
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 05 October 2021
Sec. Cancer Immunity and Immunotherapy
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.736943
 Chen Chen1,2†
Chen Chen1,2† Yixin Zhou2,3†
Yixin Zhou2,3† Xuanye Zhang2,4†
Xuanye Zhang2,4† Yuhong Wang2,5
Yuhong Wang2,5 Li-na He2,4
Li-na He2,4 Zuan Lin2,6
Zuan Lin2,6 Tao Chen2,7
Tao Chen2,7 Yongluo Jiang2,7
Yongluo Jiang2,7 Shaodong Hong2,4*
Shaodong Hong2,4* Li Zhang2,4*
Li Zhang2,4*Background: More and more immune-oncology trials have been conducted for treating various cancers, yet it is unclear what the reporting quality of immune-oncology trials is,and characteristics associated with higher reporting quality.
Objective: This study aims to evaluate the reporting quality of immune-oncology trials.
Methods: The PubMed and Cochrane library were searched to identify all English publications of clinical trials assessing immunotherapy for cancer. Reporting quality of immune-oncology trials was evaluated by a quality score with 11 points derived from the Trial Reporting in Immuno-Oncology (TRIO) statement, which contained two parts: an efficacy score of 6 points and toxicity score of 5 point. Linear regression was used to identify characteristics associated with higher scores.
Results: Of the 10,169 studies screened, 298 immune-oncology trial reports were enrolled. The mean quality score, efficacy score, and toxicity score were 6.46, 3.61, and 2.85, respectively. The most common well-reported items were response evaluation criteria (96.0%) and toxicity grade (98.7%), followed by Kaplan-Meier survival analyses (80.5%). Treatment details beyond progression (12.8%) and toxicity onset time and duration (7.7%) were poorly reported. Multivariate regression revealed that higher impact factor (IF) (IF >20 vs. IF <5, p < 0.001), specific tumor type (p = 0.018 for lung, p = 0.021 for urinary system, vs. pan cancer), and a certain kind of immune checkpoint blocking agent (p < 0.001 for anti-PD-1 or multiagents, vs. anti-CTLA-4) were independent predictors of higher-quality score. Similar independent predictive characteristics were revealed for high-efficacy score. Only IF >20 had a significant high-toxicity score (p < 0.001).
Conclusion: Immune-oncology trial reports presented an unsatisfied quality score, especially in the reporting of treatment details beyond progression and toxicity onset time and duration. High IF journals have better reporting quality. Future improvement of trial reporting was warranted to the benefit-risk assessment of immunotherapy.
With the success of immune checkpoint blockade (ICB), immunotherapy has revolutionized cancer therapy. More and more immunotherapies have been approved for treating various cancers (1, 2). The immune checkpoint blocking agents for immunotherapies include anticytotoxic T-lymphocyte antigen 4 (CTLA-4, ipilimumab, tremelimumab), antiprogrammed cell death protein 1 (PD-1, nivolumab, pembrolizumab), and antiprogrammed death-ligand 1 (PD-L1, atezolizumab, avelumab, durvalumab) agents (3, 4).
Clinical trials are considered essential to advancing and evaluating the use of ICB in cancer treatment (5). Biomedical publications of various journals are key methods for disseminating the design, conduct, results, and conclusions of these trials. The published reports should provide the reader with the ability to fully understand the trial and make informed judgments of trial results. Thus, it needs to be a unified standard to ensure the quality of the reports.
The Consolidated Standards of Reporting Trials (CONSORT) statement provides guidance to authors regarding essential items that should be included in trial reports and can be also applied to immune-oncology (IO) trials (6, 7). However, distinct mechanisms of IO therapies exhibit unique efficacy and toxicity compared with traditional cancer treatments such as chemotherapy, which may lead to additional considerations for reporting guidelines of IO clinical trials (8, 9). Based on this fact, the Trial Reporting in Immuno-Oncology (TRIO) statement is developed by American Society of Clinical Oncology (ASCO) and the Society for Immunotherapy of Cancer (SITC) to improve the interpretation and comparison across IO trials (10, 11). There have been literatures evaluating the quality of randomized clinical trials (RCT) reports based on the CONSORT statement (12–14), but no studies specifically evaluate the report quality of IO trials. Therefore, the purpose of this study is to evaluate the reporting quality of IO trials based on the TRIO statement. In addition, we also investigated the publications’ characteristics associated with higher quality in IO trial reporting.
We searched PubMed (https://pubmed.ncbi.nlm.nih.gov) and Cochrane (https://www.cochranelibrary.com) to identify all English publications of clinical trials assessing immunotherapy for cancer. The search was performed in September 16, 2019, using the keywords as follows: cancer (neoplasia, neoplasias, neoplasm, tumors, tumor, cancers, malignancy, malignancies, carcinoma, leukemia, lymphoma, melanoma, glioma); immune checkpoint inhibitor (immune checkpoint blocking agent, immune therapy, immunotherapy, immunotherapies, immuno-oncology treatment, anticytotoxic T-lymphocyte antigen 4, CTLA-4, ipilimumab, tremelimumab, antiprogrammed cell death protein 1, PD-1, nivolumab, pembrolizumab, antiprogrammed death-ligand 1, PD-L1, atezolizumab, avelumab, durvalumab); and clinical trials. Exclusion criteria included: non-article (review, editorial paper, abstract only, and conference paper), registration information and clinical trial protocols, non-clinical trials, pilot trials, post-hoc or pooled analysis of clinical trials, non-immunotherapy, and non-malignant tumor studies.
A trial reporting of immuno-oncology quality score (TRIOQS) based on TRIO statement was defined by two of the authors (CC and SH). The score was based on the recommendations of TRIO statement except the combination or sequencing of immunotherapies reporting standard (Table 1). This scoring system contained two parts, the one was efficacy score (TRIOQS-E, items 1–6 in Table 1) and the other one was toxicity score (TRIOQS-T, items 7–11 in Table 1). Each item enrolled in TRIOQS was scored as 1 if it was reported or 0 if it was not reported at all; each item was weighted with equal importance. For those recommendations with several subcomponents, a score of 1 was given if any one of them was reported. The 12th recommendation of TRIO statement was excluded because it was especially for clinical trials with combination or sequencing of immunotherapies.
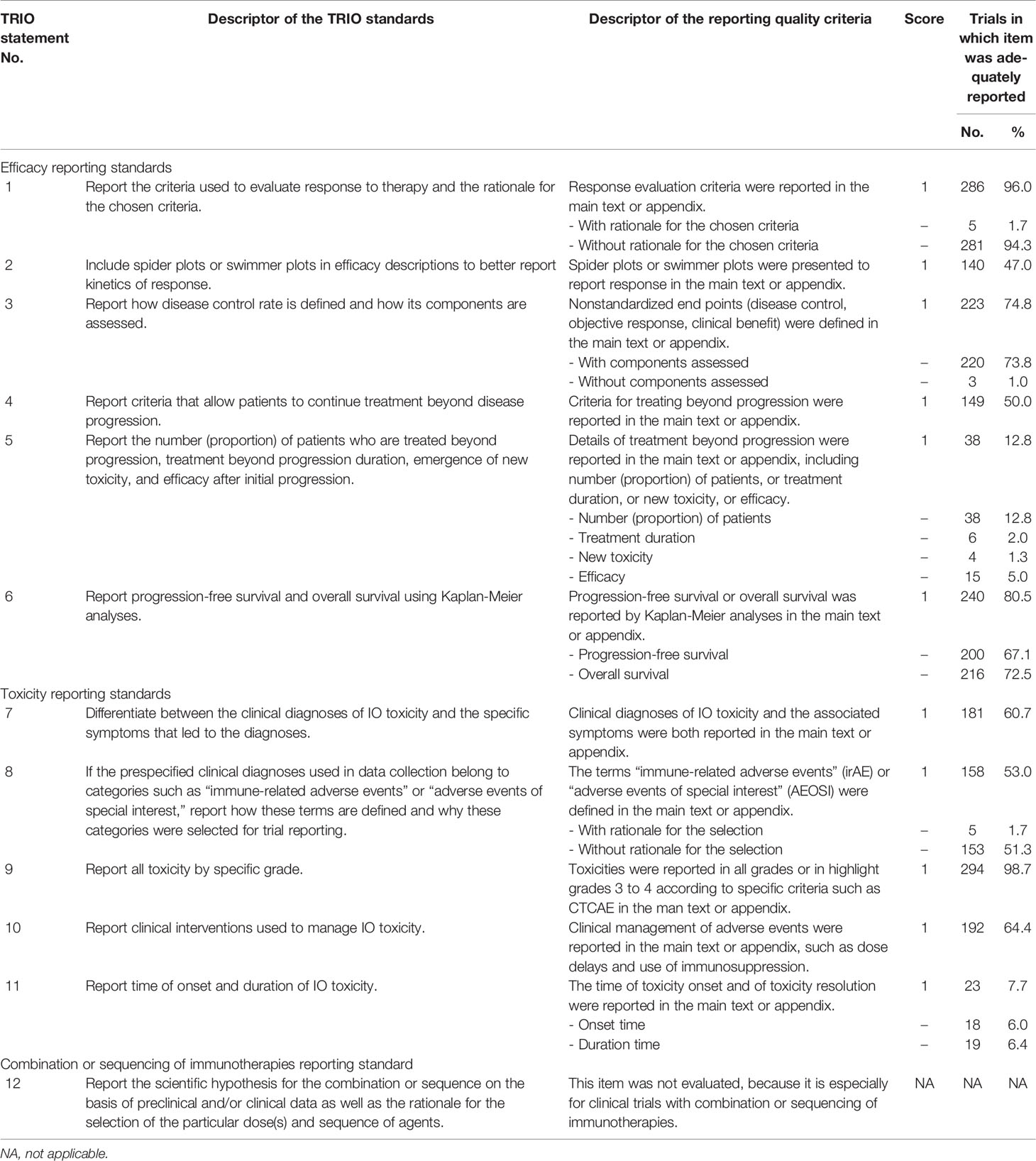
Table 1 Quality of immune-oncology clinical trial reporting using items from the Trial Reporting in Immuno-Oncology (TRIO) statement.
The scoring system was piloted on 10 randomly selected publications (110 items) by two authors (CC and YZ) who were blinded to each other’s evaluation results. Among 110 items, 6 discrepancies were identified, and all were successfully resolved by consensus. Based on this consensus, the two authors (CC and YZ) evaluated the remaining publications.
Several trial characteristics that could affect the quality score were selected. Year of publication was directly extracted as continuous variable. Journal impact factor was referred to 2018 and classified as four groups: <5, 5 to 10, 10 to 20, and >20. Trial phase was also concerned and consisted of phase I (I, or I/II), phase II (II, or II/III), and phase III (III or III/IV). Trials were considered as industry funded if they received any form of industry funding. Number of participating centers was calculated as three groups according to the median: 1 to 12, 13 to 246, and unknown group. Intercontinental trials were that recruited patients from more than one continent. Nonintercontinental trials were conducted in north America only, other regions (Asia only, Europe only, Oceania only), and unknown regions. The tumor types included in trials could be divided into the following four categories: pan cancer, lung cancer, melanoma, urinary system cancer, and other cancers. Based on the mechanism, immune checkpoint blocking agent in immunotherapy contained anti-CTLA-4, anti-PD-1, anti-PD-L1, and any mix of the above. According to the treatment strategy, immunotherapy could be used alone or combined with other therapy.
The TRIOQS was calculated as the sum of the score of items in Table 1 and expressed as an integer from 0 to 11. TRIOQS scores were descripted using mean and standard error (SE). Single-item frequencies were compared between subgroups by Chi-square tests.
Univariate and multivariate linear regression analyses were used to identify trial characteristics associated with higher TRIOQS. Given that it was deemed desirable to include as many characteristics associated with reporting quality as possible, the multivariable regression included all mentioned covariates. Violin plots were used to visually show the significant differences in TRIOQS among subgroups of statistically characteristics. Statistical analyses were performed using R software (http://www.R-project.org/). All tests were two-tailed, with p < 0.05 considered statistically significant.
From the 10,169 studies initially screened, a total of 298 immuno-oncology clinical trial reports were included in the present analysis (Figure 1; the list in the Supplementary Materials). Trials’ characteristics are listed in Table 2. The number of published trials almost monotonously increased with the year. More than half trials (n = 173, 58%) were published in journals with IF >20, including Lancet Oncology (n = 50, 16.8%), Journal of Clinical Oncology (n = 45, 15.1%), and The New England Journal of Medicine (n = 38, 12.8%). Only 45 trials were single center, and 10 trials enrolled more than 200 centers. The main treatment strategy was immunotherapy alone (n = 180, 60.4%). With the latest of publication year, the proportion of immunotherapy combined with other therapy increased (2009–2015: 23/69, 33.3%; 2016: 17/48, 35.4%; 2017: 18/68, 26.5%; 2018: 37/69, 53.6%; 2019: 23/44, 52.3%; p = 0.005), and the proportion of anti-PD-1 agent also increased (2009–2015: 21/69, 30.4%; 2016: 25/48, 52.1%; 2017: 38/68, 55.9%; 2018: 31/69, 44.9%; 2019: 26/44, 59.1%; p = 0.012).
The mean TRIOQS was 6.46 on an 11-point scale (range, 1 to 11; 95% CI, 6.23 to 6.69). Two hundred thirty-eight trials (79.9%) got a score of 5 to 9, while 27 trials (9.1%) have a score ≤3. Only two trials were found with a score of 11. The mean TRIOQS-E was 3.61 on a 6-point scale (range, 0–6; 95% CI, 3.45 to 3.77), with three trials having a score of 0 and 22 trials having a score of 6. The mean TRIOQS-T was 2.85 on a 5-point scale (range, 0–5; 95% CI, 2.72 to 2.98), with four trials having a score of 0 and 14 trials having a score of 5.
The most common well-reported items were response evaluation criteria (item 1, 96.0%) and toxicity grade (item 9, 98.7%), followed by Kaplan-Meier survival analyses (item 6, 80.5%). Spider or swimmer plots were presented more frequently by phase I trials (n = 74 of 124 trials, 59.7%) than by phase II (n = 47 of 104 trials, 45.2%) and phase III trials (n = 19 of 70 trials, 27.1%; p < 0.001). Criteria for continuous treatment beyond progression and definition of new adverse event terms (irAE or AEOSI), which were unique to immune-oncology therapy, were clearly described in 50% and 53.0% trials separately. However, treatment details beyond progression (item 5, 12.8%) and toxicity onset time and duration (item11, 7.7%) were poorly reported. It was worth noting that the reasons for the criteria selection were not fully explained in almost all trials.
The results of univariable and multivariable linear regressions are listed in Table 3. Although all characteristics were statistically significant in univariable analysis, multivariate regression only revealed that higher IF (IF >20 vs. IF <5, p < 0.001), specific tumor type (lung vs. pan cancer, p = 0.018; urinary system vs. pan cancer, p = 0.021), and a certain kind of immune checkpoint blocking agent (anti-PD-1 vs. anti-CTLA-4, p < 0.001; multiagents vs. anti-CTLA-4, p < 0.001) were independent predictors of higher TRIOQS.

Table 3 Results of linear regression analyses of trial characteristics predicting TRIOQS (0-11 scale).
Specifically, articles with IF >20 had a TRIOQS on average 1.15 points higher than those with IF <5 (Figure 2A). Publications of lung cancer and urinary system cancer had a TRIOQS that was 1.08 and 1.12 point higher than those of pan cancers separately. While publications of melanoma and other cancers respectively had a TRIOQS that was 0.81 and 0.79 point potential higher than those of pan cancers (Figure 2B). The TRIOQS of trials on anti-PD-1 agent was higher than those on anti-CTLA-4 agent by a mean of 1.17 points and trials on multiagents had an average 1.5 points higher TRIOQS than those on anti-CTLA-4 agent (Figure 2C).
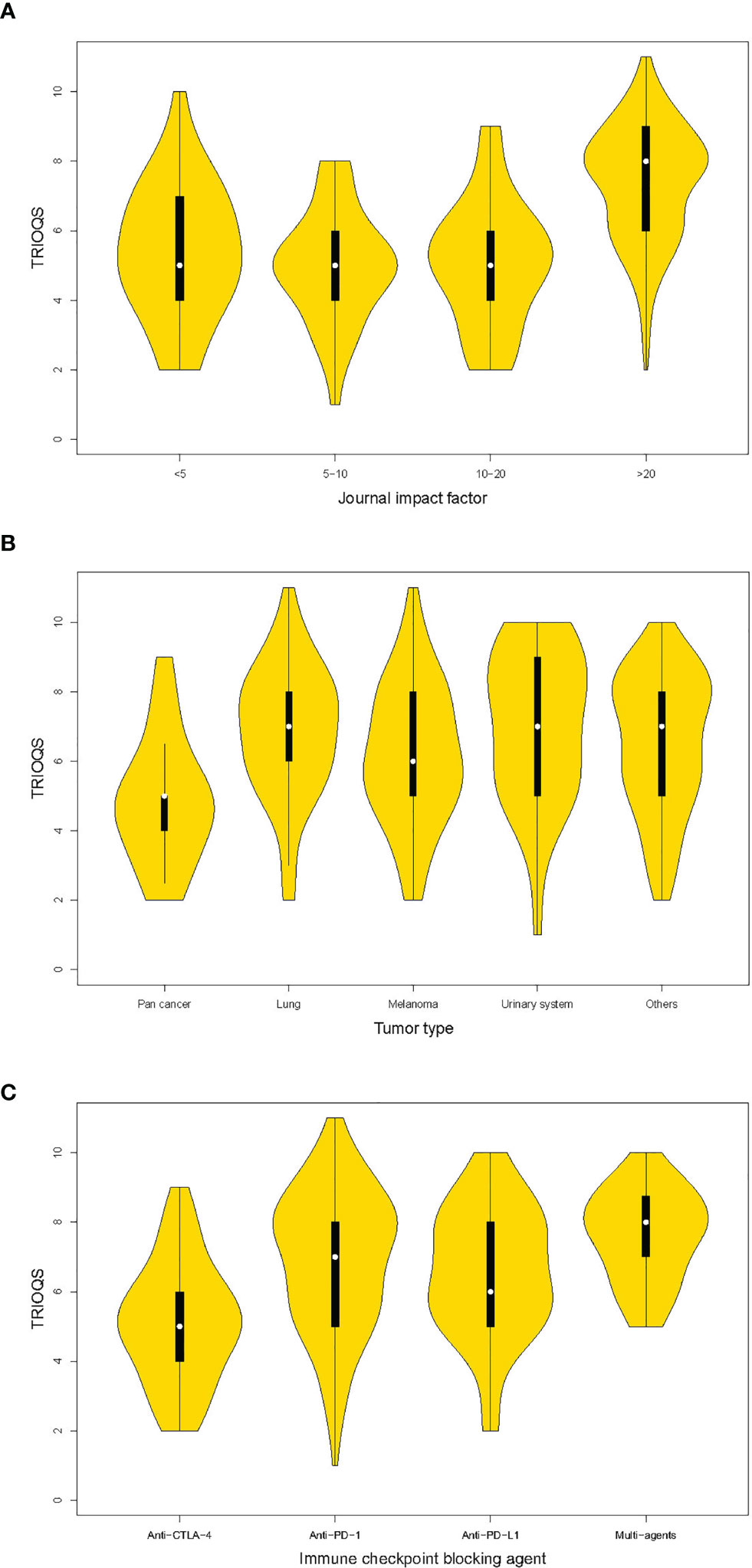
Figure 2 Violin plots of trial reporting of immuno-oncology quality score (TRIOQS) by characteristics of publications. (A) Journal impact factor; (B) tumor type; (C) immune checkpoint blocking agent. The white dot is the median value. The black box ranges from the lower quartile to the upper quartile values, and the thin black line indicates 95% confidence interval. The outer shape is the kernel density estimation.
Similar independent predictive characteristics were revealed for high TRIOQS-E in multivariable regression, including IF >20 (vs. IF <5, p = 0.034), phase II (vs. phase I, p = 0.021), specific cancer (lung cancer vs. pan cancer, p = 0.030; urinary system cancer vs. pan cancer, p = 0.013; other cancers vs. pan cancers, p = 0.012), certain kinds of immune checkpoint blocking agent (anti-PD-1, anti-PD-L1, and multiagents vs. anti-CTLA-4, all p < 0.001) (Table 4). However, only IF >20 had a significant high TRIOQS-T (p < 0.001) (Table 5).
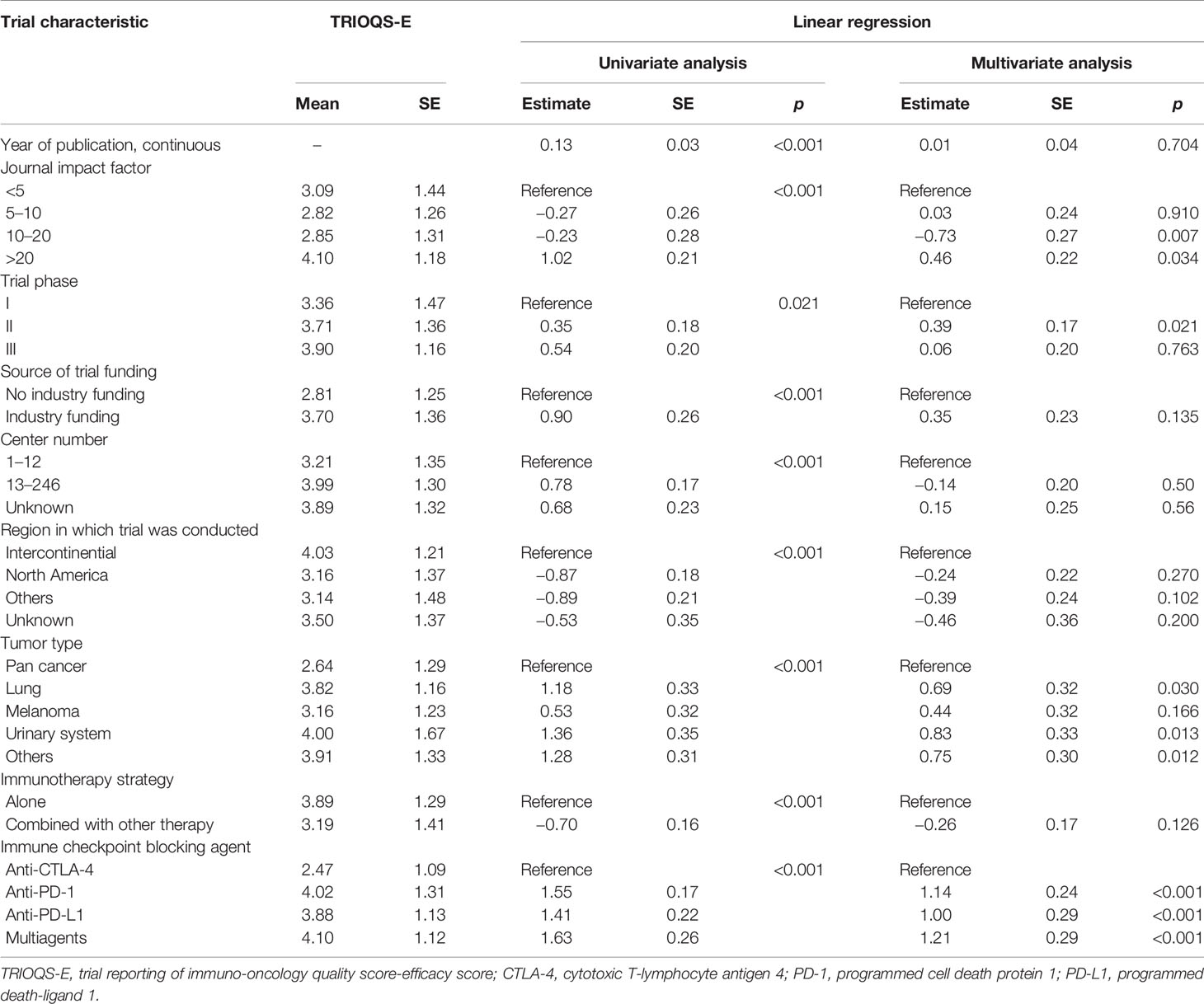
Table 4 Results of linear regression analyses of trial characteristics predicting TRIOQS-E (0–6 scale).
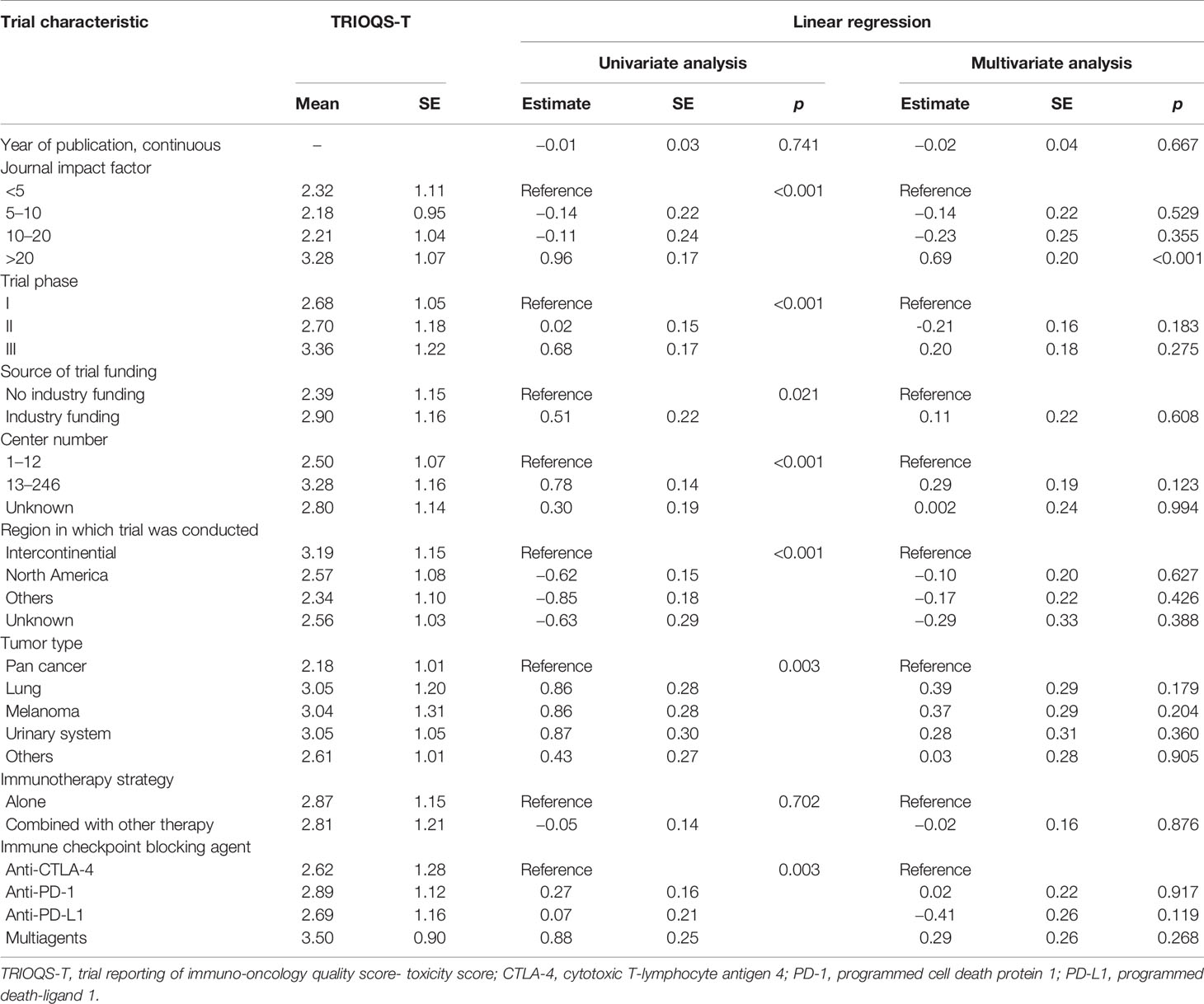
Table 5 Results of linear regression analyses of trial characteristics predicting TRIOQS-T (0–5 scale).
Due to the lack of guidelines for reports of IO clinical trials until TRIO statement (10, 11) came out more than 2 years ago, the reporting quality for IO clinical trials was unsatisfactory. Concerns have been raised that more structured and transparent approach was important to the benefit-risk assessment in the evaluation of IO treatment as a new therapy. Therefore, the standardized reporting is essential. This is the first systematic evaluation of the reporting quality of IO clinical trials of cancer treatment in accordance to the TRIO statement.
Immune-oncology trials presented an unsatisfied reporting quality score based on the specific 11-item scoring system derived from the TRIO statement, which consist of six-item efficacy score and five-item toxicity score. The most common reported items in traditional clinical trials are also well described in IO trials, such as response evaluation and toxicity grade. This may be attributing the success to the well-established CONSORT guidance for clinical trials. However, treatment details beyond progression and toxicity onset time and duration were poorly reported, which are crucial to evaluate the efficacy and toxicity of immunotherapy for the cancer.
Pseudo-progression, as a unique phenomenon in IO treatment, is an event that denotes the appearance of new lesions (usually with shrinkage of baseline index tumor burden) or an initial increase in index lesions with subsequent index lesion response by clinical or radiographic assessment (15, 16). Thus, IO clinical trials often allow patients to continue therapy beyond objective progression and half of publications have reported the criteria of continue treatment. However, the details of treatment after progression was seriously underreported (12.8% reported). This failure may be related to the insufficient awareness of authors on the importance of continuous therapy and may limit the ability to make a comprehensive benefit assessment.
Although specific toxicity items for IO therapy, definition of “immune-related adverse events” or “adverse events of special interest” and management of IO toxicity, were relatively well described in over half of publications, the onset and duration of IO toxicity were rare reported. It is worth noting that, unlike the traditional cancer treatment, the toxicity of IO therapy can be latency occurrence and long lasting (17, 18). Therefore, reporting the onset and duration of toxicity is arguably as clinically important to assess the risk-benefit and useful to design the subsequent IO trails.
Different from that the reporting quality of clinical trials of traditional chemotherapy has been fully evaluated since the CONSORT guideline proposed, the quality of IO clinical trials reporting has not been assessed according to the TRIO statement which was specifically designed for IO trials. This divergence may be related to the insufficient awareness of differences between IO and traditional chemotherapy clinical trials and slower uptake of the TRIO statement. Continued use of CONSORT cannot fully reflect the unique characteristics of IO clinical trials (19, 20). More advanced than CONSORT, TRIO adopts toxicity reporting standards at the initially proposed. Notably, only four trails have no description of toxicity. It is also interesting that more than half of trials published in journals of IF >20 (n = 173, 58%).
It is worth noting that two trial reports received the highest score of 11 points (21, 22). Both reports are multicenter randomized controlled phase III clinical studies and are published in the New England Journal of Medicine in 2015. They are both immunotherapy alone studies of nivolumab and are funded by Bristol-Myers Squibb. The tumor types they studied are melanoma (CheckMate 066) and nonsmall-cell lung cancer (CheckMate 057), respectively. Although these two trial reports meet the requirement of each item according to the TRIO statement, some subcomponents are still insufficient. Neither of them mentioned the rationale for the chosen criteria used to evaluate response to therapy and for the selection of clinical diagnoses used in data collection belong to categories such as “immune-related adverse events” or “adverse events of special interest”. Although they both reported the number (proportion) of patients who are treated beyond progression and efficacy after initial progression, they did not mention treatment beyond progression duration and emergence of new toxicity. In addition, the report of CheckMate 066 did not mention time of onset of IO toxicity.
Factors associated with higher reporting scores were also investigated. The publications in journals of IF >20 had higher quality score, either for efficacy assessment or toxicity assessment, which might be related to the original requirements and review system of the journal (13). Specific cancer, such as lung cancer and urinary system cancer had higher quality score compared with the pan cancer. Most of the trials designed for a specific cancer category aimed to confirm the clinical efficacy of the IO treatment for this disease, not just similar to the exploratory purpose in the pan cancer categories. Therefore, there were more detailed reports on the efficacy and the whole trial. Simultaneously, this would possibly reduce authors’ interest for toxicity concerns, which lead to no difference in toxicity quality score between specific cancer and pan cancer. Compared with anti-CTLA-4 agent, trials involving other agents got a better quality score, especially for efficacy score. This is largely due to the fact that other agents came out later than anti-CTLA-4 agent, when IO clinical trials were relatively mature.
Although our study comprehensively assessed the reporting quality of IO clinical trials, the limitations should also be addressed. First, this study does not compare TRIO statement with the traditional CONSORT statement, which is mainly because that the purpose of this study is to evaluate all trials of IO rather than randomized control trails. Second, the quality score in our study was given equal weight to each item on the TRIO, which may weaken some important items or overemphasize some less-important items. At last, for those recommendations with several subcomponents, we only assign values to items, not to subcomponents. This may make the evaluation criteria broad, but it is friendly and practical for most trials.
In summary, our findings show that IO trials had an unsatisfied reporting quality score assessed by TRIO statement, especially in the reporting of treatment details beyond progression and toxicity onset time and duration. High IF journals have better reporting quality. Studies focused on specific cancer and studies containing anti-PD-1 or anti-PD-L1 agents have higher efficacy quality score. As the first step toward providing an overall landscape of IO trials reporting quality, we are expecting that it may shed light into future improvement of IO trial reporting for the better benefit-risk assessment of immunotherapy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conceptualization: CC, SH, and LZ. Methodology: CC, YZ, and XZ. Software: YW and L-nH. Formal analysis: CC, YZ, and XZ: Data curation: CC, YZ, XZ, and ZL. Writing (original draft preparation: CC, YZ, and XZ. Writing (review and editing): all authors. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely appreciate Hui Yu, Mengjie Lei, and Wei Du (Sun Yat-Sen University Cancer Center) for their help in reviewing the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.736943/full#supplementary-material
1. Emens LA, Ascierto PA, Darcy PK, Demaria S, Eggermont AMM, Redmond WL, et al. Cancer Immunotherapy: Opportunities and Challenges in the Rapidly Evolving Clinical Landscape. Eur J Cancer (2017) 81:116–29. doi: 10.1016/j.ejca.2017.01.035
2. Rosenberg SA, Yang JC, Restifo NP. Cancer Immunotherapy: Moving Beyond Current Vaccines. Nat Med (2004) 10(9):909–15. doi: 10.1038/nm1100
3. Sadreddini S, Baradaran B, Aghebati-Maleki A, Sadreddini S, Shanehbandi D, Fotouhi A, et al. Immune Checkpoint Blockade Opens a New Way to Cancer Immunotherapy. J Cell Physiol (2019) 234(6):8541–9. doi: 10.1002/jcp.27816
4. Hargadon KM, Johnson CE, Williams CJ. Immune Checkpoint Blockade Therapy for Cancer: An Overview of FDA-Approved Immune Checkpoint Inhibitors. Int Immunopharmacol (2018) 62:29–39. doi: 10.1016/j.intimp.2018.06.001
5. Sharon E, Streicher H, Goncalves P, Chen HX. Immune Checkpoint Inhibitors in Clinical Trials. Chin J Cancer (2014) 33(9):434–44. doi: 10.5732/cjc.014.10122
6. Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the Quality of Reporting of Randomized Controlled Trials. The CONSORT Statement. JAMA (1996) 276(8):637–9. doi: 10.1001/jama.276.8.637
7. Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Ann Internal Med (2010) 152(11):726–32. doi: 10.7326/0003-4819-152-11-201006010-00232
8. Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(9):850–8. doi: 10.1200/JCO.2017.75.1644
9. Siu LL, Ivy SP, Dixon EL, Gravell AE, Reeves SA, Rosner GL. Challenges and Opportunities in Adapting Clinical Trial Design for Immunotherapies. Clin Cancer Res an Off J Am Assoc Cancer Res (2017) 23(17):4950–8. doi: 10.1158/1078-0432.CCR-16-3079
10. Tsimberidou AM, Levit LA, Schilsky RL, Averbuch SD, Chen D, Kirkwood JM, et al. Trial Reporting in Immuno-Oncology (TRIO): An American Society of Clinical Oncology-Society for Immunotherapy of Cancer Statement. J Clin Oncol Off J Am Soc Clin Oncol (2019) 37(1):72–80. doi: 10.1200/JCO.18.00145
11. Tsimberidou AM, Levit LA, Schilsky RL, Averbuch SD, Chen D, Kirkwood JM, et al. Trial Reporting in Immuno-Oncology (TRIO): An American Society of Clinical Oncology-Society for Immunotherapy of Cancer Statement. J Immunother Cancer (2018) 6(1):108. doi: 10.1186/s40425-018-0426-7
12. Peron J, Maillet D, Gan HK, Chen EX, You B. Adherence to CONSORT Adverse Event Reporting Guidelines in Randomized Clinical Trials Evaluating Systemic Cancer Therapy: A Systematic Review. J Clin Oncol Off J Am Soc Clin Oncol (2013) 31(31):3957–63. doi: 10.1200/JCO.2013.49.3981
13. Peron J, Pond GR, Gan HK, Chen EX, Almufti R, Maillet D, et al. Quality of Reporting of Modern Randomized Controlled Trials in Medical Oncology: A Systematic Review. J Natl Cancer Institute (2012) 104(13):982–9. doi: 10.1093/jnci/djs259
14. Tardy MP, Gal J, Chamorey E, Almairac F, Vandenbos F, Bondiau PY, et al. Quality of Randomized Controlled Trials Reporting in the Treatment of Adult Patients With High-Grade Gliomas. Oncologist (2018) 23(3):337–45. doi: 10.1634/theoncologist.2017-0196
15. Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol Off J Am Soc Clin Oncol (2015) 33(31):3541–3. doi: 10.1200/JCO.2015.61.6870
16. Reckamp KL. Real-World Pseudoprogression: An Uncommon Phenomenon. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2018) 13(7):880–2. doi: 10.1016/j.jtho.2018.05.011
17. Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, et al. Pooled Analysis Safety Profile of Nivolumab and Ipilimumab Combination Therapy in Patients With Advanced Melanoma. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(34):3815–22. doi: 10.1200/JCO.2016.72.1167
18. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(7):785–92. doi: 10.1200/JCO.2015.66.1389
19. Anagnostou V, Yarchoan M, Hansen AR, Wang H, Verde F, Sharon E, et al. Immuno-Oncology Trial Endpoints: Capturing Clinically Meaningful Activity. Clin Cancer Res an Off J Am Assoc Cancer Res (2017) 23(17):4959–69. doi: 10.1158/1078-0432.CCR-16-3065
20. Baik CS, Rubin EH, Forde PM, Mehnert JM, Collyar D, Butler MO, et al. Immuno-Oncology Clinical Trial Design: Limitations, Challenges, and Opportunities. Clin Cancer Res an Off J Am Assoc Cancer Res (2017) 23(17):4992–5002. doi: 10.1158/1078-0432.CCR-16-3066
21. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in Previously Untreated Melanoma Without BRAF Mutation. N Engl J Med (2015) 372(4):320–30. doi: 10.1056/NEJMoa1412082
Keywords: reporting quality, clinical trials, immune checkpoint inhibitor, immune therapy, cancer, evaluating
Citation: Chen C, Zhou Y, Zhang X, Wang Y, He L-n, Lin Z, Chen T, Jiang Y, Hong S and Zhang L (2021) Unsatisfied Reporting Quality of Clinical Trials Evaluating Immune Checkpoint Inhibitor Therapy in Cancer. Front. Immunol. 12:736943. doi: 10.3389/fimmu.2021.736943
Received: 06 July 2021; Accepted: 16 September 2021;
Published: 05 October 2021.
Edited by:
Carlo Gabriele Tocchetti, University of Naples Federico II, ItalyReviewed by:
Maria Rosaria Galdiero, University of Naples Federico II, ItalyCopyright © 2021 Chen, Zhou, Zhang, Wang, He, Lin, Chen, Jiang, Hong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaodong Hong, aG9uZ3NoZEBzeXN1Y2Mub3JnLmNu; Li Zhang, emhhbmdsaTZAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.