
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 11 June 2021
Sec. Cytokines and Soluble Mediators in Immunity
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.670398
 Yi Ru1,2†
Yi Ru1,2† Xiaojie Ding1,2†
Xiaojie Ding1,2† Ying Luo1,2†
Ying Luo1,2† Hongjin Li2
Hongjin Li2 Xiaoying Sun2
Xiaoying Sun2 Mi Zhou1
Mi Zhou1 Yaqiong Zhou2
Yaqiong Zhou2 Le Kuai1,2
Le Kuai1,2 Meng Xing1,2
Meng Xing1,2 Liu Liu1,2
Liu Liu1,2 Yue Luo1,2
Yue Luo1,2 Jiankun Song1,2
Jiankun Song1,2 Jiale Chen1,2
Jiale Chen1,2 Bin Li1,2,3*
Bin Li1,2,3* Xin Li1,2*
Xin Li1,2*Background: Anti-interleukin (IL)-23 agents are widely used for autoimmune disease treatment; however, the safety and risks of specific symptoms have not been systematically assessed.
Objectives: The aim of this study was to summarize the characteristics and mechanisms of occurrence of five immunological and non-immunological adverse events caused by different anti-IL-23 agents.
Methods: The Cochrane Library, EMBASE, PubMed, and Web of Science databases were searched for eligible randomized clinical trials published from inception through May 1, 2020. Randomized clinical trials that reported at least one type of adverse event after treatment were included, regardless of sex, age, ethnicity, and diagnosis. Two investigators independently screened and extracted the characteristics of the studies, participants, drugs, and adverse event types. The Cochrane Handbook was used to assess the methodological quality of the included randomized clinical trials. Heterogeneity was assessed using the I2 statistic. Meta-regression was applied to determine the sources of heterogeneity, and subgroup analysis was used to identify the factors contributing to adverse events.
Results: Forty-eight studies were included in the meta-analysis, comprising 25,624 patients treated with anti-IL-23 agents. Serious immunological or non-immunological adverse events were rare. Anti-IL-12/23-p40 agents appeared to cause adverse events more easily than anti-IL-23-p19 agents. The incidence of cancer did not appear to be related to anti-IL-23 agent treatment, and long-term medication could lead to mental diseases. The prevention of complications should be carefully monitored when administered for over approximately 40 weeks to avoid further adverse reactions, and the incidence of infection was the highest among general immunological adverse events.
Conclusions: The application of anti-IL-23 agents induced a series of immunological and non-immunological adverse events, but these agents tend to be well-tolerated with good safety profiles.
Autoimmune disorders represent a series of long-standing conditions with distinct appearances and characteristics. The mechanisms underlying central tolerance, peripheral tolerance, and adaptation ensure proper regulation of the immune system in healthy individuals to prevent autoimmunity (1). Current medical strategies for the treatment of autoimmune disorders mainly include nonsteroidal anti-inflammatory drugs, steroidal anti-inflammatory drugs, and disease-modifying anti-rheumatic drugs. However, these therapeutics are not effective in all patients, have undesirable adverse events (AEs), and fail to completely cure the diseases. Once symptoms appear, patients typically desire the resolution of pain rather than prevention of further onset. At the same time, biological agents (BAs) targeting cytokines, receptors, and signaling molecules that have been developed can overcome the limitations due to the multidrug resistance. In 1998, the Food and Drug Administration approved etanercept, a recombinant fusion protein of tumor necrosis factor receptor 2 with the Fc portion of human IgG1, as the first-generation BA for rheumatoid arthritis treatment. Since then, BAs have ushered in a new era in the treatment of autoimmune disorders. Consequently, the safety and tolerability of BAs in long-term and daily practice warrant more attention than ever.
BAs play a therapeutic role in blocking key inflammatory cytokines or cell-surface molecules (2). Their action mechanisms differ from those of chemical drugs and BAs are not digested in the gastrointestinal tract (3) Most BAs are naturally occurring proteins or humanized antibodies that can neutralize natural proteins, which can result in AEs. In contrast to those elicited by chemicals, AEs caused by BAs mainly depend on the chemistry, mode of action, metabolism, and immunogenicity (4). To distinguish the AEs caused by BAs from other adverse effects, AEs are classified into five types using the Greek alphabet (type α to ϵ) (3) (Table S1). Type α AEs occur after the abundant release of inflammatory factors with complicated and changeable symptoms. Type β AEs are immune-mediated and more serious than type α AEs. Type γ and δ AEs involve short-term and long-term toxicities, respectively, linked to the chemical structure of BAs and their metabolism. Type ϵ AEs occur during drug withdrawal, particularly when the drug is suddenly stopped.
Interleukin (IL)-23 affects inflammatory cells and relies on the ability of cytokines to indirectly counteract regulatory mechanisms. The anti-IL-23 agents has good safety and clinical curative effect (5). Ustekinumab was recognized as the most widely used anti-IL-12/23-p40 agent, which was approved in 2009 for the treatment of psoriasis (Pso) with advantages of few drug injections, high remission rates, and long-term sustainment. Although blocking the IL-23 immune axis is sufficient to treat many autoimmune disorders, the risk of serious infections and various AEs is a concern (3, 4). A phase III trial demonstrated that briakinumab, a fully human monoclonal antibody directed against IL-12/23-p40 for psoriasis treatment, caused serious complications and AEs, and the drug developer withdrew its approval application submitted to the Food and Drug Administration and European Agency for the Evaluation of Medicinal Products in 2011 (6, 7). This incident resulted in controversy and additional scrutiny of anti-IL-23 agents. In a review of four commercial BAs, secukinumab (an IL-17 inhibitor) and ustekinumab were suggested to achieve better effects (8). Another study also proposed that the probability of AEs with anti-IL-23 agents is lower than that with anti-IL-17 agents (9). However, summaries of the safety of anti-IL-23 agents are not available; thus, further analysis is necessary.
The aim of this systematic review and meta-analysis was to assess the incidence and characteristics of AEs caused by all anti-IL-23 agents currently available in the market (Table 1), and to provide a comprehensive synopsis based on existing evidence, of the efficacy and safety of anti-IL-23 agents, which will help to identify future research priorities.
This systematic review was performed following the Cochrane Handbook for Systematic Reviews of Interventions (9), and is presented according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
The inclusion and exclusion criteria were determined before initiating the study. The included studies were limited to randomized clinical trials (RCTs) that reported at least one type of AE after anti-IL-23 agent treatment, regardless of sex, age, ethnicity, and diagnosis. The exclusion criteria were as follows: (i) no anti-IL-23 agents included; (ii) no reported AEs; (iii) meeting abstracts, cell or animal studies, reviews, systematic reviews, and meta-analyses; and (iv) no full-text studies.
The primary outcomes considered were the incidence and grade of AEs according to the Common Terminology Criteria for Adverse Events v5.0 (updated November 2017) of the Department of Health and Human Services. AEs of grades ≥ 3 were considered severe. Heterogeneity and AE incidence were evaluated using meta-regression and subgroup analyses. The primary outcomes included general information of the RCTs, and the secondary outcomes were measured according to the five AE types.
The Cochrane Library, Excerpta Medica database (EMBASE), PubMed, and Web of Science databases were searched for studies published from inception through May 1, 2020, without language restrictions. The details of the clinical trials were searched at ClinicalTrials.gov until June 1, 2020. The search terms were grouped into three blocks (10).Character included “safety”, “side effects”, “adverse reactions” or “adverse events”. Clinical condition included “ustekinumab or stelara or CNTO 1275” or “briakinumab or ABT874” or “guselkumab or tremfya or CNTO1959” or “tildrakizumab or ilumya or SCH900222” or “risankizumab or skyrizi or ABBV 066” or “brazikumab or MEDI 2070” or “miriklzumab”. Trial design included clinical trial, random, and control. Vocabulary and syntax were adapted to be appropriate for each database. In total, 4,285 potential studies were identified from the electronic databases, and the detailed steps for study selection are shown in Figure S1.
The quality of the studies was assessed according to the Cochrane Handbook (9), including: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of the outcome assessment, incomplete outcome data, selective reporting, and other biases. The terms “low”, “unclear”, “high”, and “n/a” referred to low, uncertain, high risks of bias, and not applicable, respectively. The results were cross-checked by two investigators (YR, XJD) and disagreements were settled under discussion. Potential publication bias was detected by visual inspection of a funnel plot and formal testing with Egger’s test. Meta-regression was performed to explore the sources of study heterogeneity.
We estimated the incidence of AEs associated with anti-IL-23 agent treatment. Heterogeneity between studies was assessed using the Q test and I2 statistic. The random-effects model was used to calculate the average statistics of the weighted combination of multiple research statistics. All analyses were performed using R software (version 3.8.6 [2018–3–15]) with the package Meta and Metafor function, and results were assessed at a significance level of P < 0.05.
In total, 48 studies reported at least one type of AE caused by anti-IL-23 agents. The articles were retrieved from the Cochrane Library, EMBASE, PubMed, and Web of Science databases. A flowchart of the search process is presented in Figure S1. All included studies were RCTs. When a study had more than one dosage cohort, each cohort was used as an independent study for analysis.
Visual inspection of the funnel plot showed no evident asymmetry, indicating that the pooled results were not influenced by publication bias (Figure S2). Egger’s test showed P = 0.3226, indicating no evidence of publication bias (Figure S3). The overall AE incidence revealed high between-study heterogeneity with I2 = 98%; the odds ratio (OR) was 64.76% with a 95% confidence interval (CI) of 60.75–68.78 (Figure S4). The quality of most RCTs was high, according to the Cochrane quality assessment criteria (Table S2). The study population characteristics are shown in Table 2. To explore the potential sources of heterogeneity, meta-regression analysis was performed for the endpoints of AEs in different groups (Table 3).
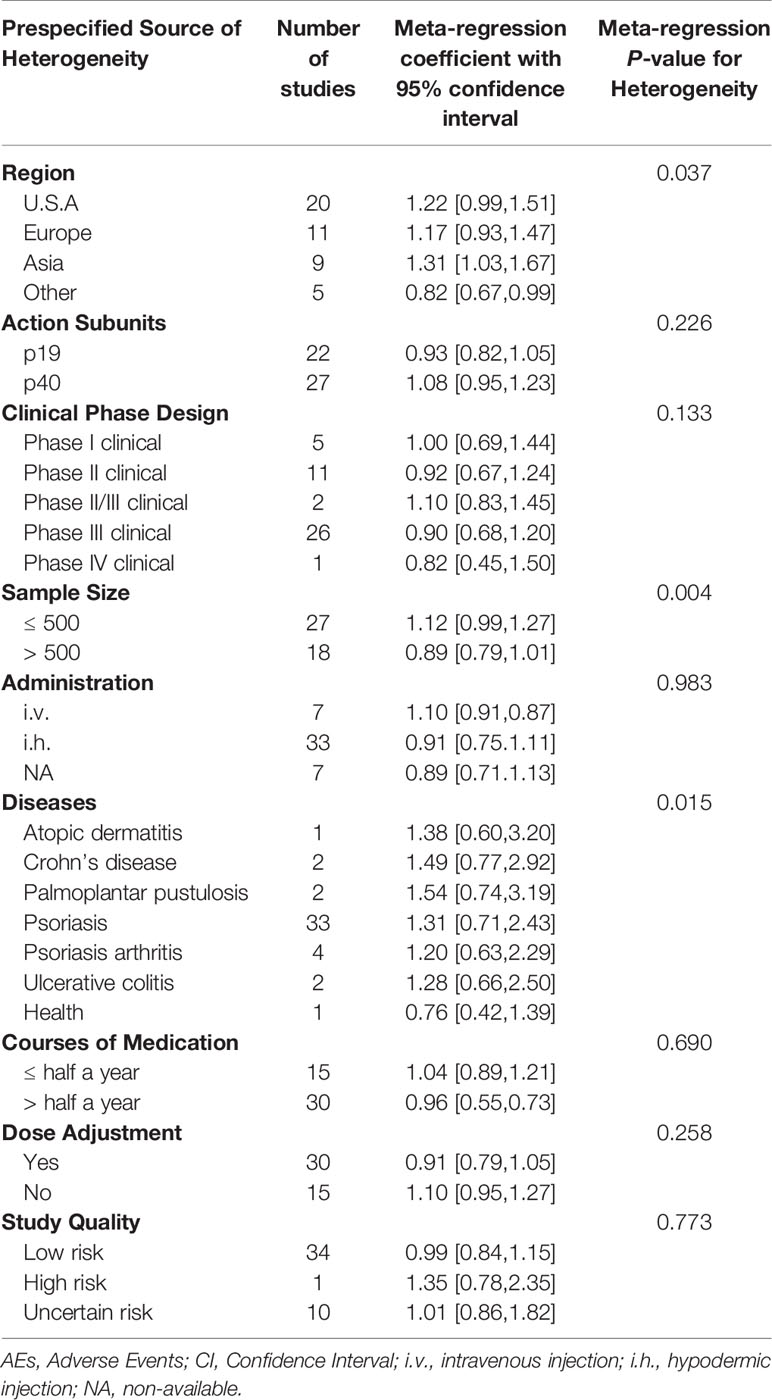
Table 3 Potential prespecified sources of heterogeneity explored among the studies reporting AEs associated with anti-IL-23 agents.
Forty-five studies proposed a clear definition of medication courses. We sorted the AE incidence and found a high degree of heterogeneity among different treatment courses (Figure S6.3). To describe the characteristics of medication courses in-depth, each interval at 12 weeks was marked, and a line chart was created with an approximately equal number of courses (Figure 1A). The AE incidence was lower when the medication period lasted within three-quarters of a year (approximately 42 weeks) (Figure 1A).
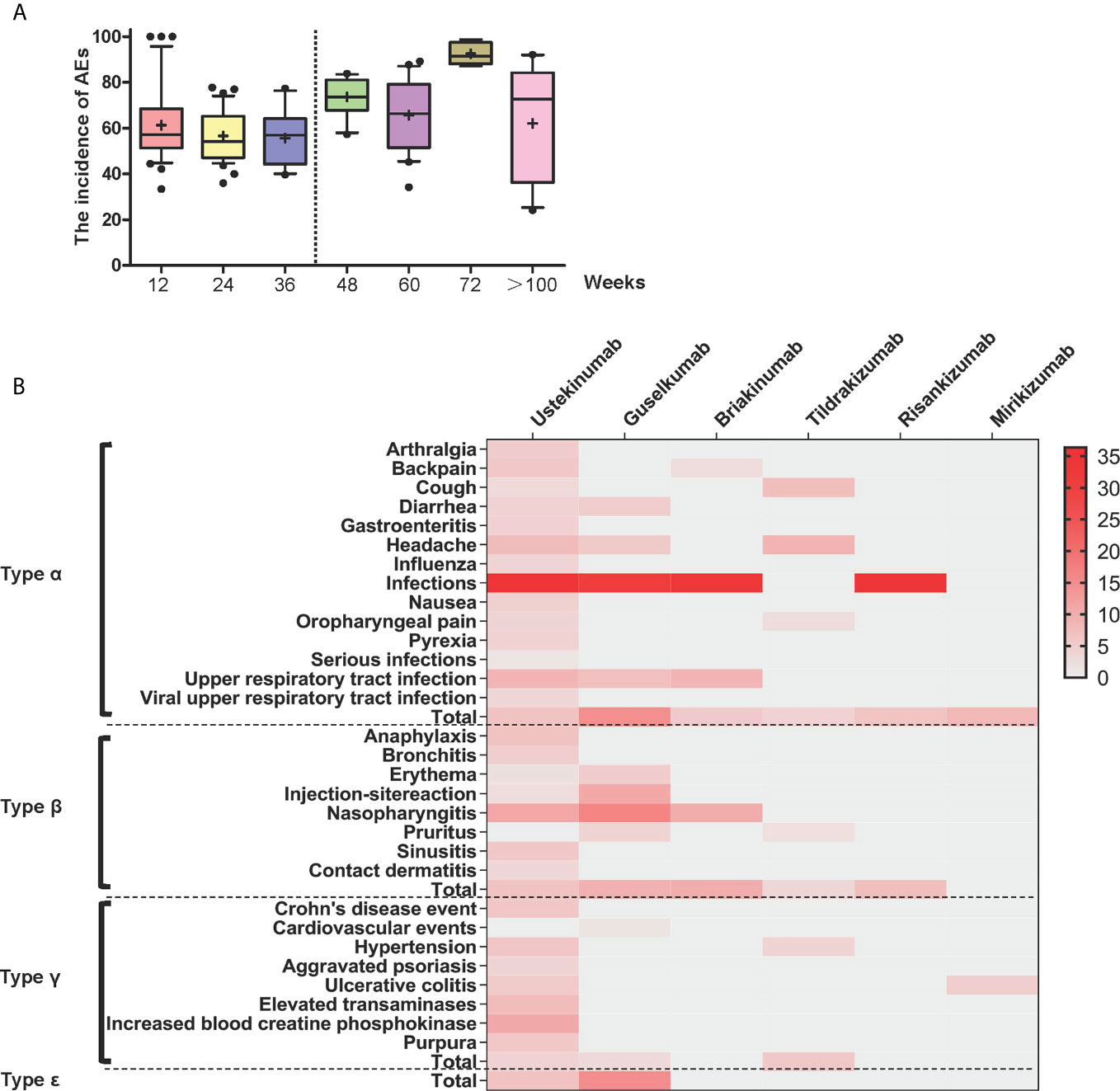
Figure 1 (A) Box diagram for subgroup analysis. Box diagram for the incidence of changes in adverse events with different medication courses. (B) Heat map of different types of symptoms caused by different drugs. Red areas indicate greater relative probability of occurrence and lighter colored areas indicate a slight or null relative probability of occurrence. All data included in the heat map are statistically significant (P < 0.05).
Further subgroup analysis showed that the AE occurrence of anti-IL-23/IL-12-p40 agents was 65.23% (95% CI 61.74–68.57, I2 = 95%), which was higher than that of anti-IL-23-p19 agents of 58.71% (95% CI 55.40–61.94, I2 = 91%) (Table S3 and Figure S6.5.1). The evident differences in AE incidence were as follows: 71.56% (95% CI 66.68–75.98, I2 = 96%) for ustekinumab, 67.07% (95% CI 55.53–76.87, I2 = 88%) for briakinumab, 65.69% (95% CI 54.44–75.42, I2 = 96%) for risankizumab, 59.13% (95% CI 53.77–64.28, I2 = 79%) for guselkumab, and 51.19% (95% CI 45.80–56.56, I2 = 89%) for tildrakizumab (Table S3 and Figure S6.5.2). However, the results were also affected by the actual clinical usage.
The pooled OR from the random-effects model for the five AE types associated with anti-IL-23 agents was analyzed. The incidence of type ϵ AEs was 13.3% (95% CI 6.23–26.17, I2 = 77%), which was higher than that of type α AEs with an incidence of 7.14% (95% CI 6.41–7.93, I2 = 97%), type β AEs at 6.57% (95% CI 5.81–7.41, I2 = 92%), and type γ AEs at 3.94% (95% CI 3.33–4.65, I2 = 80%). However, there was no significant effect on the incidence of type δ AEs.
The anti-IL-23-p19 agents tended to most strongly increase the risk for type α AEs among the five types. The most frequent type of α AE during treatment was infections with an incidence of 36.35%; serious infections showed an incidence of <1.5% (Table S3 and Figure S7.1.1). The heat map shows darker areas with a high probability of incidence and lighter areas with a low probability of occurrence (Figure 1B). Notably, other common signs of type α reactions included upper respiratory tract infection (8.53%), headache (7.12%), and viral upper respiratory tract infection (6.73%). The other frequent type of α AEs included arthralgia, backache, cough, diarrhea, gastroenteritis, influenza, nausea, oropharyngeal pain, and pyrexia (Table S3 and Figure S7.1.1).
Anti-IL-23-p19 agents were more likely to induce type β AEs. The most common symptom was nasopharyngitis, with an incidence of 12.21%. Other AEs with a higher probability of occurrence included anaphylaxis (5.00%), sinusitis (4.57%), and pruritus (4.40%). Other common type β AEs were bronchitis, erythema, injection-site reaction, and neutropenia (Table S3 and Figure S7.2.1).
Compared with the anti-IL-23-p19 agents, the anti-IL-12/23-p40 agents appeared to more frequently lead to type γ AEs. The most common type of γ AE symptom was increased transaminases (approximately 7.43%). Prolonged application of anti-IL-23 agents aggravated the original diseases, such as increasing the severity of Crohn’s disease (5.96%), hypertension (5.50%), ulcerative colitis (5.28%), Pso (3.92%), and cardiovascular events (0.76%) (Table S4 and Figure S7.3.1).
Anti-IL-23-p19 agents increased the risk of type ϵ AEs more than anti-IL-12/23-p40 agents. The most frequent symptom was depression (0.75%; Table S3 and Figure S7.4.1).
Biological therapy has evolved, owing to improved integration of knowledge of the interactions between the immune system and related cytokines, which could affect the entire pathologic disease process. Anti-IL-23 agents displayed broad range of antagonistic activities because IL-23 is a critical upstream regulator (58). IL-23 acts early in the disease inflammatory cascade, which activates downstream effectors to maintain the TH17 cell phenotype (59). Moreover, IL-12 is considered to be proatherogenic and the inhibition of IL-12/23-p40 should confer cardioprotection (60). A previous study (n = 2,447) (5) indicated that anti-IL-23 agents appear to be safer and more effective in clinical application, but the drug-specific safety information has not been explored systematically. This is the first report to classify and review the AEs caused by anti-IL-23 agents, and to provide a statistical outline.
IL-23 is secreted by several immune cells in response to microbial products and inflammatory cytokines, which essentially bridge the innate and adaptive immune responses and drive early local immunity (61, 62). IL-23 is structurally composed of the unique IL-23-p19 subunit linked to the common p40 subunit that is shared with IL-12. Moreover, IL-23 and IL-12 are responsible for driving the differentiation of naïve T helper (TH) cells to TH17 and TH1 cells, respectively (63). TH17 cells secrete several proinflammatory cytokines, stimulate the proliferation of keratinocytes, and activate downstream inflammatory signal transduction (64). TH1 cells produce inflammatory cytokines and drive the expansion of inflammation. The inhibition of IL-23 blocks the cascade of both immune and inflammatory reactions (Figure 2). Primary subgroup analysis showed significant differences in the AE incidence of briakinumab, ustekinumab, guselkumab, risankizumab, and tildrakizumab (Table S3 and Figure S6.5.2). Anti-IL-12/23-p40 agents (briakinumab and ustekinumab) were more likely to cause AEs with an incidence of 65.23% (95% CI 61.74–68.57, I2 = 95%) (Table S3 and Figure S6.5.2) than anti-IL-23-p19 treatment, which may be related to its structural features (65). Therefore, a higher rate of malignancy with IL-12/23-p40 blockade was confirmed (66); IL-12 can promote the infiltration of cytotoxic T cells and IL-23 can promote inflammatory responses (66). While our results showed that anti-IL-23 treatment was rarely associated with an increased cancer risk (Figure S3), similar those of a recent study (67). In summary, the AEs caused by anti-IL-23 agents were mainly due to their immunological effects and broad range of biological effects.
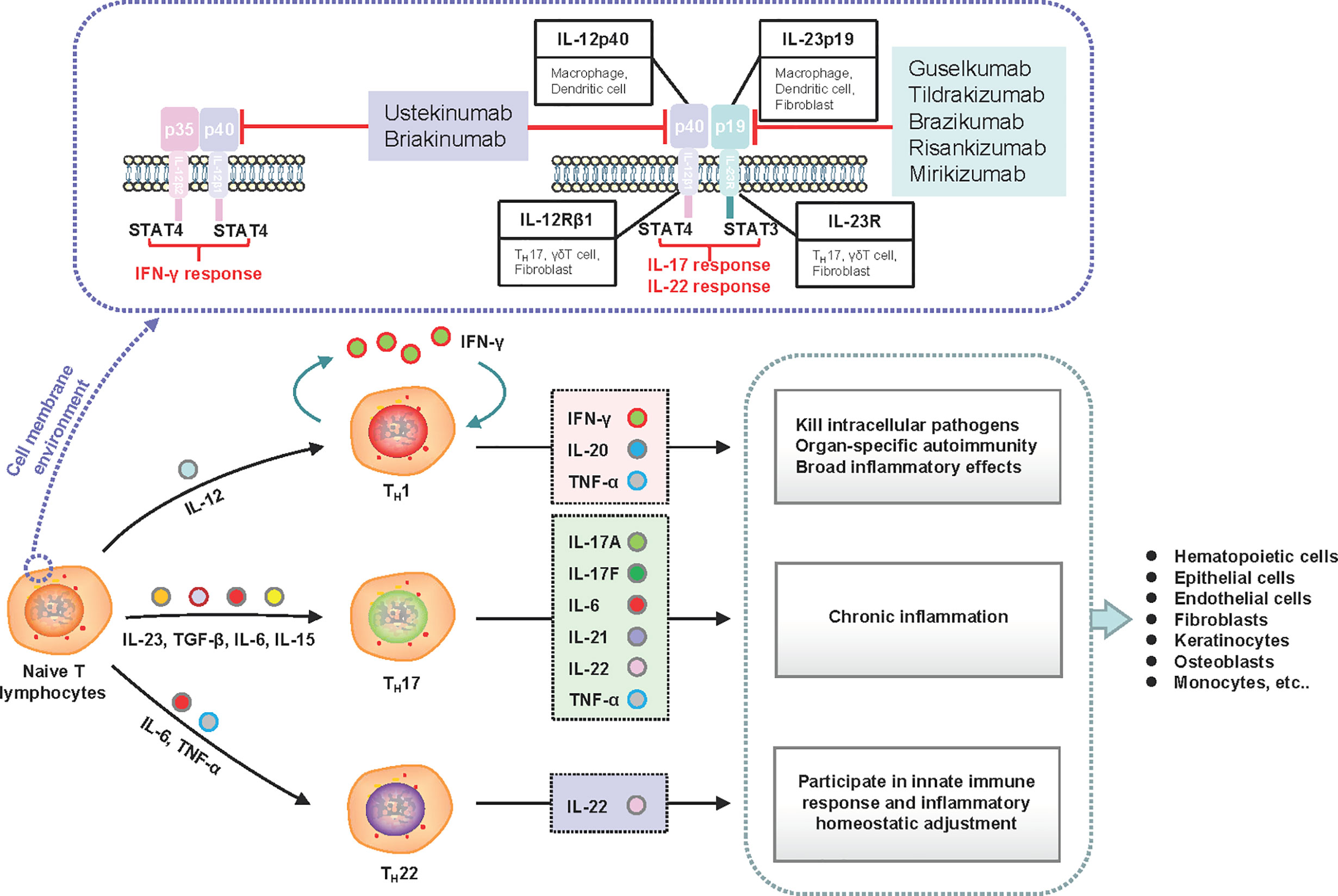
Figure 2 Schematic of the induction and effects of IL-23 subunits and their corresponding receptors and signaling molecules. IL-23 is an essential factor required for the expansion of naive CD4+ T lymphocyte populations. The functional IL-23 receptors include IL-12Rβ1 and IL-23R. The downstream signaling molecule of IL-23R is STAT3, which drives TH17 and TH22 responses. The downstream signaling molecule of IL-12Rβ1 is STAT4, which drives the TH1 response. Under stimulation with different cell factor combinations, naive CD4+ cells differentiate into T helper (TH1, TH17, and TH22) cells to produce corresponding inflammatory factors and execute their functional activities. Inhibiting the upstream subunits (IL-12Rβ1 or IL-23R) can govern both upstream and downstream processes in the cascade to improve clinical symptoms. IFN, interferon; IL, interleukin; STAT, signal transducer and activator of transcription; TH, T helper; TGF, transforming growth factor; TNF, tumor necrosis factor.
AEs induced by BAs always require specific knowledge, as they are different from the common AEs elicited by chemicals or drugs (Table S1). The most common AE identified in the included studies was type α with an incidence of 7.14% (95% CI 6.41–7.93, I2 = 97%), and the highest risk was for type ϵ AEs (13.30%, 95% CI 6.23–26.17, I2 = 89%) (Table S3 and Figure S7). Type ϵ is the only non-immunological AE form, which may be associated with long-term medication. Previous studies (68, 69) have shown that excessive release of pathological inflammatory mediators may lead to a high incidence of anxiety and depression; moreover, the application of BAs induces psychologically relevant AEs. The most common type ϵ AE was psychiatric disorder with an incidence of 0.75% (Table S3 and Figure S7.5.1). This indicates that the treat-and-extend regimens over a longer period may lead to mental problems, which may also relate to the chronic duration. In summary, clinician should pay attention to communication and psychological guidance to patients.
The curative effect is often comparable with decreasing tolerance to AEs. A drug concentration-response relationship affects the clinical efficacy, indicating that a higher BA drug dose may achieve better and longer efficacy duration in the first few weeks (70). However, no significant dose-dependent efficacy of anti-IL-23 agents was observed during long-term treatment (71). Regardless of whether drug dose affects the curative effect, dose adjustment showed no significant effect on AEs in our analysis (Table 3). Dose tapering does not lead to persistent flares or safety issues (72); however, variation in medication courses may induce heterogeneity. According to the subgroup analysis, the incidence of AEs stabilized when the medication period was maintained within 40 weeks (Figure 1A). A previous study (11) showed that long-term BA treatment may even decrease the survival rate due to serious AEs and ineffective treatments. This suggests that anti-IL-23 treatment is relatively safe in the first three quarters of medication use, and an intervention such as combined treatment for potential complications should be considered within the first 40 weeks.
Currently, with the increasing popularity of BA treatment, the development of new drugs is actively taking place. These drugs are beneficial for treating symptoms, and assessing the applicability of patients for potential AEs is an essential step; however, the assessment method remains unclear, and there is currently no consensus on the most appropriate treatment duration of BAs for different diseases. In addition, BAs cannot prevent the entire disease mechanism, which has raised the question as to whether BAs can change the natural course of the disease. Moreover, BAs are relatively expensive and therefore increase healthcare costs, which could be resolved in the near future with the approaching expiration of patents. Finally, no study has discussed the risk of biologics solely in specific subpopulations (such as pregnant women, children, and the elderly), which should be the subject of future research.
This study has several strengths. First, several authors reviewed all available studies independently for data retrieval and analysis to reduce information bias or missing data. Second, the included studies were RCTs of generally high quality, which minimized the selection bias associated with differences between researchers and medical settings to some extent. Third, long-term follow-up studies were included with significant implications that were found by collating rare or non-immune AEs caused by anti-IL-23 agents. Fourth, no industry was involved in this work. However, there were also some limitations to this study. The different treatment strategies had different time points used as safety measures, which prevented inclusion of placebo control data for analysis. To resolve the ambiguity of AEs caused by BAs, we prolonged the observation periods and used five defined types to screen for AEs caused by anti-IL-23 agents. In addition, we used type ϵ analysis to detect long-term and rare AEs, but this was not an in-depth exploration. Further studies should focus on the differences in AEs associated with autoimmune disorders over long-term treatment, which would certainly help to tackle remaining questions in this field.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conceptualization: YR, XD, and BL. Data curation: XD and YiL. Formal analysis: LK, XS, and LL. Funding acquisition: XL and BL. Investigation: YR and YZ. Methodology: YR, XD, and YiL. Project Administration: HL and YuL. Resources: JS and MZ. Software: MX and LL. Supervision: BL and XL. Validation: LL and JC. Visualization: XL and BL. Writing – Original Draft Preparation: YR and XD. Writing – Review & Editing: XL and BL. All authors contributed to the article and approved the submitted version.
This work was supported by the National Nature Science Foundation of China [grant numbers: 82074427,81874470, 81973860], National Key Research and Development Program of China [grant number: 2018YFC1705305], Science and Technology Commission of Shanghai Municipality [grant number: 19ZR1458700], the Shanghai Pujiang Talent Plan (No. 2020PJD067), Shanghai Development Office of TCM [No. ZY(2018-2020)-FWTX-1008], and Shanghai Municipal Key Clinical Specialty (No. shslczdzk05001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.670398/full#supplementary-material
Supplementary Figure 1 | Flow diagram of the literature search and selection.
Supplementary Figure 2 | Funnel plot
Supplementary Figure 3 | Egger’s test
Supplementary Figure 4 | Incidence of total adverse events with different anti-IL-23 agents in all patients.
Supplementary Figure 5 | Incidence of cancers with different anti-IL-23 agents in all patients.
Supplementary Figure 6.1 | Incidence of adverse events in different regions
Supplementary Figure 6.2 | Incidence of adverse events with different. diagnoses in all patients
Supplementary Figure 6.3 | Incidence of adverse events with different courses of medication in all patients.
Supplementary Figure 6.4.1 | Incidence of adverse events with different therapeutic doses in all patients.
Supplementary Figure 6.4.2 | Incidence of adverse events with different frequency of application in all patients.
Supplementary Figure 6.4.3 | Incidence of adverse events in the induction and maintenance periods in all patients.
Supplementary Figure 6.5.1 | Incidence of adverse events with different targeted subunits in all patients.
Supplementary Figure 6.5.2 | Incidence of adverse events with different anti-IL-23 agents in all patients.
Supplementary Figure 7 | Incidence of adverse events of all the symptoms using different anti-IL-23 agents.
Supplementary Figure 7.1.1 | Incidence of type α adverse events symptoms using different anti-IL-23 agents in all patients.
Supplementary Figure 7.1.2 | Incidence of type α adverse events using different targeted subunits in all patients.
Supplementary Figure 7.1.3 | Incidence of type α adverse events using different anti-IL-23 agents in all patients.
Supplementary Figure 7.2.1 | Incidence of type β adverse events symptoms using different anti-IL-23 agents in all patients.
Supplementary Figure 7.2.2 | Incidence of type β adverse events using different targeted subunits in all patients.
Supplementary Figure 7.2.3 | Incidence of type β adverse events using different anti-IL-23 agents in all patients.
Supplementary Figure 7.3.1 | Incidence of type γ adverse events symptoms using different anti-IL-23 agents in all patients.
Supplementary Figure 7.3.2 | Incidence of type γ adverse events using different targeted subunits in all patients.
Supplementary Figure 7.3.3 | Incidence of type γ adverse events using different anti-IL-23 agents in all patients.
Supplementary Figure 7.4.1 | Incidence of type δ adverse events symptoms using different anti-IL-23 agents in all patients.
Supplementary Figure 7.4.2 | Incidence of type δ adverse events using different targeted subunits in all patients.
Supplementary Figure 7.4.3 | Incidence of type δ adverse events using different anti-IL-23 agents in all patients.
Supplementary Figure 7.5.1 | Incidence of type ϵ adverse events symptoms using different anti-IL-23 agents in all patients.
Supplementary Figure 7.5.2 | Incidence of type ϵ adverse events using different targeted subunits in all patients.
Supplementary Figure 7.5.3 | Incidence of type ϵ adverse events using different anti-IL-23 agents in all patients.
Pso, psoriasis; RA, rheumatoid arthritis; AS, ankylosing spondylitis; SLE, systemic lupus erythematosus; CD, Crohn’s disease; NSAIDs, nonsteroidal anti-inflammatory drugs; SAID, steroid anti-inflammatory drugs; DMARDs, disease-modifying anti-rheumatic drugs; AEs, adverse events; BAs, biological agents; FDA, food and drug administration; TNFR2, tumor necrosis factor receptor 2; IL-23, Interleukin-23; EMEA, European Agency for the Evaluation of Medicinal Products; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; RCT, randomized clinical trial; EMBASE, Excerpta Medica data BASE; WOS, Web of Science; CI, confidence interval; OR, the odds ratio.
1. Fragoulis GE, Siebert S, McInnes IB. Therapeutic Targeting of IL-17 and IL-23 Cytokines in Immune-Mediated Diseases. Annu Rev Med (2016) 67:337–53. doi: 10.1146/annurev-med-051914-021944
2. Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR Recommendations for the Management of Rheumatoid Arthritis With Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2013 Update. Ann Rheum Dis (2014) 73(3):492–509.
3. Pichler WJ. Adverse Side-Effects to Biological Agents. Allergy (2006) 61(8):912–20. doi: 10.1111/j.1398-9995.2006.01058.x
4. Aubin F, Carbonnel F, Wendling D. The Complexity of Adverse Side-Effects to Biological Agents. J Crohns Colitis (2013) 7(4):257–62. doi: 10.1016/j.crohns.2012.06.024
5. Loft ND, Vaengebjerg S, Halling AS, Skov L, Egeberg A. Adverse Events With IL-17 and IL-23 Inhibitors for Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-Analysis of Phase III Studies. J Eur Acad Dermatol Venereol (2020) 34(6):1151–60. doi: 10.1111/jdv.16073
6. Gordon KB, Langley RG, Gottlieb AB, Papp KA, Krueger GG, Strober BE, et al. A Phase III, Randomized, Controlled Trial of the Fully Human IL-12/23 mAb Briakinumab in Moderate-to-Severe Psoriasis. J Invest Dermatol (2012) 132(2):304–14. doi: 10.1038/jid.2011.304
7. Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA. Efficacy and Safety Results From a Phase III, Randomized Controlled Trial Comparing the Safety and Efficacy of Briakinumab With Etanercept and Placebo in Patients With Moderate to Severe Chronic Plaque Psoriasis. Br J Dermatol (2011) 165(3):661–8. doi: 10.1111/j.1365-2133.2011.10419.x
8. Wu D, Yue J, Tam LS. Efficacy and Safety of Biologics Targeting Interleukin-6, -12/23 and -17 Pathways for Peripheral Psoriatic Arthritis: A Network Meta-Analysis. Rheumatology (Oxford) (2018) 57(3):563–71. doi: 10.1093/rheumatology/kex452
9. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Cochrane Collaboration Website (2011). Available at: training.cochrane.org/handbook.
10. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the Quality of Reports of Meta-Analyses of Randomised Controlled Trials: The QUOROM Statement. Quality of Reporting of Meta-analyses. Lancet (1999) 354(9193):1896–900. doi: 10.1016/S0140-6736(99)04149-5
11. Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A Human interleukin-12/23 Monoclonal Antibody for the Treatment of Psoriasis. N Engl J Med (2007) 356(6):580–92. doi: 10.1056/NEJMoa062382
12. Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and Safety of Ustekinumab, a Human interleukin-12/23 Monoclonal Antibody, in Patients With Psoriasis: 52-Week Results From a Randomised, Double-Blind, Placebo-Controlled Trial (PHOENIX 2). Lancet (2008) 371(9625):1675–84. doi: 10.1016/S0140-6736(08)60726-6
13. Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and Safety of Ustekinumab, a Human Interleukin-12/23 Monoclonal Antibody, in Patients With Psoriasis: 76-Week Results From a Randomised, Double-Blind, Placebo-Controlled Trial (PHOENIX 1). Lancet (2008) 371(9625):1665–74. doi: 10.1016/S0140-6736(08)60725-4
14. Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of Ustekinumab and Etanercept for Moderate-to-Severe Psoriasis. N Engl J Med (2010) 362(2):118–28. doi: 10.1056/NEJMoa0810652
15. Igarashi A, Kato T, Kato M, Song M, Nakagawa H. Japanese Ustekinumab Study Group. Efficacy and Safety of Ustekinumab in Japanese Patients With Moderate-to-Severe Plaque-Type Psoriasis: Long-Term Results From a Phase 2/3 Clinical Trial. J Dermatol (2012) 39(3):242–52. doi: 10.1111/j.1346-8138.2011.01347.x
16. Tsai TF, Ho JC, Song M, Szapary P, Guzzo C, Shen YK, et al. Efficacy and Safety of Ustekinumab for the Treatment of Moderate-to-Severe Psoriasis: A Phase III, Randomized, Placebo-Controlled Trial in Taiwanese and Korean Patients (PEARL). J Dermatol Sci (2011) 63(3):154–63. doi: 10.1016/j.jdermsci.2011.05.005
17. Kimball AB, Gordon KB, Fakharzadeh S, Yeilding N, Szapary PO, Schenkel B, et al. Long-Term Efficacy of Ustekinumab in Patients With Moderate-to-Severe Psoriasis: Results From the PHOENIX 1 Trial Through Up to 3 Years. Br J Dermatol (2012) 166(4):861–72. doi: 10.1111/j.1365-2133.2012.10901.x
18. Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, et al. Ustekinumab Induction and Maintenance Therapy in Refractory Crohn’s Disease. N Engl J Med (2012) 367(16):1519–28. doi: 10.1056/NEJMoa1203572
19. Kimball AB, Papp KA, Wasfi Y, Chan D, Bissonnette R, Sofen H, et al. Long-Term Efficacy of Ustekinumab in Patients With Moderate-to-Severe Psoriasis Treated for Up to 5 Years in the PHOENIX 1 Study. J Eur Acad Dermatol Venereol (2013) 27(12):1535–45. doi: 10.1111/jdv.12046
20. McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and Safety of Ustekinumab in Patients With Active Psoriatic Arthritis: 1 Year Results of the Phase 3, Multicentre, Double-Blind, Placebo-Controlled PSUMMIT 1 Trial. Lancet (2013) 382(9894):780–9. doi: 10.1016/S0140-6736(13)60594-2
21. Zhu Y, Wang Q, Frederick B, Bouman-Thio E, Marini JC, Keen M, et al. Comparison of the Pharmacokinetics of Subcutaneous Ustekinumab Between Chinese and Non-Chinese Healthy Male Subjects Across Two Phase 1 Studies. Clin Drug Investig (2013) 33(4):291–301. doi: 10.1007/s40261-013-0072-2
22. Langley RG, Lebwohl M, Krueger GG, Szapary PO, Wasfi Y, Chan D, et al. Long-Term Efficacy and Safety of Ustekinumab, With and Without Dosing Adjustment, in Patients With Moderate-to-Severe Psoriasis: Results From the PHOENIX 2 Study Through 5 Years of Follow-Up. Br J Dermatol (2015) 172(5):1371–83. doi: 10.1111/bjd.13469
23. Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, et al. Efficacy and Safety of the Anti-IL-12/23 p40 Monoclonal Antibody, Ustekinumab, in Patients With Active Psoriatic Arthritis Despite Conventional Non-Biological and Biological Anti-Tumour Necrosis Factor Therapy: 6-Month and 1-Year Results of the Phase 3, Multicentre, Double-Blind, Placebo-Controlled, Randomised PSUMMIT 2 Trial. Ann Rheum Dis (2014) 73(6):990–9. doi: 10.1136/annrheumdis-2013-204655
24. Sofen H, Smith S, Matheson RT, Leonardi CL, Calderon C, Brodmerkel C, et al. Guselkumab (an IL-23-specific mAb) Demonstrates Clinical and Molecular Response in Patients With Moderate-to-Severe Psoriasis. J Allergy Clin Immunol (2014) 133(4):1032–40. doi: 10.1016/j.jaci.2014.01.025
25. Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, et al. A Phase 2 Trial of Guselkumab Versus Adalimumab for Plaque Psoriasis. N Engl J Med (2015) 373(2):136–44. doi: 10.1056/NEJMoa1501646
26. Kavanaugh A, Puig L, Gottlieb AB, Ritchlin C, Li S, Wang Y, et al. Maintenance of Clinical Efficacy and Radiographic Benefit Through Two Years of Ustekinumab Therapy in Patients With Active Psoriatic Arthritis: Results From a Randomized, Placebo-Controlled Phase III Trial. Arthritis Care Res (Hoboken) (2015) 67(12):1739–49. doi: 10.1002/acr.22645
27. Kopp T, Riedl E, Bangert C, Bowman EP, Greisenegger E, Horowitz A, et al. Clinical Improvement in Psoriasis With Specific Targeting of Interleukin-23. Nature (2015) 521(7551):222–6. doi: 10.1038/nature14175
28. Landells I, Marano C, Hsu MC, Li S, Zhu Y, Eichenfield LF, et al. Ustekinumab in Adolescent Patients Age 12 to 17 Years With Moderate-to-Severe Plaque Psoriasis: Results of the Randomized Phase 3 CADMUS Study. J Am Acad Dermatol (2015) 73(4):594–603. doi: 10.1016/j.jaad.2015.07.002
29. Papp K, Thaçi D, Reich K, Riedl E, Langley RG, Krueger JG, et al. Tildrakizumab (MK-3222), an Anti-Interleukin-23p19 Monoclonal Antibody, Improves Psoriasis in a Phase IIb Randomized Placebo-Controlled Trial. Br J Dermatol (2015) 173(4):930–9. doi: 10.1111/bjd.13932
30. Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab Is Superior to Ustekinumab in Clearing Skin of Subjects With Moderate to Severe Plaque Psoriasis: CLEAR, a Randomized Controlled Trial. J Am Acad Dermatol (2015) 73(3):400–9. doi: 10.1016/j.jaad.2015.05.013
31. Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, et al. Secukinumab Is Superior to Ustekinumab in Clearing Skin of Subjects With Moderate-to-Severe Plaque Psoriasis Up to 1 Year: Results From the CLEAR Study. J Am Acad Dermatol (2017) 76(1):60–69.e9. doi: 10.1016/j.jaad.2016.08.008
32. Blauvelt A, Papp KA, Griffiths CE, Randazzo B, Wasfi Y, Shen YK, et al. Efficacy and Safety of Guselkumab, An Anti-Interleukin-23 Monoclonal Antibody, Compared With Adalimumab for the Continuous Treatment of Patients With Moderate to Severe Psoriasis: Results From the Phase III, Double-Blinded, Placebo- and Active Comparator-Controlled VOYAGE 1 Trial. J Am Acad Dermatol (2017) 76(3):405–17. doi: 10.1016/j.jaad.2016.11.041
33. Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med (2016) 375(20):1946–60. doi: 10.1056/NEJMoa1602773
34. Zhuang Y, Calderon C, Marciniak SJ Jr, Bouman-Thio E, Szapary P, Yang TY, et al. First-in-Human Study to Assess Guselkumab (Anti-IL-23 mAb) Pharmacokinetics/Safety in Healthy Subjects and Patients With Moderate-to-Severe Psoriasis. Eur J Clin Pharmacol (2016) 72(11):1303–10. doi: 10.1007/s00228-016-2110-5
35. Blauvelt A, Ferris LK, Yamauchi PS, Qureshi A, Leonardi CL, Farahi K, et al. Extension of Ustekinumab Maintenance Dosing Interval in Moderate-to-Severe Psoriasis: Results of a Phase IIIb, Randomized, Double-Blinded, Active-Controlled, Multicentre Study (PSTELLAR). Br J Dermatol (2017) 177(6):1552–61. doi: 10.1111/bjd.15722
36. Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab Versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis. N Engl J Med (2017) 376(16):1551–60. doi: 10.1056/NEJMoa1607017
37. Reich K, Pinter A, Lacour JP, Ferrandiz C, Micali G, French LE, et al. Comparison of Ixekizumab With Ustekinumab in Moderate-to-Severe Psoriasis: 24-Week Results From IXORA-S, a Phase III Study. Br J Dermatol (2017) 177(4):1014–23. doi: 10.1111/bjd.15666
38. Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and Safety of Guselkumab, An Anti-Interleukin-23 Monoclonal Antibody, Compared With Adalimumab for the Treatment of Patients With Moderate to Severe Psoriasis With Randomized Withdrawal and Retreatment: Results From the Phase III, Double-Blind, Placebo- and Active Comparator-Controlled VOYAGE 2 Trial. J Am Acad Dermatol (2017) 76(3):418–31. doi: 10.1016/j.jaad.2016.11.042
39. Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab Versus Placebo or Etanercept for Chronic Plaque Psoriasis (reSURFACE 1 and reSURFACE 2): Results From Two Randomised Controlled, Phase 3 Trials. Lancet (2017) 390(10091):276–88. doi: 10.1016/S0140-6736(17)31279-5
40. Saeki H, Kabashima K, Tokura Y, Murata Y, Shiraishi A, Tamamura R, et al. Efficacy and Safety of Ustekinumab in Japanese Patients With Severe Atopic Dermatitis: A Randomized, Double-Blind, Placebo-Controlled, Phase II Study. Br J Dermatol (2017) 177(2):419–27. doi: 10.1111/bjd.15493
41. Deodhar A, Gottlieb AB, Boehncke WH, Dong B, Wang Y, Zhuang Y, et al. Efficacy and Safety of Guselkumab in Patients With Active Psoriatic Arthritis: A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Study. Lancet (2018) 391(10136):2213–24. doi: 10.1136/annrheumdis-2018-eular.2059
42. Nemoto O, Hirose K, Shibata S, Li K, Kubo H. Safety and Efficacy of Guselkumab in Japanese Patients With Moderate-to-Severe Plaque Psoriasis: A Randomized, Placebo-Controlled, Ascending-Dose Study. Br J Dermatol (2018) 178(3):689–96. doi: 10.1111/bjd.16236
43. Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, An Anti-Interleukin-23 Monoclonal Antibody, for the Treatment of Moderate to Severe Plaque-Type Psoriasis in Japanese Patients: Efficacy and Safety Results From a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study. J Dermatol (2018) 45(9):1053–62. doi: 10.1111/1346-8138.14504
44. Paul C, Griffiths CEM, van de Kerkhof PCM, Puig L, Dutronc Y, Henneges C, et al. Ixekizumab Provides Superior Efficacy Compared With Ustekinumab Over 52 Weeks of Treatment: Results From IXORA-S, a Phase 3 Study. J Am Acad Dermatol (2019) 80(1):70–79.e3. doi: 10.1016/j.jaad.2018.06.039
45. Terui T, Kobayashi S, Okubo Y, Murakami M, Hirose K, Kubo H. Efficacy and Safety of Guselkumab, an Anti-Interleukin 23 Monoclonal Antibody, for Palmoplantar Pustulosis: A Randomized Clinical Trial. JAMA Dermatol (2018) 154(3):309–16. doi: 10.1001/jamadermatol.2017.5937
46. Ferris LK, Ott E, Jiang J, Hong HC, Li S, Han C, et al. Efficacy and Safety of Guselkumab, Administered With a Novel Patient-Controlled Injector (One-Press), for Moderate-to-Severe Psoriasis: Results From the Phase 3 ORION Study. J Dermatolog Treat (2020) 31(2):152–9. doi: 10.1080/09546634.2019.1587145
47. Lee MG, Huang YH, Lee JH, Lee SC, Kim TG, Aw DC, et al. Secukinumab Demonstrates Superior Efficacy and a Faster Response in Clearing Skin in Asian Subjects With Moderate to Severe Plaque Psoriasis Compared With Ustekinumab: Subgroup Analysis From the CLEAR Study. J Dermatol (2019) 46(9):752–8. doi: 10.1111/1346-8138.15004
48. Ohtsuki M, Fujita H, Watanabe M, Suzaki K, Flack M, Huang X, et al. Efficacy and Safety of Risankizumab in Japanese Patients With Moderate to Severe Plaque Psoriasis: Results From the SustaIMM Phase 2/3 Trial. J Dermatol (2019) 46(8):686–94. doi: 10.1111/1346-8138.14941
49. Reich K, Rich P, Maari C, Bissonnette R, Leonardi C, Menter A, et al. Efficacy and Safety of Mirikizumab (LY3074828) in the Treatment of Moderate-to-Severe Plaque Psoriasis: Results From a Randomized Phase II Study. Br J Dermatol (2019) 181(1):88–95. doi: 10.1111/bjd.17628
50. Sandborn WJ, Ferrante M, Bhandari BR, Berliba E, Feagan BG, Hibi T, et al. Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients With Ulcerative Colitis. Gastroenterology (2020) 158(3):537–49.e10. doi: 10.1053/j.gastro.2019.08.043
51. Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med (2019) 381(13):1201–14. doi: 10.1056/NEJMoa1900750
52. Terui T, Kobayashi S, Okubo Y, Murakami M, Zheng R, Morishima H, et al. Efficacy and Safety of Guselkumab in Japanese Patients With Palmoplantar Pustulosis: A Phase 3 Randomized Clinical Trial. JAMA Dermatol (2019) 155(10):1153–61. doi: 10.1001/jamadermatol.2019.1394
53. Blauvelt A, Papp K, Gottlieb A, Jarell A, Reich K, Maari C, et al. A Head-to-Head Comparison of Ixekizumab vs. Guselkumab in Patients With Moderate-to-Severe Plaque Psoriasis: 12-Week Efficacy, Safety and Speed of Response From a Randomized, Double-Blinded Trial. Br J Dermatol (2020) 182(6):1348–58. doi: 10.1111/bjd.19072
54. Gelfand JM, Shin DB, Alavi A, Torigian DA, Werner T, Papadopoulos M, et al. Randomized, Double-Blind, Placebo-Controlled Crossover Study of the Effects of Ustekinumab on Vascular Inflammation in Psoriasis (the VIP-U Trial). J Invest Dermatol (2020) 140(1):85–93.e2. doi: 10.1016/j.jid.2019.07.679
55. Reich K, Warren RB, Iversen L, Puig L, Pau-Charles I, Igarashi A, et al. Long-Term Efficacy and Safety of Tildrakizumab for Moderate-to-Severe Psoriasis: Pooled Analyses of Two Randomized Phase III Clinical Trials (reSURFACE 1 and reSURFACE 2) Through 148 Weeks. Br J Dermatol (2020) 182(3):605–17. doi: 10.1111/bjd.18232
56. Thaçi D, Pinter A, Sebastian M, Termeer C, Sticherling M, Gerdes S, et al. Guselkumab is Superior to Fumaric Acid Esters in Patients With Moderate-to-Severe Plaque Psoriasis Who are Naive to Systemic Treatment: Results From a Randomized, Active-Comparator-Controlled Phase IIIb Trial (POLARIS). Br J Dermatol (2020) 183(2):265–75. doi: 10.1111/bjd.18696
57. Blauvelt A, Leonardi CL, Gooderham M, Papp KA, Philipp S, Wu JJ, et al. Efficacy and Safety of Continuous Risankizumab Therapy vs Treatment Withdrawal in Patients With Moderate to Severe Plaque Psoriasis: A Phase 3 Randomized Clinical Trial. JAMA Dermatol (2020) 156(6):1–11. doi: 10.1001/jamadermatol.2020.0723
58. Stritesky GL, Yeh N, Kaplan MH. IL-23 Promotes Maintenance But Not Commitment to the Th17 Lineage. J Immunol (2008) 181:5948–55. doi: 10.4049/jimmunol.181.9.5948
59. Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 Stimulates Epidermal Hyperplasia Via TNF and IL-20R2-Dependent Mechanisms With Implications for Psoriasis Pathogenesis. J Exp Med (2006) 203(12):2577–87. doi: 10.1084/jem.20060244
60. Hauer AD, Uyttenhove C, De Vos P, Stroobant V, Renauld JC, van Berkel TJ, et al. Blockade of Interleukin-12 Function by Protein Vaccination Attenuates Atherosclerosis. Circulation (2005) 112(7):1054–62. doi: 10.1161/CIRCULATIONAHA.104.533463
61. Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal Role of Dermal IL-17-producing γδ T Cells in Skin Inflammation. Immunity (2011) 35(4):596–610. doi: 10.1016/j.immuni.2011.08.001
62. McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 Immune Pathway. Trends Immunol (2006) 27(1):17–23. doi: 10.1016/j.it.2005.10.003
63. Patel D, Kuchroo V. Th17 Cell Pathway in Human Immunity: Lessons From Genetics and Therapeutic Interventions. Immunity (2015) 43(6):1040–51. doi: 10.1016/j.immuni.2015.12.003
64. Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T Cell Trafficking and Development by IFN-Gamma: Mechanism and Pathological Relevance in Psoriasis. J Immunol (2008) 181(7):4733–41. doi: 10.4049/jimmunol.181.7.4733
65. Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 Promotes Tumour Incidence and Growth. Nature (2006) 442(7101):461–5. doi: 10.1038/nature04808
66. Lebwohl MG, Papp KA, Marangell LB, Koo J, Blauvelt A, Gooderham M, et al. Psychiatric Adverse Events During Treatment With Brodalumab: Analysis of Psoriasis Clinical Trials. J Am Acad Dermatol (2018) 78(1):81–89.e5. doi: 10.1016/j.jaad.2017.08.024
67. Schmidt C. Suicidal Thoughts End Amgen’s Blockbuster Aspirations for Psoriasis Drug. Nat Biotechnol (2015) 33(9):894–5. doi: 10.1038/nbt0915-894b
68. Esse S, Mason KJ, Green AC, Warren RB. Melanoma Risk in Patients Treated With Biologic Therapy for Common Inflammatory Diseases: A Systematic Review and Meta-Analysis. JAMA Dermatol (2020) 156(7):1–8. doi: 10.1001/jamadermatol.2020.1300
69. Van den Berghe N, De Keyser E, Soenen R, Meuleman L, Lanssens S, Gils A, et al. Clinical Response Correlates With 4-Week Postinjection Ustekinumab Concentrations in Patients With Moderate-to-Severe Psoriasis. Br J Dermatol (2020) 182(2):390–7. doi: 10.1111/bjd.18016
70. Khalilieh S, Hodsman P, Xu C, Tzontcheva A, Glasgow S, Montgomery D. Pharmacokinetics of Tildrakizumab (MK-3222), An Anti-IL-23 Monoclonal Antibody, After Intravenous or Subcutaneous Administration in Healthy Subjects. Basic Clin Pharmacol Toxicol (2018) 123(3):294–300. doi: 10.1111/bcpt.13001
71. Atalay S, van den Reek JMPA, den Broeder AA, van Vugt LJ, Otero ME, Njoo MD, et al. Comparison of Tightly Controlled Dose Reduction of Biologics With Usual Care for Patients With Psoriasis: A Randomized Clinical Trial. JAMA Dermatol (2020) 156(4):393–400. doi: 10.1001/jamadermatol.2019.4897
Keywords: anti-IL-23, adverse events, meta-analysis, systematic review, biologics
Citation: Ru Y, Ding X, Luo Y, Li H, Sun X, Zhou M, Zhou Y, Kuai L, Xing M, Liu L, Luo Y, Song J, Chen J, Li B and Li X (2021) Adverse Events Associated With Anti-IL-23 Agents: Clinical Evidence and Possible Mechanisms. Front. Immunol. 12:670398. doi: 10.3389/fimmu.2021.670398
Received: 21 February 2021; Accepted: 24 May 2021;
Published: 11 June 2021.
Edited by:
Jian-Ping Liu, Beijing University of Chinese Medicine, ChinaReviewed by:
Saikat Majumder, University of Pittsburgh, United StatesCopyright © 2021 Ru, Ding, Luo, Li, Sun, Zhou, Zhou, Kuai, Xing, Liu, Luo, Song, Chen, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, MTM2NjE5NTYzMjZAMTYzLmNvbQ==; Bin Li, MTg5MzA1NjgxMjlAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.