
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 16 December 2021
Sec. Viral Immunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.762782
This article is part of the Research TopicImmunometabolic Mechanisms Underlying the Severity of COVID-19View all 25 articles
 Daniel Elieh Ali Komi1†
Daniel Elieh Ali Komi1† Yaghoub Rahimi1†
Yaghoub Rahimi1† Rahim Asghari2†‡
Rahim Asghari2†‡ Reza Jafari2,3†‡
Reza Jafari2,3†‡ Javad Rasouli4†
Javad Rasouli4† Mehdi Mohebalizadeh5†
Mehdi Mohebalizadeh5† Ata Abbasi6†
Ata Abbasi6† Rahim Nejadrahim7†
Rahim Nejadrahim7† Farzin Rezazadeh8†
Farzin Rezazadeh8† Vahid Shafiei-Irannejad1*†
Vahid Shafiei-Irannejad1*†Coagulopathy is a frequently reported finding in the pathology of coronavirus disease 2019 (COVID-19); however, the molecular mechanism, the involved coagulation factors, and the role of regulatory proteins in homeostasis are not fully investigated. We explored the dynamic changes of nine coagulation tests in patients and controls to propose a molecular mechanism for COVID-19-associated coagulopathy. Coagulation tests including prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen (FIB), lupus anticoagulant (LAC), proteins C and S, antithrombin III (ATIII), D-dimer, and fibrin degradation products (FDPs) were performed on plasma collected from 105 individuals (35 critical patients, 35 severe patients, and 35 healthy controls). There was a statically significant difference when the results of the critical (CRT) and/or severe (SVR) group for the following tests were compared to the control (CRL) group: PTCRT (15.014) and PTSVR (13.846) (PTCRL = 13.383, p < 0.001), PTTCRT (42.923) and PTTSVR (37.8) (PTTCRL = 36.494, p < 0.001), LACCRT (49.414) and LACSVR (47.046) (LACCRL = 40.763, p < 0.001), FIBCRT (537.66) and FIBSVR (480.29) (FIBCRL = 283.57, p < 0.001), ProCCRT (85.57%) and ProCSVR (99.34%) (ProCCRL = 94.31%, p = 0.04), ProSCRT (62.91%) and ProSSVR (65.06%) (ProSCRL = 75.03%, p < 0.001), D-dimer (p < 0.0001, χ2 = 34.812), and FDP (p < 0.002, χ2 = 15.205). No significant association was found in the ATIII results in groups (ATIIICRT = 95.71% and ATIIISVR = 99.63%; ATIIICRL = 98.74%, p = 0.321). D-dimer, FIB, PT, PTT, LAC, protein S, FDP, and protein C (ordered according to p-values) have significance in the prognosis of patients. Disruptions in homeostasis in protein C (and S), VIII/VIIIa and V/Va axes, probably play a role in COVID-19-associated coagulopathy.
Coagulation is a dynamic process that is driven by the regulated proteolytic activation of zymogens (commonly known as coagulation factors) in injured vessels. Coagulation factors, except for FVIII, which is produced by liver sinusoidal endothelial cells and lymphatic tissue, are all produced by hepatocytes (1). The main mechanisms (pathways) that trigger blood clotting include intrinsic and extrinsic pathways, each including a set of coagulation proteins in which factors I, II, IX, X, XI, and XII are the main factors in the intrinsic pathway and factors I, II, VII, and X are the factors described in the extrinsic pathway. Activated partial thromboplastin time (aPTT) and prothrombin time (PT) tests primarily measure the activity of the factors involved in the intrinsic and extrinsic pathways, respectively (2, 3). Moreover, the common pathway is composed of factors I, II, V, VIII, and X (4). The proper proteolytic activation of coagulation factors controlled by a variety of regulatory proteins results in the conversion of soluble fibrinogen to insoluble fibrin strands (5). Fibrinogen, a 340-kDa glycoprotein, is an acute-phase protein consisting of three polypeptide chains, Aα, Bβ, and γ, and becomes upregulated in response to injury and inflammation (5). The term lupus anticoagulant (LAC) is used to determine heterogeneous immunoglobulins, their function resulting in the inhibition of phospholipid-dependent coagulation reactions (6). Moreover, LACs can prolong the PTT test; therefore, a LAC test is used to evaluate prolonged PTT (7). Coagulation regulatory proteins such as antithrombin III (ATIII), protein C, and D-dimer are involved in the normal function and homeostasis of the coagulation system. ATIII, a crucial anticoagulant molecule in mammalian blood, benefits from its cofactor, heparin, to inhibit the coagulation proteases, mainly thrombin and factor Xa (8). Proteins C and S are vitamin K-dependent glycoproteins. Protein S, the cofactor for protein C, supports the activated protein C in the presence of phospholipids and calcium in the inactivation of membrane-bound factors V (FVa) and FVIIIa (9). The mechanistic pathways through which protein C exerts its effects on the coagulation cascades include degrading factors V/Va and VIII/VIIIa, releasing a tissue-type plasminogen activator, and stimulating fibrinolysis by interacting with the plasminogen activator inhibitor (10). Fibrinolysis is an essential step in homeostasis that is finely controlled by a set of cofactors and inhibitors. Plasmin acts as the primary fibrinolysin and is activated from plasminogen in the presence of a tissue plasminogen activator (tPA) or urokinase (uPA) (11). Plasmin, after being produced, lyses the cross-linked fibrin polymers and consequently forms fibrin degradation products (FDPs) such as D-dimer, which is widely used as a specific marker for thrombosis and physiological fibrinolysis (12) (Figure 1). The coagulopathy and abnormal results in coagulation tests have become common features reported in patients with COVID-19 from the very early days of the emergence of the new coronavirus strain. We listed both the common coagulation tests, including PT, PTT, fibrinogen, and D-dimer, and those rarely investigated, such as regulatory proteins C and S as well as ATIII, in patients with COVID-19 along with the main results in Table 1. COVID-19-dependent coagulopathy gained attention when PT, aPTT, fibrinogen, and D-dimer tests were recommended by researchers to evaluate the proper homeostasis of the system associated with the prognosis of patients. Moreover, the prophylactic use of anticoagulants was proven to be effective in lowering the mortality rate and highlighted the role of the coagulation system in COVID-19 (31). The link between thrombosis and COVID-19 as an inflammatory disease has been investigated (32, 33). In the present study, we used a coagulation panel of nine coagulation tests to assess the coagulation pathways in 105 included individuals to determine the molecular mechanism through which COVID-19 disrupts the homeostasis of the coagulation system.
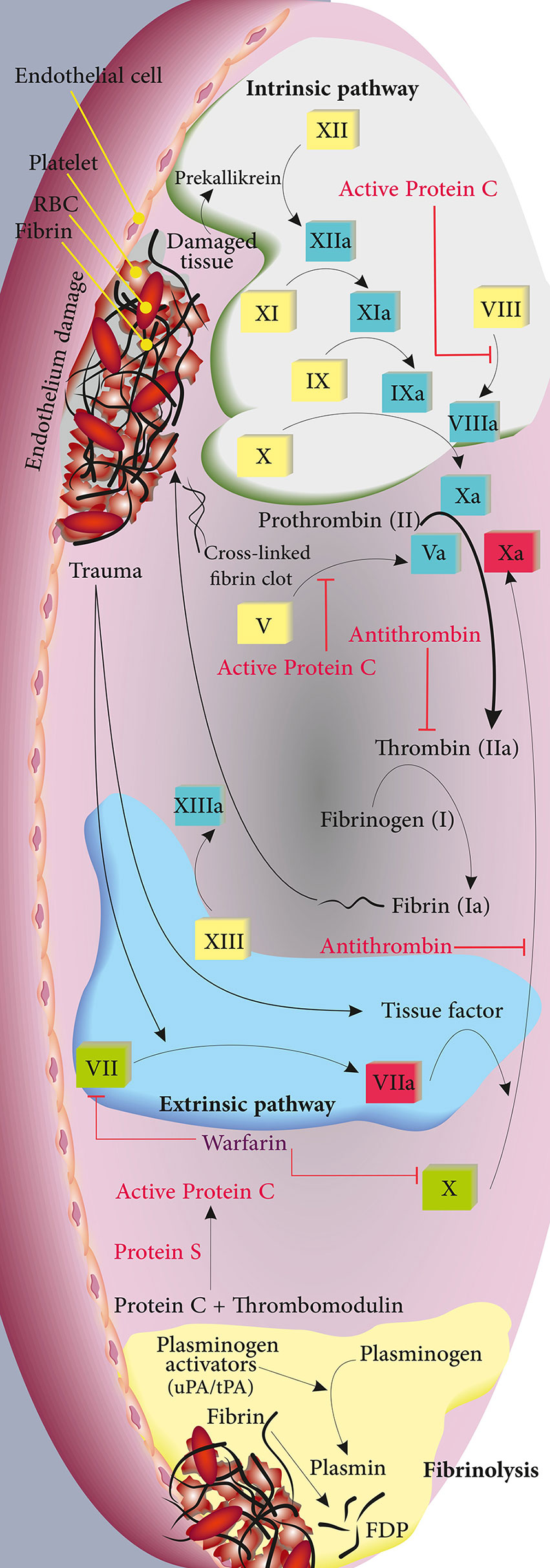
Figure 1 Depiction of the intrinsic, extrinsic, and fibrinolysis pathways in green, blue, and yellow, respectively. The involved coagulation factors for each pathway are shown. Regulatory proteins and their target molecules are shown in red.

Table 1 Brief literature review of the clinical laboratory coagulation indexes previously reported in coronavirus disease 2019 (COVID-19) patients.
We followed the guidelines for Corona Virus Disease 2019 edited by the Iranian National Health Commission (similar to the WHO guidelines and the New Coronavirus Pneumonia Prevention and Control Program, 7th edition, published by the National Health Commission of China) to classify the patients into critical and severe groups (34, 35). The criteria used for the inclusion of individuals into each group are summarized in Table 2. All 70 included patients had a positive result of the nucleic acid test of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by RT-PCR using primers targeting the RNA-dependent RNA polymerase (RdRP) and either nucleocapsid (N), envelope (E), or spike (S) genes. A negative result (using the same probes) was used as the main inclusion criterion for the control (CRL) group. All patients were tested for lung involvement by CT imaging. Moreover, individuals in the CRL group had no physical features of COVID-19 such as fever or coughing and never had a positive RT-PCR result before. We also checked immediate family history to exclude those who have and/or had a family member with a positive PCR test to exclude the possibility of including asymptomatic carriers as healthy controls. Considering that almost all coagulation factors are produced in the liver, any functional disorder in the organ may result in abnormal plasma levels of the factors; therefore, we performed liver functional tests (LFTs) for all 105 included individuals.
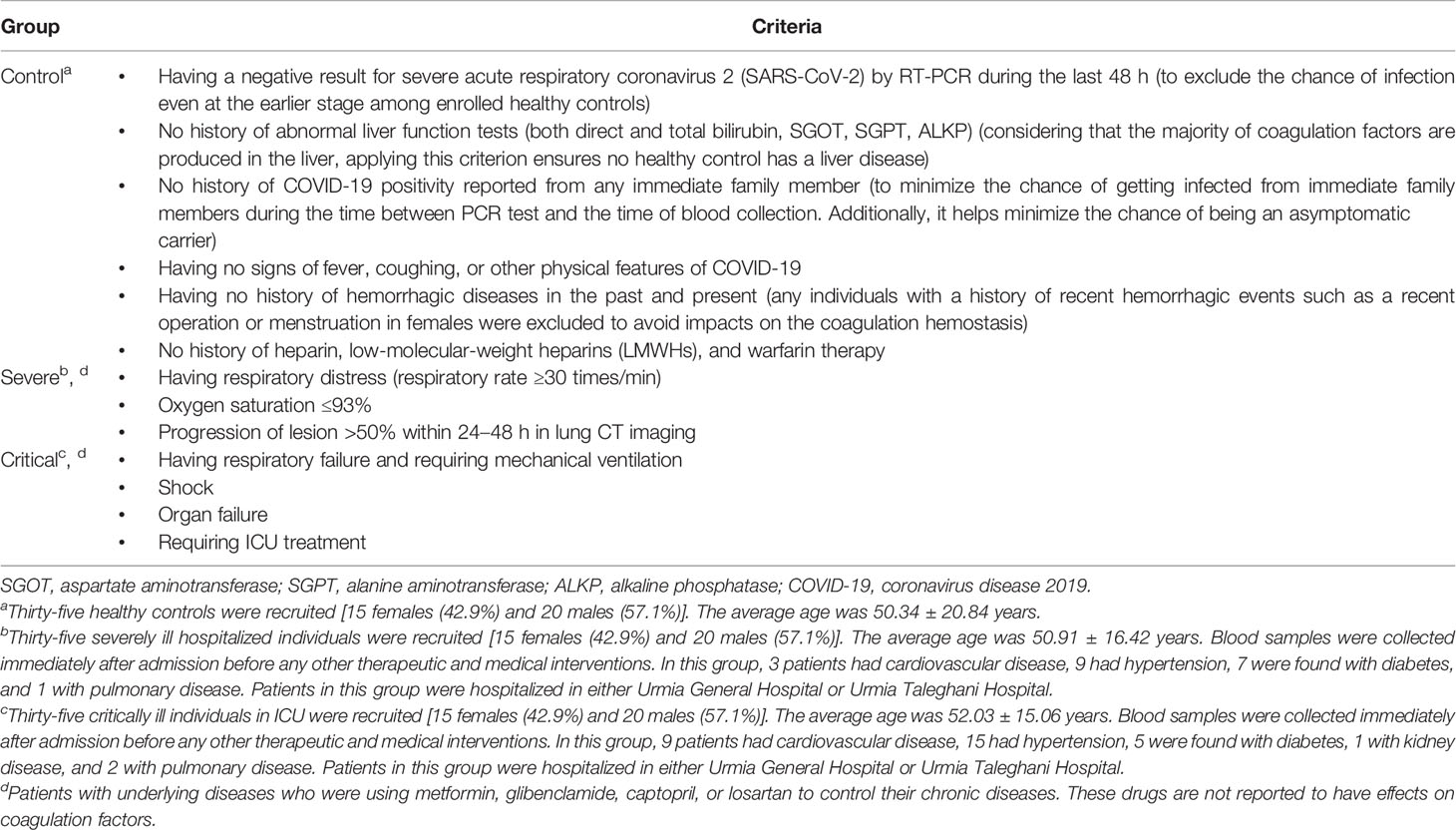
Table 2 Inclusion criteria for the recruitment of individuals into the control (CRL), severe (SVR), and critical (CTL) groups.
In this case–control study, 105 individuals were included and classified into three groups (critical, severe, and control), each consisting of 35 individuals [20 males (57.1%) and 15 females (42.9%)]. The demographic data are presented in Table 3. The mean ages in the three groups were 52.03 (SD = 15.06), 50.91 (SD = 16.42), and 50.34 years (SD = 20.84), respectively. In the critical (CTL) group, the mean weight and height were 72.51 ± 14.75 kg and 163.26 ± 13.88 cm (BMI = 27.86 ± 9.35), while the same parameters in the severe group were measured at 79.14 ± 9.95 kg and 171.80 ± 7.50 cm (BMI = 26.83 ± 3.09), respectively. In the severe (SVR) group, 3 patients had cardiovascular disease, 9 had hypertension, 7 were found with diabetes, and one had pulmonary disease. Furthermore, in the CTL group, 9 had cardiovascular disease, 15 had hypertension, 5 were found with diabetes, one with kidney disease, and two with pulmonary disease. These patients were using metformin, glibenclamide, captopril, or losartan to control their chronic diseases, which have no effects on the coagulation factors. Due to the prophylactic guidelines for the administration of anticoagulation drugs to patients with poor health conditions, we collected the samples at admission before any medical intervention. Moreover, we checked for history of any drug use that could potentially interfere with our results by referring to medical insurance records and through collecting information using an enrollment form. Assuming an α value set at 0.05 (type I error), β at 0.10 (type II errors), a dropout rate of 5%, and considering the results of Gao et al. (15), the sample size was set at a minimum of 35 patients in each group to compare the differences between the means. Patients in the severe and critical groups were selected from hospitalized patients in COVID and ICU wards, respectively, from either Urmia General Hospital or Taleghani Hospital in Urmia, Iran.
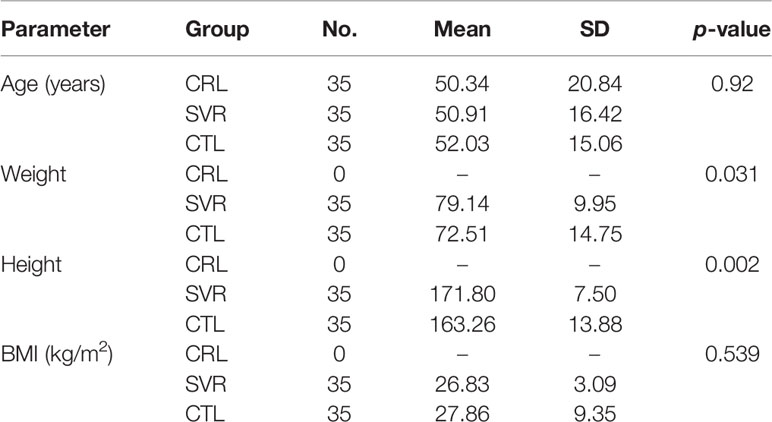
Table 3 Demographic features of the patients in control (CRL), severe (SVR), and critical (CTL) groups.
Approximately 1.8 ml of peripheral blood was collected into tubes containing 0.2 ml sodium citrate (3.2%). The tubes were immediately gently mixed and centrifuged (1,200 × g, 10 min), and the appearance of the plasma was checked to exclude icteric (abnormal function of the liver), lipemic (as a preclinical error especially in photometric assays) (36), and hemolyzed specimens (37) or tubes with micro-clots (38). Considering that prolonged storage of plasma specimens negatively affects the results of coagulation tests (39), we managed to perform this study at the peak of the fifth wave of the disease in Iran in order to include as many patients as possible. This strategy helped us collect all the required samples rapidly and to perform the coagulation tests quickly without freezing the plasma samples. All tests were run within 3 h after sample collection. According to the partially low stability of D-dimer in plasma (40), and using semi-quantitative kits to measure D-dimer and FDPs, we performed these tests before the other tests.
The PT (NeoPTimal), aPTT (C.K. PREST), CaCL2 (0.025 M), fibrinogen (STA-Liquid Fib), anti-lupus coagulant, fibrinogen, protein C (STACLOT), protein S (STACLOT), antithrombin III (STACHROM), Owren–Koller, Desorb-U, D-dimer, and FDP kits were purchased from Stago Co., Asnières sur seine, France. All tests, except for the D-dimer and FDPs, were performed using the fully automated STA Compact® System (Diagnostica Stago, Asnières sur seine, France). We used semi-quantitative D-dimer and FDP kits (benefiting from latex particles coated with monoclonal antibodies to D-dimer or FDP, respectively). Moreover, Toshiba Alexion 16-slice (Toshiba, Japan) and GE BrightSpeed Elite 16 Slice (Chicago, IL, USA) were used for CT scans of the patient groups, and micPCR Biomolecular Systems (Upper Coomera, Australia) was used for the PCR testing of all individuals.
After obtaining the samples, we checked them twice before and after putting them on the mixer to exclude any samples with visible signs of clotting or micro-clots. After 5 min of mixing the samples, they were centrifuged and the obtained plasma samples were analyzed for D-dimer and FDPs; then, the plasma samples were poured into conventional plastic tubes and loaded into the autoanalyzer. To perform semi-quantitative D-dimer and FDP tests, we used the glycine buffer to dilute each plasma sample in plastic test tubes. For the D-dimer test, we added 20 ml of reagent 1 (including ready-to-use latex particles coated with mouse anti-human D-dimer monoclonal antibody) to 20 ml of undiluted/diluted plasma samples of each individual, mixed gently, and assessed the agglutination. The interpretation of the results was done using the protocol provided in Table 4. A similar protocol was used for the FDP test, but with only two diluted concentrations/titers (1:2 and 1:8) assessed for agglutination according to the instruction of the manufacturer. We performed the rest of the tests using a fully automated STA Compact® System (Diagnostica Stago, France) in a duplicate manner. We did quality control testing for each run using STA-System Control N+P.
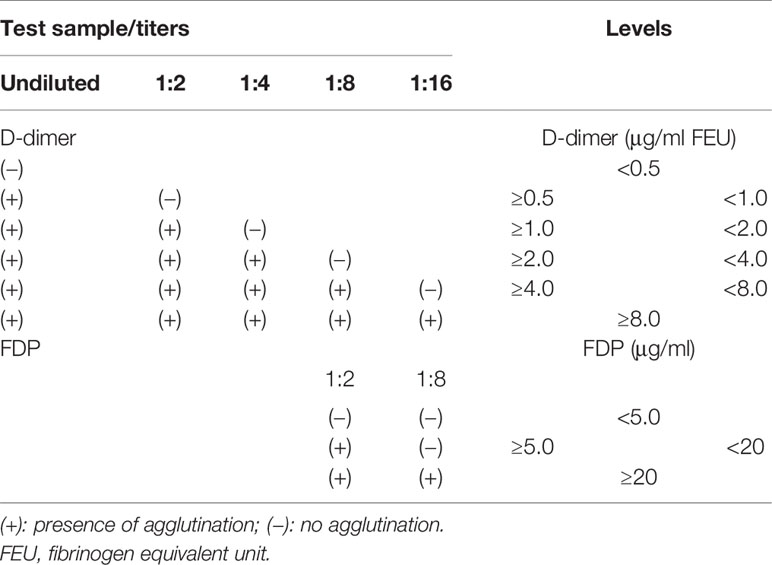
Table 4 Approximate diluted/undiluted concentrations and the titers for the D-dimer and fibrin degradation product (FDP) test.
The data obtained from the autoanalyzer for PT, PTT, fibrinogen, lupus anticoagulant, proteins C and S, and ATIII (quantitative tests) and for D-dimer and FDPs (semi-quantitative tests) were reported. Statistical analysis was performed using SPSS (ver. 21; IBM, Armonk, NY, USA). To compare the groups, we used one-way ANOVA, Fisher’s exact test, and chi-square tests. A p-value <0.05 was considered to be statistically significant.
According to the results, there was no association between the age, weight, and BMI of individuals (p = 0.92, 0.03, 0.54, respectively). The elevated PT test results have been frequently reported in previous investigations worldwide. Table 5 summarizes the statistical analysis of the data obtained from the STA Compact® system. Our results showed that while the average PT result in the CRL group was 13.38 ± 0.73 [the international sensitivity index (ISI) of the kit was 1.05; international normalized ratio (INR) = 1.03 ± 0.06], it was significantly elevated in both patient groups, in which the mean PT in the SVR group was 13.85 ± 1.12s (INR = 1.07 ± 0.09) and that in the CTL group was 15.01 ± 1.68s (INR = 1.17 ± 0.14, p < 0.001). Notably, the one-way ANOVA results showed a significant difference in the mean PT results between the patient groups (p < 0.001). The general trend for the results of the PTT test was similar to that of the PT test, in which the mean results for the test (expressed in seconds) in the CRL group was 36.50 ± 2.64, while it was 37.80 ± 3.73 in the SVR group and 42.92 ± 6.62 in the CTL group. Interestingly, the difference between the two patient groups was statistically significant (p < 0.001). For a better presentation of the obtained data, we showed the results of each patient independently. According to Figures 2A–C, the PT results showed an increasing trend from the CRL to the SVR and CRL groups. Non-survivors have been marked by black circles. The INR results for each included individual are presented in Figures 2D, F. The PTT test results followed a similar trend (minimum in the CRL group and maximum in the CRL group) (Figures 2G–I). Analysis of the results for the anti-lupus coagulant test revealed an increasing trend among the groups, in which the average for the controls was 40.76 ± 3.48 s; however, it increased to 47.05 ± 8.25 s in the SVR group and to 49.41 ± 9.24 s in the CTL group. The results for the fibrinogen test provided solid evidence that the fibrinogen levels vary between patients and controls significantly. It is clear that, while the average level of fibrinogen was 283.57 ± 70.51 mg/dl in the CRL group, it went up to 480.29 ± 129.60 mg/dl in the SVR group (p < 0.001) and to 537.66 ± 142.68 mg/dl in the CTL group (p < 0.001). According to the results of the ATIII test, there was no significant difference among the three studied groups (p = 0.321), where the average activity of ATIII in the CRL group was 98.74 ± 10.40%, in SVR group was 99.63 ± 11.56%, and in the CTL group was 95.71 ± 11.96%. The results for each individual for the anti-lupus coagulant test are represented in Figures 3A–C. The CRL group had the lowest levels, while the CTL group had the highest levels. Investigation of the fibrinogen levels showed that the CRL group had the lowest levels. The fibrinogen levels were significantly increased in the SVR group; however, the CTL group had the highest levels among all groups (Figures 3D–F). The results for ATIII, unlike other tests, revealed that there was no significant difference among the three studied groups (Figures 3G–I). Additionally, analysis of the results for protein C showed that the activity of this regulatory protein was 94.31 ± 17.07% in the CRL group, while it increased to 99.34 ± 31.6% in the SVR group. The activity levels of this protein were found to be lower in the CTL group (85.57 ± 15.79%, p = 0.04). The ANOVA results showed that the difference between the activity levels of protein C between the CTL and CRL groups was statistically significant, but not to that of the previous tests (p = 0.032). Moreover, our result showed that protein S, the cofactor of protein C, had lower activity in the patient groups compared to the CRL group, in which the highest activity was reported in the CRL group (75.03 ± 9.39%), while its activity dropped to 65.06 ± 12.76% in the SVR group. The lowest activity of protein S was observed in the CTL group (62.91 ± 12.32%, p < 0.001). According to Figures 4A–C, the minimum activity of protein C was observed in the CTL group. A similar trend was observed when we investigated the protein S levels (Figures 4D–F). According to the results regarding the D-dimer test, of the 35 individuals in the CRL group, 31 (88.6%) had D-dimer levels <0.5 μg/ml and 4 (11.4%) had D-dimer levels between 0.5 and 1.0 μg/ml. Twenty-four (68.6%) patients in the SVR group were found to have D-dimer levels below 0.5 μg/ml, and 11 (31.4%) had levels between 0.5 and 1.0 μg/ml. In the CTL group, 25 patients had D-dimer levels below 1.0 μg/ml, whereas, only 2 (5.7%) patients had D-dimer levels of 2–4 μg/ml. The same numbers were found to have D-dimer levels over 8 μg/ml [χ2(8) = 34.81, p = 0.0001] (Table 6). Data regarding the D-Dimer test results for each individual are represented in Figures 5A–C. There was a significant difference among the three studied groups, in which the CTL group had the highest levels of D-dimer, whereas the CRL group had the lowest levels. The results for FDP also revealed that the majority of healthy controls (34 out of 35) had FDP levels below 5 μg/ml, while only 1 was found to have FDP levels between 5 and 20 μg/ml. In the SVR group, 31 (88.6%) patients had FDP levels below 5 μg/ml and 4 (11.4%) had FDP levels between 5 and 20 μg/ml. In contrast, only 23 patients in the CTL group had FDP levels below 5 μg/ml, and 9 (25.7%) were found to have levels between 5 and 20 μg/ml. There were 3 (8.6%) patients with FDP levels over 20 μg/ml. According to Figures 5D–F, the results for each group showed that patients in the CTL group had the highest levels of FDP, while healthy controls had the lowest levels.
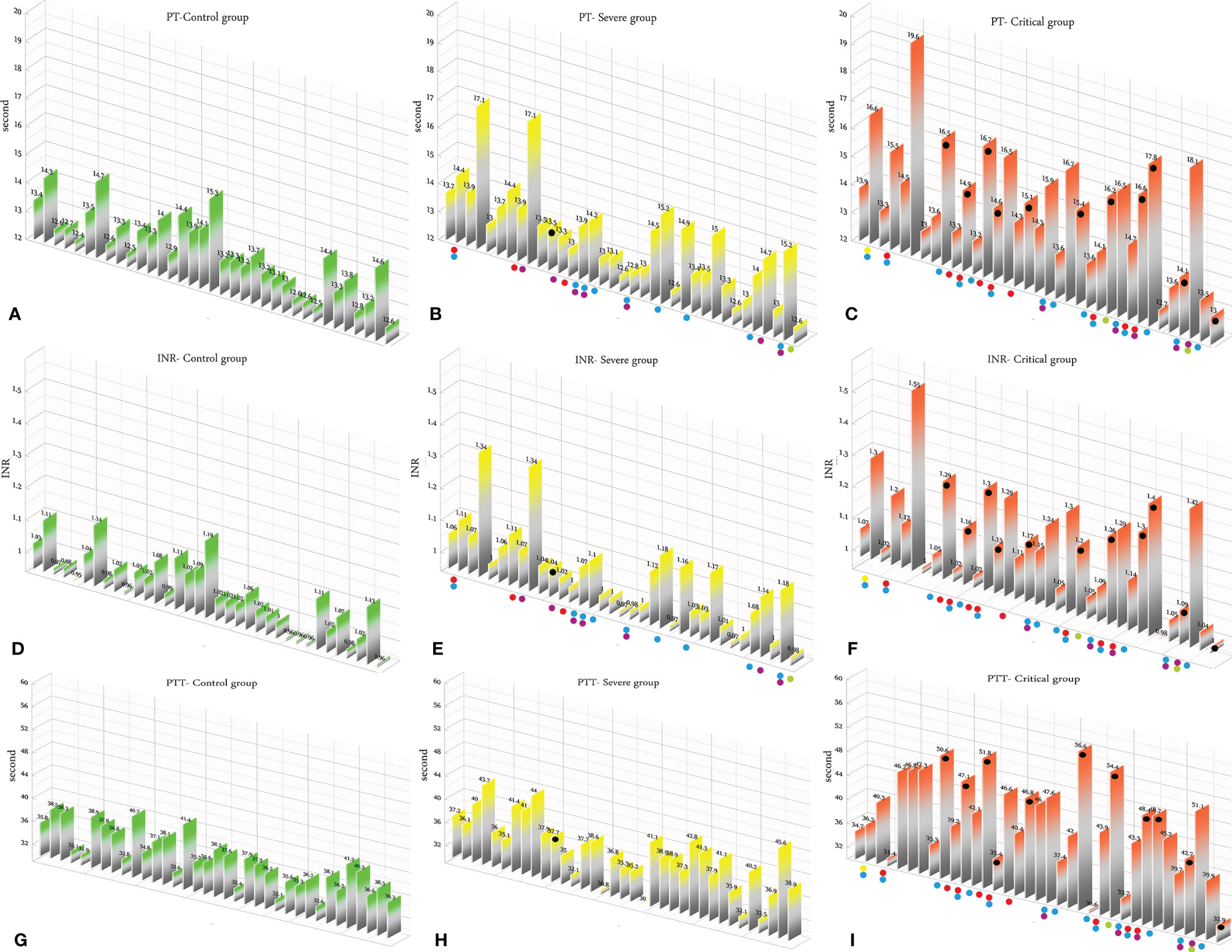
Figure 2 (A–C) Prothrombin time (PT) test results in the control (CRL, green), severe (SVR, yellow), and critical (CTL, red) groups. (D–F) International normalized ratio (INR) results in the CRL, SVR, and CTL groups. (G–I) Partial thromboplastin time (PTT) test results in the CRL, SVR, and CTL groups. Expired individuals in the SVR and CTL groups are shown with a black bullet point. Bullet points in red, blue, purple, green, and yellow indicate cardiovascular disease, hypertension, diabetes, pulmonary disease, and kidney disease, respectively, in the SVR and CTL groups.
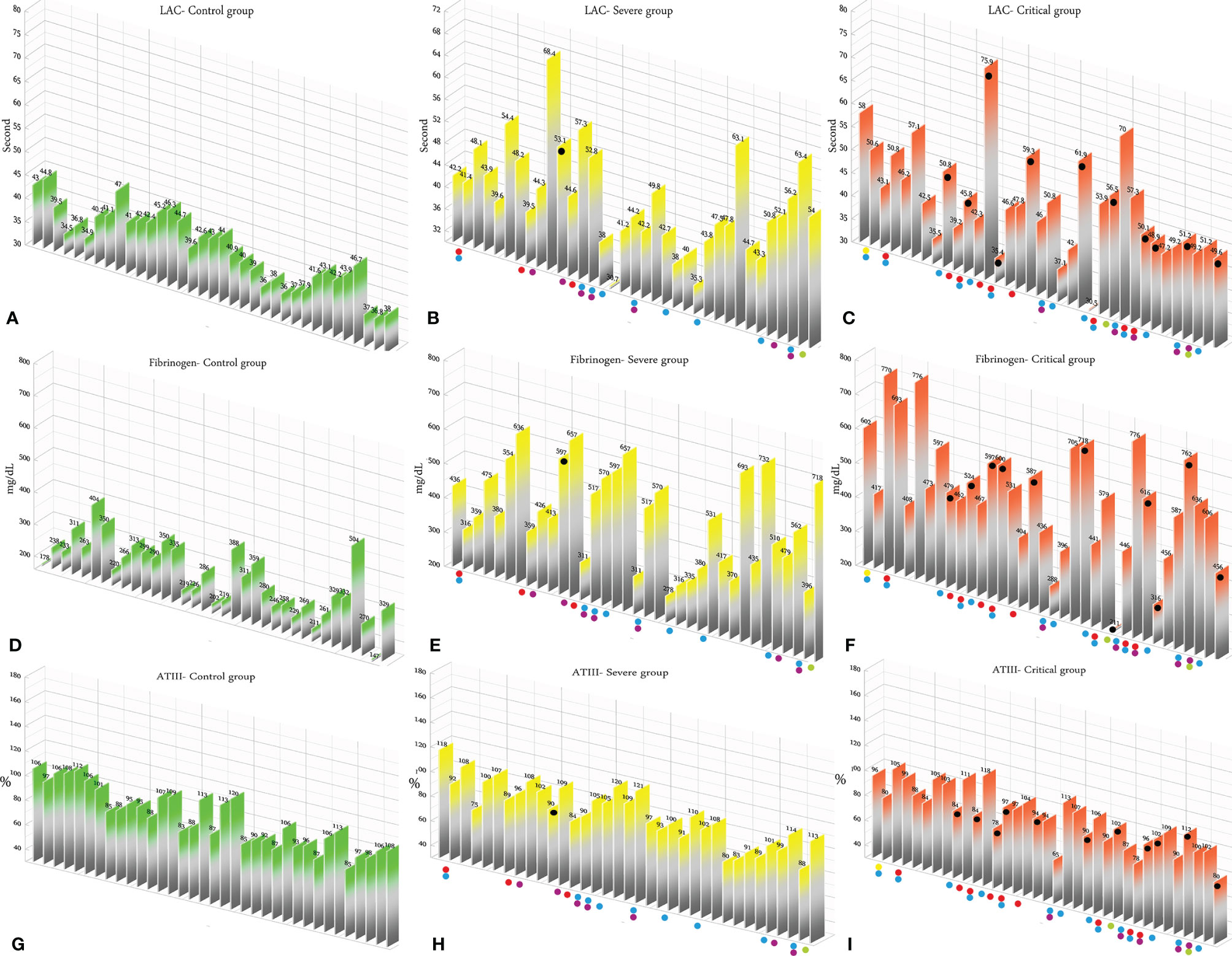
Figure 3 (A–C) Lupus anticoagulant test results in the control (CRL, green), severe (SVR, yellow), and critical (CTL, red) groups. (D–F) Fibrinogen results in the CRL, SVR, and CTL groups. (G–I) Antithrombin III (ATIII) test results in the CRL, SVR, and CTL groups. Expired individuals in the SVR and CTL groups are shown with a black bullet point. Bullet points in red, blue, purple, green, and yellow indicate cardiovascular disease, hypertension, diabetes, pulmonary disease, and kidney disease, respectively, in the SVR and CTL groups.
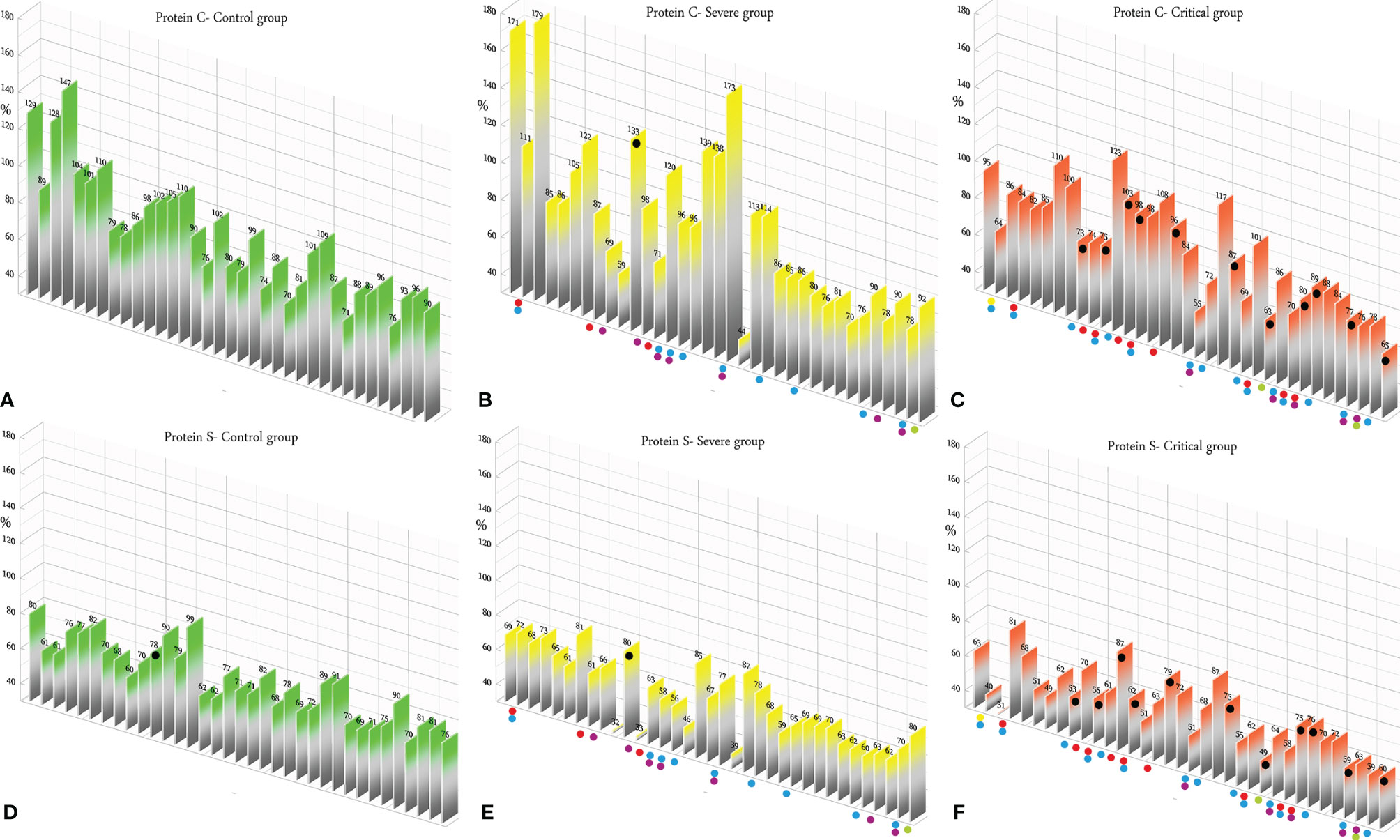
Figure 4 (A–C) Protein C test results in the control (CRL, green), severe (SVR, yellow), and critical (CTL, red) groups. (D–F) Protein C results in the CRL, SVR, and CTL groups. Expired individuals in the SVR and CTL groups are shown with a black bullet point. Bullet points in red, blue, purple, green, and yellow indicate cardiovascular disease, hypertension, diabetes, pulmonary disease, and kidney disease, respectively, in the SVR and CTL groups.
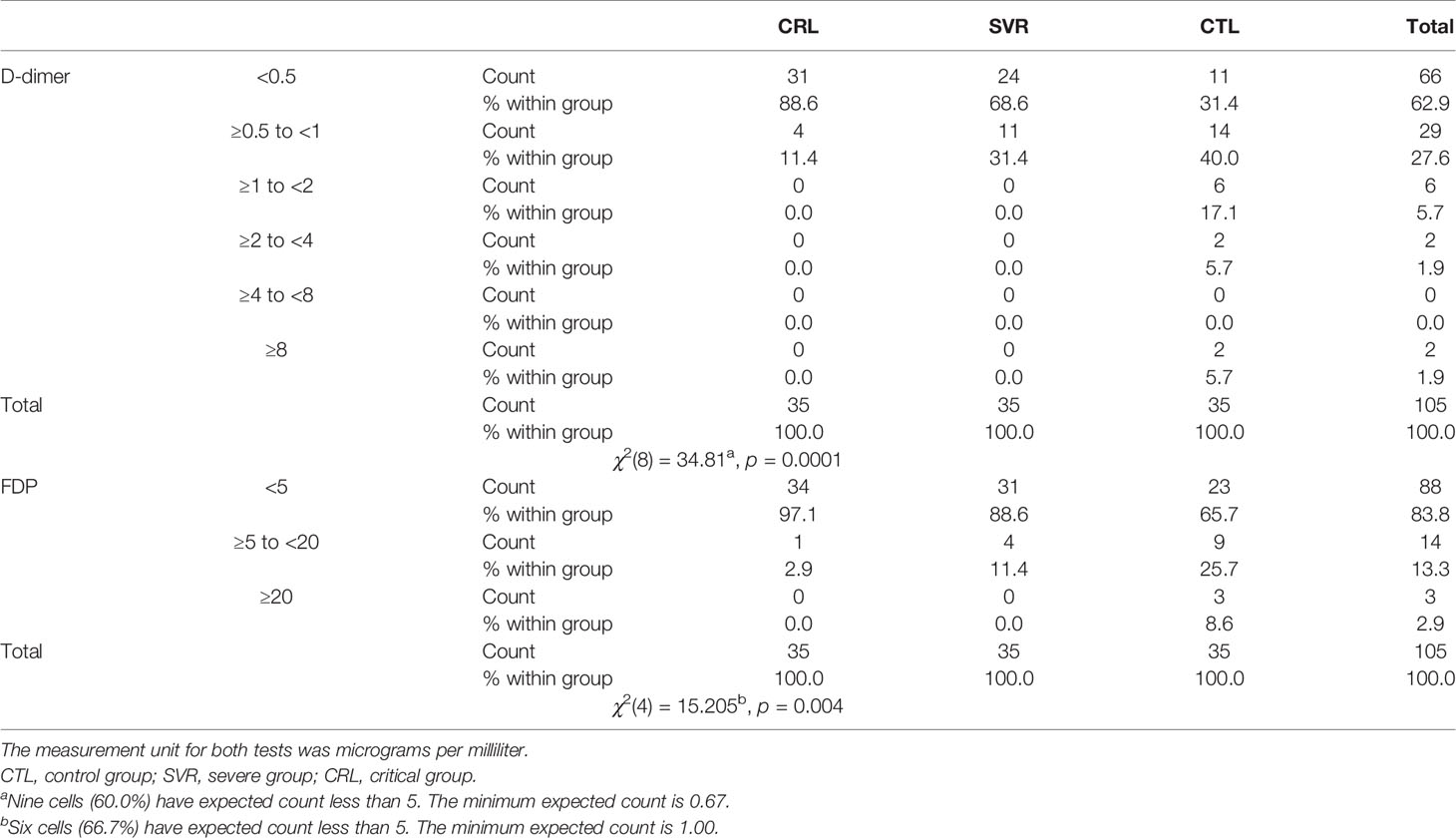
Table 6 Results of the semi-quantitative tests including D-dimer and fibrin degradation products (FDPs).
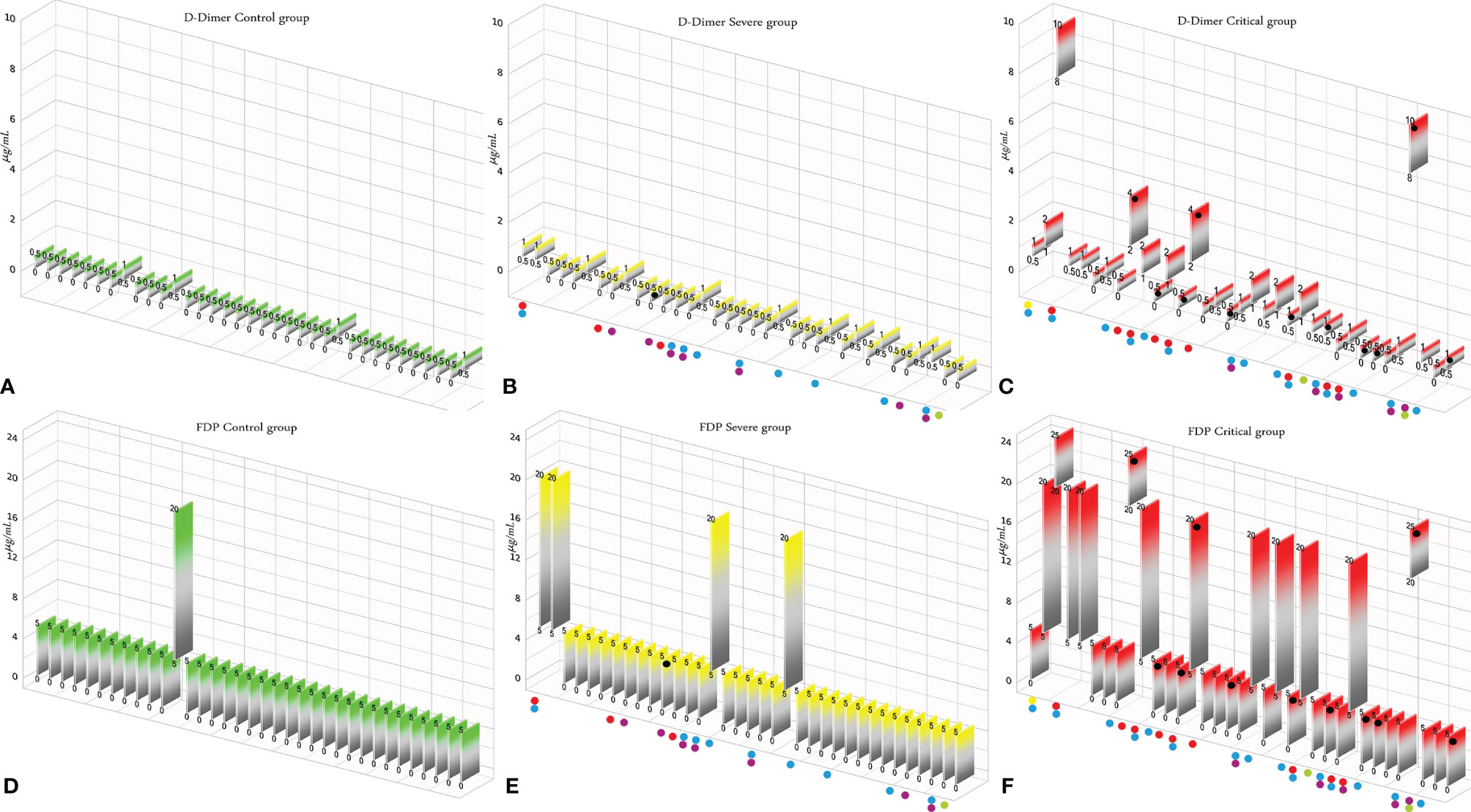
Figure 5 (A–C) D-dimer test results in the control (CRL, green), severe (SVR, yellow), and critical (CTL, red) groups. (D–F) Fibrin degradation product (FDP) test results in the CRL, SVR, and CTL groups. Expired individuals in the SVR and CTL groups are shown with a black bullet point. Bullet points in red, blue, purple, green, and yellow indicate cardiovascular disease, hypertension, diabetes, pulmonary disease, and kidney disease, respectively, in the SVR and CTL groups.
We analyzed the data for the deceased individuals (11 out of 35 in the CTL group) and compared them with those of survivors in the same group in order to obtain a better understanding of the impact of abnormal coagulation test results on the fate of the patients. We marked these patients with black bullet points in Figures 2–5. According to Figures 2C, F, I and Table 7, the expired individuals in the CTL group had higher mean values for PT, INR, and PTT when compared to all individuals in the CTL group. Additionally, according to Figures 3C, F, I and Table 7, the expired individuals in the CTL group had higher mean values for LAC, but lower values for the ATIII test. They had slightly lower mean values in the fibrinogen test than the rest of the CTL group. Moreover, according to Figures 4C, F, the expired individuals had lower mean values of protein C, but higher protein S, when compared to the mean values in the CTL group. Finally, according to Figures 5C, F, individuals who expired were among those with the highest values both in the D-dimer test [≥8 (n = 1), 2< to ≥4 (n = 2)] and in the FDP test [≥20 (n = 2), ≥5 to <20 (n = 1)].

Table 7 Comparison of the mean values of coagulation tests between expired individuals and all individuals in the critical (CTL) group.
In this section, we provide recommendations for further investigations (Table 8) and address our limitations. We included 35 individuals in each group. Recruiting more patients will provide more accurate results in prospective studies. The enrollment of more patients provides the opportunity to determine cutoffs and design an alarm panel to be used in ICUs. We also applied a single-sampling strategy; however, monitoring the results by obtaining at least 2–3 samples in the CTL and SVR groups could more effectively monitor the test results and their association with the outcomes. One limitation of this study was the use of latex-based semi-quantitative kits to assess D-dimer and FDPs. The results will be more reliable when both tests are performed using fully automated methods. Although the biofunctions of proteins S and C as biological regulators of factors V and VIII are well documented, we did not assess these two factors.
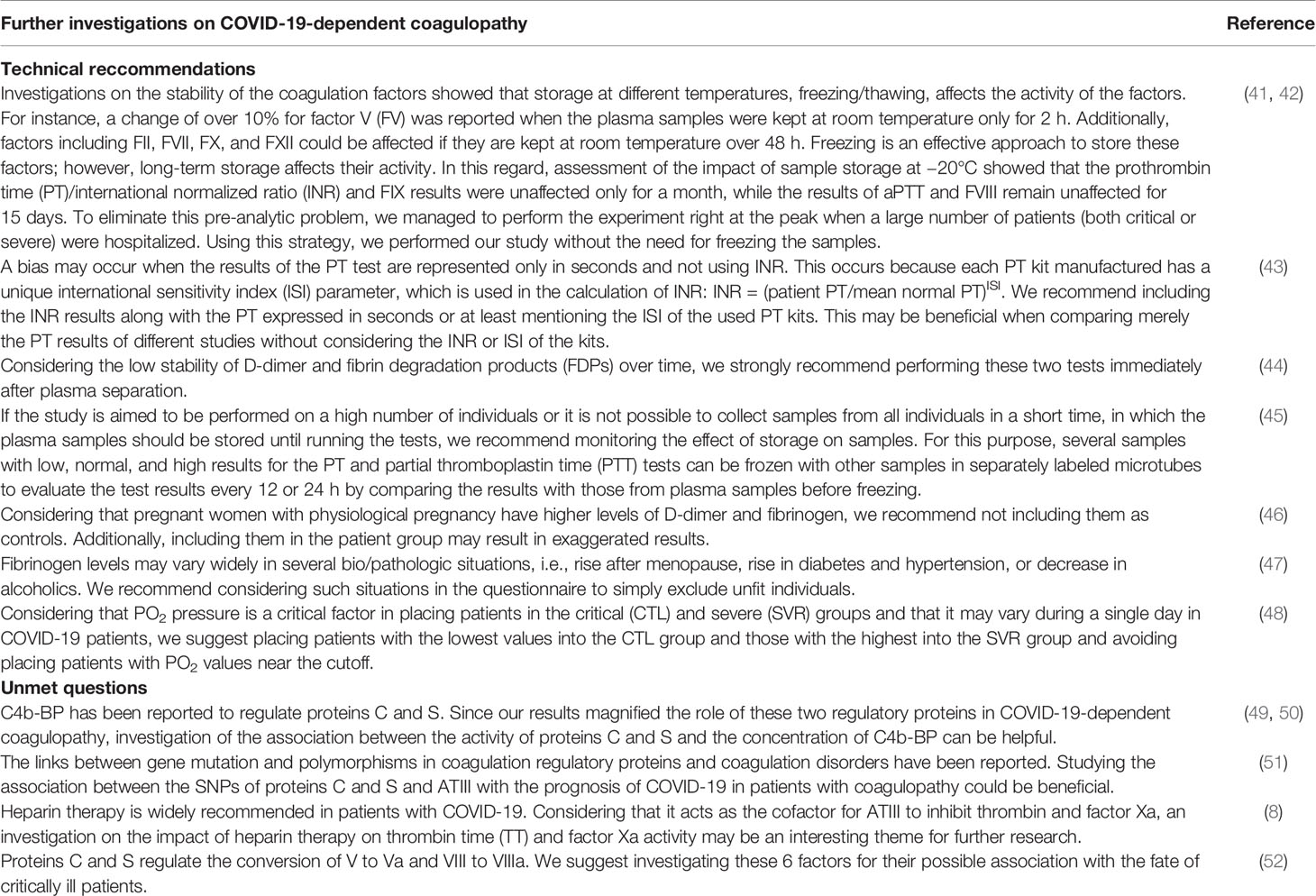
Table 8 Technical recommendations and unmet questions awaiting further investigation in COVID-19-associated coagulopathy.
In the present study, we investigated the dynamic changes in 9 coagulation tests on 105 individuals classified into CTL, SVR, and CRL groups. Our study revealed significant aberrant coagulation changes among the studied groups in 4 aspects: extrinsic and intrinsic pathways, fibrinolysis, and the regulatory factors. Our results were consistent with those of the majority of previously published papers. A brief literature review for all tests with consistency levels is represented in Table 1. The CTL group had higher PT test (therefore INR) results when compared to the SVR and CRL groups, indicating a disruption in the extrinsic coagulation pathway. In addition, the prolonged PTT results in the CTL group and also similar results in the LAC test showed that not only the extrinsic pathway but even the intrinsic pathway was dysregulated. It should be considered that, in critically ill patients, lupus anticoagulant could be positive. An elevated fibrinogen level was one of the main findings in COVID-19-associated coagulopathy. We showed that there was a significant difference in the fibrinogen levels among the three groups and that the CTL group had the highest levels. It can be used as a common biomarker to predict the severity of the disease; however, the analysis of fibrinogen levels in deceased patients in the CTL group with the whole group showed that it had no significance in predicting death. Investigation of ATIII revealed that its activity was not significantly interrupted in COVID-19 patients (p = 0.321). However, proteins C and S, the other regulatory proteins, showed a significant decrease in their activity levels (p = 0.04 and p < 0.001, respectively). The difference between the reported p-values for these proteins was probably due to the low number of individuals recruited in each group; increasing the sample size will provide more accurate data. Considering that proteins C and S regulate the conversion of factors V and VIII to their active forms, we conclude that the disruption of homeostasis in protein C (and S) regulating the conversion of factors V and VIII to their active form could be a mechanism for COVID-19-associated coagulopathy. The fibrinolysis pathway was also affected in the presence of SARS-COV-2, in which the production of FDPs, mainly D-dimer, was accelerated, and according to our results, deceased patients were found to have significantly higher FDP and D-dimer levels when compared to survivors. The majority of coagulation factors are produced in the liver; to prevent the effects of hepatopathy on the levels of the coagulation factors and the corresponding tests, we enrolled normal controls and patients whose liver function tests were normal. Interestingly, factors including FVIII and vWF (which act as markers of endothelial activation) (53) were produced in the endothelial cells. Investigation of the levels of these factors in COVID-19 patients revealed that their levels increased and may correlate with poorer prognosis (54–56). We showed that D-dimer, fibrinogen, PT, PTT, LAC, protein S, FDPs, and protein C (ordered according to their p-values) could effectively be used in the prognosis of the severity of the disease and that disruptions in proteins C and S regulating the conversion of factors V and VIII to their active form may interfere the homeostasis of the coagulation system.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Urmia Medical University (IR.UMSU.REC.1399.264). The patients/participants provided written informed consent to participate in this study.
DEAK designed the study, performed the tests, generated the figures, and prepared the manuscript. YR contributed to performing semiquantitative tests. RA and RJ supervised the recruitment of patients and the control group. JR analyzed the data. MM performed sample preparation. AA supervised quality control. RN and FR collected clinical data. VS-I supervised the research progression. All authors contributed to the article and approved the submitted version.
This study was supported by the Cellular and Molecular Research Center, Cellular and Molecular Medicine Institute, Urmia University of Medical Sciences (fund no. 1399.264).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the Cellular and Molecular Research Center, Cellular and Molecular Medicine Institute, Urmia University of Medical Sciences for the financial support.
COVID-19, coronavirus disease 2019; PT, prothrombin time; aPTT, activated partial thromboplastin time; ATIII, antithrombin III; LAC, lupus anticoagulant; ISI, international sensitivity index; INR, international normalized ratio; ICU, Intensive care unit; CRL group, control group; SVR group, severe group; CTL group, critical group.
1. Winter WE, Greene DN, Beal SG, Isom JA, Manning H, Wilkerson G, et al. Clotting Factors: Clinical Biochemistry and Their Roles as Plasma Enzymes. Adv Clin Chem (2020) 94:31–84. doi: 10.1016/bs.acc.2019.07.008
2. O’Connor SD, Taylor AJ, Williams EC, Winter TC. Coagulation Concepts Update. AJR Am J Roentgenol (2009) 193(6):1656–64. doi: 10.2214/ajr.08.2191
3. Grover SP, Mackman N. Intrinsic Pathway of Coagulation and Thrombosis. Arterioscler Thromb Vasc Biol (2019) 39(3):331–8. doi: 10.1161/atvbaha.118.312130
4. Chaudhry R, Usama SM, Babiker HM. Physiology, Coagulation Pathways. In: StatPearls. Treasure Island (FL): StatPearls Publishing LLC. (2021). StatPearls Publishing Copyright © 2021.
5. Weisel JW, Litvinov RI. Fibrin Formation, Structure and Properties. Subcell Biochem (2017) 82:405–56. doi: 10.1007/978-3-319-49674-0_13
6. Hoxha A, Banzato A, Ruffatti A, Pengo V. Detection of Lupus Anticoagulant in the Era of Direct Oral Anticoagulants. Autoimmun Rev (2017) 16(2):173–8. doi: 10.1016/j.autrev.2016.12.010
7. Winkler AM, Zimring JC. Chapter 141 - Laboratory Support for Heparin Monitoring. In: Hillyer CD, Shaz BH, Zimring JC, Abshire TC, editors. Transfusion Medicine and Hemostasis. San Diego: Academic Press (2009). doi: 10.1016/B978-0-12-374432-6.00141-X
8. Quinsey NS, Greedy AL, Bottomley SP, Whisstock JC, Pike RN. Antithrombin: In Control of Coagulation. Int J Biochem Cell Biol (2004) 36(3):386–9. doi: 10.1016/s1357-2725(03)00244-9
9. Wypasek E, Undas A. Protein C and Protein S Deficiency - Practical Diagnostic Issues. Adv Clin Exp Med (2013) 22(4):459–67.
10. Kini RM. Serine Proteases Affecting Blood Coagulation and Fibrinolysis From Snake Venoms. Pathophysiol Haemost Thromb (2005) 34(4-5):200–4. doi: 10.1159/000092424
11. Chapin JC, Hajjar KA. Fibrinolysis and the Control of Blood Coagulation. Blood Rev (2015) 29(1):17–24. doi: 10.1016/j.blre.2014.09.003
12. Chandler WL. Chapter 140 - Fibrinolytic Testing. In: Shaz BH, Hillyer CD, Roshal M, Abrams CS, editors. Transfusion Medicine and Hemostasis, 2nd ed. San Diego: Elsevier (2013). doi: 10.1016/B978-0-12-397164-7.00140-3
13. Long H, Nie L, Xiang X, Li H, Zhang X, Fu X, et al. D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis. BioMed Res Int (2020) 2020:6159720. doi: 10.1155/2020/6159720
14. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients With COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet (2020) 395(10229):1054–62. doi: 10.1016/s0140-6736(20)30566-3
15. Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic Utility of Clinical Laboratory Data Determinations for Patients With the Severe COVID-19. J Med Virol (2020) 92(7):791–6. doi: 10.1002/jmv.25770
16. Liu Y, Gao W, Guo W, Guo Y, Shi M, Dong G, et al. Prominent Coagulation Disorder is Closely Related to Inflammatory Response and Could be as a Prognostic Indicator for ICU Patients With COVID-19. J Thromb Thromb (2020) 50(4):825–32. doi: 10.1007/s11239-020-02174-9
17. Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent Changes in Blood Coagulation of Patients With SARS-CoV-2 Infection. Clin Chem Lab Med (2020) 58(7):1116–20. doi: 10.1515/cclm-2020-0188
18. Fu J, Kong J, Wang W, Wu M, Yao L, Wang Z, et al. The Clinical Implication of Dynamic Neutrophil to Lymphocyte Ratio and D-Dimer in COVID-19: A Retrospective Study in Suzhou China. Thromb Res (2020) 192:3–8. doi: 10.1016/j.thromres.2020.05.006
19. Hasan Ali O, Bomze D, Risch L, Brugger SD, Paprotny M, Weber M, et al. Severe COVID-19 Is Associated With Elevated Serum IgA and Antiphospholipid IgA-Antibodies. Clin Infect Dis (2020) 73(9):2869–74. doi: 10.1093/cid/ciaa1496
20. Siguret V, Voicu S, Neuwirth M, Delrue M, Gayat E, Stépanian A, et al. Are Antiphospholipid Antibodies Associated With Thrombotic Complications in Critically Ill COVID-19 Patients? Thromb Res (2020) 195:74–6. doi: 10.1016/j.thromres.2020.07.016
21. Fan BE, Ng J, Chan SSW, Christopher D, Tso ACY, Ling LM, et al. COVID-19 Associated Coagulopathy in Critically Ill Patients: A Hypercoagulable State Demonstrated by Parameters of Haemostasis and Clot Waveform Analysis. J Thromb Thromb (2021) 51(3):663–74. doi: 10.1007/s11239-020-02318-x
22. Tabatabai A, Rabin J, Menaker J, Madathil R, Galvagno S, Menne A, et al. Factor VIII and Functional Protein C Activity in Critically Ill Patients With Coronavirus Disease 2019: A Case Series. A A Pract (2020) 14(7):e01236. doi: 10.1213/xaa.0000000000001236
23. Gerotziafas GT, Sergentanis TN, Voiriot G, Lassel L, Papageorgiou C, Elabbadi A, et al. Derivation and Validation of a Predictive Score for Disease Worsening in Patients With COVID-19. Thromb Haemost (2020) 120(12):1680–90. doi: 10.1055/s-0040-1716544
24. Stoichitoiu LE, Pinte L, Balea MI, Nedelcu V, Badea C, Baicus C. Anticoagulant Protein S in COVID-19: Low Activity, and Associated With Outcome. Rom J Intern Med (2020) 58(4):251–8. doi: 10.2478/rjim-2020-0024
25. Martín-Rojas RM, Pérez-Rus G, Delgado-Pinos VE, Domingo-González A, Regalado-Artamendi I, Alba-Urdiales N, et al. COVID-19 Coagulopathy: An in-Depth Analysis of the Coagulation System. Eur J Haematol (2020) 105(6):741–50. doi: 10.1111/ejh.13501
26. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 Patients in Intensive Care Unit: A Report of Thromboelastography Findings and Other Parameters of Hemostasis. J Thromb Haemost (2020) 18(7):1738–42. doi: 10.1111/jth.14850
27. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5
28. Kim Y, Khose S, Abdelkhaleq R, Salazar-Marioni S, Zhang GQ, Sheth SA. Predicting In-Hospital Mortality Using D-Dimer in COVID-19 Patients With Acute Ischemic Stroke. Front Neurol (2021) 12:702927. doi: 10.3389/fneur.2021.702927
29. He X, Yao F, Chen J, Wang Y, Fang X, Lin X, et al. The Poor Prognosis and Influencing Factors of High D-Dimer Levels for COVID-19 Patients. Sci Rep (2021) 11(1):1830. doi: 10.1038/s41598-021-81300-w
30. Tang N, Li D, Wang X, Sun Z. Abnormal Coagulation Parameters are Associated With Poor Prognosis in Patients With Novel Coronavirus Pneumonia. J Thromb Haemost (2020) 18(4):844–7. doi: 10.1111/jth.14768
31. Ayerbe L, Risco C, Ayis S. The Association Between Treatment With Heparin and Survival in Patients With Covid-19. J Thromb Thromb (2020) 50(2):298–301. doi: 10.1007/s11239-020-02162-z
32. Hariyanto TI, Japar KV, Kwenandar F, Damay V, Siregar JI, Lugito NPH, et al. Inflammatory and Hematologic Markers as Predictors of Severe Outcomes in COVID-19 Infection: A Systematic Review and Meta-Analysis. Am J Emerg Med (2021) 41:110–9. doi: 10.1016/j.ajem.2020.12.076
33. Winata S, Kurniawan A. Coagulopathy in COVID-19: A Systematic Review. Medicinus (2021) 8:72–80. doi: 10.19166/med.v8i2.3444
34. Rochwerg B, Agarwal A, Siemieniuk RA, Agoritsas T, Lamontagne F, Askie L, et al. A Living WHO Guideline on Drugs for Covid-19. Bmj (2020) 370:m3379. doi: 10.1136/bmj.m3379
35. Jin X, Duan Y, Bao T, Gu J, Chen Y, Li Y, et al. The Values of Coagulation Function in COVID-19 Patients. PloS One (2020) 15(10):e0241329. doi: 10.1371/journal.pone.0241329
36. Negrini D, Bernardi D, Antonelli G, Plebani M. Interference of Lipemia in Samples for Routine Coagulation Testing Using a Sysmex CS-5100 Coagulometer. Int J Lab Hematol (2019) 41(6):772–7. doi: 10.1111/ijlh.13108
37. Lippi G, Avanzini P, Zobbi V, Ippolito L. Influence of Mechanical Hemolysis of Blood on Two D-Dimer Immunoassays. Blood Coagul Fibrinolysis (2012) 23(5):461–3. doi: 10.1097/MBC.0b013e3283549696
38. Pappas AA, Palmer SK, Meece D, Fink LM. Rapid Preparation of Plasma for Coagulation Testing. Arch Pathol Lab Med (1991) 115(8):816–7.
39. Lippi G, Salvagno GL, Montagnana M, Lima-Oliveira G, Guidi GC, Favaloro EJ. Quality Standards for Sample Collection in Coagulation Testing. Semin Thromb Hemost (2012) 38(6):565–75. doi: 10.1055/s-0032-1315961
40. Böhm-Weigert M, Wissel T, Muth H, Kemkes-Matthes B, Peetz D. Long- and Short-Term In Vitro D-Dimer Stability Measured With INNOVANCE D-Dimer. Thromb Haemost (2010) 103(2):461–5. doi: 10.1160/th09-04-0230
41. Linskens EA, Devreese KMJ. Pre-Analytical Stability of Coagulation Parameters in Plasma Stored at Room Temperature. Int J Lab Hematol (2018) 40(3):292–303. doi: 10.1111/ijlh.12784
42. Zhao Y, Feng G, Zhang J, Gong R, Cai C, Feng L. Effects of Preanalytical Frozen Storage Time and Temperature on Screening Coagulation Tests and Factors VIII and IX Activity. Sci Rep (2017) 7(1):12179. doi: 10.1038/s41598-017-11777-x
43. Taberner DA, Poller L, Thomson JM, Darby KV. Effect of International Sensitivity Index (ISI) of Thromboplastins on Precision of International Normalised Ratios (INR). J Clin Pathol (1989) 42(1):92–6. doi: 10.1136/jcp.42.1.92
44. Karakeçe E. Evaluation of The Effect of Storage Temperature on D-Dimer Stability, Using Two Different Techniques. J Microbiol Exp (2016) 3. doi: 10.15406/jmen.2016.03.00095
45. Woodhams B, Girardot O, Blanco MJ, Colesse G, Gourmelin Y. Stability of Coagulation Proteins in Frozen Plasma. Blood Coagul Fibrinolysis (2001) 12(4):229–36. doi: 10.1097/00001721-200106000-00002
46. Siennicka A, Kłysz M, Chełstowski K, Tabaczniuk A, Marcinowska Z, Tarnowska P, et al. Reference Values of D-Dimers and Fibrinogen in the Course of Physiological Pregnancy: The Potential Impact of Selected Risk Factors-A Pilot Study. BioMed Res Int (2020) 2020:3192350. doi: 10.1155/2020/3192350
47. Folsom AR. Epidemiology of Fibrinogen. Eur Heart J (1995) 16(Suppl A):21–3; discussion 23-4. doi: 10.1093/eurheartj/16.suppl_a.21
48. He B, Wang J, Wang Y, Zhao J, Huang J, Tian Y, et al. The Metabolic Changes and Immune Profiles in Patients With COVID-19. Front Immunol (2020) 11:2075. doi: 10.3389/fimmu.2020.02075
49. Hessing M. The Interaction Between Complement Component C4b-Binding Protein and the Vitamin K-Dependent Protein S Forms a Link Between Blood Coagulation and the Complement System. Biochem J (1991) 277( Pt 3):581–92. doi: 10.1042/bj2770581
50. Rezende SM, Simmonds RE, Lane DA. Coagulation, Inflammation, and Apoptosis: Different Roles for Protein S and the Protein S-C4b Binding Protein Complex. Blood (2004) 103(4):1192–201. doi: 10.1182/blood-2003-05-1551
51. Wang LP, Qiu YW, Yin AL, Ma YY, Liu KL, Xiong L, et al. Denaturing High-Performance Liquid Chromatography for Screening Antithrombin III Gene Mutation and Polymorphisms in Patients With Cerebral Venous Thrombosis. Nan Fang Yi Ke Da Xue Xue Bao (2009) 29(10):1982–6.
52. Dahlbäck B, Villoutreix BO. Regulation of Blood Coagulation by the Protein C Anticoagulant Pathway: Novel Insights Into Structure-Function Relationships and Molecular Recognition. Arterioscler Thromb Vasc Biol (2005) 25(7):1311–20. doi: 10.1161/01.Atv.0000168421.13467.82
53. Mostmans Y, De Smedt K, Richert B, Elieh Ali Komi D, Maurer M, Michel O. Markers for the Involvement of Endothelial Cells and the Coagulation System in Chronic Urticaria: A Systematic Review. Allergy (2021) 76(10):2998–3016. doi: 10.1111/all.14828
54. Ward SE, Fogarty H, Karampini E, Lavin M, Schneppenheim S, Dittmer R, et al. ADAMTS13 Regulation of VWF Multimer Distribution in Severe COVID-19. J Thromb Haemost (2021) 19(8):1914–21. doi: 10.1111/jth.15409
55. Al Otair H, AlSaleh K, AlQahtany FS, Al Ayed K, Al Ammar H, Al Mefgai N, et al. The Level of vWF Antigen and Coagulation Markers in Hospitalized Patients With Covid-19. J Blood Med (2021) 12:809–17. doi: 10.2147/jbm.S318940
Keywords: COVID-19, coagulopathy, fibrinogen, protein C (PC), protein S, antithrombin III (ATIII), D-dimer (DD)
Citation: Elieh Ali Komi D, Rahimi Y, Asghari R, Jafari R, Rasouli J, Mohebalizadeh M, Abbasi A, Nejadrahim R, Rezazadeh F and Shafiei-Irannejad V (2021) Investigation of the Molecular Mechanism of Coagulopathy in Severe and Critical Patients With COVID-19. Front. Immunol. 12:762782. doi: 10.3389/fimmu.2021.762782
Received: 22 August 2021; Accepted: 26 November 2021;
Published: 16 December 2021.
Edited by:
Vishwanath Venketaraman, Western University of Health Sciences, United StatesReviewed by:
Ahmed Abdel-Razik, Mansoura University, EgyptCopyright © 2021 Elieh Ali Komi, Rahimi, Asghari, Jafari, Rasouli, Mohebalizadeh, Abbasi, Nejadrahim, Rezazadeh and Shafiei-Irannejad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vahid Shafiei-Irannejad, U2hhZmllaS52QHVtc3UuYWMuaXI=
†ORCID: Daniel Elieh Ali Komi, orcid.org/0000-0003-0546-5280
Yaghoub Rahimi, orcid.org/0000-0003-2320-6303
Rahim Asghari, orcid.org/0000-0002-7174-6446
Reza Jafari, orcid.org/0000-0003-2036-9043
Javad Rasouli, orcid.org/0000-0003-1467-9969
Mehdi Mohebalizadeh, orcid.org/0000-0001-9062-4869
Ata Abbasi, orcid.org/0000-0001-8000-8819
Rahim Nejadrahim, orcid.org/0000-0003-1676-9722
Farzin Rezazadeh, orcid.org/0000-0003-3088-9026
Vahid Shafiei-Irannejad, orcid.org/0000-0003-2088-8986
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.