
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 21 July 2021
Sec. Viral Immunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.695674
This article is part of the Research Topic CD4 T cells in HIV: A Friend or Foe? View all 16 articles
CD4+ T-cell depletion is pathognomonic for AIDS in both HIV and simian immunodeficiency virus (SIV) infections. It occurs early, is massive at mucosal sites, and is not entirely reverted by antiretroviral therapy (ART), particularly if initiated when T-cell functions are compromised. HIV/SIV infect and kill activated CCR5-expressing memory and effector CD4+ T-cells from the intestinal lamina propria. Acute CD4+ T-cell depletion is substantial in progressive, nonprogressive and controlled infections. Clinical outcome is predicted by the mucosal CD4+ T-cell recovery during chronic infection, with no recovery occurring in rapid progressors, and partial, transient recovery, the degree of which depends on the virus control, in normal and long-term progressors. The nonprogressive infection of African nonhuman primate SIV hosts is characterized by partial mucosal CD4+ T-cell restoration, despite high viral replication. Complete, albeit very slow, recovery of mucosal CD4+ T-cells occurs in controllers. Early ART does not prevent acute mucosal CD4+ T-cell depletion, yet it greatly improves their restoration, sometimes to preinfection levels. Comparative studies of the different models of SIV infection support a critical role of immune activation/inflammation (IA/INFL), in addition to viral replication, in CD4+ T-cell depletion, with immune restoration occurring only when these parameters are kept at bay. CD4+ T-cell depletion is persistent, and the recovery is very slow, even when both the virus and IA/INFL are completely controlled. Nevertheless, partial mucosal CD4+ T-cell recovery is sufficient for a healthy life in natural hosts. Cell death and loss of CD4+ T-cell subsets critical for gut health contribute to mucosal inflammation and enteropathy, which weaken the mucosal barrier, leading to microbial translocation, a major driver of IA/INFL. In turn, IA/INFL trigger CD4+ T-cells to become either viral targets or apoptotic, fueling their loss. CD4+ T-cell depletion also drives opportunistic infections, cancers, and comorbidities. It is thus critical to preserve CD4+ T cells (through early ART) during HIV/SIV infection. Even in early-treated subjects, residual IA/INFL can persist, preventing/delaying CD4+ T-cell restoration. New therapeutic strategies limiting mucosal pathology, microbial translocation and IA/INFL, to improve CD4+ T-cell recovery and the overall HIV prognosis are needed, and SIV models are extensively used to this goal.
Even before HIV was formally established as the cause of AIDS, CD4+ T-cell depletion was identified as a key feature of HIV infection. Indeed, lymphocytopenia was among the first biological findings described in the early days of the AIDS pandemic (1). Lymphocytopenia was notably due to a depletion of CD4+ T cells and, in addition to their decrease in absolute number and percentage of total T cells, residual CD4+ T cells were dysfunctional in AIDS patients (1). Later, CD4 was identified as HIV/SIV receptor (2, 3), and CD4+ T cell counts in peripheral blood were reported to predict the risk of progression to AIDS (4).
However, the notions of CD4+ T-cell depletion and restoration encompass processes that are vastly different for different CD4+ T-cell subsets and according to their tissue location (5–8). As longitudinal studies cannot access and sample all body compartments, reasonable knowledge on CD4+ T-cell dynamics during HIV infection was obtained from descriptive studies of cohorts of HIV-1 infected patients and experimental studies of simian immunodeficiency virus (SIV) infection in nonhuman primates (NHP). Depending on whether or not an NHP is a natural host of SIV (9), the infection will be nonpathogenic or pathogenic, respectively, and CD4+ T-cell dynamics will also vary accordingly.
Here, we will review the general features of the depletion of the different CD4+ T-cell subsets and their restoration during pathogenic and nonpathogenic HIV/SIV infections, as well as the consequences of CD4+ T-cell depletion, and the potential approaches that could help reverse CD4+ T-cell depletion and prevent its deleterious consequences.
Different CD4+ T-cell subsets are defined, according to their differentiation status or phenotype. CD4+ T cells from humans and NHP are classified as naïve (CD45RA+ CCR7+ CD28+ CD95neg) or memory T cells (CD45RO+ CD95+) (10, 11). Memory T cells are subdivided into stem cell memory (Tscm; CD45RA+ CCR7+ CD28+ CD95+) (12, 13), central memory (Tcm; CD45RAneg CCR7+ CD62L+) (11), transitional memory (Ttm; CD45RAneg CCR7neg CD95+ CD62L+), effector memory (Tem; CD45RAneg CCR7neg CD95+ CD28neg CD62Lneg) (14), terminal effector (Tte; CD45RA+ CCR7neg CD95+ CD28neg CD62Lneg) (14) and resident memory (Trm; CD45RAneg CCR7neg CD69+ ± CD103+) (15) T cells. Meanwhile, based on their functional status, CD4+ T cells can be classified as Th1 (IFN-γ producing; transcription factor: T-bet), Th2 (IL-4 producing; GATA3), Th17 (IL-17 producing; RORγt), regulatory T cells (Tregs) (suppressive function; FoxP3) and follicular helper T cells (Tfh) (IL-21 producing; Bcl6).
Additional phenotypic markers can be used to differentiate CD4+ T cells, notably CCR5, the main coreceptor of HIV/SIV (16–18), and markers of cell proliferation (Ki-67, BrdU), activation (HLA-DR, CD38, CD69), exhaustion (PD-1, CTLA-4, Tim-3) or senescence (CD57, KLRG-1) (19).
Finally, CD4+ T cells can also be subdivided according to their metabolic status, with memory cells tending to have higher metabolic activities, notably glycolysis and oxidative phosphorylation (20).
The multiple CD4+ T cell populations defined using any of these features are differentially infected, depleted, and restored. Note that during the course of the HIV/SIV infection other immune cells (CD4+CD8+ T cells, γδTCR T cells, innate lymphoid cells type 2 and 3, circulating dendritic cells) (21–26) can be depleted and restored, but their dynamics will not be detailed in this review.
When CD4+ T-cell depletion was first investigated in people with HIV (PWH), research focused on CD4+ T cells dynamics in blood and superficial LNs, notably tonsils, as they were more accessible. Circulating CD4+ T cells are moderately depleted during acute HIV or SIV infection (21, 27, 28). A slight increase in the CD4+ T cell counts occurs as a consequence of the postacute partial control of viral replication and establishment of the steady-state set-points (27, 29). During chronic HIV/SIV infection, a slow and continuous decline of circulating CD4+ T cells is observed, eventually leading to severe lymphopenia, rendering persons living with HIV susceptible to opportunistic infections and eventually leading to AIDS (30). Annual rate of CD4+ T cell decline in circulation in untreated patients is correlated to plasma viral loads, and was found to be higher in persons living with HIV-1 than with HIV-2 (-15.9% vs. -4.1% per year, respectively), due to major differences in the levels of viral replication between these two infections (31). Meanwhile, in the superficial and mesenteric lymph nodes, CD4+ T-cell depletion is minimal during acute HIV or SIV infection (32–35). However, during the very advanced stages of HIV/SIV infection, CD4+ T-cell depletion may occur even in lymph nodes and is associated with lymphadenopathy and fibrosis (34, 36, 37).
With the discovery that HIV-1, HIV-2, and most of the SIV use the chemokine receptor CCR5 as a coreceptor (16–18), and therefore preferentially infect memory T cells (38, 39), numerous studies then aimed at describing the dynamics of the different CD4+ T-cell subsets in PWH and SIV-infected NHPs. In young, uninfected humans, most CD4+ T cells in peripheral circulation and the lymph nodes are naïve, while the fraction of naïve CD4+ T cells declines in older individuals (40). Naïve T cells also represent the majority of CD4+ T cells in blood and lymph nodes of young (less than 4 years) rhesus macaques (RMs), the animal model of reference for HIV infection (11, 30). In Indian RMs, it has been estimated that, on average, less than 15% of CD4+ T cells from circulation and the lymph nodes express CCR5 (30, 32, 41), while over 75% of them express CXCR4. In both humans and macaques, the vast majority of CCR5+ CD4+ T cells are memory T cells (30, 42, 43). As a result, the majority of circulating CD4+ T cells are not direct targets for HIV and SIV, as emphasized by the low fraction of circulating CD4+ T cells that are HIV-infected (44). This could explain why the loss of peripheral CD4+ T cells is limited to 50-60% in most patients during acute HIV infection, with a median nadir of CD4+ T cells ranging between 340 and 510/mm3 (27, 45, 46). Similar decline in circulating CD4+ T cells is also observed in SIV-infected NHP during acute pathogenic (21), non-pathogenic (47) and controlled SIV infections (48). In addition to this total CD4+ T-cell depletion, a preferential depletion of the memory CD4+ T cells, especially those expressing CCR5, can be seen as early as 7-14 days postinfection (dpi) in the lymph nodes and periphery, whereas naïve T cells are preserved (6, 28, 32, 49).
Once the strategies for the detailed characterization of the different memory CD4+ T-cell subsets, notably Tcm, Ttm, Tem, became available, studies were performed on sorted CD4+ T-cell subsets to assess the frequency of infection, in addition to monitoring the evolution of each of those subsets throughout HIV/SIV infection. These studies established that, in blood, Tcms represent the major cellular reservoir in HIV-1 infected individuals (50), while Ttms and/or Tems form the bulk of the reservoir in HIV-1 long term nonprogressors, SIVsmm-infected sooty mangabeys (a prototypic nonpathogenic infection) and HIV-2-infected individuals (51–53). In pathogenic hosts of SIV, the frequency of HIV/SIV infected cells is also high in the recently described Tscm subset, which expresses high levels of CCR5 (5, 54). In patients with progressive infection, as a result of a prolonged and continuous depletion of the target Tcm and Tem cells, due to cell death and reduced proliferation of Tcm, the majority of the remaining CD4+ T cells are naïve (5, 6).
Dynamics of the CD4+ T cells with specific functions have also been probed. Both HIV and SIV preferentially infect Th1 and Th17 cells (55). As a result, HIV and SIV infections are characterized by a switch from Th1 to Th2 phenotype (56), and a significant depletion of Th17 cells is observed among circulating lymphocytes throughout the infection (7). HIV-1 can also infect Tregs (57), and, although reduced (7, 58–60), stable, and increased (61, 62) absolute circulating Treg counts have all been reported during chronic infections, a decreased Th17/Treg ratio is commonly observed in pathogenic infections and was linked to immune activation and disease progression (7, 62). Furthermore, a selective depletion of circulating CD4+ T cells with gut homing potential (i.e., expressing the α4β7 integrin) preferentially occurs in untreated PWH (63) and in SIV-infected RMs in which those cells are selectively infected in the first days of infection (8). Most CD4+ T cells expressing α4β7 integrin are Tcm with a Th17 phenotype, and their dynamics in blood reflects the evolution of intestinal CD4+ T cells in jejunum (8, 64).
A subset of CD4+ T cells T follicular helper (Tfh), identified based on the expression of the surface markers CXCR5+ PD-1high, and preferentially found in B follicles in lymph nodes and spleen, can also be infected by HIV/SIV and is slightly depleted during acute infection, before accumulating during chronic infection (65–68). This chronic accumulation of Tfh might be due to a lack of regulation by the local Tregs, the follicular regulatory T cells (Tfr), as suggested by the decreased Tfr/Tfh ratio (69). Tfh cells are depleted during the AIDS stage (65). Signals provided by Tfh cells are crucial for the development of memory B cells, and the expansion of Tfh cells has been associated with B cell dysregulation, notably a reduced number of antigen-specific memory B cells, increased germinal center B cells, hypergammaglobulinemia, and lower Env-specific antibody titers (67, 70).
Finally, in vivo, CD4+ T cells can be also selectively infected according to their metabolic status (71). HIV-1 tends to infect CD4+ T cells with high oxidative phosphorylation and glycolysis, two metabolic activities more frequently enhanced in memory CD4+ T cells (71). The dynamics of CD4+ T cells during HIV/SIV infection according to their metabolic activities remain to be determined.
While the circulating lymphocytes only account for 2 to 5% of the total lymphocytes, intestinal lymphocytes represent a tremendous fraction of total lymphocytes (over 60%) in both humans and NHPs. In the GI tract, lymphocytes exist in three major forms: (i) intraepithelial lymphocytes (IEL), (ii) lamina propria lymphocytes (LPL), and iii) lymphocytes organized in lymphoid formations (i.e., the Peyer’s patches and the solitary lymphoid follicles). There are strong similarities between human and NHPs’ gut-associated lymphoid tissue (GALT) regarding the distribution of the immune cells, with the CD4/CD8 ratios being about 1:2 and 1:1 in IEL and LPL, respectively (72).
In the years following the identification of AIDS, prompted partly by the high frequency of enteropathies in PWH (73), histological studies identified a loss of CD4+ T cells in gastrointestinal biopsies of PWH (74–79). Lim and colleagues also proved that, similar to circulation, memory CD4+ T cells were the preferentially depleted cell subset in the gut during HIV infection (80). However, results were sometimes contradictory, some studies reported that CD4+ T-cell depletion only affected the LPLs, while others described CD4+ T-cell depletion as impacting both LPLs and IELs (77, 81). Furthermore, almost all intestinal biopsies were obtained from clinically indicated procedures in patients presenting with AIDS or late-stage disease, limiting the insights on the dynamics of CD4+ T-cell depletion at this site (75).
It was only a decade later that, due to research performed in SIV-infected RMs, the early dynamics of CD4+ T-cell depletion in GI tissues were described (21, 33, 82). These studies reported that a massive CD4+ T-cell depletion occurs as early as 7 dpi in SIVmac-infected RMs, leading to more than 90% of intestinal CD4+ T cells being lost at 14-21 dpi (21, 33, 83) (Figure 1). SIV-infected cells can be detected within 7 dpi in the gut (21, 33, 93). The peak of viral replication in the gut occurs approximately 10 dpi and the vast majority of the SIV-infected cells during the acute infection are found within the lamina propria, the remaining infected cells being mainly detected in organized lymphoid tissues and macrophages (33, 77, 94). CD4+ T-cell depletion occurs earlier in the jejunum than in the ileum and colon, and affects mostly LPL (33), probably because most of the lymphocytes in jejunum are found within the lamina propria, while organized lymphoid tissues are more common to the ileum and colon. The first studies reporting this massive and rapid CD4+ T-cell depletion in the gut were performed on animals intravenously inoculated, but later the same pathogenic features were confirmed in studies in which animals were infected either intrarectally or intravaginally (83, 94).
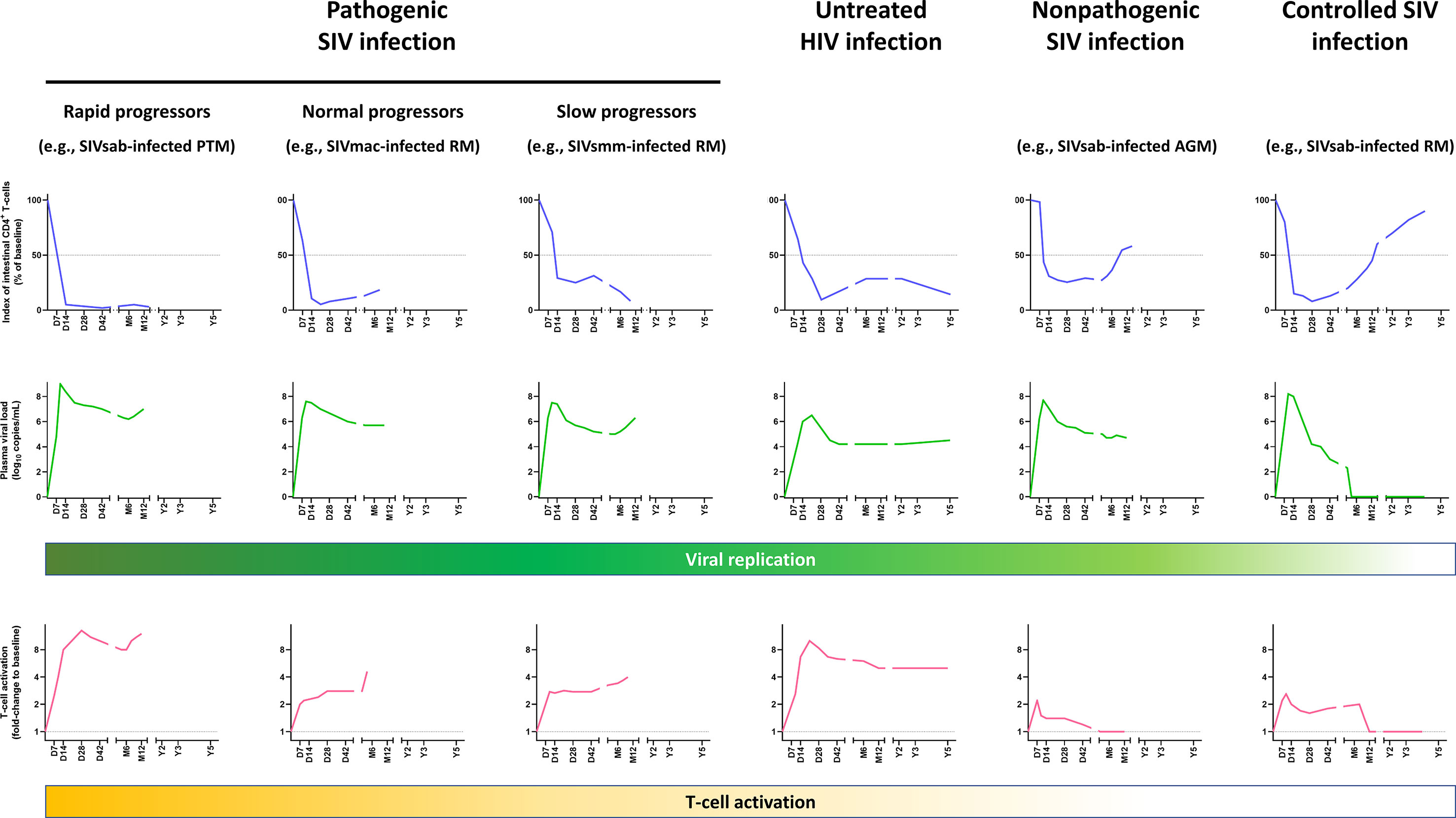
Figure 1 Comparative dynamics of intestinal CD4+ T-cell depletion, plasma viral loads and immune activation/inflammation in untreated HIV and SIV infections. Schematic representation of intestinal CD4+ T-cell depletion, viral replication and immune activation is inferred from data reported in references (21, 33, 48, 84–91). Longitudinal data are presented, with the X axis representing days (D), months (M) and years (Y) postinfection. The Y axis illustrates the magnitude of lamina propria CD4+ T-cell depletion (upper panels, blue), viral replication (middle panels, green), and the levels of T-cell activation (lower panels, yellow). Intestinal CD4+ T-cell depletion is illustrated as the index of lamina propria CD4+ T cells (i.e., percentage of CD4+ T cell fraction within the CD3+ T cell population, divided by this percentage at baseline). Viral replication is represented as plasma viral loads. From the plethora of biomarkers of immune activation/inflammation, we selected the fold-change of HLA-DR+ CD8+ T cells in SIV-infected NHPs, compared to the baseline preinfection levels, except for persons living with HIV, for which the fold-changes of HLA-DR+ CD38+ CD8+ T cells were used. Note that in other primate models of rapid progressors, T-cell activation might be more blunted (92). AGM, African green monkeys; PTM, Pigtailed macaques; RM, Rhesus macaques.
Phenotypic analyses of the intestinal CD4+ T cells enabled further characterization of the CD4+ T-cell subsets that were preferentially depleted in the gut. Contrary to circulating lymphocytes, most of the CD4+ T LPL have an activated (HLA-DR+ CD25+) and memory (CD45RAneg CD95+) phenotype (49, 95). Activated, CCR5-expressing memory CD4+ T cells, which are the preferential targets of HIV and SIV, are frequent in the gut (30, 49, 96). The predominance of this activated, memory CD4+ T cell phenotype at the mucosal sites is common to both humans and macaques, being observed even in the newborns (95), and likely occurs as a consequence of exposure to antigens in utero. Interestingly, mucosal CD4+ T cells of humans and NHPs that are not natural hosts of SIV express higher levels of CCR5 compared to African natural host species (30, 41, 47, 84). At 14 dpi, virtually all memory CCR5+ CD4+ T cells are lost in the lamina propria and effector sites of the gut of SIV-infected RMs, with the spared intestinal CCR5+CD4+ T cells being naïve T cells (30). This swift depletion of CD4+ T cells has been confirmed to occur as well during acute HIV-1 infections (35, 97–99), and intestinal CD4+ T-cell depletion persists through the course of the disease in untreated PWH (35) (Figure 1).
As described for the circulating CD4+ T cells, several experiments have shown that the initial CD4+ T-cell depletion in the gut is driven by the coreceptor usage of the virus. NHP infection with X4-tropic viruses resulted in a rapid, profound depletion of the naïve CXCR4+ CD4+ T cells from the circulation and the lymph nodes, instead of the canonical depletion of memory CD4+ T cells in the GALT, typical of the R5-tropic viruses (100–102).
Furthermore, mucosal CD4+ T-cell depletion in the gut directly affects cell subsets that are involved in the maintenance of the mucosal barrier. As such, HIV/SIV infection can disrupt the production of IL-17 and IL-22, two cytokines that are essential for maintaining the tight epithelial barrier of the GI tract and gut integrity, by selectively depleting the lymphocytes producing those cytokines. Indeed, while Th1 and Th17 cell subsets are equally depleted in peripheral blood and they did not differ in frequency of infected cells, Th17 memory CD4+ T cells are selectively depleted from the lamina propria and effector sites of the GI tract of PWH and SIV-infected RMs, as early as 2 weeks postinfection (55, 62, 85, 103). lL-22 producing lymphocytes are also depleted during SIV (104, 105) and HIV infections (85). Other IL-17 and/or IL-22 producing cells exist in the GI tract, but for most of them a preferential depletion and/or a reduced IL-17 production has been reported during SIV infection (25, 105–107). Moreover, IL-21-producing CD4+ T lymphocytes are also depleted during SIV infection, thus limiting Th17 differentiation pathways, which are controlled by IL-21 in vivo (108).
Despite being susceptible to HIV and SIV infections, intestinal Tregs are increased during chronic infections, leading to a decreased Th17/Treg ratio (60, 62, 109). This could be due to limited productive infection and reduced cell death in the Tregs, as well as to an increased differentiation of naïve CD4+ T cells into Tregs in the GI tract (109–112).
Note that, in addition to CD4+ T-cell loss, RMs infected with SIVmac were reported to suffer a massive loss of the “double positive” (CD4+ CD8+) T cells, which express high levels of CCR5 and are highly activated (113), within days following SIV infection (30, 62).
CD4+ T-cell depletion is not limited to the GI tract, lymph nodes, or circulation. It also occurs in the spleen and in the liver of NHP, within 21 dpi in the spleen and during the AIDS stage in the liver (67, 114, 115). In the bone marrow, the reduction of the pool of CD4+ T cells reflects decreases in circulating CD4+ T cells, with the loss affecting mainly memory cells (116). Meanwhile, CD4+ T cell counts in the bronchoalveolar lavages (BAL) have been used as a proxy for the CD4+ T cell counts in the lung parenchyma. Most CD4+ T cells in the lung are of memory phenotype and express CCR5. A nearly complete loss of the CD4+ T cells was observed in the BAL by 3 weeks postinfection (114), while other teams reported that both memory CCR5+ CD4+ T cells and Th17 cell subsets were maintained in the lungs (55, 117). Recently, a study in humanized mice demonstrated that SIV and HIV infections lead to a rapid loss of resident-memory CD4+ T cells from the lung interstitium in the first weeks postinfection, which could participate in the increased susceptibility to pulmonary infections (118).
Finally, CD4+ T-cell depletion can also be detected in the genital tract. As for CD4+ T cells in the GI tract, most of the CD4+ T cells found in the vaginal mucosa display an activated, memory phenotype (119). Differently from the intestinal CD4+ T cells, almost all CD4+ T cells from the vaginal mucosa express CXCR4, while CCR5 is expressed by only half of them. Within 14 dpi, depletion of CD4+ T cells, particularly those with CCR5+ expression, occurs in the vaginal mucosa of SIV-infected RMs, and lasts throughout the follow-up, until progression to AIDS (119). In HIV-infected women, vaginal CD4+ T-cell depletion is strongly correlated to the depletion of circulating CD4+ T cells (120). Regarding the male genital tract, CD4+ T cells are depleted in the semen of PWH (121) and of SIV-infected cynomolgus macaques during acute and chronic infections (122).
CD4+ T-cell dynamics during nonpathogenic and controlled SIV infections have been extensively studied. Acute SIV infection induces a slight decline in the CD4+ T cell counts from the lymph nodes and circulation in natural hosts (e.g., sooty mangabeys, African green monkeys, mandrills etc.), which is followed by a return to virtually preinfection levels at both sites within the first year (47, 123). As a result, the levels of circulating CD4+ T cells are virtually normal in chronically SIV-infected African NHPs (124–127). Meanwhile, acute SIV infection induces a massive CD4+ T-cell depletion at the mucosal sites, which largely exceeds the number of CCR5-expressing CD4+ T cells that is particularly low at the mucosal sites in the natural hosts of SIVs (47, 84). This excess of CD4+ T-cell depletion is not due to a different coreceptor usage by SIVs compared to HIV-1, as most SIV use CCR5 (128). The exceptions are strains of SIVsab that naturally infect sabaeus AGMs in West Africa and SIVmnd-1 that infects mandrills, which were reported to also use CXCR4 and/or CXCR6 (129–131), and SIVrcm that naturally infects red-capped mangabeys in West-Central African that was reported to exclusively use CCR2 (132–134). However, in vivo, SIVsab was shown to use CCR5 and preferentially deplete CCR5-expressing CD4+ T cells (84).
Thus, severe acute CD4+ T-cell depletion in GALT is not specific to pathogenic infection, nor is it predictive of the virulence of a retroviral infection, as shown by Pandrea et al., who proposed that the magnitude of the CD4+ T-cell restoration was a better predictor of disease progression (84). This conclusion was also supported by studies in rhesus macaques (6). Interestingly, when cross-species infections of rhesus macaques with SIVsmm (47) and SIVagm (48) were performed, they resulted in pathogenic and controlled infections, respectively. Yet, despite these completely opposite outcomes, in both instances, a severe mucosal CD4+ T-cell depletion occurred during acute and early chronic infection (47, 48) (Figure 1). However, later on in the follow-up, a nearly complete restoration to baseline levels was observed in SIVagm-infected rhesus macaques (48), similarly to long-term nonprogressors (135), while SIVsmm-infected rhesus macaques experienced a progressive loss of intestinal CD4+ T cells (47) (Figure 1). The SIVsmm-infected rhesus macaques eventually progressed to AIDS (47), and were classified as slow progressors, in comparison to rapid progressors (86, 136) and normal progressors (21, 33). In Figure 1, the rapidly progressive and normal progressive SIV infections are illustrated by the dynamics observed in SIVagm-infected pigtailed macaques and SIVmac-infected rhesus macaques, respectively. Note that such different patterns of disease progression can be observed in multiple species.
The presentation of SIV infection in natural hosts is intermediate between the two extreme patterns described above (i.e., pathogenic and controlled infections), consisting of a massive intestinal CD4+ T-cell depletion during acute infection followed by a partial CD4+ T-cell restoration during chronic SIV infection (Figure 1). This pattern is characteristic to SIVsmm infection of sooty mangabeys, SIVagm infection of African green monkeys and patas monkeys, and SIVmnd infection of mandrills (47, 84, 126, 137, 138). A relatively limited impact of the SIV infection on the mucosal CD4+ T cells can also be observed in a subset of animals with pathogenic infections, the long-term nonprogressors which restore intestinal CD4+ T lymphocytes and CCR5+ memory T cells to higher values than normal progressors (97, 139), as long as viral replication is limited (Figure 1).
The relatively robust mucosal CD4+ T-cell restoration occurs in natural hosts of SIV in the context of the control of chronic T-cell activation and inflammation. Thus, while T-cell activation and inflammation transiently increase during acute SIV infection, immune activation and inflammation are resolved during the transition between acute and chronic SIV infection, in spite of a relatively sustained, robust viral replication (47, 84, 123, 126, 140). This supports a paradigm in which acute CD4+ T-cell depletion is driven in natural hosts of SIV by both viral replication and increased inflammation and immune activation, while partial recovery of intestinal CD4+ T cells during chronic infection is enabled by the control of immune activation and inflammation, with the remaining mucosal CD4+ T-cell loss being due to the persistent viral replication.
Two important lessons can be drawn from nonpathogenic and controlled SIV infections. First, nonpathogenic SIV infections highlight that a moderate mucosal CD4+ T-cell depletion has no discernible pathogenic consequences if immune activation and inflammation are kept at bay. Second, when immune activation, inflammation and viral replication are entirely contained, such as in the controlled SIV infections, total recovery of intestinal CD4+ T cells is achievable, although it might take years to reach the preinfection levels (48) (Figure 1).
In the natural hosts, the control of the deleterious consequences of SIV infection (which include a moderate chronic CD4+ T-cell depletion) resulted from multiple host adaptations that occurred over millions of years of host coevolution with their species-specific viruses (141). One of the keys to this exquisite control of the deleterious consequences of SIV infection in natural hosts of SIVs is the maintenance of the epithelial gut integrity via enhanced repair mechanisms (142, 143) and the absence of consequent microbial translocation, which is the main trigger of chronic T-cell activation in pathogenic infections (144). Some of the other host adaptations to elude SIV pathogenicity involve protection from CD4+ T-cell depletion, either by preserving the pool of precursors, or by limiting the number of target cells. Interestingly, species which are natural SIV hosts usually present a reduced expression of CCR5 on circulating and mucosal CD4+ T cells (41). It has also been reported that Tcm from sooty mangabeys were less frequently infected, potentially due to their lower CCR5 expression (52). By sparing Tcm precursors, as well as Tscm, sooty mangabeys might preserve their capacity to restore the pool of intestinal CD4+ T cells (5, 52). Furthermore, lower levels of immune activation and apoptosis of the CD4+ T cells from the LNs and circulation may help protect the immune system of the natural SIV hosts from the immune exhaustion described in the late-stage diseases of pathogenic HIV/SIV infections (92, 140, 145). This might be partly due to difference in the dynamics of type 1 interferons. Type 1 interferons are beneficial in the control of SIV infection during acute infection (146, 147), but persistent, dysregulated production is known to contribute to immune activation (147, 148), to induce the expression of proapoptotic markers in uninfected cells (149), and to be associated with disease progression (146). Interestingly, during chronic SIV infection, type 1 interferon response returns to preinfection levels in natural SIV hosts, while it remains elevated during pathogenic infections (62, 150, 151). Thus, the early control of type 1 interferon production in natural SV hosts might also play a role in preventing disease progression, by limiting immune activation and apoptosis of nearby uninfected CD4+ T cells.
Additionally, limited CD4+ T cell proliferation was described in AGMs, sooty mangabeys, and mandrills, notably among Tcm, with limited to no increase in proliferating CD4+ T cells after acute infection (137, 152–156). Additionally, during SIV infection, CCR5 expression is not upregulated on memory CD4+ T cells in sooty mangabeys, limiting new rounds of infection (52). By limiting bystander apoptosis, controlling cell proliferation after acute infection, and by limiting upregulation of CCR5 expression on the surface of the CD4+ T cells, natural hosts of SIVs limit the production of new susceptible cells which might slow down the pace of CD4+ T cell destruction.
Another consequence of the limited expression of CCR5 on the surface of target cells at the mucosal sites is the reduction in the virus ability to initiate mucosal infection (157). Limited expression of CCR5 by the CD4+ T cells in the GI mucosa may also significantly impact the rates of maternal-to-infant transmission. CCR5 expression on the CD4+ T cells is extremely low at birth and increases with age in both pathogenic and nonpathogenic hosts (156). However, this increase is delayed in natural hosts of SIVs, and memory CD4+ T cells from the newborns express lower percentages of CCR5+ compared to non-natural SIV hosts, which creates the premise for a reduced rate of maternal-to-infant transmission rates of SIV in natural hosts (about 5%, compared to 20-25% in HIV-1, prior to antiretroviral therapy) (9, 158).
Another significant particularity of several African NHP species is their ability to downregulate CD4 receptor expression at the surface of their CD4+ T cells when they enter in the memory pool, rendering them resistant to SIV infection (137, 159, 160).
Through these mechanisms, natural hosts of SIVs spare specific CD4+ T-cell subsets, which could contribute to the control of inflammation and maintenance of gut integrity, despite high viral replication during chronic nonpathogenic infections. Multiple immune cell populations are involved in these processes. As an anti-inflammatory milieu, notably containing TGFβ, is rapidly established, this enhances Treg production, thus preventing the chronic immune activation (87). Furthermore, Th17 cells are spared in both gut and blood of SIVsmm-infected SMs and SIVagm-infected AGMs (55, 62, 104). The Th17/Treg ratio remains stable during SIV infection in natural SIV hosts, while it correlates with disease progression in pathogenic infections (62). Similarly, Th17 cells, as well as β7hi CD4+ T cells, are maintained in the blood and in the colon of HIV-1 long-term nonprogressors (135). Moreover, the CD4neg CD8αdim T cells and the CD4neg CD8neg (DN) T cells are able to retain some of the helper T cells functions in the African NHPs that are natural hosts of SIV (34, 160, 161).
The loss of CD4+ T cells is caused by different intertwined mechanisms (162). Viral replication significantly contributes at least to the initial CD4+ T cell loss, which occurs rapidly in infected individuals and animal models during the acute stage of infection and mirror that of the dynamics of viral replication. Several mechanisms of cell death are directly induced by the infection of those cells by the virus: (i) cytolysis due to increased permeability of cell membrane after viral budding and/or syncytium formation (163), (ii) targeting by HIV/SIV-specific cytotoxic T lymphocytes (164, 165), and (iii) programmed cell death of cells undergoing productive infection, due to caspase-3 and/or Bax activation (166–168) (Figure 2). Antibody-dependent or complement-mediated mechanisms are also involved in the destruction of HIV/SIV-infected cells [antibody-dependent cellular cytotoxicity (ADCC) (169), antibody-dependent phagocytosis (170), complement-mediated phagocytosis and lysis (171)], although escape mechanisms have been described for HIV and SIV (172–174).
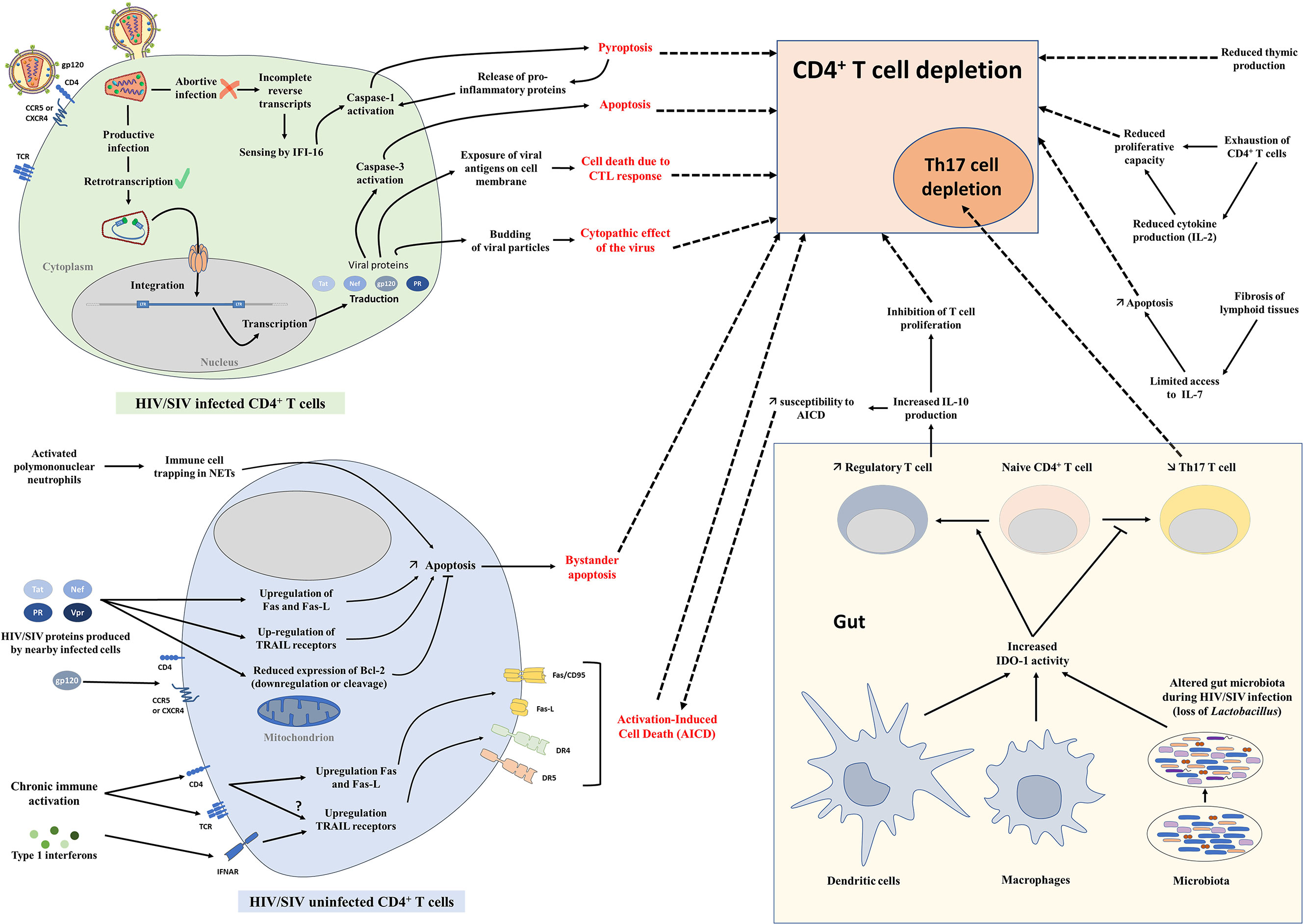
Figure 2 Mechanisms of CD4+ T-cell depletion. Schematic representation of different mechanisms involved in CD4+ T-cell depletion during HIV and SIV infections. AICD, Activation-induced cell death; CTL, Cytotoxic T lymphocytes; IDO-1, Indoleamine 2,3-dioxygenase 1; NETs, Neutrophil extracellular traps.
There are several lines of evidence to support this direct impact of viral replication on CD4+ T-cell depletion. First, there is a clear temporal association between viral loads and CD4+ T-cell depletion, with the most prominent depletion in the gut closely following the peak of viral replication, which occurs circa one to two weeks postinfection (33, 83, 175) (Figure 1). Moreover, there is a clear correlation between the levels of viral replication during acute infection and the magnitude of the CD4+ T-cell depletion, particularly in the gut (94). Studies have shown that mucosal depletion is minimal if peak viral loads are below 106 vRNA copies/ml of plasma (48, 137). Furthermore, despite their exquisite ability to finely tune inflammation and T-cell immune activation, NHP species that are natural hosts of SIVs also experience a residual CD4+ T-cell depletion during chronic infection when inflammation and immune activation are controlled (47, 84), highlighting the role of viral replication in the persistence of intestinal CD4+ T-cell depletion.
Despite this proven impact of viral replication on CD4+ T cells at every stage of HIV/SIV infection, the extent of CD4+ T-cell loss during the acute infection far exceeds the number of infected lymphocytes (32, 94). Multiple mechanisms have been proposed to explain this excess of CD4+ T-cell depletion in HIV infection and pathogenic SIV infections (Figure 2): (i) Bystander apoptosis (140, 176), due to viral proteins promoting apoptosis of nearby cells, notably HIV-1 gp120 after its interactions with CD4 and CCR5 or CXCR4 coreceptor (177, 178),; note that in natural hosts of SIVs the levels of bystander apoptosis are kept at bay (84, 140, 145); (ii) Activation-Induced Cell Death (AICD) due to immune activation which induces FasL production and Fas (CD95) expression in nearby, uninfected CD4+ T cells, shortening their lifespan and increasing their sensibility to AICD (179–182); interestingly, plasma FasL expression does not significantly increase in animals with nonpathogenic SIV infections (148, 183, 184) (iii) Abortive infection leading to pyroptosis through the caspase-1 pathway, due to an accumulation of incomplete reverse transcripts and induction of antiviral and inflammatory responses (185); (iv) Trapping of immune cells in neutrophil extracellular traps (NETs) induced by SIV infection, followed by an induction of apoptosis or lysis of those trapped CD4+ T cells, as recently described (186) (Figure 2).
In addition to these general mechanisms involved in total CD4+ T-cell depletion, preferential depletion of Th17 cells could be partly due to the induction of indoleamine 2,3-dioxygenase (IDO-1), caused by sustained microbial translocation and immune activation in pathogenic infections (110, 187) (Figure 2). Catabolites produced by the degradation of tryptophan by IDO-1 enhance Treg and deplete Th17 cells (110). As adaptive Tregs can produce IL-10 that inhibits T cell proliferation (188) and increases susceptibility to AICD (180, 189), accumulation of Tregs during chronic HIV/SIV infection could also exacerbate CD4+ T-cell depletion.
Furthermore, in late stages of HIV/SIV infection, immune exhaustion plays a role in total CD4+ T-cell depletion. During chronic infection, increased expression of PD-1 and other immune check-point inhibitors is observed on CD8+ T cells, but also on CD4+ T cells. Exhausted HIV/SIV-specific CD4+ T cells, which are associated with high plasma viremia, have a decreased proliferative capacity and reduced polyfunctional cytokine response, including decreased production of IL-2 (190–192). This fuels gradual CD4+ T-cell depletion, in combination with the reduced production of naïve T cells by the thymus (193) and TGF-β-driven fibrosis of lymphoid tissues (194, 195) that are observed during HIV and SIV infections (Figure 2).
The consequences of CD4+ T-cell depletion have been widely scrutinized, highlighting the critical roles of this cell subset for disease progression and development of comorbidities during pathogenic infections (4, 196–198). The first observation made in patients and NHPs with low peripheral CD4+ T-cell counts (<200/mm3) was their extreme susceptibility to opportunistic infections (notably fungal infections including Pneumocystis jirovecii pneumonia, mycobacterial infections, and cytomegalovirus disease) (199, 200). In addition, PWH with a lower nadir of CD4+ T-cell count are also at higher risk of developing AIDS-defining cancers (non-Hodgkin lymphoma, cervical cancer, and Kaposi sarcoma) (201, 202).
However, it was reported that the profound, but transient, CD4+ T-cell depletion observed during acute nonpathogenic SIV infections and the residual mucosal CD4+ T-cell depletion persisting during chronic nonpathogenic SIV infections were not sufficient to trigger disease progression (203). As such, a new paradigm emerged in which the combination of CD4+ T-cell depletion (notably Th17 cells), inflammation and immune activation in the GI tract drive the deleterious consequences of HIV infection. During HIV/SIV infection, CD4+ T cells but also myeloid cells are killed, releasing inflammatory cytokines (204, 205), including IL-1β, thus creating an inflammatory environment (185, 206). Combined with the loss of IL-17 and IL-22-producing cells that are involved in epithelial integrity maintenance and homeostasis, as well as in antimicrobial defense (25, 104, 207), this leads to damage of the gut epithelial integrity, enteropathy and microbial translocation (83, 93, 144). The role of impaired epithelial integrity in driving microbial translocation was confirmed by the demonstration of the leakage of microbial products occurring near breaks in the epithelial lining (144). Microbial translocation can be detected in mucosal tissues (lamina propria, gut-associated lymphoid tissue, mesenteric lymph nodes), but also in distant lymph nodes and circulation (144, 208, 209). These microbial products fuel local and systemic inflammation, and macrophage activation (144, 210). Sustained inflammation and immune activation trigger a vicious cycle by attracting new CD4+ T cells, increasing the number of susceptible cells, and by reactivating proviruses in latently-infected cells (206). Newly produced viral proteins and viruses can in turn boost inflammation, tissue damage, and microbial translocation.
The importance of the maintenance of the integrity of the intestinal epithelium was demonstrated recently (209). DSS-induced colitis in SIV-infected AGMs disrupted the intestinal epithelium integrity, recapitulating the characteristics of a pathogenic SIV infection, i.e. increased local inflammation and immune activation, detection of microbial products in lymphoid tissues and increased viral replication (209). Meanwhile, in the inflammatory bowel diseases (IBD), mucosal inflammation is associated with loss of intestinal epithelial integrity and massive infiltration of immune cells, including T cells, in the lamina propria. In response to their exposure to microbial antigens, these T cells produce inflammatory cytokines (IFNγ, TNFα) which disrupt tight-junctions function and worsen intestinal epithelial integrity. However, unlike during HIV/SIV infection, local inflammation does not lead to CD4+ T-cell depletion in patients with IBD; on the contrary, most IBD patients present with increased numbers of intestinal CD4+ T cells, including Th17 cells (211). As such, comparison with IBD demonstrates that inflammation per se, in the absence of the viral trigger, can damage the gut integrity, but it is not sufficient to deplete intestinal CD4+ T cells. However, in the context of HIV/SIV infection, inflammation drives T-cell activation (209) and eventually leads to T cell loss through increased viral replication and/or activation-induced cell death (AICD). It is possible that the persistent expression of high levels of type 1 interferons during chronic, pathogenic HIV/SIV infections play a role in this T cell loss, as type 1 interferons are known to induce AICD. Conversely, treatment with type 1 interferons had been evaluated in IBD (212), due to their ability to inhibit Th17 cell differentiation (213).
Chronic inflammation has been linked to numerous non-AIDS comorbidities, notably cardiovascular diseases, liver fibrosis and thromboembolism (214–216). Inflammation and immune activation also promote a procoagulant state in infected animals (217), and they are positively correlated with disease progression (217).
Assessment of the extent of CD4+ T-cell restoration in the GI tract that can be expected in patients initiating ART during acute or chronic HIV infection is complex, as most studies focused on the total CD4+ T cell counts and only few investigated specific CD4+ T-cell subsets, such as memory or Th17 cells. Furthermore, the replenishment of mucosal CD4+ T cells can take time, requiring long follow-up of PWH or NHP.
However, there is a general consensus in the field that the efficacy of the CD4+ T-cell restoration on ART vastly depends on the stage of the infection and the degree of immunosuppression at the time of treatment initiation. Guadalupe et al., reported that when ART was initiated at 6 weeks post-HIV infection and was maintained for 14 months, the levels of mucosal CD4+ T cells were close to values observed in uninfected individuals (97). Further studies have found that, when ART was initiated in the first weeks postinfection, and viral replication was suppressed in plasma and decreased by 1,000-fold in the GALT, a significant, albeit incomplete, restoration of mucosal CD4+ T cells was observed in all humans and macaques (88, 99, 218) (Figure 3). In rhesus macaques in which ART was initiated prior to the acute mucosal CD4+ T-cell depletion (i.e., 7 days post-SIVmac251 infection), ART failed to prevent CD4+ T-cell depletion in the GALT, but enabled a virtually complete CD4+ T-cell restoration by 6 months postinfection, particularly through a significant increase in the Tcm levels (88). Meanwhile, while early ART initiation at 3 to 4 days postinfection did not prevent the establishment of the SIV reservoir in lymph nodes (222), it prevented Th17 depletion in the lymphoid tissues (61). Similarly, early treatment of acutely HIV-infected individuals (Fiebig stage I or II) could not halt mucosal CD4+ T-cell depletion in the first weeks post-treatment but generated a strong restoration of CD4+ T cells in the lamina propria at 96 weeks post-treatment (99) (Figure 3).
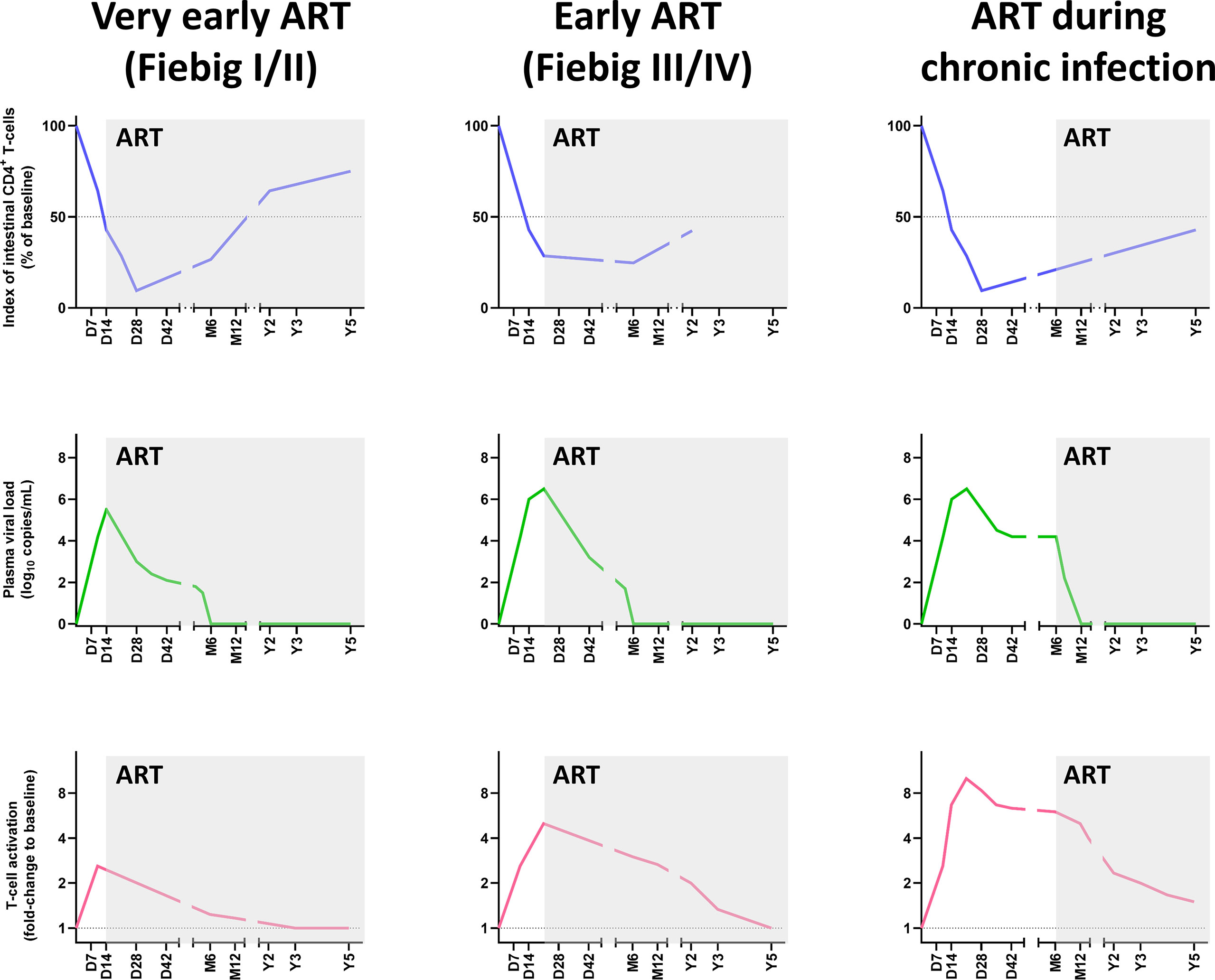
Figure 3 Comparative dynamics of intestinal CD4+ T-cell depletion, plasma viral load and immune activation/inflammation in treated HIV infections, according to the timing of initiation of antiretroviral therapy ART. Schematic representation of intestinal CD4+ T-cell depletion, viral replication and immune activation is inferred from data reported in references (85, 90, 91, 99, 219–221). Longitudinal data are presented, with the X axis representing days (D), months (M) and years (Y) postinfection. The Y axis illustrates the magnitude of mucosal CD4+ T-cell depletion (upper panels), viral replication (middle panels), and the levels of immune activation/inflammation (lower panels). Intestinal CD4+ T-cell depletion is illustrated as the index of CD4+ T cells (i.e., percentage of CD4+ T cell fraction within the CD3+ T cell population, divided by this percentage at baseline). Viral replication is represented as plasma viral loads. From the plethora of biomarkers of immune activation/inflammation, we selected the fold-change of HLA-DR+ CD38+ CD8+ T cells in persons living with HIV, compared to uninfected individuals. ART, Antiretroviral therapy.
Meanwhile, most data on patients which initiated ART during chronic HIV/SIV infection suggest a modest CD4+ T-cell restoration in the GI tract (97, 98, 219), at least when considering the relative CD4+ T cell counts (223). Finally, in patients in which ART was initiated during the AIDS stage, the immune restoration was minimal and occurred very slowly (224) (Figure 3).
In patients on ART, a more robust restoration of mucosal CD4+ T cells was observed in patients with higher frequency of Tcm in the lamina propria of the jejunum, suggesting that the maintenance and/or the restoration of this subset is critical for an important restoration of intestinal CD4+ T cells (219). Furthermore, Th17 cells were also restored in patients receiving ART, especially in those in which therapy was initiated very early in infection (85). However, Th17/Treg ratio remained reduced, as Treg cell counts in lymph nodes and in GALT did not return to baseline levels in PWH receiving ART (60, 61), which might be due to the residual viral replication and immune activation in the GALT of those patients (219).
Overall, as a near-total restoration of mucosal CD4+ T cells is observed only in early ART-treated patients, this is a strong incentive for a generalization of early antiretroviral treatment in all PWH.
Important insight on the impact of CD4+ T-cell depletion on HIV pathogenesis has been gained by directly depleting the CD4+ T cells with monoclonal antibodies, or by using knock-out models in different animal species, as well as through the study of genetic diseases in humans.
The first studies on CD4+ cell depletion in SIV-infected and SIV-uninfected NHP, performed with anti-CD4 monoclonal antibodies, were published over a decade ago (225–228), and reported that, despite an increased percentage of proliferating (Ki-67+) CD4+ T cells, reconstitution of CD4+ T cell population was slower than what was reported for CD8+ T cells in CD8-depleted animals, regardless of SIV infection status (226, 227). Interestingly, CD4+ T-cell restoration postexperimental depletion did not differ between natural and non-natural SIV hosts (227), reinforcing the previous finding that the higher restoration of intestinal CD4+ T cells in natural hosts of SIV was not due to a higher cell proliferation.
In CD4-depleted, SIV-infected NHPs, plasma viral loads decreased, in relation with the low number of CD4+ T cells (225). However, when CD4+ T-cell depletion was induced prior to SIV inoculation, this led to persisting high plasma viral loads in CD4+ T-cell-depleted monkeys, with no postpeak decline of viremia, and accelerated disease progression (229, 230).
Interestingly, microbial translocation was not increased in SIV-uninfected CD4-depleted animals (227), and CD4+ T-cell depletion was not sufficient to reactivate viral replication in CD4-depleted, ART-treated NHPs (228). This limited clinical impact of experimentally-induced CD4+ T-cell depletion might be explained by the limited CD4+ T-cell depletion at the mucosal effector sites, notably in the GI tract (<50%), in those studies during which anti-CD4 monoclonal antibodies were administered over a short period of time.
Since Treg are usually accumulating throughout chronic HIV/SIV infection, are frequently infected and suppress HIV/SIV-specific cytotoxic T cell responses (231), different Treg-specific depletion strategies, targeting either CD25 (IL-2 receptor subunit) (232–234) or CCR4 (234, 235), have been investigated. Despite achieving only partial Treg depletion with maximal effect in blood and lymph nodes, and minimal depletion in GI tract, this usually led to increased SIV-specific T cell responses (232, 234), and increased immune activation (232–234). Another strategy aimed at blocking CTLA-4 also resulted in increased SIV-specific T cell responses (236). Viral reactivation occurred in most NHPs in which Treg functions were blocked by anti-CTLA-4 monoclonal antibodies, or in which Treg were depleted (232–234, 237, 238). Higher viral loads in mucosal tissues and greater loss of CCR5+ CD4+ T cells in the rectal mucosa have been reported to occur in the NHPs receiving an anti-CTLA-4 blocking monoclonal antibody (237).
Numerous CD4 knock-out mice models have been developed (239–241). In these mice, the TCRαβ+ γδ- CD4neg CD8neg (double negative, or DN) T-cell subset is expanded (239). The TCR repertoire of those DN T cells is more polyclonal than in wild-type mice, and these cells are able to maintain part of the helper T cell functions, similarly to natural hosts of SIV (240, 241). However, it was reported that memory cytotoxic CD8+ T lymphocytes could be reduced in CD4-deficient mice (242).
In the late 1980s, a severe lymphopenia, preferentially impacting CD4+ T cells, was identified in HIV-uninfected patients with no other condition or treatment known to induce lymphocytopenia (243). This condition was termed idiopathic CD4 lymphopenia (ICL) (244). This disease is rare, with less than 0.5% of blood donors in the United States meeting the definition criteria (245, 246). Due to their low levels of circulating CD4+ T cells, ICL patients develop opportunistic infections, some similar to AIDS patients, notably fungal, nontuberculosis mycobacterial and HPV-associated infections (247, 248). A recent work suggests that ICL could have an autoimmune component linked to the production of auto-antibodies directed against CD4+ T lymphocytes (249). In some cases, genetic mutations have also been linked to ICL (250).
In addition to CD4+ T-cell lymphopenia, an increase in circulating Treg was observed and, in some patients, decreases in CD8+ T cells and/or CD19+ B cells and/or NK cell counts were also reported (247, 248). Furthermore, CD4+ T cells are more activated and proliferating in ICL patients than in controls (247, 251). No specific depletion of CD4+ T- cell subsets (Th1, Th2, Th17) was observed in peripheral blood, but a reduction of the percentage of naïve CD4+ T cells was seen, compared to controls and PWH (251). Monitoring the CD4+ T-cell counts in the mucosal tissues of ICL patients also identified a profound CD4+ T-cell loss, although less severe than in PWH, as only a 3-fold reduction in the number of intestinal CD4+ T cells was observed (252). This CD4+ T-cell loss did not affect the functionality of mucosal Th1 and Th17 cells (252). While an initial study on 10 ICL patients reported a slight increase in microbial translocation (251), a more recent study of 46 ICL patients found normal levels of LPS, and only slight increases of sCD14 (252). We can hypothesize that, similarly to natural hosts of SIV, despite CD4+ T cell loss, the maintained functionality of remaining Th17 cells and/or other IL-17 producing cells such as mucosa-associated immune T cells (MAIT) might be sufficient to preserve gut epithelial integrity and limit microbial translocation in ICL patients (253).
Genetic mutations leading to absolute CD4+ T-cell depletion have been reported in two patients, one 22-year-old female with a mutation in the translation initiation codon of the CD4 gene (254) and a 45-year-old female with a mutation in a splice acceptor site leading to the expression of a CD4 protein lacking its anchoring domain to the cellular membrane (255). In the first case, neither CD4 expression on cell membrane, nor soluble CD4 were detected, whereas for the second patient, only CD4 expression on cell membrane was abrogated, while soluble CD4 could still be detected in plasma (254, 255). The first patient was hospitalized for severe viral respiratory infection which led to the discovery of her primary immunodeficiency. Both patients presented numerous HPV-associated warts. However, in both cases, immunodeficiencies were only detected when patients were adults, later than most other primary cellular immunodeficiencies. Interestingly, in both cases, it was shown that helper T cell functions could be performed by DN T cells and/or CD8+ T cells (254, 255), similarly to what has been described in in natural hosts of SIV (160, 161) and in CD4 knock-out models in mice (240, 241). The DN T-cell subset was also expanded, similarly to CD4 KO mice. These two reports illustrate that this rescue mechanism can also be found in humans. This absence of CD4+ T cells has also been reported in one patas monkey with near-complete loss of peripheral and mucosal CD4+ T cells, which protected it from productive SIV infection when intravenously-inoculated with SIVsab (137).
Patients with hyper-IgE syndromes present with elevated IgE serum levels, decreased Th17 cells, and higher susceptibility to Staphylococcus aureus pulmonary, skin infections and Candida infections (256, 257). Multiple genetic mutations have been associated with this syndrome, and patients with DOCK8 mutation also present with HPV-associated warts, cutaneous manifestations of Molluscum contagiosum and/or Herpes simplex virus infections (258, 259). These studies highlight the importance of Th17 cells in the protection of the organism from bacterial and fungal infections, notably through the maintenance of the integrity of the intestinal barrier, as also emphasized by the increased microbial translocation observed during HIV and SIV pathogenic infections in which Th17 cells are depleted and the intestinal barrier is damaged, with visible breaches in the intestinal epithelium.
The most effective treatment currently available for preventing or limiting CD4+ T-cell depletion is the early initiation of ART, ideally during Fiebig stages I or II. This is the only treatment which has proved a high efficacy in restoring intestinal CD4+ T cell in PWH and can have additional positive impact on limiting size of viral reservoirs (Figure 3). However, as there is persistent immune activation in PWH on ART, which could cause a limited CD4+ T cell loss, early ART might not be sufficient to entirely restore mucosal CD4+ T cells in all patients. It should also be acknowledged that, even when viral replication is suppressed, restoration can take time, as evidenced in elite controllers SIVagm-infected RMs, in which complete recovery of intestinal CD4+ T cells was only observed after 4 years of absence of viral replication in plasma and tissues (48) (Figure 1). Thus, complete recovery of intestinal CD4+ T cells in PWH could take even longer, especially if the treatment could not be initiated early in the infection.
As the crucial role of IL-17 and IL-22 producing T cells in preserving the mucosal integrity emerged recently, it has been hypothesized that treatments aiming at maintaining or restoring those cell subsets could limit the deleterious impact of CD4+ T-cell depletion in HIV/SIV-infected NHPs, i.e., microbial translocation, inflammation, and immune activation. As pointed out, a long follow-up of PWH receiving those treatments will probably be necessary before being able to definitively rule on their efficacy.
IL-21 has been described to enhance several immune functions, including long-term maintenance of CD8+ T cells, differentiation of memory B cells and differentiation of naïve CD4+ T cells into Th17 cells (108, 260–262). Several studies have explored its potential to limit Th17 depletion in SIV-infected NHPs. In a preliminary study, Micci et al., observed that, after 5 weekly doses of recombinant IL-21, the frequency of circulating Th17 cells increased in chronically SIVmac-infected macaques (108). Paiardini et al., confirmed these findings in a subsequent study in rhesus macaques treated with IL-21 between weeks 2 and 6 postinfection (263). They observed no difference with respect to the total CD4+ T cell counts in circulation, lymph nodes and the GI tract, but intestinal Th17 cells were maintained at week 6 postinfection in IL-21-treated macaques, while a severe depletion was observed in controls (263). This preservation of the Th17 cell subset was associated with lower intestinal inflammation and microbial translocation, as expected (263). Unfortunately, this protective effect on Th17 depletion faded away and intestinal Th17 cell loss was similar in both groups 23 weeks postinfection (263). Similarly, in ART-treated SIV-infected macaques, treatment with IL-21 did not enhance total CD4+ T-cell restoration in the circulation, lymph nodes and GI tract, but both intestinal Th17 and IL-22- producing CD4+ T cells were restored to near-baseline levels (264). Th17 cells were more frequently polyfunctional in IL-21-treated macaques, and this effect was more robust in jejunum than in rectal biopsies (265). This was sufficient to limit neutrophil infiltration in intestinal tissues, as well as T cell activation and proliferation. However, these positive effects were also blunted over time (264, 265). Conversely, one recent study reported a reduction in immune activation and T-cell exhaustion in IL-21 treated rhesus macaques, but did not see any impact on Th17 CD4+ T cells (266).
IL-7 was among the first cytokines investigated, as it was shown to boost CD4+ T-cell regeneration (267–269). In SIV-infected rhesus macaques on ART, rsIL-7 induced a transient increase in CD4+ and CD8+ T-cell counts (270). Similarly, in virologically-suppressed PWH on ART, rhIL-7 increased CD4+ T-cell counts in circulation and in the gut (268, 271, 272). Although transient viral reactivations were detected, mainly in patients receiving high rhIL-7 doses (268, 271), and a slight increase in viral reservoir was reported (273), those preliminary results were promising for restoring T cells but clinical trials investigating IL-7 were interrupted due to the appearance of neutralizing antibodies in IL-7-treated patients and production issues.
Metabolites generated by the catabolism of tryptophan by IDO-1 (kynurenine pathway) can lead to an increase in the number of Treg while depleting Th17 CD4+ T cells (110), Specific IDO-1 inhibitors have been used in oncology, but none has been tested in PWH and only one in SIV-infected RMs (274, 275). Until now, in the HIV/SIV field, in order to reduce IDO-1 expression, most studies focused on altering gut microbiota. A recent study by Vujkovic-Cvijn and colleagues showed that dysbiosis caused by acute SIV infection, notably loss of Lactobacillus spp, increased IDO-1 activity and was correlated with Th17 depletion in peripheral blood (187). Interestingly, enhanced IDO-1 activity due to SIV infection could be thwarted by supplementing SIV-infected macaques with Lactobacillus (187). The addition of IL-21 did not further lower IDO-1 activity (187, 265). However, the beneficial effect of those probiotic treatments on Th17 cell restoration still has to be demonstrated.
Alterations of intestinal microbiota in PWH and in SIV-infected NHPs have been extensively described (276). In pigtailed macaques (PTM), prebiotics/probiotics improved intestinal CD4+ T cell counts, enhanced functionality of colonic Th17 and Th1 CD4+ T cells, but did not prevent systemic microbial translocation as shown by the presence of microbial products in peripheral lymph nodes (277). Clinical trials have suggested a potential beneficial effect of probiotics on circulating CD4+ T-cell counts or intestinal Th17 cells (278–280). However, the varying compositions of probiotic supplements hindered comparisons between studies, and most of these studies were underpowered due to a low number of included patients. Moreover, other confounding factors complicated the evaluation of those strategies: both HIV-1 and LPS induce IDO-1 expression (112, 281), and thus ART itself could reduce IDO-1 activity in PWH (282).
Another inhibitor of the kynurenine pathway has been recently evaluated in NHPs, a kynurenine 3-monooxygenase inhibitor which increased circulating CD4+ T cells but failed to increase intestinal Th17 cell restoration and to prevent microbial translocation (283). Recently, one work reported increased Th17 and Th22 populations among circulating CD4+ T cells in ART-treated, SIV-infected rhesus macaques that received a fecal microbial transplantation (284). This restoration of Th17 and Th22 subsets in the blood needs to be confirmed in intestinal tissues in further studies.
Other strategies have been suggested. One of them consists of targeting CD4+ T cells expressing α4β7 integrin, which is a gut-homing signal (285), using an anti-α4β7 monoclonal antibody, to reduce the number of susceptible cells in mucosal tissues and preserve CD4+ T cells in the GALT (286). In preliminary works, SIV-infected NHPs receiving anti-α4β7 monoclonal antibody had higher CD4+ T cell counts than controls in both peripheral blood and in intestinal tissues (286, 287). However, in PWH, despite a slight increase in the circulating CD4+ T cell counts at 10 weeks postinfusion, this increase was not sustained (288). Furthermore, even though gut homing was limited, treatment with anti-α4β7 monoclonal antibody did not prevent HIV or SIV infection, viral reservoir seeding, nor it delayed viral rebound post-treatment interruption (288, 289). Recently, an anti-caspase inhibitor administered to RMs in the first days following SIVmac infection has been shown to reduce T cell death and maintenance of CD4/CD8 T cell ratios (290). Furthermore, memory CD4+ T cells were preserved after the early administration of this inhibitor (290).
In his literary masterpiece “The Restaurant at the End of the Universe”, Douglas Adams states that “It is a curious fact, and one to which no-one knows quite how much importance to attach, that something like 85 percent of all known worlds in the Galaxy, be they primitive or highly advanced, have invented a drink called jynnan tonyx, or gee-N’N-T’N-ix, or jinond-o-nicks, [ … ] ‘chinanto/mnigs,’ [ … ] ‘tzjin-anthony-ks’”. Similarly, acute mucosal CD4+ T-cell depletion is a common feature of all HIV and SIV infections, be they pathogenic, nonpathogenic, or controlled. However, as clearly demonstrated by the data presented here, acute CD4+ T-cell depletion is only the spark that can ignite the wildfire in the woods, while chronic inflammation and immune activation that lead to comorbidities and disease progression, and the ability of the host to manage these features associated with HIV/SIV infection, are driving the prognosis.
The natural hosts of SIV seem to be also a good example of convergent evolution to develop strategies to thwart retroviral infections. These NHP species are able to constrain this fire to a limited timing by: (i) spacing the trees, i.e. limiting the number of target cells by having a reduced number of CD4+ T cells expressing CCR5 and/or down-regulating CCR5 expression when entering the memory pool, (ii) limiting the propagation of fire to unburnt trees, i.e. hampering by-stander apoptosis that is the main driver of cell death in HIV/SIV infections, (iii) preserving specific trees that protects the soil, i.e., Th17 cells that are crucial in the maintenance of gut integrity and protecting from bacterial and fungal infections, or trees that will help the regrowth of the forest, i.e. sparing Tcm cells, that have a higher expansion potential, and (iv) growing fire-resistant trees that are able to maintain wild-life in the absence of the other trees, i.e. CD3+ CD4neg CD8neg T cells that exhibit some of the helper T cell functions and that are frequent in most natural hosts of SIV.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
QLH, IP, and CA designed the manuscript and contributed to drafting. QLH, IS, AL, IP, and CA drafted and revised the manuscript. QLH, IP, and CA prepared the figures. QLH, IS, AL, IP, and CA edited the manuscript. All authors contributed to the article and approved the submitted version.
QLH, IP, and CA are supported by grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases/National Heart, Lung and Blood Institute/National Institute of Allergy and Infectious Diseases: R01DK113919 (IP/CA), R01DK119936 (CA), R01 AI119346 (CA), RO1 HL117715 (IP), R01 HL123096 (IP). The work of IS was supported by the intramural research program of NIAID/NIH. ALL is supported by UM1-AI106701 AIDS Clinical Trials Group Immunology Support Laboratory. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Adam Kleinman for helpful discussion and critical reading of the manuscript.
1. Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, Wolf RA, et al. Pneumocystis Carinii Pneumonia and Mucosal Candidiasis in Previously Healthy Homosexual Men: Evidence of a New Acquired Cellular Immunodeficiency. N Engl J Med (1981) 305:1425–31. doi: 10.1056/NEJM198112103052401
2. Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) Antigen Is an Essential Component of the Receptor for the AIDS Retrovirus. Nature (1984) 312:763–7. doi: 10.1038/312763a0
3. Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, et al. T-Lymphocyte T4 Molecule Behaves as the Receptor for Human Retrovirus LAV. Nature (1984) 312:767–8. doi: 10.1038/312767a0
4. Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, et al. The Prognostic Value of Cellular and Serologic Markers in Infection With Human Immunodeficiency Virus Type 1. N Engl J Med (1990) 322:166–72. doi: 10.1056/NEJM199001183220305
5. Cartwright EK, McGary CS, Cervasi B, Micci L, Lawson B, Elliott ST, et al. Divergent CD4+ T Memory Stem Cell Dynamics in Pathogenic and Nonpathogenic Simian Immunodeficiency Virus Infections. J Immunol (2014) 192:4666–73. doi: 10.4049/jimmunol.1303193
6. Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, Rohankhedkar M, et al. Progressive CD4+ Central Memory T Cell Decline Results in CD4+ Effector Memory Insufficiency and Overt Disease in Chronic SIV Infection. J Exp Med (2007) 204:2171–85. doi: 10.1084/jem.20070567
7. Prendergast A, Prado JG, Kang YH, Chen F, Riddell LA, Luzzi G, et al. HIV-1 Infection is Characterized by Profound Depletion of CD161+ Th17 Cells and Gradual Decline in Regulatory T Cells. AIDS (2010) 24:491–502. doi: 10.1097/QAD.0b013e3283344895
8. Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, et al. Alpha4(+)beta7(hi)CD4(+) Memory T Cells Harbor Most Th-17 Cells and are Preferentially Infected During Acute SIV Infection. Mucosal Immunol (2009) 2:439–49. doi: 10.1038/mi.2009.90
9. Pandrea I, Sodora DL, Silvestri G, Apetrei C. Into the Wild: Simian Immunodeficiency Virus (SIV) Infection in Natural Hosts. Trends Immunol (2008) 29:419–28. doi: 10.1016/j.it.2008.05.004
10. Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Buck D, Terstappen LW. Control of Lymphocyte Recirculation in Man. I. Differential Regulation of the Peripheral Lymph Node Homing Receptor L-Selectin on T Cells During the Virgin to Memory Cell Transition. J Immunol (1993) 150:1105–21.
11. Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and Homeostasis of T Cell Memory in Rhesus Macaque. J Immunol (2002) 168:29–43. doi: 10.4049/jimmunol.168.1.29
12. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A Human Memory T Cell Subset With Stem Cell-Like Properties. Nat Med (2011) 17:1290–7. doi: 10.1038/nm.2446
13. Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, et al. Superior T Memory Stem Cell Persistence Supports Long-Lived T Cell Memory. J Clin Invest (2013) 123:594–9. doi: 10.1172/JCI66327
14. Viollet L, Monceaux V, Petit F, Ho Tsong Fang R, Cumont MC, Hurtrel B, et al. Death of CD4+ T Cells From Lymph Nodes During Primary SIVmac251 Infection Predicts the Rate of AIDS Progression. J Immunol (2006) 177:6685–94. doi: 10.4049/jimmunol.177.10.6685
15. Pichyangkul S, Yongvanitchit K, Limsalakpetch A, Kum-Arb U, Im-Erbsin R, Boonnak K, et al. Tissue Distribution of Memory T and B Cells in Rhesus Monkeys Following Influenza A Infection. J Immunol (2015) 195:4378–86. doi: 10.4049/jimmunol.1501702
16. Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, et al. HIV-1 Entry Into CD4+ Cells is Mediated by the Chemokine Receptor CC-CKR-5. Nature (1996) 381:667–73. doi: 10.1038/381667a0
17. Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, et al. Identification of a Major Co-Receptor for Primary Isolates of HIV-1. Nature (1996) 381:661–6. doi: 10.1038/381661a0
18. Rucker J, Edinger AL, Sharron M, Samson M, Lee B, Berson JF, et al. Utilization of Chemokine Receptors, Orphan Receptors, and Herpesvirus-Encoded Receptors by Diverse Human and Simian Immunodeficiency Viruses. J Virol (1997) 71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997
19. Nixon DE, Landay AL. Biomarkers of Immune Dysfunction in HIV. Curr Opin HIV AIDS (2010) 5:498–503. doi: 10.1097/COH.0b013e32833ed6f4
20. Saez-Cirion A, Sereti I. Immunometabolism and HIV-1 Pathogenesis: Food for Thought. Nat Rev Immunol (2021) 21:5–19. doi: 10.1038/s41577-020-0381-7
21. Smit-McBride Z, Mattapallil JJ, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T Lymphocytes Retain High Potential for Cytokine Responses But Have Severe CD4(+) T-Cell Depletion at All Stages of Simian Immunodeficiency Virus Infection Compared to Peripheral Lymphocytes. J Virol (1998) 72:6646–56. doi: 10.1128/JVI.72.8.6646-6656.1998
22. Hermier F, Comby E, Delaunay A, Petitjean J, Favennec L, Bazin C, et al. Decreased Blood TcR Gamma Delta+ Lymphocytes in AIDS and P24-Antigenemic HIV-1-infected Patients. Clin Immunol Immunopathol (1993) 69:248–50. doi: 10.1006/clin.1993.1176
23. Kloverpris HN, Kazer SW, Mjosberg J, Mabuka JM, Wellmann A, Ndhlovu Z, et al. Innate Lymphoid Cells Are Depleted Irreversibly During Acute HIV-1 Infection in the Absence of Viral Suppression. Immunity (2016) 44:391–405. doi: 10.1016/j.immuni.2016.01.006
24. Mudd JC, Busman-Sahay K, DiNapoli SR, Lai S, Sheik V, Lisco A, et al. Hallmarks of Primate Lentiviral Immunodeficiency Infection Recapitulate Loss of Innate Lymphoid Cells. Nat Commun (2018) 9:3967. doi: 10.1038/s41467-018-05528-3
25. Xu H, Wang X, Liu DX, Moroney-Rasmussen T, Lackner AA, Veazey RS. IL-17-producing Innate Lymphoid Cells are Restricted to Mucosal Tissues and are Depleted in SIV-Infected Macaques. Mucosal Immunol (2012) 5:658–69. doi: 10.1038/mi.2012.39
26. Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, et al. Loss of Blood CD11c(+) Myeloid and CD11c(-) Plasmacytoid Dendritic Cells in Patients With HIV-1 Infection Correlates With HIV-1 RNA Virus Load. Blood (2001) 98:2574–6. doi: 10.1182/blood.V98.8.2574
27. Robb ML, Eller LA, Kibuuka H, Rono K, Maganga L, Nitayaphan S, et al. Prospective Study of Acute HIV-1 Infection in Adults in East Africa and Thailand. N Engl J Med (2016) 374:2120–30. doi: 10.1056/NEJMoa1508952
28. Mattapallil JJ, Letvin NL, Roederer M. T-Cell Dynamics During Acute SIV Infection. AIDS (2004) 18:13–23. doi: 10.1097/00002030-200401020-00002
29. Letvin NL, Daniel MD, Sehgal PK, Desrosiers RC, Hunt RD, Waldron LM, et al. Induction of AIDS-like Disease in Macaque Monkeys With T-Cell Tropic Retrovirus STLV-III. Science (1985) 230:71–3. doi: 10.1126/science.2412295
30. Veazey RS, Mansfield KG, Tham IC, Carville AC, Shvetz DE, Forand AE, et al. Dynamics of CCR5 Expression by CD4(+) T Cells in Lymphoid Tissues During Simian Immunodeficiency Virus Infection. J Virol (2000) 74:11001–7. doi: 10.1128/JVI.74.23.11001-11007.2000
31. Gottlieb GS, Sow PS, Hawes SE, Ndoye I, Redman M, Coll-Seck AM, et al. Equal Plasma Viral Loads Predict a Similar Rate of CD4+ T Cell Decline in Human Immunodeficiency Virus (HIV) Type 1- and HIV-2-infected Individuals From Senegal, West Africa. J Infect Dis (2002) 185:905–14. doi: 10.1086/339295
32. Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive Infection and Loss of Memory CD4+ T Cells in Multiple Tissues During Acute SIV Infection. Nature (2005) 434:1093–7. doi: 10.1038/nature03501
33. Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal Tract as a Major Site of CD4+ T Cell Depletion and Viral Replication in SIV Infection. Science (1998) 280:427–31. doi: 10.1126/science.280.5362.427
34. Milush JM, Mir KD, Sundaravaradan V, Gordon SN, Engram J, Cano CA, et al. Lack of Clinical AIDS in SIV-Infected Sooty Mangabeys With Significant CD4+ T Cell Loss is Associated With Double-Negative T Cells. J Clin Invest (2011) 121:1102–10. doi: 10.1172/JCI44876
35. Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T Cell Depletion During All Stages of HIV Disease Occurs Predominantly in the Gastrointestinal Tract. J Exp Med (2004) 200:749–59. doi: 10.1084/jem.20040874
36. Mangkornkanok-Mark M, Mark AS, Dong J. Immunoperoxidase Evaluation of Lymph Nodes From Acquired Immune Deficiency Patients. Clin Exp Immunol (1984) 55:581–6.
37. Janossy G, Pinching AJ, Bofill M, Weber J, McLaughlin JE, Ornstein M, et al. An Immunohistological Approach to Persistent Lymphadenopathy and its Relevance to AIDS. Clin Exp Immunol (1985) 59:257–66.
38. Willerford DM, Gale MJ Jr., Benveniste RE, Clark EA, Gallatin WM. Simian Immunodeficiency Virus is Restricted to a Subset of Blood CD4+ Lymphocytes That Includes Memory Cells. J Immunol (1990) 144:3779–83.
39. Schnittman SM, Lane HC, Greenhouse J, Justement JS, Baseler M, Fauci AS. Preferential Infection of CD4+ Memory T Cells by Human Immunodeficiency Virus Type 1: Evidence for a Role in the Selective T-cell Functional Defects Observed in Infected Individuals. Proc Natl Acad Sci USA (1990) 87:6058–62. doi: 10.1073/pnas.87.16.6058
40. Hulstaert F, Hannet I, Deneys V, Munhyeshuli V, Reichert T, De Bruyere M, et al. Age-Related Changes in Human Blood Lymphocyte Subpopulations. II. Varying Kinetics of Percentage and Absolute Count Measurements. Clin Immunol Immunopathol (1994) 70:152–8. doi: 10.1006/clin.1994.1023
41. Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, et al. Paucity of CD4+CCR5+ T Cells Is a Typical Feature of Natural SIV Hosts. Blood (2007) 109:1069–76. doi: 10.1182/blood-2006-05-024364
42. Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible Programs of Chemokine Receptor Expression on Human Polarized T Helper 1 and 2 Lymphocytes. J Exp Med (1998) 187:875–83. doi: 10.1084/jem.187.6.875
43. Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV Coreceptors CXCR4 and CCR5 are Differentially Expressed and Regulated on Human T Lymphocytes. Proc Natl Acad Sci USA (1997) 94:1925–30. doi: 10.1073/pnas.94.5.1925
44. Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, et al. Presence of an Inducible HIV-1 Latent Reservoir During Highly Active Antiretroviral Therapy. Proc Natl Acad Sci USA (1997) 94:13193–7. doi: 10.1073/pnas.94.24.13193
45. Ghosn J, Deveau C, Chaix ML, Goujard C, Galimand J, Zitoun Y, et al. Despite Being Highly Diverse, Immunovirological Status Strongly Correlates With Clinical Symptoms During Primary HIV-1 Infection: A Cross-Sectional Study Based on 674 Patients Enrolled in the ANRS Co 06 PRIMO Cohort. J Antimicrob Chemother (2010) 65:741–8. doi: 10.1093/jac/dkq035
46. Ananworanich J, Sacdalan CP, Pinyakorn S, Chomont N, de Souza M, Luekasemsuk T, et al. Virological and Immunological Characteristics of HIV-Infected Individuals at the Earliest Stage of Infection. J Virus Erad (2016) 2:43–8. doi: 10.1016/S2055-6640(20)30688-9
47. Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, et al. Severe Depletion of Mucosal CD4+ T Cells in AIDS-free Simian Immunodeficiency Virus-Infected Sooty Mangabeys. J Immunol (2007) 179:3026–34. doi: 10.4049/jimmunol.179.5.3026
48. Pandrea I, Gaufin T, Gautam R, Kristoff J, Mandell D, Montefiori D, et al. Functional Cure of SIVagm Infection in Rhesus Macaques Results in Complete Recovery of CD4+ T Cells and is Reverted by CD8+ Cell Depletion. PloS Pathog (2011) 7:e1002170. doi: 10.1371/journal.ppat.1002170
49. Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, et al. Identifying the Target Cell in Primary Simian Immunodeficiency Virus (SIV) Infection: Highly Activated Memory CD4(+) T Cells are Rapidly Eliminated in Early SIV Infection In Vivo. J Virol (2000) 74:57–64. doi: 10.1128/JVI.74.1.57-64.2000
50. Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV Reservoir Size and Persistence are Driven by T Cell Survival and Homeostatic Proliferation. Nat Med (2009) 15:893–900. doi: 10.1038/nm.1972
51. Descours B, Avettand-Fenoel V, Blanc C, Samri A, Melard A, Supervie V, et al. Immune Responses Driven by Protective Human Leukocyte Antigen Alleles From Long-Term Nonprogressors are Associated With Low HIV Reservoir in Central Memory CD4 T Cells. Clin Infect Dis (2012) 54:1495–503. doi: 10.1093/cid/cis188
52. Paiardini M, Cervasi B, Reyes-Aviles E, Micci L, Ortiz AM, Chahroudi A, et al. Low Levels of SIV Infection in Sooty Mangabey Central Memory CD(4)(+) T Cells are Associated With Limited CCR5 Expression. Nat Med (2011) 17:830–6. doi: 10.1038/nm.2395
53. Samri A, Charpentier C, Diallo MS, Bertine M, Even S, Morin V, et al. Limited HIV-2 Reservoirs in Central-Memory CD4 T-Cells Associated to CXCR6 Co-Receptor Expression in Attenuated HIV-2 Infection. PloS Pathog (2019) 15:e1007758. doi: 10.1371/journal.ppat.1007758
54. Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, et al. HIV-1 Persistence in CD4+ T Cells With Stem Cell-Like Properties. Nat Med (2014) 20:139–42. doi: 10.1038/nm.3445
55. Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, et al. Differential Th17 CD4 T-cell Depletion in Pathogenic and Nonpathogenic Lentiviral Infections. Blood (2008) 112:2826–35. doi: 10.1182/blood-2008-05-159301
56. Clerici M, Hakim FT, Venzon DJ, Blatt S, Hendrix CW, Wynn TA, et al. Changes in Interleukin-2 and Interleukin-4 Production in Asymptomatic, Human Immunodeficiency Virus-Seropositive Individuals. J Clin Invest (1993) 91:759–65. doi: 10.1172/JCI116294
57. Oswald-Richter K, Grill SM, Shariat N, Leelawong M, Sundrud MS, Haas DW, et al. HIV Infection of Naturally Occurring and Genetically Reprogrammed Human Regulatory T-Cells. PloS Biol (2004) 2:E198. doi: 10.1371/journal.pbio.0020198
58. Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, et al. Depletion of Regulatory T Cells in HIV Infection Is Associated With Immune Activation. J Immunol (2005) 174:4407–14. doi: 10.4049/jimmunol.174.7.4407
59. Andersson J, Boasso A, Nilsson J, Zhang R, Shire NJ, Lindback S, et al. The Prevalence of Regulatory T Cells in Lymphoid Tissue is Correlated With Viral Load in HIV-infected Patients. J Immunol (2005) 174:3143–7. doi: 10.4049/jimmunol.174.6.3143
60. Epple HJ, Loddenkemper C, Kunkel D, Troger H, Maul J, Moos V, et al. Mucosal But Not Peripheral FOXP3+ Regulatory T Cells are Highly Increased in Untreated HIV Infection and Normalize After Suppressive HAART. Blood (2006) 108:3072–8. doi: 10.1182/blood-2006-04-016923
61. Yero A, Farnos O, Rabezanahary H, Racine G, Estaquier J, Jenabian MA. Differential Dynamics of Regulatory T-Cell and Th17 Cell Balance in Mesenteric Lymph Nodes and Blood Following Early Antiretroviral Initiation During Acute Simian Immunodeficiency Virus Infection. J Virol (2019) 93(19):e00371-19. doi: 10.1128/JVI.00371-19
62. Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, et al. Critical Loss of the Balance Between Th17 and T Regulatory Cell Populations in Pathogenic SIV Infection. PloS Pathog (2009) 5:e1000295. doi: 10.1371/journal.ppat.1000295
63. Krzysiek R, Rudent A, Bouchet-Delbos L, Foussat A, Boutillon C, Portier A, et al. Preferential and Persistent Depletion of CCR5+ T-Helper Lymphocytes With Nonlymphoid Homing Potential Despite Early Treatment of Primary HIV Infection. Blood (2001) 98:3169–71. doi: 10.1182/blood.V98.10.3169
64. Wang X, Xu H, Gill AF, Pahar B, Kempf D, Rasmussen T, et al. Monitoring alpha4beta7 Integrin Expression on Circulating CD4+ T Cells as a Surrogate Marker for Tracking Intestinal CD4+ T-Cell Loss in SIV Infection. Mucosal Immunol (2009) 2:518–26. doi: 10.1038/mi.2009.104
65. Xu H, Wang X, Malam N, Lackner AA, Veazey RS. Persistent Simian Immunodeficiency Virus Infection Causes Ultimate Depletion of Follicular Th Cells in AIDS. J Immunol (2015) 195:4351–7. doi: 10.4049/jimmunol.1501273
66. Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, et al. CD4 T Follicular Helper Cell Dynamics During SIV Infection. J Clin Invest (2012) 122:3281–94. doi: 10.1172/JCI63039
67. Moukambi F, Rabezanahary H, Rodrigues V, Racine G, Robitaille L, Krust B, et al. Early Loss of Splenic Tfh Cells in SIV-Infected Rhesus Macaques. PloS Pathog (2015) 11:e1005287. doi: 10.1371/journal.ppat.1005287
68. Moukambi F, Rabezanahary H, Fortier Y, Rodrigues V, Clain J, Benmadid-Laktout G, et al. Mucosal T Follicular Helper Cells in SIV-Infected Rhesus Macaques: Contributing Role of IL-27. Mucosal Immunol (2019) 12:1038–54. doi: 10.1038/s41385-019-0174-0
69. Chowdhury A, Del Rio Estrada PM, Tharp GK, Trible RP, Amara RR, Chahroudi A, et al. Decreased T Follicular Regulatory Cell/T Follicular Helper Cell (TFH) in Simian Immunodeficiency Virus-Infected Rhesus Macaques may Contribute to Accumulation of TFH in Chronic Infection. J Immunol (2015) 195:3237–47. doi: 10.4049/jimmunol.1402701
70. Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, et al. Expansion of HIV-Specific T Follicular Helper Cells in Chronic HIV Infection. J Clin Invest (2012) 122:3271–80. doi: 10.1172/JCI64314
71. Valle-Casuso JC, Angin M, Volant S, Passaes C, Monceaux V, Mikhailova A, et al. Cellular Metabolism Is a Major Determinant of HIV-1 Reservoir Seeding in CD4(+) T Cells and Offers an Opportunity to Tackle Infection. Cell Metab (2019) 29:611–626 e5. doi: 10.1016/j.cmet.2018.11.015
72. Veazey RS, Rosenzweig M, Shvetz DE, Pauley DR, DeMaria M, Chalifoux LV, et al. Characterization of Gut-Associated Lymphoid Tissue (GALT) of Normal Rhesus Macaques. Clin Immunol Immunopathol (1997) 82:230–42. doi: 10.1006/clin.1996.4318
73. Kotler DP, Gaetz HP, Lange M, Klein EB, Holt PR. Enteropathy Associated With the Acquired Immunodeficiency Syndrome. Ann Intern Med (1984) 101:421–8. doi: 10.7326/0003-4819-101-4-421
74. Rodgers VD, Fassett R, Kagnoff MF. Abnormalities in Intestinal Mucosal T Cells in Homosexual Populations Including Those With the Lymphadenopathy Syndrome and Acquired Immunodeficiency Syndrome. Gastroenterology (1986) 90:552–8. doi: 10.1016/0016-5085(86)91108-X
75. Ellakany S, Whiteside TL, Schade RR, van Thiel DH. Analysis of Intestinal Lymphocyte Subpopulations in Patients With Acquired Immunodeficiency Syndrome (AIDS) and AIDS-related Complex. Am J Clin Pathol (1987) 87:356–64. doi: 10.1093/ajcp/87.3.356
76. Budhraja M, Levendoglu H, Kocka F, Mangkornkanok M, Sherer R. Duodenal Mucosal T Cell Subpopulation and Bacterial Cultures in Acquired Immune Deficiency Syndrome. Am J Gastroenterol (1987) 82:427–31.
77. Jarry A, Cortez A, Rene E, Muzeau F, Brousse N. Infected Cells and Immune Cells in the Gastrointestinal Tract of AIDS Patients. An Immunohistochemical Study of 127 Cases. Histopathology (1990) 16:133–40. doi: 10.1111/j.1365-2559.1990.tb01081.x
78. Schneider T, Jahn HU, Schmidt W, Riecken EO, Zeitz M, Ullrich R. Loss of CD4 T Lymphocytes in Patients Infected With Human Immunodeficiency Virus Type 1 Is More Pronounced in the Duodenal Mucosa Than in the Peripheral Blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut (1995) 37:524–9. doi: 10.1136/gut.37.4.524
79. Bishop PE, McMillan A, Gilmour HM. A Histological and Immunocytochemical Study of Lymphoid Tissue in Rectal Biopsies From Homosexual Men. Histopathology (1987) 11:1133–47. doi: 10.1111/j.1365-2559.1987.tb01854.x
80. Lim SG, Condez A, Lee CA, Johnson MA, Elia C, Poulter LW. Loss of Mucosal CD4 Lymphocytes Is an Early Feature of HIV Infection. Clin Exp Immunol (1993) 92:448–54. doi: 10.1111/j.1365-2249.1993.tb03419.x
81. Ullrich R, Zeitz M, Heise W, L’Age M, Ziegler K, Bergs C, et al. Mucosal Atrophy Is Associated With Loss of Activated T Cells in the Duodenal Mucosa of Human Immunodeficiency Virus (HIV)-Infected Patients. Digestion (1990) 46(Suppl 2):302–7. doi: 10.1159/000200401
82. Mattapallil JJ, Smit-McBride Z, McChesney M, Dandekar S. Intestinal Intraepithelial Lymphocytes Are Primed for Gamma Interferon and MIP-1beta Expression and Display Antiviral Cytotoxic Activity Despite Severe CD4(+) T-Cell Depletion in Primary Simian Immunodeficiency Virus Infection. J Virol (1998) 72:6421–9. doi: 10.1128/JVI.72.8.6421-6429.1998
83. Kewenig S, Schneider T, Hohloch K, Lampe-Dreyer K, Ullrich R, Stolte N, et al. Rapid Mucosal CD4(+) T-Cell Depletion and Enteropathy in Simian Immunodeficiency Virus-Infected Rhesus Macaques. Gastroenterology (1999) 116:1115–23. doi: 10.1016/S0016-5085(99)70014-4
84. Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, et al. Acute Loss of Intestinal CD4+ T Cells Is Not Predictive of Simian Immunodeficiency Virus Virulence. J Immunol (2007) 179:3035–46. doi: 10.4049/jimmunol.179.5.3035
85. Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, et al. Initiation of ART During Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation. PloS Pathog (2014) 10:e1004543. doi: 10.1371/journal.ppat.1004543
86. Mandell DT, Kristoff J, Gaufin T, Gautam R, Ma D, Sandler N, et al. Pathogenic Features Associated With Increased Virulence Upon Simian Immunodeficiency Virus Cross-Species Transmission From Natural Hosts. J Virol (2014) 88:6778–92. doi: 10.1128/JVI.03785-13
87. Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, et al. Antiinflammatory Profiles During Primary SIV Infection in African Green Monkeys are Associated With Protection Against AIDS. J Clin Invest (2005) 115:1082–91. doi: 10.1172/JCI23006
88. Verhoeven D, Sankaran S, Silvey M, Dandekar S. Antiviral Therapy During Primary Simian Immunodeficiency Virus Infection Fails to Prevent Acute Loss of CD4+ T Cells in Gut Mucosa But Enhances Their Rapid Restoration Through Central Memory T Cells. J Virol (2008) 82:4016–27. doi: 10.1128/JVI.02164-07
89. Staprans SI, Dailey PJ, Rosenthal A, Horton C, Grant RM, Lerche N, et al. Simian Immunodeficiency Virus Disease Course is Predicted by the Extent of Virus Replication During Primary Infection. J Virol (1999) 73:4829–39. doi: 10.1128/JVI.73.6.4829-4839.1999
90. Ananworanich J, Eller LA, Pinyakorn S, Kroon E, Sriplenchan S, Fletcher JL, et al. Viral Kinetics in Untreated Versus Treated Acute HIV Infection in Prospective Cohort Studies in Thailand. J Int AIDS Soc (2017) 20:21652. doi: 10.7448/IAS.20.1.21652
91. Ndhlovu ZM, Kamya P, Mewalal N, Kloverpris HN, Nkosi T, Pretorius K, et al. Magnitude and Kinetics of CD8+ T Cell Activation During Hyperacute HIV Infection Impact Viral Set Point. Immunity (2015) 43:591–604. doi: 10.1016/j.immuni.2015.08.012
92. Cumont MC, Diop O, Vaslin B, Elbim C, Viollet L, Monceaux V, et al. Early Divergence in Lymphoid Tissue Apoptosis Between Pathogenic and Nonpathogenic Simian Immunodeficiency Virus Infections of Nonhuman Primates. J Virol (2008) 82:1175–84. doi: 10.1128/JVI.00450-07
93. Heise C, Miller CJ, Lackner A, Dandekar S. Primary Acute Simian Immunodeficiency Virus Infection of Intestinal Lymphoid Tissue Is Associated With Gastrointestinal Dysfunction. J Infect Dis (1994) 169:1116–20. doi: 10.1093/infdis/169.5.1116
94. Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, et al. Peak SIV Replication in Resting Memory CD4+ T Cells Depletes Gut Lamina Propria CD4+ T Cells. Nature (2005) 434:1148–52. doi: 10.1038/nature03513
95. Wang X, Rasmussen T, Pahar B, Poonia B, Alvarez X, Lackner AA, et al. Massive Infection and Loss of CD4+ T Cells Occurs in the Intestinal Tract of Neonatal Rhesus Macaques in Acute SIV Infection. Blood (2007) 109:1174–81. doi: 10.1182/blood-2006-04-015172
96. Lapenta C, Boirivant M, Marini M, Santini SM, Logozzi M, Viora M, et al. Human Intestinal Lamina Propria Lymphocytes are Naturally Permissive to HIV-1 Infection. Eur J Immunol (1999) 29:1202–8. doi: 10.1002/(SICI)1521-4141(199904)29:04<1202::AID-IMMU1202>3.0.CO;2-O
97. Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, et al. Severe CD4+ T-Cell Depletion in Gut Lymphoid Tissue During Primary Human Immunodeficiency Virus Type 1 Infection and Substantial Delay in Restoration Following Highly Active Antiretroviral Therapy. J Virol (2003) 77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003
98. Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, et al. Primary HIV-1 Infection Is Associated With Preferential Depletion of CD4+ T Lymphocytes From Effector Sites in the Gastrointestinal Tract. J Exp Med (2004) 200:761–70. doi: 10.1084/jem.20041196
99. Deleage C, Schuetz A, Alvord WG, Johnston L, Hao XP, Morcock DR, et al. Impact of Early cART in the Gut During Acute HIV Infection. JCI Insight (2016) 1(10):e87065. doi: 10.1172/jci.insight.87065
100. Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct Pathogenic Sequela in Rhesus Macaques Infected With CCR5 or CXCR4 Utilizing SHIVs. Science (1999) 284:816–9. doi: 10.1126/science.284.5415.816
101. Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, et al. Insufficient Production and Tissue Delivery of CD4+ Memory T Cells in Rapidly Progressive Simian Immunodeficiency Virus Infection. J Exp Med (2004) 200:1299–314. doi: 10.1084/jem.20041049
102. Ho SH, Shek L, Gettie A, Blanchard J, Cheng-Mayer C. V3 Loop-Determined Coreceptor Preference Dictates the Dynamics of CD4+-T-cell Loss in Simian-Human Immunodeficiency Virus-Infected Macaques. J Virol (2005) 79:12296–303. doi: 10.1128/JVI.79.19.12296-12303.2005
103. Cecchinato V, Trindade CJ, Laurence A, Heraud JM, Brenchley JM, Ferrari MG, et al. Altered Balance Between Th17 and Th1 Cells at Mucosal Sites Predicts AIDS Progression in Simian Immunodeficiency Virus-Infected Macaques. Mucosal Immunol (2008) 1:279–88. doi: 10.1038/mi.2008.14
104. Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, et al. Loss of Mucosal CD103+ DCs and IL-17+ and IL-22+ Lymphocytes is Associated With Mucosal Damage in SIV Infection. Mucosal Immunol (2012) 5:646–57. doi: 10.1038/mi.2012.38
105. Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, et al. A Role for Mucosal IL-22 Production and Th22 Cells in HIV-associated Mucosal Immunopathogenesis. Mucosal Immunol (2012) 5:670–80. doi: 10.1038/mi.2012.72
106. Reeves RK, Rajakumar PA, Evans TI, Connole M, Gillis J, Wong FE, et al. Gut Inflammation and Indoleamine Deoxygenase Inhibit IL-17 Production and Promote Cytotoxic Potential in NKp44+ Mucosal NK Cells During SIV Infection. Blood (2011) 118:3321–30. doi: 10.1182/blood-2011-04-347260
107. Campillo-Gimenez L, Cumont MC, Fay M, Kared H, Monceaux V, Diop O, et al. AIDS Progression is Associated With the Emergence of IL-17-producing Cells Early After Simian Immunodeficiency Virus Infection. J Immunol (2010) 184:984–92. doi: 10.4049/jimmunol.0902316
108. Micci L, Cervasi B, Ende ZS, Iriele RI, Reyes-Aviles E, Vinton C, et al. Paucity of IL-21-producing CD4(+) T Cells is Associated With Th17 Cell Depletion in SIV Infection of Rhesus Macaques. Blood (2012) 120:3925–35. doi: 10.1182/blood-2012-04-420240
109. Allers K, Loddenkemper C, Hofmann J, Unbehaun A, Kunkel D, Moos V, et al. Gut Mucosal FOXP3+ Regulatory CD4+ T Cells and Nonregulatory CD4+ T Cells Are Differentially Affected by Simian Immunodeficiency Virus Infection in Rhesus Macaques. J Virol (2010) 84:3259–69. doi: 10.1128/JVI.01715-09
110. Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan Catabolism by Indoleamine 2,3-Dioxygenase 1 Alters the Balance of TH17 to Regulatory T Cells in HIV Disease. Sci Transl Med (2010) 2:32ra36. doi: 10.1126/scitranslmed.3000632
111. Jenabian MA, Patel M, Kema I, Kanagaratham C, Radzioch D, Thebault P, et al. Distinct Tryptophan Catabolism and Th17/Treg Balance in HIV Progressors and Elite Controllers. PloS One (2013) 8:e78146. doi: 10.1371/journal.pone.0078146
112. Manches O, Munn D, Fallahi A, Lifson J, Chaperot L, Plumas J, et al. HIV-Activated Human Plasmacytoid DCs Induce Tregs Through an Indoleamine 2,3-Dioxygenase-Dependent Mechanism. J Clin Invest (2008) 118:3431–9. doi: 10.1172/JCI34823
113. Pahar B, Lackner AA, Veazey RS. Intestinal Double-Positive CD4+CD8+ T Cells are Highly Activated Memory Cells With an Increased Capacity to Produce Cytokines. Eur J Immunol (2006) 36:583–92. doi: 10.1002/eji.200535520
114. Vajdy M, Veazey R, Tham I, deBakker C, Westmoreland S, Neutra M, et al. Early Immunologic Events in Mucosal and Systemic Lymphoid Tissues After Intrarectal Inoculation With Simian Immunodeficiency Virus. J Infect Dis (2001) 184:1007–14. doi: 10.1086/323615
115. Ahsan MH, Gill AF, Lackner AA, Veazey RS. Acute and Chronic T Cell Dynamics in the Livers of Simian Immunodeficiency Virus-Infected Macaques. J Virol (2012) 86:5244–52. doi: 10.1128/JVI.07080-11
116. Hoang TN, Harper JL, Pino M, Wang H, Micci L, King CT, et al. Bone Marrow-Derived CD4(+) T Cells Are Depleted in Simian Immunodeficiency Virus-Infected Macaques and Contribute to the Size of the Replication-Competent Reservoir. J Virol (2019) 93(1):e01344-18. doi: 10.1128/JVI.01344-18
117. Brenchley JM, Knox KS, Asher AI, Price DA, Kohli LM, Gostick E, et al. High Frequencies of Polyfunctional HIV-Specific T Cells Are Associated With Preservation of Mucosal CD4 T Cells in Bronchoalveolar Lavage. Mucosal Immunol (2008) 1:49–58. doi: 10.1038/mi.2007.5
118. Corleis B, Bucsan AN, Deruaz M, Vrbanac VD, Lisanti-Park AC, Gates SJ, et al. HIV-1 and SIV Infection Are Associated With Early Loss of Lung Interstitial CD4+ T Cells and Dissemination of Pulmonary Tuberculosis. Cell Rep (2019) 26:1409–1418 e5. doi: 10.1016/j.celrep.2019.01.021
119. Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T Cells Express High Levels of CCR5 and are Rapidly Depleted in Simian Immunodeficiency Virus Infection. J Infect Dis (2003) 187:769–76. doi: 10.1086/368386
120. Gumbi PP, Jaumdally SZ, Salkinder AL, Burgers WA, Mkhize NN, Hanekom W, et al. CD4 T Cell Depletion at the Cervix During HIV Infection is Associated With Accumulation of Terminally Differentiated T Cells. J Virol (2011) 85:13333–41. doi: 10.1128/JVI.05671-11
121. Politch JA, Mayer KH, Anderson DJ. Depletion of CD4+ T Cells in Semen During HIV Infection and Their Restoration Following Antiretroviral Therapy. J Acquir Immune Defic Syndr (2009) 50:283–9. doi: 10.1097/QAI.0b013e3181989870
122. Bernard-Stoecklin S, Gommet C, Corneau AB, Guenounou S, Torres C, Dejucq-Rainsford N, et al. Semen CD4+ T Cells and Macrophages are Productively Infected at All Stages of SIV Infection in Macaques. PloS Pathog (2013) 9:e1003810. doi: 10.1371/journal.ppat.1003810
123. Pandrea I, Apetrei C, Dufour J, Dillon N, Barbercheck J, Metzger M, et al. Simian Immunodeficiency Virus SIVagm.sab Infection of Caribbean African Green Monkeys: A New Model for the Study of SIV Pathogenesis in Natural Hosts. J Virol (2006) 80:4858–67. doi: 10.1128/JVI.80.10.4858-4867.2006
124. Pandrea I, Onanga R, Kornfeld C, Rouquet P, Bourry O, Clifford S, et al. High Levels of SIVmnd-1 Replication in Chronically Infected Mandrillus Sphinx. Virology (2003) 317:119–27. doi: 10.1016/j.virol.2003.08.015
125. Sumpter B, Dunham R, Gordon S, Engram J, Hennessy M, Kinter A, et al. Correlates of Preserved CD4(+) T Cell Homeostasis During Natural, Nonpathogenic Simian Immunodeficiency Virus Infection of Sooty Mangabeys: Implications for AIDS Pathogenesis. J Immunol (2007) 178:1680–91. doi: 10.4049/jimmunol.178.3.1680
126. Apetrei C, Sumpter B, Souquiere S, Chahroudi A, Makuwa M, Reed P, et al. Immunovirological Analyses of Chronically Simian Immunodeficiency Virus SIVmnd-1- and SIVmnd-2-infected Mandrills (Mandrillus Sphinx). J Virol (2011) 85:13077–87. doi: 10.1128/JVI.05693-11
127. Apetrei C, Gautam R, Sumpter B, Carter AC, Gaufin T, Staprans SI, et al. Virus Subtype-Specific Features of Natural Simian Immunodeficiency Virus SIVsmm Infection in Sooty Mangabeys. J Virol (2007) 81:7913–23. doi: 10.1128/JVI.00281-07
128. Apetrei C, Robertson DL, Marx PA. The History of SIVS and AIDS: Epidemiology, Phylogeny and Biology of Isolates From Naturally SIV Infected non-Human Primates (NHP) in Africa. Front Biosci (2004) 9:225–54. doi: 10.2741/1154
129. Pandrea I, Kornfeld C, Ploquin MJ, Apetrei C, Faye A, Rouquet P, et al. Impact of Viral Factors on Very Early In Vivo Replication Profiles in Simian Immunodeficiency Virus SIVagm-infected African Green Monkeys. J Virol (2005) 79:6249–59. doi: 10.1128/JVI.79.10.6249-6259.2005
130. Riddick NE, Wu F, Matsuda K, Whitted S, Ourmanov I, Goldstein S, et al. Simian Immunodeficiency Virus SIVagm Efficiently Utilizes Non-CCR5 Entry Pathways in African Green Monkey Lymphocytes: Potential Role for GPR15 and CXCR6 as Viral Coreceptors. J Virol (2015) 90:2316–31. doi: 10.1128/JVI.02529-15
131. Schols D, De Clercq E. The Simian Immunodeficiency Virus Mnd(GB-1) Strain Uses CXCR4, Not CCR5, as Coreceptor for Entry in Human Cells. J Gen Virol (1998) 79( Pt 9):2203–5. doi: 10.1099/0022-1317-79-9-2203
132. Chen Z, Kwon D, Jin Z, Monard S, Telfer P, Jones MS, et al. Natural Infection of a Homozygous Delta24 CCR5 Red-Capped Mangabey With an R2b-tropic Simian Immunodeficiency Virus. J Exp Med (1998) 188:2057–65. doi: 10.1084/jem.188.11.2057
133. Beer BE, Foley BT, Kuiken CL, Tooze Z, Goeken RM, Brown CR, et al. Characterization of Novel Simian Immunodeficiency Viruses From Red-Capped Mangabeys From Nigeria (SIVrcmNG409 and -NG411). J Virol (2001) 75:12014–27. doi: 10.1128/JVI.75.24.12014-12027.2001
134. Georges-Courbot MC, Lu CY, Makuwa M, Telfer P, Onanga R, Dubreuil G, et al. Natural Infection of a Household Pet Red-Capped Mangabey (Cercocebus Torquatus Torquatus) With a New Simian Immunodeficiency Virus. J Virol (1998) 72:600–8. doi: 10.1128/JVI.72.1.600-608.1998
135. Ciccone EJ, Greenwald JH, Lee PI, Biancotto A, Read SW, Yao MA, et al. CD4+ T Cells, Including Th17 and Cycling Subsets, are Intact in the Gut Mucosa of HIV-1-infected Long-Term Nonprogressors. J Virol (2011) 85:5880–8. doi: 10.1128/JVI.02643-10
136. Hirsch VM, Dapolito G, Johnson PR, Elkins WR, London WT, Montali RJ, et al. Induction of AIDS by Simian Immunodeficiency Virus From an African Green Monkey: Species-Specific Variation in Pathogenicity Correlates With the Extent of In Vivo Replication. J Virol (1995) 69:955–67. doi: 10.1128/jvi.69.2.955-967.1995
137. Apetrei C, Gaufin T, Gautam R, Vinton C, Hirsch V, Lewis M, et al. Pattern of SIVagm Infection in Patas Monkeys Suggests That Host Adaptation to Simian Immunodeficiency Virus Infection may Result in Resistance to Infection and Virus Extinction. J Infect Dis (2010) 202(Suppl 3):S371–6. doi: 10.1086/655970
138. Onanga R, Kornfeld C, Pandrea I, Estaquier J, Souquiere S, Rouquet P, et al. High Levels of Viral Replication Contrast With Only Transient Changes in CD4(+) and CD8(+) Cell Numbers During the Early Phase of Experimental Infection With Simian Immunodeficiency Virus SIVmnd-1 in Mandrillus Sphinx. J Virol (2002) 76:10256–63. doi: 10.1128/JVI.76.20.10256-10263.2002
139. Ling B, Veazey RS, Hart M, Lackner AA, Kuroda M, Pahar B, et al. Early Restoration of Mucosal CD4 Memory CCR5 T Cells in the Gut of SIV-infected Rhesus Predicts Long Term Non-Progression. AIDS (2007) 21:2377–85. doi: 10.1097/QAD.0b013e3282f08b32
140. Estaquier J, Idziorek T, de Bels F, Barre-Sinoussi F, Hurtrel B, Aubertin AM, et al. Programmed Cell Death and AIDS: Significance of T-Cell Apoptosis in Pathogenic and Nonpathogenic Primate Lentiviral Infections. Proc Natl Acad Sci USA (1994) 91:9431–5. doi: 10.1073/pnas.91.20.9431
141. Ma D, Jasinska A, Kristoff J, Grobler JP, Turner T, Jung Y, et al. SIVagm Infection in Wild African Green Monkeys From South Africa: Epidemiology, Natural History, and Evolutionary Considerations. PloS Pathog (2013) 9:e1003011. doi: 10.1371/journal.ppat.1003011
142. Raehtz KD, Barrenas F, Xu C, Busman-Sahay K, Valentine A, Law L, et al. African Green Monkeys Avoid SIV Disease Progression by Preventing Intestinal Dysfunction and Maintaining Mucosal Barrier Integrity. PloS Pathog (2020) 16:e1008333. doi: 10.1371/journal.ppat.1008333
143. Barrenas F, Raehtz K, Xu C, Law L, Green RR, Silvestri G, et al. Macrophage-Associated Wound Healing Contributes to African Green Monkey SIV Pathogenesis Control. Nat Commun (2019) 10:5101. doi: 10.1038/s41467-019-13816-9
144. Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, et al. Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections. PloS Pathog (2010) 6:e1001052. doi: 10.1371/journal.ppat.1001052
145. Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, McClure HM, et al. Nonpathogenic SIV Infection of Sooty Mangabeys Is Characterized by Limited Bystander Immunopathology Despite Chronic High-Level Viremia. Immunity (2003) 18:441–52. doi: 10.1016/S1074-7613(03)00060-8
146. Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, et al. Type I Interferon Responses in Rhesus Macaques Prevent SIV Infection and Slow Disease Progression. Nature (2014) 511:601–5. doi: 10.1038/nature13554
147. Carnathan D, Lawson B, Yu J, Patel K, Billingsley JM, Tharp GK, et al. Reduced Chronic Lymphocyte Activation Following Interferon Alpha Blockade During the Acute Phase of Simian Immunodeficiency Virus Infection in Rhesus Macaques. J Virol (2018) 92(9):e01760-17. doi: 10.1128/JVI.01760-17
148. Meythaler M, Martinot A, Wang Z, Pryputniewicz S, Kasheta M, Ling B, et al. Differential CD4+ T-Lymphocyte Apoptosis and Bystander T-Cell Activation in Rhesus Macaques and Sooty Mangabeys During Acute Simian Immunodeficiency Virus Infection. J Virol (2009) 83:572–83. doi: 10.1128/JVI.01715-08
149. Herbeuval JP, Grivel JC, Boasso A, Hardy AW, Chougnet C, Dolan MJ, et al. CD4+ T-cell Death Induced by Infectious and Noninfectious HIV-1: Role of Type 1 Interferon-Dependent, TRAIL/DR5-mediated Apoptosis. Blood (2005) 106:3524–31. doi: 10.1182/blood-2005-03-1243
150. Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, et al. Nonpathogenic SIV Infection of African Green Monkeys Induces a Strong But Rapidly Controlled Type I IFN Response. J Clin Invest (2009) 119:3544–55. doi: 10.1172/JCI40093
151. Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, et al. Downregulation of Robust Acute Type I Interferon Responses Distinguishes Nonpathogenic Simian Immunodeficiency Virus (SIV) Infection of Natural Hosts From Pathogenic SIV Infection of Rhesus Macaques. J Virol (2010) 84:7886–91. doi: 10.1128/JVI.02612-09
152. Chan ML, Petravic J, Ortiz AM, Engram J, Paiardini M, Cromer D, et al. Limited CD4+ T Cell Proliferation Leads to Preservation of CD4+ T Cell Counts in SIV-Infected Sooty Mangabeys. Proc Biol Sci (2010) 277:3773–81. doi: 10.1098/rspb.2010.0972
153. McGary CS, Cervasi B, Chahroudi A, Micci L, Taaffe J, Meeker T, et al. Increased Stability and Limited Proliferation of CD4+ Central Memory T Cells Differentiate Nonprogressive Simian Immunodeficiency Virus (SIV) Infection of Sooty Mangabeys From Progressive SIV Infection of Rhesus Macaques. J Virol (2014) 88:4533–42. doi: 10.1128/JVI.03515-13
154. Ortiz AM, Carnathan DG, Yu J, Sheehan KM, Kim P, Reynaldi A, et al. Analysis of the In Vivo Turnover of CD4+ T-Cell Subsets in Chronically SIV-Infected Sooty Mangabeys. PloS One (2016) 11:e0156352. doi: 10.1371/journal.pone.0156352
155. Chakrabarti LA, Lewin SR, Zhang L, Gettie A, Luckay A, Martin LN, et al. Normal T-Cell Turnover in Sooty Mangabeys Harboring Active Simian Immunodeficiency Virus Infection. J Virol (2000) 74:1209–23. doi: 10.1128/JVI.74.3.1209-1223.2000
156. Ma D, Jasinska AJ, Feyertag F, Wijewardana V, Kristoff J, He T, et al. Factors Associated With Siman Immunodeficiency Virus Transmission in a Natural African Nonhuman Primate Host in the Wild. J Virol (2014) 88:5687–705. doi: 10.1128/JVI.03606-13
157. Pandrea I, Parrish NF, Raehtz K, Gaufin T, Barbian HJ, Ma D, et al. Mucosal Simian Immunodeficiency Virus Transmission in African Green Monkeys: Susceptibility to Infection is Proportional to Target Cell Availability at Mucosal Sites. J Virol (2012) 86:4158–68. doi: 10.1128/JVI.07141-11
158. Pandrea I, Onanga R, Souquiere S, Mouinga-Ondeme A, Bourry O, Makuwa M, et al. Paucity of CD4+ CCR5+ T Cells may Prevent Transmission of Simian Immunodeficiency Virus in Natural Nonhuman Primate Hosts by Breast-Feeding. J Virol (2008) 82:5501–9. doi: 10.1128/JVI.02555-07
159. Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, et al. CD4 Downregulation by Memory CD4+ T Cells In Vivo Renders African Green Monkeys Resistant to Progressive SIVagm Infection. Nat Med (2009) 15:879–85. doi: 10.1038/nm.1970
160. Vinton C, Klatt NR, Harris LD, Briant JA, Sanders-Beer BE, Herbert R, et al. CD4-Like Immunological Function by CD4- T Cells in Multiple Natural Hosts of Simian Immunodeficiency Virus. J Virol (2011) 85:8702–8. doi: 10.1128/JVI.00332-11
161. Sundaravaradan V, Saleem R, Micci L, Gasper MA, Ortiz AM, Else J, et al. Multifunctional Double-Negative T Cells in Sooty Mangabeys Mediate T-Helper Functions Irrespective of SIV Infection. PloS Pathog (2013) 9:e1003441. doi: 10.1371/journal.ppat.1003441
162. Doitsh G, Greene WC. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe (2016) 19:280–91. doi: 10.1016/j.chom.2016.02.012
163. Leonard R, Zagury D, Desportes I, Bernard J, Zagury JF, Gallo RC. Cytopathic Effect of Human Immunodeficiency Virus in T4 Cells Is Linked to the Last Stage of Virus Infection. Proc Natl Acad Sci USA (1988) 85:3570–4. doi: 10.1073/pnas.85.10.3570
164. Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-Specific CD8+ Cytotoxic T-lymphocyte Activity Associated With Control of Viremia in Primary Human Immunodeficiency Virus Type 1 Infection. J Virol (1994) 68:6103–10. doi: 10.1128/jvi.68.9.6103-6110.1994
165. Jones RB, Walker BD. HIV-Specific CD8(+) T Cells and HIV Eradication. J Clin Invest (2016) 126:455–63. doi: 10.1172/JCI80566
166. Gandhi RT, Chen BK, Straus SE, Dale JK, Lenardo MJ, Baltimore D. HIV-1 Directly Kills CD4+ T Cells by a Fas-independent Mechanism. J Exp Med (1998) 187:1113–22. doi: 10.1084/jem.187.7.1113
167. Petit F, Arnoult D, Lelievre JD, Moutouh-de Parseval L, Hance AJ, Schneider P, et al. Productive HIV-1 Infection of Primary CD4+ T Cells Induces Mitochondrial Membrane Permeabilization Leading to a Caspase-Independent Cell Death. J Biol Chem (2002) 277:1477–87. doi: 10.1074/jbc.M102671200
168. Laforge M, Petit F, Estaquier J, Senik A. Commitment to Apoptosis in CD4(+) T Lymphocytes Productively Infected With Human Immunodeficiency Virus Type 1 Is Initiated by Lysosomal Membrane Permeabilization, Itself Induced by the Isolated Expression of the Viral Protein Nef. J Virol (2007) 81:11426–40. doi: 10.1128/JVI.00597-07
169. Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc Receptor But Not Complement Binding Is Important in Antibody Protection Against HIV. Nature (2007) 449:101–4. doi: 10.1038/nature06106
170. Musich T, Li L, Liu L, Zolla-Pazner S, Robert-Guroff M, Gorny MK. Monoclonal Antibodies Specific for the V2, V3, CD4-Binding Site, and gp41 of HIV-1 Mediate Phagocytosis in a Dose-Dependent Manner. J Virol (2017) 91(8):e02325-16. doi: 10.1128/JVI.02325-16
171. Solder BM, Schulz TF, Hengster P, Lower J, Larcher C, Bitterlich G, et al. HIV and HIV-infected Cells Differentially Activate the Human Complement System Independent of Antibody. Immunol Lett (1989) 22:135–45. doi: 10.1016/0165-2478(89)90180-6
172. Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, et al. Immune Escape From HIV-specific Antibody-Dependent Cellular Cytotoxicity (ADCC) Pressure. Proc Natl Acad Sci USA (2011) 108:7505–10. doi: 10.1073/pnas.1016048108
173. Schmitz J, Zimmer JP, Kluxen B, Aries S, Bogel M, Gigli I, et al. Antibody-Dependent Complement-Mediated Cytotoxicity in Sera From Patients With HIV-1 Infection Is Controlled by CD55 and CD59. J Clin Invest (1995) 96:1520–6. doi: 10.1172/JCI118190
174. Dufloo J, Guivel-Benhassine F, Buchrieser J, Lorin V, Grzelak L, Dupouy E, et al. Anti-HIV-1 Antibodies Trigger Non-Lytic Complement Deposition on Infected Cells. EMBO Rep (2020) 21:e49351. doi: 10.15252/embr.201949351
175. Mehandru S, Poles MA, Tenner-Racz K, Manuelli V, Jean-Pierre P, Lopez P, et al. Mechanisms of Gastrointestinal CD4+ T-Cell Depletion During Acute and Early Human Immunodeficiency Virus Type 1 Infection. J Virol (2007) 81:599–612. doi: 10.1128/JVI.01739-06
176. Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, et al. Apoptosis Occurs Predominantly in Bystander Cells and Not in Productively Infected Cells of HIV- and SIV-Infected Lymph Nodes. Nat Med (1995) 1:129–34. doi: 10.1038/nm0295-129
177. Boirivant M, Viora M, Giordani L, Luzzati AL, Pronio AM, Montesani C, et al. HIV-1 gp120 Accelerates Fas-mediated Activation-Induced Human Lamina Propria T Cell Apoptosis. J Clin Immunol (1998) 18:39–47. doi: 10.1016/S0165-2478(97)85516-2
178. Cicala C, Arthos J, Rubbert A, Selig S, Wildt K, Cohen OJ, et al. HIV-1 Envelope Induces Activation of Caspase-3 and Cleavage of Focal Adhesion Kinase in Primary Human CD4(+) T Cells. Proc Natl Acad Sci USA (2000) 97:1178–83. doi: 10.1073/pnas.97.3.1178
179. Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA, Herzenberg LA. Fas Antigen Stimulation Induces Marked Apoptosis of T Lymphocytes in Human Immunodeficiency Virus-Infected Individuals. J Exp Med (1995) 181:2029–36. doi: 10.1084/jem.181.6.2029
180. Estaquier J, Idziorek T, Zou W, Emilie D, Farber CM, Bourez JM, et al. T Helper Type 1/T Helper Type 2 Cytokines and T Cell Death: Preventive Effect of Interleukin 12 on Activation-Induced and CD95 (FAS/APO-1)-Mediated Apoptosis of CD4+ T Cells From Human Immunodeficiency Virus-Infected Persons. J Exp Med (1995) 182:1759–67. doi: 10.1084/jem.182.6.1759
181. Estaquier J, Tanaka M, Suda T, Nagata S, Golstein P, Ameisen JC. Fas-Mediated Apoptosis of CD4+ and CD8+ T Cells From Human Immunodeficiency Virus-Infected Persons: Differential In Vitro Preventive Effect of Cytokines and Protease Antagonists. Blood (1996) 87:4959–66. doi: 10.1182/blood.V87.12.4959.bloodjournal87124959
182. Sloand EM, Young NS, Kumar P, Weichold FF, Sato T, Maciejewski JP. Role of Fas Ligand and Receptor in the Mechanism of T-cell Depletion in Acquired Immunodeficiency Syndrome: Effect on CD4+ Lymphocyte Depletion and Human Immunodeficiency Virus Replication. Blood (1997) 89:1357–63. doi: 10.1182/blood.V89.4.1357
183. Kim N, Dabrowska A, Jenner RG, Aldovini A. Human and Simian Immunodeficiency Virus-Mediated Upregulation of the Apoptotic Factor TRAIL Occurs in Antigen-Presenting Cells From AIDS-Susceptible But Not From AIDS-Resistant Species. J Virol (2007) 81:7584–97. doi: 10.1128/JVI.02616-06
184. Diop OM, Ploquin MJ, Mortara L, Faye A, Jacquelin B, Kunkel D, et al. Plasmacytoid Dendritic Cell Dynamics and Alpha Interferon Production During Simian Immunodeficiency Virus Infection With a Nonpathogenic Outcome. J Virol (2008) 82:5145–52. doi: 10.1128/JVI.02433-07
185. Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, et al. Abortive HIV Infection Mediates CD4 T Cell Depletion and Inflammation in Human Lymphoid Tissue. Cell (2010) 143:789–801. doi: 10.1016/j.cell.2010.11.001
186. Sivanandham R, Brocca-Cofano E, Krampe N, Falwell E, Venkatraman SMK, Ribeiro RM, et al. Neutrophil Extracellular Trap Production Contributes to Pathogenesis in SIV-Infected Nonhuman Primates. J Clin Invest (2018) 128:5178–83. doi: 10.1172/JCI99420
187. Vujkovic-Cvijin I, Swainson LA, Chu SN, Ortiz AM, Santee CA, Petriello A, et al. Gut-Resident Lactobacillus Abundance Associates With IDO1 Inhibition and Th17 Dynamics in SIV-Infected Macaques. Cell Rep (2015) 13:1589–97. doi: 10.1016/j.celrep.2015.10.026
188. Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, et al. IL-10 Is Up-Regulated in Multiple Cell Types During Viremic HIV Infection and Reversibly Inhibits Virus-Specific T Cells. Blood (2009) 114:346–56. doi: 10.1182/blood-2008-12-191296
189. Clerici M, Sarin A, Berzofsky JA, Landay AL, Kessler HA, Hashemi F, et al. Antigen-Stimulated Apoptotic T-Cell Death in HIV Infection Is Selective for CD4+ T Cells, Modulated by Cytokines and Effected by Lymphotoxin. AIDS (1996) 10:603–11. doi: 10.1097/00002030-199606000-00005
190. Palmer BE, Boritz E, Wilson CC. Effects of Sustained HIV-1 Plasma Viremia on HIV-1 Gag-Specific CD4+ T Cell Maturation and Function. J Immunol (2004) 172:3337–47. doi: 10.4049/jimmunol.172.5.3337
191. Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, et al. HIV-1 Viremia Prevents the Establishment of Interleukin 2-Producing HIV-Specific Memory CD4+ T Cells Endowed With Proliferative Capacity. J Exp Med (2003) 198:1909–22. doi: 10.1084/jem.20031598
192. Fenwick C, Joo V, Jacquier P, Noto A, Banga R, Perreau M, et al. T-Cell Exhaustion in HIV Infection. Immunol Rev (2019) 292:149–63. doi: 10.1111/imr.12823
193. Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, et al. Changes in Thymic Function With Age and During the Treatment of HIV Infection. Nature (1998) 396:690–5. doi: 10.1038/25374
194. Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative Mechanisms of Lymphoid Tissue Fibrosis and T Cell Depletion in HIV-1 and SIV Infections. J Clin Invest (2011) 121:998–1008. doi: 10.1172/JCI45157
195. Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, et al. Simian Immunodeficiency Virus-Induced Lymphatic Tissue Fibrosis Is Mediated by Transforming Growth Factor Beta 1-Positive Regulatory T Cells and Begins in Early Infection. J Infect Dis (2007) 195:551–61. doi: 10.1086/510852
196. Kaplan JE, Spira TJ, Fishbein DB, Bozeman LH, Pinsky PF, Schonberger LB. A Six-Year Follow-Up of HIV-Infected Homosexual Men With Lymphadenopathy. Evidence for an Increased Risk for Developing AIDS After the Third Year of Lymphadenopathy. JAMA (1988) 260:2694–7. doi: 10.1001/jama.260.18.2694
197. Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma Viral Load and CD4+ Lymphocytes as Prognostic Markers of HIV-1 Infection. Ann Intern Med (1997) 126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003
198. Goulet JL, Fultz SL, Rimland D, Butt A, Gibert C, Rodriguez-Barradas M, et al. Aging and Infectious Diseases: do Patterns of Comorbidity Vary by HIV Status, Age, and HIV Severity? Clin Infect Dis (2007) 45:1593–601. doi: 10.1086/523577
199. Phair J, Munoz A, Detels R, Kaslow R, Rinaldo C, Saah A. The Risk of Pneumocystis Carinii Pneumonia Among Men Infected With Human Immunodeficiency Virus Type 1. Multicenter Aids Cohort Study Group. N Engl J Med (1990) 322:161–5. doi: 10.1056/NEJM199001183220304
200. Masur H, Ognibene FP, Yarchoan R, Shelhamer JH, Baird BF, Travis W, et al. CD4 Counts as Predictors of Opportunistic Pneumonias in Human Immunodeficiency Virus (HIV) Infection. Ann Intern Med (1989) 111:223–31. doi: 10.7326/0003-4819-111-3-223
201. Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer Risk in the Swiss HIV Cohort Study: Associations With Immunodeficiency, Smoking, and Highly Active Antiretroviral Therapy. J Natl Cancer Inst (2005) 97:425–32. doi: 10.1093/jnci/dji072
202. Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Adolescent Spectrum of Disease and H.I.V.O.S. Investigators, Incidence of Types of Cancer Among HIV-Infected Persons Compared With the General Population in the United States, 1992-2003. Ann Intern Med (2008) 148:728–36. doi: 10.7326/0003-4819-148-10-200805200-00005
203. Milush JM, Reeves JD, Gordon SN, Zhou D, Muthukumar A, Kosub DA, et al. Virally Induced CD4+ T Cell Depletion is Not Sufficient to Induce AIDS in a Natural Host. J Immunol (2007) 179:3047–56. doi: 10.4049/jimmunol.179.5.3047
204. Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, Sugimoto C, et al. The Level of Monocyte Turnover Predicts Disease Progression in the Macaque Model of AIDS. Blood (2009) 114:2917–25. doi: 10.1182/blood-2009-02-204263
205. Laforge M, Campillo-Gimenez L, Monceaux V, Cumont MC, Hurtrel B, Corbeil J, et al. HIV/SIV Infection Primes Monocytes and Dendritic Cells for Apoptosis. PloS Pathog (2011) 7:e1002087. doi: 10.1371/journal.ppat.1002087
206. Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, et al. Cell Death by Pyroptosis Drives CD4 T-Cell Depletion in HIV-1 Infection. Nature (2014) 505:509–14. doi: 10.1038/nature12940
207. Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, et al. Simian Immunodeficiency Virus-Induced Mucosal Interleukin-17 Deficiency Promotes Salmonella Dissemination From the Gut. Nat Med (2008) 14:421–8. doi: 10.1038/nm1743
208. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial Translocation Is a Cause of Systemic Immune Activation in Chronic HIV Infection. Nat Med (2006) 12:1365–71. doi: 10.1038/nm1511
209. Hao XP, Lucero CM, Turkbey B, Bernardo ML, Morcock DR, Deleage C, et al. Experimental Colitis in SIV-uninfected Rhesus Macaques Recapitulates Important Features of Pathogenic SIV Infection. Nat Commun (2015) 6:8020. doi: 10.1038/ncomms9020
210. Kristoff J, Haret-Richter G, Ma D, Ribeiro RM, Xu C, Cornell E, et al. Early Microbial Translocation Blockade Reduces SIV-mediated Inflammation and Viral Replication. J Clin Invest (2014) 124:2802–6. doi: 10.1172/JCI75090
211. Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. Increased Expression of Interleukin 17 in Inflammatory Bowel Disease. Gut (2003) 52:65–70. doi: 10.1136/gut.52.1.65
212. Wang Y, MacDonald JK, Benchimol EI, Griffiths AM, Steinhart AH, Panaccione R, et al. Type I Interferons for Induction of Remission in Ulcerative Colitis. Cochrane Database Syst Rev (2015) (9):CD006790. doi: 10.1002/14651858.CD006790.pub3
213. Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-Beta Inhibits Human Th17 Cell Differentiation. J Immunol (2009) 183:5418–27. doi: 10.4049/jimmunol.0803227
214. Deeks SG, Tracy R, Douek DC. Systemic Effects of Inflammation on Health During Chronic HIV Infection. Immunity (2013) 39:633–45. doi: 10.1016/j.immuni.2013.10.001
215. Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and Coagulation Biomarkers and Mortality in Patients With HIV Infection. PloS Med (2008) 5:e203. doi: 10.1371/journal.pmed.0050203
216. Calmy A, Gayet-Ageron A, Montecucco F, Nguyen A, Mach F, Burger F, et al. HIV Increases Markers of Cardiovascular Risk: Results From a Randomized, Treatment Interruption Trial. AIDS (2009) 23:929–39. doi: 10.1097/QAD.0b013e32832995fa
217. Pandrea I, Cornell E, Wilson C, Ribeiro RM, Ma D, Kristoff J, et al. Coagulation Biomarkers Predict Disease Progression in SIV-Infected Nonhuman Primates. Blood (2012) 120:1357–66. doi: 10.1182/blood-2012-03-414706
218. George MD, Reay E, Sankaran S, Dandekar S. Early Antiretroviral Therapy for Simian Immunodeficiency Virus Infection Leads to Mucosal CD4+ T-Cell Restoration and Enhanced Gene Expression Regulating Mucosal Repair and Regeneration. J Virol (2005) 79:2709–19. doi: 10.1128/JVI.79.5.2709-2719.2005
219. Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, et al. Effective CD4+ T-Cell Restoration in Gut-Associated Lymphoid Tissue of HIV-Infected Patients Is Associated With Enhanced Th17 Cells and Polyfunctional HIV-Specific T-Cell Responses. Mucosal Immunol (2008) 1:475–88. doi: 10.1038/mi.2008.35
220. van den Dries L, Claassen MAA, Groothuismink ZMA, van Gorp E, Boonstra A. Immune Activation in Prolonged Cart-Suppressed HIV Patients Is Comparable to That of Healthy Controls. Virology (2017) 509:133–9. doi: 10.1016/j.virol.2017.06.014
221. Funderburg NT, Andrade A, Chan ES, Rosenkranz SL, Lu D, Clagett B, et al. Dynamics of Immune Reconstitution and Activation Markers in HIV+ Treatment-Naive Patients Treated With Raltegravir, Tenofovir Disoproxil Fumarate and Emtricitabine. PloS One (2013) 8:e83514. doi: 10.1371/journal.pone.0083514
222. Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, et al. Rapid Seeding of the Viral Reservoir Prior to SIV Viraemia in Rhesus Monkeys. Nature (2014) 512:74–7. doi: 10.1038/nature13594
223. Ciccone EJ, Read SW, Mannon PJ, Yao MD, Hodge JN, Dewar R, et al. Cycling of Gut Mucosal CD4+ T Cells Decreases After Prolonged Anti-Retroviral Therapy and Is Associated With Plasma LPS Levels. Mucosal Immunol (2010) 3:172–81. doi: 10.1038/mi.2009.129
224. Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, et al. Positive Effects of Combined Antiretroviral Therapy on CD4+ T Cell Homeostasis and Function in Advanced HIV Disease. Science (1997) 277:112–6. doi: 10.1126/science.277.5322.112
225. Klatt NR, Villinger F, Bostik P, Gordon SN, Pereira L, Engram JC, et al. Availability of Activated CD4+ T Cells Dictates the Level of Viremia in Naturally SIV-Infected Sooty Mangabeys. J Clin Invest (2008) 118:2039–49. doi: 10.1172/JCI33814
226. Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, et al. HCV Persistence and Immune Evasion in the Absence of Memory T Cell Help. Science (2003) 302:659–62. doi: 10.1126/science.1088774
227. Engram JC, Cervasi B, Borghans JA, Klatt NR, Gordon SN, Chahroudi A, et al. Lineage-Specific T-Cell Reconstitution Following In Vivo CD4+ and CD8+ Lymphocyte Depletion in Nonhuman Primates. Blood (2010) 116:748–58. doi: 10.1182/blood-2010-01-263814
228. Kumar NA, McBrien JB, Carnathan DG, Mavigner M, Mattingly C, White ER, et al. Antibody-Mediated CD4 Depletion Induces Homeostatic CD4(+) T Cell Proliferation Without Detectable Virus Reactivation in Antiretroviral Therapy-Treated Simian Immunodeficiency Virus-Infected Macaques. J Virol (2018) 92(22):e01235-18. doi: 10.1128/JVI.01235-18
229. Ortiz AM, Klatt NR, Li B, Yi Y, Tabb B, Hao XP, et al. Depletion of CD4(+) T Cells Abrogates Post-Peak Decline of Viremia in SIV-Infected Rhesus Macaques. J Clin Invest (2011) 121:4433–45. doi: 10.1172/JCI46023
230. Micci L, Alvarez X, Iriele RI, Ortiz AM, Ryan ES, McGary CS, et al. CD4 Depletion in SIV-Infected Macaques Results in Macrophage and Microglia Infection With Rapid Turnover of Infected Cells. PloS Pathog (2014) 10:e1004467. doi: 10.1371/journal.ppat.1004467
231. Kleinman AJ, Sivanandham R, Pandrea I, Chougnet CA, Apetrei C. Regulatory T Cells As Potential Targets for HIV Cure Research. Front Immunol (2018) 9:734. doi: 10.3389/fimmu.2018.00734
232. He T, Brocca-Cofano E, Policicchio BB, Sivanandham R, Gautam R, Raehtz KD, et al. Cutting Edge: T Regulatory Cell Depletion Reactivates Latent Simian Immunodeficiency Virus (SIV) in Controller Macaques While Boosting SIV-Specific T Lymphocytes. J Immunol (2016) 197:4535–9. doi: 10.4049/jimmunol.1601539
233. Pandrea I, Gaufin T, Brenchley JM, Gautam R, Monjure C, Gautam A, et al. Cutting Edge: Experimentally Induced Immune Activation in Natural Hosts of Simian Immunodeficiency Virus Induces Significant Increases in Viral Replication and CD4+ T Cell Depletion. J Immunol (2008) 181:6687–91. doi: 10.4049/jimmunol.181.10.6687
234. Sivanandham R, Kleinman AJ, Sette P, Brocca-Cofano E, Kilapandal Venkatraman SM, Policicchio BB, et al. Nonhuman Primate Testing of the Impact of Different Regulatory T Cell Depletion Strategies on Reactivation and Clearance of Latent Simian Immunodeficiency Virus. J Virol (2020) 94(19):e00533-20. doi: 10.1128/JVI.00533-20
235. Wang Z, Pratts SG, Zhang H, Spencer PJ, Yu R, Tonsho M, et al. Treg Depletion in Non-Human Primates Using a Novel Diphtheria Toxin-Based Anti-Human CCR4 Immunotoxin. Mol Oncol (2016) 10:553–65. doi: 10.1016/j.molonc.2015.11.008
236. Hryniewicz A, Boasso A, Edghill-Smith Y, Vaccari M, Fuchs D, Venzon D, et al. CTLA-4 Blockade Decreases TGF-beta, IDO, and Viral RNA Expression in Tissues of SIVmac251-Infected Macaques. Blood (2006) 108:3834–42. doi: 10.1182/blood-2006-04-010637
237. Cecchinato V, Tryniszewska E, Ma ZM, Vaccari M, Boasso A, Tsai WP, et al. Immune Activation Driven by CTLA-4 Blockade Augments Viral Replication at Mucosal Sites in Simian Immunodeficiency Virus Infection. J Immunol (2008) 180:5439–47. doi: 10.4049/jimmunol.180.8.5439
238. Harper J, Gordon S, Chan CN, Wang H, Lindemuth E, Galardi C, et al. CTLA-4 and PD-1 Dual Blockade Induces SIV Reactivation Without Control of Rebound After Antiretroviral Therapy Interruption. Nat Med (2020) 26:519–28. doi: 10.1038/s41591-020-0782-y
239. Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, et al. Normal Development and Function of CD8+ Cells But Markedly Decreased Helper Cell Activity in Mice Lacking CD4. Nature (1991) 353:180–4. doi: 10.1038/353180a0
240. Rahemtulla A, Kundig TM, Narendran A, Bachmann MF, Julius M, Paige CJ, et al. Class II Major Histocompatibility Complex-Restricted T Cell Function in CD4-Deficient Mice. Eur J Immunol (1994) 24:2213–8. doi: 10.1002/eji.1830240942
241. Locksley RM, Reiner SL, Hatam F, Littman DR, Killeen N. Helper T Cells Without CD4: Control of Leishmaniasis in CD4-Deficient Mice. Science (1993) 261:1448–51. doi: 10.1126/science.8367726
242. von Herrath MG, Yokoyama M, Dockter J, Oldstone MB, Whitton JL. CD4-Deficient Mice Have Reduced Levels of Memory Cytotoxic T Lymphocytes After Immunization and Show Diminished Resistance to Subsequent Virus Challenge. J Virol (1996) 70:1072–9. doi: 10.1128/jvi.70.2.1072-1079.1996
243. Smith DK, Neal JJ, Holmberg SD. Unexplained Opportunistic Infections and CD4+ T-Lymphocytopenia Without HIV Infection. An Investigation of Cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med (1993) 328:373–9. doi: 10.1056/NEJM199302113280601
244. C. Centers for Disease. Unexplained CD4+ T-Lymphocyte Depletion in Persons Without Evident HIV Infection–United States. MMWR Morb Mortal Wkly Rep (1992) 41:541–5. doi: 10.1001/jama.1992.03490100048012
245. Aledort LM, Operskalski EA, Dietrich SL, Koerper MA, Gjerset GF, Lusher JM, et al. Low CD4+ Counts in a Study of Transfusion Safety. The Transfusion Safety Study Group. N Engl J Med (1993) 328:441–2. doi: 10.1056/NEJM199302113280614
246. Busch MP, Valinsky JE, Paglieroni T, Prince HE, Crutcher GJ, Gjerset GF, et al. Screening of Blood Donors for Idiopathic CD4+ T-Lymphocytopenia. Transfusion (1994) 34:192–7. doi: 10.1046/j.1537-2995.1994.34394196614.x
247. Zonios DI, Falloon J, Bennett JE, Shaw PA, Chaitt D, Baseler MW, et al. Idiopathic CD4+ Lymphocytopenia: Natural History and Prognostic Factors. Blood (2008) 112:287–94. doi: 10.1182/blood-2007-12-127878
248. Regent A, Autran B, Carcelain G, Cheynier R, Terrier B, Charmeteau-De Muylder B, et al. Idiopathic CD4 Lymphocytopenia: Clinical and Immunologic Characteristics and Follow-Up of 40 Patients. Medicine (Baltimore) (2014) 93:61–72. doi: 10.1097/MD.0000000000000017
249. Perez-Diez A, Wong CS, Liu X, Mystakelis H, Song J, Lu Y, et al. Prevalence and Pathogenicity of Autoantibodies in Patients With Idiopathic CD4 Lymphopenia. J Clin Invest (2020) 130:5326–37. doi: 10.1172/JCI136254
250. Gorska MM, Alam R. A Mutation in the Human Uncoordinated 119 Gene Impairs TCR Signaling and Is Associated With CD4 Lymphopenia. Blood (2012) 119:1399–406. doi: 10.1182/blood-2011-04-350686
251. Lee PI, Ciccone EJ, Read SW, Asher A, Pitts R, Douek DC, et al. Evidence for Translocation of Microbial Products in Patients With Idiopathic CD4 Lymphocytopenia. J Infect Dis (2009) 199:1664–70. doi: 10.1086/598953
252. Kovacs SB, Sheikh V, Thompson WL, Morcock DR, Perez-Diez A, Yao MD, et al. T-Cell Depletion in the Colonic Mucosa of Patients With Idiopathic CD4+ Lymphopenia. J Infect Dis (2015) 212:1579–87. doi: 10.1093/infdis/jiv282
253. Sortino O, Dias J, Anderson M, Laidlaw E, Leeansyah E, Lisco A, et al. Preserved MAIT Cell Numbers and Function in Idiopathic CD4 Lymphocytopenia. J Infect Dis (2020). doi: 10.1093/infdis/jiaa782
254. Lisco A, Ye P, Wong CS, Pei L, Hsu AP, Mace EM, et al. Lost in Translation: Lack of CD4 Expression Due to a Novel Genetic Defect. J Infect Dis (2021) 223(4):645–54. doi: 10.1093/infdis/jiab025
255. Fernandes RA, Perez-Andres M, Blanco E, Jara-Acevedo M, Criado I, Almeida J, et al. Complete Multilineage Cd4 Expression Defect Associated With Warts Due to an Inherited Homozygous CD4 Gene Mutation. Front Immunol (2019) 10:2502. doi: 10.3389/fimmu.2019.02502
256. Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, et al. Hyper-IgE Syndrome With Recurrent Infections–An Autosomal Dominant Multisystem Disorder. N Engl J Med (1999) 340:692–702. doi: 10.1056/NEJM199903043400904
257. Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 Cells in Hyper IgE Syndrome Due to Mutations in STAT3. J Exp Med (2008) 205:1551–7. doi: 10.1084/jem.20080218
258. Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined Immunodeficiency Associated With DOCK8 Mutations. N Engl J Med (2009) 361:2046–55. doi: 10.1056/NEJMoa0905506
259. Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large Deletions and Point Mutations Involving the Dedicator of Cytokinesis 8 (DOCK8) in the Autosomal-Recessive Form of hyper-IgE Syndrome. J Allergy Clin Immunol (2009) 124:1289–302 e4. doi: 10.1016/j.jaci.2009.10.038
260. Spolski R, Leonard WJ. Interleukin-21: A Double-Edged Sword With Therapeutic Potential. Nat Rev Drug Discov (2014) 13:379–95. doi: 10.1038/nrd4296
261. Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential Autocrine Regulation by IL-21 in the Generation of Inflammatory T Cells. Nature (2007) 448:480–3. doi: 10.1038/nature05969
262. Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 Initiates an Alternative Pathway to Induce Proinflammatory T(H)17 Cells. Nature (2007) 448:484–7. doi: 10.1038/nature05970
263. Pallikkuth S, Micci L, Ende ZS, Iriele RI, Cervasi B, Lawson B, et al. Maintenance of Intestinal Th17 Cells and Reduced Microbial Translocation in SIV-Infected Rhesus Macaques Treated With Interleukin (IL)-21. PloS Pathog (2013) 9:e1003471. doi: 10.1371/journal.ppat.1003471
264. Micci L, Ryan ES, Fromentin R, Bosinger SE, Harper JL, He T, et al. Interleukin-21 Combined With ART Reduces Inflammation and Viral Reservoir in SIV-Infected Macaques. J Clin Invest (2015) 125:4497–513. doi: 10.1172/JCI81400
265. Ortiz AM, Klase ZA, DiNapoli SR, Vujkovic-Cvijin I, Carmack K, Perkins MR, et al. IL-21 and Probiotic Therapy Improve Th17 Frequencies, Microbial Translocation, and Microbiome in ARV-Treated, SIV-Infected Macaques. Mucosal Immunol (2016) 9:458–67. doi: 10.1038/mi.2015.75
266. Mendez-Lagares G, Lu D, Merriam D, Baker CA, Villinger F, Van Rompay KKA, et al. IL-21 Therapy Controls Immune Activation and Maintains Antiviral CD8(+) T Cell Responses in Acute Simian Immunodeficiency Virus Infection. AIDS Res Hum Retroviruses (2017) 33:S81–92. doi: 10.1089/aid.2017.0160
267. Fry TJ, Connick E, Falloon J, Lederman MM, Liewehr DJ, Spritzler J, et al. A Potential Role for Interleukin-7 in T-Cell Homeostasis. Blood (2001) 97:2983–90. doi: 10.1182/blood.V97.10.2983
268. Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, et al. IL-7 Administration Drives T Cell-Cycle Entry and Expansion in HIV-1 Infection. Blood (2009) 113:6304–14. doi: 10.1182/blood-2008-10-186601
269. Nugeyre MT, Monceaux V, Beq S, Cumont MC, Ho Tsong Fang R, Chene L, et al. IL-7 Stimulates T Cell Renewal Without Increasing Viral Replication in Simian Immunodeficiency Virus-Infected Macaques. J Immunol (2003) 171:4447–53. doi: 10.4049/jimmunol.171.8.4447
270. Beq S, Nugeyre MT, Ho Tsong Fang R, Gautier D, Legrand R, Schmitt N, et al. IL-7 Induces Immunological Improvement in SIV-infected Rhesus Macaques Under Antiviral Therapy. J Immunol (2006) 176:914–22. doi: 10.4049/jimmunol.176.2.914
271. Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, et al. Enhanced T Cell Recovery in HIV-1-infected Adults Through IL-7 Treatment. J Clin Invest (2009) 119:997–1007. doi: 10.1172/JCI38052
272. Sortino O, Richards E, Dias J, Leeansyah E, Sandberg JK, Sereti I. IL-7 Treatment Supports CD8+ Mucosa-Associated Invariant T-cell Restoration in HIV-1-Infected Patients on Antiretroviral Therapy. AIDS (2018) 32:825–8. doi: 10.1097/QAD.0000000000001760
273. Vandergeeten C, Fromentin R, DaFonseca S, Lawani MB, Sereti I, Lederman MM, et al. Interleukin-7 Promotes HIV Persistence During Antiretroviral Therapy. Blood (2013) 121:4321–9. doi: 10.1182/blood-2012-11-465625
274. Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res (2017) 77:6795–811. doi: 10.1158/0008-5472.CAN-17-2285
275. Boasso A, Vaccari M, Fuchs D, Hardy AW, Tsai WP, Tryniszewska E, et al. Combined Effect of Antiretroviral Therapy and Blockade of IDO in SIV-Infected Rhesus Macaques. J Immunol (2009) 182:4313–20. doi: 10.4049/jimmunol.0803314
276. Williams B, Landay A, Presti RM. Microbiome Alterations in HIV Infection a Review. Cell Microbiol (2016) 18:645–51. doi: 10.1111/cmi.12588
277. Klatt NR, Canary LA, Sun X, Vinton CL, Funderburg NT, Morcock DR, et al. Probiotic/Prebiotic Supplementation of Antiretrovirals Improves Gastrointestinal Immunity in SIV-Infected Macaques. J Clin Invest (2013) 123:903–7. doi: 10.1172/JCI66227
278. Trois L, Cardoso EM, Miura E. Use of Probiotics in HIV-Infected Children: A Randomized Double-Blind Controlled Study. J Trop Pediatr (2008) 54:19–24. doi: 10.1093/tropej/fmm066
279. Anukam KC, Osazuwa EO, Osadolor HB, Bruce AW, Reid G. Yogurt Containing Probiotic Lactobacillus Rhamnosus GR-1 and L. Reuteri RC-14 Helps Resolve Moderate Diarrhea and Increases CD4 Count in HIV/AIDS Patients. J Clin Gastroenterol (2008) 42:239–43. doi: 10.1097/MCG.0b013e31802c7465
280. d’Ettorre G, Rossi G, Scagnolari C, Andreotti M, Giustini N, Serafino S, et al. Probiotic Supplementation Promotes a Reduction in T-Cell Activation, an Increase in Th17 Frequencies, and a Recovery of Intestinal Epithelium Integrity and Mitochondrial Morphology in ART-treated HIV-1-Positive Patients. Immun Inflamm Dis (2017) 5:244–60. doi: 10.1002/iid3.160
281. Fujigaki S, Saito K, Sekikawa K, Tone S, Takikawa O, Fujii H, et al. Lipopolysaccharide Induction of Indoleamine 2,3-Dioxygenase is Mediated Dominantly by an IFN-gamma-independent Mechanism. Eur J Immunol (2001) 31:2313–8. doi: 10.1002/1521-4141(200108)31:8<2313::AID-IMMU2313>3.0.CO;2-S
282. Byakwaga H, Boum Y 2nd, Huang Y, Muzoora C, Kembabazi A, Weiser SD, et al. The Kynurenine Pathway of Tryptophan Catabolism, CD4+ T-Cell Recovery, and Mortality Among HIV-Infected Ugandans Initiating Antiretroviral Therapy. J Infect Dis (2014) 210:383–91. doi: 10.1093/infdis/jiu115
283. Swainson LA, Ahn H, Pajanirassa P, Khetarpal V, Deleage C, Estes JD, et al. Kynurenine 3-Monooxygenase Inhibition During Acute Simian Immunodeficiency Virus Infection Lowers PD-1 Expression and Improves Post-Combination Antiretroviral Therapy Cd4(+) T Cell Counts and Body Weight. J Immunol (2019) 203:899–910. doi: 10.4049/jimmunol.1801649
284. Hensley-McBain T, Zevin AS, Manuzak J, Smith E, Gile J, Miller C, et al. Effects of Fecal Microbial Transplantation on Microbiome and Immunity in Simian Immunodeficiency Virus-Infected Macaques. J Virol (2016) 90:4981–9. doi: 10.1128/JVI.00099-16
285. Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, et al. Alpha 4 Beta 7 Integrin Mediates Lymphocyte Binding to the Mucosal Vascular Addressin Madcam-1. Cell (1993) 74:185–95. doi: 10.1016/0092-8674(93)90305-A
286. Byrareddy SN, Kallam B, Arthos J, Cicala C, Nawaz F, Hiatt J, et al. Targeting alpha4beta7 Integrin Reduces Mucosal Transmission of Simian Immunodeficiency Virus and Protects Gut-Associated Lymphoid Tissue From Infection. Nat Med (2014) 20:1397–400. doi: 10.1038/nm.3715
287. Ansari AA, Reimann KA, Mayne AE, Takahashi Y, Stephenson ST, Wang R, et al. Blocking of alpha4beta7 Gut-Homing Integrin During Acute Infection Leads to Decreased Plasma and Gastrointestinal Tissue Viral Loads in Simian Immunodeficiency Virus-Infected Rhesus Macaques. J Immunol (2011) 186:1044–59. doi: 10.4049/jimmunol.1003052
288. Sneller MC, Clarridge KE, Seamon C, Shi V, Zorawski MD, Justement JS, et al. An Open-Label Phase 1 Clinical Trial of the anti-alpha4beta7 Monoclonal Antibody Vedolizumab in HIV-Infected Individuals. Sci Transl Med (2019) 11(509):eaax3447. doi: 10.1126/scitranslmed.aax3447
289. Ziani W, Shao J, Fang A, Connolly PJ, Wang X, Veazey RS, et al. Mucosal Integrin alpha4beta7 Blockade Fails to Reduce the Seeding and Size of Viral Reservoirs in SIV-Infected Rhesus Macaques. FASEB J (2021) 35:e21282. doi: 10.1096/fj.202002235R
Keywords: human immunodeficiency virus, simian immunodeficiency virus (SIV), AIDS, microbial translocation, immune activation (IA), inflammation, CD4+ T cells
Citation: Le Hingrat Q, Sereti I, Landay AL, Pandrea I and Apetrei C (2021) The Hitchhiker Guide to CD4+ T-Cell Depletion in Lentiviral Infection. A Critical Review of the Dynamics of the CD4+ T Cells in SIV and HIV Infection. Front. Immunol. 12:695674. doi: 10.3389/fimmu.2021.695674
Received: 15 April 2021; Accepted: 09 June 2021;
Published: 21 July 2021.
Edited by:
Vijayakumar Velu, Emory University, United StatesReviewed by:
Jerome Estaquier, Laval University, CanadaCopyright © 2021 Le Hingrat, Sereti, Landay, Pandrea and Apetrei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristian Apetrei, YXBldHJlaWNAcGl0dC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.