
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 11 June 2021
Sec. Autoimmune and Autoinflammatory Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.686457
This article is part of the Research Topic Sjögren’s Syndrome: Pathogenicity, Novel targets and Antigens. View all 29 articles
 Onorina Berardicurti1
Onorina Berardicurti1 Piero Ruscitti1
Piero Ruscitti1 Paola Di Benedetto1
Paola Di Benedetto1 Settimio D’Andrea2
Settimio D’Andrea2 Luca Navarini3
Luca Navarini3 Annalisa Marino3
Annalisa Marino3 Paola Cipriani1
Paola Cipriani1 Roberto Giacomelli3*
Roberto Giacomelli3*Objective: Patients with primary Sjögren’s syndrome (pSS) may develop a potentially severe disease with extra-glandular involvement and lymphoma insurgence. Minor salivary gland biopsy is routinely used in the disease diagnosis, but its potential role as a biomarker for clinical disease presentation and prognosis is still poorly understood.
Methods: We performed a systematic review and meta-analysis on clinical presentation and prognosis in pSS patients who underwent minor salivary gland biopsy at diagnosis according to the PRISMA guidelines.
Results: We included five retrospective studies and 589 pSS patients. Ectopic GCs presence was not associated with a significant increase in the odds ratio for the clinical variables explored such as salivary gland swelling, arthritis, and Raynaud’s phenomenon. As far as serological features are concerned, ectopic GCs presence accounted for an increased ratio of antibodies anti-SSA (OR = 3.13, 95% CI: 1.25–7.85, p = 0.02, I2 = 79%), anti-SSB (OR = 3.94, 95% CI: 1.50–10.37, p = 0.0005, I2 = 80%), and RFs presence (OR = 3.12, 95% CI: 1.94–5.00, p < 0.00001, I2 = 0%).
Conclusions: This study showed that the association between ectopic GC in salivary glands identifies a clinical subset characterized by autoantibodies presence, and probably pSS patients affected from a more severe disease.
Primary Sjögren’s syndrome (pSS) is an autoimmune disorder characterized by chronic exocrine gland infiltration, sicca syndrome, extra-glandular manifestations, and an increased risk of lymphoma development (1). The disease mainly affects middle-aged women, and its incidence ranges between 3 and 11 per 100,000 individuals per year (1, 2). More than half of the affected patients develop systemic involvement (3, 4). In severe patients, the excess of mortality is mainly related to the development of B cell lymphoma and visceral involvement, like interstitial lung disease, renal failure, hypokalemic paralysis, and severe cryoglobulinemic vasculitis (5). T and B-lymphocytes, together with other immune cells, home into salivary glands, promoting the disease development and determining the specific histologic pattern, known as focal lymphocytic sialadenitis (LFS) (6), used for diagnostic and classificative purposes. Minor salivary glands biopsy (mSGB) represents the main criteria used in the disease classification. In fact, from 2002, when the American–European Consensus Group (AECG) classification criteria for pSS have been proposed, to 2016, when the last set of classification criteria were released, the presence of LFS with a focus score (FS) ≥1 remains the “gold standard” for pSS classification (7–9). The first description of the minor salivary gland infiltrate related to keratoconjunctivitis and sicca syndrome is from H. Sjögren in the 1933 (10). Successively, Chisholm and Mason, Greenspan and Daniels, and Tarpley suggested three different scoring systems for mSGB. Chisholm and Mason proposed a score based on five grades, from 0 to 4, considering the presence of slight or moderate lymphocytic infiltration and/or focus of lymphocytes (11). Greenspan and Daniels introduced in 1974 the concept of LFS and FS. The FS was defined as the number of foci in a 4 mm2 area of normal-appearing tissue, and LFS was defined as an FS ≥1 (12). Finally, Tarpley proposed a score including acinar destruction and fibrosis, associated with the number of immune cell aggregates, to graduate mSGB (13). So far, different studies analyzed the role of mSG involvement as a specific tool for diagnosis, and data from a previous systematic literature review showed a wide range of sensitivity and specificity for pSS diagnosis, from 63.5 to 93.7% and from 61.2 to 100%, respectively (14). Furthermore, a large variability was observed concerning the estimated positive predictive value of mSGB, which ranged from 74.2 to 100%, and the estimated predictive negative value, which ranged from 39.1 to 96.1% (14). Despite these wide ranges, the diagnostic role of mSGB in pSS is largely recognized and recommended, while its role in pSS patients’ stratification and systemic disease prognosis still remain poorly known.
On these bases, we designed and conducted a systematic literature review (SLR) and meta-analysis to assess the value of mSGB for pSS patients’ stratification. Furthermore, we explored the possible predictive role of mSGB in systemic disease development.
This study was conducted according to the Cochrane Collaboration and the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (15). The PRISMA checklist is presented in Table 1.
In this study, we included all peer-reviewed published articles that reported demographic, clinical, and serological characteristics of pSS according to their mSGB histology. We selected all the studies conducted in pSS patients with a confirmed diagnosis (Population) who performed a mSGB (Intervention and Control) that reported the demographic, clinical, and serological associated factors (Outcome). We did not introduce temporal limits in our search strategy. Review articles, case reports, opinion articles, letters, brief reports, non-English publications, and those with missing data were excluded.
We conducted a systematic search in MEDLINE, Cochrane Library, and SCOPUS databases to identify all relevant English-language publications, with the terms: ((“Histology”[MeSH Terms] AND “Salivary Glands”[MeSH Terms]) OR “focus score” OR “lymphomononuclear infiltrates” OR “Chisholm and Mason” OR “Tarpley”) AND “Sjogren’s syndrome”[MeSH Terms]). Two independent reviewers (OB and PR) first screened the retrieved papers based on the title and abstract (Figure 1). If it was not clear from the title and abstract whether the paper contained relevant data, the full paper was retrieved. The list of all excluded papers after full-text assessment is reported in Supplementary Table 1. Finally, we scrutinized the reference lists of the identified articles to find additional pertinent studies.
Data from the selected articles were extracted according to the first author; publication year; number of participants; number of female; clinical features [xerostomia, xeroftalmia, parotid swelling, arthritis, renal involvement, hematological involvement, lung involvement, cutaneous involvement, peripheral nervous system (PNS) involvement, central nervous system (CNS) involvement, lymphoma, muscular involvement, and Raynaud’s phenomena (RP)], and serological features [anti-SSA antibodies, anti-SSB antibodies, rheumatoid factor (RF), complement components (C3, C4), cryoglobulinemia, hypergammaglobulinemia, leukopenia and anti-nuclear antibodies (ANA)]. Wherever data were missing or inconsistent, the authors were contacted to obtain the necessary information.
The quality of studies included in the quantitative analysis was assessed using the “star system” of the Newcastle-Ottawa Quality Assessment Scale (NOS) (16). The minimum and maximum scores that could be awarded were zero stars and nine stars, respectively (Table 2). Studies that scored ≥seven stars were regarded as high quality. The quality assessment was performed by two reviewers (OB and PR), and any disagreement was resolved by a third reviewer (SD’A) who re-evaluated the original study.
The relationship between ectopic GCs presence and glandular swelling, arthritis, Raynaud’s phenomena (RP), anti-SSA antibodies, anti-SSB antibodies, or rheumatoid factor (RF) was assessed using odds ratio (OR) and 95% CI as well as Mantel–Haenszel estimates. A significant heterogeneity was expected among studies. Data were combined using random effect models, which assumes that the included studies have varying effect sizes, thus providing a conservative estimate of the overall effect. The Cochrane chi-square (Cochrane Q) test and I2 test were carried out to analyze the heterogeneity among the results of different studies. An I2 value <25% was considered indicative of no heterogeneity, while I2 >50% and/or P <0.05 indicated substantial heterogeneity (17). The extracted data were analyzed using the statistical software R (version 3.0.3; R Foundation for Statistical Computing, Vienna, Austria) and the Review Manager (RevMan) of the Cochrane Library (version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
A total of 424 articles were retrieved by using the above-mentioned search strategy, and after screening titles and abstracts, 222 articles were selected for full-text assessment. After review, nine studies were included in the qualitative and five studies were included in the quantitative analysis. Among the studies included in the quantitative analysis, two studies were conducted in Norway (18, 19); one in Italy (20); one in China (21); and one in Korea (22). All of them referred to pSS patients fulfilling the revised criteria proposed by the American-European Consensus Group (7), and all the studies were retrospective. In all the included research studies the mSGBs were performed and analyzed according to the standard procedures (12). The main characteristics of the selected studies are reported in Table 3. The overall quality of the selected studies is high, but all the retrieved studies were retrospectively designed (Table 2). The main demographic, clinical, and serologic characteristics of pSS patients are reported in Tables 3, 4.
Among clinical features, the presence of ectopic GC and its association with glandular swelling (Figure 2A), arthritis (Figure 2B), and RP (Figure 2C) were explored. Ectopic GCs presence was not associated with a significant increase in the odds ratio for all the explored variables. As far as the glandular swelling is concerned, the lack of association was maintained after the “leave one out” test with a significant reduction in the heterogeneity, from 50 to 0%, confirming the reliability of the results. Concerning the serological features, ectopic GCs presence accounted for an increased ratio of anti-SSA, anti-SSB autoantibodies, and RF presence. The ectopic GCs presence at histologic evaluation increased the anti-SSA presence probability (OR = 3.13, 95% CI: 1.25–7.85, p = 0.02), as shown in Figure 3A. A significant heterogeneity was observed among the studies (p for heterogeneity = 0.0007, I2 = 79%), which was mainly observed in the study of Carubbi et al. (20). However, removing this study from the analysis did not change the outcome confirming the association (Figure 3B). Ectopic GCs presence at the histologic evaluation increased the probability of anti-SSB antibodies’ presence (OR = 3.94, 95% CI: 1.50–10.37, p = 0.0005, I2 = 80%), and also this outcome was maintained during the “leave one out” test. Finally, ectopic GCs presence increased the probability of RFs presence in pSS patients’ sera (OR = 3.12, 95% CI: 1.94–5.00, p < 0.00001, I2 = 0%), without any heterogeneity among the selected studies (Figure 3C).
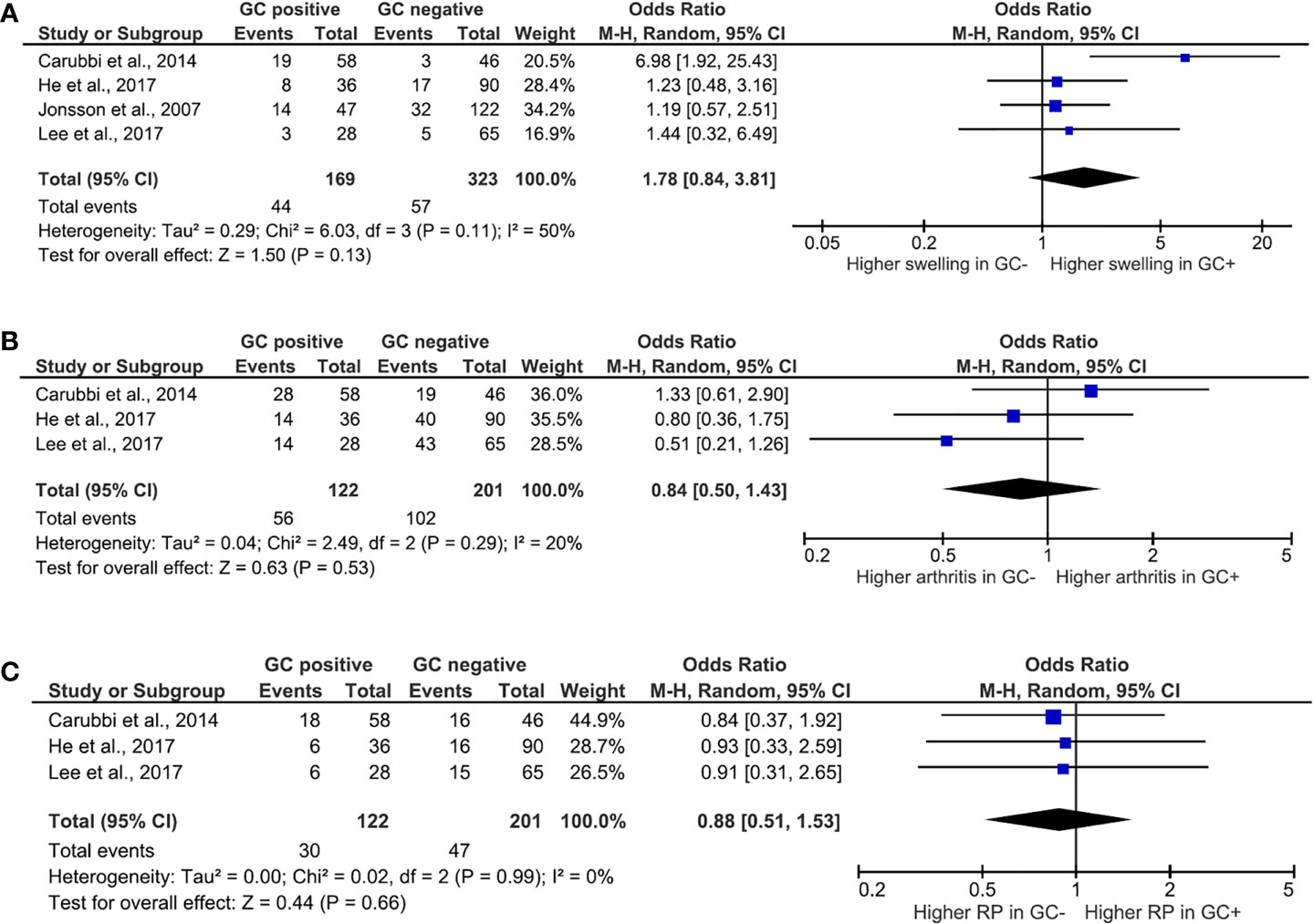
Figure 2 Meta-analysis of the presence of salivary gland swelling (A), arthritis (B), and Raynaud’s phenomena (C) between patients with Sjögren’s syndrome with or without ectopic germinal center. The size of squares is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study; diamond, the pooled estimate with 95% CI; N, the number of persons at baseline; and OR, odds ratio; GC, germinal center; RP, Raynaud’s phenomena.
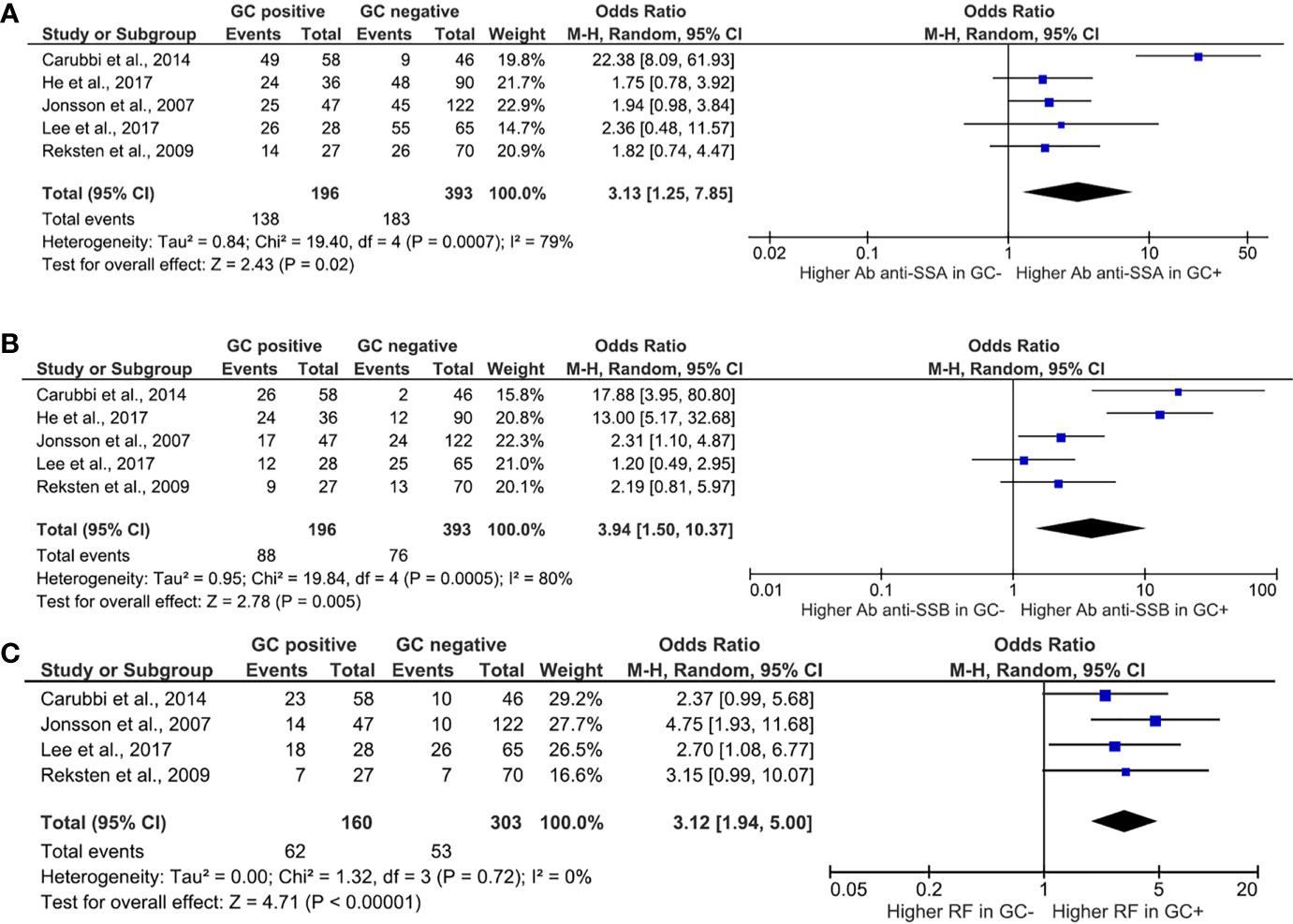
Figure 3 Meta-analysis of the presence of anti-SSA (A), anti-SSB (B), and rheumatoid factor (C) between patients with Sjögren’s syndrome with or without ectopic germinal center. The size of squares is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study; diamond, the pooled estimate with 95% CI; N, the number of persons at baseline; and OR, odds ratio; GC, germinal center; RF, rheumatoid factor.
Due to the different classification systems based on FS used in the retrieved papers, quantitative analysis was not possible. Thus we summarized the available data. Wise et al. in 1993 explored the correlation between pSS clinical features and mSGB results: this study, for the first time, pointed out the lack of correlation between FS and clinical features but unmasked the link between FS and serological markers. In fact, in this cohort, among the selected clinical features (dry eyes, Shirmer test, dry mouth, salivary swelling, pulmonary findings, renal findings, GI findings, thyroid disease, cutaneous lesions, adenopathy, neurologic findings), only the salivary swelling was significantly present in patients with FS >1. On the other hand, serologic findings, defined as ANA, anti-SSA, anti-SSB, and RFs presence, were significantly increased in patients with FS >1, when they were considered individually or as a group (23). Reksten et al., in 2009, did not confirm these results, showing that patients in the FS− group had a higher frequency of both anti-SSA and anti-SSB antibodies when compared to patients in the FS+ group, which may be related to the specific inclusion criteria used in the study (19). Successively, Daniels et al. explored the associations between SG histopathologic changes and phenotypic features in 1,726 pSS patients from the database of the Sjögren’s International Collaborative Clinical Alliance (SICCA). They found that FS >1 was significantly associated with serum anti-SSA and anti-SSB positivity, RF, but not with symptoms of dry mouth and/or dry eyes. Patients with positive anti-SSA/SSB were nine times (95% CI: 7.4–11.9) more likely to have a focus score of >1 than those without anti-SSA/SSB. Of note, patients with an unstimulated whole salivary flow rate of <0.1 ml/min were two times (95% CI 1.7–2.8) more likely to have a focus score of >1 than those with a higher flow rate (25). In 2015, Carubbi et al. confirmed the variability of pSS disease spectrum among patients with different FSs. In their experience, higher FS values were associated with a significant higher frequency of salivary gland swelling and lymphoma. Furthermore, reduction of C4, hypergammaglobulinemia, circulating monoclonal component, and double association anti-SSA and anti-SSB were more common in patients with FS ≥1 (24). The main characteristics of patients enrolled in these studies are summarized in Tables 3, 4.
In the 2014, Risselada et al. explored the prognostic role of mSGB in pSS patients’ follow-up. In a retrospective study, they analyzed the prognostic value of FS and the percentages of IgA+, IgM+, and IgG+ plasma cells in mSGBs on disease outcomes. Their results showed that mean FS was significantly higher in patients developing non-Hodgkin lymphoma (NHL) (3.0 ± 0.894 vs 2.25 ± 1.086; p = 0.021), and FS ≥3 foci had a positive predictive value of 16% for NHL and a negative predictive value of 98%. Only FS ≥3 contributed significantly and independently to NHL development in a standard multiple regression model (26). The prognostic value of FS on lymphoma development was confirmed in 2015 by Carubbi et al. In fact, in a multivariate analysis, they showed that patients with higher FS had a higher risk of developing lymphoma (OR = 1.314, 95% CI: 1.090–1.585, p = 0.004) (24). In another retrospective study, a FS ≥4 was, together with age and male gender, a risk factor for interstitial lung disease (ILD) development in pSS patients (OR = 3.954, 95% CI: 1.423–10.987, p = 0,008) (27).
mSGB is a cornerstone in pSS diagnosis, but to date, its role is underestimated in the follow-up of pSS patients, and behind its valuable role in pSS classification criteria, its potential use in different clinimetric settings is still poorly recognized. Our SLR pointed out the limited number of studies that explored the association between histologic scores and pSS clinical presentation and prognosis, confirming what was already reported (28, 29). Furthermore, in the analyzed studies, different standards and different definitions were used to evaluate the results of mSGBs and any study that independently selected the clinical features of disease presentation, making challenging the comparison of the results and the quantitative analysis. Furthermore, mSGB interpretation and GCs detection could be influenced by the pathologist’s experience.
Here, analyzing data derived from 589 pSS patients enrolled in five studies, we did not find any association between ectopic GCs presence at diagnosis and salivary gland swelling, arthritis, and RP presence. On the other hand, patients with ectopic GCs presence, in their mSGBs, showed a higher OR for anti-SSA, anti-SSB, and RF positivity. B lymphocyte accumulation in pSS salivary glands is a key feature of the disease, and ectopic GC structures promote their chronic stimulation and activation. B lymphocytes producing autoantibodies were described at the borders of ectopic GCs (30). To date, the sites involved in autoantibodies’ production during pSS are not fully elucidated, but affected salivary glands seem to contribute to the production. The presence of plasma cells with intracytoplasmic immunoglobulins with anti-SSA activity in the mSGs, the finding of autoreactive B lymphocytes in the ectopic GC structures, and the evidence of autoantibodies in the saliva contribute to support this hypothesis (30, 31). Furthermore, in vitro studies demonstrated the ability of epithelial salivary gland cells in the exposure of Ro60/TRIM, Ro52/TROVE2, and La/SSB during their death, fueling the autoimmune response (32, 33). Our data mirrors what has been already reported in the literature, in which ectopic GCs presence in mSGBs, hosting B lymphocyte chronic activation, selection, and affinity maturation (34), may be associated with serological presentation and autoimmune phenomena activation, characterizing a pSS phenotype with potentially a more severe disease. Furthermore, FLS and ectopic GC being a continuum in the inflammatory infiltrate characterizing the mSGs of pSS patients, these results reinforce the reported association between FS and serologic features (23–25), lymphoma (24, 26), and ILD development (27).
To our knowledge, this systematic review and meta-analysis is the first report to provide a comprehensive analysis of the clinical findings and laboratory abnormalities associated with histologic markers in pSS patients. Despite the low number of high-quality published data, after combining all the studies using the conservative random-effects model, the obtained results may be considered robust and reliable.
We are aware that our results may be influenced by the lack of randomized control studies as well as by the small number of available studies, mainly retrospective in nature. Therefore, the overall generalizability of our meta-analysis results may be outdated in the future when randomized studies will be published.
In conclusion, pSS patients with ectopic GC, despite exhibiting similar glandular dysfunction and clinical presentation with the patients without ectopic GCs presence, show different features across laboratory parameters, which are mainly related to B lymphocyte hyperactivity. The formation of ectopic GC within the salivary glands of pSS patients may be an important step in the process leading to lymphocytic sialadenitis. Although our study failed to identify any significant associations between the presence of ectopic GC and clinical features, ectopic GC positive pSS patients do exhibit distinct serological features, highlighting the importance of mSGBs behind pSS diagnosis. Studies specifically designed are necessary to confirm these results in larger cohorts to definitively assess the importance of these laboratory abnormalities and their relevance for the clinical outcomes.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
OB, PR, PC, and RG contributed to conception and design of the study. SD’A performed the statistical analysis. OB and PR wrote the first draft of the manuscript. PB, LN, and AM wrote sections of the manuscript and participated in the literature review. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Mrs. Federica Sensini for her technical assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.686457/full#supplementary-material
2. Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of Primary Sjögren’s Syndrome: A Systematic Review and Meta-Analysis. Ann Rheum Dis (2015) 74:1983–9. doi: 10.1136/annrheumdis-2014-205375
3. Brito-Zeròn P, Baldini C, Bootsma H, Bowman SJ, Jonsson R, Mariette X, et al. Sjogren Syndrome. Nat Rev Dis Primers (2016) 2:16047. doi: 10.1038/nrdp.2016.47
4. Brito-Zeròn P, Kostov B, Solans R, Fraile G, Suárez-Cuervo C, Casanovas A, et al. Systemic Activity and Mortality in Primary Sjögren Syndrome: Predicting Survival Using the EULAR-SS Disease Activity Index (ESSDAI) in 1045 Patients. Ann Rheum Dis (2016) 75:348–55. doi: 10.1136/annrheumdis-2014-206418
5. Singh AG, Singh S, Matteson EL. Rate, Risk Factors and Causes of Mortality in Patients With Sjögren’s Syndrome: A Systematic Review and Meta-Analysis of Cohort Studies. Rheumatology (Oxford) (2016) 55:450–60. doi: 10.1093/rheumatology/kev354
6. Bombardieri M, Lewis M, Pitzalis C. Ectopic Lymphoid Neogenesis in Rheumatic Autoimmune Diseases. Nat Rev Rheumatol (2017) 13:141–54. doi: 10.1038/nrrheum.2016.217
7. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification Criteria for Sjögren’s Syndrome: A Revised Version of the European Criteria Proposed by the American-European Consensus Group. Ann Rheum Dis (2002) 61:554–8. doi: 10.1136/ard.61.6.554
8. Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. American College of Rheumatology Classification Criteria for Sjögren’s Syndrome: A Data-Driven, Expert Consensus Approach in the Sjögren’s International Collaborative Clinical Alliance Cohort. Arthritis Care Res (Hoboken) (2012) 64:475–87. doi: 10.1002/acr.21591
9. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Ann Rheum Dis (2017) 76:9–16. doi: 10.1136/annrheumdis-2016-210571
10. Sjögren H. Zur Kenntnis Der Keratoconjunctivitis Sicca. Keratitis Filiformis Bei Hypofunktion Der Tränendrüsen [On Knowledge of Keratoconjunctivitis Sicca. Keratitis Filiformis Due to Lacrimal Gland Hypofunction]. Acta Ophthalmol (1933) 2:1–151.
11. Chisholm DM, Mason DK. Labial Salivary Gland Biopsy in Sjogren’s Disease. J Clin Pathol (1968) 21:656–60. doi: 10.1136/jcp.21.5.656
12. Greenspan JS, Daniels TE, Talal N, Sylvester RA. The Histopathology of Sjogren’s Syndrome in Labial Salivary Gland Biopsies. Oral Surg Oral Med Oral Pathol (1974) 37:217–29. doi: 10.1016/0030-4220(74)90417-4
13. Tarpley TM, Anderson LG, White CL. Minor Salivary Gland Involvement in Sjogren’s Syndrome. Oral Surg Oral Med Oral Pathol (1974) 37:64–74. doi: 10.1016/0030-4220(74)90160-1
14. Guellec D, Cornec D, Jousse-Joulin S, Marhadour T, Marcorelles P, Pers JO, et al. Diagnostic Value of Labial Minor Salivary Gland Biopsy for Sjögren’s Syndrome: A Systematic Review. Autoimmun Rev (2013) 12:416–20. doi: 10.1016/j.autrev.2012.08.001
15. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
16. Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating Non-Randomised Intervention Studies. Health Technol Assess (2003) 7(27):iii–173. doi: 10.3310/hta7270
17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta- Analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
18. Jonsson MV, Skarstein K, Jonsson R, Brun JG. Serological Implications of Germinal Center-Like Structures in Primary Sjögren’s Syndrome. J Rheumatol (2007) 34:2044–9.
19. Reksten TR, Jonsson MV, Szyszko EA, Brun JG, Jonsson R, Brokstad KA. Cytokine and Autoantibody Profiling Related to Histopathological Features in Primary Sjogren’s Syndrome. Rheumatology (Oxford) (2009) 48:1102–6. doi: 10.1093/rheumatology/kep149
20. Carubbi F, Alunno A, Cipriani P, Di Benedetto P, Ruscitti P, Berardicurti O, et al. Is Minor Salivary Gland Biopsy More Than a Diagnostic Tool in Primary Sjögren׳s Syndrome? Association Between Clinical, Histopathological, and Molecular Features: A Retrospective Study. Semin Arthritis Rheum (2014) 44:314–24. doi: 10.1016/j.semarthrit.2014.05.015
21. He J, Jin Y, Zhang X, Zhou Y, Li R, Dai Y, et al. Characteristics of Germinal Center-Like Structures in Patients With Sjögren’s Syndrome. Int J Rheum Dis (2017) 20:245–51. doi: 10.1111/1756-185X.12856
22. Lee KE, Kang JH, Yim YR, Kim JE, Lee JW, Wen L, et al. The Significance of Ectopic Germinal Centers in the Minor Salivary Gland of Patients With Sjögren’s Syndrome. J Korean Med Sci (2016) 31:190–5. doi: 10.3346/jkms.2016.31.2.190
23. Wise CM, Woodruff RD. Minor Salivary Gland Biopsies in Patients Investigated for Primary Sjögren’s Syndrome. A Review of 187 Patients. J Rheumatol (1993) 20:1515–8.
24. Carubbi F, Alunno A, Cipriani P, Bartoloni E, Baldini C, Quartuccio L, et al. A Retrospective, Multicenter Study Evaluating the Prognostic Value of Minor Salivary Gland Histology in a Large Cohort of Patients With Primary Sjögren’s Syndrome. Lupus (2015) 24:315–20. doi: 10.1177/0961203314554251
25. Daniels TE, Cox D, Shiboski CH, Schiødt M, Wu A, Lanfranchi H, et al. Associations Between Salivary Gland Histopathologic Diagnoses and Phenotypic Features of Sjögren’s Syndrome Among 1,726 Registry Participants. Arthritis Rheum (2011) 63:2021–30. doi: 10.1002/art.30381
26. Risselada AP, Kruize AA, Goldschmeding R, Lafeber FP, Bijlsma JW, van Roon JA. The Prognostic Value of Routinely Performed Minor Salivary Gland Assessments in Primary Sjögren’s Syndrome. Ann Rheum Dis (2014) 73:1537–40. doi: 10.1136/annrheumdis-2013-204634
27. Kakugawa T, Sakamoto N, Ishimoto H, Shimizu T, Nakamura H, Nawata A, et al. Lymphocytic Focus Score is Positively Related to Airway and Interstitial Lung Diseases in Primary Sjögren’s Syndrome. Respir Med (2018) 137:95–102. doi: 10.1016/j.rmed.2018.02.023
28. Giacomelli R, Afeltra A, Alunno A, Bartoloni-Bocci E, Berardicurti O, Bombardieri M, et al. Guidelines for Biomarkers in Autoimmune Rheumatic Diseases - Evidence Based Analysis. Autoimmun Rev (2019) 18:93–106. doi: 10.1016/j.autrev.2018.08.003
29. Giacomelli R, Afeltra A, Bartoloni E, Berardicurti O, Bombardieri M, Bortoluzzi A, et al. The Growing Role of Precision Medicine for the Treatment of Autoimmune Diseases; Results of a Systematic Review of Literature and Experts’ Consensus. Autoimmun Rev (2021) 20:102738. doi: 10.1016/j.autrev.2020.102738
30. Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmström P, Wahren-Herlenius M, et al. Cellular Basis of Ectopic Germinal Center Formation and Autoantibody Production in the Target Organ of Patients With Sjögren’s Syndrome. Arthritis Rheum (2003) 48:3187–201. doi: 10.1002/art.11311
31. Tengnér P, Halse AK, Haga HJ, Jonsson R, Wahren-Herlenius M. Detection of Anti-Ro/SSA and Anti-La/SSB Autoantibody-Producing Cells in Salivary Glands From Patients With Sjögren’s Syndrome. Arthritis Rheum (1998) 41:2238–48. doi: 10.1002/1529-0131(199812)41:12<2238::AID-ART20>3.0.CO;2-V
32. Abu-Helu RF, Dimitriou ID, Kapsogeorgou EK, Moutsopoulos HM, Manoussakis MN. Induction of Salivary Gland Epithelial Cell Injury in Sjogren’s Syndrome: In Vitro Assessment of T Cell-Derived Cytokines and Fas Protein Expression. J Autoimmun (2001) 17:141–53. doi: 10.1006/jaut.2001.0524
33. Ohlsson M, Jonsson R, Brokstad KA. Subcellular Redistribution and Surface Exposure of the Ro52, Ro60 and La48 Autoantigens During Apoptosis in Human Ductal Epithelial Cells: A Possible Mechanism in the Pathogenesis of Sjögren’s Syndrome. Scand J Immunol (2002) 56:456–69. doi: 10.1046/j.1365-3083.2002.01072_79.x
Keywords: Sjӧgren’s syndrome, minor salivary gland biopsy, focus score, germinal center, clinical features, serological biomarkers, autoantibodies
Citation: Berardicurti O, Ruscitti P, Di Benedetto P, D’Andrea S, Navarini L, Marino A, Cipriani P and Giacomelli R (2021) Association Between Minor Salivary Gland Biopsy During Sjӧgren’s Syndrome and Serologic Biomarkers: A Systematic Review and Meta-Analysis. Front. Immunol. 12:686457. doi: 10.3389/fimmu.2021.686457
Received: 26 March 2021; Accepted: 20 April 2021;
Published: 11 June 2021.
Edited by:
Kristi A. Koelsch, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Efstathia K. Kapsogeorgou, National and Kapodistrian University of Athens, GreeceCopyright © 2021 Berardicurti, Ruscitti, Di Benedetto, D’Andrea, Navarini, Marino, Cipriani and Giacomelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Giacomelli, ci5naWFjb21lbGxpQHVuaWNhbXB1cy5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.