- 1General ICU, The First Affiliated Hospital of Zhengzhou University, Henan Key Laboratory of Critical Care Medicine, Zhengzhou Key Laboratory of Sepsis, Henan Engineering Research Center for Critical Care Medicine, Zhengzhou, China
- 2Academy of Medical Sciences, Zhengzhou University, Zhengzhou, China
- 3College of Bioinformatics Science and Technology, Harbin Medical University, Harbin, China
- 4Department of Pharmacy, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Objective: Corticosteroids are a common option used in sepsis treatment. However, the efficacy and potential risk of corticosteroids in septic patients have not been well assessed. This review was performed to assess the efficacy and safety of corticosteroids in patients with sepsis.
Methods: PubMed, Embase, and Cochrane library databases were searched from inception to March 2021. Randomized controlled trials (RCTs) that evaluated the effect of corticosteroids on patients with sepsis were included. The quality of outcomes in the included articles was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation methodology. The data were pooled by using risk ratio (RR) and mean difference (MD). The random-effects model was used to evaluate the pooled MD or RR and 95% confidence intervals (CIs).
Results: Fifty RCTs that included 12,304 patients with sepsis were identified. Corticosteroids were not associated with the mortality in 28-day (RR, 0.94; 95% CI, 0.87–1.02; evidence rank, moderate) and long-term mortality (>60 days) (RR, 0.96; 95% CI, 0.88–1.05) in patients with sepsis (evidence rank, low). However, corticosteroids may exert a significant effect on the mortality in the intensive care unit (ICU) (RR, 0.9; 95% CI, 0.83–0.97), in-hospital (RR, 0.9; 95% CI, 0.82–0.99; evidence rank, moderate) in patients with sepsis or septic shock (evidence rank, low). Furthermore, corticosteroids probably achieved a tiny reduction in the length of hospital stay and ICU. Corticosteroids were associated with a higher risk of hypernatremia and hyperglycemia; furthermore, they appear to have no significant effect on superinfection and gastroduodenal bleeding.
Conclusions: Corticosteroids had no significant effect on the 28-day and long-term mortality; however, they decreased the ICU and hospital mortality. The findings suggest that the clinical corticosteroids may be an effective therapy for patients with sepsis during the short time.
Systematic Review Registration: https://inplasy.com/wp-content/uploads/2021/05/INPLASY-Protocol-1074-4.pdf
Introduction
Sepsis is a life-threatening organ dysfunction, which is caused by a dysregulated host response to infection (1, 2) that culminates in systematic hypoperfusion and considerable organ dysfunction. The main therapies to treat sepsis in the early phase are antibiotic administration and perfusion restoration (3). Early and aggressive treatment is associated with a mortality rate of 30%–50% in critically ill patients admitted to the intensive care unit (ICU) and induces more than 5 million deaths each year across the world (3, 4). Therefore, further investigation for the treatment of sepsis is crucial.
The pathology of sepsis is marked by a dysregulated host response to infection; therefore, immunomodulatory therapies have been used in sepsis treatment that may be effective (5). Doctors have started using corticosteroids as an adjuvant therapy for sepsis since the middle of the twentieth century (3). Corticosteroids were used to treat sepsis, especially the septic shock therapy; numerous randomized clinical trials (RCTs) were performed to evaluate the safety and efficacy of corticosteroids. However, the results of these RCTs varied. Thus, many systematic reviews have been performed to assess the safety and efficacy of corticosteroids in patients with sepsis. However, the results of the most recent reviews remain controversial (6, 7). Subsequently, several studies have further assessed whether the combination of corticosteroids, vitamin C, and thiamine as compared with corticosteroids or placebo improved the survival duration, increased the vasopressor-free time over 7 days, and reduced organ injury (8, 9). These results suggest that the use of corticosteroids in combination with other drugs did not affect the safety and efficacy of corticosteroids in patients with sepsis. Hence, resolution of this controversy regarding the latest reviews that have assessed the efficacy of corticosteroids in patients with sepsis is currently the primary problem in sepsis treatment. Therefore, this systematic review and meta-analysis were performed based on the latest reviews to reintegrate the relevant data to evaluate the effects and safety of corticosteroids in patients with sepsis.
Methods
The protocol of this systematic review and meta-analysis was registered on INPLASY (ID: INPLASY2020110122). The methodology of this study was according to items of the Cochrane Collaboration, and each outcome was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines (10).
Study Searches
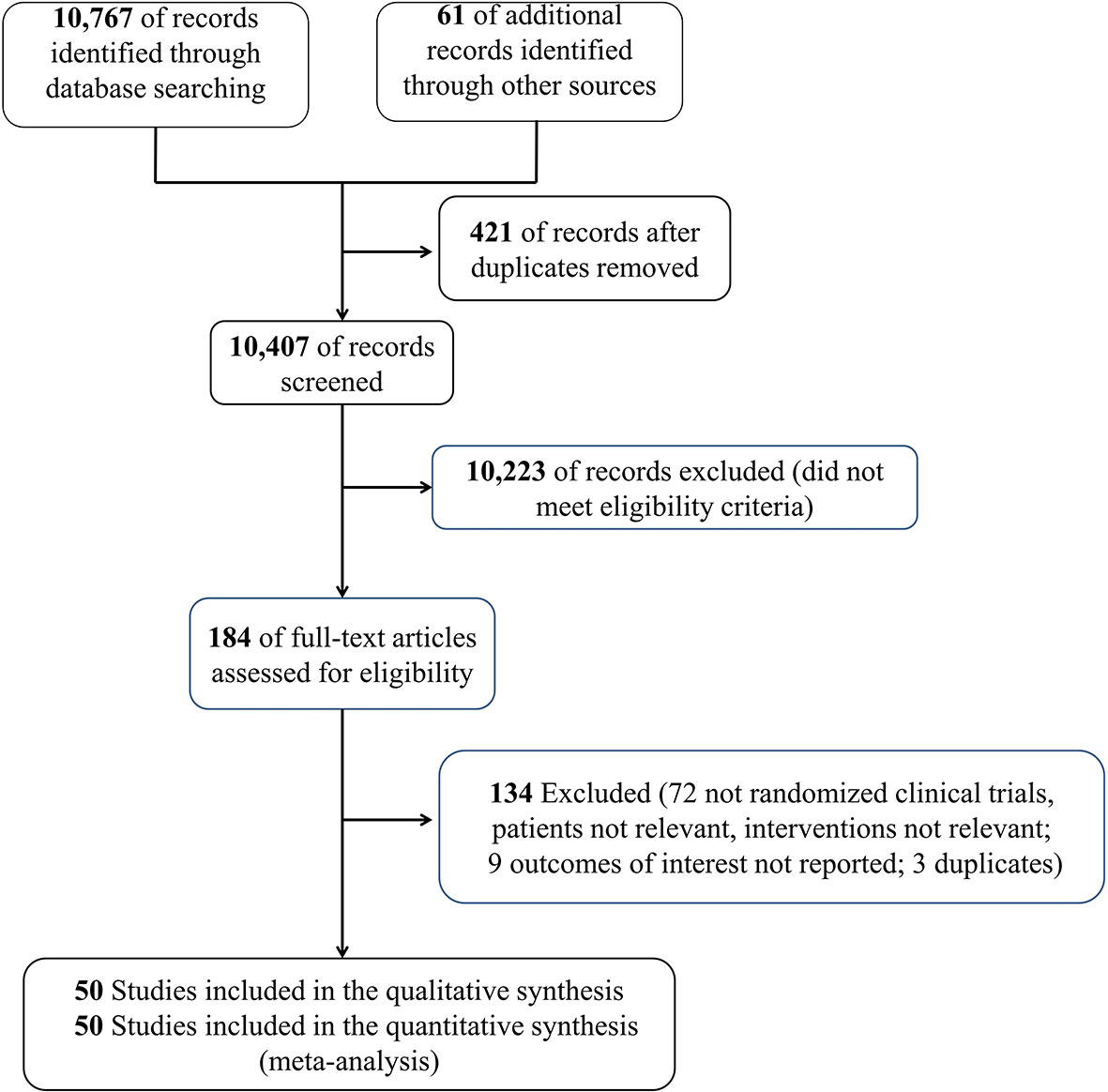
This meta-analysis was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria. Moreover, the PRISMA 2020 checklist is shown in Supplemental Table 1. PubMed, Embase, and Cochrane library databases were searched for relevant data from inception to March 2021, update to 5 July 2021, to identify RCTs that have evaluated the effect of corticosteroids on patients with sepsis. The MeSH/Emtree and title/abstract keyword combination were used to identify the eligible articles; the keyword search terms used for the English literature included the words corticosteroids and sepsis (detailed search strategy in Supplemental Table 2). It is noteworthy that we also conducted a manual search for the references of the relevant articles (study search flowchart in Figure 1).
Study Selection
Before the potential articles were searched and screened, the eligibility criteria and exclusion criteria were identified. Articles may be eligible according to the inclusion criteria in this study if they meet all of the following conditions: (1) adult patients diagnosed with sepsis, severe sepsis, or septic shock, as per the inclusion criteria during the study (11–13) [studies reporting adult patients with acute respiratory distress syndrome (ARDS) and sepsis were included]; (2) the study compared the use of corticosteroids (including hydrocortisone, methylprednisolone, betamethasone, fludrocortisone, and dexamethasone) with no use of corticosteroids; (3) the study measured and reported the outcomes in terms of 28-day and long-term mortality (>60 days), ICU mortality, in-hospital mortality, length of stay in hospital and ICU, vasopressor-free days, ventilation-free time, shock reversal at days 7 and 28, time for resolution of shock, Sequential Organ Failure Assessment (SOFA) scores at day 7, hypernatremia, hyperglycemia, superinfection, and gastroduodenal bleeding; (4) the study was an RCT or abstract and was published in English. Furthermore, the study design including case reports, case series, and observational studies or the previous unpublished studies that required the author to be contacted were excluded. All the available articles were searched by two searchers, respectively, and when disagreements occurred during the process, the third investigator should resolve these disagreements. Reviewers performed reviews in pairs to screen all relevant citations and references as per the search strategy, and the screening process included the following two stages: initial evaluation of titles and abstracts and skimming of the full text to identify the eligible studies.
Data Extraction
Researchers conducted data extraction, respectively, and in duplicate based on the eligibility and exclusion criteria. In case of disagreements, the third reviewer resolved the issue. Relevant data, including the study title, first author, study type, study period, the therapy in treatment and control groups, reported outcomes, sepsis definition, and so forth were collected. The data only for the studies that we searched including the previous review (6) were abstracted. The risk of bias for this meta-analysis was assessed by two investigators independently for every abstracted data of each article based on the Cochrane Collaboration (14) and domains including allocation concealment, blinding of participants and staff, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other biases. Additionally, the GRADE framework was used to evaluate the overall evidence rank for every outcome (15). The studies with more than six, four, to six and fewer than six items were considered high, fair, and poor quality, respectively. Importantly, the GRADE was used to assess the evidence rank of mortality and adverse events. According to the risk of bias, inconsistency, indirectness, imprecision, and publication bias, the studies were evaluated as low, moderate, or high quality.
Statistical Analyses
Mantel–Haenszel (M-H) or DerSimonian Laird (DL) methods with random-effects meta-analyses were conducted for the eligible RCTs. All the relevant data were assessed using the Review Manager (RevMan), version 5.3 (Cochrane Collaboration), STATA 16.0 (StataCorp, College Station, TX, USA). Risk ratio (RR) and mean difference (MD) were used to present the dichotomous and continuous outcomes, with 95% CI. Moreover, a Funnel plot was used to examine the potential for some small effects if the outcome included more than 10 trials, and the possibility of publication bias was assessed using the Funnel plot and Egger regression test (16). The chi-square test, I2, and visual inspection of the forest plots were used to evaluate heterogeneity among the eligible studies; when I2 was >50%, the heterogeneity was considered substantial. In addition, we performed the subgroup analyses based on the following variables: sepsis subtype [sepsis, septic shock, sepsis and ARDS, sepsis and community-acquired pneumonia (CAP), and severe COVID-19], type of corticosteroids (hydrocortisone or hydrocortisone plus fludrocortisone or methylprednisolone or prednisone or betamethasone or dexamethasone), and type of ICU [surgical, medical (internal) or surgical/medical ICU], searching the source of heterogeneity. Additionally, as the unit dose of the corticosteroids varied, relevant included studies about the use of corticosteroids were collected based on catecholamine use for qualitative analysis.
Results
Characteristics of Eligible Studies
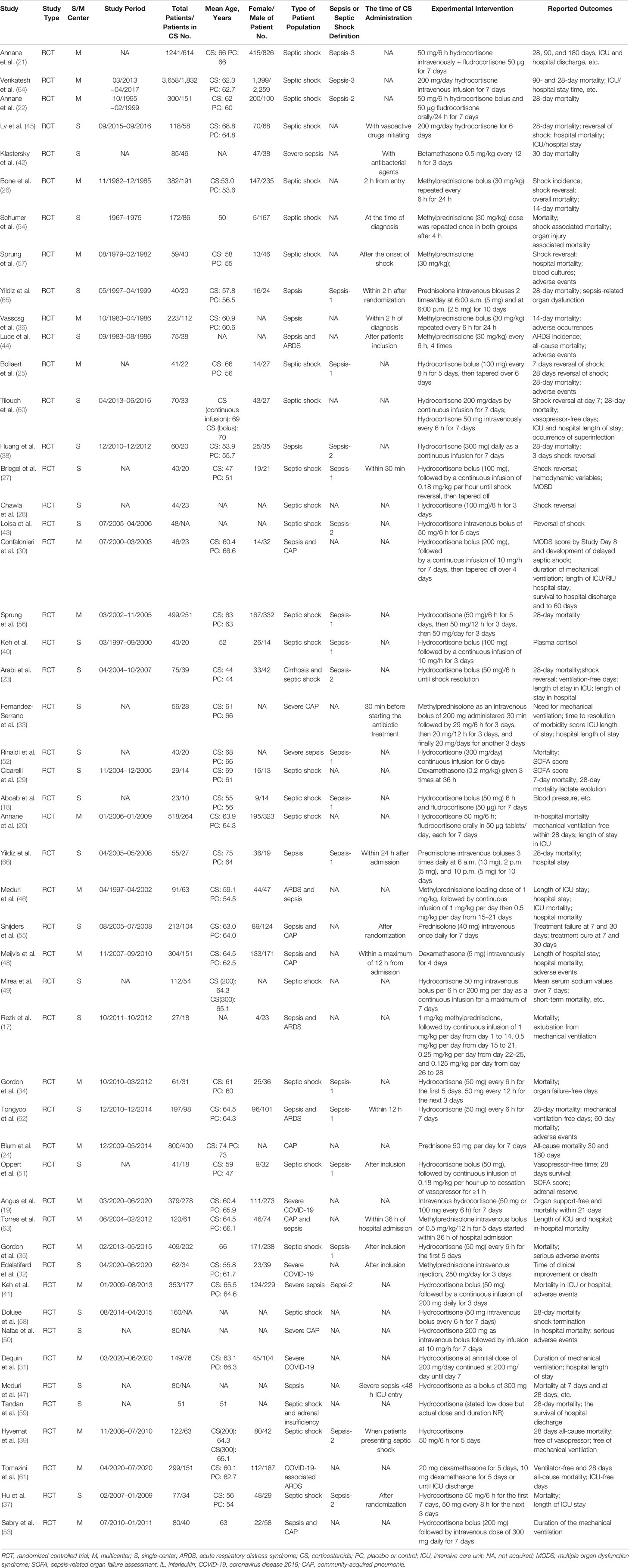
We initially identified 10,828 records, and 10,407 citations remained after the duplicate trials were removed; 184 RCTs were eligible after preliminary screening by title and abstract. Finally, 50 RCTs (17–66) that included 12,304 patients with sepsis were included in this meta-analysis (Figure 1). The characteristics of the included RCTs are listed in Table 1. Twenty-five RCTs (18, 20–23, 25–29, 34, 35, 37, 39, 40, 43, 45, 51, 54, 56–60, 64) on 8,400 patients with septic shock, 8 RCTs (36, 38, 40, 42, 47, 52, 65, 66) on 936 patients with sepsis, 4 RCTs (17, 44, 46, 62) on 390 patients with sepsis and ARDS, 8 RCTs (24, 30, 33, 48, 50, 53, 55, 63) on 1,699 patients with sepsis and community-acquired pneumonia, and 4 RCTs (19, 31, 32, 61) on 748 patients with severe COVID-19 were included. Additionally, 27 RCTs (19, 23, 25, 27, 28, 30–32, 34, 35, 37–41, 43, 45, 47, 49–53, 56, 58, 59, 64) (6,981 patients) of which were treated with hydrocortisone, 4 RCTs (18, 20–22) (2,082 patients) with hydrocortisone plus fludrocortisone, 10 RCTs (17, 26, 32, 33, 36, 44, 46, 54, 57, 63) (1,245 patients) with methylprednisolone, 4 RCTs (33, 55, 65, 66) (364 patients) with prednisolone, 3 RCTs (29, 48, 61) (408 patients) with dexamethasone, and only 1 RCT (42) with betamethasone (85 patients). Furthermore, 6 RCTs (39, 51, 60, 62, 65, 66) recruited eligible patients into the medical (internal) ICU and 3 studies (23, 29, 54) into the surgical ICU, and the remaining 40 studies were reported in the medical/surgical ICU or ICU. Moreover, 24 studies (17, 20–23, 25, 27–29, 31, 34, 35, 39, 41, 43, 45, 47, 48, 51, 56–58, 60, 61) showed corticosteroids use based on catecholamine in patients with septic shock. Specifically, 16 RCTs (20–23, 31, 34, 35, 39, 41, 43, 45, 56, 58, 60, 61) reported corticosteroids dose to be not more than 200 mg/day or 50 mg every 6 h; 8 RCTs (17, 25, 27–29, 47, 48, 51) showed a dose more than 200 mg/day (most of which were 200–300 mg/day). In addition, 21 studies (26, 27, 32, 33, 35–37, 39, 42, 44, 45, 47, 48, 51, 54, 55, 57, 62, 63, 65, 66) showed the time of corticosteroids administration in patients with sepsis, 16 RCTs (26, 27, 32, 33, 35–37, 39, 42, 44, 45, 51, 54, 55, 57, 65) of which reported the time of corticosteroids administration within 2 h for prognosis or randomization or as soon as possible and 5 RCTs (47, 48, 62, 63, 66) at 12 h or more after admission.
Primary Outcome
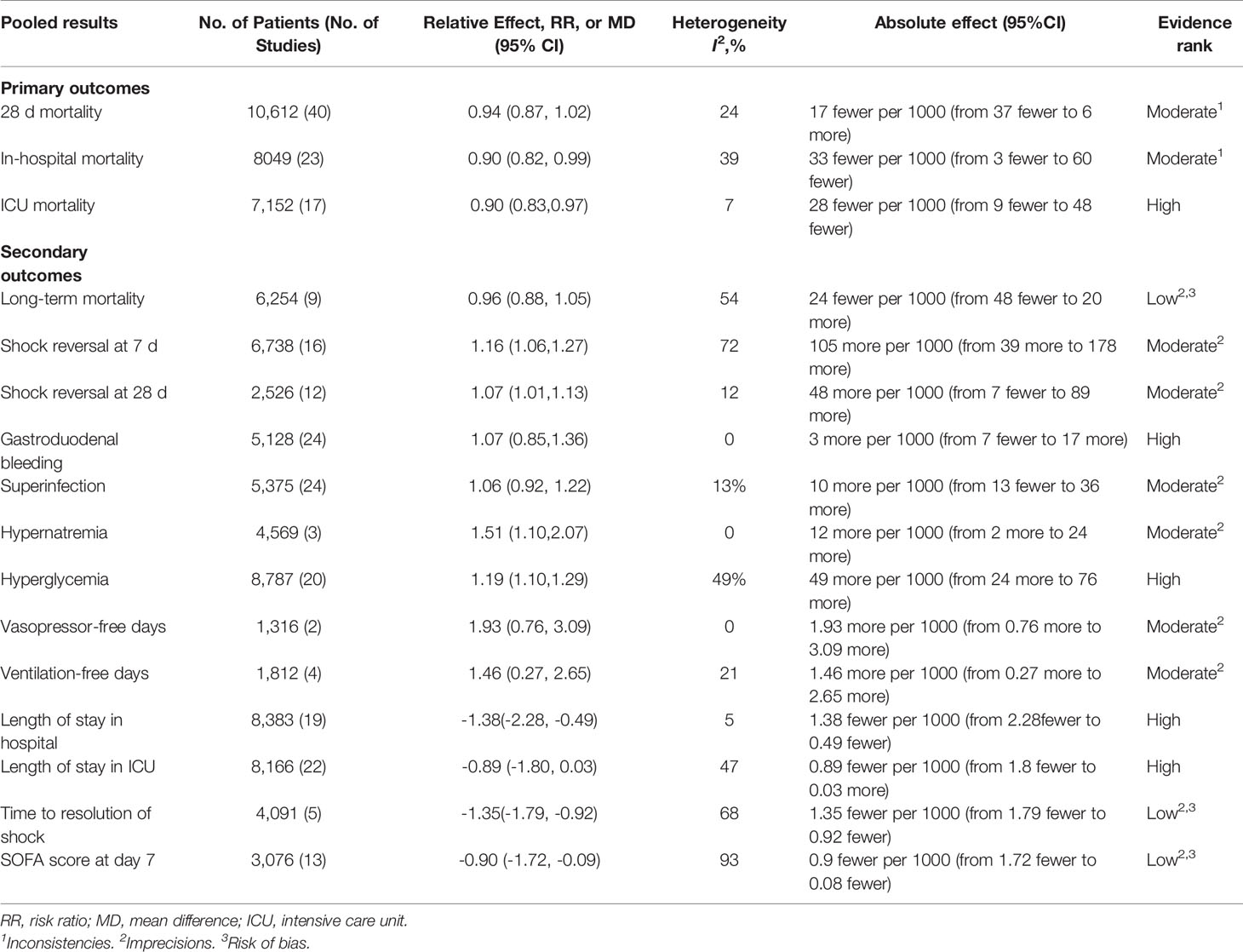
Forty trials (17, 18, 21–30, 34–38, 40–42, 44–48, 51–59, 61–66) (10,612 patients), 23 trials (21–23, 25, 27, 28, 30, 33–35, 41, 44–48, 50, 52, 56, 59, 63–65) (11,579 patients), and 17 trials (21–23, 25, 27, 28, 30, 34, 35, 37, 41, 46, 52, 53, 56, 63, 64) (7,175 patients) were included in this meta-analysis for assessing the 28-day mortality, in-hospital mortality, and ICU mortality, respectively. We used the random-effects model with RRs to assess the pooled results. Corticosteroids therapy showed no difference in the 28-day mortality (RR, 0.94; 95% CI, 0.87–1.02; evidence rank, moderate; Figure 2), with low heterogeneity among the trials (I2 = 24%). However, corticosteroids treatment resulted in a significant decrease in the in-hospital mortality (RR, 0.90; 95% CI, 0.82–0.99; evidence rank, moderate; Figure 3) and ICU mortality (RR, 0.90; 95% CI, 0.83–0.97; evidence rank, high; Figure 4) with low heterogeneity (I2 = 39% and I2 = 7%, respectively). The Funnel plot and Egger test showed no publication bias in the 28-day mortality (p = 0.11), but in-hospital mortality (p = 0.028) and ICU mortality (p = 0.054) showed potential publication bias (Supplementary Figures 1–3). The results of sensitivity analysis showed that the models of the 28-day mortality, in-hospital mortality, and ICU mortality were credible (Supplementary Figures 4–6). Furthermore, L’Abbé plot reported that the mortality in the placebo group increased significantly than the corticosteroids group, suggesting the potential effects of corticosteroids in patients with sepsis (Supplementary Figures 7–9).
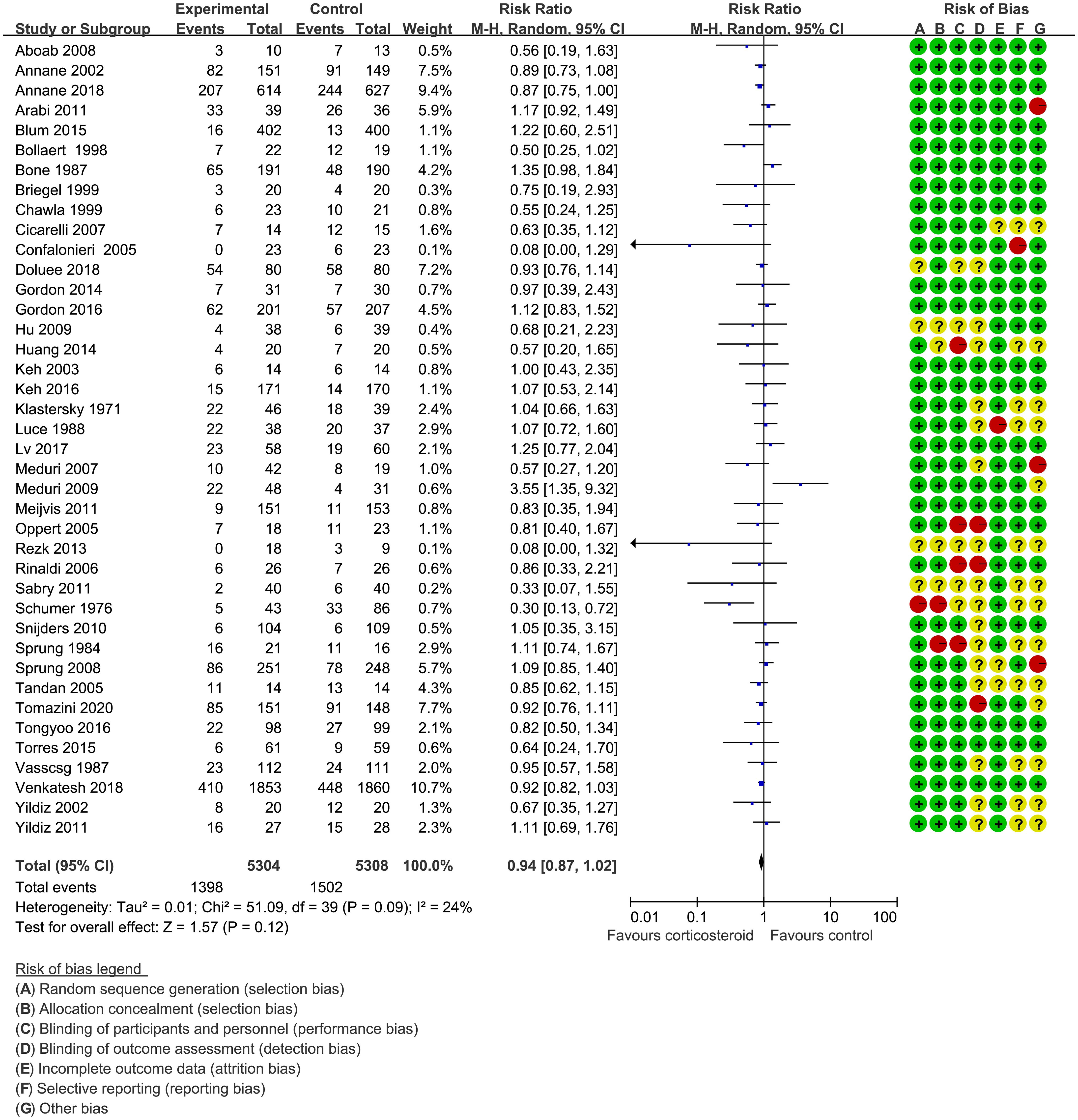
Figure 2 The 28-day mortality of patients with sepsis based on the corticosteroids treatment. The pooled effects in the forest plot were calculated by the M-H method with the random-effects model.
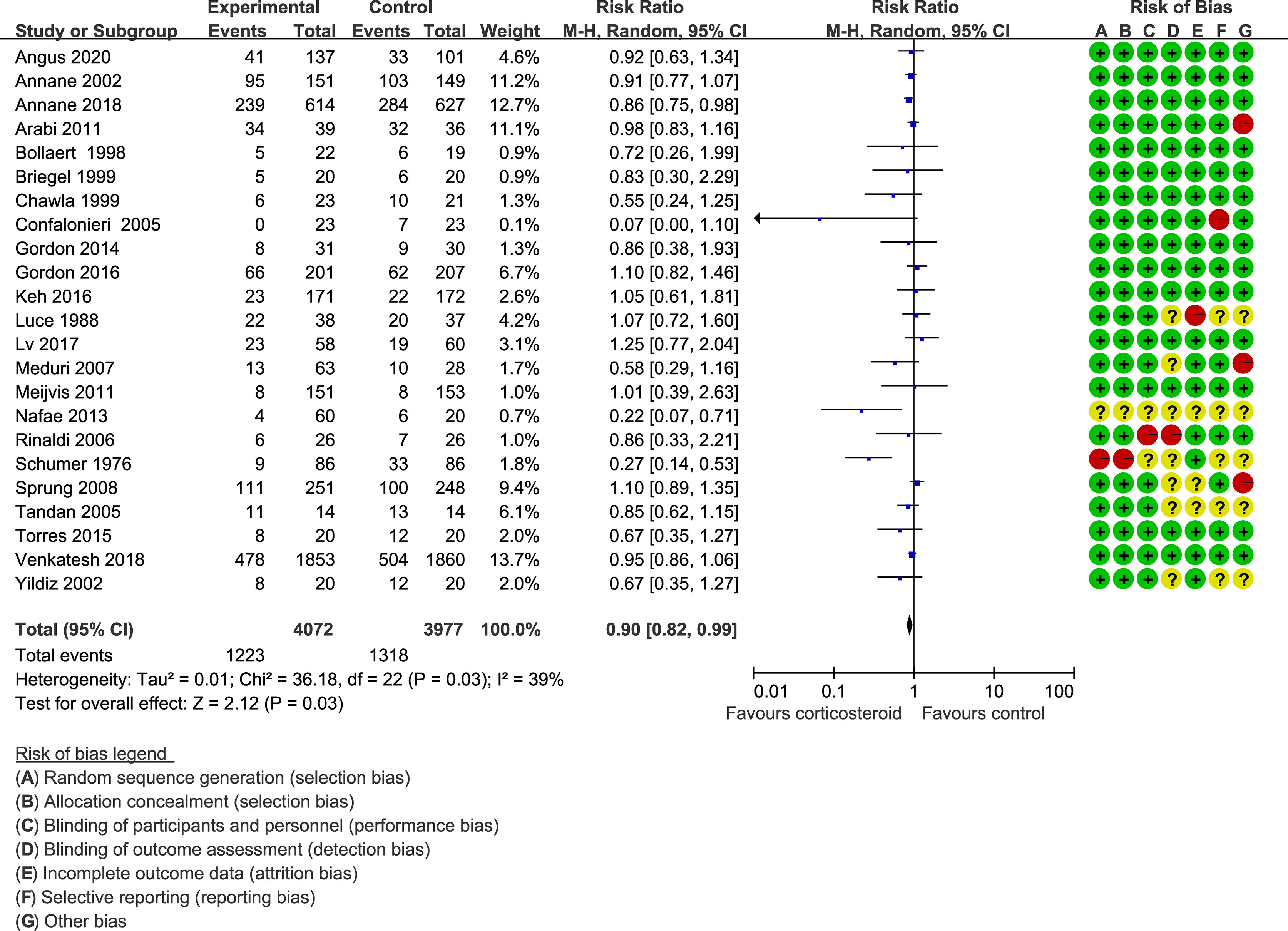
Figure 3 In-hospital mortality of patients with sepsis based on the corticosteroids treatment. The pooled effects in the forest plot were calculated by the M-H method with the random-effects model.
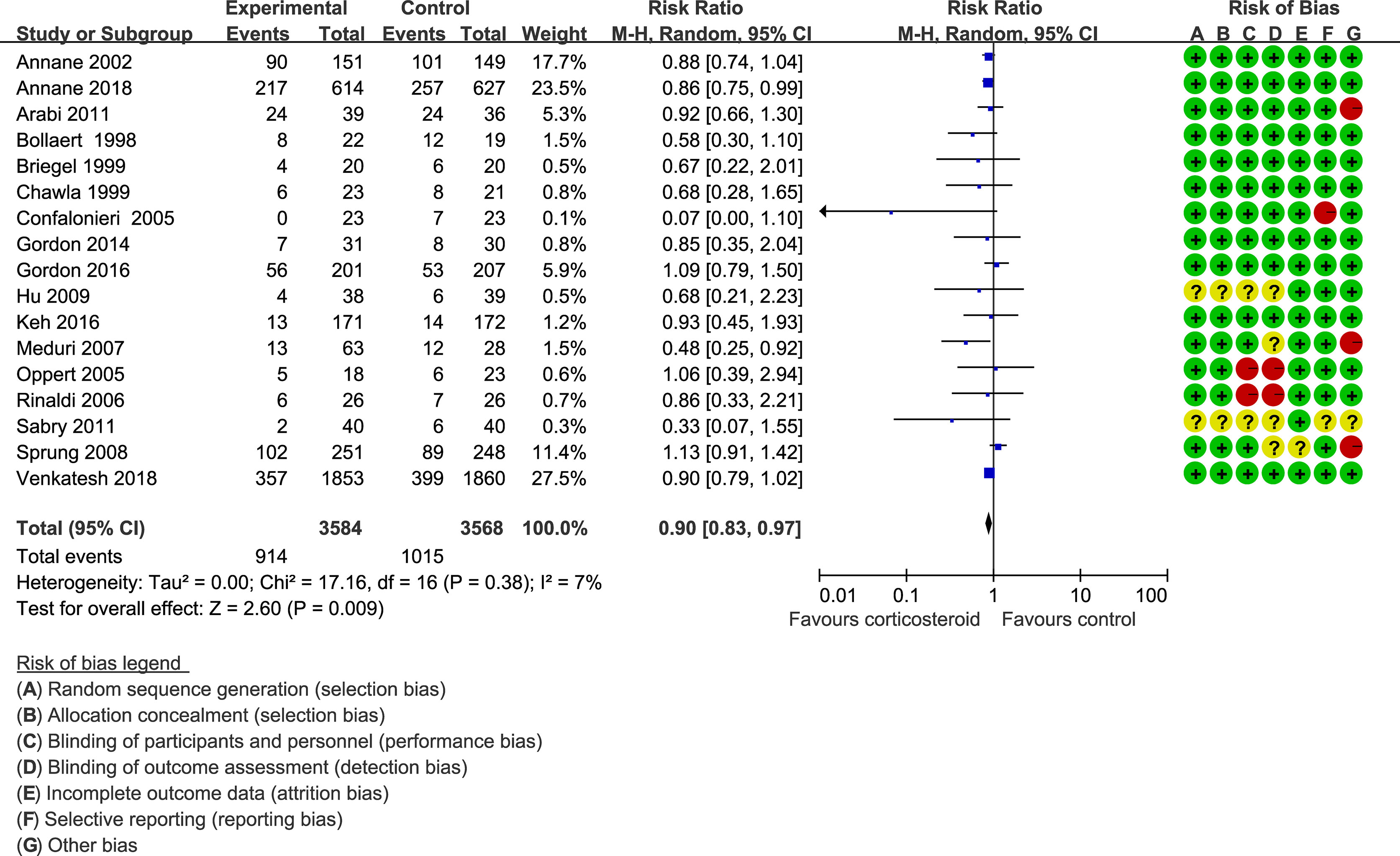
Figure 4 ICU mortality of patients with sepsis based on the corticosteroids treatment. The pooled effects in the forest plot were calculated by the M-H method with the random-effects model.
Secondary Outcomes
Supplementary Figures 10–22 present the assessment of the secondary outcomes. Corticosteroids achieved a small reduction in length of stay in hospital (MD, −1.38; 95% CI, −2.28 to −0.49; I2 = 5%; evidence rank, high), SOFA scores at day 7 (MD, −0.90; 95% CI, −1.72 to −0.09; I2 = 93%; evidence rank, low), and time to resolution of shock (MD, −1.35; 95% CI, −1.79 to −0.92; I2 = 68%; evidence rank, low) for patients with sepsis. Conversely, corticosteroids resulted in higher risk of hypernatremia (RR, 1.51; 95% CI, 1.10–2.07; I2 = 0%; evidence rank, moderate) and hyperglycemia (RR, 1.19; 95% CI, 1.10–1.29; I2 = 49%; evidence rank, high). Furthermore, corticosteroids increased the vasopressor-free days (MD, 1.93; 95% CI, 0.76–3.09; I2 = 0%; evidence rank, moderate), ventilation-free time (MD, 1.46; 95% CI, 0.27–2.65; I2 = 21%; evidence rank, moderate), and shock reversal at day 7 (RR, 1.16; 95% CI, 1.06–1.27; I2 = 72%; evidence rank, moderate) and day 28 (RR, 1.07; 95% CI, 1.01–1.13; I2 = 12%; evidence rank, moderate). Additionally, corticosteroids achieve no reduction in the long-term mortality (>60 days) (RR, 0.96; 95% CI, 0.88–1.05; I2 = 54%; evidence rank, low), length of stay in ICU (MD, −0.89; 95% CI, −1.80–0.03; I2 = 47%; evidence rank, moderate), superinfection (RR, 1.06; 95% CI, 0.92–1.22; I2 = 13%; evidence rank, moderate), and gastroduodenal bleeding (RR, 1.07; 95% CI, 0.85–1.36; I2 = 0%; evidence rank, high).
The Funnel plot and Egger test showed no publication bias in the length of stay in hospital (p = 0.99), SOFA scores at day 7 (p = 0.86), hyperglycemia (p = 0.98), the shock reversal at day 7 (p = 0.285), length of stay in ICU (p = 0.334), superinfection (p = 0.231), gastroduodenal bleeding (p = 0.867), and shock reversal at day 28 (p = 0.414) (Supplementary Figures 23–30). The results of the sensitivity analysis showed that the models of the abovementioned outcomes, including length of stay in hospital, SOFA scores at day 7, hyperglycemia, shock reversal at day 7, length of stay in ICU, superinfection, gastroduodenal bleeding, and shock reversal at day 28 were credible (Supplementary Figures 31–38).
Importantly, the risk of bias was reported in the first plot of each outcome, and the evidence rank is shown in Table 2.
Subgroup Analysis
We performed subgroup analysis based on the sepsis subtype or type of corticosteroids used for the primary outcomes or I2 >75% in the secondary outcomes with more than 10 trials for each outcome. The results of the subgroup analysis showed no effect on the 28-day mortality; however, the in-hospital and ICU mortality were significantly improved in the hydrocortisone plus fludrocortisone treatment and in the patients with septic shock, sepsis, and community-acquired pneumonia (Supplementary Figures 39–44). Moreover, the result of the subgroup in SOFA scores at day 7 represented that the main original heterogeneity may be from the trials with smaller samples who were given hydrocortisone treatment or trials on patients with sepsis shock (Supplementary Figures 45 and 46). Additionally, the subgroup based on the patients that were recruited into the surgical, medical, or surgical/medical ICU showed that corticosteroids were not associated with a 28-day mortality, SOFA scores at day 7, and in-hospital mortality but were related to lower ICU mortality in surgical/medical ICU patients (Supplementary Table 3). Importantly, the subgroup in the corticosteroids based on catecholamine use for qualitative analysis showed that 19 RCTs (21–23, 25, 28, 29, 34, 35, 39–41, 43, 45, 51, 56–58, 60, 61) reported 28-day mortality, but it was not associated with the reduced 28-day mortality, no matter what the corticosteroids dose. Moreover, 11 RCTs (21–23, 25, 27, 28, 34, 35, 41, 45, 56) showed in-hospital mortality, 8 RCTs (21–23, 34, 35, 41, 45, 56) of which reported that the dose of corticosteroids was 200 mg/day or 50 mg every 6 h; only 1 (21) showed that corticosteroids may be associated with lower in-hospital mortality. Eleven RCTs (21–23, 25, 27, 28, 34, 35, 41, 51, 56) reported ICU mortality, seven studies (21–23, 34, 35, 41, 56) of which reported the corticosteroids dose was 200 mg/day or 50 mg every 6 h; only one (21) study showed that corticosteroids may be associated with the lower ICU mortality. Furthermore, three RCTs (25, 27, 28) showed that the corticosteroids dose was more than 200 mg/day; corticosteroids was not associated with the ICU mortality. However, two RCTs (21, 23) showed that corticosteroids dose of 200 mg/day or 50 mg every 6 h may reduce the time of vasopressors use.
Discussion
This meta-analysis included 50 RCTs (12,304 patients) and demonstrated that corticosteroids failed to improve the 28-day and long-term mortality; however, there was a small reduction in the in-hospital mortality and ICU mortality. To our knowledge, this systematic review and meta-analysis is the most comprehensive review of many new RCTs; the precision of the pooled effect estimates how sepsis could be increased substantially.
We found that the corticosteroids therapy for sepsis increased the incidence of the vasopressor-free days, ventilation-free time, shock reversal at days 7 and 28, and adverse events, such as hyperglycemia and hypernatremia. Corticosteroids were associated with a decreased risk of the time for shock resolution and length of stay in the hospital. However, our study failed to report a decreased risk of corticosteroids on the length of ICU stay and adverse events, such as superinfection and gastroduodenal bleeding. Ascertainment of the adverse events in the eligible trials was also vulnerable, which may induce the evidence rank to be low. Moreover, a quantitative analysis for the effect of the time of corticosteroids administration on septic patients was made. The effect of the different time of corticosteroid administration on septic patients cannot be compared because the time of corticosteroid administration was indistinct in the included studies. Therefore, further clinical studies should explore the time of corticosteroid administration for septic patients and ensure whether it is the same as antibiotics, which is the earlier the better.
Subgroup analyses in this review showed that the results did not identify any credible effect of modification in sepsis subtype and type of corticosteroids used. Much evidence comes from the trials with hydrocortisone or methylprednisolone treatment. Our subgroup analysis results showed that the efficacy of corticosteroids on in-hospital, ICU, and short-time mortality was mainly due to the hydrocortisone plus fludrocortisone.
Mechanistically, corticosteroids could inhibit the nuclear factor kappa B (NF-κB) activation and the extensive inflammatory factors release, finally improving the inflammatory response of sepsis or pneumonia. Our previous studies reported that corticosteroids were associated with a decreased risk of ARDS and length of the disease in patients with CAP (67). Previous reviews have assessed the efficacy and safety of corticosteroids in patients with sepsis. Unfortunately, the conclusions were contradictory owing to the small number of trials included. One meta-analysis included 20 RCTs and showed no reduction in the 28-day mortality, hospital mortality, and ICU mortality in patients with severe sepsis and sepsis shock on corticosteroids treatment (68). Subsequently, a Cochrane systematic review further conducted to search the effect of corticosteroids on mortality of patients with sepsis, including a total of 33 RCTs, found a small reduction in 28-day mortality on the corticosteroids treatment (69). Simultaneously, another study included 35 RCTs and showed a converse result that corticosteroids failed to decrease the mortality (70). In 2018, Rochwerg et al. (7) examined 42 RCTs including 10,194 patients, wherein corticosteroids achieved no reduction in the short-term (28–31 days) mortality and may have a little effect on the long-term mortality. In 2019, Fang et al. (71) included 37 RCTs; this trial suggested that corticosteroids use was associated with a decrease in the 28-day mortality, ICU mortality, and in-hospital mortality. In parallel, Annane et al. (6) published a Cochrane systematic review on 40 RCTs and achieved a reduction in the 28-day mortality in patients with sepsis on the corticosteroids therapy.
The results of this meta-analysis showed that corticosteroids treatment failed to improve the 28-day mortality, in contrast with results from the previous meta-analysis. The difference in part may be due to the result reported by Annane et al. (6), in which corticosteroids therapy showed an increased risk of 28-day mortality, while the CI contained the null effect line, suggesting that corticosteroids had no effect on sepsis based on the statistics. More importantly, we included four RCTs about severe COVID-19 and showed that there was no significant difference in 28-day mortality with corticosteroids use. The data were extracted from the latest RCTs and may have helped in reinforcing the conclusions, decreasing the heterogeneity among the studies, and improving the precision with more comprehensive assessment for the therapeutic effects of corticosteroids treatment.
In this meta-analysis, the result of the qualitative analysis showed that 200 mg/day or less may have a clinical benefit, such as increasing the vasopressor-free time, improving tissue oxygen supply, and restoring circulatory homeostasis in catecholamine-dependent septic shock (21, 23, 37). More importantly, the earlier study (25) reported that supraphysiological doses of hydrocortisone could improve hemodynamics and enhance the vascular sensitivity to catecholamines, thereby reducing the dose of catecholamine (dopamine >10 µg/kg/min) in the patients with septic shock. The subsequent studies (40) also showed that under the dose of dopamine ≥6 µg/kg/min, low-dose hydrocortisone could be a better maintenance of hemodynamics by increasing vascular sensitivity to catecholamines. Furthermore, Ibarra-Estrada et al. (72) suggested that compared with bolus infusion of hydrocortisone, continuous infusion may restore the vascular sensitivity to catecholamines better. Similarly, an experimental study (73) found that fludrocortisone combined with hydrocortisone therapy dose dependently increased phenylephrine with cumulative increasing concentrations, which caused concentration-dependent contraction of isolated mesenteric arteries from septic rats. Contrarily, a prospective cohort study (74) showed that after hydrocortisone therapy, there was a significant reduction in norepinephrine in survivors, whereas higher catecholamine dosages were required for the non-survivors. However, a latest retrospective cohort study (75) showed that higher norepinephrine (24.6 mcg/min) in early hydrocortisone therapy could improve reduction in ICU mortality compared with the late hydrocortisone therapy with norepinephrine (21.3 mcg/min) in patients with sepsis shock. Based on the abovementioned results, the potential mechanisms of corticosteroids restoring the vascular sensitivity to catecholamines have been reported as follows: (1) in the septic shock, as the excess production of nitric oxide causes host catecholamine resistance (76, 77), corticosteroids could inhibit inducible NOS formation and production restoring the vascular sensitivity to catecholamines; (2) desensitization and/or downregulation of β-adrenergic receptors (78) and possibly α-adrenergic receptors (79) maybe due to the downregulation by endogenous catecholamine production in septic shock, whereas the corticosteroids may reverse receptor desensitization (80) and further allow reduced catecholamine dosage (25). Given that the evidence of the relationship between catecholamines and corticosteroids is currently inconsistent, future clinical studies should be conducted to further research the dependence of catecholamine administration on the effect of cortisone administration.
Additionally, to explore which septic patients were more responsive to the corticosteroids therapy, the ICU subgroup analysis after the type of disease and corticosteroids subgroup was conducted. The results showed that with corticosteroids use, there was no difference in the 28-day mortality, in-hospital mortality, and SOFA scores at day 7 among the surgical ICU, medical ICU, and surgical/medical ICU, but there was lower ICU mortality in patients with sepsis from surgical/medical ICU. The results of the subgroup analysis may not provide useful information mainly because ICU description is too vague in the included studies. Thus, details cannot be determined. Therefore, future clinical research should distinguish patients based on ICU type (e.g., surgical or medical ICU) to explore which sepsis primary cause is the corticosteroids therapy effective.
Corticosteroids have already been used for adjuvant therapy in sepsis for more than half a century. However, credible evidence is still lacking to guide the choice of patients, the time of corticosteroids administration, or the dose of corticosteroids for catecholamine-dependent patients. With the definition of sepsis that varies from sepsis-1.0 to sepsis-3.0, the accuracy of sepsis diagnosis has significantly improved. However, corticosteroids use also varied from sepsis-1.0 to sepsis-3.0. Specifically, only patients with septic shock used corticosteroids and suggested the use of flumetasone (50 µg/day) in sepsis-1.0 (81). The use of hydrocortisone was suggested only in children with suspected or confirmed absolute adrenal insufficiency, which was a more stringent use of corticosteroids compared with sepsis-1, in sepsis-2.0 (82), and the use of hydrocortisone (200 mg/day) was suggested only in patients with refractory septic shock wherein appropriate fluid resuscitation and vasopressor therapy cannot restore hemodynamic stability in sepsis-3.0 (3), The proposals from sepsis-1.0, sepsis-2.0, and sepsis-3.0 lack credible evidence to support the clinical use of corticosteroids. Analysis of all relevant data from available RCTs showed that the effect of corticosteroids therapy for septic patients was not consistent. However, the latest studies showed that corticosteroids may not reduce mortality in septic patients compared with the control group. Importantly, this study suggests that corticosteroids administration may not reduce the 28-day mortality, long-term mortality, and length of ICU stay but may be associated with ICU mortality, in-hospital mortality, length of hospital stay, SOFA scores at day 7, and time to shock resolution, and increased shock reversal at days 7 and 28 and vasopressor- and ventilation-free days. Furthermore, this study suggested that corticosteroids may be an effective therapy with a low dose and long-term course. However, future studies need to appropriately study the time of corticosteroids administration, the primary infection source, and dose of corticosteroids use for septic shock patients who are dependent on catecholamine in the treatment of sepsis.
This meta-analysis has several strengths. First, this study is the most comprehensive trial to assess the efficacy of corticosteroids treatment on patients with sepsis to date. Second, we performed a thorough literature search including unpublished sources, using the GRADE methodology, to evaluate the evidence rank in overall RR, a predefined illustration of potential effect variables including direction of effect and subsequent subgroup analysis to search the effect variables, and illustration including the relative and absolute effects. Third, the primary outcomes showed low or no heterogeneity among the studies, suggesting that the results were not variable. Furthermore, the heterogeneity of SOFA scores on day 7 was high, and the subgroup analysis showed that the source of heterogeneity may be the inclusion of trials with small size on patients who were given hydrocortisone treatment. Finally, the results of the sensitivity analysis for this study suggested that these conclusions were robust and reliable.
However, this meta-analysis also has certain limitations, including the significant methodological or clinical heterogeneity among the included studies, especially with respect to the SOFA score on day 7. All the included RCTs enrolled patients with sepsis as per the previous sepsis definition criteria; however, we do not know whether the efficacy and safety of corticosteroids would change using the Sepsis-3 definition criteria. Hence, the defined mortality may be essential, but the certainty is limited due to the imprecision of the included studies.
Conclusions
This is the most comprehensive systematic review and meta-analysis to describe the efficacy and safety of corticosteroids in patients with sepsis. The findings demonstrate that corticosteroids failed to reduce the 28-day, 90-day, and long-term mortalities; however, they could reduce the in-hospital and ICU mortalities. Importantly, our subgroup analyses results indicated that this efficacy of corticosteroids in patients with sepsis may be associated with the hydrocortisone plus fludrocortisone treatment. Therefore, the results suggest that corticosteroids could not improve the 28-day mortality in adult patients with sepsis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
All the authors contributed equally to the work presented in this article. TS, HuL conceived the idea of this study. XD, HoL, and GS contributed to the data extraction. SH, RZ, and XD computed and evaluated the pooled outcomes. HuL and SH contributed to the study protocol and wrote the article. QK and TS revised the article. QK and TS had full access to all of the data, and the final responsibility for the decision to submit this article for publication. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the United Fund of National Natural Science Foundation of China (Grant No. U2004110) and Leading Talents Fund in Science and Technology Innovation in Henan Province (Grant No. 194200510017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.709155/full#supplementary-material
Supplementary Figure 1 | The Funnel plot assessed the potential publication bias of pooled effect in 28-day mortality for corticosteroids vs. placebo treatment in patients with sepsis.
Supplementary Figure 2 | The Funnel plot assessed the potential publication bias of pooled effect in in-hospital mortality for corticosteroids vs. placebo treatment in patients with sepsis.
Supplementary Figure 3 | The Funnel plot assessed the potential publication bias of pooled effect in ICU mortality for corticosteroids vs. placebo treatment in patients with sepsis.
Supplementary Figure 4 | The sensitivity analysis evaluated the robustness of the pooled effect model in 28-day mortality for this meta-analysis.
Supplementary Figure 5 | The sensitivity analysis evaluated the robustness of the pooled effect model in in-hospital mortality for this meta-analysis.
Supplementary Figure 6 | The sensitivity analysis evaluated the robustness of the pooled effect model in ICU mortality for this meta-analysis.
Supplementary Figure 7 | L’Abbe plot according to the corticosteroids therapy. 40 RCTs of corticosteroids and 28-day mortality in patients with sepsis were presented in a L’Abbe plot.
Supplementary Figure 8 | L’Abbe plot according to the corticosteroids therapy. 23 RCTs of corticosteroids and in-hospital mortality in patients with sepsis were presented in a L’Abbe plot.
Supplementary Figure 9 | L’Abbe plot according to the corticosteroid therapy. 17 RCTs of corticosteroids and ICU mortality in patients with sepsis were presented in a L’Abbe plot.
Supplementary Figure 10 | The Forest plot showed the pooled effect of length of stay in hospital in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 11 | The Forest plot showed the pooled effect of SOFA scores at day 7 in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 12 | The Forest plot showed the pooled effect of time to resolution of shock in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 13 | The Forest plot showed the pooled effect of hypernatremia in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 14 | The Forest plot showed the pooled effect of hyperglycemia in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 15 | The Forest plot showed the pooled effect of vasopressor-free days in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 16 | The Forest plot showed the pooled effect of ventilation-free time in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 17 | The Forest plot showed the pooled effect of shock reversal at day 7 in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 18 | The Forest plot showed the pooled effect of shock reversal at day 28 in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 19 | The Forest plot showed the pooled effect of long-term mortality in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 20 | The Forest plot showed the pooled effect of length of stay in ICU in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 21 | The Forest plot showed the pooled effect of superinfection in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 22 | The Forest plot showed the pooled effect of gastroduodenal bleeding in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
Supplementary Figure 23 | The Funnel plot assessed the potential publication bias of pooled effect in length of stay in hospital in patients with sepsis for this meta-analysis.
Supplementary Figure 24 | The Funnel plot assessed the potential publication bias of pooled effect in SOFA scores at day 7 in patients with sepsis for this meta-analysis.
Supplementary Figure 25 | The Funnel plot assessed the potential publication bias of pooled effect in hyperglycemia in-hospital in patients with sepsis for this meta-analysis.
Supplementary Figure 26 | The Funnel plot assessed the potential publication bias of pooled effect in the shock reversal at day 7 in patients with sepsis for this meta-analysis.
Supplementary Figure 27 | The Funnel plot assessed the potential publication bias of pooled effect in length of stay in ICU in patients with sepsis for this meta-analysis.
Supplementary Figure 28 | The Funnel plot assessed the potential publication bias of pooled effect in superinfection in patients with sepsis for this meta-analysis.
Supplementary Figure 29 | The Funnel plot assessed the potential publication bias of pooled effect in gastroduodenal bleeding in patients with sepsis for this meta-analysis.
Supplementary Figure 30 | The Funnel plot assessed the potential publication bias of pooled effect in shock reversal at day 28 in patients with sepsis for this meta-analysis.
Supplementary Figure 31 | The sensitivity analysis evaluated the robustness of pooled effect model in length of stay in hospital for this meta-analysis.
Supplementary Figure 32 | The sensitivity analysis evaluated the robustness of pooled effect model in SOFA scores at day 7 for this meta-analysis.
Supplementary Figure 33 | The sensitivity analysis evaluated the robustness of pooled effect model in hyperglycemia for this meta-analysis.
Supplementary Figure 34 | The sensitivity analysis evaluated the robustness of pooled effect model in shock reversal at day 7 for this meta-analysis.
Supplementary Figure 35 | The sensitivity analysis evaluated the robustness of pooled effect model in length of stay in ICU for this meta-analysis.
Supplementary Figure 36 | The sensitivity analysis evaluated the robustness of pooled effect model in superinfection for this meta-analysis.
Supplementary Figure 37 | The sensitivity analysis evaluated the robustness of pooled effect model in gastroduodenal bleeding for this meta-analysis.
Supplementary Figure 38 | The sensitivity analysis evaluated the robustness of pooled effect model in shock reversal at day 28 for this meta-analysis.
Supplementary Figure 39 | Subgroup analysis of 28-day mortality of patients with sepsis based on the corticosteroids type.
Supplementary Figure 40 | Subgroup analysis of in-hospital mortality of patients with sepsis based on the corticosteroids type.
Supplementary Figure 41 | Subgroup analysis of ICU mortality of patients with sepsis based on the corticosteroids type.
Supplementary Figure 42 | Subgroup analysis of 28-day mortality of patients with sepsis based on the sepsis subtype.
Supplementary Figure 43 | Subgroup analysis of in-hospital mortality of patients with sepsis based on the sepsis subtype.
Supplementary Figure 44 | Subgroup analysis of ICU mortality of patients with sepsis based on the sepsis subtype.
Supplementary Figure 45 | Subgroup analysis of SOFA scores at day 7 of patients with sepsis based on the corticosteroids type.
Supplementary Figure 46 | Subgroup analysis of SOFA scores at day 7 of patients with sepsis based on the sepsis subtype.
Abbreviations
RR, risk ratio; CI, confidence interval; RCTs, randomized controlled trials; MD, mean difference; CAP, community-acquired pneumonia; ICU, intensive care unit; ARDS, acute respiratory distress syndrome; GRADE, Grading of Recommendations Assessment, Development and Evaluation; SOFA, Sequential Organ Failure Assessment.
References
1. Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA (2016) 315(8):762–74. doi: 10.1001/jama.2016.0288
2. DeMerle KM, Angus DC, Baillie JK, Brant E, Calfee CS, Carcillo J, et al. Sepsis Subclasses: A Framework for Development and Interpretation. Crit Care Med (2021) 49(5):748–59. doi: 10.1097/CCM.0000000000004842
3. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med (2017) 43(3):304–77. doi: 10.1007/s00134-017-4683-6
4. Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA (2017) 318(13):1241–9. doi: 10.1001/jama.2017.13836
5. Annane D, Pastores SM, Arlt W, Balk RA, Beishuizen A, Briegel J, et al. Critical Illness-Related Corticosteroid Insufficiency (CIRCI): A Narrative Review From a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Med (2017) 43(12):1781–92. doi: 10.1007/s00134-017-4914-x
6. Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y, et al. Corticosteroids for Treating Sepsis in Children and Adults. Cochrane Database Systematic Rev (2019) 12(12):Cd002243. doi: 10.1002/14651858.CD002243.pub4
7. Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley-Cote E, D’Aragon F, et al. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit Care Med (2018) 46(9):1411–20. doi: 10.1097/CCM.0000000000003262
8. Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA (2020) 323(5):423–31. doi: 10.1001/jama.2019.22176
9. Sevransky JE, Rothman RE, Hager DN, Bernard GR, Brown SM, Buchman TG, et al. Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator- and Vasopressor-Free Days in Patients With Sepsis: The VICTAS Randomized Clinical Trial. JAMA (2021) 325(8):742–50. doi: 10.1001/jama.2020.24505
10. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is “Quality of Evidence” and Why is it Important to Clinicians? BMJ (Clinical Res ed) (2008) 336(7651):995–8. doi: 10.1136/bmj.39490.551019.BE
11. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest (1992) 101(6):1644–55. doi: 10.1378/chest.101.6.1644
12. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med (2003) 31(4):1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B
13. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
14. Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [Updated March 2011]. Cochrane Collaboration (2011).
15. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ (Clinical Res ed) (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
16. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ (Clinical Res ed) (2011) 343:d5928. doi: 10.1136/bmj.d5928
17. Abdelsalam Rezk N, Mohamed Ibrahim A. Effects of Methyl Prednisolone in Early ARDS. Egyptian J Chest Dis Tuberculosis (2013) 62(1):167–72. doi: 10.1016/j.ejcdt.2013.02.013
18. Aboab J, Polito A, Orlikowski D, Sharshar T, Castel M, Annane D. Hydrocortisone Effects on Cardiovascular Variability in Septic Shock: A Spectral Analysis Approach. Crit Care Med (2008) 36(5):1481–6. doi: 10.1097/CCM.0b013e31816f48f2
19. Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA (2020) 324(13):1317–29. doi: 10.1001/jama.2020.17022
20. Annane D, Cariou A, Maxime V, Azoulay E, D’Honneur G, Timsit JF, et al. Corticosteroid Treatment and Intensive Insulin Therapy for Septic Shock in Adults: A Randomized Controlled Trial. JAMA (2010) 303(4):341–8. doi: 10.1001/jama.2010.2
21. Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, et al. Hydrocortisone Plus Fludrocortisone for Adults With Septic Shock. N Engl J Med (2018) 378(9):809–18. doi: 10.1056/NEJMoa1705716
22. Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, et al. Effect of Treatment With Low Doses of Hydrocortisone and Fludrocortisone on Mortality in Patients With Septic Shock. JAMA (2002) 288(7):862–71. doi: 10.1001/jama.288.7.862
23. Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, et al. Low-Dose Hydrocortisone in Patients With Cirrhosis and Septic Shock: A Randomized Controlled Trial. CMAJ: Can Med Assoc J = J l’Association medicale Can (2011) 182(18):1971–7. doi: 10.1503/cmaj.090707
24. Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, et al. Adjunct Prednisone Therapy for Patients With Community-Acquired Pneumonia: A Multicentre, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet (Lond Engl) (2015) 385(9977):1511–8. doi: 10.1016/S0140-6736(14)62447-8
25. Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of Late Septic Shock With Supraphysiologic Doses of Hydrocortisone. Crit Care Med (1998) 26(4):645–50. doi: 10.1097/00003246-199804000-00010
26. Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A Controlled Clinical Trial of High-Dose Methylprednisolone in the Treatment of Severe Sepsis and Septic Shock. N Engl J Med (1987) 317(11):653–8. doi: 10.1056/NEJM198709103171101
27. Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al. Stress Doses of Hydrocortisone Reverse Hyperdynamic Septic Shock: A Prospective, Randomized, Double-Blind, Single-Center Study. Crit Care Med (1999) 27(4):723–32. doi: 10.1097/00003246-199904000-00025
28. Chawla K, Kupfer Y, Goldman I, Tessler S. Hydrocortisone Reverses Refractory Septic Shock. Crit Care Med (1999) 27(1):33A. doi: 10.1097/00003246-199901001-00022
29. Cicarelli DD, Benseñor FE, Vieira JE. Effects of Single Dose of Dexamethasone on Patients With Systemic Inflammatory Response. Sao Paulo Med J = Rev paulista medicina (2007) 124(2):90–5. doi: 10.1590/S1516-31802006000200008
30. Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone Infusion for Severe Community-Acquired Pneumonia: A Preliminary Randomized Study. Am J Respir Crit Care Med (2005) 171(3):242–8. doi: 10.1164/rccm.200406-808OC
31. Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA (2020) 324(13):1298–306. doi: 10.1001/jama.2020.16761
32. Edalatifard M, Akhtari M. Intravenous Methylprednisolone Pulse as a Treatment for Hospitalised Severe COVID-19 Patients: Results From a Randomised Controlled Clinical Trial. Eur Respir J (2020) 56(6):2002808. doi: 10.1183/13993003.02808-2020
33. Fernández-Serrano S, Dorca J, Garcia-Vidal C, Fernández-Sabé N, Carratalà J, Fernández-Agüera A, et al. Effect of Corticosteroids on the Clinical Course of Community-Acquired Pneumonia: A Randomized Controlled Trial. Crit Care (Lond Engl) (2011) 15(2):R96. doi: 10.1186/cc10103
34. Gordon AC, Mason AJ, Perkins GD, Stotz M, Terblanche M, Ashby D, et al. The Interaction of Vasopressin and Corticosteroids in Septic Shock: A Pilot Randomized Controlled Trial. Crit Care Med (2014) 42(6):1325–33. doi: 10.1097/CCM.0000000000000212
35. Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA (2016) 316(5):509–18. doi: 10.1001/jama.2016.10485
36. VASSCS G. Effect of High-Dose Glucocorticoid Therapy on Mortality in Patients With Clinical Signs of Systemic Sepsis. N Engl J Med (1987) 317(11):659–65. doi: 10.1056/NEJM198709103171102
37. Hu B, Li JG, Liang H, Zhou Q, Yu Z, Li L, et al. [The Effect of Low-Dose Hydrocortisone on Requirement of Norepinephrine and Lactate Clearance in Patients With Refractory Septic Shock]. Zhongguo wei zhong bing ji jiu yi xue = Chin Crit Care Med = Zhongguo weizhongbing jijiuyixue (2009) 21(9):529–31. doi: 10.3760/cma.j.issn.1003-0603.2009.09.006
38. Huang R, Zhang Z, Xu M, Chang X, Qiao Q, Wang L, et al. [Effect of Sini Decoction on Function of Hypothalamic-Pituitary-Adrenal Axis in Patients With Sepsis]. Zhonghua wei zhong bing ji jiu yi xue (2014) 26(3):184–7. doi: 10.3760/cma.j.issn.2095-4352.2014.03.012
39. Hyvernat H, Barel R, Gentilhomme A, Césari-Giordani JF, Freche A, Kaidomar M, et al. Effects of Increasing Hydrocortisone to 300 Mg Per Day in the Treatment of Septic Shock: A Pilot Study. Shock (Augusta Ga) (2016) 46(5):498–505. doi: 10.1097/SHK.0000000000000665
40. Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, et al. Immunologic and Hemodynamic Effects of “Low-Dose” Hydrocortisone in Septic Shock: A Double-Blind, Randomized, Placebo-Controlled, Crossover Study. Am J Respir Crit Care Med (2003) 167(4):512–20. doi: 10.1164/rccm.200205-446OC
41. Keh D, Trips E, Marx G, Wirtz SP, Abduljawwad E, Bercker S, et al. Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis: The HYPRESS Randomized Clinical Trial. JAMA (2016) 316(17):1775–85. doi: 10.1001/jama.2016.14799
42. Klastersky J, Cappel R, Debusscher L. Effectiveness of Betamethasone in Management of Severe Infections. A Double-Blind Study. N Engl J Med (1971) 284(22):1248–50. doi: 10.1056/NEJM197106032842206
43. Loisa P, Parviainen I, Tenhunen J, Hovilehto S, Ruokonen E. Effect of Mode of Hydrocortisone Administration on Glycemic Control in Patients With Septic Shock: A Prospective Randomized Trial. Crit Care (Lond Engl) (2007) 11(1):R21. doi: 10.1186/cc5696
44. Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of High-Dose Methylprednisolone in Preventing Parenchymal Lung Injury and Improving Mortality in Patients With Septic Shock. Am Rev Respir Dis (1988) 138(1):62–8. doi: 10.1164/ajrccm/138.1.62
45. Lv QQ, Gu XH, Chen QH, Yu JQ, Zheng RQ. Early Initiation of Low-Dose Hydrocortisone Treatment for Septic Shock in Adults: A Randomized Clinical Trial. Am J Emergency Med (2017) 35(12):1810–4. doi: 10.1016/j.ajem.2017.06.004
46. Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone Infusion in Early Severe ARDS: Results of a Randomized Controlled Trial. Chest (2007) 131(4):954–63. doi: 10.1378/chest.06-2100
47. Meduri GU, Golden E, Umberger R. Prospective Double-Blind Randomized Clinical Trial on the Effects of Low-Dose Hydrocortisone Infusion in Patients With Severe Sepsis. Chest (2009) 136(4):45S. doi: 10.1016/S0012-3692(16)48001-3
48. Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, et al. Dexamethasone and Length of Hospital Stay in Patients With Community-Acquired Pneumonia: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet (Lond Engl) (2011) 377(9782):2023–30. doi: 10.1016/S0140-6736(11)60607-7
49. Mirea L, Ungureanu R, Pavelescu D, Grintescu IC, Dumitrache C, Grintescu I, et al. Continuous Administration of Corticosteroids in Septic Shock can Reduce Risk of Hypernatremia. Crit Care (2014) 18(1):P239. doi: 10.1186/cc13429
50. Nafae RM, Ragab MI, Amany FM, Rashed SB. Adjuvant Role of Corticosteroids in the Treatment of Community-Acquired Pneumonia. Egyptian J Chest Dis Tuberculosis (2013) 62(3):439–45. doi: 10.1016/j.ejcdt.2013.03.009
51. Oppert M, Schindler R, Husung C, Offermann K, Gräf KJ, Boenisch O, et al. Low-Dose Hydrocortisone Improves Shock Reversal and Reduces Cytokine Levels in Early Hyperdynamic Septic Shock. Crit Care Med (2005) 33(11):2457–64. doi: 10.1097/01.CCM.0000186370.78639.23
52. Rinaldi S, Adembri C, Grechi S, De Gaudio AR. Low-Dose Hydrocortisone During Severe Sepsis: Effects on Microalbuminuria. Crit Care Med (2006) 34(9):2334–9. doi: 10.1097/01.CCM.0000233872.04706.BB
53. Sabry NA, Omar EE-D. Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings. Pharmacol Pharm (2011) 02(02):73–81. doi: 10.4236/pp.2011.22009
54. Schumer W. Steroids in the Treatment of Clinical Septic Shock. Ann Surg (1976) 184(3):333–41. doi: 10.1097/00000658-197609000-00011
55. Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of Corticosteroids in Community-Acquired Pneumonia: A Randomized Double-Blinded Clinical Trial. Am J Respir Crit Care Med (2010) 181(9):975–82. doi: 10.1164/rccm.200905-0808OC
56. Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone Therapy for Patients With Septic Shock. N Engl J Med (2008) 358(2):111–24. doi: 10.1056/NEJMoa071366
57. Sprung CL, Caralis PV, Marcial EH, Pierce M, Gelbard MA, Long WM, et al. The Effects of High-Dose Corticosteroids in Patients With Septic Shock. A Prospective, Controlled Study. N Engl J Med (1984) 311(18):1137–43. doi: 10.1056/NEJM198411013111801
58. Talebi Doluee M, Salehi M, Mahmoudi Gharaee A, Jalalyazdi M, Reihani H. The Effect of Physiologic Dose of Intravenous Hydrocortisone in Patients With Refractory Septic Shock: A Randomized Control Trial. J Emergency Pract Trauma (2018) 4(1):29–33. doi: 10.15171/jept.2017.25
59. Tandan SM GR GN. Corticosteroidsfor Treating Sepsis in Children and Adultslow Dose Steroids and Adrenocortical Insufficiency in Septic Shock: A Double-Blind Randomised Controlled Trial From India. Proc Am Thoracic Soc Meeting (2005) A24.
60. Tilouche N, Jaoued O, Ali HBS, Gharbi R, Fekih Hassen M, Elatrous S. Comparison Between Continuous and Intermittent Administration of Hydrocortisone During Septic Shock: A Randomized Controlled Clinical Trial. Shock (Augusta Ga) (2019) 52(5):481–6. doi: 10.1097/SHK.0000000000001316
61. Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA (2020) 324(13):1307–16. doi: 10.1001/jama.2020.17021
62. Tongyoo S, Permpikul C, Mongkolpun W, Vattanavanit V, Udompanturak S, Kocak M, et al. Hydrocortisone Treatment in Early Sepsis-Associated Acute Respiratory Distress Syndrome: Results of a Randomized Controlled Trial. Crit Care (Lond Engl) (2016) 20(1):329. doi: 10.1186/s13054-016-1511-2
63. Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of Corticosteroids on Treatment Failure Among Hospitalized Patients With Severe Community-Acquired Pneumonia and High Inflammatory Response: A Randomized Clinical Trial. JAMA (2015) 313(7):677–86. doi: 10.1001/jama.2015.88
64. Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. Adjunctive Glucocorticoid Therapy in Patients With Septic Shock. N Engl J Med (2018) 378(9):797–808. doi: 10.1056/NEJMoa1705835
65. Yildiz O, Doganay M, Aygen B, Güven M, Keleştimur F, Tutuû A. Physiological-Dose Steroid Therapy in Sepsis [ISRCTN36253388]. Crit Care (Lond Engl) (2002) 6(3):251–9. doi: 10.1186/cc1498
66. Yildiz O, Tanriverdi F, Simsek S, Aygen B, Kelestimur F. The Effects of Moderate-Dose Steroid Therapy in Sepsis: A Placebo-Controlled, Randomized Study. J Res Med Sciences: Off J Isfahan Univ Med Sci (2011) 16(11):1410–21.
67. Wan YD, Sun TW, Liu ZQ, Zhang SG, Wang LX, Kan QC. Efficacy and Safety of Corticosteroids for Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis. Chest (2016) 149(1):209–19. doi: 10.1378/chest.15-1733
68. Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, et al. Corticosteroids in the Treatment of Severe Sepsis and Septic Shock in Adults: A Systematic Review. JAMA (2009) 301(22):2362–75. doi: 10.1001/jama.2009.815
69. Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for Treating Sepsis. Cochrane Database Systematic Rev (2015) 2015(12):Cd002243. doi: 10.1002/14651858.CD002243.pub3
70. Volbeda M, Wetterslev J, Gluud C, Zijlstra JG, van der Horst IC, Keus F. Glucocorticosteroids for Sepsis: Systematic Review With Meta-Analysis and Trial Sequential Analysis. Intensive Care Med (2015) 41(7):1220–34. doi: 10.1007/s00134-015-3899-6
71. Fang F, Zhang Y, Tang J, Lunsford LD, Li T, Tang R, et al. Association of Corticosteroid Treatment With Outcomes in Adult Patients With Sepsis: A Systematic Review and Meta-Analysis. JAMA Internal Med (2019) 179(2):213–23. doi: 10.1001/jamainternmed.2018.5849
72. Ibarra-Estrada MA, Chávez-Peña Q, Reynoso-Estrella CI, Rios-Zermeño J, Aguilera-González PE, García-Soto MA, et al. Timing, Method and Discontinuation of Hydrocortisone Administration for Septic Shock Patients. World J Crit Care Med (2017) 6(1):65–73. doi: 10.5492/wjccm.v6.i1.65
73. Laviolle B, Nesseler N, Massart C, Bellissant E. Fludrocortisone and Hydrocortisone, Alone or in Combination, on In Vivo Hemodynamics and In Vitro Vascular Reactivity in Normal and Endotoxemic Rats: A Randomized Factorial Design Study. J Cardiovasc Pharmacol (2014) 63(6):488–96. doi: 10.1097/FJC.0000000000000072
74. Winter W, Kamolz L, Donner A, Hoerauf K, Blaicher A, Andel H. Hydrocortisone Improved Haemodynamics and Fluid Requirement in Surviving But Not Non-Surviving of Severely Burned Patients. Burns: J Int Soc Burn Injuries (2003) 29(7):717–20. doi: 10.1016/S0305-4179(03)00148-7
75. Sacha GL, Chen AY, Palm NM, Duggal A. Evaluation of the Initiation Timing of Hydrocortisone in Adult Patients With Septic Shock. Shock (Augusta Ga) (2021) 55(4):488–94. doi: 10.1097/SHK.0000000000001651
76. Vincent JL, Zhang H, Szabo C, Preiser JC. Effects of Nitric Oxide in Septic Shock. Am J Respir Crit Care Med (2000) 161(6):1781–5. doi: 10.1164/ajrccm.161.6.9812004
77. Parker MM. Pathophysiology of Cardiovascular Dysfunction in Septic Shock. New Horizons (Baltimore Md) (1998) 6(2):130–8.
78. Silverman HJ, Penaranda R, Orens JB, Lee NH. Impaired Beta-Adrenergic Receptor Stimulation of Cyclic Adenosine Monophosphate in Human Septic Shock: Association With Myocardial Hyporesponsiveness to Catecholamines. Crit Care Med (1993) 21(1):31–9. doi: 10.1097/00003246-199301000-00010
79. McMillan M, Chernow B, Roth BL. Hepatic Alpha 1-Adrenergic Receptor Alteration in a Rat Model of Chronic Sepsis. Circulatory Shock (1986) 19(2):185–93.
80. Walker BR, Williams BC. Corticosteroids and Vascular Tone: Mapping the Messenger Maze. Clin Sci (London England: 1979) (1992) 82(6):597–605. doi: 10.1042/cs0820597
81. American College of Chest Physicians/Society of Critical Care Medicine Consensus ConferenceDefinitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Crit Care Med (1992) 20(6):864–74. doi: 10.1097/00003246-199206000-00025
Keywords: corticosteroids, sepsis, mortality, meta-analysis, systematic review
Citation: Liang H, Song H, Zhai R, Song G, Li H, Ding X, Kan Q and Sun T (2021) Corticosteroids for Treating Sepsis in Adult Patients: A Systematic Review and Meta-Analysis. Front. Immunol. 12:709155. doi: 10.3389/fimmu.2021.709155
Received: 13 May 2021; Accepted: 26 July 2021;
Published: 16 August 2021.
Edited by:
Veronique Godot, INSERM U955 Institut Mondor de Recherche Biomédicale (IMRB), FranceReviewed by:
Jan Rossaint, University of Münster, GermanyWan-Jie Gu, Nanjing Drum Tower Hospital, China
Copyright © 2021 Liang, Song, Zhai, Song, Li, Ding, Kan and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tongwen Sun, c3VudG9uZ3dlbkAxNjMuY29t; Quancheng Kan, a2FucXVhbmNoZW5nQDEyNi5jb20=
†These authors have contributed equally to this work
 Huoyan Liang1,2†
Huoyan Liang1,2† Ruiqing Zhai
Ruiqing Zhai Tongwen Sun
Tongwen Sun