
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 12 May 2021
Sec. Immunological Tolerance and Regulation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.672168
 Sandra Hellberg1,2*
Sandra Hellberg1,2* Johanna Raffetseder2
Johanna Raffetseder2 Olof Rundquist1
Olof Rundquist1 Rasmus Magnusson1
Rasmus Magnusson1 Georgia Papapavlou2
Georgia Papapavlou2 Maria C. Jenmalm2
Maria C. Jenmalm2 Jan Ernerudh3†
Jan Ernerudh3† Mika Gustafsson1†
Mika Gustafsson1†The changes in progesterone (P4) levels during and after pregnancy coincide with the temporary improvement and worsening of several autoimmune diseases like multiple sclerosis (MS) and rheumatoid arthritis (RA). Most likely immune-endocrine interactions play a major role in these pregnancy-induced effects. In this study, we used next generation sequencing to investigate the direct effects of P4 on CD4+ T cell activation, key event in pregnancy and disease. We report profound dampening effects of P4 on T cell activation, altering the gene and protein expression profile and reversing many of the changes induced during the activation. The transcriptomic changes induced by P4 were significantly enriched for genes associated with diseases known to be modulated during pregnancy such as MS, RA and psoriasis. STAT1 and STAT3 were significantly downregulated by P4 and their downstream targets were significantly enriched among the disease-associated genes. Several of these genes included well-known and disease-relevant cytokines, such as IL-12β, CXCL10 and OSM, which were further validated also at the protein level using proximity extension assay. Our results extend the previous knowledge of P4 as an immune regulatory hormone and support its importance during pregnancy for regulating potentially detrimental immune responses towards the semi-allogenic fetus. Further, our results also point toward a potential role for P4 in the pregnancy-induced disease immunomodulation and highlight the need for further studies evaluating P4 as a future treatment option.
Pregnancy represents a unique immunological condition as the maternal immune system is able to tolerate the presence of the semi-allogenic fetus. This immunological tolerance is thought to arise from extensive immune and endocrine alterations induced during pregnancy (1, 2) orchestrated by the increased levels of the steroid hormones such as progesterone (P4), which is essential for the establishment and maintenance of pregnancy (3–5). Accordingly, low levels of P4 have been associated with several pregnancy complications (6–8) and treatment with the P4-receptor antagonist Mifepristone (RU486) results in cessation of pregnancy (9), further supporting the importance of P4 during gestation. Interestingly, therapeutic use of P4 in pregnancy has been shown to reduce the risk of preterm birth in certain risk groups (10), highlighting a potential use of P4 as treatment in pregnancy complications.
The role of P4 has been mainly related to its influence on myometrial homeostasis and remodeling (11). However, an important immune-modulatory role of P4 in vivo is indicated by the pregnancy-related improvement and subsequent worsening of autoimmune diseases, such as multiple sclerosis (MS) and rheumatoid arthritis (RA), which coincide with the time points during and after pregnancy when P4 levels are the highest and lowest, respectively (12–14). In addition, differences in immune responses related to the high and low P4 levels during the menstrual cycle have also been observed (15, 16). Previous studies regarding immune-endocrine interactions have to a large extent focused on how estrogen influences the immune system. Interestingly, estrogen levels increase and decrease during pregnancy in a similar way as P4, although estrogen seems to have both immune regulatory and immune-activating properties (17). Indeed, estrogen has been suggested as a major factor explaining the increased occurrence of autoimmune disease in women (18). On the other hand, in vitro effects of P4 on different immune cell populations support a pivotal role for P4 in regulating immune responses that could be central for promoting fetal tolerance (19–24). Furthermore, a role for P4 as a potent immunosuppressor is supported by in vivo studies showing involvement of P4 in response to allogenic and xenogenic transplantation (25–27) and in graft rejection in humans (28).
CD4+ T cells are central in the immune system, serving as chief regulators of immunity and tolerance (29). The importance of CD4+ T cells during pregnancy is evident by their relative exclusion from the fetal-maternal interface in order to limit potentially detrimental activation (30), whereas regulatory T cells are enriched, thereby further limiting harmful T cell responses (31, 32). In parallel, limiting CD4+ T cell activation is an essential aspect in T cell-mediated diseases as aberrant activation of autoreactive CD4+ T cells is a central mechanism in the disease pathogenesis (33). Thus, regulation of CD4+ T cells is a common denominator that could both prevent unwanted maternal immune responses against the fetus, and also explain improvement of autoimmune diseases during pregnancy. Interestingly, P4 has been shown to limit CD4+ T cell activation (34–36). However, in-depth analysis of the precise effects of P4 on human CD4+ T cell activation and its potential involvement in disease modulation during pregnancy is still lacking.
We here report in-depth RNA sequencing data demonstrating a profound direct effect of P4 on T cell activation. More specifically, the large P4-induced transcriptomic changes were most prominently downregulatory on immune-associated genes and pathways, while mostly non-immune genes were upregulated by P4. Interestingly, these immune genes downregulated by P4 were significantly enriched for genes associated with diseases known to be modulated during pregnancy, including MS and RA. The effects on several of these disease-associated genes were further validated at the protein level using proximity extension assay. Our findings support P4 as a major immune-regulating hormone during pregnancy and suggest that P4 could be involved in mediating the pregnancy-induced improvement of certain autoimmune diseases, thereby constituting a potential avenue for future treatment options.
Blood samples were collected from thirteen healthy female volunteers (median age 32, 25-43 yrs), recruited among students and personnel at Linköping University and Linköping University Hospital, Sweden. Informed consent was obtained prior to sample collection and the study was approved by the Regional Ethics Review Board in Linköping (Regionala etikprövningsnämnden i Linköping), Sweden (approval number: M39-08). None of the women were using hormonal contraceptives or taking any other medications at the time of inclusion. The time points of sample collection were evenly distributed across the menstrual cycle (assuming a 28-day menstrual cycle; seven women were in the luteal and six in the follicular phase). There was no difference in response to P4 (based on fold decreased of CD69 expression between women in the luteal versus follicular phase (data not shown).
Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation using Lymphoprep™ (Axis-Shield, Oslo, Norway) and washed thrice in Hank’s Balanced Salt Solution (Life Technologies, Darmstadt, Germany). Magnetic activated cell sorting (MACS) was used to isolate CD4+ T cells. The PBMCs were resuspended in MACS buffer (phosphate buffered saline, PBS; Medicago, Uppsala, Sweden) supplemented with 2mM EDTA (Sigma Aldrich, Saint Louis, MO, USA) and 0.5% fetal bovine serum (FBS; Cytivia (Formerly GE Healthcare Life Sciences) HyClone™, Uppsala, Sweden) and the CD4+ cells were isolated by positive immunomagnetic selection using MS columns and a miniMACS separator (Miltenyi Biotec, Bergish Gladbach, Germany) according to the instructions provided by the manufacturer. The purity of the isolated CD4+ T cells was assessed by flow cytometry (median purity 98.5%, range 97.6-99.0%).
The isolated CD4+ T cells were pre-incubated with 10, 30 and 50 µM of P4 (water-soluble; Sigma Aldrich) or without (cell culture media alone). The cells were plated in 24-well flat-bottom plates (Costar™; Corning Inc, Corning, NY, USA) at 1.0x106 cells/ml, in a 1 ml final volume/well of Isocove’s Modified Dulbecco’s Medium (IMDM; Invitrogen, Carlsbad, CA, USA) supplemented with L-glutamine (292 mg/l; Sigma-Aldrich), MEM non-essential amino acids 100X (10 ml/l; Gibco®), penicillin (50 IE/ml), streptomycin (50 µg/ml; Cambrex-Lonza, Basel, Switzerland), and sodium bicarbonate (3.024 g/l; Sigma-Aldrich) and 5% FBS and incubated at 37°C and 5% CO2. After 20 hrs, the cells were removed from the plates, centrifuged and resuspended in cell culture media prior to in vitro stimulation. See Figure 1A for an overview of the experimental design of the study.
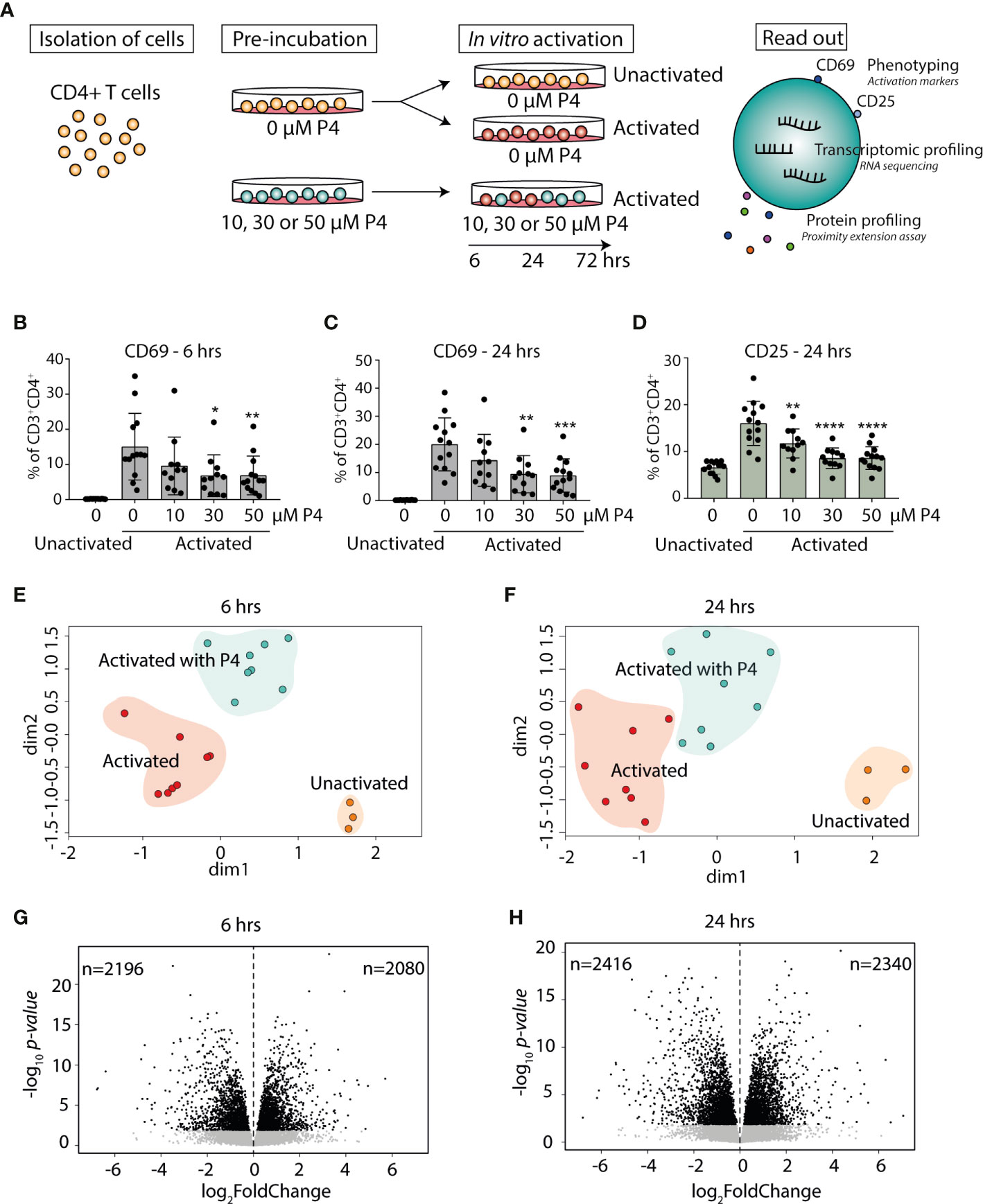
Figure 1 Progesterone dampens T cell activation and induces transcriptomic changes in activated CD4+ T cells. (A) Primary human CD4+ T cells were isolated from healthy non-pregnant women (n=13) and pre-incubated with or without different concentrations (10, 30 and 50 µM) of P4 for 20 hrs and then cultured unactivated or activated in vitro with plate-bound anti-CD3 and anti-CD28 antibodies in the presence or absence of P4 for 6-24-72 hrs. Samples cultured for 72 hrs were only used for measurement of secreted proteins. The effect of P4 on T cell activation was evaluated by flow cytometry, RNA sequencing and proximity extension assay of secreted proteins in culture supernatants. (B–D) The effect of P4 on T cell activation markers CD69 (6 and 24 hrs) and CD25 (24 hrs only) was analyzed by flow cytometry (n=11-13). Bar graphs shows the percentages of CD4+ T cells expressing the T cell activation markers. Mean ± standard deviation is shown. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. (E, F) Multidimensional scaling analysis of gene expression data generated by RNA-sequencing (n=3 unactivated, n=8 activated with and without 50 µM P4). The groups are highlighted with background color for schematic purposes only. (G, H) Volcano plots of the transcriptomic analysis of the differentially expressed genes in CD4+ T cells activated in the presence of P4 as compared to absence of P4. Black dots FDR ≤ 0.05. P4, progesterone.
Twenty-four-well flat bottom plates were coated with 0.1 µg/ml of low endotoxin anti-CD3 and anti-CD28 antibodies (clone UCHT1, clone YTH913.12; Bio-Rad AbD Serotec Limited, Hercules, CA, USA) or with PBS alone for 20 hrs at 4°C followed by washing thrice in PBS. The concentration of antibodies was chosen based on titration experiments where 0.1 µg/ml resulted in moderately increased cell surface expression of the early T cell activation marker CD69. The CD4+ T cells pre-incubated without P4 were cultured unactivated or activated (with anti-CD3/CD28 antibodies), whereas the CD4+ T cells pre-incubated with P4 were activated in the presence of the same concentrations as during the pre-incubation for 6-24-72 hrs and subsequently processed for flow cytometry, RNA extraction or measuring of secreted proteins. Cells cultured for 72 hrs were only used for protein measurements. Briefly, after culturing, the supernatants were collected, and frozen at -70°C. A portion of the cells was used for flow cytometry analysis and the rest lysed and homogenized in buffer RLT Plus (Qiagen; Hilden, Germany) supplemented with 143 mM β-mercaptoethanol (Sigma Aldrich) and homogenized using a syringe and a needle according to the instructions provided by the manufacturer. The lysates were stored at -70°C before extraction. The viability of the cells after culture was 87.6% ± 2.7 (mean ± standard deviation (SD)) after 6 hrs and 83.1% ± 3.7 after 24 hrs.
The CD4+ T cells were resuspended in LIVE/DEAD™ Fixable Dead Cell Stain (Invitrogen), diluted 1:500 in PBS+0.1%FBS and stained with mouse anti-human CD4-FITC (clone SK3), CD69-APCCy7 (clone FN50), CD25-PE (clone 2A3) and CD3-APC (clone SK7; all from BD Biosciences, Franklin Lakes, NJ, USA). The cells were incubated in the dark for 15 minutes at room temperature and washed in PBS+0.1%FBS prior to flow cytometry analysis. Ten thousand CD4+ T cells were collected and analyzed using FACS Canto II (BD Biosciences) and Kaluza software version 2.1 (Beckman Coulter, Brea, CA, USA). The cells were gated according to forward (FSC) and side scatter (SSC) and further defined as CD3+CD4+ (Figure S1). The cut-off value for CD69 expression was based on its expression in unactivated CD4+ T cells and CD25 expression was set based upon the contour of the identified negative and positive populations. Fold change of CD69 and CD25 expression was calculated based on expression of the activation markers on the cells activated without P4 (expression activated cells with P4/expression activated cells without P4). All data were analyzed using GraphPad Prism version 8.0.1 (San Diego, CA, USA). Most of the data was normally distributed and therefore analyzed using one-way ANOVA with Dunnett’s multiple comparison test. Data are expressed as mean and SD. P-values ≤ 0.05 were considered statistically significant.
RNA was extracted using the AllPrep DNA/RNA Mini kit (Qiagen) according to the protocol provided by the manufacturer and RNA was eluted with 30 µl of RNase-free water. The concentration of RNA was determined using Nanodrop® ND-1000 spectrophotometer (Nanodrop Technologies Inc; Wilmington, DE, USA). The RNA quality was controlled using Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA, USA) on an Agilent 2100 Bioanalyzer instrument (Agilent Technologies). RNA integrity numbers (RIN) were 9.5 ± 0.4 (mean ± SD). Libraries were constructed with TruSeq Stranded mRNA (Illumina, San Diego, CA, USA), which have been adapted to run on an Agilent BRAVO robot (Agilent Technologies) with 440 ng of mRNA as starting material per sample. Briefly, poly-A containing mRNA was isolated using poly dT-coated beads and broken down into 150-400 base pair fragments by chemical fragmentation and converted to cDNA with reverse transcriptase and random primer. Enzymes and shorter fragments were removed using AMPure XP beads (Beckman Coulter, Indianapolis, IN, US). The remaining fragments were adenylated followed by ligation of index-adapters and amplified with PCR. Samples were barcoded, pooled and sequenced on the Illumina NovaSeq 6000 platform with an S1 flowcell and sequenced PE2x101 bp. An average of 48.9 million reads were obtained per sample (48.9x106 ± 10.5x106, mean ± SD). Per-cycle base call files were demultiplexed and converted to FASTQ using bcl2fastq v2.19.1.403 from the CASAVA software suite (Illumina). Library preparation and sequencing was carried out at the National Genomics Infrastructure, Science for Life Laboratories, Stockholm. Paired samples from a total of eight individuals (n=38 samples in total) were used for RNA sequencing (RNA-seq).
The FASTQ files were processed with TrimGalore! to remove adapter contamination and trimming of low-quality regions. Paired-end reads were aligned and mapped to the Ensemble human reference genome GRCh37 (Genome Reference Consortium Human Build 37) using STAR (version 2.5.3.a) (37). Gene read counts were generated using StringTie (version 1.3.3) (38). Data was processed in R Studio (R version 4.0; Boston, MA, USA) using the edgeR (39, 40) and limma packages (41, 42). The mapped reads were filtered for lowly expressed genes (genes with counts per million >1 in at least 3 replicates were kept) and used for further analysis. The filtered gene counts were normalized by using the trimmed mean of M-values via calcNormFactors in edgeR. Voom-transformation was applied prior to differential expression analysis. A false discovery rate (FDR; Benjamini-Hochberg) of 0.05 was used as a threshold for differentially expressed genes (DEGs). Differential expression induced by P4 was calculated comparing CD4+ T cells activated in the presence or absence of P4 and is referred to as P4 response genes.
To explore the biological relevance of the DEGs, a gene set enrichment (GSE) analysis using gseKEGG and pathway analysis using enrichKEGG from clusterProfiler (43) based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) (44, 45) was performed. For GSE analysis, a pre-ranked list of P4-response DEGs (based on logFoldChange (logFC), n=3339 genes at 6 hrs and n=3725 genes at 24 hrs) was used as input, where genes that lacked Entrez gene ID were removed prior to analysis. An enrichment score (ES) was calculated for all gene sets and normalized for gene set size (normalized enrichment score; NES) over a mean distribution of 1000 permutations. The minimum gene set size was set to 20 and the maximum to 200 genes, thereby excluding small and very large generic data sets. An adjusted p-value ≤ 0.05 (Benjamini-Hochberg) was considered statistically significant. Gene overlap between different gene sets was calculated using the GeneOverlap package in R (46).
In order to verify central transcriptomic changes on the protein level, culture supernatants collected after 6, 24 and 72 hrs were analyzed for 92 inflammation-associated proteins (Olink inflammation panel; https://www.olink.com/products/inflammation/) with multiplex proximity extension assay (PEA) at the Clinical Biomarkers facility, Science for Life Laboratory, Uppsala University, SE-751 85 Uppsala. Briefly, 1 µl of cell supernatant was incubated with matched pairs of antibodies linked to unique oligonucleotides (proximity probes) specific for each biomarker to be measured. Close proximity of the probes bound to their targets results in hybridization which, with the addition of DNA polymerase, extends the oligonucleotides, creating a DNA amplicon that can be detected and quantified by quantitative real-time PCR (47). Four internal controls were included for quality control and for data normalization. Data was expressed as normalized protein eXpression (NPX), an arbitrary unit in log2 scale. Values below the detection limit were assigned half the value of the limit of detection. Proteins that were detected in a least 50% of the samples at each time point were included in the statistical analysis with the following exceptions: CCL20 (40% detectable at 24 hrs), transforming growth factor (TGF)-β1 (40% detectable at 72 hrs) and leukemia inhibitory factor (LIF; 47% at 24 hrs) where more than half of the samples in the activation alone group was detectable (8-10 out of 10) and significantly higher than the unactivated samples. Statistical differences were determined using Friedman test and Benjamini-Hochberg to correct for multiple comparisons. An adjusted p-value of ≤0.05 was considered as statistically significant. All data were analyzed with GraphPad Prism version 8.0.1.
Disease-associated genes for Hashimoto’s (n=141), Graves’ disease (n=245), MS (n=801), psoriasis (n=580), RA (n=1273), systemic lupus erythematosus (SLE; n=817) and systemic sclerosis (SS; n=400) were derived from DisGeNET (48). All disease genes that were not present in the background (all genes detected by RNA-seq, n=14363) were discarded from downstream analysis. Enrichment analysis of disease-associated genes among the genes affected by P4 was performed using Fisher’s exact test to compute p values and odds ratio for the overlaps. The P4 response genes that were used comprised the uniquely expressed genes combining the DEGs from both 6 and 24 hrs (in total n= 2563 downregulated and n= 2403 upregulated genes).
Transcription factor (TF)-target interactions were derived using TRRUST (49) and DoRothEA (50). The total number of interactions from TRRUST was 9396 and 6620 from DoRothEA (including interactions with confidence score A and B) but limiting the interactions to TFs that were significantly affected by P4 resulted in a total number of 3124 interactions. We used the TFs that were significantly affected by P4 and then only included those TF-target interactions where the target was also significantly downregulated by P4 and disease-associated, as established by the common genes between all diseases or between MS, psoriasis and RA. Enrichment of targets for STAT1 and STAT3 was computed using Fisher’s exact test where targets among the disease-associated genes that were downregulated by P4 were compared to the targets of the corresponding TFs among all genes downregulated by P4. For TF-mRNA-protein analysis, we combined the differentially expressed proteins (DEPs) derived from the measurement of the expression of 92 inflammation-related proteins in culture supernatants at 6-24-72 hrs (n=41). These proteins were mapped to the corresponding mRNA and, in extension, to the known interacting TFs.
In order to examine the effect of P4 on CD4+ T cells and T cell activation, we established an in vitro model where primary human CD4+ T cells were pre-incubated with P4 prior to activation, reflecting the in vivo situation where the T cells would constantly be exposed to P4 prior to antigen challenge. The cells were subsequently cultured unactivated or with the commonly used combined activation through the T cell receptor (anti-CD3) and co-stimulatory CD28 (anti-CD28) in the presence or absence of P4 for 6, 24 and 72 hrs (Figure 1A). These time points were chosen to mainly capture the influence of P4 on the cellular and transcriptomic events involved in T cell activation leading up to clonal expansion and differentiation. We used a low-to-moderate level of stimulation, as measured by the proportion of activated T cells expressing surface activation markers (6 hrs CD69, mean± SD unactivated: 0.2% ± 0.04, activated: 15%± 9.5; 24 hrs CD69, unactivated: 0.2% ± 0.07, activated: 20% ± 9.4; 24 hrs CD25, unactivated: 6.5% ± 1.4, activated: 16% ± 4.7) (Figures 1B–D). Activation of CD4+ T cells in the presence of different concentrations of P4 decreased the level of activation of the cells in a dose-dependent manner, with reduced proportion of both CD69 and CD25 expressing cells, where exposure to the highest concentration of P4 (50 µM) resulted in the largest overall decrease (6 hrs: CD69 p<0.010, 0.4 ± 0.1 fold change compared to activation without P4 p<0.0001; 24 hrs: CD69 p<0.001, 0.4 ± 0.1 fold change p<0.0001 and CD25 p<0.0001, 0.5 ± 0.1 fold change p<0.0001) (Figures 1B–D and Figure S2). Next, we performed transcriptomic analysis using RNA-Seq on CD4+ T cells activated for 6 and 24 hrs in the presence (50 µM P4) or absence of P4 along with unactivated cells, to obtain an in-depth picture of how T cell activation is affected by P4. To get an initial genome-wide understanding of the transcriptomic changes, we performed unsupervised clustering of the samples using multidimensional scaling, which showed clear differences between the cells activated in the presence or absence of P4, as well as in comparison to unactivated cells (Figures 1E, F). Indeed, differential expression analysis revealed that P4 induced significant changes in the gene expression profile of the activated CD4+ T cells at both 6 and 24 hrs (as compared to activation in the absence of P4), referred to as P4 response genes. In total, P4 exposure resulted in 4276 DEGs (FDR ≤0.05; 2080 up-regulated and 2196 down-regulated) at 6 hrs and 4756 DEGs (2340 up-regulated and 2416 down-regulated) at 24 hrs (Figures 1G, H).
To gain further insight into the functional significance of the P4 response genes, we performed GSE analysis. Several immune-related pathways associated with and downstream of T cell signaling were significantly downregulated by P4 at both 6 and 24 hrs, for example T cell receptor signaling, JAK-STAT signaling, cytokine-cytokine receptor interactions, as well as many immune-associated disease pathways (Figures 2A, B and Table S1). At 24 hrs, several pathways related to T cell differentiation (TH1, TH2 and TH17 differentiation) were also affected by P4. Strikingly, GSE analysis showed very few pathways to be upregulated by P4 even though there was an almost equal number of P4 response genes that were up- or downregulated. Furthermore, none of the upregulated pathways were immune-related (Table S1). Since P4 was found by us and others (24, 35) to have a dampening effect on T cell activation, we investigated if the changes induced by P4 were related to genes involved in the T cell activation process itself. We therefore assessed if P4 could counteract the changes induced during activation by analyzing the overlap between the P4 response genes and genes involved in T cell activation, i.e. genes being differentially expressed in activated versus unactivated T cells. Indeed, there was a striking overlap between the DEGs upregulated during T cell activation and downregulated by P4 (6 hrs: odds ratio (OR): 9.1, p=2.6x10-220; 24 hrs: OR:16.1, p=1.6x10-481; Figure 2C) and between the DEGs downregulated during T cell activation and upregulated by P4 (6 hrs: OR: 4.4, p=1.6x10-125; 24 hrs: OR: 5.1, p=5.9x10-195; Figure 2D), demonstrating that P4 significantly affects genes related to the actual T cell activation by opposing some of the changes induced during activation. Conversely, few genes were coincidingly up- or down-regulated by both P4 and T cell activation (data not shown).
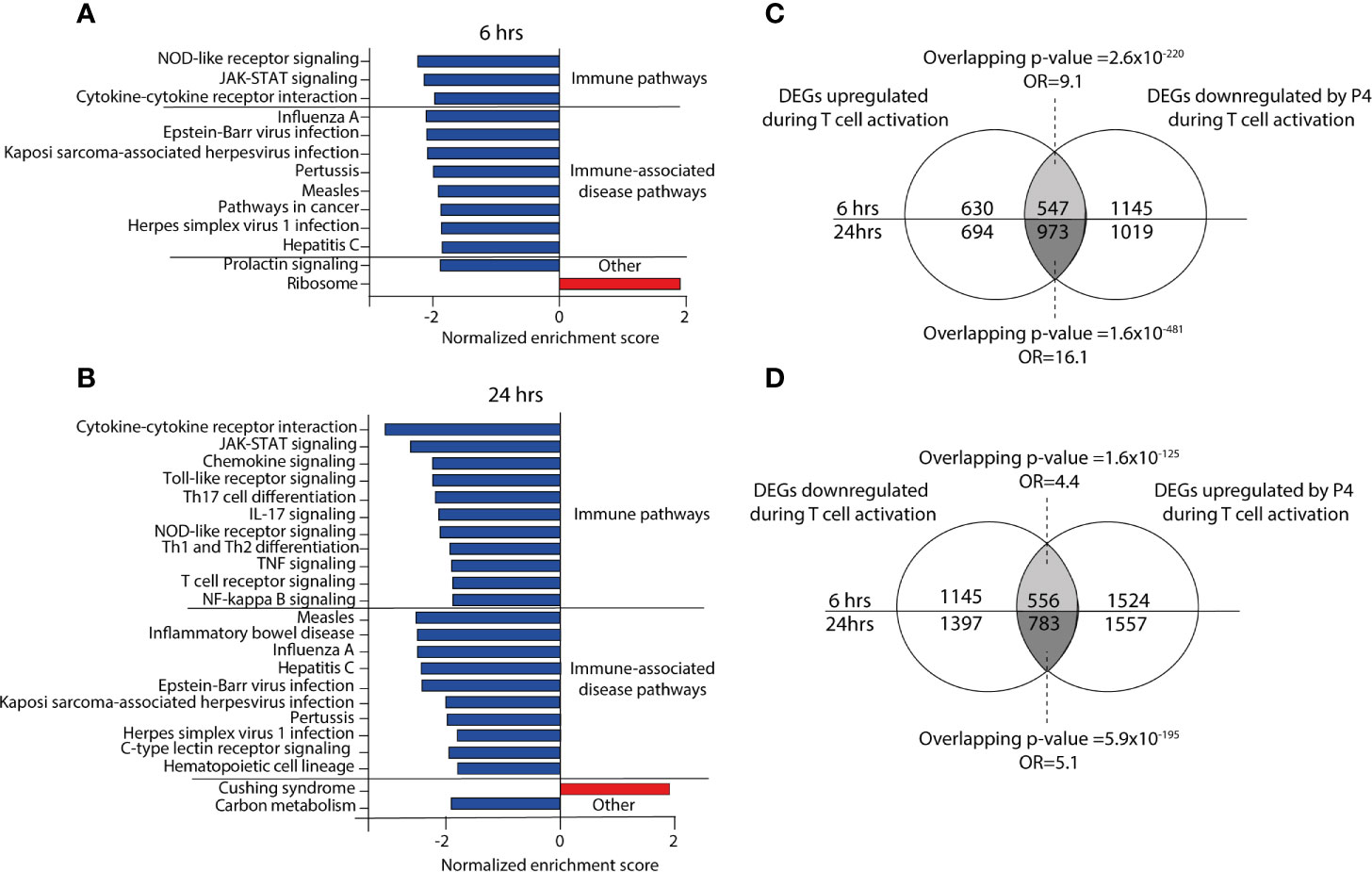
Figure 2 Progesterone down regulates immune-related pathways during activation of CD4+ T cells. (A, B) Bar graphs showing the normalized enrichments score on the x-axis from the KEGG gene set enrichment analysis of the P4 response genes (DEGs comparing activation in the presence of P4 as compared to activation alone) at 6 and 24 hrs. All pathways have an FDR adjusted p-value <0.05. (C, D) Venn diagrams of the overlapping DEGs between up-and down-regulated genes in T cells activated in the presence or absence of 50 µM P4. n=3 unactivated, n=8 activated with and without 50 µM P4. DEGs, differentially expressed genes: OR, odds ratio; P4, progesterone.
To investigate if P4 also had an effect at the proteomics level, we performed a screening of 92 inflammation-related proteins in culture supernatants collected at 6, 24 and 72 hrs by using a proximity extension assay with high sensitivity and specificity (47). At 6 hrs, only 24 out of the 92 proteins were detectable, of which 14 (58%) proteins were differentially expressed between CD4+ T cells cultured in the presence versus in the absence of P4 (Figure 3A and Table S2). At 24 hrs, 39 out of the 92 proteins were detectable and 28 (72%) of those were differentially expressed and at 72 hrs, 65 proteins were detectable but only 23 (35%) were differentially expressed (Figure 3A and Table S2). Consistent with the transcriptomic findings, most DEPs were significantly lower in supernatants collected from T cells that had been activated in the presence of P4 as compared to activation alone. Only three proteins (ADA, CXCL5 and EIF4EBP1) were upregulated at 24 and/or 72 hrs. Also at the protein level, P4 seemingly opposed the changes induced during activation, where most proteins that were significantly upregulated during T cell activation (as compared to unactivated cells) were downregulated by P4 (Figures 3B–D), except ADA (24 and 72 hrs), CCL8 (72hrs), CXCL5 (24 hrs), EIF4EBP1 (24 hrs), interleukin (IL) -6 (72 hrs) and TNFSF12 (24 hrs). There were five proteins that were consistently downregulated at all three time points: CXCL8, CCL8, IL-17A, tumor necrosis factor (TNF) and FLT3LG (Figure 3E). The dampening effect of P4 on immune-related processes at the transcriptomic levels is thus further supported by its apparent downregulatory effect on inflammation-related proteins.
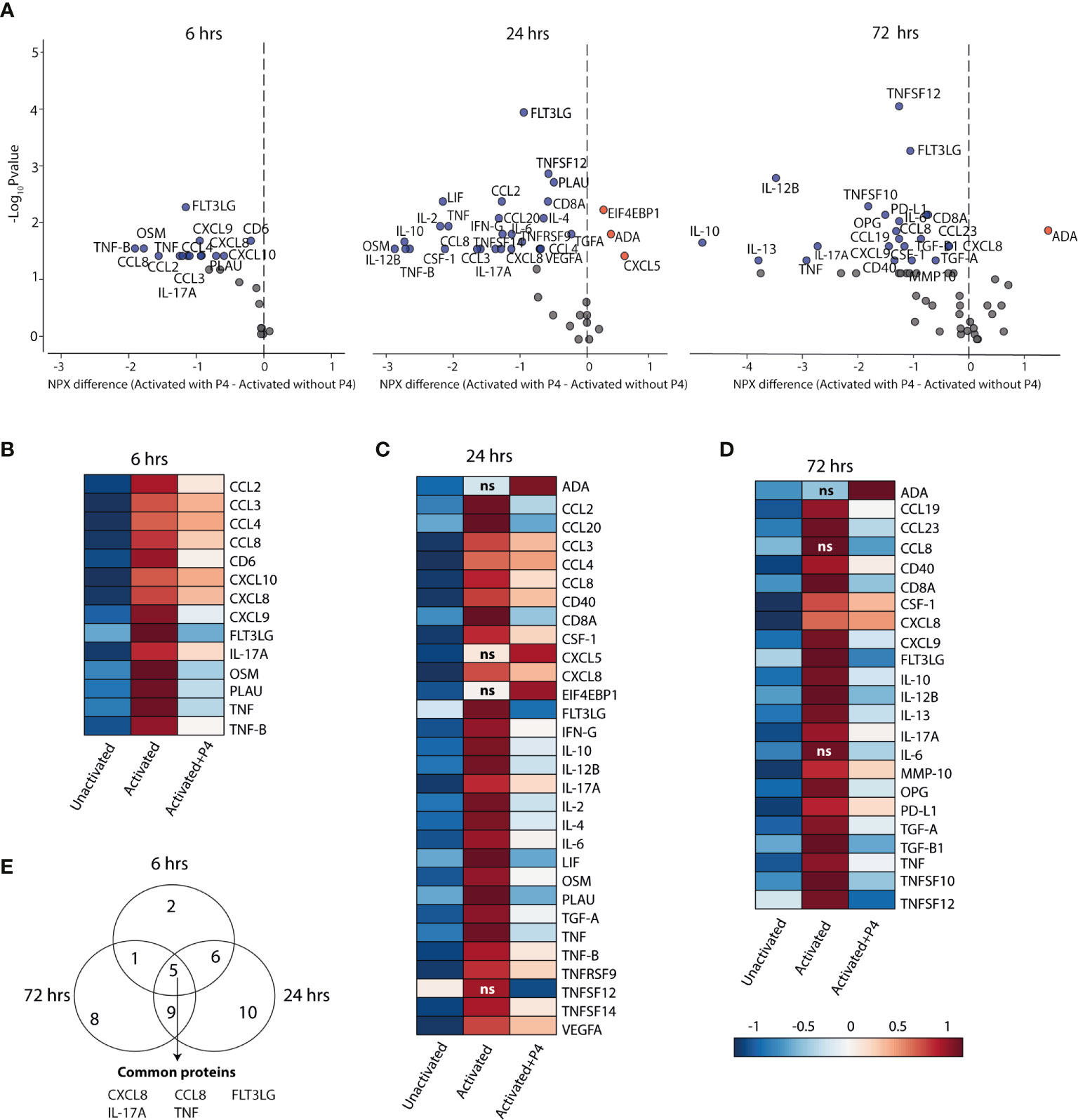
Figure 3 P4 significantly downregulates protein expression in culture supernatants. A panel of inflammation-related proteins was measured in supernatants collected from CD4+ T cells that were cultured unactivated or activated with or without 50 µM of P4 (n= 9 for 6 hrs, n=10 for 24 hrs and n=10 for 72 hrs). (A) Volcano plots of differentially expressed proteins (DEPs) at 6, 24 and 72 hrs comparing activation in the presence or absence of P4. DEPs were determined using Friedman test and Benjamini-Hochberg. Data is presented as difference in the normalized protein expression values (NPX) provided by the manufacturer. Blue dots DEPs downregulated by P4, red dots upregulated DEPs and grey dots FDR ≤ 0.05. (B–D) Heat maps of the differentially expressed proteins comparing activation in the presence of P4 as compared to activation alone. ns are proteins that were not significantly different between activation alone as compared to unactivated. Median NPX values are shown in the figure. ns, non-significant. (E) Venn diagram displaying the number of unique and common DEPs between the different time points.
The fact that P4 levels during pregnancy coincide with the clinical changes observed in several immune-mediated diseases, prompted us to investigate if the genes affected by P4, i.e. the P4 response genes, were related to established disease-associated genes. To this end, we used disease-associated genes derived from DisGeNET (48) for seven diseases that have previously been shown to be altered during pregnancy (51): Hashimoto’s disease, Graves’ disease, MS, psoriasis, RA, systemic lupus erythematosus (SLE) and systemic sclerosis. We combined the P4 response genes at both 6 and 24 hrs, resulting in a total of 2563 downregulated and 2403 upregulated genes that were differentially expressed by P4 over the course of 24 hrs. In line with the previous finding of a much more pronounced downregulatory effect of P4 on immune-related genes, the downregulated P4 response genes were significantly enriched for genes associated with all seven diseases; Hashimoto’s disease (n=51, OR:2.6, p=1.6x10-7), Graves’ disease (n=73, OR: 2.0, p=2.3x10-6), psoriasis (n=134, OR: 1.4, p=5.8x10-4), MS (n=201, OR: 1.6, p=7.0x10-8), RA (n=270, OR:1.3, p=6.8x10-4), SLE (n=212, OR: 1.7, p=1.4x10-9) and systemic sclerosis (n=96, OR:1.5, p=8.5x10-4) whereas the upregulated P4 response genes showed no significant enrichment for any disease-associated genes (Figure 4A and Table S3). Thus, only the downregulated P4 response genes were considered for the subsequent analysis. There was a total of 17 shared genes between all seven diseases that were significantly downregulated by P4: BCL2, CXCL10, FAS, FOXP3, ICAM1, IL10, IL17A, IL1A, IL1B, IL21, IL22, IL23R, IL2R, ISG20, RBM45, TGFB1 and TNF (Figure 4A and Table S3), highlighting genes of potential relevance in the disease pathogenesis.
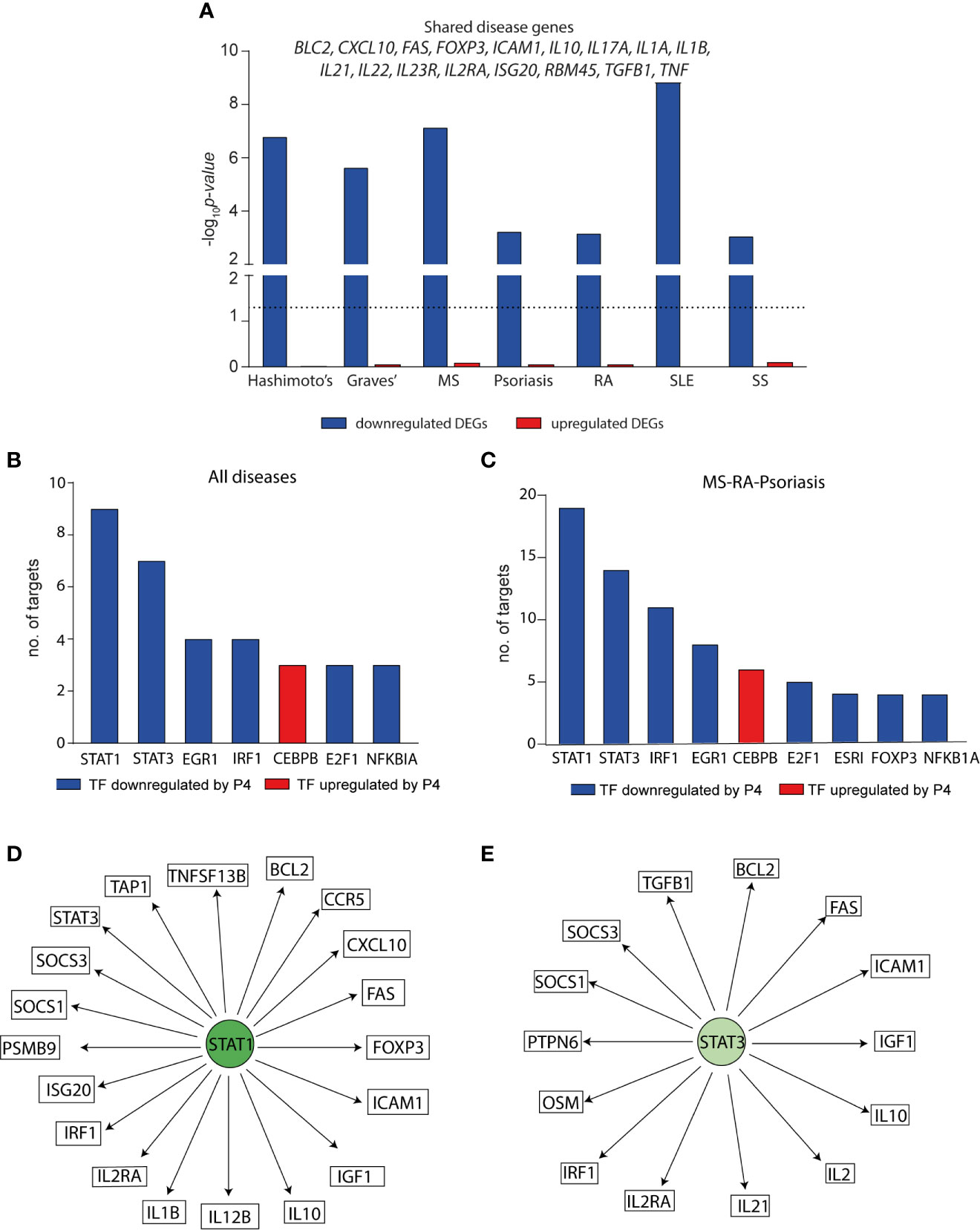
Figure 4 Progesterone downregulates disease-associated genes for several autoimmune diseases particularly through STAT1 and STAT3. The effect of P4 on disease-associated genes and upstream transcription factors was interrogated using known disease genes (derived from DisGeNET) and TF-target interactions were based on TRRUST and DoRothEA. (A) Enrichment of disease-associated genes for seven autoimmune diseases among the differentially expressed genes by P4. Downregulated DEGs = blue bars, upregulated DEGs = red bars. DEGs were defined comparing cells activated in the presence of P4 as compared to activation alone and represent the total number of uniquely expressed genes at 6 and 24 hrs combined. Dotted line shows p=0.05. The genes that are common between all seven diseases among the differentially downregulated genes by P4 are depicted above the bars. Enrichment p values were calculated by Fisher’s exact test. The TFs with the highest number of interacting genes among the disease-associated differentially downregulated genes for (B) shared genes between the seven different diseases (out of 36 TFs in total) and (C) common genes between MS, RA and psoriasis (out of 57 TFs in total). (D, E) Schematic representation of STAT1 and STAT3 and their interacting genes. STAT1 and STAT3 are significantly downregulated by P4 as are their targets depicted in the figure. DEGs, differentially expressed genes; MS; multiple sclerosis; P4, progesterone; RA, rheumatoid arthritis; TF, transcription factor.
To get a more complete understanding of the effect of P4 on disease-associated changes, we sought to identify upstream regulators of the affected disease genes. Using known validated TF- target interactions combining TRRUST (49) and DoRothEA (50), we identified a total of 36 TFs were significantly affected by P4 and together regulated 12 out of the 17 shared disease genes. STAT1 and STAT3 were significantly downregulated by P4 and had the highest number of target genes. Furthermore, the targets of STAT1 and STAT3 were significantly enriched among the disease genes (STAT1: n=9, OR: 9.5, p=0.0004; STAT3: n=7, OR: 11.8, p=0.0001) (Figure 4B and Table S4). Next, we focused on MS, RA and psoriasis, which have been shown to markedly improve during pregnancy (52–54), in order to more specifically pinpoint which TFs and genes that are affected by P4. There was a total of 62 common genes between the three diseases that were significantly downregulated by P4 (Table S5). A total of 57 different TFs were annotated to regulate 39 of these common genes (2.6 mean number of interactions per TF) where again targets for STAT1 and STAT3 were significantly enriched among the common disease-associated genes (STAT1: n=19, targeting 48% of the common genes, OR: 3.2, p=0.001; STAT3: n=14, targeting 36% of the common genes, OR: 5.6, p=3.0x10-5) (Figures 4C–E and Table S5). Thus, the downregulatory effect of P4 on disease-associated genes seems to be primarily mediated through targeting STAT1 and STAT3 and their downstream targets.
To further highlight the role of P4 in the pregnancy-associated improvement of MS, RA and psoriasis, we investigated if the disease-associated transcriptomic changes were also reflected at the protein level. We thereby identified proteins where the known interacting TFs, as per TRRUST and DoRothEA, were also significantly affected by P4, and the disease-associated gene as well as the corresponding protein were significantly downregulated by P4. We combined all proteins that were significantly downregulated by P4 at 6, 24 and 72 hrs, to get a global map of the proteomic changes, resulting in a total of 41 DEPs. Focusing on STAT1 and STAT3, we found several disease-associated proteins downstream of STAT1 and STAT3 to be significantly decreased by P4: IL-12β (STAT1), IL-10 (STAT1, STAT3), IL-2 (STAT3), CXCL10 (STAT1), OSM (STAT3) and TGF-β1 (STAT3) (Table 1). Further, we found three additional proteins that were also disease-associated whose corresponding genes were significantly downregulated by P4: TNF, TNFSF10 and IL-13 (Table 1). Thus, for nine inflammatory-related proteins, we can confirm that the transcriptomic changes induced by P4 were also mirrored at the proteomic levels. Taken together, P4 significantly affects TF expression resulting in downregulation of the interacting disease-associated genes, which in turn dampens the expression of the corresponding proteins.
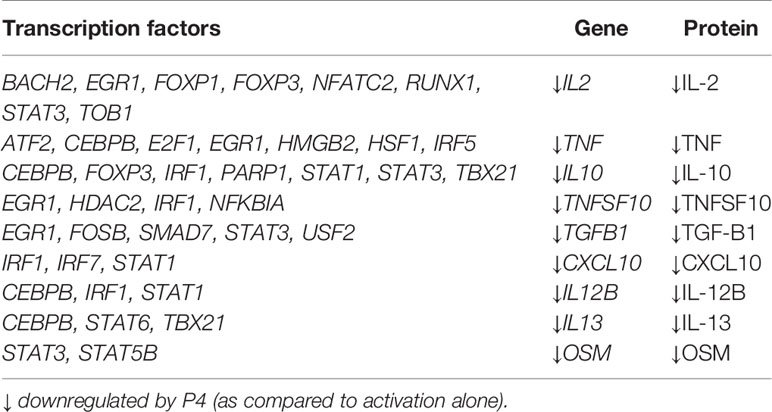
Table 1 Disease-associated genes and proteins and their corresponding transcription factors that are significantly affected by P4.
In the present study, we demonstrate that P4 significantly dampens T cell activation, thus providing a compelling explanation for how T cell responses can potentially be regulated during pregnancy when P4 levels are high and immune regulation is required. Transcriptomic analysis revealed that P4 significantly altered the gene expression profile by dampening immune-related genes and pathways such as JAK-STAT and T cell receptor signaling. Interestingly, the transcriptomic changes induced by P4 were highly enriched for genes associated with immune-mediated diseases, known to be modulated during pregnancy such as MS, RA, psoriasis and SLE. Further, STAT1 and STAT3 were highlighted as central regulators and several downstream targets were significantly downregulated by P4, also at the protein level, including several well-known and disease-relevant cytokines. Our findings extend previous knowledge of P4 as an immune-regulatory hormone and provide further knowledge on how P4 affects CD4+ T cell activation and its potential involvement in the pregnancy-associated disease modulation observed for several immune-mediated diseases. Collectively, our findings support the role of P4 as an immunosuppressive hormone and indicate that P4 plays a significant role in the pregnancy-induced immunomodulation responses. Our results open up for the exploration of new therapeutic regimes, mimicking the pregnancy situation, in immune-mediated diseases.
Previous studies have investigated the effect of P4 on lymphocyte and CD3+ T cell activation (35, 55–58) and our results are in line with a suppressive effect of P4 on immune responses. However, the present study is to our knowledge not only the first study to directly assess the effect of P4 on human CD4+ T cell activation, but also the first study to perform in-depth transcriptomic profiling to decipher the effects of P4. Using an in vitro system of T cell activation, P4 was found to consistently inhibit T cell activation, as evident from the lowering of the expression of cell surface activation markers and by the profound dampening of immune-related genes and proteins. Further, changes that were specifically related to the actual T cell activation were indeed opposed by P4, i.e. genes and proteins upregulated during T cell activation were downregulated by P4 and vice versa. T cell activation is indeed a crucial checkpoint for immune regulation in both pregnancy tolerance (59) and autoimmunity (33, 60) and administration of P4 has been shown to prevent T cell activation-induced preterm labor and preterm birth in an animal model (61), emphasizing the importance of P4 in controlling T cell activation during pregnancy.
P4 has been suggested as one potential candidate for the pregnancy-induced immune modulation of several autoimmune diseases, considering the correlation of P4 levels with disease activity during pregnancy. Indeed, pre-treatment with P4 in experimental autoimmune encephalomyelitis (EAE), an animal model of MS, attenuated disease severity and reduced inflammatory responses (62, 63). Further, even after EAE onset, P4 administration could significantly reduce disease severity (64). Our findings that the P4-induced genes were significantly enriched for disease-associated genes, related to several autoimmune diseases that are known to be modulated during pregnancy, provide additional support for a possible involvement of P4 in this disease modulating activity. Interestingly, there were several genes overlapping between all diseases, indicating potentially shared mechanisms in the pregnancy-induced modulation. It is indeed difficult to speculate which concentrations of P4 that would be relevant for the pregnancy-induced immunomodulation. We used P4 concentrations similar or higher to those found in the placenta (65), with the most prominent dampening effect at the highest concentration. The relevance of the selected concentrations for the pregnancy-induced disease modulation could be challenged considering the lower systemic concentrations of P4 (66). However, the extrapolation of in vivo levels to in vitro remains problematic. In vivo, there are several other factors and cells involved that could potentially enhance the hormonal effects. Indeed, the local cell-to-cell concentrations may be higher than currently measurable levels, which could explain why most often supra-physiological concentrations are used in vitro. Furthermore, circulating maternal immune cells are sequestered in the placenta where they come in close contact with hormone-producing fetally-derived cells (67) and thus, have the potential of being exposed to much higher levels of P4 than in the peripheral circulation.
STAT1 and STAT3, which were found to be significantly dampened by P4, have been implicated in the disease pathogenesis of several autoimmune diseases. Loss of STAT3 expression in T cells has for example been shown to confer protection against EAE (68), and high levels of STAT3 can be used to predict conversion from clinically isolated syndrome to definite MS (69). Furthermore, targeting STAT3 has been suggested as a potential therapeutic approach in diseases like MS, psoriasis and RA (70–72). STAT1 and STAT3 are central TFs in driving the differentiation of TH1 and TH17 cells. P4 has previously been shown to dampen both TH1 and TH17-related immune responses (20, 73–76) and is suggested to play a major role in the shift away from TH1/TH17 in favor of more TH2/Treg-dominated responses during pregnancy (19). Suggestively, the dampening effect of P4 on STAT1 and STAT3 could be involved in altering the balance of TH1 and TH17, immune responses that are a prominent part of the disease pathogenesis in MS, psoriasis and RA, which could be a possible mechanism for the pregnancy-induced modulation of disease activity. Our transcriptomic findings were further validated at the protein level for several highly relevant disease targets downstream of STAT1 and STAT3. Targeting IL-12β (IL12p40), which constitutes a crossroad between TH1-associated IL-12 and TH17-associated IL-23, is successfully implemented as treatment for psoriasis and is undergoing clinical trials for several other immune-mediated inflammatory diseases (77). Clearly, IL-12β plays a profound role in disease and the dampening effect of P4 on this gene and subsequent protein expression could thus constitute a possible avenue in the modulation of disease by P4. It is noteworthy that P4 did not only dampen pro-inflammatory, but also anti-inflammatory genes and proteins such as IL-10 and TGF-β, indicating that P4 induces a rather general downregulation of immune responses. It is well-known that anti-inflammatory responses are often induced as a negative feedback mechanism during inflammation to help prevent excessive inflammatory responses and help restore tissue homeostasis. Speculatively, as P4 dampens immune responses, it is only natural to assume that lesser anti-inflammatory responses would follow as a result. This is exemplified through the intricate relationship between STAT3 and IL-10, where the IL10 promoter contains a specific binding motif for STAT3, which appears to control the expression of IL10 (78). Indeed, increased expression of STAT3 in T cells from patients with SLE promoted IL-10 expression (79) and similar associations have been proposed between STAT3 and TGF-β as well (80, 81).
One potential limitation of our study is that we investigated the effect of P4 in healthy female donors. To the best of our knowledge, there are no studies that have specifically evaluated if patients with autoimmune diseases would respond differently to P4 than healthy individuals. Further, the responsiveness to P4 could vary as the P4-related receptor expression could vary throughout the menstrual cycle (82, 83). The women included in this study were sampled evenly across the menstrual cycle, which should minimize the bias in differences in receptor expression. Furthermore, cells from all included women responded to P4 in a consistent way. Additionally, as potential treatment option the effect of P4 in males would also need to be further investigated. Indeed, P4 administration has shown wide-spread physiological effects in men (84), although detailed studies regarding effects on the male immune system are lacking. Interestingly, promising results were noted in a recent pilot clinical trial where progesterone was used in male patients with COVID-19 with the aim to mitigate the overactive immune system and sometimes fatal cytokine storm. Notably, no severe side effects associated with progesterone were reported, even at progesterone levels similar to those found during pregnancy (85). Although further investigations are needed, the study highlights the potential of use of progesterone as treatment in males as well.
Another caveat which is not yet addressed is the precise mechanisms by which P4 operates. Deciphering which receptors that are involved in mediating the effects of P4 is quite complex due to the promiscuous nature of P4, potentially binding both membrane and nuclear progesterone receptors (PR) as well as glucocorticoid and mineralocorticoid receptors. In contrast to mice, most studies in humans have failed to detect expression of the nuclear PR in lymphocytes (24, 86). Also, in our data, there was no expression of nuclear PR. Instead, expression of the membrane PRs PGRMC1, PGRMC2, PAQR6 and PAQR7 were detected, which most likely rules out any possible involvement of the nuclear PR. Expression and signaling through the membrane PRs have been reported in human T cells (58, 86, 87), in agreement with the effects observed here. However, the involvement of the glucocorticoid receptor cannot be ruled out (88, 89) and the synthetic corticosteroid dexamethasone has been shown to exert similar effects on T cell differentiation as P4 (76). Most likely the observed effect of P4 is a combinatory effect mediated via the different receptors. Further studies will be needed to dissect the involvement and importance of the different receptors in P4 signaling, for example through blocking the action of the different receptors. The recently described specific glucocorticoid antagonist (CORT125134), with no PR binding (90), combined with synthetic progestins with different affinities for the different receptors could increase the understanding of the contribution of the glucocorticoid receptor versus the PRs.
In summary, we showed that the presence of P4 significantly reduced activation of CD4+ T cells and induced large transcriptomic changes in the activated cells. Most prominently P4 down-regulated immune responses associated with the T cell activation and the genes affected by P4 were significantly enriched for disease-associated genes of immune-mediated diseases that are known to be modulated during pregnancy. We conclude that our study supports a role for P4 in the immune modulation induced during pregnancy and that P4 should be further evaluated as a potential treatment option in T-cell mediated diseases.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/geo/, GSE162051.
The studies involving human participants were reviewed and approved by Regional Ethics Review Board in Linköping (Regionala etikprövningsnämnden i Linköping), Sweden (approval number: M39-08). The participants provided their written informed consent to participate in this study.
SH, JR, and GP collected samples and performed cell culture experiments and flow cytometry. SH, OR, and RM performed pre-processing, analysis of the RNA-sequencing data, and bioinformatics analysis. JE, MG and MJ contributed to the study design and overall supervision of the study. SH was responsible for preparation of figures and the writing of the manuscript with the support of MG, JE and MJ. All authors contributed to the article and approved the submitted version.
This study was funded by the Swedish Foundation for Strategic Research (SB16-0011), the Swedish Research Council (2018-02776), Swedish Foundation for MS research, NEURO Sweden, the Swedish Society of Medicine (SLS-879791) and the Swedish Lions Foundation’s research funds.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge the National Genomics Infrastructure (NGI) in Stockholm funded by Science for Life Laboratory, the Knut and Alice Wallenberg Foundation, and the Swedish Research Council, and SNIC/Uppsala Multidisciplinary Centre for Advanced Computational Science for assistance with massively parallel sequencing and access to the UPPMAX computational infrastructure. The authors would also like to acknowledge the support of the Clinical biomarker facility at SciLifeLab Sweden for providing assistance in protein analyses.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.672168/full#supplementary-material
1. Schumacher A, Costa SD, Zenclussen AC. Endocrine Factors Modulating Immune Responses in Pregnancy. Front Immunol (2014) 5:196. doi: 10.3389/fimmu.2014.00196
2. Nair RR, Verma P, Singh K. Immune-Endocrine Crosstalk During Pregnancy. Gen Comp Endocrinol (2017) 242:18–23. doi: 10.1016/j.ygcen.2016.03.003
3. Labarta E, Mariani G, Holtmann N, Celada P, Remohi J, Bosch E. Low Serum Progesterone on the Day of Embryo Transfer is Associated With a Diminished Ongoing Pregnancy Rate in Oocyte Donation Cycles After Artificial Endometrial Preparation: A Prospective Study. Hum Reprod (2017) 32:2437–42. doi: 10.1093/humrep/dex316
4. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, et al. Mice Lacking Progesterone Receptor Exhibit Pleiotropic Reproductive Abnormalities. Genes Dev (1995) 9:2266–78. doi: 10.1101/gad.9.18.2266
5. Mesiano S. Myometrial Progesterone Responsiveness and the Control of Human Parturition. J Soc Gynecol Investig (2004) 11:193–202. doi: 10.1016/j.jsgi.2003.12.004
6. Ku CW, Allen JC Jr, Lek SM, Chia ML, Tan NS, Tan TC. Serum Progesterone Distribution in Normal Pregnancies Compared to Pregnancies Complicated by Threatened Miscarriage From 5 to 13 Weeks Gestation: A Prospective Cohort Study. BMC Pregnancy Childbirth (2018) 18:360. doi: 10.1186/s12884-018-2002-z
7. Okabe H, Makino S, Kato K, Matsuoka K, Seki H, Takeda S. The Effect of Progesterone on Genes Involved in Preterm Labor. J Reprod Immunol (2014) 104-105:80–91. doi: 10.1016/j.jri.2014.03.008
8. Arck PC, Rucke M, Rose M, Szekeres-Bartho J, Douglas AJ, Pritsch M, et al. Early Risk Factors for Miscarriage: A Prospective Cohort Study in Pregnant Women. Reprod BioMed Online (2008) 17:101–13. doi: 10.1016/S1472-6483(10)60300-8
9. Couzinet B, Le Strat N, Ulmann A, Baulieu EE, Schaison G. Termination of Early Pregnancy by the Progesterone Antagonist RU 486 (Mifepristone). N Engl J Med (1986) 315:1565–70. doi: 10.1056/NEJM198612183152501
10. Jarde A, Lutsiv O, Beyene J, McDonald SD. Vaginal Progesterone, Oral Progesterone, 17-OHPC, Cerclage, and Pessary for Preventing Preterm Birth in At-Risk Singleton Pregnancies: An Updated Systematic Review and Network Meta-Analysis. BJOG (2019) 126:556–67. doi: 10.1111/1471-0528.15566
11. Wu SP, Li R, DeMayo FJ. Progesterone Receptor Regulation of Uterine Adaptation for Pregnancy. Trends Endocrinol Metab (2018) 29:481–91. doi: 10.1016/j.tem.2018.04.001
12. Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of Pregnancy-Related Relapse in Multiple Sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med (1998) 339:285–91. doi: 10.1056/NEJM199807303390501
13. Oka M. Effect of Pregnancy on the Onset and Course of Rheumatoid Arthritis. Ann Rheum Dis (1953) 12:227–9. doi: 10.1136/ard.12.3.227
14. Boyd AS, Morris LF, Phillips CM, Menter MA. Psoriasis and Pregnancy: Hormone and Immune System Interaction. Int J Dermatol (1996) 35:169–72. doi: 10.1111/j.1365-4362.1996.tb01632.x
15. Dosiou C, Lathi RB, Tulac S, Huang ST, Giudice LC. Interferon-Related and Other Immune Genes are Downregulated in Peripheral Blood Leukocytes in the Luteal Phase of the Menstrual Cycle. J Clin Endocrinol Metab (2004) 89:2501–4. doi: 10.1210/jc.2003-031647
16. Kalo-Klein A, Witkin SS. Regulation of the Immune Response to Candida Albicans by Monocytes and Progesterone. Am J Obstet Gynecol (1991) 164:1351–4. doi: 10.1016/0002-9378(91)90712-Z
17. Straub RH. The Complex Role of Estrogens in Inflammation. Endocr Rev (2007) 28:521–74. doi: 10.1210/er.2007-0001
18. Mohammad I, Starskaia I, Nagy T, Guo J, Yatkin E, Vaananen K, et al. Estrogen Receptor Alpha Contributes to T Cell-Mediated Autoimmune Inflammation by Promoting T Cell Activation and Proliferation. Sci Signal (2018) 11:eaap9415. doi: 10.1126/scisignal.aap9415
19. Shah NM, Imami N, Johnson MR. Progesterone Modulation of Pregnancy-Related Immune Responses. Front Immunol (2018) 9:1293. doi: 10.3389/fimmu.2018.01293
20. Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, et al. Progesterone Favors the Development of Human T Helper Cells Producing Th2-type Cytokines and Promotes Both IL-4 Production and Membrane CD30 Expression in Established Th1 Cell Clones. J Immunol (1995) 155:128–33.
21. Yao Y, Li H, Ding J, Xia Y, Wang L. Progesterone Impairs Antigen-non-Specific Immune Protection by CD8 T Memory Cells Via Interferon-Gamma Gene Hypermethylation. PloS Pathog (2017) 13:e1006736. doi: 10.1371/journal.ppat.1006736
22. Su L, Sun Y, Ma F, Lu P, Huang H, Zhou J. Progesterone Inhibits Toll-like Receptor 4-Mediated Innate Immune Response in Macrophages by Suppressing NF-kappaB Activation and Enhancing SOCS1 Expression. Immunol Lett (2009) 125:151–5. doi: 10.1016/j.imlet.2009.07.003
23. Lee JH, Lydon JP, Kim CH. Progesterone Suppresses the mTOR Pathway and Promotes Generation of Induced Regulatory T Cells With Increased Stability. Eur J Immunol (2012) 42:2683–96. doi: 10.1002/eji.201142317
24. Lissauer D, Eldershaw SA, Inman CF, Coomarasamy A, Moss PA, Kilby MD. Progesterone Promotes Maternal-Fetal Tolerance by Reducing Human Maternal T-cell Polyfunctionality and Inducing a Specific Cytokine Profile. Eur J Immunol (2015) 45:2858–72. doi: 10.1002/eji.201445404
25. Moriyama I, Sugawa T. Progesterone Facilitates Implantation of Xenogenic Cultured Cells in Hamster Uterus. Nat New Biol (1972) 236:150–2. doi: 10.1038/newbio236150a0
26. Majewski AC, Hansen PJ. Progesterone Inhibits Rejection of Xenogeneic Transplants in the Sheep Uterus. Horm Res (2002) 58:128–35. doi: 10.1159/000063578
27. Pavia C, Siiteri PK, Perlman JD, Stites DP. Suppression of Murine Allogeneic Cell Interactions by Sex Hormones. J Reprod Immunol (1979) 1:33–8. doi: 10.1016/0165-0378(79)90027-5
28. Fekecs T, Kalmar-Nagy K, Szakaly P, Nemeth K, Moezzi M, Zapf I, et al. Changes of Progesterone-Induced Blocking Factor in Patients After Kidney Transplantation. Transplant Proc (2011) 43:3694–6. doi: 10.1016/j.transproceed.2011.08.087
29. Sallusto F. Heterogeneity of Human CD4(+) T Cells Against Microbes. Annu Rev Immunol (2016) 34:317–34. doi: 10.1146/annurev-immunol-032414-112056
30. Bulmer JN, Williams PJ, Lash GE. Immune Cells in the Placental Bed. Int J Dev Biol (2010) 54:281–94. doi: 10.1387/ijdb.082763jb
31. Mjösberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ Regulatory T Cells and T Helper 1, T Helper 2, and T Helper 17 Cells in Human Early Pregnancy Decidua. Biol Reprod (2010) 82:698–705. doi: 10.1095/biolreprod.109.081208
32. Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA, et al. Evidence for a Selective Migration of Fetus-Specific CD4+CD25bright Regulatory T Cells From the Peripheral Blood to the Decidua in Human Pregnancy. J Immunol (2008) 180:5737–45. doi: 10.4049/jimmunol.180.8.5737
33. Dendrou CA, Fugger L, Friese MA. Immunopathology of Multiple Sclerosis. Nat Rev Immunol (2015) 15:545–58. doi: 10.1038/nri3871
34. Hughes GC, Clark EA, Wong AH. The Intracellular Progesterone Receptor Regulates CD4+ T Cells and T Cell-Dependent Antibody Responses. J Leukoc Biol (2013) 93:369–75. doi: 10.1189/jlb.1012491
35. Chien EJ, Chang CP, Lee WF, Su TH, Wu CH. Non-Genomic Immunosuppressive Actions of Progesterone Inhibits PHA-induced Alkalinization and Activation in T Cells. J Cell Biochem (2006) 99:292–304. doi: 10.1002/jcb.20858
36. Chien EJ, Liao CF, Chang CP, Pu HF, Lu LM, Shie MC, et al. The Non-Genomic Effects on Na+/H+-Exchange 1 by Progesterone and 20alpha-Hydroxyprogesterone in Human T Cells. J Cell Physiol (2007) 211:544–50. doi: 10.1002/jcp.20962
37. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: Ultrafast Universal RNA-seq Aligner. Bioinformatics (2013) 29:15–21. doi: 10.1093/bioinformatics/bts635
38. Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie Enables Improved Reconstruction of a Transcriptome From RNA-seq Reads. Nat Biotechnol (2015) 33:290–5. doi: 10.1038/nbt.3122
39. Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics (2010) 26:139–40. doi: 10.1093/bioinformatics/btp616
40. McCarthy DJ, Chen Y, Smyth GK. Differential Expression Analysis of Multifactor RNA-Seq Experiments With Respect to Biological Variation. Nucleic Acids Res (2012) 40:4288–97. doi: 10.1093/nar/gks042
41. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma Powers Differential Expression Analyses for RNA-sequencing and Microarray Studies. Nucleic Acids Res (2015) 43:e47. doi: 10.1093/nar/gkv007
42. Law CW, Alhamdoosh M, Su S, Dong X, Tian L, Smyth GK, et al. RNA-Seq Analysis is Easy as 1-2-3 With Limma, Glimma and Edger. F1000Res (2016) 5:1408. doi: 10.12688/f1000research.9005.2
43. Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS (2012) 16:284–7. doi: 10.1089/omi.2011.0118
44. Kanehisa M, Sato Y, Furumichi M, Morishima K, Tanabe M. New Approach for Understanding Genome Variations in KEGG. Nucleic Acids Res (2019) 47:D590–D5. doi: 10.1093/nar/gky962
45. Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res (2000) 28:27–30. doi: 10.1093/nar/28.1.27
46. Shen L, Sinai M. Geneoverlap: Test and Visualize Gene Overlaps. R Package Version 1.24.0 (2020). Available at: http://shenlab-sinai.github.io/shenlab-sinai/.
47. Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, et al. Homogenous 96-Plex PEA Immunoassay Exhibiting High Sensitivity, Specificity, and Excellent Scalability. PloS One (2014) 9:e95192. doi: 10.1371/journal.pone.0095192
48. Pinero J, Ramirez-Anguita JM, Sauch-Pitarch J, Ronzano F, Centeno E, Sanz F, et al. The DisGeNET Knowledge Platform for Disease Genomics: 2019 Update. Nucleic Acids Res (2020) 48:D845–D55. doi: 10.1093/nar/gkz1021
49. Han H, Cho JW, Lee S, Yun A, Kim H, Bae D, et al. TRRUST v2: An Expanded Reference Database of Human and Mouse Transcriptional Regulatory Interactions. Nucleic Acids Res (2018) 46:D380–D6. doi: 10.1093/nar/gkx1013
50. Garcia-Alonso L, Holland CH, Ibrahim MM, Turei D, Saez-Rodriguez J. Benchmark and Integration of Resources for the Estimation of Human Transcription Factor Activities. Genome Res (2019) 29:1363–75. doi: 10.1101/gr.240663.118
51. Piccinni MP, Lombardelli L, Logiodice F, Kullolli O, Parronchi P, Romagnani S. How Pregnancy can Affect Autoimmune Diseases Progression? Clin Mol Allergy (2016) 14:11. doi: 10.1186/s12948-016-0048-x
52. Vena GA, Cassano N, Bellia G, Colombo D. Psoriasis in Pregnancy: Challenges and Solutions. Psoriasis (Auckl) (2015) 5:83–95. doi: 10.2147/PTT.S82975
53. Finkelsztejn A, Brooks JB, Paschoal FM Jr, Fragoso YD. What can We Really Tell Women With Multiple Sclerosis Regarding Pregnancy? A Systematic Review and Meta-Analysis of the Literature. BJOG (2011) 118:790–7. doi: 10.1111/j.1471-0528.2011.02931.x
54. de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease Activity of Rheumatoid Arthritis During Pregnancy: Results From a Nationwide Prospective Study. Arthritis Rheum (2008) 59:1241–8. doi: 10.1002/art.24003
55. Clemens LE, Siiteri PK, Stites DP. Mechanism of Immunosuppression of Progesterone on Maternal Lymphocyte Activation During Pregnancy. J Immunol (1979) 122:1978–85.
56. Ehring GR, Kerschbaum HH, Eder C, Neben AL, Fanger CM, Khoury RM, et al. A Nongenomic Mechanism for Progesterone-Mediated Immunosuppression: Inhibition of K+ Channels, Ca2+ Signaling, and Gene Expression in T Lymphocytes. J Exp Med (1998) 188:1593–602. doi: 10.1084/jem.188.9.1593
57. Kobayashi H, Mori T, Suzuki A, Nishimura T, Nishimoto H, Harada M. Suppression of Mixed Lymphocyte Reaction by Progesterone and Estradiol-17beta. Am J Obstet Gynecol (1979) 134:255–9. doi: 10.1016/S0002-9378(16)33029-0
58. Ndiaye K, Poole DH, Walusimbi S, Cannon MJ, Toyokawa K, Maalouf SW, et al. Progesterone Effects on Lymphocytes may be Mediated by Membrane Progesterone Receptors. J Reprod Immunol (2012) 95:15–26. doi: 10.1016/j.jri.2012.04.004
59. Erlebacher A. Mechanisms of T Cell Tolerance Towards the Allogeneic Fetus. Nat Rev Immunol (2013) 13:23–33. doi: 10.1038/nri3361
60. Hellberg S, Eklund D, Gawel DR, Köpsén M, Zhang H, Nestor CE, et al. Dynamic Response Genes in CD4+ T Cells Reveal a Network of Interactive Proteins That Classifies Disease Activity in Multiple Sclerosis. Cell Rep (2016) 16:2928–39. doi: 10.1016/j.celrep.2016.08.036
61. Arenas-Hernandez M, Romero R, Xu Y, Panaitescu B, Garcia-Flores V, Miller D, et al. Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment With Progesterone. J Immunol (2019) 202:2585–608. doi: 10.4049/jimmunol.1801350
62. Garay L, Gonzalez Deniselle MC, Lima A, Roig P, De Nicola AF. Effects of Progesterone in the Spinal Cord of a Mouse Model of Multiple Sclerosis. J Steroid Biochem Mol Biol (2007) 107:228–37. doi: 10.1016/j.jsbmb.2007.03.040
63. Garay L, Gonzalez Deniselle MC, Meyer M, Costa JJ, Lima A, Roig P, et al. Protective Effects of Progesterone Administration on Axonal Pathology in Mice With Experimental Autoimmune Encephalomyelitis. Brain Res (2009) 1283:177–85. doi: 10.1016/j.brainres.2009.04.057
64. Yates MA, Li Y, Chlebeck P, Proctor T, Vandenbark AA, Offner H. Progesterone Treatment Reduces Disease Severity and Increases IL-10 in Experimental Autoimmune Encephalomyelitis. J Neuroimmunol (2010) 220:136–9. doi: 10.1016/j.jneuroim.2010.01.013
65. Feinshtein V, Ben-Zvi Z, Sheiner E, Amash A, Sheizaf B, Holcberg G. Progesterone Levels in Cesarean and Normal Delivered Term Placentas. Arch Gynecol Obstet (2010) 281:387–92. doi: 10.1007/s00404-009-1125-x
66. Shah NM, Lai PF, Imami N, Johnson MR. Progesterone-Related Immune Modulation of Pregnancy and Labor. Front Endocrinol (Lausanne) (2019) 10:198. doi: 10.3389/fendo.2019.00198
67. Kaipe H, Raffetseder J, Ernerudh J, Solders M, Tiblad E. Mait Cells At the Fetal-Maternal Interface During Pregnancy. Front Immunol (2020) 11:1788. doi: 10.3389/fimmu.2020.01788
68. Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T Cells Prevents Development of Experimental Autoimmune Diseases. J Immunol (2008) 180:6070–6. doi: 10.4049/jimmunol.180.9.6070
69. Frisullo G, Nociti V, Iorio R, Patanella AK, Marti A, Mirabella M, et al. The Persistency of High Levels of pSTAT3 Expression in Circulating CD4+ T Cells From CIS Patients Favors the Early Conversion to Clinically Defined Multiple Sclerosis. J Neuroimmunol (2008) 205:126–34. doi: 10.1016/j.jneuroim.2008.09.003
70. Oike T, Sato Y, Kobayashi T, Miyamoto K, Nakamura S, Kaneko Y, et al. Stat3 as a Potential Therapeutic Target for Rheumatoid Arthritis. Sci Rep (2017) 7:10965. doi: 10.1038/s41598-017-11233-w
71. Miyoshi K, Takaishi M, Nakajima K, Ikeda M, Kanda T, Tarutani M, et al. Stat3 as a Therapeutic Target for the Treatment of Psoriasis: A Clinical Feasibility Study With STA-21, a Stat3 Inhibitor. J Invest Dermatol (2011) 131:108–17. doi: 10.1038/jid.2010.255
72. Liu Y, Holdbrooks AT, De Sarno P, Rowse AL, Yanagisawa LL, McFarland BC, et al. Therapeutic Efficacy of Suppressing the Jak/STAT Pathway in Multiple Models of Experimental Autoimmune Encephalomyelitis. J Immunol (2014) 192:59–72. doi: 10.4049/jimmunol.1301513
73. Xu L, Dong B, Wang H, Zeng Z, Liu W, Chen N, et al. Progesterone Suppresses Th17 Cell Responses, and Enhances the Development of Regulatory T Cells, Through Thymic Stromal Lymphopoietin-Dependent Mechanisms in Experimental Gonococcal Genital Tract Infection. Microbes Infect (2013) 15:796–805. doi: 10.1016/j.micinf.2013.06.012
74. Lee JH, Ulrich B, Cho J, Park J, Kim CH. Progesterone Promotes Differentiation of Human Cord Blood Fetal T Cells Into T Regulatory Cells But Suppresses Their Differentiation Into Th17 Cells. J Immunol (2011) 187:1778–87. doi: 10.4049/jimmunol.1003919
75. AbdulHussain G, Azizieh F, Makhseed M, Raghupathy R. Effects of Progesterone, Dydrogesterone and Estrogen on the Production of Th1/Th2/Th17 Cytokines by Lymphocytes From Women With Recurrent Spontaneous Miscarriage. J Reprod Immunol (2020) 140:103132. doi: 10.1016/j.jri.2020.103132
76. Miyaura H, Iwata M. Direct and Indirect Inhibition of Th1 Development by Progesterone and Glucocorticoids. J Immunol (2002) 168:1087–94. doi: 10.4049/jimmunol.168.3.1087
77. Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, et al. Il-12 and IL-23 Cytokines: From Discovery to Targeted Therapies for Immune-Mediated Inflammatory Diseases. Nat Med (2015) 21:719–29. doi: 10.1038/nm.3895
78. Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in Lipopolysaccharide-Induced IL-10 Gene Expression. J Immunol (2000) 165:1612–7. doi: 10.4049/jimmunol.165.3.1612
79. Hedrich CM, Rauen T, Apostolidis SA, Grammatikos AP, Rodriguez Rodriguez N, Ioannidis C, et al. Stat3 Promotes IL-10 Expression in Lupus T Cells Through Trans-Activation and Chromatin Remodeling. Proc Natl Acad Sci USA (2014) 111:13457–62. doi: 10.1073/pnas.1408023111
80. Shi J, Feng J, Xie J, Mei Z, Shi T, Wang S, et al. Targeted Blockade of TGF-beta and IL-6/JAK2/STAT3 Pathways Inhibits Lung Cancer Growth Promoted by Bone Marrow-Derived Myofibroblasts. Sci Rep (2017) 7:8660. doi: 10.1038/s41598-017-09020-8
81. Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z, et al. JAK/STAT3 Signaling is Required for TGF-beta-induced Epithelial-Mesenchymal Transition in Lung Cancer Cells. Int J Oncol (2014) 44:1643–51. doi: 10.3892/ijo.2014.2310
82. Mertens HJ, Heineman MJ, Theunissen PH, de Jong FH, Evers JL. Androgen, Estrogen and Progesterone Receptor Expression in the Human Uterus During the Menstrual Cycle. Eur J Obstet Gynecol Reprod Biol (2001) 98:58–65. doi: 10.1016/S0301-2115(00)00554-6
83. Mangal RK, Wiehle RD, Poindexter AN,3, Weigel NL. Differential Expression of Uterine Progesterone Receptor Forms A and B During the Menstrual Cycle. J Steroid Biochem Mol Biol (1997) 63:195–202. doi: 10.1016/S0960-0760(97)00119-2
84. Oettel M, Mukhopadhyay AK. Progesterone: The Forgotten Hormone in Men? Aging Male (2004) 7:236–57. doi: 10.1080/13685530400004199
85. Ghandehari S, Matusov Y, Pepkowitz S, Stein D, Kaderi T, Narayanan D, et al. Progesterone in Addition to Standard of Care vs Standard of Care Alone in the Treatment of Men Hospitalized With Moderate to Severe Covid-19: A Randomized, Controlled Pilot Trial. Chest (2021). doi: 10.1016/j.chest.2021.02.024
86. Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, et al. Expression of Membrane Progesterone Receptors on Human T Lymphocytes and Jurkat Cells and Activation of G-proteins by Progesterone. J Endocrinol (2008) 196:67–77. doi: 10.1677/JOE-07-0317
87. Areia A, Vale-Pereira S, Alves V, Rodrigues-Santos P, Moura P, Mota-Pinto A. Membrane Progesterone Receptors in Human Regulatory T Cells: A Reality in Pregnancy. BJOG (2015) 122:1544–50. doi: 10.1111/1471-0528.13294
88. Engler JB, Kursawe N, Solano ME, Patas K, Wehrmann S, Heckmann N, et al. Glucocorticoid Receptor in T Cells Mediates Protection From Autoimmunity in Pregnancy. Proc Natl Acad Sci USA (2017) 114:E181–E90. doi: 10.1073/pnas.1617115114
89. Hierweger AM, Engler JB, Friese MA, Reichardt HM, Lydon J, DeMayo F, et al. Progesterone Modulates the T-cell Response Via Glucocorticoid Receptor-Dependent Pathways. Am J Reprod Immunol (2019) 81:e13084. doi: 10.1111/aji.13084
90. Hunt H, Donaldson K, Strem M, Zann V, Leung P, Sweet S, et al. Assessment of Safety, Tolerability, Pharmacokinetics, and Pharmacological Effect of Orally Administered CORT125134: An Adaptive, Double-Blind, Randomized, Placebo-Controlled Phase 1 Clinical Study. Clin Pharmacol Drug Dev (2018) 7:408–21. doi: 10.1002/cpdd.389
Keywords: CD4+ T cells, T cell activation, transcriptomics, protein profiling, progesterone
Citation: Hellberg S, Raffetseder J, Rundquist O, Magnusson R, Papapavlou G, Jenmalm MC, Ernerudh J and Gustafsson M (2021) Progesterone Dampens Immune Responses in In Vitro Activated CD4+ T Cells and Affects Genes Associated With Autoimmune Diseases That Improve During Pregnancy. Front. Immunol. 12:672168. doi: 10.3389/fimmu.2021.672168
Received: 25 February 2021; Accepted: 26 April 2021;
Published: 12 May 2021.
Edited by:
Ursula Grohmann, University of Perugia, ItalyReviewed by:
Raj Raghupathy, Kuwait University, KuwaitCopyright © 2021 Hellberg, Raffetseder, Rundquist, Magnusson, Papapavlou, Jenmalm, Ernerudh and Gustafsson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandra Hellberg, c2FuZHJhLmhlbGxiZXJnQGxpdS5zZQ==
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.