Foot-and-Mouth Disease Virus Counteracts on Internal Ribosome Entry Site Suppression by G3BP1 and Inhibits G3BP1-Mediated Stress Granule Assembly via Post-Translational Mechanisms
- 1State Key Laboratory of Agricultural Microbiology, College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, China
- 2The Cooperative Innovation Center for Sustainable Pig Production, Wuhan, China
- 3National Foot and Mouth Diseases Reference Laboratory, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, China
- 4Department of Microbiology, Immunology and Biochemistry, University of Tennessee Health Science Center, Memphis, TN, United States
By Ye X, Pan T, Wang D, Fang L, Ma J, Zhu X, Shi Y, Zhang K, Zheng H, Chen H, Li K and Xiao S (2018). Front. Immunol. 9:1142. doi: 10.3389/fimmu.2018.01142
In the original article, there was a mistake in Figure 6A as published. We checked the raw data and found that we accidentally cropped the control group, so there is a control group missing from the Western blot experiment for detecting Flag-G3BP1. Lane 1 was used to show that the specific bands detected by Western blot were the ectopic expression of Flag-G3BP1, as the same background/noise was present in lane 1 as in lanes 2-6 (compared to blank lanes), but no specific bands were detected in lane 1. The corrected Figure 6 appears below.
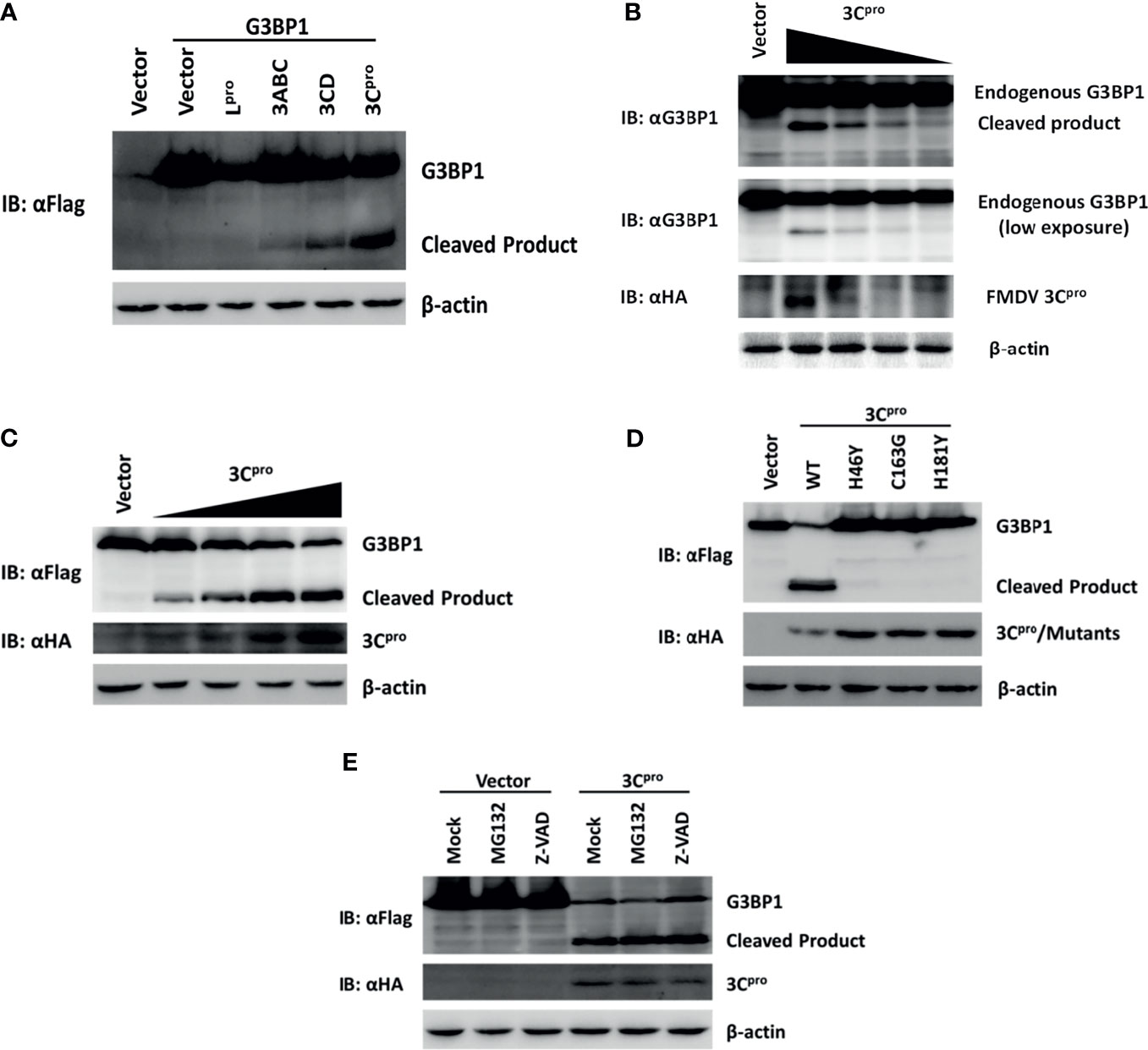
Figure 6 Foot-and-mouth disease virus 3Cpro cleaves G3BP1 by means of its protease activity. (A) Human embryonic kidney cells (HEK)-293T cells cultured in 60-mm dishes were transfected with Flag-tagged porcine G3BP1 as indicated (4 μg), along with HA-Lpro, 3Cpro, or 3Cpro-containing precursors (0.05 μg). Cell lysates were prepared 30 h post-transfection and analyzed by western blotting. (B) IBRS-2 cells were transfected with increasing quantities (0, 0.5, 1, 2, or 4 μg) of plasmid encoding 3Cpro. Cell lysates were prepared 36 h post-transfection and analyzed by western blotting. (C) HEK-293T cells were transfected with Flag-tagged wild-type porcine G3BP1 (4 μg), along with increasing quantities HA-3Cpro plasmid (0, 0.0125, 0.025, 0.05, or 0.1 μg). Cell lysates were prepared 30 h post-transfection and analyzed by western blotting. (D) HEK-293T cells were transfected with Flag-tagged porcine G3BP1 expression plasmid (4 μg), along with wild-type 3Cpro expression plasmids or its mutants (0.05 μg). Cell lysates were prepared 30 h post-transfection and analyzed by western blotting. (E) HEK-293T cells were co-transfected with Flag-tagged porcine G3BP1 expression plasmid (4 μg) and plasmid encoding 3Cpro or empty vector (0.05 μg). 24 h after transfection, MG132 or zVAD-FMK were added to a final concentration of 20 μM. Cell lysates were prepared 8 h after treatment and analyzed by western blotting.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: foot-and-mouth disease virus, phosphoproteomics, G3BP stress granule assembly factor 1, internal ribosome entry site, innate immunity
Citation: Ye X, Pan T, Wang D, Fang L, Ma J, Zhu X, Shi Y, Zhang K, Zheng H, Chen H, Li K and Xiao S (2021) Corrigendum: Foot-and-Mouth Disease Virus Counteracts on Internal Ribosome Entry Site Suppression by G3BP1 and Inhibits G3BP1-Mediated Stress Granule Assembly via Post-Translational Mechanisms. Front. Immunol. 12:702530. doi: 10.3389/fimmu.2021.702530
Received: 29 April 2021; Accepted: 20 October 2021;
Published: 04 November 2021.
Edited and reviewed by:
Zhiwei Wu, Nanjing University, ChinaCopyright © 2021 Ye, Pan, Wang, Fang, Ma, Zhu, Shi, Zhang, Zheng, Chen, Li and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dang Wang, d2FuZ2RhbmdAbWFpbC5oemF1LmVkdS5jbg==
†These authors have contributed equally to this work and should be considered as co-first authors
 Xu Ye1,2†
Xu Ye1,2† Dang Wang
Dang Wang