- 1Genesis Research Services, Broadmeadow, NSW, Australia
- 2Centre for Inflammatory Diseases, School of Clinical Sciences at Monash Health, Monash University, Clayton, VIC, Australia
- 3Ritchie Centre, Hudson Institute of Medical Research, Clayton, VIC, Australia
- 4Department of Paediatrics, Monash University, Clayton, VIC, Australia
- 5Monash Newborn, Monash Children’s Hospital, Melbourne, VIC, Australia
- 6Hunter Pain Specialists, Broadmeadow, NSW, Australia
Interleukin (IL)-37 has an important function in limiting excessive inflammation. Its expression is increased in numerous inflammatory and autoimmune conditions and correlates with disease activity, suggesting it could have potential as a disease biomarker. Nevertheless, a reference range has yet to be determined. Our aim was to establish the first reference range of circulating IL-37 levels in healthy adult humans. PubMed was searched for studies reporting blood IL-37 concentrations in healthy adult subjects as measured by enzyme-linked immunosorbent assay. Nineteen studies were included in the analysis. Mean IL-37 levels were weighted by sample sizes, and weighted mean lower and upper levels ( ± 2SD of means) were calculated to provide a weighted mean and reference range. IL-37 levels were quantified in either serum or plasma from a total of 1035 (647 serum; 388 plasma) healthy subjects. The serum, plasma and combined matrix weighted means (reference ranges) were 72.9 (41.5 – 104.4) pg/mL, 83.9 (41.1 – 126.8) pg/mL, and 77.1 (41.4 – 112.8) pg/mL, respectively. There were no significant differences between serum and plasma means and upper and lower limits. Study means and upper IL-37 levels were significantly higher in Chinese population studies. From our analysis, a preliminary reference range for circulating IL-37 levels in healthy human adults has been established. In order to determine a reliable reference range for clinical application, large, prospective, multi-ethnic, healthy population studies are necessary. In addition, demographics, sample matrix, collection, processing and storage methods potentially affecting IL-37 detection levels should be thoroughly investigated.
Introduction
Interleukin (IL)-37, formally known as IL-1 family member 7 (IL-1F7), is the second most recent addition to the IL-1 family (1–3). Unlike most IL-1 family members, which are pro-inflammatory, IL-37 has anti-inflammatory actions (4). Research over the last decade has provided detailed insights into the fundamental mechanisms of action of IL-37, and highlighted its key role in suppressing innate immune and inflammatory responses (3–6). The importance of IL-37 as an anti-inflammatory cytokine (7) is highlighted by the exponentially increasing number of peer-reviewed publications dealing with this cytokine (Figure 1).
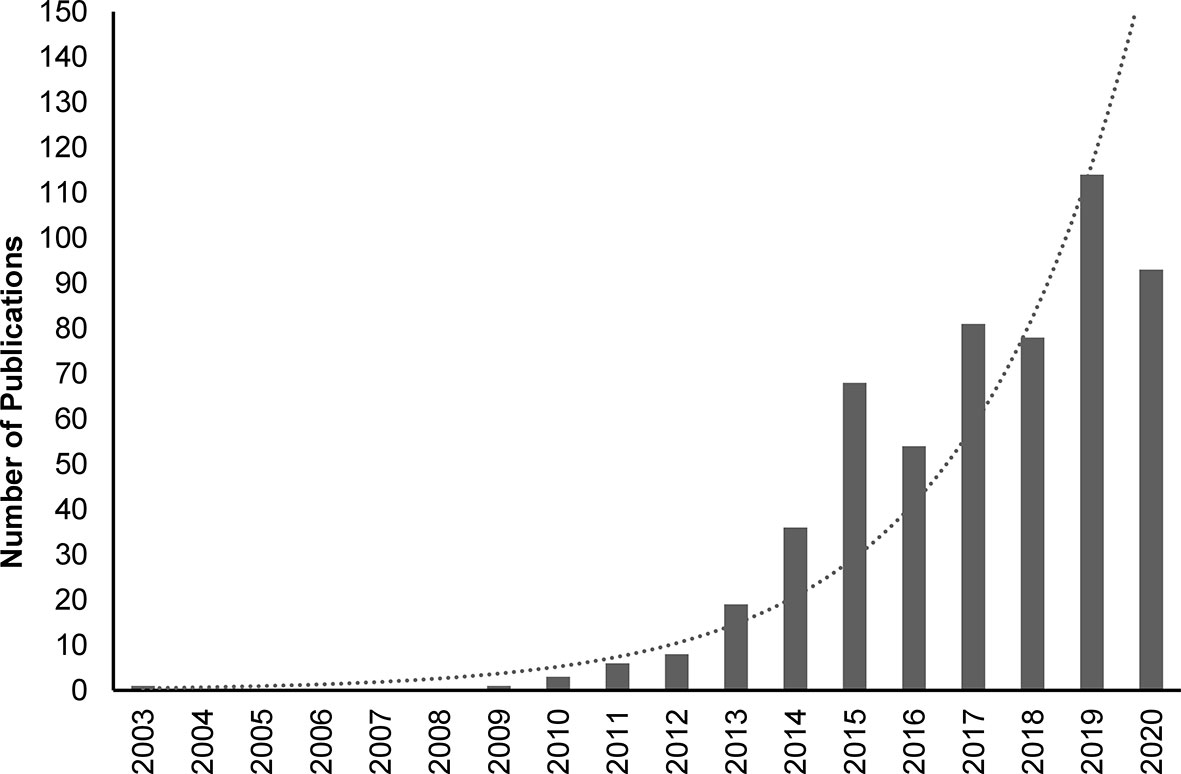
Figure 1 PubMed-indexed peer-reviewed publications on IL-37 between 2003 and 2020. Search was performed on 12th January 2021 using the following search terms: IL37, IL-37, IL 37, Interleukin-37, Interleukin37, Interleukin 37. Dotted line: exponential trendline.
The primary sources of IL-37 are circulating innate immune cells, including monocytes, dendritic cells and macrophages (8, 9). Under healthy conditions, IL-37 protein expression levels are low, as an mRNA instability sequence has been identified in the IL-37 coding region and therefore IL-37 mRNA is modulated under normal conditions (10). In peripheral blood mononuclear cells (PBMCs), IL-37 expression is upregulated by inflammatory stimuli such as IL-1ß, IL-18, tumor necrosis factor (TNF), interferon gamma (IFNγ), and toll-like receptor (TLR) ligands (3, 11, 12). In turn, IL-37 is a potent inhibitor of pro-inflammatory cytokine production, in particular, but not limited to, TNF, IL-1ß and IL-6 (6). Thus, IL-37 likely acts as a negative feedback mechanism to limit excessive inflammation (3, 13). Additionally, IL-37 acts to restore cell metabolic homeostasis during inflammation and to limit the metabolic cost of chronic inflammation (6, 13, 14). One mechanism by which IL-37 suppresses pro-inflammatory cytokine production is by “switching off” metabolic activation in monocytes (13).
Five distinct isoforms of IL-37 (a-e) have been identified, each with tissue-specific expression patterns and different biological functionality (11). Of the five, IL-37b (exons 1, 2, 4, 5 and 6) is the longest and the most biologically active isoform and has been the one mostly studied (7). Exon 1 is included in all isoforms except IL-37a, which still appears to share similar anti-inflammatory effects to IL-37b, while IL-37c and IL-37e are considered to act as regulators of IL-37a and IL-37b (7). Less is known about IL-37d, which also presents with anti-inflammatory functions and is detected in PBMCs (15). Of note, in the literature, the IL-37b isoform is often referred to as “IL-37”. Currently, there have been a total of 14 IL-37 protein variants identified, with three major variants constituting over 97% of all sequences, including “Var1”, “Var2” and “Ref” (16). Within the European population, 13.5% are heterozygous for the third major variant, “Var2”, which, compared to the two other major variants, induces a stronger, yet shorter-lived immune response due to preferential proteasome degradation (17).
Since the discovery of IL-37’s potent anti-inflammatory function, research into its role in disease pathogenesis has been growing. Compared to healthy subjects, increased circulating (serum and plasma) levels of IL-37 have been documented in various inflammatory and autoimmune conditions, including rheumatoid arthritis (18–24), ankylosing spondylitis (25), endometriosis (26–28), systemic lupus erythematosus (29–31), Guillain-Barré syndrome (32), Graves’ disease (33), and multiple sclerosis (34, 35), as well as in spinal cord injury (36) where its levels are frequently correlated with measures of disease activity. Increased IL-37 is likely one of the body’s attempts to dampen inflammation and restore immune homeostasis. Elevated circulating IL-37 levels have also been reported in several types of cancers (e.g. epithelial ovarian cancer, gastric cancer), and chronic heart failure, and were associated with poor prognosis (37–39). Conversely, decreased circulating IL-37 abundance has been observed in some inflammatory diseases, such as Behçet’s disease (40, 41) and inflammatory bowel disease (ulcerative colitis and Crohn’s disease) (42). Interestingly, in patients with inflammatory bowel disease, increased local abundance has been reported in colonic/intestinal tissues (42–44). IL-37 protein level has also been studied in other body fluids of patients suffering from inflammatory diseases, including the cerebrospinal fluid of patients with Behçet’s disease with neurological localizations (lower IL-37 levels) (45), and in those with Guillain-Barré syndrome (higher IL-37 levels) (32). A growing number of cell line studies, animal model studies, and genetic studies provide further support for involvement of IL-37 in the aforementioned and additional conditions, such as gout, in which rare genetic variants of IL-37 that result in loss of anti-inflammatory action have been identified (46), obesity-induced inflammation and metabolic syndrome (47, 48), melanoma (49), and other cancers (50).
The potential use of IL-37 as a disease biomarker is often discussed, however, such an approach would require a reference range to be established, and to the best of our knowledge, no attempts have been made yet. Importantly, bearing in mind low physiological levels of IL-37 in the circulation, while differential expression of IL-37 is reported in many inflammatory diseases compared to healthy controls, the direction of change appears inconsistent across diseases, highlighting the need for hyper sensitive assays that can determine both low and high thresholds for normal ranges. Additionally, the dynamics and magnitude of expected fluctuation of IL-37 levels over the course of each disease should be studied.
Limited reports have focused on establishing reference ranges or cut-off levels for cytokines to develop robust diagnostic or prognostic biomarkers (51). Kleiner et al. (52) recognized the unmet need for reference values to be established in physiological conditions and investigated the levels of 48 cytokines and chemokines in the serum of 72 healthy subjects, however, IL-37 was not included in their analysis. Most human cytokine biomarker studies primarily aim to identify proteins with the largest significant difference in their levels between the cohort of patients compared to healthy subjects, and to find relationships between candidate biomarkers and disease phenotype, activity, or severity. The clinical translation of such findings into new diagnostic or prognostic tools is difficult and also limited due to the lack of cytokine reference ranges or consensus cut-offs.
This study aimed to review the existing literature on circulating levels of IL-37 in healthy adult subjects and to derive a preliminary reference range using published meta-analysis-type methods (53–55).
Methods
Search Strategy
A Medline search was performed via PubMed for peer-reviewed articles published up to the 1st March 2021 that reported on blood IL-37 concentrations in healthy humans using the following search term: (il37 OR il-37 OR “IL 37” OR interleukin37 OR interleukin-37 OR “interleukin 37”) AND (healthy) AND (serum OR plasma OR blood). Search results were filtered for English language.
Study Selection
Full articles were reviewed and screened. For inclusion into the analysis, studies had to report serum or plasma levels of IL-37 from healthy adults as measured using enzyme-linked immunosorbent assays (ELISA). IL-37 levels had to be reported as mean + standard deviation (SD) (or standard error of the mean).
Studies were excluded if they met any of the following exclusion criteria: 1) only genetic or gene expression (mRNA) studies; 2) cell/tissue studies; 3) animal model studies; 4) IL-37 levels measured in non-blood matrix (e.g., urine, saliva, sputum); 5) healthy control subjects considered non ‘normal’ (e.g., pregnant females or other patients); 6) healthy subjects < 18 years of age; 7) numerical values of mean (SD) circulating IL-37 not reported/available (e.g., graphical results only); 8) only median IL-37 levels reported; 9) extrapolated IL-37 levels reported (outside of the detection limits of the ELISA kit used); 10) large standard deviations that result in calculation of negative lower limit values; 11) quantification by method other than ELISA; 12) not IL-37 (misleading article title). Additional articles listed under ‘Similar Articles’ on the article abstract pages in PubMed were also reviewed. This screening process is summarized in Figure 2.
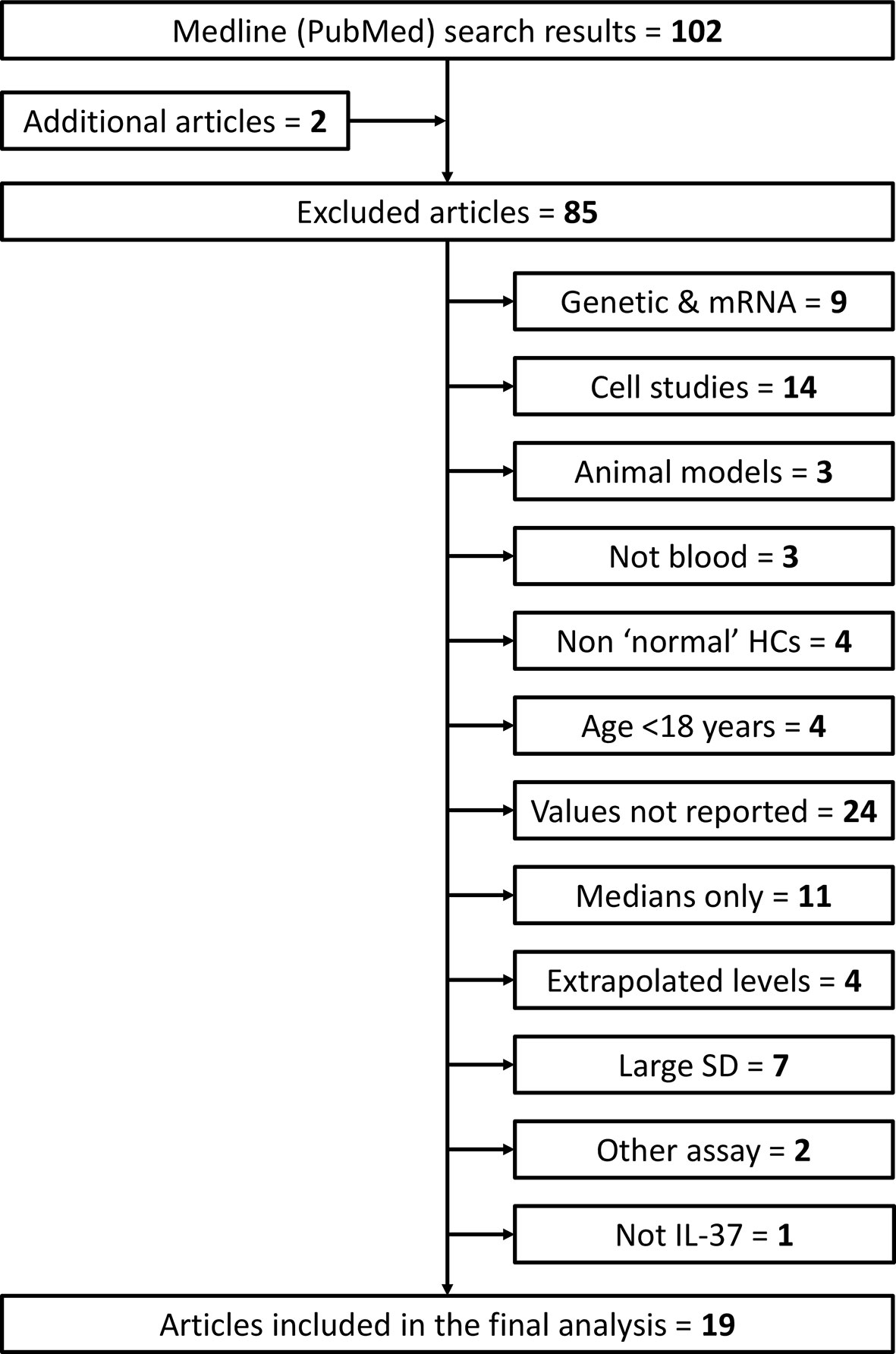
Figure 2 Flow diagram of search results and article screening, exclusion and inclusion for analysis of circulating IL-37 in healthy adult humans. Additional articles were found by reviewing the ‘Similar Articles’ listed on abstract pages on PubMed. HCs, healthy controls; SD, standard deviation.
Data Collection
The following healthy subject data was extracted: mean (SD) IL-37 blood concentration in pg/mL, sample type (serum or plasma), number of healthy subjects; and (if available): mean age (SD or range), gender frequencies. ELISA quantification kit manufacturer and the country in which the study was performed was also recorded.
Statistical Analysis
All data analyses and data visualization were performed within Microsoft Excel 2016 (Version 2102) using spreadsheets developed by Neyeloff et al. (56) for conducting meta-analyses. To calculate a reference range for circulating IL-37 in healthy adult subjects, we considered the approaches taken by Nemeth et al. (55) and Venner et al. (53) to calculate references ranges for other molecular biomarkers, and applied the methodology of Thijs et al. (54) and Staessen et al. (57, 58) to determine reference values for blood pressure.
To calculate a reference value (mean) for IL-37 levels in healthy adults, the mean of each included study (xi) was weighted by its respective sample size (ni) and the sum of the weighted means was then divided by the sum of the sample sizes to calculate a weighted mean (M) as follows:
To calculate a reference range, lower and upper limits of normal IL-37 levels were first calculated for each included study as mean ± 2 SD, and the same formula was then applied to calculate a weighted mean lower value and a weighted mean upper value. The weighted means and reference range for serum, plasma, and all studies combined are presented. A forest plot was generated using the methods and spreadsheet developed by Neyeloff et al. (56).
Results
Study Characteristics
After screening 102 articles returned by the initial PubMed search (+ additional “Similar” articles), 19 met the inclusion criteria (Figure 2). Twelve and seven studies measured IL-37 levels in serum and plasma, respectively. The total number of subjects included in the meta-analysis was 1035 (serum: n = 647; plasma: n = 388). Study characteristics and healthy subject demographics are presented in Table 1.
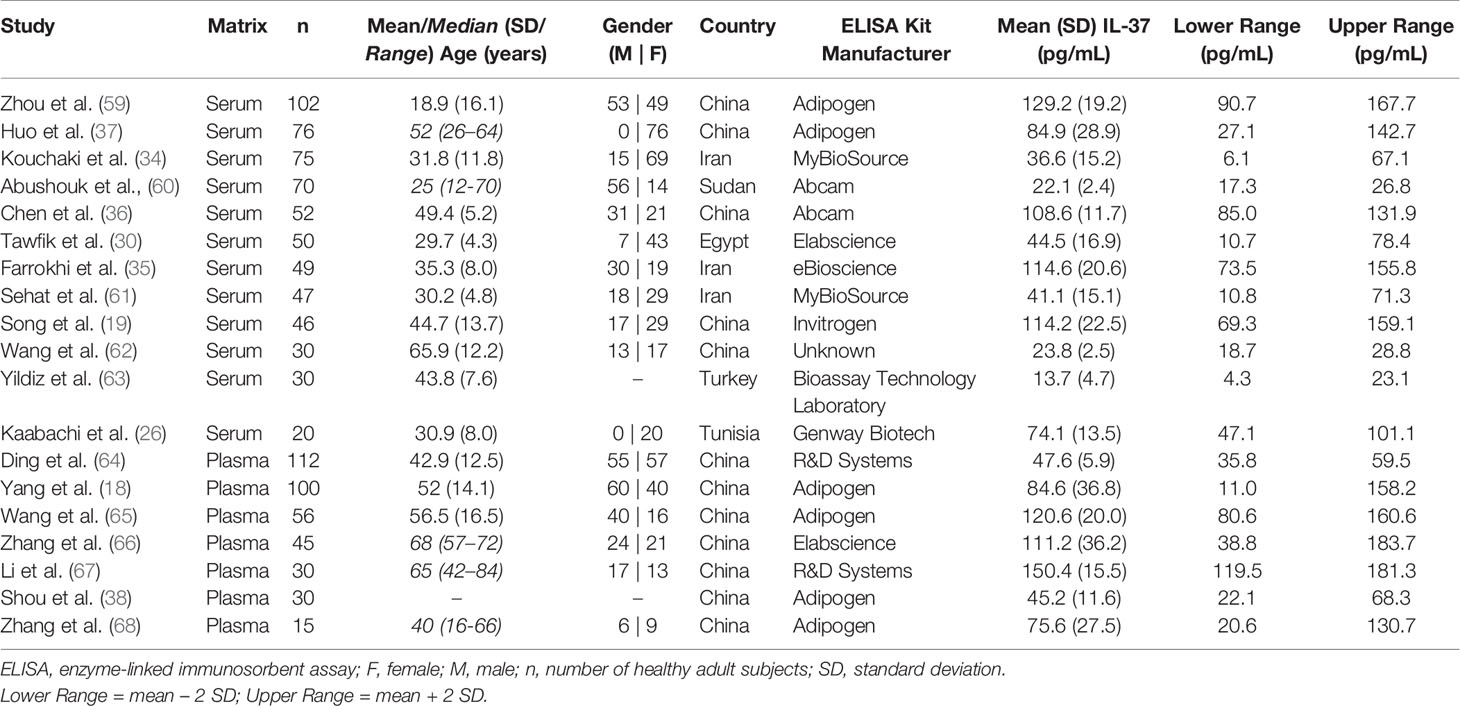
Table 1 Description of studies included in the analysis, with healthy adult subject demographics, study-reported mean IL-37 levels, and computed healthy IL-37 level ranges.
Circulating IL-37 Reference Ranges in Healthy Adults
The weighted means (reference ranges) for serum and plasma IL-37 in healthy adult subjects were 72.9 (41.5 – 104.4) pg/mL and 83.9 (41.1 – 126.8) pg/mL, respectively. The pooled (serum and plasma) weighted mean (reference range) of IL-37 in healthy adult subjects was 77.1 (41.4 – 112.8) pg/mL. Results are summarized in Table 2. Figure 3 displays a forest plot of individual study mean (± 2 SD) IL-37 levels, and the weighted means (reference ranges) for serum, plasma, and pooled serum and plasma IL-37 levels. No statistically significant difference in weighted means, and lower and upper range IL-37 levels were observed between serum and plasma groups (P = 0.24, P = 0.62, P = 0.15, respectively; unpaired two-tailed t-test).
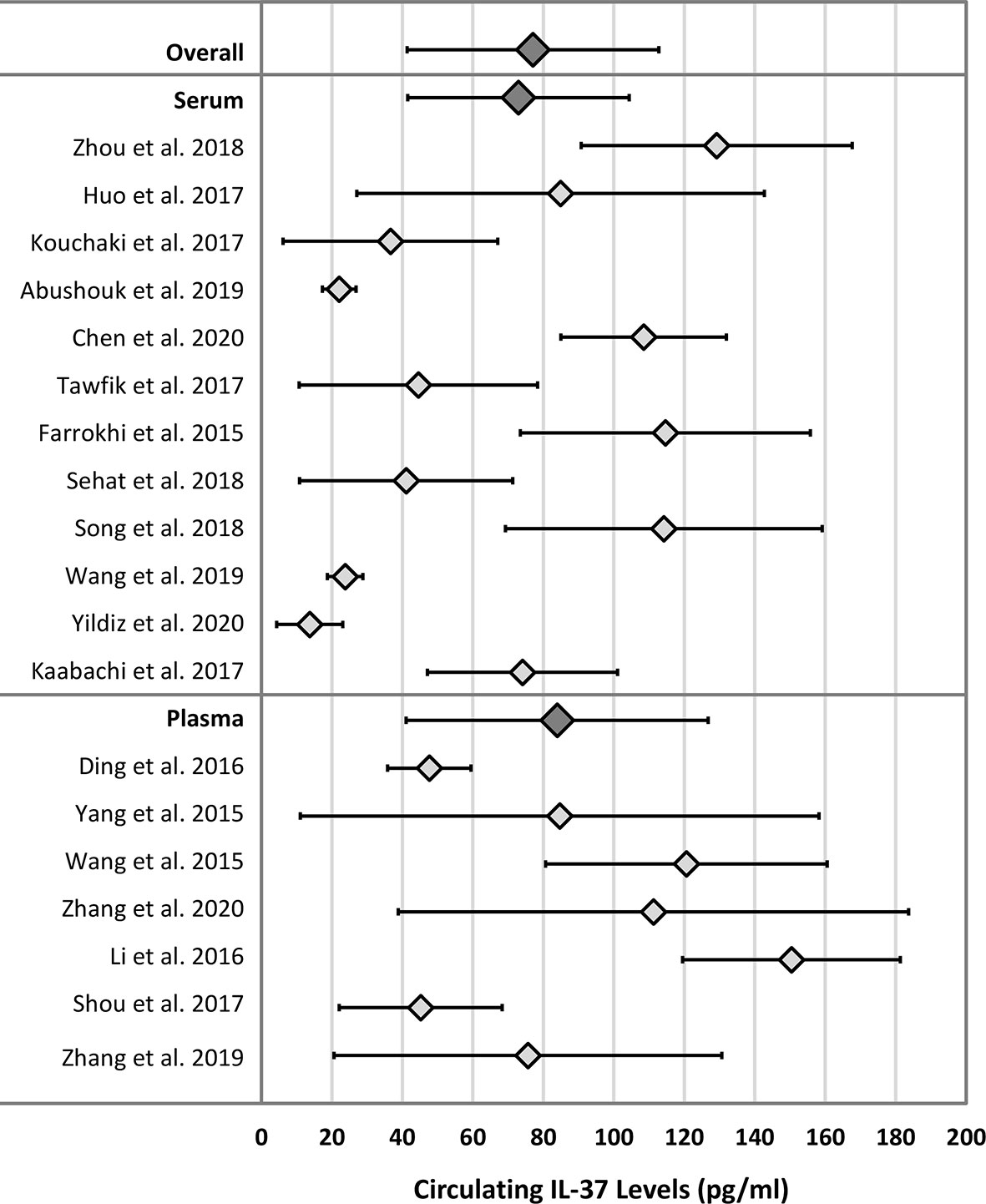
Figure 3 Forest plot of IL-37 means and ranges (± 2 SD) for individual studies and mean of weighted means and reference ranges. Dark grey diamonds: Overall - mean of weighted means and reference range of IL-37 in both serum and plasma matrix; Serum - mean of weighted means and reference range of IL-37 in serum only; Plasma - mean of weighted means and reference range of IL-37 in plasma only.
Twelve of the 19 included studies involved Chinese populations (mixed plasma and serum studies), with a weighted mean (reference range) of IL-37 of 91.9 (51.0 – 132.8) pg/mL. When compared to the non-Chinese population (serum studies only), for which the weighted mean (reference range) of IL-37 was 46.8 (21.7 – 71.9) pg/mL, mean and upper range IL-37 levels were statistically significantly higher in the Chinese populations (P = 0.03 and P = 0.03, respectively; unpaired two-tailed t-test). Results are summarized in Table 2.
There were two female population studies (endometriosis and epithelial ovarian cancer investigations), and two studies with > 85% female healthy adult subjects. For these four studies, the weighted mean (reference range) was 58.4 (18.1 – 98.7) pg/mL. No statistically significant difference in mean, lower and upper range of IL-37 levels were observed between these four studies (total 208 females and 22 males; all serum studies) and the remaining 13 studies with male and female counts reported (total 334 females and 420 males; mixed plasma and serum studies) (data not shown; P = 0.23, P = 0.15, P = 0.39, respectively; unpaired two-tailed t-test).
It is worth noting that the most commonly used ELISA kit was manufactured by AdipoGen (n = 6). The weighted mean IL-37 level for healthy subjects from studies using this kit was 98.5 pg/mL, with study means ranging from 45.2 to 120.6 pg/mL for plasma (n = 4), and 84.9 to 129.2 pg/mL for serum (n = 2) (data not shown). The remainder of the included studies used human IL-37 ELISA kits manufactured by Abcam, Bioassay Technology Laboratory, eBioSource, elabscience, GenWay, Invitrogen, MyBioSource, and R&D Systems (Table 1).
Discussion
IL-37 has recently emerged as a critical regulator of inflammation. The growing number of studies on inflammatory and autoimmune diseases that have reported significant differences in circulating IL-37 levels between patients and control subjects highlights the importance of this cytokine in disease settings, and supports its potential widespread usefulness as a disease biomarker. Additionally, numerous reports of associations between circulating IL-37 levels and inflammatory pathology parameters (i.e.: C-reactive protein, CRP; erythrocyte sedimentation rate, ESR) support the potential usefulness of circulating IL-37 levels as a biomarker of disease activity or severity. Circulating IL-37 levels have been found to positively correlate with levels of CRP and/or ESR in patients with rheumatoid arthritis (18, 21–23), systemic lupus erythematosus (30), adult-onset Still’s disease (69, 70), ankylosing spondylitis (25), gout (71), and osteoarthritis (72), as well as in patients with chronic heart failure (38). Also, the prognostic value of IL-37 has been demonstrated for several diseases, including epithelial ovarian cancer (37), gastric cancer (39), multiple sclerosis (34), spinal cord injury (36), acute ischemic stroke (66), and heart failure (38). In this respect, we have conducted a meta-analysis of 19 studies, analyzing a total of 1118 healthy adult individuals using extracted mean (SD) levels to establish, for the first time, a preliminary reference range of circulating IL-37 in both serum and plasma.
To the best of our knowledge, no published study has defined a reference range for circulating IL-37 levels in healthy individuals. Several studies have evaluated IL-37 as a disease biomarker, using receiver operating characteristic (ROC) curve analysis to assess the sensitivity and specificity of cut-off values of circulating IL-37 to distinguish patients from healthy subjects. Yuan et al. (73) reported a cut-off value of 253.86 pg/mL (plasma), with high sensitivity and specificity, to distinguish rheumatoid arthritis patients from controls. Yildiz et al. (63) reported a cut-off value 9.37 pg/mL (serum) to distinguish healthy subjects from patients with asthma, however, sensitivity was poor. For endometriosis, serum IL-37 cut-off levels of 69.84 pg/mL (28), and 5.99 pg/ml (27), both with high sensitivity and specificity, have been reported. Future research that aims to prospectively quantify IL-37 levels in serum and plasma in large, healthy populations would be of value.
According to the Clinical and Laboratory Standards Institute (CLSI) guidelines (74), each laboratory should determine the circulating levels of the analyte of interest in a minimum of 120 samples from healthy individuals for each major demographic group (e.g. age group, gender, ethnicity) for establishing optimal reference intervals. Only three of the 19 analyzed studies were of modest sample size, with n ≥ 100, yet none were ≥ 120. Ding et al. (64) conducted the largest study, including 112 healthy subjects, and reported a mean (SD) plasma IL-37 level of 47.6 (5.9) pg/mL, which computes to a range of 35.8 – 59.5 pg/mL. This range may be considered too narrow based on the results of this meta-analysis. Two other analyzed studies (60, 62) reported small SDs (apparently not standard error of the means, as indicated throughout each article), which also compute to narrow ranges. A number of excluded studies reported excessively large SDs that translated to negative lower limits. It would be valuable to know if any outliers were identified in these studies, and if sensitivity analyses were performed to assess the impact of the outliers on the study outcome.
As per the CLSI guidelines described above, it is important to consider inter- but also intra-individual variation when establishing a reference range. Blood levels of many cytokines are associated with different factors, including demographics (age, gender, ethnicity) and lifestyle factors, as shown in cross-sectional analyses (51, 52, 75). For example, Kleiner et al. (52) investigated the relationship between age and serum levels of 48 cytokines and chemokines (not including IL-37) in healthy subjects categorized by age groups (1-6, 7-17, and ≥ 18 years). They reported nine cytokines/chemokines that were significantly decreased and two that were significantly increased in adults compared to children. Of note, no significant differences between males and females across age groups were reported. Stowe et al. (75) investigated levels of IL-6, IL-10, and other cytokines, in a population-based study of Hispanic and non-Hispanic ethnic groups. They reported significantly higher levels of plasma IL-6 and TNF-r1 in subjects aged over 50 (entire cohort), and significantly higher levels of plasma IL-6 in the “non-Hispanic black” ethnic group compared to the “non-Hispanic white” ethnic group.
To our knowledge, circulating IL-37 (as measured by ELISA) has not been investigated in relation to age and ethnicity in a healthy population-based study setting. Only one of the studies included in our analysis investigated associations between gender and IL-37 levels in healthy subjects, showing no significant differences in serum IL-37 levels according to gender (35). Davarpanah et al. (76) (excluded study) reported the same findings. In light of the limited number of studies, a larger and independent healthy population study is warranted. Of note, there is limited evidence suggesting that IL-37 levels are negatively associated with age in infectious and autoimmune disease settings, including eumycetoma infection (60), Hashimoto’s thyroiditis (77), and multiple sclerosis (78). Increased serum IL-37 was also reported to be associated with Asian ethnicity in patients with systemic lupus erythematosus (79). In our analysis, we observed significantly higher mean of weighted means of IL-37 levels and calculated upper levels across the Chinese studies compared to non-Chinese population studies. These observations are, however, preliminary in light of the small number of studies in the non-Chinese group, and the potential for the matrix studied (plasma/serum) as a confounding factor. In fact, Chinese population studies included both serum (n = 5) and plasma (n = 7), while the non-Chinese studies included only serum investigations (n = 7) (Table 1). Future research is needed to confirm these preliminary findings in a prospective, large, and multi-ethnic cohort of healthy subjects.
Amongst the included studies, only cross-sectional analyses of healthy subjects were performed. Circulating levels of several other cytokines have also been shown to correlate with temporal factors such as stress, weight, diet, fasting status, and blood pressure (51, 80). In a 14-week study of cytokine serum levels (including 12 interleukins) in healthy subjects (using ultrasensitive single-molecule array assays), intra-individual variability across study visits was low and temporal stability was high for all cytokines, however, IL-37 was not measured (81). Longitudinal studies are therefore needed to investigate intra-individual variability in IL-37 levels in healthy adults.
There was a large degree of variation in circulating IL-37 levels between ELISA kits. In this regard, it is of importance that the performance of specific IL-37 detection tools always needs to be carefully validated by the user. Our team has therefore selected four different commercially available IL-37 ELISA kits for validation. PBS or murine serum spiked with recombinant IL-37 (recIL37) was used as a positive control. In every ELISA, positive controls were then tested alongside either murine serum samples from mice either injected with a bolus of recIL-37 or vehicle (negative control) (82), or systemic lupus erythematosus patient serum (79) to determine endogenous IL-37 levels. In our hands, the AdipoGen ELISA provided the most reliable results, detecting endogenous IL-37 in human serum or serum of mice injected with recombinant human IL-37. Importantly, the AdipoGen kit was also used in the majority of the studies included herein. For a comprehensive conclusion, a head-to-head comparison study of commercial ELISA kits in a broad selection of specimens would be required, which is beyond the scope of this study. Notably, our team has also extensively tested tools for the detection of IL-37 in other applications and found the IL-37 antibody clone 37D12 from eBioscience (San Diego, CA, USA) to reliably detect IL-37 by flow cytometry (8), immuno blot and immunohistochemistry (83).
Parameters other than the detection method by the kit per se, such as pre-analytics (sample collection, processing, storage) (84), assay procedure (room temperature, timing of analysis, plate reader) (85), the matrix studied (plasma vs serum), or study population heterogeneity (demographics, diet, alcohol consumption), are of importance and need to be considered as potential confounding factors. The complexity of comparing individual cytokine levels measured using different methods or platforms (including variability between assay kits from different manufacturers) has been discussed in detail by Tarrant (86), who focused on the use of blood cytokines as biomarkers of toxicity. Standardized operating procedures for pre-analytics (84) should be established by commercial kit manufacturers to optimize circulating IL-37 quantification. Sample collection and processing was poorly documented in several of the articles that we reviewed. Establishing a reference range for both serum and plasma samples would be valuable, as protein levels are known to differ according to the matrix (87). Whether this applies to IL-37 has yet to be determined. We observed no statistically significant difference in mean of weighted means, lower and upper range IL-37 levels between serum and plasma when included studies were grouped by matrix.
If circulating IL-37 is to be measured in clinical settings, then reference ranges should be established for each kit by the manufacturer, and also by the laboratory performing the test if the population tested differs from the one assessed by the manufacturer. However, it has been argued that this system does not serve the medical community well and that development of a common universal reference range is ideal (88). According to the CLSI guidelines (74), if a universal reference range that meets the criteria is established then an appropriate alternative would be for individual laboratories (and manufacturers) to verify the reference range by analyzing as little as 20 samples collected from healthy individuals recruited from the local population.
The major limitations of our study have already been highlighted. We acknowledge that the quality of the included studies potentially hinders the conclusions drawn from our analysis. Additionally, despite there being a large number of potentially useful studies in the literature, many were excluded as they did not report actual mean (SD) values for IL-37 levels in healthy subjects, presenting results graphically only, or reporting only the direction and significance level of any changes between groups. For studies that were excluded for reporting medians and minimum and maximum values only, we attempted to estimate means and standard deviations using methods by Luo et al. (89) and Wan et al. (90), however, meaningful estimations could not be achieved. Overall, this underlines the unmet need for any such study to report both mean (SD) and median (IQR) regardless of the cytokine data distribution in each of the studied groups, and to report the actual values in the text, even if the data are graphically presented. To enable meta-analysis to be performed in future research, such guidelines should be developed in collaboration with peer-reviewed journals.
Furthermore, the potential confounding effects of study population variables were not thoroughly assessed. Our aim was to provide a starting point for a reference range, the accuracy of which could be improved by performing a larger, prospective, multi-ethnic study of both serum and plasma IL-37 levels in healthy adult humans, including detailed documentation of demographic and lifestyle parameters for analysis as potential confounding factors in multivariable regression models.
The important role of IL-37 in suppressing innate immune and inflammatory responses and the growing mound of evidence for its involvement in various disease states warrants further investigations into what may be considered normal circulating levels in humans. Our study provides, for the first time, a preliminary reference range for circulating plasma and serum IL-37 levels. In order to establish a definitive reference range for both matrices, large, high-quality, prospective, longitudinal, multi-ethnic healthy population studies, including a balanced gender ratio as well as all age categories, that meet CLSI criteria, are needed.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
MAR conceptualized and designed the study. DMS collected and analyzed the data. MAR and DMS initiated the manuscript. All authors interpreted the data, contributed to the manuscript, revised the manuscript, and approved the submitted version.
Funding
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia Investigator Grant Leadership 1 [Grant 1173584] to CN-P, and the Fielding Foundation Fellowship 2017 to MN. FV is supported by a NHMRC Emerging Leadership 1 [Grant 1196112].
Conflict of Interest
Monash University, Hudson Institute (CN-P and MN) hold two patent families on IL-37, namely PCT/AU2016/050495 and EP19218657.5. No other conflicts of interest exist for these authors.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kumar S, McDonnell PC, Lehr R, Tierney L, Tzimas MN, Griswold DE, et al. Identification and Initial Characterization of Four Novel Members of the Interleukin-1 Family. J Biol Chem (2000) 275:10308–14. doi: 10.1074/jbc.275.14.10308
2. Dunn E, Sims JE, Nicklin MJ, O'Neill LA. Annotating Genes With Potential Roles in the Immune System: Six New Members of the IL-1 Family. Trends Immunol (2001) 22:533–6. doi: 10.1016/s1471-4906(01)02034-8
3. Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 Is a Fundamental Inhibitor of Innate Immunity. Nat Immunol (2010) 11:1014–22. doi: 10.1038/ni.1944
4. Li S, Amo-Aparicio J, Neff CP, Tengesdal IW, Azam T, Palmer BE, et al. Role for Nuclear Interleukin-37 in the Suppression of Innate Immunity. Proc Natl Acad Sci USA (2019) 116:4456–61. doi: 10.1073/pnas.1821111116
5. Dinarello CA, Nold-Petry C, Nold M, Fujita M, Li S, Kim S, et al. Suppression of Innate Inflammation and Immunity by Interleukin-37. Eur J Immunol (2016) 46:1067–81. doi: 10.1002/eji.201545828
6. Cavalli G, Dinarello CA. Suppression of Inflammation and Acquired Immunity by IL-37. Immunol Rev (2018) 281:179–90. doi: 10.1111/imr.12605
7. Jia H, Liu J, Han B. Reviews of Interleukin-37: Functions, Receptors, and Roles in Diseases. BioMed Res Int (2018) 2018:3058640. doi: 10.1155/2018/3058640
8. Rudloff I, Cho SX, Lao JC, Ngo D, McKenzie M, Nold-Petry CA, et al. Monocytes and Dendritic Cells Are the Primary Sources of Interleukin 37 in Human Immune Cells. J Leukoc Biol (2017) 101:901–11. doi: 10.1189/jlb.3MA0616-287R
9. Conti P, Lauritano D, Caraffa A, Gallenga CE, Kritas SK, Ronconi G, et al. Microglia and Mast Cells Generate Proinflammatory Cytokines in the Brain and Worsen Inflammatory State: Suppressor Effect of IL-37. Eur J Pharmacol (2020) 875:173035. doi: 10.1016/j.ejphar.2020.173035
10. Bufler P, Gamboni-Robertson F, Azam T, Kim SH, Dinarello CA. Interleukin-1 Homologues IL-1F7b and IL-18 Contain Functional mRNA Instability Elements Within the Coding Region Responsive to Lipopolysaccharide. Biochem J (2004) 381:503–10. doi: 10.1042/BJ20040217
11. Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, Mangelberger D, et al. IL-37: A New Anti-Inflammatory Cytokine of the IL-1 Family. Eur Cytokine Netw (2011) 22:127–47. doi: 10.1684/ecn.2011.0288
12. Wang L, Quan Y, Yue Y, Heng X, Che F. Interleukin-37: A Crucial Cytokine With Multiple Roles in Disease and Potentially Clinical Therapy. Oncol Lett (2018) 15:4711–9. doi: 10.3892/ol.2018.7982
13. Cavalli G, Tengesdal IW, Gresnigt M, Nemkov T, Arts RJW, Dominguez-Andres J, et al. The Anti-Inflammatory Cytokine Interleukin-37 Is an Inhibitor of Trained Immunity. Cell Rep (2021) 35:108955. doi: 10.1016/j.celrep.2021.108955
14. Cavalli G, Justice JN, Boyle KE, D'Alessandro A, Eisenmesser EZ, Herrera JJ, et al. Interleukin 37 Reverses the Metabolic Cost of Inflammation, Increases Oxidative Respiration, and Improves Exercise Tolerance. Proc Natl Acad Sci USA (2017) 114:2313–8. doi: 10.1073/pnas.1619011114
15. Zhao M, Li Y, Guo C, Wang L, Chu H, Zhu F, et al. IL-37 Isoform D Downregulates Pro-Inflammatory Cytokines Expression in a Smad3-Dependent Manner. Cell Death Dis (2018) 9:582. doi: 10.1038/s41419-018-0664-0
16. Kang B, Cheng S, Peng J, Yan J, Zhang S. Interleukin-37 Gene Variants Segregated Anciently Coexist During Hominid Evolution. Eur J Hum Genet (2015) 23:1392–8. doi: 10.1038/ejhg.2014.302
17. Yan J, Zhang Y, Cheng S, Kang B, Peng J, Zhang X, et al. Common Genetic Heterogeneity of Human Interleukin-37 Leads to Functional Variance. Cell Mol Immunol (2017) 14:783–91. doi: 10.1038/cmi.2016.48
18. Yang L, Zhang J, Tao J, Lu T. Elevated Serum Levels of Interleukin-37 Are Associated With Inflammatory Cytokines and Disease Activity in Rheumatoid Arthritis. APMIS (2015) 123:1025–31. doi: 10.1111/apm.12467
19. Song L, Wang Y, Sui Y, Sun J, Li D, Li G, et al. High Interleukin-37 (IL-37) Expression and Increased Mucin-Domain Containing-3 (TIM-3) on Peripheral T Cells in Patients With Rheumatoid Arthritis. Med Sci Monit (2018) 24:5660–7. doi: 10.12659/MSM.909254
20. Xia L, Shen H, Lu J. Elevated Serum and Synovial Fluid Levels of Interleukin-37 in Patients With Rheumatoid Arthritis: Attenuated the Production of Inflammatory Cytokines. Cytokine (2015) 76:553–7. doi: 10.1016/j.cyto.2015.06.005
21. Ragab D, Mobasher S, Shabaan E. Elevated Levels of IL-37 Correlate With T Cell Activation Status in Rheumatoid Arthritis Patients. Cytokine (2019) 113:305–10. doi: 10.1016/j.cyto.2018.07.027
22. Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan YX, Jiang YF. Plasma Levels of IL-37 and Correlation With TNF-Alpha, IL-17A, and Disease Activity During DMARD Treatment of Rheumatoid Arthritis. PLoS One (2014) 9:e95346. doi: 10.1371/journal.pone.0095346
23. Ye L, Jiang B, Deng J, Du J, Xiong W, Guan Y, et al. IL-37 Alleviates Rheumatoid Arthritis by Suppressing IL-17 and IL-17-Triggering Cytokine Production and Limiting Th17 Cell Proliferation. J Immunol (2015) 194:5110–9. doi: 10.4049/jimmunol.1401810
24. Xia T, Zheng XF, Qian BH, Fang H, Wang JJ, Zhang LL, et al. Plasma Interleukin-37 is Elevated in Patients With Rheumatoid Arthritis: Its Correlation With Disease Activity and Th1/Th2/Th17-Related Cytokines. Dis Markers (2015) 2015:795043. doi: 10.1155/2015/795043
25. Chen B, Huang K, Ye L, Li Y, Zhang J, Zhang J, et al. Interleukin-37 Is Increased in Ankylosing Spondylitis Patients and Associated With Disease Activity. J Transl Med (2015) 13:36. doi: 10.1186/s12967-015-0394-3
26. Kaabachi W, Kacem O, Belhaj R, Hamzaoui A, Hamzaoui K. Interleukin-37 in Endometriosis. Immunol Lett (2017) 185:52–5. doi: 10.1016/j.imlet.2017.03.012
27. Jiang J, Jiang Z, Xue M. Serum and Peritoneal Fluid Levels of Interleukin-6 and Interleukin-37 as Biomarkers for Endometriosis. Gynecol Endocrinol (2019) 35:571–5. doi: 10.1080/09513590.2018.1554034
28. Fan YY, Chen HY, Chen W, Liu YN, Fu Y, Wang LN. Expression of Inflammatory Cytokines in Serum and Peritoneal Fluid From Patients With Different Stages of Endometriosis. Gynecol Endocrinol (2018) 34:507–12. doi: 10.1080/09513590.2017.1409717
29. Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, et al. IL-37 Inhibits the Production of Inflammatory Cytokines in Peripheral Blood Mononuclear Cells of Patients With Systemic Lupus Erythematosus: its Correlation With Disease Activity. J Transl Med (2014) 12:69. doi: 10.1186/1479-5876-12-69
30. Tawfik MG, Nasef SI, Omar HH, Ghaly MS. Serum Interleukin-37: A New Player in Lupus Nephritis? Int J Rheum Dis (2017) 20:996–1001. doi: 10.1111/1756-185X.13122
31. Song L, Qiu F, Fan Y, Ding F, Liu H, Shu Q, et al. Glucocorticoid Regulates Interleukin-37 in Systemic Lupus Erythematosus. J Clin Immunol (2013) 33:111–7. doi: 10.1007/s10875-012-9791-z
32. Li C, Zhao P, Sun X, Che Y, Jiang Y. Elevated Levels of Cerebrospinal Fluid and Plasma Interleukin-37 in Patients With Guillain-Barre Syndrome. Mediators Inflamm (2013) 2013:639712. doi: 10.1155/2013/639712
33. Li Y, Wang Z, Yu T, Chen B, Zhang J, Huang K, et al. Increased Expression of IL-37 in Patients With Graves' Disease and its Contribution to Suppression of Proinflammatory Cytokines Production in Peripheral Blood Mononuclear Cells. PLoS One (2014) 9:e107183. doi: 10.1371/journal.pone.0107183
34. Kouchaki E, Tamtaji OR, Dadgostar E, Karami M, Nikoueinejad H, Akbari H. Correlation of Serum Levels of IL-33, IL-37, Soluble Form of Vascular Endothelial Growth Factor Receptor 2 (VEGFR2), and Circulatory Frequency of VEGFR2-Expressing Cells With Multiple Sclerosis Severity. Iran J Allergy Asthma Immunol (2017) 16:329–37.
35. Farrokhi M, Rezaei A, Amani-Beni A, Etemadifar M, Kouchaki E, Zahedi A. Increased Serum Level of IL-37 in Patients With Multiple Sclerosis and Neuromyelitis Optica. Acta Neurol Belg (2015) 115:609–14. doi: 10.1007/s13760-015-0491-3
36. Chen Y, Wang D, Cao S, Hou G, Ma H, Shi B. Association Between Serum IL-37 and Spinal Cord Injury: a Prospective Observational Study. BioMed Res Int (2020) 2020:6664313. doi: 10.1155/2020/6664313
37. Huo J, Hu J, Liu G, Cui Y, Ju Y. Elevated Serum Interleukin-37 Level Is a Predictive Biomarker of Poor Prognosis in Epithelial Ovarian Cancer Patients. Arch Gynecol Obstet (2017) 295:459–65. doi: 10.1007/s00404-016-4258-8
38. Shou X, Lin J, Xie C, Wang Y, Sun C. Plasma IL-37 Elevated in Patients With Chronic Heart Failure and Predicted Major Adverse Cardiac Events: A 1-Year Follow-Up Study. Dis Markers (2017) 2017:9134079. doi: 10.1155/2017/9134079
39. Zhang Y, Tang M, Wang XG, Gu JH, Zhou LN, Jin J, et al. Elevated Serum Levels of Interleukin-37 Correlate With Poor Prognosis in Gastric Cancer. Rev Esp Enferm Dig (2019) 111:941–5. doi: 10.17235/reed.2019.6460/2019
40. Bouali E, Kaabachi W, Hamzaoui A, Hamzaoui K. Interleukin-37 Expression is Decreased in Behcet's Disease and is Associated With Inflammation. Immunol Lett (2015) 167:87–94. doi: 10.1016/j.imlet.2015.08.001
41. Ye Z, Wang C, Kijlstra A, Zhou X, Yang P. A Possible Role for Interleukin 37 in the Pathogenesis of Behcet's Disease. Curr Mol Med (2014) 14:535–42. doi: 10.2174/1566524014666140414210831
42. Li Y, Wang Y, Liu Y, Wang Y, Zuo X, Li Y, et al. The Possible Role of the Novel Cytokines Il-35 and Il-37 in Inflammatory Bowel Disease. Mediators Inflamm (2014) 2014:136329. doi: 10.1155/2014/136329
43. Fonseca-Camarillo G, Furuzawa-Carballeda J, Yamamoto-Furusho JK. Interleukin 35 (IL-35) and IL-37: Intestinal and Peripheral Expression by T and B Regulatory Cells in Patients With Inflammatory Bowel Disease. Cytokine (2015) 75:389–402. doi: 10.1016/j.cyto.2015.04.009
44. Imaeda H, Takahashi K, Fujimoto T, Kasumi E, Ban H, Bamba S, et al. Epithelial Expression of Interleukin-37b in Inflammatory Bowel Disease. Clin Exp Immunol (2013) 172:410–6. doi: 10.1111/cei.12061
45. Ben Dhifallah I, Borhani-Haghighi A, Hamzaoui A, Hamzaoui K. Decreased Level of IL-37 Correlates Negatively With Inflammatory Cytokines in Cerebrospinal Fluid of Patients With Neuro-Behcet's Disease. Iran J Immunol (2019) 16:299–310. doi: 10.22034/IJI.2019.80281
46. Kluck V, van Deuren RC, Cavalli G, Shaukat A, Arts P, Cleophas MC, et al. Rare Genetic Variants in Interleukin-37 Link This Anti-Inflammatory Cytokine to the Pathogenesis and Treatment of Gout. Ann Rheum Dis (2020) 79:536–44. doi: 10.1136/annrheumdis-2019-216233
47. Ballak DB, Li S, Cavalli G, Stahl JL, Tengesdal IW, van Diepen JA, et al. Interleukin-37 Treatment of Mice With Metabolic Syndrome Improves Insulin Sensitivity and Reduces Pro-Inflammatory Cytokine Production in Adipose Tissue. J Biol Chem (2018) 293:14224–36. doi: 10.1074/jbc.RA118.003698
48. Ballak DB, van Diepen JA, Moschen AR, Jansen HJ, Hijmans A, Groenhof GJ, et al. IL-37 Protects Against Obesity-Induced Inflammation and Insulin Resistance. Nat Commun (2014) 5:4711. doi: 10.1038/ncomms5711
49. Osborne DG, Domenico J, Luo Y, Reid AL, Amato C, Zhai Z, et al. Interleukin-37 is Highly Expressed in Regulatory T Cells of Melanoma Patients and Enhanced by Melanoma Cell Secretome. Mol Carcinog (2019) 58:1670–9. doi: 10.1002/mc.23044
50. Mei Y, Liu H. IL-37: an Anti-Inflammatory Cytokine With Antitumor Functions. Cancer Rep (Hoboken) (2019) 2:e1151. doi: 10.1002/cnr2.1151
51. Monastero RN, Pentyala S. Cytokines as Biomarkers and Their Respective Clinical Cutoff Levels. Int J Inflam (2017) 2017:4309485. doi: 10.1155/2017/4309485
52. Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine Levels in the Serum of Healthy Subjects. Mediators Inflamm (2013) 2013:434010. doi: 10.1155/2013/434010
53. Venner AA, Doyle-Baker PK, Lyon ME, Fung TS. A Meta-Analysis of Leptin Reference Ranges in the Healthy Paediatric Prepubertal Population. Ann Clin Biochem (2009) 46:65–72. doi: 10.1258/acb.2008.008168
54. Thijs L, Staessen JA, Celis H, de Gaudemaris R, Imai Y, Julius S, et al. Reference Values for Self-Recorded Blood Pressure: A Meta-Analysis of Summary Data. Arch Intern Med (1998) 158:481–8. doi: 10.1001/archinte.158.5.481
55. Nemeth B, Ajtay Z, Hejjel L, Ferenci T, Abram Z, Muranyi E, et al. The Issue of Plasma Asymmetric Dimethylarginine Reference Range - A Systematic Review and Meta-Analysis. PLoS One (2017) 12:e0177493. doi: 10.1371/journal.pone.0177493
56. Neyeloff JL, Fuchs SC, Moreira LB. Meta-Analyses and Forest Plots Using a Microsoft Excel Spreadsheet: Step-by-Step Guide Focusing on Descriptive Data Analysis. BMC Res Notes (2012) 5:52. doi: 10.1186/1756-0500-5-52
57. Staessen JA, Fagard RH, Lijnen PJ, Thijs L, Van Hoof R, Amery AK. Mean and Range of the Ambulatory Pressure in Normotensive Subjects From a Meta-Analysis of 23 Studies. Am J Cardiol (1991) 67:723–7. doi: 10.1016/0002-9149(91)90529-t
58. Staessen J, Guo C, De Cort P, Fagard R, Lijnen P, Thijs L, et al. Mean and Range of the Ambulatory Pressure in Normotensive Subjects. Chin Med J (Engl) (1992) 105:328–33.
59. Zhou F, Zhu CL, Niu ZL, Xu FX, Song H, Liu XH. Influenza A Virus Inhibits Influenza Virus Replication by Inducing IL-37. J Clin Lab Anal (2019) 33:e22638. doi: 10.1002/jcla.22638
60. Abushouk A, Nasr A, Masuadi E, Allam G, Siddig EE, Fahal AH. The Role of Interleukin-1 Cytokine Family (IL-1beta, IL-37) and Interleukin-12 Cytokine Family (IL-12, IL-35) in Eumycetoma Infection Pathogenesis. PLoS Negl Trop Dis (2019) 13:e0007098. doi: 10.1371/journal.pntd.0007098
61. Sehat M, Talaei R, Dadgostar E, Nikoueinejad H, Akbari H. Evaluating Serum Levels of IL-33, IL-36, IL-37 and Gene Expression of IL-37 in Patients With Psoriasis Vulgaris. Iran J Allergy Asthma Immunol (2018) 17:179–87.
62. Wang YC, Weng GP, Liu JP, Li L, Cheng QH. Elevated Serum IL-37 Concentrations in Patients With Sepsis. Medicine (Baltimore) (2019) 98:e14756. doi: 10.1097/MD.0000000000014756
63. Yildiz H, Alp HH, Sunnetcioglu A, Ekin S, Mermit Cilingir B. Evaluation Serum Levels of YKL-40, Periostin, and Some Inflammatory Cytokines Together With IL-37, A New Anti-Inflammatory Cytokine, in Patients With Stable and Exacerbated Asthma. Heart Lung (2020) 50:177–83. doi: 10.1016/j.hrtlng.2020.04.017
64. Ding SX, Ma JB, Hu YR, Hu AR, Shen Q, Gao GS. Outcomes of Interferon/Ribavirin Therapy in Patients With HCV Defined by Expression of Plasma Soluble Human Leukocyte Antigen-G But Not IL-37. Med Sci Monit (2016) 22:1398–402. doi: 10.12659/msm.895971
65. Wang X, Cai X, Chen L, Xu D, Li J. The Evaluation of Plasma and Leukocytic IL-37 Expression in Early Inflammation in Patients With Acute ST-Segment Elevation Myocardial Infarction After PCI. Mediators Inflamm (2015) 2015:626934. doi: 10.1155/2015/626934
66. Zhang F, Zhu T, Li H, He Y, Zhang Y, Huang N, et al. Plasma Interleukin-37 is Elevated in Acute Ischemic Stroke Patients and Probably Associated With 3-Month Functional Prognosis. Clin Interv Aging (2020) 15:1285–94. doi: 10.2147/CIA.S230186
67. Li ZC, Sun MD, Zheng YQ, Fu HJ. The Low Expression of IL-37 Involved in Multiple Myeloma - Associated Angiogenesis. Med Sci Monit (2016) 22:4164–8. doi: 10.12659/msm.897451
68. Zhang F, Zhu XJ, Zhu XJ, Liu YX, Yuan T, Yao QM. Plasma Levels and Expression of Interleukin-37 in Patients With Immune Thrombocytopenia. Exp Ther Med (2019) 18:2739–45. doi: 10.3892/etm.2019.7824
69. Chi H, Liu D, Sun Y, Hu Q, Liu H, Cheng X, et al. Interleukin-37 is Increased in Adult-Onset Still's Disease and Associated With Disease Activity. Arthritis Res Ther (2018) 20:54. doi: 10.1186/s13075-018-1555-6
70. Nam SW, Kang S, Lee JH, Yoo DH. Different Features of Interleukin-37 and Interleukin-18 as Disase Activity Markers of Adult-Onset Still's Disease. J Clin Med (2021) 10:910. doi: 10.3390/jcm10050910
71. Ding L, Li H, Sun B, Wang T, Meng S, Huang Q, et al. Elevated Interleukin-37 Associated With Tophus and Pro-Inflammatory Mediators in Chinese Gout Patients. Cytokine (2021) 141:155468. doi: 10.1016/j.cyto.2021.155468
72. Ding L, Hong X, Sun B, Huang Q, Wang X, Liu X, et al. IL-37 is Associated With Osteoarthritis Disease Activity and Suppresses Proinflammatory Cytokines Production in Synovial Cells. Sci Rep (2017) 7:11601. doi: 10.1038/s41598-017-11397-5
73. Yuan ZC, Wang JM, Huang AF, Su LC, Li SJ, Xu WD. Elevated Expression of Interleukin-37 in Patients With Rheumatoid Arthritis. Int J Rheum Dis (2019) 22:1123–9. doi: 10.1111/1756-185X.13539
74. Horowitz GL. EP28: Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory (2010). Available at: https://clsi.org/standards/products/method-evaluation/documents/ep28/.
75. Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Plasma Cytokine Levels in a Population-Based Study: Relation to Age and Ethnicity. J Gerontol A Biol Sci Med Sci (2010) 65:429–33. doi: 10.1093/gerona/glp198
76. Davarpanah E, Jafarzadeh A, Nemati M, Bassagh A, Abasi MH, Khosravimashizi A, et al. Circulating Concentration of Interleukin-37 in Helicobacter Pylori-Infected Patients With Peptic Ulcer: Its Association With IL-37 Related Gene Polymorphisms and Bacterial Virulence Factor CagA. Cytokine (2020) 126:154928. doi: 10.1016/j.cyto.2019.154928
77. Ruggeri RM, Cristani M, Vicchio TM, Alibrandi A, Giovinazzo S, Saija A, et al. Increased Serum Interleukin-37 (IL-37) Levels Correlate With Oxidative Stress Parameters in Hashimoto's Thyroiditis. J Endocrinol Invest (2019) 42:199–205. doi: 10.1007/s40618-018-0903-3
78. Cavalli E, Mazzon E, Basile MS, Mammana S, Pennisi M, Fagone P, et al. In Silico and In Vivo Analysis of IL37 in Multiple Sclerosis Reveals Its Probable Homeostatic Role on the Clinical Activity, Disability, and Treatment With Fingolimod. Molecules (2019) 25:20. doi: 10.3390/molecules25010020
79. Godsell J, Rudloff I, Kandane-Rathnayake R, Hoi A, Nold MF, Morand EF, et al. Clinical Associations of IL-10 and IL-37 in Systemic Lupus Erythematosus. Sci Rep (2016) 6:34604. doi: 10.1038/srep34604
80. Kim HO, Kim HS, Youn JC, Shin EC, Park S. Serum Cytokine Profiles in Healthy Young and Elderly Population Assessed Using Multiplexed Bead-Based Immunoassays. J Transl Med (2011) 9:113. doi: 10.1186/1479-5876-9-113
81. Wu D, Dinh TL, Bausk BP, Walt DR. Long-Term Measurements of Human Inflammatory Cytokines Reveal Complex Baseline Variations Between Individuals. Am J Pathol (2017) 187:2620–6. doi: 10.1016/j.ajpath.2017.08.007
82. Ellisdon AM, Nold-Petry CA, D'Andrea L, Cho SX, Lao JC, Rudloff I, et al. Homodimerization Attenuates the Anti-Inflammatory Activity of Interleukin-37. Sci Immunol (2017) 2:eaaj1548. doi: 10.1126/sciimmunol.aaj1548
83. Cho SX, Rudloff I, Lao JC, Pang MA, Goldberg R, Bui CB, et al. Characterization of the Pathoimmunology of Necrotizing Enterocolitis Reveals Novel Therapeutic Opportunities. Nat Commun (2020) 11:5794. doi: 10.1038/s41467-020-19400-w
84. Vincent FB, Nim HT, Lee JPW, Morand EF, Harris J. Effect of Storage Duration on Cytokine Stability in Human Serum and Plasma. Cytokine (2019) 113:453–7. doi: 10.1016/j.cyto.2018.06.009
85. Ledur A, Fitting C, David B, Hamberger C, Cavaillon JM. Variable Estimates of Cytokine Levels Produced by Commercial ELISA Kits: Results Using International Cytokine Standards. J Immunol Methods (1995) 186:171–9. doi: 10.1016/0022-1759(95)00184-c
86. Tarrant JM. Blood Cytokines as Biomarkers of In Vivo Toxicity in Preclinical Safety Assessment: Considerations for Their Use. Toxicol Sci (2010) 117:4–16. doi: 10.1093/toxsci/kfq134
87. Keustermans GC, Hoeks SB, Meerding JM, Prakken BJ, de Jager W. Cytokine Assays: An Assessment of the Preparation and Treatment of Blood and Tissue Samples. Methods (2013) 61:10–7. doi: 10.1016/j.ymeth.2013.04.005
88. Jones GR, Barker A, Tate J, Lim CF, Robertson K. The Case for Common Reference Intervals. Clin Biochem Rev (2004) 25:99–104.
89. Luo D, Wan X, Liu J, Tong T. Optimally Estimating the Sample Mean From the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat Methods Med Res (2018) 27:1785–805. doi: 10.1177/0962280216669183
Keywords: biomarker, healthy, interleukin-37, plasma, reference range, serum
Citation: Santarelli DM, Vincent FB, Rudloff I, Nold-Petry CA, Nold MF and Russo MA (2021) Circulating Interleukin-37 Levels in Healthy Adult Humans – Establishing a Reference Range. Front. Immunol. 12:708425. doi: 10.3389/fimmu.2021.708425
Received: 11 May 2021; Accepted: 02 July 2021;
Published: 23 July 2021.
Edited by:
Roba M. Talaat, University of Sadat City, EgyptReviewed by:
Giulio Cavalli, Vita-Salute San Raffaele University, ItalyKamel Hamzaoui, Tunis El Manar University, Tunisia
Copyright © 2021 Santarelli, Vincent, Rudloff, Nold-Petry, Nold and Russo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc A. Russo, cGlAZ2VuZXNpc3Jlc2VhcmNoc2VydmljZXMuY29t
†These authors share senior authorship
 Danielle M. Santarelli
Danielle M. Santarelli Fabien B. Vincent
Fabien B. Vincent Ina Rudloff
Ina Rudloff Claudia A. Nold-Petry
Claudia A. Nold-Petry Marcel F. Nold
Marcel F. Nold Marc A. Russo
Marc A. Russo