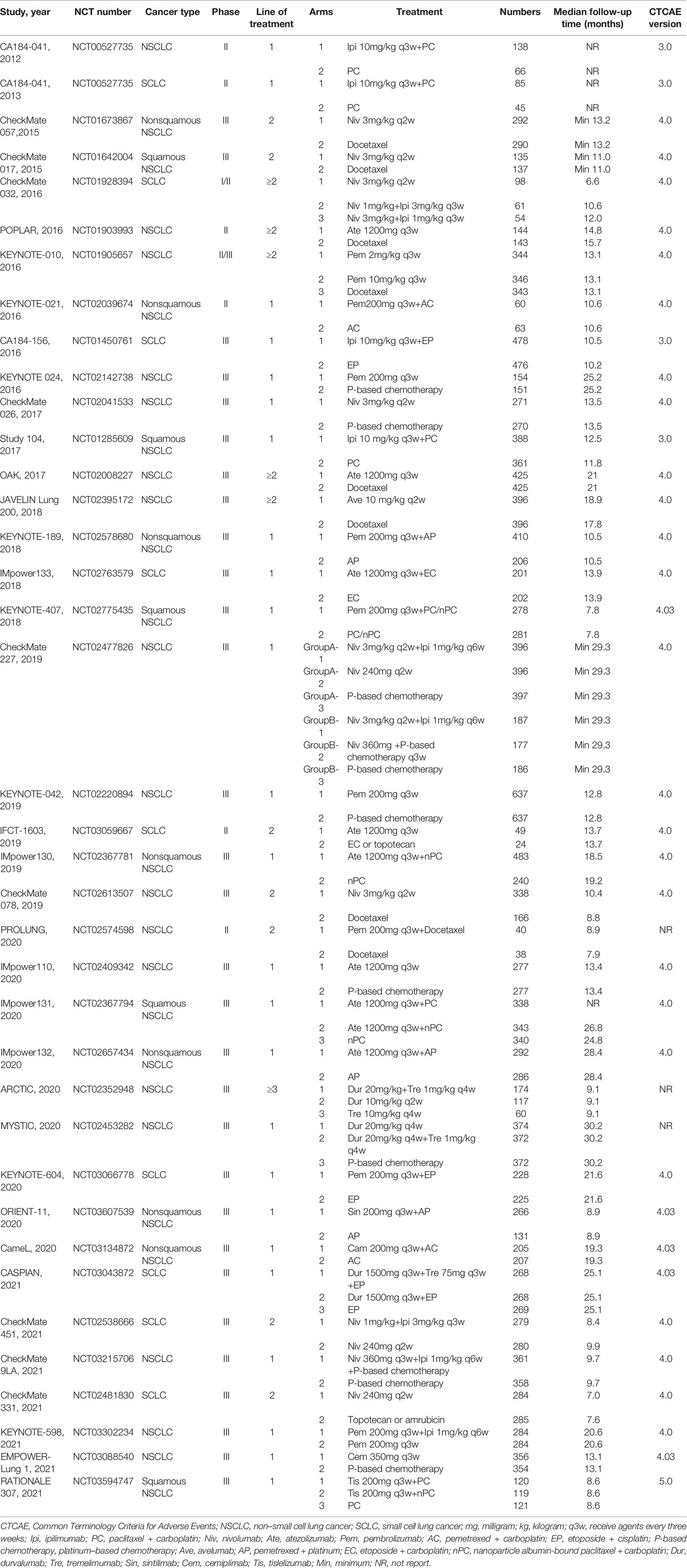
- 1Department of Pharmacy, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Oncology, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Pharmacy, The People’s Hospital of Guangxi Zhuang Autonomous Region (Guangxi Academy of Medical Sciences), Nanning, China
Background: Immune checkpoint inhibitors (ICIs) have become one of the standard treatment options for advanced lung cancer. However, adverse events (AEs), particularly immune–related AEs (irAEs), caused by these drugs have aroused public attention. The current network meta-analysis (NMA) aimed to compare the risk of AEs across different ICI–based regimens in patients with advanced lung cancer.
Methods: We systematically searched the PubMed, EMBASE, and Cochrane Library databases (from inception to 19 April 2021) for relevant randomized controlled trials (RCTs) that compared two or more treatments, with at least one ICI administered to patients with advanced lung cancer. The primary outcomes were treatment–related AEs and irAEs, including grade 1–5 and grade 3–5. The secondary outcomes were grade 1–5 and grade 3–5 irAEs in specific organs. Both pairwise and network meta-analyses were conducted for chemotherapy, ICI monotherapy, ICI monotherapy + chemotherapy, dual ICIs therapy, and dual ICIs + chemotherapy for all safety outcomes. Node–splitting analyses were performed to test inconsistencies in network. Sensitivity analyses were adopted by restricting phase III RCTs and studies that enrolled patients with non–small cell lung cancer.
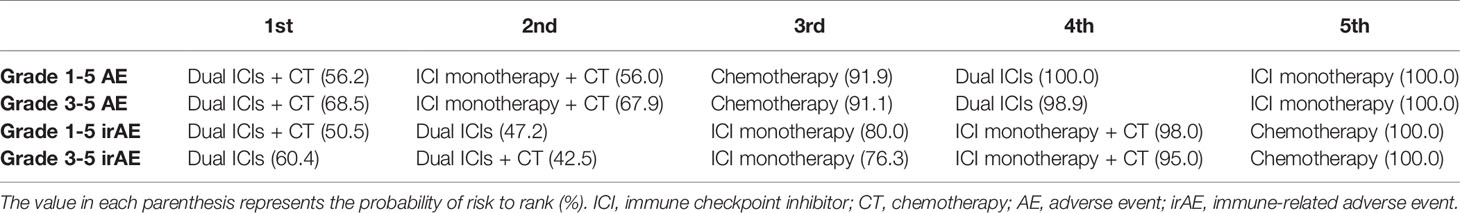
Results: Overall, 38 RCTs involving 22,178 patients with advanced lung cancer were enrolled. Both pooled incidence and NMA indicated that treatments containing chemotherapy increased the risk of treatment–related AEs when compared with ICI-based regimens without chemotherapy. As for grade 1–5 irAEs, dual ICIs + chemotherapy was associated with the highest risk of irAEs (probability in ranking first: 50.5%), followed by dual-ICI therapy (probability in ranking second: 47.2%), ICI monotherapy (probability in ranking third: 80.0%), ICI monotherapy + chemotherapy (probability in ranking fourth: 98.0%), and finally chemotherapy (probability in ranking fifth: 100.0%). In grade 3–5 irAEs, subtle differences were observed; when ranked from least safe to safest, the trend was dual ICIs therapy (60.4%), dual ICIs + chemotherapy (42.5%), ICI monotherapy (76.3%), ICI monotherapy + chemotherapy (95.0%), and chemotherapy (100.0%). Furthermore, detailed comparisons between ICI–based options provided irAE profiles based on specific organ/system and severity.
Conclusions: In consideration of overall immune–related safety profiles, ICI monotherapy + chemotherapy might be a better choice among ICI–based treatments for advanced lung cancer. The safety profiles of ICI–based treatments are various by specific irAEs and their severity.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero, identifier CRD42021268650
Introduction
Lung cancer remains the leading cause of global cancer mortality, with approximately 1.8 million deaths annually (18% of the total cancer deaths) (1). Over the past decades, platinum–based chemotherapy has become the cornerstone for managing advanced lung cancer; however, its use is of concern due to inevitable resistance and intolerable adverse events (AEs) in these fragile patients (2, 3). Recently, immunotherapy has revolutionised treatment approaches for advanced lung cancer by making longer survival times a reality (4). Unlike traditional therapy (chemotherapy and targeted therapy), immune checkpoint Inhibitor (ICI) therapies use monoclonal antibodies to inhibit the expression of proteins [cytotoxic T lymphocyte associated antigen (CTLA4), programmed death-1 receptor (PD-1), and its ligand (PD-L1)], thereby boosting T–cell activation against cancer (5). To date, a series of ICIs [pembrolizumab, nivolumab, ipilimumab, atezolizumab, and durvalumab registered by the U.S. Food and Drug Administration (FDA); camrelizumab, sintilimab, and tislelizumab approved by the National Medical Products Administration (NMPA)] have been successfully introduced for use in patients with advanced lung cancer. Recently, ICI monotherapy with or without chemotherapy, dual ICIs combination, even dual ICIs combined with chemotherapy have been clinically applied as standard first–line treatment options for advanced lung cancer.
With a dramatic increase in the availability of ICI drugs and their superior efficacy, a substantial proportion of patients with lung cancer are administered these agents. Nonetheless, concerns regarding unique treatment–specific toxicities owing to their pharmacological mechanisms, namely immune–related AEs (irAEs), associated with ICI regimens are growing (6). IrAEs are unintended effects following the activation of the immune system by ICI–mediation and can occur in any organ or system, including the gastrointestinal tract, lungs, endocrine, skin, heart, renal, liver, and muscles (7). In published randomised controlled trials (RCTs), patients administered ICIs experienced fewer AEs than those undergoing chemotherapy, while the incidence of any irAEs seemed to be distinctly higher in the ICI group (8). Without timely identification and proper management, irAEs can become severe complications, resulting in treatment discontinuation or failure, and even death (9, 10).
Previous meta-analyses have examined the risks of irAEs associated with ICI therapy; however, most of them mainly involved patients with all types of cancers (11–13). In addition, these studies did not explicitly examine the risk of individual irAEs across different ICI regimens, which may vary according to cancer type. Recently, one network meta-analysis (NMA) (14) and six traditional meta-analyses (14–19) addressed this issue in patients with lung cancer. However, they focused on one or two specific irAEs; therefore, the entire toxicity spectrum in these patients is yet to be described. Since, to the best our knowledge, head–to–head comparisons among ICI regimens are lacking in current literature, an indirect analysis could be performed to obtain comparative results and rank all possible treatments (20). In the present study, we conducted an NMA using up-to-date data from ICI–treated patients with advanced lung cancer to compare the risk of developing AEs during or following various treatment strategies.
Methods
Literature Search
This NMA was conducted according to the priori established protocol (PROSPERO: CRD42021268650; https://www.crd.york.ac.uk/prospero/#searchadvanced), and reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines (21) and its extension statement for NMA (Table S1). The PubMed, EMBASE, and Cochrane Library databases were systematically searched from inception to 19 April 2021 with language restricted to English. The search terms and their combinations used in the search strategies are shown in Table S2. We also identified potential studies listed as references in the retrieved articles and searched unpublished data from the ClinicalTrials.gov website.
Study Selection
The eligibility criteria for published studies were as follows: head-to-head phase II and III RCTs comparing two or more treatments, including at least one ICI drug in patients with advanced lung cancer. Studies published only in the form of conference abstracts, posters, and presentations of ongoing RCTs were excluded. If several studies were derived from the same trial, the study that reported comprehensive safety data was involved. Two authors (Y.Y. and J.C.) independently screened all titles and abstracts, and further assessed potentially eligible full text based on the aforementioned criteria.
Study Outcomes and Data Extraction
The primary outcomes of this study were overall safety outcomes, viz. treatment–related adverse events and immune-related adverse events as defined in each study (Table S3), including grade 1–5 and grade 3–5, respectively. The secondary outcomes were grade 1–5 and grade 3–5 irAEs in specific organ systems, including the gastrointestinal system (colitis and diarrhea), pulmonary system (pneumonitis), endocrine system (hyperthyroidism, hypothyroidism, thyroiditis, hypophysitis, and diabetes), skin (pruritus, rash, and severe skin reaction), and others (myocarditis, nephritis, hepatitis, myositis, and hypersensitivity/infusion reaction). Grading of AEs was reported according to the Common Terminology Criteria for Adverse Events (CTCAE), as reported in each study. Two authors (Y.Y. and J.C.) used a pre-designed form to extract the following data: study characteristics (study ID and publication year, NCT number, cancer type, study design, arms, treatment regimens, number of patients, follow–up time, and version of CTCAE), demographics and clinical characteristics (age, sex, PS score, brain/CNS metastasis, liver metastasis, bone metastasis, current/former smoker, prior surgery, and prior radiotherapy), and data on the aforementioned outcomes. The above information was extracted from the main text and Supplementary Materials, and only accessible data were analysed.
Quality Assessment
The methodological quality of the included trials was assessed using the Cochrane Collaboration Risk of Bias Tool (22). Low, moderate, or high risk of bias was assigned to each citation within the following five aspects: random sequence generation, allocation concealment, masking, assessment of outcomes, and selective reporting. Disagreements during study selection, data extraction, and quality assessment processes were resolved by consensus following a consultation with the corresponding investigator (Z.G.).
Statistical Analyses
To illustrate the direct and indirect comparisons among the treatments, a plot of the network geometry was generated. A pairwise meta-analysis of head-to-head comparisons was conducted to make direct estimates. Results were reported as relative risks (RRs) with 95% confidence intervals (95% CIs) using a random–effect model. Statistical heterogeneity was assessed using the I2-test, with a value > 50% representing considerable heterogeneity (23). A sensitivity analysis was performed to assess the robustness of the results by sequentially eliminating each study from the pool (24, 25). Furthermore, meta-regression analyses were performed to explore the influence of potential factors on patient outcomes (26). When a single analysis involved > 10 studies, publication bias was evaluated using funnel plots, as well as Egger’s and Begg’s tests (27). For outcomes with potential publication bias, the trim and fill method were used to estimate the number of missing studies and to provide an estimated intervention effect to perform adjustment for publication bias. In the network comparison among treatment regimens, chemotherapy was used as the reference comparator. Random effects and consistency models were used to calculate RRs and their 95% CIs; these models are thought to be the most conservative approach to dealing with between–study heterogeneity. Cumulative probabilities were used to provide a hierarchy of the treatments. According to the cumulative probabilities, treatment regimens were ranked from the worst (i.e., associated with the highest risk of AEs) to the best (i.e., associated with the lowest risk of AEs) (28). Transitivity was appraised in consistency and coherence: first, interaction analyses were used to assess the comparability between the consistency and inconsistency models; second, node–splitting analyses were performed to test coherence in the network. To further ensure the robustness of the findings, sensitivity analyses were adopted by restricting the following factors: phase III RCTs and studies that enrolled patients with non–small cell lung cancer (NSCLC). The incidences of grade 1–5 and grade 3–5 AEs were also pooled using meta-analysis (29). All data were analysed by using STATA version13.0 (Statacorp, College Station, Texas, United States), with p values < 0.05 indicating a statistically significant difference.
Results
Study Selection and Characteristics
Our initial search yielded 1,725 records from databases and 914 records from the ClinicalTrials.gov platform; 2,535 records were excluded following screening of titles and abstracts. The remaining 104 full–text articles were reviewed, and 66 articles were excluded for reasons depicted in Figure 1 and Table S4. Given that only one trial (IMpower150) involved groups of ICI + targeted + chemotherapy and ICI + targeted therapy, which had no head-to-head comparison with other five treatments, it was excluded in our network map. Finally, 38 studies (30–67) met the inclusion criteria, and their characteristics are listed in Table 1. Of these 38 studies, 30 were phase III trials, six were phase II trials, one was a phase I/II trial, and one was a phase II/III trial. As for the indication, 28 RCTs involved patients with NSCLC, and the remaining nine RCTs included patients with small cell lung cancer (SCLC). The sample sizes ranged from 73–1,739 participants, and the median follow–up time varied from 6.6–30.2 months across trials. As shown in the network map (Figure 2), a total of 22,178 patients with advanced lung cancer were included in five treatment regimens (8,768, 6,057, 4,917, 1,807, and 629 patients received chemotherapy, ICI monotherapy, ICI monotherapy + chemotherapy, dual ICIs therapy, and dual ICIs + chemotherapy, respectively). Detailed patient demographics and clinical characteristics are summarised in Table S5. The median age of patients was ranged from 50.1–66 years, and the proportion of males was 68.9%. Most of the patients were current or former smokers (84.4%), with a PS score of 0–1 (98.8%). Overall, 16.7% of the patients were reported to have metastasis (brain, liver, or bone) at baseline, while 3.4% and 4.8% of them were previously reported to have undergone surgery and radiotherapy, respectively.
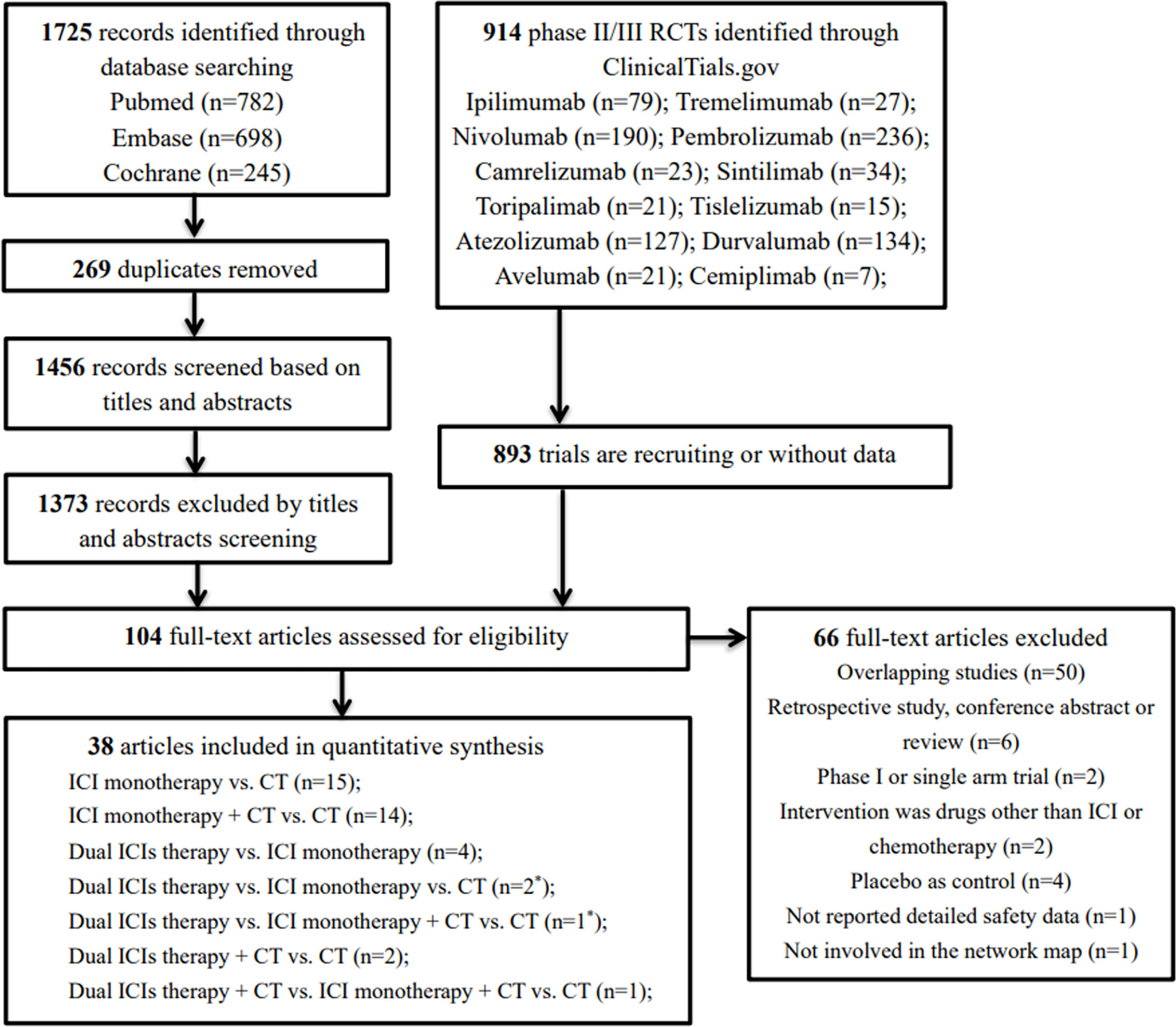
Figure 1 Flow diagram for the selection of eligible studies. ICI, immune checkpoint inhibitor; CT, chemotherapy; n, number; *, one study involved two groups, dual ICIs therapy vs. ICI monotherapy vs. CT (group A) and dual ICIs therapy vs. ICI monotherapy + CT vs. CT (group B).
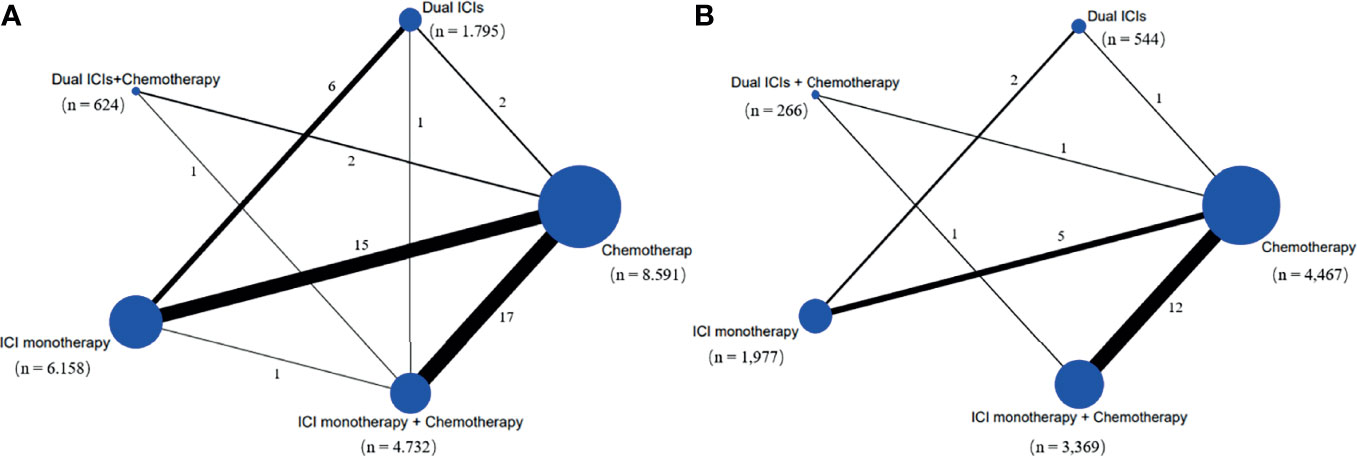
Figure 2 Network map of comparisons based on five treatments in grade 1-5 adverse events (A) and grade 1-5 immune-related adverse events (B). Each circular node represents a type of treatment. The node size is proportional to the total number of patients administering a treatment (in parentheses). Each line represents a type of head-to-head comparison. The width of lines is proportional to the total number of studies comparing the connected treatments. ICI, immune checkpoint inhibitor; n, number.
Risk-of-Bias Assessment
The included RCTs satisfied three tool items, viz. random sequence generation, incomplete outcome data, and selective reporting. Twenty–seven open–label trials did not meet the item of allocation concealment or blinding of participants and personnel, resulting in a high risk of bias in the study assessment. Overall, 27 studies exhibited a high risk of bias, and 11 studies were considered to have a low risk of bias. The details of the quality assessment are presented in Table S6.
Pairwise Meta-Analysis Based on Head-to-Head Comparisons
Direct comparisons were conducted to assess safety profiles among five treatments options (Table S7). Compared with conventional chemotherapy, three treatment regimens (ICI monotherapy, dual ICIs, and dual ICIs + chemotherapy) had a similar risk for grade 1–5 AEs, except for ICI monotherapy + chemotherapy (RR: 1.03, 95% CI: 1.01–1.04). Meanwhile, dual ICIs therapy appeared to have a higher risk for grade 1–5 AEs than ICI monotherapy (RR: 1.17, 95% CI: 1.04–1.31). Regarding grade 3–5 AEs, chemotherapy showed a noticeably higher risk compared with ICI monotherapy (RR: 0.33, 95% CI: 0.27–0.40 for ICI monotherapy vs. chemotherapy), while there was a reduced risk when compared with dual ICIs + chemotherapy (RR: 0.33, 95% CI: 0.27–0.40 for dual ICIs + chemotherapy vs. chemotherapy). Other results were consistent with those observed in grade 1–5 AEs.
In terms of irAEs, the use of ICI monotherapy (RR: 4.06, 95% CI: 2.75–5.98 for grade 1–5; RR: 5.75, 95% CI: 3.50–9.43 for grade 3–5) or ICI monotherapy + chemotherapy (RR: 2.02, 95% CI: 1.63–2.52 for grade 1–5; RR: 2.93, 95% CI: 1.98–4.34 for grade 3–5) was associated with a significantly higher risk of developing these manifestations than chemotherapy. Dual ICIs therapy had a similar risk of grade 1–5 irAEs (RR: 1.48, 95% CI: 0.77–2.84) but a superior risk of grade 3–5 irAEs (RR: 2.12, 95% CI: 1.29–3.48) compared with ICI monotherapy. Detailed data in specific irAEs are listed in Table S7. Briefly, results from organ–specific irAEs, including colitis, pneumonitis, hyper/hypothyroidism, hepatitis, and rash, were comparable with those observed in overall irAEs. Regarding heterogeneity of pairwise meta-analysis comparisons, relatively high heterogeneity was found in primary outcomes (I2: 38.2%–95.0%), except for two pairs (dual ICIs + chemotherapy vs. chemotherapy for grade 3–5 AEs and ICI monotherapy vs. chemotherapy for grade 3–5 irAEs). Overall, the general heterogeneity in individual irAEs was low to moderate.
Sensitivity analyses were conducted by sequentially removing each study. Following this, the pooled results were in line with the set primacy safety outcomes (Table S8). In addition, meta-regression analysis failed to detect any potential confounding factors affecting the primacy outcomes (Table S9). A visual inspection of the funnel plots and Begg’s test showed relative symmetry, except for grade 3–5 AE in comparison of ICI monotherapy vs. chemotherapy (P = 0.018) (Figure S1 and Table S10). However, P values of Egger’s test in several outcomes were < 0.05, suggesting that publication bias existed in this study (Table S10). The trim and fill method were adopted to mitigate publication bias, and the outcomes were consistent with our primary results (P for interaction > 0.05) (Table S10).
Network Meta-Analysis for Overall Safety
The pooled incidence for five treatments showed the following rankings: ICI monotherapy + chemotherapy had the highest incidence of AEs (93.21% for grade 1–5, 58.48% for grade 3–5), followed by dual ICIs + chemotherapy (92.02%, 54.23%), chemotherapy (88.35%, 49.75%), dual ICIs therapy (76.47%, 31.38%), ICI monotherapy (65.99%, 15.22%) (Figure 3A and Table S11). Established NMA based on the consistency model indicated that ICI monotherapy had the lowest risk of causing grade 1–5 AEs compared with chemotherapy (RR: 3.7, 95% CI: 3.05–4.48 for chemotherapy vs. ICI monotherapy), ICI monotherapy + chemotherapy (RR: 0.19, 95% CI: 0.14–0.26), dual ICIs therapy (RR: 0.55, 95% CI: 0.41–0.74), as well as dual ICIs + chemotherapy (RR: 0.18, 95% CI: 0.10–0.33) (Figure 3A). Then was dual ICIs therapy (RR: 2.03, 95% CI: 1.46–2.81 for chemotherapy vs. dual ICIs; RR: 2.89, 95% CI: 1.94–4.29 for ICI monotherapy + chemotherapy vs. dual ICIs; RR: 0.33, 95% CI: 0.18–0.63 for dual ICIs vs. dual ICIs + chemotherapy). ICI monotherapy + chemotherapy showed a higher risk of causing grade 1–5 AEs than chemotherapy (RR: 0.70, 95% CI: 0.55–0.90 for chemotherapy vs. ICI + chemotherapy), while no significant difference was seen when compared with that of the ICIs + chemotherapy group (RR: 0.96, 95% CI: 0.54–1.70). Similar results were observed in grade 3–5 AEs. The ranking probabilities based on the five treatment groups are also depicted in Figure 3A. The rankings of grade 3–5 AEs were in line with those of grade 1–5 AEs, ranging from least safe to safest as follows: ICIs + chemotherapy (probability: 56.2% for grade 1–5; 68.5% for grade 3–5), ICI monotherapy + chemotherapy (56.0%; 67.9%), chemotherapy (91.9%; 91.1%), dual ICIs therapy (100.0%; 98.9%), and ICI monotherapy (100.0%; 100.0%) (Table 2 and, Table S12).
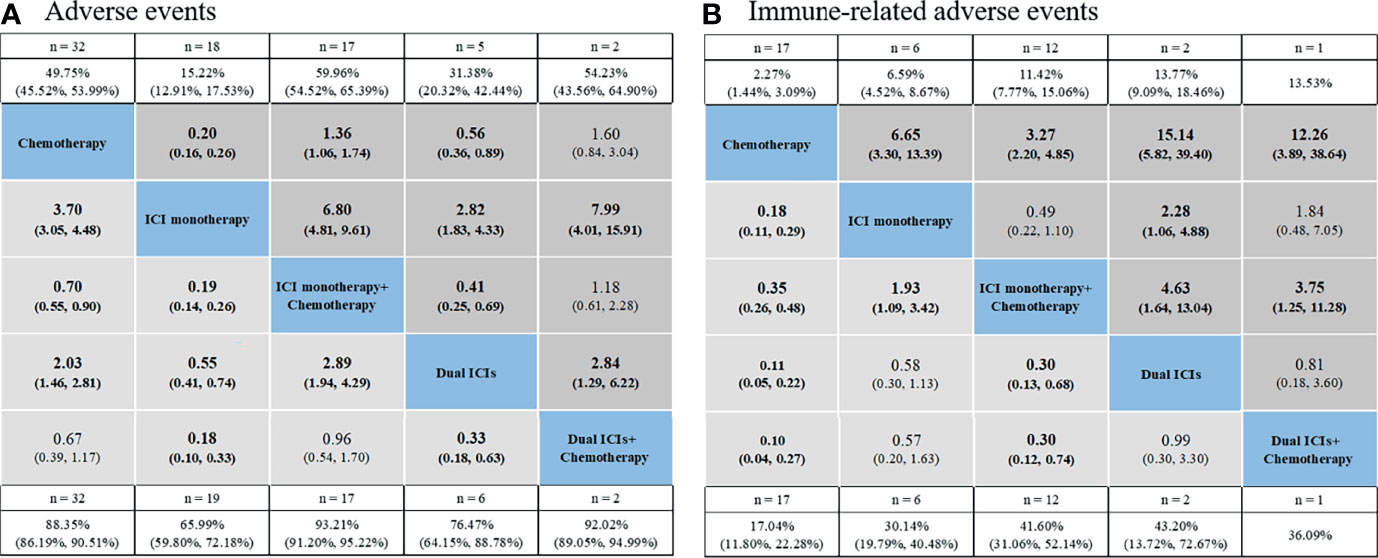
Figure 3 Safety profiles based on adverse events (A) and immune-related adverse events (B). Pooled incidences and 95% confidence intervals of grade 1–5 events for each treatment are at bottom and that of grade 3–5 events are at top of the figure. Each cell of the safety profiles contains the pooled relative risks and 95% confidence intervals for grade 1–5 (light gray cell) and grade 3–5 (dark gray cell) events; significant results are in bold. The pooled relative risks and 95% confidence intervals indicate the results of the top treatment compared with the bottom treatment. ICI, immune checkpoint inhibitor; n, number.
As for overall irAEs, the pooled incidences for chemotherapy, ICI monotherapy, ICI monotherapy + chemotherapy, dual ICIs, and dual ICIs + chemotherapy were 17.04%, 30.14%, 41.60%, 43.20%, and 36.09% for grade 1–5 irAEs, and 2.27%, 6.59%, 11.42%, 13.77%, and 13.53% for grade 3–5 irAEs, respectively. Based on NMA, the safety profiles (Figure 3B) of the five treatment choices indicated an extremely decreased risk of irAEs favoring chemotherapy over the other four treatment strategies for both grade 1–5 (RR: 0.18, 95% CI: 0.11–0.29 for chemotherapy vs. ICI monotherapy; RR: 0.35, 95% CI: 0.26–0.48 for chemotherapy vs. ICI monotherapy + chemotherapy; RR: 0.11, 95% CI: 0.05–0.22 for chemotherapy vs. dual ICI therapy; RR: 0.10, 95% CI: 0.04–0.27 for chemotherapy vs. dual ICIs + chemotherapy) and grade 3–5 events (RR: 6.65, 95% CI: 3.30–13.39 for ICI monotherapy vs. chemotherapy; RR: 3.27, 95% CI: 2.20–4.85 for ICI monotherapy + chemotherapy vs. chemotherapy; RR: 15.14, 95% CI: 5.82–39.40 for dual ICI therapy vs. chemotherapy; RR: 12.26, 95% CI: 3.89–38.64 for dual ICIs + chemotherapy vs. chemotherapy). Among ICI therapeutic schedules, ICI monotherapy + chemotherapy seemed safer than ICI monotherapy (RR: 1.93, 95% CI: 1.09–3.42 for ICI monotherapy vs. ICI monotherapy + chemotherapy in grade 1–5 irAEs; RR: 0.49, 95% CI: 0.22–1.10 in grade 3–5 irAEs), dual ICIs therapy (RR: 0.30, 95% CI: 0.13–0.68 in grade 1–5 irAEs; RR: 4.63, 95% CI: 1.64–13.04 for dual ICIs vs. ICI monotherapy + chemotherapy in grade 3–5 irAEs), and dual ICIs + chemotherapy (RR: 0.30, 95% CI: 0.12–0.74 in grade 1–5 irAEs; RR: 3.75, 95% CI: 1.25–11.28 for dual ICIs + chemotherapy vs. ICI monotherapy + chemotherapy in grade 3–5 irAEs), with one ICI being observed to be safer than two ICIs combination with regards to grade 3–5 irAEs (RR: 2.28, 95% CI: 1.06–4.48 for dual ICIs vs. ICI monotherapy). In aspect of safety ranking, dual ICIs + chemotherapy was associated with the worst ranking for grade 1–5 irAEs (probability: 50.5%), followed by dual ICIs (47.2%), ICI monotherapy (80.0%), ICI monotherapy + chemotherapy (98.0%), and finally chemotherapy (100.0%). The risk of experiencing grade 3–5 irAEs was ranked from high to low as follows: dual ICIs (60.4%), dual ICIs + chemotherapy (42.5%), ICI monotherapy (76.3%), ICI monotherapy + chemotherapy (95.0%), and chemotherapy (100.0%) (Table 2, Table S12).
The results pooled via the inconsistency model had a generally satisfactory fit compared with those calculated by the consistency model, except for minor comparisons based on chemotherapy (Table S13). Likewise, a less significant inconsistency was observed following the node–splitting analysis (Table S14).
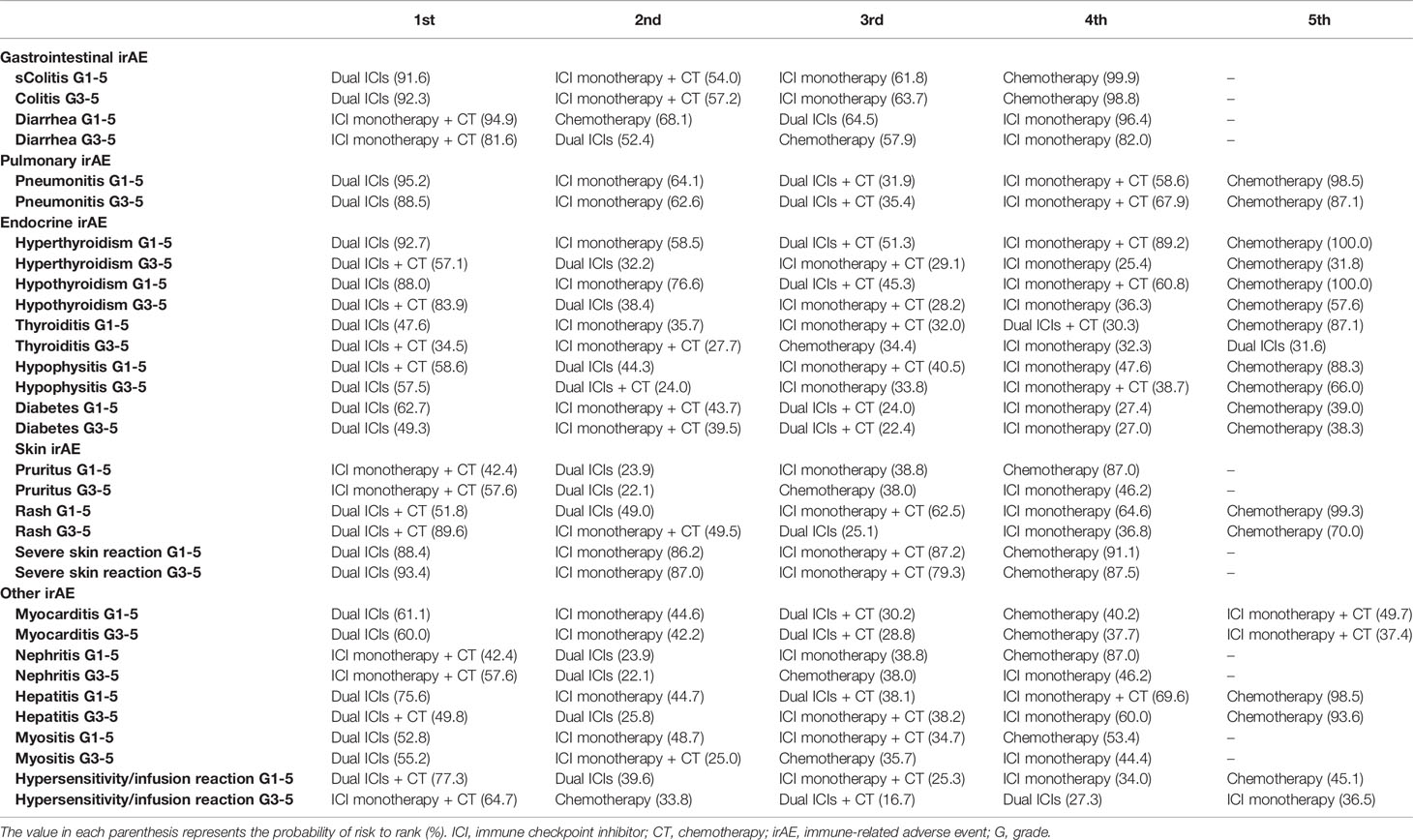
Network Meta-Analysis for Specific IrAEs
NMAs and ranking probabilities for different treatment strategies in subgroups of irAEs are depicted in Figures 4, 5 and Table 3. Although the results for individual irAEs varied by organ system and severity, traditional chemotherapy presented the lowest risk in majority of irAEs.
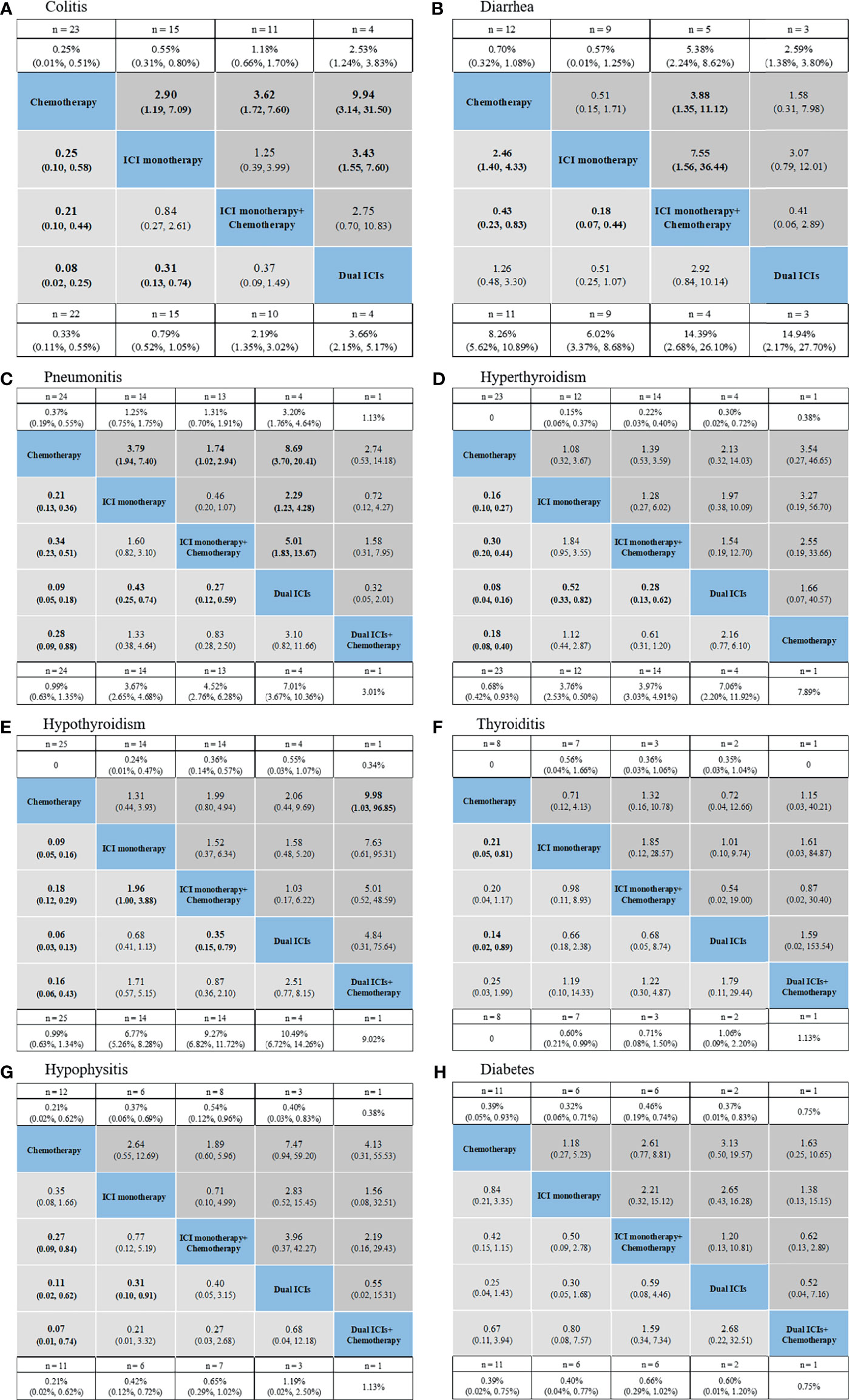
Figure 4 Safety profiles based on specific organs: colitis (A), diarrhea (B), pneumonitis (C), hyperthyroidism (D), hypothyroidism (E), thyroiditis (F), hypophysitis (G), diabetes (H). Pooled incidences and 95% confidence intervals of grade 1–5 events for each treatment are at bottom and that of grade 3–5 events are at top of the figure. Each cell of the safety profiles contains the pooled relative risks and 95% confidence intervals for grade 1–5 (light gray cell) and grade 3–5 (dark gray cell) events; significant results are in bold. The pooled relative risks and 95% confidence intervals indicate the results of the top treatment compared with the bottom treatment. ICI, immune checkpoint inhibitor; n, number.
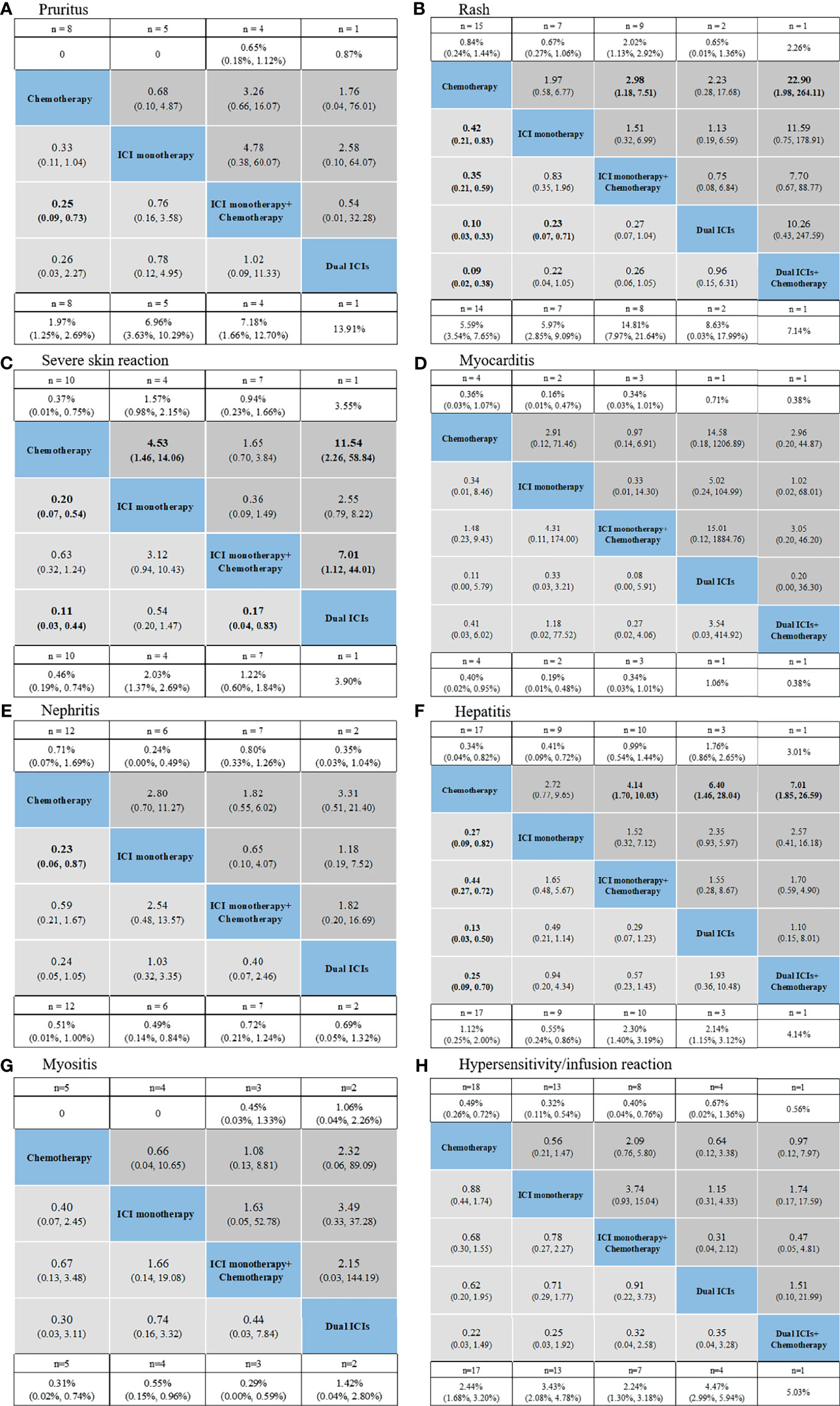
Figure 5 Safety profiles based on specific organs: pruritus (A), rash (B), severe skin reaction (C), myocarditis (D), nephritis (E), hepatitis (F), myositis (G), hypersensitivity/infusion reaction (H). Pooled incidences and 95% confidence intervals of grade 1–5 events for each treatment are at bottom and that of grade 3–5 events are at top of the figure. Each cell of the safety profiles contains the pooled relative risks and 95% confidence intervals for grade 1–5 (light gray cell) and grade 3–5 (dark gray cell) events; significant results are in bold. The pooled relative risks and 95% confidence intervals indicate the results of the top treatment compared with the bottom treatment. ICI, immune checkpoint inhibitor; n, number.
For gastrointestinal irAEs (Figures 4A, B), ICI regimens were associated with higher risk of colitis than chemotherapy, with dual ICIs showing the highest risk (incidence: 3.66% for grade 1–5 and 2.53% for grade 3–5; ranking probability: 91.6% for grade 1–5 and 92.3% for grade 3–5). Nevertheless, a different trend was detected in the case of diarrhea. ICI regimens, especially ICI monotherapy, had a lower risk of causing diarrhea than treatment strategies that included chemotherapy.
For pulmonary irAEs, pneumonitis was significantly higher in patients receiving dual ICIs than in those receiving ICI monotherapy (RR: 0.43, 95% CI: 0.25–0.74 for ICI monotherapy vs. dual ICIs in grade 1–5; RR: 2.29, 95% CI: 1.23–4.28 in grade 3–5), or ICI monotherapy + chemotherapy (RR: 0.27, 95% CI: 0.12–0.59 for ICI monotherapy + chemotherapy vs. dual ICIs in grade 1–5; RR: 5.01, 95% CI: 1.83–13.67 in grade 3–5) (Figure 4C). The ranking order of ICIs regimens was as follows: dual ICIs (probability: 95.2% for grade 1–5; 88.5% for grade 3–5), ICI monotherapy (64.1%; 62.6%), dual ICIs + chemotherapy (31.9%; 35.4%), and ICI monotherapy + chemotherapy (58.6%; 67.9%).
For endocrine irAEs, patients administering dual ICIs with or without chemotherapy seemed to be associated with a high risk of hyperthyroidism, hypothyroidism, hypophysitis, thyroiditis, and diabetes. Owing to the low incidence of serious endocrine irAEs, no positive RRs were detected among the individual ICI strategies (Figures 4D–G).
For skin irAEs, the use of ICI monotherapy + chemotherapy, dual ICIs + chemotherapy, and dual ICIs presented the highest risk of pruritus, rash, and severe skin reaction, respectively (Figures 5A–C). For other irAEs, no significant difference was observed among the ICI regimens (Figures 5D–H).
Sensitivity Analysis in Network Meta-Analysis
Thirty phase III RCTs, and 29 studies that enrolled patients with NSCLC were separately included in the sensitivity analyses. The observed ranking orders were consistent with the original NMA, irrespective of the overall AEs and irAEs (Table S15).
Discussion
Major Findings and Interpretation
The current NMA highlights the toxicity profile of ICI–based treatments among patients with advanced lung cancer based on 38 RCTs involving 22,178 patients. Our results indicated that the use of mono- or dual-ICI therapy may reduce the risk of treatment–related AEs at the expense of an increased risk of developing irAEs compared with chemotherapy. During therapy with ICI regimens, patients administering dual ICIs with chemotherapy may experience most AEs (either grade 1–5 or grade 3–5) and irAEs of any grade, and those receiving dual ICIs may have the highest risk of developing serious irAEs. There were differences observed in the toxicity spectra among the five treatment therapies. However, these results should be carefully interpreted because of the limited number of studies on groups receiving dual ICIs with and without chemotherapy.
Comparison With Previous Studies
Recently, developments in lung cancer patients with advanced stages of the disease have shown immunotherapy as a promising treatment option. However, the expanded use of ICIs has resulted in noticeable growth in adverse events, particularly irAEs (6, 68). With more treatment options with ICIs now approved for advanced lung cancer, a robust analysis is urgently required to compare the risk of safety profiles among all of the different treatment regimens.
To date, several systematic reviews and meta-analyses have been conducted to assess the safety profiles of ICIs in patients with cancer, yet few studies have focused on patients with lung cancer. The earliest meta-analysis, which pooled 22 RCTs for evaluating rare but severe irAEs resulting from the use of PD-1 and PD-L1 inhibitors in patients with NSCLC, indicated an increased all–grade pneumonitis risk from ICIs than chemotherapy (OR = 2.35, 95% CI, 1.32–4.20, P = 0.004) (15). However, this study had limited value because of the inclusion of minimal trials with a control group, which inevitably led to insufficient evidence for a conclusion; in addition, only ICI monotherapy was investigated. In 2020, an updated NMA of 25 RCTs was conducted for chemotherapy, ICI monotherapy, dual ICIs combination, and ICIs + chemotherapy, and reported a significantly reduced risk of immune–related pneumonitis in patients with lung cancer following ICIs + chemotherapy when compared with dual ICIs combination and ICI monotherapy (14). Furthermore, three other meta-analyses focused on gastrointestinal irAEs, including diarrhea and/or colitis, and consistently found that PD-1/PD-L1 inhibitors might lead to a higher risk of immune–mediated colitis but might result in a reduction in diarrhea when compared with chemotherapy (16–18). Notably, the above–mentioned studies focused on one specific irAE, making it difficult to unveil the panorama of toxicity from ICIs. Recently, Berti et al. compared overall and organ–specific irAEs between immunotherapy and immune–chemotherapy in lung cancer based on 16 phase III clinical trials and found that immunotherapy alone showed a significantly lower risk of irAEs than immunochemotherapy (69). The study investigators excluded phase II RCTs and ignored the discrepancy between ICI monotherapy and dual ICI combination therapy. Given these limitations, the current NMA comprehensively estimated the overall and organ–specific toxicity spectrum among all up to date ICI regimens by pooling all currently available phase II and III clinical trials involving patients with NSCLC and SCLC.
Safety Profile of ICI Regimens in Patients With Lung Cancer
It is well known that the incidence of overall adverse events during ICI monotherapy is lower than that for conventional chemotherapy, while exposure to immunotherapy increases the risk of irAEs, as seen in the present study. Interestingly, further analyses of ICI–based regimens found that ICI monotherapy + chemotherapy decreased the risk of grade 1–5 and grade 3–5 irAEs compared with ICI monotherapy and dual ICIs therapy. This trend was also observed in pneumonitis and myocarditis (grade1–5 and grade 3–5), as well as grade 1–5 hyperthyroidism, hypothyroidism, thyroiditis, severe skin reaction, hepatitis, myositis based on ranking. Consistent with our study, Chen et al. reported that the use of an ICI with chemotherapy led to less pneumonitis than use of ICI monotherapy or dual ICIs combination (14). One possible reason for the decreased risk in irAEs when chemotherapy is added to ICI regimens may be due to the fact that conventional chemotherapy consists of cytotoxic agents that are believed to cause chemotherapy–induced immunosuppression, augmenting stress on the entire immune system and resulting in a reduced immune function (70, 71). Another contributing factor might be the use of corticosteroids. In chemotherapy regimens containing cytotoxic agents such as platinum, pemetrexed and taxanes, which are the standard treatments for lung cancer, corticosteroids are commonly prescribed as binding pre-treatment for antiemetic and antiallergy purposes. The baseline or early use of corticosteroids at the time of initiating ICI therapy could blunt a proliferative burst of CD8-positive T cells, which are otherwise needed for the ICI therapeutic response, thus affecting the efficacy and toxicity (72, 73). In addition, corticosteroids are recommended immunosuppressive agents for various mild-to-severe irAEs such as pneumonitis, colitis, hepatitis, thyroiditis, rash, etc. (74). Accordingly, the risk of experiencing irAEs may be underestimated in circumstances with corticosteroids.
Some studies have pointed out that the incidence of irAEs in patients on a combination of two ICIs was higher than that observed with ICI monotherapy. In the present study, we did not observe any statistical differences between these two groups in terms of overall grade 1–5 irAEs, let alone for rare irAEs, such as thyroiditis, diabetes, myocarditis, nephritis, hepatitis, and myositis. Even between dual ICIs + chemotherapy and ICI monotherapy (both grade 1–5 and grade 3–5 irAEs), significant difference was not detected. However, this trend can be seen from the ranking either in grade 1–5 irAEs or grade 3–5 irAEs. Given the fact that a limited number of studies directly compared dual ICIs (with or without chemotherapy) with ICIs monotherapy, these results should be interpreted with caution. Therefore, more high–quality RCTs are needed to investigate the incidence of irAEs among different ICI–based regimens.
Clinical Implication
Our results provide possible safety speculations for clinical decision–making to tailor the best immunotherapy strategy for each patient with lung cancer. For instance, administration of an individual ICI or dual ICIs plus chemotherapy was reported to have a significantly lower risk of pneumonitis (grade 1–5 and grade 3–5) than ICI monotherapy and dual ICIs therapy and could perhaps be preferred in selected cases of lung fibrosis or severe chronic obstructive pulmonary disease. In addition, ICI monotherapy was associated with the lowest risk for both diarrhea and colitis among ICI regimens and could perhaps be preferred in selected patients in whom gastrointestinal irAEs could be a concern. Of course, these results should be proven in prospective registries or cohorts to better understand the safety of novel ICI–based options in this subset of patients.
Study Strengths and Limitations
The major strength of this study was to depict a full view of the safety profile of ICIs at different levels (risk of overall AEs of any grade and grade 3–5, any irAEs and severe irAEs by individual organs/system). Second, owing to the different toxicity spectrum based on cancer types, the study focused on a specific population of patients (i.e., with lung cancer). Third, except for combination of ICIs and targeted agents, we included all available ICI–based regimens to aid clinicians to tailor the ICI strategy for individual patient with lung cancer.
However, several limitations of this study need to be acknowledged. First, the included RCTs used different terms to describe irAEs. In clinical settings, AEs or irAEs are usually recognised and reported depending on the evaluation of the physician and are diagnosed based on their experience. Therefore, the identification of irAEs might not be completely accurate and might lead to bias in the assessment. In addition, few studies reported irAEs during the entire ICI monotherapy maintenance as separate outcome, making it difficult to investigate irAEs during this period. Second, the included studies showed heterogeneity in terms of subtype of cancer, pharmacological strategy, follow–up time, and other factors. As we focused on outlining the entire safety profile of ICI agents, subgroup analyses based on patients’ histology, specified ICIs and kind of chemotherapy were not performed. However, we performed sensitivity analyses and meta-regression in pairwise meta-analysis, as well as sensitivity analyses in NMA, to control for these possible confounders. Third, inconsistent results between direct and indirect comparisons were observed. Unlike direct comparison, network meta-analysis included both direct evidence and indirect effects from the other studies. Because of above-mentioned heterogeneity among RCTs, integrated results possibly underestimated or overestimated the actual results. Fourth, we did not obtain access to comorbidity data, which might be high–risk factors for certain irAEs. Lastly, we did not have the resources to review non-English publications. However, we enrolled studies identified following a comprehensive search of broad databases and are thus confident that this study covered the majority of trials in these special patients. Given aforementioned limitations, further studies are needed to confirm our findings.
Conclusions
In summary, this network meta-analysis contributes to clarifying the frequency and characteristics of adverse events during ICI treatment in patients with advanced lung cancer. We found that ICI monotherapy + chemotherapy had the best immune–related safety profile, followed by ICI monotherapy, dual ICIs therapy, dual ICIs + chemotherapy for grade 1–5 irAEs, and ICI monotherapy, dual ICIs + chemotherapy, dual ICIs therapy for grade 3–5 irAEs. The safety ranking of ICI-based choices is modulated by specific irAEs and severity.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
Z-CG, X-YC, and H-WL are the guarantors of the entire manuscript. Z-CG and Y-DY contributed to the study conception and design, critical revision of the manuscript for important intellectual content, and final approval of the version to be published. J-JC, JF, and Y-JS contributed to the data acquisition, analysis, and interpretation. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Ren Ji Boost Project of National Natural Science Foundation of China (RJTJ-JX-001), the Research Funds of Shanghai Health and Family Planning Commission (20184Y0022), Clinical Pharmacy Innovation Research Institute of Shanghai Jiao Tong University School of Medicine (CXYJY2019ZD001), and Shanghai “Rising Stars of Medical Talent” Youth Development Program – Youth Medical Talents – Clinical Pharmacist Program [SHWJRS (2019) 072].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank Pedzisai M for language editing about our manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.760737/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Arbour KC, Riely GJ. Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. Jama (2019) 322(8):764–74. doi: 10.1001/jama.2019.11058
3. Noronha V, Sekhar A, Patil VM, Menon N, Joshi A, Kapoor A, et al. Systemic Therapy for Limited Stage Small Cell Lung Carcinoma. J Thorac Dis (2020) 12(10):6275–90. doi: 10.21037/jtd-2019-sclc-11
4. Mielgo-Rubio X, Uribelarrea EA, Cortés LQ, Moyano MS. Immunotherapy in Non-Small Cell Lung Cancer: Update and New Insights. J Clin Transl Res (2021) 7(1):1–21. doi: 10.18053/jctres.07.202101.001
5. Zhang H, Dai Z, Wu W, Wang Z, Zhang N, Zhang L, et al. Regulatory Mechanisms of Immune Checkpoints PD-L1 and CTLA-4 in Cancer. J Exp Clin Cancer Res (2021) 40(1):184. doi: 10.1186/s13046-021-01987-7
6. Lee DJ, Lee HJ Jr, Farmer JR, Reynolds KL. Mechanisms Driving Immune-Related Adverse Events in Cancer Patients Treated With Immune Checkpoint Inhibitors. Curr Cardiol Rep (2021) 23(8):98. doi: 10.1007/s11886-021-01530-2
7. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) Clinical Practice Guideline on Immune Checkpoint Inhibitor-Related Adverse Events. J Immunother Cancer (2021) 9(6):e002435. doi: 10.1136/jitc-2021-002435
8. Gangadhar TC, Vonderheide RH. Mitigating the Toxic Effects of Anticancer Immunotherapy. Nat Rev Clin Oncol (2014) 11(2):91–9. doi: 10.1038/nrclinonc.2013.245
9. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing Toxicities Associated With Immune Checkpoint Inhibitors: Consensus Recommendations From the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer (2017) 5(1):95. doi: 10.1186/s40425-017-0300-z
10. Durrechou Q, Domblides C, Sionneau B, Lefort F, Quivy A, Ravaud A, et al. Management of Immune Checkpoint Inhibitor Toxicities. Cancer Manag Res (2020) 12:9139–58. doi: 10.2147/cmar.S218756
11. De Velasco G, Je Y, Bossé D, Awad MM, Ott PA, Moreira RB, et al. Comprehensive Meta-Analysis of Key Immune-Related Adverse Events From CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res (2017) 5(4):312–8. doi: 10.1158/2326-6066.Cir-16-0237
12. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. JAMA Oncol (2019) 5(7):1008–19. doi: 10.1001/jamaoncol.2019.0393
13. Wang Y, Kong D, Wang C, Chen J, Li J, Liu Z, et al. A Systematic Review and Meta-Analysis of Immune-Related Adverse Events of Anti-PD-1 Drugs in Randomized Controlled Trials. Technol Cancer Res Treat (2020) 19:1533033820967454. doi: 10.1177/1533033820967454
14. Chen X, Zhang Z, Hou X, Zhang Y, Zhou T, Liu J, et al. Immune-Related Pneumonitis Associated With Immune Checkpoint Inhibitors in Lung Cancer: A Network Meta-Analysis. J Immunother Cancer (2020) 8(2):e001170. doi: 10.1136/jitc-2020-001170
15. Hu YB, Zhang Q, Li HJ, Michot JM, Liu HB, Zhan P, et al. Evaluation of Rare But Severe Immune Related Adverse Effects in PD-1 and PD-L1 Inhibitors in Non-Small Cell Lung Cancer: A Meta-Analysis. Transl Lung Cancer Res (2017) 6(Suppl 1):S8–s20. doi: 10.21037/tlcr.2017.12.10
16. Lin LL, Lin GF, Luo Q, Chen XQ. The Incidence and Relative Risk of PD-1/PD-L1 Inhibitors-Related Colitis in Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. Int Immunopharmacol (2019) 77:105975. doi: 10.1016/j.intimp.2019.105975
17. Zhang C, Zhang S, Xu D, Liu R, Zhu Q, Zhao Y, et al. Incidence Risk of PD-1/PD-L1 Related Diarrhea in Non-Small Cell Lung Cancer (NSCLC) Patients: A Systematic Review and Meta-Analysis. Cancer Manag Res (2019) 11:3957–69. doi: 10.2147/cmar.S202756
18. Bishay K, Tandon P, Bourassa-Blanchette S, Laurie SA, McCurdy JD. The Risk of Diarrhea and Colitis in Patients With Lung Cancer Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Curr Oncol (2020) 27(5):e486–94. doi: 10.3747/co.27.6251
19. Garrett N, da Costa ACC, Damiani G, Vasques CI. Patients With Lung Cancer Undergoing Immune Checkpoint Inhibitors: A Meta-Analysis of Dermatological Toxicities. Crit Rev Oncol Hematol (2020) 152:102983. doi: 10.1016/j.critrevonc.2020.102983
20. Yan YD, Ding Z, Pan MM, Xia Q, Cui JJ, Wang LW, et al. Net Clinical Benefit of Direct Oral Anticoagulants in Patients With Cancer and Venous Thromboembolism: A Systematic Review and Trade-Off Analysis. Front Cardiovasc Med (2020) 7:586020. doi: 10.3389/fcvm.2020.586020
21. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
22. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
23. Higgins JP, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
24. Yan YD, Zhang C, Shen L, Su YJ, Liu XY, Wang LW, et al. Net Clinical Benefit of Non-Vitamin K Antagonist Oral Anticoagulants for Venous Thromboembolism Prophylaxis in Patients With Cancer: A Systematic Review and Trade-Off Analysis From 9 Randomized Controlled Trials. Front Pharmacol (2018) 9:575. doi: 10.3389/fphar.2018.00575
25. Gu ZC, Wei AH, Zhang C, Wang XH, Zhang L, Shen L, et al. Risk of Major Gastrointestinal Bleeding With New vs. Conventional Oral Anticoagulants: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol (2020) 18(4):792–9.e761. doi: 10.1016/j.cgh.2019.05.056
26. Qian J, Yan YD, Yang SY, Zhang C, Li WY, Gu ZC. Benefits and Harms of Low-Dose Rivaroxaban in Asian Patients With Atrial Fibrillation: A Systematic Review and Meta-Analysis of Real-World Studies. Front Pharmacol (2021) 12:642907. doi: 10.3389/fphar.2021.642907
27. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
28. Zhang P, Wei S, Zhai K, Huang J, Cheng X, Tao Z, et al. Efficacy of Left Ventricular Unloading Strategies During Venoarterial Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock: A Protocol for a Systematic Review and Bayesian Network Meta-Analysis. BMJ Open (2021) 11(10):e047046. doi: 10.1136/bmjopen-2020-047046
29. Zhao Z, Ma CL, Gu ZC, Dong Y, Lv Y, Zhong MK. Incidence and Risk of Infection Associated With Fingolimod in Patients With Multiple Sclerosis: A Systematic Review and Meta-Analysis of 8,448 Patients From 12 Randomized Controlled Trials. Front Immunol (2021) 12:611711. doi: 10.3389/fimmu.2021.611711
30. Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in Combination With Paclitaxel and Carboplatin as First-Line Treatment in Stage IIIB/IV Non-Small-Cell Lung Cancer: Results From a Randomized, Double-Blind, Multicenter Phase II Study. J Clin Oncol (2012) 30(17):2046–54. doi: 10.1200/jco.2011.38.4032
31. Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in Combination With Paclitaxel and Carboplatin as First-Line Therapy in Extensive-Disease-Small-Cell Lung Cancer: Results From a Randomized, Double-Blind, Multicenter Phase 2 Trial. Ann Oncol (2013) 24(1):75–83. doi: 10.1093/annonc/mds213
32. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
33. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
34. Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab Alone and Nivolumab Plus Ipilimumab in Recurrent Small-Cell Lung Cancer (CheckMate 032): A Multicentre, Open-Label, Phase 1/2 Trial. Lancet Oncol (2016) 17(7):883–95. doi: 10.1016/s1470-2045(16)30098-5
35. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab Versus Docetaxel for Patients With Previously Treated Non-Small-Cell Lung Cancer (POPLAR): A Multicentre, Open-Label, Phase 2 Randomised Controlled Trial. Lancet (2016) 387(10030):1837–46. doi: 10.1016/s0140-6736(16)00587-0
36. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/s0140-6736(15)01281-7
37. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and Pemetrexed With or Without Pembrolizumab for Advanced, Non-Squamous Non-Small-Cell Lung Cancer: A Randomised, Phase 2 Cohort of the Open-Label KEYNOTE-021 Study. Lancet Oncol (2016) 17(11):1497–508. doi: 10.1016/s1470-2045(16)30498-3
38. Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol (2016) 34(31):3740–8. doi: 10.1200/jco.2016.67.6601
39. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
40. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med (2017) 376(25):2415–26. doi: 10.1056/NEJMoa1613493
41. Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J Clin Oncol (2017) 35(30):3449–57. doi: 10.1200/jco.2016.71.7629
42. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab Versus Docetaxel in Patients With Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/s0140-6736(16)32517-x
43. Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab Versus Docetaxel in Patients With Platinum-Treated Advanced Non-Small-Cell Lung Cancer (JAVELIN Lung 200): An Open-Label, Randomised, Phase 3 Study. Lancet Oncol (2018) 19(11):1468–79. doi: 10.1016/s1470-2045(18)30673-9
44. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab Plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
45. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
46. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab Plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med (2018) 379(21):2040–51. doi: 10.1056/NEJMoa1810865
47. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
48. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab Versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/s0140-6736(18)32409-7
49. Pujol JL, Greillier L, Audigier-Valette C, Moro-Sibilot D, Uwer L, Hureaux J, et al. A Randomized Non-Comparative Phase II Study of Anti-Programmed Cell Death-Ligand 1 Atezolizumab or Chemotherapy as Second-Line Therapy in Patients With Small Cell Lung Cancer: Results From the IFCT-1603 Trial. J Thorac Oncol (2019) 14(5):903–13. doi: 10.1016/j.jtho.2019.01.008
50. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in Combination With Carboplatin Plus Nab-Paclitaxel Chemotherapy Compared With Chemotherapy Alone as First-Line Treatment for Metastatic Non-Squamous Non-Small-Cell Lung Cancer (IMpower130): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2019) 20(7):924–37. doi: 10.1016/s1470-2045(19)30167-6
51. Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol (2019) 14(5):867–75. doi: 10.1016/j.jtho.2019.01.006
52. Arrieta O, Barrón F, Ramírez-Tirado LA, Zatarain-Barrón ZL, Cardona AF, Díaz-García D, et al. Efficacy and Safety of Pembrolizumab Plus Docetaxel vs. Docetaxel Alone in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial. JAMA Oncol (2020) 6(6):856–64. doi: 10.1001/jamaoncol.2020.0409
53. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients With NSCLC. N Engl J Med (2020) 383(14):1328–39. doi: 10.1056/NEJMoa1917346
54. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous Non-Small-Cell Lung Cancer (IMpower131): Results From a Randomized Phase III Trial. J Thorac Oncol (2020) 15(8):1351–60. doi: 10.1016/j.jtho.2020.03.028
55. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab Plus Chemotherapy for First-Line Treatment of Non-Squamous Non-Small Cell Lung Cancer: Results From the Randomized Phase III IMpower132 Trial. J Thorac Oncol (2021) 16(4):653–64. doi: 10.1016/j.jtho.2020.11.025
56. Planchard D, Reinmuth N, Orlov S, Fischer JR, Sugawara S, Mandziuk S, et al. ARCTIC: Durvalumab With or Without Tremelimumab as Third-Line or Later Treatment of Metastatic Non-Small-Cell Lung Cancer. Ann Oncol (2020) 31(5):609–18. doi: 10.1016/j.annonc.2020.02.006
57. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab With or Without Tremelimumab vs. Standard Chemotherapy in First-Line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol (2020) 6(5):661–74. doi: 10.1001/jamaoncol.2020.0237
58. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol (2020) 38(21):2369–79. doi: 10.1200/JCO.20.00793
59. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: A Randomized, Double-Blind, Phase 3 Study (Oncology Program by InnovENT Anti-PD-1-11). J Thorac Oncol (2020) 15(10):1636–46. doi: 10.1016/j.jtho.2020.07.014
60. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab Plus Carboplatin and Pemetrexed Versus Chemotherapy Alone in Chemotherapy-Naive Patients With Advanced Non-Squamous Non-Small-Cell Lung Cancer (CameL): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Respir Med (2020) 9(3):305–14. doi: 10.1080/15384047.2020.182926510.1016/s2213-2600(20)30365-9
61. Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab Plus Ipilimumab or Placebo for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%: Randomized, Double-Blind Phase III KEYNOTE-598 Study. J Clin Oncol (2021) 39(21):2327–38. doi: 10.1200/jco.20.03579
62. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, With or Without Tremelimumab, Plus Platinum-Etoposide Versus Platinum-Etoposide Alone in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): Updated Results From a Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22(1):51–65. doi: 10.1016/s1470-2045(20)30539-8
63. Owonikoko TK, Park K, Govindan R, Ready N, Reck M, Peters S, et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J Clin Oncol (2021) 39(12):1349–59. doi: 10.1200/jco.20.02212
64. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-Line Nivolumab Plus Ipilimumab Combined With Two Cycles of Chemotherapy in Patients With Non-Small-Cell Lung Cancer (CheckMate 9LA): An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22(2):198–211. doi: 10.1016/s1470-2045(20)30641-0
65. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer With PD-L1 of at Least 50%: A Multicentre, Open-Label, Global, Phase 3, Randomised, Controlled Trial. Lancet (2021) 397(10274):592–604. doi: 10.1016/s0140-6736(21)00228-2
66. Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, et al. Second-Line Nivolumab in Relapsed Small-Cell Lung Cancer: CheckMate 331. Ann Oncol (2021) 32(5):631–41. doi: 10.1016/j.annonc.2021.01.071
67. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab Plus Chemotherapy vs. Chemotherapy Alone as First-Line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol (2021) 7(5):709–17. doi: 10.1001/jamaoncol.2021.0366
68. Fu Y, Zheng Y, Wang PP, Ding ZY. Toxicities of Immunotherapy for Small Cell Lung Cancer. Front Oncol (2021) 11:603658. doi: 10.3389/fonc.2021.603658
69. Berti A, Bortolotti R, Dipasquale M, Kinspergher S, Prokop L, Grandi G, et al. Meta-Analysis of Immune-Related Adverse Events in Phase 3 Clinical Trials Assessing Immune Checkpoint Inhibitors for Lung Cancer. Crit Rev Oncol Hematol (2021) 162:103351. doi: 10.1016/j.critrevonc.2021.103351
70. Steele TA. Chemotherapy-Induced Immunosuppression and Reconstitution of Immune Function. Leuk Res (2002) 26(4):411–4. doi: 10.1016/s0145-2126(01)00138-2
71. Krisl JC, Doan VP. Chemotherapy and Transplantation: The Role of Immunosuppression in Malignancy and a Review of Antineoplastic Agents in Solid Organ Transplant Recipients. Am J Transplant (2017) 17(8):1974–91. doi: 10.1111/ajt.14238
72. Oppong E, Cato AC. Effects of Glucocorticoids in the Immune System. Adv Exp Med Biol (2015) 872:217–33. doi: 10.1007/978-1-4939-2895-8_9
73. Cruellas M, Yubero A, Zapata M, Galvez EM, Gascon M, Isla D, et al. How Could Antibiotics, Probiotics and Corticoids Modify Microbiota and Its Influence in Cancer Immune Checkpoints Inhibitors: A Review. Infect Immun (2021) 89(9):e0066520. doi: 10.1128/IAI.00665-20
74. Neelapu SS, Adkins S, Ansell SM, Brody J, Cairo MS, Friedberg JW, et al. Society for Immunotherapy of Cancer (SITC) Clinical Practice Guideline on Immunotherapy for the Treatment of Lymphoma. J Immunother Cancer (2020) 8(2):e001235. doi: 10.1136/jitc-2020-001235
75. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Version 5. (2021). Available at: https://www.nccn.org/guidelines/guidelines-with-evidence-blocks.
Keywords: immune checkpoint inhibitors, safety, lung cancer, adverse events, network comparison
Citation: Yan Y-D, Cui J-J, Fu J, Su Y-J, Chen X-Y, Gu Z-C and Lin H-W (2021) A Network Comparison on Safety Profiling of Immune Checkpoint Inhibitors in Advanced Lung Cancer. Front. Immunol. 12:760737. doi: 10.3389/fimmu.2021.760737
Received: 18 August 2021; Accepted: 16 November 2021;
Published: 03 December 2021.
Edited by:
Xuelei Ma, Sichuan University, ChinaReviewed by:
Cleo Goyvaerts, Vrije University Brussel, BelgiumPierpaolo Correale, Azienda Ospedaliera ‘Bianchi-Melacrino-Morelli’, Italy
Huijuan Mao, Nanjing Medical University, China
Copyright © 2021 Yan, Cui, Fu, Su, Chen, Gu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Chun Gu, Z3V6aGljaHVuMjEzQDE2My5jb20=; Xiao-Yu Chen, MTUzNDc0NjI5NkBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Yi-Dan Yan1†
Yi-Dan Yan1† Ying-Jie Su
Ying-Jie Su Zhi-Chun Gu
Zhi-Chun Gu Hou-Wen Lin
Hou-Wen Lin