
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 26 September 2022
Sec. Inflammation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1000626
This article is part of the Research Topic Crosstalk between Peripheral and Local Immune Response in the Pathophysiology of Stroke and Neurodegeneration Diseases View all 30 articles
 Ying-Ying Sun1†
Ying-Ying Sun1† Mei-Qi Wang1†
Mei-Qi Wang1† Yan Wang1†
Yan Wang1† Xin Sun1
Xin Sun1 Yang Qu1
Yang Qu1 Hong-Jing Zhu1
Hong-Jing Zhu1 Si-Ji Wang1
Si-Ji Wang1 Xiu-Li Yan1
Xiu-Li Yan1 Hang Jin1
Hang Jin1 Peng Zhang1
Peng Zhang1 Yi Yang1,2*
Yi Yang1,2* Zhen-Ni Guo1,2,3*
Zhen-Ni Guo1,2,3*Background: The changes in the platelet-to-lymphocyte ratio (PLR) before and after recombinant tissue plasminogen activator (rtPA) treatment and the time point at which the PLR is a potentially valuable prognostic predictor in patients wit ischemic stroke remain largely unknown. Therefore, the purpose of this study was to explore the characteristics of the PLR and evaluate their effects on clinical outcomes before and 24 h after rtPA treatment.
Methods: This study included 741 consecutive patients with acute ischemic stroke who underwent intravenous thrombolysis with rtPA. We collected data on demographics, vascular risk factors, medication history, and other clinical information pertaining to all patients. Specifically, blood samples for PLR measurement were collected on admission and 24 h after stroke. The outcome was assessed by using the Modified Rankin Scale (mRS) at 3 months and whether death occurred within 3 months or not. Univariate and multivariate logistic regression analysis was used to assess the association of the PLR with the risks of poor outcome (mRS>2) and death. An individualized prediction model was established to predict poor outcome.
Results: Of the 741 patients, 255 (34.4%) had poor outcome, and 43 (5.8%) died. The PLR significantly increased 24 h after rtPA in patients with poor outcome and death. Logistic analysis revealed that higher PLR 24 h after rtPA was independently associated with increased risks of poor outcome and death. However, the PLR on admission was not associated with the risks of poor outcome and death. The individualized prediction model for poor outcome based on the 24-h PLR exhibited favorable discrimination (areas under the curves of the training and validation groups: 0.743 and 0.729, respectively), calibration (P > 0.05), and clinical usefulness.
Conclusions: We found the PLR to be a variable that potentially predicts the risks of poor outcome and death in patients with acute ischemic stroke 24 h after rtPA; however, it cannot make the same prediction on admission.
Acute ischemic stroke is one of the most common causes of disability and death worldwide, representing 80% of all stroke cases (1, 2). Although recombinant tissue plasminogen activator (rtPA) has been proved as the most effective treatment for acute ischemic stroke since the 1990s, limitations such as hemorrhagic transformation and narrow therapeutic window (4.5h) have posed challenges to the therapeutic qualities (3) (4, 5). Therefore, identifying the clinical indicators that potentially predict patient prognosis and determining possible means of controlling the risk factors at an earlier stage are of paramount importance.
Immune-inflammatory responses following ischemic stroke are associated with poor patient prognosis because of their role in exacerbating neuronal injury and damage to the blood-brain barrier (6). Emerging evidence suggests that platelets and lymphocytes play crucial roles in immune and inflammatory responses. Platelets are active participants in inflammatory reactions at sites of thrombosis. When a stroke occurs, platelets are activated rapidly and adhere to the injured endothelial surface. The inflammatory factors released by them can then further recruit inflammatory cells such as leukocytes to the site of injury and amplify the inflammatory response (7, 8). In contrast, lymphocytes are known to control inflammatory pathways by coordinating, healing, and repairing inflammation (9, 10). The platelet-to-lymphocyte ratio (PLR) is a bio-index combining platelets and lymphocytes, which can reflect thrombus formation and immune-inflammation pathways. The PLR has recently been reported as a potential novel biomarker in acute ischemic stroke intravenous thrombolysis treatment, playing an active role in the prediction of the functional outcomes (11, 12). However, these results were obtained from studies involving heterogeneous time points from symptom onset to the collection of blood samples for PLR calculation. The time point at which the PLR is collected is a potentially valuable predictor and the PLR changes before and after rtPA are largely unknown. Thus, this study aimed to (1) explore the changes in the PLR before and 24 h after rtPA and (2) evaluate their relationship with poor outcome and death in patients with acute ischemic stroke.
This retrospective study performed using prospectively collected data. Patients diagnosed with acute ischemic stroke who underwent intravenous thrombolysis with 0.9 mg/kg rtPA within 4.5h in our department, between April 2015 and December 2020, were performed. The exclusion criteria were as follows (1): with other severe systemic diseases such as cancer, rheumatism and hematological diseases (2); incomplete clinical data (3); incomplete follow-up data. The study flowchart is shown in Figure 1. This prospective observational study was registered (NCT05028868) and approved by the Ethics Committee of the First Hospital of Jilin University (2016–294). Written informed consent was signed by each participant or an appropriate agent.
Information on demographics (age and sex); vascular risk factors (smoking, alcohol consumption, hypertension, diabetes, coronary artery disease, atrial fibrillation and previous stroke); medication history (antihypertensive drugs, hypoglycemic, and antiplatelet agents); and other clinical information, including baseline systolic and diastolic blood pressure (SBP and DBP), baseline blood glucose, time to treatment, baseline National Institutes of Health Stroke Scale (NIHSS) score, infarction site (whether located in the anterior circulation), and the Trial of Org 10172 in Acute Stroke Treatment (TOAST) subtype were recorded. Blood samples were collected from all patients on admission and 24 h after thrombolysis for platelet- and lymphocyte-count assessment. The PLR was calculated as the ratio of the platelet count to lymphocyte count.
(A) Modified Rankin Scale (mRS): The mRS score was assessed at 3 months (13). A mRS score >2 denoted a poor outcome and that ≤2 a favorable outcome.
(B) Death was defined as a stroke related death within 3 months.
IBM SPSS Statistics 23.0 and Stata 15.0 were used for statistical analysis. The Kolmogorov–Smirnov test was used to verify the normality of the distribution. All continuous variables were non-parametric distributions. Therefore, continuous and categorical variables were compared using the Mann–Whitney U-test and chi-squared test, which was then expressed in terms of median with interquartile range (IQR)and percentages, respectively. The paired Wilcoxon signed-rank test was performed to compare the PLR before and 24 h after rtPA. Univariate and multivariate logistic regression analysis was used to assess the association between PLR and outcome. Model 1 was unadjusted, Model 2 was adjusted for variables with P<0.05, Model 3 was adjusted for all other variables using enter method. An individualized prediction model for poor outcome was applied using multivariate logistic regression analysis and selected using a backward stepwise method with Akaike’s information criterion. Of the included cases, 70% and 30% were randomly assigned to training and validation groups, respectively. The nomogram was based on the data from the training group. The area under the receiver operating characteristics curve (AUC-ROC) was applied to assess model discrimination, and the calibration curve and Hosmer–Lemeshow test were applied to assess model fit in both the training and validation groups. Additionally, to further assess clinical usefulness, decision curve analysis (DCA) was performed by calculating the net benefit and plotting the net benefit against the threshold probability to derive a “decision curve”. DCA can determine the range of threshold probabilities that the model has value, and the magnitude of benefits (14). The model was validated by using a 10-fold cross-validation in all patients. P<0.05 was considered statistically different.
In our study, 788 rtPA-treated patients were included based on the inclusion criteria. After excluding 47 patients based on the exclusion criteria, 741 were finally included (Figure 1). The baseline clinical characteristics are presented in Table 1. Of the 741 patients, 255 (34.4%) had poor outcome (mRS >2), and 43 (5.8%) died.
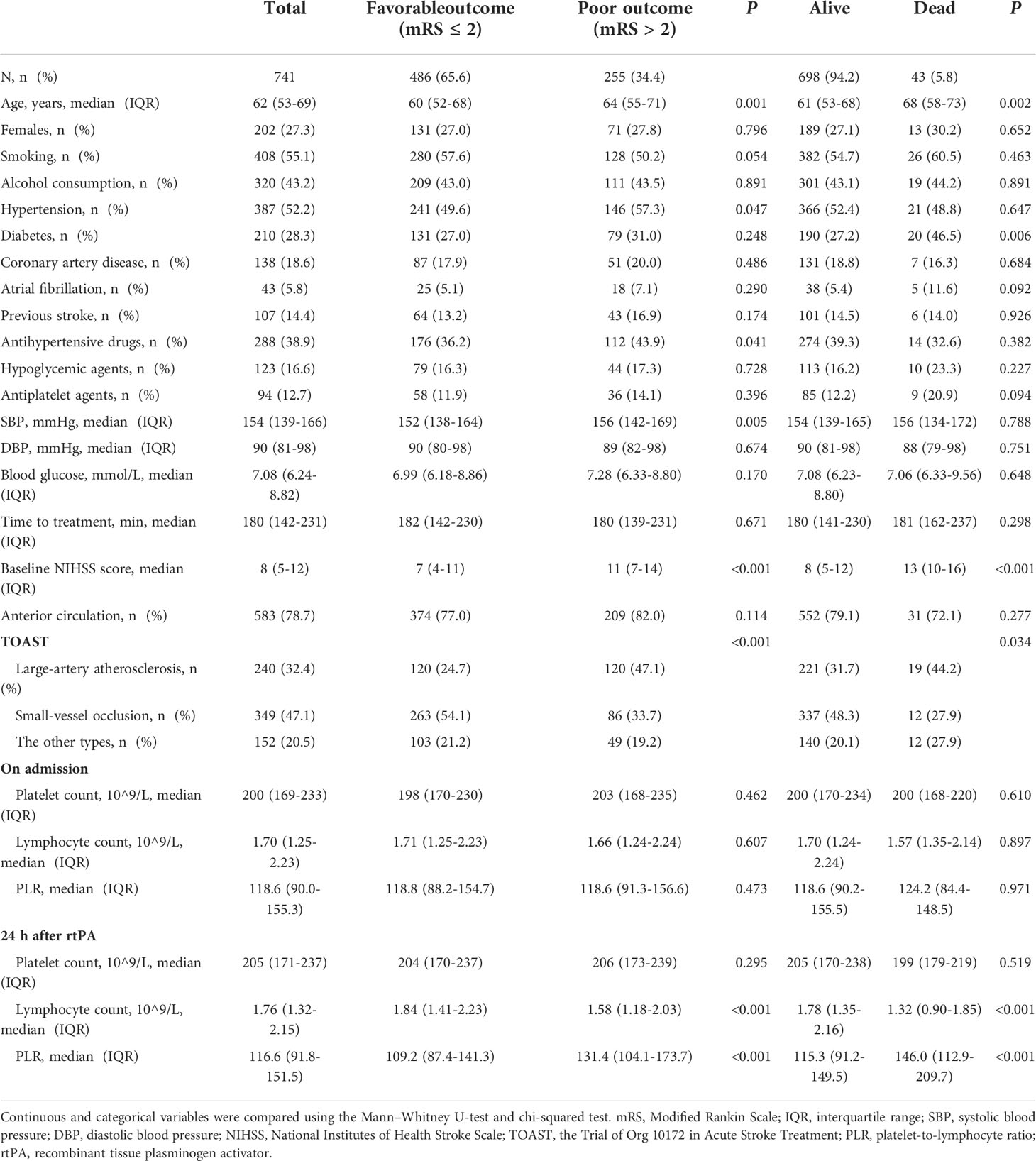
Table 1 Baseline characteristics of patients according to presence/absence of poor outcome and death.
Patients with poor outcome tended to be older and more likely to have hypertension, to be on antihypertensive therapy, have large-artery atherosclerosis, and have higher SBP and NIHSS scores (all P<0.05). Patients who died within 3 months were older and more likely to have diabetes, higher NIHSS scores, and a higher proportion of large-artery atherosclerosis (all P<0.05) (Table 1).
Overall, no significant difference was noted between the PLR on admission and that 24 h after rtPA (P=0.462). In the poor-outcome (mRS >2) group, the PLR increased significantly 24 h after rtPA treatment (median: 118.6 vs. 131.4, P=0.004). In contrast, the PLR decreased significantly 24 h after rtPA treatment compared with the value before rtPA therapy (median: 118.8 vs. 109.2, P<0.001) in the favorable-outcome group. In the deceased group, the PLR also increased significantly 24 h after rtPA treatment (median: 124.2 vs. 146.0, P=0.002) (Supplemental Table 1 and Figure 2).
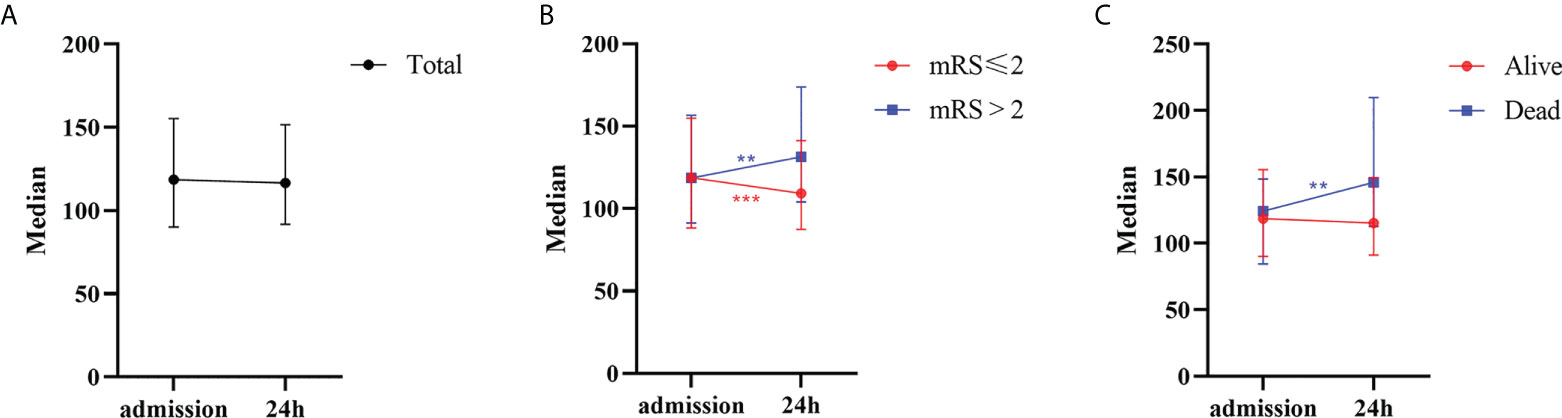
Figure 2 The 24 h dynamic change of PLR after rtPA in patients. (A) Patients in total. (B) Patients with favorable (mRS ≤ 2) or poor (mRS>2) outcome. (C) Patients with alive or dead status. PLR, platelet-to-lymphocyte ratio; rtPA, recombinant tissue plasminogen activator; mRS, Modified Rankin Scale. **P<0.01; ***P<0.001.
The 24 h PLR after rtPA therapy was significantly higher in patients with poor outcome (median: 109.2 vs. 131.4, P<0.001) and death (median: 115.3 vs. 146.0, P<0.001) than in those without (Table 1). Multivariate analysis revealed that higher PLR 24 h after rtPA was independently associated with an increased risk of poor outcome (OR=1.005; 95% CI: 1.002–1.008; P<0.001) and death (OR=1.008; 95% CI: 1.003–1.012; P=0.001). However, the PLR on admission was not associated with an increased risk of poor outcome (OR=1.001; 95% CI: 0.998–1.003; P=0.534) or death (OR=0.998; 95% CI: 0.992–1.003; P=0.421) (Table 2).
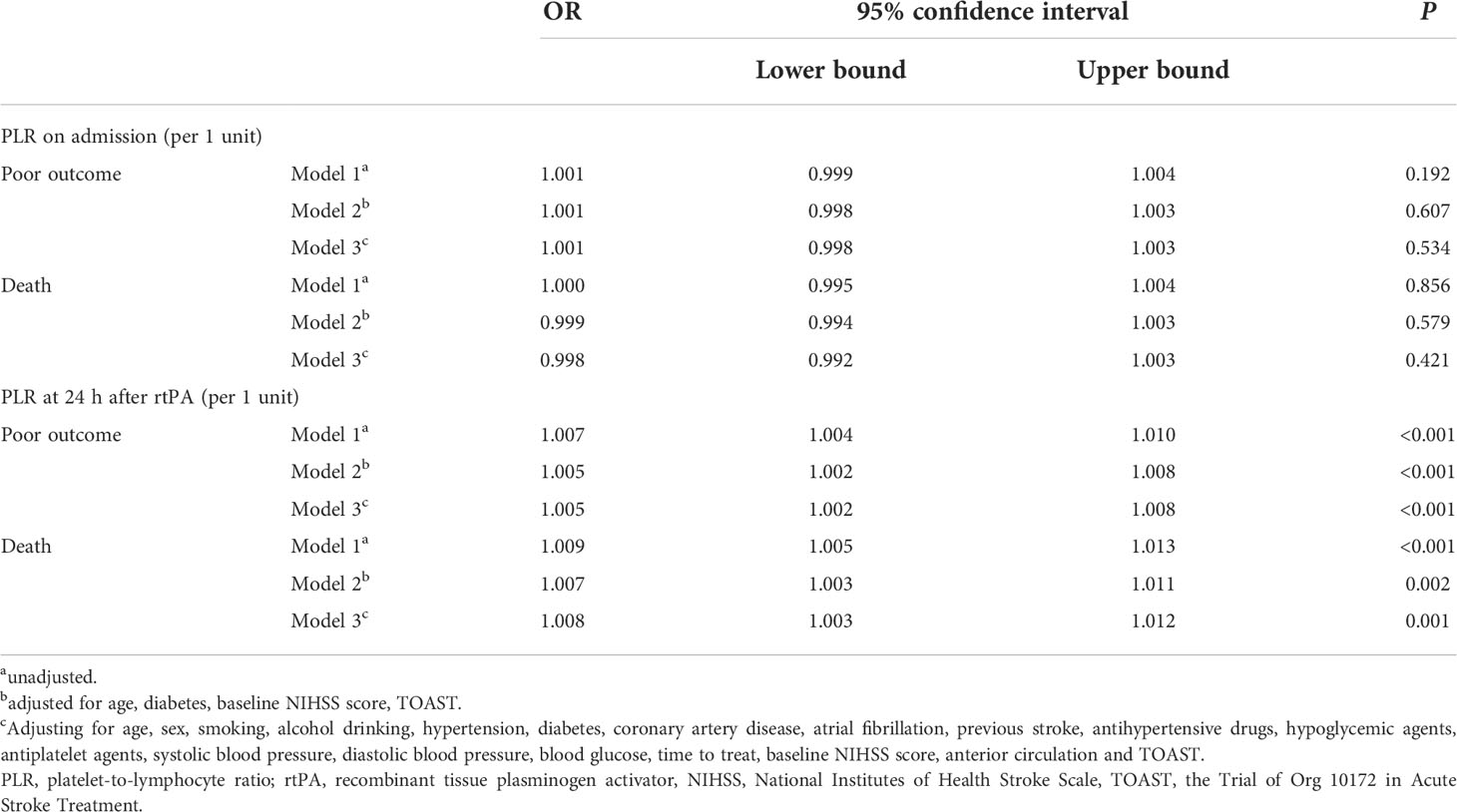
Table 2 Univariate and multivariable logistic regression analysis of PLR associated with poor outcome and death after rtPA.
A comparison of the training and validation groups is shown in Supplemental Table 2. The individualized prediction model for poor outcome comprised age (continuous variables), baseline NIHSS score (continuous variables), TOAST subtype (categorical variables), and the 24-h PLR after rtPA (continuous variables), based on the results of the multivariate logistic regression analysis. The nomogram is shown in Figure 3. The AUC-ROC values of the training and validation groups were 0.743 (95% CI: 0.692–0.787) and 0.729 (95% CI: 0.659–0.800) respectively, indicating favorable discrimination. The cutoff point for the training and validation group was 0.393 with a sensitivity of 61%, a specificity of 76%, and 0.348 with a sensitivity of 67%, a specificity of 69%, respectively. The calibration curve indicated a favorable predictive accuracy in both the training and validation groups. The Hosmer–Lemeshow test yielded P=0.977 and P=0.571 for the training and validation groups, respectively, indicating favorable calibration. DCA results were similar in the training and validation groups, reflecting a relatively favorable clinical net benefit (Figure 4). The ROC curve for the 10-fold cross-validation is given in Figure 5.
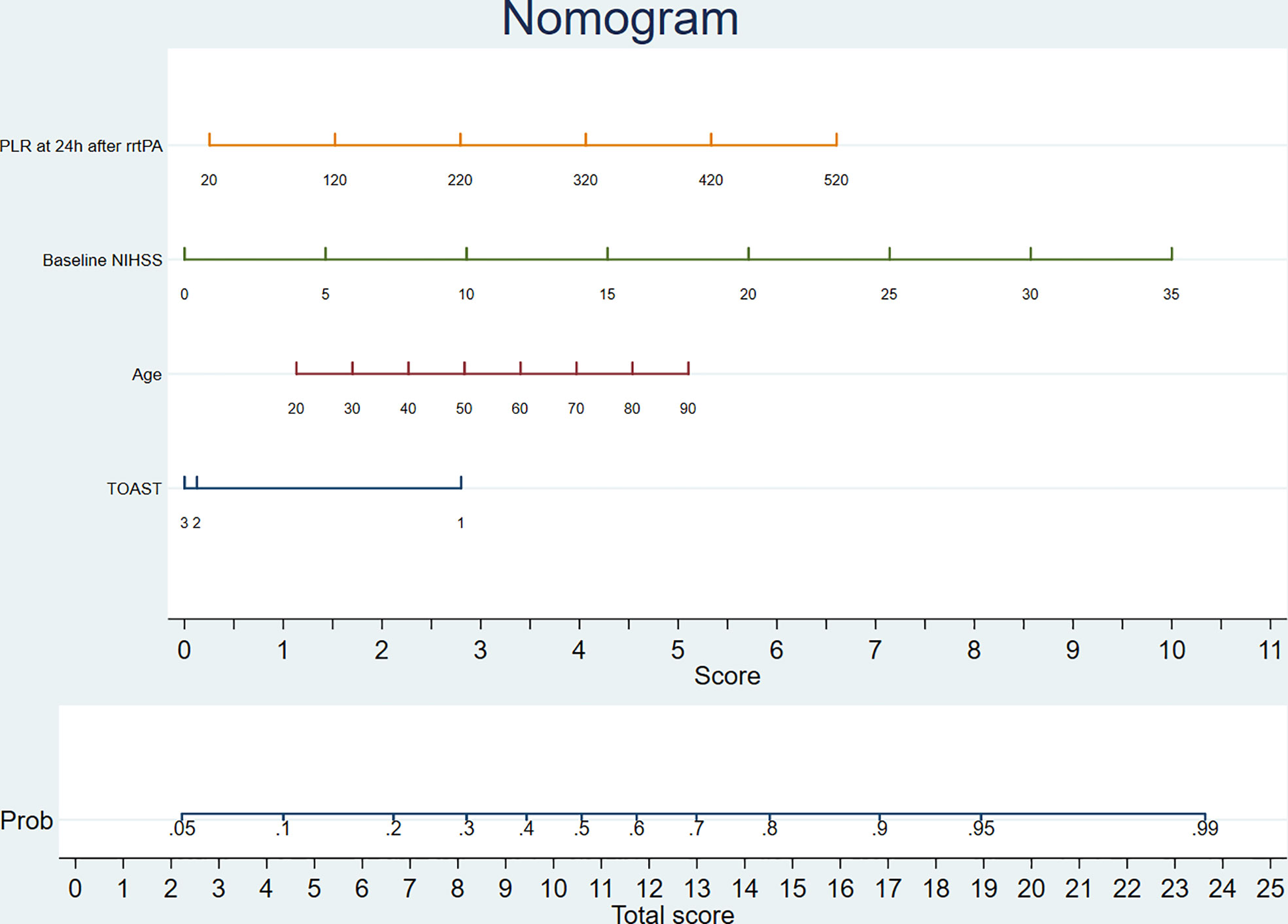
Figure 3 The nomogram for predicting poor outcome. TOAST: 1, large-artery atherosclerosis; 2, small-vessel occlusion; 3, the other types. PLR, platelet-to-lymphocyte ratio; rtPA, recombinant tissue plasminogen activator; NIHSS, National Institutes of Health Stroke Scale; TOAST, the Trial of Org 10172 in Acute Stroke Treatment.
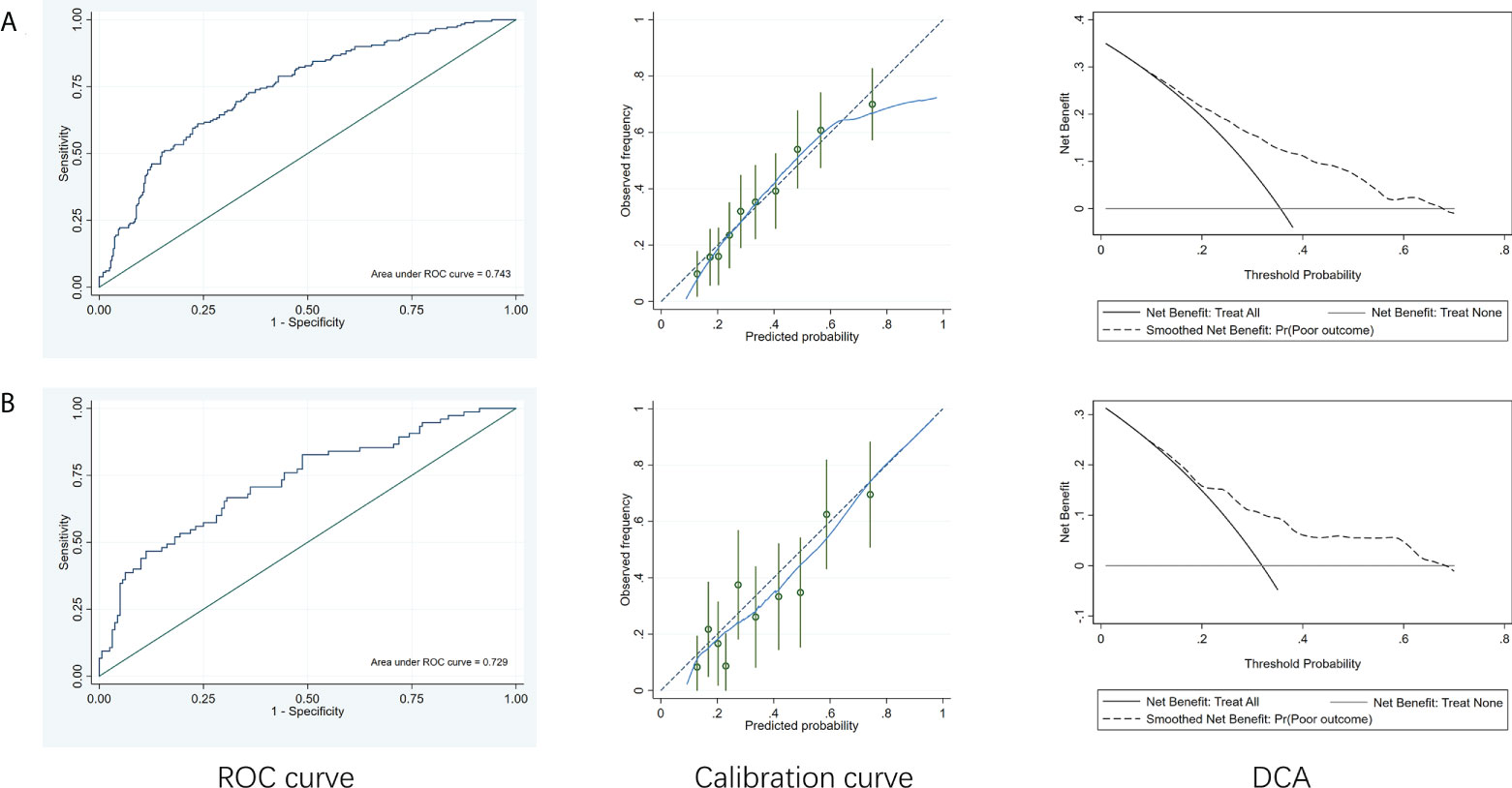
Figure 4 ROC curves, calibration curve and DCA of the model to predict poor outcome. (A) Training group. (B) Validation group. ROC, receiver operating characteristic curve; DCA, decision curve analysis.
This study attempted to explore the changes in the PLR before and 24 h after rtPA as well as evaluate their relationship with outcomes in acute ischemic stroke patients. We found the PLR increased 24 h after rtPA treatment in patients with poor outcome and death. This result indicated that instead of the PLR on admission, the 24h-PLR after rtPA was an independent risk factor for poor outcome and death. In addition, the individualized prediction model for poor outcome, which comprised age, baseline NIHSS score, TOAST subtype, and the 24-h PLR after rtPA, indicated favorable discrimination, calibration, and clinical usefulness. Our results suggest that the 24-h PLR after rtPA has prognostic value for poor outcome in patients who have undergone intravenous thrombolysis; thus, the PLR could be considered a possible target for interventions that improve outcomes in these patients.
Previous studies have shown that the PLR may be a strong biomarker used for the prognosis of acute ischemic stroke. For example, a recent study suggested that the PLR is potentially useful as an independent predictor of functional outcome at 3 months in patients with acute ischemic stroke (15). Simultaneously, a study by Altintas et al. (16) conducted in acute ischemic stroke patients undergoing endovascular therapy found the correlation between lower PLR values and a better clinical outcome (mRS ≤2). However, to the best of our knowledge, only two studies have explored the association between the PLR and outcomes in patients who have undergone intravenous thrombolysis. Xu et al. (12) collected lymphocyte- and platelet-count data within 24 h and found a higher PLR to be independently associated with poor outcome and death at 3 months in patients with stroke treated with intravenous thrombolysis. In another observational study of patients undergoing rtPA treatment, researchers found the PLR on admission before intravenous thrombolysis to be associated with early neurological improvement and deterioration (11). No study has investigated the predictive value of the PLR before intravenous thrombolysis for long-term clinical outcomes. Moreover, these previous studies exclusively reported a single time point for the PLR, and the time point at which blood samples were collected for PLR calculation was heterogeneous. Conversely, our study collected platelet- and lymphocyte-count data both on admission and 24 h after thrombolysis and found that only the 24-h PLR after rtPA was independently associated with poor outcome and death, while the PLR on admission showed no association with these outcomes.
In addition, we investigated the 24-h changes in the PLR after rtPA. Various components of the immune system change dynamically after stroke and may potentially have detrimental or beneficial effects depending on the stage of the development of ischemia. Therefore, understanding PLR changes during stroke and determining the time point at which the PLR is effective as a predictor of patient prognosis are of paramount importance (17, 18). Our study found the PLR to increase 24 h after rtPA treatment in patients with poor outcome and death; nonetheless, the trends in patients with favorable outcome were completely contrary. This mechanism may be due to the following reasons. Lymphocytes exhibit significant temporal variation after ischemic stroke (19, 20), and they accumulate in cerebral vessels at a later time within 24 h of the onset of stroke (21). A decreased lymphocyte count after ischemic stroke potentially aggravates the injury to neurons, and further exacerbate cerebral infarction and neurological deficits (22). Some subtypes of lymphocytes, such as regulatory T cells (Tregs), are known to attenuate inflammation and may exhibit brain protective qualities by producing anti-inflammatory cytokines in the process of acute stroke (19, 23). The consumption of Tregs after stroke profoundly exacerbates functional outcome (24). Furthermore, the decrease of lymphocyte count can also reflect the activation of the renin–angiotensin system, which further increases the production of proinflammatory cytokines, promoting ischemic injury (22, 25–27). In addition, lymphocytopenia may also increase the risk of infection in patients with stroke, which is associated with poor outcome (28). In contrast, studies have demonstrated that excessive activation and accumulation of platelets may reflect a greater tendency toward the inflammatory response and thrombosis, leading to hampered stroke recovery and poor prognosis (29, 30). Moreover, the mechanisms by which the prognostic value of the 24-h PLR after rtPA appears to be greater than that at baseline have not been well established. An increase in platelet count after thrombolytic therapy may be responsible for delayed thrombosis, leading to reocclusion and rethrombosis (31). A previous study revealed that rtPA potentially induces the elevation of matrix metalloproteinase-9 and chemokine ligand-2, which mediate blood–brain barrier disruption and may lead to hemorrhagic transformation. During this period, Tregs potentially eliminate the excess of these two inflammatory factors, thus decreasing the risk of brain damage and poor prognosis (24). Therefore, we speculated that the PLR at baseline may not be used as an ideal indicator to reflect the patient’s condition dynamically and comprehensively, whereas the post rtPA ratio would be a more valuable indicator.
The PLR, as an index, combines platelets and lymphocytes, which results in the unique advantage of linking key pathways both in thrombus formation and the inflammation process (32). The PLR index has potential superiority to an absolute platelet or lymphocyte count in the prediction of acute ischemic stroke prognosis. Firstly, the PLR combines two predictors which represent two inverse immunologic pathways (33). Secondly, individual platelet and lymphocyte count are not as stable as a combined ratio like PLR, as these values are fragile and potentially altered by several physiologic and pathologic conditions (34–36). Therefore, the PLR shows its reliability and rationality in predicting the risk of poor outcome and death for acute ischemic stroke patients.
In addition, we established an individualized prediction model for predicting poor outcome, which comprised age, baseline NIHSS score, TOAST subtype, and the 24-h PLR after rtPA. The AUC-ROC, Hosmer–Lemeshow test, and DCA results in the training and validation groups were similar, indicating that our model had favorable stability. Moreover, the results of the AUC-ROC, Hosmer–Lemeshow tests, and DCA suggested that our model exhibited favorable discrimination, calibration, and clinical usefulness. Thus, we considered our model based on the 24-h PLR after rtPA to have favorable prognostic value for poor outcome in patients undergoing rtPA treatment.
Notwithstanding, our study had certain limitations. First, this paper is a retrospective analysis of data from single-center hospitals. Second, we examined the PLR only at baseline and 24h after thrombolysis, where the two time points cannot fully reflect a dynamic change in the PLR throughout the entire process of stroke. In addition, the definite mechanisms for this have not been fully elucidated. Further investigation is required to explore the relevant mechanisms underlying the findings of this study.
In conclusion, we found the PLR to be a variable that is associated with the risks of poor outcome and death in patients with acute ischemic stroke 24 h after rtPA thrombolysis, rather than before. Furthermore, PLR is potentially useful as a simple, novel, and inexpensive method of predicting patient prognosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Hospital of Jilin University (2016-294). The patients/participants provided their written informed consent to participate in this study.
YY and Z-NG were responsible for study design. Material preparation, data collection and analysis were performed by Y-YS, M-QW and YW. The first draft of the manuscript was written by Y-YS. XS, YQ, H-JZ, S-JW, X-LY, HJ and PZ helped revise the manuscript. All authors read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (Grant No. 81901192) to XS, the Science and technology department of jilin province (20180623052TC), and the Jilin Provincial Key Laboratory (20190901005JC) to YY.
The authors thank the patients and their families and appreciate the study participants for their assistance in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1000626/full#supplementary-material
1. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (Ylds) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet (2012) 380(9859):2163–96. doi: 10.1016/S0140-6736(12)61729-2
2. Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener (2011) 6(1):11. doi: 10.1186/1750-1326-6-11
3. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American heart Association/American stroke association. Stroke (2019) 50(12):e344–418. doi: 10.1161/STR.0000000000000211
4. Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: A scientific statement for healthcare professionals from the American heart Association/American stroke association. Stroke (2017) 48(12):e343–e61. doi: 10.1161/STR.0000000000000152
5. Cheripelli BK, Huang X, MacIsaac R, Muir KW. Interaction of recanalization, intracerebral hemorrhage, and cerebral edema after intravenous thrombolysis. Stroke (2016) 47(7):1761–7. doi: 10.1161/STROKEAHA.116.013142
6. Javidi E, Magnus T. Autoimmunity after ischemic stroke and brain injury. Front Immunol (2019) 10:686. doi: 10.3389/fimmu.2019.00686
7. Rawish E, Nording H, Munte T, Langer HF. Platelets as mediators of neuroinflammation and thrombosis. Front Immunol (2020) 11:548631. doi: 10.3389/fimmu.2020.548631
8. Zuo K, Yang X. Decreased platelet-to-Lymphocyte ratio as predictor of thrombogenesis in nonvalvular atrial fibrillation. Herz (2018) 45(7):684–8. doi: 10.1007/s00059-018-4770-7
9. Miro-Mur F, Urra X, Gallizioli M, Chamorro A, Planas AM. Antigen presentation after stroke. Neurotherapeutics (2016) 13(4):719–28. doi: 10.1007/s13311-016-0469-8
10. Schwartz M, Moalem G. Beneficial immune activity after cns injury: Prospects for vaccination. J Neuroimmunol (2001) 113(2):185–92. doi: 10.1016/s0165-5728(00)00447-1
11. Gong P, Liu Y, Gong Y, Chen G, Zhang X, Wang S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflamm (2021) 18(1):51. doi: 10.1186/s12974-021-02090-6
12. Xu JH, He XW, Li Q, Liu JR, Zhuang MT, Huang FF, et al. Higher platelet-to-Lymphocyte ratio is associated with worse outcomes after intravenous thrombolysis in acute ischaemic stroke. Front Neurol (2019) 10:1192. doi: 10.3389/fneur.2019.01192
13. Adams HP Jr., Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline nih stroke scale score strongly predicts outcome after stroke: A report of the trial of org 10172 in acute stroke treatment (Toast). Neurology (1999) 53(1):126–31. doi: 10.1212/wnl.53.1.126
14. Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making (2006) 26(6):565–74. doi: 10.1177/0272989X06295361
15. Chen C, Gu L, Chen L, Hu W, Feng X, Qiu F, et al. Neutrophil-to-Lymphocyte ratio and platelet-to-Lymphocyte ratio as potential predictors of prognosis in acute ischemic stroke. Front Neurol (2020) 11:525621. doi: 10.3389/fneur.2020.525621
16. Altintas O, Altintas MO, Tasal A, Kucukdagli OT, Asil T. The relationship of platelet-to-Lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurol Res (2016) 38(9):759–65. doi: 10.1080/01616412.2016.1215030
17. Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory mechanisms in ischemic stroke: Focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci (2020) 21(18):6454. doi: 10.3390/ijms21186454
18. Berchtold D, Priller J, Meisel C, Meisel A. Interaction of microglia with infiltrating immune cells in the different phases of stroke. Brain Pathol (2020) 30(6):1208–18. doi: 10.1111/bpa.12911
19. Guo Z, Yu S, Xiao L, Chen X, Ye R, Zheng P, et al. Dynamic change of neutrophil to lymphocyte ratio and hemorrhagic transformation after thrombolysis in stroke. J Neuroinflamm (2016) 13(1):199. doi: 10.1186/s12974-016-0680-x
20. Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: Translational insights from experimental studies. J Cereb Blood Flow Metab (2015) 35(6):888–901. doi: 10.1038/jcbfm.2015.45
21. Xue J, Huang W, Chen X, Li Q, Cai Z, Yu T, et al. Neutrophil-to-Lymphocyte ratio is a prognostic marker in acute ischemic stroke. J stroke cerebrovasc Dis (2017) 26(3):650–7. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010
22. Ren H, Liu X, Wang L, Gao Y. Lymphocyte-to-Monocyte ratio: A novel predictor of the prognosis of acute ischemic stroke. J stroke cerebrovasc Dis (2017) 26(11):2595–602. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.019
23. Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med (2009) 15(2):192–9. doi: 10.1038/nm.1927
24. Mao L, Li P, Zhu W, Cai W, Liu Z, Wang Y, et al. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain (2017) 140(7):1914–31. doi: 10.1093/brain/awx111
25. Acanfora D, Gheorghiade M, Trojano L, Furgi G, Pasini E, Picone C, et al. Relative lymphocyte count: A prognostic indicator of mortality in elderly patients with congestive heart failure. Am Heart J (2001) 142(1):167–73. doi: 10.1067/mhj.2001.115792
26. Balci KG, Balci MM, Arslan U, Acar B, Maden O, Selcuk H, et al. Increased platelet-to-Lymphocyte ratios and low relative lymphocyte counts predict appropriate shocks in heart failure patients with icds. Acta Cardiol Sin (2016) 32(5):542–9. doi: 10.6515/acs20151012b
27. Tahsili-Fahadan P, Farrokh S, Geocadin RG. Hypothermia and brain inflammation after cardiac arrest. Brain Circ (2018) 4(1):1–13. doi: 10.4103/bc.bc_4_18
28. Vogelgesang A, Becker KJ, Dressel A. Immunological consequences of ischemic stroke. Acta Neurol Scand (2014) 129(1):1–12. doi: 10.1111/ane.12165
29. Altintas O, Tasal A, Niftaliyev E, Kucukdagli OT, Asil T. Association of platelet-to-Lymphocyte ratio with silent brain infarcts in patients with paroxysmal atrial fibrillation. Neurol Res (2016) 38(9):753–8. doi: 10.1080/01616412.2016.1210357
30. Shaik NF, Regan RF, Naik UP. Platelets as drivers of Ischemia/Reperfusion injury after stroke. Blood Adv (2021) 5(5):1576–84. doi: 10.1182/bloodadvances.2020002888
31. Marta-Enguita J, Navarro-Oviedo M, Munoz R, Olier-Arenas J, Zalba G, Lecumberri R, et al. Inside the thrombus: Association of hemostatic parameters with outcomes in Large vessel stroke patients. Front Neurol (2021) 12:599498. doi: 10.3389/fneur.2021.599498
32. Acet H, Ertas F, Bilik MZ, Akil MA, Ozyurtlu F, Aydin M, et al. The relationship between neutrophil to lymphocyte ratio, platelet to lymphocyte ratio and thrombolysis in myocardial infarction risk score in patients with St elevation acute myocardial infarction before primary coronary intervention. Postepy Kardiol Interwencyjnej (2015) 11(2):126–35. doi: 10.5114/pwki.2015.52286
33. Kurtul A, Yarlioglues M, Murat SN, Ergun G, Duran M, Kasapkara HA, et al. Usefulness of the platelet-to-Lymphocyte ratio in predicting angiographic reflow after primary percutaneous coronary intervention in patients with acute St-segment elevation myocardial infarction. Am J Cardiol (2014) 114(3):342–7. doi: 10.1016/j.amjcard.2014.04.045
34. Akboga YE, Bektas H, Anlar O. Usefulness of platelet to lymphocyte and neutrophil to lymphocyte ratios in predicting the presence of cerebral venous sinus thrombosis and in-hospital major adverse cerebral events. J neurol Sci (2017) 380:226–9. doi: 10.1016/j.jns.2017.07.036
35. Azab B, Shah N, Akerman M, McGinn JT Jr. Value of Platelet/Lymphocyte ratio as a predictor of all-cause mortality after non-St-Elevation myocardial infarction J Thromb thrombolysis (2012) 34(3):326–34 doi: 10.1007/s11239-012-0718-6
Keywords: platelet-to-lymphocyte ratio, acute ischemic stroke, intravenous thrombolysis, outcome, death
Citation: Sun Y-Y, Wang M-Q, Wang Y, Sun X, Qu Y, Zhu H-J, Wang S-J, Yan X-L, Jin H, Zhang P, Yang Y and Guo Z-N (2022) Platelet-to-lymphocyte ratio at 24h after thrombolysis is a prognostic marker in acute ischemic stroke patients. Front. Immunol. 13:1000626. doi: 10.3389/fimmu.2022.1000626
Received: 22 July 2022; Accepted: 06 September 2022;
Published: 26 September 2022.
Edited by:
Anwen Shao, Zhejiang University, ChinaReviewed by:
Xinyi Leng, The Chinese University of Hong Kong, ChinaCopyright © 2022 Sun, Wang, Wang, Sun, Qu, Zhu, Wang, Yan, Jin, Zhang, Yang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Yang, eWFuZ195aUBqbHUuZWR1LmNu; Zhen-Ni Guo, emhlbjFuaTJAamx1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.