- 1Wildfowl & Wetlands Trust, Gloucestershire, United Kingdom
- 2Animates Veterinary Clinic, Thurlby, United Kingdom
Gastropods (class Gastropoda) form the largest of the classes in the phylum Mollusca and inhabit terrestrial, fresh water and marine environments. A large number of these species are of major conservation importance and are an essential component of ecosystems. Gastropods may be deemed as pests, having a negative impact in horticulture and agriculture, whereas others may be used as a food source for human consumption and therefore are beneficial. Gastropods are susceptible to primary diseases and also act as intermediate hosts for diseases which affect other animals, including humans. The diseases described include two that are notifiable to the World Organisation for Animal Health (OIE): Xenohaliotis californiensis and Abalone viral ganglioneuritis caused by Haliotid herpesvirus-1 (HaHV-1). Research into the diseases of gastropods has often focused on those species that act as intermediate disease hosts, those that are used in research or those cultured for food. In this paper we review the viral, bacterial, fungal, parasitic and miscellaneous conditions that have been reported in gastropods and mention some of the factors that appear to predispose them to disease. The pathogenicity of a number of these conditions has not been fully ascertained and more research is needed into specifying both the etiological agent and significance in some of the diseases reported.
Introduction
Gastropods (class Gastropoda, phylum Mollusca) are comprised of more than 80,000 species and are differentiated from other classes of mollusca by the presence of a torsed body. They are separated into three subclasses: Prosobranchia, Opistobranchia, and Pulmonata. They are found in terrestrial, freshwater and marine environments. All gastropods possess a ventrally flattened foot that provides locomotion (1). Over 2000 species are reported in the International Union for Conservation of Nature (IUCN) Red List as critically endangered, endangered or vulnerable, with 14 species listed as extinct in the wild. Over 1500 other species cannot be classified due to a deficiency in data (2).
Gastropods can be used in a number of different types of research including animal and human parasites and neurobiological research (1). With such a diverse class of animals, environmental requirements differ greatly and can often be species specific. Gastopods can become predisposed to diseases due to living in adverse environmental conditions and therefore individuals dealing with them in captivity should be aware of the individual temperature, humidity, nutrition and aquarium/terrarium design requirements for their particular species. Investigations into infectious diseases of gastropods have often centred on those species cultivated as food or those that act as vectors for zoonotic diseases. A sequelae to gastropods becoming more prevalent in the pet trade and zoological collections is likely to be advancement into diagnosis and treatment of their diseases.
Viruses
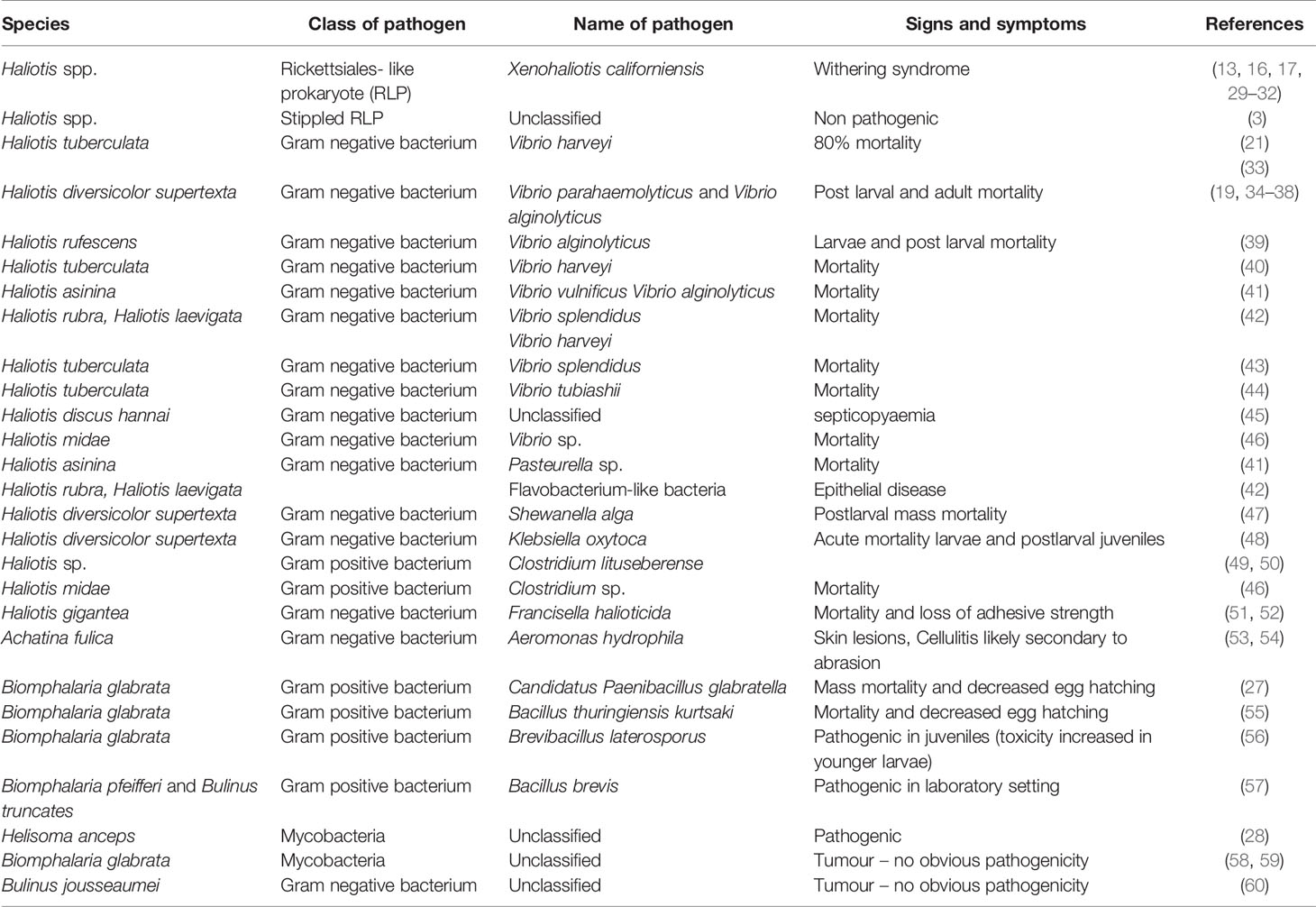
A number of viral infections have been reported in gastropods although more research is needed in many cases to identify the specific virus. Abalone viral ganglioneuritis caused by Haliotid herpesvirus-1 (HaHV-1), has been reported in farmed and free-living abalone Haliotis rubra, Haliotis laevigata and hybrid H. laevigata x H. rubra in Australia and cultured Haliotis diversicolor supertexta cohabiting with Haliotis cracherodii, in Taiwan. This disease is reportable to World Organisation for Animal Health (OIE) and the US Department of Agriculture (USDA) (3). High mortality rates (up to 90%) have been reported and death often occurs within 1-2 days (3). Histological signs indicative of intranuclear inclusion bodies, may be seen in the neurons. Confirmation is by conventional and real-time PCR (4, 5).
Following a mass mortality event in Theba pisana (an intermediate host in human and veterinary medicine), transmission electron microscopy (TEM) confirmed nuclear inclusions where unenveloped, roundish virus-like particles were observed (6) although the causal virus was not identified.
Viruses from a number of families have been identified in snails, abalone and whelks including: Bacilladnaviridae (The International Committee on Taxonomy of Viruses (7), Circoviridae, Reoviridae, Picornaviridae, Caliciviridae, Paramyxoviridae and Rhabdoviridae (8).
Bacteria
It can be complex to determine the presence of bacteria in molluscs as pathogenic as a number of species harbour a large number of commensal bacteria (9). Commensal bacteria have also been shown to be a likely source of Tetrodotoxin in Nassarius semiplicatus (10). Diseases caused by bacteria will often present differently depending on the life stage affected, with larval stages often showing high mortality whereas fewer diseases of adults have been reported (9).
Xenohaliotis californiensis, a Rickettsial- like prokaryote (RLP), causes withering syndrome in abalone. This disease is notifiable to the OIE (11). The organism invades the digestive gland and the animal exhibits a loss of condition and atrophy of the foot muscle. In laboratory studies, time from infection to signs of disease was 245 days and Haliotis cracherodii was more severely affected than Haliotis rufescens. Transmission is direct between individuals (12). Haliotis corrugata and Haliotis fulgens (13) seem to be more resistant than other abalone species (14, 15). Oxytetracycline injections have been shown to halt progression of the disease in treated animals (16). Rickettsial infections in Haliotis diversicolor supertexta (17), caused similar symptoms, but symptoms and mortality only occurred at water temperatures of 30°C. (18) also showed that at least 2 genetic variants show a different host specificity. Vibrio parahaemolyticus has also been isolated from H. diversicolor supertexta in Taiwan showing signs of withering syndrome (19, 20).
Vibrio harveyi has caused up to 80% mortality of wild and cultured Haliotis tuberculata on the coast of France (21). Travers et al. (22) showed that infection was linked to ripe or just spawned individuals in water temperatures above 18°C. Juveniles did not develop disease (23), suggested that this disease occurrence may be linked to global warming.
Potentially zoonotic bacteria have been isolated from Giant African land snails, although they do not appear to cause disease in these animals (24). P. putida and C. indologenes have been associated with infections, such as bacteraemia, in hospitalised patients (25, 26).
Biomphalaria sp. are an intermediate host for Schistosoma sp. therefore they have been the focus of substantial research into molluscicidal bacteria (27).
Mycobacteria have shown pathogenic activity (28) and experimental transmission has been shown to 6 species of fresh water snails. See Table 1 for further details of bacterial diseases.
Fungi
Only a small number of fungal conditions have been reported in the literature affecting gastropods and only minimal information is available in some cases (61), reported fungal disease (potentially linked to shell boring invertebrates) causing lesions on the inside of the shell in Haliotis iris, Haliotis australis and Haliotis virginea. The shell length of affected animals was significantly smaller than those unaffected and fatalities occurred in captive animals. The fungus has only been provisionally suggested as Deuteromycotina (62).
A fungal disease in Haliotis sieboldii, in Japan, showed tubercle-like swelling on the mantle and melanized lesions on the peduncle. This fungus was designated Atkinsiella awabi sp. nov. (63). Haliotis midae, Haliotis rufescens and Haliotis sieboldii, in Japan also showed white nodules on the mantle and mortality due to Halioticida noduliformans gen. et sp. nov. by phylogenetic analysis (64).
(65) also reported lung nodules in Pomacea canaliculata likely caused by Poterioochromonas sp., a species of golden algae, although the pathogenicity of this finding was unclear.
Fungal disease has also been reported in Haliotis sieboldii including Haliphthoros milfoldensis (66), Halocrusticida awabi (63) and Atkinsiella dubia (67) The mycelium was always observed in the lesions of diseased abalone with flat or tubercle-like swelling (68).
Parasites
Ectoparasites
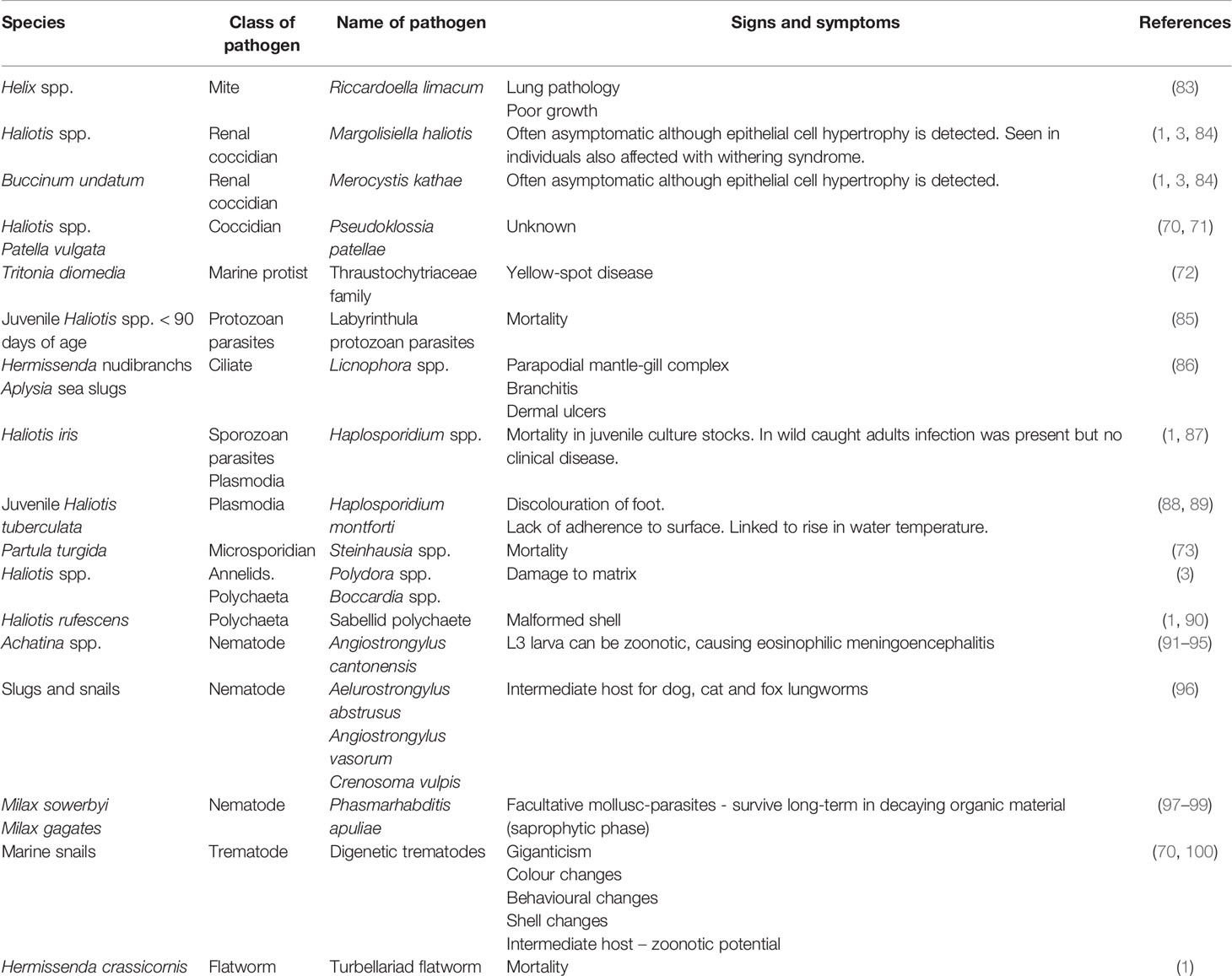
Mites can parasitise Giant African land snails and their pathogenicity varies with the species (69). High mite burdens can lead to debilitation of the snail.
Endoparasites
Protozoa
Pseudoklossia patellae, a coccidian, has been detected in the epithelial cells of the intestine, kidney, and digestive cells of the limpet, Patella vulgata, prosobranchs and Haliotis spp. (70, 71). However, the intermediate hosts from gastropods are not known and no treatment has been described (70).
Ciliates were observed in tissues of two nudibranchs. A flagellate parasitising egg masses of doridacean nudibranchs and a parasite in the Thraustochytriaceae family of marine protists, producing Yellow-spot disease in Tritonia diomedia, a dendronotacean nudibranch (72). This family of marine protists is frequently included in the lower fungi. The amoebocytes of the gastropod become flattened and form a lamellated wall around the parasitic cells to form a necrotic thick-walled acellular capsule (72).
Invertebrates are often intermediate hosts for a variety of metazoan parasites with gastropods being one of the genera that Licnophora spp. infests. Opportunistic infections may arise and their importance in causing disease varies on environmental conditions and the host species (3).
Partula turgida, is especially notable as it succumbed to a microsporidian parasite (Steinhausia sp.), and is claimed to be the first extinction caused by an infectious disease (73). The colony had declined from 296 individuals over 21 months, and post-mortem examinations showed the microsporidian present, although absence from other species suggested that it might be specific: indeed, Cunningham and Daszak raised the possibility that the parasite might also have caused its own demise.
Annelida
Shells of abalone may be infested by Annelids, worms that burrow into the matrix of the host’s shell and form tunnels, compromising the host shell’s protective and supportive functions (3).
Nematoda and Trematoda
Gastropod-borne parasites may be of concern for human and animal health. Cornu aspersum, an edible gastropod of Mediterranean origin, is an intermediate host for several metastrongylid nematodes (74–77). Of veterinary significance, is the increasing number of cases of Angiostrongylus vasorum, the dog lungworm, seen in the UK because of a decline in preventative treatment in 2020 as a result of Covid-19 restrictions (78). Dogs become infected by ingesting slugs and snails which are the intermediate hosts.
Gastropods are also sole hosts of Rhabditida, Mermithida and Ascaridida nematodes (77). The opportunistic parasite of slugs, Phasmarhabditis hermaphrodita has been formulated and developed into a biocontrol agent against slugs and commercialised.
Aquatic gastropods contribute to the distribution of trematodes, e.g. Schistosoma, that risk human health. The infectivity of Schistosoma mansoni to Biomphalaria glabrata has been shown to vary depending on life stage of the snail (79) and temperature (80). Brachylaima, an avian trematode, transmitted by the gastropod Monacha, is also zoonotic (81).
Occasionally gastropods may serve as a final host for trematodes and these parasites can be seen in the kidney or the lumen of the digestive gland e.g. Proctoeces buccini was described in the nephridial lumen of the dog whelk (70, 82).
Turbellaria
Turbellariad (flatworm) infections have been described in the haemocoel of aquaria held gastropods (1) and in the dilated renal lumen and mantle cavity in free-living dog whelk in the North Sea (70).
Copepoda
Splanchnotrophidae are endoparasitic copepods and can affect nudibranchs by producing egg sacs under the external body wall, or which project through the host’s body wall (1). There is paucity in the literature describing lesions and mortality caused by these parasites. See Table 2 for further details of bacterial diseases.
Other
Shell lesions of unknown etiology, leading to reduced growth rate, have been reported in Haliotis iris (101).
Gas bubble disease was reported by (86) in Aplysia caused by exposure to seawater supersaturated with air. Air bubbles have also been identified in the body and cerata of captive Hermissenda. Death (probably caused by pressure necrosis of vital organs by the air bubbles) usually occurred (102).
Neoplasia is rarely described in invertebrates, but has been reported in H.discus (glioma of the pleuropedal nerve cord), Ampullarius australis (papilloma of the epidermis and adenoma of the digestive gland) and Chiton tuberculatus (papilloma of the gastrointestinal tract) (103).
Parry and Pipe (104) found that exposure to three stressors (copper, temperature, bacteria) could alter certain aspects of molluscan immune function and produce complex results.
Discussion
Anthropogenic activities that pollute the environment can affect molluscan physical parameters (105). Increased ammonia or nitrite increased mortality of H. diversicolor infected with V. parahaemolyticus by reducing immune function (106, 107) and in (23), a difference of only 1°C in temperature had a highly significant impact on mortality level.
Life stage can also be an important factor in pathogenicity of gastropod infectious disease, e.g. immature abalone were insensitive to V. harveyi, while ripe or postspawning abalone were susceptible to infection and mortality (23). The abalone reproductive cycle was also an important factor associated with mortalities in (21, 108, 109), also suggested that susceptibility to this pathogen is driven by both climatic factors and reproductive physiology while (42), showed that stress factors had likely precipitated Vibrio sp. outbreaks among H. rubra, H. laevigata and their hybrids.
Life stage (79) and temperature (80) have also been shown to affect the infectivity of Schistosoma mansoni to Biomphalaria glabrata, which may lead to potential consequences for human health linked to global warming.
There have also been differences noted in mortality between wild and cultured abalone. In (23), the mortality rate due to V. harveyi was faster for the farmed than for the wild abalone. The reasons for this difference could include increased stress or reduced genetic diversity in farmed populations (110).
Dang et al. (111) suggested that, in H. laevigata, diet may enhance antibacterial activity against Vibrio anguillarum wheras (41) found that Vibrio sp. was transmitted from the seaweed (Gracilaria changii) used as food for the abalone which led to a mortality event in H. asinina. This shows that good farming and management practices as well as appropriate husbandry are vital in reducing the spread of pathogenic diseases.
As the number and variety of species of gastropod kept in captivity increases, and the conservation status of further wild populations becomes more critical, research into gastropod diseases, mitigation factors and greater depth of knowledge of those diseases already reported but not fully categorised will take place. This will benefit all gastropods, and hopefully in particular those of conservation importance.
Author Contributions
The two authors (MO’B and SP) have contributed equally to this work. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smolowitz R. Gastropods. In: Lewbart G, editor. Invertebrate Medicine, 2nd edn. Ames, Iowa: Wiley Blackwell (2012). p. 95–111.
2. IUCN. (2021). Available at: https://www.iucnredlist.org/search?query=gastropod&searchType=species.
3. Newton AL, Smolowitz R. Invertebrates. In: Terio KA, McAloose D, St. Leger J, editors. Pathology of Wildlife and Zoo Animals. London: Academic Press (2018). p. 1011–43.
4. Crane M, Corbeil S. Infection With Abalone Herpes-Like Virus. In: Sara Linnane, editor/s. OIE Manual of Diagnostic Tests for Aquatic Animals. Paris: Office International des Epizooties, Paris, France. (2012) 441–451. Available at: http://hdl.handle.net/102.100.100/98414?index=1.
5. Hooper C, Hardy-Smith P, Handlinger J. Ganglioneuritis Causing High Mortalities in Farmed Australian Abalone (Haliotis Laevigata and Haliotis Rubra). Aust Vet J (2007) 85:188–93. doi: 10.1111/j.1751-0813.2007.00155.x
6. De Vico G, Tate R, Maio N, Costantino A, Guida V, Villari G, et al. Early Evidence for a Virus-Like Agent Infecting the Pest Snail Theba Pisana (Gastropoda: Pulmonata) in Southern Italy. J Invertebrate Pathol (2017) 148:10–3. doi: 10.1016/j.jip.2017.05.005
7. The International Committee on Taxonomy of Viruses (ICTV). (2017). Available at: http://talk.ictvonline.org/taxonomy (Accessed 31st May 2021).
8. McAloose D, Chang TY. Viral Families and Documented Diseases. In: Terio KA, McAloose D, St. Leger J, editors. Pathology of Wildlife and Zoo Animals. London: Academic Press (2018). p. 1045–65.
9. Paillard C, Le Roux F, Borrego JJ. Acterial Diseases in Marine Bivalves, a Review of Recent Studies: Trends and Evolution. Aquat Living Resour (2004) 17:477–98. doi: 10.1051/alr:2004054
10. Wang X, Yu R, Luo X, Zhou M, Lin X. Toxin-Screening and Identification of Bacteria Isolated From Highly Toxic Marine Gastropod Nassarius Semiplicatus. Toxicon (2008) 52:55–61. doi: 10.1016/j.toxicon.2008.04.170
11. OIE. Manual of Diagnostic Tests for Aquatic Animals. Chapter 2.4.8. Infection With Xenohaliotis Californiensis (2019). Available at: https://www.oie.int/fileadmin/Home/eng/Health_standards/aahm/current/chapitre_xenohaliotis_californiensis.pdf (Accessed 28 May 2021).
12. Moore JD, Robbins TT, Hedrick RP, Friedman CS. Transmission of the Rickettsiales-Like Prokaryote “Candidatus Xenohaliotis Californiensis” and its Role in Withering Syndrome of California Abalone, Haliotis Spp. J Shellfish Res (2001) 20:867–74.
13. Moore JD, Finley CA, Robbins TT, Friedman CS. Withering Syndrome and Restoration of Southern California Abalone Populations. CalCOFI Rep (2002) 43:112–7.
14. Moore JD, Juhasz CI, Robbins TT, Vilchis LI. Green Abalone, Haliotis Rufescens Infected With the Agent of Withering Syndrome do Not Express Disease Signs Under a Temperature Regime Permissive for Red Abalone, Haliotis Rufescens. Marine Biol (2009) 156:2325–30. doi: 10.1007/s00227-009-1260-8
15. Cruz-Flores R, Caceres-Martınez J, Munoz-Flores M, Vasquez- Yeomans R, Hernandez Rodriguez M, Del Rıo-Portilla MA, et al. Hyperparasitism by the Bacteriophage (Caudovirales) Infecting Candidatus Xenohaliotis Californiensis (Rickettsiales-Like Prokaryote) Parasite of Wild Abalone Haliotis Fulgens and Haliotis Corrugata From the Peninsula of Baja California, Mexico. J Invertebrate Pathol (2016) 140:58–67. doi: 10.1016/j.jip.2016.09.001
16. Friedman CS, Trevelyan G, Robbins TT, Mulder EP, Fields R. Development of an Oral Administration of Oxytetracycline to Control Losses Due to Withering Syndrome in Cultured Red Abalone Haliotis Rufescens. Aquaculture (2003) 224:1 – 23. doi: 10.1016/S0044-8486(03)00165-0
17. Chang PH, Yang MC, Kuo ST, Chen MH, Cheng CH. Occurrence of a Rickettsia - Like Prokaryote in the Small Abalone,Haliotis Diversicolor Supertexta, Cultured in Taiwan. Bull Eur Ass Fish Pathol (2008) 28(2):52 – 57.
18. Nishioka T, Kamaishi T, Kurita J, Mekata T, Kiryu I, Yuasa K, et al. Pathogenicity of Two Candidatus Xenohaliotis Californiensis Genetic Variants Against Three Abalone Species (the Genus Haliotis). Fish Pathol (2016) 51:54–9. doi: 10.3147/jsfp.51.54
19. Liu PC, Chen YC, Huang CY, Lee KK. Virulence of Vibrio Parahaemolyticus Isolated From Cultured Small Abalone Haliotis Diversicolor Supertexta, With Withering Syndrome. Lett Appl Microbiol (2000) 31:433–7. doi: 10.1046/j.1365-2672.2000.00843.x
20. Huang C-Y, Liu P-C, Lee K-K. Withering Syndrome of the Small Abalone, Haliotis Diversicolor Supertexta, Is Caused by Vibrio Parahaemolyticus and Associated With Thermal Induction. Zeitschrift für Naturforschung. (2001) 56:898–901.
21. Nicolas JL, Basuyaux O, Mazurie J, Thebault A. Vibrio Carchariae, a Pathogen of the Abalone Haliotis Tuberculata. Dis Aquat Organ (2002) 50:35 – 43. doi: 10.3354/dao050035
22. Travers M, Le Goïc N, Huchette S, Koken M, Paillard C. Summer Immune Depression Associated With Increased Susceptibility of the European Abalone, Haliotis Tuberculata to Vibrio Harveyi Infection. Fish Shellfish Immunol (2008) 25:800 – 808. doi: 10.1016/j.fsi.2008.08.003
23. Travers M, Basuyaux O, Le Goïc N, Huchette S, Nicolas J, Koken M, et al. Influence of Temperature and Spawning on Haliotis Tuberculata Mortalities Caused by Vibrio Harveyi: An Example of Emerging Vibriosis Linked to Global Warming. Glob Change Biol (2009) 15:1365 – 1376. doi: 10.1111/j.1365-2486.2008.01764.x
24. Williams D, Haverson V, Chandler M. Proceedings Veterinary Invertebrate Society Summer Scientific Meeting 2017. Zeitschrift für Naturforschung (2017) Cambridge, UK.
25. Bayraktar MR, Aktas E, Ersoy Y, Cicek A, Durmaz R. Postoperative Chryseobacterium Indologenes Bloodstream Infection Caused by Contamination of Distillate Water. Infection Control and Hospital Epidemiology (2007) 28(3):368–9.
26. Yoshino Y, Kitazawa T, Kamimura M, Tatsuno M, Ota K, Yotsuyanagi Y, et al. Pseudomonas Putida Bacteremia in Adult Patients: Five Case Reports and a Review of the Literature. J Infect Chemother (2011) 17:278–82.
27. Duval D, Galinier R, Mouahid G, Toulza E, Allienne JF, Portela J, et al. A Novel Bacterial Pathogen of Biomphalaria Glabrata: A Potential Weapon for Schistosomiasis Control? PloS Negl Trop Dis (2015) 9(2):e0003489. doi: 10.1371/journal.pntd.0003489
28. Michelson EH. An Acid-Fast Pathogen of Fresh-Water Snails. Am J Trop Med Hyg (1961) 10(3):423–33. doi: 10.4269/ajtmh.1961.10.423
29. Cruz-Flores R, Caceres-Martınez J. Rickettsiales-Like Organisms in Bivalves and Marine Gastropods: A Review. Aquaculture (2020) 12(4):1–17. doi: 10.1111/raq.12419
30. Moore JD, Robbins TT, Friedman CS. The Role of a Rickettsialike Prokaryote in Withering Syndrome in California Red Abalone, Haliotis Rufescens. J Shellfish Res (2000) 19:525–6.
31. Finley CA, Wendell F, Friedman CS. Geographic Distribution of Candidatus Xenohaliotis Californiensis in Northern California Abalone. In: Devoe R, editor. Aquaculture 2001: Book of Abstracts. Baton Rouge, LA: World Aquaculture Society, Louisiana State University (2001). p. 225.
32. Friedman CS, Biggs W, Shield JD, Hedrick RP. Transmission of Withering Syndrome in Black Abalone, Haliotis Cracherodii Leach. J Shellfish Res (2002) 21:817 – 824 https://scholarworks.wm.edu/vimsarticles/471.
33. Huchette SMH, Clavier J. Status of the Ormer (Haliotis Tuberculata L.) Industry in Europe. J Shellfish Res (2004) 23:951–5 link.gale.com/apps/doc/A132270244/AONE?u=anon∼5f574294&sid=googleScholar&xid=07697bcd.
34. Liu PC, Chen YC, Lee KK. Pathogenicity of Vibrio Alginolyticus Isolated From Diseased Small Abalone Haliotis Diversicolor Supertexta. Microbios (2001) 104:71–7.
35. Lee KK, Liu PC, Chen YC, Huang CY. The Implication of Ambient Temperature With the Outbreak of Vibriosis in Cultured Small Abalone Haliotis Diversicolor Supertexta Lischke. J Thermal Biol (2001) 26:585–7. doi: 10.1016/S0306-4565(01)00004-3
36. Cai J, Han Y, Wang Z. Isolation of Vibrio Parahaemolyticus From Abalone (Haliotis Diversicolor Supertexta L.) Post-Larvae Associated With Mass Mortalities. Aquaculture (2006) 257:161–6. doi: 10.1016/j.aquaculture.2006.03.007
37. Cai J, Han H, Song Z, Li C, Zhou J. Isolation and Characterization of Pathogenic Vibrio Alginolyticus From Diseased Postlarval Abalone, Haliotis Diversicolor Supertexta (Lischke). Aquacult Res (2006) 37:1222–6. doi: 10.1111/j.1365-2109.2006.01552.x
38. Cheng L, Huang J, Shi C, Thompson KD, Mackey B, Cai J. Vibrio Parahaemolyticus Associated With Mass Mortality of Postlarval Abalone, Haliotis Diversicolor Supertexta (L.), in Sanya, China. J World Aquacult Soc (2008) 39(6):746–57. doi: 10.1111/j.1749-7345.2008.00210.x
39. Anguiano-Beltran C, Searcy-Bernal R, Lizarraga-Partida ML. Pathogenic Effects of Vibrio Alginolyticus on Larvae and Post-Larvae of the Red Abalone, Haliotis Rufescens. Dis Aquat Organisms (1998) 33:119–22. doi: 10.3354/dao033119
40. Pichon D, Cudennec B, Huchette S, Djediat C, Renault T, Paillard C, et al. Characterization of Abalone Haliotis Tuberculata-Vibrio Harveyi Interactions in Gill Primary Cultures. Cytotechnology (2013) 65:759–72. doi: 10.1007/s10616-013-9583-1
41. Kua BC, Ramly R, Devakie M, Groman D, Berthe CJF. Investigating a Mortality in Hatchery Cultured Tropical Abalone, Haliotis Asinina Linnaeu. Dis Asian Aqua (2011) 7:103–9.
42. Handlinger JJ, Donachie CL, Gabor L, Taylor D. Bacterial Infection in Tasmanian Farmed Abalone: Causes, Pathology, Farm Factors and Control Options. Dis Asian Aqua (2005) 5:289–99.
43. Saulnier D, De Decker S, Haffner P, Cobret L, Robert M, Garcia C. A Large-Scale Epidemiological Study to Identify Bacteria Pathogenic to Pacific Oyster Crassostrea Gigas and Correlation Between Virulence and Metalloprotease-Like Activity. Microbial Ecol (2010) 59:787–98. doi: 10.1007/s00248-009-9620-y
44. Travers M-A, Mersni Achour R, Haffner P, Tourbiez D, Cassone A-L, Morga B, et al. First Description of French V. Tubiashii Strains Pathogenic to Mollusk: I. Characterization of Isolates and Detection During Mortality Events. J Invertebrate Pathol (2014) 123:38–48. doi: 10.1016/j.jip.2014.04.009
45. Ma J, Wang Q, Ma F, Liu M. A Pathogen of Septicopyaemia in the Abalone Haliotis Discus Hannai Ino. Dalian, China (Translation of Title From Wang Et Al. 2004). J Fisheries China (1996) 4:332–6.
46. Dixon MG, Hecht T, Brandt CR. Identification and Treatment of a Clostridium and Vibrio Infection in South African Abalone, Haliotis Midae L. J Fish Dis (1991) 14:693–95. doi: 10.1111/j.1365-2761.1991.tb00629.x
47. Cai J, Chen H, Thompson KD, Li C. Isolation and Identification of Shewanella Alga and its Pathogenic Effects on Post-Larvae of Abalone Haliotis Diversicolor Supertexta. J Fish Dis (2006) 29:505–8. doi: 10.1111/j.1365-2761.2006.00732.x
48. Cai J, Wang Z, Cai C, Zhou Y. Characterization and Identifi Cation of Virulent Klebsiella Oxytoca Isolated From Abalone (Haliotis Diversicolor Supertexta) Postlarvae With Mass Mortality in Fujian, China. J Invertebr Pathol (2008) 97:70 – 75. doi: 10.1016/j.jip.2007.07.005
49. Bower SM. Update on Emerging Abalone Diseases and Techniques for Health Assessment. J Shellfish Res (2003) 22:805–10.
50. Bower SM. Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Bacterial Diseases of Abalone (2017). Available at: https://www.dfo-mpo.gc.ca/science/aah-saa/diseases-maladies/vibrioab-eng.html (Accessed 31st May 2021).
51. Kamaishi T, Miwa S, Goto E, Matsuyama T, Oseko N. Mass Mortality of Giant Abalone Haliotis Gigantea Caused by a Francisella Sp. Bacterium. Dis Aquat Organisms (2010) 89:145–54. doi: 10.3354/dao02188
52. Brevik ØJ, Ottem KF, Kamaishi T, Watanabe K, Nylund A. Francisella Halioticida Sp. Nov., a Pathogen of Farmed Giant Abalone (Haliotis Gigantea) in Japan. J Appl Microbiol (2011) 111:1044–56. doi: 10.1111/j.1365-2672.2011.05133.x
54. Williams D. (2001). Effects of Micro and Macroalgal Diet Supplementations on Growth and Immunity of Greenlip Abalone, Haliotis Laevigata. Exotic Animal Practice 4(2):309–20.
55. Gamalat YO, Ahmed MM, Ahmed AK, Asmaa AM. Biological Studies on Biomphalaria Alexandrina Snails Treated With Furcraea Selloa Marginata Plant (Family: Agavaceae) and Bacillus Thuringiensis Kurstaki (Dipel-2x). J Appl Pharm Sci (2011) 01:47–55.
56. de Oliveira EJ, Rabinovitch L, Monnerat R, Passos L, Zahner V. Molecular Characterization of Brevibacillus Laterosporus and its Potential Use in Biological Control. Appl Environ Microbiol (2004) 70:6657–64. doi: 10.1128/AEM.70.11.6657-6664.2004
57. Singer S, Bair T, Hammill TB, Berte AM, Correa-Ochoa MM, et al. Fermentation and Toxin Studies of the Molluscicidal Strains of Bacillus Brevis. J Ind Microbiol (1994) 13:112–9. doi: 10.1007/BF01584108
58. Pan C. Studies on the Biological Control of Schistosome-Bearing Snails: A Preliminary Report on Pathogenic Microorganisms Found in Australorbis Glabratus. J Parasitol (1956) 42(4):33.
59. Bean-Knudsen DE, Uhazy LS, Wagner JE, Young BM. Systemic Infection of Laboratory Reared Biomphalaria Glabrata (Mollusca: Gastropoda) With an Acid-Fast Bacillus. J Invertebr Pathol (1988) 51:291–3. doi: 10.1016/0022-2011(88)90039-0
60. Cole RM, Richards CS, Popkin TJ. Novel Bacterium Infecting an African Snail. J Bacteriol (1977) 132:950–66. doi: 10.1128/jb.132.3.950-966.1977
61. Grindley RM, Keogh JA, Friedman CS. Shell Lesions in New Zealand Haliotis Spp. (Mollusca, Gastropoda). J Shellfish Res (1998) 17:805–11.
62. Friedman CS, Grindley RM, Keogh JA. Isolation of a Fungus From Shell Lesions of New Zealand Abalone, Haliotis Iris Martyn and H. Australis Gmelin Molluscan Res (1997) 18:313–24. doi: 10.1080/13235818.1997.10673704
63. Kitancharoen N, Nakamura K, Wada S, Hatai K. Atkinsiella Awabi Sp. Nov. From Stocked Abalone, Haliotis Siebolodii. Mycoscience (1994) 35:265–70. doi: 10.1007/BF02268448
64. Muraosa Y, Morimoto K, Nishimura ASK, Hatai K. A New Peronosporomycete, Halioticida Noduliformans Gen. Et Sp. Nov., Isolated From White Nodules in the Abalone Haliotis Spp. From Japan. Mycoscience (2009) 50:106–15. doi: 10.1007/S10267-008-0462-0
65. Guo Y, Zhou HC, Dong Y, Zhang T, Sun YY, Zhong JF, et al. New Nodule Type Found in the Lungs of Pomacea Canaliculata, an Intermediate Host of Angiostrongylus Cantonensis. Iran J Parasitol (2018) 13(3):362–8.
66. Hatai K. On the Fungus Haliphthoros Milfordensis Isolated From Temporarily Held Abalone (Haliotis Sieboldii). Fish Pathol (1982) 17:199–204. doi: 10.3147/jsfp.17.199
67. Nakamura K, Hatai K. Atkinsiella Dubia and its Related Species. Mycoscience (1995) 36:431–8. doi: 10.1007/BF02268628
68. Mabuhay-Omar JA, Cayabo GDB, Nuñala IJP, Habal SE, Creencia LA. Microbial and Microparasite Abundance in Cage-Cultured Abalone Haliotis Asinine. J Shellfish Res (2020) 38(2):405–11. doi: 10.2983/035.038.0223
70. Lauckner G. Diseases of Mollusca: Gastropoda. In: Kinne O, editor. Diseases of Marine Animals. New York: John Wiley and Sons (1980). p. 311–424.
71. Friedman CS, Gardner GR, Hedrick RP, Stephenson M, Casthorn RJ, Upton SJ. Pseudplklossia Haliotis Sp. N. (Apicomplexa) From the Kidney of California Abalone, Haliotis Spp. (Mollusca). J Invertebrate Pathol (1995) 66:33–8. doi: 10.1006/jipa.1995.1057
72. McLean N, Porter D. The Yellow-Spot Disease of Tritonia Diomedia Bergh 1894 (Mollusca: Gastropoda: Nudibranchia): Encapsulation of the Thraustochytriaceous Parasite by Host Amoebocytes. J Parasitol (1982) 68(2):243–52. doi: 10.2307/3281182
73. Cunningham AA, Daszak P. Extinction of a Species of Land Snail Due to Infection with a Microsporidian Parasite. Conservation Biology (1998) 12(5):1139–41.
74. Seneviratna P. Studies on Anafilaroides Rostratus Gerichtein Cats. The Adult and its First Stage Larva. J Helminthol (1959) 33:99–108. doi: 10.1017/S0022149X00033356
75. Anderson RC. Nematode Parasites of Vertebrates: Their Development and Transmission. CAB Int (2000) 41–230. doi: 10.1079/9780851994215.0000
76. Giannelli A, Colella V, Abramo F, Ramos RAD, Falsone L, Brianti E, et al. Release of Lungworm Larvae From Snails in the Environment: Potential for Alternative Transmission Pathways. PloS Neglect Trop Dis (2015) 9(4):e0003722. doi: 10.1371/journal.pntd.0003722
77. Ivanova E, Clausi M, Sparacio I, Spiridonov S. Preliminary Data on the Parasite Survey of Terrestrial Gastropods of Sicily. Russian J Nematol (2019) 27(1):37–45. doi: 10.24411/0869-6918-2019-10005
78. Stokes L, Wright I. Parasites in the UK: Trends and Themes of 2020. Companion Anim (2021) 26(3):62–5. doi: 10.12968/coan.2021.0012
79. Richards CS, Minchella DJ. Transient non-Susceptibility to Schistosoma Mansoni Associated With Atrial Amoebocytic Accumulations in the Snail Host Biomphalaria Glabrata. Parasitology (1987) 95(3):499–505. doi: 10.1017/S0031182000057929
80. Ittiprasert W, Knight M. Reversing the Resistance Phenotype of the Biomphalaria Glabrata Snail Host Schistosoma Mansoni Infection by Temperature Modulation. PloS Pathog (2012) 8(4):e1002677. doi: 10.1371/journal.ppat.1002677
81. Butcher AR, Grove DI. Description of the Life-Cycle Stages of Brachylaima Cribbi N. Sp. (Digenea: Brachylaimidae) Derived From Eggs Recovered From Human Faeces in Australia. Syst Parasitol (2001) 49:211–21. doi: 10.1023/A:1010616920412
82. Loos-Frank FB. Zwei Adulte Trematoden Aus Nordsee-Mollusken: Proctoeces Buccini N. Sp. Und P. Scrobiculariae N. Sp. 324-340: Z Parasitenkd 32 (1969).
83. Elmslie LJ. Snail Collection and Small-scale production in Africa and Europe. In: Ecological Implications of Minilivestock. Potential of Insects, Rodents, Frogs and Sails Paoletti MG, editor. Potential of Insects, Rodents, Frogs and Sails. Boca Raton:CRC Press (2005). p. 93–123. doi: 10.1201/9781482294439
84. Gonzàlez R, Lohrmann KB, Pizarro J, Brokordt K. Differential Susceptibility to the Withering Syndrome Agent and Renal Coccidia in Juvenile Haliotis Rufescens, Haliotis Discus Hannai and the Interspecific Hybrid. J Invertebrate Pathol (2014) 116:13–7. doi: 10.1016/j.jip.2013.12.002
85. Bower SM. The Life Cycle and Ultrastructure of a New Species of Thraustochytrid (Protozoa: Labyrinthomorpha) Pathogenic to Small Abalone. Aquaculture (1987) 67:269–70. doi: 10.1016/0044-8486(87)90054-8
86. Leibovitz L, Capo TR. Diseases of a Mass Cultured Marine Laboratory Animal, the Sea Hare, Aplysia Californica. In: Perkins FO, Cheng TC, editors. Third International Colloquium on Pathology of Marine Aquaculture. Gloucester Point, VA: Virginia Institute of Marine Science (1988).
87. Diggles BK, Nichole J, Hine PM, Wakefield S, Cochennec-Laureau N, Roberts RD, et al. Pathology of Cultured Paua Haliotis Iris Infected With a Novel Haplosporidian Parasite, With Some Observations on the Course of the Disease. Dis Aquat Organ (2002) 50:219–31. doi: 10.3354/dao050219
88. Balseiro P, Aranguren R, Gestal C, Novoa B, Figueras A. Candidatus Xenohaliotis Californiensis and Haplosporidium Montfori Associated With Mortalities of Haliotis Tuberculata Cultured in Europe. Aquaculture (2006) 258:63–72. doi: 10.1016/j.aquaculture.2006.03.046
89. Azevedo C, Balseiro P, Casal G, Gestal C, Aranguren R, Stokes NA, et al. Ultrastructural and Molecular Characterization of Haplosporidium Montfori N. Sp., Parasite of the European Abalone Haliotis Tuberculata. J Invertebrate Pathol (2006) 92:23–32. doi: 10.1016/j.jip.2006.02.002
90. Oakes FR, Fields RC. Infestation of Haliotis Rufescens Shells by a Sabellid Polychaete. Aquaculture (1996) 140:139–43. doi: 10.1016/0044-8486(95)01190-0
91. Kim DY, Stewart TB, Bauer RW, Mitchell M. Parastrongylus (=Angiostrongylus) Cantonensis Now Endemic in Louisiana Wildlife. J Parasitol (2002) 88(5):1024–6. doi: 10.1645/0022-3395(2002)088[1024:PACNEI]2.0.CO;2
92. Toma H, Matsumura S, Oshiro C, Hidaka T, Sato Y. Ocular Angiostrongyliasis Without Meningitis Symptoms in Okinawa Japan. J Parasitol (2002) 88(1):211–3. doi: 10.1645/0022-3395(2002)088[0211:OAWMSI]2.0.CO;2
93. Neuhauss E, Fitarelli M, Romanzini J, Teixeira CG. Low Susceptibility of Achatina Fulica From Brazil to Infection With Angiostrongylus Costaricensis and A Cantonensis. Memorias do Inst Oswaldo Cruz (2007) 102(1):49–52. doi: 10.1590/S0074-02762007000100007
94. Latonio AA. The Giant African Land Snail. Achatina Fulica. A New Threat to Public Health. Trans R Soc Trop Med Hyg (1971) 65:22.
95. Moreira VLC, Giese EG, Melo FTV, Simões RO, Thiengo SC, Maldonado JA, et al. Endemic Angiostrongyliasis in the Brazilian Amazon: Natural Parasitism of Angiostrongylus Cantonensis in Rattus Rattus and R. Norvegicus, and Sympatric Giant African Land Snails, Achatina Fulica. Acta Tropica (2013) 125(1):90–7. doi: 10.1016/j.actatropica.2012.10.001
96. Lange MK, Penagos-Tabares F, Hirzmann J, Failing K, Schaper R, Van Bourgonie YR, et al. Prevalence of Angiostrongylus Vasorum, Aelurostrongylus Abstrusus and Crenosoma Vulpis Larvae in Native Slug Populations in Germany. Vet Parasitol (2018) 254:120–30. doi: 10.1016/j.vetpar.2018.03.011
97. Wilson MJ, Glen DM, George SK. The Rhabditid Nematode Phasmarhabditis Hermaphrodita as a Potential Biological Control Agent for Slugs. Biocontrol Sci Technol (1993) 3:503–11. doi: 10.1080/09583159309355306
98. Rae R, Verdun C, Grewal P, Robertson JF, Wilson MJ. Biological Control of Terrestrial Molluscs Using Phasmarhabditis Hermaphrodita Progress and Prospects. Pest Manage Sci (2007) 63:1153–64. doi: 10.1002/ps.1424
99. Nermut J, Půža V, Mráček Z. Phasmarhabditis Aquliae N. Sp. (Nematoda: Rhabditidae), a New Rhabditid Nematode From Milacid Slugs. Nematology (2016) 18:1095–112. doi: 10.1163/15685411-00003017
101. Nollens HH, Keogh JA, Probert PK. Effects of Shell Lesions on Survival, Growth, Condition and Reproduction in the New Zealand Blackfoot Abalone Haliotis Iris. Dis Aquat Organ (2003) 57:127 – 133. doi: 10.3354/dao057127
102. Dow I. Update on Hermissenda Ailments (2003). Available at: http://www.seaslugforum.net/hermcras.htmm10478 (Accessed on 3rd July 2021).
103. Peters EC, Smolowitz RM, Reynolds TL. “Neoplasia” in Invertebrate Medicine. 2nd edn. Lewbart G, editor. Ames, Iowa: Wiley Blackwell (2012) p. 431–40.
104. Parry HE, Pipe RK. Interactive Effects of Temperature and Copper on Immunocompetence and Disease Susceptibility in Mussels (Mytilus Edulis). Aquat. Toxicol (2004) 69:311–25.
105. Morley NJ. Interactive Effects of Infectious Diseases and Pollution in Aquatic Molluscs. Aquatic Toxicology (2010) 96:27–36.
106. Cheng W, Hsiao I-S, Chen J-C. Effect of Ammonia on the Immune Response of Taiwan Abalone Haliotis Diversicolor Supertexta and its Susceptibility to Vibrio Parahaemolyticus. Fish Shellfish Immunol (2004) 17:193–202. doi: 10.1016/j.fsi.2004.03.004
107. Cheng W, Hsiao I-S, Chen J-C. Effect of Nitrite on Immune Response of Taiwan Abalone Haliotis Diversicolor Supertexta and its Susceptibility to Vibrio Parahaemolyticus. Dis Aquat Org (2004) 60:157–64. doi: 10.3354/dao060157
108. Nishimori E, Hasegawa O, Numata T, Wakabayashi. Vibrio Carchariae Causes Mass Mortalities in Japanese Abalone, Sulculus Diversicolor Supratexta. Fish Pathol (1998) 33:495–502. doi: 10.3147/jsfp.33.495
109. Burge CA, Eakin CM, Friedman CS, Froelich B, Hershberger PK, Hofmann EE, et al. Climate Change Influences on Marine Infectious Diseases: Implications for Management and Society. Annu Rev Marine Sci (2014) 6:249–77. doi: 10.1146/annurev-marine-010213-135029
110. Gagnaire B, Soletchnik P, Faury N, Kerdudou N, Le Moine O, Renault T. Analysis of Hemocyte Parameters in Pacific Oysters, Crassostrea Gigas, Reared in the Field – Comparison of Hatchery Diploids and Diploids From Natural Beds. Aquaculture (2007) 264:449–56. doi: 10.1016/j.aquaculture.2006.12.041
Keywords: gastropod disease, mollusc, virus, bacteria, fungi
Citation: O’Brien MF and Pellett S (2022) Diseases of Gastropoda. Front. Immunol. 12:802920. doi: 10.3389/fimmu.2021.802920
Received: 27 October 2021; Accepted: 08 December 2021;
Published: 13 January 2022.
Edited by:
Mathilde Knight, George Washington University, United StatesReviewed by:
Daniel Horton, University of Stirling, United KingdomCopyright © 2022 O’Brien and Pellett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle F. O’Brien, bWljaGVsbGUub2JyaWVuQHd3dC5vcmcudWs=
 Michelle F. O’Brien
Michelle F. O’Brien Sarah Pellett2
Sarah Pellett2