- 1Department of Rheumatology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
- 2Radiology Department, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
Ankylosing spondylitis is a complicated consequence of genetic predisposition and environmental factors. Enthesitis is believed to be the hallmark of ankylosing spondylitis, and the chronic inflammatory state of this disease is perpetuated by the disturbances of both the innate immune system and the acquired immune system. To clarify the alteration of immune system in patients with AS, we conducted a meta-analysis concerning the proportions of major lymphocyte subsets in the peripheral blood of AS patients. We systematically searched PubMed and China National Knowledge Infrastructure (CNKI) for articles related to this subject. A total of 95 articles involving 4,020 AS patients and 3,065 healthy controls were included in the analysis. This meta-analysis is performed on R platform using R package “meta”, and Egger’s tests were used to determine the presence of publication bias. Results showed that the percentages of T cells, NK cells and NKT cells were not significantly different between AS patients and healthy controls, but B cells were significantly increased. Among the subsets of T cells, the proportions of CD4+ T cells, Th17 cells, Tfh cells as well as Th1/Th2 ratio were significantly increased, while Tregs were significantly decreased. Subgroup analysis showed that the proportions of Th17 among both PBMCs, T cells and CD4+ T cells were significantly elevated, while Tregs were only significantly lower in PBMCs. Subgroup analysis also demonstrated that Tregs defined by “CD4+CD25+FoxP3+”, “CD4+CD25+CD127low”or “CD4+CD25+CD127-”were significantly downregulated, indicating that the selection of markers could be critical. Further study is warranted in order to elucidate the complicated interactions between different lymphocyte subsets in AS patients. This study implied that the disequilibrium between Th17 and Tregs, as well as between Th1 and Th2 could contribute to the pathogenesis of ankylosing spondylitis, further cementing the understanding that ankylosing spondylitis is a consequence of disrupted balance of innate immune system and acquired immune system.
Introduction
Ankylosing spondylitis belong to the group of diseases known as spondyloarthrpathies, which is a spectrum of diseases encompassing psoriatic arthritis, reactive arthritis and undifferentiated spondyloarthritis (1). Clinical manifestations of ankylosing spondylitis include articular manifestations and extra-articular manifestions. The articular manifestations mainly involve axial skeleton presenting as inflammatory back pain, with peripheral oligoarthritis present in some of the patients, while the extra-articular manifestations include uveitis, gut inflammation and dactylitis (2–4). To date, the pathogenesis of ankylosing spondylitis has not yet been fully elucidated. Previous studies have revealed that ankylosing spondylitis is a consequence of genetic background and environmental factors, with HLA-B27 stepping into the limelight of research upon the discovery that HLA-B27 can be present in as many as 90% of patients with AS (5).
How HLA-B27 causes the disease of ankylosing spondylitis remains unclear, though several hypotheses have been put forward attempting to connect the dots (6, 7). Yet, it is undisputed that the disturbances of the immune system eventually perpetuate this disease (8–10). Unlike autoimmune diseases like systemic lupus erythematosus and rheumatoid arthritis, it is not the autoreactive B cells secreting auto-antibodies that should be held accountable, since no antibody is widely acknowledged to be detected in patients with ankylosing spondylitis (11). Instead, such disturbances in the immune system in patients with AS are the result of complicated interactions between the innate immune system and the adaptive immune systems (10). The successful application of biologics, especially TNF-α inhibitors, provide substantial evidence that by blocking cytokines characteristic of the innate immune system, the inflammatory status can be greatly alleviated (12, 13). On the other hand, numerous studies have added to the confirmation of the fact that AS is driven by the imbalances of lymphocyte subsets, especially the Th17/Tregs and Th1/Th2 imbalances, disrupting the equilibrium of the immune system (14, 15). The specific CD4+ T cell subset of Tregs possess immunosuppressive features (16), and the incapability of Tregs may allow the over-secretion of pro-inflammatory cytokines, especially IL-17, which is a potent pro-inflammatory cytokine secreted by Th17 and plays an important role in mediating bone damage (17). Meanwhile, the hyperactivation of the Th1 effector T cell lineage may secrete abundant IFNγ and TNF-α (18), leading to the chronic inflammatory state of the disease.
However, different studies have provided conflicting data regarding the direction and extent of the imbalance of lymphocytes. Most studies suggested that the percentages of Tregs were significantly decreased in patients with AS, yet a few studies found that Tregs might be increased in the peripheral blood of AS patients, arguing that the increase of Tregs might be the result of an attempt to enhance immune tolerance to control the immune response. More intriguingly, the proportions of NK cells is the peripheral blood of AS patients were heavily debated. It has been hypothesized that KIR3DL2, an inhibitory receptor expressed on NK cells, might inhibit apoptosis of NK cells once ligated with HLA-B27, leading to an excess of NK cells in the peripheral blood. In the meanwhile, a few studies observed a significant decrease in the proportions of NK cells in AS patients. Based on previous studies, we hypothesized that the elevation of Th17 and the downregulation of Tregs were pivotal in the pathogenesis of AS, while the th1/th2 polarization might also be involved. In order to clarify the actual proportions of different subsets of lymphocytes, we conducted a meta-analysis concerning the lymphocyte imbalances in the peripheral blood in patients with AS, with healthy donors as the control.
Methods
Data Sources and Searches
We searched the relevant studies using PubMed, Cochrane, Medline and China National Knowledge Infrastructure (CNKI). The literature search strategy used the following terms: (“ankylosing spondylitis”) AND (“lymphocyte subsets” OR “T cell” OR “B cell” OR “Th1” OR “Th2” OR “Th17” OR “Treg” OR “NK cell” OR “NKT cell” OR “gamma delta T cell” OR “flow cytometry”). The publication date was set before April 1, 2021, and all potential eligible studies were screened except for animal experiments or reviews. Some of the studies listed in the reference were retrieved through reference literature in related articles.
Study Selection
The inclusion criteria were as follows: (a) original research; (b) human research; (c) studies with full text available; (d)studies that provided data concerning proportions of certain lymphocyte subsets in peripheral blood of AS patients; (e) studies that provided information concerning flow cytometry experiment protocol and subject characteristics.
The following criteria is used to exclude studies from the final analysis: (a) Studies that did not provide data in the form of mean and standard deviation, or data that could not be transformed; (b) Studies focusing on certain tissue instead of peripheral blood; (c)Duplicates already included once in the analysis.
Two independent researchers (Dong Liu and Budian Liu) extracted data from eligible articles according to the inclusion criteria, while a third investigator settled any disagreements (Churong Lin). Extracted data included author’s name, publication year, baseline characteristics, number of patients and healthy controls, markers of lymphocytes, diagnostic criteria and proportions of each lymphocyte subset in PBMC or T cells or CD4+ T cells. Data were recorded as mean and standard deviation. If the percentages of lymphocytes were presented as median or interquartile range yet no obvious skewing is identified, the data is transformed to mean and standard deviation. (SD = IQR/1.35) The Newcastle-Ottawa Quality Assessment Scale was used to assess the quality of studies.
Statistical Analysis
This meta-analysis was perfomed on the R platform, using R package “meta” [v4.13-0; (19)]. The Cochrane chi-squared test was used to assess the heterogeneity of the included studies. If the heterogeneity of the studies were high (), then the random-effects model was employed to conduct the analysis. Subgroup analysis was performed when it was deemed necessary to break down the analysis on levels of comparison (PBMC, T cells or CD4+ T cells) or based on different markers. Considering the heterogeneity of the literature since different classification criteria were applied, and disease activity of the patients varied across studies, we also conducted subgroup analysis based on classification criteria and disease activity. Publication bias was assessed by the Egger’s test (p≥0:05). Sensitivity analyses was conducted to test the robustness of the results.
Results
Study Characteristics
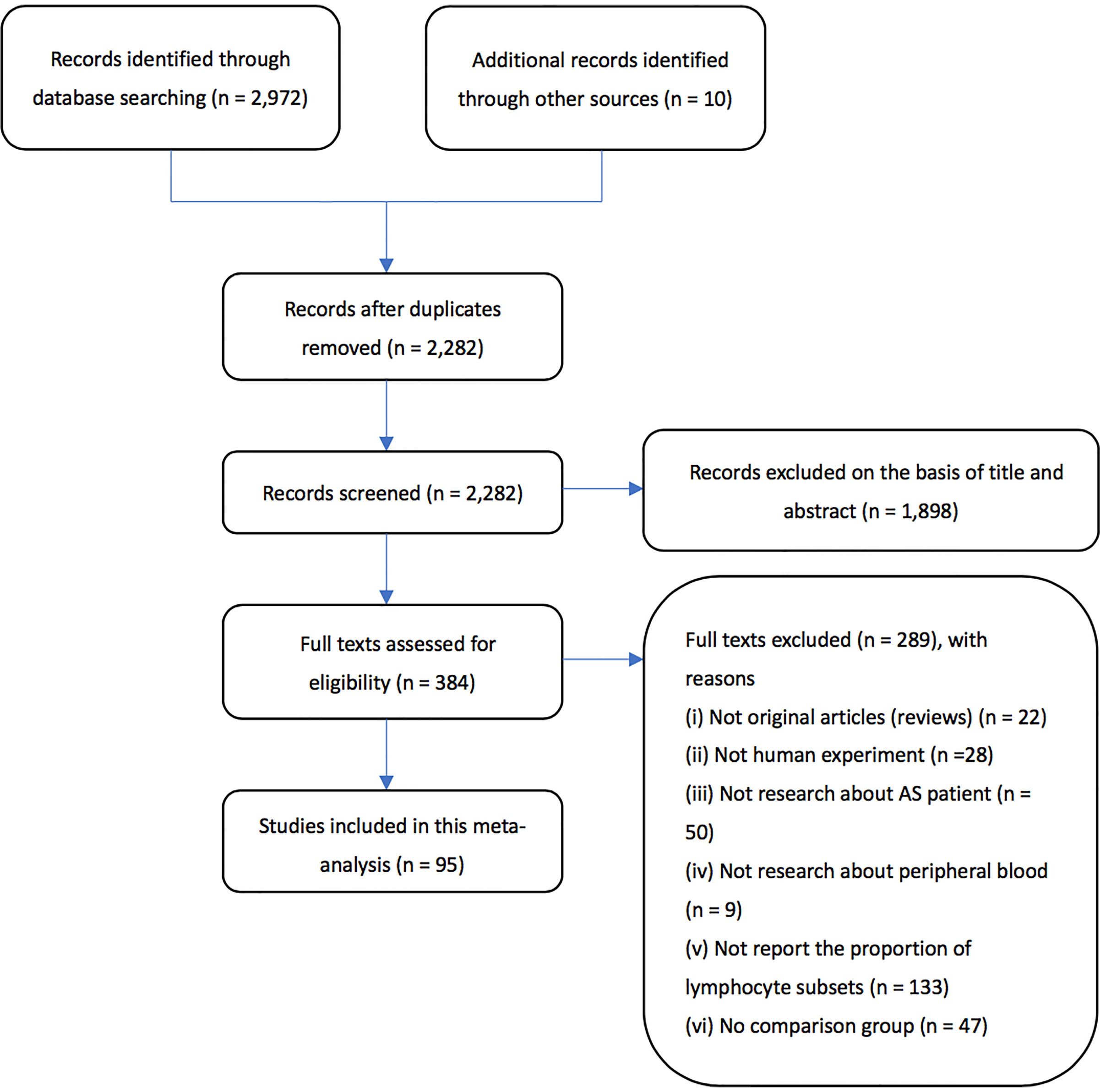
Based on the methods stated above, a total of 2,982 articles were retrieved. We excluded 700 articles since they were duplicates. Next, through screening the abstracts of the articles, a total of 384 articles were included. After carefully examining the articles, articles without full text or failing to provide original data were excluded, leaving 95 articles eligible to be included in the final meta-analysis. Flow chart of the literature search process can be seen in Figure 1.
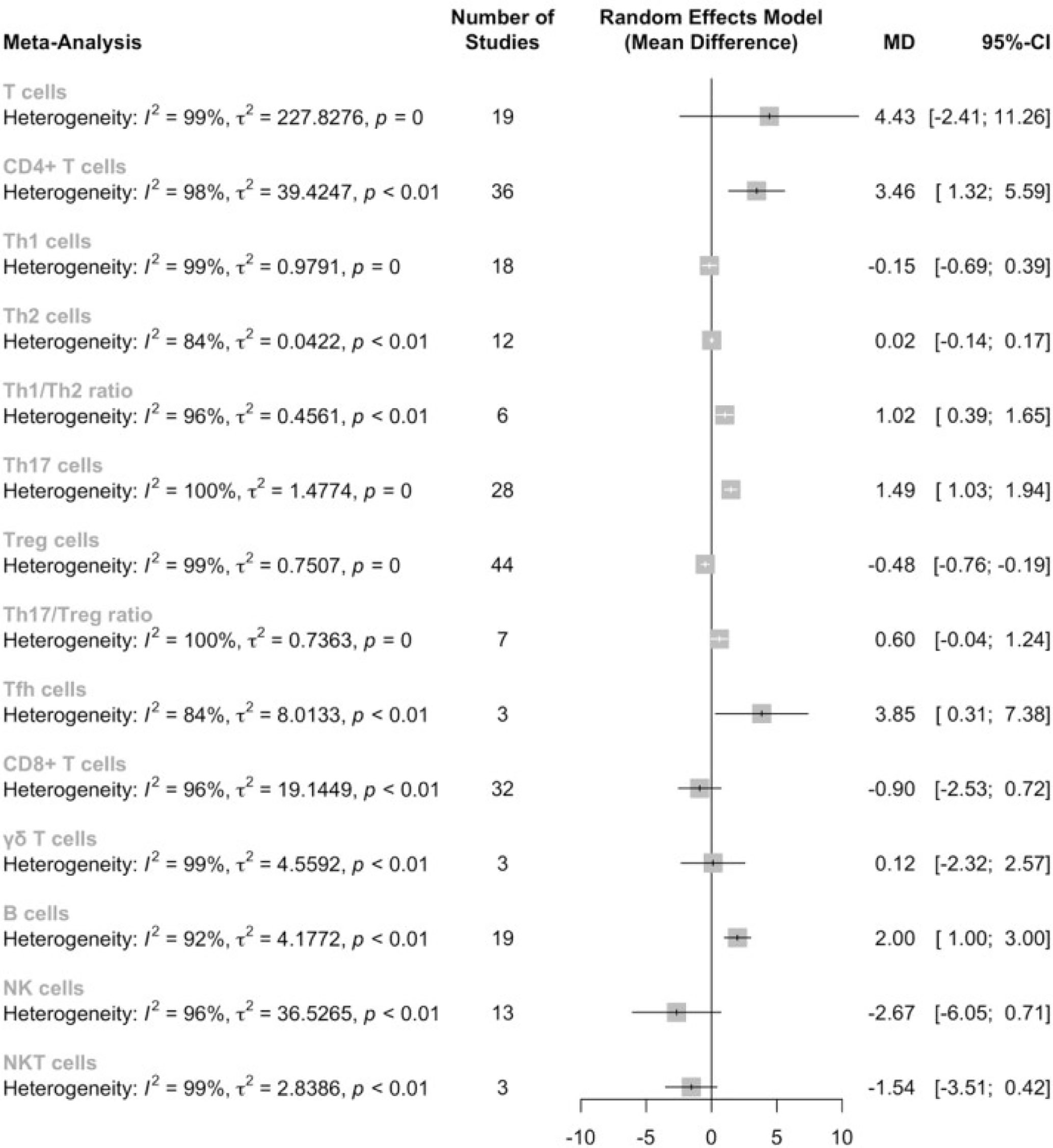
This meta-analysis included 4020 AS patients and 3065 healthy controls from 95 eligible studies. The features of these studies can be seen in Table 1. Of all the studies, 19, 19, 13 and 3 studies provided data on the proportion of T cells, B cells, NK cells and NKT cells. As for the subsets of T cells, 37, 32 and 3 studies focused on CD4+ T cells, CD8+ T cells and γδ T cells. Delving into the CD4+ T cells, 19, 12, 28, 46 and 3 studies presented data on the proportions of Th1, Th2, Th17, Tregs and Tfh cells. Six and 7 studies further discussed the Th1/Th2 proportions and Th17/Treg proportions. All studies had a NOS score of 3-7; the qualities of these studies were moderate. The original data can be seen in Supplemental Material 1.
Proportions of T Cells
Firstly, we conducted a meta-analysis on the proportions of T cells in PBMC between AS patients and healthy controls, as well as the subsets of T cells in the corresponding category (Figure 2). Results showed that there is no significant difference in the T cell proportion between AS patients and healthy controls [4.43, (-2.41,11.26), p<0.01]; however, the proportion of CD4+ T cells was significantly elevated [3.32. (1.21,5.43), p<0.01]. When examining the subsets of the CD4+ T cells, we identified significant increases in the proportion of Th17 cells[1.49, (1.03,1.65), p<0.01], Tfh cells[3.85, (0.31,7.38), p<0.01] and Th1/Th2 ratio[1.02, (0.39,1.65), p<0.01], while the proportion of Tregs was significantly decreased[-0.43, (-0.71,-0.15), p<0.01]. However, sensitivity analysis indicated that the significantly lower proportions of Tfh cells could be insignificant by omitting either Long et al, or Wu et al. No significant difference was found in the level of Th1, Th2 cells. Noteworthy, Th17/Treg ratio was increased but did not reach statistical significance [0.60, (-0.04,1.24), p<0.01]. According to the sensitivity analysis, if omitting Wang et al, the Th17/Treg ratio could be significantly elevated (See Supplemental Material 2).
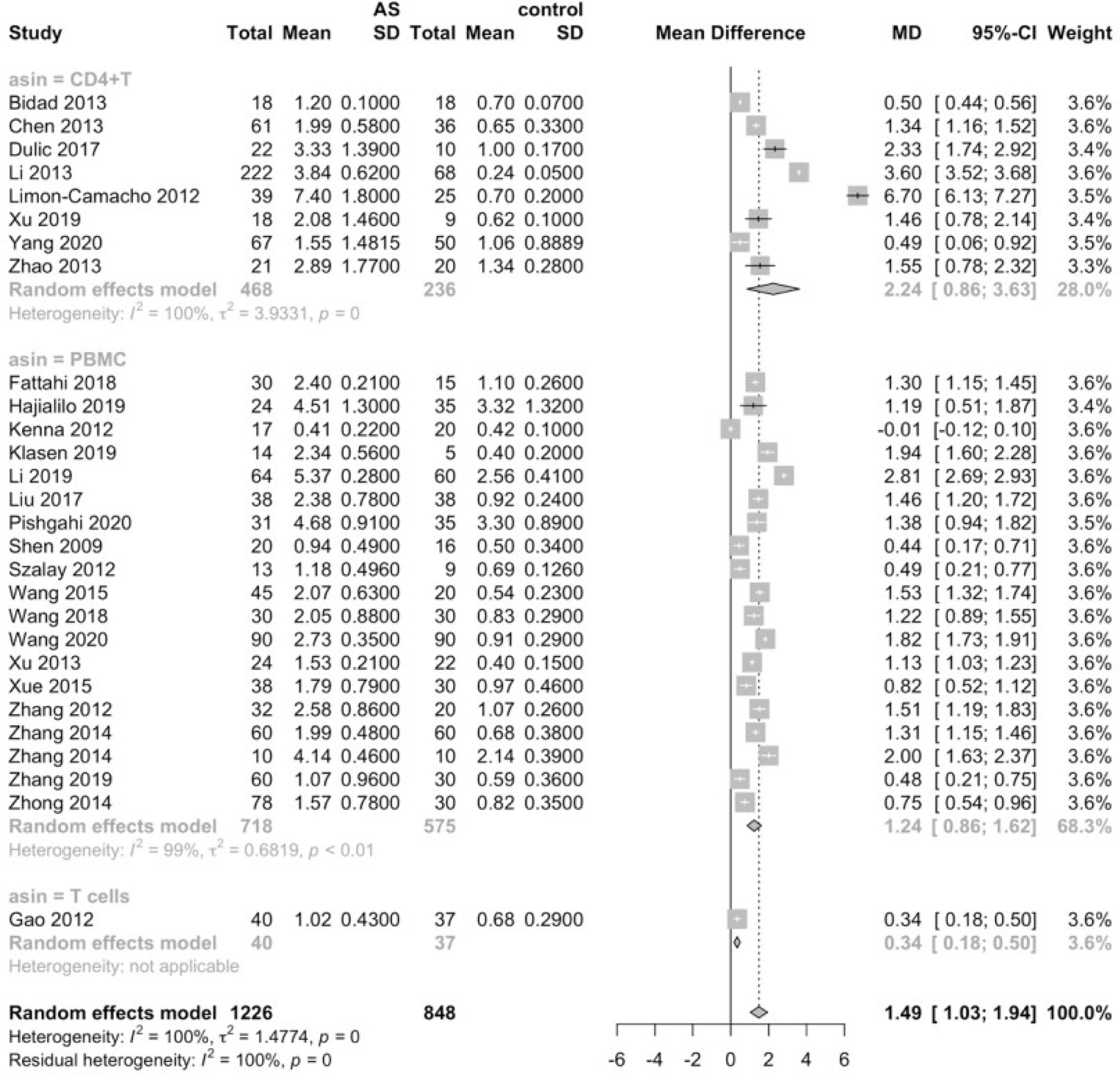
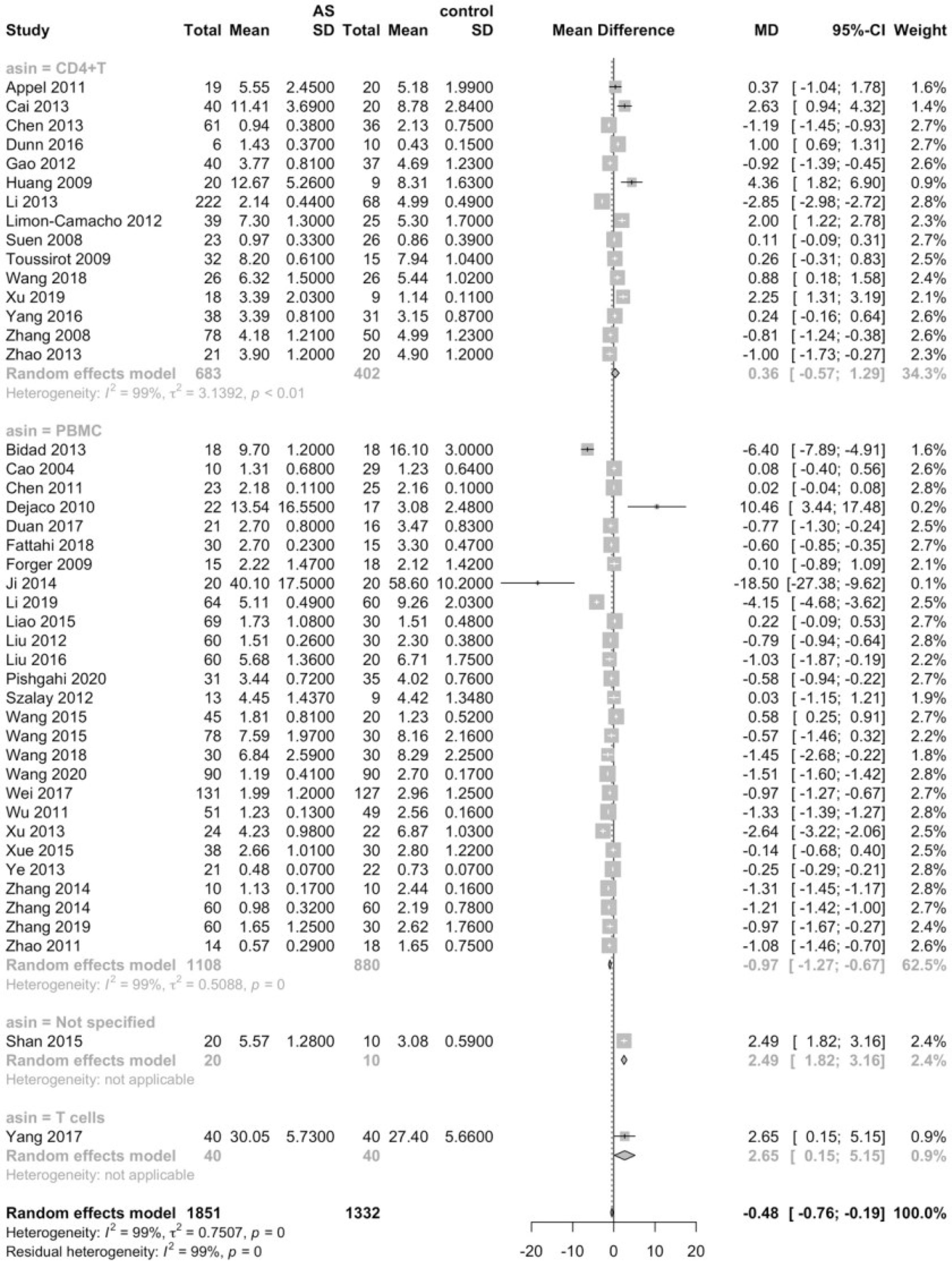
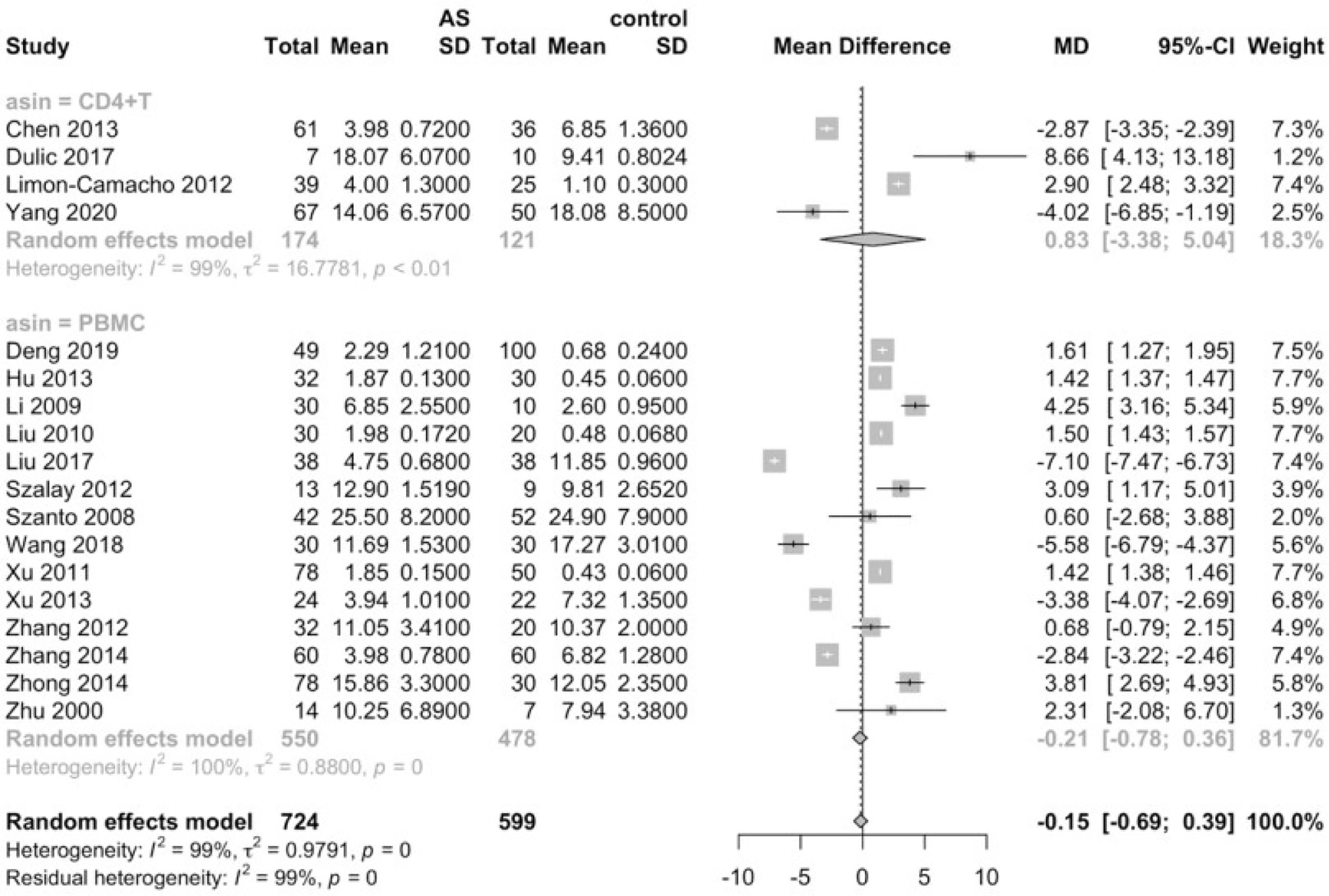
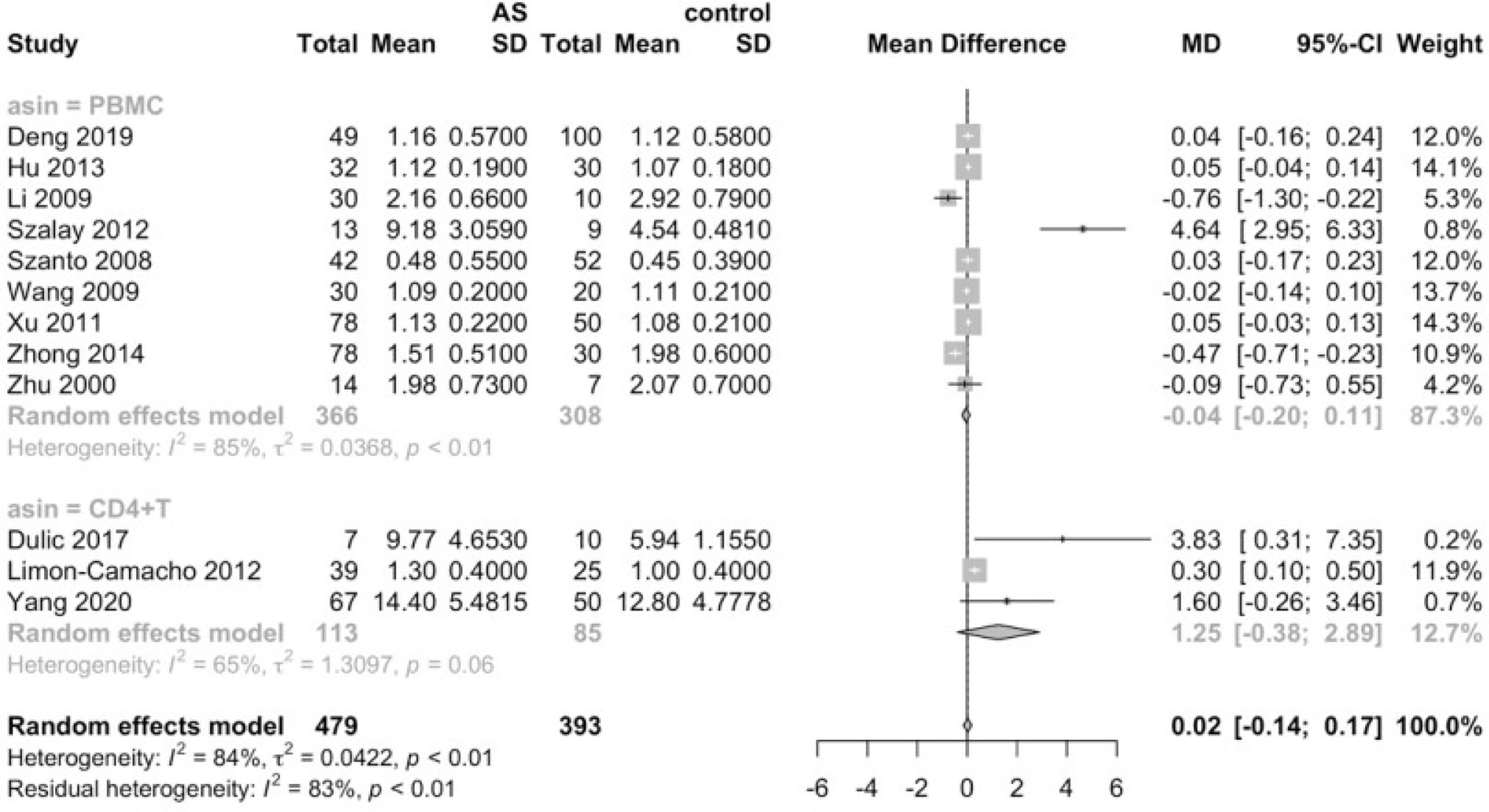
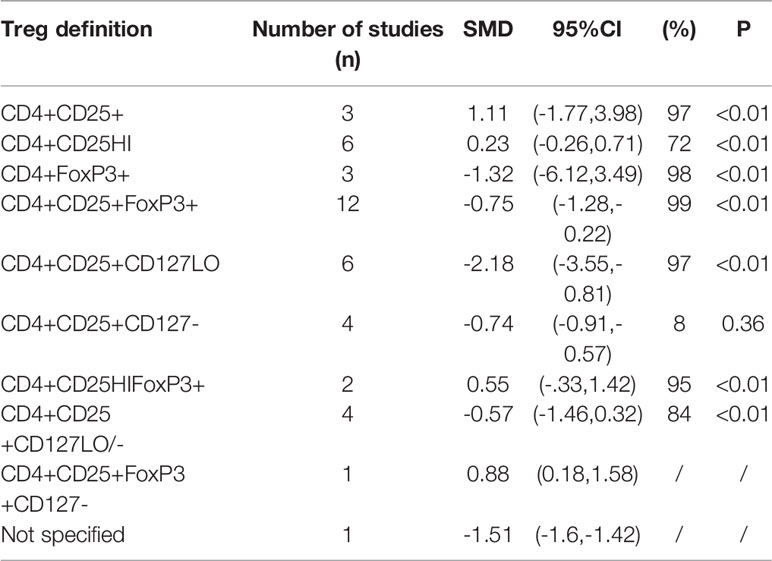
Considering that the proportions of these lymphocytes were compared to PBMCs, T cells or CD4+ cells, we deemed it necessary to conduct subgroup analysis based on the level of comparisons. Subgroup analysis revealed that Th17 cells were increased on all the levels of PBMC, T cells and CD4+ cells (Figure 3), while Tregs were only significantly decreased on the level of PBMC (Figure 4). Still no significant difference was found in the proportions of Th1 and Th2 cells on all levels (Figures 5, 6). On the other hand, due to the heterogeneity of the markers used to define Tregs, we also conducted a subgroup analysis of Tregs (Table 2). Results showed that Tregs defined by “CD4+CD25+FoxP3+”, “CD4+CD25+CD127low”or “CD4+CD25+CD127-”were significantly downregulated. No significant difference was detected in the proportions of CD8+ T cells and γδ T cells.
Subgroup analysis further suggested that T cells were significantly elevated in patients with high disease activity, and that CD4+ T cells were still significantly increased in AS patients strictly defined by 1984 modified New York criteria. Furthermore, the proportion of Th17 cells remained elevated regardless of the classification criteria or disease activity, indicating the robustness of this result. Tregs were only significantly decreased in AS patients strictly defined by 1984 modified New York criteria. Intriguingly, though previous analysis failed to detect any alterations of Th1 proportions, subgroup analysis revealed that the Th1 lineage was elevated in AS patients with high disease activity, Still no alterations were observed in the proportions of Th2 cells and CD8+ T cells in AS patients (See Supplemental Material 2).
Egger’s tests showed that there was no obvious publication bias in all the subgroups of lymphocytes (Table 3).
Proportion of B cells
According to the results of the meta-analysis, the proportion of B cells was significantly increased [2.00, (1.00,3.00), p<0.01]. Egger’s test found no publication bias in this result (Table 3). Sensitivity analysis indicated that this result was robust.
Proportions of NK Cells and NKT Cells
No significant difference was found in the proportions of NK cells [-2.67, (-6.05,0.71)] and NKT cells[-1.54, (-3.51,0.42)]. Subgroup analysis based on classification criteria and disease activity still failed to detect any differences in the proportions of NK cells between AS patients and healthy controls (See Supplemental Material 2). Egger’s test found no publication bias in this result (Table 3).
Results of the sensitivity analyses can be found in the Supplemental Material 2.
Discussion
This is the first meta-analysis to systemically examine the skewing of functional subgroups of lymphocytes, encompassing the major lymphocyte subsets, namely T cells, B cells, NK cells and NKT cells. Previous meta-analyses done by Li et al. and Lai et al. focused on regulatory T cells, arriving at the conclusion that the proportions of Tregs are significantly lower in both PBMCs and CD4+ T cells in patients with AS, though the markers used to define Tregs may have an impact on the proportions of Tregs (113, 114). In line with the results of previous studies, our study further confirmed that the levels of Tregs significantly decreased in patients with AS in PBMCs.
Tregs have been recognized as the essential subgroup of lymphocytes in charge of maintaining immune homeostasis and preventing autoimmunity. Immunosuppressive cytokines such as TGF-β and IL-10 secreted by Tregs may function as a negative regulator of immune responses and down-regulate excessive inflammatory status (115). For example, IL-10 secreted by Tregs may act directly on the IL-10 receptor on Th17 cells, thereby inhibiting the expansion of the inflammatory Th17 cells, or suppress the antigen-presenting cells and eventually suppress the responses of effector T cells (116). It has been reported that in patients with active AS, Tregs in peripheral blood fail to utilize IL-2 and cannot suppress naïve T cell proliferation (117). Moreover, application of TNF-α inhibitors can restore the proportion of Tregs, and the increase in Tregs is positively correlated with the decrease in CRP levels (94). Of note, different markers have been employed to identify the subgroup of Tregs, which may exert an influence on the proportions of Tregs measured in different studies, sometimes yielding contradictory results. Initially, Tregs is defined as CD4+CD25+, yet it was disputed since CD25 may also be expressed on cells without regulatory functions (118). Afterwards, the intracellular transcription factor (FOXP3) was proved to be exclusively expressed in Tregs and indispensable in the development of Tregs (119). The most common marker used to identify Tregs currently is CD4+CD25highCD127low or CD4+CD25highCD127-, of which CD127 is considered to be down-regulated on Tregs (113, 120). In our meta-analysis, we discovered that merely CD4+CD25+ did not produce significant outcomes regarding the proportions of Tregs, while Tregs defined by CD4+CD25+FoxP3+ and CD4+CD25+CD127low or CD4+CD25+CD127- were significantly lowered. This result of the subgroup analysis indicated that the CD127 could be a specific marker when trying to identify Tregs.
Upon the discovery of IL-23/IL-17 axis, the Th17 cells are moving center stage in the research of pathogenesis of spondyloarthropathies (121, 122). It has been widely acknowledged that enthesitis is the hallmark of spondyloarthropathies including AS, and recent research revealed that enthesitis is likely to be driven by the IL-23/IL-17 axis (123). IL-23, produced by myeloid cells either enthesis-resident or tissue infiltrating, may bind to the IL-23 receptors on Th17 cells as well as other lymphoid populations, and the activated Th17 cells can secrete IL-17, a powerful pro-inflammatory cytokine (123). Of the IL-17 family, IL-17A/IL-17F may act on stromal cells and other lymphocytes, which initiates the inflammatory process (17). It has also been reported that IL-17A may mediate bone damage by inducing the expression of RANK on the cell surface of osteoclasts, while also increasing the production of RANKL from mesenchymal stem cells (124). Apart from IL-17, Th17 lymphocytes are known to produce other pro-inflammatory mediators, such as IL-22, GM-CSF and TNF (125). All these studies further cemented the significance of Th17 cells in the pathogenesis of enthesitis, and, in a bigger picture, spondyloarthropathies. Our study substantiated that the levels of Th17 cells were significantly elevated, adding more concrete evidence to the critical role Th17 lineage plays in the pathogenesis in AS. Subgroup analysis further verified the robustness of this result, since Th17 cells were elevated on all levels of comparison, regardless of the classification criteria applied or disease activity of the patients.
In addition to Th17 cells, γδ T cells may also participate in the IL-23/IL-17 axis (123). γδ T cells are a specific population of T lymphocytes characterized by the highly diverse TCR on the cell surface, formulating TCR repertoire (126). Studies show that there is a 3-fold increase in the proportions of IL-23R-positive γδ T cells in AS patients, and such γδ T cells are also heavily skewed towards IL-17 production (48). Another study shows that IL-23R+ γδ T cells are the main producers of IL-17 in a mice model (127). More recent studies have revealed that IL-17 may also be produced in an IL-23-independent fashion (128). Therapies targeting IL-23 have failed in patients with SpA, while the downstream inhibition of IL-17 by IL-17A inhibitor Secukinumab and IL-17A/IL-17F inhibitor bimekizumab has yielded promising results in patients with SpA (129–131). Such phenomenon pointed to a possible pathway that IL-17 may be secreted without the stimulus of IL-23. It has been proved that γ/δ T cells may still secrete IL-17 despite the homozygous deletion of IL-23R (128). However, our study failed to recognize any alteration in the levels of γ/δ T cells. It could be attributed to the limited number of studies included, or that it was not the elevated number but the hyperactivity that was to blame for the IL-23 independent IL-17 secretion. Furthermore, RORγt+ iNKT cells were also reported to be able to secrete IL-17 with and without the effect of IL-23 (132).
In the meanwhile, the Th1/Th2 polarization of T helper cells is also a widely researched area in the immunity of AS (9). Th1 cells are known to mount immune responses against intracellular pathogens via secretion of IFNγ, which acts as a macrophage-activating factor (133). In addition to IFNγ, Th1 cells are also capable of producing IL-2, IL-10 and TNF-α, many of which participate in the inflammatory process (134, 135). Th2 cells, on the other hand, mainly assist in the humoral immune response (136). Cytokines secreted by Th2 cells include IL-4, IL-5 and IL-13, which facilitate the isotope switching of antibodies, mucus secretion and eosinophilia (137). Data concerning the Th1/Th2 skewing in the peripheral blood of AS patients has been highly inconsistent. Some studies reported that T helper cells in AS were skewed towards Th1 lineage suggesting that Th1 cells contributed to the excessive inflammation (56), while others failed to observe such elevations in proportions of Th1 cells (73). Our meta-analysis concluded that there was no significant alteration in the proportions of Th1 and Th2 cells overall, yet subgroup analysis revealed a significant increase in the percentages in AS patients with high disease activity, indicating that the Th1 lineage might be relevant in the acute phase. Meanwhile, the Th1/Th2 ratio was also significantly elevated. More recent research provides evidence that the plasticity of Th17 cells allows this subset of CD4+ T cells to partly assume phenotype of Th1 lineage or Th2 lineage, blurring the boundaries between Th1, Th2 and Th17 cells. It has been argued that the categorical dichotomy of Th1/Th2 should be rendered obsolete.
Another intriguing finding of our study is that the proportions of Tfh cells and B cells are significantly elevated in the peripheral blood of AS patients. Both Tfh cells and B cells participate in humoral immunity (136). After migrating to the B cell follicles, CD40L expressed on the cell surface of Tfh cells may interact with the CD40 on B cells serving as a stimulus signal, thereby facilitating the formation of germinal center, differentiation of B cells and ultimately the production of antibodies (63, 138). The relevance of humoral immunity in the pathogenesis of ankylosing spondylitis has long been underestimated, since no auto-antibody is universally acknowledged as the specific marker of AS (11). Although several studies have put forward that anti-CD74 antibody may serve as a potential biomarker for AS, its diagnostic utility awaits further confirmation (139). Another possible mechanism of the B lymphocyte involvement in the pathogenesis of AS is that B lymphocytes might mediate bone destruction through production of RANKL, as was previously reported in rheumatoid arthritis (140), How Tfh cells and B cells are involved in the pathogenesis of AS still requires more research.
As a pivotal component in the innate immunity, NK cells possess cytotoxic activity and the ability of producing pro-inflammatory cytokines, such as IFNγ (141). There is mounting evidence that NK cells, with its expression of KIR superfamily on the cell surface, may contribute to the pathogenesis of ankylosing spondylitis. Different KIRs may interact with HLA alleles in various forms, creating sophisticated genotypes of NK cells. It is hypothesized that the HLA-Bw4 group of alleles, notably HLA-B27, may bind to the KIR antigens with varying affinities, displaying inhibitory or stimulatory activities through downstream signal pathways (141–143). In particular, being an inhibitory receptor, KIR3DL2 ligation with HLA-B27 may inhibit apoptosis of NK cells and protect them from activation-induced cell death (142). However, studies regarding the frequency of NK cells in the peripheral blood of AS patients have been highly inconsistent. Azuz-Lieberman et al. found that AS patients have significantly higher percentages of NK cells in PB, while the inhibitory receptor CEACAM1 is highly expressed on the surface indicating suppressed function of NK cells (144). Another study also confirmed a higher frequency of NK cells expressing KIR3DL1 in SpA patients, with an impaired IFN-γ intracellular production in stimulated NK cells (145). However, such alteration of NK cell proportions was not observed in another study by Park, et al. (146) A more recent study found that NK cells in the peripheral blood was significantly reduced (84). Due to the inconsistencies of the data, our study failed to recognize a shift in the proportions of NK cells in the peripheral blood of AS patients.
There is no denying that there were some limitations to this study. Though being an all-encompassing meta-analysis attempting to include all the major subsets of lymphocytes, this study failed to conduct a more in-depth look into the more subtle minor subsets of lymphocytes, such as Th22 in CD4+ T cells, Tc1 and Tc2 in CD8+ T cells, Bregs, naïve B cells and memory B cells in the B cell lineage. This study originally intended to include Th22 subset in the meta-analysis, considering recent discovery that Th22 cells might have the capacity to promote osteoclast differentiation though production o IL-22 (147). To our chagrin, there were not enough studies to conduct an appropriate analysis. Second, lymphocytes may assume complicated phenotypes by expressing various antigens on the cell surface. Therefore, this crude classification of lymphocytes may not be adequate to explain the exact shifting of immune system in AS patients. However, further investigation was impeded by the insufficiency of the data. Third, there was notable heterogeneity in the studies considering the selected patients might have undergone different treatments and might be in different phases, it might be more appropriate to look into the effects of different treatments on the lymphocyte subsets. Moreover, this study only targeted lymphocytes in the peripheral blood, which could not adequately reflect the inflammatory status of the tissue.
In conclusion, our meta-analysis concluded that CD4+ T, Th17, Tfh and B cells were significantly elevated in the peripheral blood of AS patients, while Tregs were significantly reduced. Our study further cemented the understanding that the nature of ankylosing spondylitis is a hybrid of innate immunity and acquired immunity dysfunction.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
JG conceived of the presented idea. DL and BL conducted the literature search, while DL performed the analytic calculation and drafted the final version of this manuscript. CL settled any disagreements concerning the inclusion of literature. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.696973/full#supplementary-material
References
1. Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The Development of Assessment of SpondyloArthritis International Society Classification Criteria for Axial Spondyloarthritis (Part II): Validation and Final Selection. Ann Rheum Dis (2009) 68(6):777–83. doi: 10.1136/ard.2009.108233
2. Choi EY, Lee M, Lee CS. Uveitis Occurrence in Patients With Ankylosing Spondylitis According to the Type of Tumour Necrosis Factor Inhibitor: A Cohort Study of 175 Patients. Clin Exp Rheumatol (2020) 38(6):1132–7.
3. Rudwaleit M, Baeten D. Ankylosing Spondylitis and Bowel Disease. Best Pract Res Clin Rheumatol (2006) 20(3):451–71. doi: 10.1016/j.berh.2006.03.010
4. Rudwaleit M, Metter A, Listing J, Sieper J, Braun J. Inflammatory Back Pain in Ankylosing Spondylitis: A Reassessment of the Clinical History for Application as Classification and Diagnostic Criteria. Arthritis Rheum (2006) 54(2):569–78. doi: 10.1002/art.21619
5. Brown MA, Kenna T, Wordsworth BP. Genetics of Ankylosing Spondylitis - Insights Into Pathogenesis. Nat Rev Rheumatol (2016) 12(2):81–91. doi: 10.1038/nrrheum.2015.133
6. Bowness P, Ridley A, Shaw J, Chan AT, Wong–Baeza I, Fleming M, et al. Th17 Cells Expressing KIR3DL2+ and Responsive to HLA-B27 Homodimers are Increased in Ankylosing Spondylitis. J Immunol (2011) 186(4):2672–80. doi: 10.4049/jimmunol.1002653
7. Turner MJ, Sowders DP, DeLay ML, Mohapatra R, Bai S, Smith JA, et al. Hla-B27 Misfolding in Transgenic Rats is Associated With Activation of the Unfolded Protein Response. J Immunol (2005) 175(4):2438–48. doi: 10.4049/jimmunol.175.4.2438
8. Ranganathan V, Gracey E, Brown MA, Inman RD, Haroon N. Pathogenesis of Ankylosing Spondylitis - Recent Advances and Future Directions. Nat Rev Rheumatol (2017) 13(6):359–67. doi: 10.1038/nrrheum.2017.56
9. Rezaiemanesh A, Abdolmaleki M, Abdolmohammadi K, Aghaei H, Pakdel FD, Fatahi Y, et al. Immune Cells Involved in the Pathogenesis of Ankylosing Spondylitis. BioMed Pharmacother (2018) 100:198–204. doi: 10.1016/j.biopha.2018.01.108
10. Watad A, Bridgewood C, Russell T, Marzo–Ortega H, Cuthbert R, WaMcGonagletad D. The Early Phases of Ankylosing Spondylitis: Emerging Insights From Clinical and Basic Science. Front Immunol (2018) 9:2668. doi: 10.3389/fimmu.2018.02668
11. Brown MA, Li Z, Cao KL. Biomarker Development for Axial Spondyloarthritis. Nat Rev Rheumatol (2020) 16(8):448–63. doi: 10.1038/s41584-020-0450-0
12. Callhoff J, Sieper J, Weiss A, Zink A, Listing J. Efficacy of TNFalpha Blockers in Patients With Ankylosing Spondylitis and non-Radiographic Axial Spondyloarthritis: A Meta-Analysis. Ann Rheum Dis (2015) 74(6):1241–8. doi: 10.1136/annrheumdis-2014-205322
13. Landewe RB, van der Heijde D, Dougados M, Baraliakos X, Van den Bosch FE, Gaffney K, et al. Maintenance of Clinical Remission in Early Axial Spondyloarthritis Following Certolizumab Pegol Dose Reduction. Ann Rheum Dis (2020) 79(7):920–8. doi: 10.1136/annrheumdis-2019-216839
14. Dulic S, Vasarhelyi Z, Bajnok A, Szalay B, Toldi G, Kovacs L, et al. The Impact of Anti-TNF Therapy on CD4+ and CD8+ Cell Subsets in Ankylosing Spondylitis. Pathobiology (2018) 85(3):201–10. doi: 10.1159/000484250
15. Szalay B, Meszaros G, Cseh A, Acs L, Deak M, Kovacs L, et al. Adaptive Immunity in Ankylosing Spondylitis: Phenotype and Functional Alterations of T-cells Before and During Infliximab Therapy. Clin Dev Immunol (2012) 2012:808724. doi: 10.1155/2012/808724
16. Guo H, Zheng M, Zhang K, Yang F, Zhang X, Han Q, et al. Functional Defects in CD4(+) CD25(High) FoxP3(+) Regulatory Cells in Ankylosing Spondylitis. Sci Rep (2016) 6:37559. doi: 10.1038/srep37559
17. Schinocca C, Rizzo C, Fasano S, Grasso G, La Barbera L, Ciccia F, et al. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front Immunol (2021) 12:637829. doi: 10.3389/fimmu.2021.637829
18. Butcher MJ, Zhu J. Recent Advances in Understanding the Th1/Th2 Effector Choice. Fac Rev (2021) 10:30. doi: 10.12703/r/10-30
19. Balduzzi S, Rücker G, Schwarzer G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid Based Ment Health (2019) 22(4):153–60. doi: 10.1136/ebmental-2019-300117
20. An H, Li X, Li F, Gao C, Li X, Luo J. The Absolute Counts of Peripheral T Lymphocyte Subsets in Patient With Ankylosing Spondylitis and the Effect of Low-Dose Interleukin-2. Med (Baltimore) (2019) 98(15):e15094. doi: 10.1097/MD.0000000000015094
21. Appel H, Wu P, Scheer R, Kedor C, Sawitzki B, Thiel A, et al. Synovial and Peripheral Blood CD4+Foxp3+ T Cells in Spondyloarthritis. J Rheumatol (2011) 38(11):2445–51. doi: 10.3899/jrheum.110377
22. Bautista-Caro MB, Arroyo–Villa I, Castillo–Gallego C, de Miguel E, Peiteado D, Plasencia–Rodríguez C, et al. Decreased Frequencies of Circulating Follicular Helper T Cell Counterparts and Plasmablasts in Ankylosing Spondylitis Patients Naïve for TNF Blockers. PloS One (2014) 9(9):e107086. doi: 10.1371/journal.pone.0107086
23. Bidad K, Salehi E, Jamshidi A, Saboor–Yaraghi AA, Oraei M, Meysamie A, et al. Effect of All-Transretinoic Acid on Th17 and T Regulatory Cell Subsets in Patients With Ankylosing Spondylitis. J Rheumatol (2013) 40(4):476–83. doi: 10.3899/jrheum.121100
24. Brand JM, Neustock P, Kruse A, Alvarez–Ossorio L, Schnabel A, Kirchner H. Stimulation of Whole Blood Cultures in Patients With Ankylosing Spondylitis by a Mitogen Derived From Mycoplasma Arthritidis (MAS) and Other Mitogens. Rheumatol Int (1997) 16(5):207–11. doi: 10.1007/BF01330297
25. Cai CS, Xiao P. Expression of Regulatory T Cells in the Peripheral Blood of Patients With Ankylosing Spondylitis. J Chin Pract Diagnosis Ther (2013) 27(12):1192–4. doi: 10.11756/j.issn.1674-3474.2013.12.018
26. Cai PW, Lin Y, Dou M, Chen JH, Lin Y. Expression of CD40-CD40L on Peripheral Blood Lymphocytes of Patients With Ankylosing Spondylitis. Immunol J (2005) 21(06):507–513. doi: 10.13431/j.cnki.immunol.j.20050148
27. Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. Cd25brightCD4+ Regulatory T Cells are Enriched in Inflamed Joints of Patients With Chronic Rheumatic Disease. Arthritis Res Ther (2004) 6(4):R335–46. doi: 10.1186/ar1192
28. Chen SZ, Bai JP, Xie YH, You YQ, Su ML, Xu XX, et al. Expression of Transcription Factor Th17, Treg and Th1 in Peripheral Blood From Patients With Ankylosing Spondylitis and its Correlation With Disease Activity. Chin J Immunol (2013) 29(08):834–8+847. doi: 10.3969/j.issn.1000-484X.2013.08.012
29. Chen MH, Chen WS, Lee HT, Tsai CY, Chou CT. Inverse Correlation of Programmed Death 1 (PD-1) Expression in T Cells to the Spinal Radiologic Changes in Taiwanese Patients With Ankylosing Spondylitis. Clin Rheumatol (2011) 30(9):1181–7. doi: 10.1007/s10067-011-1721-6
30. Cheng F. CD4+CD25+Regulatory T Cells in Peripheral Blood of Patients With Ankylosing Spondylitis. The Second Military Medical University (2007).
31. Dejaco C, Duftner C, Klauser A, Schirmer M. Altered T-cell Subtypes in Spondyloarthritis, Rheumatoid Arthritis and Polymyalgia Rheumatica. Rheumatol Int (2010) 30(3):297–303. doi: 10.1007/s00296-009-0949-9
32. Deng L, Chen YP, Sun YL. Expression of miR⁃138 in Peripheral Blood Mononuclear Cells of Patients With Ankylosing Spondylitis and its Relationship With Th1/Th2 Imbalance. J Trop Med (2019) 19(08):1008–11+1051. doi: 10.3969/j.issn.1672-3619.2019.08.017
33. Deng JH, Li ZQ, Zhang R, Li YM. The Correlation Ananlysis of HLA-B27 Expression and Lymphocytes Subsets, Cytokines in the Patients With Ankylosing Spondylitis. Int J Lab Med (2018) 39(16):1976–9. doi: 10.3969/j.issn.1673-4130.2018.16.011
34. Dong Q, Yang DR, Liu H. The Test of Immune Function and Hemorheologic Changes in HLA-B27 Positive Patients With Ankylosing Spondylitis. Chin J Hemorheol (2006) 16(02):273–4+324. doi: 10.3969/j.issn.1009-881X.2006.02.046
35. Duan Z, Gui Y, Li C, Lin J, Gober HJ, Qin J, et al. The Immune Dysfunction in Ankylosing Spondylitis Patients. Biosci Trends (2017) 11(1):69–76. doi: 10.5582/bst.2016.01171
36. Fattahi MJ, Ahmadi H, Jafarnezhad–Ansariha F, Mortazavi–Jahromi SS, Rehm BHA, Cuzzocrea S, et al. Oral Administration Effects of Beta-D-Mannuronic Acid (M2000) on Th17 and Regulatory T Cells in Patients With Ankylosing Spondylitis. BioMed Pharmacother (2018) 100:495–500. doi: 10.1016/j.biopha.2018.02.059
37. Förger F, Villiger PM, Ostensen M. Pregnancy in Patients With Ankylosing Spondylitis: Do Regulatory T Cells Play a Role? Arthritis Rheum (2009) 61(2):279–83. doi: 10.1002/art.24161
38. Gao Y, Song Y, Fan YX, Chen M, Xiao N, Pan LZ, et al. The Alteration of TH17cells and CD4+CD25+FoxP3+ Regulatory T Cell in Patients With Ankylosing Spondylitis. Chin J Microbiol Immunol (2012) 32(04):318–22. doi: 10.3760/cma.j.issn.0254-5101.2012.04.006
39. Guo L, Hou Q, Kou R. Application of Combined Detection of T Lymphocyte Subsets and Ferritin in Patients With Ankylosing Spondylitis. Int J Lab Med (2012) 33(12):1436–7. doi: 10.3969/j.issn.1673-4130.2012.12.012
40. Hajialilo M, Dolati S, Abdolmohammadi–Vahid S, Ahmadi M, Kamrani A, Eghbal–Fard S, et al. Nanocurcumin: A Novel Strategy in Treating Ankylosing Spondylitis by Modulating Th17 Cells Frequency and Function. J Cell Biochem (2019) 120(07):12027–38. doi: 10.1002/jcb.28488
41. Han YX, Zhang SH, Wu JB. Expressions of B7 and CD28 in Peripheral Blood Lymphocytes of Patients With Ankylosing Spondylitis and Their Significance. J Wenzhou Med Univ (2006) 36(04):356–8. doi: 10.3969/j.issn.1000-2138.2006.04.018
42. He YH, Wu XM, Wu LJ. Analysis of Lymphocyte Subsets in Patients With Ankylosing Spondylitis. Int J Lab Med (2012) 33(02):141–145. doi: 10.3969/j.issn.1673-4130.2012.006
43. Hu W, Wang ML, Qiu W, Chen SM, Liu DS. Preliminary Study on Immunological Indicators of Patients With Ankylosing Spondylitis in Suqian. Contemp Med (2019) 25(10):1–3. doi: 10.3969/j.issn.1009-4393.2019.10.001
44. Hu B, Cheng J, Liu SQ. Analysis of T Cell Subsets in Peripheral Blood in Patients With Ankylosing Spondylitis. Modern Med J (2013) 41(08):543–5. doi: 10.3969/j.issn.1671-7562.2013.08.006
45. Huang H, Wang YD, Sun YY, Sui WG. Expression Regulatory T Cells in Peripheral Blood of Ankylosing Spondyligis Patients. China Trop Med (2009) 9(10):1992–3.
46. Huang F, Cai XH, Shi GY, Chen XM, Cheng QL, Dong K, et al. A Study of Cellular Immune Function in Patients With Ankylosing Spondylitis. Acad J Chin PLA Med School (1991) 12(02):102–5.
47. Ji W, Li H, Gao F, Chen Y, Zhong L, Wang D. Effects of Tripterygium Glycosides on interleukin-17 and CD4(+)CD25(+)CD127(low) Regulatory T-cell Expression in the Peripheral Blood of Patients With Ankylosing Spondylitis. BioMed Rep (2014) 2(4):517–20. doi: 10.3892/br.2014.262
48. Kenna TJ, Davidson SI, Duan R, Bradbury LA, McFarlane J, Smith M, et al. Enrichment of Circulating interleukin-17-secreting interleukin-23 Receptor-Positive γ/δ T Cells in Patients With Active Ankylosing Spondylitis. Arthritis Rheum (2012) 64(5):1420–9. doi: 10.1002/art.33507
49. Kim TJ, Lee SJ, Cho YN, Park SC, Jin HM, Kim MJ, et al. Immune Cells and Bone Formation in Ankylosing Spondylitis. Clin Exp Rheumatol (2012) 30(4):469–75.
50. Klasen C, Meyer A, Wittekind PS, Waque I, Nabhani S, Kofler DM. Prostaglandin Receptor EP4 Expression by Th17 Cells is Associated With High Disease Activity in Ankylosing Spondylitis. Arthritis Res Ther (2019) 21(1):159. doi: 10.1186/s13075-019-1948-1
51. Li WQ. Expression of Mi-155 in Peripheral Blood of Patients With Ankylosing Spondylitis and its Relationship With Th17/Treg Balance. J Shanxi Med Univ (2019) 50(02):235–40. doi: 10.13753/j.issn.1007-6611.2019.02.023
52. Xueyi L, Lina C, Zhenbiao W, Qing H, Qiang L, Zhu P. Levels of Circulating Th17 Cells and Regulatory T Cells in Ankylosing Spondylitis Patients With an Inadequate Response to Anti-TNF-α Therapy. J Clin Immunol (2013) 33(1):151–61. doi: 10.1007/s10875-012-9774-0
53. Li JX, Zhang LY, Huo YH, Li XF. Effect of Methylprednisolone on the Th1/Th2 Balance and Cytokines in Patients With Refractong Ankylosing Spondylitis. Chin J Allergy Clin Immunol (2009) 3(01):28–33. doi: 10.3969/j.issn.1673-8705.2009.01.007
54. Li HX, Sun GR, Cao YX, Wang JB. Expression and Significance of CD8+CD28-T Cells in the Peripheral Blood of Patients With as. Chin J Rheumatol (2008) 12(05):333–5+361. doi: 10.3321/j.issn:1007-7480.2008.05.011
55. Liao HT, Lin YF, Tsai CY, Chou CT. Regulatory T Cells in Ankylosing Spondylitis and the Response After Adalimumab Treatment. Joint Bone Spine (2015) 82(6):423–7. doi: 10.1016/j.jbspin.2015.03.003
56. Limón-Camacho L, Vargas–Rojas MI, Vázquez–Mellado J, Casasola–Vargas J, Moctezuma JF, Burgos–Vargas R, et al. In Vivo Peripheral Blood Proinflammatory T Cells in Patients With Ankylosing Spondylitis. J Rheumatol (2012) 39(4):830–5. doi: 10.3899/jrheum.110862
57. Lin Q, Lin ZM, Gu JR, Huang F, Li TW, Wei QJ, et al. Changes of T Lymphocyte Subsets and Expression of Costimulatory Molecule CD154 on T-cells in Peripheral Blood From Patients With Ankylosing Spondylitis. Chin J Rheumatol (2008) 12(05):309–13. doi: 10.3321/j.issn:1007-7480.2008.05.006
58. Lin Q, Gu JR, Li TW, Zhang FC, Lin ZM, Liao ZT, et al. Value of the Peripheral Blood B-cells Subsets in Patients With Ankylosing Spondylitis. Chin Med J (Engl) (2009) 122(15):1784–9. doi: 10.3760/cma.j.issn.0366-6999.2009.15.013
59. Liu EC, Feng YX. Relationship of the Balance Between Leptin and Th17 and Th1 With Ankylosing Spondycitis Patients. Med Recapitulate (2017) 23(01):187–9. doi: 10.3969/j.issn.1006-2084.2017.01.044
60. Liu L, Liu J, Wan L. The Changes of Platelet Parameters, BTLA and Treg in Peripheral Blood in Patients With Ankylosing Spondylitis. Chin J Clin Healthcare (2016) 19(01):8–11. doi: 10.3969/j.issn.1672.6790.2016.01.003
61. Liu J, Wang SH, Wan L, Zhang JS, Yang J, Zong RK, et al. Changes of Regulatory T Cells in Peripheral Blood in Ankylosing Spondylitis Patients and the Influence of Chinese Medicine Spleen-Strengthening Unit Therapy. Chin J Clin Healthcare (2012) 15(01):1–4+113. doi: 10.3969/j.issn.1672-6790.2012.01.001
62. Liu XC, Wang JX, Wei P. The Function of Helper T Lymphocytes in Ankylosing Spondylitis. Tianjin Med J (2010) 38(12):1047–9. doi: 10.3969/j.issn.0253-9896.2010.12.009
63. Long S, Zhang X, Zhang N, Zhao Y, Song LT, et al. High Frequency of Circulating Follicular Helper T Cells is Correlated With B Cell Subtypes in Patients With Ankylosing Spondylitis. Exp Ther Med (2018) 15(5):4578–86. doi: 10.3892/etm.2018.5991
64. Ma XH, Zhang X, Wang N, Gu Y, Gu LT, et al. Detection of Lymphocyte Subsets in Peripheral Blood in Patients With Ankylosing Spondylitis and its Clinical Meaning. Chin J Lab Diagnosis (2011) 15(10):1765–6. doi: 10.3969/j.issn.1007-4287.2011.10.060
65. Ma L, Zhang Y, Wang ZQ, Gu J, et al. A Study of Subsets and Activation of Lymphocytes in Patients With Ankylosing Spondylitis. J Clin Res (2011) 28(10):1963–4. doi: 10.3969/j.issn.1671-7171.2011.10.044
66. Ma L, Yang J, Li H. Study of the Activated State of TH1/TH2 Cytokines on Ankylosing Spondylitis. Chin J Immunol (2004) 20(08):572–4.
67. Meng JH, Wei P, Chen HY, Wang JX, Xie JL, Zhang Y, et al. Change of Cytotoxic T-lymphocytes in Patients With Ankylosing Spondylitis. J Hebei Med Univ (2015) 36(05):543–6. doi: 10.3969/j.issn.1007-3205.2015.05.015
68. Mo JF, Shan DP, Bao Y, Ye Q, Yan WH, et al. Percentages of Immune Cells in Peripheral Blood in Patients With Ankylosing Spondylitis and the Expression Level of CXCR6. Curr Immunol (2019) 39(03):217–21.
69. Pishgahi A, Abolhasan R, Danaii S, Amanifar B, Soltani–Zangbar MS, Zamani M, et al. Immunological and Oxidative Stress Biomarkers in Ankylosing Spondylitis Patients With or Without Metabolic Syndrome. Cytokine (2020) 128:155002. doi: 10.1016/j.cyto.2020.155002
70. Shan Y, Qi C, Zhao J, Liu Y, Gao H, Zhao D, et al. Higher Frequency of Peripheral Blood Follicular Regulatory T Cells in Patients With New Onset Ankylosing Spondylitis. Clin Exp Pharmacol Physiol (2015) 42(2):154–61. doi: 10.1111/1440-1681.12330
71. Shen H, Goodall JC, Hill Gaston JS. Frequency and Phenotype of Peripheral Blood Th17 Cells in Ankylosing Spondylitis and Rheumatoid Arthritis. Arthritis Rheum (2009) 60(6):1647–56. doi: 10.1002/art.24568
72. Suen JL, Li HT, Jong YJ, Chiang BL, Yen JH, et al. Altered Homeostasis of CD4(+) FoxP3(+) Regulatory T-cell Subpopulations in Systemic Lupus Erythematosus. Immunology (2009) 127(2):196–205. doi: 10.1111/j.1365-2567.2008.02937.x
73. Szántó S, Aleksza M, Mihály E, Lakos G, Szabó Z, Végvári A, et al. Intracytoplasmic Cytokine Expression and T Cell Subset Distribution in the Peripheral Blood of Patients With Ankylosing Spondylitis. J Rheumatol (2008) 35(12):2372–5. doi: 10.3899/jrheum.070839
74. Thoen J, Førre O, Waalen K, Pahle J, et al. Phenotypes and Spontaneous Cell Cytotoxicity of Mononuclear Cells From Patients With Seronegative Spondyloarthropathies: Ankylosing Spondylitis, Psoriatic Arthropathy and Pauciarticular Juvenile Chronic Arthritis–Analysis of Mononuclear Cells From Peripheral Blood, Synovial Fluid and Synovial Membranes. Clin Rheumatol (1988) 7(1):95–106. doi: 10.1007/BF02284064
75. Toussirot E, Saas E, Deschamps P, Pouthier M, Perrot F, Perruche L, et al. Increased Production of Soluble CTLA-4 in Patients With Spondylarthropathies Correlates With Disease Activity. Arthritis Res Ther (2009) 11(4):R101. doi: 10.1186/ar2747
76. Wang YF, Wang M, Song AF. Evaluation Value of Peripheral Th17/Treg Balance in Patients With Ankylosing Spondylitis. Int J Lab Med (2020) 41(07):842–5. doi: 10.3969/j.issn.1673-4130.2020.07.017
77. Wang CL, Li KZ, Cui W. Study on Expression Ofth1, Th17, Treg Cells and Related Cytokines in Ankylosing Spondylitis Patients. Chronic Pathematol J (2018) 19(09):1154–1156+1160. doi: 10.16440/j.cnki.1674-8166.2018.09.003
78. Wang M, Liu C, Bond A, Yang J, Zhou X, Wang J, et al. Dysfunction of Regulatory T Cells in Patients With Ankylosing Spondylitis is Associated With a Loss of Tim-3. Int Immunopharmacol (2018) 59:53–60. doi: 10.1016/j.intimp.2018.03.032
79. Wang H, Sun N, Li K, Tian J, Li H, et al. Assay of Peripheral Regulatory Vδ1 T Cells in Ankylosing Spondylitis and its Significance. Med Sci Monit (2016) 22:3163–8. doi: 10.12659/MSM.897126
80. Wang ZL, Zhong NF, Ma L. A Study on the Clinical Value and Correlation of Treg and Th17 Cells Among Different Active Stages of Ankylosing Spondylitis. J Guizhou Med Univ (2015) 40(01):68–71+75.
81. Wang C, Liao Q, Hu Y, Zhong D, et al. T Lymphocyte Subset Imbalances in Patients Contribute to Ankylosing Spondylitis. Exp Ther Med (2015) 9(1):250–6. doi: 10.3892/etm.2014.2046
82. Wang YF, Xu LH, Jiang LX, Qi CP, Wang Y, et al. Clinical Significance of Detecting Immune Functions on Patients With Ankylosing Spondylitis. J Modern Lab Med (2012) 27(06):132–4. doi: 10.3969/j.issn.1671-7414.2012.06.043
83. Wang JX, Wei P, Meng JH, Liu XC, Liu YJ, Gu G, et al. Expression and Significance of ThlTh2 Cytokines in Ankylosing Spondylitis. Clin Med China (2008) 24(10):989–90. doi: 10.3760/cma.j.issn.1008-6315.2008.10.010
84. Wei YY, Han ZJ, Huang HY, Du W, Ren TL, Gao MZ, et al. Analysis of Treg Cells and Lymphocyte Subgroup in 131 Patients With Ankylosing Spondylitis. China Med Herald (2017) 14(28):46–8.
85. Wu SS. Association of Follicular Helper T Cells and Ankylosing Spondylitis. Anhui Medical University (2014).
86. Wu Y, Ren M, Yang R, Liang X, Ma Y, Tang Y, et al. Reduced Immunomodulation Potential of Bone Marrow-Derived Mesenchymal Stem Cells Induced CCR4+CCR6+ Th/Treg Cell Subset Imbalance in Ankylosing Spondylitis. Arthritis Res Ther (2011) 13(1):R29. doi: 10.1186/ar3257
87. Wu HK, Zhou L, Zhang LZ, Zhong RQ, et al. The Expression Research of B Lymphocyte Subsets, B-cell Activating Factor and its Receptor BR3 in Peripheral Blood From Patients With Ankylosing Spondylitis. Lab Med (2011) 26(12):818–22. doi: 10.3969/j.issn.1673-8640.2011.12.007
88. Xu F, Guanghao C, Liang Y, Jun W, Wei W, Baorong H, et al. Treg-Promoted New Bone Formation Through Suppressing TH17 by Secreting Interleukin-10 in Ankylosing Spondylitis. Spine (Phila Pa 1976) (2019) 44(23):E1349–55. doi: 10.1097/BRS.0000000000003169
89. Xu WL, Luo Y, Li K, Liao CZ, Lin YH, Zhang HD, et al. Eepression and Significance of T Lymphocyte Subgroup and Natural Killer T Cells in Peripheral Blood of Ankylosing Spondylitis Patients. Lab Med Clinic (2018) 15(02):192–4. doi: 10.3969/j.issn.1672-9455.2018.02.015
90. Xu XX. The Differentiation of Th1/Th17/Treg Cells and Their Expression of Associated Transcription Factors and Cytokines in Patients With Ankylosing Spondylitis. Fujian Medical University (2013).
91. Xu XF, Jiang LH, Gao WH, Tao L, Huang LJ, Xu QB, et al. Detection of HLA-B27 and T Lymphocyte Subsets in Patients With Ankylosing Spondylitis and its Meaning. Lab Med Clinic (2011) 8(19):2366–8. doi: 10.3969/j.issn.1672-9455.2011.19.034
92. Xue GH, Hua L, Liu XF, Chen XL, Dong L, Pan J, et al. Frequencies of Human Regulatory B Cells in PBMC in Ankylosing Spondylitis Patients and its Clinical Significance. Chin J Clin Lab Sci (2015) 33(09):662–7. doi: 10.13602/j.cnki.jcls.2015.09.07
93. Xue YH, Cai YT, Xie KC. Expression of CD28/CD152: CD80/CD86 on Peripheral Blood Lymphocytes of Patients With Ankylosing Spondylitis. Lab Med (2008) 23(05):478–80. doi: 10.3969/j.issn.1673-8640.2008.05.012
94. Yang M, Lv Q, Wei Q, Jiang Y, Qi J, Xiao M, et al. TNF-Alpha Inhibitor Therapy can Improve the Immune Imbalance of CD4+ T Cells and Negative Regulatory Cells But Not CD8+ T Cells in Ankylosing Spondylitis. Arthritis Res Ther (2020) 22(1):149. doi: 10.1186/s13075-020-02226-8
95. Yang WH, Shu R, Han YX, Yuan W, Yu P, Li N, et al. Levels of Natural Killer Cells in Patients With Ankylosing Spondylitis and its Clinical Meaning. Guangxi Med J (2018) 40(10):1241–1242+1245. doi: 10.11675/j.issn.0253-4304.2018.10.36
96. Yang X. Changes and Clinical Significance of CD8+ Regulatory T Cells in the Peripheral Blood of Patients With Ankylosing Spondylitis. Anhui Medical University (2017).
97. Yang FF, Zhang X, Zhu P. Percentage of Peripheral Effector T Cells in Active Phase Patients With Ankylosing Spondylitis is Increased While Level of PD-L1 is Decreased. Chin J Cell Mol Immunol (2016) 32(05):676–9. doi: 10.13423/j.cnki.cjcmi.007764
98. Yang GM, Wang YF, Ma YF, Li SQ, Tan Y, et al. Study on Lymphocytes and CD28CD40 in Peripheral Blood of Patients With Ankylosing Spondylitis. Chin J Lab Diagnosis (2007) 11(11):1486–9. doi: 10.3969/j.issn.1007-4287.2007.11.022
99. Ye L, Zhang L, Goodall J, Gaston H, Xu H, et al. Altered Frequencies of Regulatory T-Cell Subsets in Ankylosing Spondylitis and Rheumatoid Arthritis Patients and Their Response to anti-TNF Therapy. Rheumatol (United Kingdom) (2013) 52:i135–6. doi: 10.1093/rheumatology/ket195
100. Zhang CQ, Fang LH, Liu XP, Li R, Cui LP, Wang J, et al. Differential Expression and Meaning of Th17 and Tregs in Patients With Ankylosing Spondylitis and Psoriatic Arthritis. Chin Remedies Clinics (2019) 19(01):34–6. doi: 10.11655/zgywylc2019.01.016
101. Zhang Y, Ma L, Shen X, Fang ZY, Lin J, et al. An Observation of Changes in Counts and Percentages of Peripheral Lymphocyte Subsets in Patients With Ankylosing Spondylitis. Shandong Med J (2019) 59(24):82–5. doi: 10.3969/j.issn.1002-266X.2019.24.022
102. Zhang HL, Zhang JY, Jin XY, Niu JX, Li ZJ, Zhang FL, et al. Study on the Correlation of the Imbalance of Th17 Cells, Th1 Cell, Egulatory T Cells With Ankylosing Spondylitis Disease Activity Score. Med Recapitulate (2014) 20(24):4545–4546+4555. doi: 10.3969/j.issn.1006-2084.2014.24.052
103. Zhang X, Wang P, Wu YF, Yang R, Huang L, Tang Y, et al. Allogeneic Blood Transfusion Alleviates Hip Joint Pain Induced by Ankylosing Spondylitis. Chin J Tissue Eng Res (2014) 18(09):1465–70. doi: 10.3969/j.issn.2095-4344.2014.09.026
104. Zhang L, Li YG, Li YH, Qi L, Liu XG, Yuan CZ, et al. Increased Frequencies of Th22 Cells as Well as Th17 Cells in the Peripheral Blood of Patients With Ankylosing Spondylitis and Rheumatoid Arthritis. PloS One (2012) 7(4):e31000. doi: 10.1371/journal.pone.0031000
105. Zhang SH, Han YX, Wu JB, Hu XX, Chen D, et al. The Alteration of CD4 Regulatory T Cells in Patients Th Ankylosing Spondylitis. Chin J Microbiol Immunol (2008) 28(05):445–9. doi: 10.3321/j.issn:0254-5101.2008.05.015
106. Zhao JT, Li YJ. The Comparison of the Ratio of Thl7Treg Cells in Patients With Ankylosing Spondytitis and the Normal Controls. Chin J Rheumatol (2013) 17(07):481–4. doi: 10.3760/cma.j.issn.1007-7480.2013.07.013
107. Zhao SS, Hu JW, Wang J, Lou XJ, Zhou LL, et al. Inverse Correlation Between CD4+CD25highCD127low/- Regulatory T-cells and Serum Immunoglobulin A in Patients With New-Onset Ankylosing Spondylitis. J Int Med Res (2011) 39(5):1968–74. doi: 10.1177/147323001103900543
108. Zhao XZ, Song HC, Cui LF. Clinical Significance and Expression of T Cell Subsets in Peripheral Blood From Patients Witb Ankylosing Spondylitis. Clin Med China (2009) 25(03):286–8. doi: 10.3760/cma.j.issn.1008-6315.2009.03.023
109. Zhong NF, Ma L. Clinical Significance of Th1, Th2 and Th17 Cell Determinations Among Different Active Stage Ankylosing Spondylitis Patients. Lab Med (2014) 29(05):477–82. doi: 10.3969/j.issn.1673-8640.2014.05.011
110. Zhu YY, Zhang L, Sun R, Zhao X, Xu YZ, Wu T, et al. Changes in T Lymphocyte Subsets and Related Immune Molecules in Peripheral Blood in Patients With Ankylosing Spondylitis. Curr Immunol (2017) 37(01):14–9.
111. Zhu LY, Yang WJ, Yu P, Wang J, Li N, Wang JR, et al. Expression of Natural Killer Cell in Patients With Ankylosing Spondylitis and its Significance. Chin J Coal Industry Med (2016) 19(07):989–92. doi: 10.11723/mtgyyx1007-9564201607014
112. Zhu J, Liu XY, Huang F. Th1/Th2 Balance and Ankylosing Spondylitis. Chin J Rheumatol (2000) 4(04):202–5. doi: 10.3760/j:issn:1007-7480.2000.04.003
113. Lai NL, Zhang SX, Wang J, Zhang JQ, Wang CH, Gao C, et al. The Proportion of Regulatory T Cells in Patients With Ankylosing Spondylitis: A Meta-Analysis. J Immunol Res (2019) 2019:1058738. doi: 10.1155/2019/1058738
114. Li M, Zhou X, Zhou L, Yu Z, Fu L, Yang P, et al. Meta-Analysis of Changes in the Number and Proportion of Regulatory T Cells in Patients With Ankylosing Spondylitis. BioMed Res Int (2020) 2020:8709804. doi: 10.1155/2020/8709804
115. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu Rev Immunol (2012) 30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623
116. Neumann C, Scheffold A, Rutz S. Functions and Regulation of T Cell-Derived Interleukin-10. Semin Immunol (2019) 44:101344. doi: 10.1016/j.smim.2019.101344
117. Ge L, Wang J, Zhu BQ, Zhang ZS, et al. Effect of Abnormal Activated B Cells in Patients With Ankylosing Spondylitis and its Molecular Mechanism. Eur Rev Med Pharmacol Sci (2018) 22(9):2527–33. doi: 10.26355/eurrev_201805_14941
118. Letourneau S, Krieg C, Pantaleo G, Boyman O, et al. Il-2- and CD25-dependent Immunoregulatory Mechanisms in the Homeostasis of T-cell Subsets. J Allergy Clin Immunol (2009) 123(4):758–62. doi: 10.1016/j.jaci.2009.02.011
119. Lu L, Barbi J, Pan F. The Regulation of Immune Tolerance by FOXP3. Nat Rev Immunol (2017) 17(11):703–17. doi: 10.1038/nri.2017.75
120. Sun J, Tang DN, Fu T, Sharma P, et al. Identification of Human Regulatory T Cells in the Setting of T-cell Activation and anti-CTLA-4 Immunotherapy on the Basis of Expression of Latency-Associated Peptide. Cancer Discov (2012) 2(2):122–30. doi: 10.1158/2159-8290.CD-11-0236
121. Gravallese EM, Schett G. Effects of the IL-23-IL-17 Pathway on Bone in Spondyloarthritis. Nat Rev Rheumatol (2018) 14(11):631–40. doi: 10.1038/s41584-018-0091-8
122. Yasuda K, Takeuchi Y, Hirota K. The Pathogenicity of Th17 Cells in Autoimmune Diseases. Semin Immunopathol (2019) 41(3):283–97. doi: 10.1007/s00281-019-00733-8
123. Bridgewood C, Sharif K, Sherlock J, Watad A, McGonagle D, et al. Interleukin-23 Pathway at the Enthesis: The Emerging Story of Enthesitis in Spondyloarthropathy. Immunol Rev (2020) 294(1):27–47. doi: 10.1111/imr.12840
124. Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 Functions as an Osteoclastogenic Helper T Cell Subset That Links T Cell Activation and Bone Destruction. J Exp Med (2006) 203(12):2673–82. doi: 10.1084/jem.20061775
125. Xu S, Cao X. Interleukin-17 and its Expanding Biological Functions. Cell Mol Immunol (2010) 7(3):164–74. doi: 10.1038/cmi.2010.21
126. Hayday AC. Gammadelta T Cell Update: Adaptate Orchestrators of Immune Surveillance. J Immunol (2019) 203(2):311–20. doi: 10.4049/jimmunol.1800934
127. Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdorfer L, et al. Interleukin-23-Dependent Gamma/Delta T Cells Produce Interleukin-17 and Accumulate in the Enthesis, Aortic Valve, and Ciliary Body in Mice. Arthritis Rheumatol (2016) 68(10):2476–86. doi: 10.1002/art.39732
128. Lee JS, Tato CM, Joyce–Shaikh B, Gulen MF, Cayatte C, Chen Y, et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity (2015) 43(4):727–38. doi: 10.1016/j.immuni.2015.09.003
129. Deodhar A, Gensler LS, Sieper J, Clark M, Calderon C, Wang Y, et al. Three Multicenter, Randomized, Double-Blind, Placebo-Controlled Studies Evaluating the Efficacy and Safety of Ustekinumab in Axial Spondyloarthritis. Arthritis Rheumatol (2019) 71(2):258–70. doi: 10.1002/art.40728
130. Pavelka K, Kivitz A, Dokoupilova E, Blanco R, Maradiaga M, Tahir H, et al. Efficacy, Safety, and Tolerability of Secukinumab in Patients With Active Ankylosing Spondylitis: A Randomized, Double-Blind Phase 3 Study, MEASURE 3. Arthritis Res Ther (2017) 19(1):285. doi: 10.1186/s13075-017-1490-y
131. van der Heijde D, Gensler LS, Deodhar A, Baraliakos X, Poddubnyy D, Kivitz A, et al. Dual Neutralisation of interleukin-17A and interleukin-17F With Bimekizumab in Patients With Active Ankylosing Spondylitis: Results From a 48-Week Phase IIb, Randomised, Double-Blind, Placebo-Controlled, Dose-Ranging Study. Ann Rheum Dis (2020) 79(5):595–604. doi: 10.1136/annrheumdis-2020-216980
132. Venken K, Jacques P, Mortier C, Labadia ME, Decruy T, Coudenys J, et al. Rorgammat Inhibition Selectively Targets IL-17 Producing iNKT and Gammadelta-T Cells Enriched in Spondyloarthritis Patients. Nat Commun (2019) 10(1):9. doi: 10.1038/s41467-018-07911-6
133. Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH, et al. Distinct Effects of T-bet in TH1 Lineage Commitment and IFN-gamma Production in CD4 and CD8 T Cells. Science (2002) 295(5553):338–42. doi: 10.1126/science.1065543
134. Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG, et al. The Majority of Epidermal T Cells in Psoriasis Vulgaris Lesions can Produce Type 1 Cytokines, Interferon-Gamma, interleukin-2, and Tumor Necrosis Factor-Alpha, Defining TC1 (Cytotoxic T Lymphocyte) and TH1 Effector Populations: A Type 1 Differentiation Bias is Also Measured in Circulating Blood T Cells in Psoriatic Patients. J Invest Dermatol (1999) 113(5):752–9. doi: 10.1046/j.1523-1747.1999.00749.x
135. Mitchell RE, Hassan M, Burton BR, Britton G, Hill EV, Verhagen J, et al. IL-4 Enhances IL-10 Production in Th1 Cells: Implications for Th1 and Th2 Regulation. Sci Rep (2017) 7(1):11315. doi: 10.1038/s41598-017-11803-y
136. Chatzileontiadou DSM, Sloane H, Nguyen AT, Gras S, Grant EJ, et al. The Many Faces of CD4(+) T Cells: Immunological and Structural Characteristics. Int J Mol Sci (2020) 22(1). doi: 10.3390/ijms22010073
137. Fulkerson PC, Schollaert KL, Bouffi C, Rothenberg ME, et al. IL-5 Triggers a Cooperative Cytokine Network That Promotes Eosinophil Precursor Maturation. J Immunol (2014) 193(8):4043–52. doi: 10.4049/jimmunol.1400732
138. Crotty S. T Follicular Helper Cell Differentiation, Function, and Roles in Disease. Immunity (2014) 41(4):529–42. doi: 10.1016/j.immuni.2014.10.004
139. Riechers E, Baerlecken N, Baraliakos X, Achilles–Mehr Bakhsh K, Aries P, Bannert B, et al. Sensitivity and Specificity of Autoantibodies Against CD74 in Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol (2019) 71(5):729–35. doi: 10.1002/art.40777
140. Iwata S, Zhang M, Hajime M, Ohkubo N, Sonomoto K, Torimoto K, et al. Pathological Role of Activated mTOR in CXCR3+ Memory B Cells of Rheumatoid Arthritis. Rheumatol (Oxford) (2021). doi: 10.1093/rheumatology/keab229
141. Kucuksezer UC, Aktas Cetin E, Esen F, Tahrali I, Akdeniz UN, Gelmez MY, et al. The Role of Natural Killer Cells in Autoimmune Diseases. Front Immunol (2021) 12:622306. doi: 10.3389/fimmu.2021.622306
142. Chan AT, Kollnberger SD, Wedderburn LR, Bowness P, et al. Expansion and Enhanced Survival of Natural Killer Cells Expressing the Killer Immunoglobulin-Like Receptor KIR3DL2 in Spondylarthritis. Arthritis Rheum (2005) 52(11):3586–95. doi: 10.1002/art.21395
143. Parham P, Guethlein LA. Genetics of Natural Killer Cells in Human Health, Disease, and Survival. Annu Rev Immunol (2018) 36:519–48. doi: 10.1146/annurev-immunol-042617-053149
144. Azuz-Lieberman N, Markel G, Mizrahi S, Gazit R, Hanna J, Achdout H, et al. The Involvement of NK Cells in Ankylosing Spondylitis. Int Immunol (2005) 17(7):837–45. doi: 10.1093/intimm/dxh270
145. Scrivo R, Morrone S, Spadaro A, Santoni A, Valesini G, et al. Evaluation of Degranulation and Cytokine Production in Natural Killer Cells From Spondyloarthritis Patients at Single-Cell Level. Cytometry B Clin Cytom (2011) 80(1):22–7. doi: 10.1002/cyto.b.20549
146. Park YW, Kee SJ, Cho YN, Lee EH, Lee HY, Kim EM, et al. Impaired Differentiation and Cytotoxicity of Natural Killer Cells in Systemic Lupus Erythematosus. Arthritis Rheum (2009) 60(6):1753–63. doi: 10.1002/art.24556
Keywords: Ankylosing spondylitis, lymphocyte, immune system, flow cytometry, Th17 & Treg cells
Citation: Liu D, Liu B, Lin C and Gu J (2021) Imbalance of Peripheral Lymphocyte Subsets in Patients With Ankylosing Spondylitis: A Meta-Analysis. Front. Immunol. 12:696973. doi: 10.3389/fimmu.2021.696973
Received: 18 April 2021; Accepted: 07 June 2021;
Published: 06 July 2021.
Edited by:
Giuseppe Lopalco, University of Bari Aldo Moro, ItalyReviewed by:
Vincenzo Venerito, University of Bari Aldo Moro, ItalyWon-Woo Lee, Seoul National University, South Korea
Copyright © 2021 Liu, Liu, Lin and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jieruo Gu, Z3VqaWVydW9AMTYzLmNvbQ==
 Dong Liu
Dong Liu Budian Liu
Budian Liu Churong Lin
Churong Lin Jieruo Gu
Jieruo Gu