- 1Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Biological Sciences Greenhouse, College of Art & Sciences, The Ohio State University, Columbus, OH, United States
- 3Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Pregnant women are generally more susceptible to viral infection. Although the impact of SARS-CoV-2 on pregnant women remains to be determined, evidence indicates that risks of adverse clinical outcomes are similar in pregnancy to the general population. Here we analyzed clinical symptoms and outcomes of 20 pregnant and 299 reproductive-aged non-pregnant female COVID-19 patients who were hospitalized during the same period. Laboratory measurements were compared among mild cases and healthy pregnant women. Our study found that pregnant patients showed enhanced innate immune response evident by higher neutrophils and C-reactive protein. Cytokines, chemokines, and growth factors (CCGFs) profiles from 11 pregnant and 4 non-pregnant COVID-19 patients and 10 healthy pregnant female patients, and lymphocyte subsets analysis of 7 pregnant patients and 19 non-pregnant patients, indicate suppressed cytokine storm and potential enhanced CD8+ T cell and NK cell activity in pregnant patients with COVID-19, which may be essential in contributing to the unique anti-SARS-CoV-2 response in pregnancy.
Introduction
Since December 2019, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been spreading rapidly worldwide. As of March 3, 2021, a total of 115,302,067 COVID-19 cases had been diagnosed globally, with a cumulative death toll of 2,560,638 (1).
SARS-CoV-2 enters the cell via the angiotensin-converting enzyme type 2 (ACE-2) receptors, which mainly expressed on pulmonary epithelial cells, but also on lymphocytes and other cell types. The spread of the virus among cells triggers both innate and adaptive immune responses, which then causes the production of large amounts of pro-inflammatory cytokines and chemokines (2, 3). In some patients, the activation is massive and develops into a “cytokine storm,” which induces severe inflammatory responses and causing tissue damage or even death (4, 5). Although all age groups are generally susceptible, the susceptibility and clinical outcomes under SARS-CoV-2 are highly heterogeneous due to differences in immune responses of the hosts.
During the COVID-19 pandemic, there was additional concern surrounding pregnant women due to their unique immunological status, which requires adaptions to ensure tolerance to the fetus while preserving immunoprotective functions. Immune states during pregnancy are often characterized by alterations in the cellular composition and the functions of immune cells. T cell-mediated immunity and humoral responses are suppressed in normal pregnancy particularly during the third trimester (6–8). Elevated IL-4, IL-10 and reduced IL-2, IFN-γ production in peripheral blood mononuclear cells (PBMCs) (9, 10) are also observed in pregnancy. It was reported that influenza A virus infection during pregnancy exaggerated the inflammatory response with high levels of pro-inflammatory and anti-inflammatory cytokines (11, 12). Therefore, immune characteristics and clinical outcomes of pregnant women are expected to be different to non-pregnant women with COVID-19 infections.
This heterogeneity has been reported in previous studies indicating that pregnant women are at increased risk of morbidity and mortality from respiratory viral infections, including influenza A virus, severe acute respiratory syndrome associated coronavirus 1 (SARS-CoV-1), and Middle East respiratory syndrome associated coronavirus (MERS-CoV) (13, 14). However, regarding the SARS-CoV-2 infection, studies have shown that pregnant women do not have a greater risk of death than non-pregnant COVID-19 patients (15–17). The largest dataset available so far was published by the Centers for Disease Control and Prevention (CDC), which included 8,207 pregnant women with COVID-19 and indicated that they were more likely to be hospitalized and had increased rates of ICU and mechanical ventilation admissions compared with non-pregnant women with COVID-19, but their risk for death is similar (18). The relatively low risk of death has been attributed to immunological and hormonal factors (19, 20). However, the protective effects are mainly based on speculation, as few studies have investigated the immunological features and underling mechanisms of pregnant patients suffering from COVID-19.
Therefore, we designed this study to compare the clinical and immunological features between pregnant and non-pregnant COVID-19 patients. These findings may help expand our understanding of the immune system’s response to SARS-CoV-2 infection and explore its potential protective mechanisms in pregnant COVID-19 patients, and propose reasonable options of care and treatment.
Materials and Methods
Participants and Data Collection
In this retrospective single-center study, 20 pregnant women and 299 non-pregnant women of reproductive age with confirmed COVID-19 infection (according to the 7th edition of China’s Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia) were recruited from January 19 to April 20, 2020 from Tongji Hospital in Wuhan, China. The participants’ demographic data, medical history, chronic disease history, laboratory findings, chest computed tomography (CT) scan findings, and outcomes were reviewed from the patient database. Ten healthy pregnant women hospitalized during the same period were also included as a control group. The flowchart of study design and data analysis was shown in Figure 1.

Figure 1 Flowchart of patient recruitment and data analysis procedure. A total of 3,331 COVID-19 patients from Tongji Hospital were screened and a total of 319 reproductive-aged female patients with complete medical recording were enrolled. Patients were grouped as pregnant (n = 20) and non-pregnant (n = 299), and compared by clinical characteristics, laboratory findings, and cytokines. Ten healthy pregnant women were included as control.
Definitions
All of the enrolled patients had confirmed COVID-19 according to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (7th edition). In brief, a patient is diagnosed with COVID-19 if the results of the SARS-CoV-2 RNA or specific antibody test is positive. During their hospital stay, patients are categorized as (1) mild cases if they have mild clinical symptoms with or without typical CT imaging of viral pneumonia; (2) severe cases if their oxygen saturation is ≤93% at rest, they have respiratory distress with a respiration rate (RR) <30 times/min, or their arterial partial pressure of oxygen (PaO2)/oxygen absorption concentration (FiO2) is ≤300 mmHg; or (3) critical cases if the patient suffers from respiratory failure requiring mechanical ventilation, shock, or organ failure requiring intensive care.
Abnormal hepatic function was defined as the alanine aminotransferase level >66 U/L. Abnormal renal function was defined as having a blood creatinine level >84 µmol/L or a blood urea nitrogen concentration >7.5 mmol/L.
Lymphopenia was defined as peripheral blood lymphocyte count <1 × 109/L.
Patients can be discharged if they meet the following conditions specified in the 7th edition of the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia: 1. Body temperature has returned to normal and remained normal for more than 3 days; 2. Respiratory symptoms have improved significantly; 3. CT scan shows significant improvement in acute exudative lesions; and 4. Two consecutive sputum and nasopharyngeal swabs, and other respiratory tract specimens have tested negative for nucleic acid (sampling time interval of at least 24 h). Discharge time was defined as the first day the patient met the discharge criteria.
Blood Specimen Collection and Cytokine Assessment
For the cytokine assays, plasma samples from pregnant patients and non-pregnant patients were collected from the specimen bank at Tongji Hospital. The concentrations of 48 cytokines were measured using the 152 Bio-Plex Pro Human Cytokine Screening Panel (Bio-Rad) as reported previously (21). Samples, standards, blank, and controls were added and followed by detection antibodies. Raw data were generated using the Bio-Plex Manager software. Concentrations below the detection range were considered as zero. Concentrations above the detection range were converted to the highest value of the standard curve. All cytokines were expressed as pg/ml and extrapolated from the standard curves (individual for each cytokine).
Statistical Analysis
We performed statistical analyses using SPSS version 26.0 (IBM) and R version 3.6.0. Continuous variables were described as median (interquartile range, IQR), and categorical variables were described as number (percentage). For comparison of continuous variables, we used the Mann-Whitney U test for two groups and the Kruskal-Wallis H test for three groups. Pairwise comparison of the Kruskal-Wallis H test was further used to detect the statistic difference between every two groups. The Chi-squared test and Fisher’s exact test (when the theoretical frequency was less than 5 or the sample size is less than 40) were used to compare the categorical variables. This study was approved by the Ethical Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (ID: TJ-IRB2020401). The participants provided their written informed consent to participate in this study.
Results
Demographic and Clinical Characteristics of Pregnant Patients and Non-Pregnant Female Patients
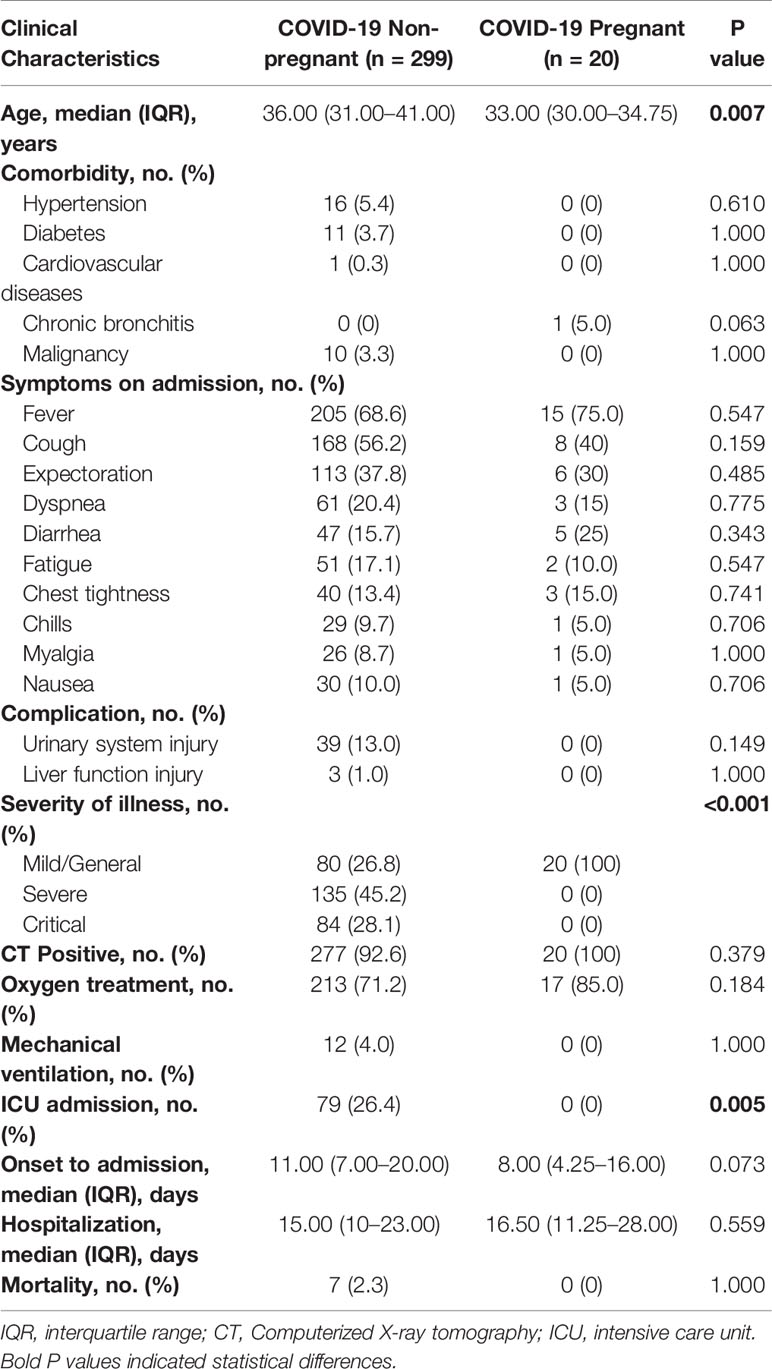
Between January 19 to April 20, 2020, 20 pregnant and 299 non-pregnant female COVID-19 patients (from 15 to 45 years old) were admitted to Tongji Hospital. The clinical symptoms were listed in Table 1. No significant difference was found in comorbidities, onset symptoms, and complications between pregnant patients and non-pregnant counterparts (Table 1). In our cohort, none of pregnant patients developed severe COVID-19 symptoms, which was significantly lower than non-pregnant group (Table 1). Therefore, we performed subsequent analysis between the pregnant patients (COVID-19 pregnant, n = 20) and female patients with mild cases (COVID-19 non-pregnant, n = 80) to illustrate the effects of pregnancy on SARS-CoV-2 infection. As clinical and immunological features may be different in normal pregnancy, we included a group of healthy pregnant women as control (Healthy pregnant, n = 10) (Table 2). Generally, the number and percentage of white blood cells and neutrophils were higher in pregnant women, while the number and percentage of myeloid cells including monocyte, basophil, eosinophil were significantly lower. And pregnant women were more anemic than their non-pregnant counterparts with less red blood cell count, lower hemoglobin level, and lower hematocrit. Laboratory tests also indicated lower levels of creatinine and higher levels of alkaline phosphatase and D-Dimer in pregnancy. The analysis of laboratory findings indicated unique physiological condition of pregnant women, which also explained the difference of clinical features between pregnant and non-pregnant women on SARS-CoV-2 infection. Notably, pregnant patients with COVID-19 had significantly higher levels of C-reactive protein (CRP) compared to the non-pregnant Covid-19 patients and healthy pregnant patients, reflecting an enhanced immune vigilance against infection during pregnancy.
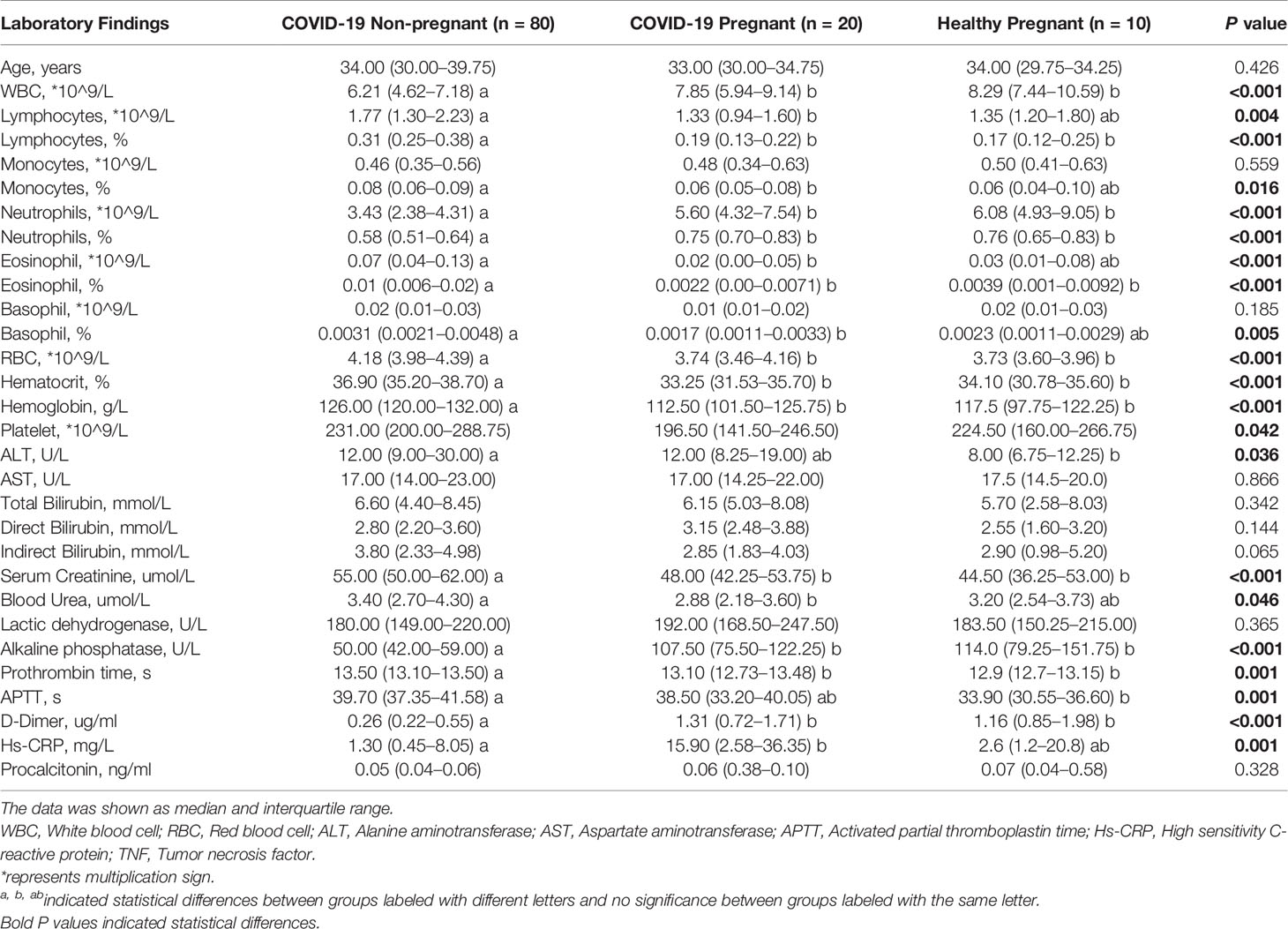
Table 2 Laboratory findings between pregnant and non-pregnant COVID-19 patients and healthy pregnant women.
Cytokines, Chemokines, and Growth Factors of Pregnant and Non-Pregnant COVID-19 Patients
To investigate the immune response of pregnant women with COVID-19 infections, a total of 11 pregnant patients and four non-pregnant patients with mild cases were compared for cytokines, chemokines, and growth factors (CCGFs). Healthy pregnant women were included as control (n = 10). Clinical characteristics were shown in Table 3, and no differences were observed in age, comorbidities, and complications. Healthy pregnant women showed high levels of interleukins including IL-2, IL-5, IL-10, and IL-12, as well as high levels of growth factors such as b-NGF and VEGF, which were largely decreased in COVID-19 pregnant and non-pregnant patients. The non-pregnant COVID-19 patients exhibited high levels of IL-4 and IL-9, and several inflammatory chemokines and growth factors including MIP-1β/CCL4, CATCK/CCL27, RANTES/CCL5, Eotaxin/CCL11, GRO-α/CXCL1, LIF, FGF, PDGF-BB, SDF-1α, and tumor necrosis factors (TNFs) were also highly expressed in non-pregnant COVID-19 group. However, the levels of these CCGFs in pregnant women with COVID-19 were not as high as non-pregnant COVID-19 patients, which may be attributed to the initial low levels of them as exhibited in healthy pregnant women (Figure 2). Our results indicated that the state of immunomodulation during pregnancy may protect pregnant COVID-19 patients from suffering from cytokine storm and progressing to acute respiratory distress syndrome.
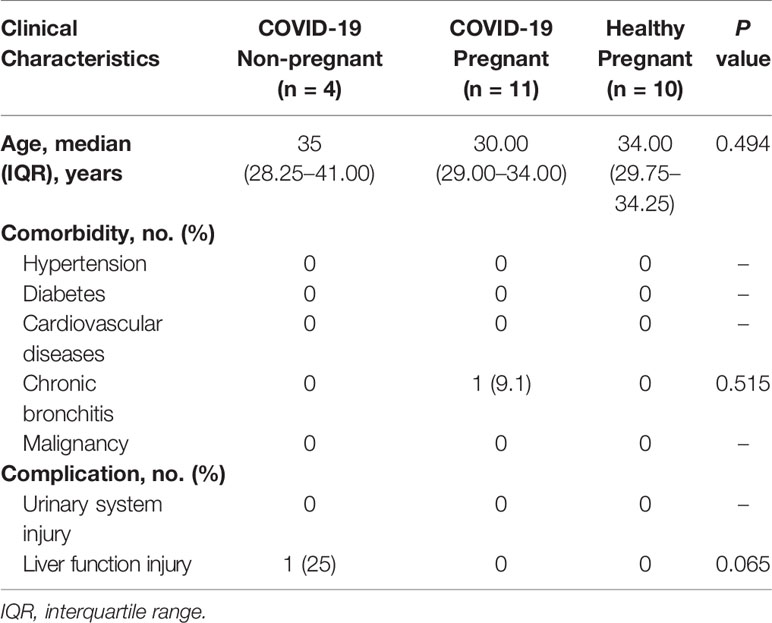
Table 3 Comparison of clinical characteristics between pregnant and non-pregnant patients tested for cytokines, chemokines, and growth factors.
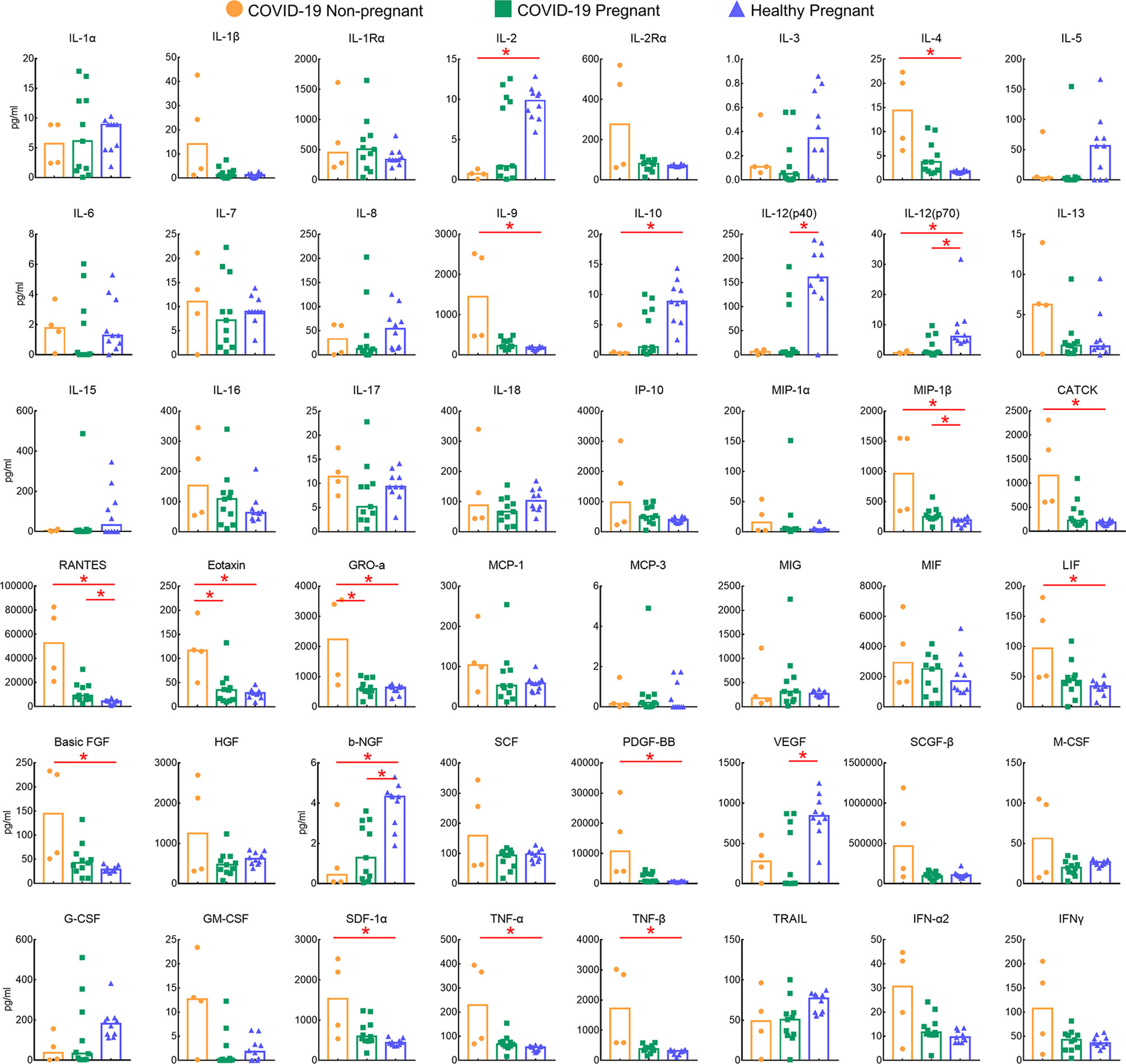
Figure 2 Comparison of plasma levels of 48 cytokines, chemokines, and growths factors between pregnant (n = 11) and non-pregnant groups (n = 4) COVID-19 patients and healthy pregnant patients (n = 10). Red lines and * above the columns indicated significant differences between the two columns.
Lymphocyte Subsets in the Peripheral Blood of Pregnant and Non-Pregnant COVID-19 Patients
Given that lymphocyte subsets were reported to be associated with COVID-19 severity and progression, we next analyzed lymphocyte composition in the peripheral blood of the pregnant group (n = 7) and non-pregnant group (n = 19) in COVID-19 patients (Figure 3). Albeit no statistical difference was found in clinical characteristics (Table 4) and lymphocyte subsets, higher counts of CD3+CD8+ T suppresser (TS) cells were observed in pregnant patients. Correspondingly, pregnant patients exhibited a lower CD4+/CD8+ ratio. In addition, NK cell showed slight high levels among pregnant group. Pregnant patients also exhibited trends of low CD19+ B cell counts, which was consistent with previous reports (22).
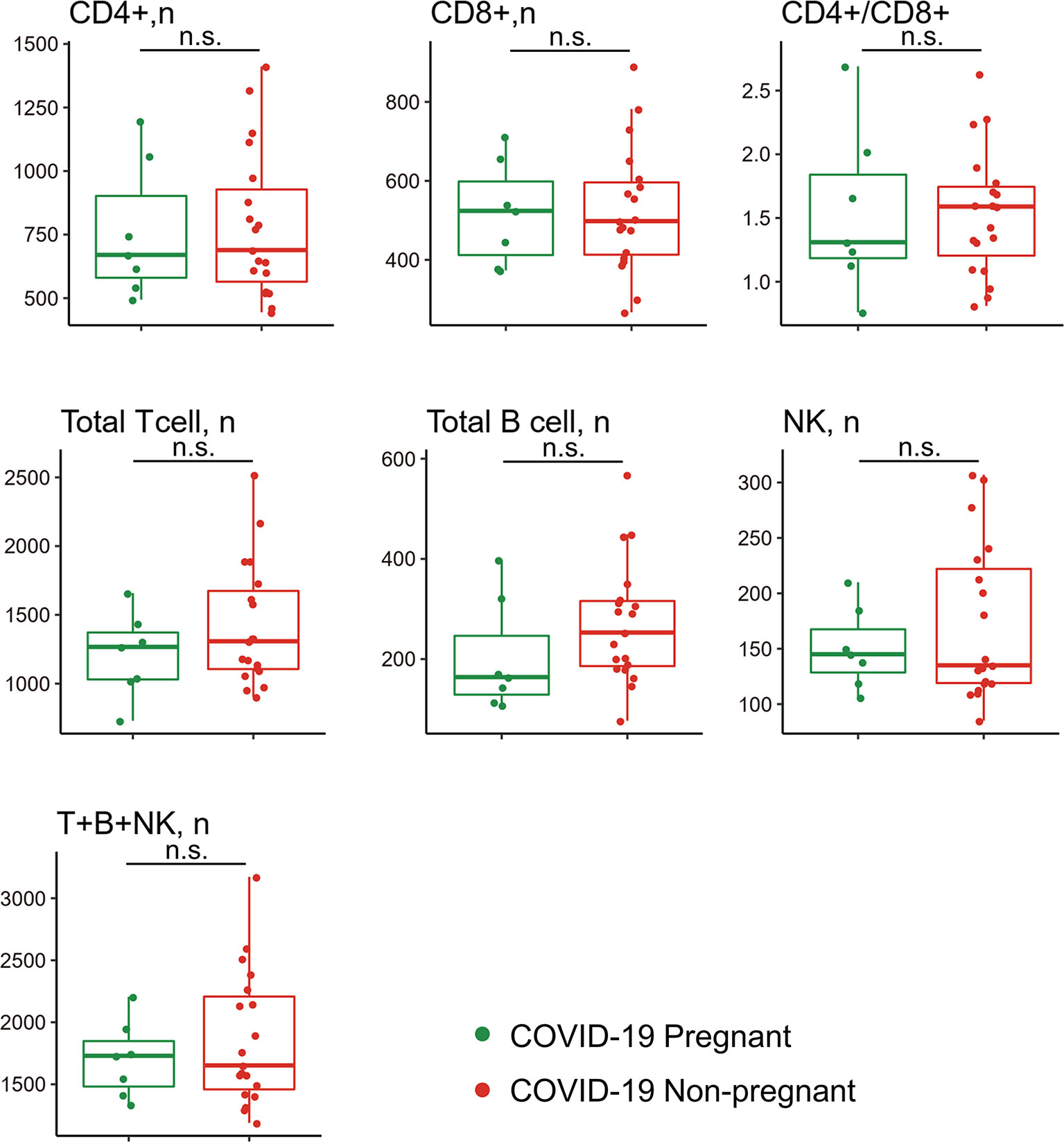
Figure 3 Comparison of peripheral lymphocyte subsets between pregnant and non-pregnant patients with COVID-19. Pregnant COVID-19 group (n = 7); non-pregnant COVID-19 group (n = 19). n.s., no significance.
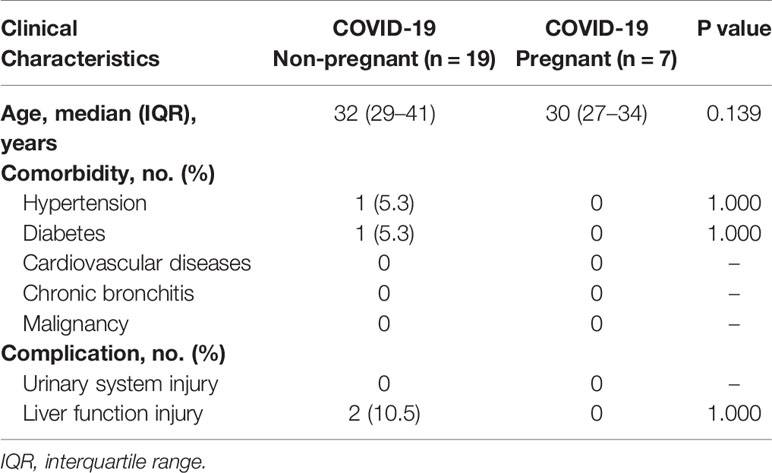
Table 4 Comparison of clinical characteristics between pregnant and non-pregnant COVID-19 patients tested for lymphocyte subsets.
Discussion
To investigate the immunological response of COVID-19 on patients during pregnancy, we performed a clinical analysis as well as CCGFs and lymphocyte subsets analysis of pregnant patients and non-pregnant patients. Our study showed deceased levels of inflammatory cytokines and chemokines of pregnant patients which may contribute to the mild symptoms experienced by these patients.
In our study, pregnant patients with COVID-19 displayed neutrophilia, lymphopenia, and high levels of CRP as well as D-dimer compared with non-pregnant COVID-19 patients, which is in consistent with previous evidence indicating high CRP and D-Dimer, leukocytosis, and elevated neutrophil ratio are more common in the COVID-19 infected pregnant women (23). However, our results also revealed that these physiological findings are typical of normal pregnancy due to adaptations to gestation (24), which may influence the clinical outcomes of pregnant women suffering from SARS-CoV-2 infection.
To investigate the immune status of pregnant patients with COVID-19, we further compared the cytokines, chemokines, and growth factors levels among pregnant COVID-19 group, non-COVID-19 group, and healthy pregnant group. Unlike increased levels of TNFα, IL-6, IL-8, IL-10, CXCL8, and IP-10 observed in H1N1 infected pregnant patients (25), COVID-19 pregnant patients showed relatively lower expressions of pro-inflammatory and anti-inflammatory cytokines. Specifically, macrophage chemokines, which are always referred to as major components of “cytokine storm” during coronaviruses infection (26, 27), including MIP-1α, CTACK, RANTES, Eotaxin, GRO-α, and TNF, showed significant low levels in pregnant patients. Meanwhile, growth factors, such as basic FGF, LIF, granulocyte colony stimulating factor (G-CSF), and PDGF-BB also showed significant low expression in pregnant group. By comparing with healthy pregnant women, we postulated that the immune adaptations during pregnancy may serve as the unique immune response to SARS-CoV-2. It was reported that the immunological response during pregnancy has a shift from a pro-inflammatory state in early stages to an anti-inflammatory state throughout the rest of pregnancy (28). Therefore, in pregnant women who already have dominant anti-inflammatory immunity, the parallel activation of pro-inflammatory and anti-inflammatory immunity in SARS-CoV-2 infection may not lead to pro-inflammatory cytokine storm (19, 29). The high levels of IL-5 and IL-10 (anti-inflammatory) and the low levels of pro-inflammatory cytokines, chemokines, and growth factors observed in our study have validated this conclusion to some extent. Taken together, cytokines response was suppressively regulated in pregnant women following SARS-CoV-2 infection, which may differentiate pregnant COVID-19 patients from patients who suffered from cytokine storm and progressed to acute respiratory distress syndrome (30).
SARS-CoV-2 infection is known to induce a decrease in CD4+ and CD8+ T cell and NK cell levels, more evidently in critically ill patients (31, 32). Therefore, we made comparison of lymphocyte subset counts in pregnant and non-pregnant COVID-19 patients. Compared with non-pregnant patients, pregnant patients showed no significant change of lymphocyte subsets in response to SARS-CoV-2 infection. However, we observed slightly increase number of CD8+ T cells and NK cells in pregnant patients with COVID-19. It is well known that both NK cells (the innate immunity) and CD8+ cytolytic T cells (the adaptive immunity) can destroy the virus-infected cells (33). And numerous clinical reports have shown that the decreased frequency of lymphocytes in the peripheral blood, including CD4+ and CD8+ T cells and NK cells, is closely related to disease severity of COVID-19 (34, 35). In addition, there is a negative relationship between high levels of cytokines and lower T cells in severe COVID-19 patients (4). Enhanced NK and CD8+ T cells response and low levels of CCGFs may protect pregnant patients from progressing to severe cases.
Our study has several limitations. First, the sample size was small because hospitalized pregnant patients accounted only for a small fraction of the sample. Second, cytokine levels may change over time, and because of the limitation of blood samples, our results may only represent a snapshot of these changes. Finally, because this was a retrospective study, the detection time of cytokines was not exactly matched.
In conclusion, our study revealed different immune responses between pregnant and non-pregnant COVID-19 patients. Pregnant patients showed intricately regulated immune response after SARS-CoV-2 infection characterized by a low expression of inflammatory cytokines and a slight increase of NK cell counts. Our study sheds light on the immunological response of pregnant COVID-19 patients, and it has significant implications for immunotherapy for COVID-19. Limited by the retrospective nature of our study, more laboratory evidence is expected to analyze the mechanisms underlying immunoregulation of SARS-CoV-2 to help improve the prognosis of severe cases and reduce mortality.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The dataset was obtained from Tongji Hospital by approval and are not available to the public. Requests to access these datasets should be directed to dGprZWtlQDEyNi5jb20=.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB2020401). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
GC collected the data, wrote, and revised the manuscript. QL analyzed the data, wrote and revised the manuscript. JA wrote and revised the manuscript. BY, HB, FL, and JC wrote and drew the figures. YC, HL, and KL designed and supervised the research. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Key Research and Development Program (2019YFC1005200, 2019YFC1005202), and the Hubei Province Health and Family Planning Scientific Research Project (WJ2019M127).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. WHO. Coronavirus Disease (COVID-19) Pandemic (2021). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
2. Napoli C, Benincasa G, Criscuolo C, Faenza M, Liberato C, Rusciano M. Immune Reactivity During COVID-19: Implications for Treatment. Immunol Lett (2021) 231:28–34. doi: 10.1016/j.imlet.2021.01.001
3. Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, et al. COVID-19 Infection: The Perspectives on Immune Responses. Cell Death Differ (2020) 27(5):1451–4. doi: 10.1038/s41418-020-0530-3
4. Wang J, Jiang M, Chen X, Montaner LJ. Cytokine Storm and Leukocyte Changes in Mild Versus Severe SARS-Cov-2 Infection: Review of 3939 COVID-19 Patients in China and Emerging Pathogenesis and Therapy Concepts. J Leukoc Biol (2020) 108(1):17–41. doi: 10.1002/JLB.3COVR0520-272R
5. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The Covid-19 Cytokine Storm; What We Know So Far. Front Immunol (2020) 11:1446. doi: 10.3389/fimmu.2020.01446
6. Watanabe M, Iwatani Y, Kaneda T, Hidaka Y, Mitsuda N, Morimoto Y, et al. Changes in T, B, and NK Lymphocyte Subsets During and After Normal Pregnancy. Am J Reprod Immunol (1997) 37(5):368–77. doi: 10.1111/j.1600-0897.1997.tb00246.x
7. Chen Y, Li Z, Zhang YY, Zhao WH, Yu ZY. Maternal Health Care Management During the Outbreak of Coronavirus Disease 2019. J Med Virol (2020) 92(7):731–9. doi: 10.1002/jmv.25787
8. Aghaeepour N, Ganio EA, McIlwain D, Tsai AS, Tingle M, Van Gassen S, et al. An Immune Clock of Human Pregnancy. Sci Immunol (2017) 2(15):eaan2946. doi: 10.1126/sciimmunol.aan2946
9. Robinson DP, Klein SL. Pregnancy and Pregnancy-Associated Hormones Alter Immune Responses and Disease Pathogenesis. Horm Behav (2012) 62(3):263–71. doi: 10.1016/j.yhbeh.2012.02.023
10. Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, et al. Characterization of Type 1 and Type 2 Cytokine Production Profile in Physiologic and Pathologic Human Pregnancy. Clin Exp Immunol (1996) 106(1):127–33. doi: 10.1046/j.1365-2249.1996.d01-809.x
11. Le Gars M, Kay AW, Bayless NL, Aziz N, Dekker CL, Swan GE, et al. Increased Proinflammatory Responses of Monocytes and Plasmacytoid Dendritic Cells to Influenza a Virus Infection During Pregnancy. J Infect Dis (2016) 214(11):1666–71. doi: 10.1093/infdis/jiw448
12. Littauer EQ, Skountzou I. Hormonal Regulation of Physiology, Innate Immunity and Antibody Response to H1N1 Influenza Virus Infection During Pregnancy. Front Immunol (2018) 9:2455. doi: 10.3389/fimmu.2018.02455
13. Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in Pregnancy and Delivery: Rapid Review. Ultrasound Obstet Gynecol (2020) 55(5):586–92. doi: 10.1002/uog.22014
14. Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, et al. H1N1 2009 Influenza Virus Infection During Pregnancy in the USA. Lancet (2009) 374(9688):451–8. doi: 10.1016/S0140-6736(09)61304-0
15. Elshafeey F, Magdi R, Hindi N, Elshebiny M, Farrag N, Mahdy S, et al. A Systematic Scoping Review of COVID-19 During Pregnancy and Childbirth. Int J Gynaecol Obstet (2020) 150(1):47–52. doi: 10.1002/ijgo.13182
16. Ryan GA, Purandare NC, McAuliffe FM, Hod M, Purandare CN. Clinical Update on COVID-19 in Pregnancy: A Review Article. J Obstet Gynaecol Res (2020) 46(8):1235–45. doi: 10.1111/jog.14321
17. Zaigham M, Andersson O. Maternal and Perinatal Outcomes With COVID-19: A Systematic Review of 108 Pregnancies. Acta Obstet Gynecol Scand (2020) 99(7):823–9. doi: 10.1111/aogs.13867
18. Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of Women of Reproductive Age With Laboratory-Confirmed SARS-Cov-2 Infection by Pregnancy Status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep (2020) 69(25):769–75. doi: 10.15585/mmwr.mm6925a1
19. Berhan Y. What Immunological and Hormonal Protective Factors Lower the Risk of COVID-19 Related Deaths in Pregnant Women? J Reprod Immunol (2020) 142:103180. doi: 10.1016/j.jri.2020.103180
20. Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, Progesterone, Immunomodulation, and COVID-19 Outcomes. Endocrinology (2020) 161(9):bqaa127. doi: 10.1210/endocr/bqaa127
21. Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in Treatment of Severe Coronavirus Disease 2019 (COVID-19): A Multicenter, Single-Blind, Randomized Controlled Trial. J Allergy Clin Immunol (2020) 146(1):137–46.e3. doi: 10.1016/j.jaci.2020.05.019
22. Li N, Han L, Peng M, Lv Y, Ouyang Y, Liu K, et al. Maternal and Neonatal Outcomes of Pregnant Women With Coronavirus Disease 2019 (COVID-19) Pneumonia: A Case-Control Study. Clin Infect Dis (2020) 71(16):2035–41. doi: 10.1093/cid/ciaa352
23. Vakili S, Savardashtaki A, Jamalnia S, Tabrizi R, Nematollahi MH, Jafarinia M, et al. Laboratory Findings of COVID-19 Infection are Conflicting in Different Age Groups and Pregnant Women: A Literature Review. Arch Med Res (2020) 51(7):603–7. doi: 10.1016/j.arcmed.2020.06.007
24. Lurie S, Rahamim E, Piper I, Golan A, Sadan O. Total and Differential Leukocyte Counts Percentiles in Normal Pregnancy. Eur J Obstet Gynecol Reprod Biol (2008) 136(1):16–9. doi: 10.1016/j.ejogrb.2006.12.013
25. Cerbulo-Vazquez A, Figueroa-Damian R, Arriaga-Pizano LA, Hernandez-Andrade E, Mancilla-Herrera I, Flores-Mejia LA, et al. Pregnant Women Infected With Pandemic H1n1pdm2009 Influenza Virus Displayed Overproduction of Peripheral Blood CD69+ Lymphocytes and Increased Levels of Serum Cytokines. PloS One (2014) 9(9):e107900. doi: 10.1371/journal.pone.0107900
26. Schulert GS, Grom AA. Pathogenesis of Macrophage Activation Syndrome and Potential for Cytokine- Directed Therapies. Annu Rev Med (2015) 66:145–59. doi: 10.1146/annurev-med-061813-012806
27. Jafarzadeh A, Chauhan P, Saha B, Jafarzadeh S, Nemati M. Contribution of Monocytes and Macrophages to the Local Tissue Inflammation and Cytokine Storm in COVID-19: Lessons From SARS and MERS, and Potential Therapeutic Interventions. Life Sci (2020) 257:118102. doi: 10.1016/j.lfs.2020.118102
28. Brann E, Edvinsson A, Rostedt Punga A, Sundstrom-Poromaa I, Skalkidou A. Inflammatory and Anti-Inflammatory Markers in Plasma: From Late Pregnancy to Early Postpartum. Sci Rep (2019) 9(1):1863. doi: 10.1038/s41598-018-38304-w
29. Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li S, Biswas A, et al. Coronavirus Disease 2019 (COVID-19) Pandemic and Pregnancy. Am J Obstet Gynecol (2020) 222(6):521–31. doi: 10.1016/j.ajog.2020.03.021
30. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5
31. Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, et al. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J Infect Dis (2020) 221(11):1762–9. doi: 10.1093/infdis/jiaa150
32. Huang W, Berube J, McNamara M, Saksena S, Hartman M, Arshad T, et al. Lymphocyte Subset Counts in COVID-19 Patients: A Meta-Analysis. Cytometry A (2020) 97(8):772–6. doi: 10.1002/cyto.a.24172
33. Soy M, Keser G, Atagunduz P, Tabak F, Atagunduz I, Kayhan S. Cytokine Storm in COVID-19: Pathogenesis and Overview of Anti-Inflammatory Agents Used in Treatment. Clin Rheumatol (2020) 39(7):2085–94. doi: 10.1007/s10067-020-05190-5
34. Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al. Lymphopenia Predicts Disease Severity of COVID-19: A Descriptive and Predictive Study. Signal Transduct Target Ther (2020) 5(1):33. doi: 10.1038/s41392-020-0148-4
Keywords: COVID-19, pregnant women, immune response, lymphocyte subtypes, cytokines
Citation: Chen G, Liao Q, Ai J, Yang B, Bai H, Chen J, Liu F, Cao Y, Liu H and Li K (2021) Immune Response to COVID-19 During Pregnancy. Front. Immunol. 12:675476. doi: 10.3389/fimmu.2021.675476
Received: 03 March 2021; Accepted: 13 April 2021;
Published: 03 May 2021.
Edited by:
Sha Wu, Southern Medical University, ChinaReviewed by:
Xiaojun Chen, Fudan University, ChinaXi Li, Oregon Health and Science University, United States
Copyright © 2021 Chen, Liao, Ai, Yang, Bai, Chen, Liu, Cao, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kezhen Li, dGprZWtlQDEyNi5jb20=; Haiyi Liu, aHlsaXVAdGpoLnRqbXUuZWR1LmNu; Yang Cao, Y2FveWFuZ2VtbWFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ge Chen1†
Ge Chen1† Qiuyue Liao
Qiuyue Liao Jing Chen
Jing Chen Kezhen Li
Kezhen Li