
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 22 July 2021
Sec. Immunological Tolerance and Regulation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.712870
This article is part of the Research TopicCharacterization, Functions, and Roles of Antigen-Specific Regulatory T Cells in Health and DiseaseView all 18 articles
Regulatory T cells (Tregs) are key mediators of peripheral self-tolerance and alterations in their frequencies, stability, and function have been linked to autoimmunity. The antigen-specific induction of Tregs is a long-envisioned goal for the treatment of autoimmune diseases given reduced side effects compared to general immunosuppressive therapies. However, the translation of antigen-specific Treg inducing therapies for the treatment or prevention of autoimmune diseases into the clinic remains challenging. In this mini review, we will discuss promising results for antigen-specific Treg therapies in allergy and specific challenges for such therapies in autoimmune diseases, with a focus on type 1 diabetes (T1D). We will furthermore discuss opportunities for antigen-specific Treg therapies in T1D, including combinatorial strategies and tissue-specific Treg targeting. Specifically, we will highlight recent advances in miRNA-targeting as a means to foster Tregs in autoimmunity. Additionally, we will discuss advances and perspectives of computational strategies for the detailed analysis of tissue-specific Tregs on the single-cell level.
The body’s immune system has evolved to effectively defeat and destroy infiltrating foreign pathogens. In order to prevent autoimmune reactions directed against the body’s own cells, our immune system employs sophisticated mechanisms of self-tolerance. On the T cell level, self-tolerance is executed in the thymus by deletion of T cells with self-reactive TCRs (central tolerance). Outside of the thymus, peripheral tolerance is maintained by specialized cells, including so-called regulatory T cells (Tregs). Tregs are characterized by the high expression of the interleukin-2-receptor-aplpha chain (CD25) and the transcription factor Foxp3, which is the master regulator of Tregs phenotype and function (1–4). The critical importance of Tregs for the maintenance of self-tolerance is illustrated by severe multi-organ autoimmunity in humans with the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) (5) and mice with Scurfy mutations (6), both resulting from mutations in the Foxp3 gene. Tregs develop in the thymus, referring to thymic Tregs (tTregs), and harbor a TCR repertoire that is skewed towards self-antigens. Additionally, Tregs can likewise be induced in the periphery in an antigen-specific manner, so called peripheral Tregs (pTregs), with a TCR repertoire different from their tTreg counterparts (7). Considerable research has been conducted in order to induce disease-relevant antigen-specific Tregs with the goal to restore mechanisms of tolerance and interfere with unwanted immune reactions in allergies and autoimmunity. Accordingly, we and others have shown that Treg induction requires stimulation via the TCR and it has become apparent that fine-tuned TCR signals are needed to efficiently induce Tregs (8–11). Here, we will discuss promising results for antigen-specific Treg therapies in allergy and specific challenges for such therapies in autoimmune diseases, with a focus on type 1 diabetes (T1D) as well as opportunities for antigen-specific Treg therapies in T1D.
Antigen-specific therapy is a long-envisioned goal for the treatment or prevention of autoimmune diseases. The ability of Tregs to regulate immune responses not only via direct inhibition of effector T cells with the same specificity but also via modulation of antigen-presenting cell (APCs), a process called bystander suppression, makes Tregs an important target for tolerizing therapies (12). Currently, approaches based either on the expansion, manipulation and transfer of autologous Tregs as well as the in vivo induction with antigen are extensively studied. While the ex vivo expansion of polyclonal Tregs has proven to be safe in the clinic the efficacy is largely dependent on disease-relevant antigen-specific Tregs. However, their very low frequency in the case of autoimmune diseases necessitates the manipulation of Tregs before transfer [reviewed in (13)]. This includes the forced expression of FOXP3 in autoantigen-specific effector T cells as well as the expression of disease relevant TCRs on isolated Tregs [reviewed in (13)]. Although results from preclinical studies are promising, the long-term fate of these engineered Tregs is not fully understood and especially the differentiation into pro-inflammatory lineages might be a safety concern. The alternative of induction of Tregs with antigen administered directly to the patients is more cost-effective and its safety has been demonstrated in a variety of clinical trials. Even though clinical translation of such tolerizing therapies has been challenging, several examples relying on different forms of antigen-delivery and tolerization protocols from pre-clinical and clinical trials highlight the potential of such strategies.
Desensitization to allergens is a common practice for the treatment of severe allergies. However, only a few studies have addressed the effect of such antigen-specific desensitization protocols on Tregs. Importantly, oral immunotherapy with peanut proteins in allergic patients led to an increase in peanut protein-specific FOXP3+ Tregs within peripheral blood mononuclear cells (PBMCs) 6 and 12 months after the treatment started (14). Interestingly, in a follow-up study focusing more specifically on Tregs, it became evident that the increased frequencies of peanut-protein specific Tregs were associated with enhanced DNA demethylation of the FOXP3 locus (15), a measure for maintenance of FOXP3 expression and therefore for the stability of the Treg phenotype (16). These findings highlight that antigen-specific therapy can not only enhance Treg frequencies but also positively affect Treg characteristics including their stability.
Autoimmune diseases like T1D affect millions of people worldwide with a steadily rising incidence. Currently, curative treatments for autoimmune diseases do not exist and available therapies rely on the treatment of symptoms often involving immunosuppressive reagents that can have severe side effects. The antigen-specific induction of disease-relevant Tregs offers the opportunity to restore natural tolerance mechanisms in the absence of immune side effects induced by general immune suppression and is therefore a long-standing goal for the treatment or prevention of autoimmune diseases. We were able to demonstrate that in the peripheral blood of children at risk to develop T1D, insulin-specific Treg frequencies are reduced during the onset of islet autoimmunity, while higher frequencies are associated with a slow progression to clinically overt T1D (17). These findings directly support the concept of inducing these insulin-specific Tregs to delay the progression to clinically symptomatic disease. However, the translation of antigen-specific Treg therapies for autoimmune diseases into the clinic remains challenging and most studies using oral insulin treatments for tolerization in T1D conducted so far failed to meet their primary outcome (18, 19). Nevertheless, post-hoc analysis revealed a delay in progression in a subset of these treated participants (20). One analytical caveat of clinical trials studying Treg therapies has been the divergence of protocols for Treg identification in peripheral blood. While in the mouse setting Foxp3 is expressed exclusively by Tregs, human effector T cells can transiently express intermediate levels of FOXP3. Accordingly, most researchers characterize human Tregs as CD25+CD127lowFOXP3+. It has become apparent though, that even those more stringently defined Tregs are heterogeneous in their composition. Not only can Tregs co-express classical effector T cell transcription factors (e.g. TBET, RORC, GATA3) which affects their migration and function, but they also vary in their activation state and functionality. This is especially evident in the divergent expression of CD45RA, with CD45RA- Tregs being antigen-experienced and having a higher suppressive activity [reviewed in (7)]. According to this heterogeneity, divergent markers have been used for the identification of Tregs in clinical trials which contributes to the difficulties in assessing translatability. Importantly, researchers are starting to analyze antigen-specific immune responses in such clinical trials in more mechanistic detail, which will help to define critical parameters, such as the optimal dosing of oral insulin. Additionally, other factors need to be critically considered, including the route of administration and the chosen antigen but also the time point of administration within the disease course.
We know from murine studies that the efficient de novo induction of Tregs from naïve T cells in vivo requires the stimulation with a strong-agonistic ligand for the TCR supplied under subimmunogenic conditions (8, 9). Higher immunogenic doses of antigen on the other hand activate the Pi3k-Akt-mTOR pathway, thereby directly inhibiting Treg induction (10). We used immunodeficient HLA-DQ8-transgenic NOD-Scid-IL2Rγ knockout (NSG) mice reconstituted with human hematopoietic stem cells to study requirements for human Treg induction in vivo. Importantly, these humanized mice develop a functional human immune system, including the positive selection of autoreactive insulin-specific CD4+ T cells in the thymus (17, 21). Using this system under steady state conditions in the absence of autoimmune activation, we were able to demonstrate that, similar to the murine setting, subimmunogenic doses of strong-agonistic insulin variants are able to induce human Tregs in vivo (17).
In contrast to the steady state, we demonstrated that during the onset of islet autoimmunity the capacity to induce Tregs from naïve T cells from peripheral blood is significantly impaired (22). Importantly, this impairment in Treg induction was not limited to the insulin-specific population, but was likewise observed for hemagglutinin-specific and polyclonal Treg induction, highlighting a broad defect in Treg induction (22). Furthermore, we were able to show that a reduction in the activation threshold of insulin-specific T cells during the onset of islet autoimmunity limits the possibility of subimmunogenic stimulation for efficient Treg induction (22). Apart from defects in Treg induction during islet autoimmunity, we likewise observed reduced Treg stability as indicated by increased DNA methylation of the conserved non-coding sequence 2 (CNS2) of the Foxp3 locus both in non-obese diabetic mice (NOD, mouse model for T1D) with islet autoimmunity as well as in children with overt T1D (23). The Foxp3 CNS2 is completely demethylated in stable Tregs, while its methylation leads to the loss of Foxp3 expression and the Treg phenotype (16). Importantly, this defect in Treg stability in NOD mice was observed already at a young age, shortly after weaning, indicating a possible causative role in disease development and progression as opposed to a mere consequence of the ongoing autoimmune process (23). The identified impairments in Treg induction and stability directly highlight the importance of considering the time point of administration of antigen-specific Treg inducing therapies. Our in vitro and ex vivo data suggest limitations in the efficacy of such treatments during the first years after development of islet autoimmunity. In addition, these findings strengthen the rationale of considering preventive strategies in genetically at-risk patients, before the onset of overt islet autoimmunity, for future antigen-specific Treg targeting in man. Accordingly, for T1D pilot results from the Pre-POINT study, the first study to administer daily oral insulin to children at risk to develop T1D, but before the start of the autoimmune reaction, resulted in enhanced frequencies of insulin-specific CD4+ T cells with regulatory features (24). These preliminary results are currently further investigated in the larger POINT study for efficacy (25).
The finding that Treg induction potential is significantly limited during onset of islet autoimmunity (22) highlights the concept that antigen-specific Treg induction in the presence of ongoing autoimmune activation will benefit from combinatorial immune targeting. Specifically, a combination with treatments that control aberrant immune activation while fostering Tregs will be critical in order to broaden the window of opportunity for Treg induction.
With the goal to understand mechanisms of impaired Treg induction, we focused on microRNAs (miRNAs). miRNAs are small non-coding RNAs that can sequence-specifically inhibit their target mRNAs. miRNAs usually target a multitude of different mRNAs, thereby regulating entire signaling pathways and complex cellular states, such as T cell activation, which makes them important targets for immunotherapies (26–28). Using miRNA sequencing of CD4+ T cells from peripheral blood of children with or without ongoing islet autoimmunity, we were able to identify several differentially regulated miRNAs and investigated three in more detail. Specifically, we focused on miRNAs that are predicted to target negative regulators of T cell activation and could therefore potentially inhibit Treg induction [reviewed in (29–31)].
We were able to demonstrate that miRNA92a-3p, a member of the miRNA17~92 cluster of miRNAs which was shown to induce lupus-like autoimmunity when overexpressed in mice (32), regulates human T follicular helper (TFH) cell differentiation (33). TFH cells are an integral part of the humoral immune response because of their ability to help B cells produce high-affinity antibodies [reviewed in (34)]. Accordingly, we found CXCR5+ insulin-specific TFH cell frequencies to be increased during onset of islet autoimmunity, which was directly correlated with miRNA92a-3p expression. Importantly, miRNA92a-3p targets negative regulators of T cell activation (e.g., PTEN, PHLPP2, FOXO1, CTLA4) and thereby simultaneously reduces Treg induction. Hence, inhibition of miRNA92a-3p enhanced while a miRNA92a-3p mimic reduced Treg induction (33).
Furthermore, we investigated miRNA181a-5p, which has been demonstrated previously to regulate the signal strength of the TCR stimulus in developing T cells in the thymus (35). In line with excessive T cell activation observed during recent onset of islet autoimmunity, we found miRNA181a-5p to be specifically increased in CD4+ T cells from peripheral blood of children with recent activation of islet autoimmunity. Importantly, we found that higher expression of miRNA181a-5p enhances the expression of Nfat5 involving mechanisms of increased TCR- and co-stimulation and that enhanced Nfat5 expression negatively affects Treg induction. Accordingly, inhibiting either miRNA181a-5p or Nfat5 augmented in vitro Treg induction, while inhibiting miRNA181a-5p in Nfat5 deficient T cells had no effect on Treg induction. These findings thereby highlight, that miRNA181a-5p mediated impairments in Treg induction are dependent on Nfat5 (22).
In a third study we used high throughput sequencing of RNA isolated by crosslinking immunoprecipitation (HITS-CLIP) to show, that miRNA142-3p directly targets the methylcytosine deoxygenase Tet2. Importantly, TET proteins catalyze the first step of DNA demethylation and can thereby impact the epigenetic landscape (36). We were able to link increased expression of miRNA142-3p and resulting reduced Tet2 expression with impairments both in Treg induction as well as in Treg stability. Accordingly, the inhibition of miRNA142-3p was able to enhance Treg induction and enable induced Tregs to retain their Foxp3 expression to a higher degree than their untreated counterparts (23).
Importantly, the inhibition of all three miRNAs or the downstream molecule Nfat5 directly in vivo in NOD mice with ongoing islet autoimmunity resulted in enhanced frequencies of Tregs accompanied by a reduction in the clinical disease score of the mice (22, 23, 33). These preliminary findings highlight the potential of miRNA-targeting as immunotherapy in T1D. Notably, a miRNA inhibitor is currently being investigated in a clinical trial as treatment for hepatitis C virus infections, thereby indicating the feasibility of miRNA modulation as immunotherapy (37). However, miRNAs are important regulators of cellular functions and can have distinct properties depending on the cell type. Therefore, the use of miRNA modulation as immunotherapy will be largely dependent on the cell type-specific targeting of the therapy. Specifically, the targeted delivery of miRNA inhibitors or mimics to immune cells or even immune cell subsets will greatly improve their use as immunotherapeutics. Here, it will be especially important to identify specific signatures for targeting defined subsets of immune cells, e.g., tissue-specific Tregs in the target organ, the pancreas.
Apart from their canonical function of immune suppression, it is now well accepted that Tregs likewise take residence in tissues, where they play important roles in maintaining tissue homeostasis. These tissue Tregs were found to express specific gene signatures that are distinct from their circulating counterparts. Such tissue specific Treg gene signatures have been identified for Tregs from specific tissues, while they have been especially well studied for Tregs in the muscle and adipose tissue [reviewed in (38)]. Importantly, some signature genes are universal for tissue Tregs while others are more unique to Tregs from distinct tissues, e.g., the expression of the transcription factor PPARγ on adipose-tissue residing Tregs (39). Apart from their gene expression signature, TCR sequencing of tissue resident Tregs has identified a distinct TCR repertoire and clonal expansion of certain TCRs, indicating the response to tissue-specific antigens (40). Importantly, treatment with the PPARγ agonist pioglitazone, which is used for the treatment of type 2 diabetes because of its positive effects on metabolic health and local inflammation, was shown to expand adipose tissue Tregs, which supports the idea of targeting tissue-specific Tregs for the treatment of diseases (39).
While Tregs in adipose tissue, muscle and the intestine have been studied extensively, only very little is known about Tregs in the pancreas. A study by the group of Christophe Benoist demonstrated that the diabetic lesions in NOD mice are enriched in CXCR3+ Tregs and that the expression of CXCR3 is dependent on Tbet. More importantly, they showed that the ablation of Tbet in Tregs accelerates the disease and overcomes the usually present sex-bias in NOD mice (41). Interestingly, Tbet+ Tregs were also found in the lamina propria of patients with inflammatory bowel disease (42) as well as in patients with multiple sclerosis (43), where Tbet+ Tregs were shown to contribute to the disease manifestation and being less suppressive (43). Importantly, the reduced suppressive activity was linked to the Ifnγ production of the Tregs which was not elevated in Tbet+ Tregs from the pancreas (41). These findings highlight the possibility of specifically targeting defined Treg subsets within the pancreas for a more tailored immune modulation. However, all studies conducted so far on pancreas residing Tregs focused solely on NOD mice with ongoing insulitis. A more detailed understanding of pancreas residing Tregs and their contribution to immune homeostasis in the steady state will be crucial to advance immune modulation targeted to the pancreas.
As one means to foster advancement in tissue-specific Treg targeting, recent years have seen tremendous progress in the simultaneous analysis of transcriptome, DNA methylation and accessibility, surface protein expression, perturbations, and receptor sequences on the single cell level. In this regard, computational strategies for integration of these complex data sets have enabled an unprecedented description of molecular behavior and identities of individual cells and therefore made it possible to move along to the next level of dissecting tissue Tregs (Figure 1).
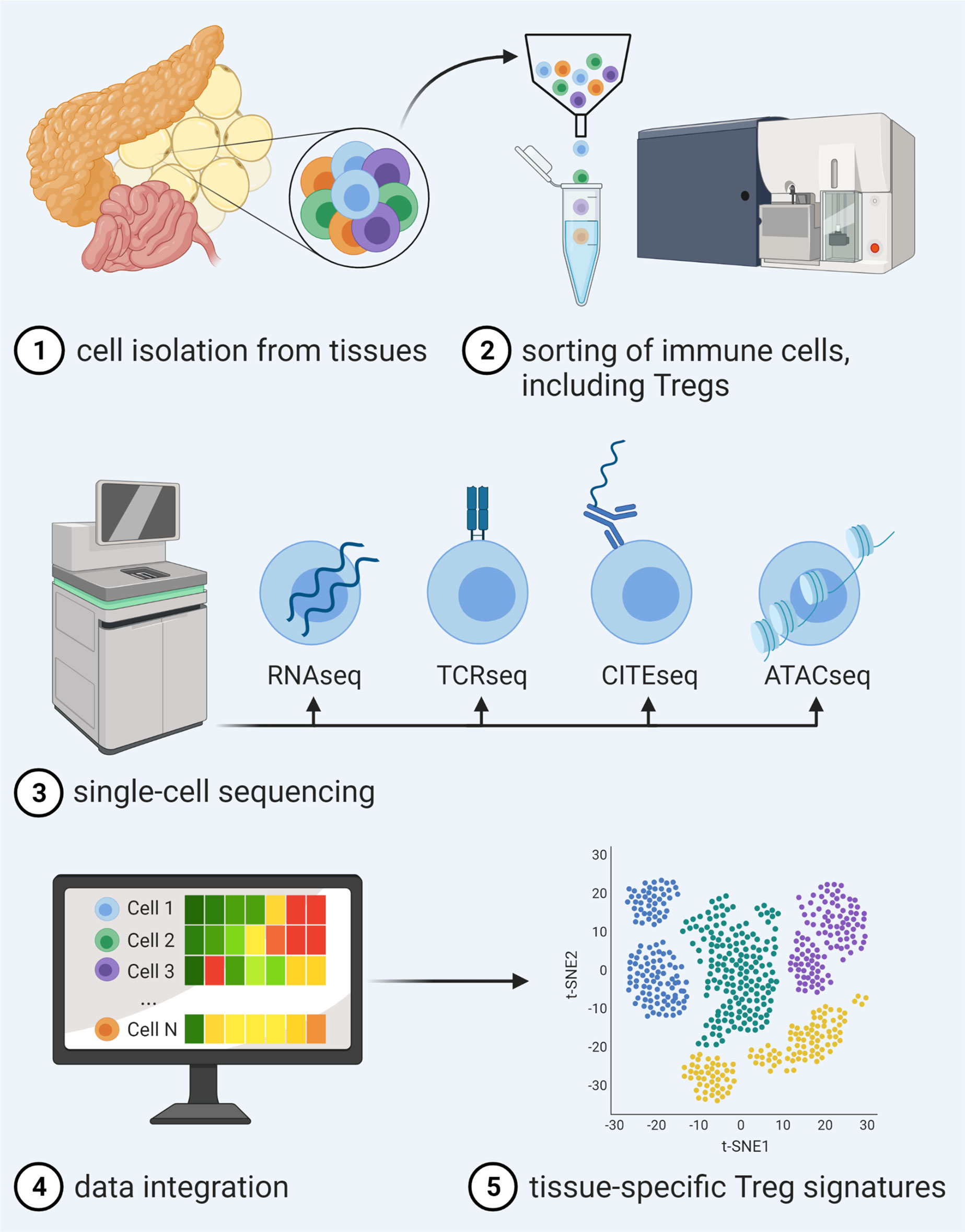
Figure 1 Advancements in single-cell multi-omics integration allow for a detailed analysis of tissue Treg signatures. After isolation and sorting of heterogeneous immune cell populations from tissues and single-cell sequencing of distinct libraries for RNAseq in combination with, e.g., TCRseq, CITEseq or ATACseq, novel computational approaches enable data integration of different traits, thereby enabling unprecedented description of molecular behavior and identities of individual cells within a certain tissue.
Current single-cell multi-omics methods can measure up to four different omics types at once [reviewed in (44–46)], with the transcriptomics layer often used to connect between the different omics types. These techniques bear high potential for medical research to study individual heterogeneity, drug resistance, or disease progression at an unprecedented level (47, 48). Especially, T cell focused immunological studies will benefit from recent developments as newly arising techniques can also simultaneously reconstruct TCR sequences and determine their specificities for a predefined set of epitopes (49–51). These methods have already greatly advanced our understanding of T cell responses in disease (50, 52–56), and lead to innovative analysis strategies such as the usage of TCR-sequence as natural barcodes to trace the cellular response pre- and post-antigen stimulation in vivo (57).
With the rise of single-cell multi-omics approaches, new computational models have been developed that can jointly analyze such multi-modal data [reviewed in (46, 58)]. Several studies used correlation-based approaches to jointly analyze copy number variations (59, 60), DNA methylation (61–63), or protein abundance (64) and gene expression data. Recently, Schattgen et al. proposed an integration approach for TCR and gene expression data based on graph analysis defined on transcriptomic and TCR distances and could uncover known and novel associations between TCR sequences and transcriptomics phenotypes (65). Others used traditional statistical approaches (66), or advanced deep learning methods (67–73) to integrate multiple data sources at once to represent the joint information of all omics-layers. Along these lines, a recent method by Zhang et al. jointly integrated TCR and transcriptomic information using Bayesian clustering based on the TCR sequence and gene expression profile (74). Through this method Zhang et al. could show that joint TCR and gene expression analysis better separates T-cell specificity and captures the antigen binding efficiency gradient better than TCR-information alone (74). Similarly, we introduced a joint TCR-transcriptome deep learning model which additionally captured transcriptional gradients within clonotypes (73). Such methods could be used to further elucidate the relationship between the TCR sequence and transcriptional information of Tregs in autoimmune diseases.
The identification of specific TCRs on tissue Tregs will help to define whether the migration of these cells to the tissue is likely antigen-driven and can also help to facilitate studies on tissue Tregs. In this regard, Diane Mathis group was able to analyze the ontogeny of visceral white adipose tissue (VAT)-residing Tregs by generating a mouse line transgenic for the TCR of an expanded VAT Treg clone (40). Additionally, the transfer of TCR transgenic Tregs has already been tested in preclinical studies for autoimmune diseases (75, 76). These studies mostly rely on the use of effector T cell derived TCRs and it is not entirely clear how that could affect Treg function, migration, and fate after transfer. The identification of tissue- and Treg-specific TCRs in the steady state as well as differences to the disease state might enable us to design such transgenic Tregs more strategically and could therefore help to increase efficacy and safety of TCR transgenic Treg infusions.
However, the identification of TCR sequences is only one side of the coin and a remaining bottleneck for T cell biology is the identification of the peptide-MHC ligands recognized by the identified TCRs. Here, recent advances have been made for experimental identification of epitopes recognized by orphan TCRs in a high-throughput screening of highly complex peptide-encoding oligo pools presented by bar-coded T cell-cytokine capturing APCs (77). Additionally, machine learning has enabled novel computational approaches to predict TCR specificity.
Sequence-based computational methods for TCR specificity analysis can be grouped into two categories: comparison and prediction. TCR comparison approaches impute antigen specificities by either allocating unknown TCRs to T-cell clusters or by assigning pairwise distance scores to TCR sequences with known antigen specificity. When several TCRs specific to the antigens of interest are known, these methods can be used to identify T cells with similar sequences likely to bind to the same antigen. The second category applies machine learning models to directly predict TCR binding to specific epitopes. Since these methods often additionally analyze the epitope sequence, they allow to predict specificity towards previously unknown antigens.
TCR sequences with common epitope specificity carry statistically enriched motifs (78, 79). Methods such as TCRdist (78) and GLIPH (79, 80) compare such common motifs to identify TCR sequences with shared antigen specificities. Other methods were proposed differing in computational approach to match TCRs using sequence similarity (81) or numeric embeddings (82, 83).
While comparison-based methods can serve as a proxy for determining TCR-specificity, such methods fail for novel epitopes without known corresponding TCRs. Machine learning methods can alleviate these issues by learning general rules that guide the T-cell epitope interaction. De Neuter et al. provided a proof of concept by predicting specificity towards one of two B*08 restricted HIV-1 epitopes based on the TCR CDR3β sequence (84). Jurtz et al. additionally incorporated the peptide sequence but observed limited generalization to unknown epitopes (85). Subsequently, different models developed on varying datasets have been proposed with limited improvements (86). In recent years, deep learning methods were introduced (52, 87–89), of which some incorporate additional information such as CDR3α, CDR1 and CDR2 sequences, HLA type, and surface protein counts leading partially to increased prediction performances (52, 86).
These tools will potentially enable the identification of Tregs associated with disease-relevant antigens by predicting the specificity for large libraries of sequenced T cells. By limiting the number of candidates, for which specificity needs to be tested, the time and cost for identifying disease-relevant Tregs will be significantly reduced. However, due to different evaluation methodologies and different datasets, these methods often cannot directly be compared. Therefore, it remains yet to be determined, which model to choose, and to what degree computational tools can be already used for the development of targeted immunotherapies. It is apparent though, that the use of multi-omics techniques for the deep characterization of tissue-specific Tregs can critically contribute to the development and advancement of Treg-based immunotherapies. TCR transgenic Tregs migrate to the site of immune activation and therefore will facilitate the development of effective and safe therapies. Additionally, identification of surface markers specific to tissue-residing Tregs will enable targeted delivery of therapeutics, e.g., miRNA inhibitors or mimics, to foster Tregs specifically at the site of the autoimmune attack.
While advances have been made for antigen-specific Treg inducing therapies e.g. to treat patients with severe peanut allergies, the success of such therapies in autoimmune T1D is still limited. A broad impairment in Treg induction in children during onset of islet autoimmunity highlights the necessity of combinatorial strategies to foster Tregs in order to open the window of opportunity for antigen-specific Treg therapies. miRNA-targeting offers the opportunity to improve Treg induction and stability in T1D. However new strategies to specifically modify miRNAs in specific cell types are needed. Identifying key signatures and characteristics of Tregs residing in the pancreas, the target organ of the disease, will be important to target therapies more specifically to those cells that are directly involved in the disease development and progression. Major advances in the use of single-cell multi-omics integration together with machine learning approaches for TCR specificity prediction have paved the way for a detailed description of individual cells from different tissues and will therefore help to bring antigen-specific Treg therapy to the next level.
IS and FD reviewed the literature and wrote the manuscript. CD and BS reviewed the literature and contributed to the conceptualization of the manuscript. All authors contributed to the article and approved the submitted version.
BS acknowledges financial support by the Postdoctoral Fellowship Program of the Helmholtz Zentrum München (https://www.helmholtz-muenchen.de/fellows/index.html). IS is supported by a Research Grant of the Deutsche Forschungsgemeinschaft (DFG, SE 3036/2-1). FD is supported by the Helmholtz Association under the joint research school “Munich School for Data Science - MUDS”. CD holds a professorship grant from the Excellence Program for Outstanding Female Scientists from the Helmholtz Association, is supported by a Research Group at Helmholtz Zentrum München, the German Center for Diabetes Research (DZD), through a membership in the CRC1054 of the Deutsche Forschungsgemeinschaft (B11), and through an award of the EFSD/JDRF/Lilly Programme on Type 1 Diabetes Research 2020.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Martin G. Scherm for preparing the figure using BioRender and all former and current members of our group for valuable input and helpful discussion.
1. Roncador G, Brown PJ, Maestre L, Hue S, Martínez-Torrecuadrada JL, Ling K-L, et al. Analysis of FOXP3 Protein Expression in Human CD4+CD25+ Regulatory T Cells at the Single-Cell Level. Eur J Immunol (2005) 35(6):1681–91. doi: 10.1002/eji.200526189
2. Hori S, Nomura T, Sakaguchi S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science (2003) 299(5609):1057–61. doi: 10.1126/science.1079490
3. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 Programs the Development and Function of CD4+CD25+ Regulatory T Cells. Nat Immunol (2003) 4(4):330–6. doi: 10.1038/ni904
4. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor Alpha-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J Immunol (1995) 155(3):1151–64.
5. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked Syndrome (IPEX) Is Caused by Mutations of FOXP3. Nat Genet (2001) 27(1):20–1. doi: 10.1038/83713
6. Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko S-A, et al. Disruption of a New Forkhead/Winged-Helix Protein, Scurfin, Results in the Fatal Lymphoproliferative Disorder of the Scurfy Mouse. Nat Genet (2001) 27(1):68–73. doi: 10.1038/83784
7. Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+) Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity (2019) 50(2):302–16. doi: 10.1016/j.immuni.2019.01.020
8. Gottschalk RA, Corse E, Allison JP. TCR Ligand Density and Affinity Determine Peripheral Induction of Foxp3 In Vivo. J Exp Med (2010) 207(8):1701–11. doi: 10.1084/jem.20091999
9. Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of Type 1 Diabetes in Mice by Tolerogenic Vaccination With a Strong Agonist Insulin Mimetope. J Exp Med (2011) 208(7):1501–10. doi: 10.1084/jem.20110574
10. Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, et al. T Cell Receptor Signaling Controls Foxp3 Expression Via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA (2008) 105(22):7797–802. doi: 10.1073/pnas.0800928105
11. Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H, et al. Inducing and Expanding Regulatory T Cell Populations by Foreign Antigen. Nat Immunol (2005) 6(12):1219–27. doi: 10.1038/ni1265
12. Miller A, Lider O, Weiner H. Antigen-Driven Bystander Suppression After Oral Administration of Antigens. J Exp Med (1991) 174(4):791–8. doi: 10.1084/jem.174.4.791
13. Raffin C, Vo LT, Bluestone JA. Treg Cell-Based Therapies: Challenges and Perspectives. Nat Rev Immunol (2020) 20(3):158–72. doi: 10.1038/s41577-019-0232-6
14. Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical Efficacy and Immune Regulation With Peanut Oral Immunotherapy. J Allergy Clin Immunol (2009) 124(2):292–300.e1-97. doi: 10.1016/j.jaci.2009.05.022
15. Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, et al. Peanut Oral Immunotherapy Results in Increased Antigen-Induced Regulatory T-Cell Function and Hypomethylation of Forkhead Box Protein 3 (FOXP3). J Allergy Clin Immunol (2014) 133(2):500–10. doi: 10.1016/j.jaci.2013.12.1037
16. Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, et al. DNA Methylation Controls Foxp3 Gene Expression. Eur J Immunol (2008) 38(6):1654–63. doi: 10.1002/eji.200838105
17. Serr I, Furst RW, Achenbach P, Scherm MG, Gokmen F, Haupt F, et al. Type 1 Diabetes Vaccine Candidates Promote Human Foxp3(+)Treg Induction in Humanized Mice. Nat Commun (2016) 7:10991. doi: 10.1038/ncomms10991
18. Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, et al. Effects of Oral Insulin in Relatives of Patients With Type 1 Diabetes: The Diabetes Prevention Trial–Type 1. Diabetes Care (2005) 28(5):1068–76. doi: 10.2337/diacare.28.5.1068
19. Chaillous L, Lefèvre H, Thivolet C, Boitard C, Lahlou N, Atlan-Gepner C, et al. Oral Insulin Administration and Residual (β-Cell Function in Recent-Onset Type 1 Diabetes: A Multicentre Randomised Controlled Trial. Lancet (2000) 356(9229):545–9. doi: 10.1016/S0140-6736(00)02579-4
20. Sosenko JM, Skyler JS, Herold KC, Schatz DA, Haller MJ, Pugliese A, et al. Slowed Metabolic Decline After 1 Year of Oral Insulin Treatment Among Individuals at High Risk for Type 1 Diabetes in the Diabetes Prevention Trial–Type 1 (Dpt-1) and TrialNet Oral Insulin Prevention Trials. Diabetes (2020) 69(8):1827–32. doi: 10.2337/db20-0166
21. Serr I, Kral M, Scherm MG, Daniel C. Advances in Human Immune System Mouse Models for Personalized Treg-Based Immunotherapies. Front Immunol (2021) 12(643544):332. doi: 10.3389/fimmu.2021.643544
22. Serr I, Scherm MG, Zahm AM, Schug J, Flynn VK, Hippich M, et al. A miRNA181a/NFAT5 Axis Links Impaired T Cell Tolerance Induction With Autoimmune Type 1 Diabetes. Sci Trans Med (2018) 10(422):eaag1782. doi: 10.1126/scitranslmed.aag1782
23. Scherm MG, Serr I, Zahm AM, Schug J, Bellusci S, Manfredini R, et al. miRNA142-3p Targets Tet2 and Impairs Treg Differentiation and Stability in Models of Type 1 Diabetes. Nat Commun (2019) 10(1):5697. doi: 10.1038/s41467-019-13587-3
24. Bonifacio E, Ziegler AG, Klingensmith G, Schober E, Bingley PJ, Rottenkolber M, et al. Effects of High-Dose Oral Insulin on Immune Responses in Children at High Risk for Type 1 Diabetes: The Pre-POINT Randomized Clinical Trial. JAMA (2015) 313(15):1541–9. doi: 10.1001/jama.2015.2928
25. Ziegler A-G, Achenbach P, Berner R, Casteels K, Danne T, Gündert M, et al. Oral Insulin Therapy for Primary Prevention of Type 1 Diabetes in Infants With High Genetic Risk: The GPPAD-POInT (Global Platform for the Prevention of Autoimmune Diabetes Primary Oral Insulin Trial) Study Protocol. BMJ Open (2019) 9(6):e028578. doi: 10.1136/bmjopen-2018-028578
26. Liston A, Lu LF, O'Carroll D, Tarakhovsky A, Rudensky AY. Dicer-Dependent microRNA Pathway Safeguards Regulatory T Cell Function. J Exp Med (2008) 205(9):1993–2004. doi: 10.1084/jem.20081062
27. Chong MM, Rasmussen JP, Rudensky AY, Littman DR. The RNAseIII Enzyme Drosha Is Critical in T Cells for Preventing Lethal Inflammatory Disease. J Exp Med (2008) 205(9):2005–17. doi: 10.1084/jem.20081219
28. Cobb BS, Nesterova TB, Thompson E, Hertweck A, O'Connor E, Godwin J, et al. T Cell Lineage Choice and Differentiation in the Absence of the RNase III Enzyme Dicer. J Exp Med (2005) 201(9):1367–73. doi: 10.1084/jem.20050572
29. Scherm MG, Serr I, Kaestner KH, Daniel C. The Role of T Cell miRNAs for Regulatory T Cell Induction in Islet Autoimmunity. Mol Metab (2019) 27S:S122–8. doi: 10.1016/j.molmet.2019.06.009
30. Scherm MG, Daniel C. Mirna Regulation of T Cells in Islet Autoimmunity and Type 1 Diabetes. Curr Diabetes Rep (2020) 20(9):41. doi: 10.1007/s11892-020-01325-9
31. Scherm MG, Daniel C. MiRNA-Mediated Immune Regulation in Islet Autoimmunity and Type 1 Diabetes. Front Endocrinol (2020) 11:914. doi: 10.3389/fendo.2020.606322
32. Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, et al. Lymphoproliferative Disease and Autoimmunity in Mice With Increased miR-17-92 Expression in Lymphocytes. Nat Immunol (2008) 9(4):405–14. doi: 10.1038/ni1575
33. Serr I, Furst RW, Ott VB, Scherm MG, Nikolaev A, Gokmen F, et al. miRNA92a Targets KLF2 and the Phosphatase PTEN Signaling to Promote Human T Follicular Helper Precursors in T1D Islet Autoimmunity. Proc Natl Acad Sci USA (2016) 113(43):E6659–68. doi: 10.1073/pnas.1606646113
34. Serr I, Daniel C. Regulation of T Follicular Helper Cells in Islet Autoimmunity. Front Immunol (2018) 9:1729. doi: 10.3389/fimmu.2018.01729
35. Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, et al. miR-181a Is an Intrinsic Modulator of T Cell Sensitivity and Selection. Cell (2007) 129(1):147–61. doi: 10.1016/j.cell.2007.03.008
36. Yue X, Trifari S, Aijo T, Tsagaratou A, Pastor WA, Zepeda-Martinez JA, et al. Control of Foxp3 Stability Through Modulation of TET Activity. J Exp Med (2016) 213(3):377–97. doi: 10.1084/jem.20151438
37. van der Ree MH, de Vree WA, Stelma WA, Willemse WA, van der Valk WA, Rietdijk WA, et al. Safety, Tolerability, and Antiviral Effect of RG-101 in Patients With Chronic Hepatitis C: A Phase 1B, Double-Blind, Randomised Controlled Trial. Lancet (2017) 389(10070):709–17. doi: 10.1016/S0140-6736(16)31715-9
38. Becker M, Levings MK, Daniel C. Adipose-Tissue Regulatory T Cells: Critical Players in Adipose-Immune Crosstalk. Eur J Immunol (2017) 47(11):1867–74. doi: 10.1002/eji.201646739
39. Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. Ppar-γ Is a Major Driver of the Accumulation and Phenotype of Adipose Tissue Treg Cells. Nature (2012) 486(7404):549–53. doi: 10.1038/nature11132
40. Li C, DiSpirito JR, Zemmour D, Spallanzani RG, Kuswanto W, Benoist C, et al. Tcr Transgenic Mice Reveal Stepwise, Multi-Site Acquisition of the Distinctive Fat-Treg Phenotype. Cell (2018) 174(2):285–99.e12. doi: 10.1016/j.cell.2018.05.004
41. Tan TG, Mathis D, Benoist C. Singular Role for T-BET+CXCR3+ Regulatory T Cells in Protection From Autoimmune Diabetes. Proc Natl Acad Sci USA (2016) 113(49):14103–8. doi: 10.1073/pnas.1616710113
42. Di Giovangiulio M, Rizzo A, Franzè E, Caprioli F, Facciotti F, Onali S, et al. Tbet Expression in Regulatory T Cells Is Required to Initiate Th1-Mediated Colitis. Front Immunol (2019) 10:2158. doi: 10.3389/fimmu.2019.02158
43. Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T Helper Type 1–Like, Foxp3+ Regulatory T Cells in Human Autoimmune Disease. Nat Med (2011) 17(6):673–5. doi: 10.1038/nm.2389
44. Chappell L, Russell AJC, Voet T. Single-Cell (Multi)Omics Technologies. Annu Rev Genomics Hum Genet (2018) 19:15–41. doi: 10.1146/annurev-genom-091416-035324
45. Lee J, Hyeon DY, Hwang D. Single-Cell Multiomics: Technologies and Data Analysis Methods. Exp Mol Med (2020) 52(9):1428–42. doi: 10.1038/s12276-020-0420-2
46. Ma A, McDermaid A, Xu J, Chang Y, Ma Q. Integrative Methods and Practical Challenges for Single-Cell Multi-Omics. Trends Biotechnol (2020) 38(9):1007–22. doi: 10.1016/j.tibtech.2020.02.013
47. Tang X, Huang Y, Lei J, Luo H, Zhu X. The Single-Cell Sequencing: New Developments and Medical Applications. Cell Biosci (2019) 9(1):53. doi: 10.1186/s13578-019-0314-y
48. Peng A, Mao X, Zhong J, Fan S, Hu Y. Single-Cell Multi-Omics and Its Prospective Application in Cancer Biology. Proteomics (2020) 20(13):e1900271. doi: 10.1002/pmic.201900271
49. Mimitou EP, Cheng A, Montalbano A, Hao S, Stoeckius M, Legut M, et al. Multiplexed Detection of Proteins, Transcriptomes, Clonotypes and CRISPR Perturbations in Single Cells. Nat Methods (2019) 16(5):409–12. doi: 10.1038/s41592-019-0392-0
50. Ma K-Y, Schonnesen AA, He C, Xia AY, Sun E, Chen E, et al. High-Throughput and High-Dimensional Single Cell Analysis of Antigen-Specific Cd8+ T Cells. bioRxiv (2021) 2021.03.04.433914. doi: 10.1101/2021.03.04.433914
51. 10xGenomics. A New Way of Exploring Immunity - Linking Highly Multiplexed Antigen Recognition to Immune Repertoire and Phenotype (2019). Available at: https://www.10xgenomics.com/resources/application-notes/a-new-way-of-exploring-immunity-linking-highly-multiplexed-antigen-recognition-to-immune-repertoire-and-phenotype/ (Accessed July 22, 2019).
52. Fischer DS, Ansari M, Wagner KI, Jarosch S, Huang Y, Mayr CH, et al. Single-Cell RNA Sequencing Reveals In Vivo Signatures of SARS-Cov-2-Reactive T Cells Through ‘Reverse Phenotyping’. medRxiv (2020) 2020.12.07.20245274. doi: 10.1101/2020.12.07.20245274
53. Zheng C, Yoo L, Guo JK, Zhang H, Guo Y, Zheng X, et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell (2017) 169(7):1342–56.e16. doi: 10.1016/j.cell.2017.05.035
54. Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir E-aD, Amadori L, et al. Single-Cell Immune Landscape of Human Atherosclerotic Plaques. Nat Med (2019) 25(10):1576–88. doi: 10.1038/s41591-019-0590-4
55. Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, et al. Defining T Cell States Associated With Response to Checkpoint Immunotherapy in Melanoma. Cell (2018) 175(4):998–1013.e20. doi: 10.1016/j.cell.2018.10.038
56. Su Y, Chen D, Yuan D, Lausted C, Choi J, Dai CL, et al. Multi-Omics Resolves a Sharp Disease-State Shift Between Mild and Moderate Covid-19. Cell (2020) 183(6):1479–95.e20. doi: 10.1016/j.cell.2020.10.037
57. Fischer DS, Wu Y, Schubert B, Theis FJ. Predicting Antigen Specificity of Single T Cells Based on TCR CDR3 Regions. Mol Syst Biol (2020) 16(8):e9416. doi: 10.15252/msb.20199416
58. Colomé-Tatché M, Theis FJ. Statistical Single Cell Multi-Omics Integration. Curr Opin Syst Biol (2018) 7:54–9. doi: 10.1016/j.coisb.2018.01.003
59. Dey SS, Kester L, Spanjaard B, Bienko M, van Oudenaarden A. Integrated Genome and Transcriptome Sequencing of the Same Cell. Nat Biotechnol (2015) 33(3):285–9. doi: 10.1038/nbt.3129
60. Han KY, Kim KT, Joung JG, Son DS, Kim YJ, Jo A, et al. SIDR: Simultaneous Isolation and Parallel Sequencing of Genomic DNA and Total RNA From Single Cells. Genome Res (2018) 28(1):75–87. doi: 10.1101/gr.223263.117
61. Angermueller C, Clark SJ, Lee HJ, Macaulay IC, Teng MJ, Hu TX, et al. Parallel Single-Cell Sequencing Links Transcriptional and Epigenetic Heterogeneity. Nat Methods (2016) 13(3):229–32. doi: 10.1038/nmeth.3728
62. Hou Y, Guo H, Cao C, Li X, Hu B, Zhu P, et al. Single-Cell Triple Omics Sequencing Reveals Genetic, Epigenetic, and Transcriptomic Heterogeneity in Hepatocellular Carcinomas. Cell Res (2016) 26(3):304–19. doi: 10.1038/cr.2016.23
63. Hu Y, Huang K, An Q, Du G, Hu G, Xue J, et al. Simultaneous Profiling of Transcriptome and DNA Methylome From a Single Cell. Genome Biol (2016) 17(1):88. doi: 10.1186/s13059-016-0950-z
64. Peterson VM, Zhang KX, Kumar N, Wong J, Li L, Wilson DC, et al. Multiplexed Quantification of Proteins and Transcripts in Single Cells. Nat Biotechnol (2017) 35(10):936–9. doi: 10.1038/nbt.3973
65. Schattgen SA, Guion K, Crawford JC, Souquette A, Barrio AM, Stubbington MJT, et al. Linking T Cell Receptor Sequence to Transcriptional Profiles With Clonotype Neighbor Graph Analysis (Conga). bioRxiv (2020) 2020.06.04.134536. doi: 10.1101/2020.06.04.134536
66. Welch JD, Kozareva V, Ferreira A, Vanderburg C, Martin C, Macosko EZ, et al. Single-Cell Multi-Omic Integration Compares and Contrasts Features of Brain Cell Identity. Cell (2019) 177(7):1873–87.e17. doi: 10.1016/j.cell.2019.05.006
67. Welch JD, Hartemink AJ, Prins JF. MATCHER: Manifold Alignment Reveals Correspondence Between Single Cell Transcriptome and Epigenome Dynamics. Genome Biol (2017) 18(1):138. doi: 10.1186/s13059-017-1269-0
68. Gayoso A, Steier Z, Lopez R, Regier J, Nazor KL, Streets A, et al. Joint Probabilistic Modeling of Single-Cel Multi-Omic Data With TotalVI. Nat Methods (2020) 18(3):272–82. doi :10.1038/s41592-020-01050-x
69. Amodio M, Krishnaswamy S. Magan: Aligning Biological Manifolds. In: Jennifer D, Andreas K, editors. Proceedings of the 35th International Conference on Machine Learning. PMLR: Proceedings of Machine Learning Research (2018). p. 215–23.
70. Liu J, Huang Y, Singh R, Vert J-P, Noble WS. Jointly Embedding Multiple Single-Cell Omics Measurements. bioRxiv (2019) 644310. doi: 10.1101/644310
71. Cao K, Bai X, Hong Y, Wan L, et al. Unsupervised Topological Alignment for Single-Cell Multi-Omics Integration. Bioinformatics (2020) 36(Supplement_1):i48–56. doi: 10.1093/bioinformatics/btaa443
72. Stanley JS III, Prog B, Gigante S, Wolf G, Krishnaswamy S. Manifold Alignment With Feature Correspondence (2019). Available at: https://www.researchgate.net/profile/Scott-Gigante-2/publication/337029752_Manifold_Alignment_by_Feature_Correspondence/links/5dc18bb5299bf1a47b18a6e1/Manifold-Alignment-by-Feature-Correspondence.pdf.
73. An Y, Drost F, Theis F, Schubert B, Lotfollahi M. Jointly Learning T-cell Receptor and Transcriptomic Information to Decipher the Immune Response. bioRxiv (2021) 2021.06.24.449733. doi: 10.1101/2021.06.24.449733
74. Zhang Z, Xiong D, Wang X, Liu H, Wang T. Mapping the Functional Landscape of T Cell Receptor Repertoires by Single-T Cell Transcriptomics. Nat Methods (2021) 18(1):92–9. doi: 10.1038/s41592-020-01020-3
75. Kim YC, Zhang AH, Su Y, Rieder SA, Rossi RJ, Ettinger RA, et al. Engineered Antigen-Specific Human Regulatory T Cells: Immunosuppression of FVIII-Specific T- and B-Cell Responses. Blood (2015) 125(7):1107–15. doi: 10.1182/blood-2014-04-566786
76. Kim YC, Zhang AH, Yoon J, Culp WE, Lees JR, Wucherpfennig KW, et al. Engineered MBP-specific Human Tregs Ameliorate MOG-Induced EAE Through IL-2-Triggered Inhibition of Effector T Cells. J Autoimmun (2018) 92:77–86. doi: 10.1016/j.jaut.2018.05.003
77. Lee MN, Meyerson M. Antigen Identification for HLA Class I– and HLA Class II–Restricted T Cell Receptors Using Cytokine-Capturing Antigen-Presenting Cells. Sci Immunol (2021) 6(55):eabf4001. doi: 10.1126/sciimmunol.abf4001
78. Dash P, Fiore-Gartland AJ, Hertz T, Wang GC, Sharma S, Souquette A, et al. Quantifiable Predictive Features Define Epitope-Specific T Cell Receptor Repertoires. Nature (2017) 547(7661):89–93. doi: 10.1038/nature22383
79. Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, et al. Identifying Specificity Groups in the T Cell Receptor Repertoire. Nature (2017) 547(7661):94–8. doi: 10.1038/nature22976
80. Huang H, Wang H, Rubelt H, Scriba H, Davis H. Analyzing the Mycobacterium Tuberculosis Immune Response by T-Cell Receptor Clustering With GLIPH2 and Genome-Wide Antigen Screening. Nat Biotechnol (2020) 38(10):1194–202. doi: 10.1038/s41587-020-0505-4
81. Thakkar N, Bailey-Kellogg C. Balancing Sensitivity and Specificity in Distinguishing TCR Groups by CDR Sequence Similarity. BMC Bioinf (2019) 20(1):241. doi: 10.1186/s12859-019-2864-8
82. Zhang H, Liu L, Zhang J, Chen J, Ye J, Shukla S, et al. Investigation of Antigen-Specific T-Cell Receptor Clusters in Human Cancers. Clin Cancer Res (2020) 26(6):1359–71. doi: 10.1158/1078-0432.CCR-19-3249
83. Valkiers S, Van Houcke M, Laukens K, Meysman P. clusTCR: A Python Interface for Rapid Clustering of Large Sets of CDR3 Sequences With Unknown Antigen Specificity. Bioinformatics (2021) btab446:1367-4803. doi: 10.1093/bioinformatics/btab446
84. De Neuter N, Bittremieux W, Beirnaert C, Cuypers B, Mrzic A, Moris P, et al. On the Feasibility of Mining CD8+ T Cell Receptor Patterns Underlying Immunogenic Peptide Recognition. Immunogenetics (2018) 70(3):159–68. doi: 10.1007/s00251-017-1023-5
85. Jurtz VI, Jessen LE, Bentzen AK, Jespersen MC, Mahajan S, Vita R, et al. NetTCR: Sequence-Based Prediction of TCR Binding to Peptide-MHC Complexes Using Convolutional Neural Networks. bioRxiv (2018) 433706. doi: 10.1101/433706
86. Jokinen E, Huuhtanen J, Mustjoki S, Heinonen M, Lähdesmäki H. Predicting Recognition Between T Cell Receptors and Epitopes With TCRGP. PloS Comput Biol (2021) 17(3):e1008814. doi: 10.1371/journal.pcbi.1008814
87. Moris P, De Pauw J, Postovskaya A, Gielis S, De Neuter N, Bittremieux W, et al. Current Challenges for Epitope-Agnostic TCR Interaction Prediction and a New Perspective Derived From Image Classification. Brief Bioinform (2020) bbaa318:1477–4054. doi: 10.1093/bib/bbaa318
88. Luu AM, Leistico JR, Miller T, Kim S, Song JS. Predicting TCR-Epitope Binding Specificity Using Deep Metric Learning and Multimodal Learning. Genes (2021) 12(4):572. doi: 10.3390/genes12040572
Keywords: antigen-specific Treg therapy, autoimmunity, T1D, microRNAs, tissue Tregs, single-cell multi-omics integration, TCR specificity prediction
Citation: Serr I, Drost F, Schubert B and Daniel C (2021) Antigen-Specific Treg Therapy in Type 1 Diabetes – Challenges and Opportunities. Front. Immunol. 12:712870. doi: 10.3389/fimmu.2021.712870
Received: 21 May 2021; Accepted: 06 July 2021;
Published: 22 July 2021.
Edited by:
Giang Tran, University of New South Wales, AustraliaCopyright © 2021 Serr, Drost, Schubert and Daniel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolin Daniel, Y2Fyb2xpbi5kYW5pZWxAaGVsbWhvbHR6LW11ZW5jaGVuLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.