2 macrophages via Tim-3, which in turn can promote the invasion of OS cells and metastasis (135). Zhang et al. showed that OS cell-derived exosomal COL6A1 can convert normal fibroblasts into CAFs by secreting proinflammatory cytokines. After activation, CAFs can mediate the TGF-β/COL6A1 pathway to enhance the migration and invasion of OS cells (132). Zhang et al. showed that exosomal LIFR-AS1 obtained from macrophages could promote the OS malignancy grade by combining with miR-29a, which promoted the NFIA level (133).
Extracellular vesicles consist of various biomolecules, which are biologically active. They circulate through systemic circulation and are also found in various body fluids capable of mediating long-distance intercellular communication (40, 136). Tumor-derived EVs are rich in biomolecules, such as proteins, nucleotides, and lipids, which indicate the origin of the pathophysiological status of the cells (137, 138). EVs can provide a specialized lipid bilayer covering, thus preventing the degradation of RNA molecules (137, 139). Hence, the detection of tumor EVs in patients provides significant advantages to liquid biopsy, and EVs might also be used for early diagnosis. EVs might also be used to develop efficacious treatment strategies and monitor the prognosis of different diseases [181.182]. A specific collection of RNAs in the EV cargo might also serve as new or supplementary biomarkers in the diagnosis and progression of OS (31). Another study showed dysregulated levels of several miRNAs and mRNAs in EVs isolated from the serum of OS patients with a poor chemotherapeutic response compared to that of patients who responded positively to chemotherapy (140). A pilot study showed a higher tumor mutation burden in the RNA isolated from the plasma samples with metastatic EVs compared to that isolated from the plasma samples with non-metastatic ones (141). These findings highlighted the clinical application of EVs in OS.
The diagnostic and prognostic assessment of OS improved considerably with the application of EVs as a biomarker for the disease. Next-generation sequencing was conducted, and eight novel miRNAs were identified from OS cells, out of which five miRNAs were present in circulating EVs among OS patients. However, the biological activity in the pathogenesis of OS and the diagnostic and therapeutic potential of these miRNAs need to be further investigated (142). The expression levels of plasma EV-miR-101 in OS patients and normal participants were determined by performing qRT–PCR. The results indicated a significant decrease in EV-miR-101 levels in OS patients relative to that in normal participants. Moreover, the EV-miR-101 plasma levels in OS patients with metastases were lower than those in patients without metastases. Hence, EV-miR-101 might be a diagnostic marker for OS (143). Ye et al. identified 57 differentially expressed miRNAs in plasma samples obtained from OS patients and normal participants via high-throughput sequencing. Among these miRNAs, 20 were upregulated, and 37 were downregulated. The expression of miR-92a-3p, miR-130a-3p, miR-195–3p, let-7i-3p, and miR-335–5p increased significantly within EVs from OS patients relative to their expression in controls. The findings suggested that these miRNAs might be used as potential diagnostic markers for OS (144). Zhang et al. reported high levels of CASC15 in OS cells and tissues along with a significant increase in the levels of CASC15 in the OS plasma EVs compared to their levels in controls (145). Cambier et al. described the significant diagnostic potential of overexpressed biomarkers such as HSATII, HSATI, Charlie 3, and LINE1-P1 at the DNA level rather than the RNA level in serum EVs from OS patients compared to their levels in serum EVs of the control (146). Huo et al. described significant upregulation of hsa_circ_0056285 in serum EVs in OS patients. They also showed the great diagnostic ability of hsa_circ_0056285 based on the ROC curve analysis (147). The expression of SENP1 obtained from plasma exosomes of OS patients was closely related to the tumor size, tumor location, necrosis rate, lung metastasis, and surgical staging. Moreover, patients with higher SENP1 expression had poorer overall survival, and disease-free survival (DFS) compared to OS patients with downregulated SENP1 (148). Han et al. analyzed EVs from plasma samples of OS patients with and without metastases and compared the results to those of normal controls using MALDI-TOF MS. They identified seven exosomal protein markers that were associated with OS lung metastasis (11). Also, noninvasive liquid biopsy using MALDI-TOF MS fingerprinting and SERS for the identification of EVs can be applied for the rapid diagnosis of OS (149).
Treatment options for OS were either surgery or radiotherapy until the 1970s. Patients with OS also showed high resistance to radiotherapy (150, 151). Clinical results showed that surgical intervention, including tumor resection and/or amputation, cannot improve the survival rate (the operative mortality was about 80%) (152). The five-year survival rate of tumor resection cases is only 20% (153). Additionally, chemotherapeutic interventions can improve the survival rate of OS and reduce the amputation rate, thus improving the limb rescue score. The long-term survival rate of OS patients without metastasis is as high as 75%, compared to 20% before the 1970s (154, 155). However, the long-term survival rate of patients with recurrence or metastasis is still low (Figure 2).
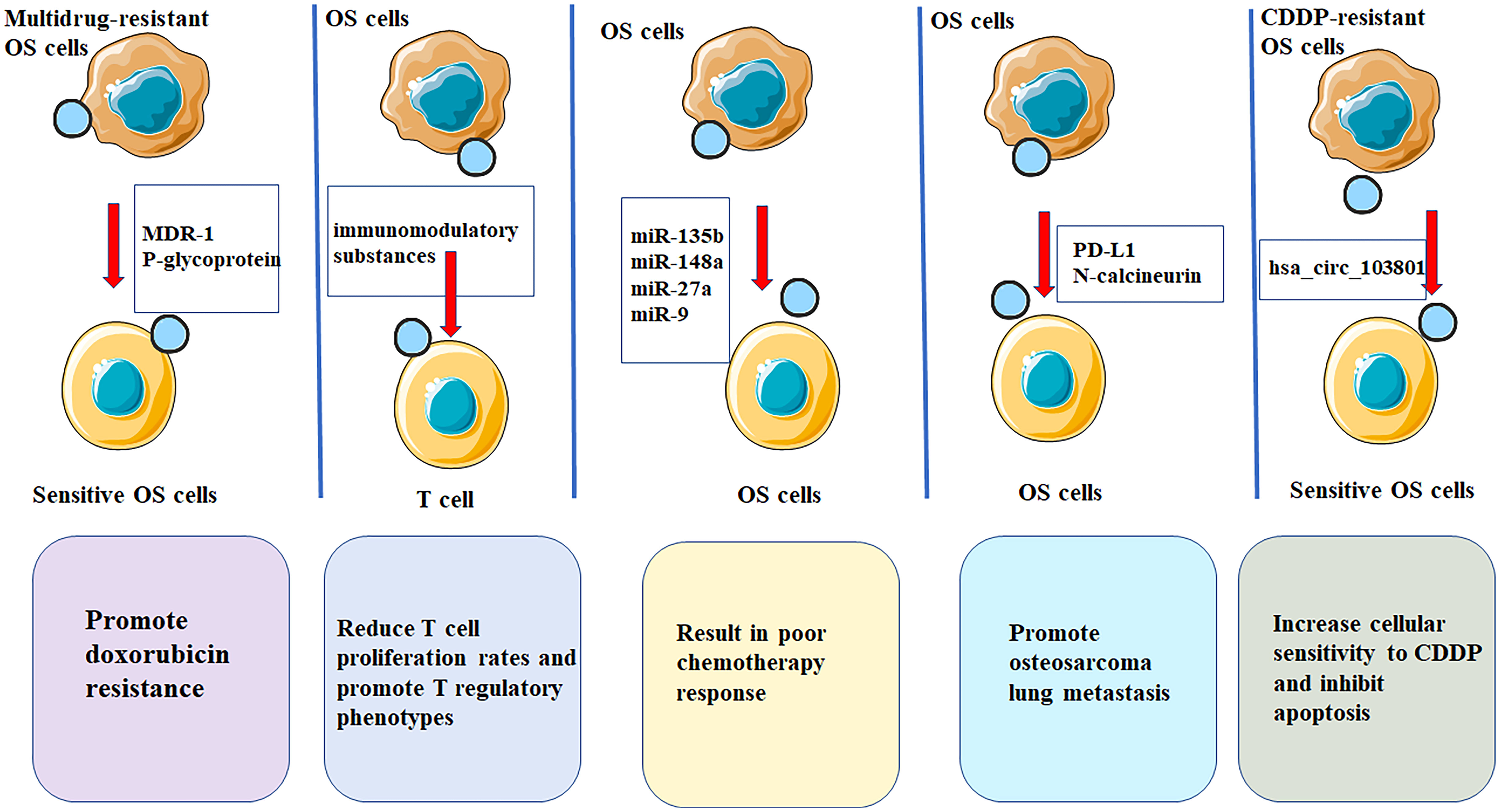
Figure 2 EVs have potential applications in treatment of OS. EVs are multifunctional nanostructured carriers which can be used as drug delivery systems with low immunogenicity as well as high biocompatibility and efficacy. OS-derived EVs contain immunomodulation properties that significantly reduces T cell proliferation rates and promote T regulatory phenotypes, thereby promoting OS progression. OS cases showing low chemosensitivity in patients showing favorable chemosensitivity. miR-9, miR-27a, miR-135b and miR-148a show marked up-regulation within serum EVs of OS patients. OS cells could promote osteosarcoma lung metastasis by releasing EVs that contained PD-L1 and N-calcineurin. EVs from cisplatin-resistant (CDDP)-resistant OS cells decreased P-glycoprotein and MDR-associated protein 1 levels in MG63 and U2OS cells, increases cellular sensitivity to CDDP and inhibits apoptosis through exosomal-hsa_circ_103801.
Kyung et al. showed that EVs have antitumor effects on osteosarcoma cells. EVs from canine macrophages can activate the apoptosis pathway of canine OS cells, which is an effective anti-cancer treatment (156). Additionally, MSC-derived EVs carrying miR-150 can reduce the proliferation and migration of osteosarcoma cells by targeting IGF2BP1 (insulin-like growth factor-2 mRNA binding protein 1) (157) Exosomes might also be used as a carrier to deliver chemotherapeutic drugs to osteosarcoma cells [188.189]. Exosomes can be directly charged with drugs [190.191].
The advanced metastasized tumors, in contrast to primary tumors, often pose a major hindrance to the success of treatment outcomes in OS and increase patient mortality. Therefore, early diagnosis is the key to improving the prognosis and survival of OS patients (123, 158). EVs are stable, diverse, nano-sized vesicles that are found in most tissues, organs, and body fluids (124, 159). Moreover, EVs containing transmembrane proteins and some intracellular proteins, such as integrins or genetic material from the cells of origin, display a high level of identity within cells. This identity is associated with the identification of the tissue of origin, suggesting the importance of EVs and their potential as biomarkers in the early diagnosis and prognosis of OS (22, 160, 161). The surface proteins of EVs can be targeted and captured by recipient cells, and the contents of EVs can alter the physiological state of recipient cells (162, 163). Tumor EVs can also modulate cancer progression, immune evasion, metastasis, and angiogenesis by interacting with other cells within the TME (125, 164, 165). Additionally, the exosome-mediated pathological processes also highlight the great potential of EVs as biomarkers. Also, a better understanding of the mechanisms of exosome action is necessary to screen, diagnose, and assess patient prognosis.
There are still many problems in the development of EVs. For example, a standardized approach is needed for the quick, easy, and specific isolation of EVs in liquid biopsy. Moreover, EVs can serve as potential biomarkers for the diagnosis of OS, predict its prognosis, and monitor real-time treatment response. Clinical studies with a small sample size have shown reproducibility of EVs (166–168). However, more multicenter trials with large sample sizes are required for developing more accurate liquid biopsies. For evaluating the biological functions of EVs, determining whether they have similar regulatory functions in vivo and in vitro is challenging. The reason for this heterogeneity is that numerous assays have been performed in vitro, however, similar culture conditions cannot be replicated in vivo. Additionally, for therapeutic purposes, exosome-derived cells need to be selected carefully to ensure safe treatment. Due to their availability and non-nucleated and non-genetic nature, erythrocytes are the most promising cells for producing exosomes.
Besides their potential as good biomarkers, EVs are promising for precise and targeted cancer therapy (169–171). The development of a novel drug-loading system is a barrier to enhancing the effectiveness of antitumor drug therapy. Therefore, as a natural therapeutic carrier, EVs might be used for their low immunogenicity and various therapeutic bioactive molecules contained within (21, 161). Moreover, exogenous drugs carried by EVs can maintain drug stability in vivo. These advantages make EVs a better drug loading system than traditional drug delivery models. Hence, EVs are important for developing precision medicine for OS and other cancers. Han et al. constructed the iRGD-Lamp2b-modified MSC fusion gene for isolating and purifying EVs, as well as, loading the anti-miRNA-221 oligonucleotides into EVs. AMO-loaded EVs are effective in inhibiting in-vitro colon cancer (CC) cell growth and clone-forming ability (172). Exosomal ANXA6 levels in the sera of TNBC patients can predict the efficacy of gemcitabine chemotherapy (173). CC cells can produce exosomal miR-208b to receptor T-cells and promote the expansion of Treg cells via programmed cell death factor 4 (PDCD4), leading to malignant tumor growth and oxaliplatin resistance (174).
In this study, we highlighted and reviewed the advancements in the research on the biological functions of EVs during the occurrence and development of OS, along with its clinical applications. Moreover, EVs from OS can promote the progression of OS by regulating cancer drug resistance, immunity, angiogenesis, and metastasis. These findings highlight the role of EVs as anti-OS targets. Additionally, due to their abnormal expression in tumor-derived exosomal inclusions and their ability to reflect the tumor status, EVs might be used as markers for the diagnosis and prognosis of OS. Exosomal drug carriers and immunomodulatory therapy are promising therapeutic strategies in the treatment of OS.
SL, XG, and BG were responsible for original drafting, supplementation, allocation as well as editing. The authors have carefully read and approved the final version for submission.
The present study was funded by Fundamental Research Funds for the Central Universities (LD202110) and Natural Science Foundation of Liaoning Province (2020-MS-058) and Shenyang Young and Middle-age Scientific and Technological Innovation Talent Support Plan (RC190456).
The present study was funded by Liaoning Cancer Hospital & Institute (Shenyang) and China Medical University (Shenyang).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
OS, Osteosarcoma; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; lncRNAs, long non-coding RNAs; miRNAs, microRNAs; mRNAs, messenger RNAs; circRNAs, circular RNAs; ASMCs, airway smooth muscle cells; NSCLC, non-small-cell lung cancer; LUAD lung adenocarcinoma; EVs, extracellular vesicles, ILVs, luminal vesicles; MVBs, multivesicular bodies; Aiix, ALG-2 interacting protein X; ESCRT, endosomal sorting complex required for transport; HGG, high-grade gliomas; PSA, prostate-specific antigen; EMT, epithelial-mesenchymal transition; MSCs, mesenchymal stem cell; CAFs, cancer-associated fibroblasts; BMSCs, bone marrow-derived mesenchymal stem cells; OS, overall survival; DFS, disease-free survival; NGS, next-generation sequencing; CDDP, cisplatin-resistant.
1. Chen Y, Cao J, Zhang N, Yang B, He Q, Shao X, et al. Advances in differentiation therapy for osteosarcoma. Drug Discovery Today (2020) 25(3):497–504. doi: 10.1016/j.drudis.2019.08.010
2. Dean DC, Shen S, Hornicek FJ, Duan Z. From genomics to metabolomics: emerging metastatic biomarkers in osteosarcoma. Cancer Metastasis Rev (2018) 37(4):719–31. doi: 10.1007/s10555-018-9763-8
3. Zheng C, Tang F, Min L, Hornicek F, Duan Z, Tu C. PTEN in osteosarcoma: Recent advances and the therapeutic potential. Biochim Biophys Acta Rev Cancer (2020) 1874(2):188405. doi: 10.1016/j.bbcan.2020.188405
4. Hattinger CM, Patrizio MP, Fantoni L, Casotti C, Riganti C, Serra M. Drug resistance in osteosarcoma: Emerging biomarkers, therapeutic targets and treatment strategies. Cancers (Basel) (2021) 13(12):2878. doi: 10.3390/cancers13122878
5. Czarnecka AM, Synoradzki K, Firlej W, Bartnik E, Sobczuk P, Fiedorowicz M, et al. Molecular biology of osteosarcoma. Cancers (Basel) (2020) 12(8):2130. doi: 10.3390/cancers12082130
6. Lin Z, Wu Z, Luo W. Chimeric antigen receptor T-cell therapy: The light of day for osteosarcoma. Cancers (Basel) (2021) 13(17):4469. doi: 10.3390/cancers13174469
7. Brookes MJ, Chan CD, Baljer B, Wimalagunaratna S, Crowley TP, Ragbir M, et al. Surgical advances in osteosarcoma. Cancers (Basel) (2021) 13(3):388. doi: 10.3390/cancers13030388
8. Gill J, Gorlick R. Advancing therapy for osteosarcoma. Nat Rev Clin Oncol (2021) 18(10):609–24. doi: 10.1038/s41571-021-00519-8
9. Huang Q, Liang X, Ren T, Huang Y, Zhang H, Yu Y, et al. The role of tumor-associated macrophages in osteosarcoma progression-therapeutic implications. Cell Oncol (Dordr) (2021) 44(3):525–39. doi: 10.1007/s13402-021-00598-w
10. Marchandet L, Lallier M, Charrier C, Baud'huin M, Ory B, Lamoureux F. Mechanisms of resistance to conventional therapies for osteosarcoma. Cancers (Basel) (2021) 13(4):683. doi: 10.3390/cancers13040683
11. Cui J, Dean D, Hornicek FJ, Chen Z, Duan Z. The role of extracelluar matrix in osteosarcoma progression and metastasis. J Exp Clin Cancer Res (2020) 39(1):178. doi: 10.1186/s13046-020-01685-w
12. Liu Y, Huang N, Liao S, Rothzerg E, Yao F, Li Y, et al. Current research progress in targeted anti-angiogenesis therapy for osteosarcoma. Cell Prolif (2021) 54(9):e13102. doi: 10.1111/cpr.13102
13. Prudowsky ZD, Yustein JT. Recent insights into therapy resistance in osteosarcoma. Cancers (Basel) (2020) 13(1):83. doi: 10.3390/cancers13010083
14. Laskar S, Kakoti S, Khanna N, Manjali JJ, Mangaj A, Puri A, et al. Outcomes of osteosarcoma, chondrosarcoma and chordoma treated with image guided-intensity modulated radiation therapy. Radiother Oncol (2021) 164:216–22. doi: 10.1016/j.radonc.2021.09.018
15. Christie JD, Appel N, Canter H, Achi JG, Elliott NM, de Matos AL, et al. Systemic delivery of TNF-armed myxoma virus plus immune checkpoint inhibitor eliminates lung metastatic mouse osteosarcoma. Mol Ther Oncolytics (2021) 22:539–54. doi: 10.1016/j.omto.2021.07.014
16. Synoradzki KJ, Bartnik E, Czarnecka AM, Fiedorowicz M, Firlej W, Brodziak A, et al. TP53 in biology and treatment of osteosarcoma. Cancers (Basel) (2021) 13(17):4284. doi: 10.3390/cancers13174284
17. Chen C, Xie L, Ren T, Huang Y, Xu J, Guo W. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett (2021) 500:1–10. doi: 10.1016/j.canlet.2020.12.024
18. Pathania AS, Prathipati P, Challagundla KB. New insights into exosome mediated tumor-immune escape: Clinical perspectives and therapeutic strategies. Biochim Biophys Acta Rev Cancer (2021) 1876(2):188624. doi: 10.1016/j.bbcan.2021.188624
19. Psaraki A, Ntari L, Karakostas C, Korrou-Karava D, Roubelakis MG. Extracellular vesicles derived from mesenchymal Stem/Stromal cells: The regenerative impact in liver diseases. Hepatology (2021) 75(6):1590–1603. doi: 10.1002/hep.32129
20. Trams EG, Lauter CJ, Salem N Jr., Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta (1981) 645(1):63–70. doi: 10.1016/0005-2736(81)90512-5
21. Bondhopadhyay B, Sisodiya S, Alzahrani FA, Bakhrebah MA, Chikara A, Kasherwal V, et al. EVs: A forthcoming era of breast cancer therapeutics. Cancers (Basel) (2021) 13(18):4672. doi: 10.3390/cancers13184672
22. Vautrot V, Bentayeb H, Causse S, Garrido C, Gobbo J. Tumor-derived EVs: Hidden players in PD-1/PD-L1 resistance. Cancers (Basel) (2021) 13(18):4537. doi: 10.3390/cancers13184537
23. Thakur A, Ke X, Chen YW, Motallebnejad P, Zhang K, Lian Q, et al. The mini player with diverse functions: Extracellular vesicles in cell biology, disease, and therapeutics. Protein Cell (2021) 13(9):631–54. doi: 10.1007/s13238-021-00863-6
24. Johnstone RM, Ahn J. A common mechanism may be involved in the selective loss of plasma membrane functions during reticulocyte maturation. BioMed Biochim Acta (1990) 49:S70–5. doi: 10.1007/BF02789143
25. Jiang C, Zhang N, Hu X, Wang H. Tumor-associated EVs promote lung cancer metastasis through multiple mechanisms. Mol Cancer (2021) 20(1):117. doi: 10.1186/s12943-021-01411-w
26. Piffoux M, Volatron J, Cherukula K, Aubertin K, Wilhelm C, Silva AKA, et al. Engineering and loading therapeutic extracellular vesicles for clinical translation: A data reporting frame for comparability. Adv Drug Delivery Rev (2021) 178:113972. doi: 10.1016/j.addr.2021.113972
27. Kim H, Jang H, Cho H, Choi J, Hwang KY, Choi Y, et al. Recent advances in exosome-based drug delivery for cancer therapy. Cancers (Basel) (2021) 13(17):4435. doi: 10.3390/cancers13174435
28. Isaac R, Reis FCG, Ying W, Olefsky JM. EVs as mediators of intercellular crosstalk in metabolism. Cell Metab (2021) 33(9):1744–62. doi: 10.1016/j.cmet.2021.08.006
29. Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y, et al. EVs derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145–5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett (2019) 442:351–61. doi: 10.1016/j.canlet.2018.10.039
30. Kim MS, Haney MJ, Zhao Y, Yuan D, Elena V. Engineering macrophage-derived EVs for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine. (2018) 14:195–204. doi: 10.1016/j.nano.2017.09.011
31. Perut F, Roncuzzi L, Baldini N. The emerging roles of extracellular vesicles in osteosarcoma. Front Oncol (2019) 9:1342. doi: 10.3389/fonc.2019.01342
32. Li F, Chen X, Shang C, Ying Q, Zhou X, Zhu R, et al. Bone marrow mesenchymal stem cells-derived extracellular vesicles promote proliferation, invasion and migration of osteosarcoma cells via the lncRNA MALAT1/miR-143/NRSN2/Wnt/β-Catenin axis. Onco Targets Ther (2021) 14:737–49. doi: 10.2147/OTT.S283459
33. Hur JY, Lee KY. Characteristics and clinical application of extracellular vesicle-derived DNA. Cancers (Basel) (2021) 13(15):3827. doi: 10.3390/cancers13153827
34. Jiang X, You L, Zhang Z, Cui X, Zhong H, Sun X, et al. Biological properties of milk-derived extracellular vesicles and their physiological functions in infant. Front Cell Dev Biol (2021) 9:693534. doi: 10.3389/fcell.2021.693534
35. Poupardin R, Wolf M, Strunk D. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv Drug Delivery Rev (2021) 176:113872. doi: 10.1016/j.addr.2021.113872
36. Burgos-Ravanal R, Campos A, Diaz-Vesga MC, Gonzalez MF, Leon D, Lobos-Gonzalez L, et al. Extracellular vesicles as mediators of cancer disease and as nanosystems in theranostic applications. Cancers (Basel) (2021) 13(13):3324. doi: 10.3390/cancers13133324
37. Mittal R, Bencie N, Langlie J, Mittal J, Eshraghi AA. EVs as drug delivery vehicles and biomarkers for neurological and auditory systems. J Cell Physiol (2021) 236(12):8035–49. doi: 10.1002/jcp.30484
38. Chen J, Zhang Q, Liu D, Liu Z. EVs: Advances, development and potential therapeutic strategies in diabetic nephropathy. Metabolism (2021) 122:154834. doi: 10.1016/j.metabol.2021.154834
39. Ruan S, Greenberg Z, Pan X, Zhuang P, Erwin N, He M. Extracellular vesicles as an advanced delivery biomaterial for precision cancer immunotherapy. Adv Healthc Mater (2021) 11(5):e2100650. doi: 10.1002/adhm.202100650
40. Sun SJ, Wei R, Li F, Liao SY, Tse HF. Mesenchymal stromal cell-derived EVs in cardiac regeneration and repair. Stem Cell Rep (2021) 16(7):1662–73. doi: 10.1016/j.stemcr.2021.05.003
41. Kostallari E, Valainathan S, Biquard L, Shah VH, Rautou PE. Role of extracellular vesicles in liver diseases and their therapeutic potential. Adv Drug Delivery Rev (2021) 175:113816. doi: 10.1016/j.addr.2021.05.026
42. Fu P, Zhang J, Li H, Mak M, Xu W, Tao Z. Extracellular vesicles as delivery systems at nano-/micro-scale. Adv Drug Delivery Rev (2021) 179:113910. doi: 10.1016/j.addr.2021.113910
43. Tang XH, Guo T, Gao XY, Wu XL, Xing XF, Ji JF, et al. Exosome-derived non-coding RNAs in gastric cancer: Functions and clinical applications. Mol Cancer (2021) 20(1):99. doi: 10.1186/S12943-021-01396-6
44. Gurunathan S, Kang MH, Qasim M, Khan K, Kim JH. Biogenesis, membrane trafficking, functions, and next generation nanotherapeutics medicine of extracellular vesicles. Int J Nanomed (2021) 16:3357–83. doi: 10.2147/IJN.S310357
45. Chivero ET, Dagur RS, Peeples ES, Sil S, Liao K, Ma R, et al. Biogenesis, physiological functions and potential applications of extracellular vesicles in substance use disorders. Cell Mol Life Sci (2021) 78(11):4849–65. doi: 10.1007/s00018-021-03824-8
46. Zhang Z, Xu R, Yang Y, Liang C, Yu X, Liu Y, et al. Micro/nano-textured hierarchical titanium topography promotes exosome biogenesis and secretion to improve osseointegration. J Nanobiotech (2021) 19(1):78. doi: 10.1186/s12951-021-00826-3
47. Song H, Liu B, Dong B, Xu J, Zhou H, Na S, et al. Exosome-based delivery of natural products in cancer therapy. Front Cell Dev Biol (2021) 9:650426. doi: 10.3389/fcell.2021.650426
48. Gurunathan S, Kang MH, Kim JH. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of EVs. Int J Nanomed (2021) 16:1281–312. doi: 10.2147/IJN.S291956
49. Chen P, Wang L, Fan X, Ning X, Yu B, Ou C, et al. Targeted delivery of extracellular vesicles in heart injury. Theranostics (2021) 11(5):2263–77. doi: 10.7150/thno.51571
50. Shehzad A, Islam SU, Shahzad R, Khan S, Lee YS. Extracellular vesicles in cancer diagnostics and therapeutics. Pharmacol Ther (2021) 223:107806. doi: 10.1016/j.pharmthera.2021.107806
51. Tosar JP, Witwer K, Cayota A. Revisiting extracellular RNA release, processing, and function. Trends Biochem Sci (2021) 46(6):438–45. doi: 10.1016/j.tibs.2020.12.008
52. Schubert A, Boutros M. Extracellular vesicles and oncogenic signaling. Mol Oncol (2021) 15(1):3–26. doi: 10.1002/1878-0261.12855
53. Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, et al. Tumor-derived EVs drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab (2021) 33(10):2040–58.e10. doi: 10.1016/j.cmet.2021.09.002
54. Sun Z, Yang J, Li H, Wang C, Fletcher C, Li J, et al. Progress in the research of nanomaterial-based exosome bioanalysis and exosome-based nanomaterials tumor therapy. Biomaterials (2021) 274:120873. doi: 10.1016/j.biomaterials.2021.120873
55. Mohammadi M, Zargartalebi H, Salahandish R, Aburashed R, Wey Yong K, Sanati-Nezhad A. Emerging technologies and commercial products in exosome-based cancer diagnosis and prognosis. Biosens Bioelectron (2021) 183:113176. doi: 10.1016/j.bios.2021.113176
56. Fraga de Andrade I, Mehta C, Bresnick EH. Post-transcriptional control of cellular differentiation by the RNA exosome complex. Nucleic Acids Res (2020) 48(21):11913–28. doi: 10.1093/nar/gkaa883
57. Kumar A, Deep G. Hypoxia in tumor microenvironment regulates exosome biogenesis: Molecular mechanisms and translational opportunities. Cancer Lett (2020) 479:23–30. doi: 10.1016/j.canlet.2020.03.017
58. Jafari D, Malih S, Eini M, Jafari R, Gholipourmalekabadi M, Sadeghizadeh M, et al. Improvement, scaling-up, and downstream analysis of exosome production. Crit Rev Biotechnol (2020) 40(8):1098–112. doi: 10.1080/07388551.2020.1805406
59. Nakamura K, Sawada K, Kobayashi M, Miyamoto M, Shimizu A, Yamamoto M, et al. Role of the exosome in ovarian cancer progression and its potential as a therapeutic target. Cancers (Basel) (2019) 11(8):1147. doi: 10.3390/cancers11081147
60. Zhao X, Wu D, Ma X, Wang J, Hou W, Zhang W. EVs as drug carriers for cancer therapy and challenges regarding exosome uptake. BioMed Pharmacother (2020) 128:110237. doi: 10.1016/j.biopha.2020.110237
61. Li C, Hou X, Zhang P, Li J, Liu X, Wang Y, et al. Exosome-based tumor therapy: Opportunities and challenges. Curr Drug Metab (2020) 21(5):339–51. doi: 10.2174/1389200221666200515103354
62. Han C, Zhang C, Wang H, Zhao L. Exosome-mediated communication between tumor cells and tumor-associated macrophages: Implications for tumor microenvironment. Oncoimmunology (2021) 10(1):1887552. doi: 10.1080/2162402X.2021.1887552
63. Shao J, Zaro J, Shen Y. Advances in exosome-based drug delivery and tumor targeting: From tissue distribution to intracellular fate. Int J Nanomed (2020) 15:9355–71. doi: 10.2147/IJN.S281890
64. He C, Li L, Wang L, Meng W, Hao Y, Zhu G. Exosome-mediated cellular crosstalk within the tumor microenvironment upon irradiation. Cancer Biol Med (2021) 18(1):21–33. doi: 10.20892/j.issn.2095-3941.2020.0150
65. Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, et al. Exosome-based liquid biopsies in cancer: Opportunities and challenges. Ann Oncol (2021) 32(4):466–77. doi: 10.1016/j.annonc.2021.01.074
66. Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol Cancer (2020) 19(1):160. doi: 10.1186/s12943-020-01278-3
67. Mughees M, Kumar K, Wajid S. Exosome vesicle as a nano-therapeutic carrier for breast cancer. J Drug Target (2021) 29(2):121–30. doi: 10.1080/1061186X.2020.1808001
68. Gonzalez MJ, Kweh MF, Biava PM, Olalde J, Toro AP, Goldschmidt-Clermont PJ, et al. Evaluation of exosome derivatives as bio-informational reprogramming therapy for cancer. J Transl Med (2021) 19(1):103. doi: 10.1186/s12967-021-02768-8
69. Kim YK, Choi Y, Nam GH, Kim IS. Functionalized exosome harboring bioactive molecules for cancer therapy. Cancer Lett (2020) 489:155–62. doi: 10.1016/j.canlet.2020.05.036
70. Wu Q, Zhou L, Lv D, Zhu X, Tang H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol (2019) 12(1):53. doi: 10.1186/s13045-019-0739-0
71. Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: Communication from a distance. Dev Cell (2019) 49(3):347–60. doi: 10.1016/j.devcel.2019.04.011
72. Meng W, Hao Y, He C, Li L, Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol Cancer (2019) 18(1):57. doi: 10.1186/s12943-019-0982-6
73. Olioso D, Caccese M, Santangelo A, Lippi G, Zagonel V, Cabrini G, et al. Serum exosomal microRNA-21, 222 and 124–3p as noninvasive predictive biomarkers in newly diagnosed high-grade gliomas: A prospective study. Cancers (Basel) (2021) 13(12):3006. doi: 10.3390/cancers13123006
74. Shin S, Park YH, Jung SH, Jang SH, Kim MY, Lee JY, et al. Urinary exosome microRNA signatures as a noninvasive prognostic biomarker for prostate cancer. NPJ Genom Med (2021) 6(1):45. doi: 10.1038/s41525-021-00212-w
75. Wang X, Qian T, Bao S, Zhao H, Chen H, Xing Z, et al. Circulating exosomal miR-363–5p inhibits lymph node metastasis by downregulating PDGFB and serves as a potential noninvasive biomarker for breast cancer. Mol Oncol (2021) 15(9):2466–79. doi: 10.1002/1878-0261.13029
76. Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther (2020) 5(1):242. doi: 10.1038/s41392-020-00359-5
77. Gholipour E, Sarvarian P, Samadi P, Talebi M, Movassaghpour A, Motavalli R, et al. Exosome: From leukemia progression to a novel therapeutic approach in leukemia treatment. Biofactors (2020) 46(5):698–715. doi: 10.1002/biof.1669
78. Peswani Sajnani SL, Zhang Y, Vllasaliu D. Exosome-based therapies for mucosal delivery. Int J Pharm (2021) 608:121087. doi: 10.1016/j.ijpharm.2021.121087
79. Li B, Cao Y, Sun M, Feng H. Expression, regulation, and function of exosome-derived miRNAs in cancer progression and therapy. FASEB J (2021) 35(10):e21916. doi: 10.1096/fj.202100294RR
80. Santos P, Almeida F. Exosome-based vaccines: History, current state, and clinical trials. Front Immunol (2021) 12:711565. doi: 10.3389/fimmu.2021.711565
81. Jan AT, Rahman S, Badierah R, Lee EJ, Mattar EH, Redwan EM, et al. Expedition into exosome biology: A perspective of progress from discovery to therapeutic development. Cancers (Basel) (2021) 13(5):1157. doi: 10.3390/cancers13051157
82. Shen M, Chen T. Mesenchymal stem cell-derived EVs and their potential agents in hematological diseases. Oxid Med Cell Longev (2021) 2021:4539453. doi: 10.1155/2021/4539453
83. Yang C, Sun J, Tian Y, Li H, Zhang L, Yang J, et al. Immunomodulatory effect of MSCs and MSCs-derived extracellular vesicles in systemic lupus erythematosus. Front Immunol (2021) 12:714832. doi: 10.3389/fimmu.2021.714832
84. Tang LB, Ma SX, Chen ZH, Huang QY, Wu LY, Wang Y, et al. Exosomal microRNAs: Pleiotropic impacts on breast cancer metastasis and their clinical perspectives. Biol (Basel) (2021) 10(4):307. doi: 10.3390/biology10040307
85. Giordano C, La Camera G, Gelsomino L, Barone I, Bonofiglio D, Ando S, et al. The biology of EVs in breast cancer progression: Dissemination, immune evasion and metastatic colonization. Cancers (Basel) (2020) 12(8):2179. doi: 10.3390/cancers12082179
86. Kim H, Lee S, Shin E, Seong KM, Jin YW, Youn H, et al. The emerging roles of EVs as EMT regulators in cancer. Cells (2020) 9(4):861. doi: 10.3390/cells9040861
87. Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. EVs: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer (2019) 18(1):75. doi: 10.1186/s12943-019-0991-5
88. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
89. Huang T, Song X, Yang Y, Wan X, Alvarez AA, Sastry N, et al. Autophagy and hallmarks of cancer. Crit Rev Oncog (2018) 23(5–6):247–67. doi: 10.1615/CritRevOncog.2018027913
90. Sasahira T, Kirita T. Hallmarks of cancer-related newly prognostic factors of oral squamous cell carcinoma. Int J Mol Sci (2018) 19(8):2413. doi: 10.3390/ijms19082413
91. Qin F, Tang H, Zhang Y, Zhang Z, Huang P, Zhu J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J Cell Physiol (2020) 235(5):4734–45. doi: 10.1002/jcp.29351
92. Zhang H, Wang J, Ren T, Huang Y, Liang X, Yu Y, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett (2020) 490:54–65. doi: 10.1016/j.canlet.2020.07.008
93. Li F, Chen X, Shang C, Ying Q, Zhou X, Zhu R, et al. Bone marrow mesenchymal stem cells-derived extracellular vesicles promote proliferation, invasion and migration of osteosarcoma cells via the lncRNA MALAT1/miR-143/NRSN2/Wnt/beta-Catenin axis. Onco Targets Ther (2021) 14:737–49. doi: 10.2147/OTT.S283459
94. Zhao W, Qin P, Zhang D, Cui X, Gao J, Yu Z, et al. Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived EVs promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183–5p. Aging (Albany NY) (2019) 11(21):9581–96. doi:10.18632/aging.102406
95. Huang Y, Liu W, He B, Wang L, Zhang F, Shu H, et al. EVs derived from bone marrow mesenchymal stem cells promote osteosarcoma development by activating oncogenic autophagy. J Bone Oncol (2020) 21:100280. doi: 10.1016/j.jbo.2020.100280
96. Wang Y, Chu Y, Li K, Zhang G, Guo Z, Wu X, et al. EVs secreted by adipose-derived mesenchymal stem cells foster metastasis and osteosarcoma proliferation by increasing COLGALT2 expression. Front Cell Dev Biol (2020) 8:353. doi: 10.3389/fcell.2020.00353
97. Li Q, Wang X, Jiang N, Xie X, Liu N, Liu J, et al. Exosome-transmitted linc00852 associated with receptor tyrosine kinase AXL dysregulates the proliferation and invasion of osteosarcoma. Cancer Med (2020) 9(17):6354–66. doi: 10.1002/cam4.3303
98. Ge X, Liu W, Zhao W, Feng S, Duan A, Ji C, et al. Exosomal transfer of LCP1 promotes osteosarcoma cell tumorigenesis and metastasis by activating the JAK2/STAT3 signaling pathway. Mol Ther Nucleic Acids (2020) 21:900–15. doi: 10.1016/j.omtn.2020.07.025
99. Sha L, Ma D, Chen C. Exosome-mediated hic-5 regulates proliferation and apoptosis of osteosarcoma via wnt/beta-catenin signal pathway. Aging (Albany NY) (2020) 12(23):23598–608. doi: 10.18632/aging.103546
100. Han F, Pu P, Wang C, Ding X, Zhu Z, Xiang W, et al. Osteosarcoma cell-derived exosomal miR-1307 promotes tumorgenesis via targeting AGAP1. BioMed Res Int (2021) 2021:7358153. doi: 10.1155/2021/7358153
101. Wu C, Li Z, Feng G, Wang L, Xie J, Jin Y, et al. Tumor suppressing role of serum-derived exosomal microRNA-15a in osteosarcoma cells through the GATA binding protein 2/murine double minute 2 axis and the p53 signaling pathway. Bioengineered (2021) 12(1):8378–95. doi: 10.1080/21655979.2021.1987092
102. Zhang H, Wang J, Ren T, Huang Y, Liang X, Yu Y, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett (2020) 490:54–65. doi: 10.1016/j.canlet.2020.07.008
103. Li Q, Wang X, Jiang N, Xie X, Liu N, Liu J, et al. Exosome-transmitted linc00852 associated with receptor tyrosine kinase AXL dysregulates the proliferation and invasion of osteosarcoma. Cancer Med (2020) 9(17):6354–66. doi: 10.1002/cam4.3303
104. Wu C, Li Z, Feng G, Wang L, Xie J, Jin Y, et al. Tumor suppressing role of serum-derived exosomal microRNA-15a in osteosarcoma cells through the GATA binding protein 2/murine double minute 2 axis and the p53 signaling pathway. Bioengineered (2021) 12(1):8378–95. doi: 10.1080/21655979.2021.1987092
105. Chong ZX, Yeap SK, Ho WY. Unraveling the roles of miRNAs in regulating epithelial-to-mesenchymal transition (EMT) in osteosarcoma. Pharmacol Res (2021) 172:105818. doi: 10.1016/j.phrs.2021.105818
106. Zhang N, Ng AS, Cai S, Li Q, Yang L, Kerr D. Novel therapeutic strategies: Targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol (2021) 22(8):e358–e68. doi: 10.1016/S1470-2045(21)00343-0
107. Gonzalez-Martinez S, Perez-Mies B, Pizarro D, Caniego-Casas T, Cortes J, Palacios J. Epithelial mesenchymal transition and immune response in metaplastic breast carcinoma. Int J Mol Sci (2021) 22(14):7398. doi: 10.3390/ijms22147398
108. Pal A, Barrett TF, Paolini R, Parikh A. Puram SV. partial EMT in head and neck cancer biology: A spectrum instead of a switch. Oncogene (2021) 40(32):5049–65. doi: 10.1038/s41388-021-01868-5
109. Chattopadhyay I, Ambati R, Gundamaraju R. Exploring the crosstalk between inflammation and epithelial-mesenchymal transition in cancer. Mediators Inflammation (2021) 2021:9918379. doi: 10.1155/2021/9918379
110. Shimbo K, Miyaki S, Ishitobi H, Kato Y, Kubo T, Shimose S, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun (2014) 445(2):381–7. doi: 10.1016/j.bbrc.2014.02.007
111. Gong L, Bao Q, Hu C, Wang J, Zhou Q, Wei L, et al. Exosomal miR-675 from metastatic osteosarcoma promotes cell migration and invasion by targeting CALN1. Biochem Biophys Res Commun (2018) 500(2):170–76. doi: 10.1016/j.bbrc.2018.04.016
112. Zhong L, Liao D, Li J, Liu W, Wang J, Zeng C, et al. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into EVs. Signal Transduct Target Ther (2021) 6(1):59. doi: 10.1038/s41392-020-00414-1
113. Mazumdar A, Urdinez J, Boro A, Arlt MJE, Egli FE, Niederost B, et al. Exploring the role of osteosarcoma-derived extracellular vesicles in pre-metastatic niche formation and metastasis in the 143-b xenograft mouse osteosarcoma model. Cancers (Basel) (2020) 12(11):3457. doi: 10.3390/cancers12113457
114. Liu CG, Li J, Xu Y, Li W, Fang SX, Zhang Q, et al. Long non-coding RNAs and circular RNAs in tumor angiogenesis: From mechanisms to clinical significance. Mol Ther Oncolytics (2021) 22:336–54. doi: 10.1016/j.omto.2021.07.001
115. Aspritoiu VM, Stoica I, Bleotu C, Diaconu CC. Epigenetic regulation of angiogenesis in development and tumors progression: Potential implications for cancer treatment. Front Cell Dev Biol (2021) 9:689962. doi: 10.3389/fcell.2021.689962
116. Martin P, Gurevich DB. Macrophage regulation of angiogenesis in health and disease. Semin Cell Dev Biol (2021) 119:101–10. doi: 10.1016/j.semcdb.2021.06.010
117. Zhu L, Yu X, Wang L, Liu J, Qu Z, Zhang H, et al. Angiogenesis and immune checkpoint dual blockade in combination with radiotherapy for treatment of solid cancers: Opportunities and challenges. Oncogenesis (2021) 10(7):47. doi: 10.1038/s41389-021-00335-w
118. Montanino A, Manzo A, Carillio G, Palumbo G, Esposito G, Sforza V, et al. Angiogenesis inhibitors in small cell lung cancer. Front Oncol (2021) 11:655316. doi: 10.3389/fonc.2021.655316
119. Yoshida A, Fujiwara T, Uotani K, Morita T, Kiyono M, Yokoo S, et al. Clinical and functional significance of intracellular and extracellular microRNA-25–3p in osteosarcoma. Acta Med Okayama (2018) 72(2):165–74. doi: 10.18926/AMO/55857
120. Tao SC, Huang JY, Wei ZY, Li ZX, Guo SC. EWSAT1 acts in concert with EVs in osteosarcoma progression and tumor-induced angiogenesis: The “Double stacking effect”. Adv Biosyst (2020) 4(9):e2000152. doi: 10.1002/adbi.202000152
121. Li Y, Lin S, Xie X, Zhu H, Fan T, Wang S. Highly enriched exosomal lncRNA OIP5-AS1 regulates osteosarcoma tumor angiogenesis and autophagy through miR-153 and ATG5. Am J Transl Res (2021) 13(5):4211–23.
122. Mao W, Wang K, Wu Z, Xu B, Chen M. Current status of research on EVs in general, and for the diagnosis and treatment of kidney cancer in particular. J Exp Clin Cancer Res (2021) 40(1):305. doi: 10.1186/s13046-021-02114-2
123. Wang M, Zhang B. The immunomodulation potential of EVs in tumor microenvironment. J Immunol Res (2021) 2021:3710372. doi: 10.1155/2021/3710372
124. Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, et al. The biology, function, and applications of EVs in cancer. Acta Pharm Sin B (2021) 11(9):2783–97. doi: 10.1016/j.apsb.2021.01.001
125. Chen H, Chengalvala V, Hu H, Sun D. Tumor-derived EVs: Nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm Sin B (2021) 11(8):2136–49. doi: 10.1016/j.apsb.2021.04.012
126. Zheng H, Siddharth S, Parida S, Wu X, Sharma D. Tumor microenvironment: Key players in triple negative breast cancer immunomodulation. Cancers (Basel) (2021) 13(13):3357. doi: 10.3390/cancers13133357
127. Whiteside TL, Diergaarde B, Hong CS. Tumor-derived EVs (TEX) and their role in immuno-oncology. Int J Mol Sci (2021) 22(12):6234. doi: 10.3390/ijms22126234
128. Raimondi L, De Luca A, Gallo A, Costa V, Russelli G, Cuscino N, et al. Osteosarcoma cell-derived EVs affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis (2020) 41(5):666–77. doi: 10.1093/carcin/bgz130
129. Wolf-Dennen K, Gordon N, Kleinerman ES. Exosomal communication by metastatic osteosarcoma cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions. Oncoimmunology (2020) 9(1):1747677. doi: 10.1080/2162402X.2020.1747677
130. Mazumdar A, Urdinez J, Boro A, Migliavacca J, Arlt MJE, Muff R, et al. Osteosarcoma-derived extracellular vesicles induce lung fibroblast reprogramming. Int J Mol Sci (2020) 21(15):5451. doi: 10.3390/ijms21155451
131. Cheng Z, Wang L, Wu C, Huang L, Ruan Y, Xue W. Tumor-derived EVs induced M2 macrophage polarization and promoted the metastasis of osteosarcoma cells through Tim-3. Arch Med Res (2021) 52(2):200–10. doi: 10.1016/j.arcmed.2020.10.018
132. Zhang Y, Liu Z, Yang X, Lu W, Chen Y, Lin Y, et al. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics (2021) 11(3):1473–92. doi: 10.7150/thno.51245
133. Zhang H, Yu Y, Wang J, Han Y, Ren T, Huang Y, et al. Macrophages-derived exosomal lncRNA LIFR-AS1 promotes osteosarcoma cell progression via miR-29a/NFIA axis. Cancer Cell Int (2021) 21(1):192. doi: 10.1186/s12935-021-01893-0
134. Liu W, Long Q, Zhang W, Zeng D, Hu B, Liu S, et al. miRNA-221–3p derived from M2-polarized tumor-associated macrophage EVs aggravates the growth and metastasis of osteosarcoma through SOCS3/JAK2/STAT3 axis. Aging (Albany NY) (2021) 13(15):19760–75. doi: 10.18632/aging.203388
135. Cheng Z, Wang L, Wu C, Huang L, Ruan Y, Xue W. Tumor-derived EVs induced M2 macrophage polarization and promoted the metastasis of osteosarcoma cells through Tim-3. Arch Med Res (2020) 52(2):200–10. doi: 10.21203/rs.2.23484/v1
136. Hwang S, Yang YM. Exosomal microRNAs as diagnostic and therapeutic biomarkers in non-malignant liver diseases. Arch Pharm Res (2021) 44(6):574–87. doi: 10.1007/s12272-021-01338-2
137. Wen H, Peng L, Chen Y. The effect of immune cell-derived EVs in the cardiac tissue repair after myocardial infarction: Molecular mechanisms and preclinical evidence. J Cell Mol Med (2021) 25(14):6500–10. doi: 10.1111/jcmm.16686
138. Hosseini R, Asef-Kabiri L, Yousefi H, Sarvnaz H, Salehi M, Akbari ME, et al. The roles of tumor-derived EVs in altered differentiation, maturation and function of dendritic cells. Mol Cancer (2021) 20(1):83. doi: 10.1186/s12943-021-01376-w
139. Huda MN, Nafiujjaman M, Deaguero IG, Okonkwo J, Hill ML, Kim T, et al. Potential use of EVs as diagnostic biomarkers and in targeted drug delivery: Progress in clinical and preclinical applications. ACS Biomater Sci Eng (2021) 7(6):2106–49. doi: 10.1021/acsbiomaterials.1c00217
140. Xu JF, Wang YP, Zhang SJ, Chen Y, Gu HF, Dou XF, et al. Exosomes containing the differential expression of microRNA and mRNA in osteosarcoma that can predict response to chemotherapy. Oncotarget. (2017) 8(44):75968–78. doi: 10.18632/oncotarget.18373
141. Ayers L, Pink R, Carter DRF, Nieuwland R. Clinical requirements for extracellular vesicle assays. J Extracell Vesicles. (2019) 8(1):1593755. doi: 10.1080/20013078.2019.1593755
142. Cuscino N, Raimondi L, De Luca A, Carcione C, Russelli G, Conti L, et al. Gathering novel circulating exosomal microRNA in osteosarcoma cell lines and possible implications for the disease. Cancers (Basel) (2019) 11(12):1924. doi: 10.3390/cancers11121924
143. Zhang K, Dong C, Chen M, Yang T, Wang X, Gao Y, et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics (2020) 10(1):411–25. doi: 10.7150/thno.33482
144. Ye Z, Zheng Z, Peng L. MicroRNA profiling of serum EVs in patients with osteosarcoma by high-throughput sequencing. J Investig Med (2020) 68(4):893–901. doi: 10.1136/jim-2019-001196
145. Zhang H, Wang J, Ren T, Huang Y, Yu Y, Chen C, et al. LncRNA CASC15 is upregulated in osteosarcoma plasma EVs and CASC15 knockdown inhibits osteosarcoma progression by regulating miR-338–3p/RAB14 axis. Onco Targets Ther (2020) 13:12055–66. doi: 10.2147/OTT.S282053
146. Cambier L, Stachelek K, Triska M, Jubran R, Huang M, Li W, et al. Extracellular vesicle-associated repetitive element DNAs as candidate osteosarcoma biomarkers. Sci Rep (2021) 11(1):94. doi: 10.1038/s41598-020-77398-z
147. Huo S, Dou D. Circ_0056285 regulates proliferation, apoptosis and glycolysis of osteosarcoma cells via miR-1244/TRIM44 axis. Cancer Manag Res (2021) 13:1257–70. doi: 10.2147/CMAR.S290645
148. Wang L, Wu J, Song S, Chen H, Hu Y, Xu B, et al. Plasma exosome-derived sentrin SUMO-specific protease 1: A prognostic biomarker in patients with osteosarcoma. Front Oncol (2021) 11:625109. doi: 10.3389/fonc.2021.625109
149. Han Z, Yi J, Yang Y, Li D, Peng C, Long S, et al. SERS and MALDI-TOF MS based plasma exosome profiling for rapid detection of osteosarcoma. Analyst (2021) 146(21):6496–505. doi: 10.1039/d1an01163d.
150. Anderson PM. Effectiveness of radiotherapy for osteosarcoma that responds to chemotherapy. Mayo Clin Proc (2003) 78:145–6. doi: 10.4065/78.2.145
151. Schwarz R, Bruland Øyvind S, Cassoni A, Schomberg P, Bielack S. The role of radiotherapy in oseosarcoma. Cancer Treat Res (2009) 152:147–64. doi: 10.1007/978-1-4419-0284-9_7
152. Majó J, Cubedo R, Pardo N. Treatment of osteosarcoma. a review. Rev Esp. Cir. Ortop. Traumatol. (2010) 54:329–36. doi: 10.1016/S1988-8856(10)70255-8
153. Saraf AJ, Fenger JM, Roberts RD. Osteosarcoma: Accelerating progress makes for a hopeful future. Front Oncol (2018) 8:4. doi: 10.3389/fonc.2018.00004
154. Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol (2007) 19:341–6. doi: 10.1097/CCO.0b013e328122d73f
155. Mialou V, Philip T, Kalifa C, Perol D, Gentet J-C, Marec-Berard P, et al. Metastatic osteosarcoma at diagnosis: Prognostic factors and long-term outcom–the French pediatric experience. Cancer (2005) 104:1100–9. doi: 10.1002/cncr.21263
156. Lee KM, An JH, Yang SJ, Park SM, Lee JH, Chae HK, et al. Influence of canine macrophage-derived extracellular vesicles on apoptosis in canine melanoma and osteosarcoma cell lines. Anticancer. Res (2021) 41:719–30. doi: 10.21873/anticanres.14823
157. Xu Z, Zhou X, Wu J, Cui X, Wang M, Wang X, et al. Mesenchymal stem cell-derived exosomes carrying microRNA-150 suppresses the proliferation and migration of osteosarcoma cells via targeting IGF2BP1. Transl Cancer Res (2020) 9:5323–35. doi: 10.21037/tcr-20-83
158. Killingsworth B, Welsh JA, Jones JC. EV translational horizons as viewed across the complex landscape of liquid biopsies. Front Cell Dev Biol (2021) 9:556837. doi: 10.3389/fcell.2021.556837
159. Clark RA, Garman ZG, Price RJ, Sheybani ND. Functional intersections between extracellular vesicles and oncolytic therapies. Trends Pharmacol Sci (2021) 42(11):883–96. doi: 10.1016/j.tips.2021.09.001
160. Gaglani S, Gonzalez-Kozlova E, Lundon DJ, Tewari AK, Dogra N, Kyprianou N. EVs as a next-generation diagnostic and therapeutic tool in prostate cancer. Int J Mol Sci (2021) 22(18):131. doi: 10.3390/ijms221810131
161. Mkhobongo B, Chandran R, Abrahamse H. The role of melanoma cell-derived EVs (MTEX) and photodynamic therapy (PDT) within a tumor microenvironment. Int J Mol Sci (2021) 22(18):131. doi: 10.3390/ijms221810131
162. Prieto-Vila M, Yoshioka Y, Ochiya T. Biological functions driven by mRNAs carried by extracellular vesicles in cancer. Front Cell Dev Biol (2021) 9:620498. doi: 10.3389/fcell.2021.620498
163. Deng Y, Sun Z, Wang L, Wang M, Yang J, Li G. Biosensor-based assay of exosome biomarker for early diagnosis of cancer. Front Med (2021) 16(2):157–75. doi: 10.1007/s11684-021-0884-z
164. Nie H, Liao Z, Wang Y, Zhou J, He X, Ou C. Exosomal long non-coding RNAs: Emerging players in cancer metastasis and potential diagnostic biomarkers for personalized oncology. Genes Dis (2021) 8(6):769–80. doi: 10.1016/j.gendis.2020.12.004
165. Jing Z, Chen K, Gong L. The significance of EVs in pathogenesis, diagnosis, and treatment of esophageal cancer. Int J Nanomed (2021) 16:6115–27. doi: 10.2147/IJN.S321555
166. Torreggiani E, Roncuzzi L, Perut F, Zini N, Baldini N. Multimodal transfer of MDR by exosomes in human osteosarcoma. Int J Oncol (2016) 49(1):189–96. doi: 10.3892/ijo.2016.3509
167. Nawaz M, Fatima F, Nazarenko I, Ekström K, Murtaza I, Anees M, et al. Extracellular vesicles in ovarian cancer: Applications to tumor biology, immunotherapy and biomarker discovery. Expert Rev Proteomics. (2016) 13:395–409. doi: 10.1586/14789450.2016.1165613
168. Mc Namee N, O'Driscoll L. Extracellular vesicles and anti-cancer drug resistance. Biochim Biophys Acta (2018) 1870:123–36. doi: 10.1016/j.bbcan.2018.07.003
169. Ng CY, Chai JY, Foo JB, Mohamad Yahaya NH, Yang Y, Ng MH, et al. Potential of EVs as cell-free therapy in articular cartilage regeneration: A review. Int J Nanomed (2021) 16:6749–81. doi: 10.2147/IJN.S327059
170. Claridge B, Lozano J, Poh QH, Greening DW. Development of extracellular vesicle therapeutics: Challenges, considerations, and opportunities. Front Cell Dev Biol (2021) 9:734720. doi: 10.3389/fcell.2021.734720
171. Matheakakis A, Batsali A, Papadaki HA, Pontikoglou CG. Therapeutic implications of mesenchymal stromal cells and their extracellular vesicles in autoimmune diseases: From biology to clinical applications. Int J Mol Sci (2021) 22(18):132. doi: 10.3390/ijms221810132
172. Han S, Li G, Jia M, Zhao Y, He C, Huang M, et al. Delivery of anti-miRNA-221 for colorectal carcinoma therapy using modified cord blood mesenchymal stem cells-derived EVs. Front Mol Biosci (2021) 8:743013. doi: 10.3389/fmolb.2021.743013
173. Li T, Tao Z, Zhu Y, Liu X, Wang L, Du Y, et al. Exosomal annexin A6 induces gemcitabine resistance by inhibiting ubiquitination and degradation of EGFR in triple-negative breast cancer. Cell Death Dis (2021) 12(7):684. doi: 10.1038/s41419-021-03963-7
Keywords: EVs, osteosarcoma, biomarkers, treatment, diagnosis
Citation: Gao X, Gao B and Li S (2022) Extracellular vesicles: A new diagnostic biomarker and targeted drug in osteosarcoma. Front. Immunol. 13:1002742. doi: 10.3389/fimmu.2022.1002742
Received: 25 July 2022; Accepted: 12 September 2022;
Published: 23 September 2022.
Edited by:
Hamid Reza Mirzaei, Tehran University of Medical Sciences, IranReviewed by:
Maryam Azimi, Iran University of Medical Sciences, IranCopyright © 2022 Gao, Gao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shenglong Li, bGlzaGVuZ2xvbmdAY2FuY2VyaG9zcC1sbi1jbXUuY29t; c2xsaUBjbXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.