- 1Department of Stem Cell and Immune Regulation, Yokohama City University Graduate School of Medicine, Yokohama, Japan
- 2Department of Infectious Diseases, Keio University School of Medicine, Tokyo, Japan
- 3Chemothrapy Center, Yokohama City University Hospital, Yokohama, Japan
Immune checkpoint inhibitor (ICI)-related myositis is a rare, potentially fatal condition that warrants further studies. Its incidence, clinical features, and prognosis remain poorly understood. To address these gaps, we conducted a systematic review and meta-analysis to evaluate the risk of myositis associated with ICI for solid tumors by analyzing phase III randomized controlled trials of anti-programmed death-1/ligand-1 (PD-1/PD-L1) and anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4). To complement this analysis with clinical data, we evaluated published ICI case reports along with cases from our institutional registry. This registry comprised 422 patients treated with ICIs alone or in combination from September 2014 to June 2021. The analysis revealed an incidence of ICI-related myositis in 6,838 patients in 18 randomized controlled trials of 0.38% (odds ratio 1.96; 95% confidence interval 1.02–3.75) for patients receiving ICIs compared with controls. Detailed analysis of 88 cases from the literature search and our registry showed that myositis induced by PD-1 inhibitors was more frequent than that induced by anti-CTLA-4 agents, revealing a clinically diverse trend including myasthenia gravis and myocarditis. Importantly, having ptosis at the time of onset was significantly associated with the development of concomitant myocarditis (odds ratio 3.81; 95% CI 1.48–9.83), which is associated with poor prognosis. Regarding treatment, most patients received glucocorticoids, and some received immunosuppressants. Our study revealed the incidence of ICI-mediated myositis and the clinical features of myocarditis, highlighting the need for recognition and early intervention.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment by exerting anti-tumor effects on various types and stages of cancer that cannot be achieved with existing drugs, and numerous clinical trials are underway to expand indications (1). However, treatment response to ICIs is variable in terms of both efficacy and adverse effects (2, 3). Autoimmune reactions to various organs—immune-related adverse events (irAEs)—are observed in patients treated with ICIs (4, 5). The phenotypes of irAEs vary and it is currently impossible to predict how often they will occur, and in which organs. ICI-related myositis is rare but has been reported to be potentially fatal (6).
A review by Kadota et al. (7) reported that myositis occurred in patients receiving anti-programmed death-1 (PD-1) alone, anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) alone, and with combination therapy. Mean time from ICI initiation to the onset of ICI-related myositis was 4 weeks. Causes of death were myocarditis, respiratory paralysis, and cancer progression. In patients without myocarditis or respiratory muscle paralysis, creatine kinase (CK) levels normalized after ICI discontinuation and administration of immunosuppressive drugs, and the prognosis of myositis was good. The review suggested that the clinical features of ICI-related myositis can be divided into two subsets: new onset of myositis as an irAE, and onset of idiopathic inflammatory myositis (IIM). However, because of its rarity, the incidence, clinical features, and prognosis of ICI-related myositis are still poorly understood.
A systematic review on myocarditis has been published (8), but, to our knowledge, no meta-analysis on this topic has been conducted. Since the 2019 publication of the Kadota et al. review (7) many additional cases have been reported, thus there is the opportunity to clarify some of the questions about this condition. With this aim, we conducted a systematic review on ICI-related myositis. To clarify the detailed clinical features of ICI-related myositis, we searched the literature for case reports together with data from the Yokohama City University ICI registry. Here, we report the incidence, clinical features, and potential predictors of fatal myocarditis in these patients.
Materials and Methods
Systematic Review and Meta-Analysis of Randomized Controlled Trials
The systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (9, 10) and was registered in the University Hospital Medical Information Network Center Clinical Trial Registry (Japan) as UMIN000044960 (11). Institutional Review Board approval and patient informed consent were waived for the systematic review and meta-analysis due to nature of the study.
In the electronic search, we systematically searched PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, and Web of Science Core Collection (up to August 16 2021) for randomized controlled trials (RCTs) reporting the risk of myositis associated with ICIs for the treatment of patients with solid tumors. Search formulae are presented in Supplementary Text 1. Two investigators (AM, KT-M) independently screened candidate articles by checking the title and abstract after uploading the citation list into Endnote X9 software (Thomson Reuters, Philadelphia, PA, USA). Inclusion criteria were as follows: (1) phase III RCT study design; (2) the experimental group was treated with at least one type of ICI with or without other systemic chemotherapy and the control group with non-ICIs; (3) three-arm studies where an ICI was included in at least one arm; (4) patients clinically diagnosed with any solid tumor; and (5) the study included the incidence of myositis. Exclusion criteria were as follows: (1) systematic review or meta-analyses; (2) retrospective analyses; (3) single prospective cohort studies with no control group; (4) ICI two-drug combination therapy; (5) republished research literature; (6) studies with no or insufficient safety results at the time of the literature search; and (7) studies published in languages other than English. Only full-text papers were used for analysis. Disagreements in assessing cases or data were resolved via discussion between the two investigators.
Odds ratios (ORs) of any-grade myositis between the ICI treatment arm and non-ICI arm were calculated using a random-effect model meta-analysis. Heterogeneity was assessed using I2 statistics and P-values. Heterogeneity was indicated by I2 wherein 0% indicated no heterogeneity and 100% indicated the strongest heterogeneity. Statistical analyses were performed using Review Manager 5.3 (Cochrane Community, London, UK). The Cochrane Risk of Bias Tool was used to evaluate the risk of bias for each RCT (12). The quality of the RCTs was independently assessed by two reviewers (AM, KT-M).
Patients and Design of the Yokohama City University Institutional Database
Consecutive cancer patients (excluding those in clinical trials) who received ICIs (nivolumab, pembrolizumab, ipilimumab, atezolizumab, or durvalumab) for approved cancer types at the collaborative research department of Yokohama City University Hospital between December 2014 and May 2021 were included. The study was approved by the ethics committee of Yokohama City University (A200500004). In this study, we included patients treated with ICI monotherapy or combination therapy. Demographic, clinical, and treatment data were obtained from clinical charts. The study followed the Ethical Guidelines for Epidemiology Research, published by the Japanese Ministry of Health, Labour and Welfare, and had an opt-out strategy.
Literature Review of Case Reports
We searched the literature for cases of ICI-related myositis using PubMed on September 7, 2021. ‘ICI-related myositis’ was defined according to the previously reported literature review (7). Cases clinically diagnosed as myositis were included in the analysis, but cases with focal myositis, such as orbital myositis, were excluded. We searched PubMed using the following terms: (pembrolizumab OR nivolumab OR ipilimumab) AND (myositis OR myopathy OR myopathies OR dermatomyositis OR polymyositis). Additional related articles were identified through a manual search of the bibliographies of the included studies to ensure that all relevant studies and recent reviews were included.
Data from case reports were divided into two groups: myositis alone, and concomitant myocarditis. Clinical characteristics, the type of ICI, autoantibody status, management, and outcome were evaluated and compared between two groups.
Clinical data were analyzed using both univariate and multivariate analysis. Comparisons of frequencies were made by Fisher’s exact test, or by chi-square test if there were three or more categories for each variable. Continuous variables were compared using Mann-Whitney U test. Logistic regression models were used to identify multivariate predictors of myocarditis, after adjusting for the effects of age and gender. Values of p<0.05 were considered significant. Statistical analyses were performed using software IBM/SPSS Statistics version 22 (Armonk, NY).
Results
Incidence and Risk of ICI-Related Myositis in the Systematic Review and Meta-Analysis
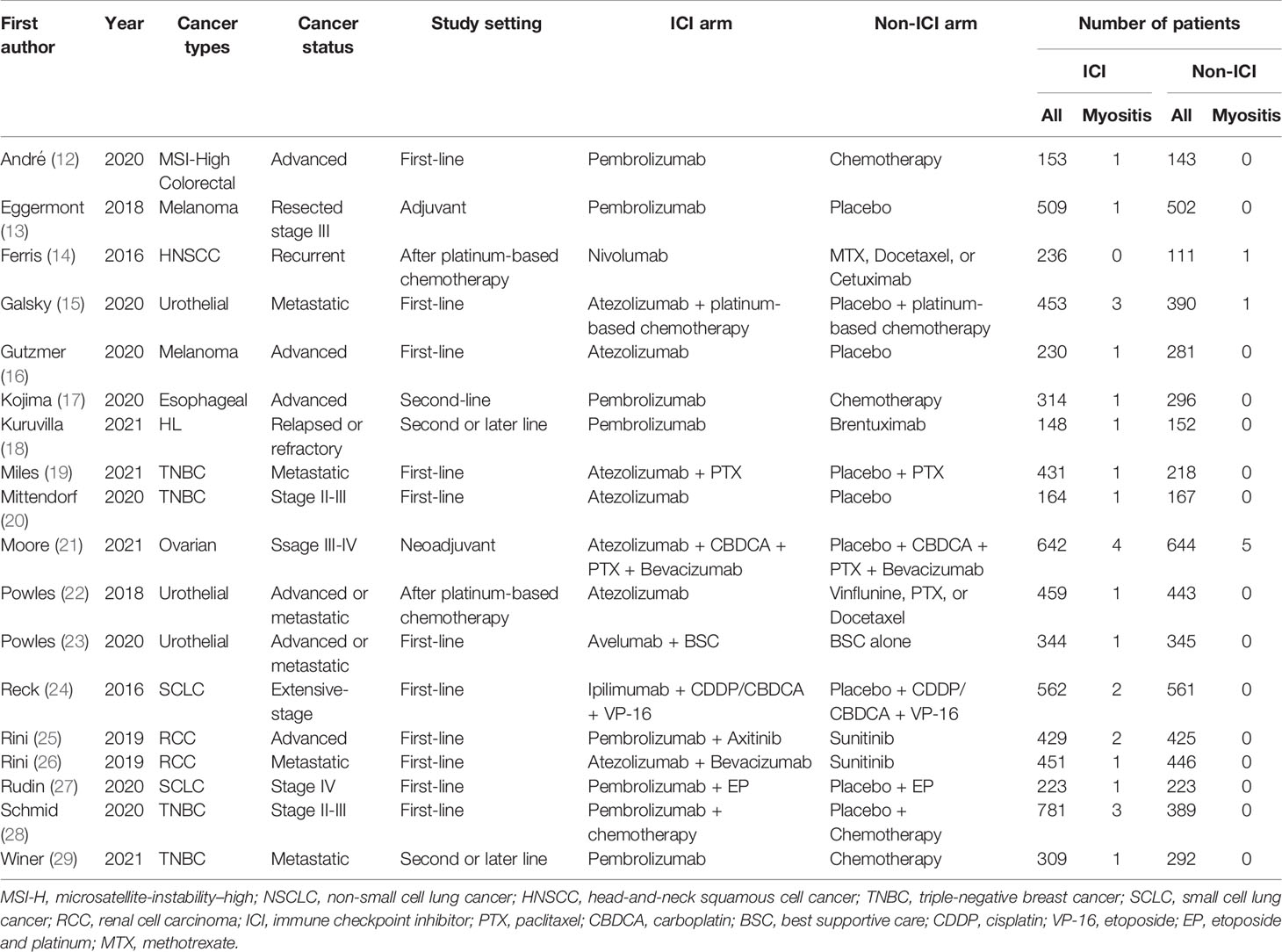
Of 5,471 candidate articles, 120 were potentially eligible after abstract and title screening. From these 120, 19 studies were excluded, and 101 articles were reviewed in detail. Studies of 93 RCTs were insufficient to assess the incidence of myositis. Finally, 18 RCTs (13–30) were included in this meta-analysis (Figure 1). Characteristics of the included studies are summarized in Table 1. Visual inspection of the funnel plot was assessed (Supplementary Figure 1). The overall risk of bias for most studies evaluated was also low (Supplementary Figure 2). There was no clear risk of publication bias in the included studies.
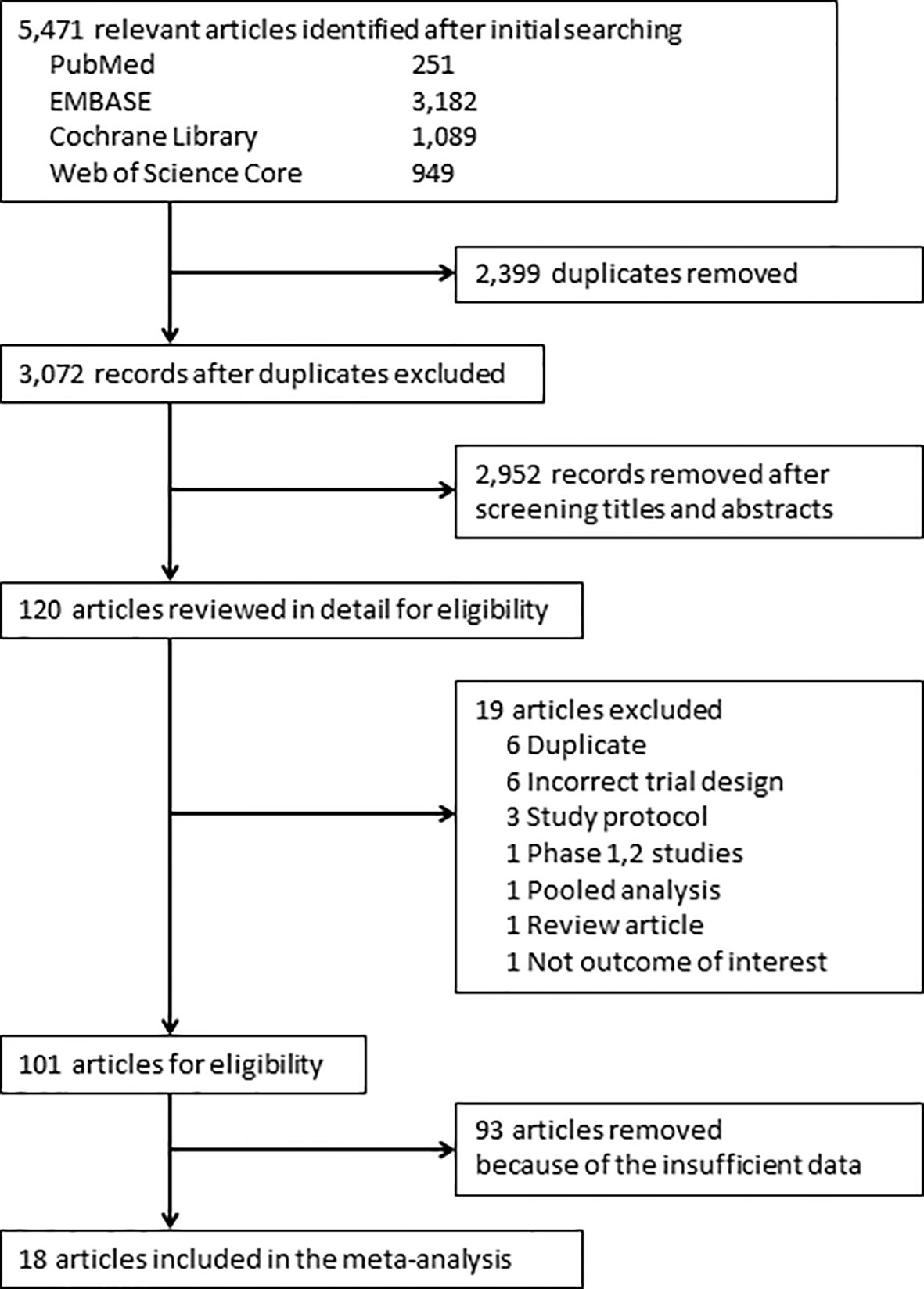
Figure 1 PRISMA flow diagram for the meta-analysis. Study selection process according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
A total of 33 events of any-grade myositis were observed in 12,866 participants (ICI treatment arm, n = 6,838; non-ICI treatment arm, n = 6,028) from 18 RCTs. Twenty-six events were observed in the ICI arm and seven events were observed in the non-ICI arm. Among the 26 ICI-related myositis, 11 events were caused by anti-PD-1, 13 by anti-programmed death ligand 1 (PD-L1), and two by anti-CTLA-4, suggesting that any ICI can elicit this condition. We calculated OR and 95% CI to compare PD-1/PD-L1 and CTLA-4 blockade therapies. There was no significant difference in frequencies of myositis between two groups (OR 1.07; 95% CI: 0.27-9.41). The overall incidence of ICI-related myositis was 0.38% (26 of 6,838), and did not seem to be affected by the cancer type or ICI regimen. We found that myositis was significantly increased with ICI treatment compared with non-ICI treatment (OR, 1.96; 95% confidence interval [CI] 1.02–3.75; p = 0.04; I2 = 0%) (Figure 2). These data suggest that ICI-related myositis may be an important component of irAEs in patients receiving ICI therapy.
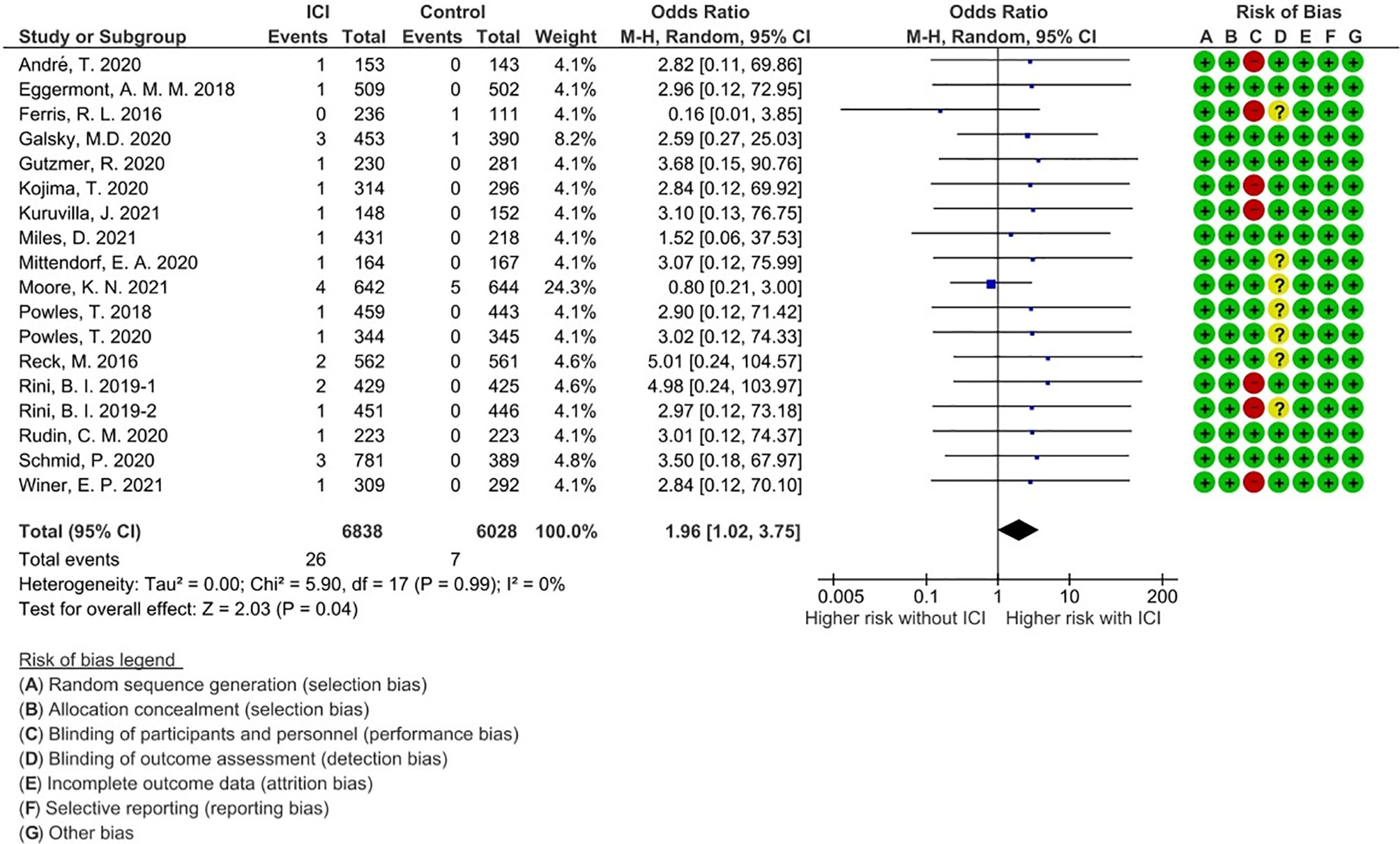
Figure 2 Forest plot with risk of bias summary for incidence of immune checkpoint inhibitor-related myositis.
Summary of Single Center Analysis
Meta-analysis provides epidemiological data such as the incidence of ICI-related myositis, but it is difficult to analyze detailed clinical characteristics. To understand the prognosis of ICI-related myositis, we first examined our institutional database. A total of 422 patients were identified. Among these, 365 cases were treated with a PD-1 or PD-L1 inhibitor alone, nine were treated with a CTLA-4 inhibitor alone, and 47 cases were treated with a PD-1 inhibitor and CTLA-4 inhibitor combination. There were three cases of ICI-related myositis (incidence 0.71%). Baseline characteristics are shown in Supplementary Table 1. All three patients were Asian Japanese and one was female, with mean age 71 ± 6.6 years. Mean treatment duration prior to ICI-related myositis was 13.7 weeks (range 3–34). These patients had muscle weakness with elevated CK with a clinical diagnosis of ICI-related myositis. All were treated with glucocorticoids and other drugs were initiated. However, two patients died (one with pneumonia and one with tumor progression). Patient details are shown in Supplementary Text 2.
Clinical Features and Prognosis of ICI-Related Myositis
To clarify the clinical features of ICI-related myositis in a larger patient population, we evaluated the clinical features of published case reports of ICI-related myositis. Of 75 candidate articles, 63 were identified as eligible (31–93). A further 14 relevant articles (7, 94–106) were added through hand searching. Among the 77 articles, 30 were from Japan, 23 were from USA, five were from France and four were from Canada and Australia (Supplementary Table 1). A total of 85 myositis cases associated with at least one ICI were identified. We combined these with the three cases from our institutional database so 88 cases were analyzed in total (precise clinical information of these three patients is noted in the Supplementary Text 2). The most frequent cancer types were lung cancer and melanoma (28 and 27 cases, respectively), followed by urothelial carcinoma. Treatment regimens were as follows: pembrolizumab (anti-PD-1; 37 cases), nivolumab (anti-PD-1; 29 cases), ipilimumab (anti-CTLA-4; four cases), and nivolumab plus ipilimumab (14 cases). Estimated onset time of myositis after ICI administration was 5.6 ± 6.1 weeks and the appearance of autoantibodies varied.
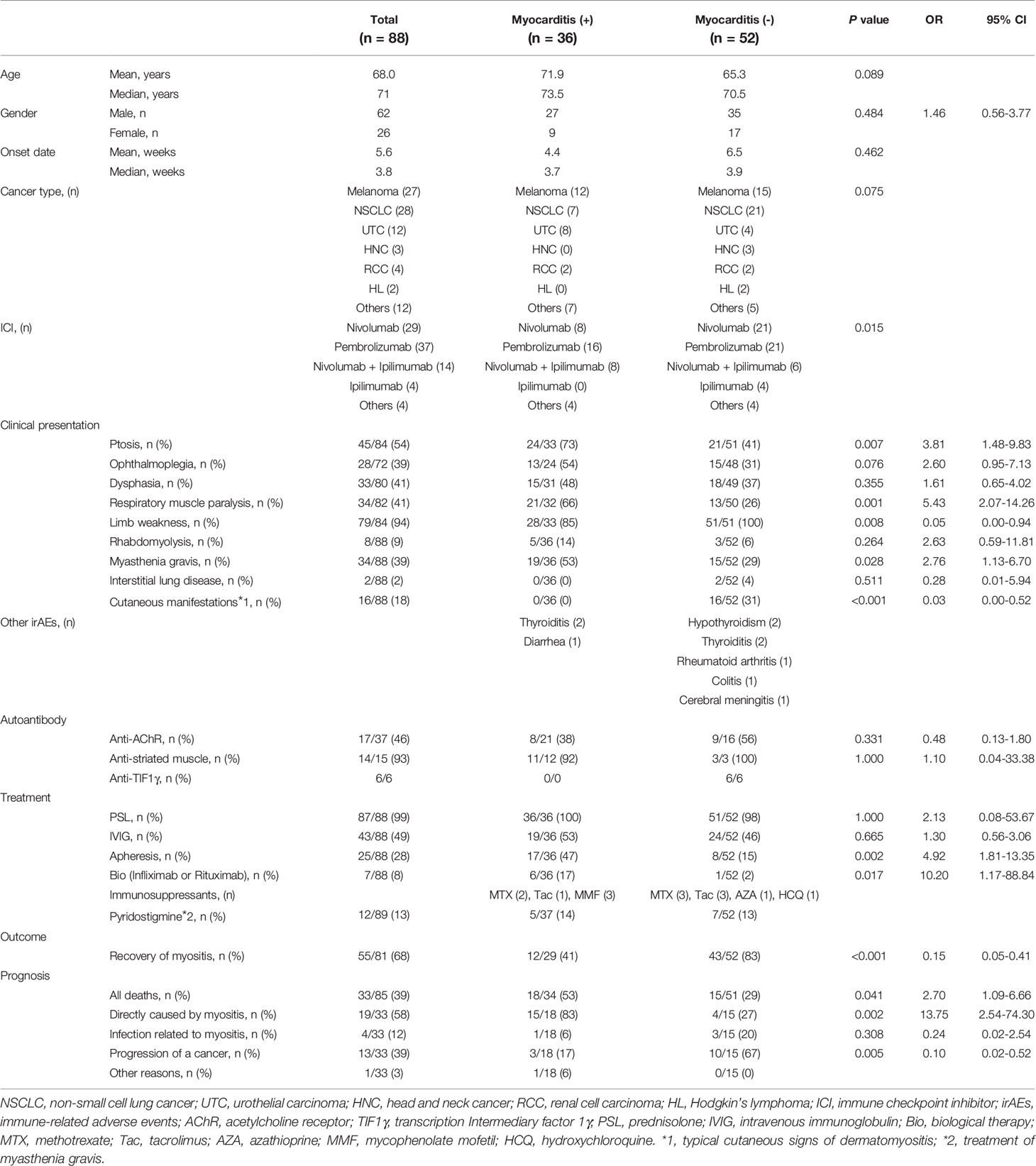
All cases except one were treated with glucocorticoids. Thirty-three patients died, of which 19 deaths were associated with myositis. Because myocarditis is known to be a frequent and fatal complication of ICI-related myositis (107), we compared clinical features of patients with and without myocarditis. Thirty-six of the 88 cases (40.9%) were complicated by myocarditis, confirming the high incidence of myocarditis in ICI-related myositis (Table 2).
There were some significant differences in clinical characteristics in the group that developed myocarditis. First, we analyzed overall ICI use and found that the occurrence of myocarditis was significantly affected by the type of ICI regimen (p=0.015), but was underpowered to detect an association with a specific regimen. Second, in terms of clinical features at the time of myositis onset, ptosis and respiratory muscle paralysis (type II respiratory failure) were significantly more frequent in the myocarditis group (p=0.007; OR 3.81; 95% CI 1.48–9.83 and p=0.001; OR 5.43; 95% CI 2.07–14.26, respectively). The ORs were calculated from multivariate logistic regression analysis, which also revealed that ptosis and respiratory muscle paralysis were independently associated with the development of myocarditis (p=0.031; OR 2.97; 95% CI 1.10–8.02 and p=0.003; OR 4.54; 95% CI: 1.68–12.23, respectively, Supplementary Table 2). Third, in terms of myositis treatment, the myocarditis group received more aggressive treatment such as plasmapheresis and biological agents (p=0.002; OR 4.92; 95% CI 1.81–13.35 and p=0.017; OR 10.20; 95% CI 1.17–88.84, respectively). Finally, in terms of prognosis, myositis symptoms including myocarditis were more refractory to treatment and myositis-related deaths were more common in the group with myocarditis (p=0.002; OR 13.75; 95% CI 2.54–74.30), while the group without myocarditis had a better response to treatment for myositis and more deaths because of cancer progression (p=0.005). These data suggest that myocarditis is a serious complication of ICI-related myositis, the onset of which can be predicted by the development of ptosis.
Discussion
Because ICI-related myositis is extremely rare, reported only in individual RCTs and case reports, its incidence and clinical phenotype are poorly understood. Here, we provide the first evidence that ICI treatment significantly increases the incidence of myositis compared with non-ICI treatment. Furthermore, detailed analysis of case reports showed that myocarditis with the poorest prognosis had ocular symptoms as the initial manifestation. These results are expected to be useful for the optimization of ICI use in clinical practice.
We show here that ICI-related myositis occurred in 0.38% of patients treated with ICIs, and was significantly more frequent in patients treated with cytotoxic anti-cancer agents. Both anti-PD-1/PD-L1 and anti-CTLA-4 antibodies can cause myositis, but anti-CTLA-4 antibodies have been reported less frequently and may cause myositis less frequently than treatment with anti-PD-1/PD-L1 antibodies. The present study was is underpowered to determine differences in myositis-inducing effects of anti-CTLA-4 and PD-1/PD-L1 antibodies. Further accumulation of cases, pathological verification, and analysis of combination therapy with these two antibodies will be of benefit.
As shown in the previous (7) and current studies, myositis resulting from ICI treatment can be complicated by myasthenia gravis and myocarditis, indicating that ICI-related myositis is a distinct phenotype from IIM (108). Therefore, prognosis in myositis may also differ from that in IIM. Although rapid diagnosis of fatal conditions—especially myocarditis—is necessary, there is currently no standardized method for diagnosing ICI-related myositis. Applying existing diagnostic criteria for IIM and myasthenia gravis to ICI-related diseases may be inappropriate and too late for diagnosis. We identified particular key features, namely ptosis and respiratory paralysis, as important indicators of myocarditis. Based on the current data, we suggest that these patients are closely monitored for myocarditis.
ICI myositis may have different clinical features from dermatomyositis and polymyositis as illustrated in Figure 3. Typical cutaneous signs of dermatomyositis were seen in 16 cases (18%) and anti-TIF1γ antibody was positive in 6 of them. These cases met the classification criteria for dermatomyositis. There were no cases in which anti-TIF1γ antibody was measured before the start of ICI treatment, and it is unclear whether anti-TIF1γ was induced by ICI-treatment or not. The complication rate of interstitial lung disease was low (2%). Although most cases of ICI-related myositis are negative for myositis-specific antibodies, some cases of positive anti-striated muscle antibodies have been reported (Supplementary Table 1), and their diagnostic significance needs to be investigated. The fact that myasthenia gravis without thymoma has been reported to be associated with a specific human leukocyte antigen suggests that there may be some immunological predisposition, although no association between irAEs and human leukocyte antigen has been identified as yet (109, 110). The time from the initiation of ICI to the onset of myositis and characteristic symptoms that overlap with myasthenia gravis, such as ptosis as reported in this study, may be important in distinguishing between ICI-related myositis and myositis because of malignancy.
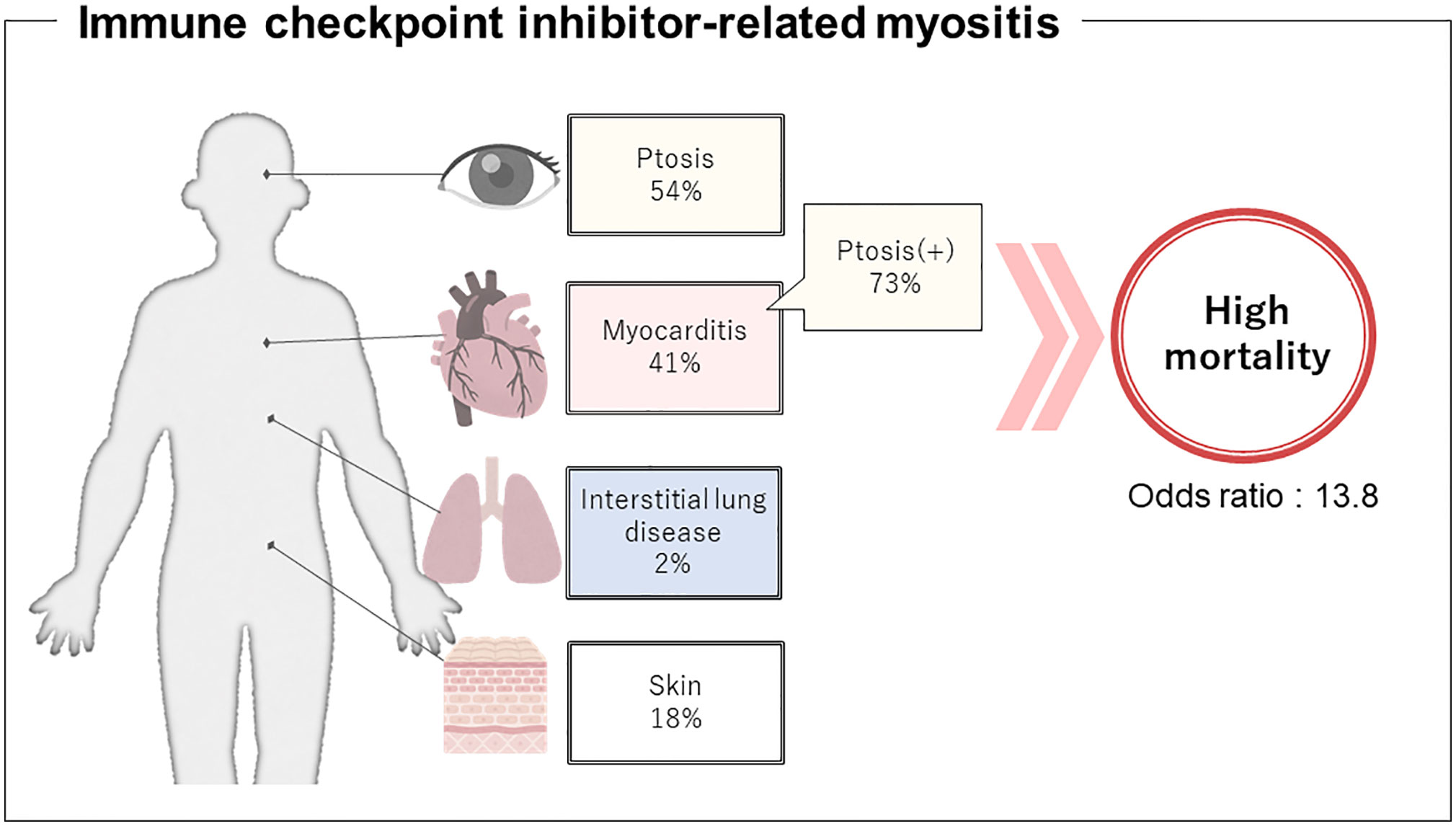
Figure 3 Presumptive clinical features regarding immune checkpoint inhibitor-related myositis. Development of concomitant myocarditis might be predicted by ptosis, therefore, can help prevent the potentially fatal outcome.
A limitation of this study is that ICI-related myositis is a rare complication, and while its incidence can be calculated from RCT data, it was not possible to extract the clinical characteristics of individual cases. Diagnosis of myositis and myasthenia gravis or myocarditis is made by clinicians, and there are no standard criteria for diagnosis. In particular, myasthenia gravis is diagnosed even in cases where anti-acetylcholine receptor antibodies are not detected, and further clarification of phenotypes are needed. Myositis-associated autoantibodies can help define subgroups of patients in terms of the clinical phenotype of IIM, but published case reports did not always provide information on myositis-specific antibodies.
Now that this study has shed light on the clinical characteristics of ICI-related myositis, further accumulation of cases from around the world will enable the development of more detailed algorithms for diagnosis and treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics committee of Yokohama City University. The patients/participants provided their written informed consent to participate in this study.
YCU irAE Working Group
The following Authors, who are listed in numerical order, contributed to the work of YCU irAE Working Group: Naoki Hamada, Department of Stem Cell and Immune Regulation, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan; Yohei Kirino, Department of Stem Cell and Immune Regulation, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan; Motohiko Tokuhisa, Department of Oncology, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan; Keiichi Kondo, Department of Urology, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan; Noboru Nakaigawa, Department of Urology, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan; Nobuaki Kobayashi, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan; Daisuke Sano, Department of Otorhinolaryngology, Head and Neck Surgery, Yokohama City University School of Medicine, Kanazawa-ku, Japan; Maki Hagihara, Department of Stem Cell and Immune Regulation, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan; Nobuhiko Oridate, Department of Otorhinolaryngology, Head and Neck Surgery, Yokohama City University School of Medicine, Kanazawa-ku, Japan; Takeshi Kaneko, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan; Yukie Yamaguchi, Department of Environmental Immuno-Dermatology, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan; Masahiro Yao, Department of Urology, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan; Yasushi Ichikawa, Department of Oncology, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan; Hideaki Nakajima, Department of Stem Cell and Immune Regulation, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Japan.
Author Contributions
NaH, AM, KT-M, and YK designed the research. NaH, AM, KT-M, HoN, and NoH conducted the research, statistical analysis, as well as the interpretation of the data. NaH, AM, KT-M, and YK drafted the manuscript. YS, RY, HoN, and HiN were involved in writing the article or revising it critically for important intellectual content, and all authors approved the final version to be published. NaH, AM and KT-M had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The study was supported from the Yokohama City University Clinical Research Promotion Project. This work was also supported by Yokohama City. This study was supported by Japanese Society for the Promotion of Science Grant-in-Aid for Scientific Research # 21K16289 (NaH).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the YCU irAE working group for advice on preparing this manuscript. We thank Helen Roberton, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.803410/full#supplementary-material
References
1. Singh S, Hassan D, Aldawsari HM, Molugulu N, Shukla R, Kesharwani P. Immune Checkpoint Inhibitors: A Promising Anticancer Therapy. Drug Discov Today (2020) 25(1):223–9. doi: 10.1016/j.drudis.2019.11.003
2. Havel JJ, Chowell D, Chan TA. The Evolving Landscape of Biomarkers for Checkpoint Inhibitor Immunotherapy. Nat Rev Cancer (2019) 19(3):133–50. doi: 10.1038/s41568-019-0116-x
3. Nakamura Y. Biomarkers for Immune Checkpoint Inhibitor-Mediated Tumor Response and Adverse Events. Front Med (Lausanne) (2019) 6:119. doi: 10.3389/fmed.2019.00119
4. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-Related Adverse Events With Immune Checkpoint Blockade: A Comprehensive Review. Eur J Cancer (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
5. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
6. Kostine M, Finckh A, Bingham CO, Visser K, Leipe J, Schulze-Koops H, et al. EULAR Points to Consider for the Diagnosis and Management of Rheumatic Immune-Related Adverse Events Due to Cancer Immunotherapy With Checkpoint Inhibitors. Ann Rheum Dis (2021) 80(1):36–48. doi: 10.1136/annrheumdis-2020-217139
7. Kadota H, Gono T, Shirai Y, Okazaki Y, Takeno M, Kuwana M. Immune Checkpoint Inhibitor-Induced Myositis: A Case Report and Literature Review. Curr Rheumatol Rep (2019) 21:10. doi: 10.1007/s11926-019-0811-3
8. Pathak R, Katel A, Massarelli E, Villaflor VM, Sun V, Salgia R. Immune Checkpoint Inhibitor-Induced Myocarditis With Myositis/Myasthenia Gravis Overlap Syndrome: A Systematic Review of Cases. Oncologist (2021). doi: 10.1002/onco.13931
9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
11. UMIN Center. UMIN Clinical Trials Registry (UMIN-CTR). Available at: https://www.umin.ac.jp/ctr/ctr_regist.htm (Accessed September 20, 2021).
12. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
13. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med (2020) 383:2207–18. doi: 10.1056/NEJMoa2017699
14. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant Pembrolizumab Versus Placebo in Resected Stage III Melanoma. N Engl J Med (2018) 378:1789–801. doi: 10.1056/NEJMoa1802357
15. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
16. Galsky MD, Arija JAA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab With or Without Chemotherapy in Metastatic Urothelial Cancer (IMvigor130): A Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet (2020) 395:1547–57. doi: 10.1016/s0140-6736(20)30230-0
17. Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, Vemurafenib, and Cobimetinib as First-Line Treatment for Unresectable Advanced BRAF V600 Mutation-Positive Melanoma (IMspire150): Primary Analysis of the Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2020) 395:1835–44. doi: 10.1016/S0140-6736(20)30934-X
18. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol (2020) 38:4138–48. doi: 10.1200/JCO.20.01888
19. Kuruvilla J, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, et al. Pembrolizumab Versus Brentuximab Vedotin in Relapsed or Refractory Classical Hodgkin Lymphoma (KEYNOTE-204): An Interim Analysis of a Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol (2021) 22:512–24. doi: 10.1016/S1470-2045(21)00005-X
20. Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, et al. Primary Results From IMpassion131, a Double-Blind, Placebo-Controlled, Randomised Phase III Trial of First-Line Paclitaxel With or Without Atezolizumab for Unresectable Locally Advanced/metastatic Triple-Negative Breast Cancer. Ann Oncol (2021) 32:994–1004. doi: 10.1016/j.annonc.2021.05.801
21. Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant Atezolizumab in Combination With Sequential Nab-Paclitaxel and Anthracycline-Based Chemotherapy Versus Placebo and Chemotherapy in Patients With Early-Stage Triple-Negative Breast Cancer (IMpassion031): A Randomised, Double-Blind, Phase 3 Trial. Lancet (2020) 396:1090–100. doi: 10.1016/S0140-6736(20)31953-X
22. Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, et al. Atezolizumab, Bevacizumab, and Chemotherapy for Newly Diagnosed Stage III or IV Ovarian Cancer: Placebo-Controlled Randomized Phase III Trial (IMagyn050/GOG 3015/ENGOT-Ov39). J Clin Oncol (2021) 39:1842–55. doi: 10.1200/JCO.21.00306
23. Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab Versus Chemotherapy in Patients With Platinum-Treated Locally Advanced or Metastatic Urothelial Carcinoma (IMvigor211): A Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. Lancet (2018) 391:748–57. doi: 10.1016/s0140-6736(17)33297-x
24. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med (2020) 383:1218–30. doi: 10.1056/NEJMoa2002788
25. Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol (2016) 34:3740–8. doi: 10.1200/JCO.2016.67.6601
26. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab Plus Axitinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380:1116–27. doi: 10.1056/NEJMoa1816714
27. Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab Plus Bevacizumab Versus Sunitinib in Patients With Previously Untreated Metastatic Renal Cell Carcinoma (IMmotion151): A Multicentre, Open-Label, Phase 3, Randomised Controlled Trial. Lancet (2019) 393:2404–15. doi: 10.1016/S0140-6736(19)30723-8
28. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol (2020) 38:2369–79. doi: 10.1200/JCO.20.00793
29. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med (2020) 382:810–21. doi: 10.1056/NEJMoa1910549
30. Winer EP, Lipatov O, Im SA, Goncalves A, Munoz-Couselo E, Lee KS, et al. Pembrolizumab Versus Investigator-Choice Chemotherapy for Metastatic Triple-Negative Breast Cancer (KEYNOTE-119): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22:499–511. doi: 10.1016/S1470-2045(20)30754-3
31. Allawh T, Quinn A, Joseph V, Khan HH. Pembrolizumab-Induced Necrotizing Polymyositis: A Case Presentation. J Clin Rheumatol (2020) 26(7):e268. doi: 10.1097/RHU.0000000000001119
32. Asano R, Shinoda K, Tsuda R, Hounoki H, Kawataka M, Makino T, et al. Anti-Tif1γ Antibody-Positive Dermatomyositis in a 15-Year-Old Boy With Epstein-Barr Virus-Related Nasopharyngeal Cancer Receiving Nivolumab. Rheumatol (Oxford) (2021) 60(6):e197–9. doi: 10.1093/rheumatology/keaa832
33. Badovinac S, Korsic M, Zarkovic K, Mursic D, Roglic M, Jakopovic M, et al. Nivolumab-Induced Synchronous Occurrence of Myositis and Hypothyroidism in a Patient With Squamous Cell Lung Cancer. Immunotherapy (2018) 10(6):427–31. doi: 10.2217/imt-2017-0174
34. Behling J, Kaes J, Münzel T, Grabbe S, Loquai C. New-Onset Third-Degree Atrioventricular Block Because of Autoimmune-Induced Myositis Under Treatment With Anti-Programmed Cell Death-1 (Nivolumab) for Metastatic Melanoma. Melanoma Res (2017) 27(2):155–8. doi: 10.1097/CMR.0000000000000314
35. Berger M, Legeay AL, Souci S, Streichenberger N, Thomas L, Dalle S. Pembrolizumab-Induced Dermatomyositis in a Patient With Metastatic Melanoma. Eur J Cancer (2018) 104:227–30. doi: 10.1016/j.ejca.2018.08.021
36. Bilen MA, Subudhi SK, Gao J, Tannir NM, Tu SM, Sharma P. Acute Rhabdomyolysis With Severe Polymyositis Following Ipilimumab-Nivolumab Treatment in a Cancer Patient With Elevated Anti-Striated Muscle Antibody. J Immunother Cancer (2016) 4:36. doi: 10.1186/s40425-016-0139-8
37. Botta C, Agostino RM, Dattola V, Cianci V, Calandruccio ND, Bianco G, et al. Myositis/Myasthenia After Pembrolizumab in a Bladder Cancer Patient With an Autoimmunity-Associated HLA: Immune-Biological Evaluation and Case Report. Int J Mol Sci (2021) 22(12). doi: 10.3390/ijms22126246
38. Bourgeois-Vionnet J, Joubert B, Bernard E, Sia MA, Pante V, Fabien N, et al. Nivolumab-Induced Myositis: A Case Report and a Literature Review. J Neurol Sci (2018) 387:51–3. doi: 10.1016/j.jns.2018.01.030
39. Chen JH, Lee KY, Hu CJ, Chung CC. Coexisting Myasthenia Gravis, Myositis, and Polyneuropathy Induced by Ipilimumab and Nivolumab in a Patient With Non-Small-Cell Lung Cancer: A Case Report and Literature Review. Medicine (2017) 96(50):e9262. doi: 10.1097/MD.0000000000009262
40. Claus J, Van Den Bergh A, Verbeek S, Wauters E, Nackaerts K. Pembrolizumab-Induced Necrotizing Myositis in a Patient With Metastatic Non-Small-Cell Lung Cancer: A Case Report. Lung Cancer Manag (2019) 8(2):Lmt10. doi: 10.2217/lmt-2018-0017
41. Diamantopoulos PT, Tsatsou K, Benopoulou O, Anastasopoulou A, Gogas H. Inflammatory Myopathy and Axonal Neuropathy in a Patient With Melanoma Following Pembrolizumab Treatment. J Immunother (2017) 40(6):221–3. doi: 10.1097/CJI.0000000000000172
42. Fazal M, Prentice DA, Kho LK, Fysh E. Nivolumab-Associated Myositis Myocarditis and Myasthenia and Anti-Striated Muscle Antibodies. Intern Med J (2020) 50(8):1003–6. doi: 10.1111/imj.14946
43. Fazel M, Jedlowski PM. Severe Myositis, Myocarditis, and Myasthenia Gravis With Elevated Anti-Striated Muscle Antibody Following Single Dose of Ipilimumab-Nivolumab Therapy in a Patient With Metastatic Melanoma. Case Rep Immunol (2019) 2019:2539493. doi: 10.1155/2019/2539493
44. Finsterer J. Pembrolizumab-Induced Hypothyroidism Manifesting as Myopathy and Psychosis. Melanoma Res (2021) 31(4):405–6. doi: 10.1097/CMR.0000000000000759
45. Fox E, Dabrow M, Ochsner G. A Case of Nivolumab-Induced Myositis. Oncologist (2016) 21(12):e3. doi: 10.1634/theoncologist.2016-0170
46. Gandiga PC, Wang AR, Gonzalez-Rivera T, Sreih AG. Pembrolizumab-Associated Inflammatory Myopathy. Rheumatol (Oxford) (2018) 57(2):397–8. doi: 10.1093/rheumatology/kex346
47. Haddox CL, Shenoy N, Shah KK, Kao JC, Jain S, Halfdanarson TR, et al. Pembrolizumab Induced Bulbar Myopathy and Respiratory Failure With Necrotizing Myositis of the Diaphragm. Ann Oncol (2017) 28(3):673–5. doi: 10.1093/annonc/mdw655
48. Hamada S, Fuseya Y, Tsukino M. Pembrolizumab-Induced Rhabdomyolysis With Myositis in a Patient With Lung Adenocarcinoma. Arch Bronconeumol (2018) 54(6):346–8. doi: 10.1016/j.arbres.2018.01.026
49. Hayakawa N, Kikuchi E, Suzuki S, Oya M. Myasthenia Gravis With Myositis Induced by Pembrolizumab Therapy in a Patient With Metastatic Urothelial Carcinoma. Int Cancer Conf J (2020) 9(3):123–6. doi: 10.1007/s13691-020-00408-4
50. Hellman JB, Traynis I, Lin LK. Pembrolizumab and Epacadostat Induced Fatal Myocarditis and Myositis Presenting as a Case of Ptosis and Ophthalmoplegia. Orbit (2019) 38(3):244–7. doi: 10.1080/01676830.2018.1490439
51. Hibino M, Maeda K, Horiuchi S, Fukuda M, Kondo T. Pembrolizumab-Induced Myasthenia Gravis With Myositis in a Patient With Lung Cancer. Respirol Case Rep (2018) 6(7):e00355. doi: 10.1002/rcr2.355
52. Hinogami H, Yamashita C, Tanaka A, Shirai H, Nakano Y, Matsuura Y. Case of Dermatomyositis During Treatment With Pembrolizumab for Lung Cancer. J Dermatol (2019) 46(11):e430–2. doi: 10.1111/1346-8138.14993
53. Huh SY, Shin SH, Kim MK, Lee SY, Son KH, Shin HY. Emergence of Myasthenia Gravis With Myositis in a Patient Treated With Pembrolizumab for Thymic Cancer. J Clin Neurol (2018) 14(1):115–7. doi: 10.3988/jcn.2018.14.1.115
54. Hunter G, Voll C, Robinson CA. Autoimmune Inflammatory Myopathy After Treatment With Ipilimumab. Can J Neurol Sci (2009) 36(4):518–20. doi: 10.1017/s0317167100007939
55. Kamo H, Hatano T, Kanai K, Aoki N, Kamiyama D, Yokoyama K, et al. Pembrolizumab-Related Systemic Myositis Involving Ocular and Hindneck Muscles Resembling Myasthenic Gravis: A Case Report. BMC Neurol (2019) 19(1):184. doi: 10.1186/s12883-019-1416-1
56. Kartolo A, Towheed T, Mates M. A Case of Successful Pembrolizumab Rechallenge in a Patient With Non-Small-Cell Lung Cancer and Grade 3 Dermatomyositis. Immunotherapy (2021) 13(6):477–81. doi: 10.2217/imt-2020-0309
57. Kim JS, Nam TS, Kim J, Kho BG, Park CK, Oh IJ, et al. Myasthenia Gravis and Myopathy After Nivolumab Treatment for Non-Small Cell Lung Carcinoma: A Case Report. Thorac Cancer (2019) 10(10):2045–9. doi: 10.1111/1759-7714.13177
58. Kimura T, Fukushima S, Miyashita A, Aoi J, Jinnin M, Kosaka T, et al. Myasthenic Crisis and Polymyositis Induced by One Dose of Nivolumab. Cancer Sci (2016) 107(7):1055–8. doi: 10.1111/cas.12961
59. Kobayashi T, Guo YM, Yamashita T, Nara M, Yoshioka T, Kameoka Y, et al. Relationship Between Clinical Course of Nivolumab-Related Myositis and Immune Status in a Patient With Hodgkin’s Lymphoma After Allogeneic Hematopoietic Stem Cell Transplantation. Int J Hematol (2019) 109(3):356–60. doi: 10.1007/s12185-018-02584-9
60. Koh B, Tuite K, Khattak A, Dyke JM. Lymphocyte Involvement in Nivolumab-Induced Autoimmune Myositis. Pathology (2019) 51(5):555–7. doi: 10.1016/j.pathol.2019.02.006
61. Kosche C, Stout M, Sosman J, Lukas RV, Choi JN. Dermatomyositis in a Patient Undergoing Nivolumab Therapy for Metastatic Melanoma: A Case Report and Review of the Literature. Melanoma Res (2020) 30(3):313–6. doi: 10.1097/CMR.0000000000000642
62. Kudo F, Watanabe Y, Iwai Y, Miwa C, Nagai Y, Ota H, et al. Advanced Lung Adenocarcinoma With Nivolumab-Associated Dermatomyositis. Intern Med (2018) 57(15):2217–21. doi: 10.2169/internalmedicine.9381-17
63. Lie G, Weickhardt A, Kearney L, Lam Q, John T, Liew D, et al. Nivolumab Resulting in Persistently Elevated Troponin Levels Despite Clinical Remission of Myocarditis and Myositis in a Patient With Malignant Pleural Mesothelioma: Case Report. Transl Lung Cancer Res (2020) 9(2):360–5. doi: 10.21037/tlcr.2020.02.05
64. Liu WK, Naban N, Kaul A, Patel N, Fusi A. Life-Threatening Polymyositis With Spontaneous Hematoma Induced by Nivolumab in a Patient With Previously Resected Melanoma. Melanoma Res (2021) 31(1):85–7. doi: 10.1097/CMR.0000000000000706
65. Matsui H, Kawai T, Sato Y, Ishida J, Kadowaki H, Akiyama Y, et al. A Fatal Case of Myocarditis Following Myositis Induced by Pembrolizumab Treatment for Metastatic Upper Urinary Tract Urothelial Carcinoma. Int Heart J (2020) 61(5):1070–4. doi: 10.1536/ihj.20-162
66. Messer A, Drozd B, Glitza IC, Lu H, Patel AB. Dermatomyositis Associated With Nivolumab Therapy for Melanoma: A Case Report and Review of the Literature. Dermatol Online J (2020) 26(8). doi: 10.5070/D3268049887
67. Nakanishi S, Nishida S, Miyazato M, Goya M, Saito S. A Case Report of Nivolumab-Induced Myasthenia Gravis and Myositis in a Metastatic Renal Cell Carcinoma Patient. Urol Case Rep (2020) 29:101105. doi: 10.1016/j.eucr.2019.101105
68. Nosaki Y, Mashimo S, Watanabe M, Yokoi T, Kobayashi Y, Iwai K. Paraspinal Muscle Involvement in Pembrolizumab-Associated Myositis. Oxf Med Case Rep (2020) 2020(2):omaa003. doi: 10.1093/omcr/omaa003
69. Okubo N, Kijima T, Nukui A, Kamai T. Immune-Related Myositis Resulting From Combination Therapy of Ipilimumab and Nivolumab in Patient With Metastatic Renal Cell Carcinoma. BMJ Case Rep (2020) 13(9). doi: 10.1136/bcr-2020-235199
70. Onda A, Miyagawa S, Takahashi N, Gochi M, Takagi M, Nishino I, et al. Pembrolizumab-Induced Ocular Myasthenia Gravis With Anti-Titin Antibody and Necrotizing Myopathy. Intern Med (2019) 58(11):1635–8. doi: 10.2169/internalmedicine.1956-18
71. Osaki M, Tachikawa R, Ohira J, Hara S, Tomii K. Anti-Transcriptional Intermediary Factor 1-γ Antibody-Positive Dermatomyositis Induced by Nivolumab for Lung Adenocarcinoma: A Case Report. Invest New Drugs (2021) 39(1):251–5. doi: 10.1007/s10637-020-00974-7
72. Pagkopoulou E, Simopoulou T, Maragkouli E, Perifanou-Sotiri S, Kotsakis A, Bogdanos DP. Arthritis and Myositis in a Patient Treated With Programmed Cell Death-1 (PD-1) Inhibitor Pembrolizumab for Lung Cancer. Mediterr J Rheumatol (2020) 31(3):355–7. doi: 10.31138/mjr.31.3.355
73. Peverelli L, De Rosa A, Domina E, Ciscato P, Sita G, Velardo D, et al. Severe Inflammatory Myopathy in a Pulmonary Carcinoma Patient Treated With Pembrolizumab: An Alert for Myologists. J Neuromuscul Dis (2020) 7(4):511–4. doi: 10.3233/JND-200504
74. Rus T, Kramberger MG, Jakob GB, Leonardis L, Meznaric M. Life-Threatening Myositis After One Dose of Nivolumab in a Patient With Nonmetastatic Completely Resected Cutaneous Melanoma. Acta Neurol Belg (2021). doi: 10.1007/s13760-021-01760-9
75. Saini L, Chua N. Severe Inflammatory Myositis in a Patient Receiving Concurrent Nivolumab and Azacitidine. Leuk Lymphoma (2017) 58(8):2011–3. doi: 10.1080/10428194.2016.1265115
76. Sanchez-Sancho P, Selva-O’Callaghan A, Trallero-Araguás E, Ros J, Montoro B. Myositis and Myasteniform Syndrome Related to Pembrolizumab. BMJ Case Rep (2021) 14(7). doi: 10.1136/bcr-2021-241766
77. Ali SS, Goddard AL, Luke JJ, Donahue H, Todd DJ, Werchniak A, et al. Drug-Associated Dermatomyositis Following Ipilimumab Therapy: A Novel Immune-Mediated Adverse Event Associated With Cytotoxic T-Lymphocyte Antigen 4 Blockade. JAMA Dermatol (2015) 151(2):195–9. doi: 10.1001/jamadermatol.2014.2233
78. Shibata C, Kato J, Toda N, Imai M, Fukumura Y, Arai J, et al. Paraneoplastic Dermatomyositis Appearing After Nivolumab Therapy for Gastric Cancer: A Case Report. J Med Case Rep (2019) 13(1):168. doi: 10.1186/s13256-019-2105-9
79. Shikano K, Kaneko K, Kaburaki K, Isobe K, Kawabe K, Homma S, et al. Nivolumab-Induced Anti-Aminoacyl-tRNA Synthetase Antibody-Positive Polymyositis Complicated by Interstitial Pneumonia in a Patient With Lung Adenocarcinoma. Scand J Rheumatol (2020) 49(1):82–3. doi: 10.1080/03009742.2019.1596309
80. Sutaria R, Patel P, Danve A. Autoimmune Myositis and Myasthenia Gravis Resulting From a Combination Therapy With Nivolumab and Ipilimumab for Metastatic Melanoma. Eur J Rheumatol (2019) 6(3):153–4. doi: 10.5152/eurjrheum.2019.18159
81. Swali R. Pembrolizumab-Induced Myositis in the Setting of Metastatic Melanoma: An Increasingly Common Phenomenon. J Clin Aesthet Dermatol (2020) 13(6):44–5.
82. Takahashi S, Mukohara S, Hatachi S, Yamashita M, Kumagai S. A Case of Myositis With Dropped Head Syndrome and Anti-Titin Antibody Positivity Induced by Pembrolizumab. Scand J Rheumatol (2020) 49(6):509–11. doi: 10.1080/03009742.2020.1760346
83. Takatsuki K, Yanagihara T, Egashira A, Ogo N, Yoshizawa S, Sunami S, et al. A Rare Case of Pembrolizumab-Induced Dermatomyositis in a Patient With Cancer of Unknown Primary Origin. Am J Case Rep (2021) 22:e930286. doi: 10.12659/AJCR.930286
84. Tan RYC, Toh CK, Takano A. Continued Response to One Dose of Nivolumab Complicated by Myasthenic Crisis and Myositis. J Thorac Oncol (2017) 12(7):e90–1. doi: 10.1016/j.jtho.2017.02.024
85. Tauber M, Cohen R, Laly P, Josselin L, André T, Mekinian A. Severe Necrotizing Myositis Associated With Long Term Anti-Neoplastic Efficacy Following Nivolumab Plus Ipilimumab Combination Therapy. Clin Rheumatol (2019) 38(2):601–2. doi: 10.1007/s10067-018-4373-y
86. Todo M, Kaneko G, Shirotake S, Shimada Y, Nakano S, Okabe T, et al. Pembrolizumab-Induced Myasthenia Gravis With Myositis and Presumable Myocarditis in a Patient With Bladder Cancer. IJU Case Rep (2019) 3(1):17–20. doi: 10.1002/iju5.12128
87. Uchio N, Unuma A, Kakumoto T, Osaki M, Zenke Y, Sakuta K, et al. Pembrolizumab on Pre-Existing Inclusion Body Myositis: A Case Report. BMC Rheumatol (2020) 4:48. doi: 10.1186/s41927-020-00144-5
88. Valenti-Azcarate R, Vazquez IE, Illan CT, Gastearena MAI, Pérez-Larraya JG. Nivolumab and Ipilimumab-Induced Myositis and Myocarditis Mimicking a Myasthenia Gravis Presentation. Neuromuscul Disord (2020) 30(1):67–9. doi: 10.1016/j.nmd.2019.10.006
89. Vallet H, Gaillet A, Weiss N, Vanhaecke C, Saheb S, Touitou V, et al. Pembrolizumab-Induced Necrotic Myositis in a Patient With Metastatic Melanoma. Ann Oncol (2016) 27(7):1352–3. doi: 10.1093/annonc/mdw126
90. Veccia A, Kinspergher S, Grego E, Peterlana D, Berti A, Tranquillini E, et al. Myositis and Myasthenia During Nivolumab Administration for Advanced Lung Aancer: A Case Report and Review of the Literature. Anticancer Drugs (2020) 31(5):540–4. doi: 10.1097/CAD.0000000000000903
91. Wiggins CJ, Chon SY. Dermatomyositis, Pembrolizumab, and Squamous Cell Carcinoma of the Lung. Proc (Bayl Univ Med Cent) (2020) 34(1):120–1. doi: 10.1080/08998280.2020.1811189
92. Yamaguchi Y, Abe R, Haga N, Shimizu H. A Case of Drug-Associated Dermatomyositis Following Ipilimumab Therapy. Eur J Dermatol (2016) 26(3):320–1. doi: 10.1684/ejd.2016.2770
93. Yoshioka M, Kambe N, Yamamoto Y, Suehiro K, Matsue H. Case of Respiratory Discomfort Due to Myositis After Administration of Nivolumab. J Dermatol (2015) 42(10):1008–9. doi: 10.1111/1346-8138.12991
94. Johnson ED, Kerrigan K, Butler K, Patel SB. Nivolumab-Induced Hypothyoidism With Consequent Hypothyroid Related Myopathy. J Oncol Pharm Pract (2020) 26(1):224–7. doi: 10.1177/1078155219835912
95. Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical Neurological Complications of Ipilimumab Therapy in Patients With Metastatic Melanoma. Neuro Oncol (2014) 16(4):589–93. doi: 10.1093/neuonc/nou001
96. Fuentes-Antrás J, Peinado P, Guevara-Hoyer K, Arco CDD, Sánchez-Ramón S, Aguado C. Fatal Autoimmune Storm After a Single Cycle of Anti-PD-1 Therapy: A Case of Lethal Toxicity But Pathological Complete Response in Metastatic Lung Adenocarcinoma. Hematol Oncol Stem Cell Ther (2020) S1658-3876(20):30098–4. doi: 10.1016/j.hemonc.2020.04.006
97. Imai R, Ono M, Nishimura N, Suzuki K, Komiyama N, Tamura T. Fulminant Myocarditis Caused by an Immune Checkpoint Inhibitor: A Case Report With Pathologic Findings. J Thorac Oncol (2019) 14:e36–8. doi: 10.1016/j.jtho.2018.10.156
98. Jeyakumar N, Etchegaray M, Henry J, Lelenwa L, Zhao B, Segura A, et al. The Terrible Triad of Checkpoint Inhibition: A Case Report of Myasthenia Gravis, Myocarditis, and Myositis Induced by Cemiplimab in a Patient With Metastatic Cutaneous Squamous Cell Carcinoma. Case Rep Immunol (2020) 2020:5126717. doi: 10.1155/2020/5126717
99. Monge C, Maeng H, Brofferio A, Apolo AB, Sathya B, Arai AE, et al. Myocarditis in a Patient Treated With Nivolumab and PROSTVAC: A Case Report. J Immunother Cancer (2018) 6:150. doi: 10.1186/s40425-018-0473-0
100. Nasr F, El Rassy E, Maalouf G, Azar C, Haddad F, Helou J, et al. Severe Ophthalmoplegia and Myocarditis Following the Administration of Pembrolizumab. Eur J Cancer (2018) 91:171–3. doi: 10.1016/j.ejca.2017.11.026
101. Sessums M, Yarrarapu S, Guru PK, Sanghavi DK. Atezolizumab-Induced Myositis and Myocarditis in a Patient With Metastatic Urothelial Carcinoma. BMJ Case Rep (2020) 13:e236357. doi: 10.1136/bcr-2020-236357
102. Shah M, Tayar JH, Abdel-Wahab N, Suarez-Almazor ME. Myositis as an Adverse Event of Immune Checkpoint Blockade for Cancer Therapy. Semin Arthritis Rheum (2019) 48:736–40. doi: 10.1016/j.semarthrit.2018.05.006
103. Shirai T, Kiniwa Y, Sato R, Sano T, Nakamura K, Mikoshiba Y, et al. Presence of Antibodies to Striated Muscle and Acetylcholine Receptor in Association With Occurrence of Myasthenia Gravis With Myositis and Myocarditis in a Patient With Melanoma Treated With an Anti-Programmed Death 1 Antibody. Eur J Cancer (2019) 106:193–5. doi: 10.1016/j.ejca.2018.10.025
104. So H, Ikeguchi R, Kobayashi M, Suzuki M, Shimizu Y, Kitagawa K. PD-1 Inhibitor-Associated Severe Myasthenia Gravis With Necrotizing Myopathy and Myocarditis. J Neurol Sci (2019) 399:97–100. doi: 10.1016/j.jns.2019.02.023
105. Xing Q, Zhang ZW, Lin QH, Shen LH, Wang PM, Zhang S, et al. Myositis-Myasthenia Gravis Overlap Syndrome Complicated With Myasthenia Crisis and Myocarditis Associated With Anti-Programmed Cell Death-1 (Sintilimab) Therapy for Lung Adenocarcinoma. Ann Transl Med (2020) 8:250. doi: 10.21037/atm.2020.01.79
106. Lipe DN, Galvis-Carvajal E, Rajha E, Wechsler AH, Gaeta S. Immune Checkpoint Inhibitor-Associated Myasthenia Gravis, Myositis, and Myocarditis Overlap Syndrome. Am J Emerg Med (2021) 46:51–5. doi: 10.1016/j.ajem.2021.03.005
107. Aldrich J, Pundole X, Tummala S, Palaskas N, Andersen CR, Shoukier M, et al. Inflammatory Myositis in Cancer Patients Receiving Immune Checkpoint Inhibitors. Arthritis Rheumatol (2021) 73:866–74. doi: 10.1002/art.41604
108. Seki M, Uruha A, Ohnuki Y, Kameda S, Noda T, Suzuki S, et al. Inflammatory Myopathy Associated With PD-1 Inhibitors. J Autoimmun (2019) 100:105–13. doi: 10.1016/j.jaut.2019.03.005
109. Ohnuki Y, Suzuki S, Shiina T, Uruha A, Watanabe Y, Suzuki S, et al. HLA-DRB1 Alleles in Immune-Mediated Necrotizing Myopathy. Neurology (2016) 87:1954–5. doi: 10.1212/WNL.0000000000003160
Keywords: immune checkpoint inhibitor (ICI), irAE, autoimmune, myocarditis, myositis
Citation: Hamada N, Maeda A, Takase-Minegishi K, Kirino Y, Sugiyama Y, Namkoong H, Horita N, Yoshimi R, Nakajima H and YCU irAE Working Group (2021) Incidence and Distinct Features of Immune Checkpoint Inhibitor-Related Myositis From Idiopathic Inflammatory Myositis: A Single-Center Experience With Systematic Literature Review and Meta-Analysis. Front. Immunol. 12:803410. doi: 10.3389/fimmu.2021.803410
Received: 27 October 2021; Accepted: 22 November 2021;
Published: 06 December 2021.
Edited by:
Shinji Sato, Tokai University, JapanReviewed by:
Takashi Matsushita, Kanazawa University, JapanKeishi Fujio, The University of Tokyo, Japan
Copyright © 2021 Hamada, Maeda, Takase-Minegishi, Kirino, Sugiyama, Namkoong, Horita, Yoshimi, Nakajima and YCU irAE Working Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaoru Takase-Minegishi, a2FvcnVfdEB5b2tvaGFtYS1jdS5hYy5qcA==; Yohei Kirino, a2lyaW5vQHlva29oYW1hLWN1LmFjLmpw
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Naoki Hamada
Naoki Hamada Ayaka Maeda
Ayaka Maeda Kaoru Takase-Minegishi
Kaoru Takase-Minegishi Yohei Kirino
Yohei Kirino Yumiko Sugiyama
Yumiko Sugiyama Ho Namkoong
Ho Namkoong Nobuyuki Horita
Nobuyuki Horita Ryusuke Yoshimi
Ryusuke Yoshimi Hideaki Nakajima
Hideaki Nakajima