- 1Department of Surgery, Starzl Transplantation Institute, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 2Liver Cancer Center, University of Pittsburgh Medical Center, Pittsburgh, PA, United States
- 3Department of Immunology, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
Liver allograft recipients are more likely to develop transplantation tolerance than those that receive other types of organ graft. Experimental studies suggest that immune cells and other non-parenchymal cells in the unique liver microenvironment play critical roles in promoting liver tolerogenicity. Of these, liver interstitial dendritic cells (DCs) are heterogeneous, innate immune cells that appear to play pivotal roles in the instigation, integration and regulation of inflammatory responses after liver transplantation. Interstitial liver DCs (recruited in situ or derived from circulating precursors) have been implicated in regulation of both ischemia/reperfusion injury (IRI) and anti-donor immunity. Thus, livers transplanted from mice constitutively lacking DCs into syngeneic, wild-type recipients, display increased tissue injury, indicating a protective role of liver-resident donor DCs against transplant IRI. Also, donor DC depletion before transplant prevents mouse spontaneous liver allograft tolerance across major histocompatibility complex (MHC) barriers. On the other hand, mouse liver graft-infiltrating host DCs that acquire donor MHC antigen via “cross-dressing”, regulate anti-donor T cell reactivity in association with exhaustion of graft-infiltrating T cells and promote allograft tolerance. In an early phase clinical trial, infusion of donor-derived regulatory DCs (DCreg) before living donor liver transplantation can induce alterations in host T cell populations that may be conducive to attenuation of anti-donor immune reactivity. We discuss the role of DCs in regulation of warm and liver transplant IRI and the induction of liver allograft tolerance. We also address design of cell therapies using DCreg to reduce the immunosuppressive drug burden and promote clinical liver allograft tolerance.
Introduction
Dendritic Cell Biology and Diversity
Our understanding of DC development and function is based largely on extensive studies in mouse models and human in vitro systems. DCs are heterogeneous innate immune cells that link innate and adaptive immunity (1). They are subdivided into conventional DCs (cDCs) that acquire, process and present antigen (Ag), and non-conventional plasmacytoid DCs (pDCs) that produce type-1 interferon (IFN) following viral stimulation. While indispensable for antiviral immunity, pDCs also promote or regulate other inflammatory/immune responses (2, 3). cDCs and pDCs arise from a common bone marrow precursor in a fms-like tyrosine kinase 3 ligand (Flt3L)-dependent manner (4–7). Mouse cDCs are further divided into two subsets,- cDC1 (CD11c+,CD103+,CD11b-) and cDC2 (CD11c+,CD103-,CD11b+) that differentiate under the influence of IFN regulatory factor (IRF) 8 and IRF4, respectively. Mouse pDCs are CD11c+, CD103-, CD11b-,B220+,Gr-1+, sialic acid-binding immunoglobulin-like lectin (Siglec) H+ (5, 6, 8–11). Human cDCs express high levels of CD11c and are subdivided into CD1c+ (blood DC Ag (BDCA)1+), CD1b+, CD11b+, CD14+ DC that promote T helper (Th)17 cells and correspond broadly to mouse cDC2s, versus CD141+ (BDCA3+) DC that promote Th1 cell responses and Ag cross-priming to CD8+ T cells, corresponding to mouse DC1s. Human pDCs express CD123 (IL-3R), CD14 and CD303 (BDCA2) and potently produce type-1 IFN. Each DC subset in mice and humans develops under the control of a specific repertoire of transcription factors involving differential levels of IRF8 and IRF4 expression (12–16).
Liver Dendritic Cells
Multiple DC subsets have been identified in the liver, although their relative abundance differs from that in peripheral blood and secondary lymphoid tissue (17–21). cDC1, cDC2 and pDCs and their functional relevance in the steady-state and liver disease have been reviewed (21). Improved understanding of liver DC heterogeneity and function in mice and humans is required to further elucidate their roles and for design of DC-directed therapeutic intervention in liver injury, transplantation and other liver disorders. Recently, single cell RNA sequencing (seq) analysis has been used to quantify liver DC subsets (cDC1, cDC2 and pDC) and to define signatures of DC-T cell interactions in draining lymph nodes under healthy conditions and in liver disease (22). Mouse liver DC heterogeneity has also been described using cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) (23). The phenotype and function of liver interstitial DCs is influenced by the hepatic microenvironment that promotes their inherent tolerogenicity in the healthy steady-state (24–26). Thus, via their production of macrophage colony-stimulating factor and other soluble and cell-cell contact factors, liver stromal cells induce regulatory cDCs that secrete high levels of IL-10 and nitric oxide (NO), but little IL-12 and inhibit T cell proliferative responses/induce activated T cell apoptosis (24, 27, 28). Exposure to gut-derived pathogen-associated microbial products e.g. bacterial lipopolysaccharide (LPS) inhibits liver cDC or pDC maturation by stimulating IL-6- signal transducer and activator of transcription 3 (STAT3) activity that upregulates expression of interleukin-1 receptor-associated kinase M (IRAK-M), an inhibitor of Toll-like receptor (TLR) signaling (29). This phenomenon, referred to as endotoxin tolerance (30), extends to several TLRs (cross-tolerance), as well as to TLRs and ischemic injury. By contrast, exposure to LPS stimulates secretion of IL-10 and IL-27 by liver cDCs that can then expand regulatory T cells (Tregs) (31, 32).
Liver cDCs also express comparatively low levels of major histocompatibility complex (MHC) class II and co-stimulatory molecules (33), but comparatively high levels of the T cell co-inhibitory molecule programed death ligand -1 (PD-L1). Compared with lymphoid tissue DCs, they also express high levels of the ectoenzyme CD39 (34) that degrades adenosine triphosphate to adenosine, and the immunoreceptor transmembrane adaptor protein DNAX activating protein of 12 kDa (DAP12) that regulates their maturation (35). Like liver cDCs, liver pDCs express comparatively high levels of DAP12 and high PD-L1:CD80/86 ratios (36, 37) and secrete IL-10. Thus, liver DCs are refractory to stimulation with microbial products and express gene products that undermine effector T cell responses, but promote Tregs (38).
We discuss below reported roles of liver DCs in regulation of liver IRI and immune responses to liver allografts. We also consider how regulatory DC (DCreg) therapy is being introduced in clinical trials to ascertain its potential to promote reduced dependency on/withdrawal of immunosuppression (IS) in liver transplantation.
Liver Ischemia Reperfusion Injury (IRI)
Graft IRI remains an understudied area in transplantation, despite its clinical significance. Hepatocellular damage associated with liver removal, storage and engraftment is critical to primary graft non-function or late dysfunction and may promote acute and chronic rejection and graft loss (39–41). IRI is a complex process that occurs when hypoxic tissue damage is increased by the inflammatory pathways that are activated during the return of blood flow and oxygen delivery, that combines elements of “warm” and “cold” injury (39, 42). Warm IRI is dominated by liver macrophage-derived cytotoxic molecule-mediated hepatocellular damage. Cold IRI, that occurs during ex-vivo organ preservation, is dominated by damage to liver sinusoidal endothelial cells (SECs) and disruption of the microcirculation (39, 43, 44).
Liver DCs and Regulation of Liver IRI
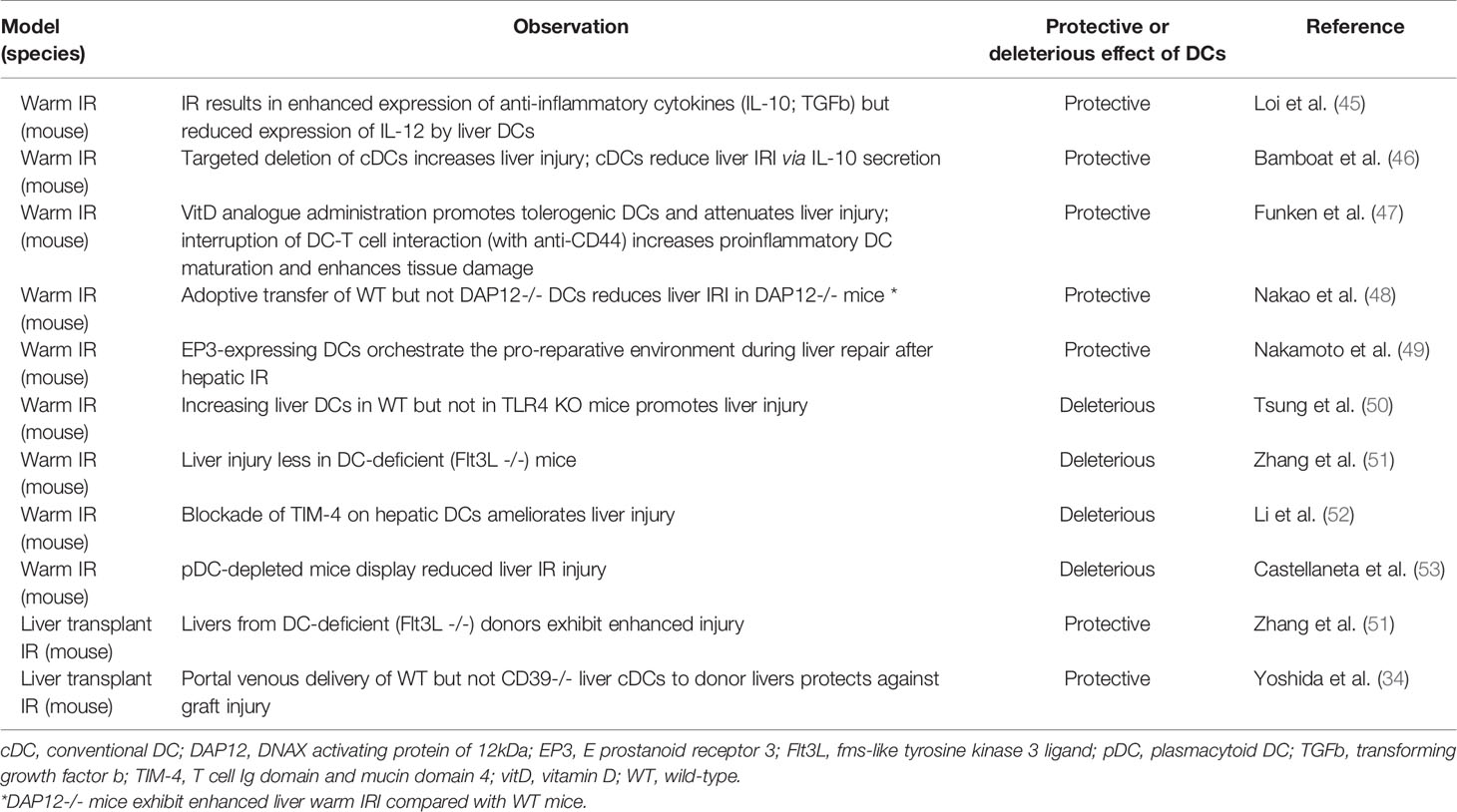
Regulatory properties of liver DCs have been described in both liver warm and cold (transplant) IRI in the mouse (Table 1).
Liver Warm IRI
Loi et al. (45) reported that liver DCs isolated after hepatic warm IR exhibited a more mature surface phenotype than those from uninjured liver, but preferentially produced the anti-inflammatory cytokines IL-10 and transforming growth factor b that might inhibit T cell and natural killer (NK) cell stimulation after IRI. It was also shown (46) that targeted deletion of cDCs by injecting CD11c-diptheria toxin (DT) receptor mice with DT 12–18 hours prior to I/R increased liver injury. Moreover, cDCs reduced liver IRI by secreting IL-10 that inhibited IL-6, tumor necrosis factor (TNF) and reactive oxygen species production by inflammatory monocytes recruited to the liver. More recent work (49) indicates that signaling via the prostaglandin E receptor EP3 in DCs promotes liver repair after warm IR by inducing IL-13-mediated switching of macrophages from pro-inflammatory to IL-10-producing, reparative cells. Vitamin D analogue administration promotes regulatory DCs and attenuates liver warm IRI, whereas interruption of DC-T cell interaction enhances proinflammatory DC maturation and tissue damage (47). Adoptive transfer of wild-type (WT) but not DAP12-/- cDCs reduces warm liver IRI in DAP12-/- mice that exhibit enhanced tissue injury compared with WT animals (48). Taken together, these findings suggest a protective role for DCs in warm liver IRI.
Other data however, conflict with this view. Fms-like tyrosine kinase 3 ligand (Flt3L) is a potent, endogenous DC poietin. Flt3L KO mice exhibit profound reductions in mDC and pDC in liver and lymphoid tissues (51, 54, 55). In these Flt3L KO mice (51), warm liver IR results in reduced hepatic injury, with less polymorphonuclear cell infiltration compared with WT animals. Absence of hepatic interstitial DC in this study also induces less upregulation of inflammatory cytokine and chemokine (TNFa, CCL2 and CXCL2) gene expression in the liver. Moreover, adoptive transfer of splenic or hepatic WT DC into Flt3L KO or WT mice increases hepatic warm IR injury. TIM-4 (T cell immunoglobulin domain and mucin domain containing 4) expression by liver cDC has been reported to play an important role in mouse segmental warm IRI (52); its blockade by anti-TIM-4 antibody reduces liver injury and inflammatory cytokine production and facilitates induction of Foxp3+ Tregs, suggesting a potential therapeutic approach. Thus, in contrast to the protective roles of liver DC described above, these findings suggest injurious effects of DC in liver warm IRI (51). In another report (50), increasing cDCs in the liver by GM-CSF hydrodynamic transfection increased liver injury after warm IR in WT but not TLR4 KO mice. With respect to liver pDCs, mice depleted of these cells using anti-pDC Ag (PDCA)-1 antibody failed to upregulate hepatic IFNa and exhibited reduced levels of hepatic IL-6, TNFa and liver injury after warm IR compared with WT controls (53).
Thus, while reports using different experimental approaches suggest both protective and deleterious effects of liver DCs in liver warm IRI, the balance of reports indicate protective properties of these cells in mouse models (53, 56–59). Further studies, taking into account liver DC heterogeneity and focused on the role of specific hepatic DC subsets, as well as the release of small extracellular vesicles with proinflammatory versus reparative properties by these cells during warm IRI (60, 61), may help elucidate these conflicting observations. Depending on microenvironmental conditions, complement system activators and inhibitors may also influence the differentiation/function of DC subsets towards immunogenicity or tolerance (62) and may be worthy of further investigation in the context of liver DCs their regulation of hepatic inflammatory responses.
Liver Transplant IRI
Several molecules have been implicated in regulation of liver transplant (cold) IRI by DCs. Absence of CD39 in liver grafts enhances cold IRI, associated with higher levels of pro‐inflammatory cytokines (IL-6, TNFa, monocyte chemoattractant protein-1, IL-12p40), compared to WT donors. In addition, these CD39-/- allografts express higher levels of DC maturation markers (CD80, CD86, MHC II) and lower levels of coinhibitory PD-L1. Moreover, adoptive transfer of WT liver mDCs exerts a protective effect against transplant-induced liver IRI, that is not achieved by CD39-/- liver mDC infusion (34, 63).
When Flt3L KO donor livers (lacking interstitial DCs) are transplanted into syngeneic WT mice with 24 hours of cold ischemia, the grafts show dramatically increased IR injury, with enhanced alanine transaminase levels, hepatic necrosis and neutrophil infiltration, indicating a protective role of liver-resident DC in WT livers (51). Thus, from the limited studies undertaken to date, liver DCs appear to have a protective role against liver transplant IRI in mice.
Liver Transplant Tolerance
The liver is considered a tolerogenic environment, as evidenced by oral tolerance, portal venous tolerance, the ability of adeno-associated viral gene therapy to induce systemic tolerance to a transgene (64), metastasis of tumors to the liver and, in animals, acceptance of liver allografts across MHC barriers, without IS therapy (65–69). Within the liver microenvironment, multiple parenchymal and non-parenchymal cell populations (including DCs, Kupffer cells, SECs and stellate cells) express gene products e.g. indoleamine dioxygenase, arginase and PD-L1, that suppress inflammatory and immune-mediated responses (70). DCs express human leukocyte Ig-like receptor B (LILRB) family members, ligation of which renders DCs tolerogenic, leading in turn, to suppression of T cell responses (71) and immune tolerance in humanized mice. Since LILRB family members are considered receptors for HLA-G, that can be produced by liver cells (hepatocytes, liver stem/progenitor cells and biliary epithelial cells) (27, 72–74), this may potentially be an additional mechanism of immune regulation within the liver environment. The liver is also considered a site in which T cells activated therein exhibit defective cytotoxic function (75), and a site of increased T cell apoptosis (76). Potential mechanisms that may mediate liver transplant tolerance have been reviewed recently (26, 77).
Liver DCs and Regulation of the Balance Between Liver Transplant Tolerance and Rejection
Hematopoietic progenitors within the liver are programed to differentiate into DCregs with comparatively low MHC II and T cell costimulatory molecule expression and high IL-10 but low IL-12 secretion. Both liver cDCs and pDCs only weakly stimulate allogeneic T cell proliferation and can promote activated T cell hyporesponsiveness/apoptosis and Tregs (32, 78–80). Together with other liver NPCs, liver DCreg appear to play key roles in the induction of liver transplant tolerance (24); reviewed in (25, 26, 70, 81). The properties of mouse and human hepatic DCs that may promote regulation of alloreactive T cell responses/tolerance induction are summarized in Table 2.
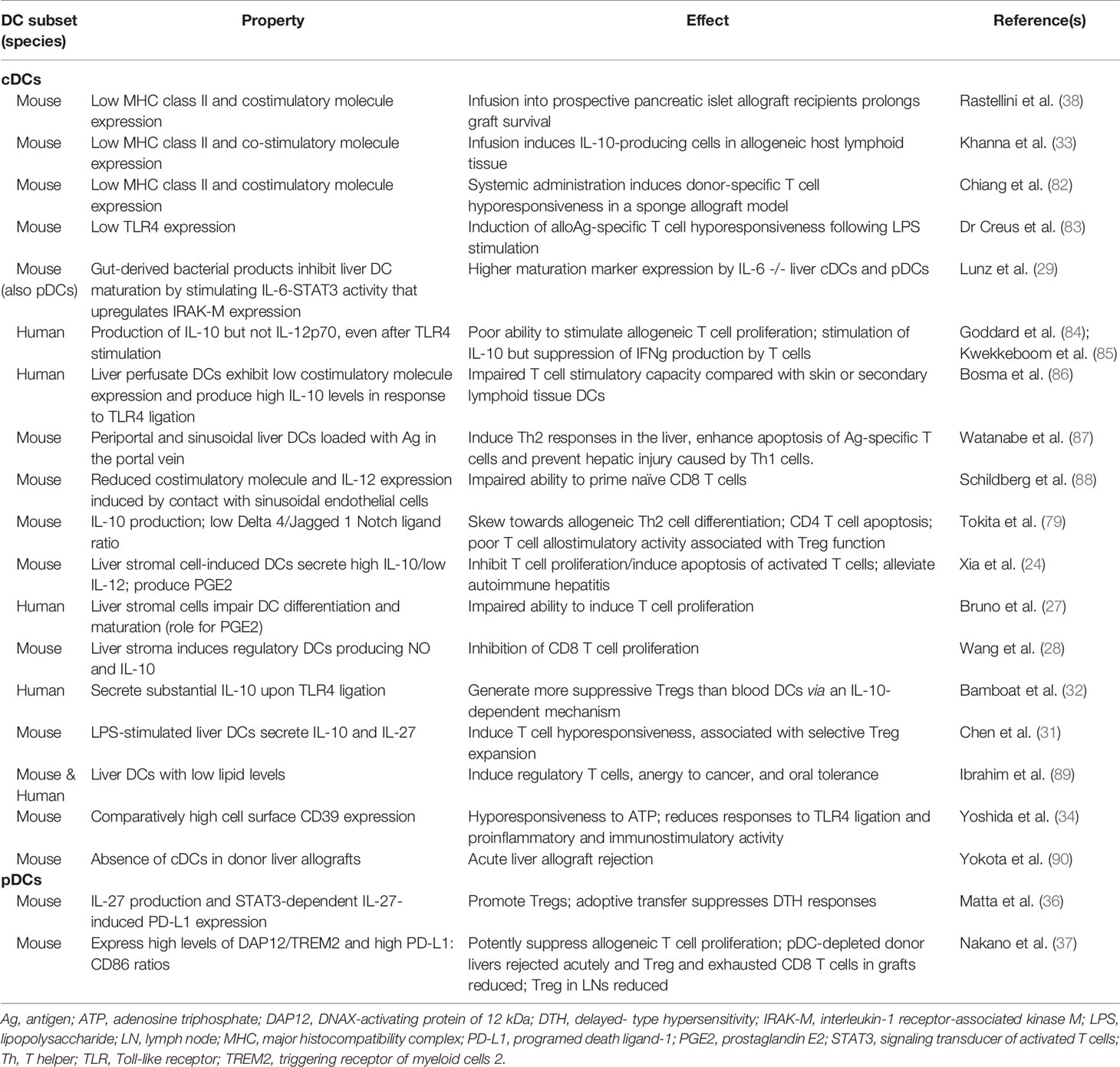
Table 2 Properties of hepatic DCs that promote their immune regulatory function and may contribute to tolerance induction.
In the liver, the coinhibitory molecule PD-L1 is expressed constitutively by DCs, Kupffer cells and SECs (91, 92). PD-L1 also can be up-regulated on both NPCs and hepatocytes following inflammatory stimulation (55, 93–95). It has been reported that transplantation of mouse liver allografts from PD-L1 KO donors, or blocking of PD-1/PD-L1 interactions using anti-PD-L1 monoclonal antibody, results in acute liver allograft rejection. This is associated with increased graft CD8+ T cell infiltration and FasL perforin, granzyme B, iNOS and OPN mRNA expression in the recipients (96).
Depletion of donor interstitial DCs before mouse liver transplantation using CD11c-diptheria toxin receptor (DTR) donor mice in which DCs are depleted by DT administration, prevents induction of spontaneous allograft tolerance (90). Moreover, donor-derived cDCs can be generated ex vivo from progenitors present in normal mouse liver. They can also be generated from lymphoid tissue of untreated recipients of liver but not heart allografts from the same donor strain that are rejected acutely (97). In addition, when adoptively transferred to prospective pancreatic islet allograft recipients, donor liver-derived cDCs prolong graft survival (38). Collectively, these and other observations have implicated donor-derived liver cDCs in the promotion of liver transplant tolerance (70).
Absence of the transmembrane adaptor protein DAP12 (that is constitutively expressed on liver DCs at higher levels that on secondary lymphoid tissue DCs) (35) in mouse liver allografts results in higher pro-inflammatory cytokine (IL-6, IL-12p40, IFNγ, and TNFα) gene expression within the graft, enhanced IFNγ production by graft-infiltrating CD8+ T cells and systemic levels of IFNγ, but reduced incidences of CD4+Foxp3+ cells, associated with acute graft rejection (98).
Non-lymphoid tissue pDCs, such as those that reside in the airways, gut and liver, play a significant role in regulating mucosal immunity and are critical for the development of tolerance to inhaled or ingested/dietary Ags (99). The liver is a site of oral Ag presentation and compared to secondary lymphoid tissue, is comparatively rich in pDCs (18) that appear to rapidly induce anergy or deletion of Ag-specific T cells (37, 79). We have reported (37) that hepatic pDCs of donor origin, that express high levels of DAP12, triggering receptor of myeloid cells 2 (TREM2) and high ratios of T cell coinhibitory PD-L1:costimulatory CD86 compared with secondary lymphoid tissue pDCs, play a key role in attenuating graft-infiltrating T effector cell responses, enhancing Foxp3+ Tregs, and promoting spontaneous acceptance of mouse liver allografts.
Recently, we have also examined the role of graft-infiltrating DCs in regulation of mouse spontaneous liver transplant tolerance. The phenomenon of plasma membrane fragment transfer or “cross-dressing” between leukocytes was reported in 1999 (100). It has been postulated that molecules acquired by acceptor APCs during this process influence subsequent T cell responses. Several recent publications (101–103) have drawn attention to an important role of cross-dressed DCs (CD-DCs) in rejection of experimental heart, kidney and skin transplants. However, our recent novel findings (104) suggest that graft-infiltrating host cDCs that acquire donor MHC Ag shortly after liver transplantation via cross-dressing, regulate anti-donor T cell responses and promote allograft tolerance.
Therapeutic Application of DCregs in Clinical Liver Transplantation
Properties of DCregs and approaches to promoting their tolerogenic functions in transplantation are depicted in Figure 1. Following the initial observation that infusion of liver-derived cDCs, one week before transplant, could promote subsequent donor-strain allograft survival in mice (38), many rodent studies have confirmed the ability of donor-derived DCs (cDCs or pDCs) with immunoregulatory properties to enhance organ allograft survival and donor-specific tolerance (105–108). In addition, the safety and efficacy of donor-derived mDCs in prolonging MHC mis-matched renal allograft survival has been demonstrated in a clinically-relevant nonhuman primate model using a minimal IS drug regimen (109). These promising findings have provided a rationale and justification for an early phase (phase 1/2; open label, non-controlled, non-randomized) clinical trial of donor-derived DCregs in an IS drug withdrawal study in adult living donor liver transplant (LDLT) patients at the University of Pittsburgh (110).

Figure 1 DCreg and promotion of their function. Center, DCreg showing cell membrane-expressed and secreted/molecules and released small extracellular vesicles (exosomes) that can regulate T cell responses and immune reactivity; left panel, approaches to targeting of DCreg in situ; upper right panel, use of immunosuppressive agents that promote DC tolerogenicity; lower right panel, adoptive transfer of DCreg in transplant recipients. DAP12, DNAX activating protein of 12 kDa; HO-1, hemoxygenase-1; PD-L1/2, programed death ligand1/2; miRNA, microRNA; TGFB, transforming growth factor beta; TREM2; triggering receptor expressed on myeloid cells 2.
This first-in-human study commenced in late 2017 (NCT 03164265),- donor-derived DCregs generated from circulating blood monocytes have been infused into 15 prospective liver transplant patients, once only, one week before transplantation, together with a half dose of mycophenolate mofetil (MMF) to minimize any low potential risk of host sensitization. The DCregs that are infused exhibit a tolerogenic gene transcriptional profile, high cell surface PD-L1 to CD86 ratios, secrete high levels of IL-10 but little of no IL-12 in response to TLR4- or and CD40 ligation, and only weakly stimulate proliferation of prospective graft recipient T cells (111). The dose of DCregs infused (2.5-10 x 106/kg body weight) is based on the dose range that proved safe and effective in the preceding NHP studies. Patients receive conventional, post-transplant IS with steroid, MMF and tacrolimus. A protocol biopsy is performed at 1 year and, if permissive, careful weaning of tacrolimus is undertaken. Target cell numbers have been achieved for each of the prospective liver recipients and no adverse events associated with DCreg infusion have been observed. In a second clinical IS drug withdrawal study, also being performed in LDLT patients at the University of Pittsburgh (NCT04208919), a single donor-derived DCreg infusion is being administered to stable graft recipients enrolled 1-3 years post-transplant following biopsy confirmation of the absence of rejection. In addition to determining the safety of the infused DCreg product, an important objective of these studies is to determine preliminary efficacy of DCreg infusion in achieving complete IS drug withdrawal. Currently, drug withdrawal can only be achieved in 10-15% of adult liver allograft recipients in the first 2 years post-transplant (112).
Mechanistic Studies
The initial trial of donor-derived DCregs in LDLT is being accompanied by mechanistic studies aimed at understanding the in vivo fate of the donor-derived DCregs and the influence of their infusion on host anti-donor immune reactivity. Following DCreg infusion one week before transplant, intact donor DCregs can be detected in host peripheral blood shortly after completion of the infusion by discriminatory MHC class I staining and flow cytometric analysis. By 3 days post infusion, no intact donor DCregs can be detected. However, in several HLA-A2 negative graft recipients given HLA-A2 positive donor cells, transiently elevated levels of both donor HLA and immunoregulatory PD-L1, CD39 and CD73 could be detected in circulating small extracellular vesicles (sEVs) (111). At the same time, flow and advanced image stream analysis revealed “cross-dressing” of host DCs in the peripheral blood and in host lymph nodes obtained at the time of surgery, before graft implantation. PD-L1 co-localization with donor HLA was observed at significantly higher levels than with recipient HLA (111). These findings resemble our observations (113) of graft-infiltrating host DCs cross-dressed with donor MHC class I Ag and co-expressing high levels of PD-L1 in mouse liver allograft recipients that accept liver allografts without IS therapy. These cross-dressed recipient DCs marked inhibited anti-donor T cell proliferation ex vivo. Our observations in patients also resemble the identification of circulating host APCs cross-dressed with donor MHC Ag in human liver allograft recipients early and transiently after transplantation (114). In our studies, between the time of donor DCreg infusion and liver transplantation, memory CD8+ T cells expressing high levels of the transcription factors T-bet and Eomesodermin (T-bethiEomeshi) decreased, whereas regulatory (CD25hiCD127-Foxp3+):T-bethiEomeshi CD8+ T cell ratios increased. Although the number of observations is small, this increase appeared to be associated with the incidence of cross-dressed DCs observed in the circulation. Thus, it appears that donor-derived DCreg infusion in prospective liver transplant recipients may induce systemic changes in host APCs and T cells that may be conducive to modulated anti-donor immune T cells responses at the time of transplantation. We postulate that the composition (quality) of sEVs rather than the density (quantity) of peptide MHC-expressing sEVs on cross-dressed DC may play an important role in the induction of peripheral tolerance.
Conclusions
Liver interstitial DCs appear to play important roles in the regulation of hepatic IRI and other inflammatory responses within the liver environment. Donor-derived DCs and more recently, graft-infiltrating host DCs that have acquired intact donor MHC Ag via cross-dressing, have been implicated in the promotion of spontaneous liver transplant tolerance in the mouse. Demonstrations that adoptive transfer of donor-derived DCregs can prolong organ transplant survival and tolerance in preclinical models has led to clinical testing of DCregs for promotion of transplant tolerance in human liver transplantation. These studies are accompanied by mechanistic investigations designed to enhance insight into the influence of these cells on host anti-donor immune reactivity.
Author Contributions
RN: drafting of the manuscript. LT, DG, CM, and DM: critical review of the manuscript. AT: concept, design and writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors’ work is supported by National Institutes of Health (NIH) grants R01 AI118777, U01 AI136779 and U19 AI131453 and by the Immune Transplant and Therapy Center of the University of Pittsburgh Medical Center. LT is supported by NIH institutional research training grant T32 AI074490 and the Physician-Scientist Institutional Award from the Burroughs Wellcome Fund held by the University of Pittsburgh.
Acknowledgments
We thank Dr Masahiko Kubo for help in generation of Figure 1.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Merad M, Sathe P, Helft J, Miller J, Mortha A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu Rev Immunol (2013) 31:563–604. doi: 10.1146/annurev-immunol-020711-074950
2. Rogers NM, Isenberg JS, Thomson AW. Plasmacytoid Dendritic Cells: No Longer an Enigma and Now Key to Transplant Tolerance? Am J Transplant (2013) 13:1125–33. doi: 10.1111/ajt.12229
3. Swiecki M, Colonna M. The Multifaceted Biology of Plasmacytoid Dendritic Cells. Nat Rev Immunol (2015) 15:471–85. doi: 10.1038/nri3865
4. Takenaka MC, Quintana FJ. Tolerogenic Dendritic Cells. Semin Immunopathol (2017) 39:113–20. doi: 10.1007/s00281-016-0587-8
5. Freitas-Lopes MA, Mafra K, David BA, Carvalho-Gontijo R, Menezes GB. Differential Location and Distribution of Hepatic Immune Cells. Cells (2017) 6:48. doi: 10.3390/cells6040048
6. Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, et al. Development of Plasmacytoid and Conventional Dendritic Cell Subtypes From Single Precursor Cells Derived In Vitro and In Vivo. Nat Immunol (2007) 8:1217–26. doi: 10.1038/ni1522
7. Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic Cells and Regulation of Graft-Versus-Host Disease and Graft-Versus-Leukemia Activity. Blood (2012) 119:5088–103. doi: 10.1182/blood-2011-11-364091
8. Liu K, Nussenzweig MC. Origin and Development of Dendritic Cells. Immunol Rev (2010) 234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x
9. Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid Dendritic Cells: Recent Progress and Open Questions. Annu Rev Immunol (2011) 29:163–83. doi: 10.1146/annurev-immunol-031210-101345
10. Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, et al. The Development of Murine Plasmacytoid Dendritic Cell Precursors Is Differentially Regulated by FLT3-Ligand and Granulocyte/Macrophage Colony-Stimulating Factor. J Exp Med (2002) 195:953–8. doi: 10.1084/jem.20020045
11. Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. BST-2/tetherin: A New Component of the Innate Immune Response to Enveloped Viruses. Trends Microbiol (2010) 18:388–96. doi: 10.1016/j.tim.2010.06.010
12. Collin M, Bigley V. Human Dendritic Cell Subsets: An Update. Immunology (2018) 154:3–20. doi: 10.1111/imm.12888
13. Lukacs-Kornek V, Schuppan D. Dendritic Cells in Liver Injury and Fibrosis: Shortcomings and Promises. J Hepatol (2013) 59:1124–6. doi: 10.1016/j.jhep.2013.05.033
14. Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-Cell RNA-Seq Reveals New Types of Human Blood Dendritic Cells, Monocytes, and Progenitors. Science (New York NY) (2017) 356:eaah4573. doi: 10.1126/science.aah4573
15. Brown CC, Gudjonson H, Pritykin Y, Deep D, Lavallee VP, Mendoza A, et al. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell (2019) 179:846–63.e24. doi: 10.1016/j.cell.2019.09.035
16. Nutt SL, Chopin M. Transcriptional Networks Driving Dendritic Cell Differentiation and Function. Immunity (2020) 52:942–56. doi: 10.1016/j.immuni.2020.05.005
17. Lian ZX, Okada T, He XS, Kita H, Liu YJ, Ansari AA, et al. Heterogeneity of Dendritic Cells in the Mouse Liver: Identification and Characterization of Four Distinct Populations. J Immunol (2003) 170:2323–30. doi: 10.4049/jimmunol.170.5.2323
18. Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver Dendritic Cells Are Less Immunogenic Than Spleen Dendritic Cells Because of Differences in Subtype Composition. J Immunol (2004) 172:1009–17. doi: 10.4049/jimmunol.172.2.1009
19. Kelly A, Fahey R, Fletcher JM, Keogh C, Carroll AG, Siddachari R, et al. CD141(+) Myeloid Dendritic Cells Are Enriched in Healthy Human Liver. J Hepatol (2014) 60:135–42. doi: 10.1016/j.jhep.2013.08.007
20. Jomantaite I, Dikopoulos N, Kroger A, Leithauser F, Hauser H, Schirmbeck R, et al. Hepatic Dendritic Cell Subsets in the Mouse. Eur J Immunol (2004) 34:355–65. doi: 10.1002/eji.200324336
21. Wirtz TH, Brandt EF, Berres ML. Liver DCs in Health and Disease. Int Rev Cell Mol Biol (2019) 348:263–99. doi: 10.1016/bs.ircmb.2019.08.001
22. Deczkowska A, David E, Ramadori P, Pfister D, Safran M, At The B, et al. XCR1(+) Type 1 Conventional Dendritic Cells Drive Liver Pathology in Non-Alcoholic Steatohepatitis. Nat Med (2021) 27:1043–54. doi: 10.1038/s41591-021-01344-3
23. Remmerie A, Martens L, Thone T, Castoldi A, Seurinck R, Pavie B, et al. Osteopontin Expression Identifies a Subset of Recruited Macrophages Distinct From Kupffer Cells in the Fatty Liver. Immunity (2020) 53:641–57.e14. doi: 10.1016/j.immuni.2020.08.004
24. Xia S, Guo Z, Xu X, Yi H, Wang Q, Cao X. Hepatic Microenvironment Programs Hematopoietic Progenitor Differentiation Into Regulatory Dendritic Cells, Maintaining Liver Tolerance. Blood (2008) 112:3175–85. doi: 10.1182/blood-2008-05-159921
25. Soysa R, Wu X, Crispe IN. Dendritic Cells in Hepatitis and Liver Transplantation. Liver Transplant (2017) 23:1433–9. doi: 10.1002/lt.24833
26. Thomson AW, Vionnet J, Sanchez-Fueyo A. Understanding, Predicting and Achieving Liver Transplant Tolerance: From Bench to Bedside. Nat Rev Gastroenterol Hepatol (2020) 17:719–39. doi: 10.1038/s41575-020-0334-4
27. Bruno S, Grange C, Tapparo M, Pasquino C, Romagnoli R, Dametto E, et al. Human Liver Stem Cells Suppress T-Cell Proliferation, NK Activity, and Dendritic Cell Differentiation. Stem Cells Int (2016) 2016:8468549. doi: 10.1155/2016/8468549
28. Wang Q, He H, Chen D, Wang C, Xu Y, Song W. Hepatic Stroma-Educated Regulatory DCs Suppress CD8(+) T Cell Proliferation in Mice. Oncotarget (2017) 8:93414–25. doi: 10.18632/oncotarget.18459
29. Lunz JG 3rd, Specht SM, Murase N, Isse K, Demetris AJ. Gut-Derived Commensal Bacterial Products Inhibit Liver Dendritic Cell Maturation by Stimulating Hepatic Interleukin-6/Signal Transducer and Activator of Transcription 3 Activity. Hepatology (2007) 46:1946–59. doi: 10.1002/hep.21906
30. Biswas SK, Lopez-Collazo E. Endotoxin Tolerance: New Mechanisms, Molecules and Clinical Significance. Trends Immunol (2009) 30:475–87. doi: 10.1016/j.it.2009.07.009
31. Chen Y, Jiang G, Yang HR, Gu X, Wang L, Hsieh CC, et al. Distinct Response of Liver Myeloid Dendritic Cells to Endotoxin Is Mediated by IL-27. J Hepatol (2009) 51:510–9. doi: 10.1016/j.jhep.2009.04.026
32. Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, et al. Human Liver Dendritic Cells Promote T Cell Hyporesponsiveness. J Immunol (2009) 182:1901–11. doi: 10.4049/jimmunol.0803404
33. Khanna A, Morelli AE, Zhong C, Takayama T, Lu L, Thomson AW. Effects of Liver-Derived Dendritic Cell Progenitors on Th1- and Th2-Like Cytokine Responses In Vitro and In Vivo. J Immunol (2000) 164:1346–54. doi: 10.4049/jimmunol.164.3.1346
34. Yoshida O, Kimura S, Jackson EK, Robson SC, Geller DA, Murase N, et al. CD39 Expression by Hepatic Myeloid Dendritic Cells Attenuates Inflammation in Liver Transplant Ischemia-Reperfusion Injury in Mice. Hepatology (2013) 58:2163–75. doi: 10.1002/hep.26593
35. Sumpter TL, Packiam V, Turnquist HR, Castellaneta A, Yoshida O, Thomson AW. DAP12 Promotes IRAK-M Expression and IL-10 Production by Liver Myeloid Dendritic Cells and Restrains Their T Cell Allostimulatory Ability. J Immunol (2011) 186:1970–80. doi: 10.4049/jimmunol.1000527
36. Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 Production and STAT3-Dependent Upregulation of B7-H1 Mediate Immune Regulatory Functions of Liver Plasmacytoid Dendritic Cells. J Immunol (2012) 188:5227–37. doi: 10.4049/jimmunol.1103382
37. Nakano R, Yoshida O, Kimura S, Nakao T, Yokota S, Ono Y, et al. Donor Plasmacytoid Dendritic Cells Modulate Effector and Regulatory T Cell Responses in Mouse Spontaneous Liver Transplant Tolerance. Am J Transplant (2021) 21:2040–55. doi: 10.1111/ajt.16412
38. Rastellini C, Lu L, Ricordi C, Starzl TE, Rao AS, Thomson AW. Granulocyte/Macrophage Colony-Stimulating Factor-Stimulated Hepatic Dendritic Cell Progenitors Prolong Pancreatic Islet Allograft Survival. Transplantation (1995) 60:1366–70.
39. Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver Ischemia and Reperfusion Injury: New Insights Into Mechanisms of Innate-Adaptive Immune-Mediated Tissue Inflammation. Am J Transplant (2011) 11:1563–9. doi: 10.1111/j.1600-6143.2011.03579.x
40. Henderson JM. Liver Transplantation and Rejection: An Overview. Hepatogastroenterology (1999) 46(Suppl 2):1482–4.
41. Howard TK, Klintmalm GB, Cofer JB, Husberg BS, Goldstein RM, Gonwa TA. The Influence of Preservation Injury on Rejection in the Hepatic Transplant Recipient. Transplantation (1990) 49:103–7. doi: 10.1097/00007890-199001000-00023
42. Klune JR, Bartels C, Luo J, Yokota S, Du Q, Geller DA. IL-23 Mediates Murine Liver Transplantation Ischemia-Reperfusion Injury Via IFN-γ/IRF-1 Pathway. Am J Physiol Gastrointest Liver Physiol (2018) 315:G991–g1002. doi: 10.1152/ajpgi.00231.2018
43. Ikeda T, Yanaga K, Kishikawa K, Kakizoe S, Shimada M, Sugimachi K. Ischemic Injury in Liver Transplantation: Difference in Injury Sites Between Warm and Cold Ischemia in Rats. Hepatology (1992) 16:454–61. doi: 10.1002/hep.1840160226
44. Huet PM, Nagaoka MR, Desbiens G, Tarrab E, Brault A, Bralet MP, et al. Sinusoidal Endothelial Cell and Hepatocyte Death Following Cold Ischemia-Warm Reperfusion of the Rat Liver. Hepatology (2004) 39:1110–9. doi: 10.1002/hep.20157
45. Loi P, Paulart F, Pajak B, Nagy N, Salmon I, Moser M, et al. The Fate of Dendritic Cells in a Mouse Model of Liver Ischemia/Reperfusion Injury. Transplant Proc (2004) 36:1275–9. doi: 10.1016/j.transproceed.2004.05.052
46. Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs Reduce Liver Ischemia/Reperfusion Injury in Mice Via IL-10 Secretion. J Clin Invest (2010) 120:559–69. doi: 10.1172/JCI40008
47. Funken D, Ishikawa-Ankerhold H, Uhl B, Lerchenberger M, Rentsch M, Mayr D, et al. In Situ Targeting of Dendritic Cells Sets Tolerogenic Environment and Ameliorates CD4(+) T-Cell Response in the Postischemic Liver. FASEB J (2017) 31:4796–808. doi: 10.1096/fj.201601358R
48. Nakao T, Ono Y, Dai H, Nakano R, Perez-Gutierrez A, Camirand G, et al. DNAX Activating Protein of 12 kDa/Triggering Receptor Expressed on Myeloid Cells 2 Expression by Mouse and Human Liver Dendritic Cells: Functional Implications and Regulation of Liver Ischemia-Reperfusion Injury. Hepatology (2019) 70:696–710. doi: 10.1002/hep.30334
49. Nakamoto S, Ito Y, Nishizawa N, Goto T, Kojo K, Kumamoto Y, et al. EP3 Signaling in Dendritic Cells Promotes Liver Repair by Inducing IL-13-Mediated Macrophage Differentiation in Mice. FASEB J (2020) 34:5610–27. doi: 10.1096/fj.201901955R
50. Tsung A, Zheng N, Jeyabalan G, Izuishi K, Klune JR, Geller DA, et al. Increasing Numbers of Hepatic Dendritic Cells Promote HMGB1-Mediated Ischemia-Reperfusion Injury. J Leukoc Biol (2007) 81:119–28. doi: 10.1189/jlb.0706468
51. Zhang M, Ueki S, Kimura S, Yoshida O, Castellaneta A, Ozaki KS, et al. Roles of Dendritic Cells in Murine Hepatic Warm and Liver Transplantation-Induced Cold Ischemia/Reperfusion Injury. Hepatology (2013) 57:1585–96. doi: 10.1002/hep.26129
52. Li J, Zhao X, Liu X, Liu H. Disruption of TIM-4 in Dendritic Cell Ameliorates Hepatic Warm IR Injury Through the Induction of Regulatory T Cells. Mol Immunol (2015) 66:117–25. doi: 10.1016/j.molimm.2015.02.004
53. Castellaneta A, Yoshida O, Kimura S, Yokota S, Geller DA, Murase N, et al. Plasmacytoid Dendritic Cell-Derived IFN-Alpha Promotes Murine Liver Ischemia/Reperfusion Injury by Induction of Hepatocyte IRF-1. Hepatology (2014) 60:267–77. doi: 10.1002/hep.27037
54. McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, et al. Mice Lacking Flt3 Ligand Have Deficient Hematopoiesis Affecting Hematopoietic Progenitor Cells, Dendritic Cells, and Natural Killer Cells. Blood (2000) 95:3489–97. doi: 10.1182/blood.V95.11.3489.011k45_3489_3497
55. Ueki S, Castellaneta A, Yoshida O, Ozaki K, Zhang M, Kimura S, et al. Hepatic B7 Homolog 1 Expression Is Essential for Controlling Cold Ischemia/Reperfusion Injury After Mouse Liver Transplantation. Hepatology (2011) 54:216–28. doi: 10.1002/hep.24360
56. González-Navajas JM, Lee J, David M, Raz E. Immunomodulatory Functions of Type I Interferons. Nat Rev Immunol (2012) 12:125–35. doi: 10.1038/nri3133
57. Crow MK. Type I Interferon in Organ-Targeted Autoimmune and Inflammatory Diseases. Arthritis Res Ther (2010) 12(Suppl 1C:/Parser/ICE/):S5. doi: 10.1186/ar2886
58. Hamerman JA, Pottle J, Ni M, He Y, Zhang ZY, Buckner JH. Negative Regulation of TLR Signaling in Myeloid Cells–Implications for Autoimmune Diseases. Immunol Rev (2016) 269:212–27. doi: 10.1111/imr.12381
59. Zhong L, Chen XF, Zhang ZL, Wang Z, Shi XZ, Xu K, et al. DAP12 Stabilizes the C-Terminal Fragment of the Triggering Receptor Expressed on Myeloid Cells-2 (TREM2) and Protects Against LPS-Induced Pro-Inflammatory Response. J Biol Chem (2015) 290:15866–77. doi: 10.1074/jbc.M115.645986
60. Freeman CM, Quillin RC 3rd, Wilson GC, Nojima H, Johnson ,BL 3rd, Sutton JM, et al. Characterization of Microparticles After Hepatic Ischemia-Reperfusion Injury. PLoS One (2014) 9:e97945. doi: 10.1371/journal.pone.0097945
61. Yang MQ, Du Q, Goswami J, Varley PR, Chen B, Wang RH, et al. Interferon Regulatory Factor 1-Rab27a Regulated Extracellular Vesicles Promote Liver Ischemia/Reperfusion Injury. Hepatology (2018) 67:1056–70. doi: 10.1002/hep.29605
62. Luque A, Serrano I, Aran JM. Complement Components as Promoters of Immunological Tolerance in Dendritic Cells. Semin Cell Dev Biol (2019) 85:143–52. doi: 10.1016/j.semcdb.2017.11.022
63. Shen XD, Ke B, Ji H, Gao F, Freitas MC, Chang WW, et al. Disruption of Type-I IFN Pathway Ameliorates Preservation Damage in Mouse Orthotopic Liver Transplantation Via HO-1 Dependent Mechanism. Am J Transplant (2012) 12:1730–9. doi: 10.1111/j.1600-6143.2012.04021.x
64. Keeler GD, Markusic DM, Hoffman BE. Liver Induced Transgene Tolerance With AAV Vectors. Cell Immunol (2019) 342:103728. doi: 10.1016/j.cellimm.2017.12.002
65. Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The Liver: A Special Case in Transplantation Tolerance. Semin Liver Dis (2007) 27:194–213. doi: 10.1055/s-2007-979471
66. Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, et al. Induction of Immunological Tolerance by Porcine Liver Allografts. Nature (1969) 223:472–6. doi: 10.1038/223472a0
67. Kamada N, Brons G, Davies HS. Fully Allogeneic Liver Grafting in Rats Induces a State of Systemic Nonreactivity to Donor Transplantation Antigens. Transplantation (1980) 29:429–31. doi: 10.1097/00007890-198005000-00021
68. Kamada N, Davies HS, Roser B. Reversal of Transplantation Immunity by Liver Grafting. Nature (1981) 292:840–2. doi: 10.1038/292840a0
69. Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine Liver Allograft Transplantation: Tolerance and Donor Cell Chimerism. Hepatology (1994) 19:916–24. doi: 10.1002/hep.1840190418
70. Thomson AW, Knolle PA. Antigen-Presenting Cell Function in the Tolerogenic Liver Environment. Nat Rev Immunol (2010) 10:753–66. doi: 10.1038/nri2858
71. Young NT, Waller EC, Patel R, Roghanian A, Austyn JM, Trowsdale J. The Inhibitory Receptor LILRB1 Modulates the Differentiation and Regulatory Potential of Human Dendritic Cells. Blood (2008) 111:3090–6. doi: 10.1182/blood-2007-05-089771
72. Creput C, Durrbach A, Menier C, Guettier C, Samuel D, Dausset J, et al. Human Leukocyte Antigen-G (HLA-G) Expression in Biliary Epithelial Cells Is Associated With Allograft Acceptance in Liver-Kidney Transplantation. J Hepatol (2003) 39:587–94. doi: 10.1016/s0168-8278(03)00354-4
73. Amiot L, Vu N, Samson M. Biology of the Immunomodulatory Molecule HLA-G in Human Liver Diseases. J Hepatol (2015) 62:1430–7. doi: 10.1016/j.jhep.2015.03.007
74. Lombard CA, Sana G, LeMaoult J, Najar M, Ravau J, Andre F, et al. Human Hepatocytes and Differentiated Adult-Derived Human Liver Stem/Progenitor Cells Display In Vitro Immunosuppressive Properties Mediated, at Least in Part, Through the Nonclassical HLA Class I Molecule HLA-G. J Immunol Res (2019) 2019:8250584. doi: 10.1155/2019/8250584
75. Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The Site of Primary T Cell Activation Is a Determinant of the Balance Between Intrahepatic Tolerance and Immunity. J Clin Invest (2004) 114:701–12. doi: 10.1172/JCI21593
76. Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The Liver as a Site of T-Cell Apoptosis: Graveyard, or Killing Field? Immunol Rev (2000) 174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x
77. McCaughan GW, Bowen DG, Bertolino PJ. Induction Phase of Spontaneous Liver Transplant Tolerance. Front Immunol (2020) 11:1908. doi: 10.3389/fimmu.2020.01908
78. Abe M, Tokita D, Raimondi G, Thomson AW. Endotoxin Modulates the Capacity of CpG-Activated Liver Myeloid DC to Direct Th1-Type Responses. Eur J Immunol (2006) 36:2483–93. doi: 10.1002/eji.200535767
79. Tokita D, Sumpter TL, Raimondi G, Zahorchak AF, Wang Z, Nakao A, et al. Poor Allostimulatory Function of Liver Plasmacytoid DC Is Associated With Pro-Apoptotic Activity, Dependent on Regulatory T Cells. J Hepatol (2008) 49:1008–18. doi: 10.1016/j.jhep.2008.07.028
80. Liu H, Bakthavatsalam R, Meng Z, Li Z, Li W, Perkins JD, et al. PD-L1 Signal on Liver Dendritic Cells Is Critical for Foxp3(+)CD4(+)CD25(+) Treg and Liver Tolerance Induction in Mice. Transplant Proc (2013) 45:1853–5. doi: 10.1016/j.transproceed.2013.03.015
81. Thomson AW, Lu L. Are Dendritic Cells the Key to Liver Transplant Tolerance? Immunol Today (1999) 20:27–32. doi: 10.1016/s0167-5699(98)01378-4
82. Chiang YJ, Lu L, Fung JJ, Qian S. Liver-Derived Dendritic Cells Induce Donor-Specific Hyporesponsiveness: Use of Sponge Implant as a Cell Transplant Model. Cell Transplant (2001) 10:343–50. doi: 10.3727/000000001783986729
83. De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 Expression by Liver Dendritic Cells Correlates With Reduced Capacity to Activate Allogeneic T Cells in Response to Endotoxin. J Immunol (2005) 174:2037–45. doi: 10.4049/jimmunol.174.4.2037
84. Goddard S, Youster J, Morgan E, Adams DH. Interleukin-10 Secretion Differentiates Dendritic Cells From Human Liver and Skin. Am J Pathol (2004) 164:511–9. doi: 10.1016/S0002-9440(10)63141-0
85. Kwekkeboom J, Boor PP, Sen E, Kusters JG, Drexhage HA, de Jong EC, et al. Human Liver Myeloid Dendritic Cells Maturate In Vivo Into Effector DC With a Poor Allogeneic T-Cell Stimulatory Capacity. Transplant Proc (2005) 37:15–6. doi: 10.1016/j.transproceed.2004.12.003
86. Bosma BM, Metselaar HJ, Mancham S, Boor PP, Kusters JG, Kazemier G, et al. Characterization of Human Liver Dendritic Cells in Liver Grafts and Perfusates. Liver Transplant (2006) 12:384–93. doi: 10.1002/lt.20659
87. Watanabe T, Katsukura H, Chiba T, Kita T, Wakatsuki Y. Periportal and Sinusoidal Liver Dendritic Cells Suppressing T Helper Type 1-Mediated Hepatitis. Gut (2007) 56:1445–51. doi: 10.1136/gut.2007.121251
88. Schildberg FA, Hegenbarth SI, Schumak B, Scholz K, Limmer A, Knolle PA. Liver Sinusoidal Endothelial Cells Veto CD8 T Cell Activation by Antigen-Presenting Dendritic Cells. Eur J Immunol (2008) 38:957–67. doi: 10.1002/eji.200738060
89. Ibrahim J, Nguyen AH, Rehman A, Ochi A, Jamal M, Graffeo CS, et al. Dendritic Cell Populations With Different Concentrations of Lipid Regulate Tolerance and Immunity in Mouse and Human Liver. Gastroenterology (2012) 143:1061–72. doi: 10.1053/j.gastro.2012.06.003
90. Yokota S, Yoshida O, Ono Y, Geller DA, Thomson AW. Liver Transplantation in the Mouse: Insights Into Liver Immunobiology, Tissue Injury, and Allograft Tolerance. Liver Transplant (2016) 22:536–46. doi: 10.1002/lt.24394
91. Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 Determines Accumulation and Deletion of Intrahepatic CD8(+) T Lymphocytes. Immunity (2004) 20:327–36. doi: 10.1016/s1074-7613(04)00050-0
92. Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 Ligation Subverts IFN-Alpha Production by Liver Plasmacytoid Dendritic Cells and Inhibits Their T Cell Allostimulatory Activity Via B7-H1 Up-Regulation. J Immunol (2009) 183:6922–32. doi: 10.4049/jimmunol.0900582
93. Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The Function of Programmed Cell Death 1 and Its Ligands in Regulating Autoimmunity and Infection. Nat Immunol (2007) 8:239–45. doi: 10.1038/ni1443
94. Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, et al. PD-L1 Is Induced in Hepatocytes by Viral Infection and by Interferon-Alpha and -Gamma and Mediates T Cell Apoptosis. J Hepatol (2006) 45:520–8. doi: 10.1016/j.jhep.2006.05.007
95. Kassel R, Cruise MW, Iezzoni JC, Taylor NA, Pruett TL, Hahn YS. Chronically Inflamed Livers Up-Regulate Expression of Inhibitory B7 Family Members. Hepatology (2009) 50:1625–37. doi: 10.1002/hep.23173
96. Morita M, Fujino M, Jiang G, Kitazawa Y, Xie L, Azuma M, et al. PD-1/B7-H1 Interaction Contribute to the Spontaneous Acceptance of Mouse Liver Allograft. Am J Transplant (2010) 10:40–6. doi: 10.1111/j.1600-6143.2009.02859.x
97. Lu L, Rudert WA, Qian S, McCaslin D, Fu F, Rao AS, et al. Growth of Donor-Derived Dendritic Cells From the Bone Marrow of Murine Liver Allograft Recipients in Response to Granulocyte/Macrophage Colony-Stimulating Factor. J Exp Med (1995) 182:379–87. doi: 10.1084/jem.182.2.379
98. Yoshida O, Kimura S, Dou L, Matta BM, Yokota S, Ross MA, et al. DAP12 Deficiency in Liver Allografts Results in Enhanced Donor DC Migration, Augmented Effector T Cell Responses and Abrogation of Transplant Tolerance. Am J Transplant (2014) 14:1791–805. doi: 10.1111/ajt.12757
99. Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid Dendritic Cells Mediate Oral Tolerance. Immunity (2008) 29:464–75. doi: 10.1016/j.immuni.2008.06.017
100. Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, et al. TCR-Mediated Internalization of Peptide-MHC Complexes Acquired by T Cells. Science (New York NY) (1999) 286:952–4. doi: 10.1126/science.286.5441.952
101. Smyth LA, Lechler RI, Lombardi G. Continuous Acquisition of MHC:Peptide Complexes by Recipient Cells Contributes to the Generation of Anti-Graft CD8(+) T Cell Immunity. Am J Transplant (2017) 17:60–8. doi: 10.1111/ajt.13996
102. Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, et al. Donor Dendritic Cell-Derived Exosomes Promote Allograft-Targeting Immune Response. J Clin Invest (2016) 126:2805–20. doi: 10.1172/jci84577
103. Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, Paster JT, LeGuern C, Germana S, et al. Donor Exosomes Rather Than Passenger Leukocytes Initiate Alloreactive T Cell Responses After Transplantation. Sci Immunol (2016) 1:aaf8759. doi: 10.1126/sciimmunol.aaf8759
104. Ono Y, Perez-Gutierrez A, Nakao T, Dai H, Camirand G, Yoshida O, et al. Graft-Infiltrating PD-L1hi Cross-Dressed Dendritic Cells Regulate Anti-Donor T Cell Responses in Mouse Liver Transplant Tolerance. Hepatology (2017) 67:1499–515. doi: 10.1002/hep.29529
105. Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, et al. Costimulatory Molecule-Deficient Dendritic Cell Progenitors (MHC Class II+, CD80DIM, CD86-) Prolong Cardiac Allograft Survival in Nonimmunosuppressed Recipients. Transplantation (1996) 62:659–65. doi: 10.1097/00007890-199609150-00021
106. Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, et al. Immature Dendritic Cells Generated With Low Doses of GM-CSF in the Absence of IL-4 Are Maturation Resistant and Prolong Allograft Survival In Vivo. Eur J Immunol (2000) 30:1813–22. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8
107. Abe M, Wang Z, de Creus A, Thomson AW. Plasmacytoid Dendritic Cell Precursors Induce Allogeneic T-Cell Hyporesponsiveness and Prolong Heart Graft Survival. Am J Transplant (2005) 5:1808–19. doi: 10.1111/j.1600-6143.2005.00954.x
108. Morelli AE, Thomson AW. Tolerogenic Dendritic Cells and the Quest for Transplant Tolerance. Nat Rev Immunol (2007) 7:610–21. doi: 10.1038/nri2132
109. Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ, et al. Regulatory Dendritic Cell Infusion Prolongs Kidney Allograft Survival in Nonhuman Primates. Am J Transplant (2013) 13:1989–2005. doi: 10.1111/ajt.12310
110. Thomson AW, Humar A, Lakkis FG, Metes DM. Regulatory Dendritic Cells for Promotion of Liver Transplant Operational Tolerance: Rationale for a Clinical Trial and Accompanying Mechanistic Studies. Hum Immunol (2018) 79:314–21. doi: 10.1016/j.humimm.2017.10.017
111. Macedo C, Tran LM, Zahorchak AF, Dai H, Gu X, Ravichandran R, et al. Donor-Derived Regulatory Dendritic Cell Infusion Results in Host Cell Cross-Dressing and T Cell Subset Changes in Prospective Living Donor Liver Transplant Recipients. Am J Transplant (2020). doi: 10.1111/ajt.16393
112. Shaked A, DesMarais MR, Kopetskie H, Feng S, Punch JD, Levitsky J, et al. Outcomes of Immunosuppression Minimization and Withdrawal Early After Liver Transplantation. Am J Transplant (2019) 19:1397–409. doi: 10.1111/ajt.15205
113. Ono Y, Perez-Gutierrez A, Nakao T, Dai H, Camirand G, Yoshida O, et al. Graft-Infiltrating PD-L1(hi) Cross-Dressed Dendritic Cells Regulate Antidonor T Cell Responses in Mouse Liver Transplant Tolerance. Hepatology (2018) 67:1499–515. doi: 10.1002/hep.29529
Keywords: liver, dendritic cells, ischemia-reperfusion injury, immune regulation, transplant tolerance
Citation: Nakano R, Tran LM, Geller DA, Macedo C, Metes DM and Thomson AW (2021) Dendritic Cell-Mediated Regulation of Liver Ischemia-Reperfusion Injury and Liver Transplant Rejection. Front. Immunol. 12:705465. doi: 10.3389/fimmu.2021.705465
Received: 05 May 2021; Accepted: 11 June 2021;
Published: 28 June 2021.
Edited by:
Georgina Clark, Anzac Research Institute, AustraliaReviewed by:
Karen O. Dixon, Harvard Medical School, United StatesVeronika Lukacs-Kornek, University of Bonn, Germany
Copyright © 2021 Nakano, Tran, Geller, Macedo, Metes and Thomson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angus W. Thomson, dGhvbXNvbmF3QHVwbWMuZWR1
 Ryosuke Nakano
Ryosuke Nakano Lillian M. Tran
Lillian M. Tran David A. Geller
David A. Geller Camila Macedo1
Camila Macedo1 Angus W. Thomson
Angus W. Thomson