- 1Department of Dermatology and Allergy, Dermatological Allergology, Allergie-Centrum-Charité, Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany
- 2Division of Allergy, University Clinic of Respiratory and Allergic Diseases Golnik, Golnik, Slovenia
- 3Department of Dermatology and Venerology, Kepler University Hospital, Linz, Austria
Introduction: Cryoproteins, such as cryoglobulins, cryofibrinogens and cold agglutinins, precipitate at low temperatures or agglutinate erythrocytes and dissolve again when warmed. Their pathogenetic and diagnostic importance in cold urticaria (ColdU) is unclear. In this study, we aimed to characterize the prevalence of cryoproteins in patients with ColdU.
Methods: We conducted 3 analyses: i) a systematic review and meta-analysis of published data using an adapted version of the Joanna Briggs Institute’s critical appraisal tool for case series, ii) a retrospective analysis of 293 ColdU patients treated at our Urticaria Center of Reference and Excellence (UCARE) from 2014 to 2019, and iii) a prospective observational study, from July 2019 to July 2020, with 49 ColdU patients as defined by the EAACI/GA2LEN/EDF/UNEV consensus recommendations.
Results: Our systematic review identified 14 relevant studies with a total of 1151 ColdU patients. The meta-analyses showed that 3.0% (19/628), 1.1% (4/357) and 0.7% (2/283) of patients had elevated levels of cryoglobulins, cryofibrinogens and cold agglutinins, respectively. Our retrospective analyses showed that cryoproteins were assessed in 4.1% (12/293) of ColdU patients. None of 9 ColdU patients had cryoglobulins, and one of 5 had cold agglutinins. In our prospective study, none of our patients had detectable cryoglobulins (0/48) or cryofibrinogens (0/48), but 4.3% (2/46) of patients had cold agglutinins (without any known underlying autoimmune or hematological disorder).
Conclusion: Our investigation suggests that only very few ColdU patients exhibit cryoproteins and that the pathogenesis of ColdU is driven by other mechanisms, which remain to be identified and characterized in detail.
Introduction
Cold urticaria (ColdU) is a subtype of chronic inducible urticaria, where wheals, angioedema or both are evoked by exposure to low temperatures (1). A reliable medical history and the results of cold stimulation tests (CSTs) such as the ice cube test or the TempTest® establish the diagnosis (2).
Two different classification approaches can be found in the published literature. The classification of ColdU based on CSTs differentiates between typical ColdU, with wheals in the CSTs, and atypical ColdU, without wheals or atypical wheals in the CSTs (3). In the other approach, the etiology-based classification, a hereditary and an acquired form are distinguished, the latter being further subdivided into primary and secondary. Secondary acquired ColdU can be caused by hematologic diseases, infections, malignancies, cryoproteins (CPs) or other diseases (4). The pathophysiology of ColdU is not well understood, and autoallergic and autoimmune mechanisms, temperature-sensitive receptors, neurogenic signals, and CPs have been speculated to play a role (5, 6).
The term cryoprotein or cold protein usually includes cryoglobulins, cryofibrinogens, cold agglutinins, and sometimes other proteins. Cryoglobulins (CGs) are immunoglobulins that precipitate in vitro at low temperatures and dissolve when warmed (7). CGs can be divided into monoclonal (Type I) and mixed (Type II and III) immunoglobulins (8) and can cause various cold-related diseases with joint, nerve, and kidney involvement, weakness, palpable purpura, Raynaud’s phenomenon, skin rashes and ulcers (9). Various infections, especially hepatitis C, immune diseases and hematological disorders have been described as possible triggers for the occurrence of CGs (9, 10). Cryofibrinogens (CFs), which only precipitate in plasma, can occur independently or in association with CGs. Malignancies, infections and autoimmune diseases are known to induce the occurrence of CFs (11, 12). CFs can cause skin manifestations such as Raynaud’s phenomenon and purpura as well as arterial and venous thromboses (11). Cryoglobulinemia and cryofibrinogenemia, i.e. the presence of the respective CP, are distinct from cryoglobulinemic or cryofibrinogenic diseases or syndromes, i.e. resulting disease entities (11, 13). Healthy individuals may have CGs (14, 15) or CFs (11).
Cold agglutinins (CAs), also known as cold(auto)antibodies, are mostly type M immunoglobulins that agglutinate erythrocytes at low temperatures and may lead to complement activation (16).
As of now, the role and relevance of CPs in the pathogenesis of ColdU remain ill-defined and many questions need to be answered. There are several published reports on the prevalence of CPs in patients with ColdU, including many case reports and small case series. These have not been systematically analyzed at the present time, and the rates of CP-positive ColdU patients, thus, are not known. Also, it is largely unknown how often ColdU patients are assessed for CPs and what drives the decision to do so.
To answer these questions and address these unmet needs, we used a three-tiered approach. First, we performed a systematic review and meta-analysis of published reports on the rates of ColdU patients who tested positive for CGs, CFs, and CAs. Second, we retrospectively assessed how often close to 300 patients with ColdU treated at our Urticaria Center of Reference and Excellence (UCARE) (17) were assessed for these CPs and why. Finally, we prospectively measured CPs in almost 50 consecutive patients with ColdU. The overall aim of our report is to provide more clarity on the prevalence, role and relevance of CPs in ColdU.
Materials and Methods
Systematic Review and Meta-Analysis of Published Reports
On June 30, 2020, we conducted a systematic PubMed literature search with the terms (“cold urticaria”) AND (“cryoglobulin*” OR “cryofibrinogen*” OR “cold agglutinin*”) in accordance with the PRISMA guidelines (18) and included all published studies between 1980 and 2019 in German, English, Spanish and French. Additionally, we manually checked references in key publications on ColdU (3, 19–21), since CPs are often only reported as part of the patient description in the full text.
To be eligible, the studies had to have measured CPs in patients with ColdU diagnosed according to established criteria such as a typical patient history or positive CSTs. Accordingly, the occurrence of CPs was not allowed to be a study exclusion criterion. We excluded studies that exclusively dealt with secondary or hereditary ColdU forms and case series with fewer than 5 patients in order to avoid bias through selective reporting and publishing.
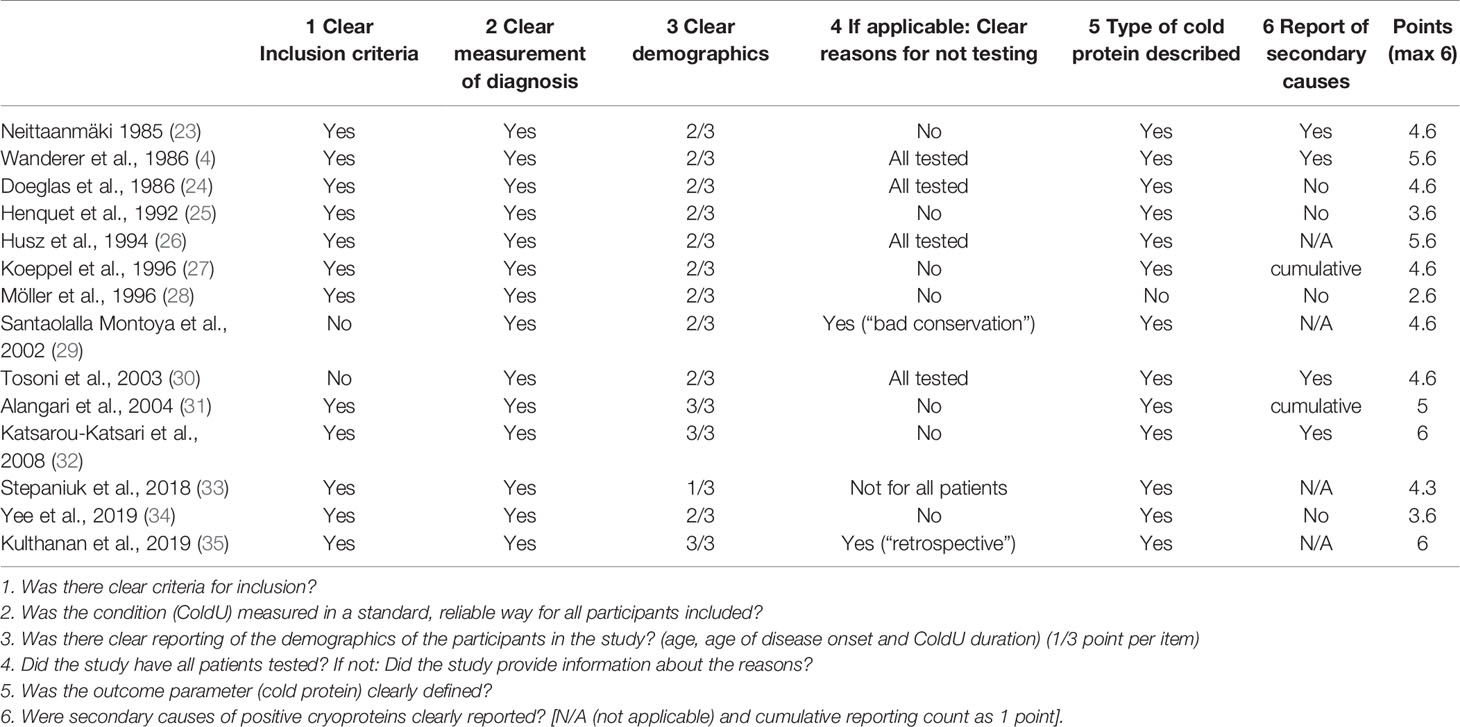
The study selection was carried out independently by two researchers based on predefined criteria. After excluding all non-relevant studies based on title and abstract screening, we procured the full texts and contacted the authors for additional information, as necessary and possible. Patient characteristics and results of CP testing were extracted independently by both researchers using a survey table. Information on inclusion criteria for the respective studies, reasons for incomplete laboratory results and reported secondary diagnoses were also collected to identify selective reporting. Where applicable, the accordance of the term “cryoglobulinemia” in the respective study with the definition used in this review was ensured. The study quality and the bias risk were then independently assessed by two researchers using a specifically adapted version of the Joanna Briggs Institute’s critical appraisal tool for case series (22) (see Table 1). Finally, the average percentage of CP-positive patients, i.e. patients who tested positive for CGs, CFs, or CAs, was calculated, weighted according to the respective study size.
Retrospective Analyses of ColdU Patients
We retrospectively searched the database of all chronic urticaria patients diagnosed with ColdU who presented at the UCARE at Charité-Universitätmedizin Berlin, Germany, between 2014 and 2019 for information on CPs. Collected data included patient age, laboratory workup, type of diagnosis, onset of ColdU and other diagnoses. Patient data were analyzed and reported anonymously in accordance with data protection regulations, and stored in a MS Excel Version 2019 based database.
Prospective Assessment of ColdU Patients
Between July 2019 and July 2020, we assessed 49 patients out of 60 consecutive patients with ColdU treated at our UCARE. Of the patients originally seen during the study period (N = 60), 4 patients were excluded because the diagnosis of ColdU was not confirmed or was questionable; one patient was excluded because he no longer had active disease. In 6 patients, a sufficient amount of blood could not be obtained due to organizational reasons, patient-related reasons or laboratory difficulties. There was no control group. Our study was approved by the local ethics committee (reference EA1/069/19), and all patients provided informed consent. Inclusion criteria were 1) diagnosed ColdU as defined by the 2016 EAACI/GA2LEN/EDF/UNEV consensus recommendations as the “recurrence of itchy wheals and/or angioedema [ … ] reproducible in response to [ … ] cold exposure” (1), 2) disease duration of 6 weeks or longer, 3) no intake of H1 antihistamines within 3 days and/or glucocorticosteroids within 7 days prior to CSTs and blood sampling. ColdU was diagnosed in all of our patients based on their typical history and CSTs. For CSTs, the TempTest 4.0® and the ice cube test with 5 minutes of cold application and reading after 10 minutes were used (36). ColdU was classified as “typical” in patients who developed a wheal at the site of the CST within 10 minutes after cold exposure and as “atypical” in patients who did not (3).
We obtained and analyzed patient demographics and the course and severity of the disease including systemic reactions to cold exposure. We also assessed patients for family history for ColdU, cold-associated complaints like wheals, pruritus or angioedema and comorbidities such as atopic diseases, infections, malignancies, connective tissue disorders, thyroid diseases. Data were collected and pseudonymously entered in an MS Excel Version 2019 based database.
Laboratory Workup
CP analyses were conducted at the central laboratory of the Charité (Labor Berlin, CGs and CFs) and the Institute of Transfusion Medicine (CAs). All peripheral-venous blood samples were collected in a standardized way with prewarmed tubes and transported directly to the laboratory, ensuring a constant transport temperature of 37°C. Since temperature deviations are a confounding factor for the analysis, a rapid and standardized transport procedure for all samples was implemented.
CAs were determined in 6ml EDTA and 10ml native venous blood, and plasma and sediment were separated at 37°C. Subsequently, samples were analyzed for CAs of blood group system I/i using foreign adult and umbilical vein erythrocytes. Reaction strength at room temperature (20°C) was evaluated by one to two observers depending on agglutination strength in categories ranging from negative (no agglutination), within physiological range (mild to moderate agglutination), borderline (more severe agglutination) to pathological/positive (massive and lumpy agglutination). At the same time, patient erythrocytes were examined with the direct Coombs test, in which in most cases only complement adhesion is expected, since any CAs detach from the erythrocytes again upon recirculation.
CFs were determined using immunoprecipitation in 2 ml EDTA, and CGs were analyzed from 2 ml serum. After centrifugation, the clear plasma or serum was examined for the formation of precipitates for 72 hours at 4°C. If precipitates or turbidity were evident, the supernatant was warmed to 37°C and, if dissolved, stored a second time at 4°C. Only in case of repeated dissolution and formation of precipitates, the sample was considered positive for CGs or CFs. Cryoprecipitates in the serum were considered as positive CGs and cryoprecipitates in the EDTA as the sum of positive CGs and positive CFs, so that both tests were always performed simultaneously for differentiation. No further differentiation or quantification of the samples was performed.
Statistical Methods
The quantitative variables reported in this study were summarized using median, range (Min, Max) and interquartile range (IQR) using R Version 3.6.3 (37).
Results
Literature Review: The Studies to Date Show a Wide Scatter of Results, but on Average the Frequency of Positive CPs in ColdU Patients was Low
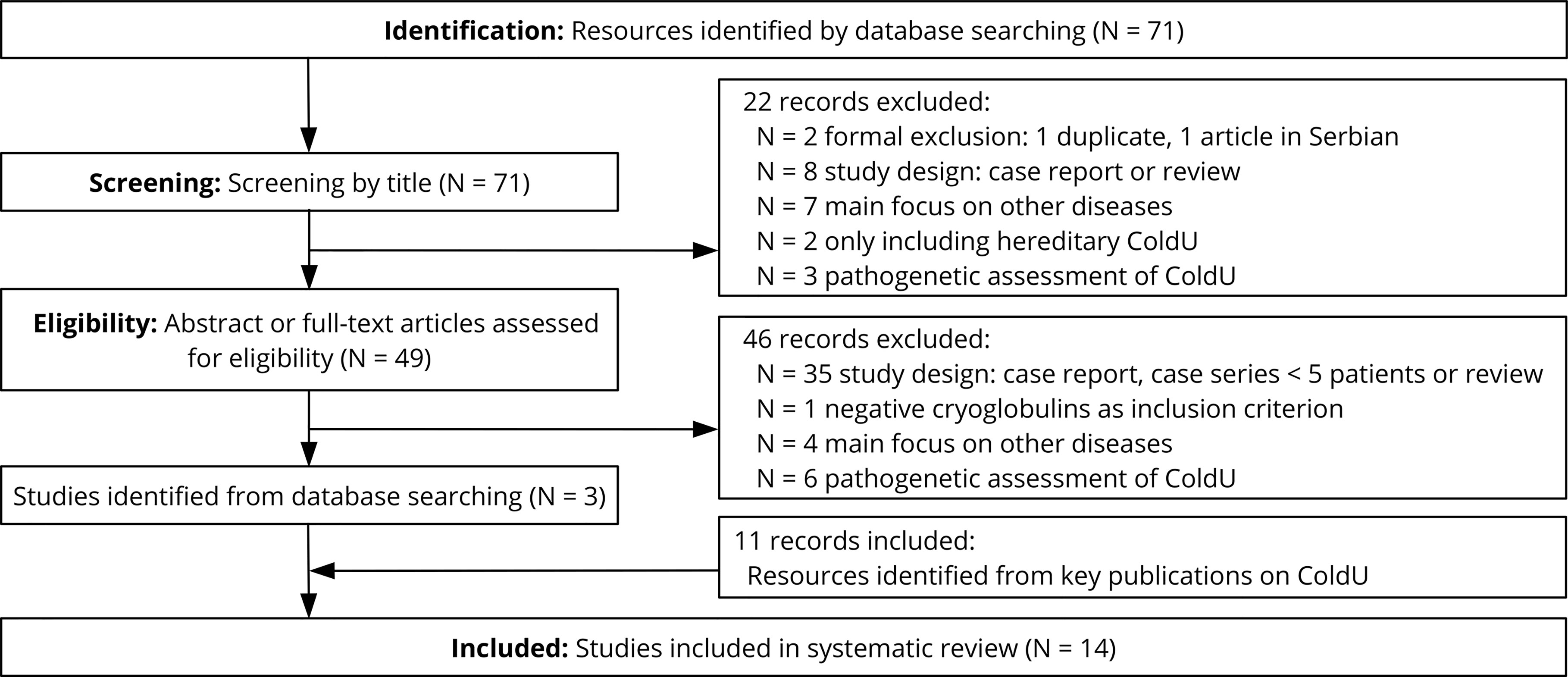
Our systemic review identified 71 publications of potential relevance (Figure 1), of which 68 were excluded after reviewing the title, abstract or full-text. In addition, we found 11 relevant publications by checking the references of key publications, resulting in a total of 14 studies from the years 1985 to 2019 that were evaluated in this review (Table 2). Most reports (11 of 14) had 4 or more points out of possible 6 on the quality score and were therefore considered to be of medium or high quality (Table 1).
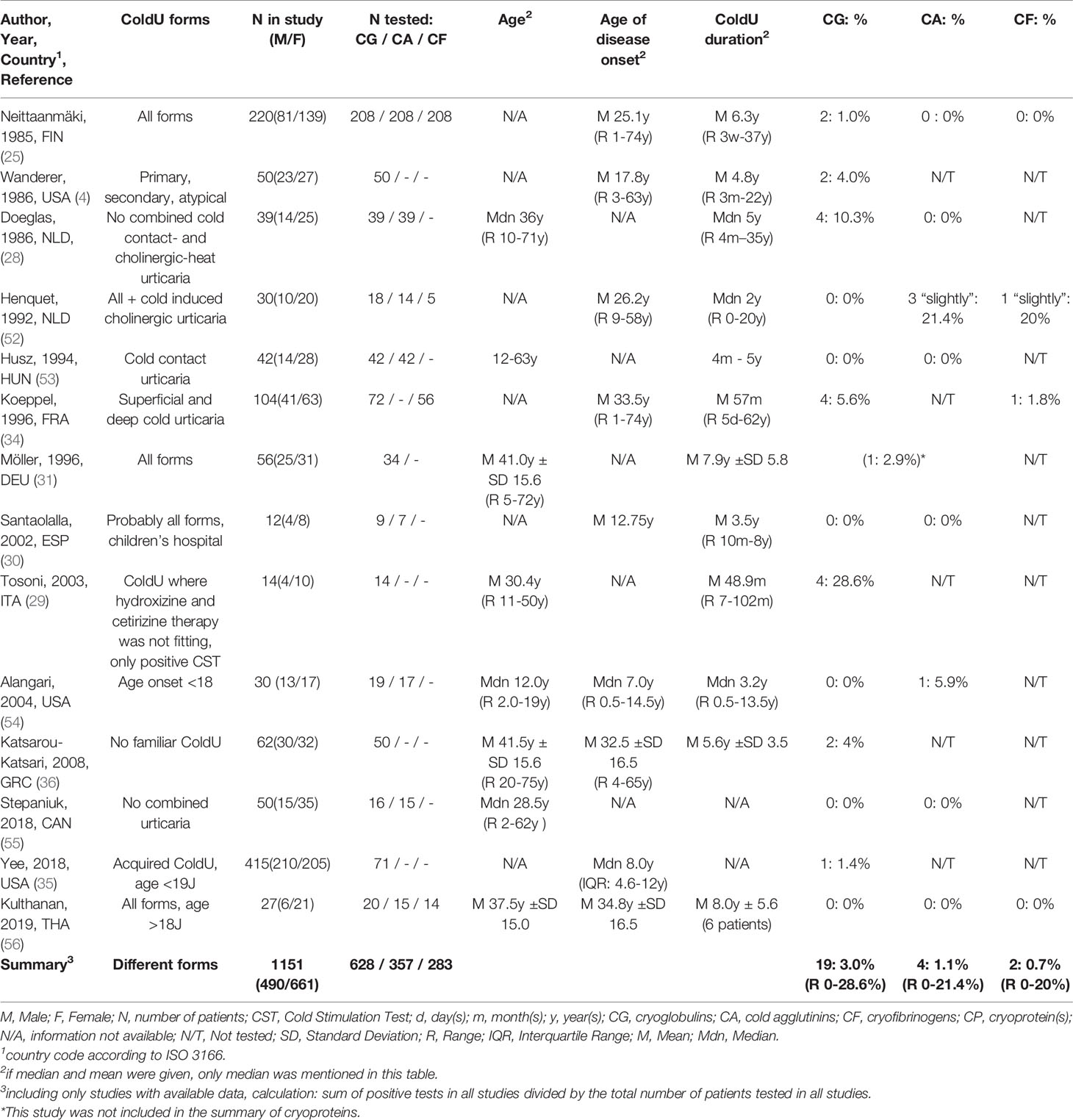
CPs had been determined in all 14 studies in patient cohorts ranging from 9 to 208 ColdU patients. In 0 to 28.6% (average summarized for all studies 3.0%) of the ColdU patients, positive CGs had been detected. This was reported to be associated with secondary disease in two of these patients, one of whom had lymphosarcoma (23) and one of whom had chronic lymphocytic leukemia (4). CFs had been determined in 4 studies in patient cohorts ranging from 5 to 208 ColdU patients. In 0 to 20% (summarized for all studies 0.7%) of the ColdU patients, positive CFs had been detected. CAs had been analyzed in 9 studies ranging from 7 to 208 patients. 0 to 21.4% (summarized for all studies: 1.1%) positive CAs were reported.
Retrospective Study: In Routine Clinical Practice, Very Few ColdU Patients Were Tested for CPs, and the Rate of Positive Tests Was Low
Between 2014 and 2019, 293 patients with various forms of ColdU were seen at our UCARE. In 12 of these patients, CPs were determined. CGs were determined in 9 patients, CA were determined in 5 patients, both CG and CA were analyzed in 2 patients. CFs were not determined in any of the patients. The main reasons for CP determination were the high disease severity (in 9 of 12 patients) and suspicion of autoimmunity or hematological disease (in 3 of 12 patients). We found no anomalies in the 9 patients tested for CGs, but one of the 5 ColdU patients tested for CAs showed a positive result. However, this patient is known to have Raynaud’s syndrome, which may be associated with the presence of CAs.
Prospective Study: Rates of CP- Positive ColdU Patients Were Very Low
Of the 49 analyzed ColdU patients, 73.5% (N = 36) were female and 26.5% (N = 13) were male, the median age was 40 years (IQR: 40 – 53 years). 69.5% (N = 34) were diagnosed with typical ColdU. Patient characteristics are described in Table 3 and anonymized data of these patients is described in the supplementary material.
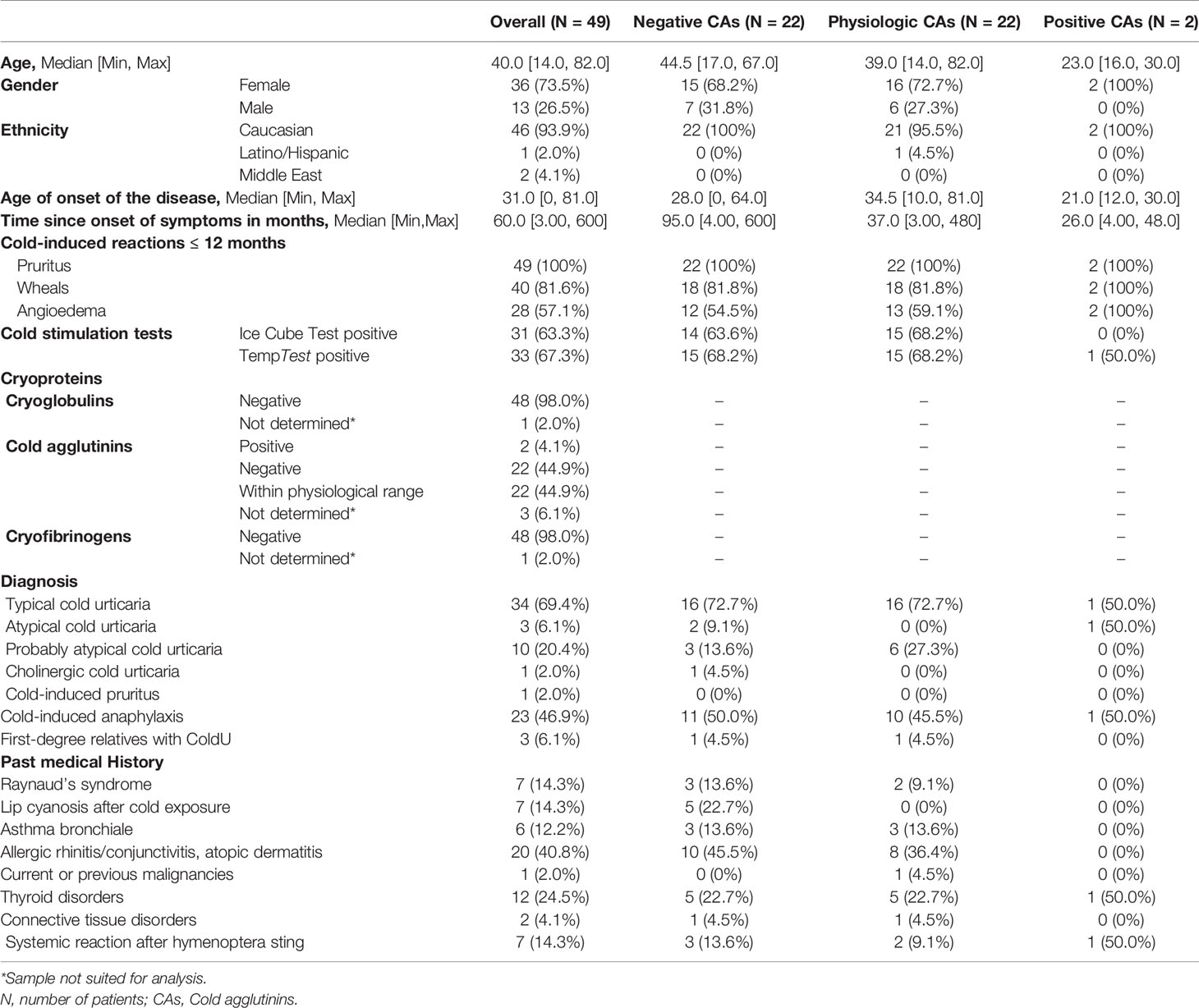
Table 3 Demographic characteristics of 49 ColdU patients included at Charité Berlin between July 2019 and July 2020.
None of 48 patients tested positive for CGs or CFs, and 2 of 46 patients (4.3%) tested positive for CAs. The two patients with positive CAs were female and aged 30 and 16 years, respectively. Both reported having experienced pruritus, wheals and angioedema within the 12 months prior to study inclusion. One woman had been diagnosed with classic acquired (ice cube test negative, TempTest positive) and the other with atypical ColdU (IceCubeTest and TempTest negative). The classic acquired ColdU patient also reported cold-induced anaphylaxis and breathing difficulties in response to cold exposure, suffered from hypothyroidism and has experienced a systemic reaction after a hymenoptera sting. The atypical ColdU patient reported no further diseases or systemic reactions. Information on the clinical differences between patients with physiological, elevated, and undetectable CAs is shown in Table 3. We could not detect major differences between patients with physiological and undetectable CAs.
Discussion
In this study, we showed that the frequency of positive CPs in ColdU patients was low with all three methods used. Moreover, ColdU patients were rarely assessed for CPs in routine clinical practice and testing positive was rarely linked to clinical features or consequences. Our results discourage routine clinical testing of ColdU patients for CPs and call for further characterization of the role and relevance of CPs in ColdU patients who test positive.
Comparison with Previous Literature
When compared to previous publications, the findings of our literature review are consistent with the review by Alain Claudy, with whose dataset we calculated a proportion of 2% positive CGs in ColdU patients (20). Contradictory results with a proportion of 20% positive CGs in ColdU patients were reported in the review by Houser et al. (38), which was cited in the teaching book by Czarnetzki (39). In order to summarize all publications until 1970, Houser et al. divided the number of publications with ColdU patients and cryoglobulinemia by the total number of publications with ColdU patients. Since the majority of the included studies are case reports, their result can be explained by the distortion due to a presumably high reporting and publication bias.
The frequency of positive CGs in the studies identified through our systematic literature review ranged between 0% and 28.6%, with strikingly high values in Doeglas (24), 10.3%, and Tonsoni (30), 28.6%. The high percentage in Doeglas et al. can be plausibly explained by the different study design, as it is the only study with a measurement of CPs in patients at 3-6 different points in time. The high proportion in Tosoni et al. of 4 patients with slightly positive CGs (28.6%) can only be partly attributed to the small number of cases (N = 14) and remains largely unexplained.
Limitations
When evaluating the literature on CPs and ColdU, limitations on several levels must be considered: On the one hand, distortion by a reporting bias should be considered, meaning that purely negative laboratory tests and cases with negative CGs may not be reported or published. This effect is reflected in the fact that the proportion of positive CGs in the case reports is much higher and decreases with increasing sample size.
The heterogeneity of the studies included in this review is a further potential constraint when summarizing the literature (see Table 1). Two studies (29, 30) did not clearly report their inclusion criteria and one (28) did not differentiate between the different CPs (28). Moreover, 7 studies did not explain the reasons for their incomplete laboratory results. Different authors included and excluded different forms of ColdU, while others did not define criteria at all. None of the papers reported on the handling of blood samples or on the laboratory procedure used, which could lead to distortions due to different measurement standards (7) and reference values (40) in different countries, as a survey of 137 European laboratories showed (41). Moreover, most publications did not report on possible underlying diseases in CP-positive patients, so that we could not distinguish between primary and secondary cryoproteinemia.
The validity of the prospective and retrospective study might be limited by the specific patient selection at our UCARE: Patients who had already been seen by a specialist before study enrollment may have been given a different diagnosis or referred directly to other departments, such as hematology, as a result. Additionally, because this study was conducted at a tertiary hospital, patients with particularly severe ColdU may have been primarily enrolled in the study. In addition, due to the study design, we could not reconstruct the exact blood collection and transport logistics in the retrospective analysis.
Nonetheless, this study analyzed a substantial number of well-characterized ColdU patients with quality-controlled sampling and transport, and only 5 other studies known to us had included similar or higher numbers of patients (4, 23, 27, 32, 34).
Explanatory Approaches for the Low Frequency of CPs and the Large Scatter of Results of Previous Publications
Our results raise the question of why, on the one hand, such disparate results were reported in the literature and why, on the other hand, we observed such a low frequency of CPs.
First, according to a theory by Wanderer (42), many laboratory results on CP could be false negatives and the actual true frequency of positive CP in ColdU patients might be much higher. However, this theory is contradicted by the fact that all studies known to us involving a large number of patients reported a very low percentage of positive CP [such as (23, 27, 34)].
More likely, therefore, is the theory that there are two manifestations of ColdU: a CP-negative and a much rarer CP-positive form. This theory is supported by the numerous case reports of patients suffering from CG-positive ColdU and additionally other diseases such as infections (38, 43–50) or hematologic disorders/malignancies (5, 51–55). In these patients, the underlying disease could have caused cryoproteinemia and as a consequence ColdU. It is possible that the underlying disease may also be diagnosed later, as Polliack and Lugassy found out (56). Furthermore, it is conceivable that the symptoms and the clinical course of CP-positive and CP-negative ColdU differ, although to our knowledge there have been no publications on this subject to date.
Another explanatory approach is that the presence of CP in a patient and the occurrence of ColdU could be completely independent of each other. To verify this theory, a comparison with the frequency of positive CPs in the general population would be helpful, but we could not find any published data on this. Furthermore, it seems possible that a third disease has caused both cryoglobulinemia and ColdU in CP-positive patients. In this case, CPs might have a diagnostical, but only an indirect pathogenical relation to ColdU.
Implications
Overall, we have found that CPs are rare in ColdU patients, suggesting that physicians may limit the measurement of CPs even more to patients with a clinical suspicion of secondary ColdU. In addition to cost savings, this automatically leads to fewer false positive findings and fewer unnecessary blood samples taken from patients. It remains to be clarified to what extent positive CPs have a diagnostic and therapeutic benefit for ColdU patients or to what extent they influence the course of the disease or the patients’ symptoms. Furthermore, we recommend the development of an international standardized protocol and defined threshold values for CP detection to reduce false negative and false positive results. The pathomechanism of ColdU with and without CPs continues to remain unclear and requires further research.
Conclusion
In summary, both through our literature review and through the analysis of our patient data, we could show that only few ColdU patients exhibit CPs and that the pathogenesis of ColdU might mainly be driven by other mechanisms, which remain to be identified and characterized in detail.
Data Availability Statement
The raw data was generated at Charité — Universitätsmedizin Berlin, Germany. All data shown in the study is in the Supplementary Material, further data supporting the findings of this study is available from the corresponding author on request.
Ethics Statement
Ethical approval and consent details: Ethical approval was obtained from the ethics committee of the Charité – Universitätsmedizin Berlin, number EA1/069/19. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KG performed the statistical analysis and drafted the manuscript. DA, KK, and SA were involved in patient recruitment and proof-reading of the manuscript. MM and MB were involved in study planning and proof-reading of the manuscript. DT-M has planned the study, coordinated the study, collected patient data, was involved in statistical analysis and drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Open access publication fund of Charité Universitätsmedizin Berlin.
Conflict of Interest
KK is or recently was a speaker and/or advisor for and/or has received research funding from Berlin Chemie, CSL Behring, Moxie, Novartis, Roche and Shire/Takeda. SA is or recently was a speaker and/or advisor for and/or has received research funding from AstraZeneca, Allakos, Sanofi, Moxie and Novartis. MM is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Celldex, Centogene, CSL Behring, FAES, Genentech, GIInnovation, Gilead, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Roche, Sanofi/Regeneron, Third HarmonicBio, UCB, and Uriach. MB is or recently was a speaker and/or advisor for Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We want to thank Prof. Pruß from the Institute for Transfusion Medicine, Charité Universitätsmedizin Berlin, for his excellent medical advice and Sophie Harms and Beate Schinzel for their excellent technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.675451/full#supplementary-material
Abbreviations
CA(s), Cold agglutinin(s); CF(s), Cryofibrinogens; CG(s), Cryoglobulin(s); ColdU, Cold Urticaria; CP(s), Cryoprotein(s); CST(s), Cold stimulation test(s); UCARE, Urticaria Center of Reference and Excellence.
References
1. Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA2LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. Allergy (2018) 73:1393–414. doi: 10.1111/all.13397
2. Siebenhaar F, Weller K, Mlynek A, Magerl M, Altrichter S, dos SRV, et al. Acquired Cold Urticaria: Clinical Picture and Update on Diagnosis and Treatment. Clin Exp Dermatol (2007) 32:241–5. doi: 10.1111/j.1365-2230.2007.02376.x
3. Maltseva N, Borzova E, Fomina D, Bizjak M, Terhorst-Molawi D, Košnik M, et al. Cold Urticaria - What We Know and What We do Not Know. Allergy (2020). doi: 10.1111/all.14674
4. Wanderer AA, Grandel KE, Wasserman SI, Farr RS. Clinical Characteristics of Cold-Induced Systemic Reactions in Acquired Cold Urticaria Syndromes: Recommendations for Prevention of This Complication and a Proposal for a Diagnostic Classification of Cold Urticaria. J Allergy Clin Immunol (1986) 78:417–23. doi: 10.1016/0091-6749(86)90027-8
5. Hauptmann G, Lang JM, North ML, Oberling F, Mayer G, Lachmann PJ. Lymphosarcoma, Cold Urticaria, IgG1 Monoclonal Cryoglobulin and Complement Abnormalities. Scand J Haematol (1975) 15:22–6. doi: 10.1111/j.1600-0609.1975.tb01051.x
6. Costanzi JJ, Coltman CA. Kappa Chain Cold Precipitable Immunoglobulin G (Igg) Associated With Cold Urticaria. I. Clinical Observations. Clin Exp Immunol (1967) 2:167–78.
7. Kolopp-Sarda M-N, Miossec P. Cryoglobulins: An Update on Detection, Mechanisms and Clinical Contribution. Autoimmun Rev (2018) 17:457–64. doi: 10.1016/j.autrev.2017.11.035
8. Brouet J-C, Clauvel J-P, Danon F, Klein M, Seligmann M. Biologic and Clinical Significance of Cryoglobulins: A Report of 86 Cases. Am J Med (1974) 57:775–88. doi: 10.1016/0002-9343(74)90852-3
9. Trejo O, Ramos-Casals M, García-Carrasco M, Yagüe J, Jiménez S, De La Red G, et al. Cryoglobulinemia: Study of Etiologic Factors and Clinical and Immunologic Features in 443 Patients From a Single Center. Med (Baltimore) (2001) 80:252–62. doi: 10.1097/00005792-200107000-00004
10. Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol (2002) 55:4–13. doi: 10.1136/jcp.55.1.4
11. Michaud M, Pourrat J. Cryofibrinogenemia. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis (2013) 19:142–8. doi: 10.1097/RHU.0b013e318289e06e
12. Moiseev S, Luqmani R, Novikov P, Shevtsova T. Cryofibrinogenaemia-a Neglected Disease. Rheumatol Oxf Engl (2017) 56:1445–51. doi: 10.1093/rheumatology/kew379
13. Roccatello D, Saadoun D, Ramos-Casals M, Tzioufas AG, Fervenza FC, Cacoub P, et al. Cryoglobulinaemia. Nat Rev Dis Primer (2018) 4:11. doi: 10.1038/s41572-018-0009-4
14. Maire MA, Mittey M, Lambert PH. The Presence of Cryoprecipitable Immunoglobulins in Normal Human Sera may Reflect Specific Molecular Interactions. Autoimmunity (1989) 2:155–64. doi: 10.3109/08916938909019952
15. Romaszko JP, Tridon A, Jouanel P, Subtil E, Dibet P, Betail G. [Detection and Analysis of Cryoglobulins: Comparative Study of a Population of Patients and One of Healthy Controls]. Pathol Biol (Paris) (1993) 41:525–9.
16. Felber N. Cold Agglutinin Disease. Postgrad Med (1976) 60:89–94. doi: 10.1080/00325481.1976.11714476
17. Maurer M, Metz M, Bindslev-Jensen C, Bousquet J, Canonica GW, Church MK, et al. Definition, Aims, and Implementation of GA(2) Len Urticaria Centers of Reference and Excellence. Allergy (2016) 71:1210–8. doi: 10.1111/all.12901
18. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
19. Kulthanan K, Hunnangkul S, Tuchinda P, Chularojanamontri L, Weerasubpong P, Subchookul C, et al. Treatments of Cold Urticaria: A systematic Review. J Allergy Clin Immunol (2019) 143:1311–31. doi: 10.1016/j.jaci.2019.02.005
20. Claudy A. Cold Urticaria. J Investig Dermatol Symp Proc (2001) 6:141–2. doi: 10.1046/j.0022-202x.2001.00028.x
21. Dressler C, Werner RN, Eisert L, Zuberbier T, Nast A, Maurer M. Chronic Inducible Urticaria: A systematic Review of Treatment Options. J Allergy Clin Immunol (2018) 141:1726–34. doi: 10.1016/j.jaci.2018.01.031
22. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. “Chapter 7: Systematic Reviews of Etiology and Risk,” In: Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute. Available at: https://reviewersmanual.joannabriggs.org/.
23. Neittaanmäki H. Cold Urticaria. Clinical Findings in 220 Patients. J Am Acad Dermatol (1985) 13:636–44. doi: 10.1016/s0190-9622(85)70208-3
24. Doeglas HM, Rijnten WJ, Schröder FP, Schirm J. Cold Urticaria and Virus Infections: A Clinical and Serological Study in 39 Patients. Br J Dermatol (1986) 114:311–8. doi: 10.1111/j.1365-2133.1986.tb02822.x
25. Henquet CJM, Martens BPM, Van Vloten WA. Cold Urticaria : A Clinico-Therapeutic Study in 30 Patients; With Special Emphasis on Cold Desensitization. Cold Urticaria Clin-Ther Study 30 Patients Spec Emphas Cold Desensitization (1992) 2:75–7.
26. Husz S, Tóth-Kása I, Kiss M, Dobozy A. Treatment of Cold Urticaria. Int J Dermatol (1994) 33:210–3. doi: 10.1111/j.1365-4362.1994.tb04956.x
27. Koeppel MC, Bertrand S, Abitan R, Signoret R, Sayag J. [Urticaria Caused by Cold. 104 Cases]. Ann Dermatol Venereol (1996) 123:627–32.
28. Möller A, Henning M, Zuberbier T, Czarnetzki-Henz BM. [Epidemiology and Clinical Aspects of Cold Urticaria]. Hautarzt Z Dermatol Venerol Verwandte Geb (1996) 47:510–4. doi: 10.1007/s001050050461
29. Santaolalla Montoya M, Martínez Molero MI, Santaolalla San Juana F, Baeza ML, Alonso Lebrero E, Zapatero Remón L. [Cold Urticaria: Review of 12 Cases]. Allergol Immunopathol (Madr) (2002) 30:259–62. doi: 10.1016/s0301-0546(02)79134-9
30. Tosoni C, Lodi-Rizzini F, Bettoni L, Toniati P, Zane C, Capezzera R, et al. Cinnarizine is a Useful and Well-Tolerated Drug in the Treatment of Acquired Cold Urticaria (Acu). Eur J Dermatol EJD (2003) 13:54–6.
31. Alangari AA, Twarog FJ, Shih M-C, Schneider LC. Clinical Features and Anaphylaxis in Children With Cold Urticaria. Pediatrics (2004) 113:e313–7. doi: 10.1542/peds.113.4.e313
32. Katsarou-Katsari A, Makris M, Lagogianni E, Gregoriou S, Theoharides T, Kalogeromitros D. Clinical Features and Natural History of Acquired Cold Urticaria in a Tertiary Referral Hospital: A 10-Year Prospective Study. J Eur Acad Dermatol Venereol JEADV (2008) 22:1405–11. doi: 10.1111/j.1468-3083.2008.02840.x
33. Stepaniuk P, Vostretsova K, Kanani A. Review of Cold-Induced Urticaria Characteristics, Diagnosis and Management in a Western Canadian Allergy Practice. Allergy Asthma Clin Immunol (2018) 14:85. doi: 10.1186/s13223-018-0310-5
34. Yee CSK, El Khoury K, Albuhairi S, Broyles A, Schneider L, Rachid R. Acquired Cold-Induced Urticaria in Pediatric Patients: A 22-Year Experience in a Tertiary Care Center (1996-2017). J Allergy Clin Immunol Pract (2019) 7:1024–31.e3. doi: 10.1016/j.jaip.2018.10.025
35. Kulthanan K, Tuchinda P, Chularojanamontri L, Kiratiwongwan R. Cold Urticaria: Clinical Features and Natural Course in a Tropical Country. Allergy Asthma Immunol Res (2019) 11:538–47. doi: 10.4168/aair.2019.11.4.538
36. Młynek A, Magerl M, Siebenhaar F, Weller K, Vieira Dos Santos R, Zuberbier T, et al. Results and Relevance of Critical Temperature Threshold Testing in Patients With Acquired Cold Urticaria. Br J Dermatol (2010) 162:198–200. doi: 10.1111/j.1365-2133.2009.09441.x
38. Houser DD, Arbesman CE, Ito K, Wicher K. Cold Urticaria. Immunologic Studies. Am J Med (1970) 49:23–33. doi: 10.1016/s0002-9343(70)80110-3
39. Czarnetzki BM. Urticaria. Berlin Heidelberg: Springer-Verlag (1986). doi: 10.1007/978-3-642-70313-3
40. Motyckova G, Murali M. Laboratory Testing for Cryoglobulins. Am J Hematol (2011) 86:500–2. doi: 10.1002/ajh.22023
41. Vermeersch P, Gijbels K, Mariën G, Lunn R, Egner W, White P, et al. A Critical Appraisal of Current Practice in the Detection, Analysis, and Reporting of Cryoglobulins. Clin Chem (2008) 54:39–43. doi: 10.1373/clinchem.2007.090134
42. Wanderer AA. A Potential New Therapy for Cold Urticaria and Chronic Idiopathic Urticaria. J Allergy Clin Immunol (2007) 119:517. doi: 10.1016/j.jaci.2006.10.041
43. Zucchi E, Cavallieri F, Giovannini G, Antonelli F, Mascia MT, Bedin R, et al. Post-Infectious Sensory Neuropathy With anti-GT1a and GQ1b Antibodies Associated With Cold Urticaria. J Clin Neurosci Off J Neurosurg Soc Australas (2018) 56:175–7. doi: 10.1016/j.jocn.2018.06.056
44. Miralles López JC, López Andreu FR, Sánchez-Gascón F, López Rodríguez C, Negro Alvarez JM. Cold Urticaria Associated With Acute Serologic Toxoplasmosis. Allergol Immunopathol (Madr) (2005) 33:172–4. doi: 10.1157/13075702
45. Morais-Almeida M, Marinho S, Gaspar A, Arêde C, Loureiro V, Rosado-Pinto J. Cold Urticaria and Infectious Mononucleosis in Children. Allergol Immunopathol (Madr) (2004) 32:368–71. doi: 10.1016/s0301-0546(04)79270-8
46. Ito A, Kazama T, Ito K, Ito M. Purpura With Cold Urticaria in a Patient With Hepatitis C Virus Infection-Associated Mixed Cryoglobulinemia Type III: Successful Treatment With Interferon-Beta. J Dermatol (2003) 30:321–5. doi: 10.1111/j.1346-8138.2003.tb00394.x
47. Lin RY, Schwartz RA. Cold Urticaria and HIV Infection. Br J Dermatol (1993) 129:465–7. doi: 10.1111/j.1365-2133.1993.tb03179.x
48. Barranco Sanz P, López Serrano C. [Cold Urticaria Associated With Serologic Markers of Hepatitis B and Cryoglobulinemia]. Allergol Immunopathol (Madr) (1987) 15:167–9.
49. Tyson CJ, Czarny D. Cold-Induced Urticaria in Infectious Mononucleosis. Med J Aust (1981) 1:33–5. doi: 10.5694/j.1326-5377.1981.tb135285.x
50. Wands JR, Perrotto JL, Isselbacher KJ. Circulating Immune Complexes and Complement Sequence Activation in Infectious Mononucleosis. Am J Med (1976) 60:269–72. doi: 10.1016/0002-9343(76)90436-8
51. Morrison JG, Hull PR, Fourie E. Erythema Elevatum Diutinum, Cryoglobulinaemia, and Fixed Urticaria on Cooling. Br J Dermatol (1977) 97:99–104. doi: 10.1111/j.1365-2133.1977.tb15435.x
52. Rawnsley HM, Shelley WB. Cold Urticaria With Cryoglobulinemia in a Patient With Chronic Lymphocytic Leukemia. Arch Dermatol (1968) 98:12–7. doi: 10.1001/archderm.98.1.12
53. Thiers H, Moulin G. Urticaria Caused by Cold and Cryoglobulinemia Revealing a Myeloma. Bull Soc Fr Dermatol Syphiligr (1969) 76:578–81.
54. Chodirker WB, Komar RR. Angioimmunoblastic Lymphadenopathy in a Child With Unusual Clinical and Immunologic Features. J Allergy Clin Immunol (1985) 76:745–52. doi: 10.1016/0091-6749(85)90681-5
55. Török L, Borka I, Szabó G. Waldenström’s Macroglobulinaemia Presenting With Cold Urticaria and Cold Purpura. Clin Exp Dermatol (1993) 18:277–9. doi: 10.1111/j.1365-2230.1993.tb02188.x
Keywords: cold urticaria, cryoglobulins, cryofibrinogens, cold agglutinins, cryoproteins
Citation: Ginter K, Ahsan DM, Bizjak M, Krause K, Maurer M, Altrichter S and Terhorst-Molawi D (2021) Cryoglobulins, Cryofibrinogens, and Cold Agglutinins in Cold Urticaria: Literature Review, Retrospective Patient Analysis, and Observational Study in 49 Patients. Front. Immunol. 12:675451. doi: 10.3389/fimmu.2021.675451
Received: 03 March 2021; Accepted: 29 April 2021;
Published: 25 May 2021.
Edited by:
Marcelo Vivolo Aun, Albert Einstein Israelite Hospital, BrazilReviewed by:
Solange Valle, Federal University of Rio de Janeiro, BrazilRosana Agondi, University of São Paulo, Brazil
Copyright © 2021 Ginter, Ahsan, Bizjak, Krause, Maurer, Altrichter and Terhorst-Molawi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcus Maurer, bWFyY3VzLm1hdXJlckBjaGFyaXRlLmRl
 Katharina Ginter
Katharina Ginter Dalia Melina Ahsan
Dalia Melina Ahsan Mojca Bizjak
Mojca Bizjak Karoline Krause
Karoline Krause Marcus Maurer
Marcus Maurer Sabine Altrichter
Sabine Altrichter Dorothea Terhorst-Molawi1
Dorothea Terhorst-Molawi1