- 1Department of Rheumatology, The Second Hospital of Shanxi Medical University, Taiyuan, China
- 2Department of Cardiology, The Second Hospital of Shanxi Medical University, Taiyuan, China
- 3Department of Neurology, The First Hospital of Shanxi Medical University, Taiyuan, China
- 4Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
Patients with rheumatoid arthritis (RA) have a significantly high risk of atrial fibrillation (AF). This study aimed to compare the absolute and relative changes in peripheral T cells in patients with RA who were also affected with and without AF. To help make an early diagnosis and prevent the initiation and progression of AF, the changes in the lymphocyte subsets were assessed in RA patients with and without AF. A propensity score matching (PSM) system (1:3) was used to perform a matched case-control study with 40 RA-AF cases and 120 RA controls. Changes in the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), anti-citrullinated peptide antibody (ACPA), and rheumatoid factor (RF) were examined. The percentage and absolute number of T, B, natural killer (NK), T helper (Th)1, Th2, Th17, and T-regulatory (Treg) cells in the peripheral blood of patients with and without RA-AF were determined using flow cytometry. Univariate and multivariate analyses were performed to determine the association between peripheral lymphocytes and RA-AF. Demographic data, ESR, CRP, ACPA, and the percentage, as well as the absolute value of B, NK, Th2, and Treg cells, showed no significant differences between the propensity score-matched groups of RA and RA-AF. Meanwhile, the absolute number and percentage of Th1 cells, the absolute number of Th17 cells, the ratio of Th1/Treg, Th17/Treg, and RF were significantly higher in patients with RA-AF than those in the control groups (P < 0.05). Univariate and multivariate logistic regression analyses also revealed that the percentage of Th1 cells, the absolute number of Th17 cells, and the ratio of Th1/Treg were associated with a significantly higher risk of AF. This PSM study demonstrated that the incidence of AF was higher in RA patients with Th cell immunological derangements.
Introduction
Rheumatoid arthritis (RA), which affects 0.1%–2.0% of adults in industrialized countries, is an autoimmune, inflammatory, and multisystem disorder characterized by chronic synovitis, systemic inflammation, and autoantibodies, particularly including the rheumatoid factor (RF) and anti-citrullinated peptide antibody (ACPA) (1). Persistent or recurrent episodes of synovitis can lead to joint damage, decreased quality of life, and even disability. However, uncontrolled active RA not only causes joint damage but also can damage other tissues such as the brain, myocardium, and autonomic nerves. Cardiovascular disease (CVD) resulting from chronic inflammation plays a vital role in increasing the economic burden and mortality of patients with RA (2). Indeed, the prevalence of atrial fibrillation (AF) is significantly higher in patients with RA than in healthy individuals (3–5), suggesting that patients with RA-AF are characterized by destabilized diffuse myocardial electrophysiology induced by chronic inflammation (6).
In 2010, AF was the most common type of arrhythmia (estimated lifetime risk of 22%–26%), as it affected ~33.5 million individuals worldwide (7). More recently, a large national survey revealed that ~7.9 million people suffer from AF in China (8). AF is associated with substantial morbidity, reduced quality of life (QOL), and increased mortality due to the combination of altered hemodynamics, progressive atrial and ventricular mechanical dysfunction, heart failure, and thromboembolic complications; thus, AF is a major burden on the healthcare systems. However, the pathophysiology of AF is complex and not fully understood. Accumulating evidence indicates that its occurrence and progression are related to immune derangement. A meta-analysis has revealed a statistically significant increased risk of subsequent development of AF among patients with RA (9). Experimental evidence has demonstrated that RA increases AF susceptibility by inducing atrial remodeling (10). Although a relationship between RA and AF has been defined, early identification and anti-inflammatory therapy that can effectively prevent CVD have not been developed. This is mainly due to the long-term and insidious impact of chronic inflammation on the atrial substrate and the fact that AF has been largely ignored in patients with RA. Therefore, the clinical features of RA-AF need to be identified and validated to facilitate early diagnosis and implement protective actions to decelerate or alter the pathogenesis of AF in patients with RA. T cells are dominant contributors to the initiation and progression of RA, and their role in triggering and developing AF cannot be ignored (11, 12). Overall, immune derangement (especially T cells) in patients with RA results in AF.
Nevertheless, the absolute and relative changes in peripheral blood lymphocytes and T subsets in RA associated with AF (RA-AF) remain unclear. In the present study, we aimed to determine how the absolute numbers and ratios (%) of peripheral blood lymphocytes and T subsets differ between patients with RA and RA-AF and to establish a novel method for detecting early AF and improving the prognosis of these patients.
Materials and Methods
Patient Information
The research was completed, and the patients were retrospectively involved in the analysis, which was developed according to previous studies and physicians’ thinking in daily clinical practice. The study was approved by the Medical Ethics Committee of Shanxi Medical University, China [ID (2021): YX No. 035].
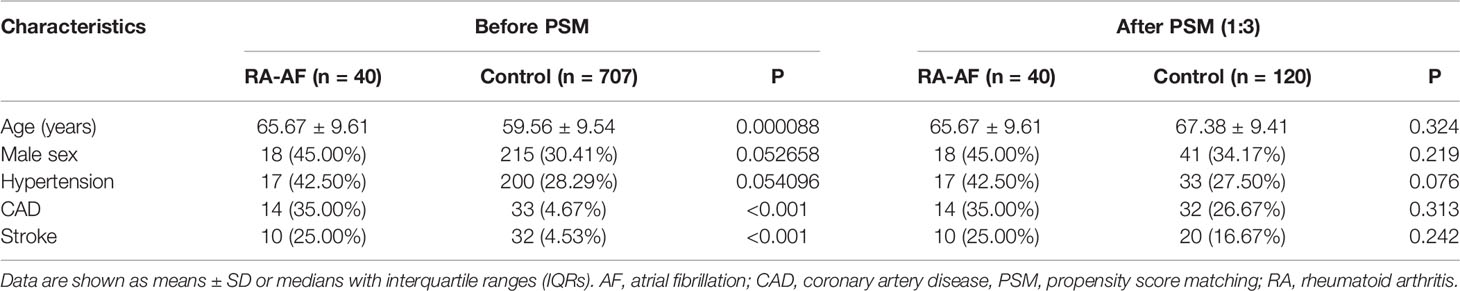
This retrospective case-control study used propensity score matching (PSM) to control for confounding factors. All data about patients were consecutively collected from the clinical database at the Department of Rheumatology, Second Hospital of Shanxi Medical University, between January 2016 and October 2020. Demographic, epidemiological, and medical information that met the RA classification standard of the American Society of Rheumatology (ACR) in 1987 was obtained from electronic medical and clinical laboratory records. The inclusion criteria comprised morning stiffness, arthritis in three or more joints, hand joints, symmetrical arthritis for at least 6 weeks (13), rheumatoid nodules, and changes in the serum rheumatoid factor and radiographical data. For classification purposes, patients who satisfied at least four of these seven criteria are considered to have RA. AF was defined according to the 2010 European Society of Cardiology guidelines (14) as surface ECG findings of absolutely irregular RR intervals and RR intervals that do not follow a repetitive profile, P-waves are not distinct on surface ECG, and usually variable atrial cycle lengths (interval between two atrial activations) <200 ms (>300 bpm). The exclusion criteria comprised valvular disease and/or mechanical heart valves; congenital heart disease; cardiomyopathy, malignant tumors; hyperthyroidism; active infection; kidney, liver, or other organ dysfunction; and long-term smoking and alcohol consumption. Screening according to the criteria selected, 747 patients with RA with information about age, sex, hypertension, coronary artery disease (CAD), stroke, erythrocyte sedimentation rate (ESR), rheumatoid factor (RF), ACPA, C-reactive protein (CRP), T-lymphocyte subsets, B-lymphocyte subsets, and natural killer (NK) cells were obtained from medical records. We finally included data from 40 and 120 patients who had RA with and without (control) AF determined by PSM (1:3).
Detection of Peripheral Lymphocytes and CD4+ T-Cell Subsets by Flow Cytometry
The essential reagents were as follows: stimulin, ionomycin, Golgi blocker, fetal bovine serum, and RPMI 1640 medium (Sigma-Aldrich Corp., St. Louis, MO, USA). Absolute count microspheres-Trucount™ tubes, hemolysin, Multitest CD3-fluorescein isothiocyanate (FITC)/CD8-PE/CD45-PercP/CD4-APC kits, Multitest CD3-FITC/CD16+56-PE/CD45-PercP/CD19-APC kits, and monoclonal antibodies to CD4-FITC, IL-4-PE, IFN-c-APC, IL-17-PE, CD25-APC, and FOXP3-PE (Becton Dickinson and Co., Franklin Lakes, NJ, USA).
In brief, 20 μl of CD3FITC/CD8PE/CD45PercP/CD4APC antibody and 20 μl of CD3FITC/CD16+56-PE/CD45PercP/CD19APC antibody were vortex-mixed with 50 μl of fully anticoagulated blood in separate Trucount tubes, then placed at room temperature for 15 min. Thereafter, the contents of each tube were mixed and incubated with 450 μl of XFACS hemolysin at room temperature for 15 min for flow cytometry. We examined 15,000 cells obtained using the MultiSET™ software (Becton Dickinson and Co.).
Culture and Detection of Th1/Th2/Th17 Cells
A mixture of phorbol myristate acetate (PMA; 10 μl, final concentration 30 ng/ml), 10 μl ionomycin (final concentration 750 ng/ml), 1 μl of BD GolgiStop™ (Becton Dickinson and Co.), and 80 μl of anticoagulated blood were incubated at 37°C for 5 h under a CO2 atmosphere. The cells were divided into tubes A and B and incubated with FITC-anti-human CD4 antibody for 30 min at room temperature in darkness. Fresh fixation/permeabilization solution was added and vortex-mixed, then the tubes were incubated at 4°C for 30 min in the dark.
Interleukin (IL)-4-PE and interferon (IFN)-c-APC were added to tube A, and anti-human IL-17-PE was added to tube B, then placed at room temperature for 30 min in the dark. The contents were washed with PBS, and cells were detected using the FACSCalibur flow cytometer (Becton Dickinson and Co.).
Culture and Detection of Treg Cells
Anticoagulated blood (80 μl) was incubated with CD4-FITC and CD25-APC at room temperature for 30 min in darkness, followed by 1 ml of freshly prepared fixation/permeabilization solution at 4°C for 30 min in darkness. Cells were stained with anti-human forkhead box P3 (FOXP3) antibody at room temperature for 30 min in the dark, washed with PBS, and detected using flow cytometry.
Flow Cytometry Assays
Cells were detected by flow cytometry within 24 h. Th (CD4+) cells were distinguished according to the forward and side scatter values. Relative ratios were analyzed using the CellQuest™ software (Becton Dickinson and Co.). The absolute numbers of cells in the subgroups were calculated as the absolute cell counts of the ratio (%) between the positive cells in each subgroup multiplied by the absolute number of CD4+ T cells/μl.
Statistical Analysis
The normality of continuous variable distribution was evaluated using Shapiro–Wilk test. Normally distributed data were expressed as means with standard deviation (SD). Non-normally distributed data were expressed as medians with interquartile ranges (IQRs). Categorical variables were described as frequencies and ratios (%). The normally distributed values were compared using unpaired Student’s t-tests between RA groups with and without AF. The non-normally distributed values were compared using Wilcoxon rank-sum or Mann–Whitney tests between RA groups with and without AF. Potential predictors of AF were initially investigated by univariate logistic regression proportional hazards regression analysis, followed by multivariate analysis to identify independent predictors and their power. All values with P < 0.05 were regarded as statistically significant.
Propensity Score-Matched Analysis
We applied PSM to minimize bias in the selection of patients who had RA with and without AF. Propensity scores were constructed for each participant using the confounding categorical variables that influenced the incidence of AF: age, sex, CAD, hypertension, and stroke. We applied optimal PSM to generate a cohort of 40 matched pairs. Rubin recommends a B value (standardized difference in mean propensity scores) of <25 for samples to be considered sufficiently balanced and the variance ratio in the propensity score between the treated and comparison groups.
Results
General Features of Patients With Rheumatoid Arthritis and Atrial Fibrillation
Figure 1 shows a flowchart of patient enrollment, and Table 1 shows the characteristics of the patients with RA and controls before and after PSM. Age, sex, CAD, hypertension, and stroke did not significantly differ between the groups (P > 0.05) after PSM (Table 1). The inflammatory markers, ESR, CRP, and ACPA, did not significantly differ between the two groups (P > 0.05), but levels of RF were significantly higher in patients with RA-AF (Table 3).
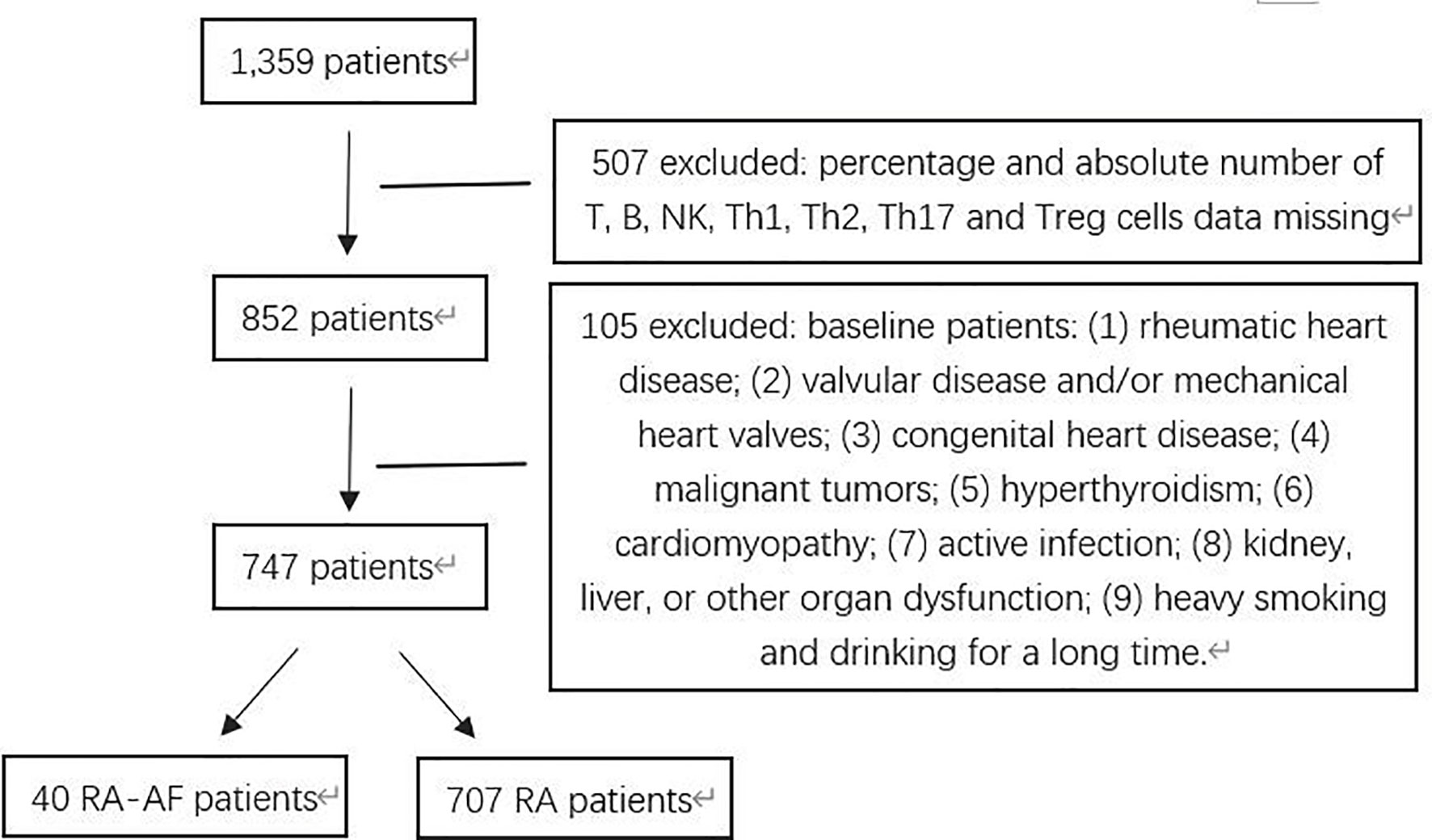
Figure 1 Flowchart of patients with rheumatoid arthritis (RA) and RA presenting atrial fibrillation (AF) enrolled in this study.
Comparison of Peripheral Lymphocyte Subsets Between RA-AF and RA Groups
For the peripheral lymphocyte subsets, the ratios (%) of T-helper (Th)1 cells, ratios of Th1/Th17, and Th17/Treg cells, as well as the absolute numbers of Th1 and Th17 cells were higher in the RA-AF than those in the RA group (Figure 2). However, levels of B, CD8+ T, and NK cells did not significantly differ between the two groups (Tables 2, 3).
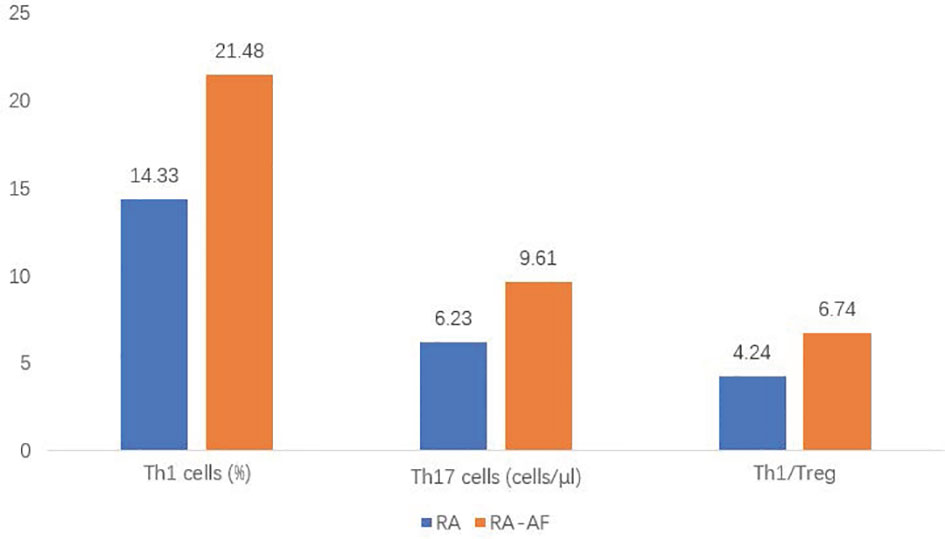
Figure 2 Comparison of proportions of T helper (Th)1, Th17, and Th1/T-regulatory (Treg) cells between rheumatoid arthritis (RA) and RA-atrial fibrillation (AF) groups.
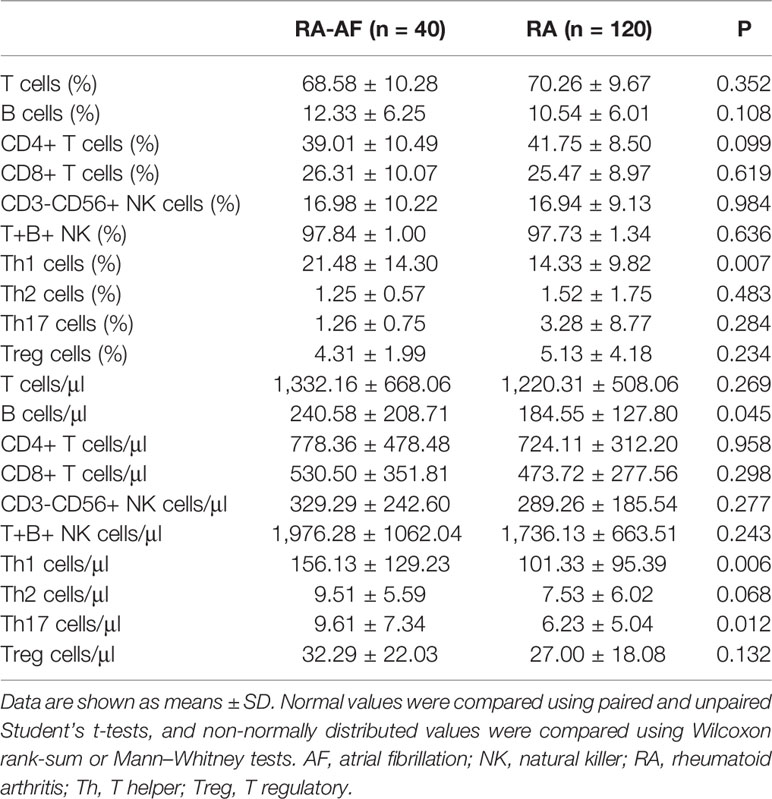
Table 2 Absolute numbers and ratios (%) of peripheral lymphocyte subsets in patients with RA-AF and RA.
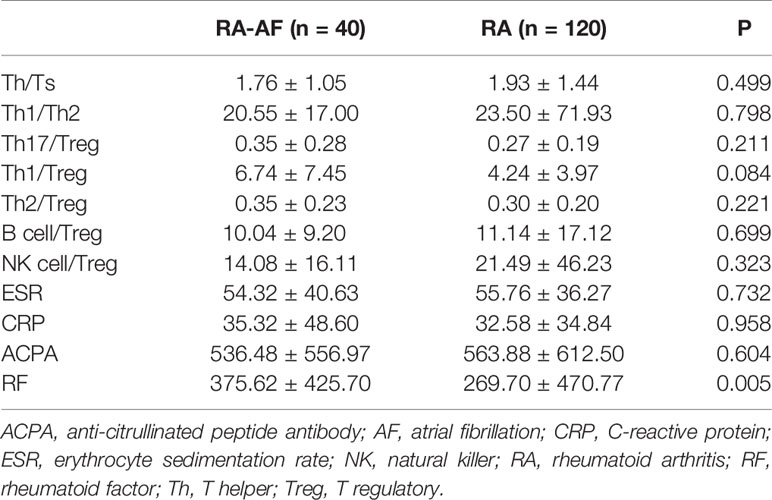
Table 3 Ratios of peripheral lymphocyte subsets and inflammatory biomarkers in patients with RA-AF and RA.
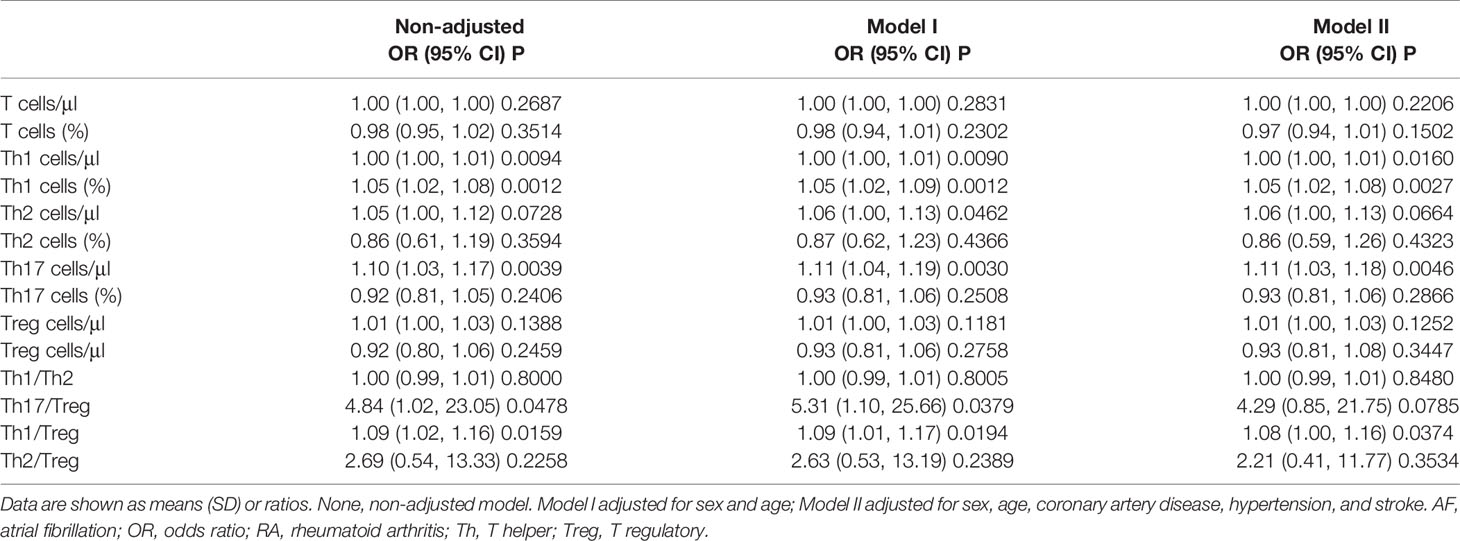
Univariate and Multivariate Analyses of Factors Associated With Rheumatoid Arthritis-Atrial Fibrillation
The results of the univariate logistic analysis showed that the ratios of Th1 cells, Th1:Th17, and Th17:Treg cells, as well as the absolute numbers of Th1 and Th17 cells were significantly associated with RA-AF. We applied multivariate logistic analysis to exclude the effects of age, sex, CAD, hypertension, and stroke. The results showed that the ratio of Th1 cells [odds ratio (OR), 1.05; 95% confidence interval (CI), 1.02–1.08, P = 0.0029], absolute number of Th17 cells (OR, 1.11; 95% CI, 1.03–1.19, P = 0.0044), and the ratio of Th1/Treg (OR, 1.08; 95% CI, 1.00–1.16, P = 0.0414) were associated with RA-AF (Table 4).
Discussion
RA is well known to increase the risk of cardiovascular events. Autopsy findings have revealed that many patients with RA had also been diagnosed with cardiac amyloidosis (15). Comprehensive cardiac magnetic resonance imaging has uncovered myocardial abnormalities in patients with RA who were asymptomatic for cardiac disease (16). Although the relationship between RA and cardiovascular events (17), such as atherosclerosis (18) and sudden cardiac death (19), has been investigated in detail, the role of RA in AF remains unclear. RA is not limited to the local synovial joints but can affect organs and vessels via peripheral blood containing CD4+ T cells, which activate numerous inflammatory cells that spread chronic inflammation throughout the body. Naive CD4+ T cells differentiate into various Th cell subsets and play various roles in autoimmune diseases. The immune response mechanism is unclear and complex in RA-AF. There is accumulating evidence that suggests that the immune response and AF are closely related (12, 20). Whether the immune response is a cause or an effect of RA-AF remains inconclusive. As a functional unit of the immune system, immune cells, especially CD4+ T cells, might play vital roles in the immunological pathogenesis of RA-AF (Figure 3).
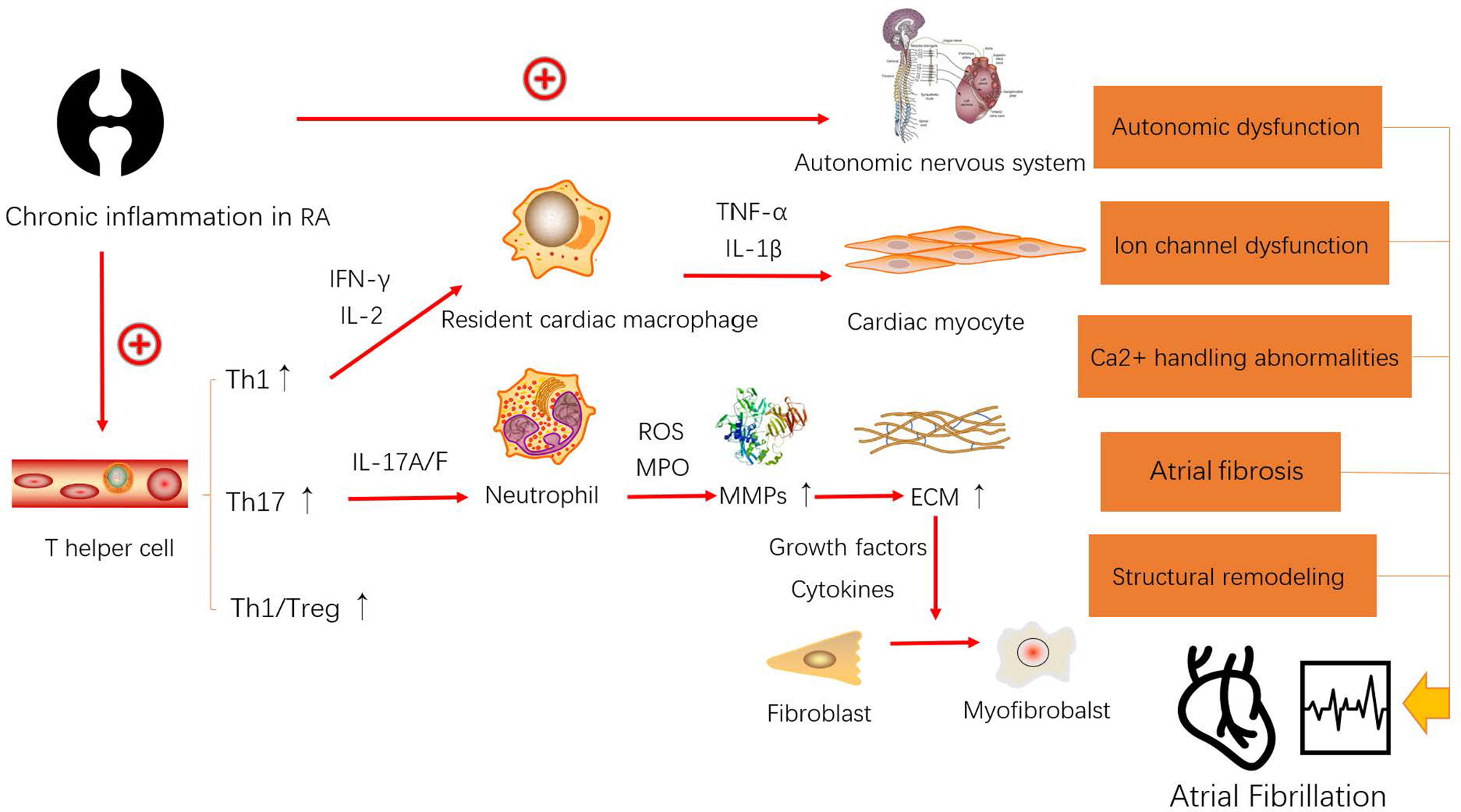
Figure 3 Model of the possible mechanism leading to the occurrence and development of atrial fibrillation (AF) caused by rheumatoid arthritis (RA).
Classically, Th1 cells that produce IFN-γ and IL-2 might play a substantial role in the synovial fluid and peripheral blood during the development of RA. The peripheral blood of patients with RA contains citrulline-specific T cells, of which ~40% of the samples are positive for the Th1 marker chemokine receptor 3 (CXCR3) (21), and plasma concentrations of IL-2 and IFN-γ, which are also biomarkers of Th1 cells, are significantly elevated in patients with AF (13, 22, 23). Among them, IL-2 activates T lymphocytes and stimulates the synthesis of TNF-α and IFN-γ, both of which exert biological effects that might promote atrial remodeling through macrophages via stimulating cytokine secretion. Thus, the major functions of Th1 cells in AF are to maximize the efficacy of macrophages and promote the inflammatory process.
Th17 cells are considered a contributing factor in the development of AF. IL-17A produced mainly by Th17 cells is a key mediator of the activation, recruitment, and migration of neutrophils. Subsequently, the pathogenic role of Th17 cells that produce IL-17A has intrigued rheumatologists. The proportion of IL-17-positive CD4+ T cells (Th17 cells) is higher in peripheral blood mononuclear cells from patients with RA compared with healthy controls, and their proportion correlates with systemic disease activity at both the onset and during the progression of RA (24, 25). Th17 cells might be involved in the pathogenesis of AF, as elevated plasma levels of Th17-associated cytokines were independently associated with the increased risk of AF, further suggesting that Th17 cells are involved in the pathogenesis of AF (26). Interleukin-17A contributes to the development of AF by promoting inflammation and cardiac fibrosis (27) and affecting protein kinase C (PKC)-β and Erk 1/2 phosphorylation, as well as nuclear factor (NF)-κB activation in fibroblasts from model mice (28). Elevated IL-17A levels are related to AF pathology that is mediated by neutrophils (29).
The core treatment of RA can complete the conversion of Th1/Th2 to Th17/Treg cells (30). However, others have also focused on Th1/Treg cells (31). Nowadays, many studies have revealed that Th1/IL-12 and Treg/IL-10 have a very close activation relationship (32, 33). IL-12 produced by cells of the innate immune system and by B cells plays a vital role in regulating naive T cells to differentiate into Th1 cells (33). Furthermore, the increase of IL-12 is highly relevant with AF by causing cardiac fibrosis (34). Meanwhile, IL-10 produced by Treg cells is a potent anti-inflammatory cytokine (33). A recent study has suggested that IL-10(-592A/C) polymorphism may have a prominent association with postoperative AF (35). Therefore, we reasonably speculate that the increase of Th1/Treg may associate with the development of RA-AF. Figure 3 shows the mechanism through which RA influences the occurrence and development of AF. Chronic inflammation in RA causes autonomic nervous system (ANS) dysfunction. Approximately 60%–80% of the patients with RA present ANS dysfunction as a sign of cardiovascular reflex impairment and altered heart rate variability, which indicates reduced and elevated cardiac parasympathetic and sympathetic activity, respectively (36). A disabled ANS is a considerable reason for triggering ectopic pacemakers in AF. Furthermore, elevated Th1 cells produce more IFN-γ to activate resident cardiac macrophages (22), which in turn produce tumor necrosis factor (TNF)-α and IL-1β, both of which can damage cardiac myocytes (37). There is considerable evidence supporting that macrophages play a core role in promoting electrical and structural remodeling by altering Ca2+ handling, shortening the action potential duration, reducing Cx40 and Cx43, causing fibrosis, and generating cardiac myocyte apoptosis and myolysis during the progress of AF (38). Th17 cells exert IF-17A/F to activate neutrophils. A theory has been introduced to explain the association between neutrophils and atrial fibrosis in AF (39). Polymorphonuclear neutrophils (PMNs) can infiltrate the myocardial interstitium and produce myeloperoxidase (MPO) and reactive oxygen species (ROS) to help convert pro-matrix metalloproteins (MMPs) to MMPs (40). Increased levels of MMPs result in the degradation of the extracellular matrix (ECM), which results in the release of growth factors [such as transforming growth factor (TGF)-β] and cytokines (such as IL-6), to assist the conversion of fibroblasts into myofibroblasts (41). Therefore, PMNs cause atrial fibrosis and progress to structural remodeling. In conclusion, elevated Th1 and Th17 levels in the peripheral blood of patients with RA putatively cause AF through overactivated resident cardiac macrophages and PMNs.
The effects of Th1 and Th17 and the anti-inflammatory effects of Tregs in RA were confirmed. We found an imbalance in CD4+ T cells in the peripheral blood of patients with RA with and without AF. This imbalance of CD4+ T cells leaning toward an unfavorable outcome was more obvious in patients with RA-AF. Thus, we concluded that a higher proportion of Th1 cells, the absolute number of Th17 cells, and the Th1/Treg ratios in the peripheral blood of patients with RA can indicate a tendency to develop AF. This suggests that RA is an important player in the development of AF, which might be associated with disordered T cells and harmful cytokines. Cardiovascular mortality is significantly associated with CD4+ CD28null cells in patients with AF [adjusted hazard ratio (HR), 1.59; 95% CI, 1.13–2.24, P = 0.008] (42). These cells can predict the development of postoperative AF after cardiac surgery (43). A risk management system should be developed to prevent RA-AF. For patients with RA at a high risk of developing AF, timely anti-inflammatory treatment should be administered to maintain a steady state of Th1, Th17, and Th1/Treg cells.
We revealed that increased frequencies of Th1, Th17, and Th1/Treg cells in the peripheral blood of patients with RA were associated with the development of AF. Our findings suggested that the immune response is involved in the pathogenesis of RA-AF, and that downregulated Th1, Th17, and Th1/Treg cells could be potential therapeutic targets for developing effective treatments against RA-AF. However, the mechanisms through which elevated Th1, Th17, and Th1/Treg cells in the peripheral blood remodel the cardiac tissues and cause AF await further investigation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The study was approved by the Medical Ethics Committee of Shanxi Medical University ID: (2021) YX No. 035. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
BL and XW, study design. YW and HF, data analysis. HF, article drafting. XY, GL, and CG, data collection. XL, article revision. BL, review and final approval. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81970391) and the Excellent Youth Foundation of Shanxi Province (grant number 201901D211504).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank the staff of Rheumatology Laboratory for their support for our work.
References
1. Klareskog LP, Catrina AIM, Paget SP. Rheumatoid Arthritis. Lancet (British edition) (2009) 373(9664):659–72. doi: 10.1016/S0140-6736(09)60008-8
2. England BR, Thiele GM, Anderson DR, Mikuls TR. Increased Cardiovascular Risk in Rheumatoid Arthritis: Mechanisms and Implications. BMJ (Clinical Res ed.) (2018) 361:k1036. doi: 10.1136/bmj.k1036
3. Bacani AK, Crowson CS, Roger VL, Gabriel SE, Matteson EL. Increased Incidence of Atrial Fibrillation in Patients With Rheumatoid Arthritis. BioMed Res Int [Journal Article; Res Support N.I.H Extramural] (2015) 2015:809514. doi: 10.1155/2015/809514
4. Kim SC, Liu J, Solomon DH. The Risk of Atrial Fibrillation in Patients With Rheumatoid Arthritis. Ann Rheum Dis [Journal Article; Res Support N.I.H Extramural] (2014) 73(6):1091–5. doi: 10.1136/annrheumdis-2013-203343
5. Lindhardsen J, Ahlehoff O, Gislason GH, Madsen OR, Olesen JB, Svendsen JH, et al. Risk of Atrial Fibrillation and Stroke in Rheumatoid Arthritis: Danish Nationwide Cohort Study. BMJ. [Journal Article; Res Support Non-U.S Gov’t] (2012) 344:e1257. doi: 10.1136/bmj.e1257
6. Lazzerini PE, Capecchi PL, Laghi-Pasini F. Systemic Inflammation and Arrhythmic Risk: Lessons From Rheumatoid Arthritis. Eur Heart J (2017) 38(22):1717–27. doi: 10.1093/eurheartj/ehw208
7. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide Epidemiology of Atrial Fibrillation: A Global Burden of Disease 2010 Study. Circ (New York N.Y.) (2014) 129(8):837–47. doi: 10.1161/CIRCULATIONAHA.113.005119
8. Du X, Guo L, Xia S, Du J, Anderson C, Arima H, et al. Atrial Fibrillation Prevalence, Awareness and Management in a Nationwide Survey of Adults in China. Heart (2021) 107(7):535–41. doi: 10.1136/heartjnl-2020-317915
9. Ungprasert P, Srivali N, Kittanamongkolchai W. Risk of Incident Atrial Fibrillation in Patients With Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Int J Rheum Dis (2017) 20(4):434–41. doi: 10.1111/1756-185X.12820
10. Dai H, Wang X, Yin S, Zhang Y, Han Y, Yang N, et al. Atrial Fibrillation Promotion in a Rat Model of Rheumatoid Arthritis. J Am Heart Assoc (2017) 6(12):e007320. doi: 10.1161/JAHA.117.007320
11. Kondo Y, Yokosawa M, Kaneko S, Furuyama K, Segawa S, Tsuboi H, et al. Review: Transcriptional Regulation of CD4+ T Cell Differentiation in Experimentally Induced Arthritis and Rheumatoid Arthritis. Arthritis Rheumatol (2018) 70(5):653–61. doi: 10.1002/art.40398
12. Liu Y, Shi Q, Ma Y, Liu Q. The Role of Immune Cells in Atrial Fibrillation. J Mol Cell Cardiol (2018) 123:198–208. doi: 10.1016/j.yjmcc.2018.09.007
13. Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheumatism (1988) 31(3):315–24. doi: 10.1002/art.1780310302
14. Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the Management of Atrial Fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J (2010) 31(19):2369–429. doi: 10.1093/eurheartj/ehq278
15. Wiland P, Wojtala R, Goodacre J, Szechinski J. The Prevalence of Subclinical Amyloidosis in Polish Patients With Rheumatoid Arthritis. Clin Rheumatol [Journal Article] (2004) 23(3):193–8. doi: 10.1007/s10067-003-0842-y
16. Kobayashi Y, Giles JT, Hirano M, Yokoe I, Nakajima Y, Bathon JM, et al. Assessment of Myocardial Abnormalities in Rheumatoid Arthritis Using a Comprehensive Cardiac Magnetic Resonance Approach: A Pilot Study. Arthritis Res Ther [Clinical Trial; J Article] (2010) 12(5):R171. doi: 10.1186/ar3131
17. England BR, Thiele GM, Anderson DR, Mikuls TR. Increased Cardiovascular Risk in Rheumatoid Arthritis: Mechanisms and Implications. BMJ. [Journal Article; Res Support N.I.H Extramural; Res Support Non-U.S Gov’t; Res Support U.S Gov’t Non-P.H.S.; Review] (2018) 361:k1036. doi: 10.1136/bmj.k1036
18. Carbone F, Bonaventura A, Liberale L, Paolino S, Torre F, Dallegri F, et al. Atherosclerosis in Rheumatoid Arthritis: Promoters and Opponents. Clin Rev Allergy Immunol [Journal Article; Review] (2020) 58(1):1–14. doi: 10.1007/s12016-018-8714-z
19. Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased Unrecognized Coronary Heart Disease and Sudden Deaths in Rheumatoid Arthritis: A Population-Based Cohort Study. Arthritis Rheumatol [Journal Article; Res Support Non-U.S Gov’t; Res Support U.S Gov’t P.H.S.; Review] (2005) 52(2):402–11. doi: 10.1002/art.20853
20. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the Pathogenesis of Atrial Fibrillation. Nat Rev Cardiol [Journal Article; Res Support Non-U.S Gov’t; Review] (2015) 12(4):230–43. doi: 10.1038/nrcardio.2015.2
21. James EA, Rieck M, Pieper J, Gebe JA, Yue BB, Tatum M, et al. Citrulline-Specific Th1 Cells Are Increased in Rheumatoid Arthritis and Their Frequency Is Influenced by Disease Duration and Therapy. Arthritis Rheumatol (2014) 66(7):1712–22. doi: 10.1002/art.38637
22. Huang J, Xiang Y, Zhang H, Wu N, Chen X, Wu L, et al. Plasma Level of Interferon-γ Predicts the Prognosis in Patients With New-Onset Atrial Fibrillation. Heart Lung Circ (2020) 29(7):e168–76. doi: 10.1016/j.hlc.2019.11.004
23. Hak A, Myśliwska J, Wickiewicz J, Szyndler K, Siebert J, Rogowski J. Interleukin-2 as a Predictor of Early Postoperative Atrial Fibrillation After Cardiopulmonary Bypass Graft (CABG). J Interferon Cytokine Res (2009) 29(6):327–32. doi: 10.1089/jir.2008.0082.2906
24. Leipe J, Grunke M, Dechant C, Reindl C, Kerzendorf U, Schulze-Koops H, et al. Role of Th17 Cells in Human Autoimmune Arthritis. Arthritis Rheumatism (2010) 62(10):2876–85. doi: 10.1002/art.27622
25. Shen H, Goodall JC, Hill Gaston JS. Frequency and Phenotype of Peripheral Blood Th17 Cells in Ankylosing Spondylitis and Rheumatoid Arthritis. Arthritis Rheumatism (2009) 60(6):1647–56. doi: 10.1002/art.24568
26. Wu N, Xu B, Liu Y, Chen X, Tang H, Wu L, et al. Elevated Plasma Levels of Th17-Related Cytokines Are Associated With Increased Risk of Atrial Fibrillation. Sci Rep (2016) 6(1):26543. doi: 10.1038/srep26543
27. Fu X, Zhao N, Dong Q, Du L, Chen X, Wu Q, et al. Interleukin-17A Contributes to the Development of Post-Operative Atrial Fibrillation by Regulating Inflammation and Fibrosis in Rats With Sterile Pericarditis. Int J Mol Med (2015) 36(1):83–92. doi: 10.3892/ijmm.2015.2204
28. Liu Y, Zhu H, Su Z, Sun C, Yin J, Yuan H, et al. IL-17 Contributes to Cardiac Fibrosis Following Experimental Autoimmune Myocarditis by a PKC /Erk1/2/NF- B-Dependent Signaling Pathway. Int Immunol (2012) 24(10):605–12. doi: 10.1093/intimm/dxs056
29. Nikoo MH, Taghavian SR, Golmoghaddam H, Arandi N, Abdi AA, Doroudchi M. Increased IL-17A in Atrial Fibrillation Correlates With Neutrophil to Lymphocyte Ratio. Iran J Immunol [Journal Article; Res Support Non-U.S Gov’t] (2014) 11(4):246–58.
30. Lee GR. The Balance of Th17 Versus Treg Cells in Autoimmunity. Int J Mol Sci (2018) 19(3):730. doi: 10.3390/ijms19030730
31. Zuo J, Yin Q, Wang YW, Li Y, Lu LM, Xiao ZG, et al. Inhibition of NF-κb Pathway in Fibroblast-Like Synoviocytes by α-Mangostin Implicated in Protective Effects on Joints in Rats Suffering From Adjuvant-Induced Arthritis. Int Immunopharmacol [Journal Article] (2018) 56:78–89. doi: 10.1016/j.intimp.2018.01.016
32. Ye J, Wang Y, Wang Z, Liu L, Yang Z, Wang M, et al. The Expression of IL-12 Family Members in Patients With Hypertension and Its Association With the Occurrence of Carotid Atherosclerosis. Mediators Inflamm [Journal Article] (2020) 2020:2369279. doi: 10.1155/2020/2369279
33. Furst DE, Emery P. Rheumatoid Arthritis Pathophysiology: Update on Emerging Cytokine and Cytokine-Associated Cell Targets. Rheumatol (Oxford) [Journal Article; Res Support Non-U.S Gov’t; Review] (2014) 53(9):1560–9. doi: 10.1093/rheumatology/ket414
34. Ye J, Wang Y, Wang Z, Liu L, Yang Z, Wang M, et al. Roles and Mechanisms of Interleukin-12 Family Members in Cardiovascular Diseases: Opportunities and Challenges. Front Pharmacol [Journal Article; Review] (2020) 11:129. doi: 10.3389/fphar.2020.00129
35. Yadav S, Akhtar S, Agarwal SK, Majumdar G, Vimal S, Sharma M. IL-10(-592a/C) Gene Variant a Predictor of Postoperative Atrial Fibrillation in the North Indian Population. J Arrhythm [Journal Article] (2018) 34(3):281–5. doi: 10.1002/joa3.12074
36. Adlan AM, Lip GY, Paton JF, Kitas GD, Fisher JP. Autonomic Function and Rheumatoid Arthritis: A Systematic Review. Semin Arthritis Rheumatol [Journal Article; Res Support Non-U.S Gov’t; Review; Systematic Review] (2014) 44(3):283–304. doi: 10.1016/j.semarthrit.2014.06.003
37. Jarrah AA, Schwarskopf M, Wang ER, LaRocca T, Dhume A, Zhang S, et al. SDF-1 Induces TNF-Mediated Apoptosis in Cardiac Myocytes. APOPTOSIS [Journal Article; Res Support N.I.H Extramural; Res Support Non-U.S Gov’t] (2018) 23(1):79–91. doi: 10.1007/s10495-017-1438-3
38. Guo Y, Lip GYH, Apostolakis S. Inflammation in Atrial Fibrillation. J Am Coll Cardiol (2012) 60(22):2263–70. doi: 10.1016/j.jacc.2012.04.063
39. Capuron L, Geisler S, Kurz K, Leblhuber F, Sperner-Unterweger B, Fuchs D. Activated Immune System and Inflammation in Healthy Ageing: Relevance for Tryptophan and Neopterin Metabolism. Curr Pharm Des [Journal Article; Review] (2014) 20(38):6048–57. doi: 10.2174/1381612820666140317110217
40. Rudolph V, Andrié RP, Rudolph TK, Friedrichs K, Klinke A, Hirsch-Hoffmann B, et al. Myeloperoxidase Acts as a Profibrotic Mediator of Atrial Fibrillation. Nat Med (2010) 16(4):470–4. doi: 10.1038/nm.2124
41. DeCoux A, Lindsey ML, Villarreal F, Garcia RA, Schulz R. Myocardial Matrix Metalloproteinase-2: Inside Out and Upside Down. J Mol Cell Cardiol (2014) 77:64–72. doi: 10.1016/j.yjmcc.2014.09.016
42. Sulzgruber P, Koller L, Winter MP, Richter B, Blum S, Korpak M, et al. The Impact of CD4(+)CD28(null) T-Lymphocytes on Atrial Fibrillation and Mortality in Patients With Chronic Heart Failure. Thromb Haemost [Comparative Study; J Article; Res Support Non-U.S Gov’t] (2017) 117(2):349–56. doi: 10.1160/TH16-07-0531
43. Sulzgruber P, Thaler B, Koller L, Baumgartner J, Pilz A, Steininger M, et al. CD4(+)CD28(null) T Lymphocytes Are Associated With the Development of Atrial Fibrillation After Elective Cardiac Surgery. Sci Rep [Clinical Trial; J Article; Res Support Non-U.S Gov’t] (2018) 8(1):9624. doi: 10.1038/s41598-018-28046-0
Keywords: T helper cell, atrial fibrillation, rheumatoid arthritis, Th1, Th17/Treg
Citation: Wang X, Fan H, Wang Y, Yin X, Liu G, Gao C, Li X and Liang B (2021) Elevated Peripheral T Helper Cells Are Associated With Atrial Fibrillation in Patients With Rheumatoid Arthritis. Front. Immunol. 12:744254. doi: 10.3389/fimmu.2021.744254
Received: 20 July 2021; Accepted: 29 September 2021;
Published: 15 October 2021.
Edited by:
Mohammad Hossein Karimi, Shiraz University of Medical Sciences, IranReviewed by:
Beatriz Tejera Segura, Insular University Hospital of Gran Canaria, SpainChih-Yao Hou, National Kaohsiung University of Science and Technology, Taiwan
Copyright © 2021 Wang, Fan, Wang, Yin, Liu, Gao, Li and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng Li, bHhmXzk4NTlAc3htdS5lZHUuY24=; Bin Liang, dHlsaWFuZ2JpbkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Xin Wang1†
Xin Wang1† Hongxuan Fan
Hongxuan Fan Chong Gao
Chong Gao Xiaofeng Li
Xiaofeng Li Bin Liang
Bin Liang