
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 05 October 2021
Sec. B Cell Biology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.733566
This article is part of the Research TopicThe Role of B and T Lymphocytes in Health, Aging and FrailtyView all 5 articles
Aging is considered to be an important risk factor for several inflammatory diseases. B cells play a major role in chronic inflammatory diseases by antibody secretion, antigen presentation and T cell regulation. Different B cell subsets have been implicated in infections and multiple autoimmune diseases. Since aging decreases B cell numbers, affects B cell subsets and impairs antibody responses, the aged B cell is expected to have major impacts on the development and progression of these diseases. In this review, we summarize the role of B cells in health and disease settings, such as atherosclerotic disease. Furthermore, we provide an overview of age-related changes in B cell development and function with respect to their impact in chronic inflammatory diseases.
Aging has a major impact on the composition and function of the immune system, thereby drastically increasing the risk for inflammatory diseases (1). Not surprisingly, the ongoing demographic shift towards an older population results in an increased incidence of infections, autoimmune diseases and fatal cardiovascular events (2), underlining the importance to enhance our understanding of the age-associated changes in the immune system, which are termed immunosenescence.
During aging, hematopoietic stem cells (HSCs) in the bone marrow show reduced self-renewal and differentiate preferentially towards the myeloid cell subsets (3). As a result, the production of neutrophils and monocytes is increased, whereas the generation of B and T lymphocytes is drastically declined. In addition to alterations in the number of immune cells, aging hallmarks, including genomic instability, telomere shortening, epigenetic dysregulation and cellular senescence, contribute to the malfunctioning of the innate and adaptive immune system (4). Several studies showed that the innate immune response in aged mice is prolonged due to the decreased phagocytic ability by neutrophils and macrophages (5, 6). Moreover, aged dendritic cells (DCs) showed an increased secretion of the pro-inflammatory cytokines IL-6 and TNF-α (7). Aging is also associated with crucial changes in the adaptive immune response. Aged T cells displayed reduced proliferative capacity and an increased production of pro-inflammatory cytokines (8, 9), thereby contributing to a chronic state of low-grade inflammation, called ‘inflammaging’. The age-related defects in CD4+ T cell helper function also impaired B cell responses (10). Moreover, aging is associated with intrinsic B cell defects, such as reduced antibody production and decreased affinity maturation in antibody responses resulting in an increased risk for infections (11). In light of the current pandemic, this also contributes to the high infection rate and poor prognosis of COVID-19 in the aged population (12). Dysfunctional B cell responses in the elderly, including increased autoantibody production, are also associated with an increased risk for autoimmune diseases and other chronic inflammatory diseases, such as atherosclerosis (13). Taken together, B cells play a major role in these diseases via antibody secretion, antigen presentation and T cell regulation. This review aims to provide an overview of the effects of aging on the functions of B cells in health and disease settings.
B cells are antigen-presenting cells (APCs) that are generated from multipotent HSCs (14–16). In the bone marrow, HSCs differentiate into B lymphocyte progenitors, which further differentiate into progenitor B cells (pro-B cells), precursor B cells (pre-B cells) and immature B cells (17). These developmental stages can be distinguished by the expression of different markers on the cell surface (Table 1). During this process, each B cell clone develops a unique B cell receptor (BCR) with a specific epitope-binding site via sequential immunoglobulin gene recombination of variable, diversity and joining genes (32). The generated heavy and light chain polypeptides, which consist of constant and variable regions, form the mature BCR (33). Together with B cell-specific membrane proteins, including CD19, BCRs form signaling complexes that activate the NF-κB, PI3K and MAPK pathways (34). These pathways, in turn, stimulate cell survival and induce the migration of transitional immature B cells to the spleen for their final stages of maturation (35). Subsequently, mature B cells migrate to the peritoneal cavity or lymphoid follicles of secondary lymphoid organs, where they can encounter foreign antigens. Upon binding of an antigen to the BCR, in combination with innate and costimulatory signals, B cells can function as APCs and differentiate into antibody-secreting plasma cells (36, 37). Under infectious conditions, antigen-specific B cells present peptides via major histocompatibility complex (MHC) II to naive CD4+ T cells, resulting in CD4+ T cell activation and follicular helper T cell (TFH) differentiation (38). Moreover, multiple studies investigating the APC function of B cells showed that B cell-derived cytokines contribute to T cell profile skewing, such as T helper (TH) 1 or TH2 (39, 40). Most B cells, however, are activated via T cell-independent (TI) or T cell-dependent (TD) mechanisms (41). During T cell-independent (TI) B cell activation, antigens with repetitive epitopes, such as polysaccharides, bind to the BCR, resulting in BCR crosslinking (42). Together with costimulation from toll-like receptors (TLRs), this leads to the development of short-lived plasma cells. In contrast, T cell-dependent B cell activation results in long-lived plasma cell differentiation and memory B cell formation (43). This type of activation requires antigen presentation to and costimulation from TH2 or TFH cells. In turn, B cell-derived plasma cells secrete immunoglobulins with heavy and light chains similar to the BCR in order to mark or neutralize the foreign antigen (44). Depending on the type of activated B cell, these antibodies can be of the IgA, IgD, IgE, IgG or IgM isotype (45).
In the early stages of B cell development, two distinct lineages arise from different precursor cells (46). The B-1 lineage, which was originally studied in mice where it develops into the B-1a and B-1b subsets, is predominant in neonates and is characterized by the expression of CD43 (18, 26). Although the exact mechanisms of B-1 development are not fully understood, the absence of nucleotides at the junctions of V and J segments in their BCR (47), which are inserted in other B cell subsets by the postnatal enzyme terminal deoxynucleotidyl transferase (48), suggests that B-1 cells are originally derived from fetal liver progenitor cells. Some studies indicated that B-1 progenitors develop prior to the existence of HSCs from pre-HSCs or fetal multipotent progenitor cells (49, 50). However, it has also been shown that fetal HSCs are responsible for B-1 development and that fetal lymphopoiesis can be restored in adult HSCs with Lin28b overexpression (51), suggesting that B-1 cells are generated in different waves. B-1 cells mainly reside in the peritoneal cavity where they can elicit antibody-producing and antigen-presenting functions (52). B-1a cells, that in contrast to B-1b cells also express the T cell surface glycoprotein CD5 (19), are responsible for most naturally occurring IgA and IgM antibodies, which are produced in the absence of antigen stimulation (53). Upon infection, B-1a cells can also respond to TI antigens and produce pathogen-specific antibodies (54). B-1b cells, however, have a broader antigen repertoire which allows them to produce bacteria-specific IgM in response to TI antigens and generate TI memory (55). In addition to antibody production, B-1 cells can activate CD4+ T cells through antigen presentation (56). Contradictive results are shown regarding the predominance of B cells in CD4+ T cell activation, and thus the exact role of B cells in antigen presentation is not fully understood (38, 57, 58). It has been previously reported that B-1a cells present antigens to both peritoneal and peripheral CD4+ T cells (59). In the peritoneal cavity, B-1a cells stimulate the activation of IL-10-, IL-4- and IFN-γ- producing T cells, whereas splenic B-1a cells induce TH17 differentiation (60). Splenic and serosal B-1a cells can also transform into granulocyte macrophage-colony stimulating factor- (GM-CSF) producing innate response activator (IRA) B cells (61). In addition to GM-CSF, which stimulates extramedullary hematopoiesis and DC activation during chronic inflammation (62), IRA B cells secrete IgM and IL-3, which in turn promotes monocyte and neutrophil production (63).
B-2 cells comprise most of the adult B cell population in peripheral tissues. In contrast to the B-1 subset, mature B-2 development and survival is dependent on the B cell activating factor (BAFF) – BAFF receptor (BAFFR) signaling pathway (64). Expression of the pro-survival receptor BAFFR starts when transitional B-2 cells undergo their final maturation stages in the spleen (65). BAFF is expressed by various cell subsets as monocytes, macrophages, DCs, stromal cells and T cells (66). Disruption of BAFF-BAFFR signaling results in dramatically decreased B-2 cell numbers (67).
B-2 cells can be further subdivided in marginal zone (MZ) and follicular (FO) B cells (68). MZ B cells are formed upon Notch-BRP-J signaling and are distinguished by very low CD23 and high CD21 expression (20, 21, 27). Upon recognition of a pathogen, short-lived MZ-derived plasma cells secrete high volumes of IgM. In addition, MZ B cells have the capacity to phagocytose the invading pathogen and present antigens to naive CD4+ T cells (69). Antigen presentation by MZ B cells induces TH1 effector differentiation and might thus be important for the generation and reactivation of memory CD4+ T cells (70). After TD activation, FO B cells can ultimately differentiate into plasma cells, which are long-lived and produce high amounts of antibodies (71). However, the majority of FO B cells interact with antigen-specific TFH cells to become GC B cells (24), which express high levels of the DNA-mutating enzyme activation-induced cytidine deaminase (AID) (30). Under the influence of CD40 ligand-mediated signals and cytokines, secreted by TFH cells, AID becomes activated and induces class switch recombination (CSR) (72). During CSR, the constant region of the BCR is hypermutated and as a result, the antibody production of the GC B cell is switched from IgM to IgG, IgA or IgE isotypes (73).
There is evidence that some B cell subpopulations have the capacity to differentiate into regulatory B cells (BREGS) under pro- and anti-inflammatory environmental conditions (74). Although the exact source of BREGS remains elusive, the similarity of cell surface markers between BREGS and various B cell subsets indicates a role for BCR signaling rather than the existence of a specific BREG precursor (75). The development of BREGS is antigen-specific and can be induced via innate and adaptive mechanisms (76). In the adaptive pathway, BREGS present antigens to CD4+ T cells and become activated through CD40-CD40L and IL-21 signals (77), whereas in the innate pathway, TLR2 and TLR9 signaling and IL-1β play important roles (78). In addition, BREGS can be induced by the anti-inflammatory cytokine IL-35 (79). BREGS can have different phenotypes (Table 2) and provide tolerance via various IL-10-dependent and IL-10-independent mechanisms (85) including cell-cell contact and the secretion of IL-35 and TGF-β (86–88). The IL-10-producing B10 subset expresses CD1d in mice and CD24 in humans (25, 31). In both species, B10 cells suppress the APC function of DCs and inhibit TH1 and TH17 differentiation. A BREG subset that inhibits effector T cells and promotes TREG differentiation through PD-L1 interaction was also identified in both mice and humans (81). Murine TIM-1+ BREGS also secrete IL-10 and mainly promote TH2 and TREG generation (80). Moreover, Kaku et al. showed that some murine BREGS mediate their immunosuppression via CD73 expression and adenosine production (82). Interestingly, in humans, a CD73low B cell subset was marked as CD4+ T cell-suppressing BREG (84). Transitional B cells, B-1a and MZ B cells can also suppress CD4+ and CD8+ T cell activation and are therefore sometimes classified as BREGS. In addition, Lundy et al. discovered that some B-1a cells exert their tolerogenic effects via the expression of the apoptotic surface molecule FasL (83). Further studies are required to elucidate if distinct BREG subpopulations are indeed derived from specialized conventional B cell subsets or whether BREGS derive from specific progenitor cells and differentiate into subsets after encounter with an antigen.
Recent studies revealed that the production of precursor B cells in the bone marrow is significantly decreased in aged mice and humans (89–92). This reduction could be attributed to age-related changes in the microenvironment of the bone marrow, including diminished levels of the pro-B cell-survival cytokine IL-7 and an enlarged bias of HSCs to produce myeloid cells instead of lymphocytes (93, 94). Despite the comparable reduction in precursor B cells in both mice and humans, conflicting results regarding peripheral B cells have been shown. In mice, the number of mature splenic B cells is maintained due to an increase in autoreactive and age-associated B cells (ABCs) (95). Although the ABC subset is also detected in humans (96), the absolute number of B cells in the periphery declines with age in humans (97). In the study of Frasca et al., the number of peripheral naive B cells was unaffected, whereas the absolute number of switch memory B cells declined upon aging. Noteworthy, the characterization of memory B cells in mice and humans is not identical. According to Reynaud et al., recirculating MZ B cells in humans are defined as long-lived IgM memory B cells in mice (98). Additionally, Benitez et al. illustrated that the dynamics of peripheral B cell production differs between humans and mice (99). In particular, the splenic FO B cell compartment was larger in humans compared to mice, which might explain the difference in observed effects of aging on B cell numbers. Nevertheless, the quality of aged peripheral B cells is reduced in both mice and humans. Frasca et al. discovered that aging downregulates CSR in human and murine peripheral B cells (97, 100), which might explain why aged murine plasma cells mainly secrete IgM, whereas young murine plasma cells mainly secrete IgG (101). Antibodies generated in aged mice also showed decreased affinity and thus provided less protection (102). Although these studies mainly investigated IgG responses, aging affects all B cell subsets (Table 3).
As mentioned above, most B-1 cells are derived from fetal or neonatal precursors and are maintained via self-replenishment during the adult life (127). According to Alter-Wolf et al., the number of B-1 progenitors in mice is unaffected upon aging and Hinkley et al. even observed an increase in murine B-1 cells due to clonal expansion (103, 104). In contrast, studies in young and aged humans showed that the percentage of B-1 cells is decreased in the elderly (105, 128), which could possibly be explained by the low capacity of the adult human bone marrow to generate B-1 cells compared to adult murine bone marrow. While the antibody-secreting capacity remained unaffected in humans, the number of spontaneously IgM-secreting B-1 cells was reduced most likely via downregulation of the transcription factors XBP-1 and Blimp-1, both involved in the proliferation and differentiation of plasma cells (129, 130), and upregulation of the plasma cell-inhibiting transcription factor PAX-5 (131). In addition to the decrease in IgM-secreting B-1 cells, the diversity of IgM antibodies was reduced (103). In line with these findings, Adler et al. illustrated that aging limited bacterial protection by IgM secreted by B-1 cells (106). This could be the effect of N-region nucleotide additions, which are found on immunoglobulins produced by aged B-1 cells (132). Together these results suggest that B-1 cell functions in humans are impaired upon aging. Although the number of B-1 cells was not decreased in aged mice, recent research showed that aging affected B-1a cells (107). Under the influence of aged myeloid cells, B-1a cells in aged mice and humans were converted into pro-inflammatory 4-1BBL expressing cells. These 4-1BBL+ B cells were shown to activate anti-tumor granzyme B-secreting CD8+ T cells via the MHC/TCR and 4-1BBL/4-1BB axes (108). This finding implies that aging also affects murine B-1 cell functionality. However, Lee-Chang et al. only studied the effects of aging on neonatal B-1a cells. Since generation of the B-1b subset seems to be more predominant in aged mice (104), B-1b cells should be further investigated in an aged setting.
Since Hu et al. reported in 1993 that aging reduced the B-2 cell antibody response to T cell-dependent antigens in mice, the effects of aging on the B-2 subset are under broad investigation (109). Initially, the loss of IgG and high affinity antibodies was thought to be induced by dysfunctional aged DCs and T cells (10, 133). DCs and T cells of aged mice were shown to be defective in the expression of B cell-activation markers, such as CD40L, resulting in diminished numbers of GC B cells and impaired isotype switching. Moreover, the number of regulatory T cells (TREGS), which can directly suppress B cell functions, is increased in aged mice (134, 135). Although contradicting results regarding the age-related effects on the immunosuppressive activity of TREGS has been shown, Sage et al. reported that the frequency and function of follicular TREGS was increased in aged mice (136), resulting in a defective antibody response. However, an adoptive transfer with young T cells and aged B cells to immunodeficient mice also resulted in low levels of high affinity antibodies, implying intrinsic B cell defects (110). Consistent with this finding, Frasca et al. concluded that isotype switching was impaired in aged B-2 cells in both mice and humans due to a reduction in E47 mRNA stability and AID transcription (111). In addition to the reduced antibody affinity, the number of progenitor cells is decreased in aged murine bone marrow (112). Despite the maintenance of peripheral B-2 cell numbers in mice, the amount and percentage of the FO B cell subset is slightly declined upon aging (113). Together with the disrupted follicle organization of aged T cells (114), this might explain the reduction in GC numbers (115). The reduction in the FO B cell subset might also be responsible for the decline in naive and memory B-2 cell numbers in aged humans (105, 116). Apart from the FO lineage, aging also affects the MZ B cell subset. Although the exact effects of aging on MZ B cells remain unclear, recent studies reported an age-associated increase in autoreactive antibodies (137). Since previous research illustrated that autoreactive MZ B cells might elude negative selection in the presence of high levels of the pro-survival factor BAFF (138), which is increased upon aging (139), Miller et al. speculated that the increase in autoreactive antibodies is caused by BAFF hypersensitivity of aged MZ B cells (113). However, Miller et al. did not investigate MZ B cell numbers in aged mice. In contrast to the hypothesis by Miller et al., both Cortegano and Birjandi et al. observed a reduction of MZ B cells in aged mice (117, 118). The age-related disruption of the marginal zone due to a decline in MZ macrophages may partially account for the reduction in MZ B cells. Besides decreased MZ B cell numbers, Turner et al. showed that antigen capture and antibody production of aged MZ B cells was defective, resulting in a diminished T cell-independent immune response (119). Altogether, these studies suggest that aging impairs B-2 cell functions in both humans and mice.
The maintenance of B-2 cell numbers in aged mice could possibly be explained by the accumulation of age-associated B cells. In 24 to 30 months-old C57BL/6 mice, half of all splenic B cells are considered to be ABCs, whereas the frequency of this population is extremely low in young mice (120). These ABCs are observed in both mice and humans and could be distinguished from other B cell subsets by their expression markers, including CD11b, CD11c and T-bet, and innate activation stimuli, such as toll-like receptor TLR7 signals (22). Although the exact cell surface markers are still under debate, ABCs do not express CD43 and CD5 that are found on B-1 cells. The ABC subset is also different from MZ and FO B cells, since ABCs do not depend on BAFF signals (121). HoweverHao et al. did show that FO B cells can transform into ABCs in both young and aged mice, suggesting that ABCs are derived from B-2 cells (121). This might also explain the reduction in the FO B cell pool upon aging (113). When Knode et al. repeated this experiment in aged mice, they concluded that ABCs arise from GC B cells following T cell-dependent antigen activation (140), thereby indicating ABCs as memory subset. In contrast, recent studies have shown that ABCs mainly arise in an antigen-limited environment and proposed that homeostatic expansion stimulates ABC formation to balance B cell progenitor loss (141–143). However, ABCs were reported to inhibit B cell generation via the production of TNF, and their depletion resulted in reactivated B lymphopoiesis (122, 144), suggesting that the loss of B cell precursors is caused rather than balanced by ABCs. Nonetheless, different subsets of ABCs have been described, indicating that ABCs can be generated via different developmental routes dependent on environmental factors, such as age and antigen load. One of these subsets, comprising almost two-thirds of all ABCs, is characterized by the expression of the TH1 transcription factor T-bet (23, 28). In addition, a T-bet negative subset, which is characterized by the expression of CXCR5, has been reported (29). The expansion of the T-bet+ subset is dependent on innate stimuli, such as TLR7 and TLR9 ligands, in an IL-21 and IFN-γ rich environment. Previous research demonstrated that the transient expression of T-bet by B cells stimulated IgG2a antibody isotype switching and a correlation between ABCs and protective IgG2a was also observed in virally infected mice (145, 146). In humans, an increase in ABCs was associated with elevated levels of IgG1 (147). Notably, Colonna-Romano et al. discovered the expansion of an ABC-like CD27- IgD- IgG+ memory B cell subset in the periphery of aged individuals, clarifying the high numbers of IgG observed in the elderly (123). Although T-bet expression was not shown for the latter double negative memory subset, Wang et al. proposed that loss of T-bet is probably necessary for the conversion of ABCs into plasma cells (148), suggesting that the double negative memory B cell might be an intermediate state from ABC to plasma cell. However, if and how plasma cells can be derived from ABCs remains poorly understood. Nonetheless, ABCs are speculated to secrete autoreactive antibodies, explaining their abundance in autoimmune diseases (149). Aside from immunoglobulin production, the antigen presenting function of ABCs is intensively investigated. Whereas Colonna-Romano et al. reported that the double negative exhausted memory B cell is incapable of antigen presentation, recent findings conclude that ABCs are efficient APCs that skew TH17 differentiation, thereby possibly explaining the increase in TH17 differentiation observed in aged mice and humans (124, 150, 151). Upon activation, TH17 cells secrete the pro-inflammatory cytokine IL-17, which plays important roles in both protective immunity and autoimmunity by the induction of neutrophil migration and activation (152), thereby contributing to inflammaging. Moreover, ABCs promote inflammaging by the production of IFN-γ and TNF-α (144). Strikingly, ABCs were also observed to secrete high levels of the anti-inflammatory cytokine IL-10, suggesting an immunoregulatory function. Ratliff et al., however, stated that this IL-10 was produced by an ABC-inhibiting regulatory FO B cell subset (120). Although many studies confirm the presence of an ABC subset in both mice and humans, the expression markers defined in these studies differ considerably. Some studies include CD11c and T-bet, whereas others focus on CD21 and CD27 or the production of IgM and IgG. These differences indicate heterogeneity in the ABC subset, suggesting that further research should examine age-related effects on distinct ABC subsets.
In the white visceral adipose tissue (VAT) of aged female obese mice, Frasca et al. discovered the accumulation of an exhausted memory B cell subset similar to ABCs (125). In addition, Frasca et al. highlighted that FO B cells in a fatty environment rapidly transform into this pro-adipogenic B cell subset, resulting in a disrupted lipid metabolism. Comparable to splenic ABCs, this subset, defined as adipose-resident age-related B cells (AABs), expressed pro-inflammatory markers and was found to be more abundant in aged female than in aged male mice (126). Since the sex-related differences in ABC content are thought to be caused by TLR7, which is located on the X-chromosome and has been shown to escape X-chromosome silencing (153), the activation mechanisms of AABs are thought to be similar to ABCs. Although the direct effects of TLR7 signaling on AAB accumulation are not investigated, Camell et al. concluded that AAB expansion is dependent on the NLRP3 inflammasome (126), which can be activated by TLR7 signaling (154). Interestingly, upon NLRP3 deficiency, Camell et al. observed a reduction in both VAT AABs as splenic ABCs, confirming comparable activation routes for these subsets. In contrast, AABs do not express T-bet and induce TH1 differentiation rather than TH17 polarization (155, 156), indicating that AABs are functionally different from ABCs. Altogether, these data illustrate that aging induces the expansion of ABCs in the spleen and the accumulation of AABs in white VAT, resulting in impaired antibody responses and disrupted lipolysis.
As observed by Ratliff et al., aging reduced the number of an IL-10-secreting regulatory FO B cell subset (120) and similar to these findings a reduction in IL-10 levels upon aging was reported recently (157). However, IL-10 is not exclusively produced by BREGS and the effects of aging on BREGS are thus far not examined. The general decrease in B cell precursors and B-1 and B-2 subsets implies a subsequent reduction in BREGS. Nevertheless, the immunosuppressive functions of TREGS were observed to be increased (158), whereas naive T cell numbers decreased upon aging (159). Since the differentiation of naive T cells into TREGS can be stimulated by BREGS (160), the immunosuppressive function of BREGS is possibly also augmented upon aging. Contributing to this hypothesis, Dang et al. showed that PD-L1 expression on B lymphocytes increased with aging in healthy donors (161). However, these PD-L1-expressing B cells were identified by CD19 only and not specifically defined as BREGS. In contrast, increased autoimmunity in the elderly might suggest an age-related loss of tolerogenic BREGS (162). Since BREGS play an important role in regulating inflammaging and balancing the pro-inflammatory responses of ABCs, it is necessary to investigate the consequences of aging on the number and functions of the BREG subset.
The age-associated changes in the immune system heavily increase the risk for bacterial and viral infections in the elderly (Figure 1) (163). In combination with the reduced responses to vaccinations in the elderly, this increase results in high hospitalization and mortality rates due to infectious diseases, such as COVID-19, pneumonia and influenza (164, 165). Although aging of the T cell repertoire and the associated TH1/TH2 imbalance, with a reduced TFH and TH2 output in the elderly (136, 166), contributes to the high frequency of influenza infection (167), intrinsic defects in aged B cells substantially impair the influenza-specific response. Firstly, the decreased ability in CSR and somatic hypermutation of aged B cells results in lower antibody titers against pathogens (168, 169). The generated antibodies in the elderly are also less protective due to their lower affinity and neutralization capacity (170). Furthermore, a recent study investigating the effectiveness of influenza vaccination at different ages observed an age-dependent increase or decrease in DNA methylation at specific CpG sites (171). These age-related epigenetic alterations could often be linked to the low responsiveness of the subject to influenza vaccination, indicating that epigenetic remodeling upon aging negatively impacts the humoral response (172). In addition, the percentage of ABCs in aged individuals negatively correlates with the responsiveness to the influenza vaccine (173). Frasca et al. reported that ABCs secrete pro-inflammatory cytokines, but do not produce antibodies against influenza antigens in aged individuals (174). However, several studies showed that influenza-specific ABCs differentiate into protective antibody-producing cells in both mice and humans (141, 175). Although the exact role of ABCs in infectious diseases is still under debate, elevated percentages of CD21- CD11c+ ABCs were also detected in severe COVID-19 cases compared to mild cases (176) and high levels of pro-inflammatory cytokines were negatively associated with the risk for pneumonia (177).
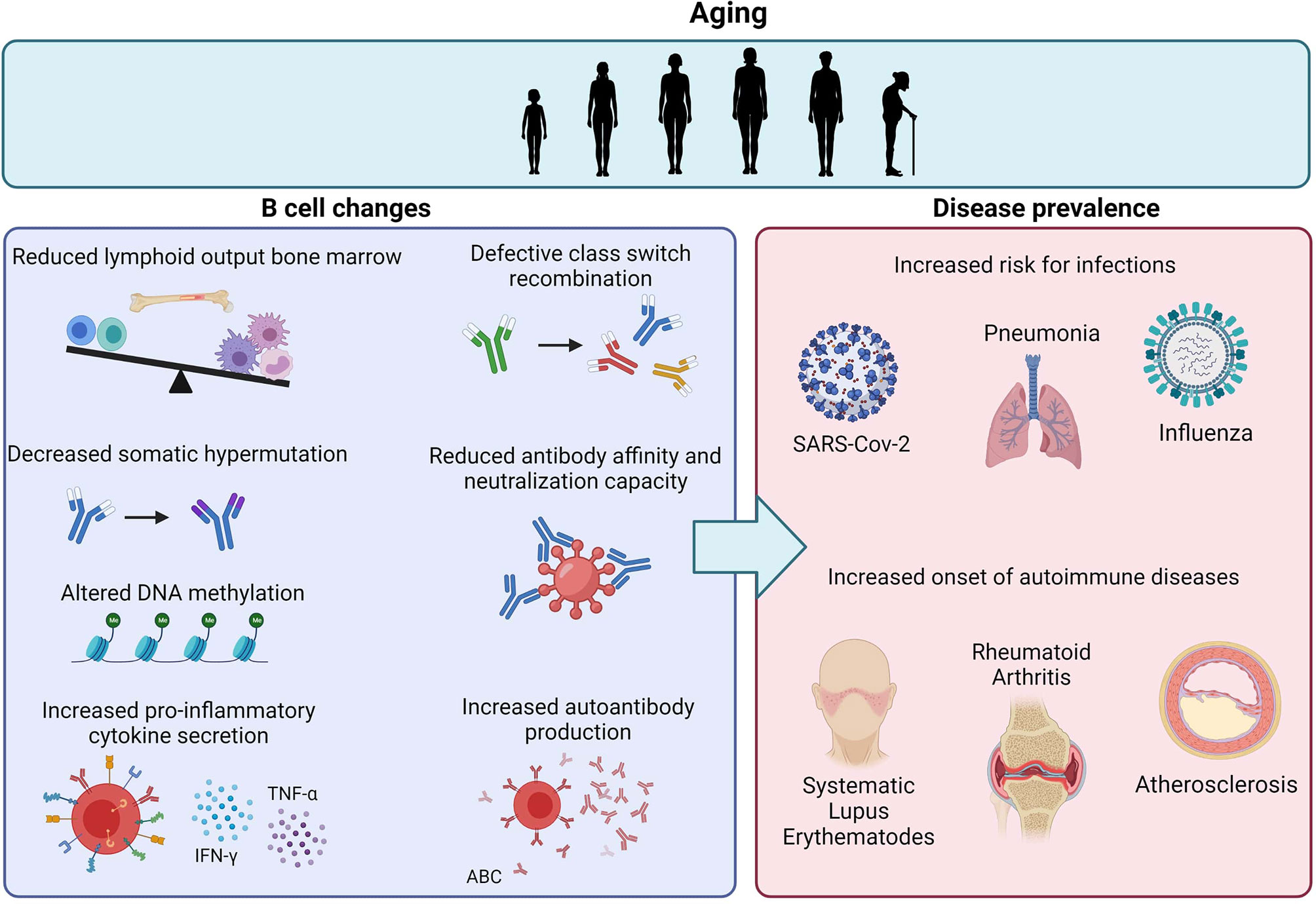
Figure 1 Age-related effects on B cells and the impact on disease prevalence. Aging is associated with reduced lymphoid output in the bone marrow and intrinsic defects in B cells, including decreased CSR, SHM and antibody affinity. The increase in ABCs also results in increased pro-inflammatory cytokine secretion and autoantibody reduction. Together, these age-related B cell changes contribute to the increased risk for infections and autoimmune diseases upon aging.
As described above in the section regarding ABCs, aging of the B cell repertoire contributes to autoimmunity (Figure 1). Autoreactive B cells are concluded to be responsible for the production of autoantibodies, the presentation of autoantigens and the secretion of pro-inflammatory cytokines (178). Upon aging, the level of self-reactive antibodies is elevated, resulting in the onset and development of autoimmune diseases (179). Since ABCs were observed to secrete autoantibodies, recent studies examined the association of this B cell subset with autoimmunity. In several autoimmune diseases, such as common variable immunodeficiency (CVID) (180), rheumatoid arthritis (RA) (181) and systemic lupus erythematosus (SLE) (148), the number of ABCs was significantly higher in both patients and autoimmune prone mice compared to healthy controls. Remarkably, Rubtsov et al. observed that the frequency of the ABC subset was significantly higher in aged female RA patients compared to young female patients and male patients of any age (28), suggesting that the previously described sex differences in ABCs contribute to the higher prevalence of autoimmune diseases in women relative to men (182). Due to the young age of investigated male and female SLE patients, peripheral ABCs in SLE were not detected by Rubtsov et al. Nevertheless, recent studies discovered the presence of ABCs, induced by TLR7 signaling, in lupus mice and patients (29, 183). Duffy et al. already reviewed that upregulated TLR7 signaling facilitated autoimmune responses (184), however, the exact mechanisms leading to TLR activation in autoimmune diseases remain unclear. Santiago-Raber et al. speculated that autoantibodies promote the uptake of self-RNA by DCs, resulting in the stimulation of TLR7 (185). Moreover, antibodies against self-DNA can give rise to the formation of immune complexes, which can subsequently activate TLR9, leading to pro-inflammatory immune responses (186). However, Nickerson et al. demonstrated that TLR9 can inhibit TLR7 and ABC development (187), indicating a regulatory role for the receptor. Nonetheless, the tolerance mechanism induced by TLR9 fails in ABCs due to the pro-inflammatory signals CD40L, IFN-γ and IL-21, resulting in autoinflammatory B cells (188).
As B cells play an important role in the lipid-driven, chronic inflammatory disease atherosclerosis (189), B cell aging is expected to also impact atherosclerosis development. Atherosclerosis, the main underlying cause of fatal cardiovascular events, leads to the formation of plaque in medium- to large-sized arteries (190). Rupture or erosion of an atherosclerotic plaque causes myocardial infarction or stroke. A hallmark step for the initiation of atherosclerosis is the retention and subsequent oxidation of circulating low-density lipoproteins (LDLs) in the subendothelial space of the arterial vessel wall. Oxidation of LDL (oxLDL) results in the formation of oxidation-specific epitopes (OSEs) (191, 192), which are recognized by the immune system and thus trigger a series of inflammatory responses starting with the recruitment of monocytes (193). In the intima, these monocytes differentiate into macrophages that phagocytose oxLDL. As a result of oxLDL uptake, macrophages convert into foam cells, which further attract immune cells to the lesion. Although these immune cells mainly include monocytes and T cells, B cells are also recruited to the vessel wall of the atherosclerotic plaque (194). B cells affect plaque formation by regulating T cell responses and producing antibodies, such as antibodies against OSEs (189). Palinski et al. reported that these anti-OSE antibodies are able to block the uptake of oxidized LDL, thereby suggesting an atheroprotective role for B cells (195). In addition, Caligiuri et al. observed a decrease in atherosclerotic lesion size upon transfer of splenic B cells to apolipoprotein E (ApoE) deficient mice (196). Although these results indicate that B cells are anti-atherogenic, several studies showed reduced atherosclerosis after B cell depletion (197, 198). These contradictory results can be attributed to differential effects exerted by different B cell subsets during atherosclerosis progression (199, 200).
B-1 cells confer an atheroprotective function by their anti-oxLDL IgM production (201). In line with these findings, Gruber et al. reported decreased atherosclerotic lesion size as a result of B cell-specific depletion of sialic acid-binding immunoglobulin-like lectin G (SIGLEC-G), which resulted in increased numbers of B-1 cells and elevated OSE-specific IgM plasma levels (202). Although B-1a and B-1b cells show similar anti-atherogenic effects (203, 204), B-1a cells can give rise to pro-atherogenic IRA B cells (61). IRA B cells secrete GM-CSF, which promotes TH1 skewing, thereby aggravating atherosclerotic lesion formation (205). Moreover, IRA B cells induce the expansion of infiltrating monocytes and neutrophils and thus promote inflammation (62, 206). In contrast to B-1 cells, Kyaw et al. observed increased atherosclerotic lesions after an adoptive transfer of 5 × 106 B-2 B cells to B lymphocyte-deficient ApoE-/- mice (207). Moreover, B-2 depletion as a result of BAFFR deficiency in ApoE-/- mice reduced atherosclerosis progression (208, 209). Similar to these findings, inhibition of BAFFR with monoclonal antibodies, which similar to BAFFR deficiency leads to increased soluble BAFF levels, decreased atherosclerotic plaque formation (210). Interestingly, it has been shown that an antibody-mediated neutralization of BAFF in atherogenic diet-fed ApoE-/- and LDLr-/- mice promoted atherosclerosis progression (211). Similar to BAFFR inhibition, pro-atherogenic B-2 cell numbers were also decreased upon BAFF neutralization, thereby suggesting an anti-inflammatory role of BAFF independent of BAFFR signaling and B cells in atherosclerosis. As mentioned previously, B-2 cells are divided in FO B cells and MZ B cells. Tay et al., reported that mature B cell-specific Blimp-1 deficiency impaired plasma cell differentiation from FO B cells, resulting in decreased IgG levels and reduced atherosclerosis development in LDLr-/- mice (212). In line with these findings, Douna et al. discovered that specific FO B cell inhibition by BTLA stimulation resulted in significantly reduced atherosclerosis (213). In contrast to FO B cells, MZ B cells exhibit atheroprotective properties by suppressing the proatherogenic TFH response, thereby inhibiting atherosclerosis development (214). Nevertheless, the exact functions of the distinct B-2 subsets in atherosclerosis need to be further investigated. Apart from B-1 and B-2 cells, BREGS have been identified in atherosclerosis (215). BREGS exhibit anti-atherogenic functions via various mechanisms, including secretion of IL-10 and IL-35, inhibition of GC B cells, apoptosis induction of effector T cells, and stimulation of the atheroprotective molecule adenosine (82, 216–218).
Although studies investigating the effects of inflammaging on cardiovascular disease (CVD) are scarce, the increased prevalence of atherosclerosis upon aging suggests an important role for age-related immune alterations in atherosclerosis. Importantly, acute cardiovascular events mostly occur in the elderly and thus treatment of CVD patients occurs in the context of an aged immune system. Recent single-cell RNA sequencing (scRNAseq) studies have shown that B cells are present in atherosclerotic plaques of aged CVD patients (219–221) and an increased understanding of the contributions of age-induced B cell impairment to atherosclerosis progression is therefore crucial for atherosclerosis immunotherapy. Similar to the human atherosclerotic plaque, transcriptomic and single-cell analysis revealed that B cells are present in high-fat diet-induced lesions of young ApoE-/- and LDLr-/- mice (199, 222, 223). Although these studies show that the number of lesional B cells is limited, B cells have also been located in the perivascular adipose tissue surrounding the atherosclerotic aorta (224). In addition, several studies identified the presence of artery tertiary lymphoid organs (ATLOs) in close proximity of the aortic lesions of aged (75-85 weeks) ApoE-/- mice (225, 226). ATLOs are lymphoid aggregates that form in the adventitia which contain high numbers of B cells, including B-1, GC and switched memory B cells (227). However, these ATLOs have only been identified in ApoE-/- mice and single cell RNA sequencing analysis has not been performed on the atherosclerotic lesions of aged ApoE-/- or LDLr-/- mice to profile local B cells. During atherosclerosis progression, apoptotic and necrotic cells accumulate (228, 229) and enhance TLR7 and TLR9 ligands in the microenvironment. Possibly, this could promote the development of age-associated B cell subsets that contribute to atherosclerosis by the production of autoreactive IgG2a antibodies and the secretion of cytokines, such as IFN-γ and IL-17. As aging studies in atherosclerosis are limited, future research focusing on age-induced changes in B cell numbers, subsets and function in atherosclerosis, as indicated in Figure 2, will provide essential fundamental knowledge regarding disease etiology and may lead to novel targets to halt atherosclerosis progression.
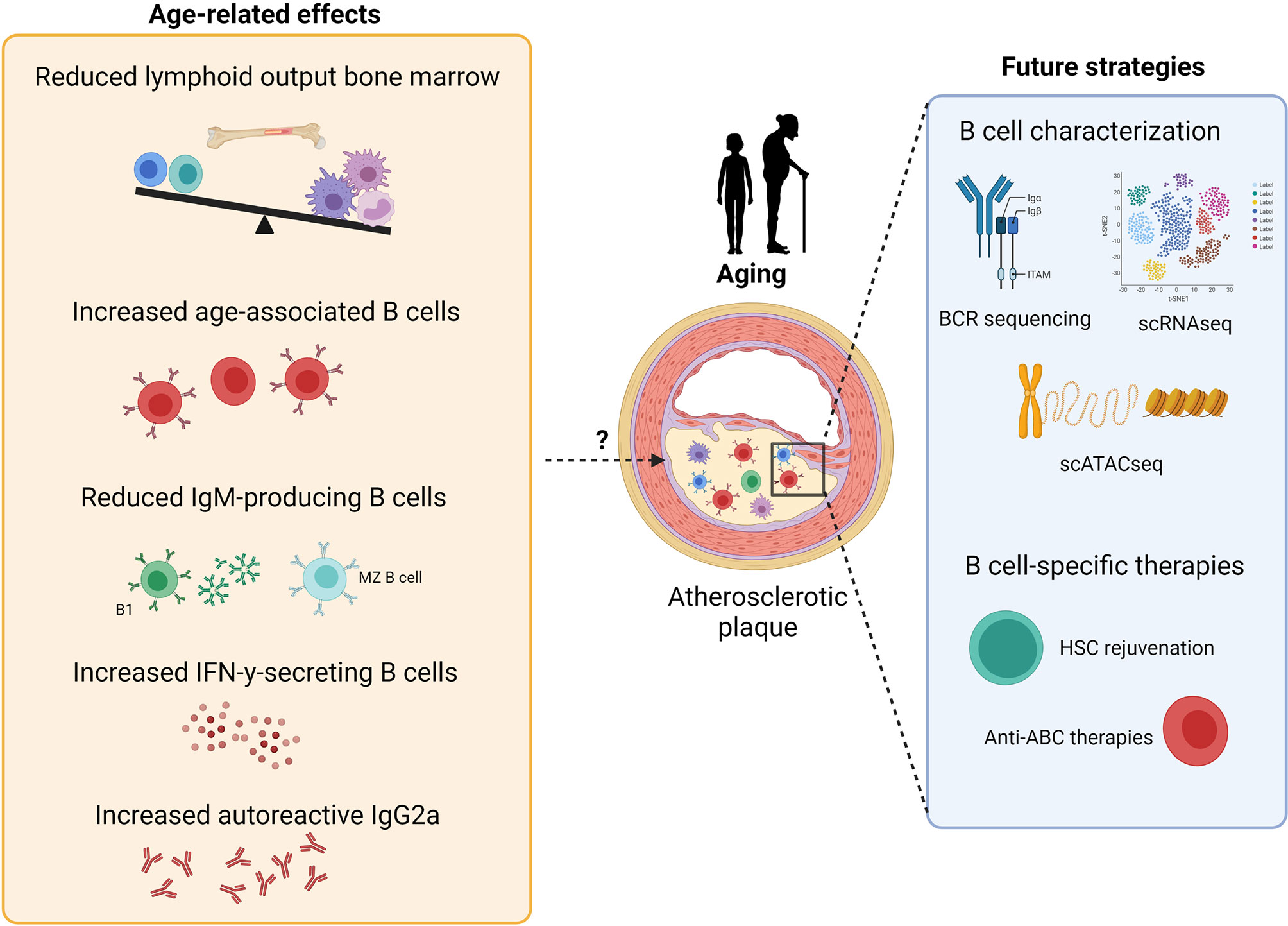
Figure 2 Potential effects of aging on atherosclerosis development and future strategies for B cell-specific treatment against atherosclerosis. B cell aging leads to reduced lymphoid output, reduced numbers of IgM-producing B cells and an increase in age-related B cell subsets, thereby resulting in both pro- and anti-atherogenic effects. Further characterization of B cells in atherosclerosis via single-cell RNA sequencing, BCR sequencing and single-cell assay for transposase-accessible chromatin sequencing should give further insights for the development of B cell-specific therapies against atherosclerosis, such as HSC rejuvenation and anti-ABC treatments.
Due to the demographic shift towards an older population and the age-related increase in chronic inflammatory diseases, it has become a major public health priority to combat age-induced maladaptive immunity. Since aging reduces the lymphoid output of HSCs, HSC rejuvenation therapies, such as FOXOs and Cdc42 activity specific inhibitor (CASIN) (230), might be interesting therapeutic strategies to restore the balance between the myeloid and lymphoid pool. FOXOs are transcription factors which are involved in longevity and aging by regulating cell survival and growth (231). Specific deletion of FOXO1 and FOXO3a in HSCs induced apoptosis (232, 233), suggesting that FOXO overexpression might increase HSC survival. In addition to FOXOs, high Cdc42 activity was associated with HSC aging and treatment of aged HSCs with CASIN increased the common lymphoid progenitor pool, restored B cell numbers and elongated lifespan after transplant (234, 235). A more B cell-specific targeting drug, rituximab, proved to be effective in reducing RA and MS (236–238). Interestingly, Novikova et al. showed that rituximab also reduced atherosclerosis development in RA patients (239), indicating that B cell lymphopoiesis should be inhibited rather than stimulated in order to halt atherosclerosis progression in this setting. Notably, rituximab inhibits all CD20-positive B cells, including anti-inflammatory B-1 cells. Therapeutic strategies specifically targeting pro-inflammatory B cell subsets, such as ABCs and AABs, might therefore be more attractive to combat age-related immune diseases. NLRP3 inflammasome inhibition might be a potential candidate for this approach. Activation of the NLRP3 inflammasome is an important signaling pathway for ABC and AAB activation (126). Deficiency of this pathway in aged mice significantly reduced ABC and AAB numbers compared to wildtype aged mice (126). Notably, NLRP3 expression is not exclusively expressed by ABCs and AABs, underlining the urgency of the characterization of aged B cells in inflammatory diseases to develop specific anti-ABC therapies. Future studies identifying unique (age-associated) B cell subset markers, such as transcription factors, growth and survival receptors and immune checkpoints, are thus crucial to develop strategies to target ABCs or other pro-inflammatory B cell subsets in age-related diseases. Deep sequencing of the BCR could also provide important details for such cell-specific therapies (240). Recent BCR sequencing in young (20-45 years old) and old (60-80 years old) adults showed decreased BCR diversity, increased BCR clonality and different expression of BCR VHJ genes in aged patients (241), indicating that the aged immune cell repertoire might respond differently to pathogens and therapeutic agents. In addition, investigating autoantibodies produced by aged B cells might result in the identification of novel auto-antigens in chronic inflammatory diseases (242). Moreover, epigenetic alterations affecting B cell development, function and responses are observed with aging (243–245) and might contribute to age-associated changes in the B cell repertoire.
With a rapidly rising life expectancy and demographic shift towards elderly, it is essential to enhance our understanding of age-associated immunity that causes disease susceptibility and mortality. In this review, we focused on age-associated alterations in B cell homeostasis in health and disease. Collectively, aging negatively affects the production of B cells in the bone marrow, resulting in decreased numbers of B-1 and B-2 cells. Moreover, antibody affinity and diversity are reduced upon aging, resulting in impaired antibody responses. Furthermore, aging induces the expansion of age-associated and adipose-resident age-related B cells, which contribute to inflammaging by the activation of pro-inflammatory T cell subsets and cytokine release. Although B cells are key drivers of autoimmune diseases, such as atherosclerosis, data on B cell aging in chronic inflammatory diseases is limited. Future studies identifying the aged B cell repertoire, including age-associated alterations in B cell numbers, subsets and antibody responses, are urgently needed in order to develop innovative B cell-specific therapies to combat chronic inflammatory diseases.
JM and ACF drafted the review and JM designed the Figures. JK, DT, and ACF provided critical feedback on the manuscript. All authors contributed to the article and approved the submitted version.
This work is supported by the European Research Area Network (ERA-CVD B-eatATHERO consortium); Dutch Heart Foundation grant number 2019T107 to ACF and Austrian Science Fund (FWF) grant number I4647 to DT.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Figures were made using BioRender.com.
APC, antigen presenting cell; ApoE, apolipoprotein E; ATLO, artery tertiary lymphoid organ; BAFF, B cell activating factor; BAFFR, B cell activating factor receptor; BCR, B cell receptor; BREG, regulatory B cell; BM, bone marrow; CASIN, Cdc42 activity specific inhibitor; CSR, class-switch recombination; CVD, cardiovascular disease; CVID, common variable immunodeficiency; DC, dendritic cell; FO, follicular; GC, germinal center; GM-CSF, granulocyte macrophage-colony stimulating factor; HSC, hematopoietic stem cell; IRA, innate response activator; MHC, major histocompatibility complex; MZ, marginal zone; oxLDL, oxidized low-density lipoprotein; pre-B cells, precursor B cells; pro-B cells, progenitor B cells; RA, rheumatoid arthritis; scATAC seq, single-cell assay for transposase-accessible chromatin sequencing; scRNAseq, single-cell RNA sequencing; SIGLEC-G, sialic acid-binding immunoglobulin-like lectin G; SLE, systemic lupus erythematosus; TD, T cell-dependent; TFH, follicular helper T cell; TH, T helper; TI, T cell-independent; TLR, toll-like receptor; TREG, regulatory T cell; VAT, visceral adipose tissue; APC, antigen presenting cell; BM, bone marrow; TH, T helper.
1. Weyand CM, Goronzy JJ. Aging of the Immune System: Mechanisms and Therapeutic Targets. Ann Am Thorac Soc [Internet] Am Thorac Soc (2016) 13(5):S422–8. doi: 10.1513/AnnalsATS.201602-095AW
2. Wang H, Naghavi M, Allen C, Barber RM, Carter A, Casey DC, et al. Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet (2016) 388(10053):1459–544. doi: 10.1016/S0140-6736(16)31012-1
3. Nishi K, Sakamaki T, Kao KS, Sadaoka K, Fujii M, Takaori-Kondo A, et al. Age-Associated Myeloid Biased Hematopoiesis Depends on Relative Decrease of Short-Term Hematopoietic Stem Cell. Blood (2019) 134(Supplement_1):2481–1. doi: 10.1182/blood-2019-122355
4. Rodrigues LP, Teixeira VR, Alencar-Silva T, Simonassi-Paiva B, Pereira RW, Pogue R, et al. Hallmarks of Aging and Immunosenescence: Connecting the Dots. Cytokine Growth Factor Rev (2021) 59:9–21. doi: 10.1016/j.cytogfr.2021.01.006
5. Wessels I, Jansen J, Rink L, Uciechowski P. Immunosenescence of Polymorphonuclear Neutrophils. The Scientific World Journal (2010) 10:145–60. doi: 10.1100/tsw.2010.14
6. Kohut ML, Senchina DS, Madden KS, Martin AE, Felten DL, Moynihan JA. Age Effects on Macrophage Function Vary by Tissue Site, Nature of Stimulant, and Exercise Behavior. Exp Gerontol (2004) 39(9):1347–60. doi: 10.1016/j.exger.2004.07.001
7. Agrawal A, Agrawal S, Cao J-N, Su H, Osann K, Gupta S. Altered Innate Immune Functioning of Dendritic Cells in Elderly Humans: A Role of Phosphoinositide 3-Kinase-Signaling Pathway. J Immunol (2007) 178(11):6912–22. doi: 10.4049/jimmunol.178.11.6912
8. Wong GCL, Strickland MC, Larbi A. Changes in T Cell Homeostasis and Vaccine Responses in Old Age. Interdiscip Top Gerontol Geriatr (2020) 43:36–55. doi: 10.1159/000504487
9. Mittelbrunn M, Kroemer G. Hallmarks of T Cell Aging. Nat Immunol Nat Res (2021) 22:687–98. doi: 10.1038/s41590-021-00927-z
10. Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-Related Defects in CD4 T Cell Cognate Helper Function Lead to Reductions in Humoral Responses. J Exp Med (2004) 200(12):1613–22. doi: 10.1084/jem.20041395
11. Frasca D, Blomberg BB. Aging Induces B Cell Defects and Decreased Antibody Responses to Influenza Infection and Vaccination. Immun Ageing BioMed Cent Ltd (2020) 17:1–10. doi: 10.1186/s12979-020-00210-z
12. Wong LSY, Loo EXL, Kang AYH, Lau HX, Tambyah PA, Tham EH. Age-Related Differences in Immunological Responses to SARS-CoV-2. J Allergy Clin Immunol Pract (2020) 8(10):3251–8. doi: 10.1016/j.jaip.2020.08.026
13. Ma S, Wang C, Mao X, Hao Y. B Cells Dysfunction Associated With Aging and Autoimmune Disease. Front Immunol Front Media S.A (2019) 10:318. doi: 10.3389/fimmu.2019.00318
14. Montecino-Rodriguez E, Dorshkind K. B-1 B Cell Development in the Fetus and Adult. Immunity (2012) 36:13–21. doi: 10.1016/j.immuni.2011.11.017
15. Hardy RR, Hayakawa K. B Cell Development Pathways (2001). Available at: www.annualreviews.org (Accessed cited 2021 Aug 20).
16. Martin VG, Wu Y-CB, Townsend CL, Lu GHC, O’Hare JS, Mozeika A, et al. Transitional B Cells in Early Human B Cell Development – Time to Revisit the Paradigm? Front Immunol (2016) 0(DEC):546. doi: 10.3389/fimmu.2016.00546
17. Hardy RR, Shinton S. Characterization of B Lymphopoiesis in Mouse Bone Marrow and Spleen. Methods Mol Biol (2004) 271:1–24. doi: 10.1385/1-59259-796-3:001
18. Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b Cells Exhibit Distinct Developmental Requirements and Have Unique Functional Roles in Innate and Adaptive Immunity to S. Pneumoniae. Immunity (2005) 23(1):7–18. doi: 10.1016/j.immuni.2005.04.011
19. Wong JB, Hewitt SL, Heltemes-Harris LM, Mandal M, Johnson K, Rajewsky K, et al. B-1a Cells Acquire Their Unique Characteristics by Bypassing the Pre-BCR Selection Stage. Nat Commun (2019) 10(1):1–15. doi: 10.1038/s41467-019-12824-z
20. Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, et al. Notch-RBP-J Signaling Is Involved in Cell Fate Determination of Marginal Zone B Cells. Nat Immunol (2002) 3(5):443–50. doi: 10.1038/ni793
21. Kanayama N, Cascalho M, Ohmori H. Analysis of Marginal Zone B Cell Development in the Mouse With Limited B Cell Diversity: Role of the Antigen Receptor Signals in the Recruitment of B Cells to the Marginal Zone. J Immunol (2005) 174(3):1438–45. doi: 10.4049/jimmunol.174.3.1438
22. Du SW, Arkatkar T, Al Qureshah F, Jacobs HM, Thouvenel CD, Chiang K, et al. Functional Characterization of CD11c + Age-Associated B Cells as Memory B Cells. J Immunol (2019) 203(11):2817–26. doi: 10.4049/jimmunol.1900404
23. Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, et al. IL4, IL21, and Ifnγ Interact to Govern TBET and CD11c Expression in TLR-Activated B Cells. J Immunol (2016) 197(4):1023. doi: 10.4049/jimmunol.1600522
24. Zhang TT, Gonzalez DG, Cote CM, Kerfoot SM, Deng S, Cheng Y, et al. Germinal Center B Cell Development has Distinctly Regulated Stages Completed by Disengagement From T Cell Help. Elife (2017) 6:19552. doi: 10.7554/eLife.19552
25. Daien CI, Gailhac S, Mura T, Audo R, Combe B, Hahne M, et al. Regulatory B10 Cells Are Decreased in Patients With Rheumatoid Arthritis and Are Inversely Correlated With Disease Activity. Arthritis Rheumatol (2014) 66(8):2037–46. doi: 10.1002/art.38666
26. Rothstein TL, Hopkins X, Guo A, María Hernández W, Li TD, Quách N, et al. Peripheral Blood Including B-1 Cells, in Human Adult Antibody-Secreting Cell Populations, Distinctions Among Circulating. J Immunol (2020) 196(3):1060–9. doi: 10.4049/jimmunol.1501843
27. Zouali M, Richard Y. Marginal Zone B-Cells, a Gatekeeper of Innate Immunity. Front Immunol (2011) 2(DEC). doi: 10.3389/fimmu.2011.00063
28. Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll-Like Receptor 7 (TLR7)-Driven Accumulation of a Novel CD11c+ B-Cell Population is Important for the Development of Autoimmunity. Blood (2011) 118(5):1305–15. doi: 10.1182/blood-2011-01-331462
29. Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, et al. Distinct Effector B Cells Induced by Unregulated Toll-Like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity (2018) 49(4):725–39.e6. doi: 10.1016/j.immuni.2018.08.015
30. Lossos IS, Levy R, Alizadeh AA. AID Is Expressed in Germinal Center B-Cell-Like and Activated B-Cell-Like Diffuse Large-Cell Lymphomas and Is Not Correlated With Intraclonal Heterogeneity. Leukemia (2004) 18(11):1775–9. doi: 10.1038/sj.leu.2403488
31. Hua C, Audo R, Yeremenko N, Baeten D, Hahne M, Combe B, et al. A Proliferation Inducing Ligand (APRIL) Promotes IL-10 Production and Regulatory Functions of Human B Cells. J Autoimmun (2016) 73:64–72. doi: 10.1016/j.jaut.2016.06.002
32. Roth DB. V(D)J Recombination: Mechanism, Errors, and Fidelity. In: Mobile DNA III. Am Soc Microbiol (2015) 2:313–24. doi: 10.1128/9781555819217.ch14
33. Schatz DG, Ji Y. Recombination Centres and the Orchestration of V(D)J Recombination. Nat Rev Immunol (2011) 11:251–63. doi: 10.1038/nri2941
34. Pierce SK, Liu W. The Tipping Points in the Initiation of B Cell Signalling: How Small Changes Make Big Differences. Nat Rev Immunol (2010) 10:767–77. doi: 10.1038/nri2853
35. Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, et al. B Cell Development in the Spleen Takes Place in Discrete Steps and Is Determined by the Quality of B Cell Receptor-Derived Signals. J Exp Med (1999) 190(1):75–89. doi: 10.1084/jem.190.1.75
36. Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma Cell Differentiation Requires the Transcription Factor XBP-1. Nature (2001) 412(6844):300–7. doi: 10.1038/35085509
37. Hua Z, Hou B. The Role of B Cell Antigen Presentation in the Initiation of CD4+ T Cell Response. Immunol Rev (2020) 296(1):24–35. doi: 10.1111/imr.12859
38. Hong S, Zhang Z, Liu H, Tang H, Hua Z, Correspondence BH. B Cells Are the Dominant Antigen-Presenting Cells That Activate Naive CD4 + T Cells Upon Immunization With a Virus-Derived Nanoparticle Antigen. Immunity (2018) 49:695–708. doi: 10.1016/j.immuni.2018.08.012
39. Wang Y, Rothstein TL. Induction of Th17 Cell Differentiation by B-1 Cells. Front Immunol (2012) 3:281/abstract(SEP). doi: 10.3389/fimmu.2012.00281/abstract
40. Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod’homme T, Varrin-Doyer M, et al. MHC Class II-Dependent B Cell APC Function Is Required for Induction of CNS Autoimmunity Independent of Myelin-Specific Antibodies. J Exp Med (2013) 210(13):2921–37. doi: 10.1084/jem.20130699
41. Barrio L, Román-García S, Díaz-Mora E, Risco A, Jiménez-Saiz R, Carrasco YR, et al. B Cell Development and T-Dependent Antibody Response Are Regulated by P38γ and P38δ. Front Cell Dev Biol (2020) 8:189. doi: 10.3389/fcell.2020.00189
42. Allman D, Wilmore JR, Gaudette BT. The Continuing Story of T-Cell Independent Antibodies. Immunol Rev (2019) 288(1):128–35. doi: 10.1111/imr.12754
43. Wang LD, Clark MR. B-Cell Antigen-Receptor Signalling in Lymphocyte Development. Immunology (2003) 110(4):411–20. doi: 10.1111/j.1365-2567.2003.01756.x
44. Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The Generation of Antibody-Secreting Plasma Cells. Nat Rev Immunol (2015) 15(3):160–71. doi: 10.1038/nri3795
45. Taubenheim N, Tarlinton DM, Crawford S, Corcoran LM, Hodgkin PD, Nutt SL. High Rate of Antibody Secretion Is Not Integral to Plasma Cell Differentiation as Revealed by XBP-1 Deficiency. J Immunol (2012) 189(7):3328–38. doi: 10.4049/jimmunol.1201042
46. Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B Cells are Distinct From Progenitors for Other B Cells. J Exp Med (1985) 161(6):1554–68. doi: 10.1084/jem.161.6.1554
47. Tornberg UC, Holmberg D. B-1a, B-1b and B-2 B Cells Display Unique VHDJH Repertoires Formed at Different Stages of Ontogeny and Under Different Selection Pressures. EMBO J (1995) 14(8):1680. doi: 10.1002/j.1460-2075.1995.tb07157.x
48. Gregoire KE, Goldschneider I, Barton RW, Bollum FJ. Ontogeny of Terminal Deoxynucleotidyl Transferase-Positive Cells in Lymphohemopoletic Tissues of Rat and Mouse. J Immunol (1979) 123(3):1347–52.
49. Ghosn EEB, Waters J, Phillips M, Yamamoto R, Long BR, Yang Y, et al. Fetal Hematopoietic Stem Cell Transplantation Fails to Fully Regenerate the B-Lymphocyte Compartment. Stem Cell Rep (2016) 6(1):137–49. doi: 10.1016/j.stemcr.2015.11.011
50. Montecino-Rodriguez E, Fice M, Casero D, Berent-Maoz B, Barber CL, Dorshkind K. Distinct Genetic Networks Orchestrate the Emergence of Specific Waves of Fetal and Adult B-1 and B-2 Development. Immun (2016) 45(3):527–39. doi: 10.1016/j.immuni.2016.07.012
51. Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b Reprograms Adult Bone Marrow Hematopoietic Progenitors to Mediate Fetal-Like Lymphopoiesis. Sci (80- ) (2012) 335(6073):1195–200. doi: 10.1126/science.1216557
52. Parra D, Rieger AM, Li J, Zhang Y-A, Randall LM, Hunter CA, et al. Pivotal Advance: Peritoneal Cavity B-1 B Cells Have Phagocytic and Microbicidal Capacities and Present Phagocytosed Antigen to CD4+ T Cells. J Leukoc Biol (2012) 91(4):525. doi: 10.1189/jlb.0711372
53. Holodick NE, Rodríguez-Zhurbenko N, Hernández AM. Defining Natural Antibodies. Front Immunol (2017) 8(JUL):872. doi: 10.3389/fimmu.2017.00872
54. Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 Cell–Derived Immunoglobulin M Antibodies Are Nonredundant Components of the Protective Response to Influenza Virus Infection. J Exp Med (2000) 192(2):271–80. doi: 10.1084/jem.192.2.271
55. Foote JB, Kearney JF. Generation of B Cell Memory to the Bacterial Polysaccharide α 1→3-Dextran. J Immunol (2009) 183(10):6359. doi: 10.4049/jimmunol.0902473
56. Rivera A, Chen C-C, Ron N, Dougherty JP, Ron Y. Role of B Cells as Antigen-Presenting Cells In Vivo Revisited: Antigen-Specific B Cells are Essential for T Cell Expansion in Lymph Nodes and for Systemic T Cell Responses to Low Antigen Concentrations. Int Immunol (2001) 13:1583–93. doi: 10.1093/intimm/13.12.1583
57. Chen X, Jensen PE. The Role of B Lymphocytes as Antigen-Presenting Cells. Archivum Immunologiae Therapiae Experimentalis (2008) 56:77–83. doi: 10.1007/s00005-008-0014-5
58. Rossetti RAM, Lorenzi NPC, Yokochi K, Rosa MBS de F, Benevides L, Margarido PFR, et al. B Lymphocytes can be Activated to Act as Antigen Presenting Cells to Promote Anti-Tumor Responses. PloS One (2018) 13(7):e0199034. doi: 10.1371/journal.pone.0199034
59. Popi AF, Longo-Maugéri IM, Mariano M. An Overview of B-1 Cells as Antigen-Presenting Cells. Front Immunol (2016) 7:138. doi: 10.3389/fimmu.2016.00138
60. Margry B, Wieland WH, van Kooten PJ, van Eden W, Broere F. Peritoneal Cavity B-1a Cells Promote Peripheral CD4+ T-Cell Activation. Eur J Immunol (2013) 43(9):2317–26. doi: 10.1002/eji.201343418
61. Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, et al. Innate Response Activator B Cells Protect Against Microbial Sepsis. Sci (80- ) (2012) 335(6068):597–601. doi: 10.1126/science.1215173
62. Wang M, Subramanian M, Abramowicz S, Murphy AJ, Gonen A, Witztum J, et al. Interleukin-3/Granulocyte Macrophage Colony-Stimulating Factor Receptor Promotes Stem Cell Expansion, Monocytosis, and Atheroma Macrophage Burden in Mice With Hematopoietic ApoE Deficiency. Arterioscler Thromb Vasc Biol (2014) 34(5):976–84. doi: 10.1161/ATVBAHA.113.303097
63. Weber GF, Chousterman BG, He S, Fenn AM, Nairz M, Anzai A, et al. Interleukin-3 Amplifies Acute Inflammation and Is a Potential Therapeutic Target in Sepsis. Sci (80- ) (2015) 347(6227):1260–5. doi: 10.1126/science.aaa4268
64. Rauch M, Tussiwand R, Bosco N, Rolink AG. Crucial Role for BAFF-BAFF-R Signaling in the Survival and Maintenance of Mature B Cells. PloS One (2009) 4(5):5456. doi: 10.1371/journal.pone.0005456
65. Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting Edge: BLyS Enables Survival of Transitional and Mature B Cells Through Distinct Mediators. J Immunol (2002) 168(12):5993–6. doi: 10.4049/jimmunol.168.12.5993
66. Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, et al. Synthesis and Release of B-Lymphocyte Stimulator From Myeloid Cells. Blood (2001) 97(1):198–204. doi: 10.1182/blood.V97.1.198
67. Smulski CR, Eibel H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front Immunol (2018) 9:2285. doi: 10.3389/fimmu.2018.02285
68. Pillai S, Cariappa A. The Follicular Versus Marginal Zone B Lymphocyte Cell Fate Decision. Nat Rev Immunol (2009) 9:767–77. doi: 10.1038/nri2656
69. Attanavanich K, Kearney JF. Marginal Zone, But Not Follicular B Cells, Are Potent Activators of Naive CD4 T Cells. J Immunol (2004) 172(2):803–11. doi: 10.4049/jimmunol.172.2.803
70. Qazi KR, Gehrmann U, Jordö ED, Karlsson MCI, Gabrielsson S. Antigen-Loaded Exosomes Alone Induce Thl-Type Memory Through a B Cell Dependent Mechanism. Blood (2009) 113(12):2673–83. doi: 10.1182/blood-2008-04-153536
71. Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity (2016) 44(1):116–30. doi: 10.1016/j.immuni.2015.12.004
72. Stavnezer J, Schrader CE. IgH Chain Class Switch Recombination: Mechanism and Regulation. J Immunol (2014) 193(11):5370–8. doi: 10.4049/jimmunol.1401849
73. Péron S, Laffleur B, Denis-Lagache N, Cook-Moreau J, Tinguely A, Delpy L, et al. AID-Driven Deletion Causes Immunoglobulin Heavy Chain Locus Suicide Recombination in B Cells. Sci (80- ) (2012) 336(6083):931–4. doi: 10.1126/science.1218692
74. Alhabbab RY, Nova-Lamperti E, Aravena O, Burton HM, Lechler RI, Dorling A, et al. Regulatory B Cells: Development, Phenotypes, Functions, and Role in Transplantation. Immunol Rev (2019) 292(1):164–79. doi: 10.1111/imr.12800
75. Rosser EC, Mauri C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity (2015) 42:607–12. doi: 10.1016/j.immuni.2015.04.005
76. Peng B, Ming Y, Yang C. Regulatory B Cells: The Cutting Edge of Immune Tolerance in Kidney Transplantation. Cell Death Dis (2018) 9:109. doi: 10.1038/s41419-017-0152-y
77. Yoshizaki A, Miyagaki T, DiLillo D, Matsushita T, Horikawa M, Kountikov E, et al. Regulatory B Cells Control T-Cell Autoimmunity Through IL-21-Dependent Cognate Interactions. Nature (2012) 491(7423):264–8. doi: 10.1038/nature11501
78. Lampropoulou V, Hoehlig K, Roch T, Neves P, Gómez EC, Sweenie CH, et al. TLR-Activated B Cells Suppress T Cell-Mediated Autoimmunity. J Immunol (2008) 180(7):4763–73. doi: 10.4049/jimmunol.180.7.4763
79. Wang R-X, Yu C-R, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 Induces Regulatory B Cells That Suppress Autoimmune Disease. Nat Med (2014) 20(6):633–41. doi: 10.1038/nm.3554
80. Xiao S, Brooks CR, Sobel RA, Kuchroo VK. Tim-1 Is Essential for Induction and Maintenance of IL-10 in Regulatory B Cells and Their Regulation of Tissue Inflammation. J Immunol (2015) 194(4):1602–8. doi: 10.4049/jimmunol.1402632
81. Zacca ER, Onofrio LI, Acosta CDV, Ferrero PV, Alonso SM, Ramello MC, et al. PD-L1+ Regulatory B Cells Are Significantly Decreased in Rheumatoid Arthritis Patients and Increase After Successful Treatment. Front Immunol (2018) 0(OCT):2241. doi: 10.3389/fimmu.2018.02241
82. Kaku H, Cheng KF, Al-Abed Y, Rothstein TL. A Novel Mechanism of B Cell–Mediated Immune Suppression Through CD73 Expression and Adenosine Production. J Immunol (2014) 193(12):5904–13. doi: 10.4049/jimmunol.1400336
83. Lundy SK, Boros DL. Fas Ligand-Expressing B-1a Lymphocytes Mediate CD4+-T-Cell Apoptosis During Schistosomal Infection: Induction by Interleukin 4 (IL-4) and IL-10. Infect Immun (2002) 70(2):812–9. doi: 10.1128/IAI.70.2.812-819.2002
84. Kim AS, Doherty TA, Karta MR, Das S, Baum R, Rosenthal P, et al. Regulatory B Cells and T Follicular Helper Cells Are Reduced in Allergic Rhinitis. J Allergy Clin Immunol (2016) 138(4):1192–5.e5. doi: 10.1016/j.jaci.2016.03.017
85. Yanaba K, Bouaziz J-D, Matsushita T, Tsubata T, Tedder TF. The Development and Function of Regulatory B Cells Expressing IL-10 (B10 Cells) Requires Antigen Receptor Diversity and TLR Signals. J Immunol (2009) 182(12):7459–72. doi: 10.4049/jimmunol.0900270
86. Boldison J, Da Rosa LC, Davies J, Wen L, Wong FS. Dendritic Cells License Regulatory B Cells to Produce IL-10 and Mediate Suppression of Antigen-Specific CD8 T Cells. Cell Mol Immunol (2019) 17:1–13. doi: 10.1038/s41423-019-0324-z
87. Mauri C, Nistala K. Interleukin-35 Takes the “B” Line. Nat Med (2014) 20:580–1. doi: 10.1038/nm.3594
88. Lee KM, Stott RT, Zhao G, Soohoo J, Xiong W, Lian MM, et al. TGF-β-Producing Regulatory B Cells Induce Regulatory T Cells and Promote Transplantation Tolerance. Eur J Immunol (2014) 44(6):1728–36. doi: 10.1002/eji.201344062
89. Miller JP, Allman D. The Decline in B Lymphopoiesis in Aged Mice Reflects Loss of Very Early B-Lineage Precursors. J Immunol (2003) 171(5):2326–30. doi: 10.4049/jimmunol.171.5.2326
90. Alter-Wolf S, Blomberg BB, Riley RL. Deviation of the B Cell Pathway in Senescent Mice Is Associated With Reduced Surrogate Light Chain Expression and Altered Immature B Cell Generation, Phenotype, and Light Chain Expression. J Immunol (2009) 182(1):138–47. doi: 10.4049/jimmunol.182.1.138
91. Guerrettaz LM, Johnson SA, Cambier JC. Acquired Hematopoietic Stem Cell Defects Determine B-Cell Repertoire Changes Associated With Aging. Proc Natl Acad Sci USA (2008) 105(33):11898–902. doi: 10.1073/pnas.0805498105
92. McKenna RW, Asplund SL, Kroft SH. Immunophenotypic Analysis of Hematogones (B-Lymphocyte Precursors) and Neoplastic Lymphoblast by 4-Color Flow Cytometry. Leuk Lymphoma (2004) 45(2):277–85. doi: 10.1080/1042819031000151950
93. Muller-Sieburg CE, Sieburg HB, Bernitz JM, Cattarossi G. Stem Cell Heterogeneity: Implications for Aging and Regenerative Medicine. Blood (2011) 119:3900–7. doi: 10.1182/blood-2011-12-376749
94. Labrie JE, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone Marrow Microenvironmental Changes Underlie Reduced RAG-Mediated Recombination and B Cell Generation in Aged Mice. J Exp Med (2004) 200(4):411–23. doi: 10.1084/jem.20040845
95. Kline GH, Hayden TA, Klinman NR. B Cell Maintenance in Aged Mice Reflects Both Increased B Cell Longevity and Decreased B Cell Generation. J Immunol (2020) 162(6):3342–9.
96. Karnell JL, Kumar V, Wang J, Wang S, Voynova E, Ettinger R. Role of CD11c+ T-Bet+ B Cells in Human Health and Disease. Cell Immunol (2017) 321:40–5. doi: 10.1016/j.cellimm.2017.05.008
97. Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, et al. Aging Down-Regulates the Transcription Factor E2A, Activation-Induced Cytidine Deaminase, and Ig Class Switch in Human B Cells. J Immunol (2008) 180(8):5283–90. doi: 10.4049/jimmunol.180.8.5283
98. Reynaud CA, Descatoire M, Dogan I, Huetz F, Weller S, Weill JC. IgM Memory B Cells: A Mouse/Human Paradox. Cell Mol Life Sci (2012) 69:1625–34. doi: 10.1007/s00018-012-0971-z
99. Benitez A, Weldon AJ, Tatosyan L, Velkuru V, Lee S, Milford T-A, et al. Differences in Mouse and Human Nonmemory B Cell Pools. J Immunol (2014) 192(10):4610–9. doi: 10.4049/jimmunol.1300692
100. Frasca D, Riley RL, Blomberg BB. Aging Murine B Cells Have Decreased Class Switch Induced by Anti-CD40 or BAFF. Exp Gerontol (2007) 42(3):192–203. doi: 10.1016/j.exger.2006.09.003
101. Han S, Yang K, Ozen Z, Peng W, Marinova E, Kelsoe G, et al. Enhanced Differentiation of Splenic Plasma Cells But Diminished Long-Lived High-Affinity Bone Marrow Plasma Cells in Aged Mice. J Immunol (2003) 170(3):1267–73. doi: 10.4049/jimmunol.170.3.1267
102. Speziali E, Santiago AF, Fernandes RM, Vaz NM, Menezes JS, Faria AMC. Specific Immune Responses But Not Basal Functions of B and T Cells are Impaired in Aged Mice. Cell Immunol (2009) 256(1–2):1–5. doi: 10.1016/j.cellimm.2009.01.010
103. Alter-Wolf S, Blomberg BB, Riley RL. Old Mice Retain Bone Marrow B1 Progenitors, But Lose B2 Precursors, and Exhibit Altered Immature B Cell Phenotype and Light Chain Usage. Mech Ageing Dev (2009) 130(6):401–8. doi: 10.1016/j.mad.2009.04.001
104. Hinkley KS, Chiasson RJ, Prior TK, Riggs JE. Age-Dependent Increase of Peritoneal B-1b B Cells in SCID Mice. Immunology (2002) 105(2):196–203. doi: 10.1046/j.1365-2567.2002.01360.x
105. Colonna-Romano G, Bulati M, Aquino A, Scialabba G, Candore G, Lio D, et al. B Cells in the Aged: CD27, CD5, and CD40 Expression. Mech Ageing Dev (2003) 124:389–93. doi: 10.1016/S0047-6374(03)00013-7
106. Adler H, Ferreira DM, Gordon SB, Rylance J. Pneumococcal Capsular Polysaccharide Immunity in the Elderly. Clin Vaccine Immunol (2017) 24:e00004–17. doi: 10.1128/CVI.00004-17
107. Lee-Chang C, Bodogai M, Moritoh K, Chen X, Wersto R, Sen R, et al. Aging Converts Innate B1a Cells Into Potent CD8 + T Cell Inducers. J Immunol (2016) 196(8):3385–97. doi: 10.4049/jimmunol.1502034
108. Lee-Chang C, Bodogai M, Moritoh K, Olkhanud PB, Chan AC, Croft M, et al. Accumulation of 4-1BBL+ B Cells in the Elderly Induces the Generation of Granzyme-B+ CD8+ T Cells With Potential Antitumor Activity. Blood (2014) 124(9):1450–9. doi: 10.1182/blood-2014-03-563940
109. Hu A, Ehleiter D, Ben-yehuda A, Schwab R, Russo C, Szabo P, et al. Effect of Age on the Expressed B Cell Repertoire: Role of B Cell Subsets. Int Immunol (1993) 5(9):1035–9. doi: 10.1093/intimm/5.9.1035
110. Yang X, Stedra J, Cerny J. Relative Contribution of T and B Cells to Hypermutation and Selection of the Antibody Repertoire in Germinal Centers of Aged Mice. J Exp Med (1996) 183(3):959–70. doi: 10.1084/jem.183.3.959
111. Frasca D, van der Put E, Riley RL, Blomberg BB. Reduced Ig Class Switch in Aged Mice Correlates With Decreased E47 and Activation-Induced Cytidine Deaminase. J Immunol (2004) 172(4):2155–62. doi: 10.4049/jimmunol.172.4.2155
112. Frasca D, Blomberg BB. Aging Impairs Murine B Cell Differentiation and Function in Primary and Secondary Lymphoid Tissues. Aging Dis (2011) 2:361–73.
113. Miller JP, Cancro MP. B Cells and Aging: Balancing the Homeostatic Equation. Exp Gerontol (2007) 42:396–9. doi: 10.1016/j.exger.2007.01.010
114. Masters AR, Jellison ER, Puddington L, Khanna KM, Haynes L. Attrition of T Cell Zone Fibroblastic Reticular Cell Number and Function in Aged Spleens. ImmunoHorizons (2018) 2(5):155–63. doi: 10.4049/immunohorizons.1700062
115. Dailey RW, Eun S-Y, Russell CE, Vogel LA. B Cells of Aged Mice Show Decreased Expansion in Response to Antigen, But Are Normal in Effector Function. Cell Immunol (2001) 214(2):99–109. doi: 10.1006/cimm.2001.1894
116. Bulati M, Buffa S, Candore G, Caruso C, Dunn-Walters DK, Pellicanò M, et al. B Cells and Immunosenescence: A Focus on IgG+IgD-CD27- (DN) B Cells in Aged Humans. Ageing Res Rev (2011) 10:274–84. doi: 10.1016/j.arr.2010.12.002
117. Cortegano I, Rodríguez M, Martín I, Prado MC, Ruíz C, Hortigüela R, et al. Altered Marginal Zone and Innate-Like B Cells in Aged Senescence-Accelerated SAMP8 Mice With Defective IgG1 Responses. Cell Death Dis (2017) 8(8):e3000. doi: 10.1038/cddis.2017.351
118. Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in Marginal Zone Macrophages and Marginal Zone B Cells in Old Mice. J Immunol (2011) 186(6):3441–51. doi: 10.4049/jimmunol.1001271
119. Turner VM, Mabbott NA. Ageing Adversely Affects the Migration and Function of Marginal Zone B Cells. Immunology (2017) 151(3):349–62. doi: 10.1111/imm.12737
120. Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL. In Senescence, Age-Associated B Cells Secrete Tnfα and Inhibit Survival of B-Cell Precursors. Aging Cell (2013) 12(2):303–11. doi: 10.1111/acel.12055
121. Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-Cell Subset Uniquely Responsive to Innate Stimuli Accumulates in Aged Mice. Blood (2011) 118(5):1294–304. doi: 10.1182/blood-2011-01-330530
122. Keren Z, Naor S, Nussbaum S, Golan K, Itkin T, Sasaki Y, et al. B-Cell Depletion Reactivates B Lymphopoiesis in the BM and Rejuvenates the B Lineage in Aging. Blood (2011) 117(11):3104–12. doi: 10.1182/blood-2010-09-307983
123. Colonna-Romano G, Bulati M, Aquino A, Pellicanò M, Vitello S, Lio D, et al. A Double-Negative (IgD-CD27-) B Cell Population is Increased in the Peripheral Blood of Elderly People. Mech Ageing Dev (2009) 130(10):681–90. doi: 10.1016/j.mad.2009.08.003
124. Lim MA, Lee J, Park JS, Jhun JY, Moon YM, La CM, et al. Increased Th17 Differentiation in Aged Mice Is Significantly Associated With High IL-1β Level and Low IL-2 Expression. Exp Gerontol (2014) 49(1):55–62. doi: 10.1016/j.exger.2013.10.006
125. Frasca D, Diaz A, Romero M, Vazquez T, Blomberg BB. Obesity Induces Pro-Inflammatory B Cells and Impairs B Cell Function in Old Mice. Mech Ageing Dev (2017) 162:91–9. doi: 10.1016/j.mad.2017.01.004
126. Camell CD, Günther P, Lee A, Goldberg EL, Spadaro O, Youm Y-H, et al. Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab (2019) 30(6):1024–39.e6. doi: 10.1016/j.cmet.2019.10.006
127. Barber CL, Montecino-Rodriguez E, Dorshkind K. Reduced Production of B-1-Specified Common Lymphoid Progenitors Results in Diminished Potential of Adult Marrow to Generate B-1 Cells. Proc Natl Acad Sci USA (2011) 108(33):13700–4. doi: 10.1073/pnas.1107172108
128. Rodriguez-Zhurbenko N, Quach TD, Hopkins TJ, Rothstein TL, Hernandez AM. Human B-1 Cells and B-1 Cell Antibodies Change With Advancing Age. Front Immunol (2019) 10(MAR):483. doi: 10.3389/fimmu.2019.00483
129. Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma Cell Differentiation and the Unfolded Protein Response Intersect at the Transcription Factor XBP-I. Nat Immunol (2003) 4(4):321–9. doi: 10.1038/ni907
130. Tellier J, Shi W, Minnich M, Liao Y, Crawford S, Smyth GK, et al. Blimp-1 Controls Plasma Cell Function Through the Regulation of Immunoglobulin Secretion and the Unfolded Protein Response. Nat Immunol (2016) 17(3):323–30. doi: 10.1038/ni.3348
131. Nera KP, Kohonen P, Narvi E, Peippo A, Mustonen L, Terho P, et al. Loss of Pax5 Promotes Plasma Cell Differentiation. Immunity (2006) 24(3):283–93. doi: 10.1016/j.immuni.2006.02.003
132. Holodick NE, Repetny K, Zhong X, Rothstein TL. Adult BM Generates CD5+ B1 Cells Containing Abundant N-Region Additions. Eur J Immunol (2009) 39(9):2383–94. doi: 10.1002/eji.200838920
133. Aydar Y, Balogh P, Tew JG, Szakal AK. Follicular Dendritic Cells in Aging, a “Bottle-Neck” in the Humoral Immune Response. Ageing Res Rev (2004) 3:15–29. doi: 10.1016/j.arr.2003.08.002
134. Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting Edge: Direct Suppression of B Cells by CD4 + CD25 + Regulatory T Cells. J Immunol (2005) 175(7):4180–3. doi: 10.4049/jimmunol.175.7.4180
135. Sharma S, Dominguez AL, Lustgarten J. High Accumulation of T Regulatory Cells Prevents the Activation of Immune Responses in Aged Animals. J Immunol (2006) 177(12):8348–55. doi: 10.4049/jimmunol.177.12.8348
136. Sage PT, Tan CL, Freeman GJ, Haigis M, Sharpe AH. Defective TFH Cell Function and Increased TFR Cells Contribute to Defective Antibody Production in Aging. Cell Rep (2015) 12(2):163. doi: 10.1016/j.celrep.2015.06.015
137. Dunn-Walters DK. The Ageing Human B Cell Repertoire: A Failure of Selection? Clin Exp Immunol (2016) 183(1):50–6. doi: 10.1111/cei.12700
138. Thien M, Phan TG, Gardam S, Amesbury M, Basten A, MacKay F, et al. Excess BAFF Rescues Self-Reactive B Cells From Peripheral Deletion and Allows Them to Enter Forbidden Follicular and Marginal Zone Niches. Immunity (2004) 20(6):785–98. doi: 10.1016/j.immuni.2004.05.010
139. Jin R, Kaneko H, Suzuki H, Arai T, Teramoto T, Fukao T, et al. Age-Related Changes in BAFF and APRIL Profiles and Upregulation of BAFF and APRIL Expression in Patients With Primary Antibody Deficieny. Int J Mol Med (2008) 21(2):233–8. doi: 10.3892/ijmm.21.2.233/abstract
140. Russell Knode LM, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, et al. Age-Associated B Cells Express a Diverse Repertoire of V H and Vκ Genes With Somatic Hypermutation. J Immunol (2017) 198(5):1921–7. doi: 10.4049/jimmunol.1601106
141. Swain SL, Kugler-Umana O, Kuang Y, Zhang W. The Properties of the Unique Age-Associated B Cell Subset Reveal a Shift in Strategy of Immune Response With Age. Cell Immunol (2017) 321:52–60. doi: 10.1016/j.cellimm.2017.05.009
142. Van Zelm MC, Szczepański T, van der Burg M, Van Dongen JJM. Replication History of B Lymphocytes Reveals Homeostatic Proliferation and Extensive Antigen-Induced B Cell Expansion. J Exp Med (2007) 204(3):645–55. doi: 10.1084/jem.20060964
143. Du SW, Arkatkar T, Jacobs HM, Rawlings DJ, Jackson SW. Generation of Functional Murine CD11c + Age-Associated B Cells in the Absence of B Cell T-Bet Expression. Eur J Immunol (2019) 49(1):170–8. doi: 10.1002/eji.201847641
144. Riley RL, Khomtchouk K, Blomberg BB. Age-Associated B Cells (ABC) Inhibit B Lymphopoiesis and Alter Antibody Repertoires in Old Age. Cell Immunol (2017) 321:61–7. doi: 10.1016/j.cellimm.2017.04.008
145. Peng SL, Szabo SJ, Glimcher LH. T-Bet Regulates IgG Class Switching and Pathogenic Autoantibody Production. Proc Natl Acad Sci USA (2002) 99(8):5545–50. doi: 10.1073/pnas.082114899
146. Barnett BE, Staupe RP, Odorizzi PM, Palko O, Tomov VT, Mahan AE, et al. Cutting Edge: B Cell–Intrinsic T-Bet Expression Is Required To Control Chronic Viral Infection. J Immunol (2016) 197(4):1017–22. doi: 10.4049/jimmunol.1500368
147. Ellebedy AH, Jackson KJL, Kissick HT, Nakaya HI, Davis CW, Roskin KM, et al. Defining Antigen-Specific Plasmablast and Memory B Cell Subsets in Human Blood After Viral Infection or Vaccination. Nat Immunol (2016) 17(10):1226–34. doi: 10.1038/ni.3533
148. Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, et al. IL-21 Drives Expansion and Plasma Cell Differentiation of Autoreactive CD11chiT-Bet+ B Cells in SLE. Nat Commun (2018) 9(1):1–14. doi: 10.1038/s41467-018-03750-7
149. Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, et al. Complement Receptor 2/CD21- Human Naive B Cells Contain Mostly Autoreactive Unresponsive Clones. Blood (2010) 115(24):5026–36. doi: 10.1182/blood-2009-09-243071
150. Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P. CD11c-Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs. J Immunol (2015) 195(1):71–9. doi: 10.4049/jimmunol.1500055
151. Ouyang X, Yang Z, Zhang R, Arnaboldi P, Lu G, Li Q, et al. Potentiation of Th17 Cytokines in Aging Process Contributes to the Development of Colitis. Cell Immunol (2011) 266(2):208–17. doi: 10.1016/j.cellimm.2010.10.007
152. Griffin GK, Newton G, Tarrio ML, Bu D, Maganto-Garcia E, Azcutia V, et al. IL-17 and TNF-α Sustain Neutrophil Recruitment During Inflammation Through Synergistic Effects on Endothelial Activation. J Immunol (2012) 188(12):6287–99. doi: 10.4049/jimmunol.1200385
153. Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, et al. TLR7 Escapes X Chromosome Inactivation in Immune Cells. Sci Immunol (2018) 3(19):eaap8855. doi: 10.1126/sciimmunol.aap8855
154. Song N, Li T. Regulation of NLRP3 Inflammasome by Phosphorylation. Front Immunol (2018) 9:2305. doi: 10.3389/fimmu.2018.02305
155. Khan S, Tsai S, Winer DA. Adipose Tissue B Cells Come of Age: The AABs of Fat Inflammation. Cell Metab (2019) 30:997–9. doi: 10.1016/j.cmet.2019.11.007
156. McDonnell ME, Ganley-Leal LM, Mehta A, Bigornia SJ, Mott M, Rehman Q, et al. B Lymphocytes in Human Subcutaneous Adipose Crown-Like Structures. Obesity (2012) 20(7):1372–8. doi: 10.1038/oby.2012.54
157. Dagdeviren S, Jung DY, Friedline RH, Noh HL, Kim JH, Patel PR, et al. IL-10 Prevents Aging-Associated Inflammation and Insulin Resistance in Skeletal Muscle. FASEB J (2017) 31(2):701–10. doi: 10.1096/fj.201600832R
158. Garg SK, Delaney C, Toubai T, Ghosh A, Reddy P, Banerjee R, et al. Aging Is Associated With Increased Regulatory T-Cell Function. Aging Cell (2014) 13(3):441–8. doi: 10.1111/acel.12191
159. Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-Related Loss of Naïve T Cells and Dysregulation of T-Cell/B-Cell Interactions in Human Lymph Nodes. Immunology (2005) 114(1):37–43. doi: 10.1111/j.1365-2567.2004.02006.x
160. Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-Evoked Regulatory B Cells Promote Breast Cancer Metastasis by Converting Resting CD4+ T Cells to T-Regulatory Cells. Cancer Res (2011) 71(10):3505–15. doi: 10.1158/0008-5472.CAN-10-4316
161. Dang L, Willard Gallo K, Garaud S, Duvillier H, Lodewyckz JN, Solinas C, et al. Aging and PD-1 & PD-L1 Gene Expression: Markers of Immunosenescence. Blood (2016) 128(22):5983. doi: 10.11852/blood.V128.22.5983.5983
162. Vadasz Z, Haj T, Kessel A, Toubi E. Age-Related Autoimmunity. BMC Med (2013) 11:94. doi: 10.1186/1741-7015-11-94
163. Kline KA, Bowdish DME. Infection in an Aging Population. Curr Opin Microbiol (2016) 29:63–7. doi: 10.1016/j.mib.2015.11.003
164. Yende S, Alvarez K, Loehr L, Folsom AR, Newman AB, Weissfeld LA, et al. Epidemiology and Long-Term Clinical and Biologic Risk Factors for Pneumonia in Community-Dwelling Older Americans Analysis of Three Cohorts. Chest (2013) 144(3):1008–17. doi: 10.1378/chest.12-2818
165. Thompson WW, Shay DK, Weintraub E, Cox N, Anderson LJ, Fukuda K. Mortality Associated With Influenza and Respiratory Syncytial Virus in the United States. J Am Med Assoc (2003) 289(2):179–86. doi: 10.1001/jama.289.2.179
166. Sakata-Kaneko S, Wakatsuki Y, Matsunaga Y, Usui T, Kita T. Altered Th1/Th2 Commitment in Human CD4+ T Cells With Ageing. Clin Exp Immunol (2000) 120(2):267–73. doi: 10.1046/j.1365-2249.2000.01224.x
167. Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, et al. Lack of Antibody Production Following Immunization in Old Age: Association With CD8 + CD28 – T Cell Clonal Expansions and an Imbalance in the Production of Th1 and Th2 Cytokines. J Immunol (2002) 168(11):5893–9. doi: 10.4049/jimmunol.168.11.5893
168. Frasca D, Diaz A, Romero M, Ferracci F, Blomberg BB. MicroRNAs miR-155 and miR-16 Decrease AID and E47 in B Cells From Elderly Individuals. J Immunol (2015) 195(5):2134–40. doi: 10.4049/jimmunol.1500520
169. Nipper AJ, Smithey MJ, Shah RC, Canaday DH, Landay AL. Diminished Antibody Response to Influenza Vaccination is Characterized by Expansion of an Age-Associated B-Cell Population With Low PAX5. Clin Immunol (2018) 193:80–7. doi: 10.1016/j.clim.2018.02.003
170. Henry C, Zheng NY, Huang M, Cabanov A, Rojas KT, Kaur K, et al. Influenza Virus Vaccination Elicits Poorly Adapted B Cell Responses in Elderly Individuals. Cell Host Microbe (2019) 25(3):357–66.e6. doi: 10.1016/j.chom.2019.01.002
171. Gensous N, Franceschi C, Blomberg BB, Pirazzini C, Ravaioli F, Gentilini D, et al. Responders and non-Responders to Influenza Vaccination: A DNA Methylation Approach on Blood Cells. Exp Gerontol (2018) 105:94–100. doi: 10.1016/j.exger.2018.01.019
172. Zimmermann MT, Oberg AL, Grill DE, Ovsyannikova IG, Haralambieva IH, Kennedy RB, et al. System-Wide Associations Between DNA-Methylation, Gene Expression, and Humoral Immune Response to Influenza Vaccination. PloS One (2016) 11(3):e0152034. doi: 10.1371/journal.pone.0152034
173. Frasca D, Diaz A, Romero M, Blomberg BB. Human Peripheral Late/Exhausted Memory B Cells Express a Senescent-Associated Secretory Phenotype and Preferentially Utilize Metabolic Signaling Pathways. Exp Gerontol (2017) 87(Pt A):113–20. doi: 10.1016/j.exger.2016.12.001
174. Frasca D, Diaz A, Romero M, Thaller S, Blomberg BB. Metabolic Requirements of Human Pro-Inflammatory B Cells in Aging and Obesity. PloS One (2019) 14(7):e0219545. doi: 10.1371/journal.pone.0219545
175. Johnson JL, Rosenthal RL, Knox JJ, Myles A, Naradikian MS, Madej J, et al. The Transcription Factor T-Bet Resolves Memory B Cell Subsets With Distinct Tissue Distributions and Antibody Specificities in Mice and Humans. Immunity (2020) 52(5):842–55.e6. doi: 10.1016/j.immuni.2020.03.020
176. Sosa-Hernández VA, Torres-Ruíz J, Cervantes-Díaz R, Romero-Ramírez S, Páez-Franco JC, Meza-Sánchez DE, et al. B Cell Subsets as Severity-Associated Signatures in COVID-19 Patients. Front Immunol (2020) 11. doi: 10.3389/fimmu.2020.611004
177. Boyd AR, Orihuela CJ. Dysregulated Inflammation as a Risk Factor for Pneumonia in the Elderly. Aging Dis (2011) 2:487–500.
178. Hampe CS. B Cells in Autoimmune Diseases. Scientifica (2012) 2012:e215308. doi: 10.6064/2012/215308
179. Attanasio R, Brasky KM, Robbins SH. Age-Related Autoantibody Production in a Nonhuman Primate Model. Clin Exp Immunol (2001) 123(3):361–5. doi: 10.1046/j.1365-2249.2001.01454.x
180. Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, et al. Circulating CD21low B Cells in Common Variable Immunodeficiency Resemble Tissue Homing, Innate-Like B Cells. Proc Natl Acad Sci USA (2009) 106(32):13451–6. doi: 10.1073/pnas.0901984106
181. Adlowitz DG, Barnard J, Biear JN, Cistrone C, Owen T, Wang W, et al. Expansion of Activated Peripheral Blood Memory B Cells in Rheumatoid Arthritis, Impact of B Cell Depletion Therapy, and Biomarkers of Response. PloS One (2015) 10(6):e0128269. doi: 10.1371/journal.pone.0128269
182. Whitacre CC. Sex Differences in Autoimmune Disease. Vol. 2, Nature Immunology. Nat Immunol (2001) 2:777–80. doi: 10.1038/ni0901-777
183. Wehr C, Eibel H, Masilamani M, Illges H, Schlesier M, Peter H-H, et al. A New CD21low B Cell Population in the Peripheral Blood of Patients With SLE. Clin Immunol (2004) 113(2):161–71. doi: 10.1016/j.clim.2004.05.010
184. Duffy L, O’Reilly SC. Toll-Like Receptors in the Pathogenesis of Autoimmune Diseases: Recent and Emerging Translational Developments. ImmunoTargets Ther (2016) 5:69–80. doi: 10.2147/ITT.S89795
185. Santiago-Raber ML, Dunand-Sauthier I, Wu T, Li QZ, Uematsu S, Akira S, et al. Critical Role of TLR7 in the Acceleration of Systemic Lupus Erythematosus in TLR9-Deficient Mice. J Autoimmun (2010) 34(4):339–48. doi: 10.1016/j.jaut.2009.11.001
186. Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, et al. Nucleic Acids of Mammalian Origin can Act as Endogenous Ligands for Toll-Like Receptors and may Promote Systemic Lupus Erythematosus. J Exp Med (2005) 202(8):1131–9. doi: 10.1084/jem.20050914
187. Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, et al. TLR9 Regulates TLR7- and MyD88-Dependent Autoantibody Production and Disease in a Murine Model of Lupus. J Immunol (2010) 184(4):1840–8. doi: 10.4049/jimmunol.0902592
188. Nickerson KM, Christensen SR, Cullen JL, Meng W, Luning Prak ET, Shlomchik MJ. TLR9 Promotes Tolerance by Restricting Survival of Anergic Anti-DNA B Cells, Yet Is Also Required for Their Activation. J Immunol (2013) 190(4):1447–56. doi: 10.4049/jimmunol.1202115
189. Srikakulapu P, McNamara CA. B Cells and Atherosclerosis. Am J Physiol - Heart Circulatory Physiol (2017) 312:H1060–7. doi: 10.1152/ajpheart.00859.2016
190. Libby P, Lichtman AH, Hansson GK. Immune Effector Mechanisms Implicated in Atherosclerosis: From Mice to Humans. Immunity (2013) 38:1092–104. doi: 10.1016/j.immuni.2013.06.009
191. Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. Early Human Atherosclerosis: Accumulation of Lipid and Proteoglycans in Intimal Thickenings Followed by Macrophage Infiltration. Arterioscler Thromb Vasc Biol (2007) 27(5):1159–65. doi: 10.1161/ATVBAHA.106.134080
192. Briley-Saebo KC, Nguyen TH, Saeboe AM, Cho YS, Ryu SK, Volkava E, et al. In Vivo Detection of Oxidation-Specific Epitopes in Atherosclerotic Lesions Using Biocompatible Manganese Molecular Magnetic Imaging Probes. J Am Coll Cardiol (2012) 59(6):616–26. doi: 10.1016/j.jacc.2011.10.881
193. Di Pietro N, Formoso G, Pandolfi A. Physiology and Pathophysiology of oxLDL Uptake by Vascular Wall Cells in Atherosclerosis. Vasc Pharmacol (2016) 84:1–7. doi: 10.1016/j.vph.2016.05.013
194. Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res (2019) 124:315–27. doi: 10.1161/CIRCRESAHA.118.313591
195. Palinski W, Hörkkö S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, et al. Cloning of Monoclonal Autoantibodies to Epitopes of Oxidized Lipoproteins From Apolipoprotein E-Deficient Mice Demonstration of Epitopes of Oxidized Low Density Lipoprotein in Human Plasma Modified Lipo-Proteins • Autoantibodies • Atherosclerosis • Immune System. J Clin Invest (1996) 98:800–14. doi: 10.1172/JCI118853
196. Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective Immunity Against Atherosclerosis Carried by B Cells of Hypercholesterolemic Mice. J Clin Invest (2002) 109(6):745. doi: 10.1172/JCI7272
197. Tay C, Liu Y-H, Hosseini H, Kanellakis P, Cao A, Peter K, et al. B-Cell-Specific Depletion of Tumour Necrosis Factor Alpha Inhibits Atherosclerosis Development and Plaque Vulnerability to Rupture by Reducing Cell Death and Inflammation. Cardiovasc Res (2016) 111(4):385–97. doi: 10.1093/cvr/cvw186
198. Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, et al. B Cell Depletion Reduces the Development of Atherosclerosis in Mice. J Exp Med (2010) 207(8):1579–87. doi: 10.1084/jem.20100155
199. Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ Res (2018) 122(12):1675–88. doi: 10.1161/CIRCRESAHA.117.312513
200. Douna H, Kuiper J. Novel B-Cell Subsets in Atherosclerosis. Curr Opin Lipidol (2016) 27:493–8. doi: 10.1097/MOL.0000000000000335
201. Tsiantoulas D, Gruber S, Binder CJ. B-1 Cell Immunoglobulin Directed Against Oxidation-Specific Epitopes. Front Immunol (2012) 3(JAN). doi: 10.3389/fimmu.2012.00415
202. Gruber S, Hendrikx T, Tsiantoulas D, Ozsvar-Kozma M, Göderle L, Mallat Z, et al. Sialic Acid-Binding Immunoglobulin-Like Lectin G Promotes Atherosclerosis and Liver Inflammation by Suppressing the Protective Functions of B-1 Cells. Cell Rep (2016) 14(10):2348–61. doi: 10.1016/j.celrep.2016.02.027
203. Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, et al. B1a B Lymphocytes are Atheroprotective by Secreting Natural IgM That Increases IgM Deposits and Reduces Necrotic Cores in Atherosclerotic Lesions. Circ Res (2011) 109(8):830–40. doi: 10.1161/CIRCRESAHA.111.248542
204. Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, et al. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circ Res (2015) 117(3):e28–39. doi: 10.1161/CIRCRESAHA.117.306044
205. Hilgendorf I, Theurl I, Gerhardt LMS, Robbins CS, Weber GF, Gonen A, et al. Innate Response Activator B Cells Aggravate Atherosclerosis by Stimulating T Helper-1 Adaptive Immunity. Circulation (2014) 129(16):1677–87. doi: 10.1161/CIRCULATIONAHA.113.006381
206. Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, et al. Extramedullary Hematopoiesis Generates Ly-6C High Monocytes That Infiltrate Atherosclerotic Lesions. Circulation (2012) 125(2):364–74. doi: 10.1161/CIRCULATIONAHA.111.061986
207. Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, et al. Conventional B2 B Cell Depletion Ameliorates Whereas Its Adoptive Transfer Aggravates Atherosclerosis. J Immunol (2010) 185(7):4410–9. doi: 10.4049/jimmunol.1000033
208. Kyaw T, Tay C, Hosseini H, Kanellakis P, Gadowski T, MacKay F, et al. Depletion of B2 But Not B1a B Cells in Baff Receptor-Deficient Apoe -/- Mice Attenuates Atherosclerosis by Potently Ameliorating Arterial Inflammation. PloS One (2012) 7(1):e29371. doi: 10.1371/journal.pone.0029371
209. Sage AP, Tsiantoulas D, Baker L, Harrison J, Masters L, Murphy D, et al. BAFF Receptor Deficiency Reduces the Development of Atherosclerosis in Mice–Brief Report. Arterioscler Thromb Vasc Biol (2012) 32(7):1573–6. doi: 10.1161/ATVBAHA.111.244731
210. Kyaw T, Cui P, Tay C, Kanellakis P, Hosseini H, Liu E, et al. BAFF Receptor mAb Treatment Ameliorates Development and Progression of Atherosclerosis in Hyperlipidemic ApoE–/– Mice. PloS One (2013) 8(4):e60430. doi: 10.1371/journal.pone.0060430
211. Tsiantoulas D, Sage AP, Göderle L, Ozsvar-Kozma M, Murphy D, Porsch F, et al. B Cell-Activating Factor Neutralization Aggravates Atherosclerosis. Circulation (2018) 138(20):2263–73. doi: 10.1161/CIRCULATIONAHA.117.032790
212. Tay C, Liu YH, Kanellakis P, Kallies A, Li Y, Cao A, et al. Follicular B Cells Promote Atherosclerosis via T Cell-Mediated Differentiation Into Plasma Cells and Secreting Pathogenic Immunoglobulin G. Arterioscler Thromb Vasc Biol (2018) 38(5):e71–84. doi: 10.1161/ATVBAHA.117.310678
213. Douna H, Amersfoort J, Schaftenaar FH, Kröner MJ, Kiss MG, Slütter B, et al. B- And T-Lymphocyte Attenuator Stimulation Protects Against Atherosclerosis by Regulating Follicular B Cells. Cardiovasc Res (2020) 116(2):295–305. doi: 10.1093/cvr/cvz129
214. Nus M, Sage AP, Lu Y, Masters L, Lam BYH, Newland S, et al. Marginal Zone B Cells Control the Response of Follicular Helper T Cells to a High-Cholesterol Diet. Nat Med (2017) 23(5):601–10. doi: 10.1038/nm.4315
215. Strom AC, Cross AJ, Cole JE, Blair PA, Leib C, Goddard ME, et al. B Regulatory Cells are Increased in Hypercholesterolaemic Mice and Protect From Lesion Development via IL-10. Thromb Haemost (2015) 114(4):835–47. doi: 10.1160/TH14-12-1084
216. Douna H, Amersfoort J, Schaftenaar FH, Kroon S, van Puijvelde GHM, Kuiper J, et al. Bidirectional Effects of IL-10+ Regulatory B Cells in Ldlr–/– Mice. Atherosclerosis (2019) 280:118–25. doi: 10.1016/j.atherosclerosis.2018.11.019
217. Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B Cells Are Critical Regulators of Humoral Immunity. Nat Commun (2015) 6:6997. doi: 10.1038/ncomms6997
218. Lundy SK, Fox DA. Reduced Fas Ligand-Expressing Splenic CD5 +B Lymphocytes in Severe Collagen-Induced Arthritis. Arthritis Res Ther (2009) 11(4):R128. doi: 10.1186/ar2795
219. Depuydt MAC, Prange KHM, Slenders L, Örd T, Elbersen D, Boltjes A, et al. Microanatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics. Circ Res (2020) 127:1437–55. doi: 10.1161/CIRCRESAHA.120.316770
220. Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, et al. Atheroprotective Roles of Smooth Muscle Cell Phenotypic Modulation and the TCF21 Disease Gene as Revealed by Single-Cell Analysis. Nat Med (2019) 25(8):1280–9. doi: 10.1038/s41591-019-0512-5
221. Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir E ad D, Amadori L, et al. Single-Cell Immune Landscape of Human Atherosclerotic Plaques. Nat Med (2019) 25(10):1576–88. doi: 10.1038/s41591-019-0590-4
222. van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slütter B, et al. NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice-Brief Report. Arterioscler Thromb Vasc Biol (2017) 37(8):1457–61. doi: 10.1161/ATVBAHA.117.309575
223. Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, et al. Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models. Circ Res (2018) 123(10):1127–42. doi: 10.1161/CIRCRESAHA.118.312804
224. Srikakulapu P, Upadhye A, Rosenfeld SM, Marshall MA, McSkimming C, Hickman AW, et al. Perivascular Adipose Tissue Harbors Atheroprotective IgM-Producing B Cells. Front Physiol (2017) 8(SEP):719. doi: 10.3389/fphys.2017.00719
225. Srikakulapu P, Hu D, Yin C, Mohanta SK, Bontha SV, Peng L, et al. Artery Tertiary Lymphoid Organs Control Multilayered Territorialized Atherosclerosis B-Cell Responses in Aged ApoE-/- Mice. Arterioscler Thromb Vasc Biol (2016) 36(6):1174–85. doi: 10.1161/ATVBAHA.115.306983
226. Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, et al. Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect Against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin β Receptors. Immunity (2015) 42(6):1100–15. doi: 10.1016/j.immuni.2015.05.015
227. Mohanta SK, Yin C, Peng L, Srikakulapu P, Bontha V, Hu D, et al. Artery Tertiary Lymphoid Organs Contribute to Innate and Adaptive Immune Responses in Advanced Mouse Atherosclerosis. Circ Res (2014) 114:1772–87. doi: 10.1161/CIRCRESAHA.114.301137
228. Petes C, Odoardi N, Gee K. The Toll for Trafficking: Toll-Like Receptor 7 Delivery to the Endosome. Front Immunol (2017) 8:1. doi: 10.3389/fimmu.2017.01075
229. Van Vré EA, Ait-Oufella H, Tedgui A, Mallat Z. Apoptotic Cell Death and Efferocytosis in Atherosclerosis. Arterioscler Thromb Vasc Biol (2012) 32(4):887–93. doi: 10.1161/ATVBAHA.111.224873
230. Akunuru S, Geiger H. Aging, Clonality, and Rejuvenation of Hematopoietic Stem Cells. Trends Mol Med (2016) 22:701–12. doi: 10.1016/j.molmed.2016.06.003
231. Martins R, Lithgow GJ, Link W. Long Live FOXO: Unraveling the Role of FOXO Proteins in Aging and Longevity. Aging Cell (2016) 15(2):196. doi: 10.1111/acel.12427
232. Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, et al. Foxo3a is Essential for Maintenance of the Hematopoietic Stem Cell Pool. Cell Stem Cell (2007) 1(1):101–12. doi: 10.1016/j.stem.2007.02.001
233. Tothova Z, Kollipara R, Huntly BJ, Le BH, Castrillon DH, Cullen DE, et al. FoxOs Are Critical Mediators of Hematopoietic Stem Cell Resistance to Physiologic Oxidative Stress. Cell (2007) 128(2):325–39. doi: 10.1016/j.cell.2007.01.003
234. Florian MC, Dörr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M, et al. CDC42 Activity Regulates Hematopoietic Stem Cell Aging and Rejuvenation. Cell Stem Cell (2012) 10(5):520. doi: 10.1016/j.stem.2012.04.007
235. Leins H, Mulaw M, Eiwen K, Sakk V, Liang Y, Denkinger M, et al. Aged Murine Hematopoietic Stem Cells Drive Aging-Associated Immune Remodeling. Blood (2018) 132(6):565–76. doi: 10.1182/blood-2018-02-831065
236. Edwards JCW, Szczepański L, Szechiński J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-Cell–Targeted Therapy With Rituximab in Patients With Rheumatoid Arthritis. N Engl J Med (2004) 350(25):2572–81. doi: 10.1056/NEJMoa032534
237. Gerlag DM, Safy M, Maijer KI, Tang MW, Tas SW, Starmans-Kool MJF, et al. Effects of B-Cell Directed Therapy on the Preclinical Stage of Rheumatoid Arthritis: The PRAIRI Study. Ann Rheum Dis (2019) 78(2):179–85. doi: 10.1136/annrheumdis-2017-212763
238. Airas L, Nylund M, Mannonen I, Matilainen M, Sucksdorff M, Rissanen E. Rituximab in the Treatment of Multiple Sclerosis in the Hospital District of Southwest Finland. Mult Scler Relat Disord (2020) 40:101980. doi: 10.1016/j.msard.2020.101980
239. Novikova DS, Popkova TV, Nasonov EL. The Effect of Anti-B-Cell Therapy on the Development of Atherosclerosis in Patients With Rheumatoid Arthritis. Curr Pharm Des (2012) 18(11):1512–8. doi: 10.2174/138161212799504768
240. Kim D, Park D. Deep Sequencing of B Cell Receptor Repertoire. BMB Rep (2019) 52:540–7. doi: 10.5483/BMBRep.2019.52.9.192
241. Zheng Y, Liu X, Le W, Xie L, Li H, Wen W, et al. A Human Circulating Immune Cell Landscape in Aging and COVID-19. Protein Cell (2020) 11(10):740–70. doi: 10.1007/s13238-020-00762-2
242. Lorenzo C, Delgado P, Busse CE, Sanz-Bravo A, Martos-Folgado I, Bonzon-Kulichenko E, et al. ALDH4A1 Is an Atherosclerosis Auto-Antigen Targeted by Protective Antibodies. Nature (2021) 589. doi: 10.1038/s41586-020-2993-2
243. Danger R, Braza F, Giral M, Soulillou JP, Brouard S. MicroRNAs, Major Players in B Cells Homeostasis and Function. Front Immunol (2014) 5:98. doi: 10.3389/fimmu.2014.00098
244. Zhang Y, Good-Jacobson KL. Epigenetic Regulation of B Cell Fate and Function During an Immune Response. Immunol Rev (2019) 288:75–84. doi: 10.1111/imr.12733
Keywords: B cells, aging, inflammaging, immunosenescence, autoimmune diseases, atherosclerosis
Citation: de Mol J, Kuiper J, Tsiantoulas D and Foks AC (2021) The Dynamics of B Cell Aging in Health and Disease. Front. Immunol. 12:733566. doi: 10.3389/fimmu.2021.733566
Received: 30 June 2021; Accepted: 16 September 2021;
Published: 05 October 2021.
Edited by:
Guobing Chen, Jinan University, ChinaReviewed by:
Yi Hao, Huazhong University of Science and Technology, ChinaCopyright © 2021 de Mol, Kuiper, Tsiantoulas and Foks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda C. Foks, YS5jLmZva3NAbGFjZHIubGVpZGVudW5pdi5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.