- 1Department of Pathology, The Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 2Department of Histology and Embryology of Basic Medical Department, Guangdong Medical University, Dongguan, China
- 3Department of Ophthalmology, The Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 4Department of Pathology, The Central Hospital of Wuhan, Huazhong University of Science and Technology, Wuhan, China
- 5Department of Medical Oncology, The Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 6Dermatology Hospital, Southern Medical University, Guangzhou, China
Background: Tumor-infiltrating lymphocytes (TILs) are considered a manifestation of the host immune response against cancer and tertiary lymphoid structures (TLS) may contribute to lymphocytes recruitment. Both of them have been reported as potential prognostic parameters in some human malignancies. However, the roles of TILs, TLS, and their correlation in Epstein-Barr Virus-associated gastric carcinoma (EBVaGC) and EBV-negative gastric carcinoma (EBVnGC) are largely unknown.
Methods: To observe the correlation among TILs, TLS, and clinicopathological characteristics and their prognostic significance in EBVaGC and EBVnGC, respectively. TILs and TLS were assessed by morphology and/or immunohistochemistry, and accompanied by clinicopathological analysis from 846 gastric cancer patients in multiple institutions.
Results: Forty-two (5.0%) cases of EBVaGC and 804 cases of EBVnGC were identified by in situ hybridization, respectively. For EBVnGC, higher TILs grade was correlated with TLS-present. EBVnGC patients with high TILs grade and TLS-present exhibited survival benefits. TILs (P = 0.001) and TLS (P = 0.003), especially TILs & TLS (P < 0.001) were independent prognostic factors in EBVnGC. A nomogram was constructed and validated for predicting the probability of overall survival and performed well with a good calibration. No significant prognostic value was detected in EBVaGC.
Conclusion: TILs and TLS, especially TILs & TLS were promising prognostic indicators for overall survival in EBVnGC. TILs and TLS were highly overlapping in their extent and prognostic abilities, and may be considered as a coindicator of prognosis of gastric cancer. The evaluations of TILs and TLS are simple and can be assessed routinely in pathological diagnosis.
Introduction
Gastric carcinoma (GC) is the fourth leading cause of cancer-related mortality worldwide and the most prevalent cancer in Eastern Asia (1, 2). Immunity plays a key role in tumor initiation and progression, with immune modulation considered to be an important strategy for cancer therapy. As the major type of infiltrating immune cells, tumor-infiltrating lymphocytes (TILs) are a heterogeneous group containing T cells, B cells, and natural killer cells, which have been reported to be related to favorable prognosis in various tumors such as melanoma, breast and nasopharyngeal carcinomas (3–5). The low TILs density could predict regional lymph node metastasis and poor prognosis for recurrence free survival in GC (6). Some suggested that TILs may direct patient selection for immune checkpoint blockade therapy in GC (7, 8). However, a large proportion of patients do not respond to immunotherapy, suggesting other possible immune factors may play a certain role in tumor microenvironment (9, 10).
Tertiary lymphoid structures (TLS), characterized by ectopic aggregated lymphocytes with high endothelial venules, have gained attention because of its correlation with prolonged patient’s survival in some tumors (11, 12). The formation and regulation of TLS involve the same chemokines and cytokines networks that orchestrate lymphoid organogenesis (13, 14). TLS have been reported to be associated with lymphocyte infiltration, represent a privileged area to provide a pathway for the recruitment of TILs, and generate the central-memory T and B cells to limit cancer progression (15, 16). Meanwhile, TLS could cooperate with TILs in a coordinated antitumor immune response (17). The exact prognostic role and the relationship between TILs and TLS in GC remain largely unknown.
Additionally, the association between Epstein-Barr virus (EBV) and GC is thought to be a predictive indicator for immunotherapy (18). Compared with EBV-negative GC (EBVnGC), EBV-associated GC (EBVaGC) has distinct clinicopathological features and most exhibit histology rich in lymphocyte infiltration and relatively favorable prognosis (19, 20).
The present study investigated TILs and TLS in the tumor tissues of patients with GC and evaluate their prognostic significance. In addition, the relationship between tumoral immune parameters such as TILs, TLS, TILs & TLS, and clinicopathological features in 42 EBVaGC and 804 EBVnGC patients was determined.
Materials and Methods
Patients and Specimens
Eight hundred forty-six cases of surgically resected GC were collected from multiple institutions including the First, Third, and Six Affiliated Hospitals of Sun Yat-sen University, from January 2001 to December 2013. An additional 86 GC patients from the Sun Yat-sen Memorial Hospital of Sun Yat-sen University (July 2008 to December 2011) were selected as a validation cohort for the nomogram. None of the patients underwent systematic chemotherapy or radiotherapy before surgery. Cases with cancer confined to mucosa were excluded because they have an excellent prognosis regardless of number of TILs.
Standard pathologic analyses were performed blindly by two experienced pathologists (CN, LP). Any discrepancy was reviewed to reach consensus at a multi-headed microscope. More than two H&E–stained section slides with tumor were obtained per case, and the mean number of slides was 4.72 (range, 3–14). In these slides, at least one slide contained the tumor invasive margin. Clinicopathological data were retrieved from the archives of the medical records and pathologic reports. All patients were restaged according to the American Joint Committee on Cancer (AJCC) Staging Manual, Seventh Edition (21).
Patients’ clinical outcomes were followed up from the date of GC resection until death or December 31, 2016. The data of patients who were alive at the last follow-up date and of those died from a cause other than GC were regarded as censored data.
This study was approved by the Institute Research Ethics Committees of the First, Third, Six Affiliated Hospitals and Sun Yat-sen Memorial Hospital, Sun Yat-sen University. All participants provided written informed consents prior to surgery.
In Situ Hybridization for EBER-1
ISH assay was performed with an EBER-1 oligonucleotide probe (PanPath, Amsterdam, Netherlands), as previously described by Chen et al. (22). Dark brown nuclear staining was considered to be a positive signal. The known EBER-1-positive nasopharyngeal carcinoma tissues were used as the positive control and a sense probe for EBER-1 was used as the negative control.
Immunohistochemistry
Immunohistochemical staining was performed on 4-µm thick sections of tissue samples using an automatic staining device (Ventana Benchmark Ultra immunostainer, Ventana Medical Systems, Inc., Tucson, USA). Antibodies were as follows: mouse anti-CD3 (clone LN10, 1:100, Novocastra), mouse anti-CD20 (clone L26, 1:250, Novocastra), and mouse anti-CD21 (clone 2G9, prediluted, Novocastra). PBS was used as the negative control. A cervical lymph node served as the positive control.
Evaluation of TILs and TLS
No current consensus exists on the morphologic evaluation of TILs in GC, so we adopted and modified the TIL scoring recommendation used in previous studies (23–25). Briefly, global TILs are defined as the mean percentage of the invasive tumor area (including the tumor bed and peri-tumoral stroma) occupied by lymphocytes and plasma cells (23, 26), which was assessed by using a continuous scale as a semiquantitative parameter in 10% increments; if less than 10%, a 1 or 5% criteria was used. All available full-face tumor sections were evaluated, with no focus on hotspots. Area with necrosis, hemorrhage, or crush artifacts was excluded for TILs evaluation.
GC with lymphoid stroma, a rare histological variant of GC with prominent lymphocytic infiltration into the tumor and surrounding stroma, has distinctive clinicopathological and molecular features and is associated with a significantly better prognosis (24, 27). Therefore, patients with TILs level of >50% were classified as a separate group, and patients with TILs level of ≤50% were subdivided into two categories based on the mean value, which was determined as a threshold for survival analysis. As a whole, TILs were divided into three groups: grade 1 (minimal, ≤10%), grade 2 (moderate, 10–50%), and grade 3 (abundant, >50%).
All available sections were screened for the presence of TLS. First, the presence of lymphoid aggregates (LAs) was confirmed, as well as their patterns of organization at the tumor invasive margin and/or within the stroma of GC. Second, LAs with the visible germinal centers were considered as TLS. Third, LAs without visible germinal center were selectively stained by immunohistochemistry. The well-organized LAs with one or more CD20+ B cells aggregations containing CD21+ FDCs, surrounded by a CD3+ T cells rich area were defined as TLS. LAs in the mucosa or submucosa of stomach were excluded (28).
Statistical Analysis
Comparisons among clinicopathologic features, EBV status, TILs, and TLS were performed by the Pearson Chi-Square test or Fisher’s exact test. Pearson correlation analysis was used to examine the correlation between TILs and TLS. Survival distribution was compared using the Kaplan-Meier method and the log-rank test. Prognostic variables associated with overall survival were examined by univariate analyses using a Cox proportional hazards regression model. Only those variables which were significantly associated with survival were enrolled into multivariate regression analyses. A nomogram was generated by R software 3.3, with the discriminative ability assessed by the concordance index (C-index), which ranges from 0.5 (no discrimination at all) to 1.0 (perfect discrimination). Calibration plots were generated to compare the predicted probability of overall survival with the observed outcome. Furthermore, the precision of survival predictions was evaluated using the area under receiver operating characteristic (ROC) curve (AUC) in the validation cohort. Two-sided P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 17.0 statistics software (SPSS Inc., Chicago, IL, USA).
Results
Clinicopathological Features of EBVaGC and EBVnGC
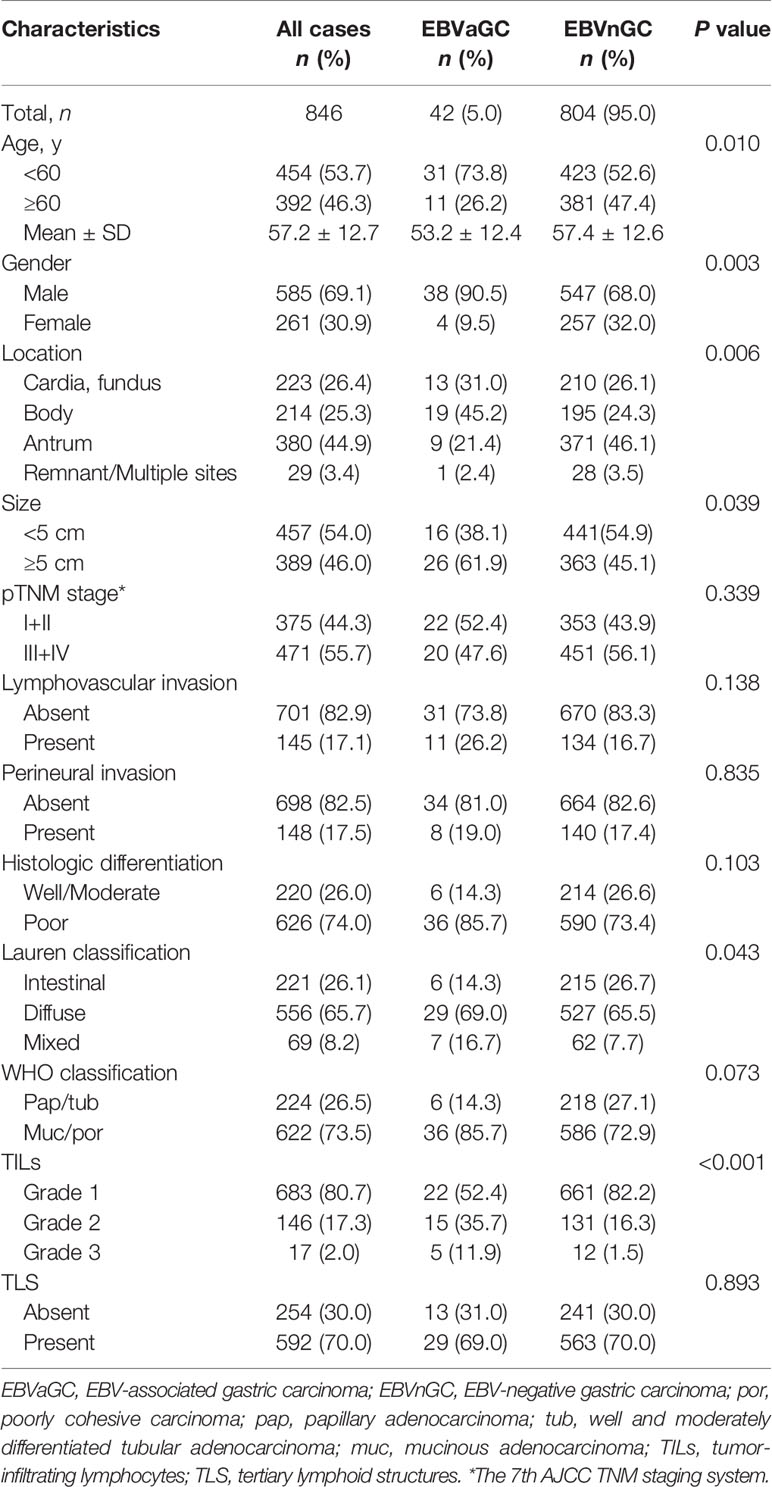
According to the ISH results (Figures 1A–D), 42 of the 846 cases (5.0%) were identified as EBVaGC. As presented in Table 1, EBVaGC displayed distinct clinicopathological features, including younger age (P = 0.010), male predominance (P = 0.003), proximal stomach location (P = 0.006), bigger in tumor size (P = 0.039), Lauren diffuse type (P = 0.043), and higher grade of TILs (P < 0.001).
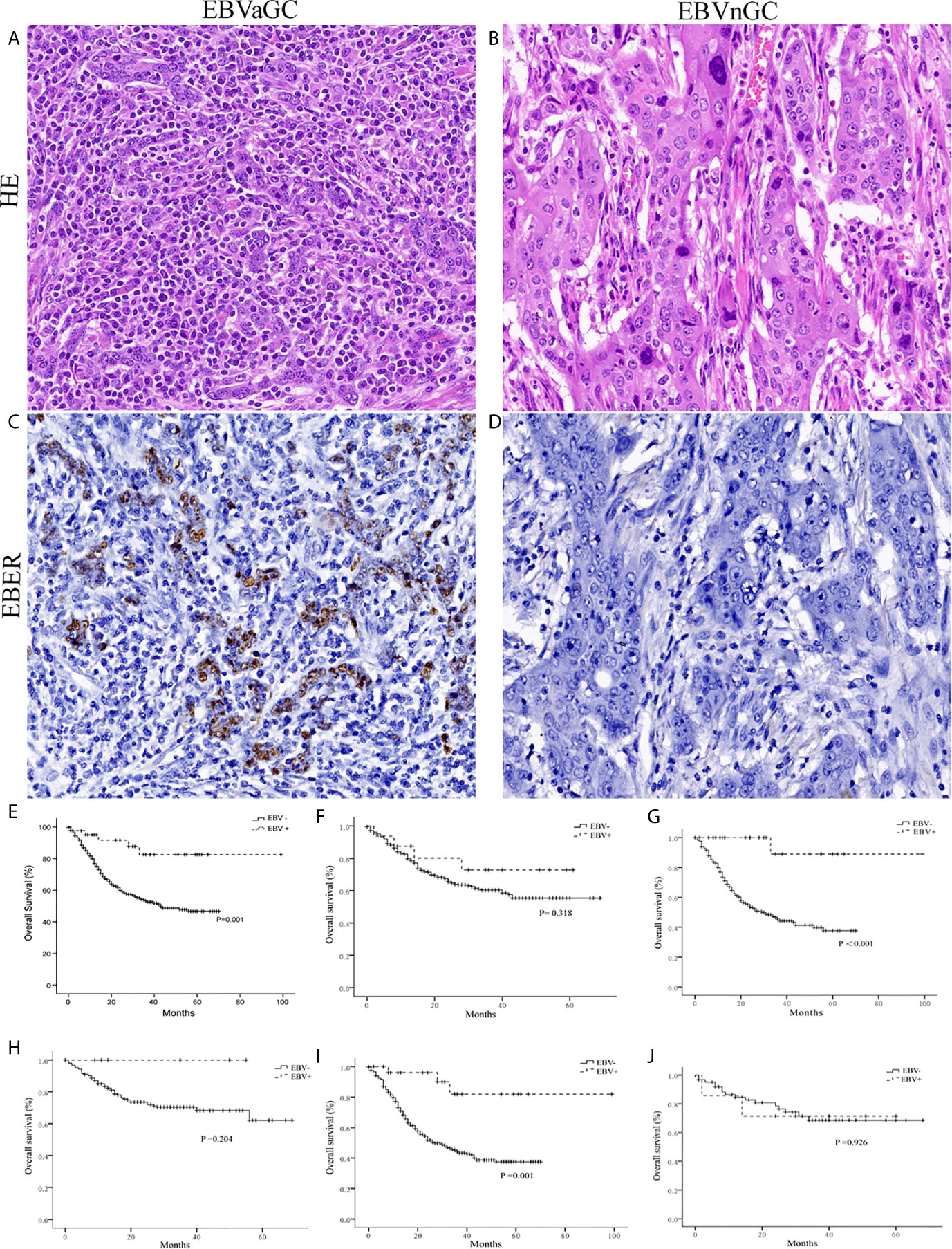
Figure 1 Histology, EBER-1 ISH, and survival curves in EBVaGC and EBVnGC. Histology of the representative cases of EBVaGC (A) and EBVnGC (B) (×400). EBER-1 ISH revealed strong nuclear staining in EBVaGC (C), but not in EBVnGC (D) (×400). (E) Kaplan-Meier survival curves for overall survival in 42 cases of EBVaGC and 804 cases of EBVnGC. EBVaGC had better prognosis than EBVnGC (P = 0.001, Log-rank test). When patients were stratified based on tumor size [<5 cm (F); >5 cm (G)] and Lauren classification [Intestinal (H), Diffuse (I), Mixed (J)], EBVaGC exhibited longer overall survival than EBVnGC in patients with tumor size >5 cm (P < 0.001) and Lauren diffuse type (P = 0.001).
During a mean of 22.1 (range, 1–99) months of follow-up, 5 (12%) patients in EBVaGC and 309 (38%) ones in EBVnGC group died. Kaplan-Meier analysis revealed that patients of EBVaGC had significantly better overall survivals than that of EBVnGC (P = 0.001, Figure 1E). While stratified by tumor size and Lauren classification, EBVaGC exhibited better overall survivals than EBVnGC in patients with tumor size >5 cm (P < 0.001, Figure 1G) and Lauren diffuse type (P = 0.001, Figure 1I). No statistically significant difference was observed in EBVaGC and EBVnGC patients with tumor size <5 cm (Figure 1F), Lauren intestinal and mixed types (Figures 1H, J).
Comparison of Clinicopathologic Characteristics According to TILs
To identify the clinicopathological significance of TILs, we divided the specimens into three groups (grade 1 TILs ≤10%, grade 2 TILs 10–50%, and grade 3 TILs >50%) (Figure 2A). For EBVnGC, a summary of the clinicopathological characteristics according to the grade of TILs is shown in Table 2. The tumor with higher grade of TILs was bigger in size (P = 0.028). According to Lauren classification, there was a significant association between the TILs density and the diffuse/mixed type (P < 0.001).
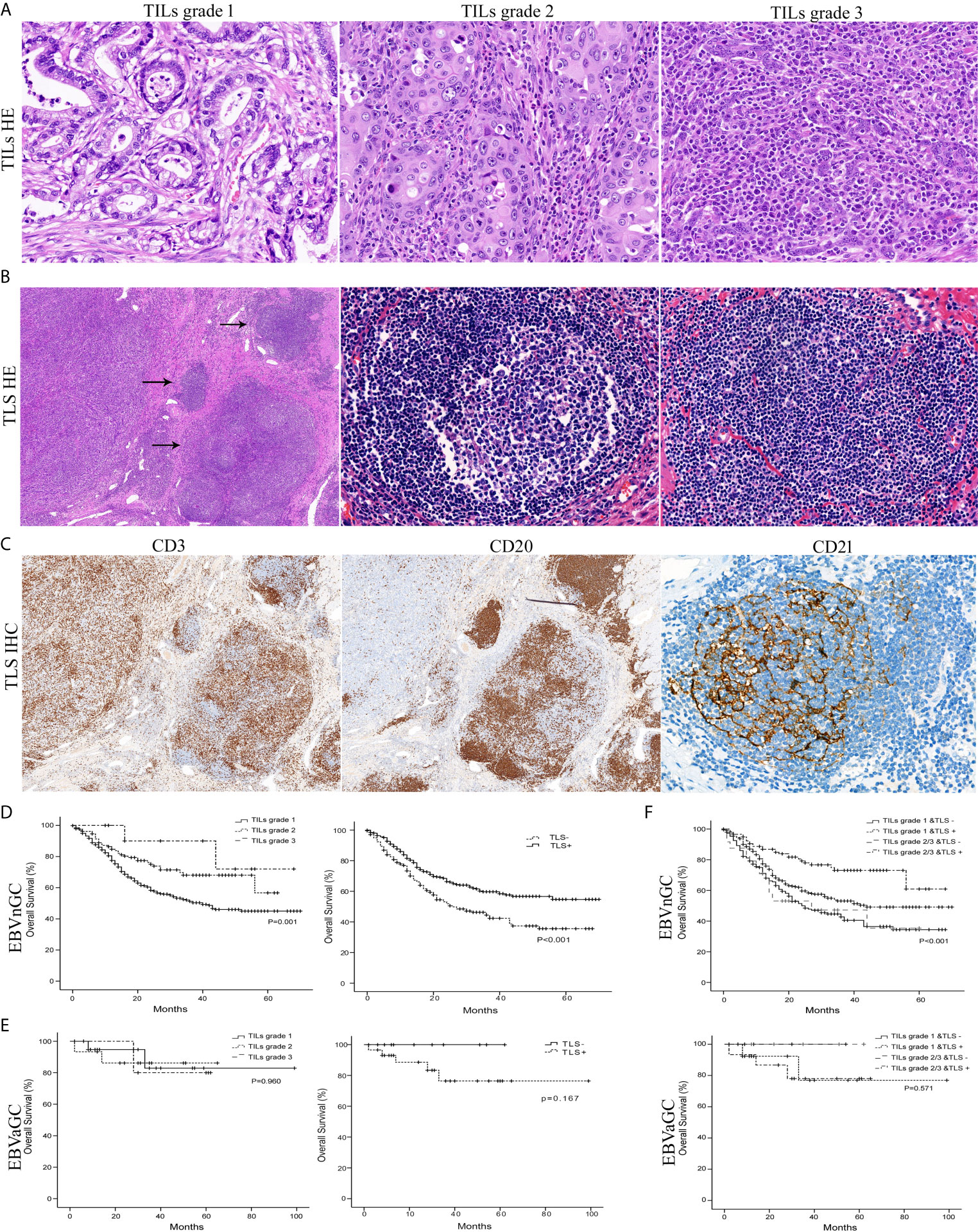
Figure 2 Histology of the TILs grade, TLS, and survival curves in EBVaGC and EBVnGC. (A) The mean percentage of the stromal area occupied by lymphocytes and plasma cells within tumor was assessed as TILs grade 1 (minimal, ≤10%), grade 2 (moderate, 10–50%), and grade 3 (abundant, >50%) (H&E, ×400). (B) TLS (arrows) with or without germinal centers (center) were mainly localized at the invasive margin of cancer (left field) (H&E, left ×50, center ×400, right ×400). (C) Whether germinal centers were visible or not, clusters of CD20+ B lymphocytes (×50) in TLS were surrounded by CD3+ T cell areas (×50) and contained a network of CD21+ FDCs (×400) by immunohistochemical staining. Kaplan-Meier survival analyses for overall survival were performed according to the TILs grade, TLS, or TILs & TLS in EBVnGC (D, F) top and EBVaGC (E, F) bottom.
For EBVaGC, no statistically significant difference was observed, except for gender. The patients with increasing TILs density were more likely to be male (P = 0.046; Supplementary Table 1). The proportion of TILs grade 2 and 3 in EBVaGC is 47.6%, significantly higher than that in EBVnGC (17.8%) (P < 0.001; Table 1).
Comparison of Clinicopathologic Characteristics According to TLS
TLS are highly organized structures with or without germinal center (Figures 2B, C). Among the total 804 EBVnGC patients, 563 (66.5%) cases showed the presence of TLS. Patients with the presence of TLS were younger age (P = 0.010), smaller in tumor size (P = 0.013), high pTNM stage (P = 0.036), poorly histologic differentiation (P = 0.007), Lauren diffuse type (P = 0.009), and WHO poorly differentiated type (P = 0.007) (Table 2).
For EBVaGC, no statistically significant difference was observed (Supplementary Table 1). However, the proportion of TLS-present patients was higher than that of TLS-absent ones (69.0% and 31.0%, respectively) (Supplementary Table 1).
Association Between TILs and TLS in EBVaGC and EBVnGC
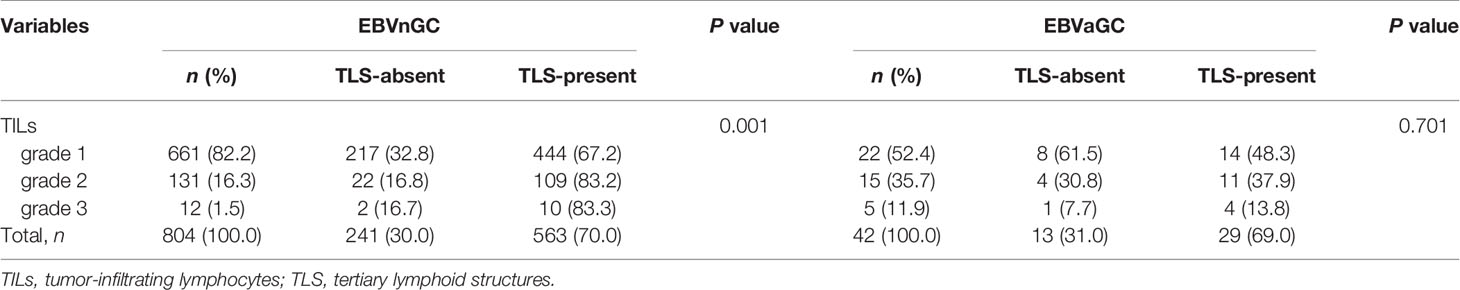
The presence of TLS in EBVnGC was related with TILs (P = 0.001; Table 3). The proportion of TILs grade 2 and 3 in TLS-present patients was 21.2%, obviously higher than that in TLS-absent ones (9.9%) (Table 3). However, there was no significant association between TILs and TLS in EBVaGC (Table 3).
Prognostic Significance of TILs and TLS in EBVaGC and EBVnGC
We detected that EBVnGC patients with higher TILs grade and the presence of TLS showed survival benefits according to Kaplan-Meier survival analysis (Figure 2D). No significant prognostic value was detected in EBVaGC (Figure 2E).
In the univariate analysis of EBVnGC, the clinical parameters of tumor location, size, pTNM stage, lymphovascular invasion, perineural invasion, histologic differentiation, Lauren classification, WHO classification, TILs, and TLS were found to be significantly associated with overall survival (Table 4). The multivariate model revealed that pTNM stage (HR 5.025; 95% CI 3.745–6.743; P < 0.001), lymphovascular invasion (HR 2.053, 95% CI 1.571–2.684, P < 0.001), perineural invasion (HR 1.649, 95% CI 1.267–2.146, P < 0.001), histologic differentiation (HR 1.817, 95% CI 1.347–2.400, P < 0.001), Lauren classification (HR 1.782, 95% CI 1.323–2.662, P < 0.001), WHO classification (HR 1.798, 95% CI 1.337–2.416, P < 0.001), TILs (HR 1.830, 95% CI 1.295–2.586, P = 0.001), and TLS (HR 1.558, 95% CI 1.228–1.977, P = 0.003) were independent prognostic factors for overall survival (Table 4).
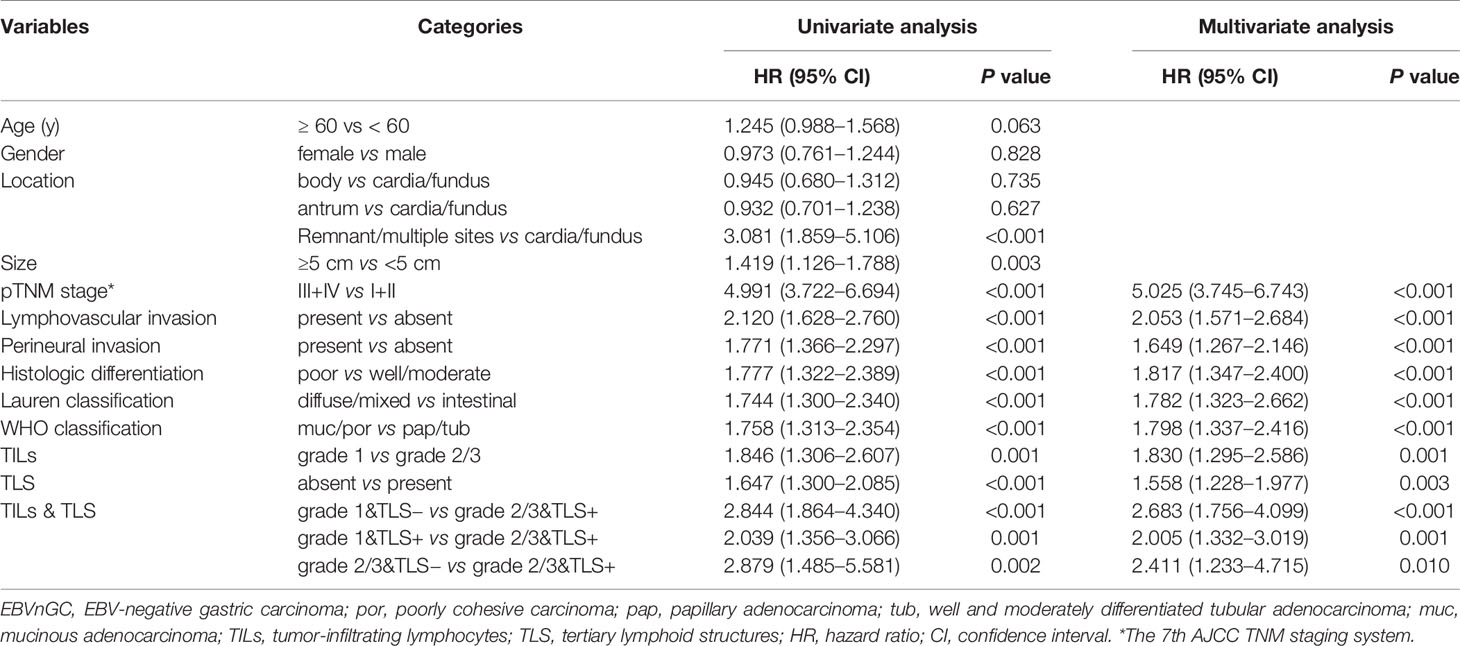
Table 4 Cox proportional hazards regression models for the predictors of overall survival in EBVnGC.
For EBVnGC, even though TILs and TLS have a certain correlation (r = 0.139, P < 0.001), some tumors with moderate to abundant TILs did not show the presence of TLS. Therefore, we divided tumors into four groups according to TILs (grade 1 vs grade 2/3) and TLS (absent or present). As shown in Figure 2F, patients with higher grades of TILs and the presence of TLS had the significantly best overall survival than the other three groups. The univariate and multivariate analysis also confirmed that TILs and TLS was significantly and independently associated with better survival.
Prognostic Nomogram in EBVnGC and Validation of Predictive Accuracy of the Nomogram for Overall Survival
A prognostic nomogram was depicted to predict 1-, 3-, and 5-year individualized absolute risk for mortality based on significant factors among all EBVnGC patients (Figure 3A). Significant attributes were selected by the multivariate stepwise regression analysis, including location, pTNM stage, TILs, and TLS (all P < 0.05). Predictive accuracy of the nomogram was good, with the C-index being 0.751 (95% CI 0.724–0.779). Calibration curves for 1-, 3-, and 5-year survival prediction indicated good agreement between predicted probabilities and actual observations (Figures 3B–D).
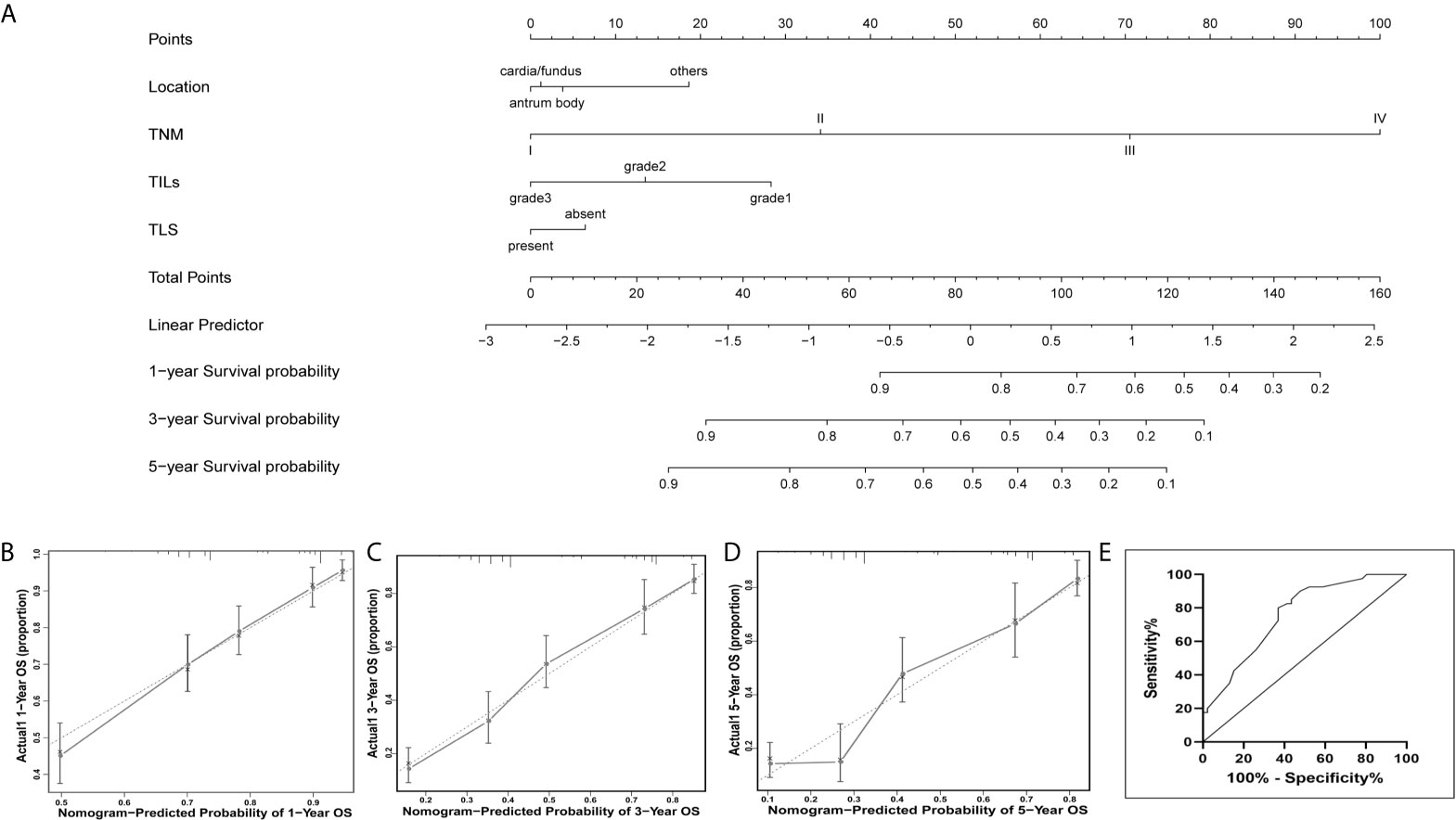
Figure 3 Nomogram for predicting prognosis in patients with EBVnGC. (A) A predictive nomogram for 1-year, 3-year, and 5-year overall survival was generated by combining significant independent prognostic factors including location, TNM stage, TILs, and TLS. To estimate the survival in a given patient, the “Total Points” score is calculated by summing the respective “Points” values corresponding to each variable. Using this “Total Points” score, the survival probabilities at 1, 3, and 5 years can be predicted according to the lower scales. Calibration plots of the nomogram for 1-year (B), 3-years (C), and 5-years (D) overall survival. Dotted Line, ideal model; vertical bars, 95% confident interval. (E) Predictive accuracy of the nomogram for overall survival were confirmed in the external validation cohort, indicating the model was reliable.
For the external validation cohort, the mean follow-up time was 32.1 months (range 1–82 months). A summary of clinicopathological characteristics was shown in Supplementary Table 2. Predictive accuracy of the nomogram for overall survival was good, with the AUC value of 0.759 (95% CI, 0.641 to 0.848), indicating the nomogram was useful for predicting survival of patients with GC (Figure 3E).
Discussion
In this study, TILs and TLS, especially TILs & TLS correlated with the clinical outcome of GC. Patients with higher TILs grade and TLS-present exhibited survival benefits in EBVnGC. TILs were associated with TLS and both were promising independent prognostic factors of EBVnGC. Moreover, we established a nomogram model that combined the TILs grade and TLS status as prognostic variables with other well-established prognostic factors in EBVnGC and found that the nomogram performed well for both calibration and external validation. The model may be the crucial determinants of clinical care for individual GC patients.
TILs were assessed on H&E sections and divided into three groups. EBVnGC patients with high TILs density showed markedly improved survival. The TILs grade was proved to be a promising independent prognostic indicator for overall survival in EBVnGC, which was in accordance with previous literature with regard to GC (23, 29) and other types of cancers (30–32). Generally, the predominance of TILs has been claimed to reflect an effective anti-tumor immune response, which was promoted by a dynamic and complex interaction between infiltrating immune cells and tumor cells, and this interaction is critical for tumor progression and clinical outcome (33, 34).
We found that the presence of TLS was a good, independent prognostic parameter for overall survival in EBVnGC. Despite heterogeneity in TLS-signatures and TLS-quantifying methods, most studies have consistently found the association between TLS and prolonged patients’ survival, suggesting the occurrence of an active immune response within TLS to tumor microenvironment (35, 36). Conversely, limited studies have detected that the presence of TLS was a negative prognostic factor and associated with more advanced disease in colorectal, breast, and hepatocellular carcinomas (37–39). The possible reason for this discrepancy was that the maintenance and function of TLS dictated by their cellular composition and the surrounding immune contexture may vary in different tumors (36).
In our study, 42 (5.0%) patients were identified as EBVaGC, with distinct clinicopathological features and significantly better prognosis. The high TILs density and the presence of TLS may be the possible reasons. Kang et al. assessed the prognostic value of TILs amongst EBVaGC and found that the TILs density was an independent predictor for recurrence free survival (6). However, in our study, limited numbers of EBVaGC patients and the uneven distribution of cases within each TILs and TLS group made little internal difference, so no significant prognostic value was found in EBVaGC. Further large-scale validation studies remain to be done to fully understand the exact prognostic role of TILs and TLS in EBVaGC.
Of note, TLS and the TILs were highly overlapping in their extent and prognostic abilities. Combination of the two has prognostic power superior to each one individually. Comparing to patients with high TILs grade but the absence of TLS, the ones with high TILs grade and the presence of TLS showed improved survival, suggesting that TLS may actively license the prognostic value of TILs. Dendritic cells or plasma cells expressing markers of antigen-specific responses within TLS were reported to be associated with increased responses of TILs, which propose that TLS may educate TILs to control tumors better (15, 40). Some studies demonstrated that TLS were correlated with TILs, contributing to TILs recruitment and cooperating with TILs in antitumor immune response in colorectal cancer (17) and breast cancer (41). Hennequin et al. found a significant correlation between the density of B cell aggregates and Tbet+ effector T cells in GC, which was also associated with better relapse-free survival, indicating that GC could be sustained through a complex network of tumor-infiltrating immune cells organized in TLS, allowing T/B cells coordination (42). The adhesion molecules, chemokines, and integrins may mediate migration of tumor-specific T cells into TLS. Meanwhile, TLS-serving HEVs may provide a gateway for the recruitment of circulating T lymphocytes into the tumor (43, 44).
Interestingly, we found a certain correlation between TILs and TLS, but some patients with moderate to abundant TILs did not develop TLS. The local tumor microenvironment including a series of signals or cytokines following the local cross-talk between TILs and resident stromal cells, may provide specific cues conducive to the formation of TLS (45, 46). We previously showed that CD3+ and CD8+ T lymphocytes as the predominant constituent cells of TILs in gastric cancer were associated with good prognosis (47), whereas tumor-infiltrating B cells especially when present in TLS, may be key players in anti-tumor immunity (48). Over half but not all diffuse type/genome stable GCs had enrichment of intratumoral TLS and exhibited different chemokine gene expression signature, reflecting signs of an initiated antitumor immune response and the different stages of lymphoid neogenesis (49). The presence of TLS may represent a privileged site where specific naïve B cells can undergo their final differentiation into effector B cells, such as memory B cells (48, 50). These suggest that TILs and TLS may interact with each other and play different roles in different stages of the anti-tumor immune response.
In conclusion, the present study indicated that high grade of TILs was associated with the presence of TLS and further elucidated that TILs and TLS, especially TILs & TLS were promising independent prognostic factors for overall survival in GC. TILs and TLS were highly overlapping in their extent and prognostic abilities, and could be considered as a coindicator of prognosis of gastric cancer. The evaluations of TILs and TLS are simple and can be assessed routinely in pathological diagnosis. TILs and TLS appear likely to be part of an adaptive immune response and may be helpful for understanding the immunobiology of the tumor microenvironment of gastric cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institute Research Ethics Committees of the First, Third, and Six Affiliated Hospital and Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CS and JC prepared the study concept and design. NC, PL, JC, and CS wrote the paper. NC and PL performed standard pathologic analysis. XZ, MD, and YZ collected the cohort data and samples. HC and PZ did data analysis and interpretation. CS and JC supervised the project. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (82073397), the Guangdong Basic and Applied Basic Research Foundation (2019A1515011455), the Natural Science Foundation of Guangdong Province (2018A030313650), the Guangzhou Science and Technology Project (202102010156, 202102010267), and the NSFC cultivating grant of The Third Affiliated Hospital, Sun Yat-sen University (2020GZRPYMS01, 2021GZRPYQN12), Guangdong Province, China.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Ling Xue from the First Affiliated Hospital, Sun Yat-Sen University, Prof. Yan Huang from the Sixth Affiliated Hospital, Sun Yat-Sen University, and Prof. Haigang Li from the Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University for providing gastric carcinoma tissue samples used in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.692859/full#supplementary-material
Abbreviations
TILs, tumor-infiltrating lymphocytes; TLS, tertiary lymphoid structures; GC, gastric carcinoma; EBVaGC, Epstein-Barr Virus-associated gastric carcinoma; EBVnGC, EBV-negative gastric carcinoma; ISH, in situ hybridization; EBER-1, EBV-encoded small RNA 1; H&E, hematoxylin and eosin; FDCs, follicular dendritic cells; AJCC, the American Joint Committee on Cancer; LAs, lymphoid aggregates.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric Cancer. Lancet (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
3. Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H, et al. Tumor-Infiltrating Lymphocyte Grade in Primary Melanomas is Independently Associated With Melanoma-Specific Survival in the Population-Based Genes, Environment and Melanoma Study. J Clin Oncol (2013) 31:4252–9. doi: 10.1200/JCO.2013.51.3002
4. Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G. Tumor-Associated Lymphocytes Predict Response to Neoadjuvant Chemotherapy in Breast Cancer Patients. J Breast Cancer (2013) 16:32–9. doi: 10.4048/jbc.2013.16.1.32
5. Wang YQ, Chen YP, Zhang Y, Jiang W, Liu N, Yun JP, et al. Prognostic Significance of Tumor-Infiltrating Lymphocytes in Nondisseminated Nasopharyngeal Carcinoma: A Large-Scale Cohort Study. Int J Cancer (2018) 142:2558–66. doi: 10.1002/ijc.31279
6. Kang BW, Seo AN, Yoon S, Bae HI, Jeon SW, Kwon OK, et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Epstein-Barr Virus-Associated Gastric Cancer. Ann Oncol (2016) 27:494–501. doi: 10.1093/annonc/mdv610
7. Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, et al. Clinicopathological Features of Programmed Death Ligand 1 Expression With Tumor-Infiltrating Lymphocyte, Mismatch Repair, and Epstein-Barr Virus Status in a Large Cohort of Gastric Cancer Patients. Gastric Cancer (2017) 20:407–15. doi: 10.1007/s10120-016-0631-3
8. Dai C, Geng R, Wang C, Wong A, Qing M, Hu J, et al. Concordance of Immune Checkpoints Within Tumor Immune Contexture and Their Prognostic Significance in Gastric Cancer. Mol Oncol (2016) 10:1551–8. doi: 10.1016/j.molonc.2016.09.004
9. Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol (2015) 33:1974–82. doi: 10.1200/JCO.2014.59.4358
10. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-Driven Biomarkers to Guide Immune Checkpoint Blockade in Cancer Therapy. Nat Rev Cancer (2016) 16:275–87. doi: 10.1038/nrc.2016.36
11. Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary Lymphoid Structures in the Era of Cancer Immunotherapy. Nat Rev Cancer (2019) 19:307–25. doi: 10.1038/s41568-019-0144-6
12. Gago DGC, van Baarsen L, Mebius RE. Tertiary Lymphoid Structures: Diversity in Their Development, Composition, and Role. J Immunol (2021) 206:273–81. doi: 10.4049/jimmunol.2000873
13. de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, et al. Characterization of Chemokines and Adhesion Molecules Associated With T Cell Presence in Tertiary Lymphoid Structures in Human Lung Cancer. Cancer Res (2011) 71:6391–9. doi: 10.1158/0008-5472.CAN-11-0952
14. Sautes-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, et al. Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front Immunol (2016) 7:407. doi: 10.3389/fimmu.2016.00407
15. Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, et al. Dendritic Cells in Tumor-Associated Tertiary Lymphoid Structures Signal a Th1 Cytotoxic Immune Contexture and License the Positive Prognostic Value of Infiltrating CD8+ T Cells. Cancer Res (2014) 74:705–15. doi: 10.1158/0008-5472.CAN-13-1342
16. Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, et al. High Endothelial Venules (Hevs) in Human Melanoma Lesions: Major Gateways for Tumor-Infiltrating Lymphocytes. Oncoimmunology (2012) 1:829–39. doi: 10.4161/onci.20492
17. Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, et al. Occurrence of Tertiary Lymphoid Tissue is Associated With T-cell Infiltration and Predicts Better Prognosis in Early-Stage Colorectal Cancers. Clin Cancer Res (2014) 20:2147–58. doi: 10.1158/1078-0432.CCR-13-2590
18. Naseem M, Barzi A, Brezden-Masley C, Puccini A, Berger MD, Tokunaga R, et al. Outlooks on Epstein-Barr Virus Associated Gastric Cancer. Cancer Treat Rev (2018) 66:15–22. doi: 10.1016/j.ctrv.2018.03.006
19. Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr Virus and Gastric Cancer (Review). Int J Oncol (2015) 46:1421–34. doi: 10.3892/ijo.2015.2856
20. Nishikawa J, Yoshiyama H, Iizasa H, Kanehiro Y, Nakamura M, Nishimura J, et al. Epstein-Barr Virus in Gastric Carcinoma. Cancers (Basel) (2014) 6:2259–74. doi: 10.3390/cancers6042259
21. Edge S, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A. eds. AJCC Cancer Staging Handbook, 7th ed. New York: Springer-Verlag (2010).
22. Chen JN, Ding YG, Feng ZY, Li HG, He D, Du H, et al. Association of Distinctive Epstein-Barr Virus Variants With Gastric Carcinoma in Guangzhou, Southern China. J Med Virol (2010) 82:658–67. doi: 10.1002/jmv.21731
23. Zhang D, He W, Wu C, Tan Y, He Y, Xu B, et al. Scoring System for Tumor-Infiltrating Lymphocytes and Its Prognostic Value for Gastric Cancer. Front Immunol (2019) 10:71. doi: 10.3389/fimmu.2019.00071
24. Cho CJ, Kang HJ, Ryu YM, Park YS, Jeong HJ, Park YM, et al. Poor Prognosis in Epstein-Barr Virus-Negative Gastric Cancer With Lymphoid Stroma is Associated With Immune Phenotype. Gastric Cancer (2018) 21:925–35. doi: 10.1007/s10120-018-0820-3
25. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The Evaluation of Tumor-Infiltrating Lymphocytes (Tils) in Breast Cancer: Recommendations by an International Tils Working Group 2014. Ann Oncol (2015) 26:259–71. doi: 10.1093/annonc/mdu450
26. Buisseret L, Desmedt C, Garaud S, Fornili M, Wang X, Van den Eyden G, et al. Reliability of Tumor-Infiltrating Lymphocyte and Tertiary Lymphoid Structure Assessment in Human Breast Cancer. Mod Pathol (2017) 30:1204–12. doi: 10.1038/modpathol.2017.43
27. Gullo I, Oliveira P, Athelogou M, Goncalves G, Pinto ML, Carvalho J, et al. New Insights Into the Inflamed Tumor Immune Microenvironment of Gastric Cancer With Lymphoid Stroma: From Morphology and Digital Analysis to Gene Expression. Gastric Cancer (2019) 22:77–90. doi: 10.1007/s10120-018-0836-8
28. Hjelmstrom P. Lymphoid Neogenesis: De Novo Formation of Lymphoid Tissue in Chronic Inflammation Through Expression of Homing Chemokines. J Leukoc Biol (2001) 69:331–9.
29. Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, et al. Prognostic Implications of Type and Density of Tumour-Infiltrating Lymphocytes in Gastric Cancer. Br J Cancer (2008) 99:1704–11. doi: 10.1038/sj.bjc.6604738
30. Almangush A, Ruuskanen M, Hagstrom J, Hirvikoski P, Tommola S, Kosma VM, et al. Tumor-Infiltrating Lymphocytes Associate With Outcome in Nonendemic Nasopharyngeal Carcinoma: A Multicenter Study. Hum Pathol (2018) 81:211–9. doi: 10.1016/j.humpath.2018.07.009
31. Rakaee M, Kilvaer TK, Dalen SM, Richardsen E, Paulsen EE, Hald SM, et al. Evaluation of Tumor-Infiltrating Lymphocytes Using Routine H&E Slides Predicts Patient Survival in Resected non-Small Cell Lung Cancer. Hum Pathol (2018) 79:188–98. doi: 10.1016/j.humpath.2018.05.017
32. Huh JW, Lee JH, Kim HR. Prognostic Significance of Tumor-Infiltrating Lymphocytes for Patients With Colorectal Cancer. Arch Surg (2012) 147:366–72. doi: 10.1001/archsurg.2012.35
33. de Visser KE, Eichten A, Coussens LM. Paradoxical Roles of the Immune System During Cancer Development. Nat Rev Cancer (2006) 6:24–37. doi: 10.1038/nrc1782
34. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The Prognostic Influence of Tumour-Infiltrating Lymphocytes in Cancer: A Systematic Review With Meta-Analysis. Br J Cancer (2011) 105:93–103. doi: 10.1038/bjc.2011.189
35. Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH. Tertiary Lymphoid Structures in Cancer and Beyond. Trends Immunol (2014) 35:571–80. doi: 10.1016/j.it.2014.09.006
36. Colbeck EJ, Ager A, Gallimore A, Jones GW. Tertiary Lymphoid Structures in Cancer: Drivers of Antitumor Immunity, Immunosuppression, or Bystander Sentinels in Disease? Front Immunol (2017) 8:1830. doi: 10.3389/fimmu.2017.01830
37. Bento DC, Jones E, Junaid S, Tull J, Williams GT, Godkin A, et al. High Endothelial Venules are Rare in Colorectal Cancers But Accumulate in Extra-Tumoral Areas With Disease Progression. Oncoimmunology (2015) 4:e974374. doi: 10.4161/2162402X.2014.974374
38. Finkin S, Yuan D, Stein I, Taniguchi K, Weber A, Unger K, et al. Ectopic Lymphoid Structures Function as Microniches for Tumor Progenitor Cells in Hepatocellular Carcinoma. Nat Immunol (2015) 16:1235–44. doi: 10.1038/ni.3290
39. Figenschau SL, Fismen S, Fenton KA, Fenton C, Mortensen ES. Tertiary Lymphoid Structures are Associated With Higher Tumor Grade in Primary Operable Breast Cancer Patients. BMC Cancer (2015) 15:101. doi: 10.1186/s12885-015-1116-1
40. Kroeger DR, Milne K, Nelson BH. Tumor-Infiltrating Plasma Cells Are Associated With Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin Cancer Res (2016) 22:3005–15. doi: 10.1158/1078-0432.CCR-15-2762
41. Lee HJ, Kim JY, Park IA, Song IH, Yu JH, Ahn JH, et al. Prognostic Significance of Tumor-Infiltrating Lymphocytes and the Tertiary Lymphoid Structures in HER2-Positive Breast Cancer Treated With Adjuvant Trastuzumab. Am J Clin Pathol (2015) 144:278–88. doi: 10.1309/AJCPIXUYDVZ0RZ3G
42. Hennequin A, Derangere V, Boidot R, Apetoh L, Vincent J, Orry D, et al. Tumor Infiltration by Tbet+ Effector T Cells and CD20+ B Cells is Associated With Survival in Gastric Cancer Patients. Oncoimmunology (2016) 5:e1054598. doi: 10.1080/2162402X.2015.1054598
43. Goc J, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characteristics of Tertiary Lymphoid Structures in Primary Cancers. Oncoimmunology (2013) 2:e26836. doi: 10.4161/onci.26836
44. Dieu-Nosjean MC, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautes-Fridman C. Tertiary Lymphoid Structures, Drivers of the Anti-Tumor Responses in Human Cancers. Immunol Rev (2016) 271:260–75. doi: 10.1111/imr.12405
45. Fridman WH, Pages F, Sautes-Fridman C, Galon J. The Immune Contexture in Human Tumours: Impact on Clinical Outcome. Nat Rev Cancer (2012) 12:298–306. doi: 10.1038/nrc3245
46. Pipi E, Nayar S, Gardner DH, Colafrancesco S, Smith C, Barone F. Tertiary Lymphoid Structures: Autoimmunity Goes Local. Front Immunol (2018) 9:1952. doi: 10.3389/fimmu.2018.01952
47. Gong LP, Chen JN, Xiao L, He Q, Feng ZY, Zhang ZG, et al. The Implication of Tumor-Infiltrating Lymphocytes in Epstein-Barr Virus-Associated Gastric Carcinoma. Hum Pathol (2019) 85:82–91. doi: 10.1016/j.humpath.2018.11.002
48. Sakimura C, Tanaka H, Okuno T, Hiramatsu S, Muguruma K, Hirakawa K, et al. B Cells in Tertiary Lymphoid Structures are Associated With Favorable Prognosis in Gastric Cancer. J Surg Res (2017) 215:74–82. doi: 10.1016/j.jss.2017.03.033
49. Derks S, de Klerk LK, Xu X, Fleitas T, Liu KX, Liu Y, et al. Characterizing Diversity in the Tumor-Immune Microenvironment of Distinct Subclasses of Gastroesophageal Adenocarcinomas. Ann Oncol (2020) 31:1011–20. doi: 10.1016/j.annonc.2020.04.011
Keywords: tumor-infiltrating lymphocytes, tertiary lymphoid structures, EBV-associated gastric carcinoma, EBV-negative gastric carcinoma, nomogram, prognosis
Citation: Cheng N, Li P, Cheng H, Zhao X, Dong M, Zhang Y, Zhao P, Chen J and Shao C (2021) Prognostic Value of Tumor-Infiltrating Lymphocytes and Tertiary Lymphoid Structures in Epstein-Barr Virus-Associated and -Negative Gastric Carcinoma. Front. Immunol. 12:692859. doi: 10.3389/fimmu.2021.692859
Received: 09 April 2021; Accepted: 14 June 2021;
Published: 01 July 2021.
Edited by:
Abbas Ghaderi, Shiraz University of Medical Sciences, IranCopyright © 2021 Cheng, Li, Cheng, Zhao, Dong, Zhang, Zhao, Chen and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunkui Shao, c2hhb2Noa0BtYWlsLnN5c3UuZWR1LmNu; Jianning Chen, Y2hqbmluZ0BtYWlsLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Na Cheng
Na Cheng Peng Li2†
Peng Li2† Huanhuan Cheng
Huanhuan Cheng Yiwang Zhang
Yiwang Zhang Peizhen Zhao
Peizhen Zhao Jianning Chen
Jianning Chen Chunkui Shao
Chunkui Shao