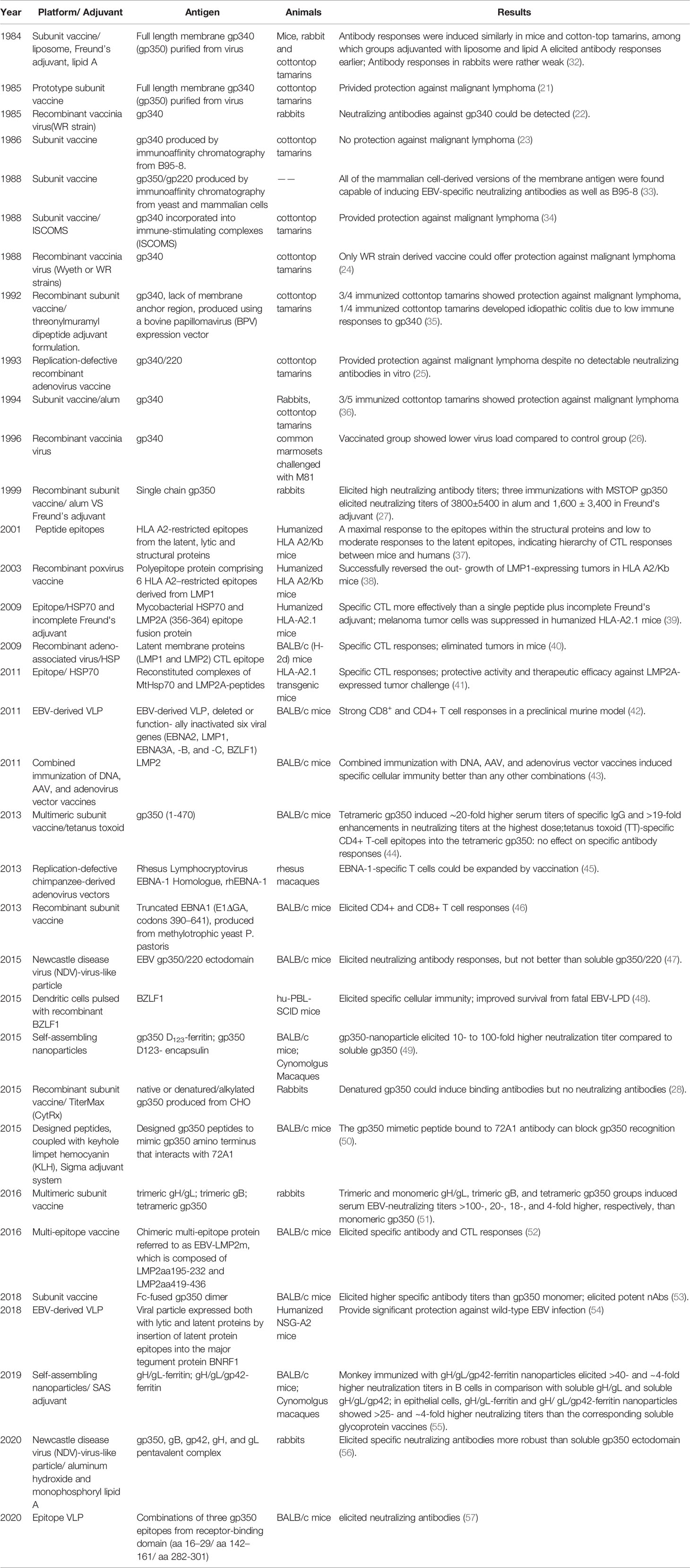
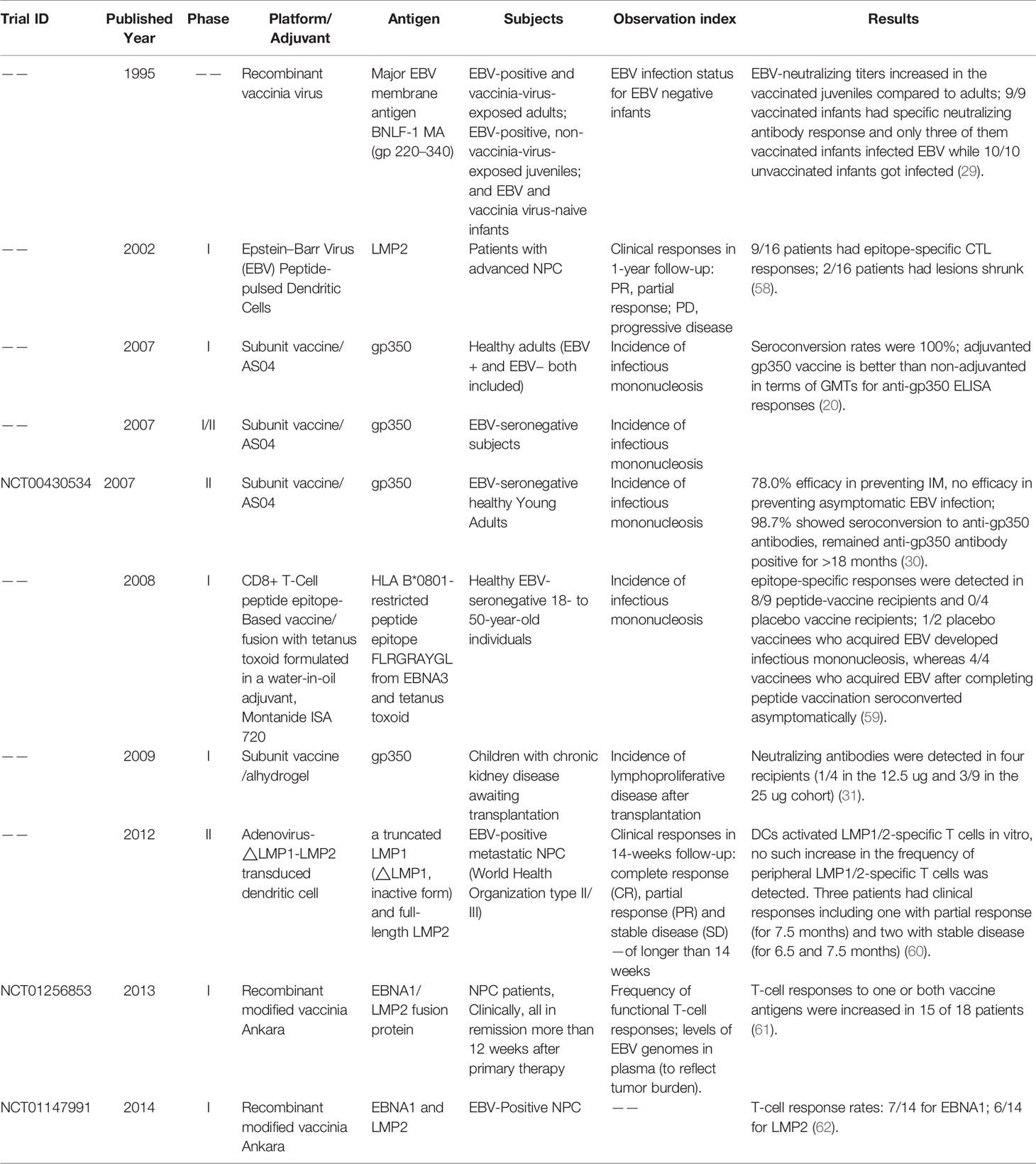
- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Department of Experimental Research, Sun Yat-sen University Cancer Center, Sun Yat-sen University, Guangzhou, China
Epstein–Barr virus (EBV) is a human herpesvirus that is common among the global population, causing an enormous disease burden. EBV can directly cause infectious mononucleosis and is also associated with various malignancies and autoimmune diseases. In order to prevent primary infection and subsequent chronic disease, efforts have been made to develop a prophylactic vaccine against EBV in recent years, but there is still no vaccine in clinical use. The outbreak of the COVID-19 pandemic and the global cooperation in vaccine development against SARS-CoV-2 provide insights for next-generation antiviral vaccine design and opportunities for developing an effective prophylactic EBV vaccine. With improvements in antigen selection, vaccine platforms, formulation and evaluation systems, novel vaccines against EBV are expected to elicit dual protection against infection of both B lymphocytes and epithelial cells. This would provide sustainable immunity against EBV-associated malignancies, finally enabling the control of worldwide EBV infection and management of EBV-associated diseases.
Introduction
Epstein–Barr virus (EBV) is a double-stranded DNA virus that belongs to the gamma herpesvirus family. It causes endemic infection in over 95% of the worldwide population (1), and is associated with diseases such as infectious mononucleosis (IM) and a broad range of lymphoid or epithelial malignancies (2, 3). It is estimated that approximately 2% of malignancies are caused by EBV infection, resulting in over 200,000 cases of EBV-associated cancer each year (4).
The transmission of EBV within the population is mainly mediated by saliva, and the infection involves both B lymphocytes and epithelial cells (5). Primary infection mostly occurs in early childhood with little or no overt symptoms (6). After the primary infection is established, EBV sustains a persistent infection in B lymphocytes, accompanied by the expression of specialized viral genes that maintain its latency, which is associated with B cell tumorigenesis (7). Therefore, a prophylactic EBV vaccine for establishing early protection against primary infection is critical for prevention both infectious diseases and EBV-associated malignancies. However, there is still no prophylactic vaccine against EBV in clinical use due to various reasons including antigen selection, vaccine platform used, and evaluation system for EBV vaccine assessment. Thus, in this review, we summarize the challenges and opportunities encountered in the development of a prophylactic EBV vaccine.
Antigen Selection for Vaccine Design
Glycoproteins
Similar to other herpesviruses, EBV is an enveloped virus, comprising a membrane decorated with envelope glycoproteins (such as gp350, gp42, gH, gL, and gB), which are crucial for receptor recognition, attachment, and virus–host membrane fusion (8). As EBV can infect both B lymphocytes and epithelial cells, the glycoproteins involved in the infection process of each cell type differ. For B cell infection, EBV gp350 interacts with CD21 or CD35 on B cells to establish viral binding, followed by the binding of gp42 in complex with gHgL to HLA class II on B cells, after which gB eventually triggers the membrane fusion in endocytic vesicles (9–12). By contrast, EBV adopts a rather different and more versatile combination of ligand–receptor paring during viral entry into epithelial cells. EBV can still use gp350 to establish attachment to CD21-expressing host cells (13), while BMRF2 (14) or the gH/gL complex binds to other host cell receptors to facilitate the infection of cells that lack CD21. Currently, integrins (15), non-muscle myosin heavy chain IIA (NMHC-IIA) (16) and ephrin receptor A2 (17, 18) (EphA2) are recognized as receptors for EBV gH/gL, while neuropilin-1 (19) (NRP1) acts as the receptor for EBV gB during epithelial cell infection, indicating a more important role of gH/gL complex and gB during the recognition and attachment in comparison to B cell infection (Figure 1).
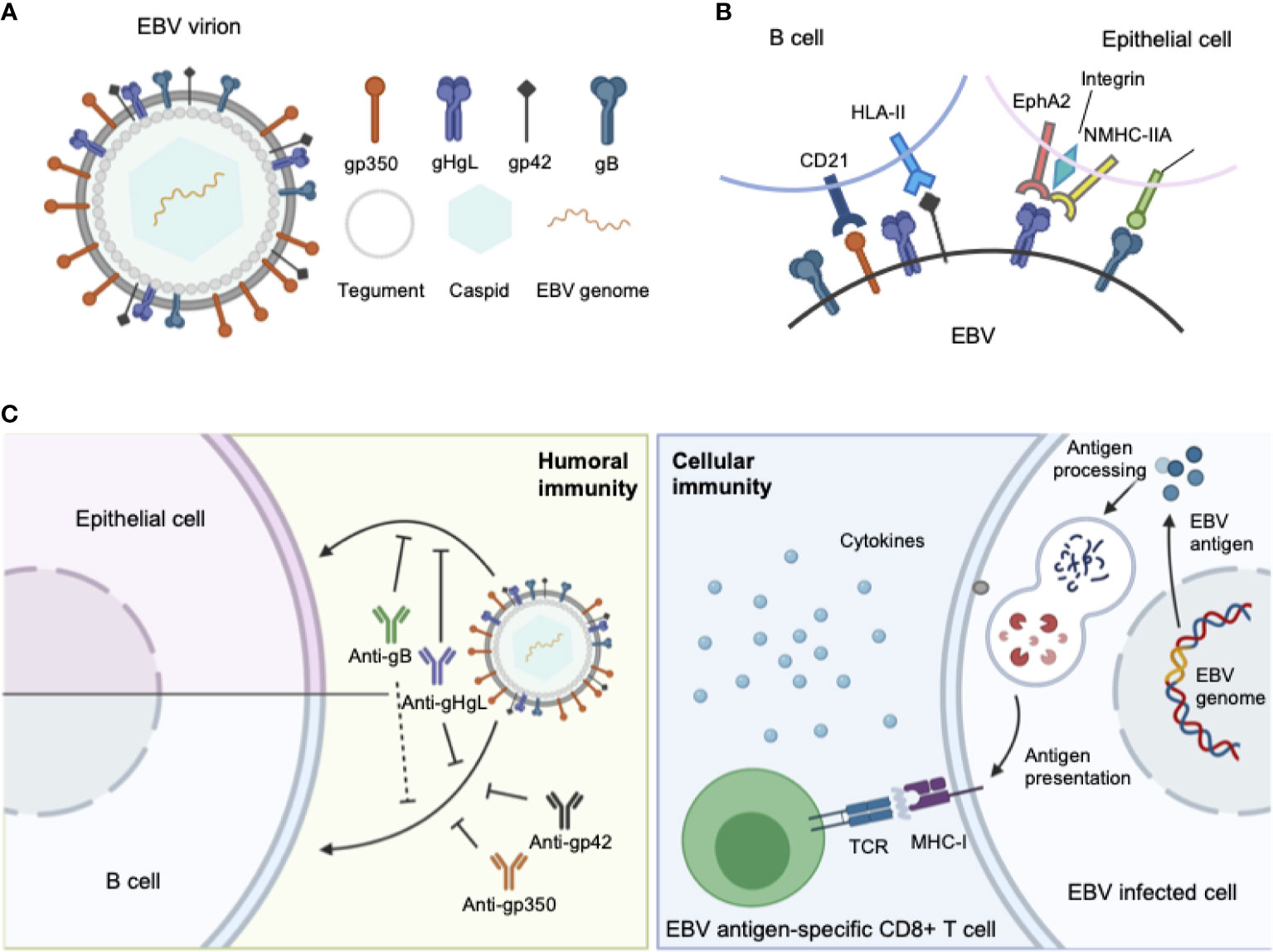
Figure 1 (A) Structure of the EBV virion. As an enveloped double-stranded DNA virus, the virion of EBV consists of a lipid membrane, tegument, viral capsid and the packed EBV genome. Glycoproteins are distributed on the virion membrane and are crucial for recognition, host cell attachment and membrane fusion. (B) The major interaction pattern of host cell receptors and the EBV membrane glycoproteins. EBV infects B lymphocytes and epithelial cells via different combinations of ligand–receptor interaction. (C) Humoral and cellular immunity against EBV infection. In humoral immunity, antibodies against various glycoproteins play different roles in the neutralization process. After infection, EBV antigen can be presented, inducing a cytotoxic CD8+ T cell response against infected cells.
The complex molecular machine of surface glycoproteins brings challenges for not only understanding the complete fusion mechanism of EBV, but also choosing appropriate antigens for vaccine development. Therefore, an ideal prophylactic vaccine against EBV should be able to elicit potent neutralizing antibodies against EBV infection in both B lymphocytes and epithelial cells, which requires careful selection of antigens during vaccine design.
gp350
As the first-isolated and most abundant EBV glycoprotein, gp350 was the most studied antigen for vaccine candidates and is the core antigen for the majority of the currently-developed EBV vaccines (20–28). The first clinical trial of a recombinant viral vector encoding gp350 performed in China in 1997 proved that gp350-specific antibodies could be elicited in both seronegative and seropositive children (29). In later studies, recombinant gp350 adjuvanted with AS04 was used as the vaccine in a phase II clinical trial among seronegative adults (30) and was shown to effectively reduce the incidence of IM compared to the placebo control group. However, this vaccine did not completely prevent EBV infection in the vaccinated population. In another phase I clinical trial gp350 was formulated with 0.2% Alhydrogel® as vaccine for reducing the risk of post-transplant lymphoproliferative disease (PTLD) (31). The vaccine failed to elicit neutralizing antibodies and control the viral titer in the majority of patients, possibly due to its low immunogenicity for immunosuppressed patients. Thus, despite the early and thorough study, gp350 exhibited only imperfect vaccination efficacy as single antigen in both preclinical and clinical trials (Figure 2A, Tables 1, 2).
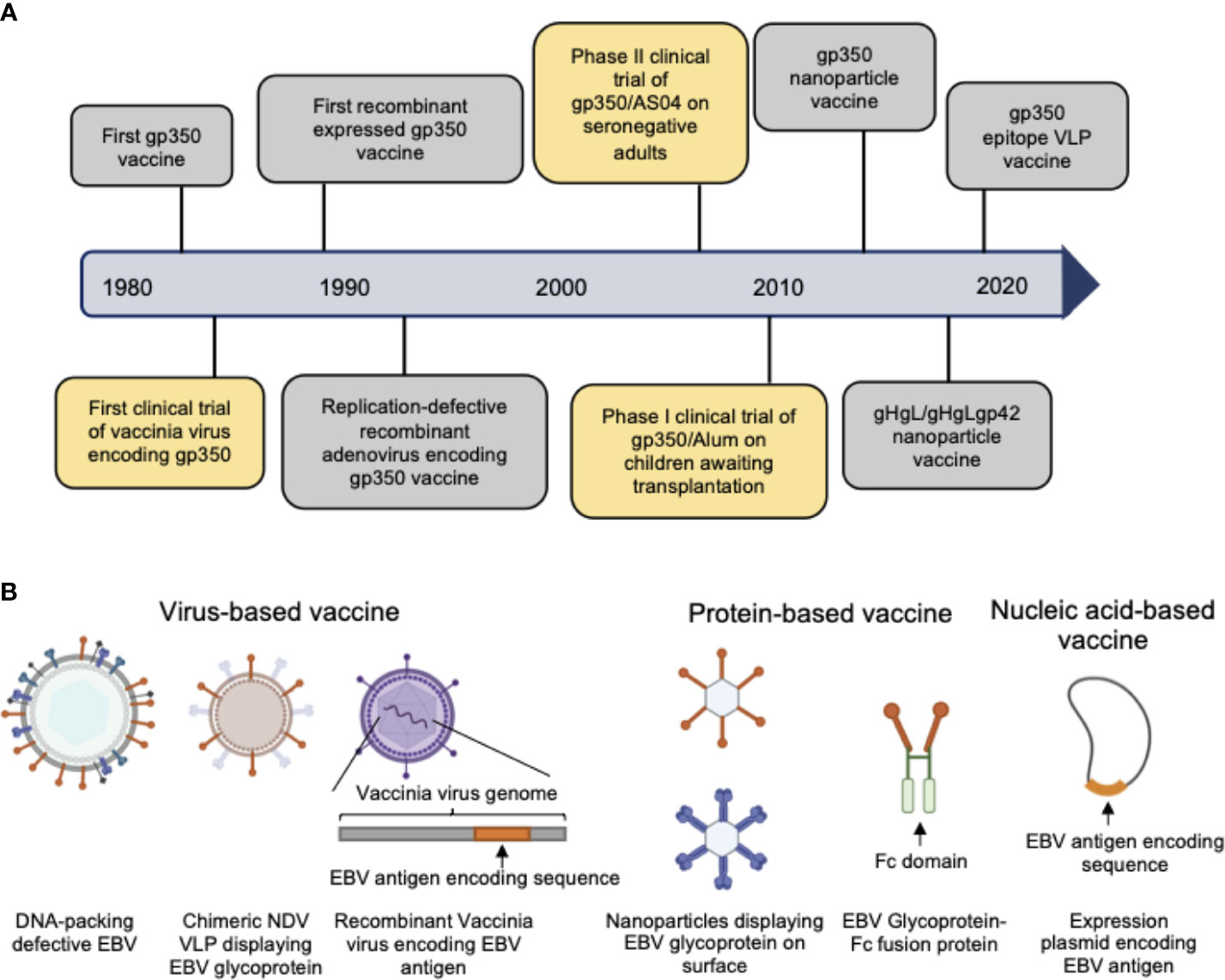
Figure 2 (A) Hallmarks of prophylactic EBV vaccine development using EBV glycoproteins as antigens. Clinical trials are marked in yellow box and others are marked in gray box. (B) Current candidate platforms for EBV vaccines, including virus-based, protein-based and nucleic-acid-based vaccines.
gH/gL
Since the gH/gL complex plays a critical role in the infection of B cells and especially epithelial cells, there is increasing focus on the gH/gL complex as the antigen for new vaccine candidates. The recently identified anti-gH/gL dual-tropic neutralizing antibody AMMO1 (63) further indicated that gH/gL may be an ideal antigen. In a study on rabbits (51), trimeric or monomeric gH/gL could elicit >100- and 18-fold higher EBV neutralizing antibody titers than monomeric gp350. Later nanoparticle vaccines displaying gH/gL or the gH/gL/gp42 complex were designed, and immunization assays in BALB/c mice demonstrated that the nanoparticle decorated with gH/gL or gH/gL/gp42 could elicit much higher neutralizing antibody titers than monomeric gH/gL or gH/gL/gp42 (55). Despite these promising results for gH/gL as a vaccine candidate, there are still no clinical trials examining whether gH/gL could provide broader protection than gp350 and possibly achieve complete protection from EBV infection.
gp42
gp42 is a subunit of the gH/gL/gp42 heterotrimer on the EBV virion membrane. It was identified as the ligand for HLA class II molecules mostly participating in B cell infection and was recently found to hinder the infection of epithelial cells (64, 65), indicating that it controls the tropism of EBV infection. The close structural connection and functional complexity suggested that a combination of gH/gL/gp42 as a complex antigen may be more potent than gp42 alone. Studies of the effect of immunization with the EBV viral fusion apparatus indicated that immunization using gH/gL in complex with gp42, either as monomers or nanoparticles, could elicit relatively higher neutralizing antibody titers against infection of both B lymphocytes and epithelial cells (55). Nevertheless, few studies investigated gp42 as the target for vaccine design. Moreover, its role in controlling the tropism of infection would complicate the protection efficacy of elicited antibodies against gp42, which may potentially influence the tropism of the original virus and enhance the efficiency of epithelial cell infection.
gB
EBV gB is the fusion protein on the viral surface mediating viral–host membrane fusion and recognizing NRP1 on epithelial cells. Most prophylactic antiviral vaccines target the viral fusion protein, such as influenza HA (66–68), HIV env (69, 70), Ebola virus GP (71, 72), and coronavirus spike protein (73, 74), as the fusion proteins in these viruses not only drive membrane fusion but also recognize host membrane factors to initiate attachment and trigger the fusion process. Thus, in these viruses the functional domain of the fusion protein is considered an ideal vulnerable site for neutralization. Hence, the similarity between EBV gB and other comprehensively studied viral fusion proteins indicates that gB could be a promising target for vaccine development. In addition to the AMMO1 antibody targeting EBV gHgL, anti-EBV gB AMMO2/3/4/5 discovered by Snijder et al. also demonstrated a strong neutralization activity against epithelial cell infection (63), supporting the use of gB as a prophylactic vaccine candidate. In addition, the previously mentioned research studying the efficacy of immunization with trimeric gH/gL in rabbits also explored gB, which was also able to elicit higher neutralizing antibody titers than gp350 (51).
With the development of protein structure analysis, the fusion status of fusion proteins becomes increasingly important for elucidating the fusion mechanism and understanding the connection between conformational changes and the fusion process. Pre-fusion status is often regarded as the natural conformation on the viral membrane (75, 76) before interacting with the host cell. The discovery of pre-fusion status and artificial modification to freeze the fusion protein in the pre-fusion conformation (77–79) greatly promoted vaccine development in recent years. During the SARS-CoV-2 pandemic, the pre-fusion-stabilized spike protein variant S-2P (80, 81) provided an ideal antigen for the design of broad-use COVID-19 vaccines. Similarly, pre-fusion-stabilized HIV env BG505-SOSIP (69) and RSV F DS-CAV1 (77, 79) also provided an impulse for vaccine development, since they could elicit much higher neutralizing antibody titers than the post-fusion conformation. Therefore, there is increasing focus on the conformation of EBV gB. However, the currently available crystal structure of gB shows a post-fusion conformation at pH8.0 (82), and there is still no high-resolution structure of any pre-fusion gB from the herpesvirus family. Although recent cryo-electron imaging studies of gB displayed on vesicles (83), pseudo-virus membranes or virions (84) were highly suggestive of a potential pre-fusion conformation of gB from other herpesviruses; more evidence and structural studies are required to define the pre-fusion form of gB from EBV, which would greatly promote the use of this antigen as a vaccine candidate.
Latent and Lytic Phase Proteins
After primary infection, EBV undergoes a short period of replication in the oropharynx, after which further infection of B cells ensues, during which glycoproteins encoded by the EBV genome become eclipsed by certain lytic and latent genes, which drive the B cell transformation and latency as summarized by a review (85). Therefore, neutralizing antibodies against glycoproteins cannot induce the clearance of latently infected cells which do not express the target, while T cell-mediated immunity would be critical for controlling EBV infection during pre-latency and latency. With a deeper understanding of the role of T cell immunity in the control of EBV infection and extensive mapping of immuno-focused T cell epitopes of EBV antigens (86–97), the application of latent or lytic phase proteins as vaccine antigens has become a topic of continuing study. Elliott et al. used the EBNA3 HLA-B8 T cell epitope FLRGRAYGL, adjuvanted with tetanus toxoid and Montanide ISA 720, as a vaccine in a phase I trial among EBV sero-negative adults (59). The results showed that despite good vaccine tolerance and reduced incidence of infectious mononucleosis, the vaccination did not protect the subjects from EBV infection. Other CTL epitopes based on LMP1 and LMP2A showed great potential in tumor treatment in preclinical studies (38–41, 52), but none displayed a clear viability as effective antigens to prevent primary infection. Thus, for prophylactic vaccine development, latent or lytic phase proteins could be used as auxiliary boosters for inducing adequate T cell responses, while the major glycoprotein antigens still play the key role in the prevention of primary infection.
Hence, during EBV vaccine development, rational and careful antigen selection is necessary to ensure both robust and comprehensive immunity against EBV infection. There is still a lot of space for extensive study on immunization efficacy of single glycoproteins, especially gH/gL or gB. Additionally, combinatorial use of multiple antigens as vaccine candidates, including glycoprotein sets or glycoprotein-latency protein combinations, deserves further study for eliciting both sufficient neutralizing antibody titers and T cell responses.
Vaccine Delivery Platforms and Formulations
Delivery Platform for Vaccine Design
The outbreak of the COVID-19 pandemic has brought significant challenges for global vaccine development, prompting a continuous stream of innovative designs of candidate vaccines against SARS-CoV-2, and thus giving great impetus to next-generation vaccine development. The rapid application of the first clinically used mRNA vaccine developed by Moderna and BioNTech (98, 99) achieved great success in combating SARS-CoV-2 and demonstrated that innovation of new vaccine designs could accelerate the procedures of vaccine development, provide more flexible platforms for antigen delivery, and improve immunization efficacy. Nevertheless, traditional platforms for vaccine development, such as weakened or inactivated virus (100–102), still account for the majority of currently available vaccines and have demonstrated their value during the COVID-19 pandemic due to their outstanding stability, immunogenicity, and convenience in distribution. Therefore, a wider array of adequate platforms for vaccine design is also critical for EBV vaccine development (Figure 2B).
Virus-Based Vaccines
Because EBV tends to establish a latent infection of host cells, a general approach to induce EBV replication and cell lysis requires complicated procedures and results in a low yield of live virus. Consequently, the development of attenuated virus or inactivated virus vaccines based on authentic EBV is challenging due to limited viral material. Thus, there are few reports on the use of inactivated or attenuated EBV as vaccine candidates. Alternatively, modification of EBV the genome for direct production of defective virions without genomic DNA could be a viable approach for EBV-derived vaccine development. EBV-derived virus-like particles (VLPs) are based on different EBV mutants with various deletions of sets of oncogenic genes or DNA packaging genes (103), produced by inducing cell lines to enter the lytic phase, followed by purification from cell supernatants by centrifugation. Multiple studies developed several EBV VLPs (delta BFLF1/BFRF1, delta BBRF1, delta BFLF2, delta TR terminal repeats) (42, 104–107) by deletion of certain critical genes to obstruct virus replication and DNA packaging. However, the possibility of repacking of EBV DNA would bring safety concerns to such designs. In addition to the construction of VLPs based on EBV itself, a Newcastle disease virus-like particle (ND VLP) platform was also used for the presentation of EBV antigens such as gp350/gp220, combinations of gHgL-EBNA1 or gB/LMP2, and even pentavalent gp350/gH/gL/gp42/gB (47, 56). It may be easier to produce VLPs by additional co-transfection of NDV-F for particle assembly, benefiting the rapid development of safe VLP vaccines.
Another approach for the development of virus-based vaccines is using viral vectors as carriers to deliver targeted antigens by generating recombinant vaccinia virus. After inserting specific sequences encoding EBV antigens into the genome of vaccinia virus, the recombinant virus can infect host cells and drive the expression of exogenous antigen in the cells, leading to antigen processing and presentation via the classic MHC-I pathway and activation of antigen-specific cytotoxic CD8+ T cells (108, 109). In addition, through maintaining the certain degree of replication function, attenuated self-replicated vaccinia virus could stimulate an even higher immune response than replication-defective virus. The vaccina virus also acted as a self-adjuvant by expressing a broad range of pathogen-associated molecular patterns (PAMPs), increasing the whole immunogenicity of vaccine. Currently, modified vaccinia virus Ankara (MVA) (61, 62, 110, 111), adenovirus (ADV) (60, 112, 113) and Varicella-zoster virus (VZV) (114) have been used as a vector to generate an EBV antigen-carrying recombinant live virus vaccine. However, this technology was more commonly used for developing therapeutic vaccines for the treatment of EBV-associated tumors due to the favorable stimulation of cellular immunity, while few trials investigated its use in prophylactic vaccines since the first human test using gp350 as antigen and smallpox-based vaccinia virus as viral vector (29) due to the uncertain safety and reported adverse events of this platform (115).
Protein-Based Vaccines
With the rapid development and great progress in structure-guided protein modification and design (116–122), recombinant proteins have gradually become an effective approach for accurate antigen immunization. As gp350 was firstly applied as antigen for EBV vaccine design, gp350 modification to promote immunization efficacy was also a focus during the early exploration of protein-based vaccines against EBV. In the late 20th century, soluble gp350 protein was successfully expressed as a vaccine antigen (33). Subsequent attempts to enhance the immunogenicity and improve the immunization efficacy aimed to increase the valency or target the protein to antigen presenting cells (APCs) using a variety of methods such as multimerization, nanoparticle assembly and fusion-protein design. For multivalency, tetrameric gp350 was designed by fusing two separate gp350 (1–470) to a C-terminal leucine-zipper with or without T cell epitopes, and the results showed that tetrameric gp350 could elicit higher neutralizing antibody titers than monomeric gp350 (51). Additionally, by fusing gp350 to ferritin or encapsulin, multivalent gp350 nanoparticles (49) were generated and immunization of mice or monkeys showed that nanoparticles elicited much higher neutralizing antibody titers than soluble monomeric gp350. Further, virus challenge experiments also demonstrated that gp350 nanoparticles provide better protection against EBV infection and improve the survival of challenged monkeys. In an effort to both increase the valency and enable APC-targeting, gp350 was fused with the Fc domain of mouse IgG2a (53, 123), rendering a dimeric antibody-like antigen which could target FcγR on antigen-presenting cells to prolong the retention time for recognition. In addition, the fused protein simplified the purification and detection.
Comparatively few studies investigated using other glycoproteins or latent phase proteins as antigens. Trimeric gHgL constructed by fusing gHgL to a C-terminal trimeric T4 bacteriophage fibritin and native trimeric gB were also tested as immunogens (51), and the results showed that trimeric gHgL could elicit higher neutralizing antibody titers than monomeric gHgL. Recently, analogous methodology was adopted to design gHgL or gHgL/gp42 nanoparticles by fusing the antigen to the 24-mer ferritin, whereby the neutralizing antibody titers of the nanoparticle-immunized groups were significantly higher than in the monomer groups as previously mentioned.
Instead of using the full length or a major segment of the protein, some studies attempted to use specific epitopes as antigens to induce site-specific immune responses and thereby achieve accurate immunization. Jerome et al. designed two 72A1-gp350 blocking peptides that mimic the interacting region of gp350 (50), which demonstrated that the neutralization epitope of the glycoprotein could be an ideal vaccine antigen. Afterwards, Zhang et al. inserted different tandem gp350 epitopes into HBC149 to construct a gp350 epitope-displaying VLP (57), and the neutralizing antibody titers of some gp350 epitope-VLP groups were even higher than that of gp350ECD123, a shortened version of the gp350 ectodomain, which compares favorably to the anti-gp350 nAb 72A1.
Nucleic-Acid Vaccines
The rapid and successful application of nucleic-acid SARS-CoV-2 vaccines demonstrated their great potential in viral vaccine development. This method, based on synthetic nucleic acids, enables large-scale manufacturing with almost perfect uniformity. Despite such advantages, the use of synthetic nucleic acids for EBV vaccine development is still in the early exploration phase. Krzysztof et al. developed DNA vaccines based on three EBV latency genes (EBNA1, LMP1 and LMP2A) (124) and found that the vaccine based on EBNA1 and LMP2A could elicit robust T cell immunity. Although mRNA vaccines are highly potent and can be rapidly manufactured, the development of an mRNA vaccine for EBV still awaits the first step. As expected, Moderna has announced its great ambitions in EBV mRNA vaccine development, with the candidate mRNA-1189 encoding all the major glycoproteins (gp350, gB, gH/gL, gp42).
Adjuvants for Vaccine Formulation
Adjuvants incorporated in components of the antigen for vaccine formulation can modulate the immune response. In addition to the original immunogenic profile of the selected antigen, a carefully selected adjuvant can broaden the use or enhance the efficacy for immunization. For vaccine platforms such as inactivated virus or protein-based subunit vaccines/VLPs, the loss of bioactivity greatly diminishes the immunogenicity of the antigen itself, which further requires powerful adjuvants for pre-stimulation of immune recognition, prolongation of antigen retention, as well as both humoral and cellular immunity enhancement (125–129).
The development of gp350-based vaccines inspired the exploration of adequate adjuvants for EBV vaccines. In the late 20th century, adjuvants such as Freund’s adjuvant, lipid A, immune-stimulating complexes (ISCOMS), and aluminum hydroxide (32, 34–36, 130) were used in the formulation of gp350 vaccines. Some may show superior immunization efficacy compared to unadjuvanted gp350 as immunogen, since an immunization trial of unadjuvanted gp350 subunit vaccine on cotton-top tamarins gave unsatisfactory results, with no protection against incidence of malignant lymphoma in spite of eliciting antibodies against gp350. With the use of more complicated adjuvant systems in recent years, a higher immunization efficacy achieved in preclinical studies supports the case for further clinical trials. However, due to the paucity of studies on other protein-based immunogens as vaccines against EBV, only a limited number of adjuvants were tested. For example, the VZV gE-based vaccine (Shingrix) was the first clinically approved herpesvirus vaccine providing protection against herpes zoster in older adults and immunosuppressed patients, while containing only VZV glycoprotein gE adjuvanted with AS01b (131). Although VZV gE was not used as a prophylactic vaccine antigen to prevent VZV infection, an appropriate combination with the adjuvant made gE into an ideal antigen (132), with benefits for controlling latent VZV infection. This result was based on a systematic screening of appropriate adjuvant systems (133). This study also offers insights for EBV vaccine development, confirming that smart selection of adjuvants can also contribute to the development of a powerful vaccine against EBV by enhancing both initial protection from primary infection and secondary protection from reactivation or expansion of latent infection.
Therefore, an appropriate platform and adjuvant systems also determine the immunization efficacy of the vaccine, and not just the antigen. The COVID-19 pandemic exemplifies the effective and rapid development of vaccines against broadly distributed infectious pathogens. Both mature, extensively tested technologies like inactivated virus (100) and emerging technologies like mRNA vaccines (98, 99) gave satisfactory results, demonstrating the unlimited opportunities of the available vaccine design platforms and encouraging further comparative studies on the use of a variety of platforms for EBV vaccine development. For virus-based vaccines, breakthroughs in the mass production of live EBV could be a solution for inactivated vaccine development, since the latency-preference and complicated induction procedures seriously hinder its manufacture. For the emerging protein- or nucleic acid-based vaccines, convenient modification of antigens to strengthen their immunogenicity and viable co-valency of multiple antigens to broaden the immune response spectrum are promising future approaches for vaccine development. Since the licensed VZV vaccine took the first step in clinical herpes virus immunization, it has brought home the lesson that appropriate adjuvants used in vaccine formulation can greatly enhance the immunization efficacy. Additionally, the rising application of specific toll-like receptor (TLR) agonists (134–136) provides additional alternatives in the selection of adjuvants to achieve specific immunization responses.
Evaluation Systems for Vaccines
Animal Models
Animal models are necessary and critical for the evaluation of infection or protection status against infectious disease pathogens and developing therapeutic drugs or vaccines. During the evaluation of vaccines against most pathogens, challenge experiments in animal models are considered the gold standard for the final assessment of vaccine efficacy (137–141). However, due to the restricted host tropism of EBV, a human herpesvirus, there is a limited range of susceptible candidate animal models (142–144) (Figure 3).
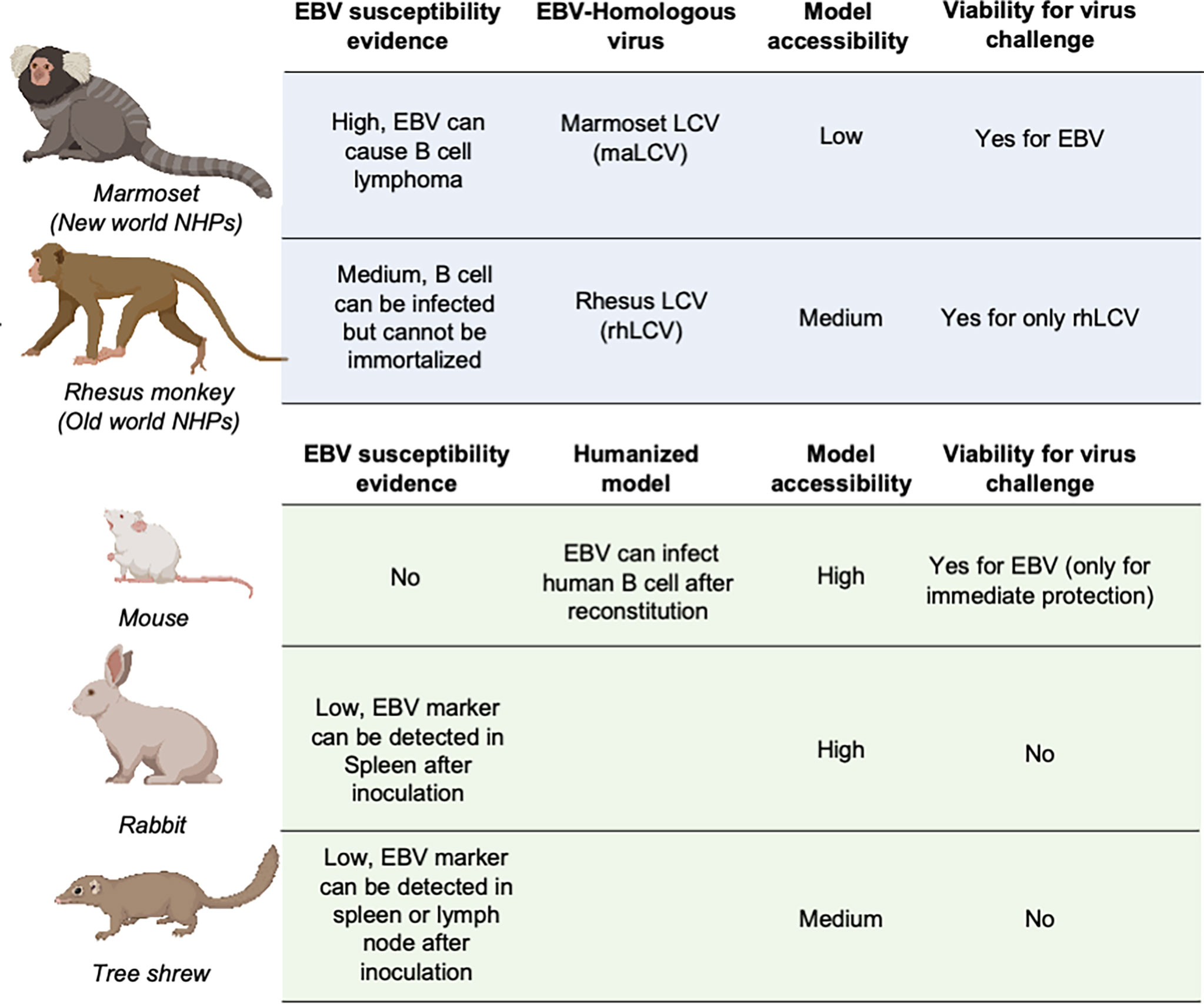
Figure 3 Animal models for EBV vaccine evaluation. Non-human primates are marked in light blue and other animal models are marked in light green. LCV, lymphocryptovirus.
Non-Human Primates
The great similarity between humans and non-human primates (NHPs) encouraged the use of NHPs as challenge models for EBV vaccine evaluation (145). The fact that New- and Old-World NHPs are naturally infected by EBV-related herpesviruses or lymphocryptoviruses (LCVs) further demonstrated the potential value of NHP in EBV vaccine evaluation.
In the late 20th century, the discoverer of EBV, Epstein et al. as well as Emini et al. used cotton-top tamarins and common marmosets (Callithrix jacchus) for gp350-based vaccine evaluation of both neutralizing antibody titers and challenge protection (146, 147). However, cotton-top tamarins are no longer a viable NHP model because of their critically endangered status, and the common marmoset is also listed on the IUCN Red List, which basically rules out these two NHPs from general use in EBV vaccine evaluation (148).
By contrast, rhesus macaque, as one of the Old World NHPs, has enjoyed broad use as an animal model for a variety of human viral infections, mostly due to its relatively larger population and successful artificial breeding. Although it is susceptible to its species-specific LCV (rhLCV), which shares a high level of genomic sequence similarity with EBV (149), EBV cannot stably infect and immortalize the B cells of rhesus macaques (150), which restricts the use of this animal model in challenge experiments. Therefore, the majority of EBV immunization studies used rhesus macaques as the animal model for evaluation of specific T cell responses (45, 151–153). And thus, instead of using EBV as challenge virus, rhLCV could be used as an equivalent virus for determining the immune protection from EBV infection. Singh et al. evaluated the protection efficacy of the anti-EBV gHgL neutralizing antibody AMMO1 via rhLCV challenge in rhesus macaques, and the protected animals showed higher plasma EBV neutralizing activity.
Other Mammalian Models
As the most widely used animal model, mice play an important role in EBV vaccine evaluation. Most prophylactic EBV vaccine studies used mouse immunization to primarily assess the serum antibody titer and neutralizing antibody titer (43, 44, 46). However, because mice cannot be naturally infected with EBV, humanized mice are used as an alternative animal model for EBV challenge experiments (37, 48, 54). These chimeric animals are constructed by transferring human CD34-positive hemopoietic stem cells into immunocompromised mice (154, 155). This model is appropriate for evaluating the efficacy of therapeutic treatment for immediate EBV challenge, rather than the eliciting of an adaptive immune response by a prophylactic EBV vaccine, since the mice have an incomplete immune system even after reconstitution and lack human epithelial cells. A humanized mouse model was also used to evaluate the protective efficacy of AMMO1 (63), and the results showed that the AMMO1 antibody could inhibit EBV infection.
Some studies also used rabbits as animal models for EBV vaccine evaluation (22, 27, 28, 51, 56, 155, 156). The anti-EBV VCA titer and EBV DNA level could be detected in the blood of most rabbits after intravenous, intranasal, or peroral inoculation. However, only a portion of rabbits showed positive EBERs, LMP1, or EBNA in splenectomized samples, and even fewer rabbits displayed sustained EBV positivity, accompanied by a heterogenous host reaction (157, 158). The uncertainty of the infection status hindered the use of rabbits as a challenge model, and most research studies only used rabbits as an immunization model for serum response evaluation (159).
Recently, it was found that the Chinese tree shrew (Tupaia belangeri subsp. chinensis) could also be a viable animal model for EBV vaccine evaluation. Following intravenous injection of virus, 8/10 tree shrews displayed symptoms of EBV infection including detectable expression of EBV-related genes and increase of anti-EBV antibodies. Despite positive results in early challenge, only a small portion of tree shrews showed EBER, LMP, and EBNA2-positive cells in spleen or mesenteric lymph node samples. The negative staining for EBV markers in the lungs and nasopharynx also indicated that epithelial cell infection might also be absent in the tree shrew animal model (160, 161).
Assessment of Immune Protection Efficacy
After confirming the design of a vaccine and immunization methods, assessment of immune protection efficacy would be critical for vaccine evaluation (162). For prophylactic vaccines against infectious pathogens, the key index revealing the efficacy of immunization protection is the neutralizing antibody (nAb) titer (163, 164), since neutralizing antibodies can efficiently block the virus from interacting with the host receptor, preventing viral attachment and membrane fusion. Therefore, a higher anti-EBV neutralizing antibody titer indicates better protection against EBV infection and could theoretically also reduce the incidence of EBV-associated malignancies. Although the presence of neutralizing antibodies is theoretically sufficient evidence for protection against viral infection, the value of this index in predicting the incidence of malignancies remained unclear (165, 166). A large cohort study conducted in Taiwan (167) indicated that EBV B cell neutralization capability of the serum or the anti-gp350 antibody titer was associated with lower risk of nasopharyngeal carcinoma. However, Zhu et al. recently performed a prospective cohort study on EBV glycoprotein-targeting neutralizing antibody titers in plasma samples from nasopharyngeal carcinoma (NPC) patients and healthy controls, which revealed that there was no significant difference in neutralizing antibody titers against EBV glycoproteins, including gp350, gHgL, gp42, and gB (168).
During the evaluation of immune reaction against EBV, the T cell response is also considered critical part, especially for eliminating latent infection and adaptive immune responses against EBV-associated tumors (58, 60–62, 169, 170). A review concluded that T cell responses participate in the control of EBV in all phases of infection (171, 172). However, the majority of vaccine studies evaluating the T cell response were based on latent-phase proteins such as LMP and EBNA, while studies on T cell responses induced by EBV glycoproteins or T cell epitope mapping for glycoproteins were relatively rare. Thus, further studies on the T cell response elicited by EBV glycoproteins for controlling both primary infection and regulating immunological surveillance against EBV-associated malignant diseases could provide guidance for improving the evaluation systems for the assessment of prophylactic vaccine efficacy.
Conclusion and Prospects
In recent years, prophylactic vaccines against EBV received significant attention, since the latest achievements in fundamental virology, vaccine technology and synthetic biology have brought new opportunities for vaccine development.
Early research studies on gp350 as a vaccine candidate revealed intrinsic shortage of gp350 in eliciting sufficient humoral immunity against primary infection. But still these studies become the forerunner for exploration of EBV glycoproteins as vaccine candidates. Recent progress in the discovery of epithelial cell receptors and elucidation of the infection mechanism of EBV highlights the critical function of gH/gL and gB during virus–host interaction and membrane fusion, indicating that they could be ideal major vaccine target for eliciting robust neutralizing antibody. Besides glycoproteins, although immunization with lytic and latent phase proteins is not able to provide protection against primary infection, the strong T cell immune response elicited by these proteins benefits the establishment of lasting immune surveillance of EBV latent infection and reinforcement of anti-EBV immunity after primary humoral defense. Additionally, an appropriate vaccine platform can improve the immunogenicity of certain antigens and enhance immune recognition. The adoption of protein modification via multimerization or fusion with immune cell-targeting domains may provide more possibilities for protein-based vaccines, while the application of synthetic nucleic acids as delivery systems could be the next milestone in the evolution of general vaccine design for not only EBV but all pathogens. Beyond vaccine design, a finer system for the evaluation of vaccine efficacy is also crucial for the development of a successful vaccine. A suitable animal model for EBV challenge is required. Further studies on the EBV-susceptibility of non-NHP models or viable NHP models would be as important as the innovation in EBV vaccine design. And it remains unclear whether T cell responses should be listed in the assessment system for determining the protection efficacy of EBV prophylactic vaccines, urging more intensive research on the connection between elicited cellular immunity and protection from both primary infection and malignancies.
Prospectively, with the advancement in understanding of immunity against EBV infection, more vaccine targets would be discovered, and using combinatorial antigens as vaccine candidate may display even promising immunization efficacy. The emerging vaccine platforms such as nanoparticle or mRNA may enjoy a broader application in development of EBV vaccines. And further studies on searching better animal models and evaluation indicators for assessment of EBV vaccine are required to assist the validation of protection efficacy after immunization.
Author Contributions
CS wrote the original manuscript and generated the figures. XC generated the summary table of animal trials and clinical trials. YK and MZ provided guidance and reviewed the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (81801645, 82030046).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with one of the authors MSZ.
References
1. Crawford DH, Rickinson A, Johannessen IL. Cancer Virus: The Story of Epstein-Barr Virus. 1st. New York: Oxford University Press, Oxford, United Kingdom (2014).
2. de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global Burden Of Cancers Attributable To Infections In 2008: A Review And Synthetic Analysis. Lancet Oncol (2012) 13:607–15. doi: 10.1016/S1470-2045(12)70137-7
3. Khan G, Hashim MJ. Global Burden Of Deaths From Epstein-barr Virus Attributable Malignancies 1990-2010. Infect Agent Cancer (2014) 9:38. doi: 10.1186/1750-9378-9-38
4. Cohen JI, Fauci AS, Varmus H, Nabel GJ. Epstein-Barr Virus: An Important Vaccine Target For Cancer Prevention. Sci Transl Med (2011) 3:107fs107. doi: 10.1126/scitranslmed.3002878
5. Laichalk LL, Hochberg D, Babcock GJ, Freeman RB, Thorley-Lawson DA. The Dispersal of Mucosal Memory B Cells: Evidence From Persistent EBV Infection. Immunity (2002) 16:745–54. doi: 10.1016/S1074-7613(02)00318-7
6. Balfour HH Jr., Odumade OA, Schmeling DO, Mullan BD, Ed JA, Knight JA, et al. Behavioral, Virologic, and Immunologic Factors Associated With Acquisition and Severity of Primary Epstein-Barr Virus Infection in University Students. J Infect Dis (2013) 207:80–8. doi: 10.1093/infdis/jis646
7. Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV Persistence in Memory B Cells In Vivo. Immunity (1998) 9:395–404. doi: 10.1016/S1074-7613(00)80622-6
8. Johannsen E, Luftig M, Chase MR, Weicksel S, Cahir-McFarland E, Illanes D, et al. Proteins of Purified Epstein-Barr Virus. Proc Natl Acad Sci USA (2004) 101:16286–91. doi: 10.1073/pnas.0407320101
9. Ogembo JG, Kannan L, Ghiran I, Nicholson-Weller A, Finberg RW, Tsokos GC, et al. Human Complement Receptor Type 1/CD35 Is an Epstein-Barr Virus Receptor. Cell Rep (2013) 3:371–85. doi: 10.1016/j.celrep.2013.01.023
10. Sathiyamoorthy K, Hu YX, Mohl BS, Chen J, Longnecker R, Jardetzky TS. Structural Basis for Epstein-Barr Virus Host Cell Tropism Mediated by gp42 and gHgL entry glycoproteins. Nat Commun (2016) 7:13557. doi: 10.1038/ncomms13557
11. Sathiyamoorthy K, Jiang JS, Hu YX, Rowe CL, Mohl BS, Chen J, et al. Assembly and Architecture of the EBV B Cell Entry Triggering Complex. PloS Pathog (2014) 10(8):e1004309. doi: 10.1371/journal.ppat.1004309
12. Fingeroth JD, Weis JJ, Tedder TF, Strominger JL, Biro PA, Fearon DT. Epstein-Barr Virus Receptor of Human B lymphocytes is the C3d Receptor CR2. Proc Natl Acad Sci USA (1984) 81:4510–4. doi: 10.1073/pnas.81.14.4510
13. Nemerow GR, Mold C, Schwend VK, Tollefson V, Cooper NR. Identification of gp350 as The Viral Glycoprotein Mediating Attachment of Epstein-Barr virus (EBV) to the EBV/C3d Receptor of B Cells: Sequence Homology of gp350 and C3 Complement Fragment C3d. J Virol (1987) 61:1416–20. doi: 10.1128/JVI.61.5.1416-1420.1987
14. Tugizov SM, Berline JW, Palefsky JM. Epstein-Barr Virus Infection of Polarized Tongue and Nasopharyngeal Epithelial Cells. Nat Med (2003) 9:307–14. doi: 10.1038/nm830
15. Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. Fusion of Epithelial Cells by Epstein-Barr Virus Proteins is trIggered by Binding of Viral Glycoproteins gHgL to Integrins Alphavbeta6 or Alphavbeta8. Proc Natl Acad Sci USA (2009) 106:20464–9. doi: 10.1073/pnas.0907508106
16. Xiong D, Du Y, Wang HB, Zhao B, Zhang H, Li Y, et al. Nonmuscle Myosin Heavy Chain IIA Mediates Epstein-Barr Virus Infection of Nasopharyngeal Epithelial Cells. Proc Natl Acad Sci USA (2015) 112:11036–41. doi: 10.1073/pnas.1513359112
17. Chen J, Sathiyamoorthy K, Zhang X, Schaller S, Perez White BE, Jardetzky TS, et al. Ephrin Receptor A2 is a Functional Entry Receptor for Epstein-Barr Virus. Nat Microbiol (2018) 3:172–80. doi: 10.1038/s41564-017-0081-7
18. Zhang H, Li Y, Wang HB, Zhang A, Chen ML, Fang ZX, et al. Ephrin Receptor A2 is an Epithelial Cell Receptor for Epstein-Barr Virus Entry. Nat Microbiol (2018) 3:1–8. doi: 10.1038/s41564-018-0155-1
19. Wang HB, Zhang H, Zhang JP, Li Y, Zhao B, Feng GK, et al. Neuropilin 1 is an Entry Factor That Promotes EBV Infection of Nasopharyngeal Epithelial Cells. Nat Commun (2015) 6:6240. doi: 10.1038/ncomms7240
20. Moutschen M, Leonard P, Sokal EM, Smets F, Haumont M, Mazzu P, et al. Phase I/II Studies To Evaluate Safety And Immunogenicity Of A Recombinant Gp350 Epstein-Barr Virus Vaccine In Healthy Adults. Vaccine (2007) 25:4697–705. doi: 10.1016/j.vaccine.2007.04.008
21. Epstein MA, Morgan AJ, Finerty S, Randle BJ, Kirkwood JK. Protection Of Cottontop Tamarins Against Epstein-Barr Virus-Induced Malignant Lymphoma By A Prototype Subunit Vaccine. Nature (1985) 318:287–9. doi: 10.1038/318287a0
22. Mackett M, Arrand JR. Recombinant Vaccinia Virus Induces Neutralising Antibodies in Rabbits Against Epstein-Barr Virus Membrane Antigen gp340. EMBO J (1985) 4:3229–34. doi: 10.1002/j.1460-2075.1985.tb04070.x
23. Epstein MA, Randle BJ, Finerty S, Kirkwood JK. Not all Potently Neutralizing, Vaccine-Induced Antibodies to Epstein-Barr Virus Ensure Protection of Susceptible Experimental Animals. Clin Exp Immunol (1986) 63:485–90.
24. Morgan AJ, Mackett M, Finerty S, Arrand JR, Scullion FT, Epstein MA. Recombinant Vaccinia Virus Expressing Epstein-Barr Virus Glycoprotein gp340 Protects Cottontop Tamarins Against EB Virus-Induced Malignant Lymphomas. J Med Virol (1988) 25:189–95. doi: 10.1002/jmv.1890250209
25. Ragot T, Finerty S, Watkins PE, Perricaudet M, Morgan AJ. Replication-Defective Recombinant Adenovirus Expressing the Epstein-Barr Virus (EBV) Envelope Glycoprotein gp340/220 Induces Protective Immunity Against EBV-Induced Lymphomas in the Cottontop Tamarin. J Gen Virol (1993) 74(Pt 3):501–7. doi: 10.1099/0022-1317-74-3-501
26. Mackett M, Cox C, Pepper SD, Lees JF, Naylor BA, Wedderburn N, et al. Immunisation of Common Marmosets With Vaccinia Virus Expressing Epstein-Barr Virus (EBV) gp340 and Challenge with EBV. J Med Virol (1996) 50:263–71. doi: 10.1002/(SICI)1096-9071(199611)50:3<263::AID-JMV9>3.0.CO;2-7
27. Jackman WT, Mann KA, Hoffmann HJ, Spaete RR. Expression of Epstein-Barr Virus gp350 as a Single Chain Glycoprotein for an EBV Subunit Vaccine. Vaccine (1999) 17:660–8. doi: 10.1016/S0264-410X(98)00248-5
28. Servat E, Ro BW, Cayatte C, Gemmell L, Barton C, Rao E, et al. Identification of the Critical Attribute(s) of EBV gp350 Antigen Required for Elicitation of a Neutralizing Antibody Response In Vivo. Vaccine (2015) 33:6771–7. doi: 10.1016/j.vaccine.2015.10.024
29. Gu SY, Huang TM, Ruan L, Miao YH, Lu H, Chu CM, et al. First EBV Vaccine Trial in Humans Using Recombinant Vaccinia Virus Expressing the Major Membrane Antigen. Dev Biol Stand (1995) 84:171–7.
30. Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Leonard P, Moreels A, et al. Recombinant gp350 Vaccine For Infectious Mononucleosis: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Safety, Immunogenicity, and Efficacy of an Epstein-Barr Virus Vaccine in Healthy Young Adults. J Infect Dis (2007) 196:1749–53. doi: 10.1086/523813
31. Rees L, Tizard EJ, Morgan AJ, Cubitt WD, Finerty S, Oyewole-Eletu TA, et al. A Phase I Trial of Epstein-Barr Virus Gp350 Vaccine for Children With Chronic Kidney Disease Awaiting Transplantation. Transplantation (2009) 88:1025–9. doi: 10.1097/TP.0b013e3181b9d918
32. Morgan AJ, Epstein MA, North JR. Comparative Immunogenicity Studies on Epstein-Barr Virus Membrane Antigen (MA) gp340 With Novel Adjuvants in Mice, Rabbits, and Cotton-Top Tamarins. J Med Virol (1984) 13:281–92. doi: 10.1002/jmv.1890130310
33. Emini EA, Schleif WA, Armstrong ME, Silberklang M, Schultz LD, Lehman D, et al. Antigenic Analysis of the Epstein-Barr Virus Major Membrane Antigen (gp350/220) Expressed in Yeast and Mammalian Cells: Implications for the Development of a Subunit Vaccine. Virology (1988) 166:387–93. doi: 10.1016/0042-6822(88)90509-0
34. Morgan AJ, Finerty S, Lovgren K, Scullion FT, Morein B. Prevention of Epstein-Barr (EB) Virus-Induced Lymphoma in Cottontop Tamarins by Vaccination with the EB Virus Envelope Glycoprotein gp340 Incorporated Into Immune-Stimulating Complexes. J Gen Virol (1988) 69( Pt 8):2093–6. doi: 10.1099/0022-1317-69-8-2093
35. Finerty S, Tarlton J, Mackett M, Conway M, Arrand JR, Watkins PE, et al. Protective Immunization Against Epstein-Barr Virus-Induced Disease in Cottontop Tamarins Using the Virus Envelope Glycoprotein gp340 Produced From a Bovine Papillomavirus Expression Vector. J Gen Virol (1992) 73( Pt 2):449–53. doi: 10.1099/0022-1317-73-2-449
36. Finerty S, Mackett M, Arrand JR, Watkins PE, Tarlton J, Morgan AJ. Immunization of Cottontop Tamarins and Rabbits with a Candidate Vaccine Against the Epstein-Barr Virus Based on the Major Viral Envelope Glycoprotein gp340 and Alum. Vaccine (1994) 12:1180–4. doi: 10.1016/0264-410X(94)90240-2
37. Bharadwaj M, Sherritt M, Khanna R, Moss DJ. Contrasting Epstein-Barr Virus-Specific Cytotoxic T Cell Responses to HLA A2-Restricted Epitopes in Humans and HLA Transgenic Mice: Implications for Vaccine Design. Vaccine (2001) 19:3769–77. doi: 10.1016/S0264-410X(01)00085-8
38. J D, M S, S T, J T, L C, G C, et al. Therapeutic LMP1 Polyepitope Vaccine for EBV-Associated Hodgkin Disease and Nasopharyngeal Carcinoma. Blood (2003) 101:3150–6. doi: 10.1182/blood-2002-10-3092
39. Liu G, Yao K, Wang B, Chen Y, Zhou F, Guo Y, et al. Immunotherapy of Epstein-Barr Virus Associated Malignancies Using Mycobacterial HSP70 and LMP2A356-364 Epitope Fusion Protein. Cell Mol Immunol (2009) 6:423–31. doi: 10.1038/cmi.2009.54
40. Pan J, Zhang Q, Zhou J, Ma D, Xiao X, Wang DW. Recombinant Adeno-Associated Virus Encoding Epstein-Barr Virus Latent Membrane Proteins Fused With Heat Shock Protein as a Potential Vaccine for Nasopharyngeal Carcinoma. Mol Cancer Ther (2009) 8:2754–61. doi: 10.1158/1535-7163.MCT-08-1176
41. Liu G, Yao K, Wang B, Zhou F, Chen Y, Li L, et al. Reconstituted Complexes of Mycobacterial HSP70 and EBV LMP2A-Derived Peptides Elicit Peptide-Specific Cytotoxic T Lymphocyte Responses and Anti-Tumor Immunity. Vaccine (2011) 29:7414–23. doi: 10.1016/j.vaccine.2011.07.063
42. Ruiss R, Jochum S, Wanner G, Reisbach G, Hammerschmidt W, Zeidler R. A Virus-Like Particle-Based Epstein-Barr Virus Vaccine. J Virol (2011) 85:13105–13. doi: 10.1128/JVI.05598-11
43. Wang Z, Yang S, Zhou L, Du H, Mo W, Zeng Y. Specific Cellular Immune Responses in Mice Immunized with DNA, Adeno-Associated Virus and Adenoviral Vaccines of Epstein-Barr Virus-LMP2 Alone or in Combination. Sci China Life Sci (2011) 54:263–6. doi: 10.1007/s11427-011-4147-0
44. Cui X, Cao Z, Sen G, Chattopadhyay G, Fuller DH, Fuller JT, et al. A Novel Tetrameric gp350 1-470 as a Potential Epstein-Barr Virus Vaccine. Vaccine (2013) 31:3039–45. doi: 10.1016/j.vaccine.2013.04.071
45. Silveira EL, Fogg MH, Leskowitz RM, Ertl HC, Wiseman RW, O’Connor DH, et al. Therapeutic Vaccination Against the Rhesus Lymphocryptovirus EBNA-1 Homologue, rhEBNA-1, Elicits T Cell Responses to Novel Epitopes in Rhesus Macaques. J Virol (2013) 87:13904–10. doi: 10.1128/JVI.01947-13
46. Wang M, Jiang S, Liu X, Wang Y. Expression, Purification, and Immunogenic Characterization of Epstein-Barr Virus Recombinant EBNA1 Protein in Pichia pastoris. Appl Microbiol Biotechnol (2013) 97:6251–62. doi: 10.1007/s00253-013-4967-x
47. Ogembo JG, Muraswki MR, McGinnes LW, Parcharidou A, Sutiwisesak R, Tison T, et al. A Chimeric EBV gp350/220-Based VLP Replicates the Virion B-Cell Attachment Mechanism and Elicits Long-Lasting Neutralizing Antibodies in Mice. J Transl Med (2015) 13:50. doi: 10.1186/s12967-015-0415-2
48. Hartlage AS, Liu T, Patton JT, Garman SL, Zhang X, Kurt H, et al. The Epstein-Barr Virus Lytic Protein BZLF1 as a Candidate Target Antigen for Vaccine Development. Cancer Immunol Res (2015) 3:787–94. doi: 10.1158/2326-6066.CIR-14-0242
49. M K, W B, MG J, G M, JR W, U B, et al. Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site. Cell (2015) 162:1090–100. doi: 10.1016/j.cell.2015.07.043
50. Tanner JE, Coincon M, Leblond V, Hu J, Fang JM, Sygusch J, et al. Peptides Designed To Spatially Depict the Epstein-Barr Virus Major Virion Glycoprotein gp350 Neutralization Epitope Elicit Antibodies That Block Virus-Neutralizing Antibody 72A1 Interaction with the Native gp350 Molecule. J Virol (2015) 89:4932–41. doi: 10.1128/JVI.03269-14
51. Cui X, Cao Z, Chen Q, Arjunaraja S, Snow AL, Snapper CM. Rabbits immunized with Epstein-Barr virus gH/gL or gB Recombinant Proteins Elicit Higher Serum Virus Neutralizing Activity Than gp350. Vaccine (2016) 34:4050–5. doi: 10.1016/j.vaccine.2016.06.021
52. Lin X, Chen S, Xue X, Lu L, Zhu S, Li W, et al. Chimerically Fused Antigen Rich of Overlapped Epitopes From Latent Membrane Protein 2 (LMP2) of Epstein-Barr Virus as a Potential Vaccine and Diagnostic Agent. Cell Mol Immunol (2016) 13:492–501. doi: 10.1038/cmi.2015.29
53. Zhao B, Zhang X, Krummenacher C, Song S, Gao L, Zhang H, et al. Immunization With Fc-Based Recombinant Epstein-Barr Virus gp350 Elicits Potent Neutralizing Humoral Immune Response in a BALB/c Mice Model. Front Immunol (2018) 9:932. doi: 10.3389/fimmu.2018.00932
54. van Zyl DG, Tsai MH, Shumilov A, Schneidt V, Poirey R, Schlehe B, et al. Immunogenic Particles With a Broad Antigenic Spectrum Stimulate Cytolytic T Cells and Offer Increased Protection Against EBV Infection Ex Vivo and in Mice. PloS Pathog (2018) 14:e1007464. doi: 10.1371/journal.ppat.1007464
55. Bu W, Joyce MG, Nguyen H, Banh DV, Aguilar F, Tariq Z, et al. Immunization with Components of the Viral Fusion Apparatus Elicits Antibodies That Neutralize Epstein-Barr Virus in B Cells and Epithelial Cells. Immunity (2019) 50:1305–16.e1306. doi: 10.1016/j.immuni.2019.03.010
56. Escalante GM, Foley J, Mutsvunguma LZ, Rodriguez E, Mulama DH, Muniraju M, et al. A Pentavalent Epstein-Barr Virus-Like Particle Vaccine Elicits High Titers of Neutralizing Antibodies Against Epstein-Barr Virus Infection in Immunized Rabbits. Vaccines (Basel) (2020) 8(2):169. doi: 10.3390/vaccines8020169
57. Zhang X, Zhao B, Ding M, Song S, Kang Y, Yu Y, et al. A Novel Vaccine Candidate Based on Chimeric Virus-Like Particle Displaying Multiple Conserved Epitope Peptides Induced Neutralizing Antibodies Against EBV Infection. Theranostics (2020) 10:5704–18. doi: 10.7150/thno.42494
58. Lin CL, Lo WF, Lee TH, Ren Y, Hwang SL, Cheng YF, et al. Immunization with Epstein-Barr virus (EBV) Peptide-Pulsed Dendritic Cells Induces Functional CD8+T-Cell Immunity and May Lead to Tumor Regression in Patients with EBV-Positive Nasopharyngeal Carcinoma. Cancer Res (2002) 62:6952–8.
59. Elliott SL, Suhrbier A, Miles JJ, Lawrence G, Pye SJ, Le TT, et al. Phase I Trial of a CD8(+) T-Cell Peptide Epitope-Based Vaccine for Infectious Mononucleosis. J Virol (2008) 82:1448–57. doi: 10.1128/JVI.01409-07
60. Chia WK, Wang WW, Teo M, Tai WM, Lim WT, Tan EH, et al. A Phase II Study Evaluating the Safety and Efficacy of an Adenovirus-ΔLMP1-LMP2 Transduced Dendritic Cell Vaccine in Patients with Advanced Metastatic Nasopharyngeal Carcinoma. Ann Oncol (2012) 23:997–1005. doi: 10.1093/annonc/mdr341
61. Hui EP, Taylor GS, Jia H, Ma BBY, Chan SL, Ho R, et al. Phase I Trial of Recombinant Modified Vaccinia Ankara Encoding Epstein-Barr Viral Tumor Antigens in Nasopharyngeal Carcinoma Patients. Cancer Res (2013) 73:1676–88. doi: 10.1158/0008-5472.CAN-12-2448
62. Taylor GS, Jia H, Harrington K, Lee LW, Turner J, Ladell K, et al. A Recombinant Modified Vaccinia Ankara Vaccine Encoding Epstein-Barr Virus (EBV) Target Antigens: A Phase I Trial in UK Patients with EBV-Positive Cancer. Clin Cancer Res (2014) 20:5009–22. doi: 10.1158/1078-0432.CCR-14-1122-T
63. Snijder J, Ortego MS, Weidle C, Stuart AB, Gray MD, McElrath MJ, et al. An Antibody Targeting the Fusion Machinery Neutralizes Dual-Tropic Infection and Defines a Site of Vulnerability on Epstein-Barr Virus. Immunity (2018) 48:799–+. doi: 10.1016/j.immuni.2018.03.026
64. Borza CM, Hutt-Fletcher LM. Alternate Replication in B Cells and Epithelial Cells Switches Tropism of Epstein-Barr Virus. Nat Med (2002) 8:594–9. doi: 10.1038/nm0602-594
65. Kirschner AN, Omerovic J, Popov B, Longnecker R, Jardetzky TS. Soluble Epstein-Barr Virus Glycoproteins gH, gL, and gp42 Form a 1:1:1 Stable Complex That Acts Like Soluble gp42 in B-cell Fusion But Not in Epithelial Cell Fusion. J Virol (2006) 80:9444–54. doi: 10.1128/JVI.00572-06
66. Yassine HM, Boyington JC, McTamney PM, Wei CJ, Kanekiyo M, Kong WP, et al. Hemagglutinin-Stem Nanoparticles Generate Heterosubtypic Influenza Protection. Nat Med (2015) 21:1065–70. doi: 10.1038/nm.3927
67. Pardi N, Parkhouse K, Kirkpatrick E, McMahon M, Zost SJ, Mui BL, et al. Nucleoside-Modified mRNA Immunization Elicits Influenza Virus Hemagglutinin Stalk-Specific Antibodies. Nat Commun (2018) 9:3361. doi: 10.1038/s41467-018-05482-0
68. Demminger DE, Walz L, Dietert K, Hoffmann H, Planz O, Gruber AD, et al. Adeno-Associated Virus-Vectored Influenza Vaccine Elicits Neutralizing and Fcγ Receptor-Activating Antibodies. EMBO Mol Med (2020) 12:e10938. doi: 10.15252/emmm.201910938
69. Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, et al. Structure and Immune Recognition of Trimeric Pre-Fusion HIV-1 Env. Nature (2014) 514:455–61. doi: 10.1038/nature13808
70. Brouwer PJM, Antanasijevic A, Berndsen Z, Yasmeen A, Fiala B, Bijl TPL, et al. Enhancing and Shaping the Immunogenicity of Native-Like HIV-1 Envelope Trimers With a Two-Component Protein Nanoparticle. Nat Commun (2019) 10:4272. doi: 10.1038/s41467-019-12080-1
71. Agnandji ST, Fernandes JF, Bache EB, Obiang Mba RM, Brosnahan JS, Kabwende L, et al. Safety and Immunogenicity of rVSVΔG-ZEBOV-GP Ebola Vaccine in Adults and Children in Lambaréné, Gabon: A Phase I Randomised Trial. PloS Med (2017) 14:e1002402. doi: 10.1371/journal.pmed.1002402
72. Clarke DK, Xu R, Matassov D, Latham TE, Ota-Setlik A, Gerardi CS, et al. Safety and Immunogenicity of a Highly Attenuated rVSVN4CT1-EBOVGP1 Ebola Virus Vaccine: A Randomised, Double-Blind, Placebo-Controlled, Phase 1 Clinical Trial. Lancet Infect Dis (2020) 20:455–66. doi: 10.1016/S1473-3099(19)30614-0
73. Amanat F, Krammer F. SARS-CoV-2 Vaccines: Status Report. Immunity (2020) 52:583–9. doi: 10.1016/j.immuni.2020.03.007
74. Dai L, Gao GF. Viral Targets for Vaccines Against COVID-19. Nat Rev Immunol (2021) 21:73–82. doi: 10.1038/s41577-020-00480-0
76. Harrison SC. Viral Membrane Fusion. Virology (2015) 479–80:498–507. doi: 10.1016/j.virol.2015.03.043
77. McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, et al. Structure-Based Design of A Fusion Glycoprotein Vaccine for Respiratory Syncytial Virus. Science (2013) 342:592–8. doi: 10.1126/science.1243283
78. Krarup A, Truan D, Furmanova-Hollenstein P, Bogaert L, Bouchier P, Bisschop IJM, et al. A Highly Stable Prefusion RSV F Vaccine Derived From Structural Analysis of the Fusion Mechanism. Nat Commun (2015) 6:8143. doi: 10.1038/ncomms9143
79. Marcandalli J, Fiala B, Ols S, Perotti M, de van der Schueren W, Snijder J, et al. Induction of Potent Neutralizing Antibody Responses by a Designed Protein Nanoparticle Vaccine for Respiratory Syncytial Virus. Cell (2019) 176:1420–31.e1417. doi: 10.1016/j.cell.2019.01.046
80. Hsieh CL, Goldsmith JA, Schaub JM, DiVenere AM, Kuo HC, Javanmardi K, et al. Structure-Based Design of Prefusion-Stabilized SARS-CoV-2 Spikes. Science (2020) 369:1501–5. doi: 10.1126/science.abd0826
81. Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, et al. SARS-CoV-2 mRNA Vaccine Design Enabled by Prototype Pathogen Preparedness. Nature (2020) 586:567–71. doi: 10.1038/s41586-020-2622-0
82. Backovic M, Longnecker R, Jardetzky TS. Structure of a Trimeric Variant of the Epstein-Barr Virus Glycoprotein B. Proc Natl Acad Sci USA (2009) 106:2880–5. doi: 10.1073/pnas.0810530106
83. Zeev-Ben-Mordehai T, Vasishtan D, Siebert CA, Whittle C, Grunewald K. Extracellular Vesicles: A Platform for the Structure Determination of Membrane Proteins by Cryo-EM. Structure (2014) 22:1687–92. doi: 10.1016/j.str.2014.09.005
84. Si Z, Zhang J, Shivakoti S, Atanasov I, Tao CL, Hui WH, et al. Different Functional States of Fusion Protein gB Revealed on Human Cytomegalovirus by Cryo Electron Tomography With Volta Phase Plate. PloS Pathog (2018) 14:e1007452. doi: 10.1371/journal.ppat.1007452
85. C M. Latency and Lytic Replication in Epstein-Barr Virus-Associated Oncogenesis. Nat Rev Microbiol (2019) 17:691–700. doi: 10.1038/s41579-019-0249-7
86. Burrows SR, Sculley TB, Misko IS, Schmidt C, Moss DJ. An Epstein-barr Virus-Specific Cytotoxic T Cell Epitope In Ebv Nuclear Antigen 3 (EBNA 3). J Exp Med (1990) 171:345–9. doi: 10.1084/jem.171.1.345
87. Schmidt C, Burrows SR, Sculley TB, Moss DJ, Misko IS. Nonresponsiveness to an Immunodominant Epstein-Barr Virus-Encoded Cytotoxic T-Lymphocyte Epitope in Nuclear Antigen 3A: Implications for Vaccine Strategies. Proc Natl Acad Sci USA (1991) 88:9478–82. doi: 10.1073/pnas.88.21.9478
88. Wallace LE, Wright J, Ulaeto DO, Morgan AJ, Rickinson AB. Identification of Two T-Cell Epitopes on the Candidate Epstein-Barr Virus Vaccine Glycoprotein gp340 Recognized by CD4+ T-Cell Clones. J Virol (1991) 65:3821–8. doi: 10.1128/JVI.65.7.3821-3828.1991
89. Murray RJ, Kurilla MG, Brooks JM, Thomas WA, Rowe M, Kieff E, et al. Identification of Target Antigens for the Human Cytotoxic T Cell Response to Epstein-Barr Virus (EBV): Implications for the Immune Control of EBV-Positive Malignancies. J Exp Med (1992) 176:157–68. doi: 10.1084/jem.176.1.157
90. Levitskaya J, Coram M, Levitsky V, Imreh S, Steigerwald-Mullen PM, Klein G, et al. Inhibition of Antigen Processing by the Internal Repeat Region of the Epstein-Barr Virus Nuclear Antigen-1. Nature (1995) 375:685–8. doi: 10.1038/375685a0
91. Blake N, Lee S, Redchenko I, Thomas W, Steven N, Leese A, et al. Human CD8+ T Cell Responses to EBV EBNA1: HLA Class I Presentation of the (Gly-Ala)-Containing Protein Requires Exogenous Processing. Immunity (1997) 7:791–802. doi: 10.1016/S1074-7613(00)80397-0
92. Khanna R, Burrows SR, Neisig A, Neefjes J, Moss DJ, Silins SL. Hierarchy of Epstein-Barr Virus-Specific Cytotoxic T-Cell Responses in Individuals Carrying Different Subtypes of an HLA Allele: Implications for Epitope-Based Antiviral Vaccines. J Virol (1997) 71:7429–35. doi: 10.1128/JVI.71.10.7429-7435.1997
93. Rickinson AB, Moss DJ. Human Cytotoxic T Lymphocyte Responses to Epstein-Barr Virus Infection. Annu Rev Immunol (1997) 15:405–31. doi: 10.1146/annurev.immunol.15.1.405
94. Khanna R, Burrows SR. Role of Cytotoxic T Lymphocytes in Epstein-Barr virus-Associated Diseases. Annu Rev Microbiol (2000) 54:19–48. doi: 10.1146/annurev.micro.54.1.19
95. Lautscham G, Mayrhofer S, Taylor G, Haigh T, Leese A, Rickinson A, et al. Processing of a Multiple Membrane Spanning Epstein-Barr Virus Protein for CD8(+) T Cell Recognition Reveals a Proteasome-Dependent, Transporter Associated with Antigen Processing-Independent Pathway. J Exp Med (2001) 194:1053–68. doi: 10.1084/jem.194.8.1053
96. Kuzushima K, Hayashi N, Kudoh A, Akatsuka Y, Tsujimura K, Morishima Y, et al. Tetramer-Assisted Identification and Characterization of Epitopes Recognized by HLA A*2402-Restricted Epstein-Barr Virus-Specific CD8+ T cells. Blood (2003) 101:1460–8. doi: 10.1182/blood-2002-04-1240
97. Tellam J, Connolly G, Green KJ, Miles JJ, Moss DJ, Burrows SR, et al. Endogenous Presentation of CD8+ T Cell Epitopes From Epstein-Barr Virus-Encoded Nuclear Antigen 1. J Exp Med (2004) 199:1421–31. doi: 10.1084/jem.20040191
98. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
99. Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA Vaccine Against SARS-CoV-2 - Preliminary Report. N Engl J Med (2020) 383:1920–31. doi: 10.1056/NEJMoa2022483
100. Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine, BBV152: A Double-Blind, Randomised, Phase 1 Trial. Lancet Infect Dis (2021) 21(5):637–46. doi: 10.1016/S1473-3099(20)30942-7
101. Sanchez-Felipe L, Vercruysse T, Sharma S, Ma J, Lemmens V, Van Looveren D, et al. A Single-Dose Live-Attenuated YF17D-Vectored SARS-CoV-2 Vaccine Candidate. Nature (2021) 590:320–5. doi: 10.1101/2020.07.08.193045
102. Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. A Systematic Review of SARS-CoV-2 Vaccine Candidates. Signal Transduct Target Ther (2020) 5:237. doi: 10.1038/s41392-020-00352-y
103. Noad R, Roy P. Virus-Like Particles As Immunogens. Trends Microbiol (2003) 11:438–44. doi: 10.1016/S0966-842X(03)00208-7
104. Feederle R, Shannon-Lowe C, Baldwin G, Delecluse HJ. Defective Infectious Particles And Rare Packaged Genomes Produced By Cells Carrying Terminal-Repeat-Negative Epstein-Barr Virus. J Virol (2005) 79:7641–7. doi: 10.1128/JVI.79.12.7641-7647.2005
105. Hettich E, Janz A, Zeidler R, Pich D, Hellebrand E, Weissflog B, et al. Genetic Design of an Optimized Packaging Cell Line For Gene Vectors Transducing Human B Cells. Gene Ther (2006) 13:844–56. doi: 10.1038/sj.gt.3302714
106. Granato M, Feederle R, Farina A, Gonnella R, Santarelli R, Hub B, et al. Deletion of Epstein-Barr Virus BFLF2 Leads to Impaired Viral DNA Packaging And Primary Egress as Well as to the Production of Defective Viral Particles. J Virol (2008) 82:4042–51. doi: 10.1128/JVI.02436-07
107. Pavlova S, Feederle R, Gärtner K, Fuchs W, Granzow H, Delecluse HJ. An Epstein-Barr Virus Mutant Produces Immunogenic Defective Particles Devoid of Viral DNA. J Virol (2013) 87:2011–22. doi: 10.1128/JVI.02533-12
108. Ewer KJ, Lambe T, Rollier’ CS, Spencer AJ, Hill AVS, Dorrell L. Viral vEctors as Vaccine Platforms: From Immunogenicity to Impact. Curr Opin Immunol (2016) 41:47–54. doi: 10.1016/j.coi.2016.05.014
109. Small JC, Ertl HCJ. Viruses - From Pathogens to Vaccine Carriers. Curr Opin Virol (2011) 1:241–5. doi: 10.1016/j.coviro.2011.07.009
110. Ruhl J, Citterio C, Engelmann C, Haigh T, Dzionek A, Dreyer J, et al. Heterologous Prime-Boost Vaccination Protects Against EBV Antigen-Expressing Lymphomas. J Clin Invest (2019) 129:2071–87. doi: 10.1172/JCI125364
111. Taylor GS, Haigh TA, Gudgeon NH, Phelps RJ, Lee SP, Steven NM, et al. Dual stimulation of Epstein-Barr Virus (EBV)-Specific CD4+- and CD8+-T-Cell Responses by a Chimeric Antigen Construct: Potential Therapeutic Vaccine for EBV-Positive Nasopharyngeal Carcinoma. J Virol (2004) 78:768–78. doi: 10.1128/JVI.78.2.768-778.2004
112. Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained Complete Responses in Patients with Lymphoma Receiving Autologous Cytotoxic T Lymphocytes Targeting Epstein-Barr Virus Latent Membrane Proteins. J Clin Oncol (2014) 32:798–808. doi: 10.1200/JCO.2013.51.5304
113. Gottschalk S, Edwards OL, Sili U, Huls MH, Goltsova T, Davis AR, et al. Generating CTLs Against the Subdominant Epstein-Barr Virus LMP1 Antigen for the Adoptive Immunotherapy of EBV-Associated Malignancies. Blood (2003) 101:1905–12. doi: 10.1182/blood-2002-05-1514
114. Lowe RS, Keller PM, Keech BJ, Davison AJ, Whang Y, Morgan AJ, et al. Varicella-Zoster Virus as a Live Vector for the Expression of Foreign Genes. Proc Natl Acad Sci USA (1987) 84:3896–900. doi: 10.1073/pnas.84.11.3896
115. Condit RC, Williamson AL, Sheets R, Seligman SJ, Monath TP, Excler JL, et al. Unique Safety Issues Associated with Virus-Vectored Vaccines: Potential for and Theoretical Consequences of Recombination with Wild Type Virus Strains. Vaccine (2016) 34:6610–6. doi: 10.1016/j.vaccine.2016.04.060
116. Mascola JR, Fauci AS. Novel Vaccine Technologies for the 21st Century. Nat Rev Immunol (2020) 20:87–8. doi: 10.1038/s41577-019-0243-3
117. Correia BE, Ban YE, Holmes MA, Xu H, Ellingson K, Kraft Z, et al. Computational Design of Epitope-Scaffolds Allows Induction of Antibodies Specific for a Poorly Immunogenic HIV Vaccine Epitope. Structure (2010) 18:1116–26. doi: 10.1016/j.str.2010.06.010
118. Ofek G, Guenaga FJ, Schief WR, Skinner J, Baker D, Wyatt R, et al. Elicitation of Structure-Specific Antibodies by Epitope Scaffolds. Proc Natl Acad Sci USA (2010) 107:17880–7. doi: 10.1073/pnas.1004728107
119. Konduru K, Bradfute SB, Jacques J, Manangeeswaran M, Nakamura S, Morshed S, et al. Ebola Virus Glycoprotein Fc Fusion Protein Confers Protection Against Lethal Challenge In Vaccinated Mice. Vaccine (2011) 29:2968–77. doi: 10.1016/j.vaccine.2011.01.113
120. Azoitei ML, Ban YE, Julien JP, Bryson S, Schroeter A, Kalyuzhniy O, et al. Computational Design of High-Affinity Epitope Scaffolds by Backbone Grafting of a Linear Epitope. J Mol Biol (2012) 415:175–92. doi: 10.1016/j.jmb.2011.10.003
121. Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, et al. Proof of Principle for Epitope-Focused Vaccine Design. Nature (2014) 507:201–6. doi: 10.1038/nature12966
122. Sesterhenn F, Yang C, Bonet J, Cramer JT, Wen X, Wang Y, et al. De Novo Protein Design Enables the Precise Induction of RSV-Neutralizing Antibodies. Science (2020) 368(6492):eaay5051. doi: 10.1126/science.aay5051
123. Moyle PM. Biotechnology Approaches to Produce Potent, Self-Adjuvanting Antigen-Adjuvant Fusion Protein Subunit Vaccines. Biotechnol Adv (2017) 35:375–89. doi: 10.1016/j.biotechadv.2017.03.005
124. Wojtak K, Perales-Puchalt A, Weiner DB. Novel Synthetic DNA Immunogens Targeting Latent Expressed Antigens of Epstein-Barr Virus Elicit Potent Cellular Responses and Inhibit Tumor Growth. Vaccines (Basel) (2019) 7(2):44. doi: 10.3390/vaccines7020044
125. Guy B. The Perfect Mix: Recent Progress in Adjuvant Research. Nat Rev Microbiol (2007) 5:505–17. doi: 10.1038/nrmicro1681
126. Schijns VE, Lavelle EC. Trends in vaccine adjuvants. Expert Rev Vaccines (2011) 10:539–50. doi: 10.1586/erv.11.21
127. Reed SG, Bertholet S, Coler RN, Friede M. New Horizons in Adjuvants for Vaccine Development. Trends Immunol (2009) 30:23–32. doi: 10.1016/j.it.2008.09.006
128. Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New Adjuvants for Human Vaccines. Curr Opin Immunol (2010) 22:411–6. doi: 10.1016/j.coi.2010.04.004
129. Tom JK, Albin TJ, Manna S, Moser BA, Steinhardt RC, Esser-Kahn AP. Applications of Immunomodulatory Immune Synergies to Adjuvant Discovery and Vaccine Development. Trends Biotechnol (2019) 37:373–88. doi: 10.1016/j.tibtech.2018.10.004
130. Klasse PJ, Nixon DF, Moore JP. Immunogenicity of Clinically Relevant SARS-CoV-2 Vaccines in Non-Human Primates and Humans. Sci Adv (2021) 7(12):eabe8065. doi: 10.1126/sciadv.abe8065
131. Cunningham AL, Heineman TC, Lal H, Godeaux O, Chlibek R, Hwang SJ, et al. Immune Responses to a Recombinant Glycoprotein E Herpes Zoster Vaccine in Adults Aged 50 Years or Older. J Infect Dis (2018) 217:1750–60. doi: 10.1093/infdis/jiy095
132. Heineman TC, Cunningham A, Levin M. Understanding the Immunology of Shingrix, a Recombinant Glycoprotein E Adjuvanted Herpes Zoster Vaccine. Curr Opin Immunol (2019) 59:42–8. doi: 10.1016/j.coi.2019.02.009
133. Frazer IH, Levin MJ. Paradigm Shifting Vaccines: Prophylactic Vaccines Against Latent Varicella-Zoster Virus Infection and Against HPV-Associated Cancer. Curr Opin Virol (2011) 1:268–79. doi: 10.1016/j.coviro.2011.07.007
134. Li JK, Balic JJ, Yu L, Jenkins B. TLR Agonists as Adjuvants for Cancer Vaccines. Adv Exp Med Biol (2017) 1024:195–212. doi: 10.1007/978-981-10-5987-2_9
135. Hu Y, Tang L, Zhu Z, Meng H, Chen T, Zhao S, et al. A Novel TLR7 Agonist as Adjuvant to Stimulate High Quality HBsAg-Specific Immune Responses in an HBV Mouse Model. J Transl Med (2020) 18:112. doi: 10.1186/s12967-020-02275-2
136. Vreman S, McCaffrey J, Popma-de Graaf DJ, Nauwynck H, Savelkoul HFJ, Moore A, et al. Toll-Like Receptor Agonists as Adjuvants For Inactivated Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Vaccine. Vet Immunol Immunopathol (2019) 212:27–37. doi: 10.1016/j.vetimm.2019.04.008
137. Geisbert TW, Feldmann H, Broder CC. Animal Challenge Models of Henipavirus Infection and Pathogenesis. Curr Top Microbiol Immunol (2012) 359:153–77. doi: 10.1007/82_2012_208
138. Chu YK, Ali GD, Jia F, Li Q, Kelvin D, Couch RC, et al. The SARS-CoV Ferret Model in an Infection-Challenge Study. Virology (2008) 374:151–63. doi: 10.1016/j.virol.2007.12.032
139. Gurumurthy CB, Quadros RM, Richardson GP, Poluektova LY, Mansour SL, Ohtsuka M. Genetically Modified Mouse Models to Help Fight COVID-19. Nat Protoc (2020) 15:3777–87. doi: 10.1038/s41596-020-00403-2
140. Hatziioannou T, Evans DT. Animal Models for HIV/AIDS Research. Nat Rev Microbiol (2012) 10:852–67. doi: 10.1038/nrmicro2911
141. Berggren KA, Suzuki S, Ploss A. Animal Models Used in Hepatitis C Virus Research. Int J Mol Sci (2020) 21(11):3869. doi: 10.3390/ijms21113869
142. Gujer C, Chatterjee B, Landtwing V, Raykova A, McHugh D, Munz C. Animal Models of Epstein Barr Virus Infection. Curr Opin Virol (2015) 13:6–10. doi: 10.1016/j.coviro.2015.03.014
143. Chatterjee B, Leung CS, Munz C. Animal Models of Epstein Barr Virus Infection. J Immunol Methods (2014) 410:80–7. doi: 10.1016/j.jim.2014.04.009
144. Munz C. Probing Reconstituted Human Immune Systems in Mice With Oncogenic gamma-Herpesvirus Infections. Front Immunol (2020) 11:581419. doi: 10.3389/fimmu.2020.581419
145. Wang F. Nonhuman Primate Models for Epstein-Barr Virus Infection. Curr Opin Virol (2013) 3:233–7. doi: 10.1016/j.coviro.2013.03.003
146. Wedderburn N, Edwards JM, Desgranges C, Fontaine C, Cohen B, de Thé G. Infectious Mononucleosis-Like Response in Common Marmosets Infected with Epstein-Barr Virus. J Infect Dis (1984) 150:878–82. doi: 10.1093/infdis/150.6.878
147. Miller G, Shope T, Coope D, Waters L, Pagano J, Bornkamn G, et al. Lymphoma in Cotton-Top Marmosets After Inoculation with Epstein-Barr Virus: Tumor Incidence, Histologic Spectrum Antibody Responses, Demonstration of vIral DNA, and Characterization of Viruses. J Exp Med (1977) 145:948–67. doi: 10.1084/jem.145.4.948
148. UCN Red List of Threatened Species. Available at: https://www.iucn.org/resources/conservation-tools/iucn-red-list-threatened-species.
149. Estes JD, Wong SW, Brenchley JM. Nonhuman Primate Models of Human Viral Infections. Nat Rev Immunol (2018) 18:390–404. doi: 10.1038/s41577-018-0005-7
150. Moghaddam A, Koch J, Annis B, Wang F. Infection of Human B Lymphocytes with Lymphocryptoviruses Related to Epstein-Barr Virus. J Virol (1998) 72:3205–12. doi: 10.1128/JVI.72.4.3205-3212.1998
151. Leskowitz R, Fogg MH, Zhou XY, Kaur A, Silveira EL, Villinger F, et al. Adenovirus-Based Vaccines Against Rhesus Lymphocryptovirus EBNA-1 Induce Expansion of Specific CD8+ and CD4+ T Cells in Persistently Infected Rhesus Macaques. J Virol (2014) 88:4721–35. doi: 10.1128/JVI.03744-13
152. Leskowitz RM, Zhou XY, Villinger F, Fogg MH, Kaur A, Lieberman PM, et al. CD4+ and CD8+ T-Cell Responses to Latent Antigen EBNA-1 and Lytic Antigen BZLF-1 During Persistent Lymphocryptovirus Infection of Rhesus Macaques. J Virol (2013) 87:8351–62. doi: 10.1128/JVI.00852-13
153. Rivailler P, Carville A, Kaur A, Rao P, Quink C, Kutok JL, et al. Experimental Rhesus Lymphocryptovirus Infection in Immunosuppressed Macaques: An Animal Model for Epstein-Barr Virus Pathogenesis in the Immunosuppressed Host. Blood (2004) 104:1482–9. doi: 10.1182/blood-2004-01-0342
154. Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a Human Adaptive Immune System in Cord Blood Cell-Transplanted Mice. Science (2004) 304:104–7. doi: 10.1126/science.1093933
155. Singh S, Homad LJ, Akins NR, Stoffers CM, Lackhar S, Malhi H, et al. Neutralizing Antibodies Protect Against Oral Transmission of Lymphocryptovirus. Cell Rep Med (2020) 1(3):100033. doi: 10.1016/j.xcrm.2020.100033
156. Heeke DS, Lin R, Rao E, Woo JC, McCarthy MP, Marshall JD. Identification of GLA/SE as an Effective Adjuvant for the Induction of Robust Humoral and Cell-Mediated Immune Responses to EBV-gp350 in Mice and Rabbits. Vaccine (2016) 34:2562–9. doi: 10.1016/j.vaccine.2016.04.012
157. Okuno K, Takashima K, Kanai K, Ohashi M, Hyuga R, Sugihara H, et al. Epstein-Barr Virus Can Infect Rabbits by the Intranasal or Peroral Route: An Animal Model for Natural Primary EBV Infection in Humans. J Med Virol (2010) 82:977–86. doi: 10.1002/jmv.21597
158. Takashima K, Ohashi M, Kitamura Y, Ando K, Nagashima K, Sugihara H, et al. A New Animal Model for Primary and Persistent Epstein-Barr Virus Infection: Human EBV-Infected Rabbit Characteristics Determined Using Sequential Imaging and Pathological Analysis. J Med Virol (2008) 80:455–66. doi: 10.1002/jmv.21102
159. Kanai K, Kato K, Sano H, Nagata K, Okuno K, Kuwamoto S, et al. In vitro Epstein-Barr Virus Infection Model of Rabbit Lymphocytes From Peripheral Blood or Spleen. Intervirology (2011) 54:17–24. doi: 10.1159/000318882
160. Wang Z, Yi X, Du L, Wang H, Tang J, Wang M, et al. A study of Epstein-Barr Virus Infection in the Chinese Tree Shrew(Tupaia belangeri chinensis). Virol J (2017) 14:193. doi: 10.1186/s12985-017-0859-5
161. Xiao J, Liu R, Chen CS. Tree shrew (Tupaia belangeri) as a Novel Laboratory Disease Animal Model. Zool Res (2017) 38:127–37. doi: 10.24272/j.issn.2095-8137.2017.033
162. Amanna IJ, Messaoudi I, Slifka MK. Protective Immunity Following Vaccination: How Is it Defined? Hum Vaccin (2008) 4:316–9. doi: 10.4161/hv.4.4.5751
163. Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, et al. Hemagglutination Inhibition Antibody Titers as a Correlate of Protection for Inactivated Influenza Vaccines in Children. Pediatr Infect Dis J (2011) 30:1081–5. doi: 10.1097/INF.0b013e3182367662
164. Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A Systematic Review of Anti-Rotavirus Serum IgA Antibody Titer as a Potential Correlate of Rotavirus Vaccine Efficacy. J Infect Dis (2013) 208:284–94. doi: 10.1093/infdis/jit166
165. Ackerman ME, Barouch DH, Alter G. Systems Serology for Evaluation of HIV Vaccine Trials. Immunol Rev (2017) 275:262–70. doi: 10.1111/imr.12503
166. Cortese M, Sherman AC, Rouphael NG, Pulendran B. Systems Biological Analysis of Immune Response to Influenza Vaccination. Cold Spring Harb Perspect Med (2020) a038596. doi: 10.1101/cshperspect.a038596
167. Coghill AE, Bu W, Nguyen H, Hsu WL, Yu KJ, Lou PJ, et al. High Levels of Antibody that Neutralize B-cell Infection of Epstein-Barr Virus and that Bind EBV gp350 Are Associated with a Lower Risk of Nasopharyngeal Carcinoma. Clin Cancer Res (2016) 22:3451–7. doi: 10.1158/1078-0432.CCR-15-2299
168. Zhu QY, Kong XW, Sun C, Xie SH, Hildesheim A, Cao SM, et al. Association Between Antibody Responses to Epstein-Barr Virus Glycoproteins, Neutralization of Infectivity, and the Risk of Nasopharyngeal Carcinoma. mSphere (2020) 5(6):e00901-20. doi: 10.1128/mSphere.00901-20
169. Tashiro H, Brenner MK. Immunotherapy Against Cancer-Related Viruses. Cell Res (2017) 27:59–73. doi: 10.1038/cr.2016.153
170. Si Y, Deng Z, Lan G, Du H, Wang Y, Si J, et al. The Safety and Immunological Effects of rAd5-EBV-LMP2 Vaccine in Nasopharyngeal Carcinoma Patients: A Phase I Clinical Trial and Two-Year Follow-Up. Chem Pharm Bull (2016) 64:1118–23. doi: 10.1248/cpb.c16-00114
171. Long HM, Meckiff BJ, Taylor GS. The T-cell Response to Epstein-Barr Virus-New Tricks From an Old Dog. Front Immunol (2019) 10:2193. doi: 10.3389/fimmu.2019.02193
Keywords: Epstein–Barr virus, vaccine, virus immunology, adjuvant, animal model
Citation: Sun C, Chen X, Kang Y and Zeng M (2021) The Status and Prospects of Epstein–Barr Virus Prophylactic Vaccine Development. Front. Immunol. 12:677027. doi: 10.3389/fimmu.2021.677027
Received: 07 March 2021; Accepted: 20 May 2021;
Published: 08 June 2021.
Edited by:
Lawrence S. Young, University of Warwick, United KingdomReviewed by:
Chang-Han Lee, Seoul National University, South KoreaDuo Xu, University of California, Riverside, United States
Copyright © 2021 Sun, Chen, Kang and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mu-sheng Zeng, zengmsh@sysucc.org.cn
 Cong Sun
Cong Sun Xin-chun Chen
Xin-chun Chen Yin-feng Kang
Yin-feng Kang Mu-sheng Zeng
Mu-sheng Zeng