
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 09 July 2021
Sec. B Cell Biology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.695220
 Mark A. Hutchinson1†
Mark A. Hutchinson1† Han-Sol Park1†
Han-Sol Park1† Kimberly J. Zanotti1
Kimberly J. Zanotti1 Juan Alvarez-Gonzalez1
Juan Alvarez-Gonzalez1 Jing Zhang2
Jing Zhang2 Li Zhang2
Li Zhang2 Richard Telljohann2
Richard Telljohann2 Mingyi Wang2
Mingyi Wang2 Edward G. Lakatta2
Edward G. Lakatta2 Patricia J. Gearhart1
Patricia J. Gearhart1 Robert W. Maul1*
Robert W. Maul1*Current models stipulate that B cells and antibodies function during atherosclerosis in two distinct ways based on antibody isotype, where IgM is protective and IgG is inflammatory. To examine this model, we generated ApoE-/- Aid-/- mice, which are unable to produce IgG antibodies due to the absence of activation-induced deaminase (AID) but maintain high plasma cholesterol due to the absence of apolipoprotein E (APOE). We saw a dramatic decrease in plaque formation in ApoE-/- Aid-/- mice compared to ApoE-/- mice. Rigorous analysis of serum antibodies revealed both ApoE-/- and ApoE-/- Aid-/- mice had substantially elevated titers of IgM antibodies compared to C57BL/6J controls, suggesting a more complex dynamic than previously described. Analysis of antigen specificity demonstrated that ApoE-/- Aid-/- mice had elevated titers of antibodies specific to malondialdehyde-oxidized low density lipoprotein (MDA-oxLDL), which has been shown to block macrophage recruitment into plaques. Conversely, ApoE-/- mice showed low levels of MDA-oxLDL specificity, but had antibodies specific to numerous self-proteins. We provide evidence for a hierarchical order of antibody specificity, where elevated levels of MDA-oxLDL specific IgM antibodies inhibit plaque formation. If the level of MDA-oxLDL specific IgM is insufficient, self-reactive IgM and IgG antibodies are generated against debris within the arterial plaque, resulting in increased inflammation and further plaque expansion.
During atherosclerosis, low density lipoproteins are oxidized (oxLDL) and activate endothelial cells to release cytokines, which then recruit circulating monocytes to invade the arterial wall and uptake oxLDL (1). These inflammatory signals also promote the recruitment of other innate and adaptive immune cells to the plaque (2). This includes B cells, which have been identified in the adventitia and surrounding adipose tissue (3). Depletion of total B cells has been shown to alter atherosclerosis in mouse models (4, 5), leading to proposals that different B cell subsets can either prevent or promote disease. The introduction of B-1 cells into B cell-deficient mice decreased plaque and inflammation (6, 7), presumably by secreting IgM antibodies that bind to oxLDL (8). IgM antibodies could prevent disease by sequestering oxLDL away from macrophages and inhibiting plaque initiation (9–11). In contrast, depletion of the more abundant B-2 cells decreased atherosclerosis (12, 13), suggesting that B-2 cells and their antibodies are inflammatory. Upon activation, B-2 cells form germinal centers and fine-tune their antigen binding through somatic hypermutation and class switch recombination to produce high affinity IgG antibodies. Disruption of germinal center generation substantially decreased atherosclerosis (14, 15). Although B-2 cells express both IgM and IgG, the IgG isotype may bind to atherosclerotic antigens and attach to Fcγ receptors on macrophages and vascular smooth muscle cells to increase foam cell development and initiate hyperplasia, respectively (16–20).
While cellular manipulation indicated a demarcation between B-1 and B-2 cells in inhibiting or promoting atherosclerosis, the mechanisms could simply be ascribed to the isotypes and specificities of antibodies. For example, mice deficient in the ability to produce high levels of serum IgM (sIgM-/- or Xbp1-/-) had increased atherosclerosis (21–23). However, this may be more complicated, as different plasma cell-deficient models (Prdm1-/-) reduced circulating antibodies but decreased plaque (15, 20). Direct examination of antibody function using antibody transfer or immunization also produced inconsistent findings. Treatment of mice with anti-phosphorylcholine-specific IgM diminished plaque development (24), and treatment with IgG from ApoE-/- mice boosted atherosclerosis (15, 20), supporting the theory that IgM is helpful and IgG is harmful. However, immunization with either plaque material or malondialdehyde-oxidized LDL (MDA-oxLDL) significantly prevented atherosclerosis by boosting IgG but not IgM titers (25, 26). These numerous conflicting reports raise essential questions about the role of antibody isotypes and antigen specificity during atherosclerosis.
To address this controversy, we generated double-knockout mice which are deficient in both apolipoprotein E (ApoE) and activation-induced deaminase (AID) to rigorously analyze the function of antibody isotypes. The APOE protein is a component of very-low density lipoprotein (VLDL) and is important for proper binding of VLDL to the LDL receptor in the liver. ApoE deficiency results in elevated levels of serum cholesterol, which is exacerbated by a high-fat diet. AID is a cytosine DNA deaminase expressed in activated B cells to initiate somatic hypermutation and class switch recombination of antibodies (27). Thus, AID deficiency results in a complete block in the production of IgG, IgE, and IgA classes of antibodies, which allows for a direct examination of antibody isotype on atherosclerosis. Additionally, we performed a comprehensive analysis of B cell and antibody identification to address the effects of atherosclerosis on B cell function. Compared to ApoE-/- mice, we found that ApoE-/- Aid-/- mice displayed an 80% reduction in lipid content on the aorta while retaining high levels of serum cholesterol. Meanwhile, examination of antibody concentrations revealed that ApoE-/- mice have a 2.8-fold and 6-fold increase in IgM and IgG1 titers respectively, while ApoE-/- Aid-/- mice had a 4-fold increase in IgM compared to C57BL/6J mice. Surprisingly, the antibodies from ApoE-/- Aid-/- mice showed specificity to malondialdehyde (MDA)-oxLDL, while ApoE-/- mice displayed low levels of MDA-oxLDL binding. Instead, using protein array screening, we identified that the high IgM and IgG1 titers in ApoE-/- mice bound numerous self-proteins. Thus, our results convincingly demonstrate that MDA-oxLDL-specific IgM had a striking effect on inhibiting plaque development. If MDA-oxLDL-specific IgM titers are insufficient, plaque accumulation promotes the generation of autoantibodies driving inflammation and defines a distinct role for antigen specificity during the development and progression of atherosclerosis. Our data support the hypothesis that immunization strategies promoting oxLDL-specific antibody responses will prevent atherosclerosis development.
C57BL/6J and ApoE-/- (B6.129P2-Apoetm1Unc/J) mice were purchased from The Jackson Laboratory. ApoE-/- Aid-/- double-knockout mice were generated in our mouse colony. Male mice were raised on chow diet until they reached full adult maturity at 12 weeks of age and switched to high-fat diet (#D12079B, Research Diets Inc., 21% fat and 0.15% cholesterol) until 28 weeks of age to exacerbate plaque formation. Only male mice were used to eliminate any effects of sex on disease severity (28). Cholesterol levels were measured from animals that fasted overnight using an HDL and LDL/VLDL Quantitation Kit (Sigma) per manufacturer’s recommendations. All animal protocols were reviewed and approved by the Animal Care and Use Committee of the National Institute on Aging.
For Oil Red O staining, freshly dissected intact aortas were fixed with 4% paraformaldehyde. Samples were mixed with propylene glycol to remove water and incubated with 0.5% Oil Red O in propylene glycol solution (Sigma Aldrich). Excess stain was washed with 85% propylene glycol, and stained aortas were imaged with a Leica M165 FC microscope. The staining area was calculated using HALO software (Indica Labs). Aortas were fixed with 4% paraformaldehyde and set in paraffin blocks. Samples were then sectioned and stained with hematoxylin and eosin, or Verhoeff-Van Gieson by AML Laboratories. Images were generated using a BZ-X800 microscope (Keyence).
Spleen cells were obtained by mechanical separation followed by lysis of red blood cells and centrifugation. Lymphocytes were isolated from the peritoneal cavity by exposing the membrane and injecting 5 mL of RPMI 1640 media (Thermo Fisher) into the cavity. The fluid was then collected and centrifuged to obtain lymphocytes. The aorta and its perivascular fat were collected from mice through manual isolation. The tissue was mechanically separated and enzymatically digested [0.8 mg/mL Dispase (Gibco), 0.2 mg/mL Collagenase IA (Sigma), 0.2 mg/mL Collagenase XI (Sigma), and 10 U/mL DNase I (Ambion)] for 20 mins at 37°C to isolate lymphocytes. For analysis, cells were treated with TruStain FcX™ block (Biolegend) and stained using the following antibody panels from Biolegend except where noted. Total B cells: CD19+ (clone 6D5); germinal center B cells: B220+ (clone RA3-6B2), CD38- (clone 90) and GL7+ (clone GL-7); follicular B cells: CD19+, GL7-, CD3e- (clone 145-2C11), GR-1- (clone RB6-8C5), CD93- (clone AA4.1), CD138- (clone 281-2), CD43- (clone S7 from BD Pharmingen), F4/80- (clone BM8), CD21lo (clone 7E9) and CD23+ (clone B3B4); marginal zone B cells: CD19+, GL7-, CD3e-, GR-1-, CD93-, CD138-, CD43-, F4/80-, CD21hi and CD23-; B-1a cells: CD19+, CD43+ and CD5+ (clone 53-7.3); and B-1b cells: CD19+, CD43+ and CD5-.
Malondialdehyde bis(dimethyl acetal) (122 mmol, Sigma Aldrich) was activated by incubation in 1 M HCl for 1 hour and quenched with the addition of 6 M NaOH until it equilibrated at pH 10. Activated MDA precipitate was washed with acetone and dried under vacuum. 0.5 mg APOB100 protein (Abcam) was incubated with 0.5 mg activated MDA for 24 hrs at 37°C and dialyzed into PBS.
Antibody isotype concentrations were detected using a 1:10,000 dilution of serum with a Mouse isotyping panel 1 kit (Meso Scale Diagnostics). Values for mouse IgG2a were not reported due to the C57BL/6J background containing the IgG2c allotype. Additionally, values for IgG3 and IgE were below detectable levels. Lipid-specific antibody levels were detected by coating polystyrene 96-well plates (Biolegend) with antigen containing 1 µg/mL of human LDL, MDA-modified human LDL (Academy Bio-Medical Co), APOB100 (Abcam), or MDA-modified APOB100 in coating buffer (100 µL/well; 35 mmol/L sodium carbonate, 68 mmol/L sodium bicarbonate) and incubated overnight at 4°C. Serum antibodies were analyzed at a dilution of 1:1000 for IgM and 1:200 for IgG1 diluted in 5% BSA and 1x PBS. Detection antibodies (goat anti-mouse IgM-HRP or goat anti-mouse IgG1-HRP, Southern Biotech) were added at 1:10,000 dilution, and signal was detected with TMB peroxidase substrate (Vector Labs). oxLDL serum levels were measured using a 1:20 dilution of serum with the mouse oxidized low density lipoprotein ELISA kit (Mybiosource) per manufacture’s recommendation. Plates were read using a SpectraMax M2 plate reader (Molecular Devices) at 450 nm.
Lipid antigens were screened using PIP Strips (Echelon Biosciences). Each membrane was incubated with a 1:100 dilution of serum and detected with a 1:10000 dilution of α-IgH+L-HRP (Southern Biotech). Signal was detected by addition of 5 mL TMB substrate (Vector Laboratories) per manufacturers recommendation. Protein antigens were determined through the utilization of a human proteome microarray (CDI Labs, HuProt Human Proteome Microarray, v 4.0) (29). Serum (1:1000 dilution) was added to the HuProt array and antibody binding identified after incubation with a fluorescent secondary antibody; Alexa647-anti-mouse-IgM and Cy3-anti-mouse-IgG. Fluorescence was detected and antibody-protein binding assessed, and specificity analyzed. Non-specific hits that directly bound to secondary antibodies were eliminated from the analysis of samples. CDI software was used to quantify the specificities of each sample to specific proteins on the array based on z-score. Z-score is the average of the duplicate spots of a given protein calculated as:
Significant targets were determined by averaging the z-score for each protein among all mice (ApoE-/-, n = 15; C57BL/6J, n = 10) with a cut-off of average z-score > 3. P-values were calculated by two-tailed T-test comparing ApoE-/- with C57BL/6J.
To systematically examine the mechanisms of IgM and IgG on atherosclerosis, 12-wk old C57BL/6J, ApoE-/-, and ApoE-/- Aid-/- mice were placed on a high-fat diet for 16 additional weeks (Figure S1A). We first determined the effects of AID deficiency on diet-induced hypercholesterolemia in the ApoE-/- background. Compared to C57BL/6J controls, ApoE-/- and ApoE-/- Aid-/- mice had a significant 7.2-fold and 5.4-fold increase in total cholesterol levels, respectively (Figure S1B). Thus, the loss of AID function had little influence on total cholesterol accumulation in APOE-deficient mice, in that both strains had higher levels than C57BL/6J. These results reinforce the appropriateness of comparing the two ApoE-/- models for analyzing the effect of IgM vs. switched isotypes on atherosclerosis.
In ApoE-/- mice, plaque development occurs at sites in which blood flow is disrupted. These areas include the aortic valves, aortic arch, and abdominal aorta. To examine the effects of class switching on plaque development, aortas from 28-wk old mice were stained with Oil Red O to analyze lipid accumulation. Consistent with previous reports (30–32), diet-induced atherosclerosis was prevalent in the aortic arch of ApoE-/- mice but not in C57BL/6J controls (Figure 1A). In striking contrast, the loss of class switching drastically reduced plaque levels in ApoE-/- Aid-/- mice. This reduction occurred in both the aortic arch and the abdominal aorta (Figures 1A, B). In addition to total reduction in staining, ApoE-/- Aid-/- mice also had reduced plaque volume, with smaller expansion into the vessel lumen (Figure S2). Quantification of plaque staining showed that ApoE-/- mice displayed detectable levels of Oil Red O staining in 22% of the aortic surface, while ApoE-/- Aid-/- mice had significantly reduced staining covering only 4% of the total aorta (Figure 1C). These data robustly demonstrate that inhibiting antibody diversification substantially altered atherosclerosis even in the presence of hypercholesterolemia.
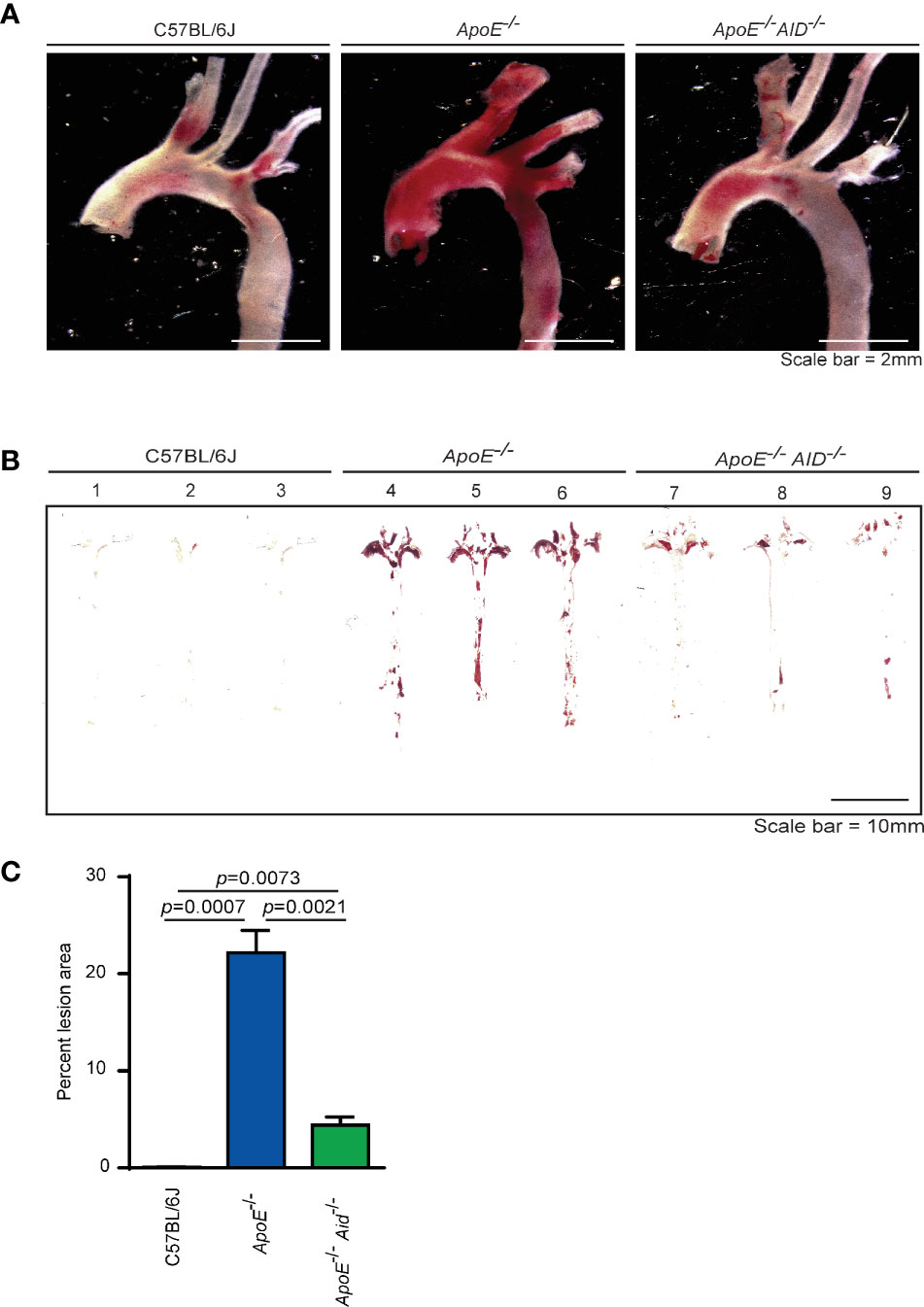
Figure 1 Lipid accumulation in aortas from mice fed a high fat diet for 4 months. (A) Representative images of Oil Red O-stained aortic arch and branches and (B) en face thoracic and abdominal aortas from 3 mice each from C57BL/6J, ApoE-/- and ApoE-/- Aid-/- genotypes. (C) Quantification of Oil Red O staining area from en face images using HALO software. Error-bars represent standard deviation from a total of 11-15 mice per genotype. Statistics were calculated by two-tailed T-test.
It has been speculated that the severity of atherosclerosis can be modulated by altering specific populations of B cells. For example, it was reported that splenic marginal zone B cells increased plaque development when depleted (33), peritoneal B-1a cells either promoted atherosclerosis when cells were depleted or inhibited plaque size when cells were expanded (6, 7, 34), and a novel splenic subset termed IRA B cells which express GM-CSF was associated with the disease (35). Therefore, we systematically examined alterations in lymphocyte populations from C57BL/6J, ApoE-/-, and ApoE-/- Aid-/- mice on the high-fat diet. The splenic populations had the same percentage of total B cells (Figure 2A). Germinal center B cells were significantly increased in ApoE-/- and ApoE-/- Aid-/- mice, suggesting that the elevated serum cholesterol in both strains (Figure S1) could activate B cells systemically (Figure 2B). Examination of splenic B cells revealed no statistical changes in follicular and marginal zone cells between the three strains (Figures 2C, D). We were unable to evaluate IRA B cells, since they only appeared in appreciable amounts in mice with severe dermatitis, which were excluded from the study. Notably, the B-1a and B-1b populations in the peritoneal cavity had similar levels among the three strains, and were not significantly increased in ApoE-/- Aid-/- mice (Figures 2E, F).
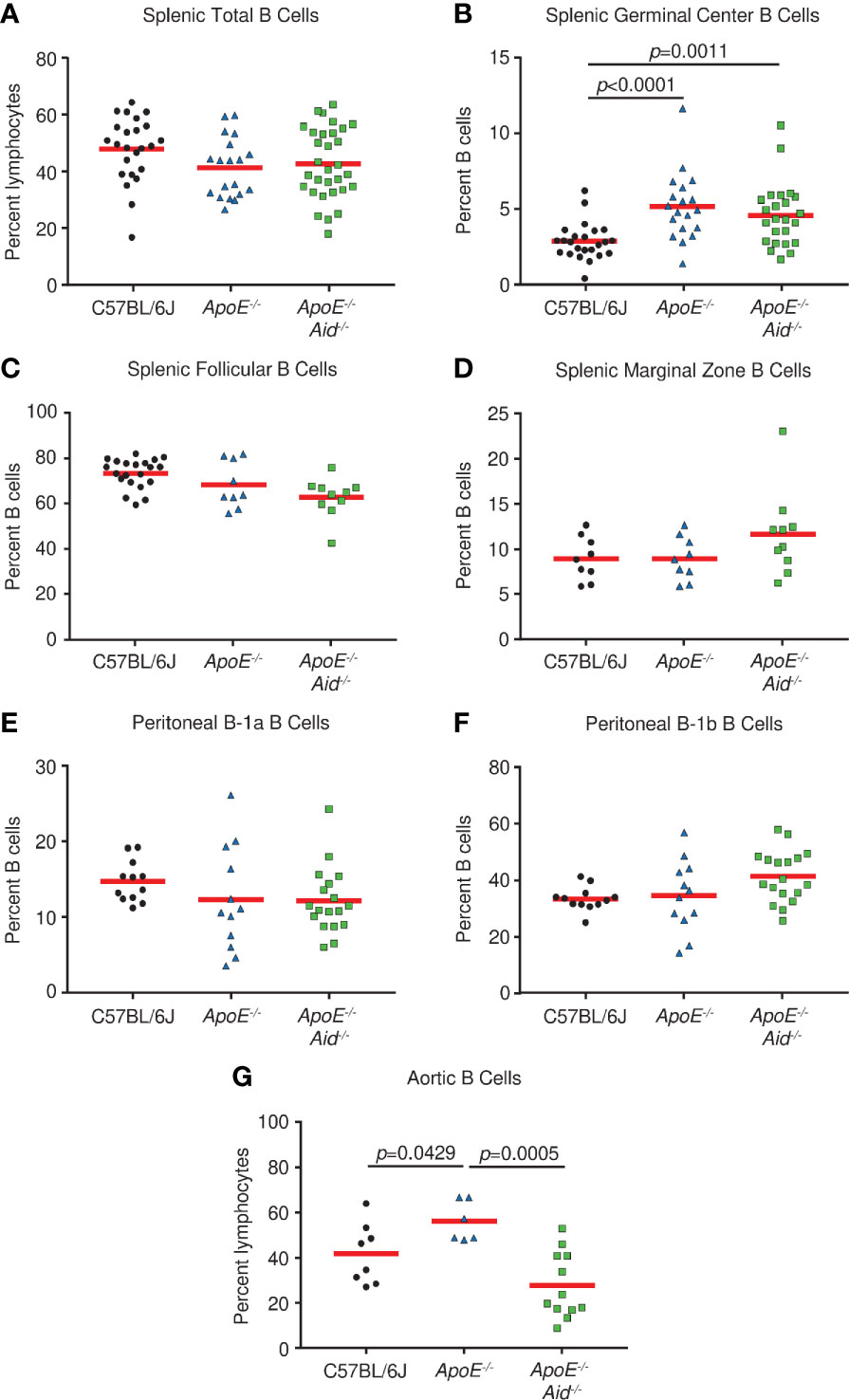
Figure 2 B cell subsets in spleen, peritoneal cavity, and aorta from C57BL/6J, ApoE-/- and ApoE-/- Aid-/- mice. (A) Total, (B) germinal center, (C) follicular, and (D) marginal zone B cells were analyzed from spleens of mice at 28 weeks. (E) B1a and (F) B-1b cells were isolated from the peritoneal cavity. (G) Aortic B cells were isolated from the aorta and adjacent fat layer. Symbols represent percentages in individual mice with red line displaying the mean. p-values represent two-tailed T-test.
Although rampant atherosclerosis in the arteries from ApoE-/- mice did not appear to alter B cell populations from spleen or peritoneum, it may affect B cells at the site of inflammation. B cells have recently been identified in the adipose tissue surrounding the aorta (3); therefore we examined the aorta and surrounding fatty tissue for lymphocyte content. Compared to C57BL/6J controls, ApoE-/- mice had increased numbers of B cells (Figure 2G). However, this phenotype was absent in ApoE-/- Aid-/- mice, which shows that atherosclerosis itself was associated with local B cell expansion. Our data demonstrates that atherosclerosis in ApoE-/- mice affected B cell content on the aorta and not in the spleen, whereas serum hypercholesterolemia in ApoE-/- and ApoE-/- Aid-/- mice appeared to systemically activate splenic germinal center B cells.
To test the hypothesis that IgM and IgG antibodies function in opposing roles during atherosclerosis, we examined the effects of hypercholesterolemia and AID deficiency on IgM and IgG titers. Sera was collected at the start of the high-fat diet at 12 weeks of age, midway at 20 weeks, and the end at 28 weeks. Immunoglobulin concentrations were measured by multiplex ELISA for IgM, IgG1, IgG2b, and IgA (Figures 3A, B, S3A, B). For IgM levels (Figures 3A, S3C), C57BL/6J mice increased from 0.34 mg/mL to 1.35 mg/mL, and ApoE-/- mice expanded from 1.46 to 3.72 mg/mL. This indicates that diet and the resulting development of atherosclerosis boosts IgM titers, presumably by reacting to cholesterol epitopes. In the absence of AID, IgM titers were substantially elevated by the inability to class switch, as previously seen in Aid-/- mice (36) and humans with hyper-IgM syndrome (37). Consistent with this genetic block, ApoE-/- Aid-/- had 4.20 mg/mL IgM prior to the start of the high-fat diet, which increased to 5.45 mg/mL.
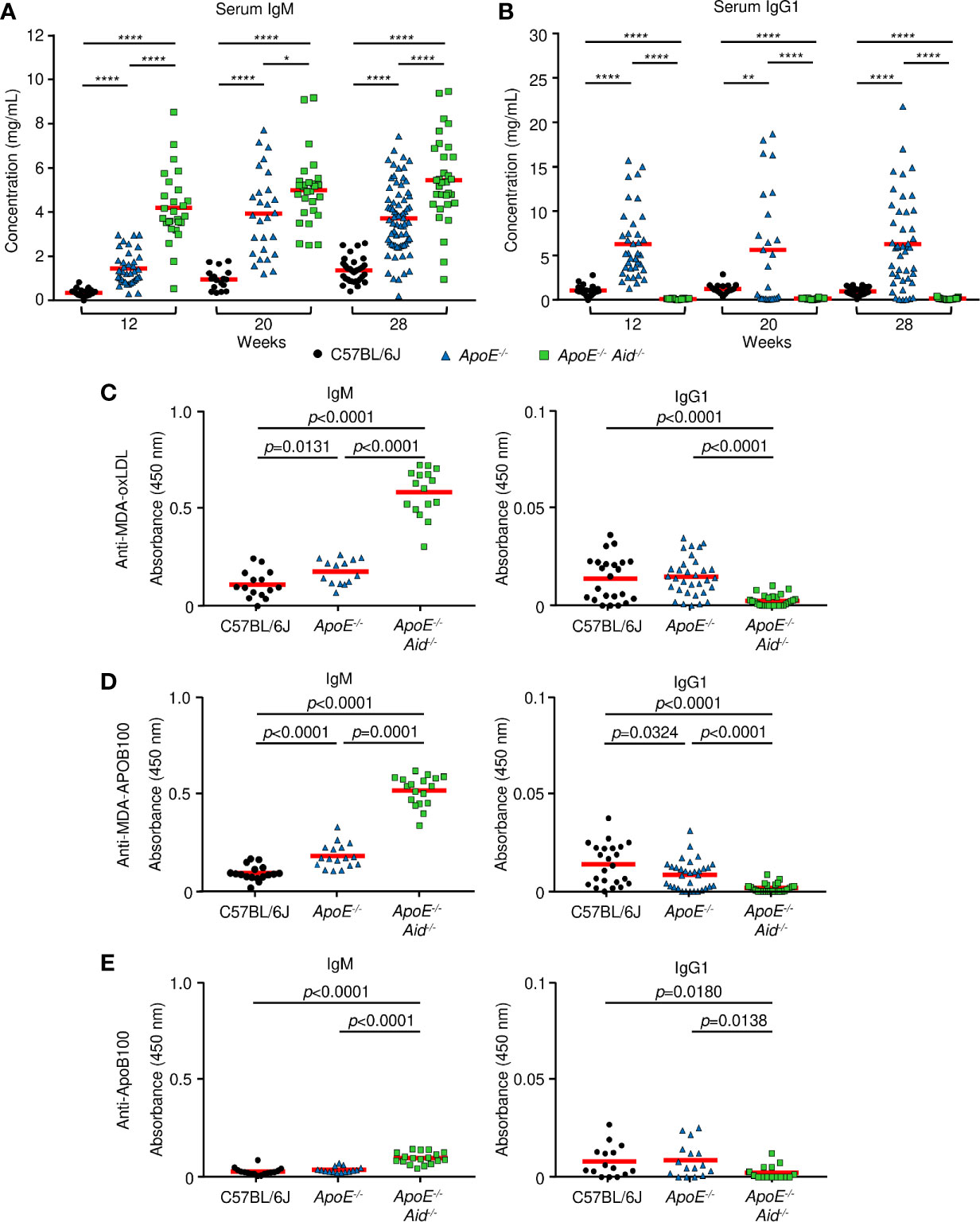
Figure 3 Serum antibody concentrations from mice. (A, B) total immunoglobulin measured by ELISA at 12, 20 and 28 weeks on a high fat diet. (A) IgM and (B) IgG1 from C57BL/6J (black circle), ApoE-/- (blue triangle) or ApoE-/- Aid-/- (green square) mice. Red line represents the mean from 17 or more independent mice. *0.05 > p > 0.01, **0.01 > p > 0.001, ***0.001 > p > 0.0001, ****p < 0.0001. (C–E), IgM and IgG1 antibody titers measured by ELISA at 28 weeks on the diet. (C) MDA-oxLDL, (D) MDA-ApoB100, and (E) ApoB100. IgM sera was diluted 1:1000, and IgG1 sera was diluted 1:200 for analysis. Symbols represent percentages in individual mice with red line displaying the mean from 14-16 mice per genotype. Significance was determined by two-tailed T-test.
For the switched isotypes, IgG1 is the predominant isotype expressed in ApoE-/- serum, relative to IgG2b and IgA. For IgG1 levels, C57BL/6J mice had consistent levels of about 1 mg/mL throughout the diet, and ApoE-/- mice had consistent levels of around 6 mg/mL (Figure 3B). Thus, at multiple time points, ApoE-/- mice had very high levels of IgG1 compared to C57BL/6J mice. As expected, ApoE-/- Aid-/- mice had no IgG1. IgG2b levels were relatively constant at about 1.5 mg/mL in C57BL/6J and ApoE-/- sera from 12-28 weeks (Figures S3A, C). IgA levels were constant in C57BL/6J mice at 0.14 mg/mL and were slightly elevated in ApoE-/- mice at around 0.5 mg/mL (Figures S3B, C). ApoE-/- Aid-/- mice had only background levels of class-switched serum antibodies (Figure S3C). This serum analysis shows that in ApoE-/- mice, both IgM and IgG1 were elevated, yet the kinetics show that high IgG1 titers occurred prior to the high-fat diet while IgM titers were further boosted by the diet. The observation that both the ApoE-/- Aid-/- and ApoE-/- mice have elevated IgM titers, suggests a more complex dynamic where IgM titers by themselves are insufficient at controlling atherosclerosis.
Do IgM antibodies from ApoE-/- Aid-/- mice bind different antigens than IgM antibodies from ApoE-/- mice? To examine antigen specificity, we determined if serum antibodies could bind to MDA-oxLDL, which has been implicated in protection during atherosclerosis (38). IgM titers to MDA-oxLDL were modestly elevated in ApoE-/- serum compared to C57BL/6J controls (Figure 3C). Strikingly, the MDA-oxLDL specific antibody titers were further increased by 3-fold in the ApoE-/- Aid-/- mice (Figure 3C). In contrast, only modest and similar levels of IgG1 to MDA-oxLDL was seen in C57BL/6J and ApoE-/- serum (Figure 3C), consistent with previous observations (26, 39). This indicates that although the ApoE-/- mice had high levels of circulating IgG1, it was directed against different antigens than those recognized by IgM from ApoE-/- Aid-/- mice. Similar results were observed for IgM and IgG1 binding to MDA-modified APOB100 protein, suggesting the antibody response is directed against the protein component of LDL (Figure 3D). These antibodies were specific for the oxidized epitope, as they only bound at low levels to unmodified ApoB100 (Figure 3E) or LDL (data not shown). To test whether the different levels of MDA-LDL antibodies were due to altered oxLDL levels in each strain of mice, we analyzed serum oxLDL concentrations. ApoE-/- mice had the highest levels of oxLDL at 88.4 ng/mL while ApoE-/- Aid-/- mice had 84.0 ng/mL and C57BL/6J had only 75 ng/mL. Thus, the differences in antibody specificity were not a result of lower antigen exposure in ApoE-/- mice.
When the modest levels of MDA-specific antibodies are compared to the drastic elevation of total serum Ig in ApoE-/- mice, it becomes clear that other antigens are driving most B cell responses during plaque formation. To identify these antigens, we first looked at antibodies specific to phospholipids as they have been suggested to play a role in atherosclerosis (40). Analysis of 15 different lipid molecules produced no significant signal indicating that the majority of antibodies are not against lipids (data not shown). Due to the established link between autoimmune disorders and heart disease (41), we then investigated whether these antibodies were generated against self-proteins on a microarray containing 81% of the human proteome. To control for cross-species binding, serum was tested from the C57BL/6J high-fat diet control mice. Analysis of 23,120 protein targets showed that IgM from ApoE-/- mice significantly bound (average z-score > 3) 221 proteins (Data File S1), while IgM from C57BL/6J mice bound 120 proteins (Data File S2), with an overlap of 50 (Figure 4A). To identify proteins which were specific to ApoE-/- IgM, the δ z-score values was calculated for all targets which displayed significant IgM binding in either ApoE-/- or C57BL/6J serum (291 proteins). Plotting δ z-score vs -log(p-value) showed that there were 121 targets that were significantly different (p < 0.01), of which 75 bound to more IgM in the ApoE-/- serum compared to C57BL/6J (Figure 4B). One target, Cytoplasmic polyadenylation element-binding protein 3 (CPEB3), appeared multiple times, suggesting antibody binding to conserved regions of the various CPEB3 isoforms on the array, further verifying accuracy of the technique. Furthermore, analysis of z-score values from individual mice confirmed that levels of IgM antibodies to CREB3, Nectin-3 (PVRL3), Vasculin (GPBP1), and 40S ribosomal protein SA (RPSA), were consistently higher in ApoE-/- serum compared to C57BL/6J serum (Figure 4C). Similar analysis of IgG1 protein targets revealed 212 significant proteins for ApoE-/- IgG1 (Data File S3) and 124 for C57BL/6J IgG1 (Data File S4), with 47 shared proteins (Figure 4D). Analysis of δ z-score vs -log(p-value) values for the 289 proteins identified, 99 were significantly different between the two strains. 59 targets were found more often in ApoE-/- serum, including RSPA, PVRL3, GPBP1, and Family with sequence similarity 131 member C (FAM131c), and were consistently higher when looking at individual mice (Figures 4E, F). To examine if ApoE-/- Aid-/- mice also produced self-antibodies, we analyzed IgM z-scores for the top ApoE-/- targets identified in Figure 4B. Compared to C57BL/6J serum, ApoE-/- Aid-/- IgM antibodies do display elevated binding to these antigens, but they are lower than the ApoE-/- serum, consistent with reduced plaque burden in the absence of AID (Figure S4).
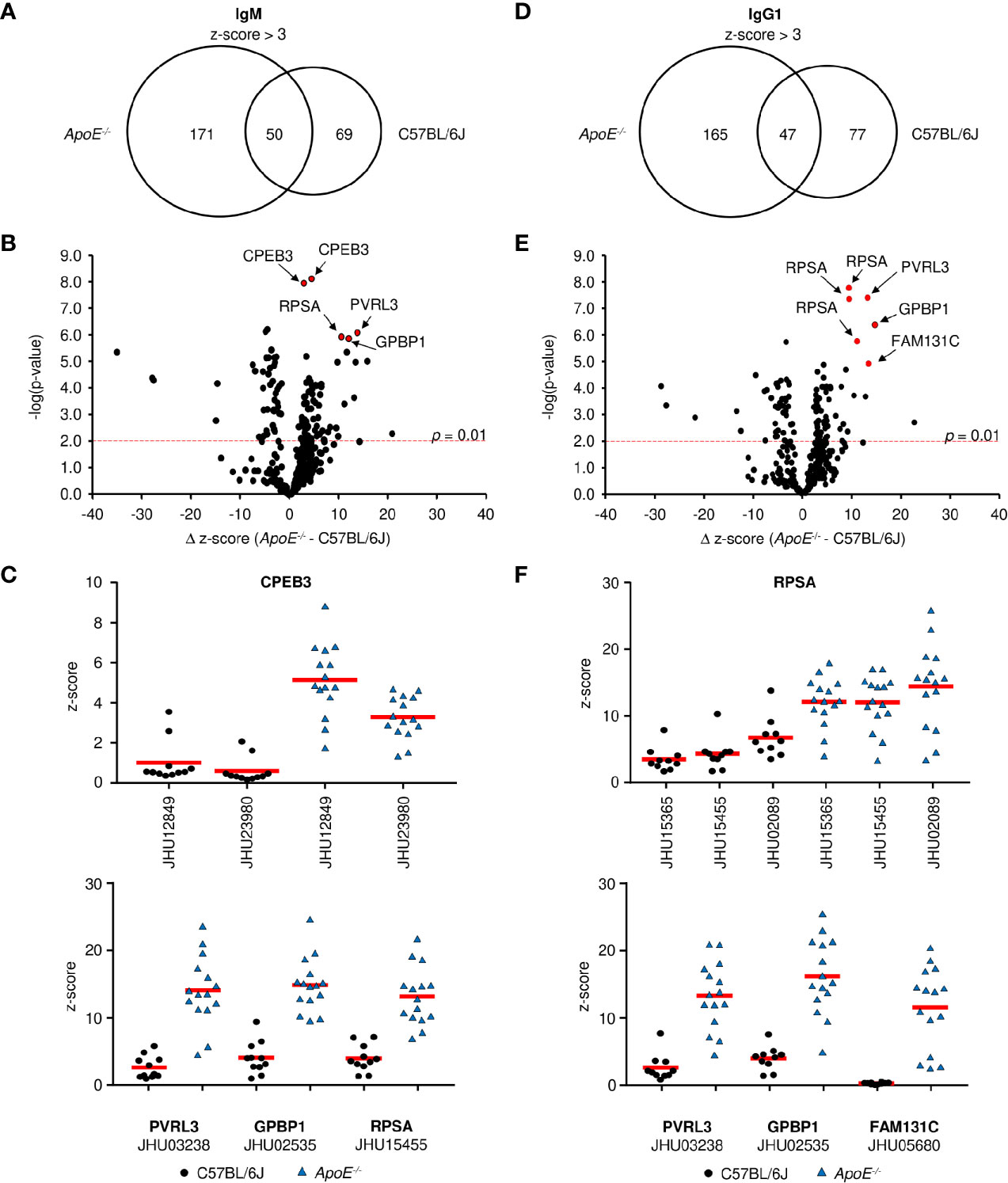
Figure 4 Antigen identification in serum from ApoE-/- and C57BL/6J mice. (A, D) Venn diagrams showing the overlap of (A) IgM and (D) IgG1 antigen targets in ApoE-/- and C57BL/6J mice. Volcano plots examined the change in z-score between targets in ApoE-/- and C57BL/6J mice and the relative significance of those differences for (B) IgM and (E) IgG1. Red dashed line represents a p-value of 0.01 (two-tailed T-test). (C, F) Z-score values for representative antigens identified by red circles in (B, E) in all individual mice for (C) IgM and (F) IgG1. Values were calculated from 10 C57BL/6J and 15 ApoE-/- mice. Red solid line represents mean value.
The observation that the ApoE-/- IgM and IgG1 protein targets identified similar proteins (i.e. RPSA, PVRL3, GPBP1) suggests that the B-2 antibody response is not isotype specific. To address this, we analyzed average z-scores for all protein targets and found that 72% of targets (z-score > 3) are shared between IgM and IgG1 for ApoE-/- mice (Figure 5A), with only 20 targets showing statistically significant differences (p < 0.01). The observation is also found with C57BL/6J mice where 72% of all targets are shared between IgM and IgG1 (Figure 5B) and only 18 being statistically different. These results confirm that in atherosclerotic mice, antibody responses are generated against self-proteins and are independent of isotype.
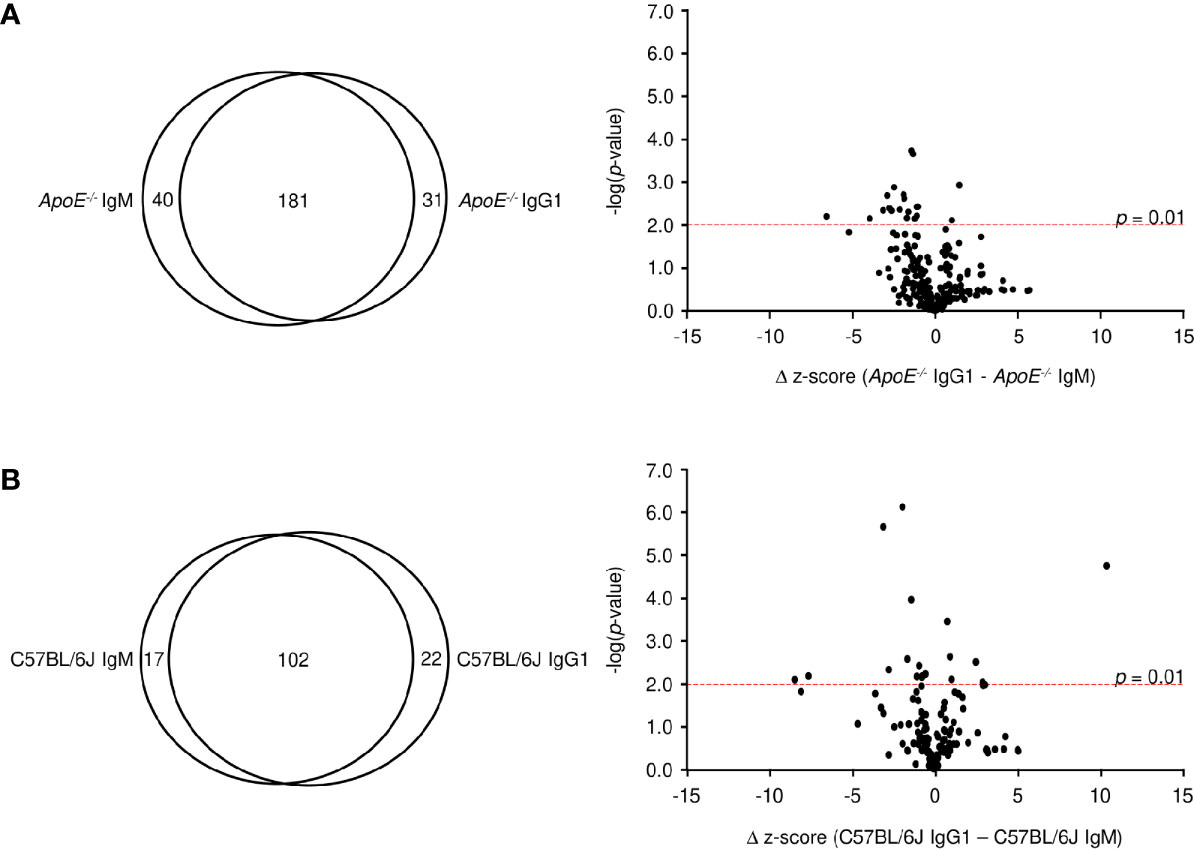
Figure 5 Comparative analysis of IgM and IgG1 antibodies. Venn diagram and volcano plots comparing significant protein targets for IgM and IgG within (A) ApoE-/- and (B) C57BL/6J serum. Red dashed line represents a p-value of 0.01 determined by two-tailed T test.
To characterize the role of B cells and antibodies in atherosclerosis, we generated ApoE-/- Aid-/- mice which develop hypercholesterolemia and are deficient in generating class-switched antibodies. In the absence of switched isotypes, these mice had dramatically less arterial plaque than their ApoE-/- counterparts. Previous experiments have shown that germline-encoded IgM produced by B-1 cells is protective (6, 7). By increasing the presence of B-1a or B-1b cells in a mouse, through adoptive transfer or genetic manipulation, oxLDL-specific IgM antibody titers were increased and disease was reduced. However, in our study, B-1 cells were not increased in ApoE-/- mice compared to C57BL/6J mice. More importantly, the protective MDA-oxLDL specific IgM titers only modestly increased in ApoE-/- compared to C57BL6J mice and were insufficient at controlling disease development with the increased lipid content. Thus, our data imply that B-1 cells are providing a basal level of antibody protection, but are unable to produce sufficient levels of antibody to control hyperlipidemia. In contrast, IgM serum levels were greatly raised in ApoE-/- Aid-/- mice, with elevated titers of antibody against MDA-oxLDL and its protein component MDA-APOB100, suggesting the B-2 cell response can provide protection when directed toward the correct antigen. However, in ApoE-/- mice, IgM and IgG1 did not bind to MDA-oxLDL at high levels but bound to numerous self-proteins, presumably increasing inflammation and promoting atherosclerosis consistent with previous studies (12, 13). These antibodies were detected against intracellular proteins, suggesting the environment of the plaque is driving the B cell adaptive response, consistent with our observation of increased B cells around the aorta.
These data support a hierarchy in appearance of antibody specificity to their cognate antigens, where the balance between oxLDL and oxLDL-specific antibodies is a key regulator of disease progression. When MDA-oxLDL specific antibody titers are low (as in ApoE-/- mice) they cannot block oxLDL uptake by macrophages and plaques develop (9, 18). Once established, macrophages convert to foam cells and rupture, releasing self-proteins which could be oxidized/modified in the necrotic environment, and may be the source of the elusive epitopes which elevate IgM and IgG1 titers in ApoE-/- mice. This theory is consistent with data showing a correlation between autoimmune diseases which generate high levels of self-antibodies and which develop accelerated atherosclerosis (41). B cells specific for these antigens expand and produce autoantibodies, which may bind to Fcγ receptors on macrophages (16–19) and vascular smooth muscle cells (16, 20), to release inflammatory cytokines and set up a virtual war zone in the artery. It will be interesting to see if the autoantibodies against a specific protein (i.e., CPEB3 or RPSA) promote a particular inflammatory response in the aorta, or if any self-protein or combination of self-proteins can generate inflammation and plaque formation.
Why do ApoE-/- Aid-/- mice produce high titers of MDA-oxLDL specific antibodies? In addition to CSR deficiency, the mice also have no somatic hypermutation, causing only germline-encoded antibodies to be expressed, which may bind to the lipids with sufficient affinity (6). Although ApoE-/- mice produce a low level of MDA-oxLDL antibodies, it is possible that mutated antibodies drift away from binding MDA-oxLDL and decrease the response. Alternatively, during atherosclerosis, more prominent self-antigens (i.e., RPSA) are released to hijack the immune response and drive the generation of high-affinity auto-antibodies. Both possibilities may not occur in ApoE-/- Aid-/- mice due to the absence of SHM.
In conclusion, we show that high levels of oxLDL antibodies prevent atherosclerosis, consistent with recent experiments showing an antigen-specific Fab molecule can regulate plaque formation (40). However, in ApoE-/- mice, the oxLDL antibody response is inadequate and the response is directed towards self-antigens potentially coming from the plaque environment. Strategies which boost antibody responses to MDA-oxLDL or block self-antigen recognition, could prevent lipid accumulation and decrease atherosclerosis.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
All animal protocols were reviewed and approved by the Animal Care and Use Committee of the National Institute on Aging.
The concept was conceived by MW, EL, PG, and RM. The overall study design was developed by MH, H-SP, and RM. Additional experiments were performed by KZ, JA-G, JZ, LZ, and RT. Manuscript was written by MH, PG, and RM. All authors contributed to the article and approved the submitted version.
This work was supported entirely through the Intramural Research Program at the National Institutes of Health (NIH), National Institute on Aging (AG000777). The authors have no disclosures to report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.695220/full#supplementary-material
1. Gimbrone MA Jr., Garcia-Cardena G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res (2016) 118:620–36. doi: 10.1161/CIRCRESAHA.115.306301
2. Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, et al. Immune Activation Caused by Vascular Oxidation Promotes Fibrosis and Hypertension. J Clin Invest (2016) 126:1607. doi: 10.1172/JCI87425
3. Srikakulapu P, Upadhye A, Rosenfeld SM, Marshall MA, McSkimming C, Hickman AW, et al. Perivascular Adipose Tissue Harbors Atheroprotective IgM-Producing B Cells. Front Physiol (2017) 8:719. doi: 10.3389/fphys.2017.00719
4. Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective Immunity Against Atherosclerosis Carried by B Cells of Hypercholesterolemic Mice. J Clin Invest (2002) 109:745–53. doi: 10.1172/JCI7272
5. Major AS, Fazio S. And Linton MF. B-Lymphocyte Deficiency Increases Atherosclerosis in LDL Receptor-Null Mice. Arterioscler Thromb Vasc Biol (2002) 22:1892–8. doi: 10.1161/01.ATV.0000039169.47943.EE
6. Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, et al. B1a B Lymphocytes Are Atheroprotective by Secreting Natural IgM That Increases IgM Deposits and Reduces Necrotic Cores in Atherosclerotic Lesions. Circ Res (2011) 109:830–40. doi: 10.1161/CIRCRESAHA.111.248542
7. Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, et al. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circ Res (2015) 117:e28–39. doi: 10.1161/CIRCRESAHA.117.306044
8. Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B Cells and Humoral Immunity in Atherosclerosis. Circ Res (2014) 114:1743–56. doi: 10.1161/CIRCRESAHA.113.301145
9. Boullier A, Gillotte KL, Horkko S, Green SR, Friedman P, Dennis EA, et al. The Binding of Oxidized Low Density Lipoprotein to Mouse CD36 Is Mediated in Part by Oxidized Phospholipids That Are Associated With Both the Lipid and Protein Moieties of the Lipoprotein. J Biol Chem (2000) 275:9163–9. doi: 10.1074/jbc.275.13.9163
10. Shaw PX, Horkko S, Tsimikas S, Chang MK, Palinski W, Silverman GJ, et al. Human-Derived Anti-Oxidized LDL Autoantibody Blocks Uptake of Oxidized LDL by Macrophages and Localizes to Atherosclerotic Lesions In Vivo. Arterioscler Thromb Vasc Biol (2001) 21:1333–9. doi: 10.1161/hq0801.093587
11. Tsimikas S, Miyanohara A, Hartvigsen K, Merki E, Shaw PX, Chou MY, et al. Human Oxidation-Specific Antibodies Reduce Foam Cell Formation and Atherosclerosis Progression. J Am Coll Cardiol (2011) 58:1715–27. doi: 10.1016/j.jacc.2011.07.017
12. Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, et al. B Cell Depletion Reduces the Development of Atherosclerosis in Mice. J Exp Med (2010) 207:1579–87. doi: 10.1084/jem.20100155
13. Kyaw T, Tay C, Hosseini H, Kanellakis P, Gadowski T, MacKay F, et al. Depletion of B2 But Not B1a B Cells in BAFF Receptor-Deficient ApoE Mice Attenuates Atherosclerosis by Potently Ameliorating Arterial Inflammation. PloS One (2012) 7:e29371. doi: 10.1371/journal.pone.0029371
14. Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of Atherosclerosis in Mice by Inhibition of CD40 Signalling. Nature (1998) 394:200–3. doi: 10.1038/28204
15. Tay C, Liu YH, Kanellakis P, Kallies A, Li Y, Cao A, et al. Follicular B Cells Promote Atherosclerosis via T Cell-Mediated Differentiation Into Plasma Cells and Secreting Pathogenic Immunoglobulin G. Arterioscler Thromb Vasc Biol (2018) 38:e71–84. doi: 10.1161/ATVBAHA.117.310678
16. Hernandez-Vargas P, Ortiz-Munoz G, Lopez-Franco O, Suzuki Y, Gallego-Delgado J, Sanjuan G, et al. Fcgamma Receptor Deficiency Confers Protection Against Atherosclerosis in Apolipoprotein E Knockout Mice. Circ Res (2006) 99:1188–96. doi: 10.1161/01.RES.0000250556.07796.6c
17. Ng HP, Zhu X, Harmon EY, Lennartz MR, Nagarajan S. Reduced Atherosclerosis in apoE-Inhibitory FcgammaRIIb-Deficient Mice Is Associated With Increased Anti-Inflammatory Responses by T Cells and Macrophages. Arterioscler Thromb Vasc Biol (2015) 35:1101–12. doi: 10.1161/ATVBAHA.115.305290
18. Zhu X, Ng HP, Lai YC, Craigo JK, Nagilla PS, Raghani P, et al. Scavenger Receptor Function of Mouse Fcgamma Receptor III Contributes to Progression of Atherosclerosis in Apolipoprotein E Hyperlipidemic Mice. J Immunol (2014) 193:2483–95. doi: 10.4049/jimmunol.1303075
19. Mendez-Fernandez YV, Stevenson BG, Diehl CJ, Braun NA, Wade NS, Covarrubias R, et al. The Inhibitory FcgammaRIIb Modulates the Inflammatory Response and Influences Atherosclerosis in Male Apoe(-/-) Mice. Atherosclerosis (2011) 214:73–80. doi: 10.1016/j.atherosclerosis.2010.10.018
20. Centa M, Jin H, Hofste L, Hellberg S, Busch A, Baumgartner R, et al. Germinal Center-Derived Antibodies Promote Atherosclerosis Plaque Size and Stability. Circulation (2019) 139:2466–82. doi: 10.1161/CIRCULATIONAHA.118.038534
21. Tsiantoulas D, Bot I, Ozsvar-Kozma M, Goderle L, Perkmann T, Hartvigsen K, et al. Increased Plasma IgE Accelerate Atherosclerosis in Secreted IgM Deficiency. Circ Res (2017) 120:78–84. doi: 10.1161/CIRCRESAHA.116.309606
22. Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M Is Required for Protection Against Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient Mice. Circulation (2009) 120:417–26. doi: 10.1161/CIRCULATIONAHA.109.868158
23. Sage AP, Nus M, Bagchi Chakraborty J, Tsiantoulas D, Newland SA, Finigan AJ, et al. X-Box Binding Protein-1 Dependent Plasma Cell Responses Limit the Development of Atherosclerosis. Circ Res (2017) 121:270–81. doi: 10.1161/CIRCRESAHA.117.310884
24. Faria-Neto JR, Chyu KY, Li X, Dimayuga PC, Ferreira C, Yano J, et al. Passive Immunization With Monoclonal IgM Antibodies Against Phosphorylcholine Reduces Accelerated Vein Graft Atherosclerosis in Apolipoprotein E-Null Mice. Atherosclerosis (2006) 189:83–90. doi: 10.1016/j.atherosclerosis.2005.11.033
25. Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL Immunization Induces T-Cell-Dependent Antibody Formation and Protection Against Atherosclerosis. Arterioscler Thromb Vasc Biol (2001) 21:108–14. doi: 10.1161/01.ATV.21.1.108
26. Freigang S, Horkko S, Miller E, Witztum JL, Palinski W. Immunization of LDL Receptor-Deficient Mice With Homologous Malondialdehyde-Modified and Native LDL Reduces Progression of Atherosclerosis by Mechanisms Other Than Induction of High Titers of Antibodies to Oxidative Neoepitopes. Arterioscler Thromb Vasc Biol (1998) 18:1972–82. doi: 10.1161/01.ATV.18.12.1972
27. Maul RW, Gearhart PJ. AID and Somatic Hypermutation. Adv Immunol (2010) 105:159–91. doi: 10.1016/S0065-2776(10)05006-6
28. Caligiuri G, Nicoletti A, Zhou X, Tornberg I, Hansson GK. Effects of Sex and Age on Atherosclerosis and Autoimmunity in apoE-Deficient Mice. Atherosclerosis (1999) 145:301–8. doi: 10.1016/S0021-9150(99)00081-7
29. Venkataraman A, Yang K, Irizarry J, Mackiewicz M, Mita P, Kuang Z, et al. A Toolbox of Immunoprecipitation-Grade Monoclonal Antibodies to Human Transcription Factors. Nat Methods (2018) 15:330–8. doi: 10.1038/nmeth.4632
30. Reddick RL, Zhang SH, Maeda N. Atherosclerosis in Mice Lacking Apo E. Evaluation of Lesional Development and Progression. Arterioscler Thromb (1994) 14:141–7. doi: 10.1161/01.ATV.14.1.141
31. Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous Hypercholesterolemia and Arterial Lesions in Mice Lacking Apolipoprotein E. Science (1992) 258:468–71. doi: 10.1126/science.1411543
32. Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-Deficient Mice Develop Lesions of All Phases of Atherosclerosis Throughout the Arterial Tree. Arterioscler Thromb (1994) 14:133–40. doi: 10.1161/01.ATV.14.1.133
33. Nus M, Sage AP, Lu Y, Masters L, Lam BYH, Newland S, et al. Marginal Zone B Cells Control the Response of Follicular Helper T Cells to a High-Cholesterol Diet. Nat Med (2017) 23:601–10. doi: 10.1038/nm.4315
34. Hosseini H, Yi L, Kanellakis P, Cao A, Tay C, Peter K, et al. Anti-TIM-1 Monoclonal Antibody (RMT1-10) Attenuates Atherosclerosis By Expanding IgM-Producing B1a Cells. J Am Heart Assoc (2018) 7(13):e008447. doi: 10.1161/JAHA.117.008447
35. Hilgendorf I, Theurl I, Gerhardt LM, Robbins CS, Weber GF, Gonen A, et al. Innate Response Activator B Cells Aggravate Atherosclerosis by Stimulating T Helper-1 Adaptive Immunity. Circulation (2014) 129:1677–87. doi: 10.1161/CIRCULATIONAHA.113.006381
36. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class Switch Recombination and Hypermutation Require Activation-Induced Cytidine Deaminase (AID), a Potential RNA Editing Enzyme. Cell (2000) 102:553–63. doi: 10.1016/S0092-8674(00)00078-7
37. Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, et al. Activation-Induced Cytidine Deaminase (AID) Deficiency Causes the Autosomal Recessive Form of the Hyper-IgM Syndrome (HIGM2). Cell (2000) 102:565–75. doi: 10.1016/S0092-8674(00)00079-9
38. Binder CJ, Chou MY, Fogelstrand L, Hartvigsen K, Shaw PX, Boullier A, et al. Natural Antibodies in Murine Atherosclerosis. Curr Drug Tar (2008) 9:190–5. doi: 10.2174/138945008783755520
39. Dimayuga PC, Zhao X, Yano J, Chyu KY. Changes in Immune Responses to Oxidized LDL Epitopes During Aging in Hypercholesterolemic Apoe(-/-) Mice. Am J Physiol Regul Integr Comp Physiol (2006) 291:R1644–50. doi: 10.1152/ajpregu.00511.2005
40. Que X, Hung MY, Yeang C, Gonen A, Prohaska TA, Sun X, et al. Oxidized Phospholipids Are Proinflammatory and Proatherogenic in Hypercholesterolaemic Mice. Nature (2018) 558:301–6. doi: 10.1038/s41586-018-0198-8
Keywords: AID, atherosclerosis, B cells, antibodies, antigens
Citation: Hutchinson MA, Park H-S, Zanotti KJ, Alvarez-Gonzalez J, Zhang J, Zhang L, Telljohann R, Wang M, Lakatta EG, Gearhart PJ and Maul RW (2021) Auto-Antibody Production During Experimental Atherosclerosis in ApoE-/- Mice. Front. Immunol. 12:695220. doi: 10.3389/fimmu.2021.695220
Received: 14 April 2021; Accepted: 21 June 2021;
Published: 09 July 2021.
Edited by:
Lee Ann Garrett-Sinha, University at Buffalo, United StatesReviewed by:
Jürgen Bernhagen, Ludwig Maximilian University of Munich, GermanyCopyright © 2021 Hutchinson, Park, Zanotti, Alvarez-Gonzalez, Zhang, Zhang, Telljohann, Wang, Lakatta, Gearhart and Maul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert W. Maul, bWF1bHJ3QG1haWwubmloLmdvdg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.