
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Immunol. , 30 March 2021
Sec. Microbial Immunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.669616
This article is part of the Research Topic Advances in Immunotherapeutic Approaches to Tuberculosis View all 17 articles
This article is a correction to:
Activation of M1 Macrophages in Response to Recombinant TB Vaccines With Enhanced Antimycobacterial Activity
A Corrigendum on
Activation of M1 Macrophages in Response to Recombinant TB Vaccines With Enhanced Antimycobacterial Activity
By Shiu-Ju Yang, Yih-Yuan Chen, Chih-Hao Hsu, Chia-Wei Hsu, Chun-Yu Chang, Jia-Ru Chang, Horng-Yunn Dou (2020) Front. Immunol. 23 June 2020 doi: 10.3389/fimmu.2020.01298
In the original article, there was a mistake in Figure 4G. When analyzing the original data, the authors mistakenly took raw data of the second image of 4G, which caused the third and second images to be duplicated. The corrected Figure 4 appears below.
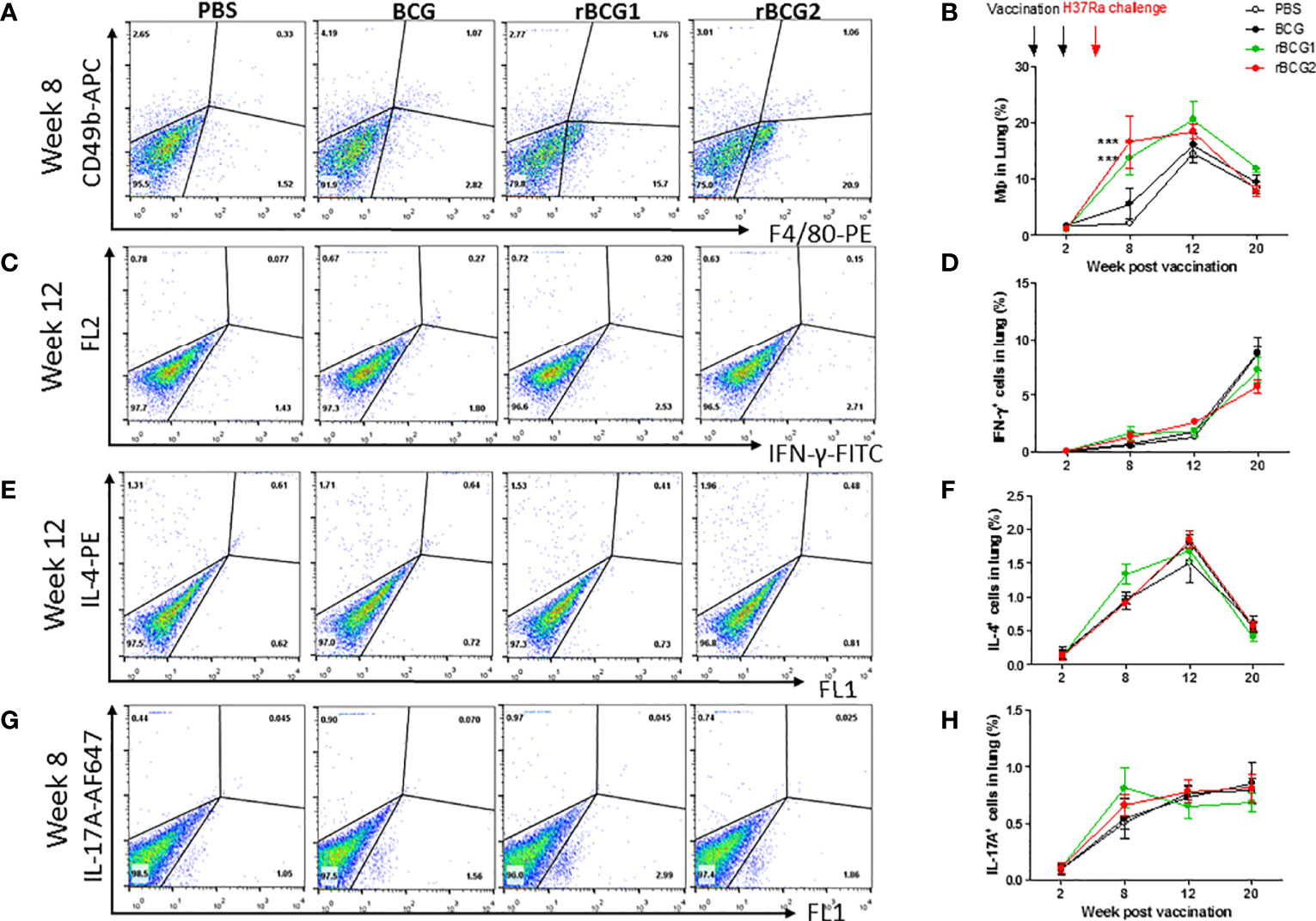
Figure 4 Innate and adaptive immune cell profiles pulsed with tuberculosis-specific peptides from mice immunized with BCG, rBCG1, or rBCG2. C57BL/6 mice were immunized with BCG, rBCG1 or rBCG2 at weeks 0 and 2, and then challenged with H37Ra at week 4 (n = 6 to 7, in two independent experiments). At 2, 8, 12, and 20 weeks, the mice were sacrificed, and lungs were homogenized to single-cell suspensions. The cells were stimulated with tuberculosis-specific TB peptides (described in the Materials and Methods) for 68 h and then Golgi-stop for 4 h, stained for surface and intracellular markers, and then subjected to flow cytometry to determine the percentage of cytokine-producing cells within CD4+ T cells. (A) Percentage of macrophages (F4/80+ cells) in lung post-vaccination at week 8. (B) Percentage of macrophages (F4/80+ cells) in lung post-vaccination at weeks 2 to 20. (C) Percentage of IFN-γ+ cells in lung post-vaccination at week 12. (D) Percentage of IFN-γ+ cells in lung post-vaccination at weeks 2 to 20. (E) Percentage of IL-4+ cells in lung post-vaccination at week 12. (F) Percentage of IL-4+ cells in lung post-vaccination at weeks 2 to 20. (G) Percentage of IL-17+ T cells in lung post-vaccination at week 8. (H) Percentage of IL-17+ T cells in lung post-vaccination at weeks 2 to 20. Differences among groups were determined by one-way or two-way ANOVA with a Tukey or Bonferroni post hoc test (***P < .001).
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Keywords: recombinant Bacille Calmette–Guérin, Mycobacterium tuberculosis, innate immunity, macrophage, vaccine
Citation: Yang S-J, Chen Y-Y, Hsu C-H, Hsu C-W, Chang C-Y, Chang J-R and Dou H-Y (2021) Corrigendum: Activation of M1 Macrophages in Response to Recombinant TB Vaccines With Enhanced Antimycobacterial Activity. Front. Immunol. 12:669616. doi: 10.3389/fimmu.2021.669616
Received: 19 February 2021; Accepted: 16 March 2021;
Published: 30 March 2021.
Edited and reviewed by: Juraj Ivanyi, King’s College London. United Kingdom
Copyright © 2021 Yang, Chen, Hsu, Hsu, Chang, Chang and Dou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Horng-Yunn Dou, aHlkb3VAbmhyaS5lZHUudHc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.