
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 06 December 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1001414
This article is part of the Research Topic Community Series in Immunotherapy with Checkpoint Inhibitors for Non-small Cell Lung Cancer, Colon Cancer and Esophageal Cancer, volume II View all 19 articles
Background: Pulmonary lymphoepithelioma-like carcinoma (LELC) exhibits a unique immune microenvironment, including high PD-L1 expression and abundant infiltrating-immune cells. However, the availability of PD-1/PD-L1 inhibitors in patients with LELC is still not determined.
Methods: A total of 36 cases of pulmonary LELC treated with PD-1/PD-L1 inhibitors were reviewed, including 10 cases from our institute and 26 cases included from the literature. The Kaplan-Meier method and log-rank test were utilized to analyze the survival outcomes of LELC patients receiving immunotherapy, and the factors related to immunotherapy response were further examined.
Results: Of the 10 patients from our institute, the median age was 53.5 years, adrenal glands and distant lymph nodes were the most common metastatic sites, and 4 of 8 (50%) patients had a PD-L1 TPS ≥50%. The median progression-free survival and overall survival in patients from our institute and from the literature were 11.6 and 27.3 months, 17.2 months and not reached, respectively. In all 36 patients, the objective response rate was as high as 57.6%. Patients with higher PD-L1 expression were more likely to have a tumor response, but the association of PD-L1 expression with survival time remains to be determined.
Conclusions: PD-1/PD-L1 inhibitors in patients with pulmonary LELC demonstrated a promising efficacy in retrospective cohorts, and deserve further validation in prospective studies administrating in front-line setting.
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) is a rare subtype of non-small cell lung cancer (NSCLC) predominantly affecting younger non-smokers in Southeast Asia where nasopharyngeal cancer (NPC) prevails (1, 2). First reported in 1987, it is a non-keratinizing, poorly differentiated, pulmonary-originated squamous cell carcinoma associated with Epstein-Barr Virus (EBV) infection (3). Recently, a genetic study using whole-exome sequencing has revealed that pulmonary LELC possesses a distinct genomic profile from other lung cancers but shares similar alterations with NPC, including constitutive activation of inflammatory nuclear factor kappa B (NF-ĸB) signaling driven by EBV-encoded oncoprotein latent infection membrane protein 1 (LMP1) and crippled innate antiviral immunity due to losses of type I interferon (IFN) genes (4). In addition, programmed cell death-ligand 1 (PD-L1) upregulation in pulmonary LELC has been recognized as a major culprit to blame for undermining adaptive immune response (4, 5). Indeed, pulmonary LELC is uniquely featured by its inflamed environment but effective immune evasion.
Optimal treatment for advanced pulmonary LELC has not been well established. Our previous study had identified that platinum-based combination chemotherapy with or without radiotherapy could achieve good initial responses (6). However, the majority of advanced tumors eventually progressed from upfront treatments and there are limited therapeutic options to choose from after their resistance to chemotherapy. Actionable oncogenic driver mutations in NSCLC, such as epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement, rarely exist in pulmonary LELC (7). PD-1 blockade or its combination with chemotherapy has been proven effective in treating patients with advanced NPC (8–10). Based on the histologic and genetic resemblance of pulmonary LELC to NPC (4, 5, 11), one might naturally surmise that PD-1/PD-L1 inhibitors would emerge as another promising weapon in the therapeutic arsenal against this rare tumor.
Yet, only a handful of case reports have explored the efficacy of anti-PD-1/PD-L1 antibodies in pulmonary LELC (12–21). The rarity of this lethal disease makes it impossible to conduct any convincing clinical trial for this matter. Here, we reported a cohort of ten patients who received anti-PD-1 therapy after routine management failed. To our knowledge, this represents the first and the largest cohort to test PD-1 inhibitors as late-line treatment in patients with advanced pulmonary LELC. We also performed a focused search of the literature with Pubmed to identify studies of blocking PD-1/PD-L1 in pulmonary LELC and attempted to determine predictors of efficacy from collected clinicopathological traits in this enriched population.
From July 2017 to September 2020, 10 patients received anti-PD-1 antibodies after progression from previous chemotherapy in the Centro Hospitalar Conde de Sao Januario (CHCSJ), Macau for advanced pulmonary LELCs. Data were collected from the hospital information system. All these cases were confirmed by Epstein-Barr encoding region (EBER) positivity. Otolaryngologists’ consultations with nasopharyngoscopy check-ups and imaging tests were applied to rule out NPC or other origins of LELCs. Flat dosing of nivolumab (240mg every 2 weeks) or pembrolizumab (200mg every 3 weeks) were given until progressive disease or intolerable toxicity. Tumor assessments were performed by computed tomography before the anti-PD-1 treatment and every 6 or 9 weeks thereafter. Treatment response was determined according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST) (22). Progression-free survival (PFS) was calculated from the date of starting anti-PD-1 treatment to disease progression or death due to any cause. Overall survival (OS) was calculated from the date of starting anti-PD-1 treatment to death due to any cause.
PD-L1 expressions of 8 patients were assessed, based on sample deriving from primary tumor, by the PD-L1 immunohistochemical (IHC) staining 22C3 pharmDx assay (Dako North America, CarpinteriaCA) that has been approved as a companion diagnostic for use in non–small-cell lung cancer (23). The staining protocol used in this study was as described in the instructions for the commercial assay. Expression was scored using a tumor proportion score (TPS) which is defined as the number of positive tumor cells divided by the total number of viable tumor cells multiplied by 100%.
We conducted a literature search for reports of pulmonary LELCs in the Pubmed database and collected 191 studies. We then screened out 11 studies that focused on using PD-1/PD-L1 inhibitors monotherapy or their combination to treat advanced diseases. 10 studies were finally recruited after removing 1 case that was duplicated in the CHCSJ cohort. Studies that contained original information on clinical results of PD-1/PD-L1 inhibitor treatment were screened, including original researches and case reports. Clinicopathological factors, PD-L1 expression status, tumor response, and survival data were collected.
The Mann-Whitney U test was used to analyze the relationship between PD-L1 expression and tumor responses to anti-PD-1/PD-L1 therapy. PFS and OS were assessed using Kaplan-Meier method. Patients were divided into two groups (low/high) according to their PD-L1 expression level and based on the optimal cut-off value of PFS calculated by the “survminer” package of R software. Univariate and multivariate analyses were applied to identify prognostic factors of PFS. R (version 3.6.1, http://www.r-project.org) was used for statistical analyses. Two-tailed value of p < 0.05 was considered to be statistically significant.
The clinicopathological features of the CHCSJ cohort are described in Table 1. There were 4 males and 6 females. The median age of this cohort was 53.5 years (range, 46-67 years). Adrenal glands and distant lymph nodes were the most common sites of metastases (n=3, respectively). 8 patients had their PD-L1 expression tested: 4 (50%) were with TPS ≥50%, 3 with TPS 1~49%, and 1 with TPS <1%. 6 patients received pembrolizumab while 4 others nivolumab. Anti-PD-1 antibodies were applied in the 2nd line setting in 3 patients, the 3rd line setting in 3 patients, and the 4th line setting in 4 patients. The median duration of anti-PD-1 treatment was 10.5 cycles (range, 1 to 30 cycles) and detailed descriptions of the duration of each treatment were shown in Figure 1.
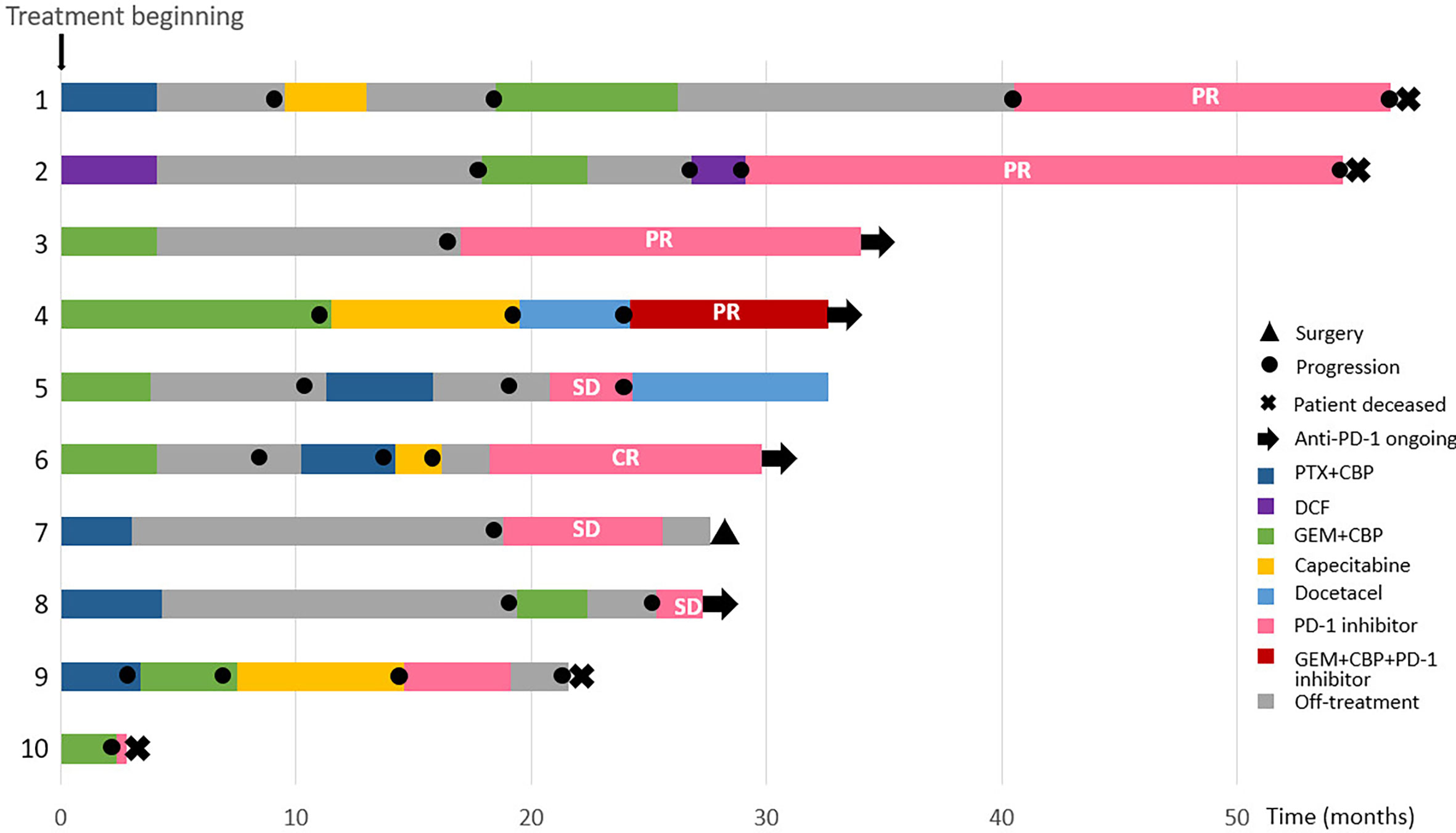
Figure 1 Summary of treatment reactions to chemotherapy and anti-PD-1 antibodies in the CHCSJ cohort.
8 patients were available for response rate analysis and 2 others died before an imaging-based assessment could be carried out. The objective response rate (ORR) was 62.5% (5/8), while disease control rate (DCR) was 100%. One male patient (12.5%) receiving nivolumab monotherapy as 3rd line treatment achieved a complete response (CR). Partial response (PR) was reached in 4 (50.0%) patients who took pembrolizumab or its combination with chemotherapy in the 2nd to 4th line. 3 others achieved stable disease (SD).
During a median follow-up time of 18.5 months, 7 patients had progressed from PD-1 inhibitors or died from pulmonary LELC. The median PFS was 11.6 months (95% CI 8.8-NR [not reached]) and the median OS was 27.3 months (95% CI 17.4-NR) (Supplementary Figure S1).
We finally recruited ten reports from 2017 to 2021 and gathered a total of 26 pulmonary LELC patients who were treated with PD-1/PD-L1 inhibitors for their unresectable or metastatic diseases (Table 2). 42.3% of patients received PD-1/PD-L1 inhibitors monotherapy, 34.6% of patients anti-PD-1 antibodies with chemotherapy, and 23.1% of patients anti-PD-1 antibodies with vascular endothelial growth factor receptor (VEGFR)-targeted tyrosine kinase inhibitors (TKIs). 19.2% of patients took immunotherapy in the 1st line, 57.7% of patients in the 2nd line, 23.1% of patients in the 3rd or later lines. Of 25 patients whose responses had been clearly reported, 56% achieved PR and 40% SD. Only 1 patient experienced quick progression after nivolumab. The median PFS was 17.2 months (95% CI 7.7-NR). The median OS was not reached (Supplementary Figure S2).
Altogether, the CHCSJ cohort and literature review contributed 36 pulmonary LELC patients. 30 patients had PD-L1 expression analyzed using TPS. 33 patients had clear tumor response data of anti-PD-1/PD-L1-based therapy. Of 27 patients with both available PD-L1 expression and immunotherapy response data, those who achieved PR had a significantly higher level of PD-L1 expression compared to those who achieved SD (median PD-L1 expression: 80% vs 22.5%, p=0.002, Figure 2A). Utilizing TPS 30% as the cut-off value determined by the “survminer” package of R software, patients with high PD-L1 expression (>30%) had higher ORR (14/17, 82.4% vs 1/10, 10.0%, p<0.001, Figure 2B) than those with low PD-L1 expression (≤30%). Likewise, the PFS of patients with high PD-L1 expression in the CHCSJ cohort was 25.4 months (95% CI 16.2-NR), significantly longer than that of those with low PD-L1 expression (6.2 months, 95% CI 3.5-NR, p=0.027, Figure 3A). However, such survival advantage in patients with high-level PD-L1 expression was not seen in OS analysis (27.3 vs 3.5 months, p=0.628, Figure 3B). Furthermore, there were no significant differences in PFS (17.2 vs 8.8 months, p=0.177, Figure 3C) and OS (22.0 months vs NR, p=0.541, Figure 3D) between those with high and low PD-L1 expression when the two cohorts combined.
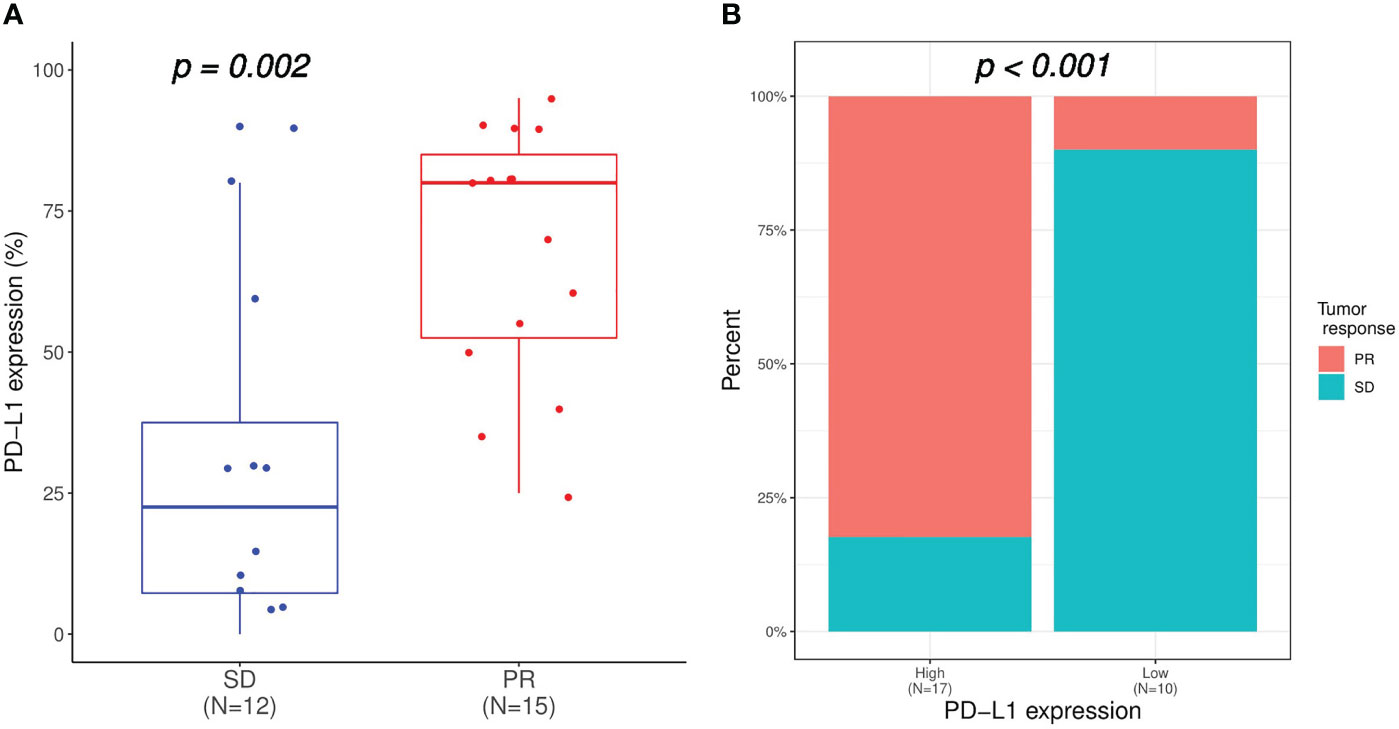
Figure 2 Relation of PD-L1 expression with immunotherapy response. (A) Comparison of PD-L1 expression between patients achieved SD and PR. (B) Comparison of immunotherapy response between patients with high (TPS ≥30%) and low (TPS <30%) PD-L1 expression. SD, stable disease; PR, partial response; TPS, tumor proportion score.
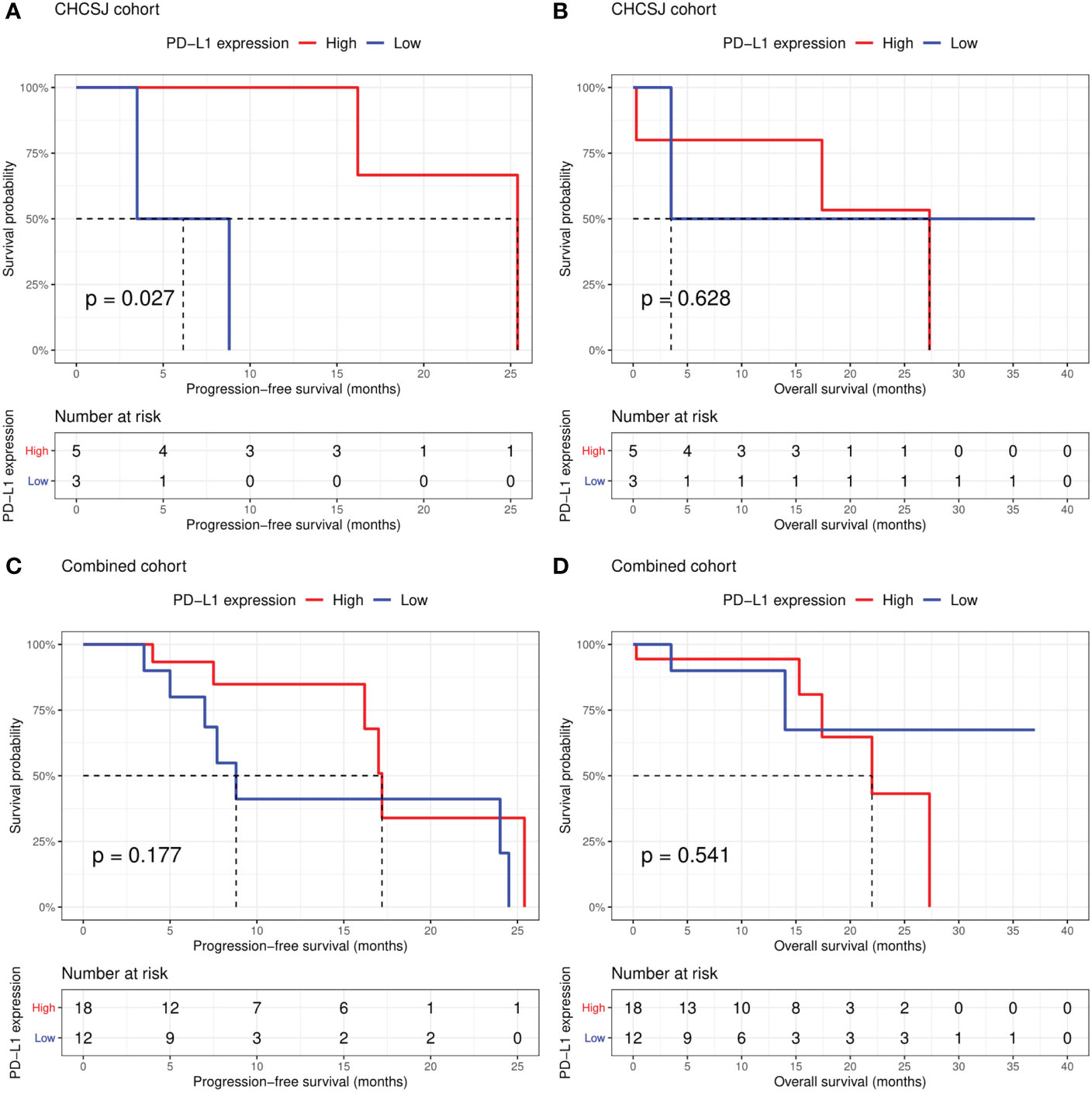
Figure 3 Comparison of progression-free survival (A, C) and overall survival (B, D) between patients with high (TPS ≥30%) and low (TPS <30%) PD-L1 expression in the CHCSJ and combined cohorts. TPS, tumor proportion score.
During a median follow-up time of 10.5 months (range, 0.3-37 months), 18 patients experienced disease progression and 8 patients died of pulmonary LELCs among 36 patients. The Cox regression analysis was conducted to assess the impact of other clinical factors on PFS and OS. As shown in Table 3, univariate analysis showed that, compared to non-smoking patients, former or current smokers tended to have poor PFS (HR=12.08, p=0.053). However, neither smoking history nor treatment modality became independent predictors in multivariate analysis. In contrast, the difference in OS between never-smokers and ever-smokers was statistically significant (HR 30.74, 95% CI 2.11-447.25, p=0.012). However, other factors including sex, age (≥60y vs <60y), tumor stage (locally advanced vs metastatic), treatment modality (monotherapy or combination), liver metastasis (yes vs no), treatment scenario (<2nd line vs ≥3rd line) and PD-L1 expression (TPS >30% vs ≤30%) did not seem to exert any significant influence on the OS of patients with pulmonary LELCs (Table 4). Furthermore, compared with patients receiving immunotherapy alone, those who received immunotherapy combined with chemotherapy or targeted therapy had statistically non-significant improved PFS (8.8 vs 17.2 months, p=0.128) and OS (15.3 months vs NR, p=0.541, Supplementary Figure S3).

Table 3 Univariate and multivariate analyses of factors for progression-free survival in the combined two cohorts.
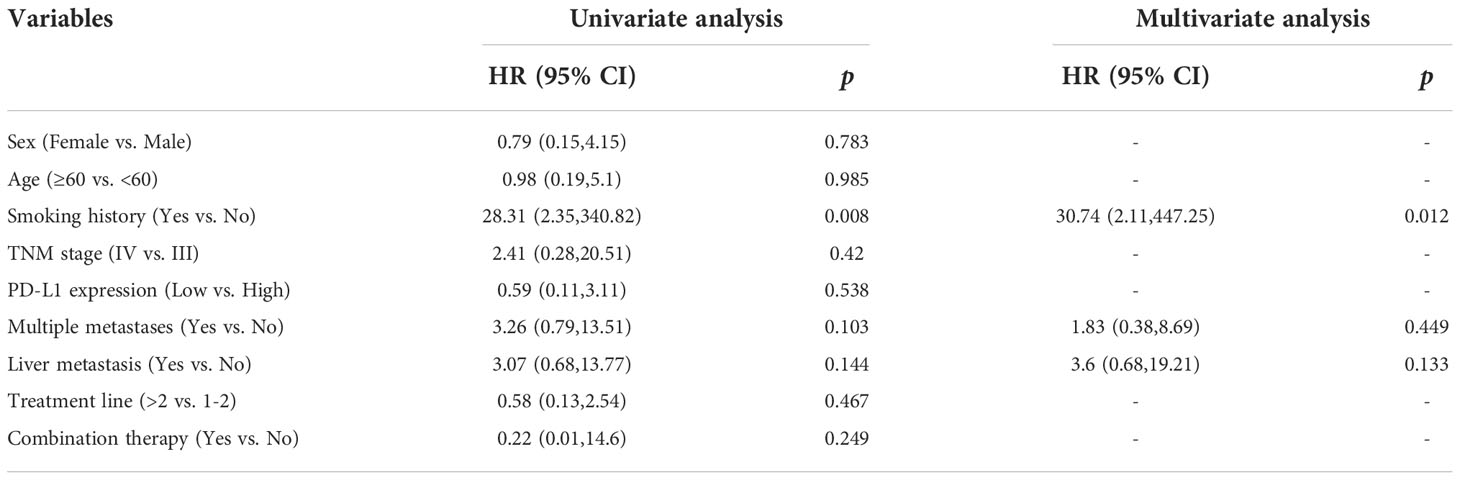
Table 4 Univariate and multivariate analyses of factors for overall survival in the combined two cohorts.
Pulmonary LELC is a rare subtype of NSCLC characterized by EBV infection and abundant lymphocyte infiltration. EBV infection has been linked to upregulation of PD-L1 expression in malignancies (24). On the other hand, a high level of lymphocyte infiltration and PD-L1 expression are believed to be associated with immunotherapy response (25). However, the efficacy of PD-1/PD-L1 inhibitors in pulmonary LELC needs to be further verified.
In the present study, we described 10 previously treated LELC patients receiving PD-1 inhibitors, with an ORR as high as 62.5%. Of note, in a retrospective study previously reported by our institution, 41 LELC patients treated by 2nd or above chemotherapy had an ORR of 20-25% (6). Likewise, previously treated LELC patients receiving immunotherapy also tended to have longer PFS and OS (6). Furthermore, we performed a literature review, and found that pulmonary LELC tends to have a higher PD-L1 expression and desirable immunotherapy response compared with other subtypes of NSCLC (26). Consistently, in the CHCSJ cohort, our results suggested that the PFS of patients with PD-L1 TPS >30% was significantly longer than those with TPS ≤30%. In contrast, the association of prolonged PFS with higher PD-L1 expression was not observed in patients retrieved from literature or when two cohorts were combined. Furthermore, the prognostic significance (both PFS and OS) of PD-L1 expression levels in LELC patients treated with chemotherapy is also controversial (27–29). Intriguingly, when we focused on the patients of Chinese descent, those with high PD-L1 expression (TPS >30%) tended to have a prolonged PFS (n=29, p=0.09, data not shown). Even when the study was limited to the patients who received PD-1/PD-L1 inhibitors in China, high PD-L1 expression was significantly associated with prolonged PFS (n=28, p=0.049, data not shown). These results suggest the possibility that patient pedigree and environmental factors may influence the efficacy of immunotherapy in pulmonary LELC. On all accounts, given the desirable efficacy of PD-1/PL-L1 inhibitors in lung and nasopharyngeal cancer, our results support the administration of immunotherapy in patients with pulmonary LELC.
Notably, our results suggested non-smoking patients with pulmonary LELC may have better survival outcomes when compared to ever-smokers. However, the effect of smoking on the tumor immune microenvironment is complicated. On the one hand, smoking has been associated with elevated tumor mutation burden and PD-L1 expression, suggesting better immunotherapy results (30). On the other hand, smoking may impair PD-1/PD-L1 response by inhibiting immune cell infiltration into tumors (31). Studies support that smoking can improve the efficacy of checkpoint inhibitor monotherapy, but does not significantly affect the response of NSCLC to immunochemotherapy combination (32). Specifically, a previous study reported that smoking was an independent predictor of unfavorable survival in patients with pulmonary LELC (33). Overall, the effect of smoking status on immunotherapy outcomes in patients with LELC needs to be further confirmed in larger cohorts.
To the best of our knowledge, the present study reports the largest cohort to date of pulmonary LELC patients treated with PD-1 inhibitors, and our results support the administration of immunotherapy in LELC patients. Nevertheless, several limitations need to be highlighted. Firstly, the relatively small scale of the CHCSJ and literature review cohorts might lead to selection bias. Although we did a thorough screening in Pubmed, only 1 case whose disease progressed after the application of nivolumab was found. This may be the case, meaning the vast majority of advanced pulmonary LELCs responded well or at least with stable disease to anti-PD-1/PD-L1 therapy, or cases that didn’t benefit from immunotherapy hadn’t been reported and included in this study. Unavailability of the responses in several cases was another reason why the efficacy of anti-PD-1/PD-L1 antibodies should not be overestimated in daily practice. Secondly, some of the public data were lacking. For example, patient fitness and treatment-related adverse events were untouched in this study due to incomplete data and it may have an impact on clinical outcomes of pulmonary LELCs. PD-L1 expressions in some cases were inaccessible and different antibodies such as SP142 or 22C3 were used for TPS evaluation in public data. All may interfere with our endeavor to identify the relationship between PD-L1 expression and the efficacy of anti-PD-1/PD-L1 antibodies, for blueprint study showed interchangeability of 22C3 and other antibodies but lower sensitivity of SP142 in NSCLC TPS IHC assay (34). Thirdly, the study was puzzled by various anti-PD-1/PD-L1 regimens and their combinations with chemotherapy or targeted therapy. However, all these PD-1/PD-L1 inhibitors have been proven to be comparably effective in treating driver mutation-negative NSCLC either on their own or joined by other active agents (35). Nevertheless, combining public data and Macau cases, our analysis made a good summary of how PD-1/PD-L1 antagonists performed in LELCs and proved a positive correlation between PD-L1 expression on the efficacy of immunotherapy. It also raised the evidence of using PD-1/PD-L1 inhibitors in advanced pulmonary LELC to a higher level rather than individual experiences.
In conclusion, our results preliminarily examine the efficacy of anti-PD-1 antibodies in patients with pulmonary LELC. Further validation was also warranted for solid conclusions supplementary to the scarce specified data about immunotherapy in pulmonary LELC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of CHCSJ. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
NZ, YYW and YZW conceived the study. NZ, HT, YL and YZW reviewed medical records and analyzed the data. SY performed pathological review. NZ, HT and YZW drafted the manuscript. YYW revised the draft. All authors contributed to the article and approved the submitted version.
This study was supported by National High Level Hospital Clinical Research Funding (2022-PUMCH-A-215, 2022-PUMCH-A-213).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1001414/full#supplementary-material
1. Tay CK, Chua YC, Takano A, Min Chee MY, Lim WT, Lim C, et al. Primary pulmonary lymphoepithelioma-like carcinoma in Singapore. Ann Thorac Med (2018) 13(1):30–5. doi: 10.4103/atm.ATM_304_17
2. Liang Y, Wang L, Zhu Y, Lin Y, Liu H, Rao H, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer (2012) 118(19):4748–58. doi: 10.1002/cncr.27452
3. Bégin LR, Eskandari J, Joncas J, Panasci L. Epstein-Barr Virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol (1987) 36(4):280–3. doi: 10.1002/jso.2930360413
4. Bruce JP, To KF, Lui VWY, Chung GTY, Chan YY, Tsang CM, et al. Whole-genome profiling of nasopharyngeal carcinoma reveals viral-host co-operation in inflammatory NF-κB activation and immune escape. Nat Commun (2021) 12(1):4193. doi: 10.1038/s41467-021-24348-6
5. Hong S, Liu D, Luo S, Fang W, Zhan J, Fu S, et al. The genomic landscape of Epstein-Barr virus-associated pulmonary lymphoepithelioma-like carcinoma. Nat Commun (2019) 10(1):3108. doi: 10.1038/s41467-019-10902-w
6. Zhou N, Lin Y, Peng X, Wang Y, Wang Y. Thorough survey and analysis of pulmonary lymphoepithelioma-like carcinoma in Macau and multimodality treatment for advanced disease. Lung Cancer. (2019) 138:116–23. doi: 10.1016/j.lungcan.2019.10.004
7. Chau SL, Tong JH, Chow C, Kwan JS, Lung RW, Chung LY, et al. Distinct molecular landscape of Epstein-Barr virus associated pulmonary lymphoepithelioma-like carcinoma revealed by genomic sequencing. Cancers (Basel). (2020) 12(8):2065. doi: 10.3390/cancers12082065
8. Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol (2021) 22(8):1162–74. doi: 10.1016/s1470-2045(21)00302-8
9. Xu R-h, Mai H-Q, Chen Q-Y, Chen D, Hu C, Yang K, et al. JUPITER-02: Randomized, double-blind, phase III study of toripalimab or placebo plus gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (NPC). Journal of Clinical Oncology. (2021) 39(18_suppl):LBA2–2. doi: 10.1200/JCO.2021.39.15_suppl.LBA2
10. Zhang L, Yang Y, J-j P, Chen X, Sun Y, Wang H, et al. RATIONALE-309: Updated progression-free survival (PFS), PFS after next line of treatment, and overall survival from a phase 3 double-blind trial of tislelizumab versus placebo, plus chemotherapy, as first-line treatment for recurrent/metastatic nasopharyngeal cancer. Journal of Clinical Oncology. (2022) 40(36_suppl):384950–0. doi: 10.1200/JCO.2022.40.36_suppl.384950
11. Lin DC, Meng X, Hazawa M, Nagata Y, Varela AM, Xu L, et al. The genomic landscape of nasopharyngeal carcinoma. Nat Genet (2014) 46(8):866–71. doi: 10.1038/ng.3006
12. Darrason M, Martin A, Soussan M, Chouahnia K, Pailler MC, Boudabous H, et al. Immunotherapy for LELC: Case report and a focused review. Clin Lung Cancer. (2019) 20(3):e393–401. doi: 10.1016/j.cllc.2018.12.008
13. Narayanan A, Knollmann FD, Walby JAS, Lim S, Gandara DR, Riess JW. EBV-positive primary pulmonary lymphoepithelioma-like carcinoma response to PD-L1 blockade. Clin Lung Cancer. (2019) 20(3):e238-e241. doi: 10.1016/j.cllc.2018.12.015
14. Wu Z, Xian X, Wang K, Cheng D, Li W, Chen B. Immune checkpoint blockade therapy may be a feasible option for primary pulmonary lymphoepithelioma-like carcinoma. Front Oncol (2021) 11:626566. doi: 10.3389/fonc.2021.626566
15. Kumar V, Dave V, Harris J, Huang Y. Response of advanced stage recurrent lymphoepithelioma-like carcinoma to nivolumab. Immunotherapy (2017) 9(12):955–61. doi: 10.2217/imt-2017-0067
16. Chen MT, Wang Z, Yuan M, Fang XJ, Lin TY. Treatment of pembrolizumab and chemotherapy results in pulmonary lymphoepithelioma-like carcinoma progression through harboring secondary amplification of PI3KCA and IL-7R. Lung Cancer. (2020) 144:87–9. doi: 10.1016/j.lungcan.2020.03.018
17. Tang Z, Fang R, Tong G, Liu P, Ou Z, Tang Y. Overcoming resistance to anti-PD-1 immunotherapy in lymphoepithelioma-like carcinoma: A case report and review of the literature. Lung Cancer. (2020) 146:335–40. doi: 10.1016/j.lungcan.2020.06.027
18. Xie Z, Liu L, Lin X, Xie X, Gu Y, Liu M, et al. A multicenter analysis of genomic profiles and PD-L1 expression of primary lymphoepithelioma-like carcinoma of the lung. Mod Pathol (2020) 33(4):626–38. doi: 10.1038/s41379-019-0391-9
19. Fu Y, Zheng Y, Wang PP, Chen YY, Ding ZY. Pulmonary lymphoepithelioma-like carcinoma treated with immunotherapy or chemotherapy: A single institute experience. Onco Targets Ther (2021) 14:1073–81. doi: 10.2147/ott.S290113
20. Qiu ZX, Zhou P, Wang K. Primary pulmonary lymphoepithelioma-like carcinoma response favorably to nivolumab: A case report. Onco Targets Ther (2019) 12:8595–600. doi: 10.2147/ott.S219512
21. Kim C, Rajan A, DeBrito PA, Giaccone G. Metastatic lymphoepithelioma-like carcinoma of the lung treated with nivolumab: a case report and focused review of literature. Transl Lung Cancer Res (2016) 5(6):720–6. doi: 10.21037/tlcr.2016.11.06
22. Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-update and clarification: From the RECIST committee. Eur J Cancer. (2016) 62:132–7. doi: 10.1016/j.ejca.2016.03.081
23. Batenchuk C, Albitar M, Zerba K, Sudarsanam S, Chizhevsky V, Jin C, et al. A real-world, comparative study of FDA-approved diagnostic assays PD-L1 IHC 28-8 and 22C3 in lung cancer and other malignancies. J Clin Pathol (2018) 71(12):1078–83. doi: 10.1136/jclinpath-2018-205362
24. Li X, Zhang W. Expression of PD-L1 in EBV-associated malignancies. Int Immunopharmacol. (2021) 95:107553. doi: 10.1016/j.intimp.2021.107553
25. Ren D, Hua Y, Yu B, Ye X, He Z, Li C, et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol Cancer. (2020) 19(1):19. doi: 10.1186/s12943-020-1144-6
26. Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol (2019) 14(5):867–75. doi: 10.1016/j.jtho.2019.01.006
27. Jiang L, Wang L, Li PF, Zhang XK, Chen JW, Qiu HJ, et al. Positive expression of programmed death ligand-1 correlates with superior outcomes and might be a therapeutic target in primary pulmonary lymphoepithelioma-like carcinoma. Onco Targets Ther (2015) 8:1451–7. doi: 10.2147/ott.S84234
28. Zhang M, Li G, Wang Y, Wang Y, Zhao S, Haihong P, et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep (2017) 7(1):10255. doi: 10.1038/s41598-017-10925-7
29. Chen B, Zhang Y, Dai S, Zhou P, Luo W, Wang Z, et al. Molecular characteristics of primary pulmonary lymphoepithelioma-like carcinoma based on integrated genomic analyses. Signal Transduct Target Ther (2021) 6(1):6. doi: 10.1038/s41392-020-00382-6
30. Zaleskis G, Pasukoniene V, Characiejus D, Urbonas V. Do the benefits of being a smoker hint at the existence of PD-1/PD-L1 sensitizers for patients on single-agent immunotherapy? J Immunother Cancer. (2021) 9(8):e003191. doi: 10.1136/jitc-2021-003191
31. de la Iglesia JV, Slebos RJC, Martin-Gomez L, Wang X, Teer JK, Tan AC, et al. Effects of tobacco smoking on the tumor immune microenvironment in head and neck squamous cell carcinoma. Clin Cancer Res (2020) 26(6):1474–85. doi: 10.1158/1078-0432.Ccr-19-1769
32. Kim J, Ha H, Park J, Cho J, Lim JH, Lee MH. Association of smoking status with efficacy of first-line immune checkpoint inhibitors in advanced non-small cell lung cancers: A systematic review and meta-analysis. J Cancer. (2022) 13(2):364–72. doi: 10.7150/jca.65374
33. Chang YL, Yang CY, Lin MW, Wu CT, Yang PC. PD-L1 is highly expressed in lung lymphoepithelioma-like carcinoma: A potential rationale for immunotherapy. Lung Cancer. (2015) 88(3):254–9. doi: 10.1016/j.lungcan.2015.03.017
34. Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: Results of blueprint phase 2 project. J Thorac Oncol (2018) 13(9):1302–11. doi: 10.1016/j.jtho.2018.05.013
Keywords: lung cancer, Lymphoepithelioma-like carcinoma, rare subtype, efficacy, immunotherpay
Citation: Zhou N, Tang H, Yu S, Lin Y, Wang Y and Wang Y (2022) Anti-PD-1 antibodies, a novel treatment option for advanced chemoresistant pulmonary lymphoepithelioma carcinoma. Front. Immunol. 13:1001414. doi: 10.3389/fimmu.2022.1001414
Received: 23 July 2022; Accepted: 17 November 2022;
Published: 06 December 2022.
Edited by:
Hubing Shi, Sichuan University, ChinaCopyright © 2022 Zhou, Tang, Yu, Lin, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingyi Wang, d2FuZ3lpbmd5aUBwdW1jaC5jbg==; Yuzhou Wang, WXV6aG91d0B5YWhvby5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.