
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 29 April 2021
Sec. Vaccines and Molecular Therapeutics
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.664998
Inflammatory bowel disease (IBD) increases the risk of colorectal cancer, and it has the potential to diminish the quality of life. Recent clinical and experimental evidence demonstrate protective aspects of parasitic helminth infection against IBD. Reports have highlighted the potential use of helminths and their byproducts as potential treatment for IBD. In the current study, we studied the effect of a newborn larvae-specific serine protease from Trichinella spiralis (TsSp) on the host immune and inflammatory responses. A 49-kDa recombinant TsSp (rTsSp) was expressed in Escherichia coli BL21 (DE3) and purified. The cytotoxicity of rTsSp was analyzed. The immune protective effect of rTsSp was studied by using dextran sodium sulfate (DSS)-induced mouse colitis model. The result illustrated that rTsSp has no toxic effects on cells. We further demonstrated that administration of the rTsSp without the additional adjuvant before the induction of DSS-induced colitis reduced the severity of intestinal inflammation and the disease index; it suppressed macrophage infiltration, reduced TNF-α secretion, and induced IL-10 expression. Our findings suggest therapeutic potential of rTsSp on colitis by altering the effect of macrophages. Data also suggest immunotherapy with rTsSp holds promise for use as an additional strategy to positively modulate inflammatory processes involved in IBD.
Inflammatory bowel disease (IBD) is characterized by mucosal damage and ulceration that encompasses Crohn’s disease (CD) and ulcerative colitis (UC) (1). The increasing prevalence of IBD will likely diminish quality of life and increase the risk of colorectal cancer (2, 3). IBD inflammation results from a complex innate and adaptive cytokine network (1). Although the exact cause of IBD remains unknown, past investigations have suggested that the pathogenesis of IBD involve genetic factors, changes in the gut microbiome, and immune pathways including cytokines and immune cells (4).
Evidence from epidemiological studies of the IBD hygiene hypothesis indicate that helminth infections have modulatory effects in certain immune-mediated diseases, including IBD (5). Helminths, characterized by their ability to maintain long-standing, chronic infections in humans, have infected more than 2 billion people worldwide (6).
Helminth infections lead to modulated immune responses with a strong Th2 bias and a major immunoregulatory component, involving the secretion of interleukin (IL)-4, IL-5, IL-9, IL-10 and IL-13, and accompanied by the hyperplasia of mast cells, eosinophils, goblet cells and innate lymphoid cells (ILCs) (7, 8). Also involved is the expansion of both peripheral and thymic regulatory T cells (Tregs) (9). The regulation of parasite-specific immune response affects not only helminth antigens, but also unrelated antigens. In recent years, clinical and experimental evidence has demonstrated that infection with parasitic helminth protects hosts from IBD; for example, a case control study in South Africa demonstrated protective association of childhood helminth infection against the development of IBD (10); pre-existing helminth Echinococcus multilocularis (11), Heligmosomoides polygyrus (12), or Hymenolepis diminuta (13), etc., infection have been shown to reduce chemically-induced colitis in mice by modulating the immune responses of their hosts.
The helminth Trichinella has a unique life cycle: unlike other helminths, all stages in the life cycle of larval and adult occur in the same host (14). The immunomodulatory properties of T. spiralis have been validated in several models of immunopathology, and experiments have shown that Trichinella infection can ameliorate some immuno-mediated disease pathology. For example, T. spiralis infection ameliorates experimental dextran sodium sulfate (DSS)-induced colitis in mice. The amelioration results from downregulation of pro-inflammatory cytokines- IL-6 and interferon gamma (IFN-γ) and upregulation of regulatory cytokines- IL-10 and transforming growth factor (TGF)-β by spleen lymphocytes and mesenteric lymph nodes (MLN). This is also accompanied by increased CD4+CD25+Foxp3+ Treg cell recruitment in spleen lymphocytes and MLN (15). Moreover, the effects of Excretion–Secretion products released by Trichinella in the modulation of experimental colitis have also been described (16).
Although parasitic helminthes and helminth products are regarded as potential therapy for autoimmune and inflammatory disorders in humans (17, 18), treatment with live worms is not a very attractive notion. Unanswered questions regarding the use of helminths in intestinal disease, such as appropriate dosing regimens, optimal timing of treatment, the role of host genetics, diet and environment warrant investigation (18). Moreover, helminth products are composed of a complex mixture of molecules, so it is still unclear on exact mechanisms of their protective effects. Therefore, the individual molecules responsible for the protective effects are to be identified to address these questions. Early study has shown that infection with T. spiralis resulted in the attenuation of experimental colitis in mice (19). One superfamily of enzymatic proteins, serine proteases (SPs), has been found in all stages of the T. spiralis (20). From the previous studies, we know that larvae-specific serine protease of TsSp has a key immunodominant region involved in protective immunity against Trichinella infection in mice by promoting a balance between types 1 and 2 immune responses (21, 22). Here, we examined the effect of treating the female C57BL/6 mice with recombinant T. spiralis newborn larvae-specific Serine protease (rTsSp) on experimentally DSS-induced colitis. Among various chemical induced colitis models, DSS-induced colitis model is widely used because of its simplicity and many similarities with human UC (23). We hypothesized that immunization with T. spiralis derived protein-rTsSp could induce an immuno-regulatory response that reduces the development of colitis in the mice gut.
In the present study, we provide evidence that administration of rTsSp without the addition of adjuvant before the induction of colitis with DSS reduces the severity of intestinal inflammation and the disease index, and results in modulation of macrophage responses associated with regulatory cytokine expression. These observations suggest that the unique helminth derived protein rTsSp is able to ameliorate disease signs in an acute model of colitis via its immunomodulatory properties.
Pathogen-free, 8-week-old female C57BL/6 mice were purchased from the Experimental Animal Center of Henan Province. They were fed autoclaved food and water. The Institutional Subcommittee on Research Animal Care approved all animal experiments.
The TsSp- pET-22b (+) recombinant plasmid was provided by Prof. Mingyuan Liu from Jilin University. Recombinant proteins (rTsSp) were expressed in Escherichia coli BL21 (DE3), purified with Ni-NTA Spin Kit (QIAGEN, Catalog No. 31314), and analyzed with the sodium dodecyl sulfate–poly acrylamide gel electrophoresis (SDS-PAGE) as previous studies (24, 25) and silver staining as the manufacturer’s instruction (Sigma, Product Code PROT-SIL1). The rTsSp concentration was detected by using BCA Protein Assay Reagent (Thermo Fisher Scientific, Catalog No. 23223) and Synergy 2 (Bio-Rad).
Prior to incubation, Raw 264.7 cells were seeded at 106 cells/ml in 12-well cell culture plates overnight. Cells were washed twice with PBS and incubated with 5 μg/ml, 10 μg/ml, and 20 μg/ml rTsSp for 6, 12, and 24 h. On the indicated time, cells were collected. Cell count and viability measurements using trypan blue stain (0.4%, Gibco, REF# 15250-061) were performed by Countess Automated Cell Counter (Invitrogen).
To differentiate between apoptotic and necrotic cells, we used carboxyfluorescein (FAM)- fluorochrome-labeled inhibitors of caspases (FLICA) in vitro Caspase Detection Kit (ImmunoChemistry Technologies’, Catalog # 92) to assess cell death in vitro. Cells labeled with FAM-FLICA can be counter-stained with the red live/dead stains Propidium Iodide (PI, included in FAM-FLICA kits) to distinguish apoptosis from necrosis. Cells at 5 × 105 cells/mL per sample were used for FLICA-PI staining following the manufacturer’s recommendations. 30× FLICA were added at a ratio of 1:30. Cells were incubated at 37°C protected from light for 2 h and then washed by 1 mL 1× apoptosis wash buffer (included in FAM-FLICA kits). To avoid cells from settling on the bottom of the tubes, the tubes were gently resuspended by swirling cells every 20 min to ensure an even distribution of FLICA. The samples were centrifuged at 1200 rpm for 5 min at room temperature to pellet cells, supernatants were aspirated. Cell pellets were resuspended with 0.5% v/v of PI and incubated at RT protected from light for 5 min, then washed two times by 1× apoptosis wash buffer and fixed by Fixative (included in FAM-FLICA kits) at a ratio of 1:10. The stained samples were analyzed with a BD FACSCanto. Debris was excluded from analysis via size exclusion. Four populations of cells were revealed: cells in early apoptosis fluoresce green with FAM-FLICA, cells in late apoptosis are dually stained with FAM-FLICA and PI, necrotic cells fluoresce red and unstained live cells have minimal fluorescence.
Raw 264.7 cells were maintained at 37°C in 5% CO2 in cDMEM. The cells were seeded at 106 cells/ml in 24-well cell culture plates. Cells were pre-treated with/without rTsSp 1 day before or at the same time when stimulated with LPS. After 5/10 hours stimulation, the supernatants were collected, and the levels of TNF-α present were detected via ELISA.
C57BL/6 mice were divided into four groups (1. PBS; 2. PBS-DSS; 3. rTsSp; 4. rTsSp-DSS), immunized three times with intraperitoneal injection at weekly intervals between each injection; three independent experiments were performed (5 mice per group). Groups 3 and 4 were immunized with 50 μg rTsSp in 100 μl phosphate-buffered saline (PBS) without any adjuvant. Groups 1 and 2 were immunized with 100 μl PBS as control. The last injection was performed 1 week before DSS treatment. About 50 μl of tail blood of immunized mice were collected at day 0. The levels of the specific antibodies in serum were determined by ELISA as previous studies (24). Experimental colitis was induced in groups 2 and 4 by administering a 2.5% (w/v) solution of DSS (M.W. 36000–50000 kDa; MP Biomedicals, Cat. no. 106110) to mice as a substitute for autoclaved water for 7 days. Mice were monitored daily for clinical signs including weight loss, piloerection, mobility, and fecal consistency/bleeding. At day 7 post DSS administration, the 2.5% DSS solution was replaced by autoclaved water. After additional 3 days of autoclaved water administration, the mice were sacrificed and samples were obtained and processed (Figure 1).
The colon tissues (from cecum to rectum) were separated after sacrificing mice. Colon length was recorded, a 0.5- to 1-cm colon piece (cecum side) was collected for the measurement of tissue cytokine expression by using real-time qPCR, the rest of the colon tissue was cut longitudinally and rolled with a toothpick for histological assessment of inflammatory infiltration.
At necropsy, colon tissues were collected, formalin-fixed and embedded in paraffin. The process tissues was sectioned into 5-μm-thick slices and stained with hematoxylin and eosin (H&E) to assess the pathology.
The slides were blocked with avidin/biotin agent (Vector Laboratories). To analyze the abundance of infiltrating cells, colon slides were stained with FITC-labeled anti-mouse CD11b (Biolegen, 1:200). Nucleus were stained and mounted using the 4′, 6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). Sections were analyzed by microscopy and the quantification was performed by ImageJ.
Peritoneal macrophages were isolated from normal and rTsSp-immunized mice by incubating total peritoneal cells in complete Dulbecco’s modified Eagle’s medium (cDMEM contains 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, and 1 mM sodium pyruvate) at 37°C for 2 h. Non-adherent cells were removed by washing with PBS, the adherent macrophages were treated with lipopolysaccharides (LPS, 100 ng/ml, Sigma), PBS was used as control. After 5 h, supernatants were collected and analyzed for the levels of TNF-α present via enzyme-linked immunosorbent assay (ELISA).
Intracellular Reactive oxygen species (ROS) generation was measured using 2′,7′-dichlorofluorescin diacetate (DCFDA) Cellular ROS Detection Assay Kit (Abcam, Ab113851) in accordance with the manufacturer’s instructions. Peritoneal cells were collected by washing the peritoneal cavity of wild type (WT) or rTsSp pre immunized mice with ice-cold PBS. First, Peritoneal macrophages were identified with anti-F4/80 (eBioscience) and CD11b (abcom) monoclonal antibody (mAb). Subsequently, cells were incubated with 200 nM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and 20 μM DCFDA at 37°C for 30 min. Cells without PMA treatment were used as controls. The stained samples were analyzed with a BD FACSCanto. Dead cells and debris were excluded from analysis via size exclusion. Results are expressed as fold change of mean fluorescent intensity after background subtraction between control and treated cells. Each experiment was performed in triplicate.
Total RNA prepared from colon tissues was reverse transcribed into cDNA. cDNA was synthesized by using 1 μg total RNA. The cDNA samples were then tested for the expression of IL-10 (F, CCACAAAGCAGCCTTGCA; R, AGTAAGAGCAGGCAGCATAGCA) and TNF-α (F, CCCTCACACTCAGATCATCTTCT; R, GCTACGACGTGGGCTACAG) by real-time quantitative PCR using SYBR Green PCR Master Mix (Apex Bioresearch Product) on a 7500 Fast Real-Time PCR system (Applied Biosystems). GAPDH (F, GGTTGTCTCCTGCGACTTCA; R, TGGTCCAGGG TTTCTTACTCC) was used as the housekeeping control. The gene expression level was normalized by subtracting the expression level of GAPDH of the same group, and the different expression levels were calculated using the comparative Ct (2-ΔΔCt) method.
All results were expressed as the mean ± SEM. Statistical differences were determined using a two-tailed Student t test or One-way ANOVA with SPSS 21.0 software. A P value <0.05 was considered significant.
The cDNA encoding full-length TsSp is 1600 bp, encodes 430 amino acids with a predicted molecular weight of 49 kDa. Recombinant proteins expressed in Escherichia coli BL21 (DE3) and purified using Ni-NTA Spin Kit. The SDS-PAGE and silver staining (Supplementary Figure 1) analysis showed that an individual protein band with a molecular weight around 49 kDa was observed (Figure 2A) indicating the recombinant TsSp (rTsSp).
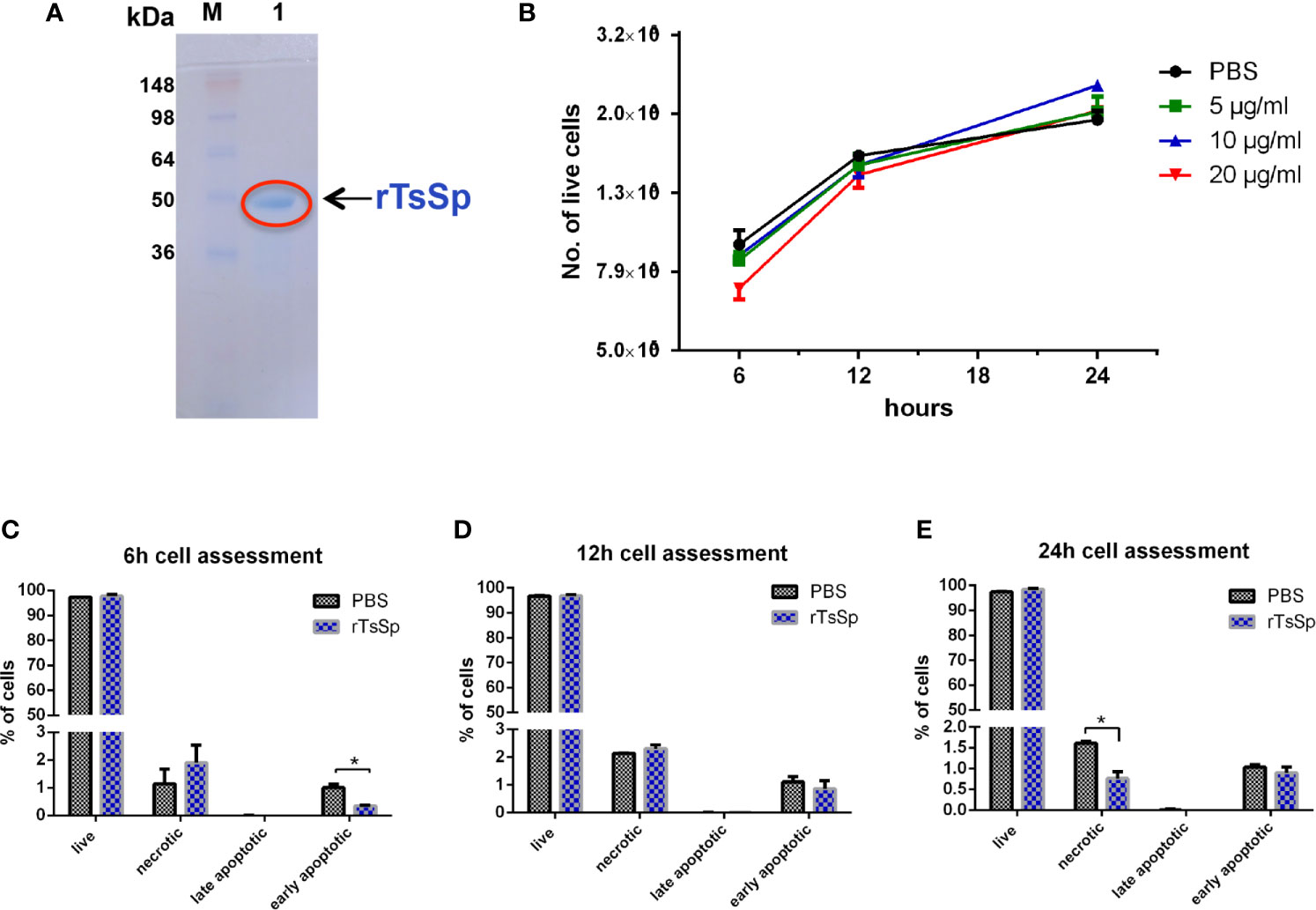
Figure 2 Cytotoxicity assay for the rTsSp. (A) SDS-PAGE analysis of rTsSp protein. M: protein molecular weight marker; 1: rTsSp purified by Ni-NTA Spin Kit. (B) Cell viability testing with trypan blue exclusion: Raw264.7 cells were treated with different concentrations of the rTsSp in different time points, then collected and analyzed using Countess Automated Cell Counter. (C–E) FAM-FLICA in vitro caspase detection: Raw264.7 cells were treated with 10 μg/ml rTsSp in different time points, then collected and stained with fluorochrome-labeled inhibitors of caspases (FLICA) and Propidium Iodide (PI). Stained Cells were analyzed on the BD FACSCanto. Four populations of cells were detected: FLICA-PI- indicated unstained live cells; FLICA-PI+ necrotic cells; FLICA+PI+ cells in late apoptosis; FLICA+PI- cells in early apoptosis. The data shown are means ± SEM from three separate experiments. *P < 0.05.
Trypan blue is a stain that leaves nonviable cells with a distinctive blue color, while viable cells appear unstained. To determine if there was any cytotoxicity from rTsSp, we did cell viability testing with trypan blue exclusion. Raw 264.7 cells were incubated with 5 μg/ml, 10 μg/ml, and 20 μg/ml rTsSp for 6, 12, and 24 h. On the indicated time, cells were collected and the number of the live cells were counted. Results showed live cells increased with time in both the absence and presence of rTsSp, thus indicating that rTsSp had no influence on the cell viability (Figure 2B). The cell viability testing with trypan blue exclusion is a method that tests the membrane integrity of the cells, it cannot differentiate whether the cells are truly nonviable or just damaged a little bit. To differentiate between apoptotic and necrotic cells, we used FAM-FLICA in vitro Caspase Detection Kit to assess cell death by detecting apoptosis in vitro. Cells labeled with FAM-FLICA can be counter-stained with the red live/dead stains Propidium Iodide (PI) to distinguish apoptosis from necrosis. The fluorescence-activated cell sorting (FACS) data showed that the percent of early apoptosis cells was decreased in cells incubated with rTsSp when compared to the control cells at the time point of 6 h (Figure 2C), and the percent of necrotic cells decreased in cells incubated with rTsSp in a statistically significant manner when compared to control cells at the time point of 24 h (Figure 2D). However, the overall percent of unstained live cells in different time points did not appear to be either diminished or amplified in cells incubated with rTsSp when compared to control cells incubated with PBS (Figures 2C–E). These results demonstrated that rTsSp had no toxic effects on cells, in fact, it suppressed early apoptosis and necrosis.
To analyze the effect of rTsSp on macrophage responses, the TNF-α production from Raw 264.7 cells was evaluated in vitro. Specifically, Raw 264.7 cells were pre-treated with/without rTsSp 1 day before or at the same time when the cells were stimulated with LPS. After 5- or 10-h stimulation, the supernatants were collected to detect the cytokine by ELISA (Figure 3A). Results showed that LPS-treatment induced a marked increase in macrophage TNF-α production. A significant reduction of LPS-induced TNF-α production was detected in cells that were pre-treated with rTsSp. Cells treated with rTsSp alone did not induce TNF-α production (Figures 3B, C).
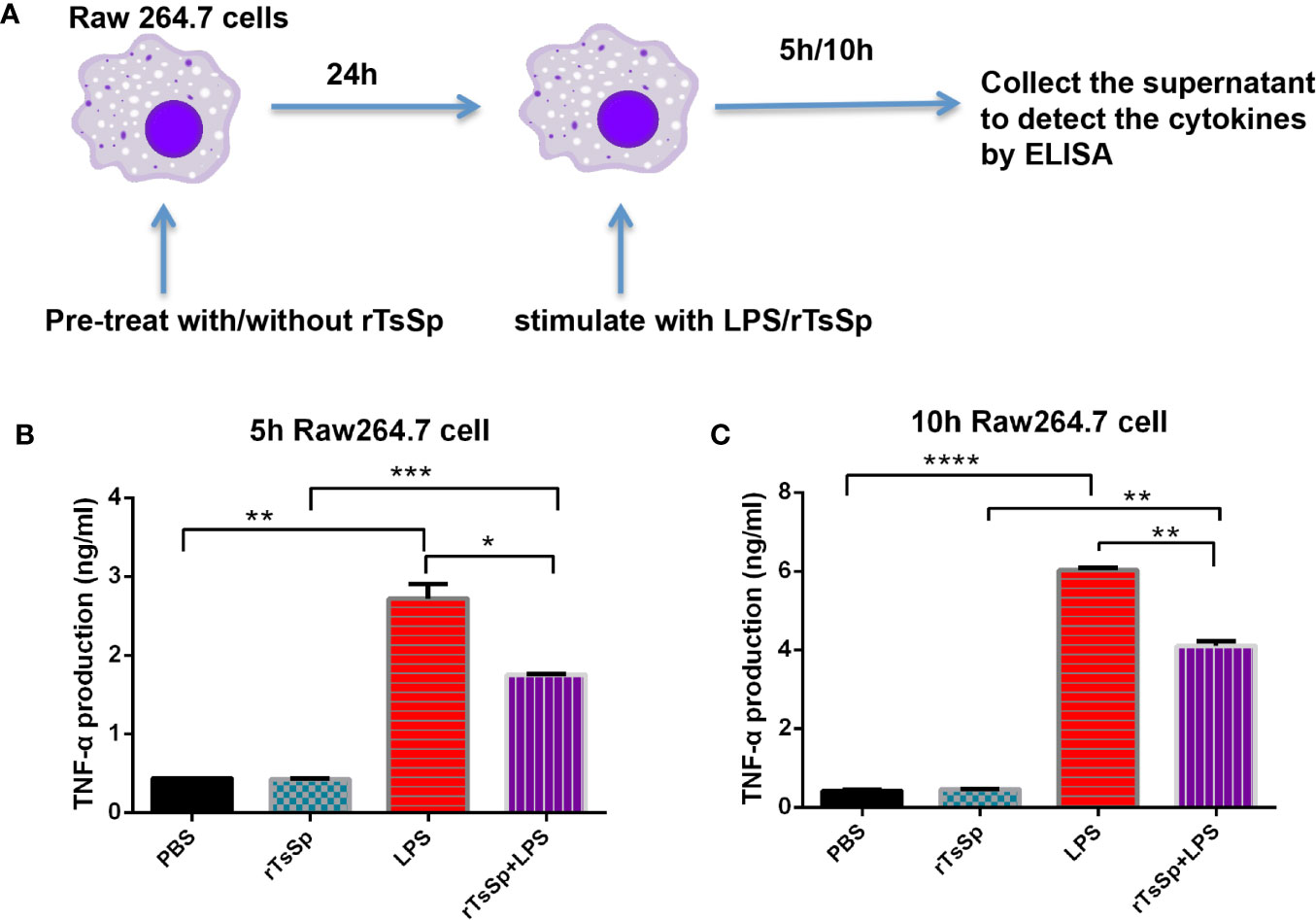
Figure 3 rTsSp reduced TNF-α production by macrophages. (A) Raw 264.7 cells were pre-treated with/without rTsSp 1 day before or at the same time when stimulated with LPS; after 5 or 10 h stimulated, the supernatant was collected; the TNF-α production of the supernatants of (B) 5 h and (C) 10 h were detected by ELISA. The data shown are means ± SEM from one of three separate experiments performed showing similar results. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
DSS acute colitis model was used to assess whether rTsSp was able to produce the modulation of intestinal inflammation. The mice were immunized with rTsSp three times, prior to DSS administration. Anti-rTsSP specific antibody levels in mice immunized with rTsSP increased following the immunization (Supplementary Figure 2). As expected, the DSS-positive control group exhibited visible signs of inflammation characterized by a hunched back, weight loss, piloerection, inactivity, and displayed important macroscopic and histological lesions, while pre-administration of rTsSp led to a significant decrease in weight loss and disease symptoms in DSS colitis. PBS and rTsSp-immunized mice showed no weight loss or clinical disease symptoms. Mice treated with DSS showed weight loss starting on day 5 after DSS administration; previous immunization with rTsSp showed weight loss starting on day 7 after DSS administration, and resulted in a statistically relevant decrease in body weight loss when compared to DSS positive control group (Figure 4A). The body weights of mice in the PBS control and rTsSp immunization alone groups remained stable. Further, to confirm that rTsSp was a particular T. spiralis protein that had the immune protective effects in mouse colitis model, we observed the body weight change of mice from another recombinant protein (a newborn larvae-specific cysteine protease inhibitor from Trichinella spiralis, with a predicted molecular weight of 46.9 kDa, expressed in pET-22b (+) plasmid and purified in our lab) pre-administration-DSS group or protein non-derived from helminth pre-administration-DSS group, and found that there was no immune protective effect stemming from those proteins (Supplementary Figure 3). When the mice were sacrificed, we separated the colon tissues and measured the length, found that colons were thinner and shorter in mice treated with DSS alone, compared to those of control and rTsSp-immunized mice (Figures 4B, C). Histological examination of the colon showed that DSS administration (positive control) induced significant colonic damage as characterized by epithelial erosion, ulceration, crypt abscess, edema, loss of the mucus layer and substantial polymorphonuclear infiltrate into the lamina propria (Figure 4D), and rTsSp pre-immunization significantly prevented the damage in the colons produced by DSS. PBS control and rTsSp immunization alone did not result in microscopic damage of the colon (Figure 4D). These results demonstrate that rTsSp pre-immunization suppressed clinical signs in DSS-induced colitis in mice.
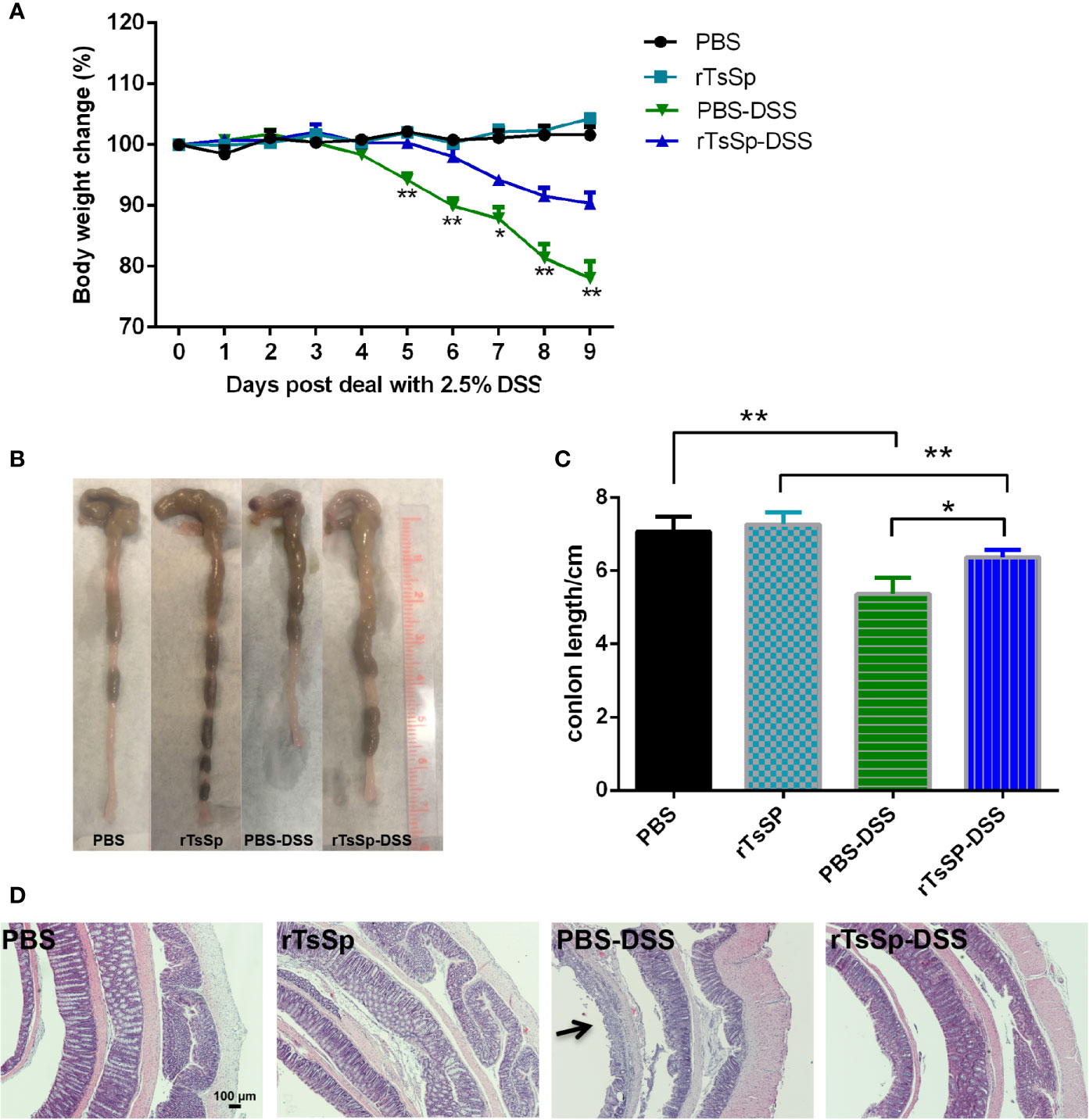
Figure 4 Immunization of the unique Trichinella-derived protein-rTsSp prevents DSS-induced colitis in mice. (A) Weight change in percent, (B) macroscopic characteristics of the colon, (C) colon length and (D) H&E stain of colonic sections were evaluated. The data shown are means ± SEM (5 mice per group) from one of three separate experiments performed showing similar results. *P < 0.05, **P < 0.01.
F4/80 (indicates macrophages) was detected to assess the macrophages in colon tissues. F4/80 was labeled red, and nuclei were stained with DAPI. DSS treatment resulted in statistically significant quantities of F4/80 positive macrophages in both the absence and presence of the rTsSp pre immunization when compared to their respective controls, PBS or rTsSp pre immunization alone (Figure 5A). Meanwhile, there was a statistically significant increase in the quantity of macrophages in the colon of DSS-treated mice when compared to the mice in the concurrent presence of rTsSp pre immunization and DSS treatment (Figure 5B). These results indicated that pre immunization with rTsSp reduced macrophage recruitment to the DSS induced colitis of the colon.
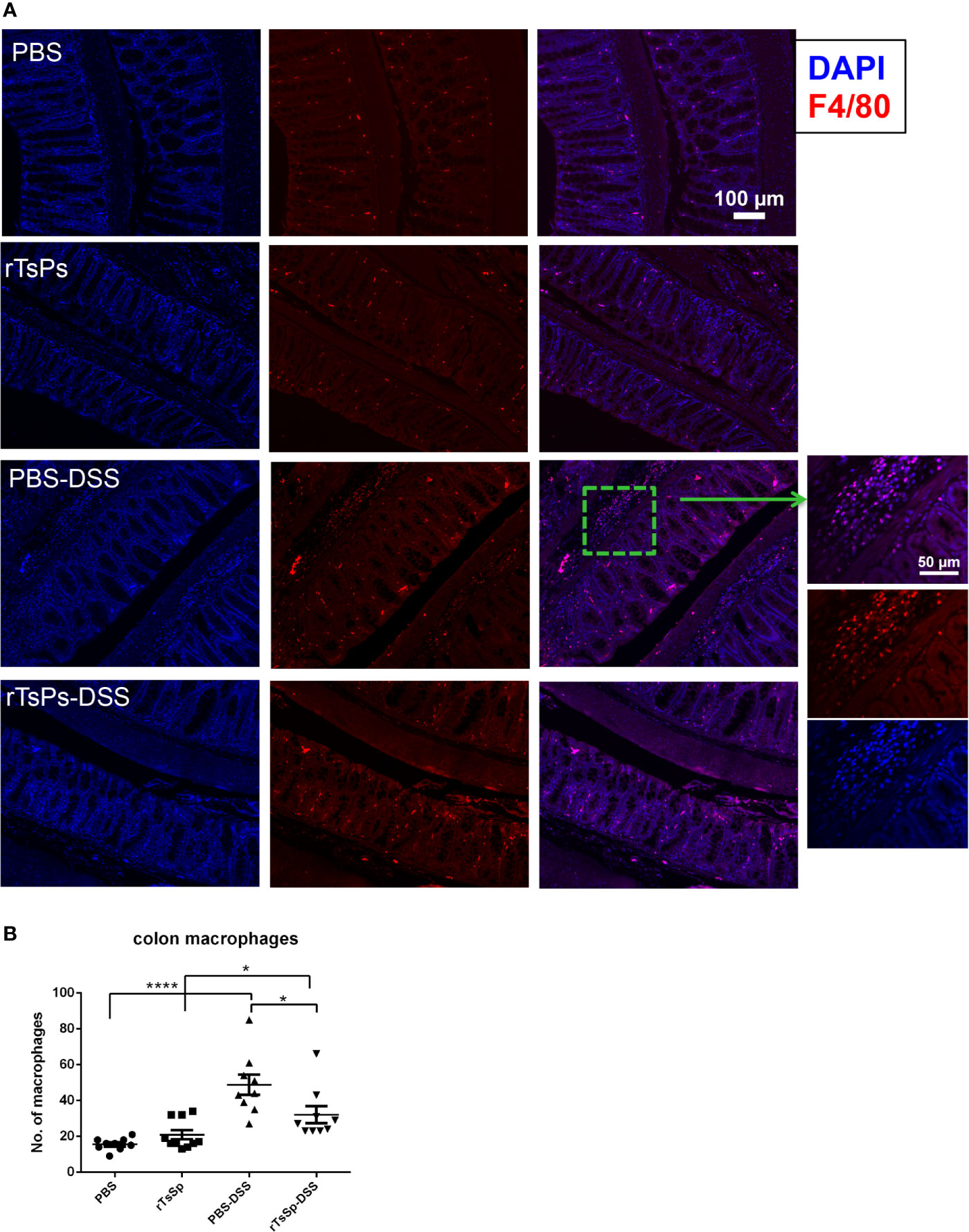
Figure 5 Pre immunization with rTsSp reduces macrophages recruitment to the DSS induced colitis of the colon. (A) Colon slides from PBS control, rTsSp pre immunization, DSS treatment, rTsSp pre immunization and DSS treatment mice were stained with anti-F4/80 antibody for macrophages. The number of F4/80+ cells (B) detected in each high power field was determined by counting 10 fields from each sample (samples from three mice per group were counted) using the Image J software. The data shown are means ± SEM. *P < 0.05, ****P < 0.0001.
To further analyze the possible association of the effect of rTsSp on mouse innate immune cells, we separated peritoneal macrophages, identified the cells, and analyzed the production of intracellular ROS and TNF-α. First, the mice were immunized with rTsSp (3 times, 7 days/time), PBS intraperitoneal injection was used as control. 21 days later, peritoneal macrophages were isolated from control and rTsSp-immunized mice. The cells were identified with anti-F4/80 and CD11b mAb, and the result showed that pre immunization with rTsSp resulted in a statistically relevant decrease in the population of F4/80+CD11b+ macrophages present in the peritoneal cavity when compared to WT control (Figure 6A). The DCFDA Cellular ROS Detection Assay Kit was used to detect the impact of rTsSp immunization on the intracellular ROS production of peritoneal macrophages. Cells were treated with PMA and stained in the DCFDA solution. The result showed that rTsSp pre immunization did not appear to have an impact on peritoneal macrophage ROS production (Figure 6B). For detecting TNF-α production, the cells were stimulated with LPS/PBS, after 5 h incubation, supernatants were collected and the levels of TNF-α production were analyzed. As shown in Figure 6C, intraperitoneal immunization with rTsSp resulted in suppression of TNF-α production. Significantly high levels of TNF-α production were detected from LPS stimulated peritoneal macrophage isolated from control mice, compared to the PBS stimulated, and both of PBS and LPS incubated peritoneal macrophages isolated from rTsSp immunized mice. These results demonstrated that rTsSp treatment reduced the population of F4/80+CD11b+ macrophages in the peritoneal cavity and suggested that rTsSp had the potential for modulating macrophage response.
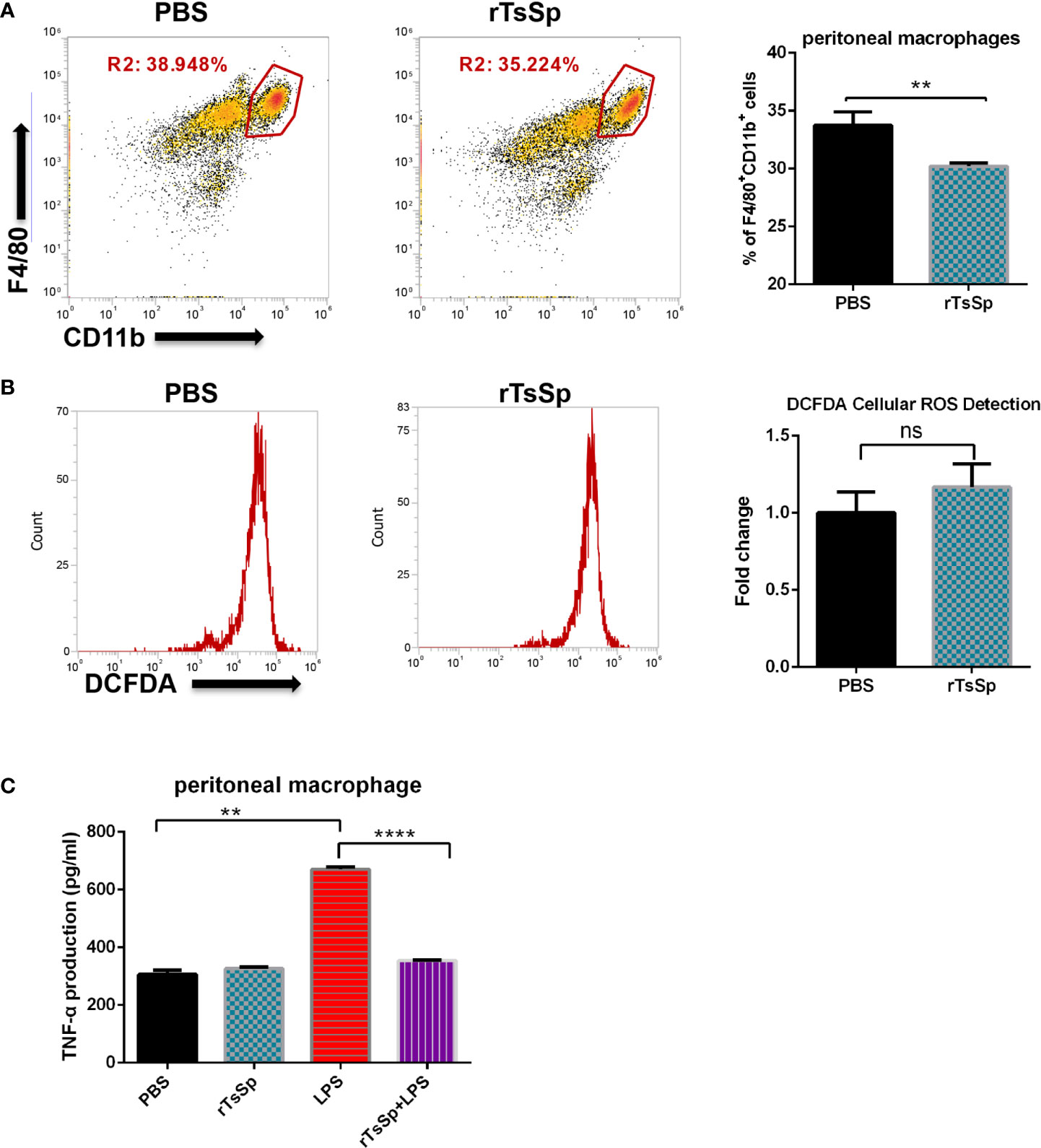
Figure 6 The effect of rTsSp on peritoneal macrophage population, ROS and TNF-α production. (A) Peritoneal macrophages were isolated from wild type and rTsSp pre-treated mice, identified with anti-F4/80 and CD11b mAb. (B) Intracellular ROS production was measured using the DCFDA. The stained samples were analyzed with BD FACSCanto. Results are expressed as fold change of mean fluorescent intensity after background subtraction between control and treated cells. (C) Immunization with rTsSp results in suppression of the TNF-α production by peritoneal macrophages. Peritoneal macrophages were isolated from PBS control and rTsSp-immunized mice and stimulated with LPS/PBS. The data shown are means ± SEM from one of three separate experiments performed showing similar results. **P < 0.01; ****P < 0.0001; ns, no significant.
To investigate the impact of rTsSp on the inflammatory response, the expression of IL-10 and TNF-α was detected using the real-time quantitative RT-PCR. The results showed that rTsSp pre-treatment reduced the colon tissue TNF-α expression of mice with DSS induced colitis (Figure 7A). In contrast, the rTsSp pre-treatment increased IL-10 expression in the colon tissues of mice with DSS induced colitis (Figure 7B).
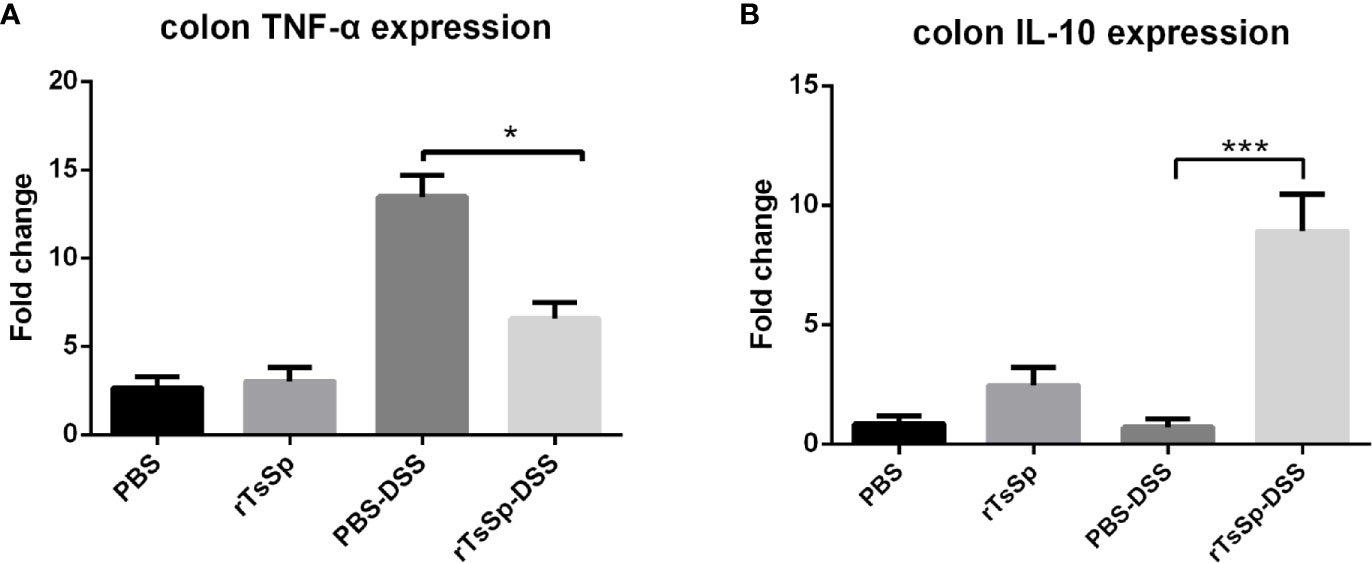
Figure 7 Prior rTsSp treatment has differential impact on inflammatory mediators. Colon tissues were collected. Total RNA was isolated. IL-10 and TNF-α expression was determined using RT-qPCR. Values are the relative expression compared to the PBS control group. The data shown are means ± SD from three independent experiments. *P < 0.05, ***P < 0.001.
Millions of people worldwide currently suffer from parasitic helminth infections. The long-lasting infections of the helminth parasites result in the manipulation of the host immune responses causing serious health implications among people (26, 27). The immunomodulatory properties of T. spiralis have been validated in several models. Infection with living T. spiralis ameliorates experimental DSS-induced colitis or acetic acid-induced colitis in mice. T. spiralis muscle larval ES-treated dendritic cells ameliorates TNBS- induced colitis in mice (16).
Among various chemically induced colitis models, DSS- induced colitis model is widely used because of its simplicity (23) and most closely resembles human UC (28), which is clearly a global disease as its incidence is rising in nations around the world (29). Like UC, the mechanism by which DSS induces intestinal inflammation is also unclear. An important caveat pertaining to DSS colitis is that T and B cells are not required for development of colitis (23). Hence, the acute DSS colitis model is particularly useful when studying the contribution of the innate immune system to the development of intestinal inflammation.
Several helminthes, helminth extracts or helminth derived individual molecules, including recombinant proteins from Ascaris lumbricoides (30), Wuchereria Bancroft (31), Brugia malayi (32, 33), attenuate colitis in DSS models, by inducing anti-inflammatory cytokine-IL-10 and TGF-β expression and downregulating pro-inflammatory- IFN-γ, IL-6, IL-17 and TNF-α cytokines expression by colonic tissue/splenocytes. However, the cell types directly responsible for protective effects of the helminth protein in colitis have not been definitively resolved. In our present study, we expressed and purified the rTsSp and demonstrated that rTsSp has no toxic effects by cytotoxicity assay. Additionally in vitro TNF-α production by Raw 264.7 cells pre-incubated with rTsSp was reduced. We found that immunization of mice with rTsSp significantly attenuated macroscopical and histological features of DSS-induced colonic inflammation that were accompanied by decreased macrophage recruitment in the colon and reduced TNF-α mRNA levels in the colon; in contrast, the expression level of IL-10 increased in the colon tissue.
Macrophages are important in the host’s immunological and inflammatory responses. The intestine contains the largest pool of macrophages in the body, which are essential for maintaining mucosal homeostasis but are also involved in the pathology of IBD (34). The resident intestinal macrophages cannot be easily induced to mediate acute inflammatory responses. When there is perturbation of homeostasis, the composition of the intestinal macrophage pool changes considerably. In the mucosa of Crohn’s disease and ulcerative colitis patients, there is an increase in the mucosal macrophage population derived from circulating monocytes; these cells have the ability to produce many mediators that are important in the proinflammatory responses, such as IL-1, IL-6, TNF-α, reactive metabolites of oxygen and nitrogen and proteases, and the expression of iNOS (34–38). Colitis pathology has been shown to be ameliorated in mice deficient (39) or depleted (40) of these cells. Thus, the ability to reduce the monocyte/macrophage recruitment to the inflammation location is the protective mechanism of the rTsSp in the DSS-induced colitis model.
It is well known that the cytokine responses are the key pathophysiological elements that govern the inflammation of IBD. TNF-α is an important mediator of inflammation; it plays a critical role in the pathogenesis of IBD. Studies have shown that systemic TNF-α level is elevated in DSS-induced colitis (41, 42) and in IBD patients (43). Varol C. et al. (44) reported that TNF-α production by recruited macrophages appears to be central to disease progression, as the severity of DSS-induced colitis is reduced in mice in which Ly6Chi monocytes are deficient in TNF-α production. And therapeutic monoclonal antibodies against TNF (anti-TNF therapy) are emerging as a common treatment for acute severe UC (45). In the present study, the TNF-α production by peritoneal macrophages of rTsSp immunized mice was reduced. IL-10 is a pre-dominantly anti-inflammatory cytokine with an essential role in maintaining gastrointestinal homeostasis, which is produced by hematopoietically derived cells, including T cells, B cells, dendritic cells, and macrophages (46), and potently inhibits production of most inducible chemokines that are involved in inflammation (47). Tomoyose et al. (48) reported that administration of systemic IL-10 ameliorates colitis in mice by inhibiting intestinal inflammation and suppressing TNF-α and IL-1β production. In our results, mice immunized with rTsSp produced high level of IL-10 in the presence of DSS administration. Toll-like receptors (TLRs) are essential components of the innate immune system. Almost all TLRs (except TLR3) signal via a MyD88-dependent signaling pathway (49). Previous research findings have shown that helminth and helminth-associated protein up-regulated the expression of TLR2 in mice (50, 51), and ligands recognized by TLR2 stimulated Th2 or Treg responses and IL-10 production (51–53). The rTsSp may carry the function of reducing the production of TNF-α and inducing the expression of IL-10 by stimulating the TLR2/MyD88 signaling pathway. However, our present data are not sufficient to confirm whether the induction by rTsSp is due to stimulation of TLR2 on APC, which will require further analysis.
In summary, our data indicate that immunization with the individual recombinant protein of T. spiralis- rTsSp ameliorates the DSS-induced colitis in mice by decreasing the infiltration of macrophages, reducing the production of TNF-α and inducing the expression of IL-10. These findings suggest that rTsSp may have therapeutic potential on colitis, and that immunotherapy with rTsSp represents an additional strategy to improve inflammatory processes of IBD.
The unique helminth derived protein- rTsSp is able to ameliorate disease signs in an acute model of colitis via its immunomodulatory properties, which suggests that rTsSp have therapeutic potential on colitis, and immunotherapy with rTsSp represents an additional strategy to improve inflammatory processes of IBD.
The data sets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, AY491941.
The animal study was reviewed and approved by The Life Science Ethics Committee of Zhengzhou University.
SL and RL performed experiments. SL, ZW, and C-WS designed experiments. SL and DK analyzed data and wrote the paper. ZW, C-WS, and DK participated in editing and provided conceptual advice. All authors contributed to the article and approved the submitted version.
This work was supported by grants from National Key Research and Development Program of China (2017YFD0501302) and China Postdoctoral Science Foundation (2020M672287).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Prof. Mingyuan Liu from Jilin University for providing recombinant plasmid.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.664998/full#supplementary-material
IBD, inflammatory bowel disease; rTsSp, recombinant T. spiralis newborn larvae-specific Serine protease; DSS, dextran sodium sulfate; MLN, mesenteric lymph nodes; TNF-α, tumor necrosis factor alpha; IL-10, interleukin-10; UC, ulcerative colitis; ROS, reactive oxygen species; PMA, phorbol 12-myristate 13-acetate; LPS, lipopolysaccharide; ELISA, enzyme-linked immunosorbent assay; PBS, phosphate-buffered saline; cDMEM, complete Dulbecco’s modified Eagle’s medium; DCFDA, 2′,7′-Dichlorofluorescin Diacetate; WT, wild type; DAPI, 4′, 6-diamidino- 2-phenylindole; PI, Propidium Iodide; FLICA, fluorochrome-labeled inhibitors of caspases.
1. Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and Adaptive Immunity in Inflammatory Bowel Disease. Autoimmun Rev (2014) 13(1):3–10. doi: 10.1016/j.autrev.2013.06.004
2. Ananthakrishnan AN. Epidemiology and Risk Factors for IBD. Nat Rev Gastroenterol Hepatol (2015) 12(4):205–17. doi: 10.1038/nrgastro.2015.34
3. Subramanya SB, Chandran S, Almarzooqi S, Raj V, Al Zahmi AS, Al Katheeri RA, et al. Frondanol, a Nutraceutical Extract From Cucumaria frondosa, Attenuates Colonic Inflammation in a DSS-induced Colitis Model in Mice. Mar Drugs (2018) 16(5):148. doi: 10.3390/md16050148
4. Lee SH, Kwon JE, Cho ML. Immunological Pathogenesis of Inflammatory Bowel Disease. Intest Res (2018) 16(1):26–42. doi: 10.5217/ir.2018.16.1.26
5. Weinstock JV, Elliott DE. Helminths and the IBD Hygiene Hypothesis. Inflammation Bowel Dis (2009) 15(1):128–33. doi: 10.1002/ibd.20633
6. Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth Infections: The Great Neglected Tropical Diseases. J Clin Invest (2008) 118(4):1311–21. doi: 10.1172/JCI34261
7. Allen JE, Maizels RM. Diversity and Dialogue in Immunity to Helminths. Nat Rev Immunol (2011) 11(6):375–88. doi: 10.1038/nri2992
8. Grencis RK. Immunity to Helminths: Resistance, Regulation, and Susceptibility to Gastrointestinal Nematodes. Annu Rev Immunol (2015) 33:201–25. doi: 10.1146/annurev-immunol-032713-120218
9. Metenou S, Nutman TB. Regulatory T Cell Subsets in Filarial Infection and Their Function. Front Immunol (2013) 4:305. doi: 10.3389/fimmu.2013.00305
10. Chu KM, Watermeyer G, Shelly L, Janssen J, May TD, Brink K, et al. Childhood Helminth Exposure is Protective Against Inflammatory Bowel Disease: A Case Control Study in South Africa. Inflammation Bowel Dis (2013) 19(3):614–20. doi: 10.1097/MIB.0b013e31827f27f4
11. Wang J, Goepfert C, Mueller N, Piersigilli A, Lin R, Wen H, et al. Larval Echinococcus Multilocularis Infection Reduces Dextran Sulphate Sodium-Induced Colitis in Mice by Attenuating T Helper Type 1/Type 17-Mediated Immune Reactions. Immunology (2018) 154(1):76–88. doi: 10.1111/imm.12860
12. Hang L, Blum AM, Setiawan T, Urban JP Jr, Stoyanoff KM, Weinstock JV. Heligmosomoides polygyrus bakeri Infection Activates Colonic Foxp3+ T Cells Enhancing Their Capacity to Prevent Colitis. J Immunol (2013) 191(4):1927–34. doi: 10.4049/jimmunol.1201457
13. Wang A, Arai T, Campbell A, Reyes JL, Lopes F, McKay DM. Triggering Immunological Memory Against the Tapeworm Hymenolepis diminuta to Protect Against Colitis. Parasite Immunol (2017) 39(11):e12490. doi: 10.1111/pim.12490
14. Bruschi F, Chiumiento L. Immunomodulation in Trichinellosis: Does Trichinella Really Escape the Host Immune System? Endocr Metab Immune Disord Drug Targets (2012) 12(1):4–15. doi: 10.2174/187153012799279081
15. Cho MK, Park MK, Kang SA, Choi SH, Ahn SC, Yu HS. Trichinella spiralis Infection Suppressed Gut Inflammation With CD4(+)CD25(+)Foxp3(+) T Cell Recruitment. Korean J Parasitol (2012) 50(4):385–90. doi: 10.3347/kjp.2012.50.4.385
16. Jin X, Yang Y, Bai X, Shi H, Zhang W, Zhang Z, et al. Dendritic Cells Treated by Trichinella spiralis Muscle Larval Excretory/Secretory Products Alleviate TNBS-induced Colitis in Mice. Int Immunopharmacol (2019) 70:378–86. doi: 10.1016/j.intimp.2019.02.028
17. Helmby H. Human Helminth Therapy to Treat Inflammatory Disorders - Where do We Stand? BMC Immunol (2015) 16:12. doi: 10.1186/s12865-015-0074-3
18. Sipahi AM, Baptista DM. Helminths as an Alternative Therapy for Intestinal Diseases. World J Gastroenterol (2017) 23(33):6009–15. doi: 10.3748/wjg.v23.i33.6009
19. Khan WI, Blennerhasset PA, Varghese AK, Chowdhury SK, Omsted P, Deng Y, et al. Intestinal Nematode Infection Ameliorates Experimental Colitis in Mice. Infect Immun (2002) 70(11):5931–7. doi: 10.1128/IAI.70.11.5931-5937.2002
20. Yang Y, Wen Y, Cai YN, Vallée I, Boireau P, Liu MY, et al. Serine Proteases of Parasitic Helminths. Korean J Parasitol (2015) 53(1):1–11. doi: 10.3347/kjp.2015.53.1.1
21. Liu MY, Wang XL, Fu BQ, Li CY, Wu XP, Le Rhun D, et al. Identification of Stage-Specifically Expressed Genes of Trichinella spiralis by Suppression Subtractive Hybridization. Parasitology (2007) 134(Pt 10):1443–55. doi: 10.1017/S0031182007002855
22. Xu J, Bai X, Wang LB, Shi HN, Van Der Giessen JWB, Boireau P, et al. Immune Responses in Mice Vaccinated With a DNA Vaccine Expressing Serine Protease-Like Protein From the New-Born Larval Stage of Trichinella spiralis. Parasitology (2017) 144(6):712–9. doi: 10.1017/S0031182016002493
23. Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr Protoc Immunol (2014) 104:15.25.11–15.25.14. doi: 10.1002/0471142735.im1525s104
24. Long SR, Wang ZQ, Liu RD, Liu LN, Li LG, Jiang P, et al. Molecular Identification of Trichinella spiralis Nudix Hydrolase and its Induced Protective Immunity Against Trichinellosis in BALB/c Mice. Parasit Vectors (2014) 7:600. doi: 10.1186/s13071-014-0600-9
25. Song YY, Wang LA, Na Ren H, Qi X, Sun GG, Liu RD, et al. Cloning, Expression and Characterisation of a Cysteine Protease From Trichinella spiralis. Folia Parasitol (Praha) (2018) 65:007. doi: 10.14411/fp.2018.007
26. Elliott DE, Weinstock JV. Helminth-Host Immunological Interactions: Prevention and Control of Immune-Mediated Diseases. Ann N Y Acad Sci (2012) 1247:83–96. doi: 10.1111/j.1749-6632.2011.06292.x
27. Weinstock JV, Elliott DE. Helminth Infections Decrease Host Susceptibility to Immune-Mediated Diseases. J Immunol (2014) 193(7):3239–47. doi: 10.4049/jimmunol.1400927
28. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A Novel Method in the Induction of Reliable Experimental Acute and Chronic Ulcerative Colitis in Mice. Gastroenterology (1990) 98(3):694–702. doi: 10.1016/0016-5085(90)90290-H
29. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology (2012) 142(1):46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001
30. Coronado S, Barrios L, Zakzuk J, Regino R, Ahumada V, Franco L, et al. A Recombinant Cystatin From Ascaris lumbricoides Attenuates Inflammation of DSS-induced Colitis. Parasite Immunol (2017) 39(4):e12425. doi: 10.1111/pim.12425
31. Togre N, Bhoj P, Amdare N, Goswami K, Tarnekar A, Shende M. Immunomodulatory Potential of Recombinant Filarial Protein, rWbL2, and its Therapeutic Implication in Experimental Ulcerative Colitis in Mouse. Immunopharmacol Immunotoxicol (2018) 40(6):483–90. doi: 10.1080/08923973.2018.1431925
32. Khatri V, Amdare N, Tarnekar A, Goswami K, Reddy MV. Brugia malayi Cystatin Therapeutically Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice. J Dig Dis (2015) 16(10):585–94. doi: 10.1111/1751-2980.12290
33. Khatri V, Amdare N, Yadav RS, Tarnekar A, Goswami K, Reddy MV. Brugia malayi Abundant Larval Transcript 2 Protein Treatment Attenuates Experimentally-Induced Colitis in Mice. Indian J Exp Biol (2015) 53(11):732–9.
34. Bain CC, Mowat AM. Macrophages in Intestinal Homeostasis and Inflammation. Immunol Rev (2014) 260(1):102–17. doi: 10.1111/imr.12192
35. Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and Pro-Inflammatory Macrophages in the Colon Represent Alternative Context-Dependent Fates of the Same Ly6Chi Monocyte Precursors. Mucosal Immunol (2013) 6(3):498–510. doi: 10.1038/mi.2012.89
36. Mahida YR. The Key Role of Macrophages in the Immunopathogenesis of Inflammatory Bowel Disease. Inflamm Bowel Dis (2000) 6(1):21–33. doi: 10.1097/00054725-200002000-00004
37. Rugtveit J, Nilsen EM, Bakka A, Carlsen H, Brandtzaeg P, Scott H. Cytokine Profiles Differ in Newly Recruited and Resident Subsets of Mucosal Macrophages From Inflammatory Bowel Disease. Gastroenterology (1997) 112(5):1493–505. doi: 10.1016/S0016-5085(97)70030-1
38. Thiesen S, Janciauskiene S, Uronen-Hansson H, Agace W, Högerkorp CM, Spee P, et al. CD14(Hi)HLA-DR(dim) Macrophages, With a Resemblance to Classical Blood Monocytes, Dominate Inflamed Mucosa in Crohn’s Disease. J Leukoc Biol (2014) 95(3):531–41. doi: 10.1189/jlb.0113021
39. Platt AM, Bain CC, Bordon Y, Sester DP, Mowat AM. An Independent Subset of TLR Expressing CCR2-dependent Macrophages Promotes Colonic Inflammation. J Immunol (2010) 184(12):6843–54. doi: 10.4049/jimmunol.0903987
40. Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, et al. Ly6C Hi Monocytes in the Inflamed Colon Give Rise to Proinflammatory Effector Cells and Migratory Antigen-Presenting Cells. Immunity (2012) 37(6):1076–90. doi: 10.1016/j.immuni.2012.08.026
41. Ali MN, Choijookhuu N, Takagi H, Srisowanna N, Nguyen Nhat Huynh M, Yamaguchi Y, et al. The HDAC Inhibitor, SAHA, Prevents Colonic Inflammation by Suppressing Pro-Inflammatory Cytokines and Chemokines in DSS-induced Colitis. Acta Histochem Cytochem (2018) 51(1):33–40. doi: 10.1267/ahc.17033
42. Yue B, Ren YJ, Zhang JJ, Luo XP, Yu ZL, Ren GY, et al. Anti-Inflammatory Effects of Fargesin on Chemically Induced Inflammatory Bowel Disease in Mice. Molecules (2018) 23(6):1380. doi: 10.3390/molecules23061380
43. Singh UP, Singh NP, Murphy EA, Price RL, Fayad R, Nagarkatti M, et al. Chemokine and Cytokine Levels in Inflammatory Bowel Disease Patients. Cytokine (2016) 77:44–9. doi: 10.1016/j.cyto.2015.10.008
44. Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, et al. Intestinal Lamina Propria Dendritic Cell Subsets Have Different Origin and Functions. Immunity (2009) 31(3):502–12. doi: 10.1016/j.immuni.2009.06.025
45. Rosen MJ, Minar P, Vinks AA. Review Article: Applying Pharmacokinetics to Optimise Dosing of anti-TNF Biologics in Acute Severe Ulcerative Colitis. Aliment Pharmacol Ther (2015) 41(11):1094–103. doi: 10.1111/apt.13175
46. Li B, Alli R, Vogel P, Geiger TL. Il-10 Modulates DSS-induced Colitis Through a macrophage-ROS-NO Axis. Mucosal Immunol (2014) 7(4):869–78. doi: 10.1038/mi.2013.103
47. Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 Receptor. Annu Rev Immunol (2001) 19:683–765. doi: 10.1146/annurev.immunol.19.1.683
48. Tomoyose M, Mitsuyama K, Ishida H, Toyonaga A, Tanikawa K. Role of interleukin-10 in a Murine Model of Dextran Sulfate Sodium-Induced Colitis. Scand J Gastroenterol (1998) 33(4):435–40. doi: 10.1080/00365529850171080
49. Kwissa M, Kasturi SP, Pulendran B. The Science of Adjuvants. Expert Rev Vaccines (2007) 6(5):673–84. doi: 10.1586/14760584.6.5.673
50. Wojtkowiak-Giera A, Derda M, Wandurska-Nowak E, Jagodziński PP, Kolasa-Wołosiuk A, Kosik-Bogacka D, et al. Changes in the Expression of TLR2 During the Intestinal Phase of Trichinellosis. J Vet Res (2020) 64(2):269–74. doi: 10.2478/jvetres-2020-0027
51. Wang X, Zhou S, Chi Y, Wen X, Hoellwarth J, He L, et al. Cd4+Cd25+ Treg Induction by an HSP60-derived Peptide SJMHE1 From Schistosoma japonicum is TLR2 Dependent. Eur J Immunol (2009) 39(11):3052–65. doi: 10.1002/eji.200939335
52. Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, et al. A Toll-like Receptor 2 Ligand Stimulates Th2 Responses In Vivo, Via Induction of Extracellular Signal-Regulated Kinase Mitogen-Activated Protein Kinase and c-Fos in Dendritic Cells. J Immunol (2004) 172(8):4733–43. doi: 10.4049/jimmunol.172.8.4733
53. Alameddine J, Godefroy E, Papargyris L, Sarrabayrouse G, Tabiasco J, Bridonneau C, et al. Faecalibacterium prausnitzii Skews Human DC to Prime IL10-Producing T Cells Through TLR2/6/JNK Signaling and IL-10, Il-27, CD39, and IDO-1 Induction. Front Immunol (2019) 10:143. doi: 10.3389/fimmu.2019.00143
Keywords: Trichinella spiralis, colitis, dextran sodium sulfate, macrophage, immune protection
Citation: Long SR, Liu RD, Kumar DV, Wang ZQ and Su C-W (2021) Immune Protection of a Helminth Protein in the DSS-Induced Colitis Model in Mice. Front. Immunol. 12:664998. doi: 10.3389/fimmu.2021.664998
Received: 06 February 2021; Accepted: 08 April 2021;
Published: 29 April 2021.
Edited by:
Bertrand Kaeffer, Le nouvel Institut national de recherche sur l’agriculture, L’alimentation et L’environnement en France INRAE, FranceReviewed by:
Bernd Lepenies, University of Veterinary Medicine Hannover, GermanyCopyright © 2021 Long, Liu, Kumar, Wang and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chien-Wen Su, Q1NVMkBtZ2guaGFydmFyZC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.