- 1Department of Rheumatology and Immunology, West China Hospital, Sichuan University, Chengdu, China
- 2Health Management Center, West China Hospital, Sichuan University, Chengdu, China
Objective: The effectiveness of rituximab in anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis (DM) with interstitial lung disease (ILD) has been explored only in isolated case reports and small series. This paper aims to review the current evidence regarding rituximab (RTX) use in the treatment of ILD related to anti-MDA5 DM (anti-MDA5 DM-ILD).
Methods: We conducted a review by searching PubMed, Web of Science, Embase, and Cochrane for articles with information on patients with anti-MDA5 DM and RTX treatment, published until August 2021, in English language. The selected studies listed variation in chest high-resolution computed tomography (HRCT) and/or pulmonary function test (PFT) as a primary outcome, in patients with anti-MDA5 DM-related ILD after using RTX.
Results: Of the 145 potentially eligible articles, 17 were selected. The information gathered from a total of 35 patients with anti-MDA5 DM-ILD was reviewed, including 13 men and 22 women. Patient age at onset was 47.60 ± 13.72 years old. A total of 11.43% (4/35) of the patients were found to have chronic ILD (C-ILD) and 88.57% (31/30) exhibited rapidly progressive ILD (RP-ILD). Most patients (29/30) had typical DM rashes. Prior to RTX administration, the majority of patients (27/35) were treated with medium- or high-dose glucocorticoids and at least one additional immunotherapeutic agent. With regard to RTX efficacy for ILD in anti-MDA5 DM, 71.43% (25/35) of the patients responded to treatment. Skin rash also improved in more than half of the patients after RTX treatment. The most common side effects were infections, reported by 37.14% (13/35) of the patients after using RTX.
Conclusion: As a CD20 targeting drug, RTX is a promising therapeutic tool for anti-MDA5 DM-ILD, although the risk of infections should be considered before treatment. Further prospective controlled studies are required to evaluate the optimal RTX treatment regimen.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021289714, identifier CRD42021289714.
Introduction
Dermatomyositis (DM) is an autoimmune inflammatory disease that predominantly affects the muscles of the proximal extremities and the skin. DM is clinically heterogeneous both regarding patient symptoms and the severity of the disease. Myositis-specific autoantibodies (MSAs) are a class of recently discovered biomarkers that are associated to a unique clinical subset of myositis, and are therefore of great value in the classification of the disease, assessment of prognosis, and formulation of treatment plans (1). An important example of MSAs is the anti-melanoma differentiation-associated gene 5 autoantibody (anti-MDA5 autoAb). Patients positive for anti-MDA5 autoantibody (autoAb) typically exhibit cutaneous manifestations and mild or even no myopathy, but frequently are diagnosed with interstitial lung disease (ILD) (2). The prevalence of ILD in patients positive for anti-MDA5 autoAb has been estimated to be 50%–72.7% in reports from Europe and America (3–5) and 82%–100% in studies from Asia (6–10). In addition, the prevalence of patients that develop rapidly progressing ILD (RP-ILD) among the population of patients with anti-MDA5 DM-related ILD (anti-MDA5 DM-ILD) can be as high as 100% (11). More importantly, patients with anti-MDA5 DM usually have a high mortality rate due to relentless RP-ILD and lack of effective treatment. A recent study revealed that the 6-month survival rate of anti-MDA5 DM-ILD patients was only 33% even when treated with immunosuppressants (12).
Currently, there is no universal treatment for anti-MDA5 DM-ILD. Empiric treatment primarily focuses on glucocorticoid administration combined with the commonly used immunosuppressants - cyclophosphamide (CYC), calcineurin inhibitor (CNI) (12). However, there is still a large group of patients with anti-MDA5 DM-ILD who respond poorly to treatment with glucocorticoids and conventional immunosuppressants (13, 14). Hence, there is an urgent need to identify new treatment options for improving the therapeutic effect and prognosis.
CD20 is a transmembrane antigen selectively expressed on pre-B and mature B lymphocytes and is lost when B cells differentiate into plasma cells. In previous years, rituximab (RTX), a chimeric anti-CD20 monoclonal antibody, has been used in the management of B-cell malignancies (15), antineutrophil cytoplasmic antibody (ANCA)-associated renal vasculitis (16), rheumatoid arthritis (RA) (17), and systemic lupus erythematosus (SLE) (18). Although no guidelines on the treatment of myositis-related ILD have been published by the American College of Rheumatology (ACR) or by the European Alliance of Associations for Rheumatology (EULAR), RTX has also been used off-label in patients who did not respond to conventional therapy, based on a postulated pathogenetic role for B cells in anti-MDA5 DM-ILD. It is important to notice that the evaluation of therapeutic efficacy may be challenging in these conditions, since there are only a few case reports and small series in which RTX has been used in anti-MDA5 DM-ILD treatment.
The present paper aims to systematically review the currently available evidence regarding the use of RTX in anti-MDA5 DM-ILD.
Methods
Literature Search Strategy
This systematic review was registered in PROSPERO (CRD42021289714) and performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations (19). Two reviewers conducted a search on PubMed, Web of Science, Embase, and Cochrane databases, in an independent and simultaneous manner, for information on case reports, case series, and case-control studies of patients positive for anti-MDA5 DM and/or RTX treatment, reported until August 2021, in English language. The key retrieval terms included dermatomyositis, anti-MDA5, interstitial lung disease, Rituximab, and CD20 targeting. The detailed search strategy is documented in the supplementary file. The reference list of selected papers was also screened to identify additional studies to be included. Figure 1 shows the flowchart of the paper selection process.
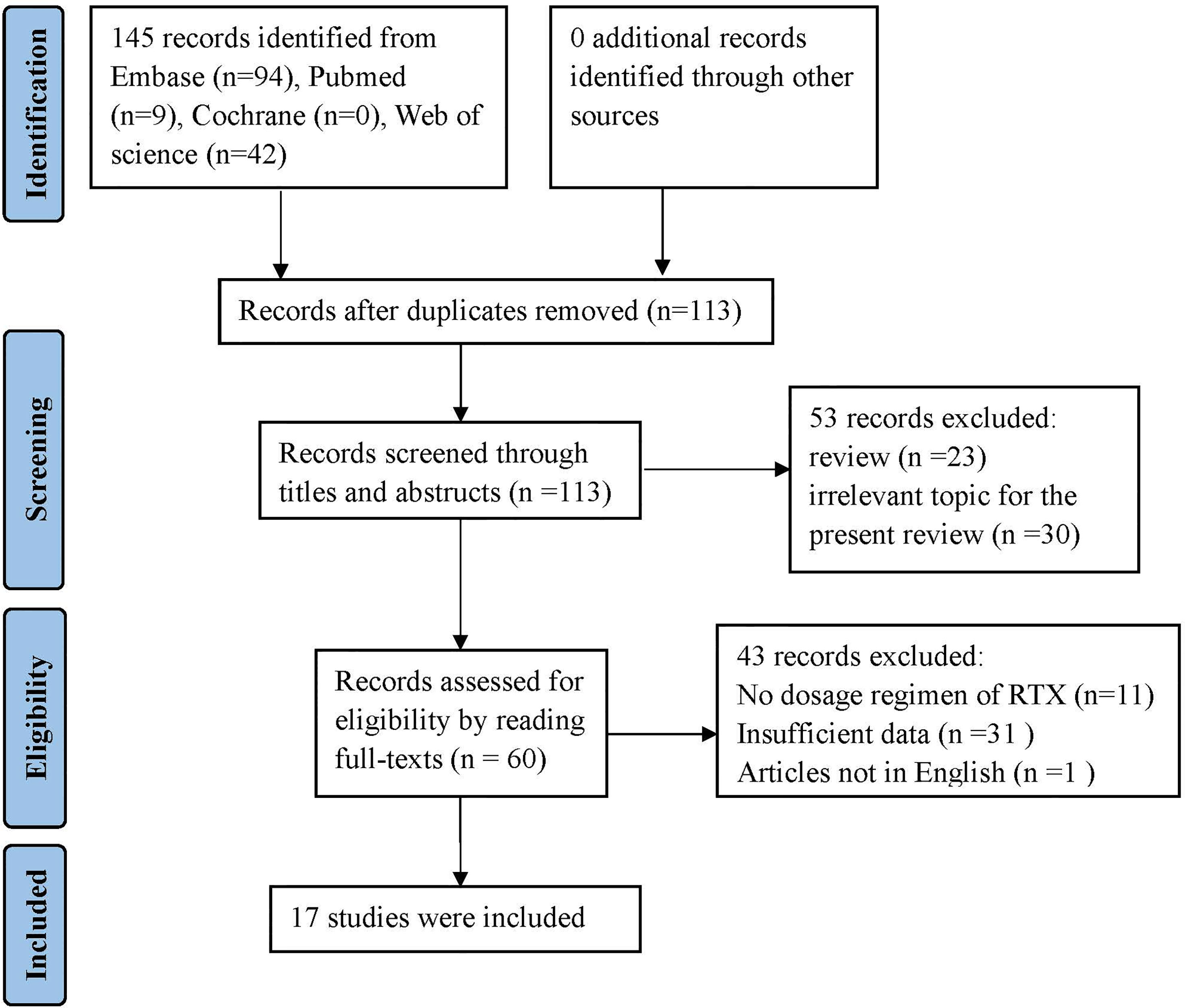
Figure 1 Flow diagram according to Prisma guidelines (20).
Inclusion and Exclusion Criteria
Eligible patients in our review fulfilled the following criteria: i) met the Bohan and Peter criteria of DM (21) or Sontheimer’s criteria of CADM (22); ii) had positive anti-MDA5 antibody confirmed by immunoblotting assay or ELISA; iii) had confirmed interstitial lung diseases (ILD), defined as ground-glass changes and/or fibrosis noted on high-resolution computed tomography (HRCT) (23). iv) had receive RTX treatment. Subsequently, only cases with complete epidemiological data and therapeutic information were selected. Study designs were either randomized control trials (RCTs), cohort studies, case-control studies, case or case series. Exclusion criteria included suffering from malignancy or other overlapping rheumatic diseases, such as systemic lupus erythematosus (SLE) and systemic sclerosis (SSc).
Data Extraction and Quality Assessment
Data recorded included age, sex, clinical manifestation (pulmonary involvement and/or extrapulmonary manifestations), laboratory data at disease presentation, and administered treatment (e.g., prednisolone, immunosuppressive agents, intravenous immunoglobulin, plasma exchange) previous to RTX administration were also recorded. Additional information collected included the RTX treatment schedule, RTX adverse effects, and clinical response to RTX. Two independent researchers assessed the methodological quality of all studies using the Newcastle-Ottawa Scale (NOS) criteria (24), classified according to the selection of the study groups, the comparability of the groups, and the ascertainment of the outcome. The classification scale ranges from 0 (poor methodological quality) to 9 (optimal methodological quality). Any discrepancies were resolved via discussion or consultation with a third researcher.
Response Criteria
Patients were defined as responders if at least one of two criteria were met: i) ≥ 10% increase in forced vital capacity (FVC) and/or ≥ 15% increase in diffusing capacity of carbon monoxide (DLCO) (25); ii) improved outcome of lung imaging by either chest X-rays or HRCT (reviewed by a radiologist blinded to the design of the study) (23).
Statistical Analysis
Data are expressed as the mean ± SD. Differences in quantitative parameters between two groups were assessed using the Mann-Whitney U-test. Fisher’s exact test was used to compare the trends between groups of qualitative variables. All statistical analyses were performed using Prism 8.3 software (GraphPad Software, La Jolla, CA, USA) and a two-sided p value of 0.05 or less was considered significant.
Results
The bibliographic search conducted to identify cases of anti-MDA5 DM-ILD treated with RTX yielded 145 articles. After screening and eligibility check were conducted, 128 articles were excluded, as detailed in Figure 1. Of the 17 articles selected to be included in this review, 16 were case reports and case series, and one was a retrospective case-control study. Clinical data was extracted relating to 35 patients with anti-MDA5 DM-ILD meeting the inclusion criteria (23, 26–41). The demographic characteristics, clinical manifestations, laboratory data, treatment regimen, and outcomes are shown in Tables 1, 2.
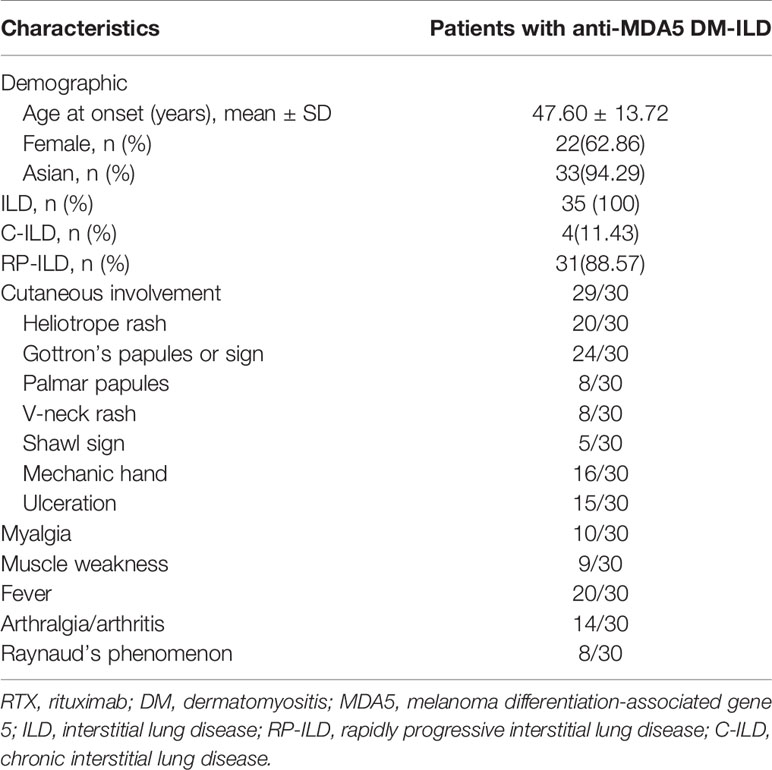
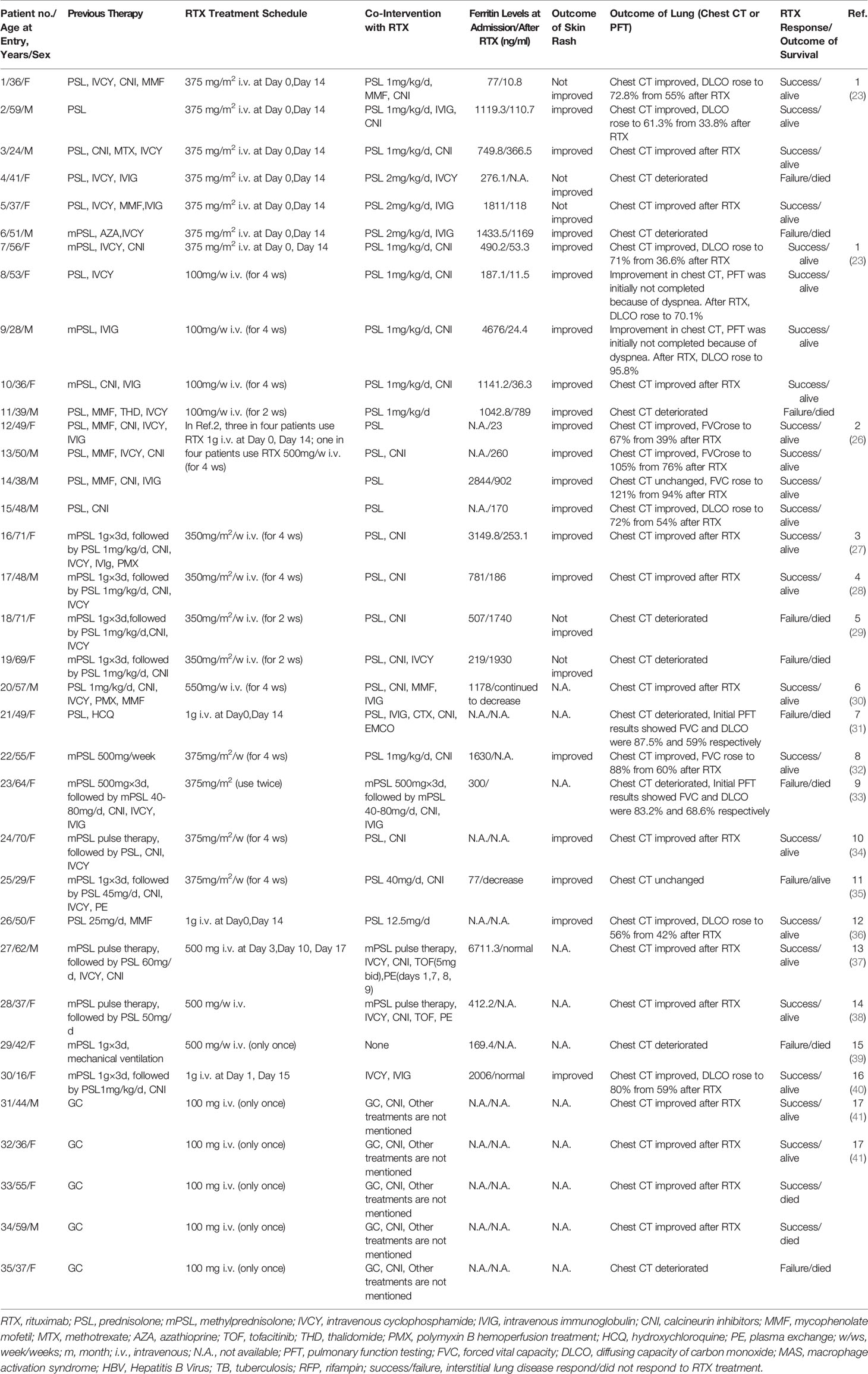
Table 1 Summary of the general characteristics of 35 patients with anti-MDA5 DM-ILD treated with RTX.
Clinical Characteristics of Anti-MDA5 DM-ILD Patients Receiving RTX Treatment
The mean age at diagnosis was 47.6 ± 13.72 years. Approximately 62.86% (22/35) of the patients in our study were female and 94.29% (33/35) of patients were Asian. Patients with polymyositis (PM) and/or DM aggravated by ILD can be divided into two main clinical patterns: chronic (C-ILD) and rapidly progressive ILD (RP-ILD) (42), the latter being associated with poorer prognosis. In this study, all enrolled patients had ILD, from which 88.57% (31/35) had RP-ILD, and 11.43% (4/35) had C-ILD. Clinical manifestation data of 30 patients were obtained. Most patients (29/30) had typical DM rashes, such as heliotrope sign (20/30), Gottron’s sign (24/30), palmar papules (8/30), V-neck rash (8/30), shawl sign (5/30), mechanic hand (16/30), and ulceration (15/30). Myalgia and muscle weakness were observed in 10 and 9 patients, respectively. There were 20 patients with fever and 14 patients with arthralgia/arthritis. Raynaud’s phenomenon was observed in 8 patients (Table 1).
Differential Treatment Regimen Across the Anti-MDA5 DM-ILD Patients
Prior to RTX administration, the majority of patients (27/35) in our study were treated with medium or high doses of glucocorticoids and at least one additional immunotherapy treatments, including intravenous cyclophosphamide (18/35, 51.43%), calcineurin inhibitor (18/35, 51.43%), intravenous immunoglobulin (8/35, 22.86%), mycophenolate mofetil (8/35, 22.86%), polymyxin B hemoperfusion treatment (2/35, 5.71%), plasmapheresis (1/35, 2.86%), methotrexate (1/35, 2.86%), azathioprine (1/35, 2.86%), hydroxychloroquine (1/35, 2.86%), and thalidomide (1/35, 2.86%). In this study, 19 patients were treated with two or more immunotherapy treatments. Remarkably, three patients received five treatment modalities. Approximately 42.86% (15/35) of patients were treated with the lymphoma schedule or lymphoma-like schedule (350–375 mg/m2 every 1 or 2 weeks) and 17.14% (6/35) of patients received the rheumatology schedule (500–1000 mg every 2 weeks). Other schedules included 500–550 mg RTX every week in 5 (14.29%) patients. In addition to the above conventional regimens, 9 anti-MDA5 DM-ILD patients were treated with a novel low-dose RTX regimen (100 mg per week)(Table 2).
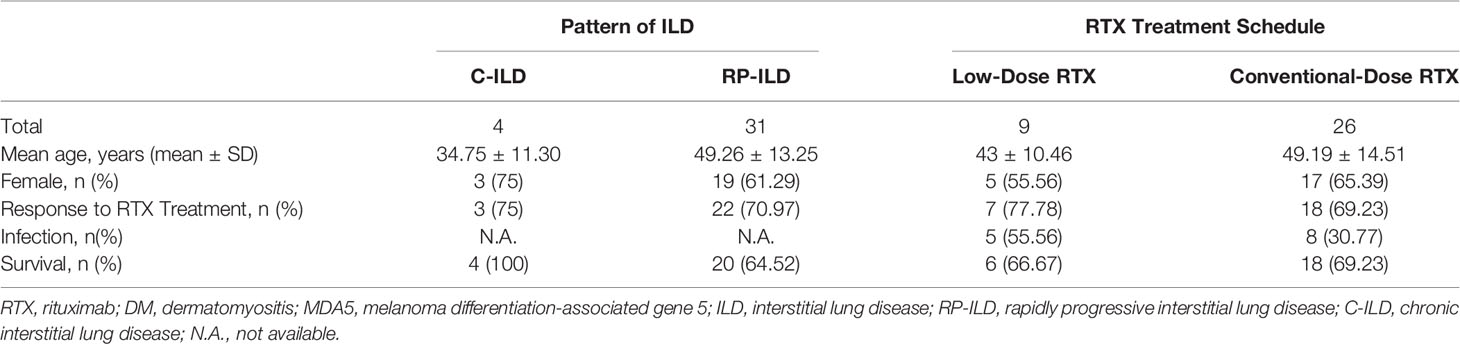
Efficacy of RTX for Anti-MDA5 DM-ILD Patients
Considering the efficiency of RTX treatment for ILD in anti-MDA5 DM patients, 71.43% (25/35) of patients presented a response to treatment (assessed by chest HRCT and/or PFT). In the C-ILD subgroup, 75% (3/4) of the patients presented a response to RTX, while 70.97% (22/34) of patients in the RP-ILD subgroup presented a response to RTX. Among the low-dose and conventional-dose RTX subgroups, the response rate to treatment was 77.78% (7/9) and 69.23% (18/31), respectively (Table 3). Ferritin data were collected from 24 patients in this study. The levels of ferritin were significantly higher in the RTX treatment responsive subgroup than in the non-responsive subgroup (P=0.0196)(supplementary Fig 1). Seventeen patients had decreased ferritin levels after RTX treatment. A decrease of anti-MDA5 autoAb titers observed in patients 17, 25 and 27 after RTX treatment, while data from other patients were not available. Considering the outcome of cutaneous involvement, 19 patients showed improvement, including reduced degree and size of skin rash or healing of skin ulcers.
Safety of RTX in Treatment of Anti-MDA5 DM-ILD Patients
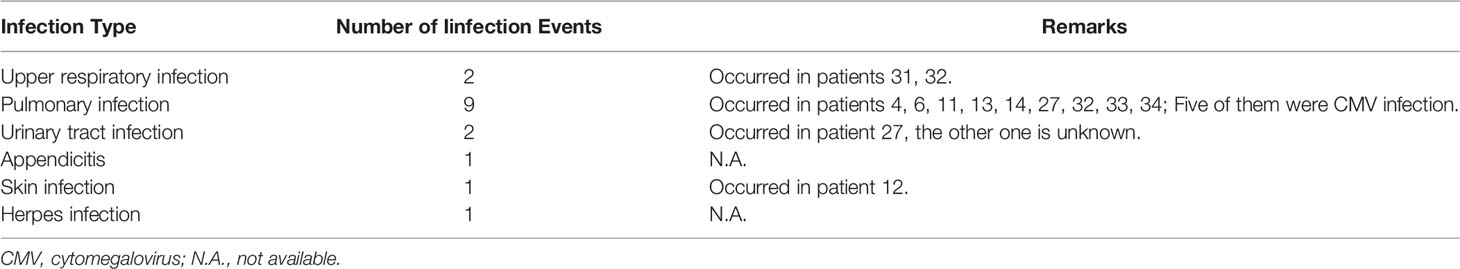
With regard to safety of RTX in anti-MDA5 DM-ILD patients, infusion reactions were not reported in any of the cases under study, and the most common side effects were infections. A total of 16 infection were reported by 13 patients, after RTX. Noteworthy, 56.25% (9/16) of the infection events were pulmonary infections, and more than half of them were caused by cytomegalovirus (CMV) (Table 4). The infection rate of patients in the low-dose and conventional-dose RTX subgroups was 55.56% (5/9) and 30.77% (8/26), respectively. The survival rates among patients with C-ILD and RP-ILD were 100% (4/4) and 64.52% (20/31), respectively. The survival rates of patients in the low-dose and conventional-dose RTX subgroups were 66.67% (6/9) and 69.23% (18/26), respectively (Table 3). The survival rates of patients in the RTX treatment responsive and non-responsive subgroups were 92% (23/25) and 10% (1/10), respectively (Table 5).
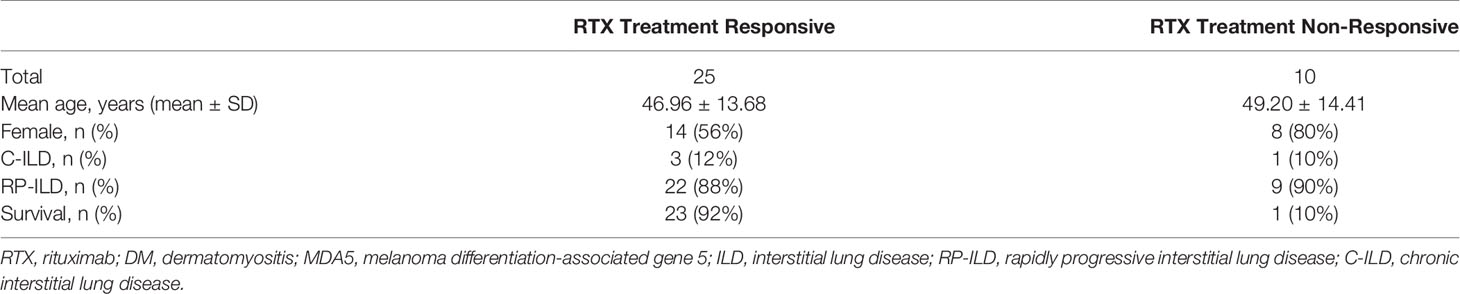
Table 5 Clinical data between RTX treatment responsive and non-responsive subgroups in 35 patients with anti-MDA5 DM-ILD.
Discussion
The hallmarks of anti-MDA5 DM are the presence of autoantibodies targeting MDA5 and unique cutaneous features, as well as an elevated risk of ILD. The underlying pathogenesis is not yet fully understood. T and B lymphocytes (23, 43–47), neutrophils (48, 49), macrophages (50, 51), type I interferon (IFN-I) (52, 53) and “Cytokines storm” [which is similarity with SARS-CoV-2 infection (54)] were thought to be involved in the development of the disease (Figure 2). MDA5 protein, as a pattern recognition receptor (PRR), can recognizes viral double-stranded RNA (dsRNA) then activates IFN-I pathway (55) and induces the production of proinflammatory cytokines by the cell. Indeed, the aberrant activation of the type I interferon system has been demonstrated in anti-MDA5 DM in previous studies (52, 53). While MDA5 protein is also an IFN-inducible protein (56), so activation of the IFN-I system can further promote the production of MDA5 protein. Abnormal accumulation of MDA5 protein may lead to a loss of tolerance to MDA5, resulting in the production of anti-MDA5 autoAb. As for how anti-MDA5 autoAbs play their potentially pathogenic role is less well understood and is only briefly described here in light of a recent review on the subject (47). In pathological contexts, MDA5 is strong expressed in skin tissue of DM patients (57) and detectable in cytoplasmic, cell surface, and secretory vesicles in neutrophils (58). Hence, some scholars have speculated that anti-MDA5 Abs can bind to MDA5 on cell surface and induce an inappropriate activation of MDA5, resulting in chronic activation of the IFN-I pathway. Apart from binding to MDA5 expressed on cell surface, they suggested anti-MDA5 Abs could also form immune complexes with the MDA5 proteins released from apoptotic cell and interact with cytoplasmic MDA5, which may similar to other Abs described previously (59, 60). In the lungs, the chemokine CX3CL1 can be produced by vascular endothelial cells when exposed to IFN-I and induce recruitment of CX3CR1+ M2 macrophages (61, 62). Local production of transformation growth factor-β (TGF-β) by M2 macrophages directly promotes pulmonary fibrosis. Moreover, there is a CD4+CXCR4+ T cell subset in anti-MDA5 DM-ILD, which can produce profibrotic agents (TGF-β, α-smooth muscle actin (α-SMA), collagen I, and IL-21) (46). In addition, the process of activated neutrophils releasing neutrophil extracellular traps (NETs) could expose autoantigens that have the potential to break immune tolerance and lead to production of autoantibodies (63). In recent years, accumulating evidence suggested that B lymphocytes may play a critical role in the pathogenesis of anti-MDA5 DM-ILD. First, plasma cells that secrete antibodies derive from B lymphocytes, while multiple studies have already demonstrated that anti-MDA5 autoantibodies are closely related to disease activity and ILD in patients with anti-MDA5 DM (64, 65); Secondly, B cell activating factor (BAFF) is a member of the tumor necrosis factor (TNF) superfamily, playing a key role in the survival and balance of peripheral B cells and plasma cells. It not only promotes the maturation of B cells, but also regulates immunoglobulin class-switching (66, 67). Data from Kobayashi et al. showed that juvenile dermatomyositis (JDM) patients with a high titer of anti-MDA5 autoantibodies had higher levels of BAFF than those with low titers (68). Another study noted that higher levels of BAFF were detected in patients positive for anti-MDA5 autoantibodies, when compared to negative patients, and that the levels of BAFF correlated positively with the titers of anti-MDA5 autoantibodies. The same study also indicates that the BAFF level of anti-MDA5 DM patients aggravated by ILD was significantly higher than anti-MDA5 DM patients not suffering from ILD, and the level of BAFF was parallel with KL-6, an indicator of the severity of ILD, suggesting that patients with anti-MDA5 DM complicated with severe ILD tend to have higher levels of serum BAFF (52); Thirdly, a recent study by Shuang Ye et al. revealed that the peripheral percentage of CD4+CXCR4+T cells is relevant to the severity of ILD in idiopathic inflammatory myopathy (IIM), especially to anti-MDA5 DM-ILD. Furthermore, they confirmed that the circulating CD4+CXCR4+T cell subset expresses high levels of IL-21 (46), which can induce the differentiation of B cells into plasmablasts by binding to the IL-21 receptor on the surface of B cells (69).
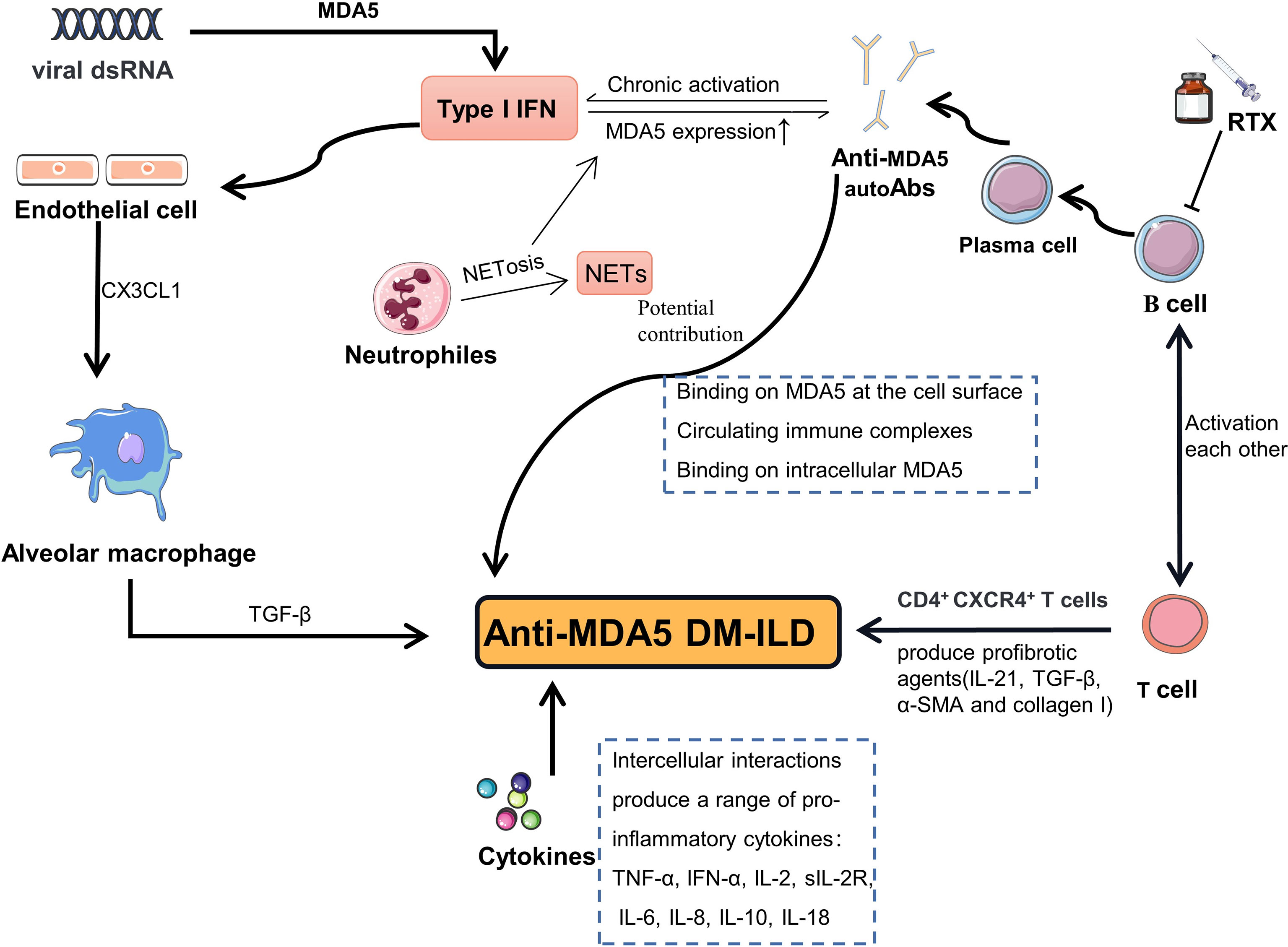
Figure 2 Schematic representation of the hypothesized pathogenesis in anti-MDA5 DM-ILD. MDA5 protein can recognizes viral dsRNA then activates IFN-I pathway. Activation of the IFN-I system promote the production of MDA5 and cause MDA5 overexpression. Abnormal accumulation of MDA5 protein may lead to a loss of immune tolerance, resulting in the production of anti-MDA5 autoAb. Anti-MDA5 autoAbs may potential contribute to the pathogenesis through binding to MDA5 on cell surface, forming immune complexes, and interacting with cytoplasmic MDA5. In the lungs, CX3CL1 can be produced by endothelial cells when exposed to IFN-I and induce recruitment of alveolar M2 macrophages. Local production of TGF-β by M2 macrophages directly promotes pulmonary fibrosis. CD4+CXCR4+ T cell subset in anti-MDA5 DM-ILD can produce profibrotic agents (TGF-β, α-SMA, collagen I, and IL-21). The process of activated neutrophils releasing NETs could expose MDA5 autoantigens. MDA5, melanoma differentiation-associated gene 5; DM, dermatomyositis; ILD, interstitial lung disease; autoAb, autoantibody; RTX, rituximab; dsRNA, double-stranded ribose nucleic acid; NETs, neutrophil extracellular traps; NETosis, neutrophil extracellular traps externalization process; IFN-I, type I interferon; TGF-β, transformation growth factor-β; α-SMA, α-smooth muscle actin.
Based on the possible causative role of B cells, targeting CD20 in the treatment of anti-MDA5 DM-ILD seems to be of great importance. Hence, RTX is empirically used as a therapeutic agent for patients with anti-MDA5 DM-ILD. All currently published studies on the efficacy of RTX in the treatment of anti-MDA5 DM-ILD are case reports, case series, or case-control studies. The present work offers a systematic review of the effects of RTX in 35 anti-MDA5 DM-ILD patients. The clinical response after using RTX for ILD could be defined by the improvement of chest HRCT and/or PFT. After RTX treatment, 71.43% (25/35) of patients responded positively, according to chest HRCT and/or PFT. According to the data here analyzed, anti-MDA5 DM patients with RP-ILD (22/31, 70.97%) had a lower rate of response to RTX than C-ILD (3/4, 75.0%) patients. We also observed that patients in the low-dose RTX subgroup (7/9, 77.78%) had a higher response rate than patients in the conventional-dose RTX subgroup (18/26, 69.23%). In addition, patients with anti-MDA5 DM usually exhibit typical cutaneous manifestations. Previous studies that assessed the efficacy of RTX for cutaneous lesions in anti-MDA5 DM patients have demonstrated different therapeutic effects (70, 71). In our study, skin rash improved in more than half of the patients that used RTX, whereas the rate of response to RTX treatment on cutaneous lesions in anti-MDA5 DM patients cannot be accurately calculated due to the unavailability of information on some cases. Further large-sample studies are required to evaluate the efficacy of RTX on the cutaneous lesions of patients with anti-MDA5 DM. Similarly, we observed that ferritin levels decreased in more than half of the patients, after RTX treatment. A study by Gono et al. showed that ferritin level is a poor prognostic factor in RP-ILD patients with anti-MDA5 DM, and also indicated that ferritin concentrations are useful for the evaluation of the response to treatment in patients with anti-MDA5 DM-ILD (72). This indicates that the condition of most patients in our study improved after RTX. Considering the results described above and the fact that all patients in our study received glucocorticoids and/or immunosuppressants prior to RTX treatment and had poor response to these drugs, RTX may be an effective treatment for anti-MDA5 DM-ILD resistant to glucocorticoids and multiple immunotherapies.
From a safety perspective, 37.14% (13/35) of patients developed infection after RTX therapy, and four patients died of pulmonary infection in our study. Although there seems to be an increased risk of clinical infections after RTX treatment, the rate of infection is poorly correlated with the types of immune diseases and is closely correlated with low hypogammaglobulinemia, neutropenia, CD4+ T cell dysfunction, etc. (73). Recently, a registry-based study estimated at 30% the efficacy and safety for RTX treatment in anti-synthetase syndrome, which was similar to the rate of infections after RTX administration in anti-MDA5 DM-ILD in the present review. In addition, 55.56% (5/9) of the patients in the low-dose subgroup were infected, which proved to be higher than the percentage of infections in the conventional-dose subgroup (30.77%) (8/26). This is most likely due to the fact that 6 patients who received low-dose RTX in Reference 17 were not included (due to lack of chest HRCT and/or PFT data), which led to biases in the results. Given that the response rate to RTX treatment of patients to in the low-dose subgroup (77.78%) was also higher than that of patients from the conventional-dose subgroup, and that this low-dose RTX regimen has been successfully used in several other immune diseases (74–76), we speculate that low-dose RTX (100 mg per week) may lead to a better therapeutic response than the conventional dose regimen. However, due to the limited number of patients in our study, whether low-dose RTX is recommended for anti-MDA5 DM-ILD remains to be verified in larger sample trials.
To our knowledge, the data on anti-MDA5 DM-ILD patients treated with RTX reviewed in this study were larger than those in previous studies, and we describe the detailed regimen used for RTX administration. In addition, we evaluated the changes in ILD through HRCT and/or PFT, providing more evidence for the efficacy and safety of RTX in the treatment of anti-MDA5 DM-related ILD. However, there are some limitations to this study. First, the statistical analysis of the results was limited by the small sample size, which is not easy to circumvent due to the rarity of the disease. Second, the appropriate timing of RTX administration was not demonstrated in the present study. Third, the information of anti-MDA5 autoAb titers before and after RTX treatment were not available in most cases. Finally, multiple immunosuppressants were administered prior to RTX therapy in most patients, which may prevent the attribution of improvement to RTX treatment alone.
In summary, this systematic review allows us to conclude that as a CD20 targeting drug, RTX has a good response in the treatment of ILD related to anti-MDA5 DM. Combined with the current evidence that B cells may be involved in the pathogenesis of this disease, we suggest that RTX could be a promising treatment for anti-MDA5 DM-ILD. Also, patients with anti-MDA5 DM-ILD often have a condition with worse prognosis and were treated with multiple immunosuppressants previously or simultaneously to RTX administration, the risk of infection, especially opportunistic infections, should be considered during the use of RTX, and a low dose of RTX (100 mg every week) may also be applied; In addition, belimumab, a human monoclonal antibody targeting BAFF, may also be considered as a candidate therapy for anti-MDA5 DM-ILD, since some cases in our study showed poor response to RTX treatment. Finally, further prospective controlled clinical studies are required to evaluate the status and optimal regimen of RTX in anti-MDA5 DM-ILD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
QX, GY, and CH conceived the study. CH designed the study forms. GY and QX guided this study. CH and WL searched the literature and screened the studies for inclusion, extracted data, and assessed methodological quality. CH, WL, GY, and QX organized and analyzed data. All authors drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by Sichuan Science and Technology Program (Grant number 2021JDRC0045, 2021JDRC0169, 2021YJ0472 and 2021YFS0164), and the Clinical Research Incubation Project of West China Hospital, Sichuan University (Grant number 2019HXFH038 and 2021HXFH018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.820163/full#supplementary-material
References
1. McHugh NJ, Tansley SL. Autoantibodies in Myositis. Nat Rev Rheumatol (2018) 14(5):290–302. doi: 10.1038/nrrheum.2018.56
2. Kurtzman DJB, Vleugels RA. Anti-Melanoma Differentiation-Associated Gene 5 (MDA5) Dermatomyositis: A Concise Review With an Emphasis on Distinctive Clinical Features. J Am Acad Dermatol (2018) 78(4):776–85. doi: 10.1016/j.jaad.2017.12.010
3. Hall JC, Casciola-Rosen L, Samedy LA, Werner J, Owoyemi K, Danoff SK, et al. Anti-Melanoma Differentiation-Associated Protein 5-Associated Dermatomyositis: Expanding the Clinical Spectrum. Arthritis Care Res (Hoboken) (2013) 65(8):1307–15. doi: 10.1002/acr.21992
4. Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-Melanoma Differentiation-Associated Gene 5 Is Associated With Rapidly Progressive Lung Disease and Poor Survival in US Patients With Amyopathic and Myopathic Dermatomyositis. Arthritis Care Res (Hoboken) (2016) 68(5):689–94. doi: 10.1002/acr.22728
5. Ceribelli A, Fredi M, Taraborelli M, Cavazzana I, Tincani A, Selmi C, et al. Prevalence and Clinical Significance of Anti-MDA5 Antibodies in European Patients With Polymyositis/Dermatomyositis. Clin Exp Rheumatol (2014) 32(6):891–7.
6. Cao H, Pan M, Kang Y, Xia Q, Li X, Zhao X, et al. Clinical Manifestations of Dermatomyositis and Clinically Amyopathic Dermatomyositis Patients With Positive Expression of Anti-Melanoma Differentiation-Associated Gene 5 Antibody. Arthritis Care Res (Hoboken) (2012) 64(10):1602–10. doi: 10.1002/acr.21728
7. Nakashima R, Imura Y, Kobayashi S, Yukawa N, Yoshifuji H, Nojima T, et al. The RIG-I-Like Receptor IFIH1/MDA5 is a Dermatomyositis-Specific Autoantigen Identified by the Anti-CADM-140 Antibody. Rheumatol (Oxford) (2010) 49(3):433–40. doi: 10.1093/rheumatology/kep375
8. Hamaguchi Y, Kuwana M, Hoshino K, Hasegawa M, Kaji K, Matsushita T, et al. Clinical Correlations With Dermatomyositis-Specific Autoantibodies in Adult Japanese Patients With Dermatomyositis: A Multicenter Cross-Sectional Study. Arch Dermatol (2011) 147(4):391–8. doi: 10.1001/archdermatol.2011.52
9. Motegi SI, Sekiguchi A, Toki S, Kishi C, Endo Y, Yasuda M, et al. Clinical Features and Poor Prognostic Factors of Anti-Melanoma Differentiation-Associated Gene 5 Antibody-Positive Dermatomyositis With Rapid Progressive Interstitial Lung Disease. Eur J Dermatol (2019) 29(5):511–7. doi: 10.1684/ejd.2019.3634
10. Li Y, Li Y, Wu J, Miao M, Gao X, Cai W, et al. Predictors of Poor Outcome of Anti-MDA5-Associated Rapidly Progressive Interstitial Lung Disease in a Chinese Cohort With Dermatomyositis. J Immunol Res (2020) 2020:2024869. doi: 10.1155/2020/2024869
11. Fujikawa K, Kawakami A, Kaji K, Fujimoto M, Kawashiri S, Iwamoto N, et al. Association of Distinct Clinical Subsets With Myositis-Specific Autoantibodies Towards Anti-155/140-kDa Polypeptides, Anti-140-kDa Polypeptides, and Anti-Aminoacyl tRNA Synthetases in Japanese Patients With Dermatomyositis: A Single-Centre, Cross-Sectional Study. Scand J Rheumatol (2009) 38(4):263–7. doi: 10.1080/03009740802687455
12. Tsuji H, Nakashima R, Hosono Y, Imura Y, Yagita M, Yoshifuji H, et al. Multicenter Prospective Study of the Efficacy and Safety of Combined Immunosuppressive Therapy With High-Dose Glucocorticoid, Tacrolimus, and Cyclophosphamide in Interstitial Lung Diseases Accompanied by Anti-Melanoma Differentiation-Associated Gene 5-Positive Dermatomyositis. Arthritis Rheumatol (2020) 72(3):488–98. doi: 10.1002/art.41105
13. Chen F, Li S, Wang T, Shi J, Wang G. Clinical Heterogeneity of Interstitial Lung Disease in Polymyositis and Dermatomyositis Patients With or Without Specific Autoantibodies. Am J Med Sci (2018) 355(1):48–53. doi: 10.1016/j.amjms.2017.07.013
14. Yoshida N, Okamoto M, Kaieda S, Fujimoto K, Ebata T, Tajiri M, et al. Association of Anti-Aminoacyl-Transfer RNA Synthetase Antibody and Anti-Melanoma Differentiation-Associated Gene 5 Antibody With the Therapeutic Response of Polymyositis/Dermatomyositis-Associated Interstitial Lung Disease. Respir Investig (2017) 55(1):24–32. doi: 10.1016/j.resinv.2016.08.007
15. Kimani S, Painschab MS, Kaimila B, Kasonkanji E, Zuze T, Tomoka T, et al. Safety and Efficacy of Rituximab in Patients With Diffuse Large B-Cell Lymphoma in Malawi: A Prospective, Single-Arm, Non-Randomised Phase 1/2 Clinical Trial. Lancet Glob Health (2021) 9(7):e1008–e16. doi: 10.1016/S2214-109X(21)00181-9
16. Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab Versus Cyclophosphamide in ANCA-Associated Renal Vasculitis. N Engl J Med (2010) 363(3):211–20. doi: 10.1056/NEJMoa0909169
17. Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-Cell-Targeted Therapy With Rituximab in Patients With Rheumatoid Arthritis. N Engl J Med (2004) 350(25):2572–81. doi: 10.1056/NEJMoa032534
18. Cervera R, Mosca M, Rios-Garces R, Espinosa G, Trujillo H, Bada T, et al. Treatment for Refractory Lupus Nephritis: Rituximab vs Triple Target Therapy. Autoimmun Rev (2019) 18(12):102406. doi: 10.1016/j.autrev.2019.102406
19. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
21. Bohan A, Peter JB. Polymyositis and Dermatomyositis (First of Two Parts). N Engl J Med (1975) 292(7):344–7. doi: 10.1056/NEJM197502132920706
22. Sontheimer RD. Would a New Name Hasten the Acceptance of Amyopathic Dermatomyositis (Dermatomyositis Sine Myositis) as a Distinctive Subset Within the Idiopathic Inflammatory Dermatomyopathies Spectrum of Clinical Illness? J Am Acad Dermatol (2002) 46(4):626–36. doi: 10.1067/mjd.2002.120621
23. Ge Y, Li S, Tian X, He L, Lu X, Wang G. Anti-Melanoma Differentiation-Associated Gene 5 (MDA5) Antibody-Positive Dermatomyositis Responds to Rituximab Therapy. Clin Rheumatol (2021) 40(6):2311–7. doi: 10.1007/s10067-020-05530-5
24. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
25. American Thoracic Society. Idiopathic Pulmonary Fibrosis: Diagnosis and Treatment. International Consensus Statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med (2000) 161(2 Pt 1):646–64. doi: 10.1164/ajrccm.161.2.ats3-00
26. So H, Wong VTL, Lao VWN, Pang HT, Yip RML. Rituximab for Refractory Rapidly Progressive Interstitial Lung Disease Related to Anti-MDA5 Antibody-Positive Amyopathic Dermatomyositis. Clin Rheumatol (2018) 37(7):1983–9. doi: 10.1007/s10067-018-4122-2
27. Koichi Y, Aya Y, Megumi U, Shunichi K, Masafumi S, Hiroaki M, et al. A Case of Anti-MDA5-Positive Rapidly Progressive Interstitial Lung Disease in a Patient With Clinically Amyopathic Dermatomyositis Ameliorated by Rituximab, in Addition to Standard Immunosuppressive Treatment. Modern Rheumatol (2017) 27(3):536–40. doi: 10.3109/14397595.2015.1014140
28. Ogawa Y, Kishida D, Shimojima Y, Hayashi K, Sekijima Y. Effective Administration of Rituximab in Anti-MDA5 Antibody-Positive Dermatomyositis With Rapidly Progressive Interstitial Lung Disease and Refractory Cutaneous Involvement: A Case Report and Literature Review. Case Rep Rheumatol (2017) 2017:5386797–. doi: 10.1155/2017/5386797
29. Tokunaga K, Hagino N. Dermatomyositis With Rapidly Progressive Interstitial Lung Disease Treated With Rituximab: A Report of 3 Cases in Japan. Internal Med (2017) 56(11):1399–403. doi: 10.2169/internalmedicine.56.7956
30. Hisanaga J, Kotani T, Fujiki Y, Yoshida S, Takeuchi T, Makino S. Successful Multi-Target Therapy Including Rituximab and Mycophenolate Mofetil in Anti-Melanoma Differentiation-Associated Gene 5 Antibody-Positive Rapidly Progressive Interstitial Lung Disease With Clinically Amyopathic Dermatomyositis. Int J Rheumatic Dis (2017) 20(12):2182–5. doi: 10.1111/1756-185x.13136
31. Alqatari S, Riddell P, Harney S, Henry M, Murphy G. MDA-5 Associated Rapidly Progressive Interstitial Lung Disease With Recurrent Pneumothoraces: A Case Report. BMC Pulm Med (2018) 18(1):59. doi: 10.1186/s12890-018-0622-8
32. Hoa S, Troyanov Y, Fritzler MJ, Targoff IN, Chartrand S, Mansour AM, et al. Describing and Expanding the Clinical Phenotype of Anti-MDA5-Associated Rapidly Progressive Interstitial Lung Disease: Case Series of Nine Canadian Patients and Literature Review. Scand J Rheumatol (2018) 47(3):210–24. doi: 10.1080/03009742.2017.1334814
33. Tamai K, Tachikawa R, Otsuka K, Ueda H, Hosono Y, Tomii K. Early Pulmonary Involvement of Anti-CADM-140 Autoantibody-Positive Rapidly Progressive Interstitial Lung Disease Preceding Typical Cutaneous Symptoms. Intern Med (2014) 53(21):2515–9. doi: 10.2169/internalmedicine.53.2769
34. Nishizawa T, Kida G, Tsukahara Y, Emoto K, Tsumiyama E, Kriiwa S, et al. A Case of Rapidly Progressive Interstitial Pneumonia in Association With Amyopathic Dermatomyositis With Elevated Titers of Anti-MDA-5 Antibody, Successfully Treated With Rituximab in Addition to Standard Immunosuppressive Therapy. Am J Respir Crit Care Med (2018) 197:A6475.
35. Kishida D, Sakaguchi N, Ueno K-I, Ushiyama S, Ichikawa T, Yoshinaga T, et al. Macrophage Activation Syndrome in Adult Dermatomyositis: A Case-Based Review. Rheumatol Int (2020) 40(7):1151–62. doi: 10.1007/s00296-020-04590-9
36. Scirocco C, Gubbiotti A, Sebastiani A, Sebastiani GD. Rituximab in Antimelanoma Differentiation-Associated Protein-5 Dermatomyositis With Interstitial Lung Disease. Case Rep Rheumatol (2020) 2020:8145790–. doi: 10.1155/2020/8145790
37. Machiyama T, Shirai T, Fujita Y, Sato H, Fujii H, Ishii T, et al. Successful Concomitant Therapy With Tofacitinib for Anti- Melanoma Differentiation Associated Gene 5 Antibody-Positive Rapidly Progressive Interstitial Lung Disease With Poor Prognostic Factors: A Case Report and Literature Review. Med: Case Rep Study Protoc (2021) 2(1):e0026. doi: 10.1097/md9.0000000000000026
38. Akiyama C, Shirai T, Sato H, Fujii H, Ishii T, Harigae H. Association of Various Myositis-Specific Autoantibodies With Dermatomyositis and Polymyositis Triggered by Pregnancy. Rheumatol Int (2021). doi: 10.1007/s00296-021-04851-1
39. Mehta AA, Paul T, Cb M, Haridas N. Anti-MDA5 Antibody-Positive Dermatomyositis With Rapidly Progressive Interstitial Lung Disease: Report of Two Cases. BMJ Case Rep (2021) 14(4):e240046. doi: 10.1136/bcr-2020-240046
40. Yeung T-W, Cheong K-N, Lau Y-L, Tse K-CN. Adolescent-Onset Anti-MDA5 Antibody-Positive Juvenile Dermatomyositis With Rapidly Progressive Interstitial Lung Disease and Spontaneous Pneumomediastinum: A Case Report and Literature Review. Pediatr Rheumatol (2021) 19(1):103. doi: 10.1186/s12969-021-00595-1
41. Mao M-M, Xia S, Guo B-P, Qian W-P, Zheng Z-X, Peng X-M, et al. Ultra-Low Dose Rituximab as Add-on Therapy in Anti-MDA5-Positive Patients With Polymyositis/Dermatomyositis Associated ILD. Respir Med (2020) 172:105983. doi: 10.1016/j.rmed.2020.105983
42. Ye S, Chen XX, Lu XY, Wu MF, Deng Y, Huang WQ, et al. Adult Clinically Amyopathic Dermatomyositis With Rapid Progressive Interstitial Lung Disease: A Retrospective Cohort Study. Clin Rheumatol (2007) 26(10):1647–54. doi: 10.1007/s10067-007-0562-9
43. Mukae H, Ishimoto H, Sakamoto N, Hara S, Kakugawa T, Nakayama S, et al. Clinical Differences Between Interstitial Lung Disease Associated With Clinically Amyopathic Dermatomyositis and Classic Dermatomyositis. Chest (2009) 136(5):1341–7. doi: 10.1378/chest.08-2740
44. Zou J, Li T, Huang X, Chen S, Guo Q, Bao C. Basiliximab may Improve the Survival Rate of Rapidly Progressive Interstitial Pneumonia in Patients With Clinically Amyopathic Dermatomyositis With Anti-MDA5 Antibody. Ann Rheum Dis (2014) 73(8):1591–3. doi: 10.1136/annrheumdis-2014-205278
45. Matsushita T, Kobayashi T, Kano M, Hamaguchi Y, Takehara K. Elevated Serum B-Cell Activating Factor Levels in Patients With Dermatomyositis: Association With Interstitial Lung Disease. J Dermatol (2019) 46(12):1190–6. doi: 10.1111/1346-8138.15117
46. Wang K, Zhao J, Chen Z, Li T, Tan X, Zheng Y, et al. CD4+CXCR4+ T Cells as a Novel Prognostic Biomarker in Patients With Idiopathic Inflammatory Myopathy-Associated Interstitial Lung Disease. Rheumatol (Oxford) (2019) 58(3):511–21. doi: 10.1093/rheumatology/key341
47. Nombel A, Fabien N, Coutant F. Dermatomyositis With Anti-MDA5 Antibodies: Bioclinical Features, Pathogenesis and Emerging Therapies. Front Immunol (2021) 12:773352. doi: 10.3389/fimmu.2021.773352
48. Zhang S, Shen H, Shu X, Peng Q, Wang G. Abnormally Increased Low-Density Granulocytes in Peripheral Blood Mononuclear Cells are Associated With Interstitial Lung Disease in Dermatomyositis. Mod Rheumatol (2017) 27(1):122–9. doi: 10.1080/14397595.2016.1179861
49. Peng Y, Zhang S, Zhao Y, Liu Y, Yan B. Neutrophil Extracellular Traps may Contribute to Interstitial Lung Disease Associated With Anti-MDA5 Autoantibody Positive Dermatomyositis. Clin Rheumatol (2018) 37(1):107–15. doi: 10.1007/s10067-017-3799-y
50. Gono T, Miyake K, Kawaguchi Y, Kaneko H, Shinozaki M, Yamanaka H. Hyperferritinaemia and Macrophage Activation in a Patient With Interstitial Lung Disease With Clinically Amyopathic DM. Rheumatol (Oxford) (2012) 51(7):1336–8. doi: 10.1093/rheumatology/kes012
51. Horiike Y, Suzuki Y, Fujisawa T, Yasui H, Karayama M, Hozumi H, et al. Successful Classification of Macrophage-Mannose Receptor CD206 in Severity of Anti-MDA5 Antibody Positive Dermatomyositis Associated ILD. Rheumatol (Oxford) (2019) 58(12):2143–52. doi: 10.1093/rheumatology/kez185
52. Zhang SH, Zhao Y, Xie QB, Jiang Y, Wu YK, Yan B. Aberrant Activation of the Type I Interferon System may Contribute to the Pathogenesis of Anti-Melanoma Differentiation-Associated Gene 5 Dermatomyositis. Br J Dermatol (2019) 180(5):1090–8. doi: 10.1111/bjd.16917
53. Horai Y, Koga T, Fujikawa K, Takatani A, Nishino A, Nakashima Y, et al. Serum Interferon-Alpha is a Useful Biomarker in Patients With Anti-Melanoma Differentiation-Associated Gene 5 (MDA5) Antibody-Positive Dermatomyositis. Mod Rheumatol (2015) 25(1):85–9. doi: 10.3109/14397595.2014.900843
54. Giannini M, Ohana M, Nespola B, Zanframundo G, Geny B, Meyer A. Similarities Between COVID-19 and Anti-MDA5 Syndrome: What can We Learn for Better Care? Eur Respir J (2020) 56(3):2001618. doi: 10.1183/13993003.01618-2020
55. Rehwinkel J, Gack MU. RIG-I-Like Receptors: Their Regulation and Roles in RNA Sensing. Nat Rev Immunol (2020) 20(9):537–51. doi: 10.1038/s41577-020-0288-3
56. Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. Mda-5: An Interferon-Inducible Putative RNA Helicase With Double-Stranded RNA-Dependent ATPase Activity and Melanoma Growth-Suppressive Properties. Proc Natl Acad Sci USA (2002) 99(2):637–42. doi: 10.1073/pnas.022637199
57. Zahn S, Barchet W, Rehkamper C, Hornung T, Bieber T, Tuting T, et al. Enhanced Skin Expression of Melanoma Differentiation-Associated Gene 5 (MDA5) in Dermatomyositis and Related Autoimmune Diseases. J Am Acad Dermatol (2011) 64(5):988–9. doi: 10.1016/j.jaad.2010.08.004
58. Berger M, Hsieh CY, Bakele M, Marcos V, Rieber N, Kormann M, et al. Neutrophils Express Distinct RNA Receptors in a Non-Canonical Way. J Biol Chem (2012) 287(23):19409–17. doi: 10.1074/jbc.M112.353557
59. Vlahakos D, Foster MH, Ucci AA, Barrett KJ, Datta SK, Madaio MP. Murine Monoclonal Anti-DNA Antibodies Penetrate Cells, Bind to Nuclei, and Induce Glomerular Proliferation and Proteinuria. vivo J Am Soc Nephrol (1992) 2(8):1345–54. doi: 10.1681/ASN.V281345
60. Deng SX, Hanson E, Sanz I. In Vivo Cell Penetration and Intracellular Transport of Anti-Sm and Anti-La Autoantibodies. Int Immunol (2000) 12(4):415–23. doi: 10.1093/intimm/12.4.415
61. Ishida Y, Kimura A, Nosaka M, Kuninaka Y, Hemmi H, Sasaki I, et al. Essential Involvement of the CX3CL1-CX3CR1 Axis in Bleomycin-Induced Pulmonary Fibrosis via Regulation of Fibrocyte and M2 Macrophage Migration. Sci Rep (2017) 7(1):16833. doi: 10.1038/s41598-017-17007-8
62. Nakano M, Fujii T, Hashimoto M, Yukawa N, Yoshifuji H, Ohmura K, et al. Type I Interferon Induces CX3CL1 (Fractalkine) and CCL5 (RANTES) Production in Human Pulmonary Vascular Endothelial Cells. Clin Exp Immunol (2012) 170(1):94–100. doi: 10.1111/j.1365-2249.2012.04638.x
63. Thieblemont N, Wright HL, Edwards SW, Witko-Sarsat V. Human Neutrophils in Auto-Immunity. Semin Immunol (2016) 28(2):159–73. doi: 10.1016/j.smim.2016.03.004
64. Matsushita T, Mizumaki K, Kano M, Yagi N, Tennichi M, Takeuchi A, et al. Antimelanoma Differentiation-Associated Protein 5 Antibody Level is a Novel Tool for Monitoring Disease Activity in Rapidly Progressive Interstitial Lung Disease With Dermatomyositis. Br J Dermatol (2017) 176(2):395–402. doi: 10.1111/bjd.14882
65. Chen Z, Cao M, Plana MN, Liang J, Cai H, Kuwana M, et al. Utility of Anti-Melanoma Differentiation-Associated Gene 5 Antibody Measurement in Identifying Patients With Dermatomyositis and a High Risk for Developing Rapidly Progressive Interstitial Lung Disease: A Review of the Literature and a Meta-Analysis. Arthritis Care Res (Hoboken) (2013) 65(8):1316–24. doi: 10.1002/acr.21985
66. Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, et al. BAFF and MyD88 Signals Promote a Lupuslike Disease Independent of T Cells. J Exp Med (2007) 204(8):1959–71. doi: 10.1084/jem.20062567
67. Stohl W. Biologic Differences Between Various Inhibitors of the BLyS/BAFF Pathway: Should We Expect Differences Between Belimumab and Other Inhibitors in Development? Curr Rheumatol Rep (2012) 14(4):303–9. doi: 10.1007/s11926-012-0254-6
68. Kobayashi N, Kobayashi I, Mori M, Sato S, Iwata N, Shigemura T, et al. Increased Serum B Cell Activating Factor and a Proliferation-Inducing Ligand Are Associated With Interstitial Lung Disease in Patients With Juvenile Dermatomyositis. J Rheumatol (2015) 42(12):2412–8. doi: 10.3899/jrheum.140977
69. Spolski R, Leonard WJ. Interleukin-21: A Double-Edged Sword With Therapeutic Potential. Nat Rev Drug Discov (2014) 13(5):379–95. doi: 10.1038/nrd4296
70. Girard C, Vincent T, Bessis D. [Dermatomyositis and Acute Interstitial Lung Disease Associated With MDA-5 Antibodies: An Atypical Case]. Ann Dermatol Venereol (2013) 140(10):628–34. doi: 10.1016/j.annder.2013.04.083
71. Clottu A, Laffitte E, Prins C, Chizzolini C. Response of Mucocutaneous Lesions to Rituximab in a Case of Melanoma Differentiation Antigen 5-Related Dermatomyositis. Dermatology (2012) 225(4):376–80. doi: 10.1159/000346573
72. Gono T, Sato S, Kawaguchi Y, Kuwana M, Hanaoka M, Katsumata Y, et al. Anti-MDA5 Antibody, Ferritin and IL-18 are Useful for the Evaluation of Response to Treatment in Interstitial Lung Disease With Anti-MDA5 Antibody-Positive Dermatomyositis. Rheumatol (Oxford) (2012) 51(9):1563–70. doi: 10.1093/rheumatology/kes102
73. Christou EAA, Giardino G, Worth A, Ladomenou F. Risk Factors Predisposing to the Development of Hypogammaglobulinemia and Infections Post-Rituximab. Int Rev Immunol (2017) 36(6):352–9. doi: 10.1080/08830185.2017.1346092
74. Li Y, Shi Y, He Z, Chen Q, Liu Z, Yu L, et al. The Efficacy and Safety of Low-Dose Rituximab in Immune Thrombocytopenia: A Systematic Review and Meta-Analysis. Platelets (2019) 30(6):690–7. doi: 10.1080/09537104.2019.1624706
75. Yang CS, Yang L, Li T, Zhang DQ, Jin WN, Li MS, et al. Responsiveness to Reduced Dosage of Rituximab in Chinese Patients With Neuromyelitis Optica. Neurology (2013) 81(8):710–3. doi: 10.1212/WNL.0b013e3182a1aac7
Keywords: dermatomyositis, melanoma differentiation-associated gene 5, interstitial lung disease, rituximab, targeting CD20
Citation: He C, Li W, Xie Q and Yin G (2022) Rituximab in the Treatment of Interstitial Lung Diseases Related to Anti-Melanoma Differentiation-Associated Gene 5 Dermatomyositis: A Systematic Review. Front. Immunol. 12:820163. doi: 10.3389/fimmu.2021.820163
Received: 22 November 2021; Accepted: 28 December 2021;
Published: 18 January 2022.
Edited by:
Guochun Wang, China-Japan Friendship Hospital, ChinaCopyright © 2022 He, Li, Xie and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geng Yin, eWluZ2VuZzE5NzVAMTYzLmNvbQ==; Qibing Xie, eGllcWliaW5nMTk3MUAxNjMuY29t
†These authors have contributed equally to this work
 Chenjia He
Chenjia He Wenyu Li2†
Wenyu Li2† Qibing Xie
Qibing Xie Geng Yin
Geng Yin