- 1Department of Medical Genetics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Phytochemistry Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Department of Pharmacognosy, College of Pharmacy, Hawler Medical University, Erbil, Iraq
- 4Center of Research and Strategic Studies, Lebanese French University, Erbil, Iraq
- 5Institute of Human Genetics, Jena University Hospital, Jena, Germany
- 6Skull Base Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Sepsis is resulted from a systemic inflammatory response to bacterial, viral, or fungal agents. The induced inflammatory response by these microorganisms can lead to multiple organ system failure with devastating consequences. Recent studies have shown altered expressions of several non-coding RNAs such as long non-coding RNAs (lncRNAs), microRNAs (miRNAs) and circular RNAs (circRNAs) during sepsis. These transcripts have also been found to participate in the pathogenesis of multiple organ system failure through different mechanisms. NEAT1, MALAT1, THRIL, XIST, MIAT and TUG1 are among lncRNAs that participate in the pathoetiology of sepsis-related complications. miR-21, miR-155, miR-15a-5p, miR-494-3p, miR-218, miR-122, miR-208a-5p, miR-328 and miR-218 are examples of miRNAs participating in these complications. Finally, tens of circRNAs such as circC3P1, hsa_circRNA_104484, hsa_circRNA_104670 and circVMA21 and circ-PRKCI have been found to affect pathogenesis of sepsis. In the current review, we describe the role of these three classes of noncoding RNAs in the pathoetiology of sepsis-related complications.
Introduction
Sepsis is a systemic inflammatory response to different infections, namely bacterial, viral, or fungal agents. This condition is the principal source of mortality in intensive care units (1). These infectious microorganisms can stimulate inflammatory reactions through induction of cytokines release. These reactions lead to multiple organ system failure. Other factors that contribute in this devastating condition during sepsis are systemic hypotension and abnormal perfusion of the microcirculatory system (2). No specific treatment modality has been suggested for prevention of multiple organ system failure during sepsis (2). Thus, identification of sepsis-related changes at cellular and biochemical levels is important. Currently, there is no effective pharmacological therapy for sepsis. Thus, early diagnosis, resuscitation and instant administration of suitable antibiotics are essential steps in decreasing the burden of this condition {Thompson, 2019 #562}.
Lipopolysaccharide (LPS) as the main constituent of the cell wall of Gram-negative bacteria has been found to stimulate apoptotic pathways in tubular epithelial cells of kidney (3). Moreover, it can prompt acute inflammatory responses through activation of NF-κB during the course of acute kidney injury (4). This molecular pathway is an important axis in mediation of immune-related organ damage.
Recent studies have shown altered expressions of several non-coding RNAs such as long non-coding RNAs (lncRNAs), microRNAs (miRNAs) and circular RNAs (circRNAs) during sepsis. These transcripts have also been found to participate in the pathogenesis of multiple organ system failure through different mechanisms. In the current review, we describe the role of these three classes of noncoding RNAs in the pathoetiology of sepsis-related complications.
LncRNAs and Sepsis
LncRNAs are transcripts with sizes larger than 200 nucleotides. These transcripts regulate gene expression through modulation of chromatin configuration, regulation of splicing events, serving as decoys for other transcripts and making structures for recruitment of regulatory proteins (5). These transcripts participate in the regulation of immune reactions and pathoetiology of several immune-related disorders (6).
Experiments in animal model of acute lung injury have shown down-regulation of TUG1 and induction of apoptosis and inflammation. Up-regulation of TUG1 in these animals could ameliorate sepsis-associated lung injury, apoptosis and inflammatory reactions. TUG1 could also protect lung microvascular endothelial cells from deteriorative effects of LPS. In fact, TUG1 inhibits cell apoptosis and inflammatory reactions in LPS-stimulated microvascular endothelial cells through sponging miR-34b-5p and releasing GAB1 from its inhibitory effects. Cumulatively, TUG1 ameliorates sepsis-associated inflammation and apoptosis through miR-34b-5p/GAB1 axis (7). Another study has demonstrated down-regulation of TUG1 while up-regulation of miR-223 in the plasma samples of sepsis patients. They have also reported a negative correlation between expressions of TUG1 and miR-223 in sepsis patients. Besides, expression levels of TUG1 have been negatively correlated with respiratory infection, serum creatinine, white blood cell, C-reactive protein, APACHE II score, and SOFA score. Based on these results, TUG1 has been suggested as a biomarker for prediction of course and prognosis of sepsis (8). TUG1 has also been shown to interact with miR-27a. Over-expression of TUG1 has resulted in down-regulation of TNF-α, while up-regulation of miR-27a has enhanced expression of TNF-α in cardiomyocytes. TNF-α and miR-27a up-regulation could enhance LPS-induced apoptosis of cardiomyocytes. On the other hand, TUG1 up-regulation has exerted opposite effects (9).
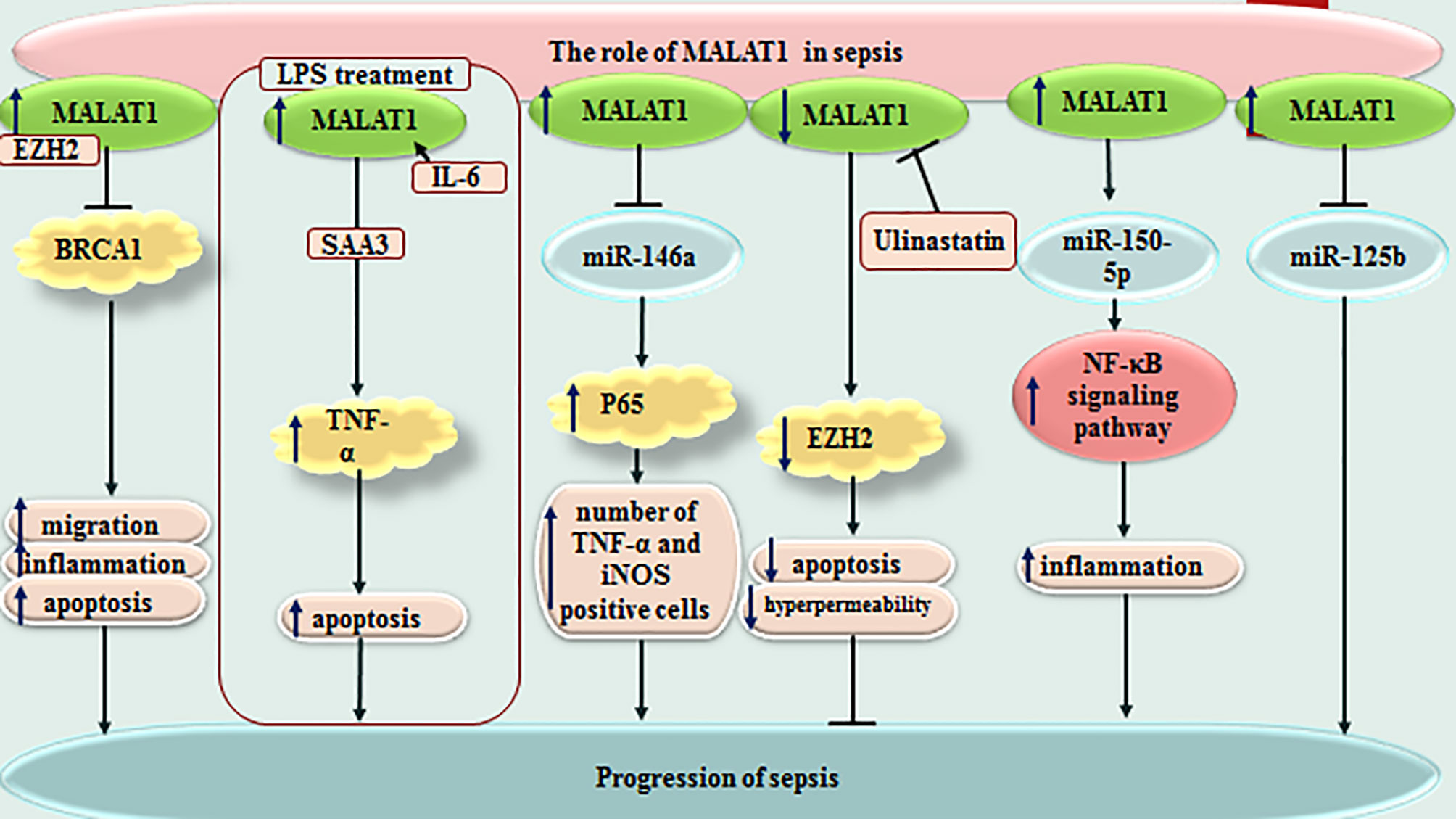
MALAT1 is another lncRNA that affects immune responses of rats with LPS-induced sepsis through influencing the miR-146a/NF-κB P65 axis (10). Moreover, MALAT1 could increase apoptosis skeletal muscle cells and sepsis-associated immune responses through down-regulating BRCA1 levels via recruitment of EZH2 (11). The miR-150-5p/NF-κB axis is another axis that mediates the effects of MALAT1 in sepsis-associated cardiac inflammation (12). In addition, the protective effects of Ulinastatin against LPS-associated dysfunction of heart microvascular endothelial cells have been shown to be exerted through down-regulation of MALAT1 (13). Most notably, MALAT1/miR-125a axis has been shown to discriminate sepsis patients based on their severity of diseases, organ damage, levels of inflammatory responses and mortality (14). Figure 1 depicts function of MALAT1 in sepsis-related events.
NEAT1 is another lncRNA whose participation in the pathophysiology of sepsis has been vastly investigated. This lncRNA could promote inflammatory responses and aggravate sepsis-associated hepatic damage through the Let-7a/TLR4 axis (15). Moreover, NEAT1 can accelerate progression of sepsis via miR-370-3p/TSP-1 axis (16). This lncRNA could also promote LPS-induced inflammatory responses in macrophages through regulation of miR-17-5p/TLR4 axis (17). NEAT1 silencing could suppress immune responses during sepsis through miR‐125/MCEMP1 axis (18). Figure 2 shows the function of NEAT1 in sepsis-related events. Several other lncRNAs have also been found to influence course of sepsis through modulation of immune responses (Table 1).
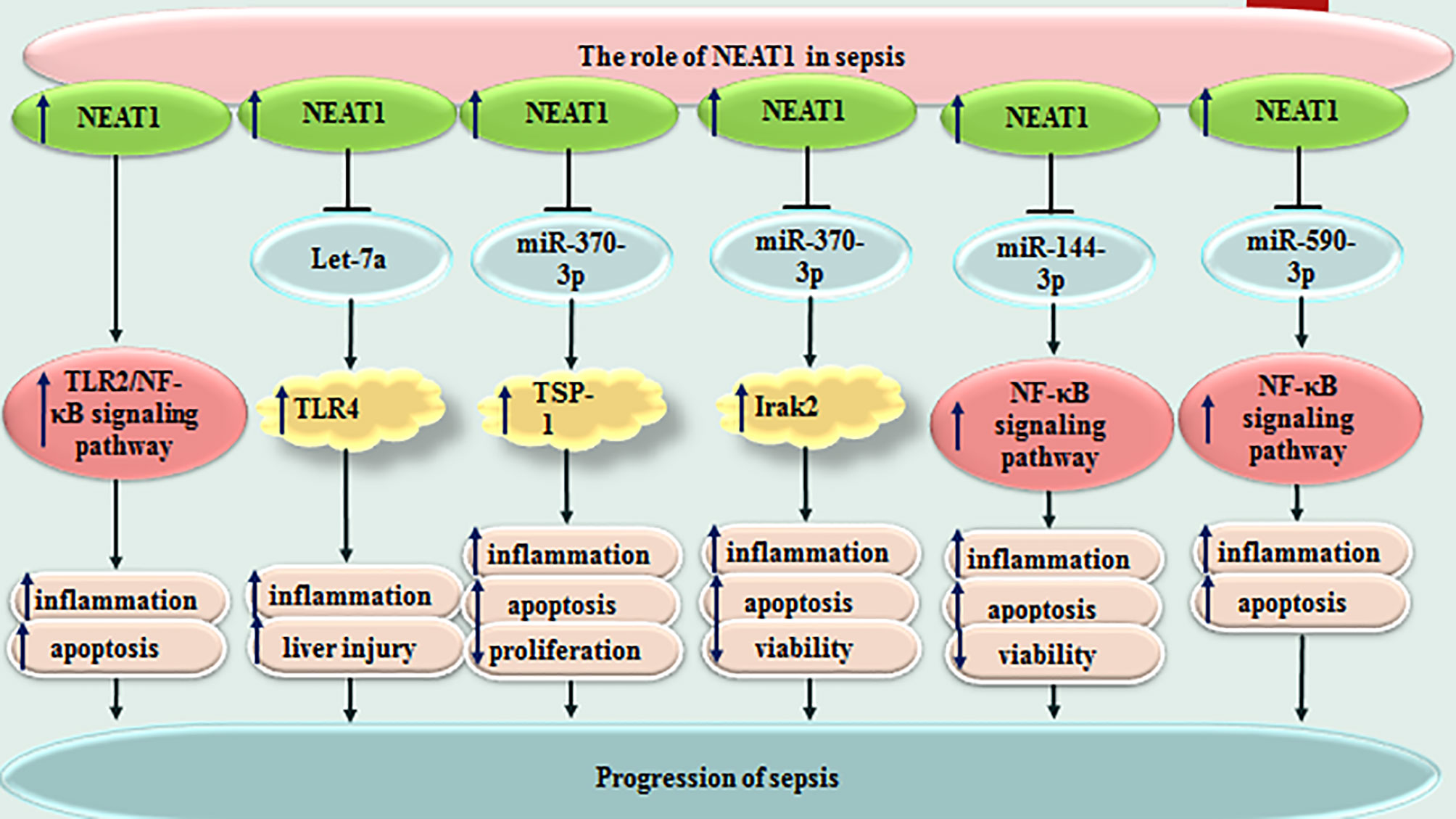
Figure 2 Function of NEAT1 in sepsis-related events. Several other lncRNAs have also been found to influence course of sepsis through modulation of immune responses (Table 1).
miRNAs and Sepsis
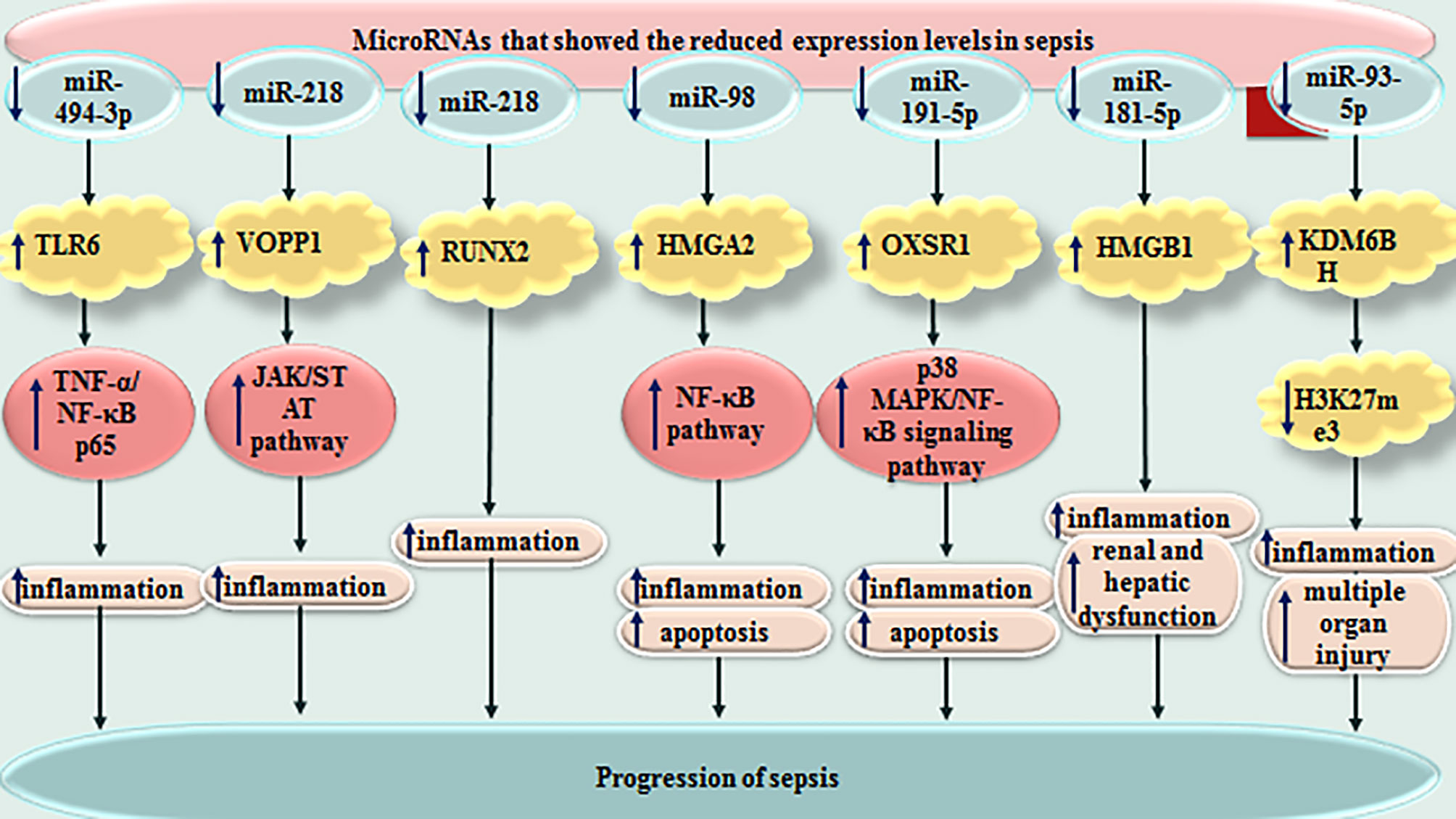
miRNAs have sizes about 22 nucleotides and regulate expression of genes through binding with different regions of target mRNAs, particularly their 3’ UTR. They can either degrade target mRNA or suppress its translation. Several miRNAs have been found to influence course of sepsis. Altered expression of these small-sized transcripts has been reported in sepsis by numerous research groups. For instance, plasma levels of miR-494-3p have been shown to be decreased in sepsis patients compared with healthy controls in correlation with up-regulation of TLR6. Expression level of miR-494-3p has been decreased in LPS-induced RAW264.7 cells, parallel with up-regulation of TLR6 and TNF-α. Forced over-expression of miR-494-3p in RAW264.7 cells could reduce TNF-α level and suppress translocation of NF-κB p65 to the nucleus. TLR6 has been shown to be targeted by miR-494-3p. Taken together, miR-494-3p could attenuate sepsis-associated inflammatory responses through influencing expression of TLR6 (132). miR-218 is another miRNA which participates in the pathoetiology of sepsis. This miRNA could reduce inflammatory responses in the sepsis through decreasing expression of VOPP1 via JAK/STAT axis (133).
miR-122 is another important miRNA in the sepsis which has superior diagnostic power compared with CRP and total leucocytes count for distinguishing sepsis from wound infection. miR-122 has also been found to be a prognostic marker for sepsis, albeit with poor specificity and accuracy values (134).
In the mice model of sepsis, decreased levels of miR-208a-5p and increased levels of SOCS2 has been associated with enhanced activity of SOD, while reduction in LDH and MDA activities. Moreover, down-regulation of miR-208a-5p has been associated with low levels TNF-α, IL-6, NF-κB p65 and HIF-1α in this animal model. miR-208a-5p silencing could decrease the extent of mitochondria swelling, and inhibit apoptosis of cardiomyocytes in animal model of sepsis. Taken together, miR-208a-5p suppression has been suggested as a modality to attenuate sepsis-related myocardial damage. This function is mediated through NF-κB/HIF-1α axis (135).
miR-21 is another miRNA whose role in sepsis has been investigated by several groups. Down-regulation of miR-21 has been shown to inhibit inflammasome activation, ASC pyroptosome, LPS-induced pyroptosis and septic shock in one study (136). On the other hand, another study in animal models of sepsis has shown that up-regulation of miR-21 reduced inflammation and apoptosis (137). Similarly, βMSCs-derived exosomes have been shown to reduce symptoms in septic mice and improve their survival rate through up-regulation of miR-21 (138).
miR-328 is another miRNA which is dysregulated in sepsis patients as well as animal models of sepsis. Serum levels of this miRNA could properly differentiate sepsis from normal conditions. Thus, miR-328 has been suggested as a diagnostic biomarker for sepsis. Moreover, down-regulation of miR-328 could amend sepsis-related heart dysfunction and inflammatory responses in this tissue (139). miR-452 is another miRNA with diagnostic applications in sepsis. Notably, serum and urinary levels of this miRNA have been suggested as possible markers for early diagnosis of sepsis-associated acute kidney injury, since expression of this miRNA has been higher in sepsis patients with acute kidney injury compared with those without this condition (140) (Table 2). Figure 3 depicts miRNAs that are down-regulated in sepsis.
CircRNAs and sepsis
CircRNAs are a recently appreciated group of non-coding RNAs with enclosed circular configuration formed by covalent bonds between two ends of linear transcripts. However, some of these transcripts have been shown to produce proteins. They mostly exert regulatory functions in the transcriptome. Impact of circRNAs in the sepsis has been assessed by several groups (303). For instance, circC3P1 has been shown to attenuate production of inflammatory cytokines and decrease cell apoptosis in sepsis-associated acute lung injury via influencing expression of miR‐21 (304).
A microarray-based has shown differential expression of 132 circRNAs between sepsis patients and healthy controls among them have been hsa_circRNA_104484 and hsa_circRNA_104670 whose up-regulation in sepsis serum exosomes has been verified been RT-PCR. Expression levels of these two circRNAs have been suggested as diagnostic biomarkers for sepsis (305).
CircVMA21 is another circRNA that has been shown to ameliorate sepsis‐related acute kidney injury through modulation of oxidative stress and inflammatory responses via miR‐9‐3p/SMG1 axis (306). Circ_0114428/miR-495-3p/CRBN axis is another molecular axis which is involved in the pathoetiology of sepsis‐related acute kidney injury (307). Moreover, expression levels of circPRKCI have been correlated with sepsis risk, severity of sepsis and mortality during a period of 28 days (308). Table 3 summarizes the role of circRNAs in sepsis.
Discussion
A vast body of literature points to the involvement of lncRNAs, miRNAs and circRNAs in the pathoetiology of sepsis-related complications. NEAT1, MALAT1, MEG3, THRIL, XIST, CRNDE, ZFAS1, HULC, MIAT and TUG1 are among lncRNAs with the strongest evidence for their participation in this process. NEAT1 as the mostly assessed lncRNA in this regard has been shown to act as a molecular sponge for let-7a, let-7b-5p, miR-370-3p, miR-124, miR-125, miR-17-5p, miR-16-5p, miR-93-5p, miR-370-3p, miR-144-3p, miR-944, miR495-3p, miR-22-3p, miR-31-5p and miR-590-3p. Through sequestering these miRNAs, NEAT1 can affect several molecular pathways in the course of sepsis. It can enhance immune responses and the related injury in target organs, thus participating in sepsis-related multiple organ damage.
Similar to lncRNAs, circRNAs influence course of sepsis mainly through acting as molecular sponges for miRNAs. circC3P1/miR-21, circVMA21/miR-9, circVMA21/miR-199a-5p, circ-PRKCI/miR-545, circPRKCI/miR-106b-5p, circDNMT3B/miR-20b-5p, circ_0114428/miR-495-3p, circ_Ttc3/miR-148a, circPRKCI/miR-454, circ-Fryl/miR-490-3p, circ_0091702/miR-182, circTLK1/miR-106a-5p, circFADS2/miR-15a-5p, circ_0091702/miR-545-3p, hsa_circ_0068,888/miR-21-5p, circPTK2/miR-181c-5p, circ-FANCA/miR-93-5p and circANKRD36/miR-330 are among circRNA/miRNA axes which are involved in the pathophysiology of sepsis-related conditions.
NF‐κB, PI3K/AKT, JAK/STAT and Wnt/β‐catenin pathways are the most important pathways being regulated by lncRNAs, circRNAs and miRNAs in the context of sepsis. These transcripts, particularly miRNAs can be used as diagnostic or prognostic markers in sepsis. Expression levels of these regulatory transcripts might be used for diagnosis of organ specific damages during the course of sepsis.
In general, the pathophysiology of sepsis is considered as an initial hyperinflammatory phase (“cytokine storm”) followed by a protracted immunosuppressive phase. Since no data is available about the differential expression of non-coding RNAs during these two distinct phases, future studies are needed to evaluate expression patterns of non-coding RNAs in these two phases. It is possible that some of the non-coding RNAs that suppress the immune response could be used as biomarkers to indicate the immunoparalysis in sepsis.
From a therapeutic point of view, several in vitro and in vivo studies have shown that up-regulation/silencing of circRNAs, lncRNAs and miRNAs can ameliorate the pathologic events in the target organs, particularly heart and kidney during sepsis. Yet, this field is still in its infancy needing verification in additional animal models and cell lines. Moreover, since sepsis is an emergency situation, any therapeutic option should be verified in terms of bioavailability, efficiency and instant amelioration of pathological events.
Since the pathoetiology of sepsis-related complications is not completely understood, high throughput sequencing strategies focusing on different classes of non-coding as well coding RNAs are necessary to find the complicated networks between these transcripts in the context of sepsis.
Author Contributions
SG-F wrote the draft and revised it. MT designed and supervised the study. NA, BH, and TK collected the data and designed the figures and tables. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zarjou A, Agarwal A. Sepsis and Acute Kidney Injury. J Am Soc Nephrol (2011) 22(6):999–1006. doi: 10.1681/ASN.2010050484
2. Rossaint J, Zarbock A. Pathogenesis of Multiple Organ Failure in Sepsis. Crit Rev Immunol (2015) 35(4):277–91. doi: 10.1615/CritRevImmunol.2015015461
3. Li C, Wu J, Li Y, Xing G. Cytoprotective Effect of Heat Shock Protein 27 Against Lipopolysaccharide-Induced Apoptosis of Renal Epithelial HK-2 Cells. Cell Physiol Biochem (2017) 41(6):2211–20. doi: 10.1159/000475636
4. Ye H-Y, Jin J, Jin L-W, Chen Y, Zhou Z-H, Li Z-Y. Chlorogenic Acid Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Inhibiting TLR4/NF-κb Signal Pathway. Inflammation (2017) 40(2):523–9. doi: 10.1007/s10753-016-0498-9
5. Zhang X, Wang W, Zhu W, Dong J, Cheng Y, Yin Z, et al. Mechanisms and Functions of Long Non-Coding Rnas at Multiple Regulatory Levels. Int J Mol Sci (2019) 20(22):5573. doi: 10.3390/ijms20225573
6. Flores-Concha M, Oñate N. Long Non-Coding Rnas in the Regulation of the Immune Response and Trained Immunity. Front Genet (2020) 11:718. doi: 10.3389/fgene.2020.00718
7. Qiu N, Xu X, He Y. Lncrna TUG1 Alleviates Sepsis-Induced Acute Lung Injury by Targeting Mir-34b-5p/GAB1. BMC Pulmonary Med (2020) 20(1):1–12. doi: 10.1186/s12890-020-1084-3
8. Li N, Wu S, Yu L. The Associations of Long Non-Coding RNA Taurine Upregulated Gene 1 and Microrna-223 With General Disease Severity and Mortality Risk in Sepsis Patients. Med (2020) 99(50). doi: 10.1097/MD.0000000000023444
9. Ge X, Liu W, Zhao W, Feng S, Duan A, Ji C, et al. Exosomal Transfer of LCP1 Promotes Osteosarcoma Cell Tumorigenesis and Metastasis by Activating the JAK2/STAT3 Signaling Pathway. Mol Ther Nucleic Acids (2020) 21:900–15. doi: 10.1016/j.omtn.2020.07.025
10. Zhou Y-L, Yang S-H, Zhang C, Zhang B, Yang X-J. Lncrna MALAT1 Modulates the Immunoreaction of Rats With Lipopolysaccharide-Induced Sepsis by Targeting the Mir-146a/NF-κb P65 Pathway. Sichuan da xue xue bao Yi xue ban= J Sichuan Univ Med Sci Ed (2018) 49(6):865–70.
11. Li Z, Liu S, Li X, Zhao W, Li J, Xu Y. Circular RNA in Schizophrenia and Depression. Front Psychiatry (2020) 11. doi: 10.3389/fpsyt.2020.00392
12. Wei S, Liu Q. Long Noncoding RNA MALAT1 Modulates Sepsis-Induced Cardiac Inflammation Through the Mir-150-5p/NF-κb Axis. Int J Clin Exp Pathol (2019) 12(9):3311.
13. Yu Z, Rayile A, Zhang X, Li Y, Zhao Q. Ulinastatin Protects Against Lipopolysaccharide-Induced Cardiac Microvascular Endothelial Cell Dysfunction via Downregulation of Lncrna MALAT1 and EZH2 in Sepsis. Int J Mol Med (2017) 39(5):1269–76. doi: 10.3892/ijmm.2017.2920
14. Liu W, Geng F, Yu L. Long Non-Coding RNA MALAT1/Microrna 125a Axis Presents Excellent Value in Discriminating Sepsis Patients and Exhibits Positive Association With General Disease Severity, Organ Injury, Inflammation Level, and Mortality in Sepsis Patients. J Clin Lab Anal (2020) 34(6):e23222. doi: 10.1002/jcla.23222
15. Zhang C-C, Niu F. Lncrna NEAT1 Promotes Inflammatory Response in Sepsis-Induced Liver Injury via the Let-7a/TLR4 Axis. Int Immunopharmacol (2019) 75:105731. doi: 10.1016/j.intimp.2019.105731
16. Wu X, Fang Y, Zheng F, Zhang Y, Li Q. Lncrna NEAT1 Facilitates the Progression of Sepsis Through Up-Regulating TSP-1 via Sponging Mir-370-3p. Eur Rev Med Pharmacol Sci (2020) 24(1):333–44. doi: 10.26355/eurrev_202001_19931
17. Li Y, Guo W, Cai Y. NEAT1 Promotes LPS-Induced Inflammatory Injury in Macrophages by Regulating Mir-17-5p/TLR4. Open Med (2020) 15(1):38–49. doi: 10.1515/med-2020-0007
18. Chen JX, Xu X, Zhang S. Silence of Long Noncoding RNA NEAT1 Exerts Suppressive Effects on Immunity During Sepsis by Promoting Microrna-125-Dependent MCEMP1 Downregulation. IUBMB Life (2019) 71(7):956–68. doi: 10.1002/iub.2033
19. Dong Y, Fan G, Li Y, Zhou Q. TUG1 Represses Apoptosis, Autophagy, and Inflammatory Response by Regulating Mir-27a-3p/SLIT2 in LPS-Treated Vascular Endothelial Cells. J Surg Res (2020) 256:345–54. doi: 10.1016/j.jss.2020.05.102
20. Xie W, Chen L, Chen L, Kou Q. Silencing of Long Non-Coding RNA MALAT1 Suppresses Inflammation in Septic Mice: Role of Microrna-23a in the Down-Regulation of MCEMP1 Expression. Inflamm Res (2020) 69(2):179–90. doi: 10.1007/s00011-019-01306-z
21. Lin L, Niu G, Zhang X. Influence of Lncrna MALAT1 on Septic Lung Injury in Mice Through P38 MAPK/P65 NF-κb Pathway. Eur Rev Med Pharmacol Sci (2019) 23(3):1296–304. doi: 10.26355/eurrev_201902_17025
22. Liang WJ, Zeng XY, Jiang SL, Tan HY, Yan MY, Yang HZ. Long Non-Coding RNA MALAT1 Sponges Mir-149 to Promote Inflammatory Responses of LPS-Induced Acute Lung Injury by Targeting Myd88. Cell Biol Int (2020) 44(1):317–26. doi: 10.1002/cbin.11235
23. Liu L, Yan L-N, Sui Z. Microrna-150 Affects Endoplasmic Reticulum Stress via MALAT1-Mir-150 Axis-Mediated NF-κb Pathway in LPS-Challenged Huvecs and Septic Mice. Life Sci (2021) 265:118744. doi: 10.1016/j.lfs.2020.118744
24. Huang X, Zhao M. High Expression of Long Non-Coding RNA MALAT1 Correlates With Raised Acute Respiratory Distress Syndrome Risk, Disease Severity, and Increased Mortality in Sepstic Patients. Int J Clin Exp Pathol (2019) 12(5):1877.
25. Zhuang Y, Xu D, Wang G, Sun J, Huang Y, Wang S. IL-6 Induced Lncrna MALAT1 Enhances TNF-α Expression in LPS-Induced Septic Cardiomyocytes via Activation of SAA3. Eur Rev Med Pharmacol Sci (2017) 21(2):302–9.
26. Geng F, Liu W, Yu L. Potential Role of Circulating Long Noncoding RNA MALAT1 in Predicting Disease Risk, Severity, and Patients' Survival in Sepsis. J Clin Lab Anal (2019) 33(8):e22968. doi: 10.1002/jcla.22968
27. Chen J, He Y, Zhou L, Deng Y, Si L. Long Non−Coding RNA MALAT1 Serves as an Independent Predictive Biomarker for the Diagnosis, Severity and Prognosis of Patients With Sepsis. Mol Med Rep (2020) 21(3):1365–73. doi: 10.3892/mmr.2020.10923
28. Gao F, Chen R, Xi Y, Zhao Q, Gao H. Long Noncoding RNA MALAT1 Regulates Sepsis in Patients With Burns by Modulating Mir−214 With TLR5. Mol Med Rep (2019) 19(5):3756–66. doi: 10.3892/mmr.2019.10028
29. Sun F, Yuan W, Wu H, Chen G, Sun Y, Yuan L, et al. Lncrna KCNQ1OT1 Attenuates Sepsis-Induced Myocardial Injury via Regulating Mir-192-5p/XIAP Axis. Exp Biol Med (2020) 245(7):620–30. doi: 10.1177/1535370220908041
30. Chen T, Zhu C, Ye C. Lncrna CYTOR Attenuates Sepsis-Induced Myocardial Injury via Regulating Mir-24/XIAP. Cell Biochem Funct (2020) 38(7):976–85. doi: 10.1002/cbf.3524
31. Liu F, Hu S, Zhao N, Shao Q, Li Y, Jiang R, et al. Lncrna-5657 Silencing Alleviates Sepsis-Induced Lung Injury by Suppressing the Expression of Spinster Homology Protein 2. Int Immunopharmacol (2020) 88:106875. doi: 10.1016/j.intimp.2020.106875
32. Han Y, Cai Y, Lai X, Wang Z, Wei S, Tan K, et al. Lncrna RMRP Prevents Mitochondrial Dysfunction and Cardiomyocyte Apoptosis via the Mir-1-5p/Hsp70 Axis in LPS-Induced Sepsis Mice. Inflammation (2020) 43(2):605–18. doi: 10.1007/s10753-019-01141-8
33. Yin J, Han B, Shen Y. Lncrna NEAT1 Inhibition Upregulates Mir-16-5p to Restrain the Progression of Sepsis-Induced Lung Injury via Suppressing BRD4 in a Mouse Model. Int Immunopharmacol (2021) 97:107691. doi: 10.1016/j.intimp.2021.107691
34. Wang S, Liu G, Xian H, Si J, Qi S, Yu Y. Lncrna NEAT1 Alleviates Sepsis-Induced Myocardial Injury by Regulating the TLR2/NF-κb Signaling Pathway. Eur Rev Med Pharmacol Sci (2019) 23(11):4898–907. doi: 10.26355/eurrev_201906_18078
35. Zhou H, Wang X, Zhang B. Depression of Lncrna NEAT1 Antagonizes LPS-Evoked Acute Injury and Inflammatory Response in Alveolar Epithelial Cells via HMGB1-RAGE Signaling. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/8019467
36. Gao C, Zou X, Chen H, Shang R, Wang B. Long Non-Coding RNA Nuclear Paraspeckle Assembly Transcript 1 (NEAT1) Relieves Sepsis-Induced Kidney Injury and Lipopolysaccharide (LPS)-Induced Inflammation in HK-2 Cells. Med Sci Monit: Int Med J Exp Clin Res (2020) 26:e921906–1. doi: 10.12659/MSM.921906
37. Wang W, Guo Z-H. Downregulation of Lncrna NEAT1 Ameliorates LPS-Induced Inflammatory Responses by Promoting Macrophage M2 Polarization via Mir-125a-5p/TRAF6/TAK1 Axis. Inflammation (2020) 43(4):1548–60. doi: 10.1007/s10753-020-01231-y
38. Yang J, Wu L, Liu S, Hu X, Wang Q, Fang L. Long Non-Coding RNA NEAT1 Promotes Lipopolysaccharide-Induced Injury in Human Tubule Epithelial Cells by Regulating Mir-93-5p/TXNIP Axis. Med Microbiol Immunol (2021) 210(2):121–32. doi: 10.1007/s00430-021-00705-6
39. Xiao T, Sun C, Xiao Y, Li Y. Lncrna NEAT1 Mediates Sepsis Progression by Regulating Irak2 via Sponging Mir-370-3p. Biol Open (2020) 9(6):bio049353. doi: 10.1242/bio.049353
40. Wei J, Wu C, Chen J, Shang F, Guo S, Zhang X, et al. Lncrna NEAT1 Promotes the Progression of Sepsis-Induced Myocardial Cell Injury by Sponging Mir-144-3p. Eur Rev Med Pharmacol Sci (2020) 24(2):851–61. doi: 10.26355/eurrev_202001_20069
41. Huang Q, Huang C, Luo Y, He F, Zhang R. Circulating Lncrna NEAT1 Correlates With Increased Risk, Elevated Severity and Unfavorable Prognosis in Sepsis Patients. Am J Emergency Med (2018) 36(9):1659–63. doi: 10.1016/j.ajem.2018.06.008
42. Chen C, Zhang H, Ge M, Ye J, Li R, Wang D. Lncrna NEAT1 Acts as a Key Regulator of Cell Apoptosis and Inflammatory Response by the Mir-944/TRIM37 Axis in Acute Lung Injury. J Pharmacol Sci (2021) 145(2):202–12. doi: 10.1016/j.jphs.2020.11.009
43. Huang S, Qian K, Zhu Y, Huang Z, Luo Q, Qing C. Diagnostic Value of the Lncrna NEAT1 in Peripheral Blood Mononuclear Cells of Patients With Sepsis. Dis Markers (2017) 2017. doi: 10.1155/2017/7962836
44. Liu B, Ren H, Chen J. Lncrna NEAT1 Correlates With Th1 and Th17 and Could Serve as an Assistant Biomarker in Sepsis. Biomarkers Med (2021) 1177–86. doi: 10.2217/bmm-2020-0906
45. Xia D, Yao R, Zhou P, Wang C, Xia Y, Xu S. Lncrna NEAT1 Reversed the Hindering Effects of Mir-495-3p/STAT3 Axis and Mir-211/PI3K/AKT Axis on Sepsis-Relevant Inflammation. Mol Immunol (2020) 117:168–79. doi: 10.1016/j.molimm.2019.10.009
46. Yang Y, Yang L, Liu Z, Wang Y, Yang J. Long Noncoding RNA NEAT 1 and its Target Microrna-125a in Sepsis: Correlation With Acute Respiratory Distress Syndrome Risk, Biochemical Indexes, Disease Severity, and 28-Day Mortality. J Clin Lab Anal (2020) 34(12):e23509. doi: 10.1002/jcla.23509
47. Liu W, Wang Y, Zheng Y, Chen X. Effects of Long Non-Coding RNA NEAT1 on Sepsis-Induced Brain Injury in Mice via NF-κb. Eur Rev Med Pharmacol Sci (2019) 23(9):3933–9. doi: 10.26355/eurrev_201905_17822
48. He F, Zhang C, Huang Q. Long Noncoding RNA Nuclear Enriched Abundant Transcript 1/Mirna-124 Axis Correlates With Increased Disease Risk, Elevated Inflammation, Deteriorative Disease Condition, and Predicts Decreased Survival of Sepsis. Med (2019) 98(32). doi: 10.1097/MD.0000000000016470
49. Feng Y, Liu J, Wu R, Yang P, Ye Z, Song F. NEAT1 Aggravates Sepsis-Induced Acute Kidney Injury by Sponging Mir-22-3p. Open Med (2020) 15(1):333–42. doi: 10.1515/med-2020-0401
50. Yang Y, Xue J, Qin L, Zhang J, Liu J, Yu J. Lncrna NEAT1 Promotes Inflammatory Response in Sepsis via the Mir-31-5p/POU2F1 Axis. Inflammation (2021) 44(4):1–11. doi: 10.1007/s10753-021-01436-9
51. Liu L, Liu F, Sun Z, Peng Z, You T, Yu Z. Lncrna NEAT1 Promotes Apoptosis and Inflammation in LPS−Induced Sepsis Models by Targeting Mir−590−3p. Exp Ther Med (2020) 20(4):3290–300. doi: 10.3892/etm.2020.9079
52. Fang Y, Hu J, Wang Z, Zong H, Zhang L, Zhang R, et al. Lncrna H19 Functions as an Aquaporin 1 Competitive Endogenous RNA to Regulate Microrna-874 Expression in LPS Sepsis. Biomed Pharmacother (2018) 105:1183–91. doi: 10.1016/j.biopha.2018.06.007
53. Shan B, Li J-Y, Liu Y-J, Tang X-B, Zhou Z, Luo L-X. Lncrna H19 Inhibits the Progression of Sepsis-Induced Myocardial Injury via Regulation of the Mir-93-5p/SORBS2 Axis. Inflammation (2021) 44(1):344–57. doi: 10.1007/s10753-020-01340-8
54. Yu B, Cui R, Lan Y, Zhang J, Liu B. Long Non-Coding RNA H19 as a Diagnostic Marker in Peripheral Blood of Patients With Sepsis. Am J Trans Res (2021) 13(4):2923.
55. Wang H-R, Guo X-Y, Liu X-Y, Song X. Down-Regulation of Lncrna CASC9 Aggravates Sepsis-Induced Acute Lung Injury by Regulating Mir-195-5p/PDK4 Axis. Inflammation Res (2020) 69(6):559–68. doi: 10.1007/s00011-020-01316-2
56. Zhang Z, Lv M, Wang X, Zhao Z, Jiang D, Wang L. Lncrna LUADT1 Sponges Mir-195 to Prevent Cardiac Endothelial Cell Apoptosis in Sepsis. Mol Med (2020) 26(1):1–8. doi: 10.1186/s10020-020-00228-5
57. Zhang Y, Zhang Y, Xia F, Yang A, Qian J, Zhao H, et al. Effect of Lncrna-MIAT on Kidney Injury in Sepsis Rats via Regulating Mir-29a Expression. Eur Rev Med Pharmacol Sci (2019) 23:10942–9. doi: 10.26355/eurrev_201912_19797
58. Xing P-C, An P, Hu G-Y, Wang D-L, Zhou M-J. Lncrna MIAT Promotes Inflammation and Oxidative Stress in Sepsis-Induced Cardiac Injury by Targeting Mir-330-5p/TRAF6/NF-κb Axis. Biochem Genet (2020) 58(5):783–800. doi: 10.1007/s10528-020-09976-9
59. Liu T, Liu J, Tian C, Wang H, Wen M, Yan M. Lncrna THRIL Is Upregulated in Sepsis and Sponges Mir-19a to Upregulate TNF-α in Human Bronchial Epithelial Cells. J Inflammation (2020) 17(1):1–7. doi: 10.1186/s12950-020-00259-z
60. Chen H, Hu X, Li R, Liu B, Zheng X, Fang Z, et al. Lncrna THRIL Aggravates Sepsis-Induced Acute Lung Injury by Regulating Mir-424/ROCK2 Axis. Mol Immunol (2020) 126:111–9. doi: 10.1016/j.molimm.2020.07.021
61. Wang Y, Fu X, Yu B, Ai F. Long Non-Coding RNA THRIL Predicts Increased Acute Respiratory Distress Syndrome Risk and Positively Correlates With Disease Severity, Inflammation, and Mortality in Sepsis Patients. J Clin Lab Anal (2019) 33(6):e22882. doi: 10.1002/jcla.22882
62. Song X, Li L, Zhao Y, Song Y. Down-Regulation of Long Non-Coding RNA XIST Aggravates Sepsis-Induced Lung Injury by Regulating Mir-16-5p. Hum Cell (2021) 34(5):1–11. doi: 10.1007/s13577-021-00542-y
63. Wang L, Cao QM. Long Non-Coding RNA XIST Alleviates Sepsis-Induced Acute Kidney Injury Through Inhibiting Inflammation and Cell Apoptosis via Regulating Mir-155-5p/WWC1 Axis. Kaohsiung J Med Sci (2021). doi: 10.1002/kjm2.12442
64. Shen C, Li J. Lncrna XIST Silencing Protects Against Sepsis-Induced Acute Liver Injury via Inhibition of BRD4 Expression. Inflammation (2021) 44(1):194–205. doi: 10.1007/s10753-020-01321-x
65. Xu G, Mo L, Wu C, Shen X, Dong H, Yu L, et al. The Mir-15a-5p-XIST-CUL3 Regulatory Axis Is Important for Sepsis-Induced Acute Kidney Injury. Ren Fail (2019) 41(1):955–66. doi: 10.1080/0886022X.2019.1669460
66. Liang D, Jin Y, Lin M, Xia X, Chen X, Huang A. Down-Regulation of Xist and Mir-7a-5p Improves LPS-Induced Myocardial Injury. Int J Med Sci (2020) 17(16):2570. doi: 10.7150/ijms.45408
67. Li M, Zhang Z, Liu B, Chen L, Wang M. Lncrna GAS5 Upregulates Mir-214 Through Methylation to Participate in Cell Apoptosis of Sepsis. Arch Physiol Biochem (2020) 1–6. doi: 10.1080/13813455.2020.1764051
68. Zhu Y, Sun A, Meng T, Li H. Protective Role of Long Noncoding RNA CRNDE in Myocardial Tissues From Injury Caused by Sepsis Through the Microrna-29a/SIRT1 Axis. Life Sci (2020) 255:117849. doi: 10.1016/j.lfs.2020.117849
69. Wang Y, Xu Z, Yue D, Zeng Z, Yuan W, Xu K. Linkage of Lncrna CRNDE Sponging Mir-181a-5p With Aggravated Inflammation Underlying Sepsis. Innate Immun (2020) 26(2):152–61. doi: 10.1177/1753425919880946
70. Sun B, Sui Y, Huang H, Zou X, Chen S, Yu Z. Effect of Lncrna CRNDE on Sepsis-Related Kidney Injury Through the TLR3/NF-κb Pathway. Eur Rev Med Pharmacol Sci (2019) 23(23):10489–97. doi: 10.26355/eurrev_201912_19688
71. Wu S, Qiu H, Wang Q, Cao Z, Wang J. Effects and Mechanism of Lncrna CRNDE on Sepsis-Induced Acute Kidney Injury. Anal Cell Pathol (2020) 2020. doi: 10.1155/2020/8576234
72. Wang J, Song J, Li Y, Shao J, Xie Z, Sun K. Down-Regulation of Lncrna CRNDE Aggravates Kidney Injury via Increasing Mir-181a-5p in Sepsis. Int Immunopharmacol (2020) 79:105933. doi: 10.1016/j.intimp.2019.105933
73. Li Y, Song J, Xie Z, Liu M, Sun K. Long Noncoding RNA Colorectal Neoplasia Differentially Expressed Alleviates Sepsis-Induced Liver Injury via Regulating Mir-126-5p. IUBMB Life (2020) 72(3):440–51. doi: 10.1002/iub.2230
74. Jiang Z, Zhang M, Fan Z, Sun W, Tang Y. Influence of Lncrna HOTAIR on Acute Kidney Injury in Sepsis Rats Through Regulating Mir-34a/Bcl-2 Pathway. Eur Rev Med Pharmacol Sci (2019) 23(8):3512–9. doi: 10.26355/eurrev_201904_17717
75. Yang W, Luo X, Liu Y, Xiong J, Xia H, Liu Y. Potential Role of Lncrna HULC/Mir−128−3p/RAC1 Axis in the Inflammatory Response During LPS−Induced Sepsis in HMEC−1 Cells. Mol Med Rep (2020) 22(6):5095–104. doi: 10.3892/mmr.2020.11601
76. Wang H, Feng Q, Wu Y, Feng L, Yuan H, Hou L, et al. Association of Circulating Long Non-Coding RNA HULC Expression With Disease Risk, Inflammatory Cytokines, Biochemical Index Levels, Severity-Assessed Scores, and Mortality of Sepsis. J Clin Lab Anal (2021) 35(3):e23656. doi: 10.1002/jcla.23656
77. Chen X, Song D. LPS Promotes the Progression of Sepsis by Activation of Lncrna HULC/Mir-204-5p/TRPM7 Network in Huvecs. Biosci Rep (2020) 40(6):BSR20200740. doi: 10.1042/BSR20200740
78. Chen Y, Fu Y, Song Y-F, Li N. Increased Expression of Lncrna UCA1 and HULC Is Required for Pro-Inflammatory Response During LPS Induced Sepsis in Endothelial Cells. Front Physiol (2019) 10:608. doi: 10.3389/fphys.2019.00608
79. Shen J, Liu L, Zhang F, Gu J, Pan G. Lncrna Tapsaki Promotes Inflammation Injury in HK-2 Cells and Urine Derived Sepsis-Induced Kidney Injury. J Pharm Pharmacol (2019) 71(5):839–48. doi: 10.1111/jphp.13049
80. Zeng Q, Wu J, Yang S. Circulating Lncrna ITSN1-2 Is Upregulated, and its High Expression Correlates With Increased Disease Severity, Elevated Inflammation, and Poor Survival in Sepsis Patients. J Clin Lab Anal (2019) 33(4):e22836. doi: 10.1002/jcla.22836
81. Wang W, Li Y, Zhi S, Li J, Miao J, Ding Z, et al. Lncrna-ROR/Microrna-185-3p/YAP1 Axis Exerts Function in Biological Characteristics of Osteosarcoma Cells. Genomics (2020) 113(1 Pt 2):450–61. doi: 10.1016/j.ygeno.2020.09.009
82. Zhang P, Yi L, Qu S, Dai J, Li X, Liu B, et al. The Biomarker TCONS_00016233 Drives Septic AKI by Targeting the Mir-22-3p/AIFM1 Signaling Axis. Mol Therapy-Nucleic Acids (2020) 19:1027–42. doi: 10.1016/j.omtn.2019.12.037
83. Qin G, Wei L, Jiang F, Li J, Zhang B, Pan D, et al. Lncrna NR024118 Is Downregulated in Sepsis and Inhibits LPS−Induced Apoptosis of Cardiomyocytes. Mol Med Rep (2021) 23(6):1–7. doi: 10.3892/mmr.2021.12073
84. Zhang C, Li J, Li H, Wang G, Wang Q, Zhang X, et al. Lncrna MIR155HG Accelerates the Progression of Sepsis via Upregulating MEF2A by Sponging Mir-194-5p. DNA Cell Biol (2021) 40(6):811–20. doi: 10.1089/dna.2021.0038
85. Wang J, Xin S, Yang R, Jiang J, Qiao Y. Knockdown of Lncrna LUCAT1 Attenuates Sepsis−Induced Myocardial Cell Injury by Sponging Mir-642a. Mamm Genome (2021) 32(6):1–9. doi: 10.1007/s00335-021-09890-4
86. Chen M, Guan Y, Li A, Zhao Y-Z, Zhang L, Zhang L, et al. Lncrna SOX2OT Mediates Mitochondrial Dysfunction in Septic Cardiomyopathy. DNA Cell Biol (2019) 38(11):1197–206. doi: 10.1089/dna.2019.4839
87. Deng J, Tan W, Luo Q, Lin L, Zheng L, Yang J. Long Non-Coding RNA MEG3 Promotes Renal Tubular Epithelial Cell Pyroptosis by Regulating the Mir-18a-3p/GSDMD Pathway in Lipopolysaccharide-Induced Acute Kidney Injury. Front Physiol (2021) 12. doi: 10.3389/fphys.2021.663216
88. Chen K, Shi X, Jin Y, Wang F, Shen Q, Xu W. High Lncrna MEG3 Expression Is Associated With High Mortality Rates in Patients With Sepsis and Increased Lipopolysaccharide−Induced Renal Epithelial Cell and Cardiomyocyte Apoptosis. Exp Ther Med (2019) 18(5):3943–7. doi: 10.3892/etm.2019.8049
89. Wu X, Chen D, Yu L. The Value of Circulating Long Non-Coding RNA Maternally Expressed Gene 3 as a Predictor of Higher Acute Respiratory Distress Syndrome Risk and 28-Day Mortality in Sepsis Patients. J Clin Lab Anal (2020) 34(11):e23488. doi: 10.1002/jcla.23488
90. Na L, Ding H, Xing E, Gao J, Liu B, Wang H, et al. Lnc-MEG3 Acts as a Potential Biomarker for Predicting Increased Disease Risk, Systemic Inflammation, Disease Severity, and Poor Prognosis of Sepsis via Interacting With Mir-21. J Clin Lab Anal (2020) 34(4):e23123. doi: 10.1002/jcla.23123
91. Du X, Tian D, Wei J, Yan C, Hu P, Wu X, et al. MEG3 Alleviated LPS-Induced Intestinal Injury in Sepsis by Modulating Mir-129-5p and Surfactant Protein D. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/8232734
92. Fang Y, Hu J, Wang Z, Zhang S, Zhang R, Sun L, et al. GAS5 Promotes Podocyte Injury in Sepsis by Inhibiting PTEN Expression. Eur Rev Med Pharmacol Sci (2018) 22(23):8423–30. doi: 10.26355/eurrev_201812_16541
93. Li L, He Y, He X-J, Bi M-R, Qi Y-H, Zhu W-W. Down-Regulation of Long Noncoding RNA LINC00472 Alleviates Sepsis-Induced Acute Hepatic Injury by Regulating Mir-373-3p/TRIM8 Axis. Exp Mol Pathol (2020) 117:104562. doi: 10.1016/j.yexmp.2020.104562
94. Wu H, Liu J, Li W, Liu G, Li Z. Lncrna-HOTAIR Promotes TNF-α Production in Cardiomyocytes of LPS-Induced Sepsis Mice by Activating NF-κb Pathway. Biochem Biophys Res Commun (2016) 471(1):240–6. doi: 10.1016/j.bbrc.2016.01.117
95. Shen J, Zhang J, Jiang X, Wang H, Pan G. Lncrna HOX Transcript Antisense RNA Accelerated Kidney Injury Induced by Urine-Derived Sepsis Through the Mir-22/High Mobility Group Box 1 Pathway. Life Sci (2018) 210:185–91. doi: 10.1016/j.lfs.2018.08.041
96. Alkhateeb T, Bah I, Kumbhare A, Youssef D, Yao ZQ, McCall CE, et al. Long Non-Coding RNA Hotairm1 Promotes S100A9 Support of MDSC Expansion During Sepsis. J Clin Cell Immunol (2020) 11(6).
97. Han D, Fang R, Shi R, Jin Y, Wang Q. Lncrna NKILA Knockdown Promotes Cell Viability and Represses Cell Apoptosis, Autophagy and Inflammation in Lipopolysaccharide-Induced Sepsis Model by Regulating Mir-140-5p/CLDN2 Axis. Biochem Biophys Res Commun (2021) 559:8–14. doi: 10.1016/j.bbrc.2021.04.074
98. Wu H, Wang J, Ma Z. Long Noncoding RNA HOXA-AS2 Mediates Microrna-106b-5p to Repress Sepsis-Engendered Acute Kidney Injury. J Biochem Mol Toxicol (2020) 34(4):e22453. doi: 10.1002/jbt.22453
99. Shi C, Zhao Y, Li Q, Li J. Lncrna SNHG14 Plays a Role in Sepsis-Induced Acute Kidney Injury by Regulating Mir-93. Mediators Inflamm (2021) 2021. doi: 10.1155/2021/5318369
100. Jia Y, Li Z, Cai W, Xiao D, Han S, Han F, et al. SIRT1 Regulates Inflammation Response of Macrophages in Sepsis Mediated by Long Noncoding RNA. Biochim Biophys Acta (BBA)-Molecular Basis Dis (2018) 1864(3):784–92. doi: 10.1016/j.bbadis.2017.12.029
101. Tan J, Fan J, He J, Zhao L, Tang H. Knockdown of Lncrna DLX6-AS1 Inhibits HK-2 Cell Pyroptosis via Regulating Mir-223-3p/NLRP3 Pathway in Lipopolysaccharide-Induced Acute Kidney Injury. J Bioenerg Biomembr (2020) 52(5):367–76. doi: 10.1007/s10863-020-09845-5
102. Wang M, Wei J, Shang F, Zang K, Ji T. Long Non−Coding RNA CASC2 Ameliorates Sepsis−Induced Acute Kidney Injury by Regulating the Mir−155 and NF−κb Pathway. Int J Mol Med (2020) 45(5):1554–62. doi: 10.3892/ijmm.2020.4518
103. Zhu L, Shi D, Cao J, Song L. Lncrna CASC2 Alleviates Sepsis-Induced Acute Lung Injury by Regulating the Mir-152-3p/PDK4 Axis. Immunol Invest (2021) 1–15. doi: 10.1080/08820139.2021.1928693
104. Xu Y, Shao B. Circulating Long Noncoding RNA ZNFX1 Antisense RNA Negatively Correlates With Disease Risk, Severity, Inflammatory Markers, and Predicts Poor Prognosis in Sepsis Patients. Med (2019) 98(9). doi: 10.1097/MD.0000000000014558
105. Chen D-D, Wang H-W, Cai X-J. Long Non-Coding RNA ZFAS1 Alleviates Sepsis-Induced Myocardial Injury via Target Mir-34b-5p/SIRT1. Innate Immun (2021) 27(5):377–87. doi: 10.1177/17534259211034221
106. Liu J-J, Li Y, Yang M-S, Chen R, Cen C-Q. SP1-Induced ZFAS1 Aggravates Sepsis-Induced Cardiac Dysfunction via Mir-590–3p/NLRP3-Mediated Autophagy and Pyroptosis. Arch Biochem Biophys (2020) 695:108611. doi: 10.1016/j.abb.2020.108611
107. An L, Yang T, Zhong Y, Yin Y, Li W, Gao H. Molecular Pathways in Sepsis-Induced Cardiomyocyte Pyroptosis: Novel Finding on Long Non-Coding RNA ZFAS1/Mir-138–5p/SESN2 Axis. Immunol Lett (2021) 238:47–56. doi: 10.1016/j.imlet.2021.07.003
108. Zhang Z, Yu T, Geng W. Long Non-Coding RNA CCHE1 Participates in Postoperative Distant Recurrence But Not Local Recurrence of Osteosarcoma Possibly by Interacting With ROCK1. BMC Musculoskeletal Disord (2020) 21(1):462. doi: 10.1186/s12891-020-3184-x
109. Xu X, Xu Y, Tao X, Liang G. Lncrna Mirt2 Upregulates Mir-1246 Through Methylation to Suppress LPS-Induced Lung Cell Apoptosis. Immun Inflamm Dis (2021) 9(3):695–701. doi: 10.1002/iid3.422
110. Liu X, Zhu N, Zhang B, Xu SB. Long Noncoding RNA TCONS_00016406 Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Regulating the Mir-687/PTEN Pathway. Front Physiol (2020) 11:622. doi: 10.3389/fphys.2020.00622
111. Zhang H, Li L, Xu L, Zheng Y. Clinical Significance of the Serum Lncrna NORAD Expression in Patients With Neonatal Sepsis and its Association With Mir-410-3p. J Inflammation Res (2021) 14:4181. doi: 10.2147/JIR.S315985
112. Gao Z, Huang D. Lncrna GAS5−Mediated Mir−23a−3p Promotes Inflammation and Cell Apoptosis by Targeting TLR4 in a Cell Model of Sepsis. Mol Med Rep (2021) 24(1):1–9. doi: 10.3892/mmr.2021.12149
113. Gui F, Peng H, Liu Y. Elevated Circulating lnc-ANRIL/Mir-125a Axis Level Predicts Higher Risk, More Severe Disease Condition, and Worse Prognosis of Sepsis. J Clin Lab Anal (2019) 33(6):e22917. doi: 10.1002/jcla.22917
114. Liu Y, Peng H, Gui F. Long Noncoding Plasmacytoma Variant Translocation 1 Facilitates the Surveillance of Acute Respiratory Distress Syndrome and Mortality Prediction in Sepsis. Biomarkers Med (2021) 15(6):401–12. doi: 10.2217/bmm-2020-0506
115. Zheng S, Li W, Liao W, Huang C, Zhou M, Zheng Y, et al. Silencing of Lncrna-PVT1 Ameliorates Lipopolysaccharide-Induced Inflammation in THP-1-Derived Macrophages via Inhibition of the P38 MAPK Signaling Pathway. Ann palliative Med (2021) 10(6):6410–8. doi: 10.21037/apm-21-1078
116. Deng L-T, Wang Q-L, Yu C, Gao M. Lncrna PVT1 Modulates NLRP3−Mediated Pyroptosis in Septic Acute Kidney Injury by Targeting Mir−20a−5p. Mol Med Rep (2021) 23(4):1–. doi: 10.3892/mmr.2021.11910
117. Luo Y-Y, Yang Z-Q, Lin X-F, Zhao F-L, Tu H-T, Wang L-J, et al. Knockdown of Lncrna PVT1 Attenuated Macrophage M1 Polarization and Relieved Sepsis Induced Myocardial Injury via Mir-29a/HMGB1 Axis. Cytokine (2021) 143:155509. doi: 10.1016/j.cyto.2021.155509
118. Chen J, Gu X, Zhou L, Wang S, Zhu L, Huang Y, et al. Long Non−Coding RNA−HOTAIR Promotes the Progression of Sepsis by Acting as a Sponge of Mir−211 to Induce IL−6R Expression. Exp Ther Med (2019) 18(5):3959–67. doi: 10.3892/etm.2019.8063
119. Ni S-Y, Xu W-T, Liao G-Y, Wang Y-L, Li J. Lncrna HOTAIR Promotes LPS-Induced Inflammation and Apoptosis of Cardiomyocytes via Lin28-Mediated PDCD4 Stability. Inflammation (2021) 44(4):1–12. doi: 10.1007/s10753-021-01431-0
120. Huang J, Liu Y, Xie Q, Liang G, Kong H, Liu M, et al. Expression Profiling of Long Noncoding RNA and Messenger RNA in a Cecal Ligation and Puncture-Induced Colon Injury Mouse Model. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/8925973
121. Zhang X, Huang Z, Wang Y, Wang T, Li J, Xi P. Long Non-Coding RNA RMRP Contributes to Sepsis-Induced Acute Kidney Injury. Yonsei Med J (2021) 62(3):262. doi: 10.3349/ymj.2021.62.3.262
122. Gao H, Ma H, Gao M, Chen A, Zha S, Yan J. Long Non-Coding RNA GAS5 Aggravates Myocardial Depression in Mice With Sepsis via the Microrna-449b/HMGB1 Axis and the NF-κb Signaling Pathway. Biosci Rep (2021) 41(4):BSR20201738. doi: 10.1042/BSR20201738
123. Han X, Yuan Z, Jing Y, Zhou W, Sun Y, Xing J. Knockdown of Lncrna Tapsaki Alleviates LPS-Induced Injury in HK-2 Cells Through the Mir-205/IRF3 Pathway. Open Med (2021) 16(1):581–90. doi: 10.1515/med-2021-0204
124. Sun J, Xin K, Leng C, Ge J. Down-Regulation of SNHG16 Alleviates the Acute Lung Injury in Sepsis Rats Through Mir-128-3p/HMGB3 Axis. BMC Pulmonary Med (2021) 21(1):1–14. doi: 10.1186/s12890-021-01552-0
125. Zhao H, Chen B, Li Z, Wang B, Li L. Long Noncoding RNA DANCR Suppressed Lipopolysaccharide-Induced Septic Acute Kidney Injury by Regulating Mir-214 in HK-2 Cells. Med Sci monitor: Int Med J Exp Clin Res (2020) 26:e921822–1. doi: 10.12659/MSM.921822
126. Hu Q, Zen W, Zhang M, Wang Z, Cui W, Liu Y, et al. Long Non-Coding RNA CASC2 Overexpression Ameliorates Sepsis-Associated Acute Kidney Injury by Regulating Mir-545-3p/PPARA Axis. J Surg Res (2021) 265:223–32. doi: 10.1016/j.jss.2021.03.047
127. Luo S, Huang X, Liu S, Zhang L, Cai X, Chen B. Long Non-Coding RNA Small Nucleolar RNA Host Gene 1 Alleviates Sepsis-Associated Myocardial Injury by Modulating the Mir-181a-5p/XIAP Axis In Vitro. Ann Clin Lab Sci (2021) 51(2):231–40.
128. Yang N, Wang H, Zhang L, Lv J, Niu Z, Liu J, et al. Long Non-Coding RNA SNHG14 Aggravates LPS-Induced Acute Kidney Injury Through Regulating Mir-495-3p/HIPK1. Acta Biochim Biophys Sin (2021) 53(6):719–28. doi: 10.1093/abbs/gmab034
129. Hu M, Wei J, Yang L, Xu J, He Z, Li H, et al. Linc-KIAA1737–2 Promoted LPS-Induced HK-2 Cell Apoptosis by Regulating Mir-27a-3p/TLR4/NF-κb Axis. J Bioenerg Biomembr (2021) 53(4):1–11. doi: 10.1007/s10863-021-09897-1
130. Fu D, Zhou K, Liu J, Zheng P, Li P, Cheng W, et al. Long Non-Coding RNA Plncrna-1 Regulates Cell Proliferation, Apoptosis, and Autophagy in Septic Acute Kidney Injury by Regulating BCL2. Int J Clin Exp Pathol (2018) 11(1):314.
131. Wang B, Sun Q, Ye W, Li L, Jin P. Long Non-Coding RNA CDKN2B-AS1 Enhances LPS-Induced Apoptotic and Inflammatory Damages in Human Lung Epithelial Cells via Regulating the Mir-140-5p/TGFBR2/Smad3 Signal Network. BMC pulmonary Med (2021) 21(1):1–12. doi: 10.1186/s12890-021-01561-z
132. Wang H, Li Y, Wang Y, Li H, Dou L. Microrna-494-3p Alleviates Inflammatory Response in Sepsis by Targeting TLR6. Eur Rev Med Pharmacol Sci (2019) 23(7):2971–7. doi: 10.26355/eurrev_201904_17578
133. Li J, Zhang H, Zuo Y. Microrna-218 Alleviates Sepsis Inflammation by Negatively Regulating VOPP1 via JAK/STAT Pathway. Eur Rev Med Pharmacol Sci (2018) 22(17):5620–6. doi: 10.26355/eurrev_201809_15827
134. Abou El-Khier NT, Zaki ME, Alkasaby NM. Study of Microrna-122 as a Diagnostic Biomarker of Sepsis. Egypt J Immunol (2019) 26(2):105–16.
135. Ouyang H, Tan Y, Li Q, Xia F, Xiao X, Zheng S, et al. Microrna-208-5p Regulates Myocardial Injury of Sepsis Mice via Targeting SOCS2-Mediated NF-κb/Hif-1α Pathway. Int Immunopharmacol (2020) 81:106204. doi: 10.1016/j.intimp.2020.106204
136. Xue Z, Xi Q, Liu H, Guo X, Zhang J, Zhang Z, et al. Mir-21 Promotes NLRP3 Inflammasome Activation to Mediate Pyroptosis and Endotoxic Shock. Cell Death Dis (2019) 10(6):1–13. doi: 10.1038/s41419-019-1713-z
137. Jia P, Wu X, Dai Y, Teng J, Fang Y, Hu J, et al. Microrna-21 Is Required for Local and Remote Ischemic Preconditioning in Multiple Organ Protection Against Sepsis. Crit Care Med (2017) 45(7):e703–e10. doi: 10.1097/CCM.0000000000002363
138. Yao M, Cui B, Zhang W, Ma W, Zhao G, Xing L. Exosomal Mir-21 Secreted by IL-1β-Primed-Mesenchymal Stem Cells Induces Macrophage M2 Polarization and Ameliorates Sepsis. Life Sci (2021) 264:118658. doi: 10.1016/j.lfs.2020.118658
139. Sun B, Luan C, Guo L, Zhang B, Liu Y. Low Expression of Microrna-328 can Predict Sepsis and Alleviate Sepsis-Induced Cardiac Dysfunction and Inflammatory Response. Braz J Med Biol Res (2020) 53. doi: 10.1590/1414-431x20209501
140. Liu Z, Yang D, Gao J, Xiang X, Hu X, Li S, et al. Discovery and Validation of Mir-452 as an Effective Biomarker for Acute Kidney Injury in Sepsis. Theranostics (2020) 10(26):11963. doi: 10.7150/thno.50093
141. Zhou M, Zhang L, Song M, Sun W. Microrna-218 Prevents Lung Injury in Sepsis by Inhibiting RUNX2. Eur Rev Med Pharmacol Sci (2018) 22(23):8438–46. doi: 10.26355/eurrev_201812_16543
142. Na L, Ding H, Xing E, Zhang Y, Gao J, Liu B, et al. The Predictive Value of Microrna-21 for Sepsis Risk and its Correlation With Disease Severity, Systemic Inflammation, and 28-Day Mortality in Sepsis Patients. J Clin Lab Anal (2020) 34(3):e23103. doi: 10.1002/jcla.23103
143. Lin R, Hu H, Li L, Chen G, Luo L, Rao P. The Potential of Microrna-126 in Predicting Disease Risk, Mortality of Sepsis, and its Correlation With Inflammation and Sepsis Severity. J Clin Lab Anal (2020) 34(9):e23408. doi: 10.1002/jcla.23408
144. Wang Q, Feng Q, Zhang Y, Zhou S, Chen H. Decreased Microrna 103 and Microrna 107 Predict Increased Risks of Acute Respiratory Distress Syndrome and 28-Day Mortality in Sepsis Patients. Med (2020) 99(25). doi: 10.1097/MD.0000000000020729
145. Gao Y, Zhang N, Lv C, Li N, Li X, Li W. Lncrna SNHG1 Knockdown Alleviates Amyloid-β-Induced Neuronal Injury by Regulating ZNF217 via Sponging Mir-361-3p in Alzheimer’s Disease. J Alzheimer's Dis (2020) Preprint):1–14. doi: 10.3233/JAD-191303
146. Zhu J, Lin X, Yan C, Yang S, Zhu Z. Microrna-98 Protects Sepsis Mice From Cardiac Dysfunction, Liver and Lung Injury by Negatively Regulating HMGA2 Through Inhibiting NF-κb Signaling Pathway. Cell Cycle (2019) 18(16):1948–64. doi: 10.1080/15384101.2019.1635869
147. Li S, Zhao D, Cui J, Wang L, Ma X, Li Y. Correlation of Microrna-125a/B With Acute Respiratory Distress Syndrome Risk and Prognosis in Sepsis Patients. J Clin Lab Anal (2020) 34(3):e23098. doi: 10.1002/jcla.23098
148. Liu Y, Guan H, Zhang J-L, Zheng Z, Wang H-T, Tao K, et al. Acute Downregulation of Mir-199a Attenuates Sepsis-Induced Acute Lung Injury by Targeting SIRT1. Am J Physiol-Cell Physiol (2018) 314(4):C449–C55. doi: 10.1152/ajpcell.00173.2017
149. Guo H, Tang L, Xu J, Lin C, Ling X, Lu C, et al. Microrna-495 Serves as a Diagnostic Biomarker in Patients With Sepsis and Regulates Sepsis-Induced Inflammation and Cardiac Dysfunction. Eur J Med Res (2019) 24(1):1–9. doi: 10.1186/s40001-019-0396-3
150. Shen Y, Yu J, Jing Y, Zhang J. Mir-106a Aggravates Sepsis-Induced Acute Kidney Injury by Targeting THBS2 in Mice Model1. Acta cirurgica Bras (2019) 34. doi: 10.1590/s0102-865020190060000002
151. Song Y, Dou H, Li X, Zhao X, Li Y, Hou Y. Exosomal Mir-146a Contributes to the En-Hanced Therapeutic Efficacy of IL-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells (2017) 35(5):1208–21. doi: 10.1002/stem.2564
152. Sun W, Li H, Gu J. Up-Regulation of Microrna-574 Attenuates Lipopolysaccharide-or Cecal Ligation and Puncture-Induced Sepsis Associated With Acute Lung Injury. Cell Biochem Funct (2020) 38(7):847–58. doi: 10.1002/cbf.3496
153. Zheng P, Feng X, Deng Q, Guo R, Li W, Hayakumo S, et al. Study on the Efficacy of Nanoantibiotics in Rats With Sepsis Based on Microrna-195 and TGF-β1/Smads Signaling Pathway. J Nanosci Nanotechnol (2021) 21(2):1357–64. doi: 10.1166/jnn.2021.18646
154. Chen L, Xie W, Wang L, Zhang X, Liu E, Kou Q. Mirna-133a Aggravates Inflammatory Responses in Sepsis by Targeting SIRT1. Int Immunopharmacol (2020) 88:106848. doi: 10.1016/j.intimp.2020.106848
155. Qin Y, Wang G, Peng Z. Microrna-191-5p Diminished Sepsis-Induced Acute Kidney Injury Through Targeting Oxidative Stress Responsive 1 in Rat Models. Biosci Rep (2019) 39(8):BSR20190548. doi: 10.1042/BSR20190548
156. Chen L, Yu L, Zhang R, Zhu L, Shen W. Correlation of Microrna-146a/B With Disease Risk, Biochemical Indices, Inflammatory Cytokines, Overall Disease Severity, and Prognosis of Sepsis. Medicine (2020) 99(22):e19754. doi: 10.1097/MD.0000000000019754
157. Zou Q, Zhao S, Wu Q, Wang H, He X, Liu C. Correlation Analysis of Microrna-126 Expression in Peripheral Blood Lymphocytes With Apoptosis and Prognosis in Patients With Sepsis. Zhonghua wei Zhong Bing ji jiu yi xue (2020) 32(8):938–42. doi: 10.3760/cma.j.cn121430-20200213-00181
158. Dang CP, Leelahavanichkul A. Over-Expression of Mir-223 Induces M2 Macrophage Through Glycolysis Alteration and Attenuates LPS-Induced Sepsis Mouse Model, the Cell-Based Therapy in Sepsis. PloS One (2020) 15(7):e0236038. doi: 10.1371/journal.pone.0236038
159. Zhang N, Gao Y, Yu S, Sun X, Shen K. Berberine Attenuates Aβ42-Induced Neuronal Damage Through Regulating Circhdac9/Mir-142-5p Axis in Human Neuronal Cells. Life Sci (2020) 117637. doi: 10.1016/j.lfs.2020.117637
160. Gao M, Yu T, Liu D, Shi Y, Yang P, Zhang J, et al. Sepsis Plasma-Derived Exosomal Mir-1-3p Induces Endothelial Cell Dysfunction by Targeting SERP1. Clin Sci (2021) 135(2):347–65. doi: 10.1042/CS20200573
161. Yao L, Liu Z, Zhu J, Li B, Chai C, Tian Y. Clinical Evaluation of Circulating Microrna-25 Level Change in Sepsis and its Potential Relationship With Oxidative Stress. Int J Clin Exp Pathol (2015) 8(7):7675.
162. Visitchanakun P, Tangtanatakul P, Trithiphen O, Soonthornchai W, Wongphoom J, Tachaboon S, et al. Plasma Mir-370-3p as a Biomarker of Sepsis-Associated Encephalopathy, the Transcriptomic Profiling Analysis of Microrna-Arrays From Mouse Brains. Shock (2020) 54(3):347–57. doi: 10.1097/SHK.0000000000001473
163. Pan T, Jia P, Chen N, Fang Y, Liang Y, Guo M, et al. Delayed Remote Ischemic Preconditioning Confersrenoprotection Against Septic Acute Kidney Injury via Exosomal Mir-21. Theranostics (2019) 9(2):405. doi: 10.7150/thno.29832
164. Zhang J, Liu Y, Liu L. Hyperoside Prevents Sepsis-Associated Cardiac Dysfunction Through Regulating Cardiomyocyte Viability and Inflammation via Inhibiting Mir-21. Biomed Pharmacother (2021) 138:111524. doi: 10.1016/j.biopha.2021.111524
165. De Melo P, Alvarez ARP, Ye X, Blackman A, Alves-Filho JC, Medeiros AI, et al. Macrophage-Derived Microrna-21 Drives Overwhelming Glycolytic and Inflammatory Response During Sepsis via Repression of the PGE2/IL-10 Axis. J Immunol (2021) 207(3):902–12. doi: 10.4049/jimmunol.2001251
166. Lin Z, Liu Z, Wang X, Qiu C, Zheng S. Mir-21-3p Plays a Crucial Role in Metabolism Alteration of Renal Tubular Epithelial Cells During Sepsis Associated Acute Kidney Injury via AKT/CDK2-FOXO1 Pathway. BioMed Res Int (2019) 2019. doi: 10.1155/2019/2821731
167. Jiang Q, Wu C, Zhang Q. Microrna-34a Participates in Lipopolysaccharide Mediated Sepsis Related Renal Function Impairment via Kruppel-Like Factor 4. Zhonghua wei zhong bing ji jiu yi xue (2018) 30(4):351–4. doi: 10.3760/cma.j.issn.2095-4352.2018.04.013
168. Leng C, Sun J, Xin K, Ge J, Liu P, Feng X. High Expression of Mir-483-5p Aggravates Sepsis-Induced Acute Lung Injury. J toxicol Sci (2020) 45(2):77–86. doi: 10.2131/jts.45.77
169. Ma X, Qin J, Guo X. Mir-181-5p Protects Mice From Sepsis via Repressing HMGB1 in an Experimental Model. Eur Rev Med Pharmacol Sci (2020) 24(18):9712–20. doi: 10.26355/eurrev_202009_23063
170. Wang J, Tao Y, Wang Z, Mao Q. Mir-20a Promotes Kidney Injury in Sepsis Rats Through Autophagy. J Biol Regulators Homeostatic Agents (2020) 34(4):1277–83. doi: 10.23812/20-174-A
171. Wang Y, Wang H, Zhang C, Zhang C, Yang H, Gao R, et al. Plasma Hsa-Mir-92a-3p in Correlation With Lipocalin-2 is Associated With Sepsis-Induced Coagulopathy. BMC Infect Dis (2020) 20(1):1–9. doi: 10.1186/s12879-020-4853-y
172. He Z, Wang H, Yue L. Endothelial Progenitor Cells-Secreted Extracellular Vesicles Containing Microrna-93-5p Confer Protection Against Sepsis-Induced Acute Kidney Injury via the KDM6B/H3k27me3/TNF-α Axis. Exp Cell Res (2020) 395(2):112173. doi: 10.1016/j.yexcr.2020.112173
173. Liu D, Wang Z, Wang H, Ren F, Li Y, Zou S, et al. The Protective Role of Mir-223 in Sepsis-Induced Mortality. Sci Rep (2020) 10(1):1–10. doi: 10.1038/s41598-020-74965-2
174. Chen S, Ding R, Hu Z, Yin X, Xiao F, Zhang W, et al. Microrna-34a Inhibition Alleviates Lung Injury in Cecal Ligation and Puncture Induced Septic Mice. Front Immunol (2020) 11:1829. doi: 10.3389/fimmu.2020.01829
175. Yuan FH, Chen YL, Zhao Y, Liu ZM, Nan CC, Zheng BL, et al. Microrna-30a Inhibits the Liver Cell Proliferation and Promotes Cell Apoptosis Through the JAK/STAT Signaling Pathway by Targeting SOCS-1 in Rats With Sepsis. J Cell Physiol (2019) 234(10):17839–53. doi: 10.1002/jcp.28410
176. Zhu XG, Zhang TN, Wen R, Liu CF. Overexpression of Mir-150-5p Alleviates Apoptosis in Sepsis-Induced Myocardial Depression. BioMed Res Int (2020) 2020. doi: 10.1155/2020/3023186
177. Liu L, Li T-M, Liu X-R, Bai Y-P, Li J, Tang N, et al. Microrna-140 Inhibits Skeletal Muscle Glycolysis and Atrophy in Endotoxin-Induced Sepsis in Mice via the WNT Signaling Pathway. Am J Physiol-Cell Physiol (2019) 317(2):C189–C99. doi: 10.1152/ajpcell.00419.2018
178. Wang X, Wang Y, Kong M, Yang J. Mir-22-3p Suppresses Sepsis-Induced Acute Kidney Injury by Targeting PTEN. Biosci Rep (2020) 40(6):BSR20200527. doi: 10.1042/BSR20200527
179. Zhou W, Wang J, Li Z, Li J, Sang M. Microrna-205−5b Inhibits HMGB1 Expression in LPS-Induced Sepsis. Int J Mol Med (2016) 38(1):312–8. doi: 10.3892/ijmm.2016.2613
180. Liu Y, Xiao J, Sun J, Chen W, Wang S, Fu R, et al. ATG7 Promotes Autophagy in Sepsis−Induced Acute Kidney Injury and Is Inhibited by Mir−526b. Mol Med Rep (2020) 21(5):2193–201. doi: 10.3892/mmr.2020.11001
181. Wu Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Zingarelli B, et al. Mir-145a Regulation of Pericyte Dysfunction in a Murine Model of Sepsis. J Infect Dis (2020) 222(6):1037–45. doi: 10.1093/infdis/jiaa184
182. Zhao D, Li S, Cui J, Wang L, Ma X, Li Y. Plasma Mir-125a and Mir-125b in Sepsis: Correlation With Disease Risk, Inflammation, Severity, and Prognosis. J Clin Lab Anal (2020) 34(2):e23036. doi: 10.1002/jcla.23036
183. Rahmel T, Schäfer ST, Frey UH, Adamzik M, Peters J. Increased Circulating Microrna-122 Is a Biomarker for Discrimination and Risk Stratification in Patients Defined by Sepsis-3 Criteria. PloS One (2018) 13(5):e0197637. doi: 10.1371/journal.pone.0197637
184. Zheng G, Pan M, Jin W, Jin G, Huang Y. Microrna-135a Is Up-Regulated and Aggravates Myocardial Depression in Sepsis via Regulating P38 MAPK/NF-κb Pathway. Int Immunopharmacol (2017) 45:6–12. doi: 10.1016/j.intimp.2017.01.029
185. Qin L, Wang M, Zhang H. Mir-133a Alleviates Renal Injury Caused by Sepsis by Targeting BNIP3L. Eur Rev Med Pharmacol Sci (2020) 24(5):2632–9. doi: 10.26355/eurrev_202003_20532
186. Colbert JF, Ford JA, Haeger SM, Yang Y, Dailey KL, Allison KC, et al. A Model-Specific Role of Microrna-223 as a Mediator of Kidney Injury During Experimental Sepsis. Am J Physiol-Renal Physiol (2017) 313(2):F553–F9. doi: 10.1152/ajprenal.00493.2016
187. Han Y, Li Y, Jiang Y. The Prognostic Value of Plasma Microrna-155 and Microrna-146a Level in Severe Sepsis and Sepsis-Induced Acute Lung Injury Patients. Clin Lab (2016) 62(12):2355–60. doi: 10.7754/Clin.Lab.2016.160511
188. Zhai Y, Ding N. Microrna-194 Participates in Endotoxemia Induced Myocardial Injury via Promoting Apoptosis. Eur Rev Med Pharmacol Sci (2018) 22(7):2077–83. doi: 10.26355/eurrev_201804_14739
189. Shangxun Z, Junjie L, Wei Z, Yutong W, Wenyuan J, Shanshou L, et al. ADAR1 Alleviates Inflammation in a Murine Sepsis Model via the ADAR1-Mir-30a-SOCS3 Axis. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/9607535
190. Sun J, Sun X, Chen J, Liao X, He Y, Wang J, et al. Microrna-27b Shuttled by Mesenchymal Stem Cell-Derived Exosomes Prevents Sepsis by Targeting JMJD3 and Downregulating NF-κb Signaling Pathway. Stem Cell Res Ther (2021) 12(1):1–15. doi: 10.1186/s13287-020-02068-w
191. Lv X, Zhang Y, Cui Y, Ren Y, Li R, Rong Q. Inhibition of Microrna−155 Relieves Sepsis−Induced Liver Injury Through Inactivating the JAK/STAT Pathway. Mol Med Rep (2015) 12(4):6013–8. doi: 10.3892/mmr.2015.4188
192. Zhou Y, Song Y, Shaikh Z, Li H, Zhang H, Caudle Y, et al. Microrna-155 Attenuates Late Sepsis-Induced Cardiac Dysfunction Through JNK and β-Arrestin 2. Oncotarget (2017) 8(29):47317. doi: 10.18632/oncotarget.17636
193. Vasques-Nóvoa F, Laundos TL, Cerqueira RJ, Quina-Rodrigues C, Soares-dos-Reis R, Baganha F, et al. Microrna-155 Amplifies Nitric Oxide/Cgmp Signaling and Impairs Vascular Angiotensin II Reactivity in Septic Shock. Crit Care Med (2018) 46(9):e945–54. doi: 10.1097/CCM.0000000000003296
194. Wang Q, Zhao C, Cai Q, Zhu H. Expression of Microrna-155 and Regulative T Cell in Sepsis Patients and Their Relationship. Zhonghua wei zhong bing ji jiu yi xue (2014) 26(3):179–83. doi: 10.3760/cma.j.issn.2095-4352.2014.03.011
195. Wang Z-F, Yang Y-M, Fan H. Diagnostic Value of Mir-155 for Acute Lung Injury/Acute Respiratory Distress Syndrome in Patients With Sepsis. J Int Med Res (2020) 48(7):0300060520943070. doi: 10.1177/0300060520943070
196. Zhang B, Yu L, Sheng Y. Clinical Value and Role of Microrna-29c-3p in Sepsis-Induced Inflammation and Cardiac Dysfunction. Eur J Med Res (2021) 26(1):1–7. doi: 10.1186/s40001-021-00566-y
197. Sisti F, Wang S, Brandt SL, Glosson-Byers N, Mayo LD, Son YM, et al. Nuclear PTEN Enhances the Maturation of a Microrna Regulon to Limit Myd88-Dependent Susceptibility to Sepsis. Sci Signaling (2018) 11(528):eaai9085. doi: 10.1126/scisignal.aai9085
198. Gao N, Dong L. Microrna-146 Regulates the Inflammatory Cytokines Expression in Vascular Endothelial Cells During Sepsis. Die Pharmazie-An Int J Pharm Sci (2017) 72(11):700–4. doi: 10.1691/ph.2017.7600
199. Zhang YY, Liu X, Zhang X, Zhang J. Shikonin Improve Sepsis-Induced Lung Injury via Regulation of Mirna-140-5p/TLR4—a Vitro and Vivo Study. J Cell Biochem (2020) 121(3):2103–17. doi: 10.1002/jcb.28199
200. Ma H, Wang X, Ha T, Gao M, Liu L, Wang R, et al. Microrna-125b Prevents Cardiac Dysfunction in Polymicrobial Sepsis by Targeting TRAF6-Mediated Nuclear Factor κb Activation and P53-Mediated Apoptotic Signaling. J Infect Dis (2016) 214(11):1773–83. doi: 10.1093/infdis/jiw449
201. Ling Y, Li Z-Z, Zhang J-F, Zheng X-W, Lei Z-Q, Chen R-Y, et al. Microrna-494 Inhibition Alleviates Acute Lung Injury Through Nrf2 Signaling Pathway via NQO1 in Sepsis-Associated Acute Respiratory Distress Syndrome. Life Sci (2018) 210:1–8. doi: 10.1016/j.lfs.2018.08.037
202. Gao M, Wang X, Zhang X, Ha T, Ma H, Liu L, et al. Attenuation of Cardiac Dysfunction in Polymicrobial Sepsis by Microrna-146a Is Mediated via Targeting of IRAK1 and TRAF6 Expression. J Immunol (2015) 195(2):672–82. doi: 10.4049/jimmunol.1403155
203. Benz F, Tacke F, Luedde M, Trautwein C, Luedde T, Koch A, et al. Circulating Microrna-223 Serum Levels do Not Predict Sepsis or Survival in Patients With Critical Illness. Dis Markers (2015) 2015. doi: 10.1155/2015/384208
204. Li Y, Ke J, Peng C, Wu F, Song Y. Microrna-300/NAMPT Regulates Inflammatory Responses Through Activation of AMPK/Mtor Signaling Pathway in Neonatal Sepsis. Biomed Pharmacother (2018) 108:271–9. doi: 10.1016/j.biopha.2018.08.064
205. Zhang X, Wang X, Fan M, Tu F, Yang K, Ha T, et al. Endothelial HSPA12B Exerts Protection Against Sepsis-Induced Severe Cardiomyopathy via Suppression of Adhesion Molecule Expression by Mir-126. Front Immunol (2020) 11:566. doi: 10.3389/fimmu.2020.00566
206. Zheng G, Qiu G, Ge M, Meng J, Zhang G, Wang J, et al. Mir-10a in Peripheral Blood Mononuclear Cells Is a Biomarker for Sepsis and has Anti-Inflammatory Function. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/4370983
207. Bai X, Zhang J, Cao M, Han S, Liu Y, Wang K, et al. Microrna-146a Protects Against LPS-Induced Organ Damage by Inhibiting Notch1 in Macrophage. Int Immunopharmacol (2018) 63:220–6. doi: 10.1016/j.intimp.2018.07.040
208. Hong J, Hu B-C, Xu L, Zheng Y, Shao Z-Q, Zhang R, et al. Microrna-19a Targets Fibroblast Growth Factor-Inducible Molecule 14 and Prevents Tubular Damage in Septic AKI. Anal Cell Pathol (2020) 2020. doi: 10.1155/2020/2894650
209. Ge C, Liu J, Dong S. Mirna-214 Protects Sepsis-Induced Myocardial Injury. Shock (2018) 50(1):112–8. doi: 10.1097/SHK.0000000000000978
210. Meng L, Cao H, Wan C, Jiang L. Mir-539-5p Alleviates Sepsis-Induced Acute Lung Injury by Targeting ROCK1. Folia histochem cytobiologica (2019) 57(4):168–78. doi: 10.5603/FHC.a2019.0019
211. Liu J, Shi K, Chen M, Xu L, Hong J, Hu B, et al. Elevated Mir-155 Expression Induces Immunosuppression via CD39+ Regulatory T-Cells in Sepsis Patient. Int J Infect Dis (2015) 40:135–41. doi: 10.1016/j.ijid.2015.09.016
212. Zhang J, Ding C, Shao Q, Liu F, Zeng Z, Nie C, et al. The Protective Effects of Transfected Microrna-146a on Mice With Sepsis-Induced Acute Lung Injury In Vivo. Zhonghua wei zhong bing ji jiu yi xue (2015) 27(7):591–4. doi: 10.3760/cma.j.issn.2095-4352.2015.07.010
213. Zhang W, Jia J, Liu Z, Si D, Ma L, Zhang G. Circulating Micrornas as Biomarkers for Sepsis Secondary to Pneumonia Diagnosed via Sepsis 3.0. BMC pulmonary Med (2019) 19(1):1–8. doi: 10.1186/s12890-019-0836-4
214. Jiang Y, Zhou H, Ma D, Chen ZK, Cai X. Microrna-19a and CD22 Comprise a Feedback Loop for B Cell Response in Sepsis. Med Sci monitor: Int Med J Exp Clin Res (2015) 21:1548. doi: 10.12659/MSM.894321
215. Liang G, Wu Y, Guan Y, Dong Y, Jiang L, Mao G, et al. The Correlations Between the Serum Expression of Mir-206 and the Severity and Prognosis of Sepsis. Ann Palliative Med (2020) 9(5):3222–34. doi: 10.21037/apm-20-1391
216. Funahashi Y, Kato N, Masuda T, Nishio F, Kitai H, Ishimoto T, et al. Mir-146a Targeted to Splenic Macrophages Prevents Sepsis-Induced Multiple Organ Injury. Lab Invest (2019) 99(8):1130–42. doi: 10.1038/s41374-019-0190-4
217. Xu H, Liu X, Ni H. Clinical Significance of Mir-19b-3p in Patients With Sepsis and its Regulatory Role in the LPS-Induced Inflammatory Response. Eur J Med Res (2020) 25(1):1–7. doi: 10.1186/s40001-020-00408-3
218. Yang P, Xiong W, Chen X, Liu J, Ye Z. Overexpression of Mir-129-5p Mitigates Sepsis-Induced Acute Lung Injury by Targeting High Mobility Group Box 1. J Surg Res (2020) 256:23–30. doi: 10.1016/j.jss.2020.05.101
219. Zhang W, Lu F, Xie Y, Lin Y, Zhao T, Tao S, et al. Mir-23b Negatively Regulates Sepsis-Induced Inflammatory Responses by Targeting ADAM10 in Human THP-1 Monocytes. Mediators Inflamm (2019) 2019. doi: 10.1155/2019/5306541
220. Liu Q, Wang Y, Zheng Q, Dong X, Xie Z, Panayi A, et al. Microrna-150 Inhibits Myeloid-Derived Suppressor Cells Proliferation and Function Through Negative Regulation of ARG-1 in Sepsis. Life Sci (2021) 278:119626. doi: 10.1016/j.lfs.2021.119626
221. Sheng B, Zhao L, Zang X, Zhen J, Chen W. Mir-375 Ameliorates Sepsis by Downregulating Mir-21 Level via Inhibiting JAK2-STAT3 Signaling. Biomed Pharmacother (2017) 86:254–61. doi: 10.1016/j.biopha.2016.11.147
222. Zhan C-Y, Chen D, Luo J-L, Shi Y-H, Zhang Y-P. Protective Role of Down-Regulated Microrna-31 on Intestinal Barrier Dysfunction Through Inhibition of NF-κb/Hif-1α Pathway by Binding to HMOX1 in Rats With Sepsis. Mol Med (2018) 24(1):1–14. doi: 10.1186/s10020-018-0053-2
223. McClure C, Brudecki L, Ferguson DA, Yao ZQ, Moorman JP, McCall CE, et al. Microrna 21 (Mir-21) and Mir-181b Couple With NFI-a to Generate Myeloid-Derived Suppressor Cells and Promote Immunosuppression in Late Sepsis. Infect Immun (2014) 82(9):3816–25. doi: 10.1128/IAI.01495-14
224. Roderburg C, Luedde M, Vargas Cardenas D, Vucur M, Scholten D, Frey N, et al. Circulating Microrna-150 Serum Levels Predict Survival in Patients With Critical Illness and Sepsis. PloS One (2013) 8(1):e54612. doi: 10.1371/journal.pone.0054612
225. Zhou Y, Han W, Song D, Li Z, Ding H, Zhou T, et al. Effect of Mir-10a on Sepsis-Induced Liver Injury in Rats Through TGF-β1/Smad Signaling Pathway. Eur Rev Med Pharmacol Sci (2020) 24(2):862–9. doi: 10.26355/eurrev_202001_20070
226. Ma F, Li Z, Cao J, Kong X, Gong G. A TGFBR2/SMAD2/DNMT1/Mir-145 Negative Regulatory Loop Is Responsible for LPS-Induced Sepsis. Biomed Pharmacother (2019) 112:108626. doi: 10.1016/j.biopha.2019.108626
227. Vasilescu C, Rossi S, Shimizu M, Tudor S, Veronese A, Ferracin M, et al. Microrna Fingerprints Identify Mir-150 as a Plasma Prognostic Marker in Patients With Sepsis. PloS One (2009) 4(10):e7405. doi: 10.1371/journal.pone.0007405
228. Zhou YP, Xia Q. Inhibition of Mir-103a-3p Suppresses Lipopolysaccharide-Induced Sepsis and Liver Injury by Regulating FBXW7 Expression. Cell Biol Int (2020) 44(9):1798–810. doi: 10.1002/cbin.11372
229. Han Y, Dai Q-C, Shen H-L, Zhang X-W. Diagnostic Value of Elevated Serum Mirna-143 Levels in Sepsis. J Int Med Res (2016) 44(4):875–81. doi: 10.1177/0300060516645003
230. Cao X, Zhang C, Zhang X, Chen Y, Zhang H. Mir-145 Negatively Regulates TGFBR2 Signaling Responsible for Sepsis-Induced Acute Lung Injury. Biomed Pharmacother (2019) 111:852–8. doi: 10.1016/j.biopha.2018.12.138
231. Rajput C, Tauseef M, Farazuddin M, Yazbeck P, Amin M-R, Avin BRV, et al. Microrna-150 Suppression of Angiopoetin-2 Generation and Signaling Is Crucial for Resolving Vascular Injury. Arteriosclerosis thrombosis Vasc Biol (2016) 36(2):380–8. doi: 10.1161/ATVBAHA.115.306997
232. He SY, Wang G, Pei YH, Zhu HP. Mir-34b-3p Protects Against Acute Kidney Injury in Sepsis Mice via Targeting Ubiquitin-Like Protein 4A. Kaohsiung J Med Sci (2020) 36(10):817–24. doi: 10.1002/kjm2.12255
233. Wang H, Bei Y, Shen S, Huang P, Shi J, Zhang J, et al. Mir-21-3p Controls Sepsis-Associated Cardiac Dysfunction via Regulating SORBS2. J Mol Cell Cardiol (2016) 94:43–53. doi: 10.1016/j.yjmcc.2016.03.014
234. Du X, Tian D, Wei J, Yan C, Hu P, Wu X, et al. Mir-199a-5p Exacerbated Intestinal Barrier Dysfunction Through Inhibiting Surfactant Protein D and Activating NF-κb Pathway in Sepsis. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/8275026
235. Su Y, Song X, Teng J, Zhou X, Dong Z, Li P, et al. Mesenchymal Stem Cells-Derived Extracellular Vesicles Carrying Microrna-17 Inhibits Macrophage Apoptosis in Lipopolysaccharide-Induced Sepsis. Int Immunopharmacol (2021) 95:107408. doi: 10.1016/j.intimp.2021.107408
236. Zhu X. Mir-125b But Not Mir-125a Is Upregulated and Exhibits a Trend to Correlate With Enhanced Disease Severity, Inflammation, and Increased Mortality in Sepsis Patients. J Clin Lab Anal (2020) 34(3):e23094. doi: 10.1002/jcla.23094
237. Ling L, Zhang S-H, Zhi L-D, Li H, Wen Q-K, Li G, et al. Microrna-30e Promotes Hepatocyte Proliferation and Inhibits Apoptosis in Cecal Ligation and Puncture-Induced Sepsis Through the JAK/STAT Signaling Pathway by Binding to FOSL2. Biomed Pharmacother (2018) 104:411–9. doi: 10.1016/j.biopha.2018.05.042
238. Liu J, Liu Y, Zhang L, Chen Y, Du H, Wen Z, et al. Down-Regulation of Circdmnt3b Is Conducive to Intestinal Mucosal Permeability Dysfunction of Rats With Sepsis via Sponging Mir-20b-5p. J Cell Mol Med (2020) 24(12):6731–40. doi: 10.1111/jcmm.15324
239. Wang X, Yu Y. Mir-146b Protect Against Sepsis Induced Mice Myocardial Injury Through Inhibition of Notch1. J Mol Histol (2018) 49(4):411–7. doi: 10.1007/s10735-018-9781-4
240. Yao Y, Sun F, Lei M. Mir-25 Inhibits Sepsis-Induced Cardiomyocyte Apoptosis by Targetting PTEN. Biosci Rep (2018) 38(2). doi: 10.1042/BSR20171511
241. McClure C, McPeak MB, Youssef D, Yao ZQ, McCall CE, El Gazzar M. Stat3 and C/Ebpβ Synergize to Induce Mir-21 and Mir-181b Expression During Sepsis. Immunol Cell Biol (2017) 95(1):42–55. doi: 10.1038/icb.2016.63
242. Ji Z-R, Xue W-L, Zhang L. Schisandrin B Attenuates Inflammation in LPS-Induced Sepsis Through Mir-17-5p Downregulating TLR4. Inflammation (2019) 42(2):731–9. doi: 10.1007/s10753-018-0931-3
243. Yu J, Chen J, Yang H, Chen S, Wang Z. Overexpression of Mir−200a−3p Promoted Inflammation in Sepsis−Induced Brain Injury Through ROS−Induced NLRP3. Int J Mol Med (2019) 44(5):1811–23. doi: 10.3892/ijmm.2019.4326
244. Szilágyi B, Fejes Z, Póliska S, Pócsi M, Czimmerer Z, Patsalos A, et al. Reduced Mir-26b Expression in Megakaryocytes and Platelets Contributes to Elevated Level of Platelet Activation Status in Sepsis. Int J Mol Sci (2020) 21(3):866. doi: 10.3390/ijms21030866
245. Chen X, Chen Y, Dai L, Wang N. Mir-96-5p Alleviates Inflammatory Responses by Targeting NAMPT and Regulating the NF-κb Pathway in Neonatal Sepsis. Biosci Rep (2020) 40(7):BSR20201267. doi: 10.1042/BSR20201267
246. Wang Z, Ruan Z, Mao Y, Dong W, Zhang Y, Yin N, et al. Mir-27a Is Up Regulated and Promotes Inflammatory Response in Sepsis. Cell Immunol (2014) 290(2):190–5. doi: 10.1016/j.cellimm.2014.06.006
247. Zou Z, Lin Q, Yang H, Liu Z, Zheng S. Nrp-1 Mediated Plasmatic Ago2 Binding Mir-21a-3p Internalization: A Novel Mechanism for Mir-21a-3p Accumulation in Renal Tubular Epithelial Cells During Sepsis. BioMed Res Int (2020) 2020. doi: 10.1155/2020/2370253
248. Wang H, Meng K, jun Chen W, Feng D, Jia Y, Xie L. Serum Mir-574-5p: A Prognostic Predictor of Sepsis Patients. Shock (2012) 37(3):263–7. doi: 10.1097/SHK.0b013e318241baf8
249. Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, et al. Microrna-181b Regulates NF-κb–Mediated Vascular Inflammation. J Clin Invest (2012) 122(6):1973–90. doi: 10.1172/JCI61495
250. Du X, Wei J, Tian D, Wu M, Yan C, Hu P, et al. Mir-182-5p Contributes to Intestinal Injury in a Murine Model of Staphylococcus Aureus Pneumonia-Induced Sepsis via Targeting Surfactant Protein D. J Cell Physiol (2020) 235(1):563–72. doi: 10.1002/jcp.28995
251. Zheng D, Yu Y, Li M, Wang G, Chen R, Fan G-C, et al. Inhibition of Microrna 195 Prevents Apoptosis and Multiple-Organ Injury in Mouse Models of Sepsis. J Infect Dis (2016) 213(10):1661–70. doi: 10.1093/infdis/jiv760
252. Zhang Y, Xia F, Wu J, Yang A, Zhang Y, Zhao H, et al. Mir-205 Influences Renal Injury in Sepsis Rats Through HMGB1-PTEN Signaling Pathway. Eur Rev Med Pharmacol Sci (2019) 23:10950–6. doi: 10.26355/eurrev_201912_19798
253. Wu S-Y, Zhang H, Wu W, Wu Y-Y. Value of Serum Mir-21-3p in Predicting Acute Kidney Injury in Children With Sepsis. Zhongguo Dang dai er ke za zhi= Chin J Contemp Pediatr (2020) 22(3):269–73. doi: 10.7499/j.issn.1008-8830.2020.03.016
254. Yi H-X, Jiang S-Y, Yu L-H, Chen K, Yang Z-X, Wu Q. Mir-181a-2-3p Alleviates the Apoptosis of Renal Tubular Epithelial Cell via Targeting GJB2 in Sepsis-Induced Acute Kidney Injury. Mol Cell Biol (2021) 41(7):MCB. 00016–21. doi: 10.1128/MCB.00016-21
255. Fu D, Dong J, Li P, Tang C, Cheng W, Xu Z, et al. Mirna-21 has Effects to Protect Kidney Injury Induced by Sepsis. Biomed Pharmacother (2017) 94:1138–44. doi: 10.1016/j.biopha.2017.07.098
256. Pan Y, Wang J, Xue Y, Zhao J, Li D, Zhang S, et al. GSKJ4 Protects Mice Against Early Sepsis via Reducing Proinflammatory Factors and Up-Regulating Mir-146a. Front Immunol (2018) 9:2272. doi: 10.3389/fimmu.2018.02272
257. Liu Y, Cao D, Mo G, Zhang L. Effects of Microrna-294 on Inflammatory Factor of Sepsis by Targeting Triggering Receptor Expressed on Myeloid Cells-1. Zhonghua wei zhong bing ji jiu yi xue (2014) 26(9):661–5. doi: 10.3760/cma.j.issn.2095-4352.2014.09.01
258. Wang L, Wang K, Tian Z. Mir-128-3p Inhibits NRP1 Expression and Promotes Inflammatory Response to Acute Kidney Injury in Sepsis. Inflammation (2020) 43:1772–9. doi: 10.1007/s10753-020-01251-8
259. An R, Feng J, Xi C, Xu J, Sun L. Mir-146a Attenuates Sepsis-Induced Myocardial Dysfunction by Suppressing IRAK1 and TRAF6 via Targeting Erbb4 Expression. Oxid Med Cell longevity (2018) 2018. doi: 10.1155/2018/7163057
260. Puimège L, Van Hauwermeiren F, Steeland S, Van Ryckeghem S, Vandewalle J, Lodens S, et al. Glucocorticoid-Induced Microrna-511 Protects Against TNF by Down-Regulating TNFR 1. EMBO Mol Med (2015) 7(8):1004–17. doi: 10.15252/emmm.201405010
261. Liu Z, Tang C, He L, Yang D, Cai J, Zhu J, et al. The Negative Feedback Loop of NF-κb/Mir-376b/NFKBIZ in Septic Acute Kidney Injury. JCI Insight (2020) 5(24). doi: 10.1172/jci.insight.142272
262. Wang Z-H, Liang Y-B, Tang H, Chen Z-B, Li Z-Y, Hu X-C, et al. Dexamethasone Down-Regulates the Expression of Microrna-155 in the Livers of Septic Mice. PloS One (2013) 8(11):e80547. doi: 10.1371/journal.pone.0080547
263. Tacke F, Roderburg C, Benz F, Cardenas DV, Luedde M, Hippe H-J, et al. Levels of Circulating Mir-133a Are Elevated in Sepsis and Predict Mortality in Critically Ill Patients. Crit Care Med (2014) 42(5):1096–104. doi: 10.1097/CCM.0000000000000131
264. Ling L, Lu H-T, Wang H-F, Shen M-J, Zhang H-B. Microrna-203 Acts as a Potent Suppressor in Septic Shock by Alleviating Lung Injury via Inhibition of VNN1. Kidney Blood Pressure Res (2019) 44(4):565–82. doi: 10.1159/000500484
265. Wu X, Yang J, Yu L, Long D. Plasma Mirna-223 Correlates With Risk, Inflammatory Markers as Well as Prognosis in Sepsis Patients. Med (2018) 97(27). doi: 10.1097/MD.0000000000011352
266. Möhnle P, Schütz SV, van der Heide V, Hübner M, Luchting B, Sedlbauer J, et al. Microrna-146a Controls Th1-Cell Differentiation of Human CD4+ T Lymphocytes by Targeting Prkcϵ. Eur J Immunol (2015) 45(1):260–72. doi: 10.1002/eji.201444667
267. Karam RA, Zidan HE, Karam NA, Abdel Rahman DM, El-Seifi OS. Diagnostic and Prognostic Significance of Serum Mirna-146-a Expression in Egyptian Children With Sepsis in a Pediatric Intensive Care Unit. J Gene Med (2019) 21(11):e3128. doi: 10.1002/jgm.3128
268. Cheng D-L, Fang H-X, Liang Y, Zhao Y, Shi C-S. Microrna-34a Promotes Inos Secretion From Pulmonary Macrophages in Septic Suckling Rats Through Activating STAT3 Pathway. Biomed Pharmacother (2018) 105:1276–82. doi: 10.1016/j.biopha.2018.06.063
269. Yang Q, Cao K, Jin G, Zhang J. Hsa-Mir-346 Plays a Role in the Development of Sepsis by Downregulating SMAD3 Expression and Is Negatively Regulated by Lncrna MALAT1. Mol Cell Probes (2019) 47:101444. doi: 10.1016/j.mcp.2019.101444
270. Sang Z, Dong S, Zhang P, Wei Y. Mir−214 Ameliorates Sepsis−Induced Acute Kidney Injury via PTEN/AKT/Mtor−Regulated Autophagy. Mol Med Rep (2021) 24(4):1–11. doi: 10.3892/mmr.2021.12322
271. Xue W-L, Bai X, Zhang L. Rhtnfr: Fc Increases Nrf2 Expression via Mir-27a Mediation to Protect Myocardium Against Sepsis Injury. Biochem Biophys Res Commun (2015) 464(3):855–61. doi: 10.1016/j.bbrc.2015.07.051
272. Yang W, Wu H, Zhang H, Liu H, Wei Y, Shi B. Prognostic Value of Picco Monitoring Combined With Plasma Microrna-150 Detection in Septic Shock Patients. Zhejiang da xue xue bao Yi xue ban= J Zhejiang Univ Med Sci (2015) 44(6):659–64.
273. Bai X, Li J, Li L, Liu M, Liu Y, Cao M, et al. Extracellular Vesicles From Adipose Tissue-Derived Stem Cells Affect Notch-Mir148a-3p Axis to Regulate Polarization of Macrophages and Alleviate Sepsis in Mice. Front Immunol (2020) 11:1391. doi: 10.3389/fimmu.2020.01391
274. Zhang T, Xiang L. Honokiol Alleviates Sepsis-Induced Acute Kidney Injury in Mice by Targeting the Mir-218-5p/Heme Oxygenase-1 Signaling Pathway. Cell Mol Biol Lett (2019) 24(1):1–15. doi: 10.1186/s11658-019-0142-4
275. Wang H-J, Deng J, Wang J-Y, Zhang P-J, Xin Z, Xiao K, et al. Serum Mir-122 Levels Are Related to Coagulation Disorders in Sepsis Patients. Clin Chem Lab Med (CCLM) (2014) 52(6):927–33. doi: 10.1515/cclm-2013-0899
276. Xin Y, Tang L, Chen J, Chen D, Wen W, Han F. Inhibition of Mir−101−3p Protects Against Sepsis−Induced Myocardial Injury by Inhibiting MAPK and NF−κb Pathway Activation via the Upregulation of DUSP1. Int J Mol Med (2021) 47(3):1–. doi: 10.3892/ijmm.2021.4853
277. Pan W, Wei N, Xu W, Wang G, Gong F, Li N. Microrna-124 Alleviates the Lung Injury in Mice With Septic Shock Through Inhibiting the Activation of the MAPK Signaling Pathway by Downregulating MAPK14. Int Immunopharmacol (2019) 76:105835. doi: 10.1016/j.intimp.2019.105835
278. Luo N, Gao H, Wang Y, Li H, Li Y. Mir-942-5p Alleviates Septic Acute Kidney Injury by Targeting FOXO3. Eur Rev Med Pharmacol Sci (2020) 24(11):6237–44. doi: 10.26355/eurrev_202006_21521
279. Liu S, Liu C, Wang Z, Huang J, Zeng Q. Microrna-23a-5p Acts as a Potential Biomarker for Sepsis-Induced Acute Respiratory Distress Syndrome in Early Stage. Cell Mol Biol (2016) 62(2):31–7.
280. Ma J, Xu L-Y, Sun Q-H, Wan X-Y. Inhibition of Mir-1298-5p Attenuates Sepsis Lung Injury by Targeting SOCS6. Mol Cell Biochem (2021) 476(10):1–12. doi: 10.1007/s11010-021-04170-w
281. Zheng G, Qu H, Li F, Ma W, Yang H. Propofol Attenuates Sepsis-Induced Acute Kidney Injury by Regulating Mir-290-5p/CCL-2 Signaling Pathway. Braz J Med Biol Res (2018) 51. doi: 10.1590/1414-431x20187655
282. Paik S, Choe JH, Choi G-E, Kim J-E, Kim J-M, Song GY, et al. Rg6, a Rare Ginsenoside, Inhibits Systemic Inflammation Through the Induction of Interleukin-10 and Microrna-146a. Sci Rep (2019) 9(1):1–15. doi: 10.1038/s41598-019-40690-8
283. Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K, et al. Exosomal Mir-223 Contributes to Mesenchymal Stem Cell-Elicited Cardioprotection in Polymicrobial Sepsis. Sci Rep (2015) 5(1):1–16. doi: 10.1038/srep13721
284. Gu W, Wen D, Lu H, Zhang A, Wang H, Du J, et al. Mir-608 Exerts Anti-Inflammatory Effects by Targeting ELANE in Monocytes. J Clin Immunol (2020) 40(1):147–57. doi: 10.1007/s10875-019-00702-8
285. Sun Y, Li Q, Gui H, Xu D-P, Yang Y-L, Su D-F, et al. Microrna-124 Mediates the Cholinergic Anti-Inflammatory Action Through Inhibiting the Production of Pro-Inflammatory Cytokines. Cell Res (2013) 23(11):1270–83. doi: 10.1038/cr.2013.116
286. Zhang J, Wang C, Tang X, Wei Y. Urinary Mir-26b as a Potential Biomarker for Patients With Sepsis-Associated Acute Kidney Injury: A Chinese Population-Based Study. Eur Rev Med Pharmacol Sci (2018) 22(14):4604–10. doi: 10.26355/eurrev_201807_15518
287. Ni J, He J, Kang L, Zhong Z, Wang L, Yin S. Effects of Dexmedetomidine Pretreatment on Rats With Sepsis-Induced Acute Kidney Injury and Mir-146a Expression. Cell Mol Biol (2020) 66(2):93–8. doi: 10.14715/cmb/2020.66.2.15
288. Huo R, Dai M, Fan Y, Zhou J-Z, Li L, Zu J. Predictive Value of Mirna-29a and Mirna-10a-5p for 28-Day Mortality in Patients With Sepsis-Induced Acute Kidney Injury. Nan fang yi ke da xue xue bao= J South Med Univ (2017) 37(5):646–51. doi: 10.3969/j.issn.1673-4254.2017.05.13
289. Cao Y-Y, Wang Z, Wang Z-H, Jiang X-G, Lu W-H. Inhibition of Mir-155 Alleviates Sepsis-Induced Inflammation and Intestinal Barrier Dysfunction by Inactivating NF-κb Signaling. Int Immunopharmacol (2021) 90:107218. doi: 10.1016/j.intimp.2020.107218
290. Ma F, Liu F, Ding L, You M, Yue H, Zhou Y, et al. Anti-Inflammatory Effects of Curcumin Are Associated With Down Regulating Microrna-155 in LPS-Treated Macrophages and Mice. Pharm Biol (2017) 55(1):1263–73. doi: 10.1080/13880209.2017.1297838
291. Wu Z-J, Chen Y-F, Wang H-D, Gao F-H. Expression of Plasma Mirna-497 in Children With Sepsis-Induced Myocardial Injury and its Clinical Significance. Zhongguo dang dai er ke za zhi= Chin J Contemp Pediatr (2018) 20(1):32–6. doi: 10.7499/j.issn.1008-8830.2018.01.007
292. Lou W, Yan J, Wang W. Downregulation of Mir-497-5p Improves Sepsis-Induced Acute Lung Injury by Targeting IL2RB. BioMed Res Int (2021) 2021. doi: 10.1155/2021/6624702
293. Wang Y, Li T, Wu B, Liu H, Luo J, Feng D, et al. STAT1 Regulates MD-2 Expression in Monocytes of Sepsis via Mir-30a. Inflammation (2014) 37(6):1903–11. doi: 10.1007/s10753-014-9922-1
294. Ma Y, Liu Y, Hou H, Yao Y, Meng H. Mir-150 Predicts Survival in Patients With Sepsis and Inhibits LPS-Induced Inflammatory Factors and Apoptosis by Targeting NF-κb1 in Human Umbilical Vein Endothelial Cells. Biochem Biophys Res Commun (2018) 500(3):828–37. doi: 10.1016/j.bbrc.2018.04.168
295. Brudecki L, Ferguson DA, McCall CE, El Gazzar M. Microrna-146a and RBM4 Form a Negative Feed-Forward Loop That Disrupts Cytokine Mrna Translation Following TLR4 Responses in Human THP-1 Monocytes. Immunol Cell Biol (2013) 91(8):532–40. doi: 10.1038/icb.2013.37
296. Banerjee S, Meng J, Das S, Krishnan A, Haworth J, Charboneau R, et al. Morphine Induced Exacerbation of Sepsis Is Mediated by Tempering Endotoxin Tolerance Through Modulation of Mir-146a. Sci Rep (2013) 3(1):1–12. doi: 10.1038/srep01977
297. Yang Q, Zhang D, Li Y, Li Y, Li Y. Paclitaxel Alleviated Liver Injury of Septic Mice by Alleviating Inflammatory Response via Microrna-27a/TAB3/NF-κb Signaling Pathway. Biomed Pharmacother (2018) 97:1424–33. doi: 10.1016/j.biopha.2017.11.003
298. Wang J-F, Yu M-L, Yu G, Bian J-J, Deng X-M, Wan X-J, et al. Serum Mir-146a and Mir-223 as Potential New Biomarkers for Sepsis. Biochem Biophys Res Commun (2010) 394(1):184–8. doi: 10.1016/j.bbrc.2010.02.145
299. Mei L, He M, Zhang C, Miao J, Wen Q, Liu X, et al. Paeonol Attenuates Inflammation by Targeting HMGB1 Through Upregulating Mir-339-5p. Sci Rep (2019) 9(1):1–15. doi: 10.1038/s41598-019-55980-4
300. Wang X, Hao L, Bu H-F, Scott AW, Tian K, Liu F, et al. Spherical Nucleic Acid Targeting Microrna-99b Enhances Intestinal MFG-E8 Gene Expression and Restores Enterocyte Migration in Lipopolysaccharide-Induced Septic Mice. Sci Rep (2016) 6(1):1–13. doi: 10.1038/srep31687
301. Yao Y, Xu K, Sun Y, Tian T, Shen W, Sun F, et al. Mir-215-5p Inhibits the Inflammation Injury in Septic H9c2 by Regulating ILF3 and LRRFIP1. Int Immunopharmacol (2020) 78:106000. doi: 10.1016/j.intimp.2019.106000
302. Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie L-X. Evidence for Serum Mir-15a and Mir-16 Levels as Biomarkers That Distinguish Sepsis From Systemic Inflammatory Response Syndrome in Human Subjects. Clin Chem Lab Med (2012) 50(8):1423–8. doi: 10.1515/cclm-2011-0826
303. Geng X, Jia Y, Zhang Y, Shi L, Li Q, Zang A, et al. Circular RNA: Biogenesis, Degradation, Functions and Potential Roles in Mediating Resistance to Anticarcinogens. Epigenomics (2020) 12(3):267–83. doi: 10.2217/epi-2019-0295
304. Jiang WY, Ren J, Zhang XH, Lu ZL, Feng HJ, Yao XL, et al. Circc3p1 Attenuated Pro-Inflammatory Cytokine Production and Cell Apoptosis in Acute Lung Injury Induced by Sepsis Through Modulating Mir-21. J Cell Mol Med (2020) 24(19):11221–9. doi: 10.1111/jcmm.15685
305. Tian C, Liu J, Di X, Cong S, Zhao M, Wang K. Exosomal Hsa_Circrna_104484 and Hsa_Circrna_104670 may Serve as Potential Novel Biomarkers and Therapeutic Targets for Sepsis. Sci Rep (2021) 11(1):1–18. doi: 10.1038/s41598-021-93246-0
306. Shi Y, Sun CF, Ge WH, Du YP, Hu NB. Circular RNA VMA21 Ameliorates Sepsis-Associated Acute Kidney Injury by Regulating Mir-9-3p/SMG1/Inflammation Axis and Oxidative Stress. J Cell Mol Med (2020) 24(19):11397–408. doi: 10.1111/jcmm.15741
307. He Y, Sun Y, Peng J. Circ_0114428 Regulates Sepsis-Induced Kidney Injury by Targeting the Mir-495-3p/CRBN Axis. Inflammation (2021) 44(4):1–14. doi: 10.1007/s10753-021-01432-z
308. Wei B, Yu L. Circular RNA PRKCI and Microrna-545 Relate to Sepsis Risk, Disease Severity and 28-Day Mortality. Scand J Clin Lab Invest (2020) 80(8):659–66. doi: 10.1080/00365513.2020.1827291
309. Liu S, Zhang D, Liu Y, Zhou D, Yang H, Zhang K, et al. Circular RNA Circ_0001105 Protects the Intestinal Barrier of Septic Rats by Inhibiting Inflammation and Oxidative Damage and YAP1 Expression. Gene (2020) 755:144897. doi: 10.1016/j.gene.2020.144897
310. Ma X, Zhu G, Jiao T, Shao F. Effects of Circular RNA Ttc3/Mir-148a/Rcan2 Axis on Inflammation and Oxidative Stress in Rats With Acute Kidney Injury Induced by Sepsis. Life Sci (2021) 272:119233. doi: 10.1016/j.lfs.2021.119233
311. Shi X, Ma W, Li Y, Wang H, Pan S, Pan Y, et al. Circprkci Relieves Lipopolysaccharide-Induced HK2 Cell Injury by Upregulating the Expression of Mir-545 Target Gene ZEB2. BioFactors (2020) 46(3):475–86. doi: 10.1002/biof.1620
312. Xiong H, Wang H, Yu Q. Circular RNA Circ_0003420 Mediates Inflammation in Sepsis-Induced Liver Damage by Downregulating Neuronal PAS Domain Protein 4. Immunopharmacol Immunotoxicology (2021) 43(3):271–82. doi: 10.1080/08923973.2021.1887212
313. Shen W, Zhao X, Li S. Exosomes Derived From Adscs Attenuate Sepsis-Induced Lung Injury by Delivery of Circ-Fryl and Regulation of the Mir-490-3p/SIRT3 Pathway. Inflammation (2021) 1–12. doi: 10.1007/s10753-021-01548-2
314. Zhang X, Dong S. Circ_0091702 Relieves Lipopolysaccharide (LPS)-Induced Cell Injury by Regulating the Mir-182/PDE7A Axis in Sepsis. Biosci Biotechnol Biochem (2021) 85(9):1962–70. doi: 10.1093/bbb/zbab100
315. Li X, Li R, Gong Q, Shi D, Song L, Song Y. Circular RNA Circvma21 Ameliorates Lipopolysaccharide (LPS)-Induced Acute Kidney Injury by Targeting the Mir-199a-5p/NRP1 Axis in Sepsis. Biochem Biophys Res Commun (2021) 548:174–81. doi: 10.1016/j.bbrc.2021.02.028
316. Xu H-P, Ma X-Y, Yang C. Circular RNA TLK1 Promotes Sepsis-Associated Acute Kidney Injury by Regulating Inflammation and Oxidative Stress Through Mir-106a-5p/HMGB1 Axis. Front Mol Biosci (2021) 8. doi: 10.3389/fmolb.2021.660269
317. Hong X, Li S, Wang J, Zhao Z, Feng Z. Circular RNA Circfads2 Is Overexpressed in Sepsis and Suppresses LPS-Induced Lung Cell Apoptosis by Inhibiting the Maturation of Mir-15a-5p. BMC Immunol (2021) 22(1):1–7. doi: 10.1186/s12865-021-00419-7
318. Tan M, Bei R. Circ_0091702 Serves as a Sponge of Mir-545-3p to Attenuate Sepsis-Related Acute Kidney Injury by Upregulating THBS2. J Mol Histol (2021) 52(4):1–12. doi: 10.1007/s10735-021-09991-z
319. Wei W, Yao Y, Bi H, Xu W, Gao Y. Circular RNA Circ_0068, 888 Protects Against Lipopolysaccharide-Induced HK-2 Cell Injury via Sponging Microrna-21–5p. Biochem Biophys Res Commun (2021) 540:1–7. doi: 10.1016/j.bbrc.2020.12.018
320. Li M, Hu J, Peng Y, Li J, Ren R. Circptk2-Mir-181c-5p-HMGB1: A New Regulatory Pathway for Microglia Activation and Hippocampal Neuronal Apoptosis Induced by Sepsis. Mol Med (2021) 27(1):1–15. doi: 10.1186/s10020-021-00305-3
321. Li H, Zhang X, Wang P, Zhou X, Liang H, Li C. Knockdown of Circ-FANCA Alleviates LPS-Induced HK2 Cell Injury via Targeting Mir-93-5p/OXSR1 Axis in Septic Acute Kidney Injury. Diabetol Metab Syndrome (2021) 13(1):1–14. doi: 10.1186/s13098-021-00625-8
322. Lin Q, Liang Q, Qin C, Li Y. Circankrd36 Knockdown Suppressed Cell Viability and Migration of LPS-Stimulated RAW264. 7 Cells by Sponging Mir-330. Inflammation (2021) 44(5):1–10. doi: 10.1007/s10753-021-01480-5
Keywords: lncRNA, miRNA, sepsis, expression, biomarker
Citation: Ghafouri-Fard S, Khoshbakht T, Hussen BM, Taheri M and Arefian N (2021) Regulatory Role of Non-Coding RNAs on Immune Responses During Sepsis. Front. Immunol. 12:798713. doi: 10.3389/fimmu.2021.798713
Received: 20 October 2021; Accepted: 19 November 2021;
Published: 09 December 2021.
Edited by:
Smita Kulkarni, Texas Biomedical Research Institute, United StatesReviewed by:
Hamed Shoorei, Birjand University of Medical Sciences, IranKun Yang, East Tennessee State University, United States
Copyright © 2021 Ghafouri-Fard, Khoshbakht, Hussen, Taheri and Arefian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, bW9oYW1tYWRfODIzQHlhaG9vLmNvbQ==; Normohammad Arefian, bmFyZWZpYW5AeWFob28uY29t
 Soudeh Ghafouri-Fard
Soudeh Ghafouri-Fard Tayyebeh Khoshbakht
Tayyebeh Khoshbakht Bashdar Mahmud Hussen
Bashdar Mahmud Hussen Mohammad Taheri
Mohammad Taheri Normohammad Arefian6*
Normohammad Arefian6*