
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 07 May 2021
Sec. Inflammation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.691249
 Runzhen Zhao1†
Runzhen Zhao1† Zhenlei Su2†
Zhenlei Su2† Andrey A. Komissarov1,3
Andrey A. Komissarov1,3 Shan-Lu Liu4
Shan-Lu Liu4 Guohua Yi5
Guohua Yi5 Steven Idell1,3
Steven Idell1,3 Michael A. Matthay6,7
Michael A. Matthay6,7 Hong-Long Ji1,3*
Hong-Long Ji1,3*Background: Dynamic D-dimer level is a key biomarker for the severity and mortality of COVID-19 (coronavirus disease 2019). How aberrant fibrinolysis influences the clinical progression of COVID-19 presents a clinicopathological dilemma challenging intensivists.
Methods: We performed meta-analysis and meta regression to analyze the associations of plasma D-dimer with 106 clinical variables to identify a panoramic view of the derangements of fibrinolysis in 14,862 patients of 42 studies. There were no limitations of age, gender, race, and country. Raw data of each group were extracted separately by two investigators. Individual data of case series, median and interquartile range, and ranges of median or mean were converted to SDM (standard deviation of mean).
Findings: The weighted mean difference of D-dimer was 0.97 µg/mL (95% CI 0.65, 1.29) between mild and severe groups, as shown by meta-analysis. Publication bias was significant. Meta-regression identified 58 of 106 clinical variables were associated with plasma D-dimer levels. Of these, 11 readouts were negatively related to the level of plasma D-dimer. Further, age and gender were confounding factors. There were 22 variables independently correlated with the D-dimer level, including respiratory rate, dyspnea plasma K+, glucose, SpO2, BUN (blood urea nitrogen), bilirubin, ALT (alanine aminotransferase), AST (aspartate aminotransferase), systolic blood pressure, and CK (creatine kinase).
Interpretation: These findings support elevated D-dimer as an independent predictor for both mortality and complications. The identified D-dimer-associated clinical variables draw a landscape integrating the aggregate effects of systemically suppressive and pulmonary hyperactive derangements of fibrinolysis, and the D-dimer-associated clinical biomarkers, and conceptually parameters could be combined for risk stratification, potentially for tracking thrombolytic therapy or alternative interventions.
The sustained COVID-19 pandemic has oversaturated the emergency and intensive critical care resources globally. Hypercoagulability has been evidenced in most critically ill patients by elevated D-dimer and fibrin degradation products (FDP), a decrease in platelet count, an incremental increase in the prothrombin time, and a rise in fibrinogen (1–9). Of these, patients with increased D-dimer are more vulnerable to worsen clinical consequences of COVID-19, with more severe complications, including requirements of ICU support (1–9).
Thromboembolism of COVID-19 patients is the fatal sequelae of hypercoagulation and fibrinolytic abnormalities. Pulmonary embolism (PE) and deep vein thrombosis (DVT) can cause respiratory failure in severely ill patients with COVID-19 (10–14). Postmortem pathology shows that small fibrinous thrombi in small pulmonary arterioles are very common. Activation of the coagulation cascade is further supported by endothelial tumefaction, pulmonary megakaryocytes in the capillaries, and endotheliitis (15–19). Elevated D-dimer is an indicator of the activation of the fibrinolysis system and removal of clots or extravascular collections of fibrin by plasmin. Compared with the consistent coagulopathy, however, the clinical ramifications of deranged fibrinolysis are not well studied and reviewed systematically.
Increased D-dimer level has not consistently been observed by all COVID-19 clinical studies, although it is a broadly applied biomarker for prognosis and outcomes of anti-thrombosis (20). The current explanations for the elevated D-dimer in critically ill patients are multiple, including “suppression of fibrinolysis”, “secondarily hyperactive fibrinolysis”, “consumption of fibrinolysis”, “fibrinolysis resistance”, and “fibrinolysis shutdown” (21, 22). To restore the coagulopathic changes of COVID-19, two diametrically different therapeutic regimes are in practice: fibrinolytic (alteplase-tPA) (10, 11, 23–28) and antifibrinolytic therapies (nafamostat and tranexamic acid (TXA)) (29–31). It is therefore imperative to clarify the role of pathophysiologic derangements of fibrinolysis in clinical outcomes that occur in COVID-19 patients. We, therefore, performed both meta-analyses and meta-regressions to explore the relationships between the plasma D-dimer level on admission with demographics, laboratory tests, fatal cardiopulmonary function, radiology, interventions, complications, and outcomes.
We conducted a systematic review of the literature in accordance with the methods recommended in the PRISMA guidelines (Figure 1).
Two independent investigators searched the potential studies in the NCBI PubMed, EMBASE, Scopus, Web of Science, Google Scholar, and some preprint platforms, including the medRxiv, Preprint, and bioRxiv. The search strategy was (D-dimer OR fibrin OR proteolytic OR fibrinolysis OR coagulation OR thrombin OR platelet OR plasmin OR tPA OR fibrinolytic OR thrombolytic) AND (COVID-19 OR 2019-nCoV OR SARS-nCoV OR Wuhan OR SARS-CoV-2). Sorted COVID-19 and SARS-CoV-2 preprints were screened if available. The hits were limited to publication in the year 2019-2020. Studies published in some high-impact journals focusing on the fibrinolysis and coagulation systems were summarized (Table 1). Related articles published during the preparation of this manuscript were discussed.
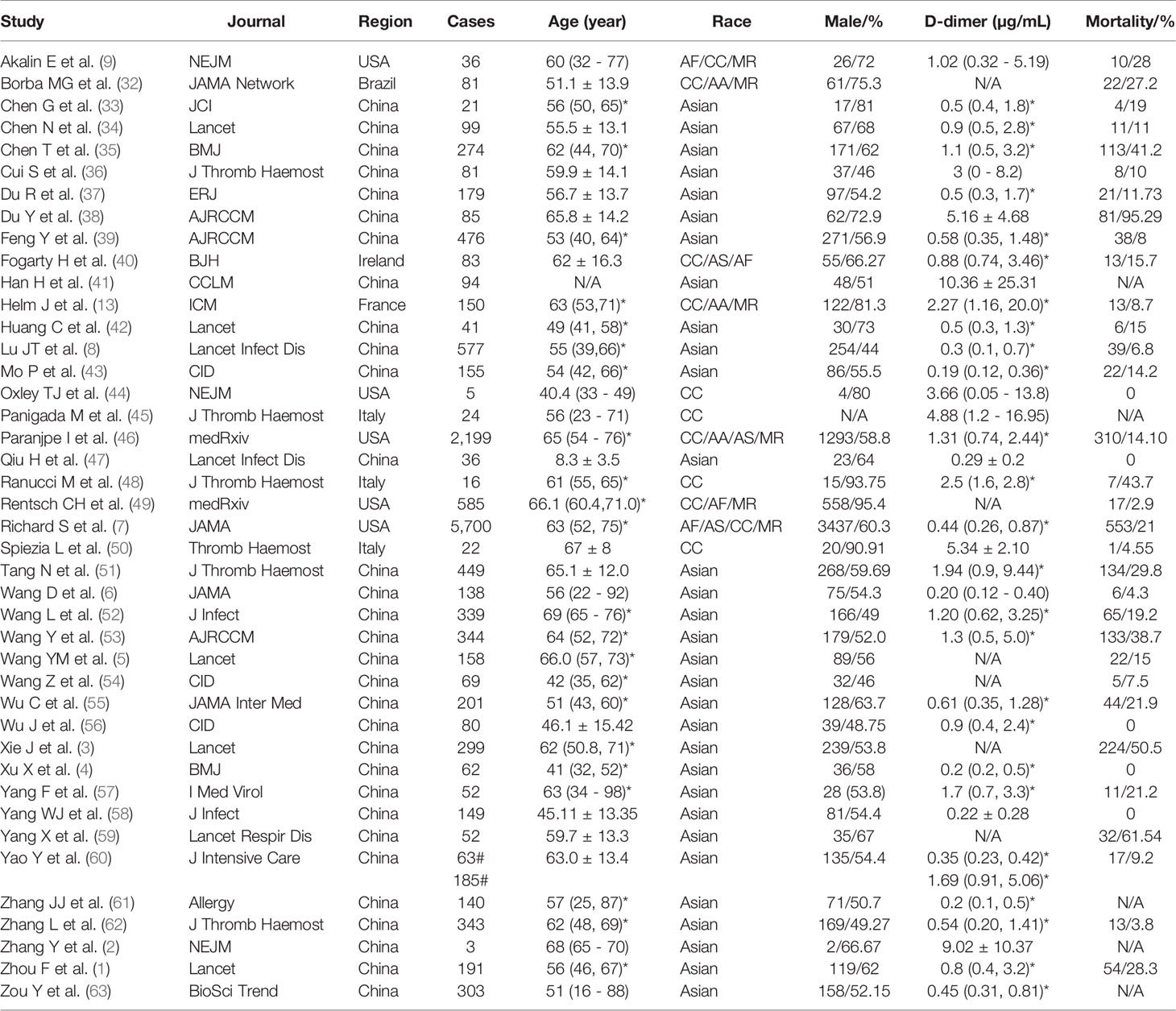
Table 1 Demographic features of the analyzed 42 studies. *IQR. Mean ± S.D. # normal and D-dimer elevated group. (-) range from minimum to maximum.
All eligible studies meeting the following criteria were included: 1) the species was human, 2) the publications were original clinical investigations, and 3) the results were presented as or could be converted or digitized to mean ± SD (SDM) or percentage. Studies were excluded if they were: 1) reviews or editorial, single case reports, commentaries, or preclinical studies; 2) results that could not be converted or digitized to SDM or percentage; and 3) full articles or clinical data that were not available. Raw data of each sub-group at admission were extracted by ZLS and RZZ. Individual data of case series (2, 44), median and interquartile range (1, 4–8, 13, 33, 35, 39, 42, 43, 46, 48, 49, 52–54, 56, 57, 61, 62), and ranges of median or mean (9, 45, 63) were converted to SDM as described previously (64, 65). Percentages and SDMs were extracted directly from studies if available.
To perform meta-analysis with the STATA v.16.1, the studies with two groups or more were pooled to compute weighted mean differences (WMD) and 95% confidence intervals (95% CI) (64). Different units and methodologies were converted to unified ones; for example, ng/ml, mcg/ml, µ;g/L for D-dimer were computed to µ;g/mL for all studies. The stages of COVID-19 were defined by the original studies mostly based on the WHO Interim Guidance. Publication bias between selected studies was assessed with both the Egger’s and Begg’s tests using the metabias program. The stability of the results was confirmed by the random-effects trim and fill analyses using the metatrim program.
The associations between D-dimer and demographic features, comorbidities, laboratory tests, radiographic results, treatments, hospitalization, outcomes, and complications were analyzed. D-dimer was considered as a covariate of other clinical variables. The standard errors of D-dimer were used to indicate the within-study variability, and the random-effect ReML method was applied. If observations were lesser than six, the results of this parameter were removed. All defined complications/diagnoses, i.e., ARDS, DIC, sepsis, were originally reported by the included studies.
Following the PRISMA guideline, we included 42 key studies for meta-analysis and meta-regression (Figure 1). The demographic features, D-dimer, ARDS, and mortality were summarized in Table 1. In total, there were 14,862 laboratory-confirmed patients: 31 studies from China (5,961cases), 5 from the USA (8,525 cases), 3 from Italy (62 cases), and 1 from Ireland (83 cases), France (150 cases), and Brazil (81 cases), respectively. The age was from 1 to 98-year-old. The races included Asian, African, Caucasian, and mixed with an incidence of ARDS ranged from 0 to 100%, and a fatality ranged from 0 to 95.3%. D-dimer level ranged from 0 to 35.7 µ;g/mL, and 9 studies reported a normal value (< 0.5 µ;g/mL).
COVID-19, as a newly emerging infectious disease, the included clinical studies had divergent designs. We compared the D-dimer level between mild and severe groups of 23 studies (Figure 2A). Only a small increase of D-dimer (normal range <0.5 µ;g/mL), 0.97 µ;g/mL (95% CI 0.65, 1.29) was observed in the relatively severe group with significant publication bias (92.5%), which was corroborated by both the Begg’s (P=0.009) and Egger’s tests (P<0.001) (Figure 2B) and the random-effects filled funnel plot (Figure 2C, P<0.001). Similarly, significant variations caused by retrospective studies and case series were observed for both age (Figure S1) and mortality (Figure S2). Thus, it was considered to be inappropriate to perform meta-analysis without well-designed RCT (randomized controlled trials) studies. Instead, we hypothesized that D-dimer could serve as a critical covariate for clinical features.
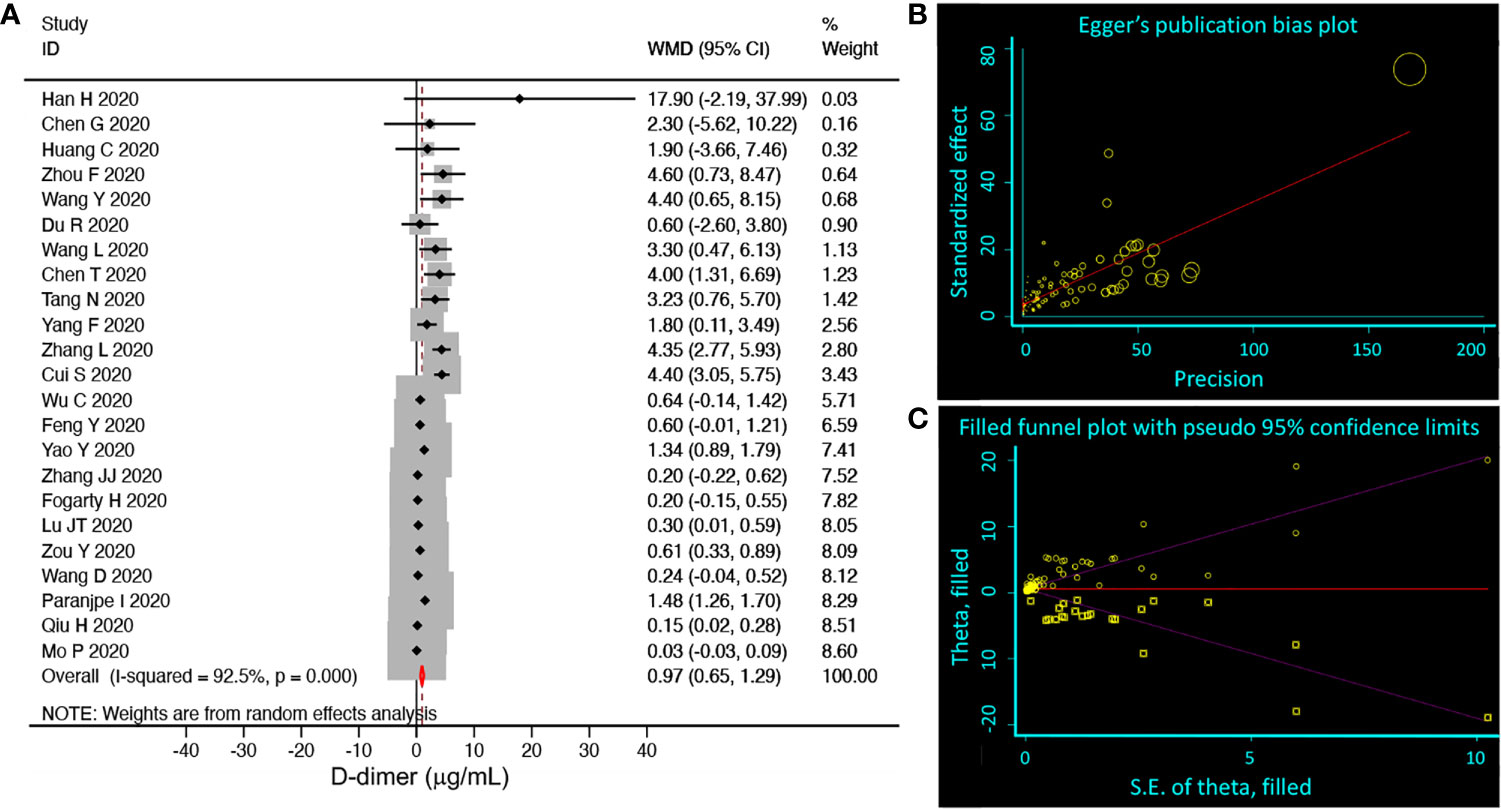
Figure 2 Random-effects meta-analysis of D-dimer level. (A) Forest plot. We selected studies that had subgroups, which could be divided into mild (including normal) and severe groups. We pooled weighted mean differences (WMD, black diamond, and gray square) and 95% CI (horizontal lines through the diamonds) of D-dimer from eligible 23 studies. Studies with only one group or moderate group were excluded. The red diamond represents the overall WMD. (B) Egger’s publication bias plot. N=89, P<0.001. (C) Filled funnel plot. P<0.001. Circle, raw data; square, pseudo data needed for symmetric distribution.
We performed meta-regression to detect the potential associations of D-dimer with 36 demographic characteristics of COVID-19 patients (Table S2). Of these, preexisting medical conditions, including any comorbidity, hypertension, diabetes, chronic lung diseases, and cerebrovascular diseases, were positively associated with D-dimer (P<0.05). Age, gender, blood pressure, and dyspnea/tachypnea positively correlated with D-dimer (P<0.05, Table 2). Moreover, the percentage of female and diastolic pressure was negatively correlated to D-dimer. Subgroup analysis showed a cutoff age was <65 years in the studies with two groups (<65 or ≥65), and further <50 in the studies with four groups (<50, ≥50, <60, ≥60, and ≥70) for a negative coefficient value (P<0.05, Table S3).
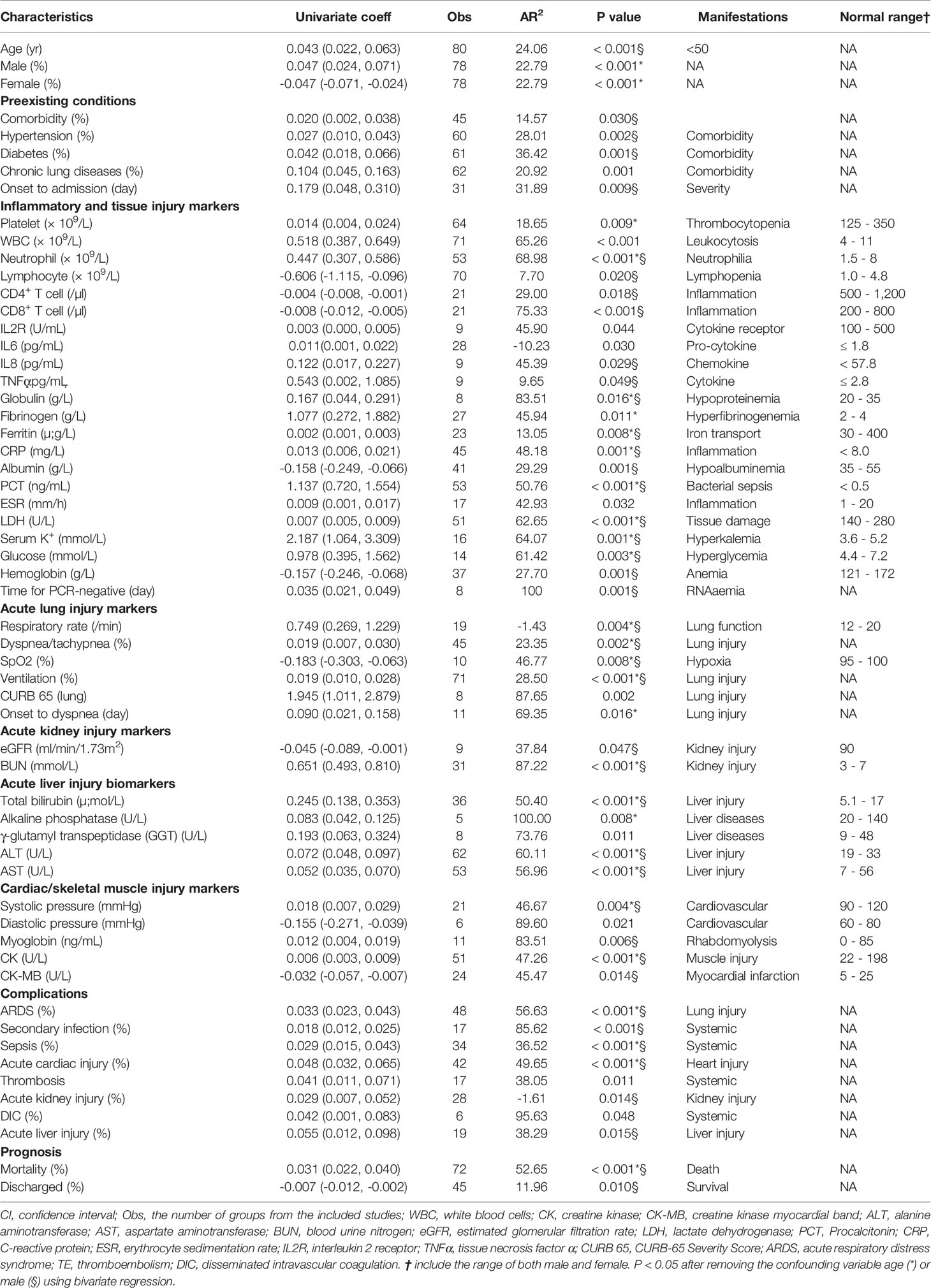
Table 2 D-dimer associated clinical variables (58 of 106 in total with a sample size ≥ 5, P < 0.05) identified by univariate-regression.
To analyze D-dimer’s correlation with 62 laboratory tests and radiological readouts, meta-regression was conducted for individual variables and summarized in Table S4. There were 32 laboratory tests significantly associated with D-dimer (Table S4). In addition, FDP tended to associate with D-dimer levels (P=0.058, N=10). These tests could be roughly grouped as 1) inflammation and tissue injury markers, 2) acute lung injury markers, 3) acute kidney injury markers, 4) acute liver injury markers, and 5) cardiac/skeletal muscle injury markers (Table 2).
To evaluate the relationship between interventions and D-dimer, we associated 11 therapies with the D-dimer level (Tables S5 and 2). Interestingly, mechanical ventilation was positively associated with D-dimer (N=71, P<0.001, Figure 3). Immune enhancement therapy (P=0.084, N=24) was also found to correlate with D-dimer if additional observations were available to increase the sample size.
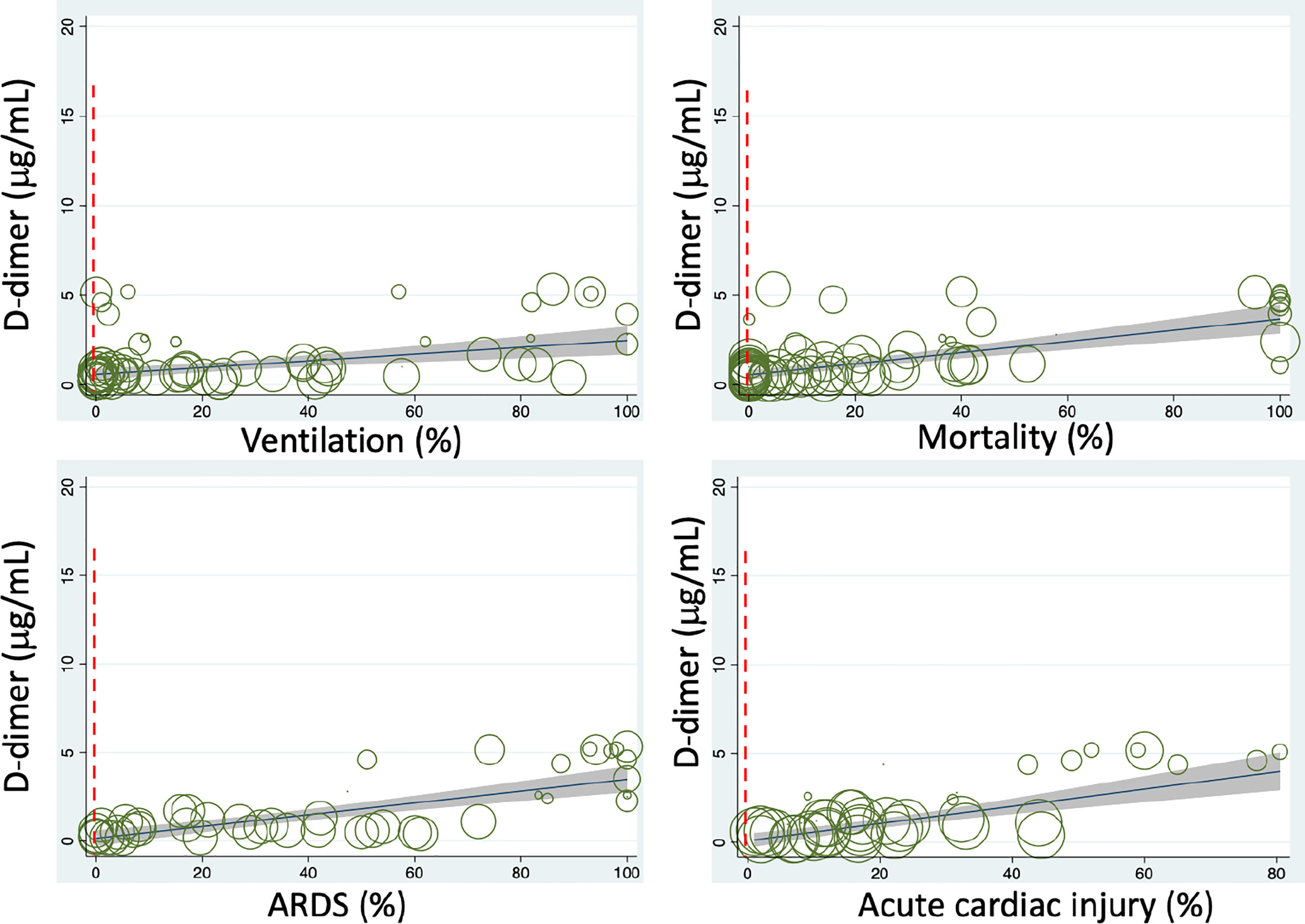
Figure 3 Meta-regressions of plasma D-dimer level on admission with some clinical variables. Dashed vertical red lines indicate the normal range.
To examine if D-dimer is an independent risk factor for deadly complications, we analyzed the dependence of fatal organ injury and systemic disorders using the metareg program. The results were summarized in Tables S6 and 2. Acute lung (ALI/ARDS), heart, kidney, and liver injuries were significantly associated with D-dimer (P<0.05, Figure 3). In addition, four systemic complications, i.e., sepsis, secondary infection, disseminated intravascular coagulation (DIC), and coagulopathy, showed significant associations with D-dimer. Acute brain injury and acidosis showed a tendency to associate with the D-dimer. Together, both acute fatal organ injury and systemic complications could be predicted by D-dimer.
Given the correlation of D-dimer with the demographic features, abnormal laboratory tests, interventions, and severe complications, we hypothesized that D-dimer is an independent indicator for these disease progression (CURB 65 score, onset to admission, onset to dyspnea), hospitalization (discharged, time is taken to turn SARS-CoV-2 PCR negative), and mortality. We analyzed the correlation of D-dimer and these clinical readouts and summarized in Tables S7 and 2. D-dimer was positively associated with the severity of lung injury (CURB 65), the days from the onset to admission, onset to dyspnea, time is taken to be PCR negative, and overall mortality (Figure 3). In contrast, the discharge rate was negatively related to D-dimer, demonstrating D-dimer’s capability to serve as a prognostic variate for outcomes.
Age and gender have been identified as preexisting medical conditions associated with COVID-19 resulting in higher mortality (3, 35, 62). We eliminated their effects on the association of D-dimer with 61 identified variables in bivariate meta-regression analyses. Age was a significant confounding factor of 27 variables, and 26 variables were still associated with the D-dimer independent of age (Table S8). In contrast, 31 variables were disassociated with the D-dimer. By comparison with age, the male gender was a much weaker confounding covariate (Table S9). Males were significantly associated with 11 variables and results in the dissociation of 14 variables with D-dimer. The association of 28 and 42 variables with D-dimer was still significant after considering age and male as a covariate, respectively (Table 2). Age-affected 18 variables include comorbidity, hypertension, diabetes, lymphocyte, CD4+ and CD8+ T cells, IL8, TNFα, eGFR, hemoglobin, albumin, CK-MB, onset to admission, onset to PCR negative, discharged, secondary infection, acute kidney injury, and acute liver injury. There were 10 variables confounded by both age and male, including diastolic pressure, chronic lung diseases, WBC, IL2R, IL6, ESR, GGT, CURB 65 score, coagulopathy, and DIC. These four variables were markedly affected by covariate male: platelets, fibrinogen, alkaline phosphatase, and onset to dyspnea. Finally, 22 variables were significantly associated with D-dimer: respiratory rate, systolic pressure, dyspnea, serum K+, neutrophils, globulin, CRP, ferritin, LDH, PCT, SpO2, blood glucose, BUN, total bilirubin, ALT, AST, CK, mortality, ventilation, ARDS, sepsis, and acute cardiac injury (Figure 4).
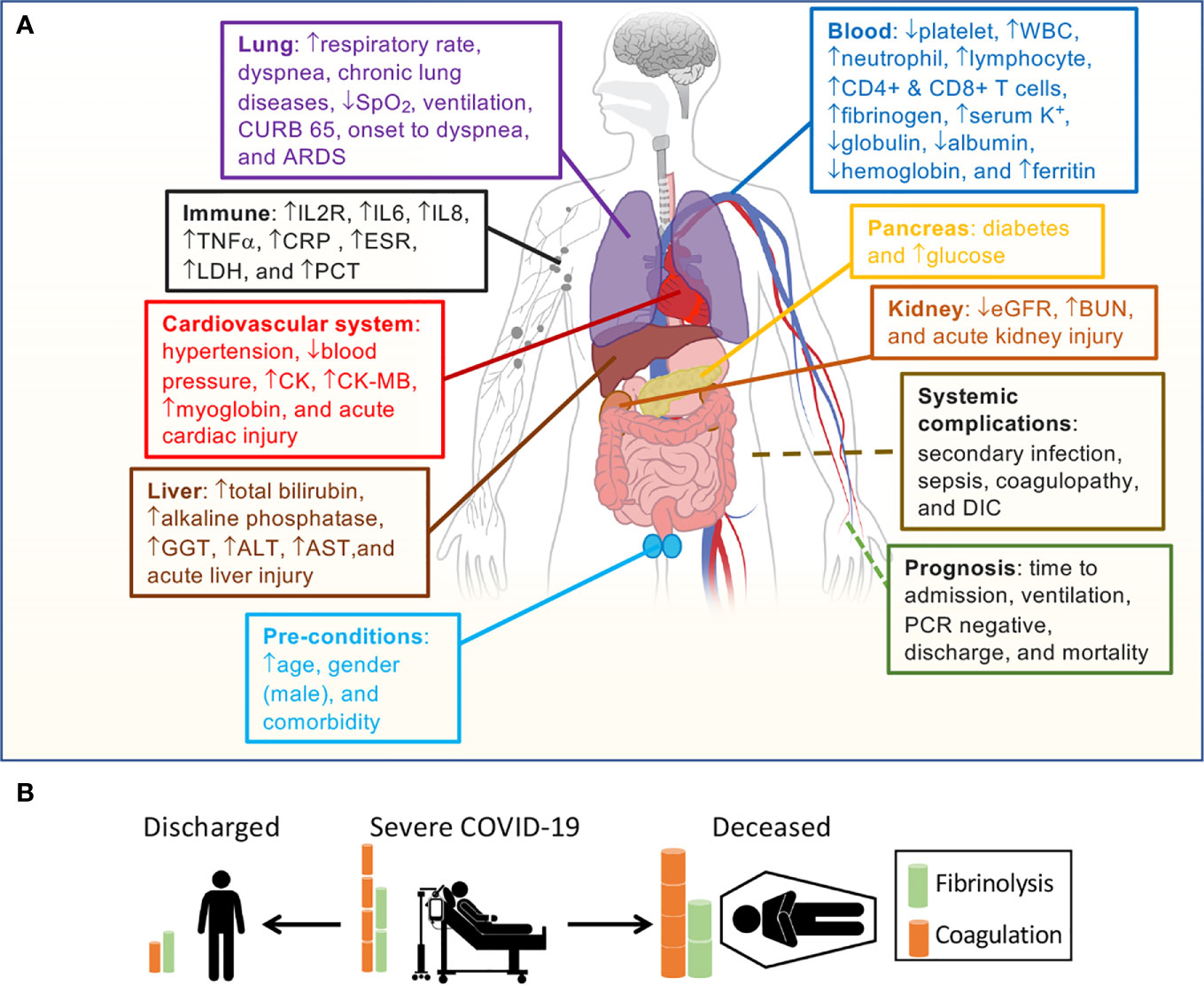
Figure 4 Correlation of D-dimer elevations and aberrant fibrin deposition with organ dysfunction. (A) D-dimer-associated clinical parameters sorted by the system and clinical relevance. (B) Outcomes correlate with D-dimer levels based on our hypothesis.
We aimed to systematically analyze the relationships between circulating D-dimer level and clinical variables in critically ill COVID-19 patients. There is a range of plasma D-dimer levels on hospital admission. The directions of dynamically changed FDPs for hospitalized patients are different between discharged and deceased cohorts. Our meta-regression analysis revealed that plasma D-dimer is associated with comorbidities, demographics, some laboratory tests, radiology, hospitalization, complications, and outcomes. These results suggest that in addition to serving as an independent predictor for fatality, severity and could potentially serve as a marker for daily monitoring of thrombolytic therapy, D-dimer is a specific biomarker that interacts with other coagulation molecules, inflammatory cytokines, and markers for organ/tissue injury. Of note, the interplay of acute-phase proteins with fibrinogen and D-dimer suggests that infection-induced inflammation (cytokines and chemokines) initiates a state of hyperfibrinolysis. This notion is supported by D-dimer’s disassociation with the entire coagulation panel (PT, APTT, factor VIII and XI, TAT) (66, 67). In general, hyperfibrinolytic homeostasis maintains vascular patency and normal organ function under physiological conditions (Figure 4B). SARS-CoV-2 and co-bacterial infection initiate a hypercoagulable state followed by hyperfibrinolysis in COVID-19. If hyperfibrinolysis can counter excessive coagulopathy, then the patients could be protected against thrombosis. Otherwise, insufficient local hyperfibrinolysis in the lung of non-survivors will be exhausted.
Age is associated with an increased D-dimer level in COVID-19 patients at admission as a covariate or independent prognostic marker for the outcomes of COVID-19. The cutoff value of D-dimer (0.5 µ;g/mL) is age-dependent for healthy cohorts (68, 69). Our subgroup analysis is consistent with the concept that adults older than 50 approach the threshold of the D-dimer levels seen in normalcy (Table S9). The difference in D-dimer between men and women is minor in a healthy population (69). D-dimer’s positive association with the percentage of male patients in COVID studies suggests more severe cases in men than women when admitted.
Soluble fibrinogen is synthesized in the liver (1.7-5g/d) primarily and others, including the bone marrow, brain, lung, and gastrointestinal epithelium (Figure 5A). It mainly distributes in the plasma (75%), interstitial fluid (16%), platelets, and lymph (70, 71). IL6 and other proinflammatory cytokines/chemokines, steroids, and miRNAs upregulate the fibrinogen synthesis up to 10-fold during the acute phase of injury and infection. Fibrinogen (2-3%) can be turned over to fibrin monomers by thrombin and cleaved by plasmin, and the process termed fibrinogenolysis (70, 72). Fibrinogen degradation products (FgDP) are 2- or 3-fold that of plasma D-dimer but with a shorter half lifetime (2.8 h vs 16 h for D-dimer) (70, 72). Crosslinked fibrin is formed in the presence of FXIIIa. At the endothelial cell surface of injured blood vessels, fibrin(ogen) “glues” the plugs formed by the aggregation of platelets to develop thrombi (clots). Excessive fibrin deposition and inflammation activate endothelial cells to produce tPA and urokinase plasminogen activator (uPA) (70, 71). Either tPA or uPA is capable of cleaving hepatocyte-derived plasminogen to activate plasmin. Plasmin proteolytically cleaves fibrin within the thrombi into FDP and D-dimers, the end products of fibrinolysis. Given the very short half lifetime (in seconds or minutes) of endogenous thrombin, tPA, uPA, and plasmin, and the overwhelming antithrombin, plasminogen activator inhibitor 1 (PAI-1), and plasmin inhibitors with a much longer lifetime in the plasma (73), the primary cleavage of plasminogen and fibrin may predominately take place at the surface of clots. Eventually, FgDP and FDP (D-dimer) will be catabolized in the liver and captured by the reticuloendothelial system and excreted to the bile (70, 71). Another clearance pathway is via the kidney to excreted to urine (70, 71). The D-dimer assay has routinely been applied for excluding PE, deep venous thromboembolism, and DIC, as well as a marker for monitoring the effects of fibrinolytic/thrombolytic therapy.
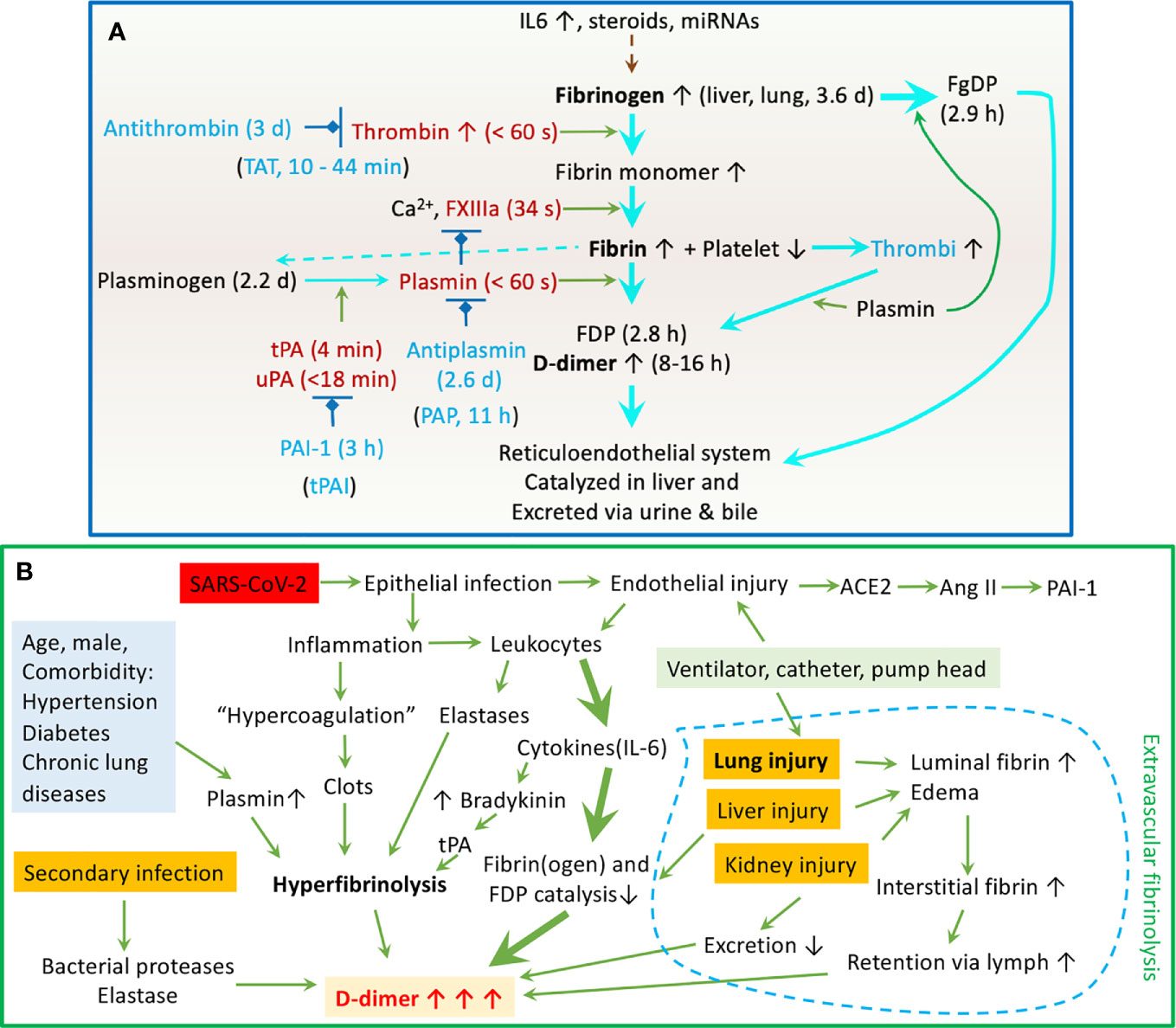
Figure 5 Schematic mechanisms for the dynamic D-dimer level in COVID-19 patients. (A) Regulation of the fibrinolysis system. The half lifetime for crucial components is given in followed brackets. (B) Clinicopathological mechanisms for elevated plasma D-dimer in COVID-19 patients.
Our regression analysis reveals that elevated D-dimer is associated with a broad spectrum of immune responses to SARS-CoV-2 infection, including increased pro-inflammatory cytokines (IL2R, IL6, IL8, and TNFα), acute phase proteins (CRP/C-reactive protein, fibrinogen, ferritin, and albumin), and inflammation indicators (ESR-erythrocyte sedimentation rate, PCT-procalcitonin, globulin, white blood cells, neutrophil, lymphocyte, and CD4+ & CD8+ T cells). Moreover, the days for reversion of the PCR test to negative is related to the D-dimer level. These correlations support the concept that interactions between high levels of circulating cytokines and hyperfibrinolysis may be functionally correlated. The binding of the spike proteins of SARS-CoV-2 to the ACE2 receptor in host respiratory epithelial cells downregulates the protective ACE2/Ang1-7/Mas axis, leading to increased expression of PAI-1 (74). Airway and lung epitheliitis releases pro-inflammatory cytokines to attract leukocytes from the blood. Moreover, infiltrated immune cells are activated and unleashed to attach normal lung tissues by releasing overwhelming cytokines. These cytokines upregulate positive acute-phase protein (i.e., fibrinogen), TF, and trypsin expression and inhibit negative proteins (i.e., albumin). Trypsin-activated matrix metalloproteinases break down the basolateral membrane and interstitial extracellular matrix. Further, endotheliopathy occurs in infected capillaries to initiate a local hypercoagulable state. The kinin-bradykinin is activated by IL6 to stimulate tPA expression in endothelial cells (74). Deposition of fibrin (clotting) activates endothelial cells to express more IL8, which suppresses clot lysis time (75). This, combined with the hypoxia-causing eryptosis, maybe the reason for IL8 associating with high mortality. ESR is associated with the severity of COVID-19 patients (76). In addition to carrying oxygen, erythrocyte-bound streptokinase and tPA break down the clots. Extrathyroidal produced PCT, if maintained at an elevated level by cytokines, is an indicator of poor outcomes of COVID-19 (77). It has been used as a marker for co-bacterial infection in septic shock and influenza. Neutrophil extracellular traps (NETs) may contribute to organ damage and promote thrombosis and fibrinolysis via elastase, so do lymphocytes/macrophages. Hepatocyte-synthesized globulins and albumin are involved in liver function, coagulation, and anti-inflammation. Their reduced levels by vast consumption predict a poor outcome. Albumin acts as an anticoagulant and antiplatelets and increases vascular permeability (78), which seems to explain the link between elevated D-dimer and hypoalbuminemia (Figure 5B).
The association of laboratory tests and complications can be explained by cell death. The lysis of platelets, erythrocytes, and other cells may contribute to higher serum K+ levels and reduced hemoglobin. This is supported by the association of D-dimer with LDH level and serum K+ and hemoglobin. At the organ level, D-dimer is associated with acute lung injury/ARDS, including hypoxia (respiratory rate, dyspnea, SpO2, CURB 65, and the onset of dyspnea). As a key early target organ of SARS-CoV-2 infection, both intra- and extravascular fibrin degradation in the alveolar sacs and interstitium could be the major site responsible for these associations. Hypoxia occurs in patients with the lungs with diffuse alveolar damage, a result in part of fibrin deposition and degradation. Moreover, mechanical ventilation with positive pressure could facilitate the retention of extravascular generated D-dimers. Associations of D-dimer with liver, kidney, and cardiac injury have been reported for other diseases (15, 17, 18, 79, 80). In addition, Pancreatic function and or metabolism of glucose may be dysfunctional, as shown by the association of D-dimer and blood glucose level. Our results demonstrate that D-dimer may serve as a biomarker to predict the damage of these organs by COVID-19 infection. Furthermore, the associations of D-dimer with systemic complications, including bacterial co-infection, septic shock, DIC, and thromboembolic events in large blood vessels, indicate high incidences and poor outcomes in critically ill patients.
Our results showed a strong positive association of elevated D-dimer on admission with mortality, indicating the prognostic value of an elevated D-dimer for the high risk of death. This is further corroborated by the positive correlation between D-dimer and days from onset to admission, the need for ventilation and the days are taken for PCR test reversion to negative. Another line of supportive evidence is the adverse relation of D-dimer and discharge probability. This data shows that prompt admission and clearance of the SARS-CoV-2 virus may alleviate the severity and reduce fatal events by preventing hyperfibrinolysis and inflammation. D-dimer, as a prognostic marker, is supported by other reviews (81–84) and clinical studies (1, 62). Patients with severe COVID-19 maybe those who were ill for a longer period than mild controls. This is supported by the association of the D-dimer level and the days from onset to admission (Table 2). Alternatively, the progression of the disease may be much quicker in critically ill patients at admission.
We have systematically reviewed the literature using meta-regressions and meta-analyses to define associations between elevated D-dimer and clinical multi-variants for the first time. Our study demonstrates: 1) D-dimer and other clinical variables’ associations indicate either a relationship that could be cause-effect or indirect. 2) Few D-dimer-associated variables have been confirmed or could be prognostic biomarkers for developing fatal events and in-hospital mortality. 3) Extremely elevated plasma D-dimer seems to be the consequence of hyperfibrinolysis predominately in the pulmonary capillaries and other organs. 4) A dynamic increase in the D-dimer level may be associated with thromboembolism and higher fatality, while we infer that a continuous decline by daily testing will generally lead to recovery.
Because COVID-19 is a newly emerging disease, most of the included studies are descriptive, case series, retrospective, single-center, and observational. Most of the studies included are from China/Asia, where the pandemic was discovered. Diversity in cohorts exists from children to seniors associated with variant comorbidities and complications. Inconsistent grouping strategies between studies pose a challenge for meta-analysis, for example, ICU vs non-ICU, ARDS vs non-ARDS, control vs COVID-19, survivor vs non-survivor, severe vs non-severe, VTE vs non-VTE, death vs recovered, mild vs moderate, moderate vs severe, normal vs abnormal D-dimer, etc. We cannot exclude the potential pre- and post-test deviations regarding the methodology for D-dimer assays. Meta-regression but not meta-analysis may partially mitigate these deviations. Also, a cutoff of D-dimer level could not be computed. Of note, D-Dimer level on admission could not be a biomarker associated with all clinical readouts.
We systematically analyzed the associations of D-dimer on admission with more than 100 clinical readouts. Our results demonstrate that elevated D-dimer could be inter-regulated by a spectrum of clinical variables, including preexisting conditions, inflammation, organ injury, abnormal glucose, complications, and outcomes. The clinical relevance of elevated D-dimer may be multifaceted.
H-LJ, ZS, and RZ were responsible for searching the literature, reviewing extracted data, meta-analyzing data, and preparing drafts of this manuscript. H-LJ, SI, AK, S-LL, MM, and GY improved the final manuscript, and all authors approved submission.
This study was funded in part by the grants: HL87017 (HLJ), HL095435 (MAM), HL134828 (MAM), HL130402 (AK & SI), AI112381(SLL), AI150473 (SLL), HL154103 (SI & AK), and HL14285301 (SI). The funders had no role in study design, data ex- traction, data analysis, data interpretation, or writing of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Mr. Cong Liu, Ms. Bingxin Guo, and Ms. Qihang Yin for their technical support at the beginning of searching the literature.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.691249/full#supplementary-material
1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients With COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet (2020) 395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3
2. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and Antiphospholipid Antibodies in Patients With COVID-19. N Engl J Med (2020) 382(17):e38. doi: 10.1056/NEJMc2007575
3. Xie J, Hungerford D, Chen H, Abrams ST, Li S, Wang G, et al. Development and External Validation of a Prognostic Multivariable Model on Admission for Hospitalized Patients With COVID-19. medRxiv (2020) 2020:03.28.20045997. doi: 10.1101/2020.03.28.20045997
4. Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical Findings in a Group of Patients Infected With the 2019 Novel Coronavirus (SARS-Cov-2) Outside of Wuhan, China: Retrospective Case Series. BMJ (2020) 368:m606. doi: 10.1136/bmj.m606
5. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in Adults With Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet (2020) 395(10236):1569–78. doi: 10.1016/S0140-6736(20)31022-9
6. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA (2020) 323(11):1061–9. doi: 10.1001/jama.2020.1585
7. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA (2020) 323(20):2052–9. doi: 10.1001/jama.2020.6775
8. Lu J, Hu S, Fan R, Liu Z, Yin X, Wang Q, et al. ACP Risk Grade: A Simple Mortality Index for Patients With Confirmed or Suspected Severe Acute Respiratory Syndrome Coronavirus 2 Disease (COVID-19) During the Early Stage of Outbreak in Wuhan, China. medRxiv (2020) 2020:02.20.20025510. doi: 10.1101/2020.02.20.20025510
9. Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. COVID-19 and Kidney Transplantation. N Engl J Med (2020) 382(25):2475–7. doi: 10.1056/NEJMc2011117
10. Bona RD, Valbusa A, Malfa G, Giacobbe DR, Ameri P, Patroniti N, et al. Systemic Fibrinolysis for Acute Pulmonary Embolism Complicating Acute Respiratory Distress Syndrome in Severe COVID-19: A Case Series. Eur Heart J Cardiovasc Pharmacother (2020) 7(1):78–80. doi: 10.1093/ehjcvp/pvaa087
11. Ly A, Alessandri C, Skripkina E, Meffert A, Clariot S, de Roux Q, et al. Rescue Fibrinolysis in Suspected Massive Pulmonary Embolism During SARS-Cov-2 Pandemic. Resuscitation (2020) 152:86–8. doi: 10.1016/j.resuscitation.2020.05.020
12. Chan KH, Slim J, Shaaban HS. Pulmonary Embolism and Increased Levels of D-Dimer in Patients With Coronavirus Disease. Emerg Infect Dis (2020) 26(8):1941–3. doi: 10.3201/eid2610.202127
13. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High Risk of Thrombosis in Patients With Severe SARS-Cov-2 Infection: A Multicenter Prospective Cohort Study. Intensive Care Med (2020) 46(6):1089–98. doi: 10.1007/s00134-020-06062-x
14. Connors JM, Levy JH. COVID-19 and Its Implications for Thrombosis and Anticoagulation. Blood (2020) 135(23):2033–40. doi: 10.1182/blood.2020006000
15. Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological Study of the 2019 Novel Coronavirus Disease (COVID-19) Through Postmortem Core Biopsies. Mod Pathol (2020) 33(6):1007–14. doi: 10.1038/s41379-020-0536-x
16. Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira EP, Saldiva PHN, et al. Pathological Evidence of Pulmonary Thrombotic Phenomena in Severe COVID-19. J Thromb Haemost (2020) 18(6):1517–9. doi: 10.1111/jth.14844
17. Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, Märkl B, et al. Postmortem Examination of Patients With COVID-19. JAMA (2020) 323(24):2518–20. doi: 10.1001/jama.2020.8907
18. Beigmohammadi MT, Jahanbin B, Safaei M, Amoozadeh L, Khoshavi M, Mehrtash V, et al. Pathological Findings of Postmortem Biopsies From Lung, Heart, and Liver of 7 Deceased COVID-19 Patients. Int J Surg Pathol (2021) 29(2):135–45. doi: 10.1177/1066896920935195
19. Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and Ultrastructural Findings of Fatal COVID-19 Infections in Washington State: A Case Series. Lancet (2020) 396(10247):320–32. doi: 10.1016/S0140-6736(20)31305-2
20. Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis Risk Associated With COVID-19 Infection. a Scoping Review. Thromb Res (2020) 192:152–60. doi: 10.1016/j.thromres.2020.05.039
21. Marchandot B, Sattler L, Jesel L, Matsushita K, Schini-Kerth V, Grunebaum L, et al. COVID-19 Related Coagulopathy: A Distinct Entity? J Clin Med (2020) 9(6):1651. doi: 10.3390/jcm9061651
22. Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, et al. Fibrinolysis Shutdown Correlation With Thromboembolic Events in Severe COVID-19 Infection. J Am Coll Surg (2020) 231(2):193–203.e1. doi: 10.1016/j.jamcollsurg.2020.05.007
23. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue Plasminogen Activator (Tpa) Treatment for COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS): A Case Series. J Thromb Haemost (2020) 18(7):1752–5. doi: 10.1111/jth.14828
24. Barrett CD, Oren-Grinberg A, Chao E, Moraco AH, Martin MJ, Reddy SH, et al. Rescue Therapy for Severe COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS) With Tissue Plasminogen Activator (Tpa): A Case Series. J Trauma Acute Care Surg (2020) 89(3):453–7. doi: 10.1097/TA.0000000000002786
25. Christie DB,3, Nemec HM, Scott AM, Buchanan JT, Franklin CM, Ahmed A, et al. Early Outcomes With Utilization of Tissue Plasminogen Activator in COVID-19 Associated Respiratory Distress: A Series of Five Cases. J Trauma Acute Care Surg (2020) 89(3):448–52. doi: 10.1097/TA.0000000000002787
26. Papamichalis P, Papadogoulas A, Katsiafylloudis P, Skoura AL, Papamichalis M, Neou E, et al. Combination of Thrombolytic and Immunosuppressive Therapy for Coronavirus Disease 2019: A Case Report. Int J Infect Dis (2020) 97:90–3. doi: 10.1016/j.ijid.2020.05.118
27. Poor HD, Ventetuolo CE, Tolbert T, Chun G, Serrao G, Zeidman A, et al. COVID-19 Critical Illness Pathophysiology Driven by Diffuse Pulmonary Thrombi and Pulmonary Endothelial Dysfunction Responsive to Thrombolysis. Clin Transl Med (2020) 10(2):e44. doi: 10.1002/ctm2.44
28. Arachchillage DJ, Stacey A, Akor F, Scotz M, Laffan M. Thrombolysis Restores Perfusion in COVID 19 Hypoxia. Br J Haematol (2020) 190(5):e270–4. doi: 10.1111/bjh.17050
29. Asakura H, Ogawa H. Potential of Heparin and Nafamostat Combination Therapy for COVID-19. J Thromb Haemost (2020) 18(6):1521–2. doi: 10.1111/jth.14858
30. Doi K, Ikeda M, Hayase N, Moriya K, Morimura N. Nafamostat Mesylate Treatment in Combination With Favipiravir for Patients Critically Ill With COVID-19: A Case Series. Crit Care (2020) 24(1):392. doi: 10.1186/s13054-020-03078-z
31. Thierry AR. Anti-Protease Treatments Targeting Plasmin(Ogen) and Neutrophil Elastase May Be Beneficial in Fighting COVID-19. Physiol Rev (2020) 100(4):1597–8. doi: 10.1152/physrev.00019.2020
32. Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of High Vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-Cov-2) Infection: A Randomized Clinical Trial. JAMA Netw Open (2020) 3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857
33. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J Clin Invest (2020) 130(5):2620–9. doi: 10.1172/JCI137244
34. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet (2020) 395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7
35. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical Characteristics of 113 Deceased Patients With Coronavirus Disease 2019: Retrospective Study. BMJ (2020) 368:m1091. doi: 10.1136/bmj.m1091
36. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of Venous Thromboembolism in Patients With Severe Novel Coronavirus Pneumonia. J Thromb Haemost (2020) 18(6):1421–4. doi: 10.1111/jth.14830
37. Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of Mortality for Patients With COVID-19 Pneumonia Caused by SARS-Cov-2: A Prospective Cohort Study. Eur Respir J (2020) 55(5):2000524. doi: 10.1183/13993003.00524-2020
38. Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical Features of 85 Fatal Cases of COVID-19 From Wuhan. a Retrospective Observational Study. Am J Respir Crit Care Med (2020) 201(11):1372–9. doi: 10.1164/rccm.202003-0543OC
39. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 With Different Severities: A Multicenter Study of Clinical Features. Am J Respir Crit Care Med (2020) 201(11):1380–8. doi: 10.1164/rccm.202002-0445OC
40. Fogarty H, Townsend L, Ni Cheallaigh C, Bergin C, Martin-Loeches I, Browne P, et al. COVID19 Coagulopathy in Caucasian Patients. Br J Haematol (2020) 189(6):1044–9. doi: 10.1111/bjh.16749
41. Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent Changes in Blood Coagulation of Patients With SARS-Cov-2 Infection. Clin Chem Lab Med (2020) 58(7):1116–20. doi: 10.1515/cclm-2020-0188
42. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
43. Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. Clinical Characteristics of Refractory COVID-19 Pneumonia in Wuhan, China. Clin Infect Dis (2020). doi: 10.1093/cid/ciaa270
44. Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-Vessel Stroke as a Presenting Feature of COVID-19 in the Young. N Engl J Med (2020) 382(20):e60. doi: 10.1056/NEJMc2009787
45. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 Patients in Intensive Care Unit. a Report of Thromboelastography Findings and Other Parameters of Hemostasis. J Thromb Haemost (2020) 18(7):1738–42. doi: 10.1111/jth.14850
46. Paranjpe I, Russak A, De Freitas JK, Lala A, Miotto R, Vaid A, et al. Clinical Characteristics of Hospitalized COVID-19 Patients in New York City. medRxiv (2020) 2020:04.19.20062117. doi: 10.1101/2020.04.19.20062117
47. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and Epidemiological Features of 36 Children With Coronavirus Disease 2019 (COVID-19) in Zhejiang, China: An Observational Cohort Study. Lancet Infect Dis (2020) 20(6):689–96. doi: 10.1016/S1473-3099(20)30198-5
48. Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, et al. The Procoagulant Pattern of Patients With COVID-19 Acute Respiratory Distress Syndrome. J Thromb Haemost (2020) 18(7):1747–51. doi: 10.1111/jth.14854
49. Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. COVID-19 Testing, Hospital Admission, and Intensive Care Among 2,026,227 United States Veterans Aged 54-75 Years. medRxiv (2020) 2020:04.09.20059964. doi: 10.1101/2020.04.09.20059964
50. Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, et al. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb Haemost (2020) 120(6):998–1000. doi: 10.1055/s-0040-1710018
51. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant Treatment is Associated With Decreased Mortality in Severe Coronavirus Disease 2019 Patients With Coagulopathy. J Thromb Haemost (2020) 18(5):1094–9. doi: 10.1111/jth.14817
52. Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus Disease 2019 in Elderly Patients: Characteristics and Prognostic Factors Based on 4-Week Follow-Up. J Infect (2020) 80(6):639–45. doi: 10.1016/j.jinf.2020.03.019
53. Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, et al. Clinical Course and Outcomes of 344 Intensive Care Patients With COVID-19. Am J Respir Crit Care Med (2020) 201(11):1430–4. doi: 10.1164/rccm.202003-0736LE
54. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis (2020) 71(15):769–77. doi: 10.1093/cid/ciaa272
55. Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A New Coronavirus Associated With Human Respiratory Disease in China. Nature (2020) 579(7798):265–9. doi: 10.1038/s41586-020-2008-3
56. Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical Characteristics of Imported Cases of COVID-19 in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis (2020) 71(15):706–12. doi: 10.1093/cid/ciaa199
57. Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Clinical Characteristics and Outcomes of Cancer Patients With COVID-19. J Med Virol (2020) 92(10):2067–73. doi: 10.1002/jmv.25972
58. Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical Characteristics and Imaging Manifestations of the 2019 Novel Coronavirus Disease (COVID-19):a Multi-Center Study in Wenzhou City, Zhejiang, China. J Infect (2020) 80(4):388–93. doi: 10.1016/j.jinf.2020.02.016
59. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical Course and Outcomes of Critically Ill Patients With SARS-Cov-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir Med (2020) 8(5):475–81. doi: 10.1016/S2213-2600(20)30079-5
60. Yao Y, Cao J, Wang Q, Liu K, Luo Z, Yu K, et al. D-Dimer as a Biomarker for Disease Severity and Mortality in COVID-19 Patients: A Case Control Study. J Intensive Care (2020) 8:49. doi: 10.1186/s40560-020-00466-z
61. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical Characteristics of 140 Patients Infected With SARS-Cov-2 in Wuhan, China. Allergy (2020) 75(7):1730–41. doi: 10.1111/all.14238
62. Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D-Dimer Levels on Admission to Predict in-Hospital Mortality in Patients With Covid-19. J Thromb Haemost (2020) 18(6):1324–9. doi: 10.1111/jth.14859
63. Zou Y, Guo H, Zhang Y, Zhang Z, Liu Y, Wang J, et al. Analysis of Coagulation Parameters in Patients With COVID-19 in Shanghai, China. Biosci Trends (2020) 14(4):285–9. doi: 10.5582/bst.2020.03086
64. Su Z, Zhu L, Wu J, Zhao R, Ji HL. Systematic Review and Meta-Analysis of Nasal Potential Difference in Hypoxia-Induced Lung Injury. Sci Rep (2016) 6:30780. doi: 10.1038/srep30780
65. Zhao R, Su Z, Wu J, Ji HL. Serious Adverse Events of Cell Therapy for Respiratory Diseases: A Systematic Review and Meta-Analysis. Oncotarget (2017) 8(18):30511–23. doi: 10.18632/oncotarget.15426
66. Coccheri S. COVID-19: The Crucial Role of Blood Coagulation and Fibrinolysis. Intern Emerg Med (2020) 15(8):1369–73. doi: 10.1007/s11739-020-02443-8
67. Martín-Rojas RM, Pérez-Rus G, Delgado-Pinos VE, Domingo-González A, Regalado-Artamendi I, Alba-Urdiales N, et al. COVID-19 Coagulopathy: An in-Depth Analysis of the Coagulation System. Eur J Haematol (2020) 105(6):741–50. doi: 10.1111/ejh.13501
68. Olson JD. D-Dimer: An Overview of Hemostasis and Fibrinolysis, Assays, and Clinical Applications. Adv Clin Chem (2015) 69:1–46. doi: 10.1016/bs.acc.2014.12.001
69. Haase C, Joergensen M, Ellervik C, Joergensen MK, Bathum L. Age- and Sex-Dependent Reference Intervals for D-Dimer: Evidence for a Marked Increase by Age. Thromb Res (2013) 132(6):676–80. doi: 10.1016/j.thromres.2013.09.033
70. Weisel JW, Litvinov RI. Fibrin Formation, Structure and Properties. Subcell Biochem (2017) 82:405–56. doi: 10.1007/978-3-319-49674-0_13
71. Takeda Y. Studies of the Metabolism and Distribution of Fibrinogen in Healthy Men With Autologous 125-I-Labeled Fibrinogen. J Clin Invest (1966) 45(1):103–11. doi: 10.1172/JCI105314
72. Favresse J, Lippi G, Roy PM, Chatelain B, Jacqmin H, Ten Cate H, et al. D-Dimer: Preanalytical, Analytical, Postanalytical Variables, and Clinical Applications. Crit Rev Clin Lab Sci (2018) 55(8):548–77. doi: 10.1080/10408363.2018.1529734
73. Rühl H, Berens C, Winterhagen A, Müller J, Oldenburg J, Pötzsch B. Label-Free Kinetic Studies of Hemostasis-Related Biomarkers Including D-Dimer Using Autologous Serum Transfusion. PloS One (2015) 10(12):e0145012. doi: 10.1371/journal.pone.0145012
74. Kwaan HC. Coronavirus Disease 2019: The Role of the Fibrinolytic System From Transmission to Organ Injury and Sequelae. Semin Thromb Hemost (2020) 46(7):841–4. doi: 10.1055/s-0040-1709996
75. Bester J, Matshailwe C, Pretorius E. Simultaneous Presence of Hypercoagulation and Increased Clot Lysis Time Due to IL-1β, IL-6 and IL-8. Cytokine (2018) 110:237–42. doi: 10.1016/j.cyto.2018.01.007
76. Lapić I, Rogić D, Plebani M. Erythrocyte Sedimentation Rate is Associated With Severe Coronavirus Disease 2019 (COVID-19): A Pooled Analysis. Clin Chem Lab Med (2020) 58(7):1146–8. doi: 10.1515/cclm-2020-0620
77. Lippi G, Plebani M. Procalcitonin in Patients With Severe Coronavirus Disease 2019 (COVID-19): A Meta-Analysis. Clin Chim Acta (2020) 505:190–1. doi: 10.1016/j.cca.2020.03.004
78. Violi F, Ceccarelli G, Cangemi R, Alessandri F, D’Ettorre G, Oliva A, et al. Hypoalbuminemia, Coagulopathy, and Vascular Disease in COVID-19. Circ Res (2020) 127(3):400–1. doi: 10.1161/CIRCRESAHA.120.317173
79. Schefold JC, Gerber JL, Angehrn MC, Müller M, Messmer AS, Leichtle AB, et al. Renal Function-Adjusted D-Dimer Levels in Critically Ill Patients With Suspected Thromboembolism. Crit Care Med (2020) 48(4):e270–6. doi: 10.1097/CCM.0000000000004204
80. Simes J, Robledo KP, White HD, Espinoza D, Stewart RA, Sullivan DR, et al. D-Dimer Predicts Long-Term Cause-Specific Mortality, Cardiovascular Events, and Cancer in Patients With Stable Coronary Heart Disease: LIPID Study. Circulation (2018) 138(7):712–23. doi: 10.1161/CIRCULATIONAHA.117.029901
81. Shah S, Shah K, Patel SB, Patel FS, Osman M, Velagapudi P, et al. Elevated D-Dimer Levels are Associated With Increased Risk of Mortality in COVID-19: A Systematic Review and Meta-Analysis. Cardiol Rev (2020) 28(6):295–302. doi: 10.1097/CRD.0000000000000330
82. Vidali S, Morosetti D, Cossu E, Luisi MLE, Pancani S, Semeraro V, et al. D-Dimer as an Indicator of Prognosis in SARS-Cov-2 Infection: A Systematic Review. ERJ Open Res (2020) 6(2):00260–2020. doi: 10.1183/23120541.00260-2020
83. Li Q, Chen L, Li Q, He W, Yu J, Chen L, et al. Cancer Increases Risk of in-Hospital Death From COVID-19 in Persons <65 Years and Those Not in Complete Remission. Leukemia (2020) 34(9):2384–91. doi: 10.1038/s41375-020-0986-7
Keywords: COVID-19, D-dimer, fibrinolysis, fibrinogenolysis, comorbidity, meta-regression
Citation: Zhao R, Su Z, Komissarov AA, Liu S-L, Yi G, Idell S, Matthay MA and Ji H-L (2021) Associations of D-Dimer on Admission and Clinical Features of COVID-19 Patients: A Systematic Review, Meta-Analysis, and Meta-Regression. Front. Immunol. 12:691249. doi: 10.3389/fimmu.2021.691249
Received: 05 April 2021; Accepted: 23 April 2021;
Published: 07 May 2021.
Edited by:
Guo-Chang Fan, University of Cincinnati, United StatesReviewed by:
Qiang Ding, University of Alabama at Birmingham, United StatesCopyright © 2021 Zhao, Su, Komissarov, Liu, Yi, Idell, Matthay and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Long Ji, amFtZXMuamlAdXRoY3QuZWR1
†These authors have equally contributed to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.