- Department of Medicine, Vanderbilt University Medical Center and Vanderbilt Ingram Cancer Center, Nashville, TN, United States
The addition of immune checkpoint inhibitors (ICIs) to the therapeutic armamentarium for solid malignancies has resulted in unprecedented improvements in patient outcomes in many cancers. The landscape of ICIs continues to evolve with novel approaches such as dual immune checkpoint blockade and combination therapies with other anticancer agents including cytotoxic chemotherapies and/or antiangiogenics. However, there is significant heterogeneity seen in antitumor responses, with certain patients deriving durable benefit, others experiencing initial benefit followed by acquired resistance necessitating change in therapy, and still others who are primarily refractory to ICIs. While generally better tolerated than traditional cytotoxic chemotherapy, ICIs are associated with unique toxicities, termed immune-related adverse events (irAEs), which can be severe or even lethal. As a disease of aging, older individuals make up a large proportion of patients diagnosed with cancer, yet this population is often underrepresented in clinical trials. Because ICIs indirectly target malignant cells through T cell activation, it has been hypothesized that age-related changes to the immune system may impact the efficacy and toxicity of these drugs. In this review, we discuss differences in the clinical efficacy and toxicity of ICIs in patients at the extremes of age.
Introduction
Since the development and successful application of immune checkpoint inhibitors (ICIs) across various cancer types, efforts to understand predictors of response to ICIs and likelihood of developing toxicities have been ongoing. Patients treated with ICIs can exhibit vastly different responses, with complete, durable responses on one end of the spectrum and primary resistance to therapy on the other end. The development of toxicities is similarly variable between patients, with no reliable predictors of severe immune-related adverse events (irAEs).
Cancer is classically understood as a “disease of aging,” as rates of cancer within a population generally increase with age (1). Despite this, data on how patients at the extremes of age respond to ICIs are scarce. Older patients are more likely to be excluded from participation in clinical trials, largely due to exclusionary comorbidities, prior cancer diagnoses, and reduced functional status (2, 3). Within the United States, patients over the age of 65 account for nearly 60% of cancer incidence, but only approximately 40% of cancer clinical trial participation. This incongruence is amplified with increasing age; patients over the age of 80 account for 16% of cancer incidence, but only 4% of cancer clinical trial participants (4). In the context of chemotherapy, studies have suggested that older patients more frequently have co-morbidities and are often treated less aggressively, but have similar outcomes to younger patients when fit enough to undergo comparable treatment (5). When compared to traditional cytotoxic chemotherapy, ICIs generally have a more favorable toxicity profile, making these therapies a potentially appealing option for older patients with limited functional reserve. Here, we aim to discuss age-related changes to the immune system that may impact ICI efficacy and toxicity, efficacy of ICIs in younger versus older patients, and age-specific considerations of ICI toxicities.
Aging and the Immune System
Aging is the most significant non-modifiable risk factor for cancer development. It has been postulated to impact ICI efficacy and toxicity. The mechanism of action for ICIs largely relies on the patient’s own immune system to reach a balance between ability to mount an effective anti-cancer response and risk of autoimmunity resulting in potentially severe irAEs. Immunosenescence, conceptually defined as the declining function of the immune system with increasing age, could lead to a differential response to ICIs between age groups. This topic has been most extensively explored in the context of adaptive immune response to vaccines in older adult patients; numerous studies have demonstrated decreased or insufficient antibody responses after common vaccines given to patients over the age of 60 (6–8). However, research in this area has been limited by lack of consensus definition as well as validated methods to measure immunosenescence. Importantly, an individual’s chronological age is not necessarily reflective of a patient’s physiological age or underlying immune system. The point at which the immune system demonstrates changes associated with being “old” is not only variable between individuals, but also between populations as a result of underlying genetics, lifestyle, and pathogenic exposures (9).
Immunosenescence is characterized by a decrease in peripheral naïve T cells with a relative increase in memory T cells (10, 11), decrease in T cell receptor repertoire (12–14), and changes in the composition of regulatory T cell populations (15). Memory T cells generated from aged naïve T cells demonstrate lower proliferation rates and effector cytokine production, thus resulting in an inferior immune response compared to those generated from young naïve T cells (16). Epigenetic alterations in aging immune cells and cancer cells also play a role in anti-cancer response, with studies of epigenetic biomarkers and combined epigenetic therapy with immunotherapy underway (17–19). Targeted pathway inhibition, however, could potentially reverse these changes in preclinical studies (20). Taken together, immunosenescence is thought to result in a decreased ability to respond to antigenic stimulation and simultaneous, paradoxical chronic low-level inflammation and autoimmunity (21).
Efficacy
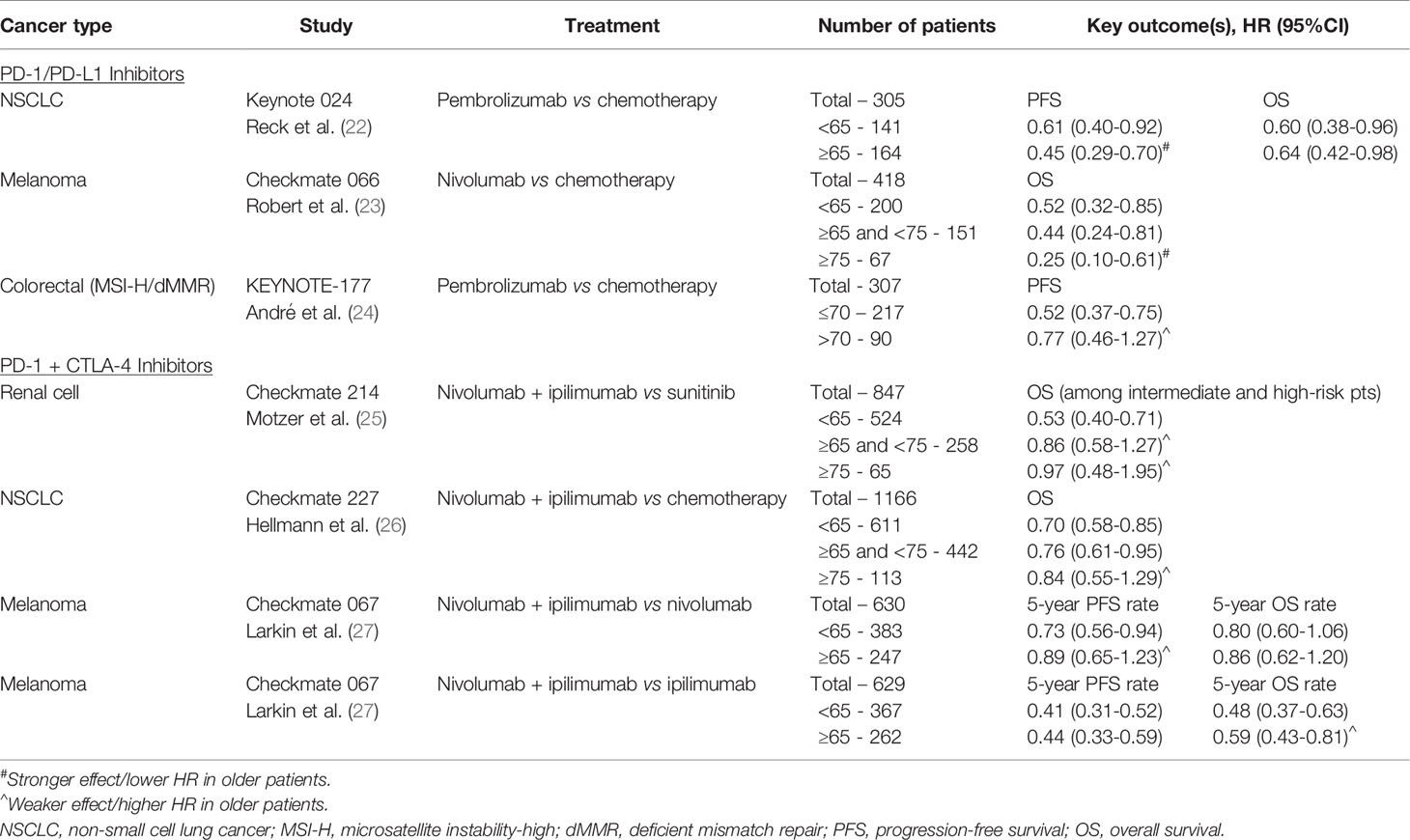
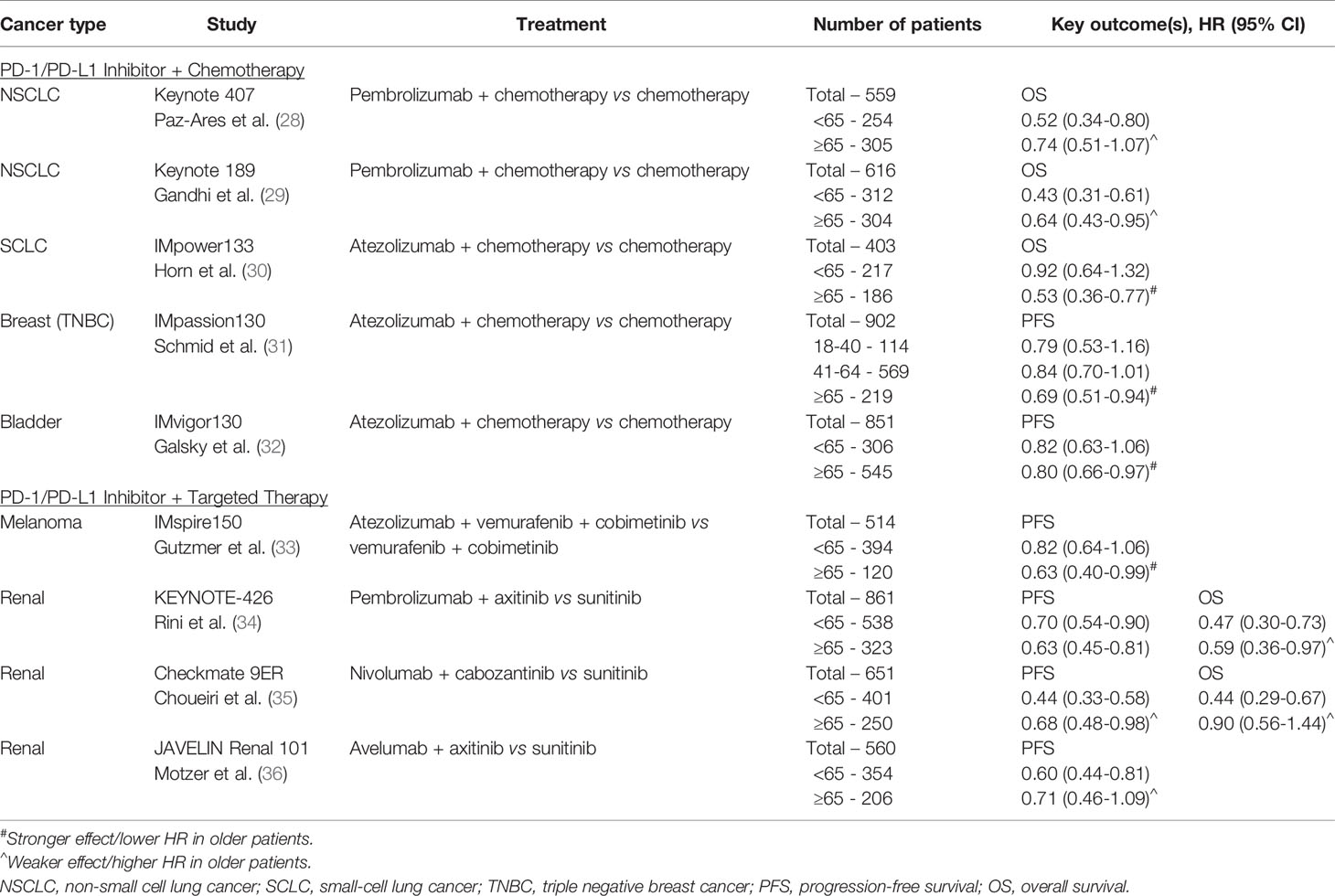
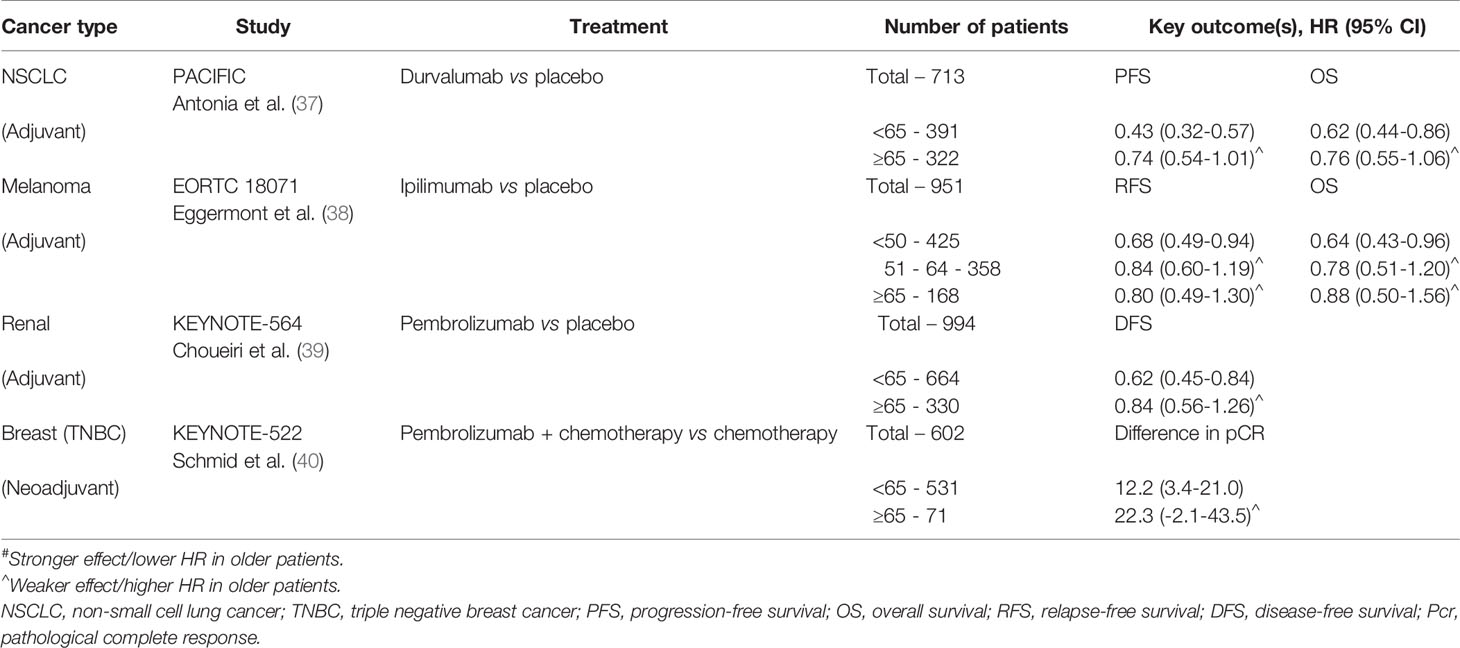
Despite the striking proliferation of ICI-based clinical trials in the past decade, there remains a gap in knowledge regarding the impact of age on the efficacy of ICIs. The ability to draw conclusions from clinical trial data is limited by the small proportion of older participants, all of whom are also highly selected for fitness and unlikely representative of the general older population. Existing knowledge on ICIs in older patients has been derived from data across tumor types and drawn from pooled meta-analyses of clinical trials, retrospective studies, and case reports. We have summarized key outcomes from select phase III clinical trials in Tables 1–3. Although ultimately inconclusive on the basis of subgroup analyses of data from single clinical trials, we have highlighted trends suggestive of age-related differences in ICI efficacy in the tables.
To elucidate the impact of age on efficacy of ICIs, several meta-analyses have been performed with 65 and 75 years as the most commonly used age cut-offs to differentiate between older versus younger patients. One of the largest meta-analyses was reported by Huang et al. in 2019, including 34 studies and containing over 20,000 patients across various advanced tumor types. Survival analyses were done using subgroup analyses with cutoffs of either 65 years and 75 years, depending on each individual study. ICIs were associated with statistically significant improvement in overall survival (OS) in patients <65 years, ≥ 65 years, and <75 years compared to their respective control groups, but less so for patients ≥75 years (HR 0.88 compared with control for ≥75 years, 95%CI 0.67-1.16, p=0.377). As for progression-free survival (PFS), improvement over control groups was seen for patients <65 years and ≥ 65 years, but not in subgroup analyses for patients <75 years or ≥75 years. Importantly, the authors noted that analyses of the ≥75 year patients were limited by the relatively small number of patients in this age cohort (~3%), and relatively fewer studies using age of 75 years as a cutoff for subgroup analyses (41). Several other meta-analyses have also demonstrated no statistically significant difference in treatment efficacy of ICI-based therapy between younger and older patients using the age cut-off of 65 years (7, 8, 42, 43), with potentially less benefit among patients 75 years and older (6, 44–47).
Real-world retrospective analyses have similarly demonstrated comparable outcomes across age groups with respect to efficacy and safety, although drawing definitive conclusions remains challenging given the different age cut-offs used across studies and the relatively small proportion of patients over the age of 70-75 (48–50). A large retrospective review of 410 patients treated with single-agent ICI across lung cancer, melanoma, and genitourinary cancer (150 patients aged 70 or older and 185 patients 69 or younger) found no significant difference between age groups in regard to PFS, OS, or grade 3 or higher irAEs (49). Ibrahim et al. evaluated older patients with a retrospective single-institution cohort study of 99 patients aged 75 and up (median age of 80 years) treated with ICI monotherapy for metastatic melanoma, demonstrating effectiveness and safety despite advanced age (51). More recently, a study of 45 patients 80 years or older (median age of 85) with non-small cell lung cancer (NSCLC) similarly determined single-agent ICI to be a reasonable option with disease control rate of 60% and PFS of 3.4 months (52). Also, among very elderly patients, a retrospective study by our group found that NSCLC, melanoma and genitourinary cancer patients aged ≥85 years experienced similar efficacy of single-agent checkpoint inhibitors when compared to patients aged 80-85 years. Objective response rate among NSCLC (n=276), melanoma (n=280), and genitourinary cancer patients (n=126) over the age of 80 years was 32%, 39% and 26%, respectively (53). Data among nonagenarian patients become even more limited. One case report summarizes the clinical course of three patients over the age of 90 treated for metastatic melanoma: two patients treated with single-agent ICI and one patient who received combination ICI. Although one patient required high-dose corticosteroids for grade 2 hepatitis while on combination nivolumab and ipilimumab, she was able to resume single-agent nivolumab following resolution of the irAE. Among the three patients, two achieved complete or partial response while one other had prolonged stable disease (54).
The specific ICI target axis (PD-1/PD-L1 versus CTLA-4) may potentiate the effect of age on treatment efficacy due to underlying changes associated with immunosenescence. A pooled analysis of 24 randomized trials by Ninomiya et al. found no age-dependent difference in survival benefit from ICIs in patients younger versus older than 65 years (HR 0.76 versus 0.78, p=0.82), but subgroup analyses evaluating the impact of ICI type suggested less survival benefit for older patients compared to their younger counterparts among those treated with an anti-CTLA-4 ICI (HR 0.90 vs 0.77, p=0.26). This difference in efficacy between the two age groups was not seen with PD-1/PD-L1 inhibitors, HR 0.74 vs 0.74, p=0.96 (42). While the CTLA-4 pathway is thought to operate earlier in the immune response by regulating autoimmunity at the initial stage of naïve T cell activation, PD-1 regulates already activated T cells in peripheral tissues (55). Among older patients, thymic involution, decreased naïve T cell output, and ultimately lower levels of circulating naïve T cells may contribute to a differential age-related survival benefit between CTLA-4 and PD-1/PD-L1 inhibitors (11, 56).
Finally, there have been relatively sparse data regarding extremely young patients. Small prospective and retrospective studies have suggested that pediatric patients may respond to ICIs, including with CNS tumors, lymphoma, and solid tumors (57–59). One study of pembrolizumab showed that 9 of 15 pediatric patients with Hodgkin lymphoma responded to treatment, broadly similar to adult data. Responses across a range of PD-L1-positive solid tumors though, were infreuqent in this study (59). It is unclear whether age is related to these findings, or whether other factors like histology or low mutational burden play a larger role. Similarly, there have been few studies in younger adults (e.g., under 40 years), so it remains unclear whether ICI has comparable efficacy in this population. The generally high response rates generated by ICI in many cancers that affect young adults (melanoma, MSI-high cancers, Hodgkin lymphoma) suggest that many patients do experience responses.
Toxicity
Despite the success of ICIs, the risks of irAEs remain an important consideration in the assessment and counselling of patients for therapy (60). The risk, type, and severity of toxicities are variable depending on individual patient characteristics as well as the ICI regimen used. Up to 10-30% of patients can develop a grade 3 or higher irAE with single-agent PD-1/PD-L1 or CTLA-4 inhibitors, and this increases to over 50% for those receiving combination ICI therapy (61). Though mild to moderate irAEs can often be managed with the initiation of systemic corticosteroids and/or discontinuation of the offending ICI, severe irAEs can require additional immunosuppressive therapies and are potentially life-threatening.
A 2021 update of the FDA adverse event reporting system found an increased rate of irAEs in adults ages ≥65 as compared to 18-64 (41.55% versus 33.3%) among those treated with ICI monotherapy or combinations (62). Data from a retrospective analysis suggested that patients more likely to suffer fatal irAEs tended to be older (median age of 70 vs 62 years) (63). A retrospective case-control study of patients with melanoma, renal cell carcinoma or NSCLC compared 185 patients <65 years, 154 patients 65-74 years and 109 patients ≥75 years. This study found no significant difference in any-grade irAE rates between age cohorts. Endocrine toxicity was found to be more common in patients <65 years, while dermatologic toxicity was more common in patients ≥75 years. Interestingly, older patients were found to be less likely to discontinue ICI treatment due to toxicity (discontinuation rate 7.4% among patients ≥75 years versus 20.5% among patients <65 years, p=0.006) (64). Additional retrospective series have suggested that rheumatologic irAEs are more common in older patients, whereas hepatitis and colitis may be more prevalent in younger patients (48). Shah et al. found a similar rates of any-grade irAE, but decreased rate of severe toxicity and hospitalization rate in older adults with melanoma as compared to younger patients. Older adults hospitalized for irAEs, however, experienced longer hospital stays and increased risk of death from irAE. This finding was confirmed with a validation pharmacoviligance dataset (65).
Prompted by concerns that chronologic age alone does not adequately predict treatment tolerance, geriatric-specific assessment indices have been developed to better stratify older adult patients into distinct functional groups for the purpose of predicting treatment tolerance. Developed by geriatricians, these indices assess functional status, comorbidities, cognitive function, nutritional status, psychological state, social support, and concurrent medications, and may be patient- or provider-administered (66, 67). Previously developed tools have demonstrated validity in stratifying older adult patients into predicted outcome groups based upon treatment with traditional cytotoxic chemotherapy and/or radiation, but such tools have not yet been well validated in the era of immunotherapy (68). One small study of 28 older cancer patients has suggested that a high prevalence of impairment in geriatric assessment domains is associated with shorter duration of treatment with ICI, although it was unclear whether this correlated with increased irAEs or worse survival outcome (69). Sarcopenia, which is more common in older adults, has also been reported as correlating with inferior outcomes in many cancer therapies. However, studies are conflicting whether this correlates with ICI responses (70–72).
Another consideration for geriatric adults is whether they have sufficient functional reserve to recover from severe toxicities. Several common irAEs may impose substantial physiologic strain and critical illness in some cases (e.g., pneumonitis, colitis, myocarditis), potentially limiting some patients’ ability to recover. High-dose steroids, the treatment for severe irAEs, also carry the risks of delirium, arrhythmias, hyperglycemia, and infection, all of which may particularly impact older patients.
When weighing the potential risks and benefits of ICIs, there are unique challenges faced by younger patients that are distinct from those of their older counterparts. ICI-related infertility is one such issue that has recently garnered attention, albeit still an area that is not well studied. Fertility can be affected by primary hypogonadism (via direct impacts on the gonads such as orchitis and impaired spermatogenesis/oogenesis) or secondary hypogonadism as a result of hypophysitis (73). ICI-mediated inflammation of the gonads can uncommonly result in hypogonadism and possibly impaired spermatogenesis, as several cases of epididymo-orchitis (unilateral and bilateral) have been reported (74–76). Subclinical injury may also occur in the absence of overt inflammation; a retrospective cohort autopsy study by Scovell et al. of 13 men (median age of 54, range 23-78 years) with metastatic melanoma found that 6 out of 7 men who received ICI therapy had histopathologic evidence of impaired spermatogenesis compared to 2 of 6 men who were treatment-naïve (77).
Infertility can also result from dysregulation of the pituitary gland. Hypophysitis is an irAE that appears to be more common with anti-CTLA-4 agents than PD-1/PD-L1 inhibitors (78), with rates of hypophysitis up to 11% among individuals receiving ipilimumab (79, 80). Given the critical role of the pituitary in downstream hormone regulation and endocrine homeostasis, its impairment carries the risk of premature menopause in women and impaired sperm production in men.
Pregnancy is another challenging situation unique to younger patients with cancer treated with ICIs. Of note, PD-L1 is highly expressed on syncytiotrophoblast cells in the placenta and likely plays a critical role in maintaining fetal tolerance (81). There is only limited evidence for outcomes apart from several case reports of pregnancy during ICI therapy (82–84). While there have been case reports of inadvertent but successful conception during treatment with ICI, the United Stated Food and Drug Administration (FDA) has assigned pregnancy category C (risk not ruled out) to ipilimumab, category D (positive evidence of risk) to pembrolizumab, and category X (contraindicated in pregnancy) for durvalumab (other FDA-approved ICIs remain unassigned at time of publication). Because melanoma is the most common malignancy diagnosed during pregnancy, further studies dedicated to understanding this patient population are needed (85).
Conclusion
ICIs appear to have comparable efficacy for younger and older patients, although meta-analysis and retrospective data suggest that the magnitude of benefit may be smaller in those over the age of 75 and in the setting of anti-CTLA-4 ICIs, potentially due to underlying changes associated with immunosenescence. Younger patients have particular considerations related to toxicities, including infertility and contraception. Given the expanding role of ICIs in the armamentarium of cancer therapies, targeted clinical trials are needed to prospectively deepen our understanding of these agents in older adults.
Author Contributions
All authors contributed to writing and editing the manuscript.
Funding
Funded by the Susan and Luke Simons Directorship in Melanoma Fund.
Conflict of Interest
DJ advises/consults for BMS, Catalyst, Jansen, Iovance, Merck, Mosaic BioEngineering, Novartis, Oncosec, Pfizer, and Targovax, and receives research funding from BMS and Incyte.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Group UCSW. United States Cancer Statistics: 1999–2010 Incidence and Mortality Web-Based Report. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute (2013). p. 201.
2. Denson AC, Mahipal A. Participation of the Elderly Population in Clinical Trials: Barriers and Solutions. Cancer Control (2014) 21(3):209–14. doi: 10.1177/107327481402100305
3. Sedrak MS, Mohile SG, Sun V, Sun CL, Chen BT, Li D, et al. Barriers to Clinical Trial Enrollment of Older Adults With Cancer: A Qualitative Study of the Perceptions of Community and Academic Oncologists. J Geriatr Oncol (2020) 11(2):327–34. doi: 10.1016/j.jgo.2019.07.017
4. Singh H, Kanapuru B, Smith C, Fashoyin-Aje LA, Myers A, Kim G, et al. FDA Analysis of Enrollment of Older Adults in Clinical Trials for Cancer Drug Registration: A 10-Year Experience by the U.S. Food and Drug Administration. J Clin Oncol (2017) 35(15_suppl):10009–9. doi: 10.1200/JCO.2017.35.15_suppl.10009
5. George M, Smith A, Sabesan S, Ranmuthugala G. Physical Comorbidities and Their Relationship With Cancer Treatment and Its Outcomes in Older Adult Populations: Systematic Review. JMIR Cancer (2021) 7(4):e26425. doi: 10.2196/26425
6. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Comparative Analysis of Immune Checkpoint Inhibitors and Chemotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. Med (Baltimore) (2018) 97(33):e11936. doi: 10.1097/MD.0000000000011936
7. Elias R, Giobbie-Hurder A, McCleary NJ, Ott P, Hodi FS, Rahma O. Efficacy of PD-1 & PD-L1 Inhibitors in Older Adults: A Meta-Analysis. J Immunother Cancer (2018) 6(1):26. doi: 10.1186/s40425-018-0336-8
8. Poropatich K, Fontanarosa J, Samant S, Sosman JA, Zhang B. Cancer Immunotherapies: Are They as Effective in the Elderly? Drugs Aging (2017) 34(8):567–81. doi: 10.1007/s40266-017-0479-1
9. Pawelec G. Hallmarks of Human "Immunosenescence": Adaptation or Dysregulation? Immun Ageing (2012) 9(1):15. doi: 10.1186/1742-4933-9-15
10. Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, et al. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front Immunol (2019) 10:2247. doi: 10.3389/fimmu.2019.02247
11. Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint J-P, Labalette M. Accumulation of Memory T Cells From Childhood to Old Age: Central and Effector Memory Cells in CD4+ Versus Effector Memory and Terminally Differentiated Memory Cells in CD8+ Compartment. Mech Ageing Dev (2006) 127(3):274–81. doi: 10.1016/j.mad.2005.11.001
12. Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB, et al. Age-Related Decrease in TCR Repertoire Diversity Measured With Deep and Normalized Sequence Profiling. J Immunol (2014) 192(6):2689–98. doi: 10.4049/jimmunol.1302064
13. Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-Related CD8 T Cell Clonal Expansions Constrict CD8 T Cell Repertoire and Have the Potential to Impair Immune Defense. J Exp Med (2004) 200(10):1347–58. doi: 10.1084/jem.20040437
14. Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The Influence of Age on T Cell Generation and TCR Diversity. J Immunol (2005) 174(11):7446–52. doi: 10.4049/jimmunol.174.11.7446
15. Jagger A, Shimojima Y, Goronzy JJ, Weyand CM. Regulatory T Cells and the Immune Aging Process: A Mini-Review. Gerontology (2014) 60(2):130–7. doi: 10.1159/000355303
16. Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T Cell Memory Derived From Young Naive Cells Functions Well Into Old Age, But Memory Generated From Aged Naive Cells Functions Poorly. Proc Natl Acad Sci U S A (2003) 100(25):15053–8. doi: 10.1073/pnas.2433717100
17. Dunn J, Rao S. Epigenetics and Immunotherapy: The Current State of Play. Mol Immunol (2017) 87:227–39. doi: 10.1016/j.molimm.2017.04.012
18. Keenan CR, Allan RS. Epigenomic Drivers of Immune Dysfunction in Aging. Aging Cell (2019) 18(1):e12878. doi: 10.1111/acel.12878
19. Villanueva L, Álvarez-Errico D, Esteller M. The Contribution of Epigenetics to Cancer Immunotherapy. Trends Immunol (2020) 41(8):676–91. doi: 10.1016/j.it.2020.06.002
20. Lanna A, Gomes DC, Muller-Durovic B, McDonnell T, Escors D, Gilroy DW, et al. A Sestrin-Dependent Erk-Jnk-P38 MAPK Activation Complex Inhibits Immunity During Aging. Nat Immunol (2017) 18(3):354–63. doi: 10.1038/ni.3665
21. Goronzy JJ, Li G, Yang Z, Weyand CM. The Janus Head of T Cell Aging - Autoimmunity and Immunodeficiency. Front Immunol (2013) 4:131. doi: 10.3389/fimmu.2013.00131
22. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
23. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in Previously Untreated Melanoma Without BRAF Mutation. N Engl J Med (2015) 372(4):320–30. doi: 10.1056/NEJMoa1412082
24. André T, Shiu K-K, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N Engl J Med (2020) 383(23):2207–18. doi: 10.1056/NEJMoa2017699
25. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab Plus Ipilimumab Versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med (2018) 378(14):1277–90. doi: 10.1056/NEJMoa1712126
26. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
27. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. Five-Year Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2019) 381(16):1535–46. doi: 10.1056/NEJMoa1910836
28. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab Plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med (2018) 379(21):2040–51. doi: 10.1056/NEJMoa1810865
29. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab Plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
30. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
31. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med (2018) 379(22):2108–21. doi: 10.1056/NEJMoa1809615
32. Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab With or Without Chemotherapy in Metastatic Urothelial Cancer (IMvigor130): A Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet (London England) (2020) 395(10236):1547–57. doi: 10.1016/S0140-6736(20)30230-0
33. Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, Vemurafenib, and Cobimetinib as First-Line Treatment for Unresectable Advanced BRAF(V600) Mutation-Positive Melanoma (IMspire150): Primary Analysis of the Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (London England) (2020) 395(10240):1835–44. doi: 10.1016/S0140-6736(20)30934-X
34. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab Plus Axitinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380(12):1116–27. doi: 10.1056/NEJMoa1816714
35. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab Plus Cabozantinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2021) 384(9):829–41. doi: 10.1056/NEJMoa2026982
36. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab Plus Axitinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380(12):1103–15. doi: 10.1056/NEJMoa1816047
37. Antonia SJ, Villegas A, Daniel D, Vicente SJ, Murakami S, Hui R, et al. Overall Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC. N Engl J Med (2018) 379(24):2342–50. doi: 10.1056/NEJMoa1809697
38. Eggermont AMM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant Ipilimumab Versus Placebo After Complete Resection of Stage III Melanoma: Long-Term Follow-Up Results of the European Organisation for Research and Treatment of Cancer 18071 Double-Blind Phase 3 Randomised Trial. Eur J Cancer (Oxford England: 1990) (2019) 119:1–10. doi: 10.1016/j.ejca.2019.07.001
39. Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang Y-H, et al. Adjuvant Pembrolizumab After Nephrectomy in Renal-Cell Carcinoma. N Engl J Med (2021) 385(8):683–94. doi: 10.1056/NEJMoa2106391
40. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med (2020) 382(9):810–21. doi: 10.1056/NEJMoa1910549
41. Huang XZ, Gao P, Song YX, Sun JX, Chen XW, Zhao JH, et al. Efficacy of Immune Checkpoint Inhibitors and Age in Cancer Patients. Immunotherapy (2020) 12(8):587–603. doi: 10.2217/imt-2019-0124
42. Ninomiya K, Oze I, Kato Y, Kubo T, Ichihara E, Rai K, et al. Influence of Age on the Efficacy of Immune Checkpoint Inhibitors in Advanced Cancers: A Systematic Review and Meta-Analysis. Acta Oncologica (2020) 59(3):249–56. doi: 10.1080/0284186X.2019.1695062
43. Yan X, Tian X, Wu Z, Han W. Impact of Age on the Efficacy of Immune Checkpoint Inhibitor-Based Combination Therapy for Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front Oncol (2020) 10:1671. doi: 10.3389/fonc.2020.01671
44. Nishijima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of Efficacy of Immune Checkpoint Inhibitors (ICIs) Between Younger and Older Patients: A Systematic Review and Meta-Analysis. Cancer Treat Rev (2016) 45:30–7. doi: 10.1016/j.ctrv.2016.02.006
45. Wu Y, Ju Q, Qian B, Zhang F, Shi H. The Effectiveness of PD-1 Inhibitors in Non-Small Cell Lung Cancer (NSCLC) Patients of Different Ages. Oncotarget (2018) 9(8):7942–8. doi: 10.18632/oncotarget.23678
46. Zhang L, Sun L, Yu J, Shan F, Zhang K, Pang X, et al. Comparison of Immune Checkpoint Inhibitors Between Older and Younger Patients With Advanced or Metastatic Lung Cancer: A Systematic Review and Meta-Analysis. BioMed Res Int (2019) 2019:9853701. doi: 10.1155/2019/9853701
47. Zheng SY, Cui HJ, Duan H, Peng YM, Li Q, Sun CY, et al. The Efficacy and Safety of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer Patients of Different Age Groups: A Meta-Analysis. Clin Transl Oncol (2020) 22(7):1146–54. doi: 10.1007/s12094-019-02241-5
48. Betof AS, Nipp RD, Giobbie-Hurder A, Johnpulle RAN, Rubin K, Rubinstein SM, et al. Impact of Age on Outcomes With Immunotherapy for Patients With Melanoma. Oncologist (2017) 22(8):963–71. doi: 10.1634/theoncologist.2016-0450
49. Corbaux P, Maillet D, Boespflug A, Locatelli-Sanchez M, Perier-Muzet M, Duruisseaux M, et al. Older and Younger Patients Treated With Immune Checkpoint Inhibitors Have Similar Outcomes in Real-Life Setting. Eur J Cancer (2019) 121:192–201. doi: 10.1016/j.ejca.2019.08.027
50. Galli G, De Toma A, Pagani F, Randon G, Trevisan B, Prelaj A, et al. Efficacy and Safety of Immunotherapy in Elderly Patients With Non-Small Cell Lung Cancer. Lung Cancer (2019) 137:38–42. doi: 10.1016/j.lungcan.2019.08.030
51. Ibrahim T, Mateus C, Baz M, Robert C. Older Melanoma Patients Aged 75 and Above Retain Responsiveness to Anti-PD1 Therapy: Results of a Retrospective Single-Institution Cohort Study. Cancer Immunol Immunother (2018) 67(10):1571–8. doi: 10.1007/s00262-018-2219-8
52. Saito Z, Fujita K, Okamura M, Ito T, Yamamoto Y, Kanai O, et al. Efficacy and Safety of Immune Checkpoint Inhibitors in Patients With Non-Small Cell Lung Cancer Aged 80 Years or Older. Cancer Rep, e1405. doi: 10.1002/cnr2.1405
53. Nebhan CA, Cortellini A, Ma W, Ganta T, Song H, Ye F, et al. Clinical Outcomes and Toxic Effects of Single-Agent Immune Checkpoint Inhibitors Among Patients Aged 80 Years or Older With Cancer: A Multicenter International Cohort Study. JAMA Oncol (2021). doi: 10.1001/jamaoncol.2021.4960
54. Johnpulle RA, Conry RM, Sosman JA, Puzanov I, Johnson DB. Responses to Immune Checkpoint Inhibitors in Nonagenarians. Oncoimmunology (2016) 5(11):e1234572. doi: 10.1080/2162402X.2016.1234572
55. Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol (2016) 39(1):98–106. doi: 10.1097/COC.0000000000000239
56. Palmer DB. The Effect of Age on Thymic Function. Front Immunol (2013) 4:316. doi: 10.3389/fimmu.2013.00316
57. Cacciotti C, Choi J, Alexandrescu S, Zimmerman MA, Cooney TM, Chordas C, et al. Immune Checkpoint Inhibition for Pediatric Patients With Recurrent/Refractory CNS Tumors: A Single Institution Experience. J Neurooncol (2020) 149(1):113–22. doi: 10.1007/s11060-020-03578-6
58. Davis KL, Fox E, Merchant MS, Reid JM, Kudgus RA, Liu X, et al. Nivolumab in Children and Young Adults With Relapsed or Refractory Solid Tumours or Lymphoma (ADVL1412): A Multicentre, Open-Label, Single-Arm, Phase 1-2 Trial. Lancet Oncol (2020) 21(4):541–50. doi: 10.1016/S1470-2045(20)30023-1
59. Geoerger B, Kang HJ, Yalon-Oren M, Marshall LV, Vezina C, Pappo A, et al. Pembrolizumab in Paediatric Patients With Advanced Melanoma or a PD-L1-Positive, Advanced, Relapsed, or Refractory Solid Tumour or Lymphoma (KEYNOTE-051): Interim Analysis of an Open-Label, Single-Arm, Phase 1-2 Trial. Lancet Oncol (2020) 21(1):121–33. doi: 10.1016/S1470-2045(19)30671-0
60. Johnson DB, Chandra S, Sosman JA. Immune Checkpoint Inhibitor Toxicity in 2018. JAMA (2018) 320(16):1702–3. doi: 10.1001/jama.2018.13995
61. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat Rev Clin Oncol (2019) 16(9):563–80. doi: 10.1038/s41571-019-0218-0
62. Chen C, Wu B, Zhang C, Xu T. Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors: An Updated Comprehensive Disproportionality Analysis of the FDA Adverse Event Reporting System. Int Immunopharmacol (2021) 95:107498. doi: 10.1016/j.intimp.2021.107498
63. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol (2018) 4(12):1721–8. doi: 10.1001/jamaoncol.2018.3923
64. Samani A, Zhang S, Spiers L, Mohamed AA, Merrick S, Tippu Z, et al. Impact of Age on the Toxicity of Immune Checkpoint Inhibition. J Immunother Cancer (2020) 8(2). doi: 10.1136/jitc-2020-000871
65. Shah KP, Song H, Ye F, Moslehi JJ, Balko JM, Salem JE, et al. Demographic Factors Associated With Toxicity in Patients Treated With Anti-Programmed Cell Death-1 Therapy. Cancer Immunol Res (2020) 8(7):851–5. doi: 10.1158/2326-6066.CIR-19-0986
66. Caillet P, Canoui-Poitrine F, Vouriot J, Berle M, Reinald N, Krypciak S, et al. Comprehensive Geriatric Assessment in the Decision-Making Process in Elderly Patients With Cancer: ELCAPA Study. J Clin Oncol (2011) 29(27):3636–42. doi: 10.1200/JCO.2010.31.0664
67. Hurria A, Gupta S, Zauderer M, Zuckerman EL, Cohen HJ, Muss H, et al. Developing a Cancer-Specific Geriatric Assessment: A Feasibility Study. Cancer (2005) 104(9):1998–2005. doi: 10.1002/cncr.21422
68. Ferrat E, Audureau E, Paillaud E, Liuu E, Tournigand C, Lagrange JL, et al. Four Distinct Health Profiles in Older Patients With Cancer: Latent Class Analysis of the Prospective ELCAPA Cohort. J Gerontol A Biol Sci Med Sci (2016) 71(12):1653–60. doi: 10.1093/gerona/glw052
69. Welaya K, Loh KP, Messing S, Szuba E, Magnuson A, Mohile SG, et al. Geriatric Assessment and Treatment Outcomes in Older Adults With Cancer Receiving Immune Checkpoint Inhibitors. J Geriatr Oncol (2020) 11(3):523–8. doi: 10.1016/j.jgo.2019.05.021
70. Akce M, Liu Y, Zakka K, Martini DJ, Draper A, Alese OB, et al. Impact of Sarcopenia, BMI, and Inflammatory Biomarkers on Survival in Advanced Hepatocellular Carcinoma Treated With Anti-PD-1 Antibody. Am J Clin Oncol (2021) 44(2):74–81. doi: 10.1097/COC.0000000000000787
71. Minami S, Ihara S, Tanaka T, Komuta K. Sarcopenia and Visceral Adiposity Did Not Affect Efficacy of Immune-Checkpoint Inhibitor Monotherapy for Pretreated Patients With Advanced Non-Small Cell Lung Cancer. World J Oncol (2020) 11(1):9–22. doi: 10.14740/wjon1225
72. Young AC, Quach HT, Song H, Davis EJ, Moslehi JJ, Ye F, et al. Impact of Body Composition on Outcomes From Anti-PD1 +/- Anti-CTLA-4 Treatment in Melanoma. J Immunother Cancer (2020) 8(2). doi: 10.1136/jitc-2020-000821
73. Özdemir BC. Immune Checkpoint Inhibitor-Related Hypogonadism and Infertility: A Neglected Issue in Immuno-Oncology. J Immunother Cancer (2021) 9(2). doi: 10.1136/jitc-2020-002220
74. Salzmann M, Tosev G, Heck M, Schadendorf D, Maatouk I, Enk AH, et al. Male Fertility During and After Immune Checkpoint Inhibitor Therapy: A Cross-Sectional Pilot Study. Eur J Cancer (2021) 152:41–8. doi: 10.1016/j.ejca.2021.04.031
75. Brunet-Possenti F, Opsomer MA, Gomez L, Ouzaid I, Descamps V. Immune Checkpoint Inhibitors-Related Orchitis. Ann Oncol (2017) 28(4):906–7. doi: 10.1093/annonc/mdw696
76. Quach HT, Robbins CJ, Balko JM, Chiu CY, Miller S, Wilson MR, et al. Severe Epididymo-Orchitis and Encephalitis Complicating Anti-PD-1 Therapy. Oncologist (2019) 24(7):872–6. doi: 10.1634/theoncologist.2018-0722
77. Scovell JM, Benz K, Samarska I, Kohn TP, Hooper JE, Matoso A, et al. Association of Impaired Spermatogenesis With the Use of Immune Checkpoint Inhibitors in Patients With Metastatic Melanoma. JAMA Oncol (2020) 6(8):1297–9. doi: 10.1001/jamaoncol.2020.1641
78. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
79. Albarel F, Gaudy C, Castinetti F, Carré T, Morange I, Conte-Devolx B, et al. Long-Term Follow-Up of Ipilimumab-Induced Hypophysitis, a Common Adverse Event of the Anti-CTLA-4 Antibody in Melanoma. Eur J Endocrinol (2015) 172(2):195–204. doi: 10.1530/EJE-14-0845
80. Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, et al. Ipilimumab-Induced Hypophysitis: A Detailed Longitudinal Analysis in a Large Cohort of Patients With Metastatic Melanoma. J Clin Endocrinol Metab (2014) 99(11):4078–85. doi: 10.1210/jc.2014-2306
81. Veras E, Kurman RJ, Wang TL, Shih IM. PD-L1 Expression in Human Placentas and Gestational Trophoblastic Diseases. Int J Gynecol Pathol (2017) 36(2):146–53. doi: 10.1097/PGP.0000000000000305
82. Bucheit AD, Hardy JT, Szender JB, Glitza Oliva IC. Conception and Viable Twin Pregnancy in a Patient With Metastatic Melanoma While Treated With CTLA-4 and PD-1 Checkpoint Inhibition. Melanoma Res (2020) 30(4):423–5. doi: 10.1097/CMR.0000000000000657
83. Burotto M, Gormaz JG, Samtani S, Valls N, Silva R, Rojas C, et al. Viable Pregnancy in a Patient With Metastatic Melanoma Treated With Double Checkpoint Immunotherapy. Semin Oncol (2018) 45(3):164–9. doi: 10.1053/j.seminoncol.2018.03.003
84. Xu W, Moor RJ, Walpole ET, Atkinson VG. Pregnancy With Successful Foetal and Maternal Outcome in a Melanoma Patient Treated With Nivolumab in the First Trimester: Case Report and Review of the Literature. Melanoma Res (2019) 29(3):333–7. doi: 10.1097/CMR.0000000000000586
Keywords: age, geriatric, PD-1, Nivolumab, Pembrolizumab, ipilimumab, toxicity, young
Citation: Wong SK, Nebhan CA and Johnson DB (2021) Impact of Patient Age on Clinical Efficacy and Toxicity of Checkpoint Inhibitor Therapy. Front. Immunol. 12:786046. doi: 10.3389/fimmu.2021.786046
Received: 29 September 2021; Accepted: 29 October 2021;
Published: 16 November 2021.
Edited by:
Joshua B. Rubin, Washington University School of Medicine in St. Louis, United StatesReviewed by:
Anne Caignard, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceJacques A. Nunes, INSERM U1068 Centre de Recherche en Cancérologie de Marseille (CRCM), France
Copyright © 2021 Wong, Nebhan and Johnson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Douglas B. Johnson, RG91Z2xhcy5iLmpvaG5zb25AdnVtYy5vcmc=
 Selina K. Wong
Selina K. Wong Caroline A. Nebhan
Caroline A. Nebhan Douglas B. Johnson
Douglas B. Johnson