- Department of Hematology, Zhongnan Hospital, Wuhan University, Wuhan, China
Natural killer (NK) cells are prominent cytotoxic and cytokine-producing components of the innate immune system representing crucial effector cells in cancer immunotherapy. Presently, various NK cell-based immunotherapies have contributed to the substantial improvement in the reconstitution of NK cells against advanced-staged and high-risk AML. Various NK cell sources, including haploidentical NK cells, adaptive NK cells, umbilical cord blood NK cells, stem cell-derived NK cells, chimeric antigen receptor NK cells, cytokine-induced memory-like NK cells, and NK cell lines have been identified. Devising innovative approaches to improve the generation of therapeutic NK cells from the aforementioned sources is likely to enhance NK cell expansion and activation, stimulate ex vivo and in vivo persistence of NK cells and improve conventional treatment response of myeloid leukemia. The tumor-promoting properties of the tumor microenvironment and downmodulation of NK cellular metabolic activity in solid tumors and hematological malignancies constitute a significant impediment in enhancing the anti-tumor effects of NK cells. In this review, we discuss the current NK cell sources, highlight ongoing interventions in enhancing NK cell function, and outline novel strategies to circumvent immunosuppressive factors in the tumor microenvironment to improve the efficacy of NK cell-based immunotherapy and expand their future success in treating myeloid leukemia.
Highlights
● NK cell-based immunotherapy continues to expand in clinical practice.
● Malignantly transformed cells and tumor progression lead to an immunosuppressed microenvironment, limiting the efficacy of various immunotherapies.
● Strategizing methods to enhance NK cell expansion, in vivo persistence and cytotoxicity may improve the treatment response of conventional chemotherapy.
Introduction
Acute myeloid leukemia (AML) is an aggressive malignant disease of high heterogeneity that remains a deterring challenge to clinicians due to shortened remission duration and high relapse rates associated with the disease. Advanced sequencing technologies have revealed the impact of several aberrant chromosomes, genomic mutations, and myeloid transcription factor dysregulation on the pathogenesis of AML (1). Over the past 30 years, the treatment of AML has not invoked drastic evolvement, with the standard of treatment being chemotherapy. Chemotherapy employs the pro-oxidant approach to induce apoptosis but causes high toxicity, chemo-refractory and disease relapse, resulting in increased morbidity, and mortality of patients with AML (2).
Chronic myeloid leukemia (CML) is a myeloproliferative malignancy genetically characterized by a translocation (3, 4) in the hematopoietic stem cell resulting in constitutively active BCR-ABL1 oncokinase. The majority of patients present with abnormal immune cells (5). On the basis of the critical role of BCR-ABL1 oncokinase activity, tyrosine kinase inhibitors (TKIs) remain the standard therapy (6, 7). Although targeted therapy such as imatinib effectively induce and sustain remissions, molecular disease often persists after years of treatment (8, 9). Mahon et al. showed that imatinib could be discontinued without relapse in only a subset of patients, indicating a possibility of a cure with TKIs (10).
In recent years, the pullulating of novel anticancer therapies to enhance immune response has pushed natural killer (NK) cells into the spotlight (3, 11). NK cells are crucial cytotoxic and cytokine-producing components of the innate immune system that represent crucial effector cells in cancer immunotherapy. As an integral part of the innate lymphoid cells (ILCs), NK cells play vital roles in antiviral and anti-tumor defense (12). ILCs are a heterogenous group of immune cells that maintain homeostasis and respond to pathogen invasion through an immune-mediated response (13). NK cells are derived from common lymphoid progenitors differently from T and B cells and do not express antigen-specific receptors. However, NK cells express a remarkedly wide array of germline-encoded inhibitory and activating receptors on their surface, mediating immune response (14).
NK cell-based anticancer therapies have been highly exploited in hematological malignancies in present-day practice (15). In acute leukemia, particularly in children given human leucocyte antigen (HLA)-haploidentical hematopoietic transplantation, infusion of mature NK cells through graft manipulation based on the selective depletion of T cells and CD19+B cells improved outcome (16–18). An increased proportion of mature NK cells was associated with molecular relapse-free survival in CML patients. Ilander et al. showed that NK cells were important in sustaining remission with imatinib discontinuation (8, 19). Similarly, Dasatinib discontinuation in patients with CML who maintained deep molecular response was successful in 50% of patients (20). In the IMMUNOSTIM study, non-relapsing patients had higher numbers of NK cells of the CD56dim subset than relapsing patients (21).
In the recent past, strategies to optimize NK cell function have aimed at improving NK cell targeting, anti-tumor response, and limiting NK cell inhibition. Various sources of NK cells such as chimeric antigen receptor (CAR) NK cells, NK cells derived from haploidentical donors (i.e. having half HLA matched), stem cell-derived NK cells, NK cell lines, umbilical cord blood (UCB) NK cells, adaptive NK cells, and cytokine-induced memory-like (CIML) NK cells have been exploited in clinical practice (22). Enhancement of NK cell generation from various sources and modulation of NK cell expansion, cytotoxicity, and in vivo survival have changed the treatment paradigm for AML.
Despite breakthroughs in novel NK cell sources, NK cells remain subject to prominent immunosuppressive barriers present in the tumor microenvironment (TME), resulting in the dysfunction and exhaustion of NK cells. Devising methods to overcome these immunosuppressive factors to enhance NK cell function and achieve the desired outcome from therapies is imperative (22). In this review, we discuss the current NK cell sources, highlight ongoing interventions in enhancing NK cell function, and outline novel strategies to circumvent immunosuppressive factors in the TME to improve the efficacy of NK cell therapy and expand their success in future research.
NK Cells in Myeloid Leukemia
NK cells express rapid and efficient cytolytic activity in recognizing and killing both transformed and virally infected cells. Through the release of cytoplasmic cytotoxic granules containing perforin, granulysin, and granzymes A and B (4), or by using effector molecules such as tumor necrosis factor, tumor-related apoptosis-inducing ligand (TRAIL), and Fas ligand, NK cells can induce apoptosis to target cells. NK cell activation in the host promotes pro-inflammatory cytokine production that affects both hematopoietic and non-hematopoietic cell function (23). Through secretion of cytokines and chemokines and direct cell-to-cell contact, NK cells promote activation and effector functions of other innate and adaptive immune cells contributing to homeostasis of the immune system. NK cells express activating and inhibitory receptors, that distinguish normal from transformed cells and recognize altered protein expression on target cells, thereby controlling the cytolytic function (24).
Evidence shows that NK cells can exhibit anti-tumor activity against leukemic blasts from patients with AML, CML, or MDS. More recent studies have mainly focused on exploiting NK cells to target AML. Cytokine-mediated activation of NK cells with IL-2 or IL-15 is commonly employed and is currently extensively studied (25). In recent investigations, the pre-activation of mouse and human NK cells with a cocktail of IL-12/15/18 (26) had enhanced and sustained anti-tumor functions in vitro and in vivo after infusion. Similarly, Romee et al. reported that CIML NK cells exhibited enhanced antileukemia functionality and that human memory-like NK cells had enhanced interferon-γ (IFN-γ) production and cytotoxicity against leukemia cell lines or primary human AML blasts in vitro (27, 28).
Additional studies investigated the beneficial effect of ex-vivo expanded NK cells against well-established cell lines such as K562 cells (27, 29). Hasmim and colleagues further investigated the in vivo anti-leukemic capacity of ex vivo-expanded NK cells against patient-derived AML cells. The study concluded that the surface expression of CD94 on ex vivo-differentiated NK cells was a potential indicator of in vitro and in vivo killer cell functionality (30).
Early clinical trials have demonstrated the overall safety and efficacy of NK cell-based therapeutic approaches, such as the use of antibodies or cytokines to enhance the NK cell function, the generation of CAR-modified NK cells or the adoptive transfer of NK cells (or their precursors) from haploidentical donors in treating AML (22, 31). Still, the effort to devise new methods of generating more feasible clinical scales of NK cells is ongoing. Recent technology is exploiting active molecules/cytokine delivery, imaging, and physicochemical properties of nanoparticles to overcome the challenges of NK cell cancer immunotherapy (32).
Role of NK Cells in Treating Myeloid Malignancies
Early evidence showed that NK cells could kill AML cells, illustrated by the absence of relapsed AML disease after hematopoietic cell transplantation containing alloreactive NK cells (3, 33). High IFN-γ-producing capacity of NK cells was associated with improved anti-tumor immunity (34). In AML, the antileukemic activity of NK cells negatively reflects on disease progression, with NK cells being suppressed at diagnosis, restored at remission, and suppressed again at relapse. Similarly, in CML, the number of NK cells decreases with disease progression, respond less to stimuli, and exhibit reduced cytolytic activity (35). CML patients in complete remission (CR) with a deep molecular response to TKIs have restored NK cell cytolytic activity (36). Furthermore, a high percentage of NK cells at TKI discontinuation was associated with improved long-term outcomes. Comparatively, higher cytolytic activity of NK cells in AML predicts an improved long-term outcome in patients at diagnosis and in remission (37, 38). Therefore, enhancing the cytotoxic activity of NK cells in myeloid malignancies plays a significant role in counteracting disease progression.
Impaired NK Cell Function in the Tumor Microenvironment
The TME plays a critical role in NK-AML recognition. AML cells create an immunosuppressive microenvironment that results in decreased NK cell function, promoting immune escape. As stated earlier, several mechanisms are implicated in the impaired function of NK cells in the TME (Figure 1). Modulation of the NK receptor repertoire inhibits NK cell activity in the TME (3). Leukemia cells defectively express ligands for NK cell activating/inhibitory receptors altering NK cell antileukemia response, i.e. decreased expression of NKG2DLs on AML cells caused by aberrant epigenetic mutations renders AML cells resistant to NK cell killing (39, 40).
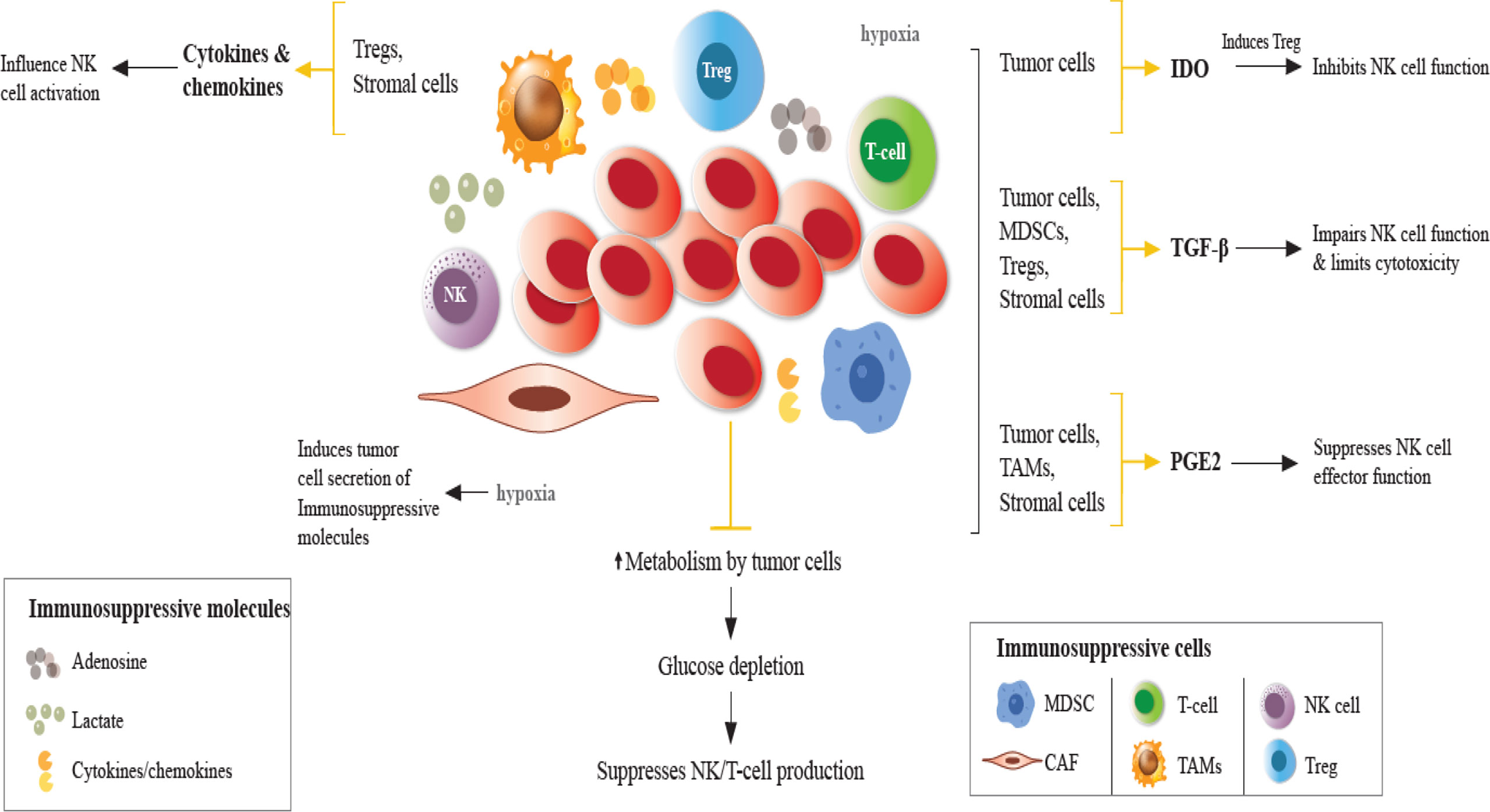
Figure 1 Impaired NK cell function in the TME. In the TME, immunosuppressive cells and molecules, in the presence of hypoxia and glucose depletion negatively regulate the maturation, proliferation, activation, and effector function of NK cells. Hypoxia induces tumor cells to secrete immunosuppressive molecules, such as TGF-β, IL-10, VEGF, galectins, and CC-chemokine ligands, contributing to the accumulation of TAMs, Tregs, MDSCs, which suppress DCs, T and NK cells. Under hypoxic conditions, NK cells fail to upregulate the expression of activating receptors. Cytokines/chemokines produced by Tregs and stromal cells influence NK cell activity. Tumor cells directly exert immunosuppressive effects on NK cells through the secretion of soluble factors. TGF-β production by tumor cells, Tregs, MDSCs, and other stromal cells impairs NK cell function. IDO induces Treg which inhibits NK cell function. IDO expression induces growth arrest of NK cells and promotes tumor progression. PGE2 production in the TME suppresses the effector function of NK cells. Adenosine secretion by tumor cells and lactate accumulation reduces NK cell cytotoxicity promoting immunosuppression by MDSCs, Tregs and TAMs. TGF-β, transforming growth factor-β; PGE2, prostaglandin E2; IDO, indoleamine 2,3 dioxygenase; NKG2D, natural killer group 2D; MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells (Tregs); TAMs, tumor-associated macrophages.
During AML development, the repertoire of NK cells is modified, reducing the expression of activating receptors, NKG2D, DNAX accessory molecule-1 (DNAM-1), natural cytotoxicity triggering receptor 3 (NKp30), NKp46 (41), and increasing that of inhibitory receptors, killer Ig-like receptors (KIRs), and natural killer group 2A (NKG2A). Moreover, the expression of the ligands of the activating receptors is also modulated (3). Ligands for NKG2D-activating receptor (NKG2DL) can be regulated by DNA methylation (39), reducing the level of expression of NKG2D on the surface of AML cells or is released as soluble activating ligands into the TME, promoting ligand degradation and loss of NK cell cytotoxicity capacity (3, 42).
A study has shown that leukemic blasts can avoid NK cell recognition by decreasing the expression levels of ligands for natural cytotoxicity receptors (NCRs) and natural killer group 2 member D (NKG2D) (35). The down-regulation of ligands for DNAM-1 on the leukemic cell surface promotes resistance to NK cell targeting (43). DNAM-1 receptor has a central role in NK cell-mediated killing of myelodysplastic syndrome (MDS) blasts (44). Additionally, NKG2D and DNAM-1 receptors as well as NCRs play key roles in targeting AML, CML, and acute lymphoblastic leukemia (ALL) blasts (45). Furthermore, NK cell abnormalities, due to interactions with tumor cells or with suppressive immune cells, can favor the escape from immune surveillance in myeloid malignancies (34).
Functional NK cell maturation is defined by differential expression of several markers, including CD56, CD16, CD57, KIR, and NKG2A. In humans, NK cells are divided into two subtypes; CD56brightCD16dim and CD56dimCD16bright, which differ in their homing properties (46). Early study by Lanier and colleagues suggested that the CD56dim NK-cell subset is derived directly from the CD56bright NK subset (47), similarly supported by more recent studies (48–50). Romagnani and colleagues further hypothesized that the differentiation of NK cells corresponded to sequential steps and that secondary lymphoid organs were the sites of NK cell final maturation (48).
Mature NK cells with the CD56dimCD16a+CD57+ subset have great cytolytic potential against tumor target cells and are predominant in peripheral blood (PB). The CD56brightCD16a−CD57− subset in immature NK cells has high cytokine-producing potential and is more abundant in secondary lymphoid organs (51). CD56dim expresses high levels of cytolytic granules, containing perforin and granzymes that are poorly expressed in CD56bright cells (52). Recently, it has been shown that the cytolytic CD56dim cells produce high levels of IFN‐γ and other cytokines upon receptor‐induced NK cell triggering (53). Moreover, CD56dim NK cells abundantly express CD16 or the low-affinity Fcγ receptor IIIA, which can bind to the Fc region of immunoglobulin. The receptor-ligand binding results in antibody-dependent cellular cytotoxicity (ADCC) which is a dominant component of effective antitumor activity (54).
Defective maturation of NK cells is implicated in chronic viral infections and cancer progression. Multiple lineages of tumors interrupt NK cell functional maturation and impair the antitumor capacity of NK cells (55, 56). Recently, a study by Khaznadar et al. showed that the presence of hypomature NK cells and reduced perforin expression in hypermature NK cells is reflective of defective NK cell maturation (37). In AML, elevated levels of miR-29b reduce the cytotoxic ability of hypermature NK cells, indicating a lesser degree of recognition of AML cells and consequently, increasing the number of hypomature cells (35, 57). MicroRNA miR-29b is a regulator of T-bet and EOMES transcription factors that are essential for terminal NK cell differentiation (57). Upregulation of miR-29b downregulates the expression of T-bet resulting in low perforin levels in mature NK cells, thereby reducing NK cytolytic ability and damage to the development of intermediate NK cells that require expression of both T-bet and EOMES. Therefore, both factors play a role in NK cell generation (58).
Chretien and colleagues reported that in myeloid malignancies, NK cell maturation was perturbed by loss of immature NK cells accompanied by an increase in mature NK cells in the PB of AML patients (59). The study further divided AML patients into three distinct groups based on NK maturation profiles. It demonstrated that AML patients with hypomaturation profiles had reduced relapse-free and overall survival, suggesting that disease-related alterations in NK maturation affected patient outcomes (60).
Three different NK cell maturation stages are reported in AML: hypomaturation (CD56bright/dim KIRs− CD57−), intermediate (CD56dim KIR−/+ CD57−/+) and hypermaturation (CD56dim KIRs+ CD57+) (3). In AML patients at first remission, an increased percentage of immature (CD56bright) NK cells was observed, resulting from reconstitution of NK cells after intense chemotherapy (35). In CML, differentiation of NK cells was observed with TKI dasatinib treatment. Upon achieving molecular response, an increased proportion of mature cytolytic NK cells (CD57+CD62L−) were seen, indicating restoration of NK cell function (36).
Immune checkpoint molecules maintain adequate immune response and play a critical role in self-tolerance and avoidance of cell lysis. The immune checkpoints programmed death 1 (PD-1), TIM3 and TIGIT regulate NK cell activity. PD-1, TIM3 and TIGIT, expressed on NK cell surface recognize their ligands, PD-L1, Gal-9, or CD112/CD155, respectively, expressed on the cell surface of AML cells. As a result, inhibitory signaling occurs in activating pathways involved in NK cell regulation, PI3K, ERK, and PKCΘ, promoting tumor immune escape (61, 62).
High CD200 expression on AML cells has been shown to inhibit antitumor response in AML patients and therefore, is associated with poor overall survival. Coles et al. demonstrated that upregulation of CD200 expression on AML cells suppressed NK cell cytolytic activity and influenced leukemia cell escape from NK cell-mediated lysis (40, 63). As indicated, decreased levels of NKG2D and NCRs, have been reported in patients with AML. A study showed that the overall NCR expression was lower in patients with high CD200 expression on AML blasts, which resulted in CD200-mediated NK cell suppression and contributed to decreased leukemic cell recognition by NK cells (63).
CD200-CD200R interaction on NK cell surface results in immunosuppressive effects that influence responses to pathogens, autoimmunity, transplant tolerance, and cancer surveillance. Targeting CD200 on leukemia blasts presents an insightful strategy to reduce relapse rates and improve outcomes in AML patients (64, 65). A phase I study investigated the therapeutic use of samalizumab (ALXN6000) as a CD200-CD200R immune checkpoint inhibitor in chronic lymphocytic leukemia (CLL) and multiple myeloma. Results showed favorable safety and reduced tumor burden in a majority of patients with advanced CLL with samalizumab treatment (66). Similarly, Herbrich and colleagues identified CD200 as a stem cell-specific immunomodulatory target that aids in establishing an immunosuppressive microenvironment by significantly suppressing cytokine secretion in response to AML stem cell activity (67). Direct contact between AML cells and NK cells, high expression of CD200 on AML cells, soluble NKG2DLs in the sera and suppressive TME are factors that lead to defective receptor expression changes (40).
Immunosuppressive Factors in the Tumor Microenvironment
Bone Marrow Microenvironment in Leukemia
In both AML and CML, the bone marrow (BM) microenvironment promotes leukemogenesis through interaction with leukemia stem cells which alter the microenvironment making it hostile for normal hematopoiesis. Targeting these interactions may provide an ample opportunity to treat leukemia more effectively (68). Leukemia cells interfere with the function of the hematopoietic niche and remodel the niche into a favorable environment for expansion or leukaemic transformation (69). Direct cell-to-cell interactions between AML cells and mesenchymal stromal cells in the TME affect the susceptibility of AML cells to NK cells (70).
In the BM, AML cells as well as their neighboring stromal cells, normal hematopoietic cells, and infiltrating immunocompetent cells produce survival and growth-regulatory chemokines and express a wide range of chemokine receptors (71). CCL and CXCL chemokines are the two major chemokine subclasses, which interact with CCR and CXCR membrane receptors, respectively (72). Several cytokines, chemokines and soluble factors have been implicated in the AML/CML BM niche bidirectional crosstalk including CXCR2, CXCR4, IL6R, LFA, VLA4, RANK and FAT/CD36 (73). Bruserud et al. further classified AML patients in three distinct subsets according to chemokine responsiveness and chemokine release profile: CCL2-4/CXCL1/8, CCL5/CXCL9-11, and CCL13/17/22/24/CXCL5 (72).
The tumor microenvironment (TME) and cancer cells exchange signals through a mechanistic process involving soluble factors, such as signaling molecules, and microvesicles. Among soluble factors, chemokines, which induce chemotaxis of target cells, and cytokines play important roles. CXCL12 produced by osteoblasts in BM serves as a ligand for CXCR4 (74, 75). Leukemia stem cells which are also attracted by CXCL12, alter the niche and secrete another cytokine, stem cell factor or KIT ligand, which contributes to the disruption of normal HSC function (76, 77). IL-6 is another cytokine implicated in promoting resistance to chemotherapy in epithelial cancers and hematological stroma-mediated drug resistance (78).
Growth factors are reported to play a role in leukemia-microenvironment crosstalk. TGF-β induces quiescence of HSCs in the niche (79). However, leukemia cells are resistant to TGF-β inhibition despite increased production of TGF-β by BM stromal cells (80). Basic fibroblast growth factor (bFGF) and VEGF are said to promote leukemia development and BM-mediated resistance to chemotherapy (81).
AML blasts induce activation of the vascular endothelium through secretion of inflammatory cytokines, such as TNF-α and IL-10. These induce expression of cell surface molecules on endothelial cells, including ICAM-1, VCAM-1, and E-selectin, promoting adhesion of leukemia blasts to the endothelium (82). Hatfield et al. demonstrated that endothelial cells provide direct support to enhance leukemic proliferation (83). Moreover, interactions between leukemic cells and BM stromal cells induce chemotherapy resistance in AML (84, 85). Various strategies to limit the microenvironment-leukemia crosstalk have been investigated i.e., CXCR4/CXCL12 inhibitors (74, 86), TGF-β neutralizing antibodies (87), and blockage of IL-6 with mAbs (88).
The BM microenvironment also contributes to leukemic cell survival through microvesicles such as exosomes. The TME and tumor cells interact via exosomes which are responsible for trafficking proteins and microRNAs that can promote proliferation, metastasis, and apoptosis (89). Additionally, exosomes can suppress hematopoiesis in AML (90). Hong et al. hypothesized that elevated levels of exosomes isolated from pre-therapy plasma of refractory/relapsed AML interfered with anti-leukemia functions of immune cells. The study further showed that AML exosomes could reprogram NK-92 cells, interfering with their anti-leukemia functions and reducing the therapeutic potential of adoptive cell transfers (91). Recently, Dai et al. proposed that the proportion of NK cells in the BM of AML could predict patient prognosis, with a lower proportion of NK cells associated with worse prognosis (92).
AML cells exist under hypoxic conditions in the BM. A hypoxic microenvironment has pivotal prosurvival effects on AML cells through activation of the PI3K-Akt-mTOR pathway and Pim-1 expression (93). Also, leukemia cells actively reduce glucose utilization by healthy cells to increase their bioavailability, resulting in glucose depletion (94). Reactive oxygen species have also been shown to alter NK cell function, affecting antitumor immune response and promoting immune escape within the TME (95–97). In cancer cells obtained from CML patients, reactive oxygen species were implicated in NK cell dysfunction, of which NK cell cytotoxic activity could be restored with catalase suppressive effects of the TME (95, 97).
TME Suppressive Factors
The TME is a major impediment in enhancing the anti-tumor effects of NK cells. As a complex network, it comprises TAMs, MDSCs, Tregs, regulatory γδT cells, soluble factors, the extracellular matrix, and suppressive molecules expressed on tumor cells (24, 98–101). Tumor cells evade immune escape by establishing an immunosuppressive microenvironment, promoting tumor progression and metastasis (102). Tumor-infiltrating NK cells exhibit poor cytotoxic capacity, accompanied by downregulation of activating receptors, upregulation of inhibitory receptors, and upregulation of immune checkpoint receptors, compared with NK cells from healthy tissues (103). The development of novel approaches such as monoclonal antibodies (mAbs) to block inhibitory receptors enhances NK cell function and its anti-tumor capacity (104). Despite efforts to combat immunosuppressive effects, multiple factors in the TME still limit NK cell function.
Hypoxia, low glucose concentration, cytokines, tumor-derived metabolites, such as adenosine and lactate, tumor cell-derived factors such as IL-6, IL-10, TGF-β, IDO, prostaglandin E2 (PGE2), and vascular endothelial growth factor (VEGF) (105, 106), including co-inhibitory molecules in the TME such as tryptophan catabolites, dickkopf-related protein 2 (DKK2), soluble HLA-G, soluble NKG2D ligands, and galactin-3 (soluble inhibitory receptor for NKp30) inhibit NK cell activity (107).
In solid malignancies, tumor growth promotes the expansion of immunosuppressive cells, including Tregs, MDSCs, and TAMs. Chemokines, such as C–X–C motif chemokine ligand 8 (CXCL8) or C–C motif chemokine ligand 2 (CCL2) secreted by tumor cells also promote the accumulation of immunosuppressive cells at the tumor site. Through production of TGF-β and IL-10, or via direct cell-to-cell interaction, immunosuppressive cells inhibit intratumoral NK cell cytotoxicity (108–110). Tregs directly inhibit NK cell cytolytic function through TGF-β production and downregulate NKG2D expression and NKp30 through membrane-bound TGF-β (111, 112). Furthermore, MDSCs inhibit NK cell cytotoxicity and cytokine secretion through membrane-bound TGF-β on MDSCs in a cell-contact-dependent manner or dependent on NKp30 on NK cells (24, 113, 114). TGF-β, IDO, nitric oxide, and adenosine (108, 115, 116) have also been reported to contribute to MDSC-mediated NK cell inhibition, resulting in reduced NK cell cytotoxicity, decreased NKG2D or NCRs expression, and reduced IFN-γ production (117). TGFβ inhibition with neutralizing antibodies, ligand traps, small-molecule kinase inhibitors, and antisense oligonucleotides are currently under investigation (118).
Expression of high levels of inhibitory molecules, including PD-L1 or PD-L2, on tumor cells, immunosuppressive cells, antigen-presenting cells, and stromal cells in the TME prevent NK cell activation by binding with their respective inhibitory receptors on NK cells, resulting in NK cell exhaustion and dysfunction (119, 120). Cancer-associated fibroblasts (CAFs) are the major stromal cells that affect the antitumor capacity of NK cells. CAFs secrete IDO or PGE2 that downregulate NKG2D expression and secrete TGF-β to reduce the expression of NKG2D, NKp30, and NKp44 and decreasing perforin/granzyme B release (121–123).
In the TME, NK cell metabolism and antitumor responses are impaired (124) due to nutrient and oxygen deprivation and the release of tumor-derived metabolic end-products such as lactate and adenosine, which impede NK cell effector and cytotoxic function (107, 125, 126). Lactate accumulation in the TME results from metabolic reprogramming of cancer cells, characterized by the use of glucose for glycolytic metabolism against metabolizing via oxidative phosphorylation (127). Hypoxia leads to decreased pH in the TME and is associated with poor prognosis and resistance to conventional therapies (128, 129). Parodi et al., recently demonstrated that a hypoxic environment could highly influence NK cell infiltration and its effects on immune-mediated responses within tumor tissues (130). Moreover, hypoxia failed to induce CXCL8, VEGF, and MIF secretion by NK cells suggesting that target-specific translational regulation could shape NK cell response to hypoxia. Similar findings were reported by Velasquez and colleagues (131).
Hypoxia also promotes the catalytic conversion of extracellular adenosine triphosphate to adenosine. Stimulation of NK cells through the adenosine A2A receptors suppresses NK cell maturation and impairs anti-tumor immune responses (132). Similarly, in the presence of adenosine, NK cells generate high IFN-γ production reducing NK cell cytotoxicity. Furthermore, hypoxia downregulates NKp30, NKp44, NKp46, NKG2D, perforin, and granzyme B. Treatment with IL-2 has been shown to restore NK cell cytotoxicity by increasing NKG2D in some hematological malignancies (133). Solocinski and colleagues showed that high-affinity NK cells could resist hypoxia through IL-2-mediated prevention of signal transducer and activator of transcription 3 (STAT3) activation, preserving NK cell function (134). On the contrarily, the function of healthy donor NK cells and NK cells from cancer patients were inhibited under hypoxia (135).
Nitric oxide is a critical player in the TME known to cause damage by metabolic reprogramming and promotion of immunosuppressive phenotypes at low concentrations (136). To date, a complete understanding of the mechanisms by which nitric oxide contributes to tumorigenesis is lacking. However, it is known to play a pivotal role in regulating cancer progression in hematological malignancies (137). Dysregulated S-nitrosylation/denitrosylation has been explored as a common mechanism by which NO signaling reprograms metabolism (138).
NK cells utilize glucose to generate ATP and NADPH required for normal function. In a glucose-deprived TME, metabolic competition impairs NK cell glycolytic activity. Amino acid depletion resulting from increased consumption by tumor cells which also synergizes with tumor-associated cells creates a nutrient-depleted microenvironment (139). Other factors are also known to influence NK cell metabolism. Obesity affects metabolic response following cytokine stimulation. Chronic inflammation and infection cause NK cell exhaustion limiting the anti-tumor/infection potential of NK cells. In hematological malignancies, inflammatory cytokines trigger a reshaping of the microenvironment. Further study is required to understand the negative impact of the TME on NK cell metabolism and cancer progression (119).
Targeting metabolic vulnerabilities in AML remains a challenge due to the magnitude of metabolic adaptation in AML cells compared with solid tumors (94, 140). Table 1. Gives a summary of various strategies applicable in clinical practice to overcome immunosuppression in the microenvironment.
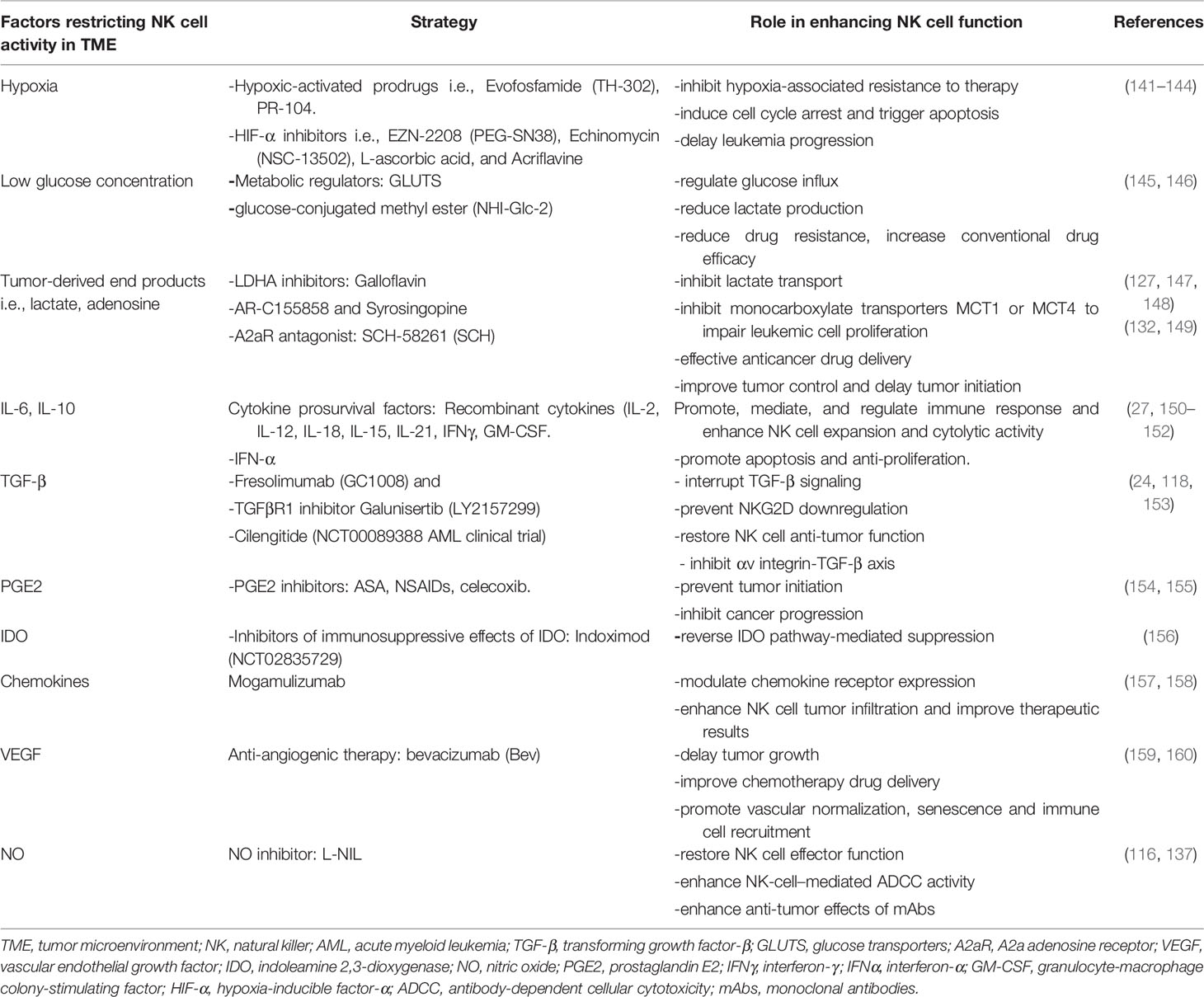
Table 1 Strategies to circumvent immunosuppressive factors in the TME and their role in restoring NK cell function.
Improved Therapeutic NK Cell Immunotherapy
Adoptive Transfer of Autologous and Haploidentical NK Cells
Autologous NK cells were the earliest major focus of adoptive NK cell therapy in cancer patients as a direct approach to restoring and improving immune function at low risk of graft-versus-host disease (GvHD). However, limited anti-tumor activity was reported against hematological and solid tumors, owing to the inhibitory activity of interactions between inhibitory receptors on autologous NK cells and matched self-major histocompatibility complex (MHC) class I presented on tumor cells. Unfavorably, patients were heavily pretreated before collection and in an immunosuppressive state resulting in reduced anti-tumor capability of the derived NK cells. For this reason, allogeneic NK cell therapy came into focus for hematological malignancies (161).
Currently, HLA-matched allogeneic hematopoietic stem cell transplant (HSCT) remains the only curative approach for high-risk AML. Still, the widespread use of HLA haploidentical (half-matched) or partially matched umbilical cord blood (UCB) grafts is ongoing. It has been reported that 25% of patients requiring an allograft have an HLA-identical sibling, while two-thirds of patients have a suitable HLA-matched unrelated donor. For remaining patients lacking an HLA-matched donor, alternative sources are being employed (162–164). Ruggeri et al. suggested that donor-recipient NK cell alloreactivity resulting mainly from KIR ligand incompatibility had decreased relapse rates and graft rejection in AML patients undergoing haploidentical HSCT (165). This concept was termed “ligand-ligand mismatch” (166). Similar studies supported the importance of NK cell alloreactivity in AML patients undergoing HSCT (167–169). Alloreactive NK cells contribute to the graft-versus-leukemia (GvL) effect, which results from the missed interaction of KIRs on donor NK cells and their ligands, HLA class I molecules, on recipient antigen-presenting cells (170). KIR ligand mismatch has a role in the successful engraftment of cord blood and haploidentical transplants (171).
Investigations regarding adoptive NK cell therapy to augment the GvL effect are continual. Miller et al. demonstrated that infusion of haploidentical NK cells after chemotherapy could induce remission in poor-prognosis AML (25). Similarly, Rubnitz et al. investigated the safety of KIR-mismatched NK cell infusion as post-remission consolidation therapy for children with AML. The study reported sustained CR in the 10 patients treated (172). Conclusively, KIR-ligand mismatch correlated positively with NK cell alloreactivity which was protective against leukemia relapse.
Farag et al. however, reported conflicting findings. KIR ligand incompatibility in unrelated donor HCT lacked significant benefit. Unrelated donor HCT showed that HLA mismatch was associated with higher treatment-related mortality, treatment failure, and overall mortality regardless of the presence or absence of KIR ligand mismatch (173). Similar findings report a lack of advantage for KIR ligand incompatibility in clinical setting (174, 175) with inferior outcomes shown with KIR ligand mismatching for hematological malignancies (176). Tanaka et al. evaluated the effect of KIR ligand mismatch on outcomes of AML and ALL patients in CR after single cord blood transplantation. No difference in outcomes of KIR ligand-compatible and incompatible transplantations in acute leukemia patients was reported (177). In an additional study, GvH directed KIR ligand mismatch was associated with graft failure (178). Furthermore, KIR-ligand mismatch seemed to provoke adverse effects in unrelated donor HSCT with reduced overall survival and increased risk for high-grade acute GVHD (170). The heterogeneity of these findings in improving overall survival without increasing GvHD in patients with CR evokes the need for more trials with the application of high-resolution genotyping and phenotyping to measure KIR and HLA of donor and recipient.
Preparatory guidelines for allogeneic NK cell infusion can enhance NK cell expansion, persistence, and efficacy. Non-myeloablative conditioning chemotherapy is administered to deplete endogenous host lymphocyte populations that may compete with the infused NK cells in patients with solid and hematological malignancies (179). Conditioning chemotherapy can potentiate NK cell function in eliminating disease as it serves to directly deplete tumor cells. However, lymphodepletion is associated with increased toxicity including neutropenia. In AML, non-myeloablative therapy administration before infusion of haploidentical NK cells induces CR outside the HSCT setting (25). Following the promising outcomes from the application of NK cells in haploidentical-HSCT, Locatelli and colleagues suggested that their benefit could be extended to curing high-risk acute leukemia and in elderly patients (17).
Miller et al. investigated the safety and in vivo expansion of adoptively transferred haploidentical NK cells in a non-HSCT setting. The study demonstrated that that adoptively transferred NK cells derived from haploidentical related donors could be expanded in vivo. Adequate lymphodepletion with high-dose cyclophosphamide and fludarabine followed by IL-2 led to in vivo NK cell expansion with persistence of donor NK cells for up to 4 weeks without inducing GvHD (25). An inverse correlation between lymphocyte count and IL-15 after lymphodepletion chemotherapy indicated that successful NK cell expansion was secondary to both clearing of alloreactive recipient cells and IL-15 stimulation of NK cells (25, 180, 181).
Recently, Nguyen et al. designed a study to assess the efficacy of adoptive therapy with haploidentical and KIR–HLA-mismatched NK cells for consolidation therapy of pediatric AML. Adoptive transfer of haploidentical and KIR–HLA-mismatched NK cells did not decrease the cumulative incidence of relapse and did not improve event-free or overall survival in children with intermediate-risk AML. However, the study concluded that during earlier phases of treatment or in combination with other immunotherapies, repeated NK cell infusions could reduce tumor burden and induce remission (182). The lack of benefit or long-term remission resulted from insufficient numbers and limited persistence of alloreactive donor NK cells which affected tumor clearance and enabled the re-emergence of leukemic cell clones in patients with relapse (182).
Exploiting Alternative Sources of Allogeneic NK Cells
Umbilical cord blood (UCB) is a rich source of NK cells. NK cells constitute up to 30% of lymphocytes in UCB and 10% of lymphocytes in PB counterparts. Currently, UCB is regarded as an “off-the-shelf,” allogeneic source of NK cells that is readily obtainable and conveniently stored. UCB-derived NK cells are younger with a stronger proliferation potential compared to PB-derived NK cells. UCB-derived NK cells express CD3-CD56+, further classified as the less differentiated CD56bright and mature CD56dim NK cells (183). Although the degranulation function on UCB-derived NK cells is identical to PB-derived NK cells, their cytotoxicity against K562 leukemia cells is weaker than those acquired from PB (184). However, when stimulated by cytokine activity, the cytotoxicity of UCB and PB-acquired NK cells is comparable.
The infusion of non-modified or modified UCB-derived NK cells as maintenance therapy after chemotherapy or combined with HSCT or UCB stem cell transplantation has shown encouraging results (185). A notable limitation with UCB-derived NK cell infusion is shortened in vivo response duration with a gradual decrease in donor NK cells. Developing strategies to improve the persistence of UCB-derived NK cells and maintain relatively high levels of donor NK cells in vivo is imperative (186, 187).
Stem cell-derived NK cells have recently come into the limelight as an unlimited source of NK cells owing to their homogenous and clinically scalable manner. Furthermore, stem cell-derived NK cells can be readily genetically modified on a clonal level, providing a platform to produce uniform and consistent NK cells with enhanced activity (188). Most adoptive NK cell therapies involve the use of primary NK cells isolated from PB, UCB and NK-92 cells, which have donor-dependent variability, blood collection delays, and challenges in genetic engineering, making product standardization and multiple-dosing strategies difficult.
NK-92 cells, though from a single source, lack many conventional NK cell markers and, as a transformed cell, must be mitotically inactivated before infusion to prevent uncontrolled proliferation (189). NK cells produced from human pluripotent stem cells, both human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs), are being used as standardized “off-the-shelf” therapies for any patient (15). Matsubara et al. showed that hPSC-derived NK cells exhibited cytotoxic properties and could suppress tumor growth in vivo without exogenous IL-2 or IL-15 against the leukemic K562 cell line (190). Furthermore, the derived NK cells had similar morphology and surface marker expressions as those of PB NK cells.
CAR NK cells have the benefit of easy feasibility as readily available “off-the-shelf” products allowing for multiple infusions in individuals. NK cells of various origins can be genetically modified using CAR constructs that redirect NK cell specificity against antigens expressed on tumor cells (15). Currently, several CAR-NK cells are being tested in phase I/II clinical trials, mostly targeting CD19 but also other antigens including CD7, CD33, BMCA, and CD22 on hematopoietic malignancies (191). Some CAR-NK cells target metastatic solid tumors expressing tumor-associated antigens like HER2, PSMA, mesothelin, ROBO1, or MUC1 (192). CAR NK cells can be used universally without requiring HLA-matching or prior exposure to tumor-associated antigens. Additionally, they cause little or no GvHD while providing therapeutic effectiveness, enhanced cytotoxicity, innate functionality, and in vivo persistence in hematological malignancies (11, 193).
CAR NK cells derived from both UCB or NK cell lines such as NK-92 cells can be used as “off-the-shelf” products (194). A recent “first-in-man” clinical trial targeting CD33 tested the safety of CAR NK-92 cells for relapsed/refractory AML patients (195). The trial showed no obvious clinical efficacy. Although multiple infusions of CD33 CAR-NK-92 appeared safe following salvage chemotherapy in relapsed AML, it did not infer an anti-leukaemic effect (195, 196). Liu et al. reported the significant benefit of “off-the-shelf” CAR NK cells in relapsed/refractory CD19-positive lymphoma and leukemia supporting its application in future trials (193, 197).
Notwithstanding that CAR-NK cells have multiple advantages, still several difficulties such as a hostile TME, loss of targeted antigen, and tumor heterogeneity, need to be addressed to maximize the efficacy of CAR-NK based AML immunotherapy (192). Recently, Kararoudi and colleagues described an approach for gene targeting in NK cells using Cas9/ribonucleoprotein complexes. Targeted gene insertion into a safe-harbor locus via homologous repair using CRISPR/Cas9 gene editing in combination with adeno-associated virus (AAV)-mediated gene delivery was used to generate primary CD33 CAR-NK cells with confirmation of enhanced anti-AML activity (198).
Human NK cell lines have since long served as attractive sources of adoptive therapy. Eight clonal NK-cell lines have been established over the past 20 years, including NK-92, YTS, NK3.3, and NKL. These commonly used NK cell lines have phenotypic and functional differences (199). Specifically, NK-92 remains the only cell line extensively tested in clinical trials for safety and efficacy assessment. NK-92 expresses a phenotype associated with the CD56bright NK cell subset, while both YTS and NKL appear more CD56dim-like. Recently, Yang et al. reported on a novel NK cell line, NK101, which produced higher levels of pro-inflammatory cytokines, IFN-γ and TNF-α, than NK-92 and demonstrated an agile immunostimulatory potential and substantial scalability (51).
NK-92 cells are easily expanded in vivo using several manufacturing technologies. The safety and efficiency of NK-92 cells with other cell lines such as engineered CAR NK-92 cell lines in both hematologic and solid malignancies has been investigated (200, 201). NK-92 cells are cytotoxic to leukemia cells both in vitro and in vivo (202). Although several phase I clinical trials have shown the safety and tolerability of irradiated NK-92 cells (203), results of their clinical benefit are still unsatisfying (204). The phase I clinical trial (NCT00900809) of adoptive transfer of NK-92 cells in refractory/relapsed AML reported no antitumor response post-infusion (205, 206).
Exploiting NK Cell Memory: Priming and Expansion
Adaptive NK Cell Therapy
Adaptive NK cells are naturally occurring cell populations that expand in humans upon human cytomegalovirus (CMV) infection or reactivation and represent an imminent source of therapeutic NK cells (207). Adaptive NK cell therapy can reduce the incidence of AML relapse through the surface expression of the maturation marker CD5r7 and the activating receptor NKG2C (208). Adaptive NK cells have extended durability, are more resistant to immune suppression than canonical NK cells, and exhibit properties of immune memory. A study by merino et al. reported that the expansion of adaptive NK cells using NKG2C-agonist antibodies resulted in increased killing abilities and enhanced cytokine secretion, making adaptive NK cells ideal candidates for adoptive cell transfer in hematologic malignancies (209).
Recently Sarhan et al. showed that a subset of “adaptive’ CD57+/NKG2C+ NK cells obtained from individuals previously exposed to the human CMV exhibited unique properties of immunological memory, potent mediating of ADCC, and resistance to tumor suppression (169). In patients with refractory AML treated with high-dose cyclophosphamide/fludarabine with “adaptive” iv FATE-NK100 NK cell infusion, in vivo persistence and function activity of NK cells were observed, with patients achieving clearance of refractory AML by day 14 (210). FATE-NK100 is an investigational, first-in-class, novel NK cell-mediated therapy comprised of adaptive memory NK cells. It is a highly specialized, functionally distinct subset of activated NK cells expressing the maturation marker CD57. FATE-NK100 is currently under investigation for use in lymphodepleted patients with advanced AML, refractory ovarian cancer (210, 211), advanced solid tumors.
The extended durability, in vivo persistence, and potency of ‘adaptive” NK cell immunotherapy presents an exciting antileukemia approach. Cichocki et al. demonstrated that human CMV reactivation in HSCT recipients with leukemia was associated with the expansion of adaptive NK cells and correlated with improved post-HSCT (212). In conclusion, the cultivation and expansion of adaptive NK cells ex vivo and in vivo is likely to yield promising results.
Cytokine-Induced Memory-Like NK Cell Therapy
Cytokine-induced memory-like (CIML) NK cells are an alternative for allogeneic NK cell therapy and are known to exhibit antileukemia functionality. Following stimulation with viruses, haptens, or cytokines, NK cells demonstrate innate memory or memory-like responses (213). The use of pre-activation regimens prior to adoptive transfer has been reported to improve NK cell function and enhance antitumor activity. CIML NK cells are generated ex vivo through brief priming with IL-12, IL-15, and IL-18 (214). Other cytokines commonly used to activate NK cells, with or without anti-CD3 stimulation (215), include IL-2 and IL-21 (216). Among these cytokines, IL-2 and IL-15 are drivers of NK cell proliferation and enhance function (217). IL-15 is more selective for NK cell proliferation, while IL-2 is associated with increased NKG2D expression (218, 219). Additionally, IL-18 was shown to stimulate IFN-γ production (220) by NK cells and provide co-stimulatory activation, while IL-21 could enhance NK cell maturation without promoting proliferation (221, 222). When comparing feeder cells employed for expansion and activation, chronic stimulation by K562 cells expressing membrane-bound IL-15 and 4-1BB ligand (223) induced senescence, which was not observed with membrane-bound IL-21 (224).
Human memory-like NK cells have enhanced IFN-γ production and cytotoxicity against leukemia cell lines or primary human AML blasts in vitro (27). CIML NK cells exhibit enhanced responses against AML blasts regardless of inhibitory KIR to KIR-ligand interactions. Romee et al. demonstrated that the long-lasting increase in functional capacity afforded by memory-like NK cell differentiation, combined with improved AML recognition, enhanced in vivo expansion and antileukemia responses (27).
Currently, a phase II clinical trial (NCT04354025) is recruiting patients to investigate the effectiveness of CIML NK cells in combination with FLAG (fludarabine, cytarabine, GCSF) chemotherapy as a treatment for refractory/relapsed AML (225). In an MHC-compatible setting, memory-like NK cells could persist longer than 2-3 weeks, demonstrating a durability and high functionality profile when stimulated with tumor targets. The ability of memory-like NK cells to overcome persistence barriers in an MHC-compatible setting presents a novel platform for NK therapies for leukemia (213).
Figure 2 illustrates novel and improved therapeutic NK cell therapies explored for the treatment of AML and their specificity in AML.
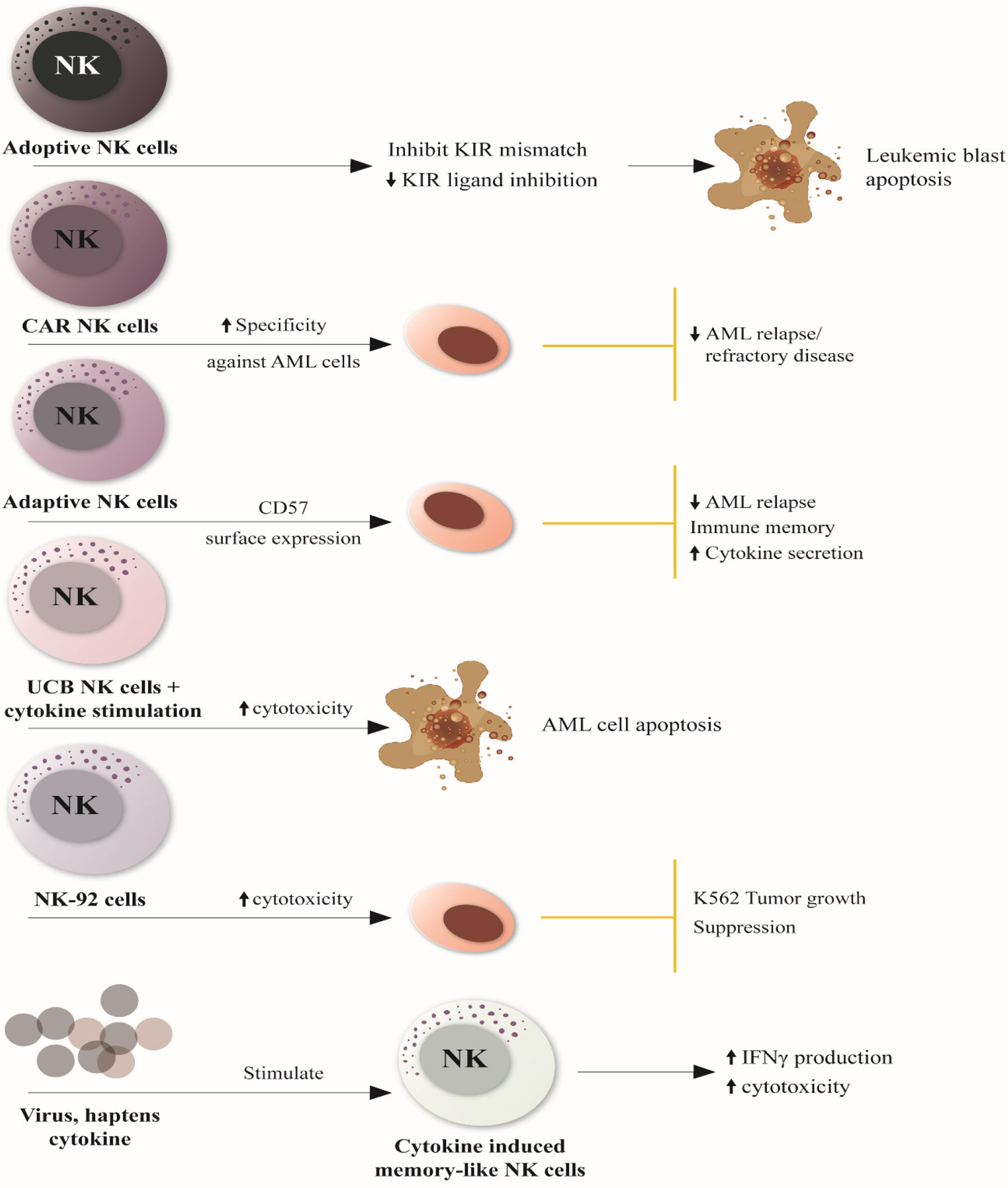
Figure 2 Novel and Improved NK cell-based therapies for AML treatment. 1. Adoptive NK cells can induce leukemic blast apoptosis by inhibiting KIR mismatch and downmodulating KIR ligand inhibitor. 2. CAR NK cells can reduce AML relapse and refractory disease by increasing NK cell specificity against antigens expressed on tumor cells 3. Adaptive NK cells restore immune memory and reduce the relapse of AML through the surface expression of the maturation marker CD57 and the activating receptor NKG2C, resulting in enhanced cytokine secretion. 4. UCB NK cells have increased cytotoxicity against AML cells when stimulated by cytokine activity, promoting cancer cell apoptosis. 5. Irradicated NK-92 cells (NK cell lines) induce tumor growth suppression through increased cytotoxic activity. 6. Various viruses, haptens and cytokines can stimulate innate memory or memory-like responses ‘cytokine-induced memory-like NK cells,” enhancing interferon-γ production and cytotoxicity against leukemia cell lines or primary human AML blasts in vitro. NK, natural killer; KIR, killer Ig-like receptor; CAR, chimeric antigen receptor; CD57, cluster of differentiation 57; UCB-NK cell, umbilical cord blood-Natural killer cells; IFN-γ, interferon-gamma.
Enhancing NK Cell Cytotoxicity Against Myeloid Leukemia
Immune Checkpoint Inhibition
NK cells have emerged as contributors to the effect of cytotoxic T lymphocyte-associated protein 4 (CTLA4), LAG3, and PD-1 in cancer patients, suggesting that immune checkpoint receptors regulate NK cell activity under pathological conditions (226). The recently identified B7 homolog 3 protein (B7H3) appears to inhibit both T- and NK-cell functions. However, more investigation regarding B7H3, particularly the discovery of its receptors on NK and T cells is needed (227, 228). TIGIT (T cell immunoreceptor with immunoglobulin and ITIM domains), CD96 (TACTILE), and (T-cell immunoglobulin and mucin domain 3) TIM-3, are immune inhibitory receptors that share regulatory functions in both NK and T cells, behaving as immune checkpoint receptors in both cell types (229).
Sialic acid-binding immunoglobulin-like lectins (Siglecs) are immunomodulatory sialic acid-binding receptors expressed on a variety of immune cells including NK cells (230). The most recent members of the inhibitory Siglecs family, Siglecs 7/9, are reported to be expressed on human NK cells. Blocking of Siglec-7/9 increases NK cell cytotoxicity (231). Another inhibitory receptor, CD200R, is expressed on T, B, NK, and myeloid cells (63). Blockade of CD200–CD200R interaction inhibits tumor growth (232). Lastly, CD47 plays an inhibitory role in NK cell-mediated anti-viral or anti-tumor cytotoxicity (233).
Immune checkpoint blockade (ICB) therapies such as anti-PD-1 and anti-CTLA-4 present a rational approach to target checkpoint molecules on NK cells and/or macrophages (234). FDA-approved ICB drugs include the anti-CTLA-4 (ipilimumab), anti-PD-1 (pembrolizumab, nivolumab, and cemiplimab), and anti-PD-L1 (atezolizumab, avelumab, and durvalumab). Antibodies block PD-1, CTLA-4 (235), TIM3, LAG3, and TIGIT counter receptors which could enhance T and NK cell functionality against cancer. In myeloid malignancies, early trial results failed to show the clinical benefit of anti-PD-1 as monotherapy in AML (236) or high-risk MDS (237) and CTLA-4 blockade using ipilimumab in high-risk MDS (238). However, ipilimumab treatment after allogeneic-HSCT showed good responses in 22 patients with various hematological malignancies including 12 AML patients. CR with durability was reported even in refractory disease, including extramedullary AML (239).
Currently, the checkpoint inhibitors, anti-TIM3 (i.e., NCT03489343), anti-LAG3 (i.e., NCT03005782), and anti-TIGIT (e.g., NCT04354246) are under investigation in phase I/II clinical trials. Adverse effects from the use of immune checkpoint inhibitors have been reported. Anti-CTLA-4 agents were associated with relatively high occurrences of colitis and hypophysitis (240). Recombinant cytokine can enhance the efficacy of mAbs. Seo et al. showed that a combination of IL-21 and checkpoint blockade facilitated the effector function of exhausted NK cells in advanced cancer patients with NK-92 class I deficient tumors (241).
NK Cell Antitumor and Effector Functions
Activating and Inhibitory Receptors
Activating and inhibitory surface receptors carry out NK cell functions through a net balance of stimulatory versus suppressive signals resulting in either a response to or tolerance of the target cells (242). In a phenomenon known as “self-recognition,” inhibitory receptors detect MHC-I ligands on normal cells, and if present, activating signals are terminated. Upon viral infection or transformation, target cells upregulate stimulatory ligands for activating NK cell receptors such as NKG2D. This interaction induces a level of activating signaling that overwhelms inhibitory signaling through inhibitory receptors such as KIRs and NKG2A, resulting in NK cell cytokine release and cytotoxicity (243). In tumor cells, downregulation of MHC-I ligands of inhibitory receptors results in the loss of inhibitory signaling and in a ”missing-self” NK cell activation.
Downregulation of MHC-I and key proteins of the antigen processing and presentation machinery (APM) is a key mechanism of cancer immune evasion (244). Cancer cells can reduce the HLA class I expression under the pressure of T cell surveillance (i.e., to escape T cell response). However, lower HLA-I expression results in reduced engagement of inhibitory KIRs and NKG2A, and consequent increase of tumor cell susceptibility to NK cell activity. In some microenvironmental conditions i.e., in the presence of IFN-γ, tumor cells may recover the original HLA class I expression, increasing their resistance to NK cells (245). In particular, the expression of non-classical HLA-E and HLA-G may play an additional role (246).
Anti-KIR mAbs evaluated in multiple indications, including AML have demonstrated favorable safety profiles (247). A study demonstrated that anti-inhibitory KIR mAb (IPH2101) could enhance antitumor effects of NK cells by blocking the major inhibitory HLA-C-specific KIR (248). In the Effikir trial, Vey et al. reported that Lirilumab as monotherapy for maintenance therapy of elderly patients with AML in first CR did not improve leukemia-free survival (249). However, in combination with anti-CD20 mAbs, anti-KIR enhanced NK cell-mediated, rituximab-dependent cytotoxicity illustrating the potential efficacy of combining a tumor-targeted therapy with an NK-cell agonist (250). Monalizumab, a humanized anti-NKG2A blocking mAb has been shown to increase degranulation and IFN-γ production by unleashing inhibition of NKG2A-expressing cells and promoting T and NK cell effector functions (251, 252).
NK Cell-Mediated ADCC
ADCC is a key mechanism of NK cell-mediated antitumor activity. The activating receptor, CD16 or FcγRIII, mainly expressed by the CD56dim NK-cell subset, is necessary for ADCC against IgG coated target cells (227). The tumor-targeting antibody drugs, rituximab, elotuzumab, and cetuximab, exert anti-tumor activity by relying on ADCC as the key mode of action to deplete tumor cells (253). Obinutuzumab, was shown to induce stronger ADCC and direct target cell death in vitro. In patients with chronic lymphocytic leukemia, obinutuzumab combined with chemotherapy prolonged progression-free survival (254).
NK-Cell-Derived Extracellular Vesicles (NKEVs)
NKEVs have been studied for application in cancer therapies and in determining actual NK cell status in patients (255). A recent study demonstrated that NKEVs express MCH I & II suggesting their potential role in MCH class I self-recognition and antigen presentation. Additionally, NKEVs express multiple cytotoxic proteins, such as granulysin, perforin, FasL, and granzyme A and B, as well as molecules that promote NK cell-mediated cytolysis (256). NKEVs also possess regulatory functions and trigger multiple killing mechanisms (257). Cytotoxic effects of NKEVs on tumor cells, including hematological malignancies, were demonstrated in ALL. NKEV treatment elicited endoplasmic reticulum stress that mediated cell death (257).
NK Cell Engagers
Bispecific or trispecific killer engagers (BiKE, TriKE) are multi-specific antibodies that directly bind NK cells and target cells, triggering NK cell activation and cell-mediated ADCC. The effectiveness of NK cell infusions against leukemic cells is limited by a lack of antigen specificity and in vivo expansion. BiKEs and TriKEs create an immunologic synapse or connection between NK cells and myeloid cells (258). In primary AML with impaired NK cell function, TriKE (CD16-IL15-CD33) restored NK cell function and induced NK cell proliferation in vitro. Furthermore, 16-15-33 TriKE exhibited superior anti-tumor activity and induced in vivo persistence and survival of NK cells (259). In a recent cohort study, Warlick et al. demonstrated that continuous infusion with GTB-3550 TriKE enhanced NK cell proliferation in relapsed AML or high-risk MDS patients and could induce remission. Although no objective responses were reported at initial dose levels, GTB-3550 drove immune activity in humans (260).
Nanoparticles in Augmenting NK Cell Therapy
Currently, clinical outcomes of NK cell immunotherapy remain unsatisfying owing to an immunosuppressive TME and low NK cell activity. Novel innovations to enhance NK cell function using nanotechnology and nanomedicine are being explored (15, 32, 261). The multifunctionality of nanomaterials and their compatibility with NK cells has shown potential in promoting ex vivo expansion of NK cells and NK cell activation. TGF-β inhibition with liposome and nano-emulsion mediated conversion of the immunosuppressive TME (262). Nanoparticles encapsulating IFN-γ were used to improve NK cell homing and infiltration (263). Nano-engagers and nanoparticles have also been applied to improve NK cell activation (264). Ortega-Sanz and colleagues supported the use of magnetic nanoparticles with external magnetic fields to promote the accumulation of cytolytic NK cells in vivo (265). Given current evidence, engineered nanoparticles could enhance NK cell activity, proliferation and migration to tumor sites.
Conclusion
NK cells have widened the treatment paradigm of AML. Despite advancements in chemotherapy, the occurrence of refractory/relapsed disease remains prevalent. Recently, NK cells demonstrated potent anti-AML effects without eliciting detrimental adverse effects, such as GvHD, neurotoxicity, and cytokine release syndrome. However, the effector functions of NK cells are suppressed by the presence of an immunosuppressive microenvironment. The complexity of the TME associated with its heterogeneity and immunosuppressive properties is a major obstacle in achieving optimal anti-tumor activity of NK cells. To achieve desired results from NK cell therapy, it is imperative to develop strategies that enhance NK cell cytotoxicity and improve target cell recognition.
Various strategies including adoptive transfer of cytokine-induced therapies, CAR-modified NK cells, mAbs, targeted nanoparticles and therapies targeting activating and inhibitory receptors in the TME might restore NK cell function and enhance their antitumor activity in AML patients. Overcoming immunosuppressive effects of the TME precipitated by various molecules and factors, through novel strategies that neutralize or block suppressive cytokines and chemokines secreted by tumor cells and inhibit the activity of immunosuppressive and stromal cells will improve future clinical outcomes.
Author Contributions
NK performed literature research and review and wrote the final manuscript. FZ conceived and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was funded by the National Natural Science Foundation of China [grant numbers: 81270597 and 81770179] for leukemia research.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the continued support of the department of hematology at Zhongnan Hospital, Wuhan University.
Abbreviations
ADCC, antibody-dependent cellular cytotoxicity; AML, acute myeloid leukemia; BiKE, bispecific killer engager; PB, peripheral blood; CAR, chimeric antigen receptor; CR, complete remission(s); CML, chronic myeloid leukemia; DCs, dendritic cells; GvHD, graft-versus-host-disease; HLA, human leucocyte antigen; HSCT, hematopoietic stem cell transplant; IL-10, interleukin-10; IFNγ, interferon-gamma; IDO, indoleamine 2,3 dioxygenase; KIRs, killer Ig-like receptors; mAbs, monoclonal antibodies; MDSCs, myeloid-derived suppressor cells; NK, natural killer; NKEVs, NK-cell-derived extracellular vesicles; NKG2DL, natural killer group 2D ligands; PGE2, prostaglandin 2; TAMs, tumor-associated macrophages; TGF-β, transforming growth factor; TME, tumor microenvironment; Tregs, regulatory T cells; TriKE, trispecific killer engager; TKI, tyrosine kinase inhibitors; UCB, umbilical cord blood.
References
1. Aziz H, Ping CY, Alias H, Ab Mutalib N-S, Jamal R. Gene Mutations as Emerging Biomarkers and Therapeutic Targets for Relapsed Acute Myeloid Leukemia. Front Pharmacol (2017) 8:897. doi: 10.3389/fphar.2017.00897
2. Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and Management of Acute Myeloid Leukemia in Adults: Recommendations From an International Expert Panel, on Behalf of the European Leukemianet. Blood (2010) 115(3):453–74. doi: 10.1182/blood-2009-07-235358
3. Baragaño Raneros A, López-Larrea C, Suárez-Álvarez B. Acute Myeloid Leukemia and NK Cells: Two Warriors Confront Each Other. OncoImmunology (2019) 8(2):e1539617. doi: 10.1080/2162402X.2018.1539617
4. Belizário JE, Neyra JM, Setúbal Destro Rodrigues MF. When and How NK Cell-Induced Programmed Cell Death Benefits Immunological Protection Against Intracellular Pathogen Infection. Innate Immun (2018) 24(8):452–65. doi: 10.1177/1753425918800200
5. Lee H-R, Baek K-H. Role of Natural Killer Cells for Immunotherapy in Chronic Myeloid Leukemia (Review). Oncol Rep (2019) 41(5):2625–35. doi: 10.3892/or.2019.7059
6. Hochhaus A, Druker B, Sawyers C, Guilhot F, Schiffer CA, Cortes J, et al. Favorable Long-Term Follow-Up Results Over 6 Years for Response, Survival, and Safety With Imatinib Mesylate Therapy in Chronic-Phase Chronic Myeloid Leukemia After Failure of Interferon-α Treatment. Blood (2008) 111(3):1039–43. doi: 10.1182/blood-2007-07-103523
7. Chen CI-U, Koschmieder S, Kerstiens L, Schemionek M, Altvater B, Pscherer S, et al. NK Cells are Dysfunctional in Human Chronic Myelogenous Leukemia Before and on Imatinib Treatment and in BCR–ABL-positive Mice. Leukemia (2012) 26(3):465–74. doi: 10.1038/leu.2011.239
8. Ilander M, Olsson-Strömberg U, Schlums H, Guilhot J, Brück O, Lähteenmäki H, et al. Increased Proportion of Mature NK Cells Is Associated With Successful Imatinib Discontinuation in Chronic Myeloid Leukemia. Leukemia (2017) 31(5):1108–16. doi: 10.1038/leu.2016.360
9. Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, et al. Persistence of Malignant Hematopoietic Progenitors in Chronic Myelogenous Leukemia Patients in Complete Cytogenetic Remission Following Imatinib Mesylate Treatment. Blood (2003) 101(12):4701–7. doi: 10.1182/blood-2002-09-2780
10. Mahon F-X, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of Imatinib in Patients With Chronic Myeloid Leukaemia Who Have Maintained Complete Molecular Remission for at Least 2 Years: The Prospective, Multicentre Stop Imatinib (STIM) Trial. Lancet Oncol (2010) 11(11):1029–35. doi: 10.1016/S1470-2045(10)70233-3
11. Sivori S, Meazza R, Quintarelli C, Carlomagno S, Della Chiesa M, Falco M, et al. Nk Cell-Based Immunotherapy for Hematological Malignancies. J Clin Med (2019) 8(10):1702. doi: 10.3390/jcm8101702
12. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of Natural Killer Cells. Nat Immunol (2008) 9(5):503–10. doi: 10.1038/ni1582
13. Cong J. Metabolism of Natural Killer Cells and Other Innate Lymphoid Cells. Front Immunol (2020) 11:1989. doi: 10.3389/fimmu.2020.01989
14. Elliott JM, Yokoyama WM. Unifying Concepts of MHC-Dependent Natural Killer Cell Education. Trends Immunol (2011) 32(8):364–72. doi: 10.1016/j.it.2011.06.001
15. Liu S, Galat V, Galat4 Y, Lee YKA, Wainwright D, Wu J. NK Cell-Based Cancer Immunotherapy: From Basic Biology to Clinical Development. J Hematol Oncol (2021) 14(1):7. doi: 10.1186/s13045-020-01014-w
16. Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, et al. Anti-Leukemia Activity of Alloreactive NK Cells in KIR Ligand-Mismatched Haploidentical HSCT for Pediatric Patients: Evaluation of the Functional Role of Activating KIR and Redefinition of Inhibitory KIR Specificity. Blood (2009) 113(13):3119–29. doi: 10.1182/blood-2008-06-164103
17. Locatelli F, Pende D, Falco M, Della Chiesa M, Moretta A, Moretta L. Nk Cells Mediate a Crucial Graft-Versus-Leukemia Effect in Haploidentical-HSCT to Cure High-Risk Acute Leukemia. Trends Immunol (2018) 39(7):577–90. doi: 10.1016/j.it.2018.04.009
18. Locatelli F, Merli P, Pagliara D, Li Pira G, Falco M, Pende D, et al. Outcome of Children With Acute Leukemia Given HLA-Haploidentical HSCT After αβ T-cell and B-cell Depletion. Blood (2017) 130(5):677–85. doi: 10.1182/blood-2017-04-779769
19. Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, et al. Safety and Efficacy of Imatinib Cessation for CML Patients With Stable Undetectable Minimal Residual Disease: Results From the TWISTER Study. Blood (2013) 122(4):515–22. doi: 10.1182/blood-2013-02-483750
20. Imagawa J, Tanaka H, Okada M, Nakamae H, Hino M, Murai K, et al. Discontinuation of Dasatinib in Patients With Chronic Myeloid Leukaemia Who Have Maintained Deep Molecular Response for Longer Than 1 Year (DADI Trial): A Multicentre Phase 2 Trial. Lancet Haematol (2015) 2(12):e528–35. doi: 10.1016/S2352-3026(15)00196-9
21. Rea D, Henry G, Khaznadar Z, Etienne G, Guilhot F, Nicolini F, et al. Natural Killer-Cell Counts are Associated With Molecular Relapse-Free Survival After Imatinib Discontinuation in Chronic Myeloid Leukemia: The IMMUNOSTIM Study. Haematologica (2017) 102(8):1368–77. doi: 10.3324/haematol.2017.165001
22. Myers JA, Miller JS. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat Rev Clin Oncol (2021) 18(2):85–100. doi: 10.1038/s41571-020-0426-7
23. Lanier LL. Natural Killer Cells. In: Ratcliffe MJH, editor. Encyclopedia of Immunobiology. Oxford: Academic Press (2016), ISBN: eBook ISBN: 9780080921525. p. 353–6.
24. Zhang C, Hu Y, Shi C. Targeting Natural Killer Cells for Tumor Immunotherapy. Front Immunol (2020) 11:60. doi: 10.3389/fimmu.2020.00060
25. Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful Adoptive Transfer and In Vivo Expansion of Human Haploidentical NK Cells in Patients With Cancer. Blood (2005) 105(8):3051–7. doi: 10.1182/blood-2004-07-2974
26. Romee R, Leong JW, Fehniger TA. Utilizing Cytokines to Function-Enable Human NK Cells for the Immunotherapy of Cancer. Science (Cairo) (2014) 2014:205796. doi: 10.1155/2014/205796
27. Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-Induced Memory-Like Natural Killer Cells Exhibit Enhanced Responses Against Myeloid Leukemia. Sci Transl Med (2016) 8(357):357ra123–357ra123. doi: 10.1126/scitranslmed.aaf2341
28. Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained Effector Function of IL-12/15/18–Preactivated NK Cells Against Established Tumors. J Exp Med (2012) 209(13):2351–65. doi: 10.1084/jem.20120944
29. Cany J, van der Waart AB, Tordoir M, Franssen GM, Hangalapura BN, de Vries J, et al. Natural Killer Cells Generated From Cord Blood Hematopoietic Progenitor Cells Efficiently Target Bone Marrow-Residing Human Leukemia Cells in NOD/SCID/IL2Rg(null) Mice. PLoS One (2013) 8(6):e64384. doi: 10.1371/journal.pone.0064384
30. Hasmim M, Khalife N, Zhang Y, Doldur M, Visentin G, Terry S, et al. Expression of CD94 by Ex Vivo-Differentiated NK Cells Correlates With the In Vitro and In Vivo Acquisition of Cytotoxic Features. Oncoimmunology (2017) 6(10):e1346763. doi: 10.1080/2162402X.2017.1346763
31. Handgretinger R, Lang P, André MC. Exploitation of Natural Killer Cells for the Treatment of Acute Leukemia. Blood (2016) 127(26):3341–9. doi: 10.1182/blood-2015-12-629055
32. Kim K-S, Kim D-H, Kim D-H. Recent Advances to Augment Nk Cell Cancer Immunotherapy Using Nanoparticles. Pharmaceutics (2021) 13(4):525. doi: 10.3390/pharmaceutics13040525
33. Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of Natural Killer Cell Alloreactivity in HLA-Mismatched Hematopoietic Stem Cell Transplantation. Blood (1999) 94(1):333–9. doi: 10.1182/blood.V94.1.333.413a31_333_339
34. Lion E, Willemen Y, Berneman Z. Natural Killer Cell Immune Escape in Acute Myeloid Leukemia. Leukemia (2012) 26: (9):2019–26. doi: 10.1038/leu.2012.87
35. Carlsten M, Järås M. Natural Killer Cells in Myeloid Malignancies: Immune Surveillance, NK Cell Dysfunction, and Pharmacological Opportunities to Bolster the Endogenous Nk Cells. Front Immunol (2019) 10:2357. doi: 10.3389/fimmu.2019.02357
36. Hughes A, Clarson J, Tang C, Vidovic L, White DL, Hughes TP, et al. CML Patients With Deep Molecular Responses to TKI Have Restored Immune Effectors and Decreased PD-1 and Immune Suppressors. Blood (2017) 129(9):1166–76. doi: 10.1182/blood-2016-10-745992
37. Khaznadar Z, Boissel N, Agaugué S, Henry G, Cheok M, Vignon M, et al. Defective NK Cells in Acute Myeloid Leukemia Patients at Diagnosis are Associated With Blast Transcriptional Signatures of Immune Evasion. J Immunol (2015) 195(6):2580–90. doi: 10.4049/jimmunol.1500262
38. Alcasid M, Ma L, Gotlib JR, Arber DA, Ohgami RS. The Clinicopathologic Significance of Lymphocyte Subsets in Acute Myeloid Leukemia. Int Jnl Lab Hem (2017) 39(2):129–36. doi: 10.1111/ijlh.12594
39. Baragaño Raneros A, Martín-Palanco V, Fernandez AF, Rodriguez RM, Fraga MF, Lopez-Larrea C, et al. Methylation of NKG2D Ligands Contributes to Immune System Evasion in Acute Myeloid Leukemia. Genes Immun (2015) 16(1):71–82. doi: 10.1038/gene.2014.58
40. Xu J, Niu T. Natural Killer Cell-Based Immunotherapy for Acute Myeloid Leukemia. J Hematol Oncol (2020) 13(1):167. doi: 10.1186/s13045-020-00996-x
41. D S-B, Mc M-L, Ea V-S, A G-H, L-S R, La M-C, et al. Overexpression of CD158 and NKG2A Inhibitory Receptors and Underexpression of NKG2D and NKp46 Activating Receptors on NK Cells in Acute Myeloid Leukemia. Arch Med Res (2016) 47(1):55–64. doi: 10.1016/j.arcmed.2016.02.001
42. Lazarova M, Steinle A. The NKG2D Axis: An Emerging Target in Cancer Immunotherapy. Expert Opin Ther Targets (2019) 23(4):281–94. doi: 10.1080/14728222.2019.1580693
43. Kearney CJ, Ramsbottom KM, Voskoboinik I, Darcy PK, Oliaro J. Loss of DNAM-1 Ligand Expression by Acute Myeloid Leukemia Cells Renders Them Resistant to NK Cell Killing. Oncoimmunology (2016) 5(8):e1196308. doi: 10.1080/2162402X.2016.1196308
44. Zhang W, Xie X, Mi H, Sun J, Ding S, Li L, et al. Abnormal Populations and Functions of Natural Killer Cells in Patients With Myelodysplastic Syndromes. Oncol Lett (2018) 15(4):5497–504. doi: 10.3892/ol.2018.8062
45. Damele L, Ottonello S, Mingari MC, Pietra G, Vitale C. Targeted Therapies: Friends or Foes for Patient’s NK Cell-Mediated Tumor Immune-Surveillance? Cancers (2020) 12(4):774. doi: 10.3390/cancers12040774
46. Cooper MA, Fehniger TA, Caligiuri MA. The Biology of Human Natural Killer-Cell Subsets. Trends Immunol (2001) 22(11):633–40. doi: 10.1016/S1471-4906(01)02060-9
47. Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The Relationship of CD16 (Leu-11) and Leu-19 (Nkh-1) Antigen Expression on Human Peripheral Blood NK Cells and Cytotoxic T Lymphocytes. J Immunol (1986) 136(12):4480–6.
48. Romagnani C, Juelke K, Falco M, Morandi B, D’Agostino A, Costa R, et al. Cd56brightCD16- Killer Ig-Like Receptor- NK Cells Display Longer Telomeres and Acquire Features of CD56dim NK Cells Upon Activation. J Immunol (2007) 178(8):4947–55. doi: 10.4049/jimmunol.178.8.4947
49. Chan A, Hong D-L, Atzberger A, Kollnberger S, Filer AD, Buckley CD, et al. CD56bright Human NK Cells Differentiate Into CD56dim Cells: Role of Contact With Peripheral Fibroblasts. J Immunol (2007) 179(1):89–94. doi: 10.4049/jimmunol.179.1.89
50. Takahashi E, Kuranaga N, Satoh K, Habu Y, Shinomiya N, Asano T, et al. Induction of CD16+ Cd56bright NK Cells With Antitumour Cytotoxicity Not Only From CD16- Cd56bright NK Cells But Also From CD16- Cd56dim NK Cells. Scand J Immunol (2007) 65(2):126–38. doi: 10.1111/j.1365-3083.2006.01883.x
51. Yang HG, Kang MC, Kim TY, Hwang I, Jin HT, Sung YC, et al. Discovery of a Novel Natural Killer Cell Line With Distinct Immunostimulatory and Proliferative Potential as an Alternative Platform for Cancer Immunotherapy. J Immunother Cancer (2019) 7(1):138. doi: 10.1186/s40425-019-0612-2
52. Del Zotto G, Marcenaro E, Vacca P, Sivori S, Pende D, Della Chiesa M, et al. Markers and Function of Human NK Cells in Normal and Pathological Conditions: Markers and Function of Human Nk Cells. Cytometry (2017) 92(2):100–14. doi: 10.1002/cyto.b.21508
53. De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting Human Natural Killer Cell Subset Function Revealed Cytolytic CD56(dim)CD16+ NK Cells as Rapid Producers of Abundant IFN-gamma on Activation. Proc Natl Acad Sci U S A (2011) 108(2):728–32. doi: 10.1073/pnas.1012356108
54. Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc Receptors Modulate In Vivo Cytotoxicity Against Tumor Targets. Nat Med (2000) 6(4):443–6. doi: 10.1038/74704
55. Richards JO, Chang X, Blaser BW, Caligiuri MA, Zheng P, Liu Y. Tumor Growth Impedes Natural-Killer-Cell Maturation in the Bone Marrow. Blood (2006) 108(1):246–52. doi: 10.1182/blood-2005-11-4535
56. Bi J, Wang X. Molecular Regulation of NK Cell Maturation. Front Immunol (2020) 11:1945. doi: 10.3389/fimmu.2020.01945
57. Mundy-Bosse BL, Scoville SD, Chen L, McConnell K, Mao HC, Ahmed EH, et al. MicroRNA-29b Mediates Altered Innate Immune Development in Acute Leukemia. J Clin Invest (2016) 126(12):4404–16. doi: 10.1172/JCI85413
58. G Sm, C J, R Lj, W J, M S, S Jc, et al. The Transcription Factors T-bet and Eomes Control Key Checkpoints of Natural Killer Cell Maturation. Immunity (2012) 36(1):55–67. doi: 10.1016/j.immuni.2011.11.016
59. Chretien A-S, Granjeaud S, Gondois-Rey F, Harbi S, Orlanducci F, Blaise D, et al. Increased NK Cell Maturation in Patients With Acute Myeloid Leukemia. Front Immunol (2015) 6:564. doi: 10.3389/fimmu.2015.00564
60. Chretien A-S, Fauriat C, Orlanducci F, Galseran C, Rey J, Bouvier Borg G, et al. Natural Killer Defective Maturation is Associated With Adverse Clinical Outcome in Patients With Acute Myeloid Leukemia. Front Immunol (2017) 8:573. doi: 10.3389/fimmu.2017.00573
61. Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the Checkpoint Receptor TIGIT Prevents NK Cell Exhaustion and Elicits Potent Anti-Tumor Immunity. Nat Immunol (2018) 19(7):723–32. doi: 10.1038/s41590-018-0132-0
62. Wang M, Bu J, Zhou M, Sido J, Lin Y, Liu G, et al. Cd8 + T Cells Expressing Both PD-1 and TIGIT But Not CD226 Are Dysfunctional in Acute Myeloid Leukemia (AML) Patients. Clin Immunol (2018) 190:64–73. doi: 10.1016/j.clim.2017.08.021
63. Coles SJ, Wang ECY, Man S, Hills RK, Burnett AK, Tonks A, et al. CD200 Expression Suppresses Natural Killer Cell Function and Directly Inhibits Patient Anti-Tumor Response in Acute Myeloid Leukemia. Leukemia (2011) 25(5):792–9. doi: 10.1038/leu.2011.1
64. Ho JM, Dobson SM, Voisin V, McLeod J, Kennedy JA, Mitchell A, et al. CD200 Expression Marks Leukemia Stem Cells in Human AML. Blood Adv (2020) 4(21):5402–13. doi: 10.1182/bloodadvances.2020001802
65. Coles SJ, Hills RK, Wang ECY, Burnett AK, Man S, Darley RL, et al. Expression of CD200 on AML Blasts Directly Suppresses Memory T-cell Function. Leukemia (2012) 26(9):2148–51. doi: 10.1038/leu.2012.77
66. Mahadevan D, Lanasa MC, Farber C, Pandey M, Whelden M, Faas SJ, et al. Phase I Study of Samalizumab in Chronic Lymphocytic Leukemia and Multiple Myeloma: Blockade of the Immune Checkpoint CD200. J Immunother Cancer (2019) 7(1):227. doi: 10.1186/s40425-019-0710-1
67. Herbrich S, Baran N, Alatrash G, Davis E, Zha D, Konopleva M. Overexpression of CD200 is a Stem Cell-Specific Mechanism of Immune Escape in AML. Blood (2020) 136(Supplement 1):14–5. doi: 10.1182/blood-2020-139434
68. Krause DS, Scadden DT. A Hostel for the Hostile: The Bone Marrow Niche in Hematologic Neoplasms. Haematologica (2015) 100: (11):1376–87. 1. doi: 10.3324/haematol.2014.113852
69. Ladikou EE, Sivaloganathan H, Pepper A, Chevassut T. Acute Myeloid Leukaemia in Its Niche: The Bone Marrow Microenvironment in Acute Myeloid Leukaemia. Curr Oncol Rep (2020) 22(3):27. doi: 10.1007/s11912-020-0885-0
70. Vasold J, Wagner M, Drolle H, Deniffel C, Kütt A, Oostendorp R, et al. The Bone Marrow Microenvironment Is a Critical Player in the NK Cell Response Against Acute Myeloid Leukaemia In Vitro. Leuk Res (2015) 39(2):257–62. doi: 10.1016/j.leukres.2014.12.001
71. Brenner AK, Reikvam H, Bruserud Ø. A Subset of Patients With Acute Myeloid Leukemia has Leukemia Cells Characterized by Chemokine Responsiveness and Altered Expression of Transcriptional as Well as Angiogenic Regulators. Front Immunol (2016) 7:205. doi: 10.3389/fimmu.2016.00205
72. Bruserud O, Ryningen A, Olsnes AM, Stordrange L, Oyan AM, Kalland KH, et al. Subclassification of Patients With Acute Myelogenous Leukemia Based on Chemokine Responsiveness and Constitutive Chemokine Release by Their Leukemic Cells. Haematologica (2007) 92(3):332–41. doi: 10.3324/haematol.10148
73. Shafat MS, Gnaneswaran B, Bowles KM, Rushworth SA. The Bone Marrow Microenvironment – Home of the Leukemic Blasts. Blood Rev (2017) 31(5):277–86. doi: 10.1016/j.blre.2017.03.004
74. Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the Hematopoietic Stem Cell Pool by CXCL12-CXCR4 Chemokine Signaling in Bone Marrow Stromal Cell Niches. Immunity (2006) 25(6):977–88. doi: 10.1016/j.immuni.2006.10.016
75. Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 Production by Bone Marrow Osteoblasts Is a Common and Critical Pathway for Cytokine-Induced Mobilization. Blood (2009) 114(7):1331–9. doi: 10.1182/blood-2008-10-184754
76. Mosteo L, Storer J, Batta K, Searle EJ, Duarte D, Wiseman DH. The Dynamic Interface Between the Bone Marrow Vascular Niche and Hematopoietic Stem Cells in Myeloid Malignancy. Front Cell Dev Biol (2021) 9:635189. doi: 10.3389/fcell.2021.635189
77. Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic Cells Create Bone Marrow Niches That Disrupt the Behavior of Normal Hematopoietic Progenitor Cells. Science (2008) 322(5909):1861–5. doi: 10.1126/science.1164390
78. Duan Z, Foster R, Bell DA, Mahoney J, Wolak K, Vaidya A, et al. Signal Transducers and Activators of Transcription 3 Pathway Activation in Drug-Resistant Ovarian Cancer. Clin Cancer Res (2006) 12(17):5055–63. doi: 10.1158/1078-0432.CCR-06-0861
79. Riether C, Schürch CM, Ochsenbein AF. Regulation of Hematopoietic and Leukemic Stem Cells by the Immune System. Cell Death Differ (2015) 22(2):187–98. doi: 10.1038/cdd.2014.89
80. Bierie B, Moses HL. Tgfβ: The Molecular Jekyll and Hyde of Cancer. Nat Rev Cancer (2006) 6(7):506–20. doi: 10.1038/nrc1926
81. Crespo LA, Zhang X, Tao J. Targeting the TumorMicroenvironment for Enhancing Chemotherapy in Hematologicmalignancies. In: Minev BR, editor. Cancer Management in Man: Chemotherapy, Biological Therapy, Hyperthermia and Supporting Measures. Dordrecht: Springer Netherlands (2011). p. 215–33. (Cancer Growth and Progression). doi: 10.1007/978-90-481-9704-0_11
82. Stucki A, Rivier A-S, Gikic M, Monai N, Schapira M, Spertini O. Endothelial Cell Activation by Myeloblasts: Molecular Mechanisms of Leukostasis and Leukemic Cell Dissemination. Blood (2001) 97(7):2121–9. doi: 10.1182/blood.V97.7.2121
83. Hatfield K, Øyan AM, Ersvaer E, Kalland K-H, Lassalle P, Gjertsen BT, et al. Primary Human Acute Myeloid Leukaemia Cells Increase the Proliferation of Microvascular Endothelial Cells Through the Release of Soluble Mediators. Br J Haematol (2009) 144(1):53–68. doi: 10.1111/j.1365-2141.2008.07411.x
84. Zhou H-S Z, Carter B, Andreeff M, Zhou H-S, Carter B Z, Andreeff M. Bone Marrow Niche-Mediated Survival of Leukemia Stem Cells in Acute Myeloid Leukemia: Yin and Yang. Cancer Biol Med (2016) 13(2):248–59. doi: 10.20892/j.issn.2095-3941.2016.0023
85. Bradstock KF, Gottlieb DJ. Interaction of Acute Leukemia Cells With the Bone Marrow Microenvironment: Implications for Control of Minimal Residual Disease. Leukemia Lymphoma (1995) 18(1–2):1–16. doi: 10.3109/10428199509064917
86. Cancilla D, Rettig MP, DiPersio JF. Targeting CXCR4 in AML and ALL. Front Oncol (2020) 10:1672. doi: 10.3389/fonc.2020.01672
87. Tabe Y, Shi YX, Zeng Z, Jin L, Shikami M, Hatanaka Y, et al. Tgf-β-Neutralizing Antibody 1d11 Enhances Cytarabine-Induced Apoptosis in AML Cells in the Bone Marrow Microenvironment. Rameshwar P, Editor. PLoS One (2013) 8(6):e62785. doi: 10.1371/journal.pone.0062785
88. Zhang TY, Dutta R, Benard B, Zhao F, Yin R, Majeti R. IL-6 Blockade Reverses Bone Marrow Failure Induced by Human Acute Myeloid Leukemia. Sci Transl Med (2020) 12(538):eaax5104. doi: 10.1126/scitranslmed.aax5104
89. Yang C, Yang H, Liu J, Zhu L, Yu S, Zhang X, et al. Focus on Exosomes: Novel Pathogenic Components of Leukemia. Am J Cancer Res (2019) 9(8):1815–29.
90. Boyiadzis M, Whiteside TL. Exosomes in Acute Myeloid Leukemia Inhibit Hematopoiesis. Curr Opin Hematol (2018) 25(4):279–84. doi: 10.1097/MOH.0000000000000439
91. Hong C-S, Sharma P, Yerneni SS, Simms P, Jackson EK, Whiteside TL, et al. Circulating Exosomes Carrying an Immunosuppressive Cargo Interfere With Cellular Immunotherapy in Acute Myeloid Leukemia. Sci Rep (2017) 7(1):14684. doi: 10.1038/s41598-017-14661-w
92. Dai Y-J, He S-Y, Hu F, Li X-P, Zhang J-M, Chen S-L, et al. Bone Marrow Infiltrated Natural Killer Cells Predicted the Anti-Leukemia Activity of MCL1 or BCL2 Inhibitors in Acute Myeloid Leukemia. Mol Cancer (2021) 20(1):8. doi: 10.1186/s12943-020-01302-6
93. Konopleva MY, Jordan CT. Leukemia Stem Cells and Microenvironment: Biology and Therapeutic Targeting. JCO (2011) 29(5):591–9. doi: 10.1200/JCO.2010.31.0904
94. Forte D, Krause DS, Andreeff M, Bonnet D, Méndez-Ferrer S. Updates on the Hematologic Tumor Microenvironment and its Therapeutic Targeting. Haematologica (2019) 104(10):1928–34. doi: 10.3324/haematol.2018.195396
95. Mellqvist U-H, Hansson M, Brune M, Dahlgren C, Hermodsson S, Hellstrand K. Natural Killer Cell Dysfunction and Apoptosis Induced by Chronic Myelogenous Leukemia Cells: Role of Reactive Oxygen Species and Regulation by Histamine. Blood (2000) 96(5):1961–8. doi: 10.1182/blood.V96.5.1961
96. Izawa S, Kono K, Mimura K, Kawaguchi Y, Watanabe M, Maruyama T, et al. H2O2 Production Within Tumor Microenvironment Inversely Correlated With Infiltration of CD56(dim) NK Cells in Gastric and Esophageal Cancer: Possible Mechanisms of NK Cell Dysfunction. Cancer Immunol Immunother (2011) 60(12):1801–10. doi: 10.1007/s00262-011-1082-7
97. Kotsafti A, Scarpa M, Castagliuolo I, Scarpa M. Reactive Oxygen Species and Antitumor Immunity—From Surveillance to Evasion. Cancers (2020) 12(7):1748. doi: 10.3390/cancers120717482
98. Farc O, Cristea V. An Overview of the Tumor Microenvironment, From Cells to Complex Networks (Review). Exp Ther Med (2021) 21(1):1–1. doi: 10.3892/etm.2020.9528
99. Zou W. Mechanistic Insights Into Cancer Immunity and Immunotherapy. Cell Mol Immunol (2018) 15(5):419–20. doi: 10.1038/s41423-018-0011-5
100. Chen H, He W. Human Regulatory γδt Cells and Their Functional Plasticity in the Tumor Microenvironment. Cell Mol Immunol (2018) 15(4):411–3. doi: 10.1038/cmi.2017.73
101. Yasinska IM, Gonçalves Silva I, Sakhnevych S, Gibbs BF, Raap U, Fasler-Kan E, et al. Biochemical Mechanisms Implemented by Human Acute Myeloid Leukemia Cells to Suppress Host Immune Surveillance. Cell Mol Immunol (2018) 15(11):989–91. doi: 10.1038/s41423-018-0047-6
102. Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, et al. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun Signaling (2020) 18(1):59. doi: 10.1186/s12964-020-0530-4
103. Habif G, Crinier A, André P, Vivier E, Narni-Mancinelli E. Targeting Natural Killer Cells in Solid Tumors. Cell Mol Immunol (2019) 16(5):415–22. doi: 10.1038/s41423-019-0224-2
104. Shi Y, Tomczak K, Li J, Ochieng JK, Lee Y, Haymaker C. Next-Generation Immunotherapies to Improve Anticancer Immunity. Front Pharmacol (2021) 11:566401. doi: 10.3389/fphar.2020.566401
105. Guerrouahen BS, Maccalli C, Cugno C, Rutella S, Akporiaye ET. Reverting Immune Suppression to Enhance Cancer Immunotherapy. Front Oncol (2020) 9:1554. doi: 10.3389/fonc.2019.01554
106. Zadka Ł, Grybowski DJ, Dzięgiel P. Modeling of the Immune Response in the Pathogenesis of Solid Tumors and its Prognostic Significance. Cell Oncol (2020) 43(4):539–75. doi: 10.1007/s13402-020-00519-3
107. Terrén I, Orrantia A, Vitallé J, Zenarruzabeitia O, Borrego F. Nk Cell Metabolism and Tumor Microenvironment. Front Immunol (2019) 10:2278. doi: 10.3389/fimmu.2019.02278
108. Cekic C, Day Y-J, Sag D, Linden J. Myeloid Expression of Adenosine A2a Receptor Suppresses T and NK Cell Responses in the Solid Tumor Microenvironment. Cancer Res (2014) 74(24):7250–9. doi: 10.1158/0008-5472.CAN-13-3583
109. Trzonkowski P, Szmit E, Myśliwska J, Dobyszuk A, Myśliwski A. Cd4+Cd25+ T Regulatory Cells Inhibit Cytotoxic Activity of T CD8+ and NK Lymphocytes in the Direct Cell-to-Cell Interaction. Clin Immunol (2004) 112(3):258–67. doi: 10.1016/j.clim.2004.04.003
110. Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-Expanded Myeloid-Derived Suppressor Cells Induce Anergy of NK Cells Through Membrane-Bound Tgf-β1. J Immunol (2009) 182(1):240–9. doi: 10.4049/jimmunol.182.1.240
111. Gonzalez-Rodriguez AP, Villa-Álvarez M, Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez S. Nk Cells in the Treatment of Hematological Malignancies. J Clin Med (2019) 8(10):1557. doi: 10.3390/jcm8101557
112. Castriconi R, Cantoni C, Chiesa MD, Vitale M, Marcenaro E, Conte R, et al. Transforming Growth Factor β1 Inhibits Expression of NKp30 and NKG2D Receptors: Consequences for the NK-Mediated Killing of Dendritic Cells. PNAS (2003) 100(7):4120–5. doi: 10.1073/pnas.0730640100
113. Hasmim M, Messai Y, Ziani L, Thiery J, Bouhris J-H, Noman MZ, et al. Critical Role of Tumor Microenvironment in Shaping Nk Cell Functions: Implication of Hypoxic Stress. Front Immunol (2015) 6:482. doi: 10.3389/fimmu.2015.00482
114. Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid Derived Suppressor Cells Inhibit Natural Killer Cells in Patients With Hepatocellular Carcinoma Via the NKp30 Receptor. Hepatology (2009) 50(3):799–807. doi: 10.1002/hep.23054
115. Zhang J, Han X, Hu X, Jin F, Gao Z, Yin L, et al. IDO1 Impairs NK Cell Cytotoxicity by Decreasing NKG2D/NKG2DLs Via Promoting Mir-18a. Mol Immunol (2018) 103:144–55. doi: 10.1016/j.molimm.2018.09.011
116. Stiff A, Trikha P, Mundy-Bosse B, McMichael E, Mace TA, Benner B, et al. Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor–Mediated Natural Killer Cell Function. Clin Cancer Res (2018) 24(8):1891–904. doi: 10.1158/1078-0432.CCR-17-0691
117. Bruno A, Mortara L, Baci D, Noonan DM, Albini A. Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-Angiogenic Activities: Roles in Tumor Progression. Front Immunol (2019) 10:771. doi: 10.3389/fimmu.2019.00771
118. Liu S, Ren J, ten Dijke P. Targeting Tgfβ Signal Transduction for Cancer Therapy. Signal Transduct Targeted Ther (2021) 6(1):8. doi: 10.1038/s41392-020-00436-9
120. Sun C, Xu J, Huang Q, Huang M, Wen H, Zhang C, et al. High NKG2A Expression Contributes to NK Cell Exhaustion and Predicts a Poor Prognosis of Patients With Liver Cancer. OncoImmunology (2017) 6(1):e1264562. doi: 10.1080/2162402X.2016.1264562
121. Li T, Yi S, Liu W, Jia C, Wang G, Hua X, et al. Colorectal Carcinoma-Derived Fibroblasts Modulate Natural Killer Cell Phenotype and Antitumor Cytotoxicity. Med Oncol (2013) 30(3):663. doi: 10.1007/s12032-013-0663-z
122. Ziani L, Chouaib S, Thiery J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front Immunol (2018) 9:414. doi: 10.3389/fimmu.2018.00414
123. Bassani B, Baci D, Gallazzi M, Poggi A, Bruno A, Mortara L. Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities Into Potent Anti-Tumor Effects. Cancers (2019) 11(4):461. doi: 10.3390/cancers11040461
124. Harmon C, Robinson MW, Hand F, Almuaili D, Mentor K, Houlihan DD, et al. Lactate-Mediated Acidification of Tumor Microenvironment Induces Apoptosis of Liver-Resident Nk Cells in Colorectal Liver Metastasis. Cancer Immunol Res (2019) 7(2):335–46. doi: 10.1158/2326-6066.CIR-18-0481
125. Gardiner CM. NK Cell Metabolism. J Leukoc Biol (2019) 105(6):1235–42. doi: 10.1002/JLB.MR0718-260R
126. Chang C-H, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell (2015) 162(6):1229–41. doi: 10.1016/j.cell.2015.08.016
127. de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, Manzo-Merino J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front Oncol (2019) 9:1143. doi: 10.3389/fonc.2019.01143
128. Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, et al. Role of Hypoxia in Cancer Therapy by Regulating the Tumor Microenvironment. Mol Cancer (2019) 18(1):157. doi: 10.1186/s12943-019-1089-9
129. Harris AL. Hypoxia — a Key Regulatory Factor in Tumour Growth. Nat Rev Cancer (2002) 2(1):38–47. doi: 10.1038/nrc704
130. Parodi M, Raggi F, Cangelosi D, Manzini C, Balsamo M, Blengio F, et al. Hypoxia Modifies the Transcriptome of Human Nk Cells, Modulates Their Immunoregulatory Profile, and Influences Nk Cell Subset Migration. Front Immunol (2018) 9:2358. doi: 10.3389/fimmu.2018.02358
131. Velásquez SY, Killian D, Schulte J, Sticht C, Thiel M, Lindner HA. Short Term Hypoxia Synergizes With Interleukin 15 Priming in Driving Glycolytic Gene Transcription and Supports Human Natural Killer Cell Activities. J Biol Chem (2016) 291(25):12960–77. doi: 10.1074/jbc.M116.721753
132. Young A, Ngiow SF, Gao Y, Patch A-M, Barkauskas DS, Messaoudene M, et al. A2ar Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment. Cancer Res (2018) 78(4):1003–16. doi: 10.1158/0008-5472.CAN-17-2826
133. Melaiu O, Lucarini V, Cifaldi L, Fruci D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front Immunol (2020) 10:3038. doi: 10.3389/fimmu.2019.03038
134. Jochems C, Hodge JW, Fantini M, Tsang KY, Vandeveer AJ, Gulley JL, et al. ADCC Employing an NK Cell Line (haNK) Expressing the High Affinity CD16 Allele With Avelumab, an Anti-PD-L1 Antibody. Int J Cancer (2017) 141(3):583–93. doi: 10.1002/ijc.30767
135. Solocinski K, Padget MR, Fabian KP, Wolfson B, Cecchi F, Hembrough T, et al. Overcoming Hypoxia-Induced Functional Suppression of NK Cells. J Immunother Cancer (2020) 8(1):e000246. doi: 10.1136/jitc-2019-000246
136. Vedenko A, Panara K, Goldstein G, Ramasamy R, Arora H. Tumor Microenvironment and Nitric Oxide: Concepts and Mechanisms. In: Birbrair A, editor. Tumor Microenvironment : Molecular Players – Part B. Cham: Springer International Publishing (2020). p. 143–58. (Advances in Experimental Medicine and Biology). doi: 10.1007/978-3-030-50224-9_10
137. Vivarelli S, Falzone L, Basile MS, Candido S, Libra M. Nitric Oxide in Hematological Cancers: Partner or Rival? Antioxid Redox Signaling (2020) 34(5):383–401. doi: 10.1089/ars.2019.7958
138. López-Sánchez LM, Aranda E, Rodríguez-Ariza A. Nitric Oxide and Tumor Metabolic Reprogramming. Biochem Pharmacol (2020) 176:113769. doi: 10.1016/j.bcp.2019.113769
139. Lukey MJ, Katt WP, Cerione RA. Targeting Amino Acid Metabolism for Cancer Therapy. Drug Discov Today (2017) 22(5):796–804. doi: 10.1016/j.drudis.2016.12.003
140. Stuani L, Sabatier M, Sarry J-E. Exploiting Metabolic Vulnerabilities for Personalized Therapy in Acute Myeloid Leukemia. BMC Biol (2019) 17(1):57. doi: 10.1186/s12915-019-0670-4
141. Irigoyen M, García-Ruiz JC, Berra E. The Hypoxia Signalling Pathway in Haematological Malignancies. Oncotarget (2017) 8(22):36832–44. doi: 10.18632/oncotarget.15981
142. Konopleva M, Thall PF, Yi CA, Borthakur G, Coveler A, Bueso-Ramos C, et al. Phase I/II Study of the Hypoxia-Activated Prodrug PR104 in Refractory/Relapsed Acute Myeloid Leukemia and Acute Lymphoblastic Leukemia. Haematologica (2015) 100(7):927–34. doi: 10.3324/haematol.2014.118455
143. Coltella N, Valsecchi R, Ponente M, Ponzoni M, Bernardi R. Synergistic Leukemia Eradication by Combined Treatment With Retinoic Acid and HIF Inhibition by EZN-2208 (Peg-SN38) in Preclinical Models of PML-Rarα and PLZF-Rarα–Driven Leukemia. Clin Cancer Res (2015) 21(16):3685–94. doi: 10.1158/1078-0432.CCR-14-3022
144. Deynoux M, Sunter N, Hérault O, Mazurier F. Hypoxia and Hypoxia-Inducible Factors in Leukemias. Front Oncol (2016) 6:41. doi: 10.3389/fonc.2016.00041
145. El Hassouni B, Granchi C, Vallés-Martí A, Supadmanaba IGP, Bononi G, Tuccinardi T, et al. The Dichotomous Role of the Glycolytic Metabolism Pathway in Cancer Metastasis: Interplay With the Complex Tumor Microenvironment and Novel Therapeutic Strategies. Semin Cancer Biol (2020) 60:238–48. doi: 10.1016/j.semcancer.2019.08.025
146. Lin X, Xiao Z, Chen T, Liang SH, Guo H. Glucose Metabolism on Tumor Plasticity, Diagnosis, and Treatment. Front Oncol (2020) 10:317. doi: 10.3389/fonc.2020.00317
147. Kishton RJ, Rathmell JC. Novel Therapeutic Targets of Tumor Metabolism. Cancer J (2015) 21(2):62–9. doi: 10.1097/PPO.0000000000000099
148. Saulle E, Spinello I, Quaranta MT, Pasquini L, Pelosi E, Iorio E, et al. Targeting Lactate Metabolism by Inhibiting MCT1 or MCT4 Impairs Leukemic Cell Proliferation, Induces Two Different Related Death-Pathways and Increases Chemotherapeutic Sensitivity of Acute Myeloid Leukemia Cells. Front Oncol (2021) 10:621458. doi: 10.3389/fonc.2020.621458
149. Sek K, Mølck C, Stewart G, Kats L, Darcy P, Beavis P. Targeting Adenosine Receptor Signaling in Cancer Immunotherapy. IJMS (2018) 19(12):3837. doi: 10.3390/ijms19123837
150. Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, et al. Cytokines in Clinical Cancer Immunotherapy. Br J Cancer (2019) 120(1):6–15. doi: 10.1038/s41416-018-0328-y
151. Daher M, Basar R, Gokdemir E, Baran N, Uprety N, Nunez Cortes AK, et al. Targeting a Cytokine Checkpoint Enhances the Fitness of Armored Cord Blood CAR-NK Cells. Blood (2021) 137(5):624–36. doi: 10.1182/blood.2020007748
152. Freund-Brown J, Chirino L, Kambayashi T. Strategies to Enhance NK Cell Function for the Treatment of Tumors and Infections. Crit Rev Immunol (2018) 38(2):105–30. doi: 10.1615/CritRevImmunol.2018025248
153. Wang Z, Guo L, Song Y, Zhang Y, Lin D, Hu B, et al. Augmented Anti-Tumor Activity of NK-92 Cells Expressing Chimeric Receptors of TGF-βr II and NKG2D. Cancer Immunol Immunother (2017) 66(4):537–48. doi: 10.1007/s00262-017-1959-1
154. Finetti F, Travelli C, Ercoli J, Colombo G, Buoso E, Trabalzini L. Prostaglandin E2 and Cancer: Insight Into Tumor Progression and Immunity. Biology (2020) 9(12):434. doi: 10.3390/biology9120434
155. Shehzad A, Lee J, Lee YS. Autocrine Prostaglandin E2 Signaling Promotes Promonocytic Leukemia Cell Survival Via COX-2 Expression and MAPK Pathway. BMB Rep (2015) 48(2):109–14. doi: 10.5483/BMBRep.2015.48.2.081
156. Emadi A, Duong VH, Pantin J, Imran M, Koka R, Singh Z, et al. Indoximod Combined With Standard Induction Chemotherapy is Well Tolerated and Induces a High Rate of Complete Remission With MRD-Negativity in Patients With Newly Diagnosed AML: Results From a Phase 1 Trial. Blood (2018) 132(Supplement 1):332–2. doi: 10.1182/blood-2018-99-117433
157. Vela M, Aris M, Llorente M, Garcia-Sanz JA, Kremer L. Chemokine Receptor-Specific Antibodies in Cancer Immunotherapy: Achievements and Challenges. Front Immunol (2015) 6:12. doi: 10.3389/fimmu.2015.00012
158. Jacquelot N, Duong CPM, Belz GT, Zitvogel L. Targeting Chemokines and Chemokine Receptors in Melanoma and Other Cancers. Front Immunol (2018) 9:2480. doi: 10.3389/fimmu.2018.02480
159. Klose R, Krzywinska E, Castells M, Gotthardt D, Putz EM, Kantari-Mimoun C, et al. Targeting VEGF-A in Myeloid Cells Enhances Natural Killer Cell Responses to Chemotherapy and Ameliorates Cachexia. Nat Commun (2016) 7:12528. doi: 10.1038/ncomms12528
160. Tamura R, Tanaka T, Akasaki Y, Murayama Y, Yoshida K, Sasaki H. The Role of Vascular Endothelial Growth Factor in the Hypoxic and Immunosuppressive Tumor Microenvironment: Perspectives for Therapeutic Implications. Med Oncol (2019) 37(1):2. doi: 10.1007/s12032-019-1329-2
161. Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front Immunol (2019) 10:1205. doi: 10.3389/fimmu.2019.01205
162. Koh L-P, Rizzieri DA, Chao NJ. Allogeneic Hematopoietic Stem Cell Transplant Using Mismatched/Haploidentical Donors. Biol Blood Marrow Transplant (2007) 13(11):1249–67. doi: 10.1016/j.bbmt.2007.08.003
163. Rocha V, Locatelli F. Searching for Alternative Hematopoietic Stem Cell Donors for Pediatric Patients. Bone Marrow Transplant (2008) 41(2):207–14. doi: 10.1038/sj.bmt.1705963
164. Mavers M, Bertaina A. High-Risk Leukemia: Past, Present, and Future Role of NK Cells. J Immunol Res (2018) 2018:e1586905. doi: 10.1155/2018/1586905
165. Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of Donor Natural Killer Cell Alloreactivity in Mismatched Hematopoietic Transplants. Science (2002) 295(5562):2097–100. doi: 10.1126/science.1068440
166. Lamb MG, Rangarajan HG, Tullius BP, Lee DA. Natural Killer Cell Therapy for Hematologic Malignancies: Successes, Challenges, and the Future. Stem Cell Res Ther (2021) 12(1):211. doi: 10.1186/s13287-021-02277-x
167. Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, et al. Survival Advantage With KIR Ligand Incompatibility in Hematopoietic Stem Cell Transplantation From Unrelated Donors. Blood (2003) 102(3):814–9. doi: 10.1182/blood-2003-01-0091
168. Ruggeri L, Mancusi A, Perruccio K, Burchielli E, Martelli MF, Velardi A. Natural Killer Cell Alloreactivity for Leukemia Therapy. J Immunother (2005) 28(3):175–82. doi: 10.1097/01.cji.0000161395.88959.1f
169. Sarhan D, Hippen KL, Lemire A, Hying S, Luo X, Lenvik T, et al. Adaptive NK Cells Resist Regulatory T-cell Suppression Driven by IL37. Cancer Immunol Res (2018) 6(7):766–75. doi: 10.1158/2326-6066.CIR-17-0498
170. Heidenreich S, Kröger N. Reduction of Relapse After Unrelated Donor Stem Cell Transplantation by KIR-Based Graft Selection. Front Immunol (2017) 8:41. doi: 10.3389/fimmu.2017.00041
171. Li L, Kolk M, Fernandez-Vina M, de Lima M, Otegbeye F. Interrogating the Impact of KIR Ligand Mismatch in Engraftment Following HLA-Disparate Stem Cell Transplantation. Bone Marrow Transplant (2020) 55(12):2294–7. doi: 10.1038/s41409-020-0957-7
172. Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. Nkaml: A Pilot Study to Determine the Safety and Feasibility of Haploidentical Natural Killer Cell Transplantation in Childhood Acute Myeloid Leukemia. JCO (2010) 28(6):955–9. doi: 10.1200/JCO.2009.24.4590
173. Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA, et al. The Effect of KIR Ligand Incompatibility on the Outcome of Unrelated Donor Transplantation: A Report From the Center for International Blood and Marrow Transplant Research, the European Blood and Marrow Transplant Registry, and the Dutch Registry. Biol Blood Marrow Transplant (2006) 12(8):876–84. doi: 10.1016/j.bbmt.2006.05.007
174. Davies SM, Ruggieri L, DeFor T, Wagner JE, Weisdorf DJ, Miller JS, et al. Evaluation of KIR Ligand Incompatibility in Mismatched Unrelated Donor Hematopoietic Transplants. Blood (2002) 100(10):3825–7. doi: 10.1182/blood-2002-04-1197
175. Bishara A, De Santis D, Witt CC, Brautbar C, Christiansen FT, Or R, et al. The Beneficial Role of Inhibitory KIR Genes of HLA Class I NK Epitopes in Haploidentically Mismatched Stem Cell Allografts may be Masked by Residual Donor-Alloreactive T Cells Causing GVHD. Tissue Antigens (2004) 63(3):204–11. doi: 10.1111/j.0001-2815.2004.00182.x
176. Schaffer M, Malmberg K-J, Ringdén O, Ljunggren H-G, Remberger M. Increased Infection-Related Mortality in KIR-Ligand–Mismatched Unrelated Allogeneic Hematopoietic Stem-Cell Transplantation. Transplantation (2004) 78(7):1081–5. doi: 10.1097/01.TP.0000137103.19717.86
177. Tanaka J, Morishima Y, Takahashi Y, Yabe T, Oba K, Takahashi S, et al. Effects of KIR Ligand Incompatibility on Clinical Outcomes of Umbilical Cord Blood Transplantation Without ATG for Acute Leukemia in Complete Remission. Blood Cancer J (2013) 3(11):e164. doi: 10.1038/bcj.2013.62
178. Otegbeye F, Li L, Kolk M, Boughan KM, Caimi P, Cooper B, et al. Kir Ligand Incompatibility in the Host Versus Graft Direction Predicts Graft Failure and Dominant Graft in Cord Blood Transplantation But Not in Haploidentical Transplantation. Biol Blood Marrow Transplant (2019) 25(3, Supplement):S217. doi: 10.1016/j.bbmt.2018.12.297
179. Medinger M, Lengerke C, Passweg J. Novel Therapeutic Options in Acute Myeloid Leukemia. Leukemia Res Rep (2016) 6:39–49. doi: 10.1016/j.lrr.2016.09.001
180. Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, et al. In Vivo Evidence for a Dependence on Interleukin 15 for Survival of Natural Killer Cells. Blood (2002) 100(10):3633–8. doi: 10.1182/blood-2001-12-0293
181. Cooley S, He F, Bachanova V, Vercellotti GM, DeFor TE, Curtsinger JM, et al. First-in-Human Trial of rhIL-15 and Haploidentical Natural Killer Cell Therapy for Advanced Acute Myeloid Leukemia. Blood Adv (2019) 3(13):1970–80. doi: 10.1182/bloodadvances.2018028332
182. Nguyen R, Wu H, Pounds S, Inaba H, Ribeiro RC, Cullins D, et al. A Phase II Clinical Trial of Adoptive Transfer of Haploidentical Natural Killer Cells for Consolidation Therapy of Pediatric Acute Myeloid Leukemia. J Immunother Cancer (2019) 7(1):81. doi: 10.1186/s40425-019-0564-6
183. Zhao X, Cai L, Hu Y, Wang H. Cord-Blood Natural Killer Cell-Based Immunotherapy for Cancer. Front Immunol (2020) 11:584099. doi: 10.3389/fimmu.2020.584099
184. Luevano M, Daryouzeh M, Alnabhan R, Querol S, Khakoo S, Madrigal A, et al. The Unique Profile of Cord Blood Natural Killer Cells Balances Incomplete Maturation and Effective Killing Function Upon Activation. Hum Immunol (2012) 73(3):248–57. doi: 10.1016/j.humimm.2011.12.015
185. Sarvaria A, Jawdat D, Madrigal JA, Saudemont A. Umbilical Cord Blood Natural Killer Cells, Their Characteristics, and Potential Clinical Applications. Front Immunol (2017) 8:329. doi: 10.3389/fimmu.2017.00329
186. Dolstra H, Roeven MWH, Spanholtz J, Hangalapura BN, Tordoir M, Maas F, et al. Successful Transfer of Umbilical Cord Blood Cd34+ Hematopoietic Stem and Progenitor-Derived Nk Cells in Older Acute Myeloid Leukemia Patients. Clin Cancer Res (2017) 23(15):4107–18. doi: 10.1158/1078-0432.CCR-16-2981
187. Shah N, Li L, McCarty J, Kaur I, Yvon E, Shaim H, et al. Phase I Study of Cord Blood-Derived Natural Killer Cells Combined With Autologous Stem Cell Transplantation in Multiple Myeloma. Br J Haematol (2017) 177(3):457–66. doi: 10.1111/bjh.14570
188. Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human Ipsc-Derived Natural Killer Cells Engineered With Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell (2018) 23(2):181–92.e5. doi: 10.1016/j.stem.2018.06.002
189. Zhu H, Blum RH, Bjordahl R, Gaidarova S, Rogers P, Lee TT, et al. Pluripotent Stem Cell–Derived NK Cells With High-Affinity Noncleavable CD16a Mediate Improved Antitumor Activity. Blood (2020) 135(6):399–410. doi: 10.1182/blood.2019000621
190. Matsubara H, Niwa A, Nakahata T, Saito MK. Induction of Human Pluripotent Stem Cell-Derived Natural Killer Cells for Immunotherapy Under Chemically Defined Conditions. Biochem Biophys Res Commun (2019) 515(1):1–8. doi: 10.1016/j.bbrc.2019.03.085
191. Kloess S, Kretschmer A, Stahl L, Fricke S, Koehl U. Car-Expressing Natural Killer Cells for Cancer Retargeting. Transfus Med Hemother (2019) 46(1):4–13. doi: 10.1159/000495771
192. Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. Car-NK Cells: A Promising Cellular Immunotherapy for Cancer. EBioMedicine (2020) 59:102975. doi: 10.1016/j.ebiom.2020.102975
193. Yilmaz A, Cui H, Caligiuri MA, Yu J. Chimeric Antigen Receptor-Engineered Natural Killer Cells for Cancer Immunotherapy. J Hematol Oncol (2020) 13(1):168. doi: 10.1186/s13045-020-00998-9
194. Hu Y, Tian Z-G, Zhang C. Chimeric Antigen Receptor (CAR)-Transduced Natural Killer Cells in Tumor Immunotherapy. Acta Pharmacol Sin (2018) 39(2):167–76. doi: 10.1038/aps.2017.125
195. Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. First-in-Man Clinical Trial of CAR Nk-92 Cells: Safety Test of CD33-CAR Nk-92 Cells in Patients With Relapsed and Refractory Acute Myeloid Leukemia. Am J Cancer Res (2018) 8(6):1083–9.
196. Gurney M, O’Dwyer M. Realizing Innate Potential: CAR-NK Cell Therapies for Acute Myeloid Leukemia. Cancers (2021) 13(7):1568. doi: 10.3390/cancers13071568
197. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med (2020) 382(6):545–53. doi: 10.1056/NEJMoa1910607
198. Naeimi Kararoudi M, Likhite S, Elmas E, Schwartz M, Sorathia K, Yamamoto K, et al. Cd33 Targeting Primary CAR-NK Cells Generated by CRISPR Mediated Gene Insertion Show Enhanced Anti-AML Activity. Blood (2020) 136(Supplement 1):3–3. doi: 10.1182/blood-2020-142494
199. Gunesch JT, Angelo LS, Mahapatra S, Deering RP, Kowalko JE, Sleiman P, et al. Genome-Wide Analyses and Functional Profiling of Human NK Cell Lines. Mol Immunol (2019) 115:64–75. doi: 10.1016/j.molimm.2018.07.015
200. Di Vito C, Mikulak J, Zaghi E, Pesce S, Marcenaro E, Mavilio D. NK Cells to Cure Cancer. Semin Immunol (2019) 41:101272. doi: 10.1016/j.smim.2019.03.004
201. Oelsner S, Friede ME, Zhang C, Wagner J, Badura S, Bader P, et al. Continuously Expanding CAR Nk-92 Cells Display Selective Cytotoxicity Against B-cell Leukemia and Lymphoma. Cytotherapy (2017) 19(2):235–49. doi: 10.1016/j.jcyt.2016.10.009
202. Zhang J, Zheng H, Diao Y. Natural Killer Cells and Current Applications of Chimeric Antigen Receptor-Modified Nk-92 Cells in Tumor Immunotherapy. IJMS (2019) 20(2):317. doi: 10.3390/ijms20020317
203. Williams BA, Law AD, Routy B, denHollander N, Gupta V, Wang X-H, et al. A Phase I Trial of NK-92 Cells for Refractory Hematological Malignancies Relapsing After Autologous Hematopoietic Cell Transplantation Shows Safety and Evidence of Efficacy. Oncotarget (2017) 8(51):89256–68. doi: 10.18632/oncotarget.19204
204. Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, et al. Nk-92: An “Off-the-Shelf Therapeutic” for Adoptive Natural Killer Cell-Based Cancer Immunotherapy. Cancer Immunol Immunother (2016) 65(4):485–92. doi: 10.1007/s00262-015-1761-x
205. Boyiadzis M, Agha M, Redner RL, Sehgal A, Im A, Hou J-Z, et al. Phase 1 Clinical Trial of Adoptive Immunotherapy Using “Off-the-Shelf” Activated Natural Killer Cells in Patients With Refractory and Relapsed Acute Myeloid Leukemia. Cytotherapy (2017) 19(10):1225–32. doi: 10.1016/j.jcyt.2017.07.008
206. Kang S, Gao X, Zhang L, Yang E, Li Y, Yu L. The Advances and Challenges of NK Cell-Based Cancer Immunotherapy. Curr Oncol (2021) 28(2):1077–93. doi: 10.3390/curroncol28020105
207. Paust S, Blish CA, Reeves RK. Redefining Memory: Building the Case for Adaptive Nk Cells. J Virol (2017) 91(20)e00169–17. doi: 10.1128/JVI.00169-17
208. Rölle A, Meyer M, Calderazzo S, Jäger D, Momburg F. Distinct HLA-E Peptide Complexes Modify Antibody-Driven Effector Functions of Adaptive Nk Cells. Cell Rep (2018) 24(8):1967–76.e4. doi: 10.1016/j.celrep.2018.07.069
209. Merino A, Dougherty P, Zhang B, Houchins JP, Miller JS, Cichocki F. Adaptive NK Cells Are Expanded By Nkg2c-Agonist Antibodies and Retain Their Cytotoxic and Secretory Properties. Blood (2018) 132(Supplement 1):3695–5. doi: 10.1182/blood-2018-99-113669
210. Cooley S, Geller M, Cichocki F, Curtsinger J, McKenna DH, Storgard C, et al. In Vivo Persistence and Function of Adaptive Nk Cell Infusions (Fate-NK100) From CMV Seropositive Haploidentical Related Donors. Biol Blood Marrow Transplant (2019) 25(3):S338. doi: 10.1016/j.bbmt.2018.12.548
211. Geller MA, Cooley SA, Wallet M, Valamehr B, Teoh DGK, DeFor TE, et al. Apollo: A Phase I Study of Adaptive Memory Natural Killer (NK) Cells in Recurrent Ovarian Cancer. JCO (2020) 38(15_suppl):6044–4. doi: 10.1200/JCO.2020.38.15_suppl.6044
212. Cichocki F, Cooley S, Davis Z, DeFor TE, Schlums H, Zhang B, et al. Cd56dimcd57+Nkg2c+ NK Cell Expansion is Associated With Reduced Leukemia Relapse After Reduced Intensity HCT. Leukemia (2016) 30(2):456–63. doi: 10.1038/leu.2015.260
213. Foltz JA, Berrien-Elliott MM, Neal C, Foster M, McClain E, Schappe T, et al. Cytokine-Induced Memory-like (Ml) NK Cells Persist for > 2 Months Following Adoptive Transfer Into Leukemia Patients With a MHC-Compatible Hematopoietic Cell Transplant (Hct). Blood (2019) 134(Supplement_1):1954–4. doi: 10.1182/blood-2019-126004
214. Berrien-Elliott MM, Wagner JA, Fehniger TA. Human Cytokine-Induced Memory-Like Natural Killer Cells. J Innate Immun (2015) 7(6):563–71. doi: 10.1159/000382019
215. Cho D, Campana D. Expansion and Activation of Natural Killer Cells for Cancer Immunotherapy. Ann Lab Med (2009) 29(2):89–96. doi: 10.3343/kjlm.2009.29.2.89
216. Wagner J, Pfannenstiel V, Waldmann A, Bergs JWJ, Brill B, Huenecke S, et al. A Two-Phase Expansion Protocol Combining Interleukin (Il)-15 and IL-21 Improves Natural Killer Cell Proliferation and Cytotoxicity Against Rhabdomyosarcoma. Front Immunol (2017) 8:676. doi: 10.3389/fimmu.2017.00676
217. Woan KV, Miller JS. Harnessing Natural Killer Cell Antitumor Immunity: From the Bench to Bedside. Cancer Immunol Res (2019) 7(11):1742. doi: 10.1158/2326-6066.CIR-19-0404
218. Lupo KB, Matosevic S. Natural Killer Cells as Allogeneic Effectors in Adoptive Cancer Immunotherapy. Cancers (Basel) (2019) 11(6):769. doi: 10.3390/cancers11060769
219. Sarkar S, Germeraad WTV, Rouschop KMA, Steeghs EMP, van Gelder M, Bos GMJ, et al. Hypoxia Induced Impairment of NK Cell Cytotoxicity Against Multiple Myeloma can be Overcome by IL-2 Activation of the NK Cells. PLoS One (2013) 8(5):e64835. doi: 10.1371/journal.pone.0064835
220. Nakanishi K. Unique Action of Interleukin-18 on T Cells and Other Immune Cells. Front Immunol (2018) 9:763. doi: 10.3389/fimmu.2018.00763
221. Brady J, Hayakawa Y, Smyth MJ, Nutt SL. Il-21 Induces the Functional Maturation of Murine NK Cells. J Immunol (2004) 172(4):2048–58. doi: 10.4049/jimmunol.172.4.2048
222. Seo H, Jeon I, Kim B-S, Park M, Bae E-A, Song B, et al. Il-21-mediated Reversal of NK Cell Exhaustion Facilitates Anti-Tumour Immunity in MHC Class I-deficient Tumours. Nat Commun (2017) 8(1):15776. doi: 10.1038/ncomms15776
223. Shah NN, Baird K, Delbrook CP, Fleisher TA, Kohler ME, Rampertaap S, et al. Acute GVHD in Patients Receiving IL-15/4-1BBL Activated NK Cells Following T-cell-depleted Stem Cell Transplantation. Blood (2015) 125(5):784–92. doi: 10.1182/blood-2014-07-592881
224. Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-Bound IL-21 Promotes Sustained Ex Vivo Proliferation of Human Natural Killer Cells. PLoS One (2012) 7(1):e30264. doi: 10.1371/journal.pone.0030264
225. Washington University School of Medicine. A Phase 2 Study of Cytokine-induced Memory-Like NK Cells in Combination With Chemotherapy in Pediatric Patients With Refractory or Relapsed AML [Internet]. Clinicaltrials.Gov (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT04354025.
226. Lanuza PM, Pesini C, Arias MA, Calvo C, Ramirez-Labrada A, Pardo J. Recalling the Biological Significance of Immune Checkpoints on NK Cells: A Chance to Overcome Lag3, PD1, and CTLA4 Inhibitory Pathways by Adoptive Nk Cell Transfer? Front Immunol (2020) 10:3010. doi: 10.3389/fimmu.2019.03010
227. Khan M, Arooj S, Wang H. Nk Cell-Based Immune Checkpoint Inhibition. Front Immunol (2020) 11:167. doi: 10.3389/fimmu.2020.00167
228. Janakiram M, Shah UA, Liu W, Zhao A, Schoenberg MP, Zang X. The Third Group of the B7-CD28 Immune Checkpoint Family: HHLA2, Tmigd2, B7x, and B7-H3. Immunol Rev (2017) 276(1):26–39. doi: 10.1111/imr.12521
229. Sanchez-Correa B, Valhondo I, Hassouneh F, Lopez-Sejas N, Pera A, Bergua JM, et al. DNAM-1 and the TIGIT/PVRIG/TACTILE Axis: Novel Immune Checkpoints for Natural Killer Cell-Based Cancer Immunotherapy. Cancers (2019) 11(6):877. doi: 10.3390/cancers11060877
230. Macauley MS, Crocker PR, Paulson JC. Siglec-Mediated Regulation of Immune Cell Function in Disease. Nat Rev Immunol (2014) 14(10):653–66. doi: 10.1038/nri3737
231. Jandus C, Boligan KF, Chijioke O, Liu H, Dahlhaus M, Démoulins T, et al. Interactions Between Siglec-7/9 Receptors and Ligands Influence NK Cell–Dependent Tumor Immunosurveillance. J Clin Invest (2014) 124(4):1810–20. doi: 10.1172/JCI65899
232. Pilch Z, Tonecka K, Skorzynski M, Sas Z, Braniewska A, Kryczka T, et al. The Pro-Tumor Effect of CD200 Expression Is Not Mimicked by Agonistic CD200R Antibodies. Chen s, Editor. PLoS One (2019) 14(1):e0210796. doi: 10.1371/journal.pone.0210796
233. Nath PR, Gangaplara A, Pal-Nath D, Mandal A, Maric D, Sipes JM, et al. Cd47 Expression in Natural Killer Cells Regulates Homeostasis and Modulates Immune Response to Lymphocytic Choriomeningitis Virus. Front Immunol (2018) 9:2985. doi: 10.3389/fimmu.2018.02985
234. Salik B, Smyth MJ, Nakamura K. Targeting Immune Checkpoints in Hematological Malignancies. J Hematol Oncol (2020) 13(1):111. doi: 10.1186/s13045-020-00947-6
235. Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front Immunol (2020) 11:940. doi: 10.3389/fimmu.2020.00940
236. Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I Safety and Pharmacokinetic Study of CT-011, a Humanized Antibody Interacting With PD-1, in Patients With Advanced Hematologic Malignancies. Clin Cancer Res (2008) 14(10):3044–51. doi: 10.1158/1078-0432.CCR-07-4079
237. Garcia-Manero G, Tallman MS, Martinelli G, Ribrag V, Yang H, Balakumaran A, et al. Pembrolizumab, a PD-1 Inhibitor, in Patients With Myelodysplastic Syndrome (MDS) After Failure of Hypomethylating Agent Treatment. Blood (2016) 128(22):345–5. doi: 10.1182/blood.V128.22.345.345
238. Zeidan AM, Knaus HA, Robinson TM, Towlerton AMH, Warren EH, Zeidner JF, et al. A Multi-center Phase I Trial of Ipilimumab in Patients With Myelodysplastic Syndromes Following Hypomethylating Agent Failure. Clin Cancer Res (2018) 24(15):3519–27. doi: 10.1158/1078-0432.CCR-17-3763
239. Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for Patients With Relapse After Allogeneic Transplantation. N Engl J Med (2016) 375(2):143–53. doi: 10.1056/NEJMoa1601202
240. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med (2018) 378:158–68. doi: 10.1056/NEJMra1703481
241. Seo H, Kim B-S, Bae E-A, Min BS, Han YD, Shin SJ, et al. Il21 Therapy Combined With PD-1 and Tim-3 Blockade Provides Enhanced NK Cell Antitumor Activity Against MHC Class I-Deficient Tumors. Cancer Immunol Res (2018) 6(6):685–95. doi: 10.1158/2326-6066.CIR-17-0708
242. Bryceson YT, March ME, Ljunggren H-G, Long EO. Activation, Coactivation, and Costimulation of Resting Human Natural Killer Cells. Immunol Rev (2006) 214(1):73–91. doi: 10.1111/j.1600-065X.2006.00457.x
243. Tremblay-McLean A, Coenraads S, Kiani Z, Dupuy FP, Bernard NF. Expression of Ligands for Activating Natural Killer Cell Receptors on Cell Lines Commonly Used to Assess Natural Killer Cell Function. BMC Immunol (2019) 20(1):8. doi: 10.1186/s12865-018-0272-x
244. Shukla A, Cloutier M, Appiya Santharam M, Ramanathan S, Ilangumaran S. The MHC Class-I Transactivator Nlrc5: Implications to Cancer Immunology and Potential Applications to Cancer Immunotherapy. Int J Mol Sci (2021) 22(4):1964. doi: 10.3390/ijms22041964
245. Malmberg K-J, Levitsky V, Norell H, de Matos CT, Carlsten M, Schedvins K, et al. IFN-Gamma Protects Short-Term Ovarian Carcinoma Cell Lines From CTL Lysis Via a CD94/NKG2A-dependent Mechanism. J Clin Invest (2002) 110(10):1515–23. doi: 10.1172/JCI0215564
246. Muntasell A, Ochoa MC, Cordeiro L, Berraondo P, López-Díaz de Cerio A, Cabo M, et al. Targeting NK-cell Checkpoints for Cancer Immunotherapy. Curr Opin Immunol (2017) 45:73–81. doi: 10.1016/j.coi.2017.01.003
247. Vey N, Karlin L, Sadot-Lebouvier S, Broussais F, Berton-Rigaud D, Rey J, et al. A Phase 1 Study of Lirilumab (Antibody Against Killer Immunoglobulin-Like Receptor Antibody KIR2D; IPH2102) in Patients With Solid Tumors and Hematologic Malignancies. Oncotarget (2018) 9(25):17675–88. doi: 10.18632/oncotarget.24832
248. Vey N, Bourhis J-H, Boissel N, Bordessoule D, Prebet T, Charbonnier A, et al. A Phase 1 Trial of the Anti-Inhibitory KIR Mab IPH2101 for AML in Complete Remission. Blood (2012) 120(22):4317–23. doi: 10.1182/blood-2012-06-437558
249. Vey N, Dumas P-Y, Recher C, Gastaud L, Lioure B, Bulabois C-E, et al. Randomized Phase 2 Trial of Lirilumab (anti-KIR Monoclonal Antibody, Mab) As Maintenance Treatment in Elderly Patients (Pts) With Acute Myeloid Leukemia (Aml): Results of the Effikir Trial. Blood (2017) 130(Suppl_1):889–9. doi: 10.1182/blood.V130.Suppl_1.889.889
250. Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, et al. Anti-KIR Antibody Enhancement of Anti-Lymphoma Activity of Natural Killer Cells as Monotherapy and in Combination With anti-CD20 Antibodies. Blood (2014) 123(5):678–86. doi: 10.1182/blood-2013-08-519199
251. van Hall T, André P, Horowitz A, Ruan DF, Borst L, Zerbib R, et al. Monalizumab: Inhibiting the Novel Immune Checkpoint NKG2A. J ImmunoTher Cancer (2019) 7(1):263. doi: 10.1186/s40425-019-0761-3
252. André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, et al. Anti-NKG2A Mab Is a Checkpoint Inhibitor That Promotes Anti-Tumor Immunity by Unleashing Both T and NK Cells. Cell (2018) 175(7):1731–43.e13. doi: 10.1016/j.cell.2018.10.014
253. Pereira NA, Chan KF, Lin PC, Song Z. The “Less-is-More” in Therapeutic Antibodies: Afucosylated Anti-Cancer Antibodies With Enhanced Antibody-Dependent Cellular Cytotoxicity. mAbs (2018) 10(5):693–711. doi: 10.1080/19420862.2018.1466767
254. Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab Plus Chlorambucil in Patients With CLL and Coexisting Conditions. United States: Massachusetts Medical Society (2014) 370(12):1101–10. doi: 10.1056/NEJMoa1313984
255. Federici C, Shahaj E, Cecchetti S, Camerini S, Casella M, Iessi E, et al. Natural-Killer-Derived Extracellular Vesicles: Immune Sensors and Interactors. Front Immunol (2020) 11:262. doi: 10.3389/fimmu.2020.00262
256. Jong AY, Wu C-H, Li J, Sun J, Fabbri M, Wayne AS, et al. Large-Scale Isolation and Cytotoxicity of Extracellular Vesicles Derived From Activated Human Natural Killer Cells. J Extracell Vesicles (2017) 6(1):1294368. doi: 10.1080/20013078.2017.1294368
257. Wu C-H, Li J, Li L, Sun J, Fabbri M, Wayne AS, et al. Extracellular Vesicles Derived From Natural Killer Cells Use Multiple Cytotoxic Proteins and Killing Mechanisms to Target Cancer Cells. null (2019) 8(1):1588538. doi: 10.1080/20013078.2019.1588538
258. Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, et al. Bispecific and Trispecific Killer Cell Engagers Directly Activate Human Nk Cells Through CD16 Signaling and Induce Cytotoxicity and Cytokine Production. Mol Cancer Ther (2012) 11(12):2674–84. doi: 10.1158/1535-7163.MCT-12-0692
259. Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, et al. Il15 Trispecific Killer Engagers (Trike) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin Cancer Res (2016) 22(14):3440–50. doi: 10.1158/1078-0432.CCR-15-2710
260. Warlick ED, Weisdorf DJ, Vallera DA, Wangen R, Lewis D, Knox J, et al. Gtb-3550 TriKETM for the Treatment of High-Risk Myelodysplastic Syndromes (MDS) and Refractory/Relapsed Acute Myeloid Leukemia (Aml) Safely Drives Natural Killer (Nk) Cell Proliferation At Initial Dose Cohorts. Blood (2020) 136(Supplement 1):7–8. doi: 10.1182/blood-2020-136398
261. Irvine DJ, Dane EL. Enhancing Cancer Immunotherapy With Nanomedicine. Nat Rev Immunol (2020) 20(5):321–34. doi: 10.1038/s41577-019-0269-6
262. Liu C, Lai H, Chen T. Boosting Natural Killer Cell-Based Cancer Immunotherapy With Selenocystine/Transforming Growth Factor-Beta Inhibitor-Encapsulated Nanoemulsion. ACS Nano (2020) 14(9):11067–82. doi: 10.1021/acsnano.9b10103
263. Park W, Gordon AC, Cho S, Huang X, Harris KR, Larson AC, et al. Immunomodulatory Magnetic Microspheres for Augmenting Tumor-Specific Infiltration of Natural Killer (Nk) Cells. ACS Appl Mater Interfaces (2017) 9(16):13819–24. doi: 10.1021/acsami.7b02258
264. Kim K-S, Han J-H, Choi SH, Jung H-Y, Park JD, An H-J, et al. Cationic Nanoparticle-Mediated Activation of Natural Killer Cells for Effective Cancer Immunotherapy. ACS Appl Mater Interfaces (2020) 12(51):56731–40. doi: 10.1021/acsami.0c16357
Keywords: acute myeloid leukemia, natural killer cells, immunotherapy, immunosuppressive microenvironment, enhancing strategies
Citation: Kaweme NM and Zhou F (2021) Optimizing NK Cell-Based Immunotherapy in Myeloid Leukemia: Abrogating an Immunosuppressive Microenvironment. Front. Immunol. 12:683381. doi: 10.3389/fimmu.2021.683381
Received: 20 March 2021; Accepted: 31 May 2021;
Published: 17 June 2021.
Edited by:
Antonio Curti, University of Bologna, ItalyReviewed by:
Massimo Vitale, Azienda Ospedaliera Universitaria San Martino (IRCCS), ItalyJun Zhang, Peking University, China
Copyright © 2021 Kaweme and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuling Zhou, emhvdWZ1bGluZ0B3aHUuZWR1LmNu
 Natasha Mupeta Kaweme
Natasha Mupeta Kaweme Fuling Zhou
Fuling Zhou