
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 09 December 2021
Sec. Multiple Sclerosis and Neuroimmunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.803504
This article is part of the Research TopicImmune Mechanism in White Matter Lesions: Clinical and Pathophysiological ImplicationsView all 6 articles
Objective: Similar white matter hyperintensities (WMH) might have different impact on the cognitive outcomes in patients with cerebral small vessel disease (CSVD). This study is to assess the possible factors related to the heterogeneity of WMH in cognitively impaired patients with CVSD.
Methods: We analyzed data from a cohort of patients with CVSD who were recruited consecutively from the Beijing Tiantan Hospital from 2015 to 2020. WMH, lacunes, enlarged perivascular space (ePVS), microbleeds and lacunar infarcts were rated on brain MRI. A score of <26 on the Montreal Cognitive Assessment (MoCA) indicated cognitive impairment. A mismatch was defined as the severity of WMH not matching the severity of cognitive dysfunction. Type-1 mismatch was defined as a mild WMH (Fazekas score = 0-1) associated with cognitive impairment, and type-2 mismatch was defined as a severe WMH (Fazekas score = 5-6) associated with normal cognitive function. Ultrasmall superparamagnetic iron oxide (USPIO)-enhanced SWI on 3-Tesla MRI was used to image the penetrating arteries in basal ganglia to explore the underlying mechanism of this mismatch. Multivariable logistic regression was used to analyze the association between the imaging features and cognitive impairment.
Results: In 156 patients, 118 (75.6%) had cognitive impairment and 37 (23.7%) showed mismatch. Twenty five (16.0%) had type-1 mismatch and 12 (7.7%) had type-2 mismatch. Regression analysis found that WMH, lacunes, microbleeds and total CSVD scores were associated with cognitive impairment and were independent of vascular risk factors. However, lacunes, microbleeds and total CSVD scores were related to the mismatch between WMH and cognitive impairment (p=0.006, 0.005 and 0.0001, respectively). Specially, age and ePVS in basal ganglia were related to type-1 mismatch (p=0.04 and 0.02, respectively); microbleeds and total CSVD scores were related to type-2 mismatch (p=0.01 and 0.03, respectively). Although the severity of WMH was similar, the injury scores of penetrating arteries were significantly different between those with and without cognitive impairment (p=0.04).
Conclusions: Heterogeneity of WMH was present in cognitively impaired patients with CSVD. Conventional imaging features and injury of penetrating arteries may account for such heterogeneity, which can be a hallmark for early identification and prevention of cognitive impairment.
Cerebral small vessel disease (CSVD) is generally caused by disorders of the intrinsic cerebral arteriolar system (1). CSVD is an important subtype of stroke and the major cause of dementia (2). White matter hyperintensities (WMH) are the most common feature of CSVD on brain magnetic resonance imaging (MRI) (3) with a prevalence up to 94% in the general population aged around 80 (4). It is associated with cognitive decline, a 90% increased risk of dementia and a 200% increased risk of stroke (5).
Several studies have focused on the association between WMH and clinical outcomes. Most of these studies found that extensive WMH burden was usually associated with increased risk of incident stroke, dementia and mortality in general population or in populations at high risk for vascular disease or dementia (6). WMH were often heterogeneous, for example, the volume of WMH could increase, remain stable, or even regress one year after a minor stroke (7–9). The microstructures within WMH, including the integrity of neural fibers, the structure of small vessels and histopathology, might vary distinctly, causing the heterogeneity of WMH (7, 10, 11). However, the effect of such heterogeneity of WMH on clinical symptoms remains uncertain.
Recently, ultrasmall superparamagnetic iron oxide (USPIO)-enhanced susceptibility-weighted imaging (SWI) on 3-Tesla MRI has enhanced the visualization of small vessels (12, 13). The structure and function of supplying vessels could directly reflect the real severity of WMH. Therefore, applying USPIO-SWI to assess the small vessels might identify factors contributing to the heterogeneity of WMH.
In this cross-sectional study of imaging features and clinical outcomes in patients with CSVD, we observed the heterogeneity of WMH severity on cognitive impairment. In order to identify the association of vascular risk factors and imaging features that contribute to this heterogeneity and its potential underlying mechanism, we used USPIO-SWI to evaluate the relevant small perforating arterioles.
Patients with CSVD were recruited prospectively and consecutively from Neurology Department of Beijing Tiantan Hospital from 2015 to 2020. All patients had a definitive diagnosis of lacunar stroke or headache or dizziness but with typical MRI features of CSVD. To qualify for enrollment, patients must meet one of the following inclusion criteria: 1) patients had lacunar stroke syndrome, and MRI showed a recent subcortical small lacunar infarction responsible for the clinical symptoms. If no such lesion was found on MRI, the clinical definition of lacunar syndromes described by Fisher (14) was used. 2) Patients underwent MRI because of headache or dizziness, although the neurological examination was negative, and had at least one of the typical MRI features of CSVD (2, 15): recent small subcortical infarcts; WMH Fazekas score ≥2; WMH Fazekas score=1, combined with at least one lacune or at least two cardiovascular risk factors (including hypertension, dyslipidemia, diabetes, smoking, or ischemic heart disease).
The exclusion criteria were as follows: 1) cortical infarct on MRI; 2) acute cerebral infarction with a diameter >20 mm on MRI; 3) acute cerebral hemorrhage or subarachnoid hemorrhage within 14 days before recruitment; 4) other WMH disorders, including those with nonvascular origin, e.g., multiple sclerosis and primary or secondary encephalitis; 5) diagnosis of neurodegenerative disease of the central nervous system, such as Alzheimer’s disease, Parkinson’s disease and so on; 6) complications with psychiatric disorders, including depression and anxiety; 7) significant stenosis (>50%) of the cervical or intracranial arteries or possible cardioembolic source (e.g., atrial fibrillation or a valvular prosthesis); and 8) failure to complete all sessions in the prospective study stream.
The diagnosis of CSVD was made by at least two trained neurologists after reviewing the clinical manifestations, MRI features and results of other diagnostic tests.
Baseline data included age, sex, and cardiovascular risk factors such as body mass index (BMI), hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, current smoking, and alcohol consumption. Hypertension was defined as the systolic blood pressure ≥140 mmHg or the diastolic blood pressure ≥90 mmHg or when the patient was on antihypertensive treatments at the entry. Diabetes mellitus was defined as the fasting blood glucose level ≥120 mg/dL or when the patient was on antidiabetic treatments at the entry. Dyslipidemia was defined as the total cholesterol level ≥240 mg/dL or the high-density lipoprotein measurement< 35 mg/dL or when the patient was on lipid-lowering treatments. Ischemic heart disease was diagnosed by the medical chart-confirmed history. Current smoking was defined as smoking at entry or quitting smoking within the previous year. Moderate to severe alcohol consumption was defined as consuming ≥ two standard alcoholic beverages per day within the last year.
All patients underwent brain MRI on a 3-Tesla scanner (Siemens MAGNETOM Prisma, Erlangen, Germany) with a Siemens 64-channel Prisma head coil.
The structural MRI included a T1-weighted sequence [repetition time (TR)/inversion time (TI)/echo time (TE) = 2300/900/2.3 ms; field of view (FOV) = 256 × 256 × 196 mm3; voxel size = 1.0× 1.0 × 1.0 mm3, flip angle = 8°] for anatomic reference and a T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence (TR/TI/TE = 5000/1800/386 ms; FOV = 256×256×196 mm3; voxel size = 1.0× 1.0 × 1.0 mm3, flip angle = 40°) for assessment of WMH, lacunes and enlarged perivascular space (ePVS). In addition, a diffusion-weighted imaging (DWI) sequence (TR/TE = 6100/65 ms; FOV = 184×226×143 mm3; voxel size = 1.6 × 2.0 × 5.0 mm3, diffusion sensitizing gradient directions b = 1200 sec/mm2) was performed for the detection of new lacunar infarcts, and a SWI sequence (TR/TE = 29/20 ms; FOV = 210×240×159 mm3; voxel size = 0.5 × 0.5 × 1.5 mm3, flip angle = 15°) was used to detect microbleeds.
The SWI sequence was performed after intravenous administration of USPIO (ferumoxytol; AMAG Pharmaceuticals, Cambridge, MA; 3 mg/kg) (12). The SWI images were acquired using a T2*-weighted, 3D-gradient echo sequence: TR/TE = 29/20 ms, FOV = 210×240×159 mm3, voxel size = 0.9 × 0.9 × 0.9 mm3, flip angle = 15°, number of slices = 176, slice oversampling 18.2%. Blood pressure was measured before the initiation of MRI scanning and within 15 minutes after scanning to monitor any signs of hypertension or hypotension.
The presence, location, and lesion size of typical CSVD imaging markers were assessed according to the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) (3) by two experienced neuroradiologists (Q.H., with 25 years of experience, and S.Y.L., with 3 years of experience) who were blinded to the patients’ clinical information. To assess the WMH severity, we used the Fazekas score (16) for visual assessment and UBO Detector (https://cheba.unsw.edu.au/group/neuroimaging-pipeline) for computational assessment. We combined the periventricular and deep Fazekas scores as the total WMH score (0–6). To conveniently reflect the severity of the WMH, we graded WMH as mild (Fazekas score=0-1), moderate (Fazekas score=3-4) or severe (Fazekas score=5-6) types. UBO Detector is a cluster-based and fully automated WMH extraction pipeline. We followed the previously published technical details to preprocess the structural images. The results of UBO Detector highly correlated with the manually traced results and were significantly associated with Fazekas scores (17). The total CSVD score (range 0-4) was calculated on the basis of the individual imaging features, and points were assigned as follows (18): 1 for any lacune, 1 for any microbleed, 1 for moderate-to-severe ePVS in the basal ganglia (BG-ePVS > 10), and 1 for WMH (deep WMH, Fazekas=2 or 3 and/or periventricular WMH, Fazekas=3). The interclass correlation coefficient for interobserver ratings for WMH was 0.94; lacunes, 0.90; BG-ePVS, 0.85; microbleeds, 0.82; and lacunar infarct, 0.96.
To assess penetrating arteries in the basal ganglia, USPIO-SWI images were first processed. Raw SWI image data were interpolated to isotropic resolution, and minimum intensity projection (mIP) was created in the coronal and axial views, with an effective slice thickness of 8 mm. The medial and lateral lenticulostriate arteries (LSAs) could be reconstructed in the coronal view according to the previous histology work of Salamon (19). Injury of the LSAs was assessed on an ordinal scale from 0-2 by counting the number of LSAs: 0 points (if 2 visible), 1 point (if 1 visible), and 2 points (if 0 visible). The medial LSAs and lateral LSAs were rated separately by two experienced radiologists who were blinded to the patients’ clinical information and the conventional imaging features. The total score was obtained by summing the 2 partial scores.
The clinical outcome was defined as cognitive impairment. Considering that the Montreal Cognitive Assessment (MoCA) has a high level of sensitivity and specificity for detecting mild cognitive impairment, we used it to assess global cognitive performance for all subjects. A MoCA score of < 26 indicated cognitive impairment and the education level was accounted for in the scoring (20–22). The previous studies demonstrated that extensive WMH was usually associated with cognitive decline (5, 6). However, we observed a “mismatch” between WMH severity and cognitive impairment, we defined it as the “mismatch type” in present study. Specifically, mild WMH (Fazekas =0-2) with cognitive impairment was defined as type-1 mismatch, and severe WMH (Fazekas =5-6) with normal cognitive function was defined as type-2 mismatch. If on the contrary, are the match types.
We used χ2 tests and Mann-Whitney tests to test for differences between patients with and without cognitive impairment. We explored the association between CSVD imaging markers and cognitive impairment by multivariable logistic regression analysis, which was adjusted for age, sex, and risk factors that are frequently associated with cognitive impairment, including hypertension, dyslipidemia, diabetes mellitus, smoking and drinking. To explore what factors contributed to the mismatch between WMH severity and cognitive impairment, we performed χ2 tests and Mann-Whitney tests to compare the differences in clinical factors, conventional imaging features of CSVD and injury scores of LSAs between patients with and without mismatch types. The results of regression analysis are presented as odds ratios (ORs) with 95% confidence intervals. All analyses were conducted with SAS software, version 9.4 (SAS Inc., Cary, N.C., USA).
Of 172 patients in the original cohort, 10 were excluded because of missing MoCA scores, and 6 were excluded because of missing MRI data. Finally, 156 patients were included in this analysis. Among them, 118 patients (75.6%) had cognitive impairment. The median age was 57.0 (50.0-65.0) years. The patients with cognitive impairment were older. Regarding the conventional MRI features, the Fazekas scores and volume of WMH, as well as the presence of lacunes, microbleeds and ePVS, were higher in patients with cognitive impairment than in those without (Table 1). The distribution of WMH severity in patients with and without cognitive impairment was showed in Figure 1.
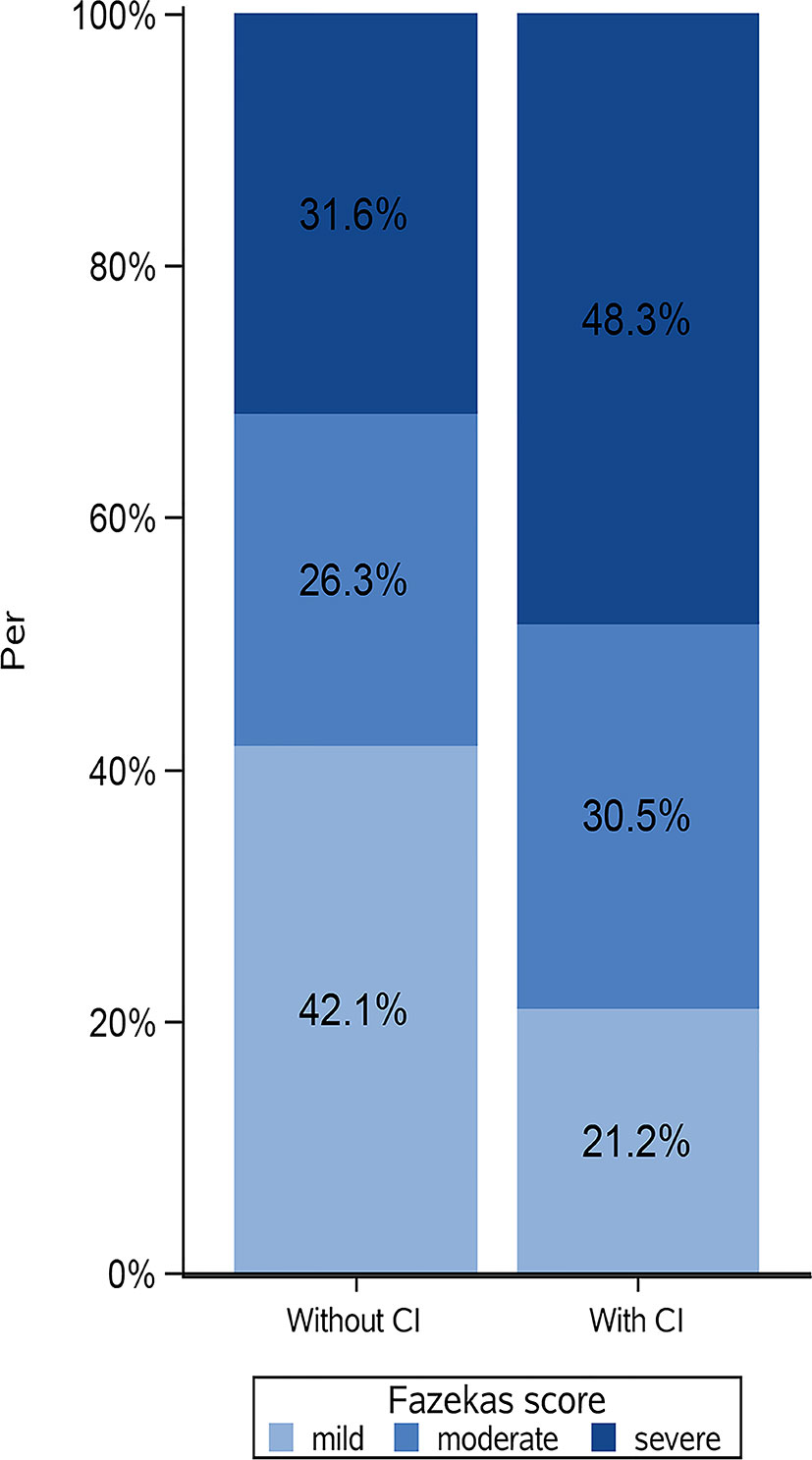
Figure 1 Distribution of white matter hyperintensities (WMH) severity in patients with and without cognitive impairment. CI, cognitive impairment; Fazekas score, mild (Fazekas score=0-2); moderate (Fazekas score=3-4); severe (Fazekas score=5-6). Patients without cognitive impairment could show severe WMH, on contrary, patients with normal cognitive function could show mild WMH.
The severity of WMH, lacunes, microbleeds, ePVS and total CSVD scores were all significantly associated with the cognitive impairment in the unadjusted multivariable logistic regression model (Table 2). In addition, compared with mild WMH (Fazekas=0-2), severe WMH (Fazekas=5-6) increased the risk of cognitive impairment by 204% (OR: 3.04, 95% confidence interval: 1.26-7.36), but moderate WMH (Fazekas=3-4) did not (OR: 2.30, 95% confidence interval: 0.90-5.90). After adjusting for age, sex, hypertension, dyslipidemia, diabetes mellitus, current smoking and alcohol consumption, the associations with the severity of WMH, lacunes, microbleeds and total CSVD scores remained significant (WMH: OR: 1.28, 95% confidence interval: 1.01-1.64; lacunes: OR: 2.49, 95% confidence interval: 1.08-5.73; microbleeds: OR: 5.29, 95% confidence interval: 2.13-13.13; total CSVD scores: OR: 1.79, 95% confidence interval: 1.28-2.52). However, the association between ePVS and cognitive impairment became insignificant, and the difference in the risk of cognitive impairment between mild WMH and severe WMH was insignificant. The above results suggested that the severity of WMH, presence of lacunes and microbleeds, and total CSVD scores were associated with the risk of cognitive impairment, which were independent of conventional vascular risk factors. Nevertheless, the risk of cognitive impairment did not increase when increased severity of WMH (moderate WMH: OR: 1.69, 95% confidence interval: 0.60-4.76; severe WMH: OR: 2.41, 95% confidence interval: 0.89-6.55).
Among all patients, 37 patients (23.7%) were observed with mismatch types and 73 patients (46.8%) had corresponding matches between imaging findings and cognitive impairment. The presence of imaging features, including lacunes, microbleeds and total CSVD scores appeared significantly different between the mismatch type and match type, which were lower in the mismatch type than in the match type (Table 3). However, recent small subcortical infarcts and BG-ePVS did not show any difference between the two types, nor did clinical factors, i.e., age, sex, and vascular risk factors, including hypertension and diabetes mellitus. Our analysis suggested that, compared with clinical factors, a lower rate of imaging features, especially lacunes and microbleeds, and lower total CSVD scores, were more likely to result in a mismatch between WMH severity and cognitive impairment.
Among all patients with mismatch types, 25 patients (16.0%) had type-1 mismatch and 12 (7.7%) with type-2 mismatch. Although lower rates of lacunes and microbleeds, as well as lower CSVD scores, showed an overall relationship to mismatch types, when examining one mismatch type, the results differed (Table 4). Age and BG-ePVS were significantly different between those in the type-1 mismatch group and match group (p=0.04 and 0.02, respectively). The patients in the type-1 mismatch group were older and had higher rates of BG-ePVS than those in the type-1 match group. On the rates of microbleeds and total CSVD scores, the patients in the type-2 mismatch group showed lower rates of microbleeds (p=0.01) and lower total CSVD scores (p=0.03) than those in the type-2 match group. The results of this analysis suggested that in patients with mild WMH, older age and BG-ePVS might increase the risk of cognitive impairment. However, among patients with severe WMH, lacking microbleeds or lower total CSVD scores might decrease the risk of cognitive impairment.
To explore the underlying mechanisms associated with heterogeneity of WMH severity on cognitive impairment, we applied USPIO-SWI to visualize the small vessels in the basal ganglia region, where the WMH were apparent and arterioles were the main supplying arteries. We performed USPIO-SWI on eight patients with severe WMH; among them, four had cognitive impairment, and the other four did not. Age, sex, presence of BG-ePVS and microbleeds were similar between the two groups. The imaging of medial and lateral LSAs was shown in Figure 2. The injury scores of the penetrating arteries were higher in patients with cognitive impairment than in those without (p<0.04, Figure 3). The results suggested that the injury degree of penetrating arteries in the basal ganglia might be responsible for the cognitive impairment in patients with similar severity of WMH, causing WMH heterogeneity in CSVD patients with cognitive impairment.
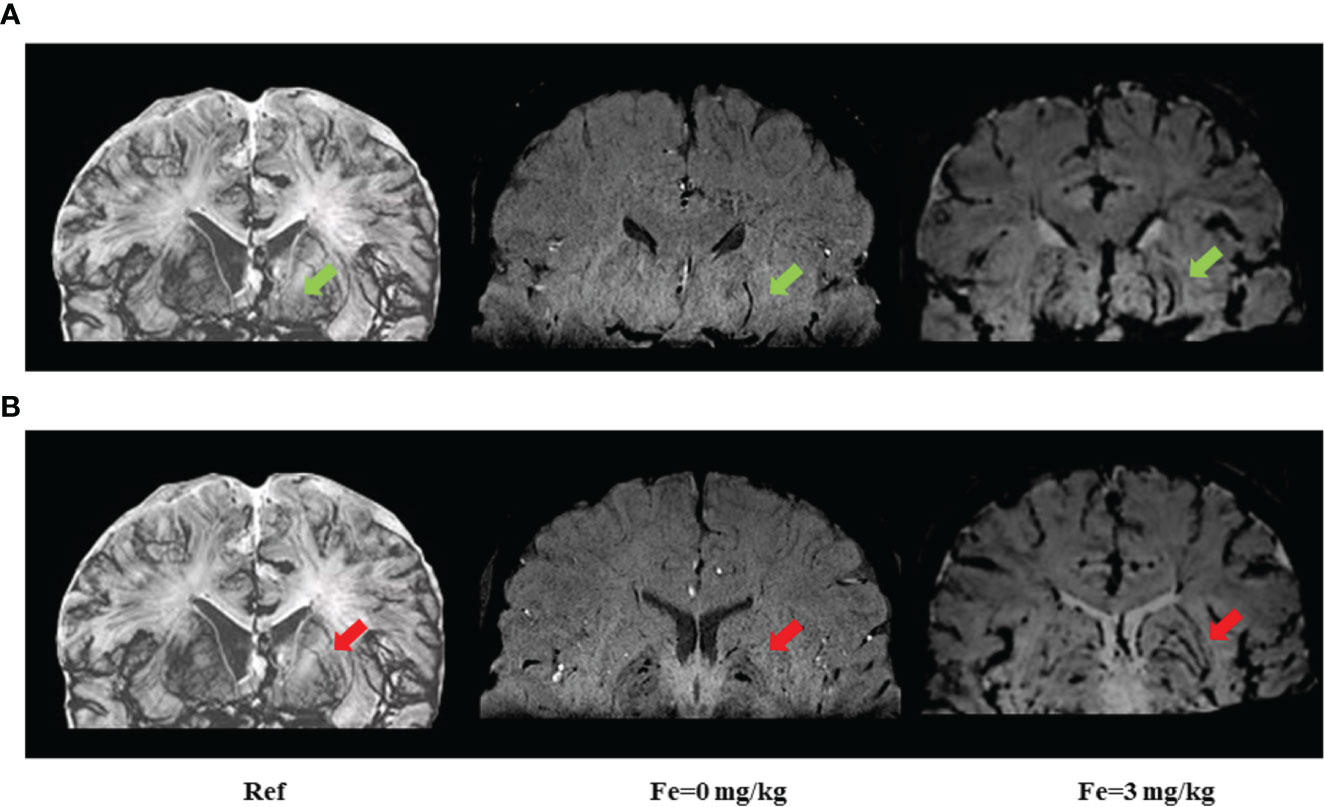
Figure 2 Imaging of medial and lateral lenticulostriate arteries (LSAs) before and after ultrasmall superparamagnetic iron oxide (USPIO) enhanced susceptibility-weighted imaging (SWI) on 3-Tesla MRI. The medial and lateral LSAs were reconstructed in the coronal view of SWI. All images were minimum intensity projected (mIP) with an effective slice thickness of 8 mm. (A) green arrows point to the medial LSAs; (B) red arrows point to lateral LSAs. Ref: reference pictures were adapted from Salamon’s histology work (19) and could also be available online at Salamon’s Neuroanatomy and Neurovasculature Web-Atlas Resource (http://www.radnet.ucla.edu/sections/DINR/index.htm, Neurovasculature > Atlas of Brain Arteries (Frontal Section) > Atlas > Slide 15); Fe=0 mg/kg: plain scan without USPIO enhancement; Fe=3 mg/kg: USPIO enhancement scan with a concentration of 3 mg/kg of Fe. USPIO-SWI imaged the LSAs more clearly compared with conventional SWI sequences.
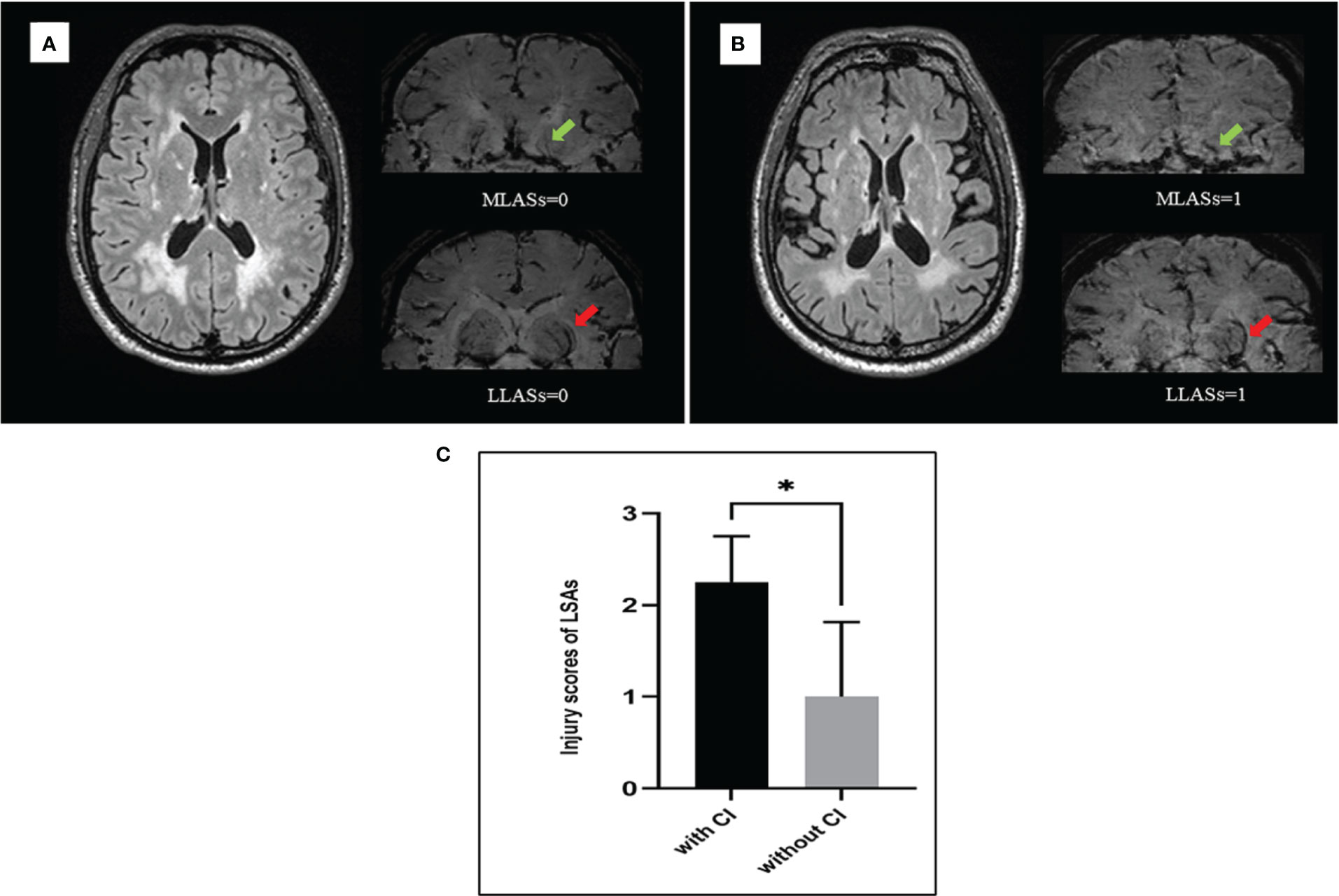
Figure 3 The injury severity of lenticulostriate arteries (LSAs) was different between patients with and without cognitive impairment when under similar white matter hyperintensities (WMH). (A) male, 50 years old, had severe WMH and normal cognitive function, the injury scores of medial and lateral LSAs were 0 separately. (B) male, 52 years old, had severe WMH and cognitive impairment, the injury scores of medial and lateral LSAs were 1 separately. (C) Eight patients with severe WMH were divided into two groups matching for sex, age, and risk factors. One group consists of four patients who had cognitive impairment and another group consists of four patients who had normal cognitive function. The injury scores of LSAs were statistically different between two groups (p=0.04). MLASs, medial lenticulostriate arteries; LLSAs, lateral lenticulostriate arteries; CI, cognitive impairment. *p < 0.05.
We demonstrated the heterogeneity of WMH in cognitively impaired CSVD patients by: 1) we observed a “mismatch” between the WMH severity and cognitive impairment. 2) We found that in addition to conventional factors, e.g. age, BG-ePVS, microbleeds and total CSVD score, the injury to penetrating arteries might also be the important contributor to this mismatch, which might increase the risk of cognitive impairment when under similar WMH. These findings can help guide prevention and treatment of cognitive impairment in at-risk CSVD patients with WMH.
CSVD has been associated with impairment in almost all major domains of cognitive ability (23). WMH were the most common imaging feature of CSVD, several studies have demonstrated that a high burden of WMH is associated with a noticeable decline in global cognitive function in both normal and high-risk populations (5). The progression of WMH is associated with further decreases in global cognitive scores (24, 25). Besides, above certain severity, WMH increased the risk of all-cause dementia (26). Our regression analysis were consistent with the findings from previous studies. In addition to WMH, we found that other imaging features of CSVD, including lacunes, ePVS in basal ganglia and microbleeds, were also associated with cognitive impairment, which was in line with the results of recent studies (27).
Heterogeneity of WMH has been reported before. Wardlaw demonstrated the progression of WMH was heterogeneous and the volume of WMH might grow and/or regress, reflected in the change of brain volume, visual scores, and mean diffusivity in WMH (7). Keun-Hwa Jung showed the variation on clinical symptoms related to WMH, that the periventricular WMH were associated with hypertension and deep WMH were associated with poor sleep quality (28). However, the heterogeneity of WMH in cognitively impaired patients has not been reported. In present study, we found a “mismatch” between the WMH severity and the decline in cognitive function. To explore the underlying mechanisms, we compared the vascular risk factors and imaging features possibly contributing to the cognitive dysfunction between patients with and without this mismatch. We found that changes on imaging features of CSVD may be important contributors of cognitive impairment, while traditional vascular risk factors, such as hypertension, diabetes mellitus, smoking, and drinking, did not play a prominent role in this mismatch. Our results suggested that the relationship between WMH severity and cognitive impairment might be independent of vascular risk factors as previously reported (26), and these factors might not show any promise for identifying patients with mismatch type.
Advances in structural and functional neuroimaging have facilitated the exploration of the mechanisms of WMH heterogeneity. Maillard investigated white matter integrity within WMH by using the diffusion tensor imaging (DTI) and found that although WMH had similar appearance on FLAIR, the diffusion metrics were significantly different and each could have different progressions over time (7, 29). In addition, the heterogeneity of white matter integrity might exist not only in WMH but also in penumbral or even remote areas. The variation in functional connectivity between different brain regions might be responsible for various clinical symptoms (11). Furthermore, the heterogeneity of blood-brain barrier (BBB) permeability might explain the mechanisms to some extent. BBB leakage tends to occur in ePVS-WMH or WMH surrounding the lacunes rather than only within WMH (30). Therefore, potential difference in BBB leakage between WMH with ePVS or lacunes and those without might be another reason why similar WMH could be associated with different clinical outcomes.
To further explore the mechanisms underlying the heterogeneity of WMH severity on cognitive impairment, we applied USPIO-SWI to visualize the penetrating arteries in the basal ganglia. USPIO-SWI has previously been used to image small vessels in vivo on a subvoxel level with reliable safety and maneuverability (12, 13, 31). Thereinto, E. Mark Haacke were achieved to visualizing the small arteries and veins in midbrain using USPIO-SWI on 3-Tesla MRI, and realized the measurement of the diameter of those small vessels (12). However, few studies have successfully imaged penetrating arteries in the basal ganglia due to the deep and complex anatomical location. We preliminarily visualized the medial and lateral LSAs on 3-Tesla USPIO-SWI and created a visual scale to assess the injury to these vessels. We found differences in numbers of these supplying arteries within WMH, and these differences might be related to the cognitive impairment. In addition to the amount, the length, diameter and stiffness of these supplying arteries might also be important metrics determining the actual severity of WMH, which need further exploration. The injury of penetrating arteries caused by arteriolosclerosis, inflammation, abnormal immunity and other etiological conditions could lead to the cerebrovascular dysfunction, such as the decrease of cerebral vasoreactivity and pulsatility, the disorder of vasomotion and even the luminal stenosis (32). The dysfunction of cerebral vessels could disturb the maintenance of the normal cerebral blood flow (CBF) regulation and BBB, which would result in the accumulation of amyloid-β proteins and impaired drainage of extravascular proteins along blood vessels (33). Furthermore, the dysfunction of CBF might lead to ischemia, which could cause the loss of myelin and axons, as well as the development of gliosis (10), disrupting the connections between cortex and the subcortex nuclei (34). These factors together contribute to the heterogeneity of WMH in clinical symptoms. Although some manual steps during the reconstruction of the penetrating arteries might influence the accuracy of the imaging, and the blooming artifact could affect the true size of these small vessels (12, 13, 31), our study provided great confidence in the utility of exploring the heterogeneity of WMH and the mechanisms of CSVD in the future.
Our study has several limitations. Firstly, the current study was a cross-sectional study and the sample size was relatively small. Larger longitudinal studies are needed to explore the heterogeneity of WMH and the progressive changes in cognitive function to better identify patients at risk of developing cognitive impairment over time. Secondly, although previous studies have shown that a MoCA score of <26 was consistent with cognitive impairment, our study could not determine the specificity and sensibility of the MoCA scores due to lack of formal neuropsychological testing. Depression, apathy or fatigue associated with CSVD might contribute to lower MoCA scores and therefore might reduce the specificity (21). Lastly, the domains of cognitive impairment might be associated with the location of WMH, it could be another factor related to the heterogeneity between WMH and cognitive impairment. Further exploration of the various locations of WMH across different cognitive domains is needed to obtain more robust evidence that the heterogeneity of WMH in cognitive impairment is clinically or physiologically relevant and shows specificity. Despite limitations, this study was innovative since the technique of imaging penetrating arteries in the basal ganglia was utilized in order to explore the mechanisms causing the heterogeneity of WMH.
Heterogeneity of WMH in cognitively impaired CSVD patients is present. Even though the WMH appeared similar on conventional imaging, the higher degree of injury to the supplying penetrating arteries in WMH or the presence of other CSVD imaging features might be indicative of cognitive impairment. The results of our study provided data to support early identification and prevention of cognitive impairment in patients with WMH. Larger population-based studies are required to determine the various types of heterogeneity of WMH and progression into different domains of cognitive function.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Boards of Beijing Tiantan Hospital. The patients/participants provided their written informed consent to participate in this study.
TW was responsible for the study concept and design, clinical data collection, MRI data processing, and manuscript drafting. AJ analyzed the data and generated the figures. YF, ZZ, and SL conducted study design, MRI data acquisition, and interpretation of data. DW revised the manuscript. YW was responsible for the study concept and design, study supervision, analysis and interpretation of data, and clinical revision of manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Our study was funded by grants from the National Natural Science Foundation of China (No. 81825007), Beijing Outstanding Young Scientist Program (No. BJJWZYJH01201910025030), Youth Beijing Scholar Program (No.010), Beijing Talent Project - Class A: Innovation and Development (No. 2018A12), “National Ten-Thousand Talent Plan”- Leadership of Scientific and Technological Innovation, National Key R&D Program of China (No. 2017YFC1307900, 2017YFC1307905) and the National Key Research and Development Program (2018YFC1312400, 2018YFC1312402) from the Ministry of Science and Technology of China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wardlaw J, Smith C, Dichgans M. Mechanisms of Sporadic Cerebral Small Vessel Disease: Insights From Neuroimaging. Lancet Neurol (2013) 12:483–97. doi: 10.1016/s1474-4422(13)70060-7
2. Pantoni L. Cerebral Small Vessel Disease: From Pathogenesis and Clinical Characteristics to Therapeutic Challenges. Lancet Neurol (2010) 9:689–701. doi: 10.1016/s1474-4422(10)70104-6
3. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging Standards for Research Into Small Vessel Disease and Its Contribution to Ageing and Neurodegeneration. Lancet Neurol (2013) 12:822–38. doi: 10.1016/s1474-4422(13)70124-8
4. Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB. Relation Between Age-Related Decline in Intelligence and Cerebral White-Matter Hyperintensities in Healthy Octogenarians: A Longitudinal Study. Lancet (London England) (2000) 356:628–34. doi: 10.1016/s0140-6736(00)02604-0
5. Debette S, Markus HS. The Clinical Importance of White Matter Hyperintensities on Brain Magnetic Resonance Imaging: Systematic Review and Meta-Analysis. Bmj (2010) 341:c3666. doi: 10.1136/bmj.c3666
6. Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-Analysis. JAMA Neurol (2019) 76:81–94. doi: 10.1001/jamaneurol.2018.3122
7. Wardlaw J, Chappell F, Valdés Hernández M, Makin S, Staals J, Shuler K, et al. White Matter Hyperintensity Reduction and Outcomes After Minor Stroke. Neurology (2017) 89:1003–10. doi: 10.1212/wnl.0000000000004328
8. Vlastra W, van den Boogert T, Krommenhoek T, Bronzwaer A, Mutsaerts H, Achterberg H, et al. Aortic Valve Calcification Volumes and Chronic Brain Infarctions in Patients Undergoing Transcatheter Aortic Valve Implantation. Int J Cardiovasc Imaging (2019) 35:2123–33. doi: 10.1007/s10554-019-01663-0
9. Cho A, Kim H, Kim W, Yang D. White Matter Hyperintensity in Ischemic Stroke Patients: It may Regress Over Time. J Stroke (2015) 17:60–6. doi: 10.5853/jos.2015.17.1.60
10. Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of Small Vessel Disease: A Systematic Review of MRI and Histopathology Correlations. J Neurol Neurosurg Psychiatry (2011) 82:126–35. doi: 10.1136/jnnp.2009.204685
11. Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw FE. Cerebral Small Vessel Disease: From a Focal to a Global Perspective. Nat Rev Neurol (2018) 14:387–98. doi: 10.1038/s41582-018-0014-y
12. Buch S, Wang Y, Park MG, Jella PK, Hu J, Chen Y, et al. Subvoxel Vascular Imaging of the Midbrain Using USPIO-Enhanced MRI. Neuroimage (2020) 220:117106. doi: 10.1016/j.neuroimage.2020.117106
13. Thrippleton MJ, Blair GW, Valdes-Hernandez MC, Glatz A, Semple SIK, Doubal F, et al. MRI Relaxometry for Quantitative Analysis of USPIO Uptake in Cerebral Small Vessel Disease. Int J Mol Sci (2019) 20:776. doi: 10.3390/ijms20030776
14. Fisher CM. Lacunar Strokes and Infarcts: A Review. Neurology (1982) 32:871–6. doi: 10.1212/wnl.32.8.871
15. Yamauchi H, Fukuda H, Oyanagi C. Significance of White Matter High Intensity Lesions as a Predictor of Stroke From Arteriolosclerosis. J Neurol Neurosurg Psychiatry (2002) 72:576–82. doi: 10.1136/jnnp.72.5.576
16. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR Signal Abnormalities at 1.5 T in Alzheimer’s Dementia and Normal Aging. AJR Am J Roentgenol (1987) 149:351–6. doi: 10.2214/ajr.149.2.351
17. Jiang J, Liu T, Zhu W, Koncz R, Liu H, Lee T, et al. UBO Detector – A Cluster-Based, Fully Automated Pipeline for Extracting White Matter Hyperintensities. NeuroImage (2018) 174:539–49. doi: 10.1016/j.neuroimage.2018.03.050
18. Staals J, Makin S, Doubal F, Dennis M, Wardlaw J. Stroke Subtype, Vascular Risk Factors, and Total MRI Brain Small-Vessel Disease Burden. Neurology (2014) 83:1228–34. doi: 10.1212/wnl.0000000000000837
19. Salamon G. Atlas of the Arteries of the Human Brain: Atlas of the Arteries of the Human Brain. Paris, France: Sandoz (1971).
20. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J Am Geriatr Soc (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
21. Pendlebury ST, Cuthbertson FC, Welch SJ, Mehta Z, Rothwell PM. Underestimation of Cognitive Impairment by Mini-Mental State Examination Versus the Montreal Cognitive Assessment in Patients With Transient Ischemic Attack and Stroke: A Population-Based Study. Stroke (2010) 41:1290–3. doi: 10.1161/strokeaha.110.579888
22. Zamboni G, Griffanti L, Jenkinson M, Mazzucco S, Li L, Küker W, et al. White Matter Imaging Correlates of Early Cognitive Impairment Detected by the Montreal Cognitive Assessment After Transient Ischemic Attack and Minor Stroke. Stroke (2017) 48:1539–47. doi: 10.1161/strokeaha.116.016044
23. Hamilton O, Backhouse E, Janssen E, Jochems A, Maher C, Ritakari T, et al. Cognitive Impairment in Sporadic Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis. Alzheimer’s Dementia (2021) 17:665–85. doi: 10.1002/alz.12221
24. Longstreth W, Arnold A, Beauchamp N, Manolio T, Lefkowitz D, Jungreis C, et al. Incidence, Manifestations, and Predictors of Worsening White Matter on Serial Cranial Magnetic Resonance Imaging in the Elderly: The Cardiovascular Health Study. Stroke (2005) 36:56–61. doi: 10.1161/01.str.0000149625.99732.69
25. van Dijk E, Prins N, Vrooman H, Hofman A, Koudstaal P, Breteler M. Progression of Cerebral Small Vessel Disease in Relation to Risk Factors and Cognitive Consequences: Rotterdam Scan Study. Stroke (2008) 39:2712–9. doi: 10.1161/strokeaha.107.513176
26. Hu HY, Ou YN, Shen XN, Qu Y, Ma YH, Wang ZT, et al. White Matter Hyperintensities and Risks of Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis of 36 Prospective Studies. Neurosci Biobehav Rev (2021) 120:16–27. doi: 10.1016/j.neubiorev.2020.11.007
27. Passiak B, Liu D, Kresge H, Cambronero F, Pechman K, Osborn K, et al. Perivascular Spaces Contribute to Cognition Beyond Other Small Vessel Disease Markers. Neurology (2019) 92:e1309–21. doi: 10.1212/wnl.0000000000007124
28. Jung KH, Stephens KA, Yochim KM, Riphagen JM, Kim CM, Buckner RL, et al. Heterogeneity of Cerebral White Matter Lesions and Clinical Correlates in Older Adults. Stroke (2021) 52:620–30. doi: 10.1161/strokeaha.120.031641
29. Maillard P, Fletcher E, Lockhart SN, Roach AE, Reed B, Mungas D, et al. White Matter Hyperintensities and Their Penumbra Lie Along a Continuum of Injury in the Aging Brain. Stroke (2014) 45:1721–6. doi: 10.1161/strokeaha.113.004084
30. Rajani RM, Ratelade J, Domenga-Denier V, Hase Y, Kalimo H, Kalaria RN, et al. Blood Brain Barrier Leakage Is Not a Consistent Feature of White Matter Lesions in CADASIL. Acta Neuropathol Commun (2019) 7:187. doi: 10.1186/s40478-019-0844-x
31. Liu S, Brisset JC, Hu J, Haacke EM, Ge Y. Susceptibility Weighted Imaging and Quantitative Susceptibility Mapping of the Cerebral Vasculature Using Ferumoxytol. J Magnetic Resonance Imaging: JMRI (2018) 47:621–33. doi: 10.1002/jmri.25809
32. Wardlaw JM, Benveniste H, Williams A. Cerebral Vascular Dysfunctions Detected in Human Small Vessel Disease and Implications for Preclinical Studies. Annu Rev Physiol (2021). doi: 10.1146/annurev-physiol-060821-014521
33. van Westen D, Lindqvist D, Blennow K, Minthon L, Nägga K, Stomrud E, et al. Cerebral White Matter Lesions - Associations With Aβ Isoforms and Amyloid PET. Sci Rep (2016) 6:20709. doi: 10.1038/srep20709
Keywords: white matter hyperintensities, cognitive impairment, cerebral small vessel disease, heterogeneity, ultrasmall superparamagnetic iron oxide (USPIO)
Citation: Wang T, Jin A, Fu Y, Zhang Z, Li S, Wang D and Wang Y (2021) Heterogeneity of White Matter Hyperintensities in Cognitively Impaired Patients With Cerebral Small Vessel Disease. Front. Immunol. 12:803504. doi: 10.3389/fimmu.2021.803504
Received: 28 October 2021; Accepted: 22 November 2021;
Published: 09 December 2021.
Edited by:
Wei Qiu, Third Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Yi Yang, First Affiliated Hospital of Jilin University, ChinaCopyright © 2021 Wang, Jin, Fu, Zhang, Li, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yilong Wang, eWlsb25nNTI4QGFsaXl1bi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.