- 1Department of Kidney Transplantation, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Graduate School of Medical and Dental Science, Department of Pathological Cell Biology, Tokyo Medical and Dental University, Tokyo, Japan
- 3Clinical Research Center for Organ Transplantation in Hunan Province, Changsha, China
- 4Clinical Immunology Center, Central South University, Changsha, China
B cells, commonly regarded as proinflammatory antibody-producing cells, are detrimental to individuals with autoimmune diseases. However, in recent years, several studies have shown that regulatory B (Breg) cells, an immunosuppressive subset of B cells, may exert protective effects against autoimmune diseases by secretion of inhibitory cytokines such as IL-10. In practice, Breg cells are identified by their production of immune-regulatory cytokines, such as IL-10, TGF-β, and IL-35, however, no specific marker or Breg cell-specific transcription factor has been identified. Multiple phenotypes of Breg cells have been found, whose functions vary according to their phenotype. This review summarizes the discovery, phenotypes, development, and function of Breg cells and highlights their potential therapeutic value in kidney diseases.
Introduction
B lymphocytes play a critical role in adaptive immune system by secreting antibodies. They also present antigens for the activation of T cells and produce several essential cytokines. A small population of B cells known as regulatory B (Breg) cells demonstrates the ability of regulating immune responses. Breg cells are a subtype of B cells that were first discovered in 1974 when two studies described the suppressive function of B cells that could delay hypersensitivity independently (1, 2). However, few studies have focused on suppressive B cells till 1996. Wolf et al. found that in the development of experimental autoimmune encephalomyelitis (EAE), commonly considered as an animal model of multiple sclerosis (MS) mediated by CD4+ T cells, EAE is inducible without the exhibition of differences in disease onset or severity between wild-type and B cell-deficient mice. However, B cell-depleted mice recovered with increased difficulty, highlighting the modulation function of B cells in acute EAE (3). Subsequently, an increasing number of studies have found that B cells with regulatory functions are involved in the development of not only EAE but also in other inflammatory diseases (4, 5). To date, these regulatory B cell-induced immune response suppression has been observed in autoimmune diseases, HIV, pregnancy, inflammatory diseases, and transplant immunity, among others. In this review, we have described the different phenotypes and functions of Breg cells and focused on their role in several types of kidney-related diseases, as well as potential challenges in the study of these cells in the future.
What Is the Phenotype of Breg Cells?
With gradual advances in the existing knowledge since their discovery, Breg cells have not been restricted to a single phenotype, but rather displaying several phenotypes, in both humans and mice.
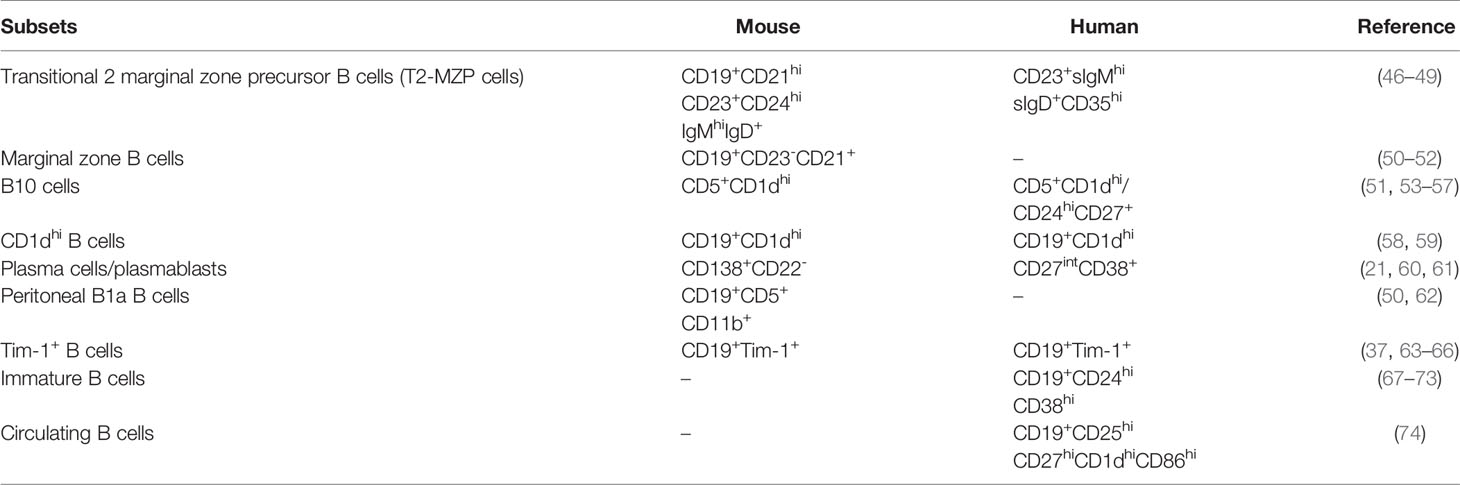
Phenotypes of Breg Cells Identified in Humans
B cells that regulate immune responses were initially found to exert their suppressive function in some specific diseases by producing interleukin-10 (IL-10). Subsequently, several studies have shown Breg cells exert suppressive functions mainly by secretion of IL-10 which also is the most important cytokine in Breg cells (6–10). It should also be noted that in certain circumstances IL-10 can serve as an enhancing role in the immune responses of B cells (11, 12) and CD8 T cells (13, 14). The most frequently observed phenotype for IL-10-producing B cells in peripheral blood is transitional CD19+CD24hiCD38hi B cells (9, 15–20), since IL-10+ B cells are abundant in this phenotype. Other phenotypes include plasmablasts (CD19+CD27intCD38+) (21) and regulatory B1 cells (Br1: CD19+CD25+CD71+CD73-) (22). Depending on the expression of CD27, IL-10+ B cells can be divided into naïve IL-10+ B cells (CD19+CD27-IL-10+) and memory IL-10+ B cells (CD19+CD27+IL-10+), wherein the ratio of naïve/memory IL-10+ B cells may hint at its function (7).
Additionally, Breg cells exhibit other markers. While transcription factor Forkhead Box P3 (Foxp3) is known to be expressed on several immune cells and is commonly regarded as a marker for Tregs, it has also been identified as a transcription factor of Breg cells, namely CD19+CD5+ B cells; in contrast, CD5- B cells do not express Foxp3 (17, 23–25). Another transcription factor should be noticed is T cell immunoglobulin and mucin domain 1 (Tim-1), a member of Tim family. It is first discovered on the cell surface of T cells and dendritic cells (DCs) and plays a crucial role in immune response regulation (26–28). More recently, B cells have also been found to express Tim-1 (29–31). Human IL-10+ B cells also express Tim-1, Tim-1+IL-10+ B cells are reported to suppress certain autoimmune diseases in human (32). Moreover, Tim-1 may prove to be a better marker for the identification of IL-10-producing B cells than CD5+CD1d+, since Tim-1 is predominantly expressed on IL-10+ B cells in humans (32). While not all of the Breg cells play a protective role in diseases, tumor-evoked Breg cells (tBreg cells), which are functionally different from conventional Breg cells, play negative roles in the occurrence of lung metastasis. The phenotype of tBreg cells is CD19+CD25+CD81hi (33). tBreg cells reportedly dampen the immune system in breast cancer and the mechanism relies not on the secretion of IL-10, IL-35, or activation of other suppressive pathways, but on the secretion of transforming growth factor-β (TGF-β) to facilitate Foxp3+ Treg cells (34).
Phenotypes of Breg Cells in Mice
The phenotypes of Breg cells vary considerably between humans and mice. In a murine study, Breg cells were mainly defined as IL-10-producing B cells, which varied from the IL-10+ B cell population in the peripheral blood and spleen. Interestingly, according to the analysis of tiger mice, a type of IL-10 reporter mouse, the majority of IL-10-producing cells in the spleen of WT mice comprise B cells, and not T cells (6). The majority of IL-10-producing T cells comprise CD4+ T cells (TCRβ+CD4+) (83% ± 2%), and most IL-10+ B cells include follicular (FO) B cells (CD19+CD23+CD21int) (41% ± 3%), while other IL-10-producing cells include neutrophils, monocytes/macrophages, and myeloid DCs. IL-10-producing B cells in the spleen include FO B cells (CD19+CD23+CD21int), marginal zone (MZ) B cells (CD19+CD23-CD21hi), transitional T1+T2 B cells (CD19+AA4/CD93+), plasma/plasmablast cells (CD19+/B220lo/-CD138+), and B1 a/b cells (CD19+CD138-CD21-CD23-CD43+CD5+/-) (6). In murine studies, the commonly observed IL-10+ B cells in the spleen are CD19+/B220lo/-CD1d+CD5+ cells (B10 cells) (35–40), since IL-10+ B cells are enriched in B10 cells.
Except for B10 cells, other markers or cytokines related to B cells may also play a regulatory role in immune responses. IL-35 secreted by B cells can inhibit inflammation in certain diseases, including inflammatory bowel disease, and such cells are referred to as IL-35-producing B cells (41–43). IL-35-treated mice showed increased abundance of CD4+CD25+Foxp3+ Tregs and IL-10+ Breg cells, indicating that IL-35 secreted by Breg cells might exert positive feedback on Breg cells (44, 45). CD9 is a cell surface glycoprotein that is encoded by a gene belonging to the tetraspanin family and is considered a key marker expressed on the cell surface of IL-10-producing B cells. Furthermore, most IL-10+ B cells (87.5% ± 1.69%) express CD9 on their cell surface. CD9+ B cells have been found to exert a stronger suppressive function than CD9- B cells, while a small proportion of CD9- B cells are known to also secrete IL-10. When CD9 expressed on B cells is subjected to blockade by anti-CD9 antibody, the suppression of T cell proliferation is inhibited, which is related to the ratio of T/B cells in a co-culture system, indicating that the suppressive function of CD9 is related to the establishment of T-B cell crosstalk in an IL-10-dependent manner (38). Tim-1 is not only expressed on human IL-10+ B cells, but also a marker for mouse IL-10+ B cells. Interestingly, the expression of Tim-1 in B cells is higher than that in T cells. B cells express Tim-1, including transitional B cells, MZ B cells, FO B cells, and B10 cells, and most IL-10+ B cells express Tim-1 on their cell surface. IL-4 and IL-10 secretion from Breg cells can be induced with the use of anti-Tim-1 antibody, demonstrating that Tim-1 ligation can induce B10 cell expansion (Table 1) (37).
Development and Function of Breg Cells
Breg cells induction in vitro under different stimuli may associate to Breg cells development in vivo. Breg cells constitute several phenotypes of B cells, including transitional B cells, FO B cells, plasmablasts and so forth. They are enriched in the spleen and peritoneal cavity, while few Breg cells exist in the blood, peripheral lymph nodes, mesenteric lymph nodes, and Peyer’s patches (53). In vitro, Breg cells can be expanded when stimulated with LPS (75), thereby inducing IL-10+ B cell differentiation into plasma/plasmablast cells after transient IL-10 production. After subjection to LPS stimulation for a period of 3 days, B10 cells gradually express CD43 and GL7 activation markers for antibody-secreting cells. Furthermore, the proportion of plasma/plasmablasts in IL-10+ B cells increased with an increase in the expression of the plasma/plasmablast-associated transcription factors, namely blimp1, xbp1, and irf4, which are related to plasma/plasmablast cell expansion (76). IL-10+ B cells can also be induced by anti-CD40 antibody (75, 77), anti-Tim-1 antibody (37), PMA/ionomycin (78), IL-21 (77, 79) and IL-35 (43, 44, 80). For example, IL-10+ B cells that mature into functional IL-10+ B cells rely on IL-21 and CD40 signaling activation, whereas IL-10 is not necessary for IL-10+ B cell development (75, 77, 79), IL-21 secreted from follicular helper T cells (Tfh) mediates the expansion of B10 cells via the phosphorylation of STAT3 (39, 40). Interestingly, IL-21-induced Breg cells exhibit a phenotype that expresses granzyme B, which is a serine protease that is expressed in the granules of NK cells and cytotoxic T cells. Granzyme B secreted by Breg cells inhibits T cell proliferation and the degradation of TCR ζ-chain (81, 82). Furthermore, IL-35 is also involved in the development and function of Breg cells. Breg cell-secreted IL-35 is a cytokine that exerts an inhibitory on the immune response. IL-35 induces the production of Treg cells, IL-10-producing Breg cells, and IL-35-producing Breg cells, indicating that Breg cell development is dependent on IL-35 signaling (43, 44, 80).
Breg cells regulate immune responses by many ways, such as anti-inflammatory cytokines IL-10, IL-35, IL-12 (83), or TGF-β (84, 85) secretion, or via the Fas-FasL (86) and PD-1/PD-L1 (86, 87) pathways. Through all these pathways, Breg cells regulate the immune system by controlling immune cell differentiation and proliferation. As the major functional cytokine secreted by Breg cells, IL-10 production is related to the B-cell linker protein (BLNK) expression, which is involved in the regulation of the immune response in allergic and autoimmune diseases (88), it regulates the differentiation of Th1 cells and Th17 cells and inhibits T cell cytokine secretion resultantly (47). IL-10+ B cells also modulate the function of Foxp3+ Tregs and CD8+ T cells. For example, the levels of IL-10 in the serum are elevated in patients with chronic hepatitis B virus (HBV) infection, and the blockade of IL-10 supports the function of virus-specific CD8+ T cells. Both the frequency of IL-10+ B cells and the secretion of IL-10 from IL-10+ B cells are facilitated in these patients, suggesting that Breg cells inhibit CD8+ T cell function in an IL-10-dependent manner (9). Additionally, IL-10+ B cells promote CD4+Foxp3+ T cell proliferation both in vivo and in vitro (89, 90) (91, 92). Evidence also indicates that IL-10 produced by Breg cells induces CD4+ T cell apoptosis (93). IL-10+ B cells function is also different among naïve IL-10+ B cells and memory IL-10+ B cells. Naïve IL-10+ B cells are involved in the prevention of immune responses in autoimmune diseases, while memory IL-10+ B cells prevent disease exacerbation (94). The ratio of naïve/memory IL-10+ B cells may be an indicator of the major function of IL-10+ B cells in specific diseases (7). Breg cells also regulate IgG production, with B10 cell depletion enhancing IgG production in WT mice (35). In a human study, Breg cells were found to impair the function of IgG4-producing B cells (22). Moreover, Breg cells inhibit the differentiation of CD4+ T cells into Tfh cells and suppress the antibody production mediated by Tfh cells (91, 92). Breg cells not only participate in the suppression of peripheral immune response but also perform inhibitory functions in the brain. They are capable of inhibiting inflammation and central nervous system damage resulting from infiltrating pro-inflammatory cells (95). Collectively, even though only 1%–2% of splenic B cells are IL-10+ B cells, they play a critical role in the regulation of immune responses (53) by suppressing immune responses and by ameliorating autoimmune diseases.
Regarding tBreg cells, which play a negative role in immune responses, anti-CD20 antibody treatment facilitates tumor escape from the immune system via the enrichment of tBreg cells that express low levels of CD20. Thus, using anti-CD20 antibody may enrich tBreg cells, which impairs the immune system and promotes breast cancer development (33).
However, the role of Breg cells in mice and human are somehow not always the same. It is also reported that CD19+CD24hiCD38hi Breg cells are enriched in some SLE patients, together with elevated serum IL-10 level from Breg cells and reduced IL-10R in circulating lymphocytes, demonstrating that IL-10 secreted from Breg cells in human is not necessarily protective in autoimmune diseases, and can be targeted in some cases (96).
What Is the Role of Breg Cells in Kidney Disease?
Breg cells exert protective effects in systemic diseases that affect the kidney, including allograft rejection (46, 97), systemic lupus erythematosus (SLE) (98), type 1 diabetes (T1D) (99, 100), anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) (101), Sjogren’s syndrome (SS) (102), Immunoglobin G4-related disease (IgG4-RD) (103), and IgA vasculitis with nephritis (IgAVN) (104), among others. Breg cell populations tend to be reduced in the above-mentioned kidney-targeting diseases. The transfer or increase of Breg cell numbers and the increased secretion of inhibitory cytokines from Breg cells alleviates disease, leading to improved kidney function. Several diseases, including AAV, T1D, and SLE, result from the generation of autoantibodies and inflammatory cytokine secretion, with or without T cell infiltration. An increased number of Breg cells reduces antibody production and CD4+ and CD8+ T cell infiltration, and promotes the infiltration of Treg cells, thereby leading to disease remission.
Kidney Transplantation
B cells play an important role in graft rejection by producing donor-specific antibodies, while increasing evidence support the role of B cells in the induction of tolerance. Long-term acceptance was more likely to be induced in kidney-transplanted rats administered with donor-derived B cells compared to donor-derived T cells (105). The importance of B cells in tolerance induction has also been demonstrated in human studies (67, 106). Based on the studies conducted using B cells collected from operational tolerant (OT) recipients, B cells were found to produce higher levels of IL-10 (107). Furthermore, the frequency of naïve B cells, memory B cells, and Breg cells increased and tended to be normal (15, 108). Breg cells may inhibit T cell function via the direct interaction of T:B cells (109). Different from most settings, IL-10 shows counter-regulatory effects in the setting of anti-CD45RB-induced tolerance. In anti-CD45RB-induced heart allograft mice model, IL-10 deficiency or IL-10 neutralization was found to improve chronic allograft vasculopathy and reduce allograft reactive antibody production. However the underlying mechanism behind this abnormality is not yet known in this specific setting (110). Breg cells in patients with tolerance were found to be similar to those in healthy individuals, while patients who experienced chronic rejection showed impaired Breg cell population (15). The immunologic injury targeting allografts is markedly related to the IL-10/TNF-α expression ratio on Breg cells (111). Therefore, elevated circulating IL-10+ Breg cells could be a marker for relatively lower risk of antibody-mediated rejection (20). More convincing evidence indicates that Breg cells are protective in inducing the tolerance or preventing the rejection of allografts in cases with IL10-/- on B cells or B cell depletion, which are more likely to be rejected; furthermore, evidence also indicates that the adoptive transfer of IL10+/+ marginal zone precursor regulatory B cells in recipients prevents rejection (46, 97). The main mechanisms involved in the induction of tolerance by Breg cells are as follows: (1) the regulation of IL-10+ Breg cells promote Foxp3+ T cell proliferation (90, 112), inhibit the proliferation (113) and induce the apoptosis (93) of CD4+ T cells, and dampen the function of CD8+ T cells (9); (2) activation of other cytokines and pathways.
Lupus Nephritis (LN)
Kidney function is commonly investigated in the prognosis of patients with SLE. As autoantibodies are the key factors in the pathogenesis of SLE and LN, increasing evidence shows the significant role of Breg cells in LN (98). Early studies have reported that the number of IL-10-producing B cells is increased in patients with SLE (114, 115). However, in patients with new-onset SLE (116) and LN patients (117), the IL-10+ B cell population is decreased. The percentages of CD19+CD24hiCD38hi subsets, including putative Breg cells in PBMCs, between SLE patients and healthy controls are similar (68, 117). However, Breg cells in the PBMCs of SLE patients secrete fewer amounts of IL-10 with an impaired CD4+ T cell suppressive capacity (118). Consistent with this finding, CD19+CD24hiCD38hi Breg cells in SLE patients produced fewer amounts of IL-10 with an impaired suppressive capacity. The suppressive effect of Breg cells on CD4+ T helper 1 cells is dependent on IL-10, CD80, and CD86, but is not TGF-β-dependent. This suppression impairment of SLE-derived Breg cells may be related to its inability to upregulate STAT3 phosphorylation upon CD40 engagement (68). Early immature B cells can produce a substantial number of self-reactive antibodies, including ssDNA-reactive antibodies (119, 120). Interestingly, IL-10-producing CD27−CD38intIgD+ pre-naïve B cells in SLE patients secrete fewer amounts of IL-10 with enhanced CD80 and CD86 expression, and this occurrence leads to the loss of self-regulation (120).
The reason for the reduced population of IL-10+ Breg cells in SLE patients may be related to the regulatory circuit between plasmacytoid dendritic cells (pDCs) and CD24+CD38hi Breg cells (69). In healthy individuals, pDCs can promote the differentiation of both Breg cells and plasmablasts by moderating IFN-α secretion and CD40 signaling. On the other hand, IL-10 produced by Breg cells can restrain the release of IFN-α by pDCs. However, in SLE patients, increased IFN-α production by hyperactive pDCs drives immature B cell differentiation with an inclination toward plasmablast generation. As a result, SLE Breg cells with reduced IL-10 production fail to restrain pDC-derived IFN-α, thus creating a vicious circle. This study also found that in SLE patients responding to rituximab, repopulated B cells contained a normal frequency of CD24+CD38hi Breg cells, and this mitigated pDC activation and restored the balance (69). Epratuzumab, which targets CD22, can reportedly inhibit B cell-derived pro-inflammatory IL-6 and TNF-α secretion while maintaining the production of IL-10 (121). This represents a potential therapeutic strategy to disrupt the vicious cycle between pDCs and Breg cells.
Another immunosuppressive agent, prednisolone, is also commonly used in SLE patients. However, the percentage of IL-10+ B cells in LN patients is negatively correlated with the daily dose of prednisolone (117) which indicates that the currently available immunosuppressive agents can affect both the effector and regulatory aspects of B cells. This might indicate that the current immunosuppressive treatment strategy failed to treat SLE/LN because of its inability to restore the natural immune balance.
Efforts have been engaged to consider and utilize Breg cells as therapeutic targets for treating LN. Human adipose-derived mesenchymal stem cells (MSCs) can trigger the expansion of IL-10-producing Breg cells in vitro and in vivo in a mouse model. After MSC treatment in the SLE mouse model, both renal histopathology improvement and autoantibody mitigation have been achieved (122). A similar effect has also been reported with the application of MDSCs, which can be subjected to blockade using an inhibitor of iNOS (89).
Type 1 Diabetes (T1D)
T1D is an autoimmune disease characterized by the destruction of cells of the islets of Langerhans, particularly beta cells. Autoantibodies, such as an anti-insulin antibody, anti-islet cell antibody, and anti-glutamic acid decarboxylase (GAD) antibody, can be detected in most T1D patients, accompanied by lymphocyte infiltration in the pancreas. Several immunosuppressive therapies have been tested, including those involving the use of cyclosporine A (CsA) and anti-CD3 monoclonal antibodies (123). Although CsA is an effective initial therapeutic strategy to confer protection to the pancreas, it is not appropriate for long-term use due to side effects (123). Although anti-CD3 antibody showed long-term effects in NOD mice, a mouse model for T1D, treatment strategies using the antibody did not achieve success in phase III clinical studies (124).
Despite considerable efforts to develop immunotherapies that target Treg or T cells for the treatment of T1D, thus far, there is a lack of effective immunotherapies. Interestingly, accumulating evidence indicates that Breg cells play an important role in the suppression of the pathology of T1D. B cell depletion by anti-CD20 antibody leads to the long-term remission of T1D in NOD mice, possibly due to the removal of autoreactive B cells and the reduction in autoantibody generation, which is critical for the development of disease in NOD mice. Another critical mechanism is the increase in the number of Foxp3+ Treg cells, which may be caused by an altered proportion of regenerated B cells (100, 125–127); however, the anti-CD20 antibody is effective only in the early stage of T1D, since pancreas-infiltrating B cells lose the ability to express CD20 (126). A similar result was obtained when B cells were subjected to depletion with the use of anti-CD22 antibody in NOD mice. When conducting the transfer of the regenerated B cells into NOD mice, Treg cells underwent expansion, anti-inflammatory cytokine secretion from Treg cells increased, and infiltrated T cell populations reduced, indicating that these regenerated B cells were regulatory and might exert a protective effect in T1D (128). Additionally, several studies have shown that Breg cells alleviate T1D pathophysiology in an IL-10-dependent manner in both mice and humans (99, 129). In a study using NOD mice of different ages, long-term normoglycemic NOD mice (average age, 30 weeks) exhibited reduced lymphoid infiltration with an increase in pancreas-infiltrating IL-10-producing B cells compared with glycemic NOD mice. To elucidate the role of IL-10+ B cells in NOD mice, a reduction in IFN-γ production by CD4+ T cells was observed when the cells were co-cultured with IL-10+ B cells and DCs. Additionally, milder disease pathology was observed when IL-10+ B cells were transferred with diabetogenic CD4+ T cells into NOD mice compared to the mice subjected to transfer with IL-10-producing B cells, demonstrating that IL-10+ B cells played a protective role in the development of T1D in NOD mice (99, 130). In a study conducted in humans, the proportion of CD5+CD1d+ B cells was positively correlated with blood C-peptide levels, a test performed to analyze insulin secretion, indicating that B10 cell reduction was related to islet destruction (129). Taken together, Breg cells seem to play a protective role in the development of type 1 diabetes in an IL-10-dependent manner, suggesting that Breg cells may be a potential target for the treatment of type 1 diabetes.
Anti-Neutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis (AAV)
ANCAs are autoantibodies that target neutrophil cytoplasmic antigens, such as proteinase 3 (PR3) and myeloperoxidase (MPO). Over 75% of the patients with AAV present with rapidly progressive glomerulonephritis, which is an important predictor of mortality (131). Studies have shown that CD19+CD24hiCD38hi cell populations are decreased in active AAV patients, while its suppressive functions and the ability to produce IL-10 are not altered (101, 132). However, during remission in AAV patients, one study found that this subset was subjected to continuous reduction (101) while another study found that the frequency of this subset did not vary from that of healthy controls. The CD19+CD24hiCD27+ subset, supposedly comprising B10 memory Breg cells, is reportedly reduced during an active disease state and restored during remission (132). Human CD5+ B cells are considered to produce IL-10 and TGF-β (133, 134). This CD5+ B cell population decreases in active AAV patients and rebounds after remission, highlighting its potential role as an indicator of disease activity, remission, and relapse (135, 136). Following B cell depletion with rituximab, a lower CD5+ percentage in B cells was correlated with a shorter time of relapse (137). However, whether CD5 alone is a practicable putative surrogate marker for Breg cells and whether its status can be considered as an indicator of AAV disease activity warrant investigation. Another study found that CD5+ B cells were inversely correlated with disease activity during relapse after treatment with rituximab; however, they were could not be used to predict the time to subsequent relapse (138).
Sjogren’s Syndrome (SS)
Sjogren’s syndrome is one of the most common autoimmune diseases, characterized by impaired exocrine function due to lymphocyte infiltration (139). The clinically overt implication of the kidney has been reported in approximately 5% of SS patients, with a low incidence of progression to end-stage disease (140, 141). Most cases of renal involvement comprise tubulointerstitial nephritis (TIN). Biopsies have shown the infiltration of T cells, B cells, and plasma cells (142). SS patients have been reported to present with a higher frequency of CD19+CD24hiCD38hi Breg cells, whose suppression ability is compromised (102, 143). However, IL-10+ B cell populations are substantially lower in both primary SS patients and SS mice models, and such a phenomenon is caused by a decreased production of IL-10 by CD19+CD24hiCD38hi cells. The adoptive transfer of IL-10-producing Breg cells can be used to ameliorate SS progression in a mouse model, thereby revealing the potential therapeutic effect of Breg cells (144). Another study found no significant difference in the percentage of CD5+ B cells compared to that observed in healthy controls; however, IL-21 receptor and Granzyme B expression in CD5+ B cells in primary SS patients were markedly enhanced, indicating the elicitation of an increased counter-regulatory reaction (145).
Immunoglobin G4-Related Disease (IgG4-RD)
IgG4-RD is a rare fibroinflammatory disease histologically characterized by lymphoplasmacytic infiltration enriched with IgG4+ plasma cells and with the occurrence of storiform fibrosis. Approximately 15% of the IgG4-RD patients exhibit clinical implications in the kidney (146). In type 1 AIP (pancreatic manifestation of IgG4-RD), CD19+CD24hiCD38hi Breg cell populations were found to be significantly increased, while the CD19+CD24hiCD27+ subset was decreased. IL-10-producing B cells were found to be similar between type 1 AIP and healthy controls (103). However, in another study involving 48 newly diagnosed IgG4-RD patients, CD19+CD24hiCD38hi Breg cells showed a marked reduction (143). This may be attributed to the heterogeneity of IgG4-RD.
IgA Vasculitis With Nephritis (IgAVN)
IgA vasculitis, formally known as Henoch-Schönlein purpura, is a type of IgA-mediated small-vessel vasculitis (147). Studies have reported that, compared with healthy controls, all CD19+CD24hiCD38hi, CD19+CD5+, and CD19+IL-10+ subsets are decreased in patients with IgAVN (54, 104). Moreover, even the concentration of IL-10 in the serum has been found to be considerably lower. After treatment, mainly with the glucocorticoid prednisolone, an increase was observed among CD5+CD1d+, CD5+CD1d+IL-10+, and IL-10+ B cell subsets, accompanied by an increase in the serum IL-10 concentration (54).
Breg Cells in Other Kidney Diseases
Numerical changes or functional alterations of Breg cells have also been observed in other immune-related kidney diseases. IgA nephropathy (IgAN) patients tend to have a lower frequency of CD5+CD1d+CD19+ Breg cells with a reduced IL-10 expression. CD5+CD1d+CD19+ Breg cells and Breg-derived IL-10 concentrations are negatively correlated with serum IgA and Gd-IgA1 levels, respectively (148). Similar findings have been reported in diabetic nephropathy (DN) patients in terms of the CD19+CD24hiCD38hi Breg subtype (149). However, among patients with hepatitis B virus-associated membranous nephropathy (HBV-MN), higher proportions of CD5+CD19+ and IL-10+CD19+ B cells and serum IL-10 levels have been reported (150). These differences could be attributed to various distinct Breg cell phenotypes or Breg cell changes that occur depending on the immunological environment. However, whether these numerical or functional changes in Breg cells are a cause or an effect of immune-related kidney diseases should be elucidated in future studies.
In addition carious studies have shown that renal fibrosis, as an inevitable outcome of nearly every kind of chronic kidney diseases, can be driven by TGF- β (151). On the other hand, IL-10 was found as a protective factor in animal studies (152–154). Presumably Breg cells might play a role in renal fibrosis through cytokine production or interactions with other immune cells such as macrophage, T cells etc. However, currently few works has been done regarding the effects of Breg cells on renal fibrosis which calls for further study.
Discussion
Breg cells regulates immune responses through the secretion of several inhibitory cytokines such as IL-10, IL-35 and TGF-β or PD1/PDL1, Fas/FasL pathways, thus facilitate Treg cells, impair CD4+ T cells, CD8+ T cells, DCs, Th1/Th17 and IgG production. These aspects are crucial to achieve successful treatment of autoimmune diseases, inflammation disease regulation, tumor growth prevention, and transplant tolerance induction. Breg cells exhibit several phenotypes, including transitional B cells, MZ B cells, and plasma/plasmablasts, among others. As a result, there is no single specific marker that can be considered for identifying effective Breg cells. Therefore, Breg cells are generally identified via their secretion of suppressive cytokines such as IL-10. Since Breg cells secrete several cytokines, including IL-10, IL-35, and TGF-β, to regulate immune responses, IL-10+ B cells may constitute a considerable population of Breg cells, but cannot represent all Breg cells.
Breg cells exert protective effects against many inflammatory diseases and tolerance induction, While IL10 KO in B cells result in deterioration of inflammatory disease conditions. The transfer of IL-10+ B cells ameliorates autoimmune responses and induces tolerance. Although Breg cells possess properties that are beneficial in the immune response, they cannot be easily utilized in clinical trials. Unlike Treg cells, Breg cell development is not well understood due to its variation in phenotypes. Thus, the identification of a surface marker pattern or lineage-specific transcription factor involved in Breg cell development remains a challenge.
In summary, Breg cells are a robust inhibitory phenotype of B cells that secrete several suppressive cytokines, such as IL-10, TGF-β, and IL-35. Among these, IL-10-producing B cells are the most extensively studied cells in research. These cells have been proven to exhibit inhibitory functions in autoimmune diseases, inflammation, and transplantation. Its role in transplantation is a topic of immense interest, with Breg cells being comprehensively studied for their crucial role in countering graft rejection and inducing tolerance. The activation of tBreg cells has also attracted attentions of many researchers in cancer immunology field. The protective role of Breg cells have been well studied in many kidney-affecting diseases such as SLE, T1D, AAV and other inflammatory diseases, also including kidney transplantation (Figure 1). Cell therapy with in vitro induction of effective Breg cells to alleviate immune responses and to induce tolerance in clinical settings could be a major focus for future studies in kidney.
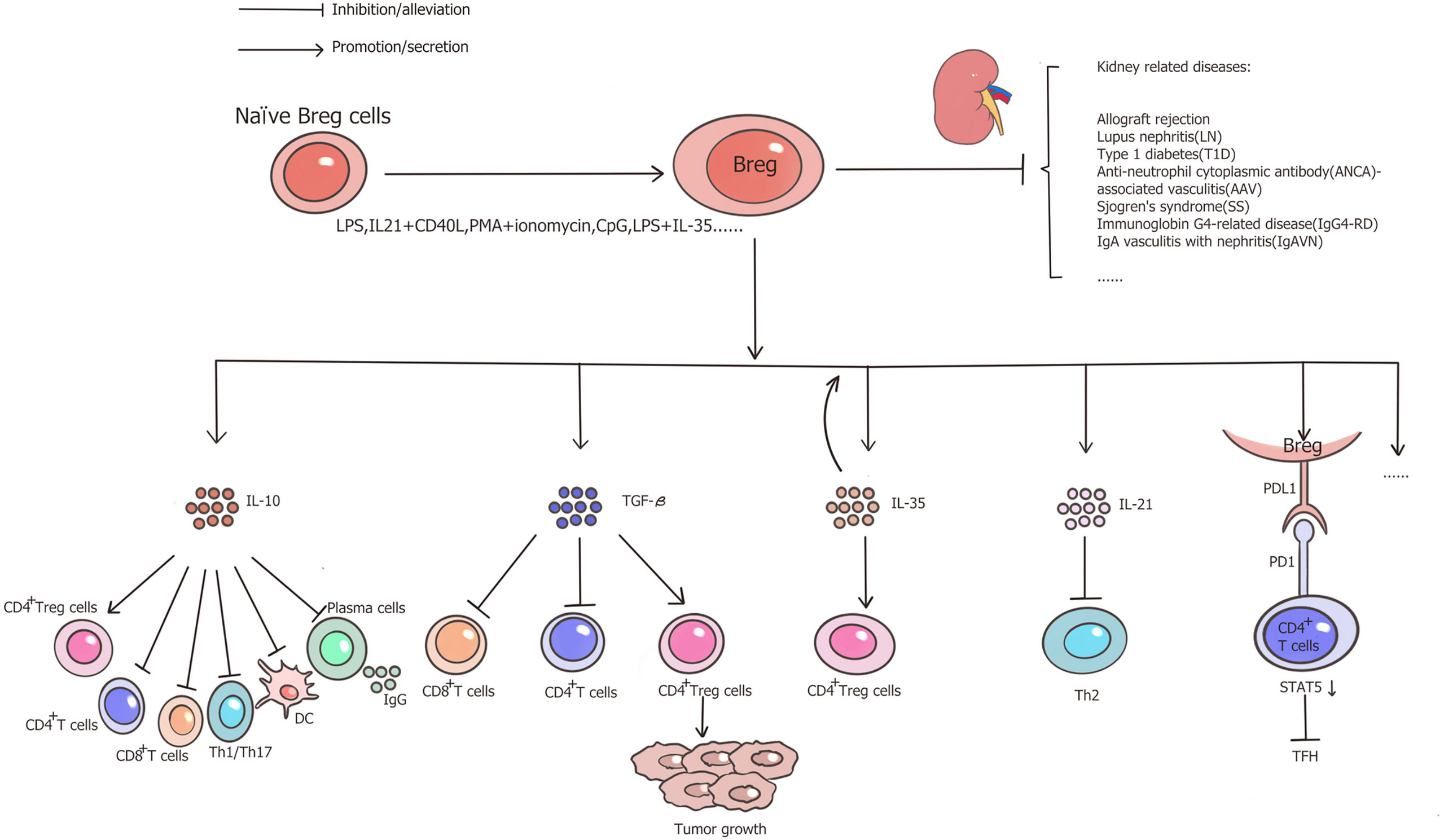
Figure 1 Functional properties of Breg cells and role in kidney-related diseases. Through the secretion of IL-10, IL-35, TGF-β, or the PD1/PDL1 pathways, Breg cells facilitate Treg cells, impair CD4+ T cells, CD8+ T cells, Th1/Th17, DC and IgG production from plasma cells, therefore, Breg cells play a protective role in allograft rejection and several kidney-related inflammatory diseases.
Author Contributions
WL and HZ drafted the manuscript. ZL generated the figure. GL, WY, and LP revised the manuscript. HD designed the outline of the manuscript and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the National Science Foundation of China (81900370, 81800664, 81970655, 82070776), Natural Science Foundation of Hunan Province of China (2019JJ50842), and Huxiang Young Talents of Hunan Province (2019RS2013).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Katz SI, Parker D, Turk JL. B-Cell Suppression of Delayed-Hypersensitivity Reactions. Nature (1974) 251(5475):550–1. doi: 10.1038/251550a0
2. Neta R, Salvin SB. Specific Suppression of Delayed Hypersensitivity: The Possible Presence of a Suppressor B Cell in the Regulation of Delayed Hypersensitivity. J Immunol (1974) 113(6):1716–25.
3. Wolf SD, Dittel BN, Hardardottir F, Janeway CA Jr. Experimental Autoimmune Encephalomyelitis Induction in Genetically B Cell-Deficient Mice. J Exp Med (1996) 184(6):2271–8. doi: 10.1084/jem.184.6.2271
4. Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF. Exacerbation of Ulcerative Colitis After Rituximab Salvage Therapy. Inflammation Bowel Dis (2007) 13(11):1365–8. doi: 10.1002/ibd.20215
5. Dass S, Vital EM, Emery P. Development of Psoriasis After B Cell Depletion With Rituximab. Arthritis Rheum (2007) 56(8):2715–8. doi: 10.1002/art.22811
6. Scapini P, Lamagna C, Hu YM, Lee K, Tang QZ, DeFranco AL, et al. B Cell-Derived IL-10 Suppresses Inflammatory Disease in Lyn-deficient Mice. Proc Natl Acad Sci USA (2011) 108(41):E823–E32. doi: 10.1073/pnas.1107913108
7. Knippenberg S, Peelen E, Smolders J, Thewissen M, Menheere P, Cohen Tervaert JW, et al. Reduction in IL-10 Producing B Cells (Breg) in Multiple Sclerosis Is Accompanied by a Reduced Naive/Memory Breg Ratio During a Relapse But Not in Remission. J Neuroimmunol (2011) 239(1-2):80–6. doi: 10.1016/j.jneuroim.2011.08.019
8. Saussine A, Tazi A, Feuillet S, Rybojad M, Juillard C, Bergeron A, et al. Active Chronic Sarcoidosis Is Characterized by Increased Transitional Blood B Cells, Increased IL-10-producing Regulatory B Cells and High BAFF Levels. PloS One (2012) 7(8):e43588. doi: 10.1371/journal.pone.0043588
9. Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, et al. IL-10-producing Regulatory B Cells in the Pathogenesis of Chronic Hepatitis B Virus Infection. J Immunol (2012) 189(8):3925–35. doi: 10.4049/jimmunol.1103139
10. Liu F, Dai W, Li C, Lu X, Chen Y, Weng D, et al. Role of IL-10-Producing Regulatory B Cells in Modulating T-helper Cell Immune Responses During Silica-Induced Lung Inflammation and Fibrosis. Sci Rep (2016) 6:28911. doi: 10.1038/srep28911
11. Rousset F, Garcia E, Defrance T, Péronne C, Vezzio N, Hsu DH, et al. Interleukin 10 Is a Potent Growth and Differentiation Factor for Activated Human B Lymphocytes. Proc Natl Acad Sci USA (1992) 89(5):1890–3. doi: 10.1073/pnas.89.5.1890
12. Itoh K, Hirohata S. The Role of IL-10 in Human B Cell Activation, Proliferation, and Differentiation. J Immunol (1995) 154(9):4341–50.
13. Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and Stimulatory Effects of IL-10 on Human CD8+ T Cells. J Immunol (1998) 160(7):3188–93.
14. Naing A, Infante JR, Papadopoulos KP, Chan IH, Shen C, Ratti NP, et al. Pegylated IL-10 (Pegilodecakin) Induces Systemic Immune Activation, Cd8. Cancer Cell (2018) 34(5):775–91.e3. doi: 10.1016/j.ccell.2018.10.007
15. Silva HM, Takenaka MC, Moraes-Vieira PM, Monteiro SM, Hernandez MO, Chaara W, et al. Preserving the B-cell Compartment Favors Operational Tolerance in Human Renal Transplantation. Mol Med (2012) 18:733–43. doi: 10.2119/molmed.2011.00281
16. Noh J, Noh G, Lee SJ, Lee JH, Kim A, Kim HS, et al. Tolerogenic Effects of Interferon-Gamma With Induction of Allergen-Specific Interleukin-10-producing Regulatory B Cell (Br1) Changes in Non-IgE-Mediated Food Allergy. Cell Immunol (2012) 273(2):140–9. doi: 10.1016/j.cellimm.2011.12.006
17. Noh J, Noh G, Kim HS, Kim AR, Choi WS. Allergen-Specific Responses of CD19(+)CD5(+)Foxp3(+) Regulatory B Cells (Bregs) and CD4(+)Foxp3(+) Regulatory T Cell (Tregs) in Immune Tolerance of Cow Milk Allergy of Late Eczematous Reactions. Cell Immunol (2012) 274(1-2):109–14. doi: 10.1016/j.cellimm.2012.01.005
18. Li X, Zhong H, Bao W, Boulad N, Evangelista J, Haider MA, et al. Defective Regulatory B-cell Compartment in Patients With Immune Thrombocytopenia. Blood (2012) 120(16):3318–25. doi: 10.1182/blood-2012-05-432575
19. Li J, Luo Y, Wang X, Feng G. Regulatory B Cells and Advances in Transplantation. J Leukoc Biol (2019) 105(4):657–68. doi: 10.1002/JLB.5RU0518-199R
20. Luo Y, Luo F, Zhang K, Wang S, Zhang H, Yang X, et al. Elevated Circulating IL-10 Producing Breg, But Not Regulatory B Cell Levels, Restrain Antibody-Mediated Rejection After Kidney Transplantation. Front Immunol (2020) 11:627496. doi: 10.3389/fimmu.2020.627496
21. Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, et al. Interleukin-10-Producing Plasmablasts Exert Regulatory Function in Autoimmune Inflammation. Immunity (2014) 41(6):1040–51. doi: 10.1016/j.immuni.2014.10.016
22. van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG, et al. IgG4 Production is Confined to Human IL-10-producing Regulatory B Cells That Suppress Antigen-Specific Immune Responses. J Allergy Clin Immunol (2013) 131(4):1204–12. doi: 10.1016/j.jaci.2013.01.014
23. Vadasz Z, Peri R, Eiza N, Slobodin G, Balbir-Gurman A, Toubi E. The Expansion of CD25 High IL-10 High FoxP3 High B Regulatory Cells Is in Association With SLE Disease Activity. J Immunol Res (2015) 2015:254245. doi: 10.1155/2015/254245
24. Park MK, Jung YO, Lee SY, Lee SH, Heo YJ, Kim EK, et al. Amelioration of Autoimmune Arthritis by Adoptive Transfer of Foxp3-Expressing Regulatory B Cells Is Associated With the Treg/Th17 Cell Balance. J Transl Med (2016) 14(1):191. doi: 10.1186/s12967-016-0940-7
25. Noh J, Choi WS, Noh G, Lee JH. Presence of Foxp3-expressing CD19(+)CD5(+) B Cells in Human Peripheral Blood Mononuclear Cells: Human CD19(+)CD5(+)Foxp3(+) Regulatory B Cell (Breg). Immune Network (2010) 10(6):247–9. doi: 10.4110/in.2010.10.6.247
26. Rennert PD. Novel Roles for TIM-1 in Immunity and Infection. Immunol Lett (2011) 141(1):28–35. doi: 10.1016/j.imlet.2011.08.003
27. Xiao S, Zhu B, Jin H, Zhu C, Umetsu DT, DeKruyff RH, et al. Tim-1 Stimulation of Dendritic Cells Regulates the Balance Between Effector and Regulatory T Cells. Eur J Immunol (2011) 41(6):1539–49. doi: 10.1002/eji.201040993
28. Curtiss M, Colgan J. The Role of the T-cell Costimulatory Molecule Tim-1 in the Immune Response. Immunologic Res (2007) 39(1-3):52–61. doi: 10.1007/s12026-007-0063-6
29. Xiao S, Brooks CR, Zhu C, Wu C, Sweere JM, Petecka S, et al. Defect in Regulatory B-cell Function and Development of Systemic Autoimmunity in T-cell Ig Mucin 1 (Tim-1) Mucin Domain-Mutant Mice. Proc Natl Acad Sci USA (2012) 109(30):12105–10. doi: 10.1073/pnas.1120914109
30. Wong SH, Barlow JL, Nabarro S, Fallon PG, McKenzie AN. Tim-1 is Induced on Germinal Centre B Cells Through B-cell Receptor Signalling But Is Not Essential for the Germinal Centre Response. Immunology (2010) 131(1):77–88. doi: 10.1111/j.1365-2567.2010.03276.x
31. Gielen AW, Lobell A, Lidman O, Khademi M, Olsson T, Piehl F. Expression of T Cell Immunoglobulin- and Mucin-Domain-Containing Molecules-1 and -3 (TIM-1 and -3) in the Rat Nervous and Immune Systems. J Neuroimmunol (2005) 164(1-2):93–104. doi: 10.1016/j.jneuroim.2005.04.004
32. Ma L, Liu B, Jiang Z, Jiang Y. Reduced Numbers of Regulatory B Cells are Negatively Correlated With Disease Activity in Patients With New-Onset Rheumatoid Arthritis. Clin Rheumatol (2014) 33(2):187–95. doi: 10.1007/s10067-013-2359-3
33. Bodogai M, Lee Chang C, Wejksza K, Lai J, Merino M, Wersto RP, et al. Anti-CD20 Antibody Promotes Cancer Escape Via Enrichment of Tumor-Evoked Regulatory B Cells Expressing Low Levels of CD20 and CD137L. Cancer Res (2013) 73(7):2127–38. doi: 10.1158/0008-5472.CAN-12-4184
34. Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-Evoked Regulatory B Cells Promote Breast Cancer Metastasis by Converting Resting CD4(+) T Cells to T-regulatory Cells. Cancer Res (2011) 71(10):3505–15. doi: 10.1158/0008-5472.CAN-10-4316
35. Poe JC, Smith SH, Haas KM, Yanaba K, Tsubata T, Matsushita T, et al. Amplified B Lymphocyte CD40 Signaling Drives Regulatory B10 Cell Expansion in Mice. PloS One (2011) 6(7):e22464. doi: 10.1371/journal.pone.0022464
36. Fang D, Kuan Y-C, Wu Y-J, Hung C-L, Sheu F. Trametes Versicolor Protein Yzp Activates Regulatory B Lymphocytes – Gene Identification Through De Novo Assembly and Function Analysis in a Murine Acute Colitis Model. PloS One (2013) 8(9):e72422. doi: 10.1371/journal.pone.0072422
37. Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B Cells are Identified by Expression of TIM-1 and can be Induced Through TIM-1 Ligation to Promote Tolerance in Mice. J Clin Invest (2011) 121(9):3645–56. doi: 10.1172/JCI46274
38. Sun J, Wang J, Pefanis E, Chao J, Rothschild G, Tachibana I, et al. Transcriptomics Identify CD9 as a Marker of Murine Il-10-Competent Regulatory B Cells. Cell Rep (2015) 13(6):1110–7. doi: 10.1016/j.celrep.2015.09.070
39. Yang X, Yang J, Chu Y, Wang J, Guan M, Zhu X, et al. T Follicular Helper Cells Mediate Expansion of Regulatory B Cells Via IL-21 in Lupus-prone MRL/lpr Mice. PloS One (2013) 8(4):e62855. doi: 10.1371/journal.pone.0062855
40. Margry B, Kersemakers SC, Hoek A, Arkesteijn GJ, Wieland WH, van Eden W, et al. Activated Peritoneal Cavity B-1a Cells Possess Regulatory B Cell Properties. PloS One (2014) 9(2):e88869. doi: 10.1371/journal.pone.0088869
41. Wang K, Gong H, Chai R, Yuan H, Chen Y, Liu J. Aberrant Frequency of IL-35 Producing B Cells in Colorectal Cancer Patients. Cytokine (2018) 102:206–10. doi: 10.1016/j.cyto.2017.10.011
42. Yu CR, Choi JK, Uche AN, Egwuagu CE. Production of IL-35 by Bregs is Mediated Through Binding of BATF-IRF-4-IRF-8 Complex to il12a and Ebi3 Promoter Elements. J Leukoc Biol (2018) 104(6):1147–57. doi: 10.1002/JLB.3A0218-071RRR
43. Dambuza IM, He C, Choi JK, Yu CR, Wang R, Mattapallil MJ, et al. IL-12p35 Induces Expansion of IL-10 and IL-35-expressing Regulatory B Cells and Ameliorates Autoimmune Disease. Nat Commun (2017) 8(1):719. doi: 10.1038/s41467-017-00838-4
44. Cai Z, Wong CK, Dong J, Chu M, Jiao D, Kam NW, et al. Remission of Systemic Lupus Erythematosus Disease Activity With Regulatory Cytokine Interleukin (IL)-35 in Murphy Roths Large (MRL)/Lpr Mice. Clin Exp Immunol (2015) 181(2):253–66. doi: 10.1111/cei.12639
45. Fonseca-Camarillo G, Furuzawa-Carballeda J, Yamamoto-Furusho JK. Interleukin 35 (IL-35) and IL-37: Intestinal and Peripheral Expression by T and B Regulatory Cells in Patients With Inflammatory Bowel Disease. Cytokine (2015) 75(2):389–402. doi: 10.1016/j.cyto.2015.04.009
46. Lal G, Nakayama Y, Sethi A, Singh AK, Burrell BE, Kulkarni N, et al. Interleukin-10 From Marginal Zone Precursor B-Cell Subset Is Required for Costimulatory Blockade-Induced Transplantation Tolerance. Transplantation (2015) 99(9):1817–28. doi: 10.1097/TP.0000000000000718
47. Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, et al. Mice Lacking Endogenous IL-10-producing Regulatory B Cells Develop Exacerbated Disease and Present With an Increased Frequency of Th1/Th17 But a Decrease in Regulatory T Cells. J Immunol (2011) 186(10):5569–79. doi: 10.4049/jimmunol.1100284
48. Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel Suppressive Function of Transitional 2 B Cells in Experimental Arthritis. J Immunol (2007) 178(12):7868–78. doi: 10.4049/jimmunol.178.12.7868
49. Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, et al. Selective Targeting of B Cells With Agonistic anti-CD40 is an Efficacious Strategy for the Generation of Induced Regulatory T2-Like B Cells and for the Suppression of Lupus in MRL/lpr Mice. J Immunol (2009) 182(6):3492–502. doi: 10.4049/jimmunol.0803052
50. Miles K, Heaney J, Sibinska Z, Salter D, Savill J, Gray D, et al. A Tolerogenic Role for Toll-like Receptor 9 is Revealed by B-cell Interaction With DNA Complexes Expressed on Apoptotic Cells. Proc Natl Acad Sci USA (2012) 109(3):887–92. doi: 10.1073/pnas.1109173109
51. Bankoti R, Gupta K, Levchenko A, Stäger S. Marginal Zone B Cells Regulate Antigen-Specific T Cell Responses During Infection. J Immunol (2012) 188(8):3961–71. doi: 10.4049/jimmunol.1102880
52. Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic Cells Protect Mice From Autoimmune Inflammation by the Induction of Regulatory B Cells. Proc Natl Acad Sci USA (2007) 104(35):14080–5. doi: 10.1073/pnas.0700326104
53. Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A Regulatory B Cell Subset With a Unique CD1dhiCD5+ Phenotype Controls T Cell-Dependent Inflammatory Responses. Immunity (2008) 28(5):639–50. doi: 10.1016/j.immuni.2008.03.017
54. Hu X, Tai J, Qu Z, Zhao S, Zhang L, Li M, et al. A Lower Proportion of Regulatory B Cells in Patients With Henoch-Schoenlein Purpura Nephritis. PloS One (2016) 11(3):e0152368. doi: 10.1371/journal.pone.0152368
55. Matsushita T, Tedder TF. Identifying Regulatory B Cells (B10 Cells) That Produce IL-10 in Mice. Methods Mol Biol (2011) 677:99–111. doi: 10.1007/978-1-60761-869-0_7
56. Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B Cells (B10 Cells) and Regulatory T Cells Have Independent Roles in Controlling Experimental Autoimmune Encephalomyelitis Initiation and Late-Phase Immunopathogenesis. J Immunol (2010) 185(4):2240–52. doi: 10.4049/jimmunol.1001307
57. Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a Rare IL-10-Competent B-Cell Subset in Humans That Parallels Mouse Regulatory B10 Cells. Blood (2011) 117(2):530–41. doi: 10.1182/blood-2010-07-294249
58. Khan AR, Amu S, Saunders SP, Hams E, Blackshields G, Leonard MO, et al. Ligation of TLR7 on CD19(+) CD1d(Hi) B Cells Suppresses Allergic Lung Inflammation Via Regulatory T Cells. Eur J Immunol (2015) 45(6):1842–54. doi: 10.1002/eji.201445211
59. van der Vlugt LE, Zinsou JF, Ozir-Fazalalikhan A, Kremsner PG, Yazdanbakhsh M, Adegnika AA, et al. Interleukin 10 (IL-10)-Producing CD1dhi Regulatory B Cells From Schistosoma Haematobium-Infected Individuals Induce IL-10-Positive T Cells and Suppress Effector T-cell Cytokines. J Infect Dis (2014) 210(8):1207–16. doi: 10.1093/infdis/jiu257
60. Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-Producing B Cells are Critical Regulators of Immunity During Autoimmune and Infectious Diseases. Nature (2014) 507(7492):366–70. doi: 10.1038/nature12979
61. Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, et al. Signaling Via the MyD88 Adaptor Protein in B Cells Suppresses Protective Immunity During Salmonella Typhimurium Infection. Immunity (2010) 33(5):777–90. doi: 10.1016/j.immuni.2010.10.016
62. Maseda D, Candando KM, Smith SH, Kalampokis I, Weaver CT, Plevy SE, et al. Peritoneal Cavity Regulatory B Cells (B10 Cells) Modulate IFN-Gamma+CD4+ T Cell Numbers During Colitis Development in Mice. J Immunol (2013) 191(5):2780–95. doi: 10.4049/jimmunol.1300649
63. Liu J, Zhan W, Kim CJ, Clayton K, Zhao H, Lee E, et al. IL-10-Producing B Cells are Induced Early in HIV-1 Infection and Suppress HIV-1-Specific T Cell Responses. PloS One (2014) 9(2):e89236. doi: 10.1371/journal.pone.0089236
64. Aravena O, Ferrier A, Menon M, Mauri C, Aguillón JC, Soto L, et al. TIM-1 Defines a Human Regulatory B Cell Population That is Altered in Frequency and Function in Systemic Sclerosis Patients. Arthritis Res Ther (2017) 19(1):8. doi: 10.1186/s13075-016-1213-9
65. Zhang Y, Zhang X, Xia Y, Jia X, Li H, Shao Z, et al. CD19+ Tim-1+ B Cells are Decreased and Negatively Correlated With Disease Severity in Myasthenia Gravis Patients. Immunol Res (2016) 64(5-6):1216–24. doi: 10.1007/s12026-016-8872-0
66. Yeung MY, Ding Q, Brooks CR, Xiao S, Workman CJ, Vignali DA, et al. TIM-1 Signaling is Required for Maintenance and Induction of Regulatory B Cells. Am J Transplant (2015) 15(4):942–53. doi: 10.1111/ajt.13087
67. Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B Cell Signature Associated With Renal Transplant Tolerance in Humans. J Clin Invest (2010) 120(6):1836–47. doi: 10.1172/JCI39933
68. Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(Hi)CD38(Hi) B Cells Exhibit Regulatory Capacity in Healthy Individuals But Are Functionally Impaired in Systemic Lupus Erythematosus Patients. Immunity (2010) 32(1):129–40. doi: 10.1016/j.immuni.2009.11.009
69. Menon M, Blair PA, Isenberg DA, Mauri C. A Regulatory Feedback Between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity (2016) 44(3):683–97. doi: 10.1016/j.immuni.2016.02.012
70. Lemoine S, Morva A, Youinou P, Jamin C. Human T Cells Induce Their Own Regulation Through Activation of B Cells. J Autoimmun (2011) 36(3-4):228–38. doi: 10.1016/j.jaut.2011.01.005
71. Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, et al. CD19+CD24HICD38HI B Cells Maintain Regulatory T Cells While Limiting TH1 and TH17 Differentiation. Sci Transl Med (2013) 5(173):173ra23. doi: 10.1126/scitranslmed.3005407
72. Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, et al. Development of a Cross-Platform Biomarker Signature to Detect Renal Transplant Tolerance in Humans. J Clin Invest (2010) 120(6):1848–61. doi: 10.1172/JCI39922
73. Liu Y, Cheng LS, Wu SD, Wang SQ, Li L, She WM, et al. IL-10-Producing Regulatory B-Cells Suppressed Effector T-Cells But Enhanced Regulatory T-Cells in Chronic HBV Infection. Clin Sci (Lond) (2016) 130(11):907–19. doi: 10.1042/CS20160069
74. Kessel A, Haj T, Peri R, Snir A, Melamed D, Sabo E, et al. Human CD19(+)CD25(High) B Regulatory Cells Suppress Proliferation of CD4(+) T Cells and Enhance Foxp3 and CTLA-4 Expression in T-Regulatory Cells. Autoimmun Rev (2012) 11(9):670–7. doi: 10.1016/j.autrev.2011.11.018
75. Kim HS, Lee JH, Han HD, Kim AR, Nam ST, Kim HW, et al. Autocrine Stimulation of IL-10 Is Critical to the Enrichment of IL-10-Producing CD40(Hi)CD5(+) Regulatory B Cells In Vitro and In Vivo. BMB Rep (2015) 48(1):54–9. doi: 10.5483/bmbrep.2015.48.1.213
76. Maseda D, Smith SH, DiLillo DJ, Bryant JM, Candando KM, Weaver CT, et al. Regulatory B10 Cells Differentiate Into Antibody-Secreting Cells After Transient IL-10 Production In Vivo. J Immunol (2012) 188(3):1036–48. doi: 10.4049/jimmunol.1102500
77. Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, et al. Regulatory B Cells Control T-Cell Autoimmunity Through IL-21-dependent Cognate Interactions. Nature (2012) 491(7423):264–8. doi: 10.1038/nature11501
78. Lighaam LC, Unger PA, Vredevoogd DW, Verhoeven D, Vermeulen E, Turksma AW, et al. In Vitro-Induced Human Il-10(+) B Cells Do Not Show a Subset-Defining Marker Signature and Plastically Co-Express IL-10 With Pro-Inflammatory Cytokines. Front Immunol (2018) 9:1913. doi: 10.3389/fimmu.2018.01913
79. Ma L, Qin J, Ji H, Zhao P, Jiang Y. Tfh and Plasma Cells are Correlated With Hypergammaglobulinaemia in Patients With Autoimmune Hepatitis. Liver Int (2014) 34(3):405–15. doi: 10.1111/liv.12245
80. Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 Induces Regulatory B Cells That Suppress Autoimmune Disease. Nat Med (2014) 20(6):633–41. doi: 10.1038/nm.3554
81. Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TF, et al. Interleukin 21-Induced Granzyme B-Expressing B Cells Infiltrate Tumors and Regulate T Cells. Cancer Res (2013) 73(8):2468–79. doi: 10.1158/0008-5472.CAN-12-3450
82. Durand J, Huchet V, Merieau E, Usal C, Chesneau M, Remy S, et al. Regulatory B Cells With a Partial Defect in CD40 Signaling and Overexpressing Granzyme B Transfer Allograft Tolerance in Rodents. J Immunol (2015) 195(10):5035–44. doi: 10.4049/jimmunol.1500429
83. Sugimoto K, Ogawa A, Shimomura Y, Nagahama K, Mizoguchi A, Bhan AK. Inducible IL-12-Producing B Cells Regulate Th2-mediated Intestinal Inflammation. Gastroenterology (2007) 133(1):124–36. doi: 10.1053/j.gastro.2007.03.112
84. Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-Activated B Cells Down-Regulate Th1 Immunity and Prevent Autoimmune Diabetes in Nonobese Diabetic Mice. J Immunol (2001) 167(2):1081–9. doi: 10.4049/jimmunol.167.2.1081
85. Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B Cells Activated by Lipopolysaccharide, But Not by Anti-Ig and Anti-CD40 Antibody, Induce Anergy in CD8+ T Cells: Role of TGF-beta 1. J Immunol (2003) 170(12):5897–911. doi: 10.4049/jimmunol.170.12.5897
86. Tang Y, Jiang Q, Ou Y, Zhang F, Qing K, Sun Y, et al. BIP Induces Mice CD19(hi) Regulatory B Cells Producing IL-10 and Highly Expressing PD-L1, Fasl. Mol Immunol (2016) 69:44–51. doi: 10.1016/j.molimm.2015.10.017
87. Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B Cells Are Critical Regulators of Humoral Immunity. Nat Commun (2015) 6:5997. doi: 10.1038/ncomms6997
88. Jin G, Hamaguchi Y, Matsushita T, Hasegawa M, Le Huu D, Ishiura N, et al. B-Cell Linker Protein Expression Contributes to Controlling Allergic and Autoimmune Diseases by Mediating IL-10 Production in Regulatory B Cells. J Allergy Clin Immunol (2013) 131(6):1674–82. doi: 10.1016/j.jaci.2013.01.044
89. Park MJ, Lee SH, Kim EK, Lee EJ, Park SH, Kwok SK, et al. Myeloid-Derived Suppressor Cells Induce the Expansion of Regulatory B Cells and Ameliorate Autoimmunity in the Sanroque Mouse Model of Systemic Lupus Erythematosus. Arthritis Rheumatol (2016) 68(11):2717–27. doi: 10.1002/art.39767
90. Mutnal MB, Hu S, Schachtele SJ, Lokensgard JR. Infiltrating Regulatory B Cells Control Neuroinflammation Following Viral Brain Infection. J Immunol (2014) 193(12):6070–80. doi: 10.4049/jimmunol.1400654
91. Achour A, Simon Q, Mohr A, Seite JF, Youinou P, Bendaoud B, et al. Human Regulatory B Cells Control the TFH Cell Response. J Allergy Clin Immunol (2017) 140(1):215–22. doi: 10.1016/j.jaci.2016.09.042
92. Zhan J, Huang L, Ma H, Chen H, Yang Y, Tan S, et al. Reduced Inflammatory Responses of Follicular Helper T Cell Promote the Development of Regulatory B Cells After Roux-en-Y Gastric Bypass. Clin Exp Pharmacol Physiol (2017) 44(5):556–65. doi: 10.1111/1440-1681.12740
93. Brosseau C, Durand M, Colas L, Durand E, Foureau A, Cheminant MA, et al. CD9(+) Regulatory B Cells Induce T Cell Apoptosis Via IL-10 and Are Reduced in Severe Asthmatic Patients. Front Immunol (2018) 9:3034. doi: 10.3389/fimmu.2018.03034
94. Rieger A, Bar-Or A. B-Cell-Derived Interleukin-10 in Autoimmune Disease: Regulating the Regulators. Nat Rev Immunol (2008) 8(6):486–7. doi: 10.1038/nri2315-c1
95. Chen Y, Bodhankar S, Murphy SJ, Vandenbark AA, Alkayed NJ, Offner H. Intrastriatal B-Cell Administration Limits Infarct Size After Stroke in B-cell Deficient Mice. Metab Brain Dis (2012) 27(4):487–93. doi: 10.1007/s11011-012-9317-7
96. Wang T, Li Z, Li X, Chen L, Zhao H, Jiang C, et al. Expression of CD19+CD24highCD38high B Cells, IL−10 and IL−10R in Peripheral Blood From Patients With Systemic Lupus Erythematosus. Mol Med Rep (2017) 16(5):6326–33. doi: 10.3892/mmr.2017.7381
97. Lal G, Kulkarni N, Nakayama Y, Singh AK, Sethi A, Burrell BE, et al. IL-10 From Marginal Zone Precursor B Cells Controls the Differentiation of Th17, Tfh and Tfr Cells in Transplantation Tolerance. Immunol Lett (2016) 170:52–63. doi: 10.1016/j.imlet.2016.01.002
98. Yap DYH, Chan TM. B Cell Abnormalities in Systemic Lupus Erythematosus and Lupus Nephritis-Role in Pathogenesis and Effect of Immunosuppressive Treatments. Int J Mol Sci (2019) 20(24):6231. doi: 10.3390/ijms20246231
99. Kleffel S, Vergani A, Tezza S, Ben Nasr M, Niewczas MA, Wong S, et al. Interleukin-10+ Regulatory B Cells Arise Within Antigen-Experienced CD40+ B Cells to Maintain Tolerance to Islet Autoantigens. Diabetes (2015) 64(1):158–71. doi: 10.2337/db13-1639
100. Boldison J, Da Rosa LC, Davies J, Wen L, Wong FS. Dendritic Cells License Regulatory B Cells to Produce IL-10 and Mediate Suppression of Antigen-Specific CD8 T Cells. Cell Mol Immunol (2020) 17(8):843–55. doi: 10.1038/s41423-019-0324-z
101. Todd SK, Pepper RJ, Draibe J, Tanna A, Pusey CD, Mauri C, et al. Regulatory B Cells are Numerically But Not Functionally Deficient in Anti-Neutrophil Cytoplasm Antibody-Associated Vasculitis. Rheumatol (Oxford) (2014) 53(9):1693–703. doi: 10.1093/rheumatology/keu136
102. Furuzawa-Carballeda J, Hernández-Molina G, Lima G, Rivera-Vicencio Y, Férez-Blando K, Llorente L. Peripheral Regulatory Cells Immunophenotyping in Primary Sjögren’s Syndrome: A Cross-Sectional Study. Arthritis Res Ther (2013) 15(3):R68. doi: 10.1186/ar4245
103. Sumimoto K, Uchida K, Kusuda T, Mitsuyama T, Sakaguchi Y, Fukui T, et al. The Role of CD19+ Cd24high CD38high and CD19+ Cd24high CD27+ Regulatory B Cells in Patients With Type 1 Autoimmune Pancreatitis. Pancreatology (2014) 14(3):193–200. doi: 10.1016/j.pan.2014.02.004
104. Yang B, Tan X, Xiong X, Wu D, Zhang G, Wang M, et al. Effect of CD40/CD40L Signaling on IL-10-producing Regulatory B Cells in Chinese Children With Henoch-Schönlein Purpura Nephritis. Immunol Res (2017) 65(3):592–604. doi: 10.1007/s12026-016-8877-8
105. Yan Y, van der Putten K, Bowen DG, Painter DM, Kohar J, Sharland AF, et al. Postoperative Administration of Donor B Cells Induces Rat Kidney Allograft Acceptance: Lack of Association With Th2 Cytokine Expression in Long-Term Accepted Grafts. Transplantation (2002) 73(7):1123–30. doi: 10.1097/00007890-200204150-00020
106. Newell KA, Asare A, Sanz I, Wei C, Rosenberg A, Gao Z, et al. Longitudinal Studies of a B Cell-Derived Signature of Tolerance in Renal Transplant Recipients. Am J Transplant (2015) 15(11):2908–20. doi: 10.1111/ajt.13480
107. Chesneau M, Pallier A, Braza F, Lacombe G, Le Gallou S, Baron D, et al. Unique B Cell Differentiation Profile in Tolerant Kidney Transplant Patients. Am J Transplant (2014) 14(1):144–55. doi: 10.1111/ajt.12508
108. Pallier A, Hillion S, Danger R, Giral M, Racape M, Degauque N, et al. Patients With Drug-Free Long-Term Graft Function Display Increased Numbers of Peripheral B Cells With a Memory and Inhibitory Phenotype. Kidney Int (2010) 78(5):503–13. doi: 10.1038/ki.2010.162
109. Mohib K, Cherukuri A, Zhou Y, Ding Q, Watkins SC, Rothstein DM. Antigen-Dependent Interactions Between Regulatory B Cells and T Cells at the T:B Border Inhibit Subsequent T Cell Interactions With DCs. Am J Transplant (2020) 20(1):52–63. doi: 10.1111/ajt.15546
110. Zhao G, Moore DJ, Lee KM, Kim JI, Duff PE, O’Connor MR, et al. An Unexpected Counter-Regulatory Role of IL-10 in B-Lymphocyte-Mediated Transplantation Tolerance. Am J Transplant (2010) 10(4):796–801. doi: 10.1111/j.1600-6143.2010.03027.x
111. Cherukuri A, Rothstein DM, Clark B, Carter CR, Davison A, Hernandez-Fuentes M, et al. Immunologic Human Renal Allograft Injury Associates With an Altered IL-10/TNF-Alpha Expression Ratio in Regulatory B Cells. J Am Soc Nephrol (2014) 25(7):1575–85. doi: 10.1681/Asn.2013080837
112. Mielle J, Audo R, Hahne M, Macia L, Combe B, Morel J, et al. IL-10 Producing B Cells Ability to Induce Regulatory T Cells Is Maintained in Rheumatoid Arthritis. Front Immunol (2018) 9:961. doi: 10.3389/fimmu.2018.00961
113. Qin Y, Zhang M, Jiang RM, Wu Q, Xu XY, Chen H, et al. B10 Cells Play a Role in the Immune Modulation of Pro- and Anti-Inflammatory Immune Responses in Mouse Islet Allograft Rejection. Cell Immunol (2016) 310:184–92. doi: 10.1016/j.cellimm.2016.09.010
114. Díaz-Alderete A, Crispin JC, Vargas-Rojas MI, Alcocer-Varela J. IL-10 Production in B Cells Is Confined to CD154+ Cells in Patients With Systemic Lupus Erythematosus. J Autoimmun (2004) 23(4):379–83. doi: 10.1016/j.jaut.2004.10.001
115. Amel Kashipaz MR, Huggins ML, Lanyon P, Robins A, Powell RJ, Todd I. Assessment of Be1 and Be2 Cells in Systemic Lupus Erythematosus Indicates Elevated interleukin-10 Producing CD5+ B Cells. Lupus (2003) 12(5):356–63. doi: 10.1191/0961203303lu338oa
116. Wang L, Zhao P, Ma L, Shan Y, Jiang Z, Wang J, et al. Increased Interleukin 21 and Follicular Helper T-like Cells and Reduced Interleukin 10+ B Cells in Patients With New-Onset Systemic Lupus Erythematosus. J Rheumatol (2014) 41(9):1781–92. doi: 10.3899/jrheum.131025
117. Heinemann K, Wilde B, Hoerning A, Tebbe B, Kribben A, Witzke O, et al. Decreased IL-10(+) Regulatory B Cells (Bregs) in Lupus Nephritis Patients. Scand J Rheumatol (2016) 45(4):312–6. doi: 10.3109/03009742.2015.1126346
118. Rosser EC, Mauri C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity (2015) 42(4):607–12. doi: 10.1016/j.immuni.2015.04.005
119. Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, et al. Control of Early Viral and Bacterial Distribution and Disease by Natural Antibodies. Science (1999) 286(5447):2156–9. doi: 10.1126/science.286.5447.2156
120. Sim JH, Kim HR, Chang SH, Kim IJ, Lipsky PE, Lee J. Autoregulatory Function of Interleukin-10-producing Pre-Naïve B Cells is Defective in Systemic Lupus Erythematosus. Arthritis Res Ther (2015) 17:190. doi: 10.1186/s13075-015-0687-1
121. Fleischer V, Sieber J, Fleischer SJ, Shock A, Heine G, Daridon C, et al. Epratuzumab Inhibits the Production of the Proinflammatory Cytokines IL-6 and TNF-α, But Not the Regulatory Cytokine IL-10, by B Cells From Healthy Donors and SLE Patients. Arthritis Res Ther (2015) 17:185. doi: 10.1186/s13075-015-0686-2
122. Park MJ, Kwok SK, Lee SH, Kim EK, Park SH, Cho ML. Adipose Tissue-Derived Mesenchymal Stem Cells Induce Expansion of Interleukin-10-producing Regulatory B Cells and Ameliorate Autoimmunity in a Murine Model of Systemic Lupus Erythematosus. Cell Transplant (2015) 24(11):2367–77. doi: 10.3727/096368914X685645
123. Bluestone JA, Herold K, Eisenbarth G. Genetics, Pathogenesis and Clinical Interventions in Type 1 Diabetes. Nature (2010) 464(7293):1293–300. doi: 10.1038/nature08933
125. Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, et al. Treatment With CD20-specific Antibody Prevents and Reverses Autoimmune Diabetes in Mice. J Clin Invest (2007) 117(12):3857–67. doi: 10.1172/JCI32405
126. Serreze DV, Chapman HD, Niens M, Dunn R, Kehry MR, Driver JP, et al. Loss of Intra-Islet CD20 Expression may Complicate Efficacy of B-cell-directed Type 1 Diabetes Therapies. Diabetes (2011) 60(11):2914–21. doi: 10.2337/db11-0705
127. Da Rosa LC, Boldison J, De Leenheer E, Davies J, Wen L, Wong FS. B Cell Depletion Reduces T Cell Activation in Pancreatic Islets in a Murine Autoimmune Diabetes Model. Diabetologia (2018) 61(6):1397–410. doi: 10.1007/s00125-018-4597-z
128. Fiorina P, Vergani A, Dada S, Jurewicz M, Wong M, Law K, et al. Targeting CD22 Reprograms B-cells and Reverses Autoimmune Diabetes. Diabetes (2008) 57(11):3013–24. doi: 10.2337/db08-0420
129. Deng C, Xiang Y, Tan T, Ren Z, Cao C, Huang G, et al. Altered Peripheral B-Lymphocyte Subsets in Type 1 Diabetes and Latent Autoimmune Diabetes in Adults. Diabetes Care (2016) 39(3):434–40. doi: 10.2337/dc15-1765
130. Hussain S, Delovitch TL. Intravenous Transfusion of BCR-Activated B Cells Protects NOD Mice From Type 1 Diabetes in an IL-10-dependent Manner. J Immunol (2007) 179(11):7225–32. doi: 10.4049/jimmunol.179.11.7225
131. Geetha D, Jefferson JA. ANCA-Associated Vasculitis: Core Curriculum 2020. Am J Kidney Dis (2020) 75(1):124–37. doi: 10.1053/j.ajkd.2019.04.031
132. Lepse N, Abdulahad WH, Rutgers A, Kallenberg CG, Stegeman CA, Heeringa P. Altered B Cell Balance, But Unaffected B Cell Capacity to Limit Monocyte Activation in Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis in Remission. Rheumatol (Oxford) (2014) 53(9):1683–92. doi: 10.1093/rheumatology/keu149
133. Karim MR, Wang YF. Phenotypic Identification of CD19. Arch Med Sci (2019) 15(5):1176–83. doi: 10.5114/aoms.2018.77772
134. Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH. Human CD5 Promotes B-cell Survival Through Stimulation of Autocrine IL-10 Production. Blood (2002) 100(13):4537–43. doi: 10.1182/blood-2002-05-1525
135. Bunch DO, McGregor JG, Khandoobhai NB, Aybar LT, Burkart ME, Hu Y, et al. Decreased CD5+ B Cells in Active ANCA Vasculitis and Relapse After Rituximab. Clin J Am Soc Nephrol (2013) 8(3):382–91. doi: 10.2215/CJN.03950412
136. Aybar LT, McGregor JG, Hogan SL, Hu Y, Mendoza CE, Brant EJ, et al. Reduced CD5(+) CD24(Hi) CD38(hi) and Interleukin-10(+) Regulatory B Cells in Active Anti-Neutrophil Cytoplasmic Autoantibody-Associated Vasculitis Permit Increased Circulating Autoantibodies. Clin Exp Immunol (2015) 180(2):178–88. doi: 10.1111/cei.12483
137. Bunch DO, Mendoza CE, Aybar LT, Kotzen ES, Colby KR, Hu Y, et al. Gleaning Relapse Risk From B Cell Phenotype: Decreased CD5+ B Cells Portend a Shorter Time to Relapse After B Cell Depletion in Patients With ANCA-associated Vasculitis. Ann Rheum Dis (2015) 74(9):1784–6. doi: 10.1136/annrheumdis-2014-206756
138. Unizony S, Lim N, Phippard DJ, Carey VJ, Miloslavsky EM, Tchao NK, et al. Peripheral CD5+ B Cells in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol (2015) 67(2):535–44. doi: 10.1002/art.38916
139. Vivino FB, Bunya VY, Massaro-Giordano G, Johr CR, Giattino SL, Schorpion A, et al. Sjogren’s Syndrome: An Update on Disease Pathogenesis, Clinical Manifestations and Treatment. Clin Immunol (2019) 203:81–121. doi: 10.1016/j.clim.2019.04.009
140. Goules AV, Tatouli IP, Moutsopoulos HM, Tzioufas AG. Clinically Significant Renal Involvement in Primary Sjögren’s Syndrome: Clinical Presentation and Outcome. Arthritis Rheum (2013) 65(11):2945–53. doi: 10.1002/art.38100
141. Ramos-Casals M, Solans R, Rosas J, Camps MT, Gil A, Del Pino-Montes J, et al. Primary Sjögren Syndrome in Spain: Clinical and Immunologic Expression in 1010 Patients. Med (Baltimore) (2008) 87(4):210–9. doi: 10.1097/MD.0b013e318181e6af
142. Jasiek M, Karras A, Le Guern V, Krastinova E, Mesbah R, Faguer S, et al. A Multicentre Study of 95 Biopsy-Proven Cases of Renal Disease in Primary Sjögren’s Syndrome. Rheumatol (Oxford) (2017) 56(3):362–70. doi: 10.1093/rheumatology/kew376
143. Lin W, Jin L, Chen H, Wu Q, Fei Y, Zheng W, et al. B Cell Subsets and Dysfunction of Regulatory B Cells in IgG4-related Diseases and Primary Sjögren’s Syndrome: The Similarities and Differences. Arthritis Res Ther (2014) 16(3):R118. doi: 10.1186/ar4571
144. Lin X, Wang X, Xiao F, Ma K, Liu L, Xu D, et al. IL-10-producing Regulatory B Cells Restrain the T Follicular Helper Cell Response in Primary Sjögren’s Syndrome. Cell Mol Immunol (2019) 16(12):921–31. doi: 10.1038/s41423-019-0227-z
145. Papp G, Gyimesi E, Szabó K, Zöld É, Zeher M. Increased IL-21 Expression Induces Granzyme B in Peripheral CD5(+) B Cells as a Potential Counter-Regulatory Effect in Primary Sjögren’s Syndrome. Mediators Inflammation (2016) 2016:4328372. doi: 10.1155/2016/4328372
146. Cortazar FB, Stone JH. IgG4-related Disease and the Kidney. Nat Rev Nephrol (2015) 11(10):599–609. doi: 10.1038/nrneph.2015.95
147. Piram M, Mahr A. Epidemiology of Immunoglobulin A Vasculitis (Henoch-Schönlein): Current State of Knowledge. Curr Opin Rheumatol (2013) 25(2):171–8. doi: 10.1097/BOR.0b013e32835d8e2a
148. Wang YY, Zhang L, Zhao PW, Ma L, Li C, Zou HB, et al. Functional Implications of Regulatory B Cells in Human IgA Nephropathy. Scand J Immunol (2014) 79(1):51–60. doi: 10.1111/sji.12128
149. Li T, Yu Z, Qu Z, Zhang N, Crew R, Jiang Y. Decreased Number of CD19. Mol Immunol (2019) 112:233–9. doi: 10.1016/j.molimm.2019.05.014
150. Liu Y, Wang H, Hu X, Qu Z, Zhang H, Crew R, et al. A Higher Frequency of IL-10-Producing B Cells in Hepatitis B Virus-Associated Membranous Nephropathy. Clin Exp Pharmacol Physiol (2016) 43(4):417–27. doi: 10.1111/1440-1681.12552
151. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: The Master Regulator of Fibrosis. Nat Rev Nephrol (2016) 12(6):325–38. doi: 10.1038/nrneph.2016.48
152. Jin Y, Liu R, Xie J, Xiong H, He JC, Chen N. Interleukin-10 Deficiency Aggravates Kidney Inflammation and Fibrosis in the Unilateral Ureteral Obstruction Mouse Model. Lab Invest (2013) 93(7):801–11. doi: 10.1038/labinvest.2013.64
153. Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, et al. Il-10 Suppresses Chemokines, Inflammation, and Fibrosis in a Model of Chronic Renal Disease. J Am Soc Nephrol (2005) 16(12):3651–60. doi: 10.1681/ASN.2005030297
Keywords: regulatory B cells, Breg, kidney disease, IL-10, transplantation
Citation: Long W, Zhang H, Yuan W, Lan G, Lin Z, Peng L and Dai H (2021) The Role of Regulatory B cells in Kidney Diseases. Front. Immunol. 12:683926. doi: 10.3389/fimmu.2021.683926
Received: 22 March 2021; Accepted: 04 May 2021;
Published: 24 May 2021.
Edited by:
Cheng Yang, Fudan University, ChinaReviewed by:
Jinfeng Li, First Affiliated Hospital of Zhengzhou University, ChinaCheng Yu, National Eye Institute (NEI), United States
Keishi Fujio, The University of Tokyo, Japan
Copyright © 2021 Long, Zhang, Yuan, Lan, Lin, Peng and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helong Dai, aGVsb25nNjg4ODhAY3N1LmVkdS5jbg==; Longkai Peng, cGVuZ2xvbmdrYWlAY3N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Wang Long1,2†
Wang Long1,2† Helong Dai
Helong Dai