
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 13 October 2021
Sec. Viral Immunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.741639
 Melanie R. Neeland1,2*
Melanie R. Neeland1,2* Samantha Bannister1,2,3
Samantha Bannister1,2,3 Vanessa Clifford1,2,3,4
Vanessa Clifford1,2,3,4 Jill Nguyen1
Jill Nguyen1 Kate Dohle1
Kate Dohle1 Isabella Overmars1
Isabella Overmars1 Zheng Quan Toh1,2
Zheng Quan Toh1,2 Jeremy Anderson1,2
Jeremy Anderson1,2 Celeste M. Donato1,2
Celeste M. Donato1,2 Sohinee Sarkar1,2
Sohinee Sarkar1,2 Lien Anh Ha Do1,2
Lien Anh Ha Do1,2 Conor McCafferty5
Conor McCafferty5 Paul V. Licciardi1,2
Paul V. Licciardi1,2 Vera Ignjatovic2,5
Vera Ignjatovic2,5 Paul Monagle2,5,6,7
Paul Monagle2,5,6,7 Julie E. Bines1,2,8
Julie E. Bines1,2,8 Kim Mulholland1,2,9
Kim Mulholland1,2,9 Nigel Curtis1,2,3
Nigel Curtis1,2,3 Sarah McNab1,2,8
Sarah McNab1,2,8 Andrew C. Steer1,2,3
Andrew C. Steer1,2,3 David P. Burgner1,2,3
David P. Burgner1,2,3 Richard Saffery1,2
Richard Saffery1,2 Shidan Tosif1,2,10†
Shidan Tosif1,2,10† Nigel W. Crawford1,2,10†
Nigel W. Crawford1,2,10†Children have reduced severity of COVID-19 compared to adults and typically have mild or asymptomatic disease. The immunological mechanisms underlying these age-related differences in clinical outcomes remain unexplained. Here, we quantify 23 immune cell populations in 141 samples from children and adults with mild COVID-19 and their PCR-negative close household contacts at acute and convalescent time points. Children with COVID-19 displayed marked reductions in myeloid cells during infection, most prominent in children under the age of five. Recovery from infection in both children and adults was characterised by the generation of CD8 TCM and CD4 TCM up to 9 weeks post infection. SARS-CoV-2-exposed close contacts also had immunological changes over time despite no evidence of confirmed SARS-CoV-2 infection on PCR testing. This included an increase in low-density neutrophils during convalescence in both exposed children and adults, as well as increases in CD8 TCM and CD4 TCM in exposed adults. In comparison to children with other common respiratory viral infections, those with COVID-19 had a greater change in innate and T cell-mediated immune responses over time. These findings provide new mechanistic insights into the immune response during and after recovery from COVID-19 in both children and adults.
Children have reduced severity of COVID-19 compared to adults, with mild or asymptomatic infection common in this age group (1, 2). Children also have lower transmission rates compared to adults, in part because asymptomatic COVID-19 is less infectious than symptomatic disease (3), and because they are less likely to be the primary index case in a household (4–6). This is in contrast to the higher prevalence and severity observed in children for most other respiratory viruses (1, 7, 8).
Understanding the immunological basis for these age-related differences (9–11), as well as the factors that contribute to protection in household contacts who remain SARS-CoV-2 PCR negative despite close and often prolonged exposure (12), would help accelerate the development of diagnostic and therapeutic strategies to control COVID-19.
In this study, we used high parameter flow cytometry to comprehensively delineate the longitudinal circulating immune cell profiles of children and adults with mild COVID-19 and their close household contacts who were repeatedly PCR negative. To investigate differences in immune responses to SARS-CoV-2 and other respiratory viruses in children, we also compared the immune response in children with SARS-CoV-2 infection alone, SARS-CoV-2 co-infection, and non-SARS-CoV-2 respiratory viral infections.
Participants were families presenting for SARS-CoV-2 testing at the Royal Children’s Hospital Melbourne Australia, between April and September 2020 (Table 1). A total of 141 samples from SARS-CoV-2-positive or SARS-CoV-2-exposed but PCR negative children and adults were included in the study (complete cohort). Acute samples were collected within 2 weeks of the first SARS-CoV-2 PCR test result and follow up samples were collected 4 to 9 weeks after first SARS-CoV-2 PCR test result. SARS-CoV-2-positive individuals were non-hospitalised patients who had a positive PCR test for SARS-CoV-2 on nasopharyngeal swab and were asymptomatic or had mild symptoms, including coryza, headaches, nausea, fever, cough, sore throat, malaise, and muscle aches (Table 1). SARS-CoV-2-exposed individuals were SARS-CoV-2 PCR negative on repeated nasopharyngeal swabs and were close contacts of confirmed SARS-CoV-2-positive patients in their households. Close contact was defined as face-to-face contact for more than 15 minutes and shared closed space with a confirmed case of COVID-19, in accordance with Victorian state guidelines. All participants in the exposed group had up to five repeat SARS-CoV-2 PCR tests at 5-to-7-day intervals for 4 weeks, all of which were negative. SARS-CoV-2 positive children with samples collected during the acute phase were younger than SARS-CoV-2 exposed children (median age 2years vs 9years, p=0.02), however no other age or sex differences were found between groups (see Extended Data). SARS-CoV-2 positive children and adults were more likely to be symptomatic relative to SARS-CoV-2 exposed children and adults (Table 1, Extended Data). In a subset of 41 participants, paired acute and follow up samples were available for longitudinal analysis (Table 1).
Additional testing for respiratory pathogens using a commercial multiplex assay (including influenza A/B, respiratory syncytial virus (RSV) A/B, rhinovirus, enterovirus, parechovirus, adenovirus, human parainfluenza viruses 1-4, human metapneumovirus, Bordetella pertussis and Mycoplasma pneumoniae) was also available for most participants (AusDiagnostics Respiratory Pathogens 16-well assay). Of these pathogens, some participants had evidence of RSV A/B, rhinovirus, enterovirus or adenovirus infection (see extended data for individual results) whilst all other pathogens were not detected in any sample. Table 1 describes the number of participants with available respiratory panel results, the number of participants in each group positive for other respiratory viruses (other than SARS-CoV-2), and the timing of sample collection relative to respiratory panel testing.
Combined oropharyngeal and nasopharyngeal (or deep nasal) swabs were collected according to national guidelines using dry FLOQSwabs® (Copan, Brescia, Italy). Briefly, FLOQSwabs were eluted in 500 μL of phosphate buffered saline (PBS) and 200 μL of eluent was used for nucleic acid extraction using the Roche Magnapure 96 extraction system (Roche, Basel, Switzerland), according to the manufacturer’s instructions. The majority of SARS-CoV-2 samples were initially tested using the LightMix® Modular SARS and Wuhan CoV E-gene kit (targeting the E-gene; sensitivity 96.5%, specificity of 98.5% (13); TIB Molbiol, Berlin, Germany) using 10 μL nucleic acid extract, according to the manufacturer’s instructions. RT-PCR was performed on the LightCycler 480 II Real-Time PCR System (Roche). Some patient samples were also tested for SARS-CoV-2 using the AusDiagnostics Respiratory Pathogens 16-well assay (Mascot, Australia), on the AusDiagnostics High-Plex 24 system [the SARS-CoV-2 target of this assay is the ORF-1 gene; 98.4% positive agreement with the Victorian Infectious Diseases Reference Laboratory (VIDRL) reference assay (14)]. The assay also detects other respiratory pathogens including influenza A/B, RSV A/B, rhinovirus, enterovirus, parechovirus, adenovirus, human parainfluenza viruses 1-4, human metapneumovirus, Bordetella pertussis and Mycoplasma pneumoniae), using 10 μL nucleic acid extract. Except in a small number of cases where there was insufficient sample, samples that were SARS-CoV-2-positive on a screening assay with a single gene target were confirmed by testing on a second assay. Ct values at diagnosis for SARS-CoV-2-positive patients are provided, when available, in the extended data.
Blood was collected in EDTA tubes from each participant and processed into peripheral blood mononuclear cells (PBMC) (15). For flow cytometry analysis of freshly isolated PBMC, cells were washed in 1 mL PBS prior to viability staining using BV510 viability dye according to manufacturer’s instructions. The viability dye reaction was stopped by the addition of FACS buffer (2% heat-inactivated FCS in 2 mM EDTA) and cells were centrifuged at 350 x g for 5 minutes. Cells were then resuspended in human FC-block according to manufacturer’s instructions for 5 minutes at room temperature. The antibody cocktail (Supplementary Table 1) made up at 2X concentration was added 1:1 with the cells and incubated for 30 minutes on ice. Following staining, cells were washed with 2 mL FACS buffer and centrifuged at 350 x g for 5 minutes. Cells were then resuspended in 2% PFA for a 20-minute fixation on ice, washed, and resuspended in 150 µl FACS buffer for acquisition using the BD LSR X-20 Fortessa (BD Biosciences, New Jersey, United States). For all flow cytometry experiments, compensation was done at the time of sample acquisition using compensation beads. Supplementary Figure 1 depicts the manual gating strategy for all samples.
Flow cytometry data were analysed using FlowJo Version 10.7.1 software. Uniform Manifold Approximation and Projection (UMAP) analyses were conducted using concatenated files containing 5,000 randomly selected live single cells per sample. Manually gated results are presented as proportion of live cells. For CD4 and CD8 T cell subset analyses, results are presented as proportion of parent gate. Data was plotted in Prism version 8.0.0.
For differential abundance analysis of all groups in cross-sectional cohorts (Figures 1, 2, 4), p-values were determined by Kruskal-Wallis rank sum test and adjusted for multiple comparisons (x23 cell populations) using the Benjamini and Hochberg approach to control the false discovery rate (FDR) (16). FDR-adjusted p<0.05 were considered significant. For cell types showing an FDR-adjusted significant difference, Dunn’s multiple comparison testing was used to find differences between clinical groups. For statistical testing of the longitudinal cohort (Figure 3), p-values were determined by two-way repeated measures analysis of variance and adjusted for multiple comparisons [x46 (23 cell populations, p(group) and p(time))] using the Benjamini and Hochberg approach to control the false discovery rate (FDR). FDR-adjusted p<0.05 were considered significant. For cell types showing an FDR-adjusted significant difference, Sidak’s multiple comparison testing was used to find differences over time. All statistical analysis was performed in Prism version 9.0.0, with multiple comparison correction performed in RStudio version 4.0.3. Boxplots show the medians, the 1st and 3rd quartiles as well as the smallest and largest values as whiskers. Individual data points are shown.
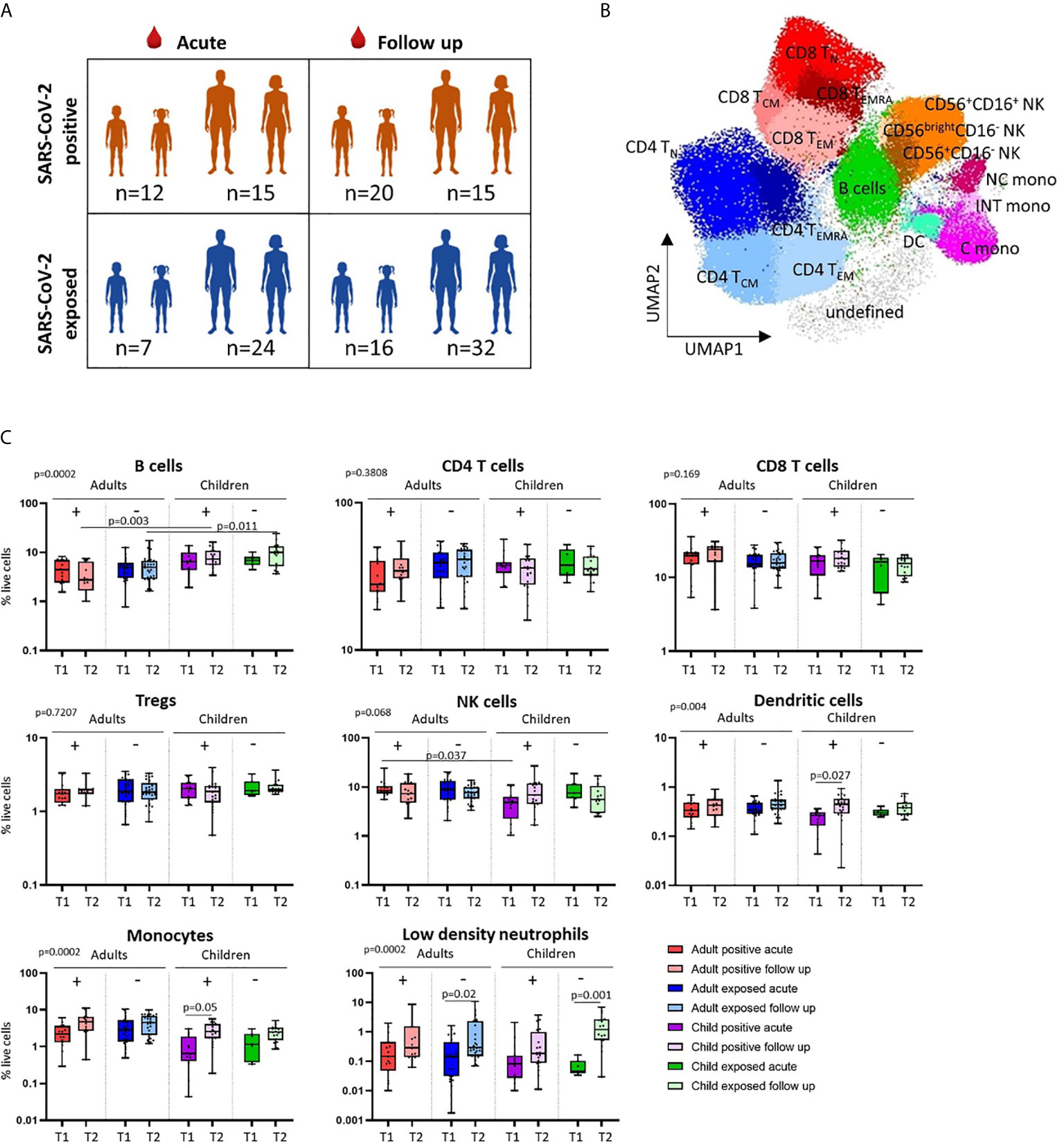
Figure 1 Cross-sectional analysis of immune cell profiles in SARS-CoV-2 positive and SARS-CoV-2 exposed children and adults. (A) Blood samples were collected during infection/exposure (acute) or 4-9 weeks following (follow up) from SARS-CoV-2 positive participants (n=12 child acute, n=20 child follow up, n=15 adult acute, n=15 adult follow up) and SARS-CoV-2 exposed participants (n=7 child acute, n=16 child follow up, n=24 adult acute, n=32 adult follow up). (B) Blood samples were processed into PBMC and analysed on the day of collection by flow cytometry. Unsupervised analysis of flow cytometry data revealed 17 clusters associated with CD4 T cells, CD8 T cells, NK cells, monocytes, dendritic cells, B cells and their cell subsets. (C) Comparison of the proportions of major cell populations in each clinical group (T1 – acute samples, T2 – follow up samples). P values by Kruskal-Wallis rank sum test and Dunn’s multiple comparison testing. FDR-adjusted P-values are reported. Boxplots show the medians, the 1st and 3rd quartile as well as the smallest and largest values as whiskers.
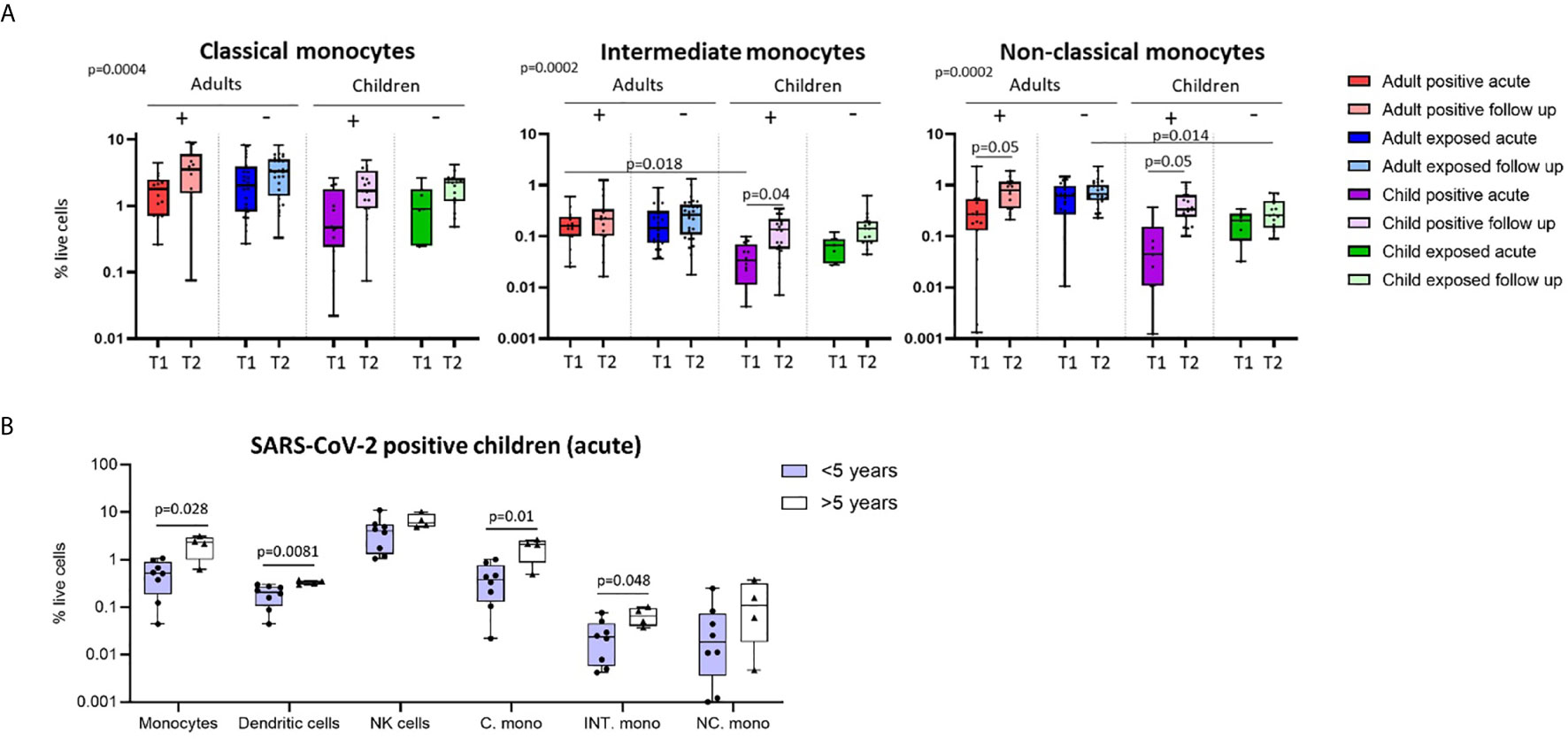
Figure 2 Innate cell responses in children and adults. (A) Classical, intermediate and non-classical monocyte proportions from SARS-CoV-2 positive participants (n=12 child acute, n=20 child follow up, n=15 adult acute, n=15 adult follow up) and SARS-CoV-2 exposed participants (n=7 child acute, n=16 child follow up, n=24 adult acute, n=32 adult follow up), T1 – acute samples, T2 – follow up samples. (B) Innate cell profiles in SARS-CoV-2 positive children during infection, stratified by age [under 5 years (n=8, median age 1.5 years), over 5 years (n=4, median age 10.5 years)]. P-values by Kruskal-Wallis rank sum test and Dunn’s multiple comparison testing (A) and by Mann-Whitney U test (B). FDR-adjusted P-values are reported. Boxplots show the medians, the 1st and 3rd quartile as well as the smallest and largest values as whiskers.
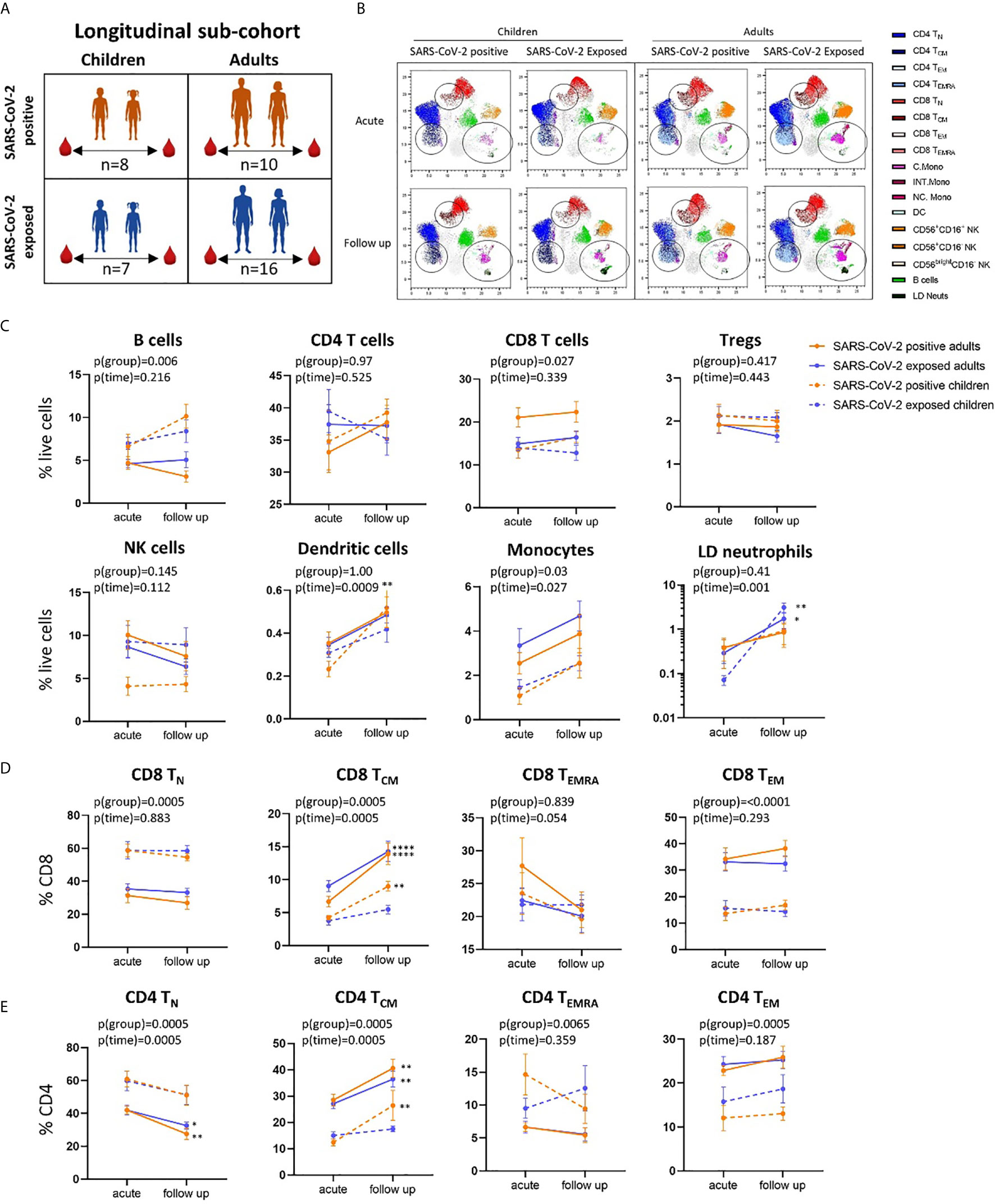
Figure 3 Longitudinal analysis of immune cell profiles in SARS-CoV-2 positive and SARS-CoV-2 exposed children and adults. (A) Paired blood samples were collected during infection/exposure and 4-9 weeks following from n=8 SARS-CoV-2 positive children, n=10 SARS-CoV-2 positive adults, n=7 SARS-CoV-2 exposed children and n=16 SARS-CoV-2 exposed adults. (B) UMAP plots consisting of cells from each individual from each clinical group. Circles highlight changes in frequency over time in key cell clusters: monocytes, low density neutrophils, CD8 TCM and CD4 TCM subsets. (C) Longitudinal analysis of major immune cell proportions (by manual gating) in each clinical group. (D) CD8 and (E) CD4 T cell subsets in each clinical group. Naive T cells (TN), central memory T cells (TCM), effector T cells (TEMRA) and effector memory T cells (TEM). P-values by two-way repeated measures analysis of variance with Sidak’s multiple comparison testing. FDR-adjusted P-values are reported, *p < 0.05, **p < 0.01, ****p < 0.0001. For (C–E), mean ± SEM are shown.
Clinical characteristics and demographics of the patients in this study are shown in Table 1. We first investigated differences in the circulating immune cell profile between children and adults using high dimensional flow cytometry and analysis on freshly isolated PBMCs. Children were aged between 1 and 17 years, and adults between 19 and 62 years (Figure 1A). UMAP analysis of all samples revealed clusters associated with CD4 T cells [naïve (TN), central memory (TCM), effector CD45RA+ (TEMRA) and effector memory (TEM)], CD8 T cells [naïve (TN), central memory (TCM), effector CD45RA+ (TEMRA) and effector memory (TEM)], B cells, NK cells (CD56+CD16+, CD56+CD16- and CD56brightCD16-), monocytes (classical: CD14+CD16-, intermediate: CD14+CD16+, non-classical: CD14lowCD16+) and dendritic cells (Figure 1B). Children had significantly higher proportions of CD4 TN and CD8 TN cells, whilst adults showed a clear shift toward CD4 and CD8 T memory cell populations (Supplementary Figure 2A). Children also had higher proportions of circulating B cells relative to adults (Figure 1C and Supplementary Figure 2A). While studies investigating the complete immune cell profile of children are rare, our findings are consistent with previous reports of cell subsets investigated in isolation that show a shift from naive to memory T cells with age, as well as higher proportions of circulating B cells in children relative to adults (17, 18).
We next explored cross-sectional differences in immune cell profiles between children and adults with or exposed to SARS-CoV-2. Samples were collected during or approximately 4-to-9 weeks post infection/exposure from SARS-CoV-2-positive and SARS-CoV-2-exposed children and adults. No significant differences in proportions of total CD4 T cells, total CD8 T cells or regulatory T cells (Tregs) were observed between children and adults with or exposed to SARS-CoV-2 (Figure 1C).
We found that SARS-CoV-2-positive children had lower proportions of dendritic cells and monocytes during the acute phase relative to follow up [median proportion in SARS-CoV-2 positive children acute vs follow up – dendritic cells: 0.23% vs 0.44% (p=0.02), and monocytes: 1.04% vs 2.75% (p=0.05)] (Figure 1C). Further analysis of monocyte subsets revealed lower proportions of both the intermediate and non-classical monocyte subsets in SARS-CoV-2-positive children during infection compared to the same group at follow up [median proportion acute vs follow up – intermediate monocytes: 0.04% vs 0.15% (p=0.04), and non-classical monocytes: 0.09% vs 0.44% (p=0.05)] (Figure 2A). SARS-CoV-2-positive adults also had reduced proportions of non-classical monocytes during the acute phase compared to follow up [median proportion of non-classical monocytes in SARS-CoV-2 positive adults acute vs follow up: 0.44% vs 0.91% (p=0.05)] (Figure 2A). This is consistent with our previous findings in an interim analysis of this cohort, revealing low circulating proportions of monocyte subsets and dendritic cells in SARS-CoV-2-positive children (19). Previous work by our team using pre-pandemic PBMCs from healthy children aged 1-15 years shows an average circulating frequency of 0.8% for dendritic cells and 4.8% for monocytes (20, 21), further highlighting the reduced proportions observed during the acute phase in SARS-CoV-2 positive children.
It should be noted that our sampling strategy for the complete cohort involved a mix of repeated sampling from the same participants as well as single sampling from participants at either timepoint, whichever was feasible. This may lead to some selection bias in the analysis for those with repeated sampling. However, we conducted a sensitivity analysis of only participants with single samples, revealing the same changes over time in these cell populations (see Extended Data).
Several other reports have also shown reduced proportions of monocytes and NK cells in the blood of adults with COVID-19, possibly reflecting redistribution of these cells to the lung (22–25). Single cell sequencing studies of bronchoalveolar lavage (BAL) samples from patients with varying COVID-19 severity provide further evidence for infiltration of innate cells into the lung during acute infection (26). Alveolar macrophages and dendritic cells have been shown to be enriched in the BAL of patients with mild COVID-19 disease, whilst severe disease is associated with infiltration of neutrophils, NK cells and monocyte-derived macrophages (27). A recent single cell analysis of lung tissue from adults with lethal COVID-19 revealed dense infiltration of monocyte-derived macrophages, impaired T cell responses, as well as monocyte and epithelial cell-derived inflammatory cytokines (28).
Combined, our results suggest that SARS-CoV-2-positive children show more pronounced changes in innate immune cell populations compared to infected adults. These results may, in part, provide a mechanistic explanation for the reduced susceptibility and severity of SARS-CoV-2 infection in children compared to adults (1). Recent data suggests further reduced severity and transmission of SARS-CoV-2 in younger children compared to adolescents (2, 4, 29). We therefore compared immune cell profiles in SARS-CoV-2-positive children under five years of age (median age 1.5 years) to those over age five (median age 10.5 years) during the acute phase of infection (Figure 2B). SARS-CoV-2-positive children under five showed significantly lower proportions of circulating monocytes and dendritic cells during infection compared to SARS-CoV-2-positive children over the age of five [median proportion during infection in children under five vs over five – monocytes: 0.5% vs 2.26% (p=0.02), and dendritic cells: 0.2% vs 0.32% (p=0.008)]. Within the monocyte subpopulations, significantly lower proportions of both classical and intermediate monocytes were observed in SARS-CoV-2-positive children under five relative to positive children over the age of five [median proportion during infection in children under five vs over five – classical monocytes: 0.38% vs 2.07% (p=0.01) and intermediate monocytes: 0.02% vs 0.06% (p=0.04)] (Figure 2B). Innate immune differences between infected children and infected adults were therefore most evident in infants and pre-school aged children.
Few studies have directly compared immune responses to SARS-CoV-2 infection in children and adults. One recent study compared cytokine, humoral and cellular immune responses in children and adults hospitalised with COVID-19. This revealed that children had a shorter length of hospital stay, decreased requirement for ventilation and lower mortality compared to adults. SARS-CoV-2 specific CD4 T cell responses and neutralising antibody titres were higher in adults compared to children (30).
A comparison of severe paediatric COVID-19, MIS-C and severe adult COVID-19 revealed that MIS-C patients had similar levels of T cell activation as severely ill adults. Robust activation of CX3CR1+ CD8 T cells was unique to MIS-C patients, whose immune activation resolved with treatment and recovery (31). Systems serology analysis of antibodies in children and the elderly revealed that whilst elderly individuals induce more class switched antibodies that target cross-reactive regions of SARS-CoV-2, infected children have antibodies with enhanced Fc functions that are more effective in clearing the virus (32).
Using longitudinally collected samples from the same patients during infection and up to 9 weeks post-infection (Figures 3A, B and Supplementary Figure 3), we show a significant increase in the proportion of dendritic cells and monocytes over time (p=0.0009 and p=0.02, respectively) (Figure 3C). Post-hoc testing revealed that SARS-CoV-2-positive children showed a significant increase in dendritic cells over time (median proportion during infection 0.23% vs follow up 0.52%, p=0.008). Changes in cell frequency over time in children and adults are highlighted by the UMAP analyses in Figure 3B.
Children and adults had significant differences in the proportion of naïve and effector CD8 and CD4 T cell populations at both time points that were independent of SARS-CoV-2 status and related to age (Figures 3D, E and Supplementary Figure 2C, D). However, central memory CD8 T cells (CD8 TCM) significantly increased over time in SARS-CoV-2-infected children and adults [median proportion of CD8 TCM acute vs follow up – SARS-CoV-2 positive children: 4.22% vs 8.99% (p=0.006), SARS-CoV-2 positive adults: 6.67 vs 13.91 (p<0.0001)] (Figure 3D). Central memory CD4 T cells (CD4 TCM) also showed the same response, significantly increasing over time in SARS-CoV-2 infected children and adults [median proportion of CD4 TCM acute vs follow up – SARS-CoV-2 positive children: 12.4% vs 26.41% (p=0.001), SARS-CoV-2 positive adults: 28.5% vs 40.59% (p=0.002)] (Figure 3E). We also report significant decreases in proportion of naïve CD4 T cells (CD4 TN) in SARS-CoV-2-positive adults between the acute and follow up phases [median proportion 41.97% vs 27.53% (p=0.001)].
These results highlight that both CD8 and CD4 central memory T cells may play key roles in the immune response to SARS-CoV-2 in both children and adults, with increases in this cell population observed during the convalescent period in participants diagnosed with COVID-19. Previous work by our team using pre-pandemic PBMCs from healthy children (11-14 years) shows an average frequency of 5.12% for CD8 TCM, further highlighting the increase in CD8 TCM in the SARS-CoV-2 positive children (20). A study analysing longitudinal samples from adults with mild COVID-19 showed the presence of SARS-CoV-2 specific memory CD8 and CD4 T cells out to 6 months post infection (33). SARS-CoV-2 specific CD8 T cells consisted primarily of TEMRA phenotype with small populations of TCM and TEM cells. SARS-CoV-2 specific CD4 T cells, however, were primarily of TCM or TEM phenotype (33). SARS-CoV-2 specific CD8 and CD4 T cells were also identified at 8 months post infection in another study comparing mild and severe disease, with greater memory CD4 T cell cytokine responses observed in severe patients relative to those of asymptomatic patients (34). Similarly, significantly larger overall T cell responses were observed in convalescent patients who had severe compared with mild disease, however mild disease was associated with greater SARS-CoV-2 specific CD8 T cell responses (35).
SARS-CoV-2-exposed but PCR-negative close contact participants also had changes in their immune cell profiles over time, despite no evidence of confirmed infection. Exposed PCR-negative children and adults both had increases in low-density immature neutrophils (defined as SSChighCD16+ cells in PBMCs) at follow up compared to acute phase [median proportion of low-density neutrophils acute vs follow up – exposed children: 0.07% vs 1.95% (p=0.001), exposed adults: 0.31% vs 1.42% (p=0.02)] (Figure 1C and Figure 3B). These neutrophils, with reduced granularity compared to conventional neutrophils and observed in mononuclear low density cell fractions, have been reported in adults with severe COVID-19 and may represent emergency myelopoiesis (36–38). CD8 TCM and CD4 TCM also increased over time in SARS-CoV-2-exposed adults [median proportion acute vs follow up – CD8 TCM: 9.04% vs 14.28% (p<0.0001), CD4 TCM: 27.10 vs 36.45 (p=0.0034)], but not in SARS-CoV-2-exposed children [median proportion acute vs follow up - CD8 TCM: 3.8% vs 5.46% (p=0.70), CD4 TCM: 15% vs 17.5% (p=0.94)] (Figures 3D, E).
As the changes in the SARS-CoV-2-exposed group over time were not antigen-specific, we next explored whether these responses were influenced by infection with other respiratory viruses in some of our SARS-CoV-2-exposed participants. We compared SARS-CoV-2 participants with or without infection with other respiratory viruses at both acute and follow up timepoints. This showed that the changes observed in key cell types over time in the exposed groups – low density neutrophils in both children (Figure 4B) and adults (Supplementary Figure 4), as well as CD8 TCM and CD4 TCM in adults (Supplementary Figure 4) – occurred in all exposed participants, independent of the presence of other respiratory viruses. In fact, changes over time in CD8 TCM and CD4 TCM cells were most evident in SARS-CoV-2-exposed adults without co-infection with other respiratory pathogens [median proportion acute vs follow up - CD8 TCM: 9.14% vs 16.00% (p=0.0034), CD4 TCM: 27.89% vs 42.54% (p=0.0001)] (Supplementary Figure 4).
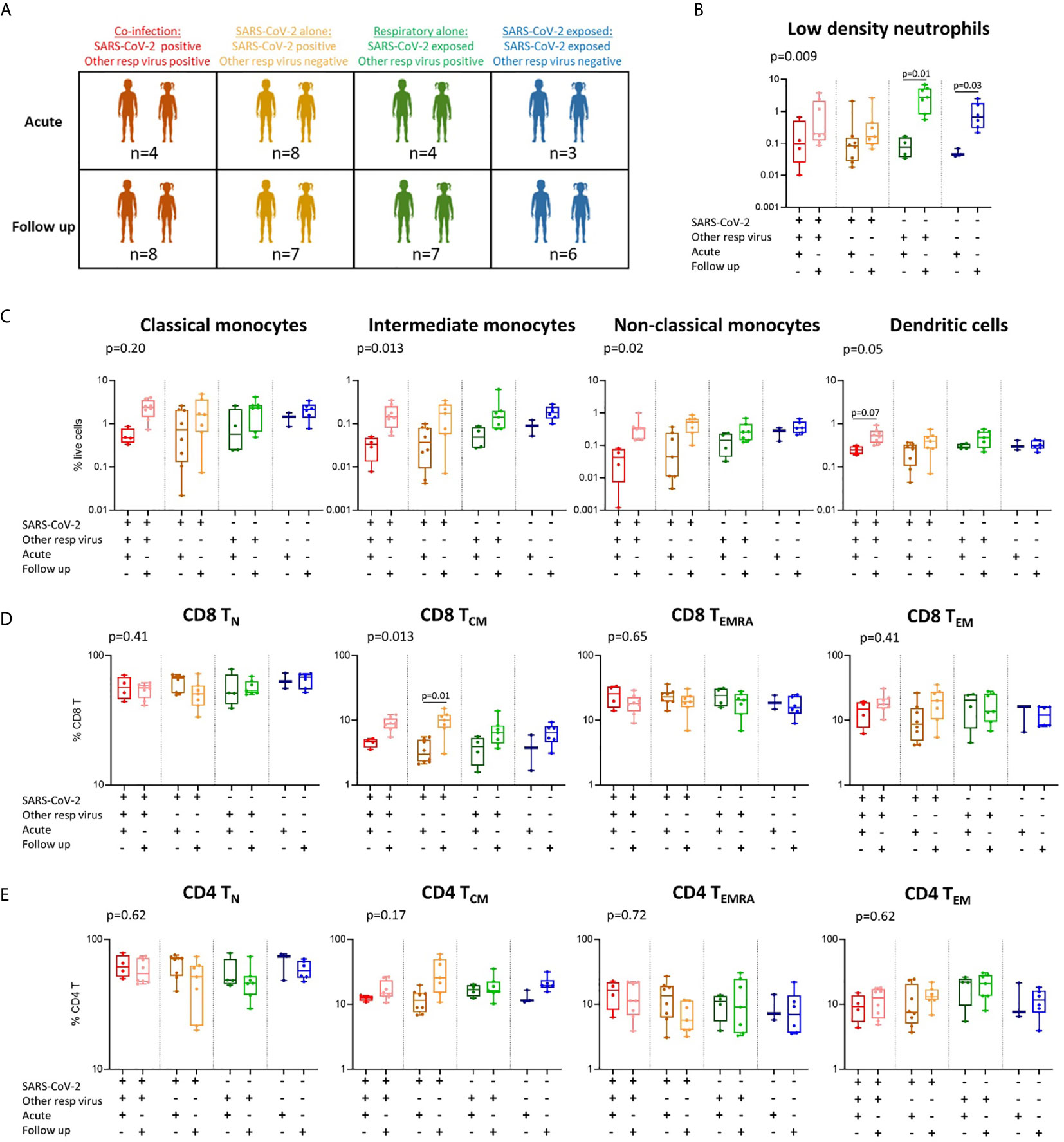
Figure 4 Immune profiles in children with SARS-CoV-2 single infection, SARS-CoV-2 co-infection, and non-SARS-CoV-2 respiratory infection. (A) A stratified analysis of samples collected from children with SARS-CoV-2 co-infection [SARS-CoV-2 positive, other respiratory virus positive (n=4 acute, n=8 follow up)], SARS-CoV-2 infection alone [SARS-CoV-2 positive, other respiratory virus negative (n=8 acute, n=7 follow up)], respiratory virus alone (SARS-CoV-2 negative (exposed), other respiratory virus positive (n=4 acute, n=7 follow up)], and SARS-CoV-2 exposed alone (SARS-CoV-2 negative, other respiratory virus negative (n=3 acute, n=6 follow up)]. (B) Proportions of low-density neutrophils. (C) Proportions of monocyte subsets and dendritic cells. (D) CD8 T cell naïve (TN), central memory (TCM), effector (TEMRA) and effector memory (TEM) populations in each group. (E) CD4 T cell naïve (TN), central memory (TCM), effector (TEMRA) and effector memory (TEM) populations in each group. P values by Kruskal-Wallis rank sum test and Dunn’s multiple comparison testing. FDR-adjusted P-values are reported. Boxplots show the medians, the 1st and 3rd quartile as well as the smallest and largest values as whiskers.
As close contact exposed participants in our cohort were all repeatedly negative upon SARS-CoV-2 PCR testing, these results suggest that exposure in the household can lead to the generation of T cell immunity even in the absence of confirmed infection. These results may also suggest that the exposed participants were infected with a low viral load that was undetectable by PCR testing. This observation is supported by a recent study exploring memory T cell responses in household close contacts (exposed but PCR negative) of confirmed COVID-19 patients, showing that both COVID-19 patients and their close contacts generate SARS-CoV-2 specific CD8 and CD4 T cell memory responses (39). The authors hypothesised that the generation of virus specific T cell immunity in SARS-CoV-2-exposed individuals in the absence of infection was either due to exposure to a limited number of viral particles or exposure for short time. In a previous case study of two parents with PCR-confirmed symptomatic SARS-CoV-2 infection and their three SARS-CoV-2 PCR negative children, we also showed that exposed children can mount a cellular immune response characterised by changes in both innate and adaptive immune cells (40).
As we were interested to explore differences in immune responses between children with COVID-19 and other respiratory infections, we compared children with SARS-CoV-2 single infection (SARS-CoV-2-positive, respiratory-negative), SARS-CoV-2 co-infection (SARS-CoV-2-positive, respiratory-positive) and children with non-SARS-CoV-2 respiratory viral infection (SARS-CoV-2 negative, respiratory-positive) (Figure 4A and Supplementary Figure 5). These results show that the SARS-CoV-2 single- and co-infected children show the greatest change in response over time in monocyte subpopulations and dendritic cells (Figure 4C), as well as CD8 TCM cells (Figure 4D), relative to non-SARS-CoV-2 respiratory-positive children. Children with SARS-CoV-2 co-infection show the lowest proportions of intermediate and non-classical monocytes at the acute timepoint (intermediate: 0.03% and non-classical: 0.04%) followed by SARS-CoV-2 single infected children (intermediate: 0.04% and non-classical: 0.12%), non-SARS-CoV-2 respiratory infected children (intermediate: 0.05% and non-classical: 0.14%), and finally SARS-CoV-2 negative respiratory-negative children (intermediate: 0.08% and non-classical: 0.25%). The lowest proportion of circulating dendritic cells was also observed in the SARS-CoV-2 co-infected children during the acute phase (Figure 4C). SARS-CoV-2 single-infected children were the only group to show a significant increase in CD8 TCM cells over time (median 3.8% acute vs 9.7% follow up), however this trend was also observed in SARS-CoV-2 co-infected children over time (median 4.5% acute vs 8.8% follow up) and non-SARS-CoV-2 respiratory infected children (3.7% acute vs 6.9% follow up) (Figure 4D). Similar results were also seen for CD4 TCM cells in SARS-CoV-2 single-infected children (median 11.8% acute vs 31.7% follow up) and co-infected children (median 12.4% acute vs 16.9% follow up) but not in non-SARS-CoV-2 respiratory infected children (median 16.5% acute vs 18.0% follow up) (Figure 4E). Combined, these data suggest that SARS-CoV-2 induces a greater change in monocyte, dendritic cell, CD8 TCM and CD4 TCM responses in children compared to other common childhood respiratory viruses. However, we acknowledge the small numbers in our groups and these results should be confirmed in larger studies.
In summary, we characterised the circulating immune cell landscape of children and adults with mild COVID-19, as well as their PCR-negative close contacts during and up to 9 weeks post infection. We directly compared immune cell profiles in children and adults, revealing markedly low proportions of circulating innate immune cells in SARS-CoV-2-positive children during acute infection, most evident in children under the age of five. These results suggest enhanced recruitment of these circulating innate immune cells to sites of infection, such as the lung. The generation of CD8 TCM and CD4 TCM cells up to 9 weeks post infection were common to both SARS-CoV-2-positive children and adults. Exposure to SARS-CoV-2 in household close contacts also resulted in a change in immune cells over time despite no evidence of confirmed SARS-CoV-2 infection and independent of the presence of other respiratory viruses. This response was characterised by increases in low-density neutrophils at follow up, as well as increases in CD8 TCM and CD4 TCM over time in exposed adults. A comparison of immune responses in children with COVID-19 to children with other common respiratory infections revealed that SARS-CoV-2 infection induced a greater change in innate and T cell mediated immune responses. Our work adds to recent studies exploring immunity and clinical outcomes in children with mild COVID-19 (19, 32, 41) and provides new mechanistic insights into the immune response during and after recovery from COVID-19 in both children and adults.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
This project was approved by The Royal Children’s Hospital Melbourne Human Research Ethics Committee (HREC): HREC/ 63666/RCHM-2019. All participants or their legal guardians provided written informed consent.
MN, AS, DB, RS, ST, and NWC designed the study. ST, KD, and VC collected the clinical data and specimens. MN, SB, and VC performed the experiments. MN, RS, ST, and NWC interpreted the findings and wrote the original manuscript. IO, JN, ZT, JA, CD, SS, LD, CM, PL, VI, PM, JB, KM, NC, and SM are study investigators and assisted in interpreting the findings. All authors contributed to the article and approved the submitted version.
The FFX study has Australian Commonwealth government support for identification of positive samples and database management. Sample analysis was supported by the Royal Children’s Hospital Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.741639/full#supplementary-material
1. Zimmermann P, Curtis N. Why is COVID-19 Less Severe in Children? A Review of the Proposed Mechanisms Underlying the Age-Related Difference in Severity of SARS-CoV-2 Infections. Arch Dis Child (2020) archdischild-2020-320338. doi: 10.1136/archdischild-2020-320338
2. Viner RM, Mytton OT, Bonell C, Melendez-Torres GJ, Ward J, Hudson L, et al. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared With Adults: A Systematic Review and Meta-Analysis. JAMA Pediatr (2021) 175(2):143–56. doi: 10.1001/jamapediatrics.2020.4573%JJAMAPediatrics
3. Sayampanathan AA, Heng CS, Pin PH, Pang J, Leong TY, Lee VJ. Infectivity of Asymptomatic Versus Symptomatic COVID-19. Lancet (2021) 397(10269):93–4. doi: 10.1016/s0140-6736(20)32651-9
4. Zhu Y, Bloxham CJ, Hulme KD, Sinclair JE, Tong ZWM, Steele LE, et al. A Meta-Analysis on the Role of Children in SARS-CoV-2 in Household Transmission Clusters. Clin Infect Dis an Off Publ Infect Dis Soc America (2020) 72(12):ciaa1825. doi: 10.1093/cid/ciaa1825
5. Galow L, Haag L, Kahre E, Blankenburg J, Dalpke AH, Lück C, et al. Lower Household Transmission Rates of SARS-CoV-2 From Children Compared to Adults. J Infect (2021) 83(1):e34–6. doi: 10.1016/j.jinf.2021.04.022
6. Soriano-Arandes A, Gatell A, Serrano P, Biosca M, Campillo F, Capdevila R, et al. Household SARS-CoV-2 Transmission and Children: A Network Prospective Study. Clin Infect Dis (2021) 73(6):e1261–9. doi: 10.1093/cid/ciab228
7. Ren G-L, Wang X-F, Xu J, Li J, Meng Q, Xie G-Q, et al. Comparison of Acute Pneumonia Caused by SARS-COV-2 and Other Respiratory Viruses in Children: A Retrospective Multi-Center Cohort Study During COVID-19 Outbreak. Mil Med Res (2021) 8(1):13. doi: 10.1186/s40779-021-00306-7
8. Song X, Delaney M, Shah RK, Campos JM, Wessel DL, DeBiasi RL. Comparison of Clinical Features of COVID-19 vs Seasonal Influenza A and B in US Children. JAMA Network Open (2020) 3(9):e2020495–e2020495. doi: 10.1001/jamanetworkopen.2020.20495
9. Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The Immunology of Multisystem Inflammatory Syndrome in Children With COVID-19. Cell (2020) 183(4):968–81.e7. doi: 10.1016/j.cell.2020.09.016
10. Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell (2020) 183(4):982–95.e14. doi: 10.1016/j.cell.2020.09.034
11. Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and Multisystem Inflammatory Syndrome in Children and Adolescents. Lancet Infect Dis (2020) 20(11):e276–88. doi: 10.1016/s1473-3099(20)30651-4
12. Ng OT, Marimuthu K, Koh V, Pang J, Linn KZ, Sun J, et al. SARS-CoV-2 Seroprevalence and Transmission Risk Factors Among High-Risk Close Contacts: A Retrospective Cohort Study. Lancet Infect Dis (2021) 21(3):333–43. doi: 10.1016/s1473-3099(20)30833-1
13. Procop GW, Brock JE, Reineks EZ, Shrestha NK, Demkowicz R, Cook E, et al. A Comparison of Five SARS-CoV-2 Molecular Assays With Clinical Correlations. Am J Clin Pathol (2020) 155(1):69–78. doi: 10.1093/ajcp/aqaa181
14. Attwood LO, Francis MJ, Hamblin J, Korman TM, Druce J, Graham M. Clinical Evaluation of AusDiagnostics SARS-CoV-2 Multiplex Tandem PCR Assay. J Clin Virol (2020) 128:104448. doi: 10.1016/j.jcv.2020.104448
15. Neeland MR, Andorf S, Manohar M, Dunham D, Lyu SC, Dang TD, et al. Mass Cytometry Reveals Cellular Fingerprint Associated With IgE+ Peanut Tolerance and Allergy in Early Life. Nat Commun (2020) 11(1):1091. doi: 10.1038/s41467-020-14919-4
16. Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B-Stat Method (1995) 57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
17. Holcar M, Goropevšek A, Ihan A, Avčin T. Age-Related Differences in Percentages of Regulatory and Effector T Lymphocytes and Their Subsets in Healthy Individuals and Characteristic STAT1/STAT5 Signalling Response in Helper T Lymphocytes. J Immunol Res (2015) 2015:352934–4. doi: 10.1155/2015/352934
18. Blanco E, Pérez-Andrés M, Arriba-Méndez S, Contreras-Sanfeliciano T, Criado I, Pelak O, et al. Age-Associated Distribution of Normal B-Cell and Plasma Cell Subsets in Peripheral Blood. J Allergy Clin Immunol (2018) 141(6):2208–19.e16. doi: 10.1016/j.jaci.2018.02.017
19. Neeland MR, Bannister S, Clifford V, Dohle K, Mulholland K, Sutton P, et al. Innate Cell Profiles During the Acute and Convalescent Phase of SARS-CoV-2 Infection in Children. Nat Commun (2021) 12(1):1084. doi: 10.1038/s41467-021-21414-x
20. Neeland MR, Andorf S, Dang TD, McWilliam VL, Perrett KP, Koplin JJ, et al. Altered Immune Cell Profiles and Impaired CD4 T-Cell Activation in Single and Multi-Food Allergic Adolescents. Clin Exp Allergy (2021) 51:674–84. doi: 10.1111/cea.13857
21. Neeland MR, Koplin JJ, Dang TD, Dharmage SC, Tang ML, Prescott SL, et al. Early Life Innate Immune Signatures of Persistent Food Allergy. J Allergy Clin Immunol (2018) 142(3):857–64.e3. doi: 10.1016/j.jaci.2017.10.024
22. Maucourant C, Filipovic I, Ponzetta A, Aleman S, Cornillet M, Hertwig L, et al. Natural Killer Cell Immunotypes Related to COVID-19 Disease Severity. Sci Immunol (2020) 5(50):eabd6832. doi: 10.1126/sciimmunol.abd6832
23. Jiang Y, Wei X, Guan J, Qin S, Wang Z, Lu H, et al. COVID-19 Pneumonia: CD8(+) T and NK Cells are Decreased in Number But Compensatory Increased in Cytotoxic Potential. Clin Immunol (2020) 218:108516. doi: 10.1016/j.clim.2020.108516
24. Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martinez-Colon GJ, McKechnie JL, et al. A Single-Cell Atlas of the Peripheral Immune Response in Patients With Severe COVID-19. Nat Med (2020) 26(7):1070–6. doi: 10.1038/s41591-020-0944-y
25. Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, van de Sandt CE, et al. Breadth of Concomitant Immune Responses Prior to Patient Recovery: A Case Report of Non-Severe COVID-19. Nat Med (2020) 26(4):453–5. doi: 10.1038/s41591-020-0819-2
26. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-Cell Landscape of Bronchoalveolar Immune Cells in Patients With COVID-19. Nat Med (2020) 26(6):842–4. doi: 10.1038/s41591-020-0901-9
27. Bost P, Giladi A, Liu Y, Bendjelal Y, Xu G, David E, et al. Host-Viral Infection Maps Reveal Signatures of Severe COVID-19 Patients. Cell (2020) 181(7):1475–1488.e12. doi: 10.1016/j.cell.2020.05.006
28. Melms JC, Biermann J, Huang H, Wang Y, Nair A, Tagore S, et al. A Molecular Single-Cell Lung Atlas of Lethal COVID-19. Nature (2021) 595(7865):114–9. doi: 10.1038/s41586-021-03569-1
29. Hall JA, Harris RJ, Zaidi A, Woodhall SC, Dabrera G, Dunbar JK. HOSTED-England’s Household Transmission Evaluation Dataset: Preliminary Findings From a Novel Passive Surveillance System of COVID-19. Int J Epidemiol (2021) 50(3):743–52. doi: 10.1093/ije/dyab057
30. Pierce CA, Preston-Hurlburt P, Dai Y, Aschner CB, Cheshenko N, Galen B, et al. Immune Responses to SARS-CoV-2 Infection in Hospitalized Pediatric and Adult Patients. Sci Transl Med (2020) 12(564):eabd5487. doi: 10.1126/scitranslmed.abd5487%JScienceTranslationalMedicine
31. Vella LA, Giles JR, Baxter AE, Oldridge DA, Diorio C, Kuri-Cervantes L, et al. Deep Immune Profiling of MIS-C Demonstrates Marked But Transient Immune Activation Compared to Adult and Pediatric COVID-19. Sci Immunol (2021) 6: (57):eabf7570. doi: 10.1126/sciimmunol.abf7570%JScienceImmunology
32. Selva KJ, van de Sandt CE, Lemke MM, Lee CY, Shoffner SK, Chua BY, et al. Systems Serology Detects Functionally Distinct Coronavirus Antibody Features in Children and Elderly. Nat Commun (2021) 12(1):2037. doi: 10.1038/s41467-021-22236-7
33. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological Memory to SARS-CoV-2 Assessed for Up to 8 Months After Infection. Science (2021) 371(6529):eabf4063. doi: 10.1126/science.abf4063%JScience
34. Kang CK, Kim M, Lee S, Kim G, Choe PG, Park WB, et al. Longitudinal Analysis of Human Memory T-Cell Response According to the Severity of Illness Up to 8 Months After SARS-CoV-2 Infection. J Infect Dis (2021) 224(1):39–48. doi: 10.1093/infdis/jiab159
35. Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, et al. Oxford Immunology Network Covid-19 Response and I. C. Investigators: Broad and Strong Memory CD4+ and CD8+ T Cells Induced by SARS-CoV-2 in UK Convalescent Individuals Following COVID-19. Nat Immunol (2020) 21(11):1336–45. doi: 10.1038/s41590-020-0782-6
36. Schulte-Schrepping J, Reusch N, Paclik D, Baßler K, Schlickeiser S, Zhang B, et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell (2020) 182(6):1419–40.e23. doi: 10.1016/j.cell.2020.08.001
37. Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil Extracellular Traps Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood (2020) 136(10):1169–79. doi: 10.1182/blood.2020007008
38. Morrissey SM, Geller AE, Hu X, Tieri D, Cooke EA, Ding C, et al. : Emergence of Low-Density Inflammatory Neutrophils Correlates With Hypercoagulable State and Disease Severity in COVID-19 Patients. medRxiv (2020) 2020:5.22.20106724. doi: 10.1101/2020.05.22.20106724
39. Wang Z, Yang X, Zhong J, Zhou Y, Tang Z, Zhou H, et al. Exposure to SARS-CoV-2 Generates T-Cell Memory in the Absence of a Detectable Viral Infection. Nat Commun (2021) 12(1):1724. doi: 10.1038/s41467-021-22036-z
40. Tosif S, Neeland MR, Sutton P, Licciardi PV, Sarkar S, Selva KJ, et al. Immune Responses to SARS-CoV-2 in Three Children of Parents With Symptomatic COVID-19. Nat Commun (2020) 11(1):5703. doi: 10.1038/s41467-020–19545-8
Keywords: COVID - 19, pediatrics, non-COVID-19 respiratory virus, household contact, cell profile
Citation: Neeland MR, Bannister S, Clifford V, Nguyen J, Dohle K, Overmars I, Toh ZQ, Anderson J, Donato CM, Sarkar S, Do LAH, McCafferty C, Licciardi PV, Ignjatovic V, Monagle P, Bines JE, Mulholland K, Curtis N, McNab S, Steer AC, Burgner DP, Saffery R, Tosif S and Crawford NW (2021) Children and Adults in a Household Cohort Study Have Robust Longitudinal Immune Responses Following SARS-CoV-2 Infection or Exposure. Front. Immunol. 12:741639. doi: 10.3389/fimmu.2021.741639
Received: 15 July 2021; Accepted: 21 September 2021;
Published: 13 October 2021.
Edited by:
Carl G. Feng, The University of Sydney, AustraliaReviewed by:
Anno Saris, Leiden University Medical Center, NetherlandsCopyright © 2021 Neeland, Bannister, Clifford, Nguyen, Dohle, Overmars, Toh, Anderson, Donato, Sarkar, Do, McCafferty, Licciardi, Ignjatovic, Monagle, Bines, Mulholland, Curtis, McNab, Steer, Burgner, Saffery, Tosif and Crawford. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melanie R. Neeland, bWVsYW5pZS5uZWVsYW5kQG1jcmkuZWR1LmF1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.