- 1Department of Epidemiology, Erasmus University Medical Center, Rotterdam, Netherlands
- 2Department of Internal Medicine, Division of Clinical Immunology, Erasmus University Medical Center, Rotterdam, Netherlands
- 3Department of Internal Medicine, Division of Nephrology, Erasmus University Medical Center, Rotterdam, Netherlands
- 4Department of Internal Medicine, Division of Endocrinology, Erasmus University Medical Center, Rotterdam, Netherlands
- 5Department of Immunology, Erasmus University Medical Center, Rotterdam, Netherlands
Background: An up-to-date overview of determinants of serum immunoglobulins in adults is pivotal for clinical practice and research, but currently lacking. We therefore performed a systematic review and meta-analysis to identify determinants of serum immunoglobulin levels.
Methods: Embase, Web of Science, Medline, Cochrane, and Google Scholar were searched from inception to July 11th, 2019 for articles reporting on determinants of serum immunoglobulin A, G or M (IgA, IgG or IgM) in adult humans. Random and fixed effect models were applied to obtain pooled mean differences (MDs) and 95% confidence intervals (CIs) for the association of age and sex with serum immunoglobulins.
Results: We retrieved 117 articles reporting on determinants of serum immunoglobulins, of which 28 could be meta-analyzed. Older compared to younger individuals had higher IgA (MD: 0.38; CI: 0.18 – 0.58), but lower IgM levels (MD: -0.40; 95%: -0.66 – -0.14). Men had higher IgA (MD: 0.22; CI: 0.03 – 0.42), but lower IgM levels (MD: -0.21; CI: -0.32 – -0.10) than women. Age and sex did not influence IgG. Caucasian ethnicity was associated with lower IgA, IgG, and IgM. Smoking and corticosteroid use were associated with lower IgG. Positive associations were reported of probiotics with IgG, alcohol with IgA, hypertension with IgA and IgG, and acute psychological stress with IgA, IgG, and IgM.
Conclusions: Older age and male sex are associated with higher IgA, but lower IgM, and urge investigation of age- and sex-specific reference ranges of immunoglobulins. Other identified determinants were ethnicity, diet, lifestyle and cardio-metabolic factors.
Introduction
Serum immunoglobulins are part of the adaptive immune system and comprise five classes, including immunoglobulin A, G, and M (IgA, IgG, and IgM). IgM provides a rapid immune response and is involved in tissue homeostasis, whereas IgG and IgA are long-lasting high-affinity antibodies, the latter mainly providing mucosal immunity (1). Immunoglobulin measurements are used for diagnosis and monitoring of various diseases, including primary immunodeficiencies and autoimmune diseases. Reference ranges of immunoglobulins are based on the 2.5th and 97.5th percentiles in healthy adults. However, several potential determinants of serum immunoglobulins, including age and sex, are not generally considered in the interpretation of immunoglobulin levels.
Aging is associated with an increased ratio of memory to naive B-cells (2), which may lead to lower IgM and higher IgA and IgG levels in older compared to younger individuals (3). Furthermore, previous population-based studies have demonstrated lower IgG (4, 5) and IgM (4), but higher IgA (4, 5) levels in men compared to women. Among others, body mass index (BMI) and lifestyle related factors such as alcohol consumption and smoking may impact serum immunoglobulin levels as well (6, 7).
However, studies performed thus far have various limitations, limiting interpretability for the general population. Most studies were cross-sectional (3–7), had a small sample size (3, 5), did not adjust for possible confounders (4, 5, 7), or had conflicting results (3, 6, 7). The last overview of factors possibly influencing serum immunoglobulin levels dates back to 1976 (8). However, this review only described a limited number of determinants, was not performed in a systematic manner, and additional literature has been published since.
In this systematic review and meta-analysis, we aim to provide an overview of determinants of serum IgA, IgG, and IgM for adequate interpretation of immunoglobulin levels in clinical practice. This could aid in defining different reference ranges for certain populations, thus changing the universal cut-off, which is currently applied to all adults. Our overview of determinants can furthermore facilitate selection of potential confounders and mediators in immunoglobulin-related research.
Methods
This systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We have provided the PRISMA checklist in Supplementary Table S1.
Search and Eligibility Criteria
We searched Embase, Web of Science, Medline, Cochrane, and Google Scholar from inception to July 11th, 2019 with the help of the Erasmus MC medical library, for articles reporting on the association between specific determinants (i.e. factors that influence) and serum levels of IgA, IgG, or IgM in adult human beings. The definition of a determinant was deliberately kept broad and encompassed a variety of factors, such as demographic features, lifestyle related factors, and interventions. No language or date restrictions were applied during the search.
We included all study types with the exception of case series, case reports, and conference papers. Relevant reviews were included to screen the reference list for potential additional articles of interest. We excluded articles performed solely in a specific patient population (e.g. studies that correlated specific diseases or treatments to serum immunoglobulin levels), genetic or family studies, studies conducted in pregnant or lactating women, and studies focusing on rare occupational exposures as determinant, as these would limit extrapolation of results to the general adult population. A detailed search strategy and full in- and exclusion criteria can be found in the Supplementary Material.
Study Selection, Data Extraction, and Quality Assessment
Titles and abstracts of the retrieved articles were screened in Endnote based on predefined in- and exclusion criteria (Supplementary Material). Similarly, full text articles were screened and if full texts were not available, we contacted first authors. We used a predefined data extraction form to extract relevant information of included studies on study design and setting, participants, included determinants, immunoglobulin assessment and serum levels, and study quality. For cross-sectional studies an adapted version of the Newcastle Ottawa Scale was used, as previously described by Modesti et al. (9). The Cochrane risk of bias tool was implemented for (non)randomized controlled clinical trials (RCTs), whereas we used the NIH quality assessment tool for before-after studies (10, 11). Screening and extraction were performed by two independent reviewers (SRK, ACB) and discussed with a third reviewer (LC) in case of disagreement.
Meta-Analyses
When ≥2 comparable studies assessed the association of a certain determinant with immunoglobulins, and when means and standard deviations (SDs) were provided or could be calculated and converted to grams per liter (g/l) based on the given information, we included these in subsequent meta-analyses. Both random (DerSimonian-Laird) and fixed effect models were used to pool mean differences (MDs) and 95% confidence intervals (CIs), and the random effect models were reported as main results. Pooled results were shown in forest plots, and an I2 statistic was calculated for heterogeneity. Publication bias was assessed through funnel plots and the Egger test. All analyses were performed in R [metacont and metafor packages, R-project, The R Foundation for Statistical Computing (2019), version 3.5.3].
Sensitivity and Stratified Analyses
We performed predefined sensitivity analyses by excluding outliers in the funnel plots and stratification analyses by mean publication year, ethnicity, World Health Organization (WHO) region, and older vs younger age groups provided that these were possible based on the retrieved information.
Results
Study Selection
We identified 9742 records after removing duplicates and added 16 articles identified through the references of retrieved reviews. Of these, 226 were eligible for full text screening. Finally, 117 articles were included in the systematic review and 28 could be meta-analyzed (Supplementary Figure S1).
Study Characteristics
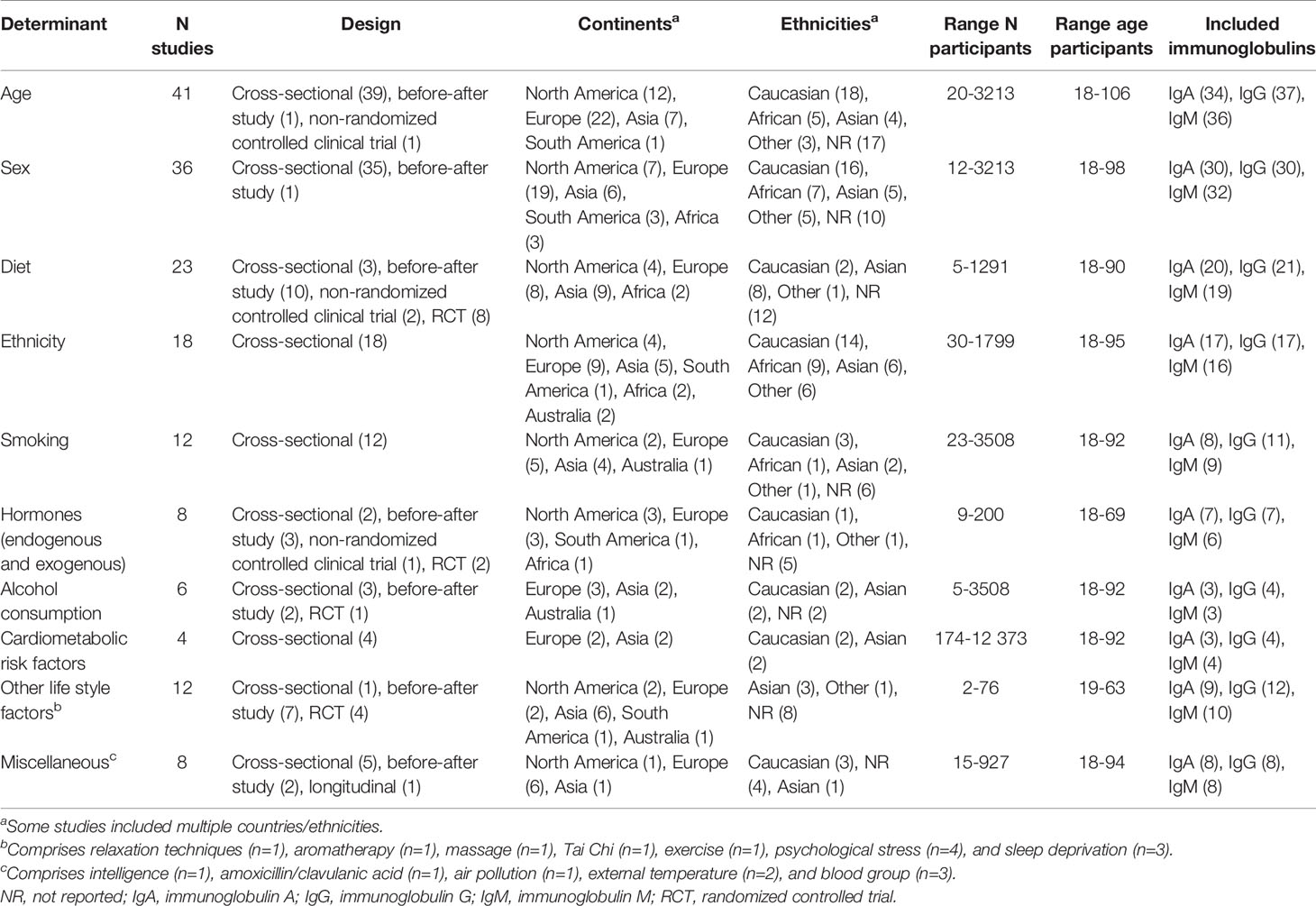
The included 117 articles were published between 1966 and 2019, with sample sizes ranging between 2 and 12 373 and mean age ranging between 21 and 74 years. Participants were either randomly drawn from the community, blood donors, or university/hospital employees, although population source was not always reported. Most studies were cross-sectional, 18 were RCTs, and 24 were before-after studies. Included studies were performed in Europe (n=48), Asia (n=28), North-America (n=25), Australia (n=5), Africa (n=4), and South-America (n=3). Remaining studies combined geographical sites (Table 1). Most studies assessed the association of age or sex with serum immunoglobulin levels. Other determinants included diet, ethnicity, smoking, alcohol consumption, cardio-metabolic risk factors, and other lifestyle related factors, with most studies including multiple determinants. A summary of characteristics of the included studies is provided in Table 1 and a complete overview including quality scores is provided in Supplementary Table S2.
Association of Age and Sex with Serum Immunoglobulins
Age was included in 41 of the identified studies (4, 6, 12–50). Studies compared means or medians with SDs/ranges between older and younger age groups, or provided a correlation coefficient/beta for the relationship between age and immunoglobulins. Studies used different cut-off values for their younger (19 to 54 years) and older (40 to >100 years) age groups. Some studies employed multiple age groups with varying intervals (4, 19, 21, 23, 25, 30, 33, 39, 40, 43, 46, 49).
Most studies reported higher serum IgA in the older compared to younger individuals (4, 6, 13–16, 20, 22, 23, 25–28, 33, 36, 39, 40, 42, 43, 45–49). Results were heterogeneous for IgG, with 19 studies not reporting an association between age and IgG (14–18, 20, 22, 26, 28, 31–34, 38, 41, 44–46, 50), and 15 studies reporting higher IgG levels (4, 6, 12, 25, 27, 30, 35–37, 40, 42, 43, 47–49) in older compared to younger individuals. Overall, no association was found between age and serum IgM (6, 14–18, 20, 25, 26, 28, 30, 31, 35, 36, 44–46, 48, 49).
Thirty-six articles assessed differences in serum immunoglobulin levels between men and women (4–6, 13, 15, 16, 18, 19, 24, 26, 28, 31, 33–35, 39, 40, 42–44, 48, 50–64). Most studies did not report an association of sex with IgA or IgG (16, 18, 26, 28, 31, 33, 35, 39, 43, 44, 48, 51–54, 56–64). Most studies reported lower IgM levels in men compared to women (6, 15, 19, 24, 28, 31, 33–35, 39, 40, 42, 43, 48, 54, 57, 60, 61).
Twenty-eight studies reporting on age and/or sex were suitable for inclusion in the meta-analysis (Supplementary Figure S1).
Association of Diet With Serum Immunoglobulins
The association of a dietary determinant with serum immunoglobulin levels was assessed in 23 studies. The majority reported on supplementation of a micro-/macronutrient or probiotic (22, 29, 65–80), and few on nutritional status or fasting in relation to immunoglobulin levels (56, 81–84).
Five studies assessed the association of probiotics with serum immunoglobulins and generally found higher immunoglobulin levels (mostly IgG) after probiotic use compared to baseline (Supplementary Table S2) (71–73, 78, 80). Ascorbate (vitamin C) supplementation did not affect serum IgA and IgG, however one study reported an increase in serum IgM (29, 66, 74, 77). Ramadan fasting was associated with lower IgG levels compared to the preceding month (83, 84). Most dietary components were not or positively associated with immunoglobulin levels (Supplementary Table S2) (22, 67–69, 79). Consumption of Lycium Barbarum juice (65), resistant corn starch (75), or saffron tablets (70) was associated with higher IgG levels, whereas saffron supplementation and roots of North American ginseng were associated with lower IgM and IgA levels respectively (70, 76). Three observational studies assessed the relation between dietary components and serum immunoglobulins (56, 81, 82) and only established a positive correlation of dietary energy and carbohydrates with IgA (81), and a negative association between 25-hydroxyvitamin D levels and IgA (82).
Association of Ethnicity With Serum Immunoglobulins
Eighteen studies described the influence of ethnicity on serum immunoglobulin levels (17, 28, 33, 42, 45, 47, 50, 55, 59, 63, 85–92). Caucasians had lower immunoglobulin levels than Africans, Asians, Amazonians, or Melanesians (17, 33, 42, 50, 59, 63, 86–89, 91, 92). An Afghan study reported higher immunoglobulin levels in the Hazaras compared to other tribes (85). Two studies compared immunoglobulin levels between inhabitants of large Asian (28) or European (45) cities, but did not find any differences. One study compared mean immunoglobulin levels between inhabitants of various cities throughout the world and found highest IgG and IgM levels in Nigeria, and lowest IgM levels in Mexico city (90). Two studies reported different immunoglobulin levels in various ethnic groups stratified by sex (Supplementary Table S2) (47, 55).
Association of Smoking With Serum Immunoglobulins
Twelve studies assessed the association of smoking with serum immunoglobulin levels (6, 51, 89, 93–101). The definition of smoking was self-reported and heterogeneous (Supplementary Table S2).
Three studies reported lower IgA levels in smokers compared to controls (98, 100) or compared to secondhand smokers (97). Five studies did not report an association of smoking with IgA (6, 51, 93, 99, 101). Seven studies reported lower IgG levels in smokers compared to non-smokers (6, 89, 94, 95, 98–100) or compared to ex-smokers (94), regardless of the number of daily cigarettes smoked and smoking duration (89). Although nicotine replacement therapy was associated with lower IgG levels than smokeless tobacco, there were no differences between these and the non-nicotine using control group (101). Three studies did not report an association between smoking and IgG (61, 93, 97). Seven studies did not find an association between smoking and serum IgM (6, 51, 93, 97, 99–101), whereas two studies reported lower IgM levels in smokers compared to non-smokers (96, 98).
Association of Alcohol With Serum Immunoglobulins
The relation between alcohol consumption and serum immunoglobulins was described in six studies (6, 95, 96, 102–104). A positive association was found between alcohol consumption and IgA levels (6, 102, 103). Two studies established lower IgG levels in drinkers compared to non-drinkers (6, 95), one study found no association (104), and another study reported higher IgG levels after alcohol consumption compared to abstention (103). Results for IgM were heterogeneous, with alcohol consumption being associated with lower (96) or higher (103) serum IgM, or not having an effect at all (6).
Association of Hormones With Serum Immunoglobulins
Eight studies assessed the association of hormones, either endogenous or exogenous (predominantly contraceptives or corticosteroids) with immunoglobulin levels (15, 53, 105–110).
The association of contraceptives with serum immunoglobulins was heterogeneous (Supplementary Table S2) (15, 108, 109). Menstrual phase did not affect serum immunoglobulins (53). Dehydroepiandrosterone (DHEA) did not affect immunoglobulin levels either (107). Oral corticosteroids were associated with lower IgG levels (105, 110), and a longer treatment duration led to a slower recovery of serum IgG afterwards (105). The prostaglandin E1 analog misoprostol did not change serum immunoglobulin levels (106).
Association of Cardio-Metabolic Risk Factors With Serum Immunoglobulins
Four studies described the association of cardio-metabolic risk factors (blood pressure and anthropometric measures such as weight and BMI) with serum immunoglobulin levels (6, 18, 56, 111). Although different definitions of high blood pressure were applied (Supplementary Table S2), high compared to normal blood pressure was generally associated with higher IgA and IgG levels (6, 111) and no difference in IgM levels (6, 18). Two studies reported on the association of various anthropometric measures with serum immunoglobulins (Supplementary Table S2) and found positive associations of obesity with IgA and IgG (6), abdominal obesity with IgA (6), and triceps skinfold thickness with IgM (56).
Association of Other Lifestyle Factors With Serum Immunoglobulins
Twelve studies described among others, the influence of physical activity, psychological stress, or sleep (112–123). Psychological stress, either due to blood donation (117) or a university examination (118, 119), was associated with increased levels of IgA (117–119), IgG, and IgM (117, 119). Furthermore, a positive association was established between job strain and serum IgG (120). Sleep deprivation (SD) was associated with increased serum IgA, IgG, and IgM levels in one study (121), while no differences were observed in another study (122), and serum IgA even decreased during rapid eye movement SD (REM-SD) in a third study (123). While various relaxation techniques and tai chi increased all serum immunoglobulin levels (112, 115), and an increase in IgA and IgG was seen after combined aerobic and resistance exercise respectively a full body Swedish massage (114, 116), aromatherapy did not influence serum immunoglobulins (113).
Association of Miscellaneous Determinants With Serum Immunoglobulins
Eight studies included determinants that could not be combined into demographic or lifestyle related groups (35, 44, 46, 58, 81, 94, 124, 125). Three studies described the influence of a hematological factor on serum immunoglobulins and found a positive association between transferrin and IgM (81), and lower IgA levels in HLA-B8 DR+ compared to HLA-B8 DR- subjects (35), while no association was found between AB0 blood group and immunoglobulins (44). Two studies investigated the influence of outside temperature and concluded that serum IgA and IgM were lowest in samples that were stored at -20C for three months compared to fresh sera or sera stored for three or four weeks (46), while sauna heat exposure increased immunoglobulin levels (58). Although mean immunoglobulin levels were comparable between inhabitants of city areas with different degrees of air pollution, IgA levels of ≥300 mg/dl (≥3.0 g/l) were more prevalent in the more polluted areas (94). Two weeks after treatment with the antibiotic amoxicillin/clavulanic acid, serum IgG was lower compared to the level two weeks before start of treatment (124). Intelligence, as measured by the Wechsler adult intelligence scale (WAIS) score was negatively associated with serum IgG, even after adjustment for age, sex, and race (125).
Meta-Analyses
Due to the large amount of heterogeneity in the definition of included determinants or a limited number of studies investigating a certain determinant, we could only meta-analyze results for age and sex.
Pooled Association of Age With Immunoglobulins
Nineteen studies reporting on age were included in the meta-analysis. We included 13 studies that reported MDs in serum immunoglobulins in older versus younger individuals (12, 14–16, 18–20, 29, 30, 32, 37, 38, 50). To ensure minimum overlap between the older and younger age groups as defined in the included studies, we employed a cut-off of 45 years. Estimates of multiple age groups were combined into overall estimates for the older and younger age groups. In addition, we separately meta-analyzed the results of six other studies that provided correlation coefficients for age and serum immunoglobulins (22, 26, 31, 35, 39, 45).
Older individuals had higher IgA (pooled MD: 0.38; 95% CI: 0.18 – 0.58), but lower IgM levels (pooled MD: -0.40; 95% CI: -0.66 – -0.14) compared to the younger individuals. There was a trend for lower IgG levels (pooled MD: -0.30; 95% CI: -2.00 – 1.40), but this only reached significance in the fixed-effect model (pooled MD: -2.10; 95% CI: -2.36 – -1.84) (Figure 1). There was a substantial amount of heterogeneity for IgG (I2: 97%) and IgM (I2: 95%), and in lesser extent for IgA (I2: 71%) as well. The fixed-effect meta-analyses for a pooled correlation coefficient for age and serum immunoglobulins, yielded comparable results. We found a positive correlation of age with IgA, no correlation with IgG, and a negative correlation with IgM (Supplementary Figure S2).
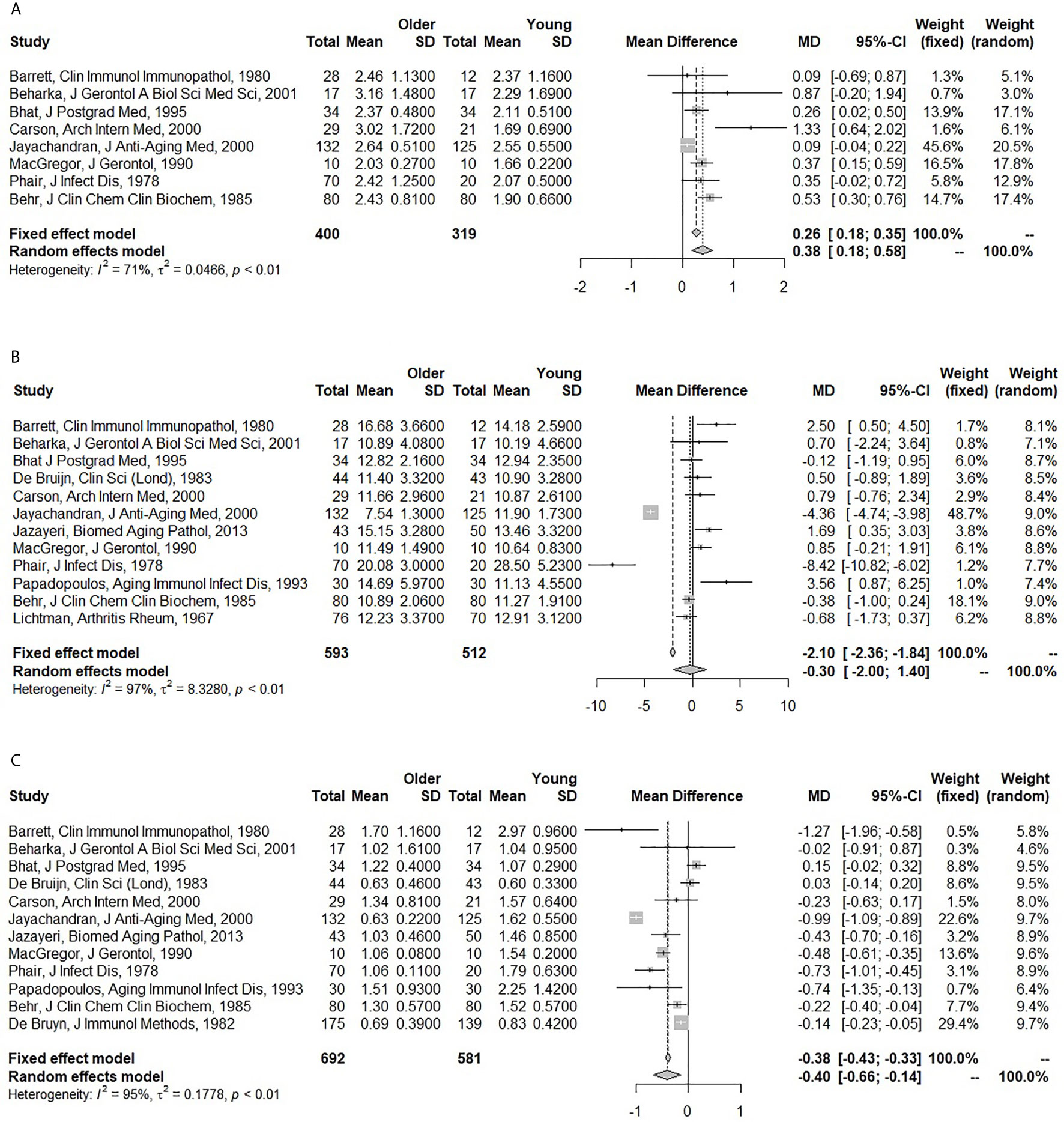
Figure 1 Forest plots for the association between age and serum immunoglobulin levels. (A) Association of age with serum immunoglobulin A (IgA) (g/l). (B) Association of age with serum immunoglobulin G (IgG) (g/l). (C) Association of age with serum immunoglobulin M (IgM) (g/l). The closed squares with horizontal lines depict the mean differences in serum immunoglobulin levels between older (≥45 years) and young (<45 years) subjects with 95% confidence intervals. The diamonds depict the pooled mean differences between the older and young age groups. The random effect model was taken as primary model.
Pooled Association of Sex With Immunoglobulins
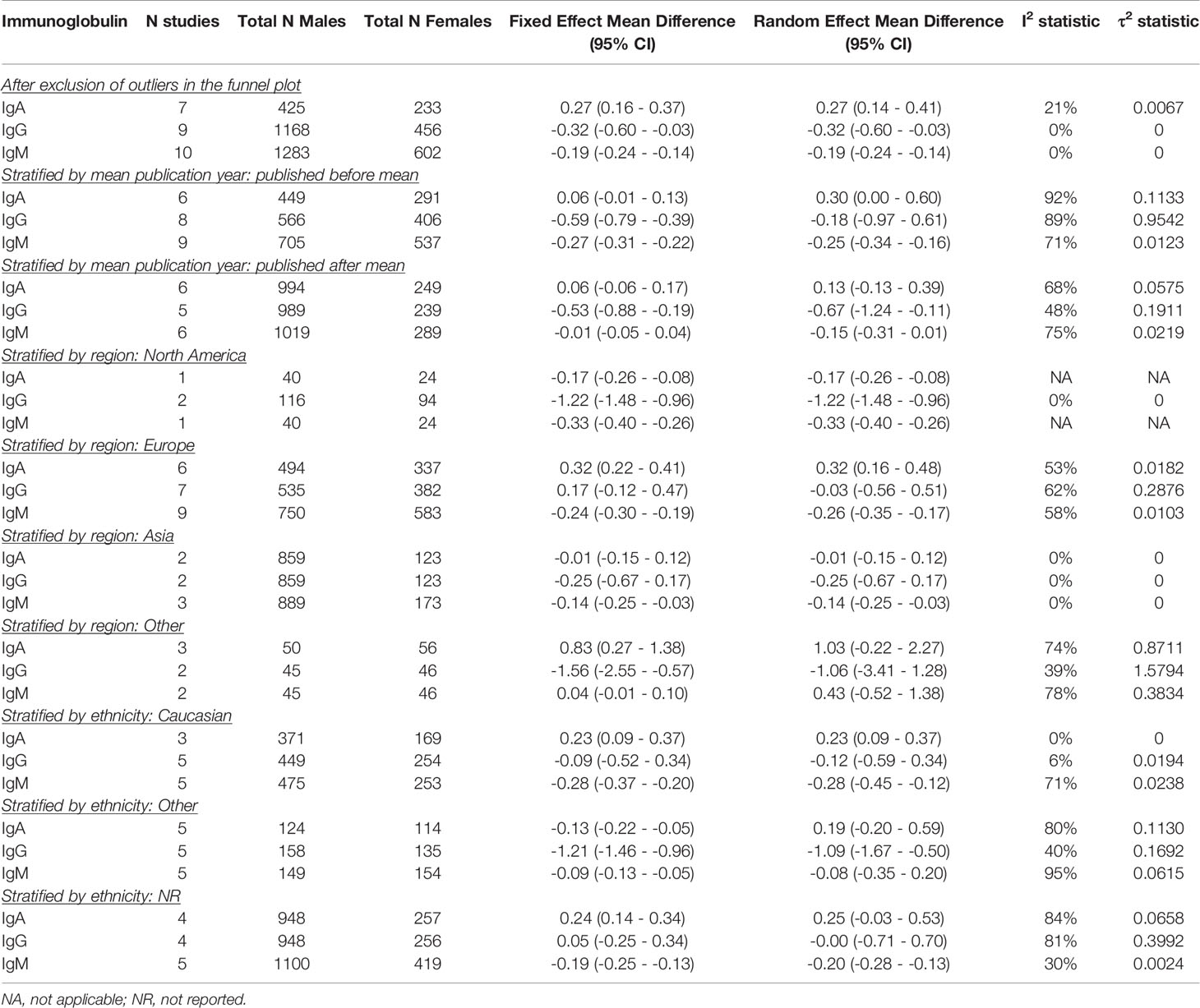
Seventeen articles were included to obtain pooled MDs for men compared to women (5, 15, 16, 18, 19, 26, 31, 35, 50, 52–54, 57, 58, 63, 64, 103).
Men had higher IgA (pooled MD: 0.22; 95% CI: 0.03 – 0.42), but lower IgM levels (pooled MD: -0.21; 95% CI: -0.32 – -0.10) than women. No difference was observed for IgG (pooled MD: -0.39; 95% CI: -0.91 – 0.12) (Figure 2). In the fixed effect model, the association of sex and IgA was lost (pooled MD: 0.06; 95% CI: 0.00 – 0.12), while IgG became lower in men compared to women (pooled MD: -0.57; 95% CI: -0.75 – -0.40). A large amount of heterogeneity was observed (I2: 86% for IgA; 83% for IgG; 88% for IgM).
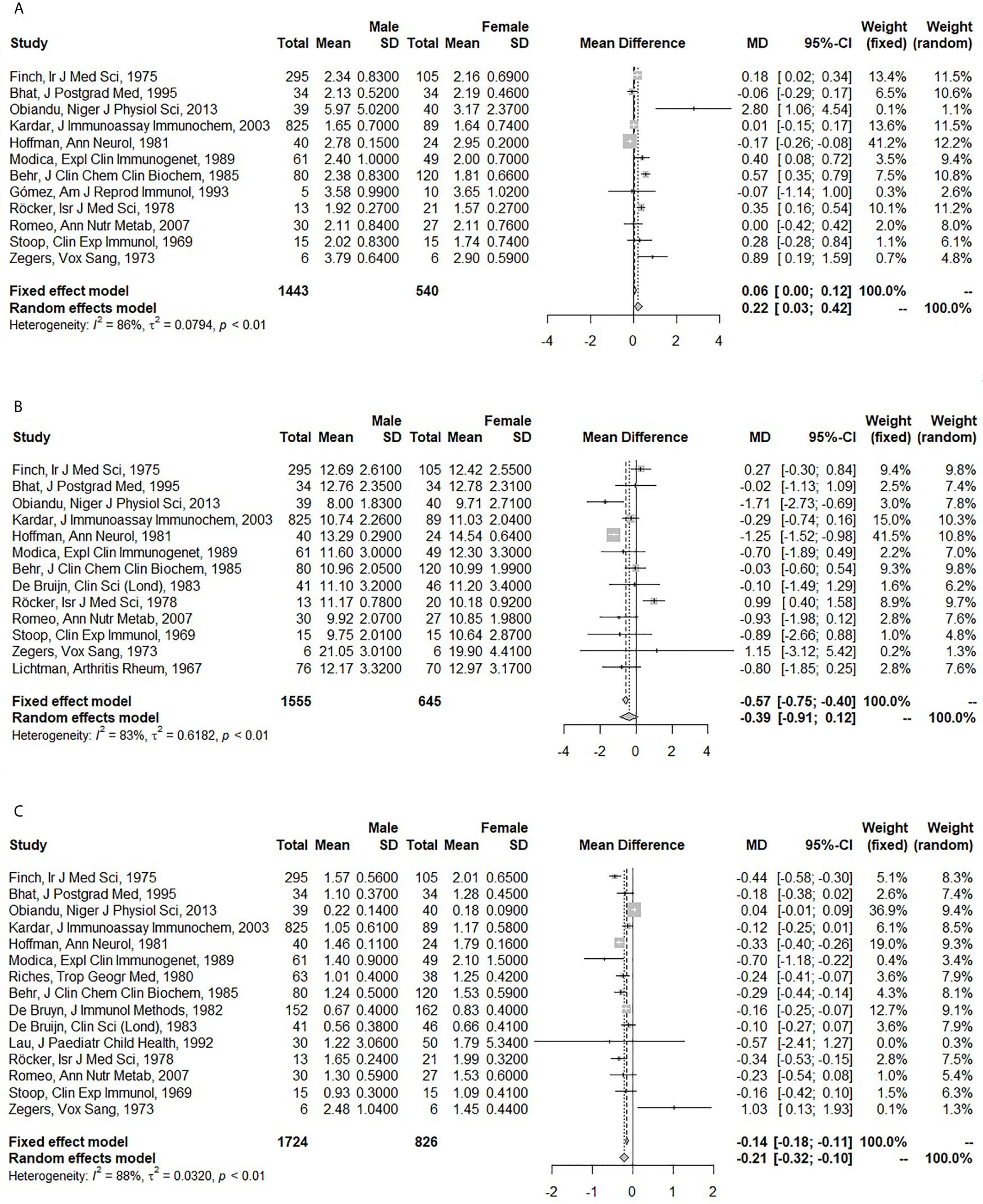
Figure 2 Forest plots for the association between sex and serum immunoglobulin levels. (A) Association of sex with serum immunoglobulin A (IgA) (g/l). (B) Association of sex with serum immunoglobulin G (IgG) (g/l). (C) Association of sex with serum immunoglobulin M (IgM) (g/l). The closed squares with horizontal lines depict the mean differences in serum immunoglobulin levels between men and women with 95% confidence intervals. The diamonds depict the pooled mean differences between men and women. The random effect model was taken as primary model.
Assessment of Publication Bias
The Egger test was significant for the association of age (P = 0.002) and sex (P = 0.013) with IgA. No statistical indications of publication bias were found for IgG and IgM. The funnel plots indicated three outliers for the association between age and IgA (15, 20, 29). For the association between age and IgG six outliers were found (12, 29, 30, 32, 37, 38), whereas for age and IgM there were seven outliers (12, 15, 16, 18, 19, 29, 38) (Supplementary Figure S3). In the funnel plots for sex, there were five outliers for IgA (5, 15, 16, 26, 31), four for IgG (5, 26, 52, 58), and five for IgM (5, 26, 35, 52, 63) (Supplementary Figure S4).
Sensitivity and Stratified Analyses
Employing a Different Cut-off for Age
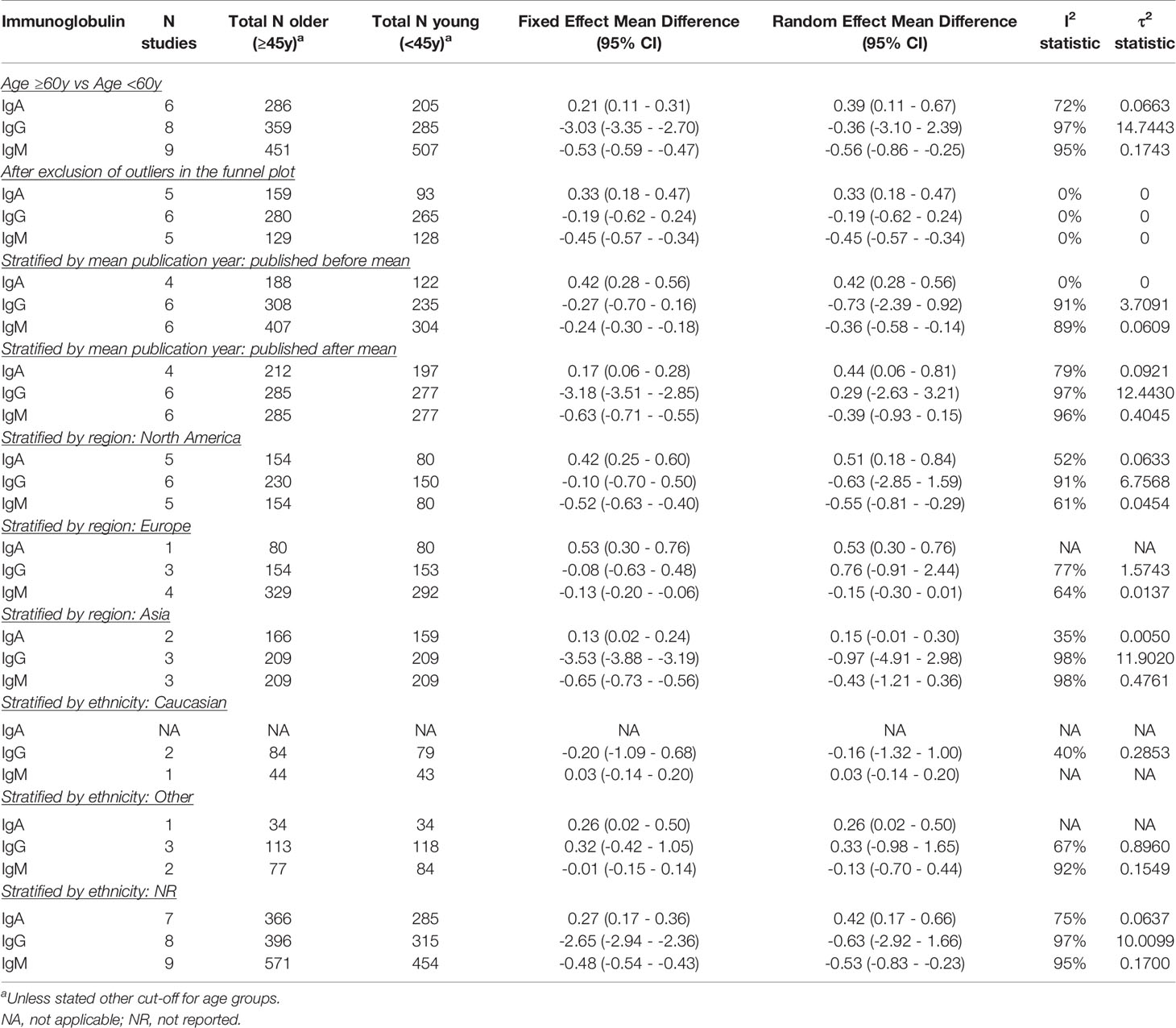
Using a cut-off of 60 instead of 45 years to compare older vs younger individuals, yielded similar results and did not impact heterogeneity (Table 2A).
Excluding Outliers in the Funnel Plot
While excluding the outliers in the funnel plots eliminated heterogeneity, it did not affect effect estimates for the association of age with IgA, IgG, and IgM. For sex however, exclusion of the outliers led to a negative association with IgG (pooled MD: -0.32; 95% CI: -0.60 – -0.03). No differences were found for the effect of sex on IgA and IgM, although heterogeneity was greatly reduced or eliminated (Table 2B). We also excluded the outliers one at a time, but could not identify a particular study that explained most of the heterogeneity (data not shown).
Stratifying by Mean Publication Year
When stratified by mean publication year, the negative association between age and serum IgM was lost in the more recently published studies. The fixed effect model furthermore yielded a strong negative association between age and IgG in the more recently published studies (pooled MD: -3.18; 95% CI: -3.51 – -2.85) (Table 2A). For sex, stratification by mean publication year did not impact IgA. Serum IgG was lower in men compared to women in the more recently published studies (pooled MD: -0.67; 95% CI: -1.24 – -0.11), whereas the association of sex and IgM was lost in these studies (Table 2B).
Stratifying by WHO Region
Age was not associated with IgG in any WHO region. The positive association of age with IgA was lost in Asia (pooled MD: 0.15; 95% CI: -0.01 – 0.30) and the negative association of age and IgM only remained in North America (pooled MD: -0.55; 95% CI: -0.81 – -0.29) (Table 2A). No relation was found between sex and IgA in Asian and other regions (excluding North America and Europe). In Europe however, men compared to women had higher IgA levels (pooled MD: 0.32; 95% CI: 0.16 – 0.48). The opposite association was found in North America (pooled MD: -0.17; 95% CI: -0.26 – -0.08) although only one study was performed in that region. IgG was lower in men compared to women in the North American studies (pooled MD: -1.22; 95% CI: -1.48 – -0.96), whereas no association between sex and IgG was found in the other WHO regions. IgM was lower in men than women in all North American, European and Asian studies (pooled MDs ranging from -0.33 to -0.14). Only one study was performed in another WHO region and did not report a relation between sex and IgM (Table 2B).
Stratifying by Ethnicity
The positive association of age with IgA was not impacted by ethnicity, although most studies did not report the ethnicity of participants. While the effect estimates for the association of age with IgG and IgM were opposite in Caucasians vs subjects of other ethnicities, none of these associations reached significance. IgM levels were lower in older compared to younger subjects of no reported ethnicity (pooled MD: -0.53; 95% CI: -0.83 – -0.23) (Table 2A). Stratification by ethnicity only yielded an association between sex and IgA in Caucasians (pooled MD: 0.23; 95% CI: 0.09 – 0.37). Men compared to women of non-Caucasian ethnicity had lower IgG levels (pooled MD: -1.09; 95% CI: -1.67 – -0.50). Men furthermore had lower IgM levels than women, expect for the ones of non-Caucasian ethnicity (Table 2B).
Summary of Identified Determinants
A graphic overview of identified determinants per immunoglobulin, both through the systematic review and meta-analyses, has been provided in Supplementary Figure S5.
Discussion
In this study we have provided an up-to-date overview of published determinants of serum immunoglobulins, while also being the first to meta-analyze reported results. Age, sex, ethnicity, smoking, and psychological stress were identified as potentially important determinants. Heterogeneous and inconclusive results were found for the effect of diet, alcohol, hormones, and cardio-metabolic risk factors.
Pooled results showed 0.38 g/l higher IgA, but 0.40 g/l lower IgM levels in older compared to younger individuals. Our findings could be explained by a decline in IgM-producing B-cells at older age (3), although studies included in a recent review have shown a decline of naïve, IgM-memory, and switched-memory B-cells in the elderly (126). Increased immunoglobulin levels in elderly could indicate inflammatory disorders (e.g. Sjögren syndrome or rheumatoid arthritis) (127) or monoclonal gammopathy of undetermined significance (MGUS), an asymptomatic premalignant condition whose prevalence increases with age (128).
Furthermore, in our meta-analyses we showed 0.22 g/l higher IgA and 0.21 g/l lower IgM levels in men compared to women. This could partly be explained by hormonal differences, as testosterone application to human peripheral blood mononuclear cells led to decreased IgG and IgM production, whereas estradiol application had the opposite effect (129, 130). IgM-regulating properties of the X-chromosome were hypothesized to lead to higher levels in women, but results of family studies were inconclusive (131, 132). A recent study showed a positive effect of testosterone and a negative effect of estradiol on mucosal immunity in Amazonian adolescents, which could explain the higher IgA levels we found in men (133).
Caucasians had lower serum immunoglobulin levels than Africans, Asians, Native Americans, or Melanesians. This could be explained by environmental (lower microbial exposure) (134, 135) or genetic differences, as a study of black and white families from Richmond showed high heritability values for serum immunoglobulins, especially in white subjects (136). Furthermore, in admixed Latin-Americans, ancestry-specific single nucleotide polymorphisms regulated innate and adaptive immune responses (137). Genetic differences could also explain the higher immunoglobulin levels in Hazaras compared to other large Afghan tribes. Extensive genome analyses on worldwide human populations revealed that Hazaras were genetically more identical to Turkic populations in Central-Asia than to local populations (138). However, exploration of genetic determinants is beyond the scope of this systematic review.
The majority of included studies found that smoking was associated with lower serum IgG, and fewer studies also reported decreased IgA and IgM levels. Nicotine could stimulate the release of immunosuppressive hormones such as glucocorticoids and catecholamines (139). Furthermore, lymphocytes express nicotinic acetylcholine receptors (nAChR) and smoking reduces the expression of the α7 nAChR subunit known to regulate B-cell development, activation, and antibody production (140). A small study also indicated DNA methylation changes and upregulation of certain genes in leukocytes and lymphocytes of smokers (141).
All included studies on psychological stress reported increased immunoglobulin levels. This was expected, since these studies included acute stressors and in the initial stress response glucocorticoids and catecholamines exert immunostimulating rather than immunosuppressive effects (142). Long-term psychological stress was associated with decreased serum IgG-antibody production in mice and with decreased salivary IgA secretion in a population-based cohort of middle-aged and elderly individuals (143, 144).
We could not identify clear dietary determinants, possibly due to large heterogeneity and included studies on average having a sample size of <100 subjects and a short follow-up of a few weeks. However, most studies on probiotic use reported an increase of serum immunoglobulins. Probiotics can positively influence immune function, depending on the probiotic strain and dose and the consumer’s age (145). Studies on alcohol consumption reported an increase of IgA levels, while results for IgG and IgM were inconclusive. This could be due to a predominantly mucosal immune response, as animal studies showed alcohol-induced damage of the intestinal mucosa and disruption of the intestinal barrier function (146).
Studies reporting on the association of hypertension with serum immunoglobulins generally showed a positive association. Hypertension can activate the adaptive immune system, possibly through formation of neoantigens (147). Mouse models furthermore showed an increase in plasma cell count and serum IgG after angiotensin II administration (148). However, various immunological pathways have been described in the pathophysiology of hypertension, suggesting a bidirectional association (147, 149).
Our biggest strength lies in provision of an up-to-date qualitative (systematic review) and quantitative (meta-analysis) overview of serum immunoglobulin determinants. We were therefore able to present coherent and comparable data, indicating consistent associations of age, sex, and key environmental factors with serum immunoglobulin levels. Our results will furthermore encourage clinicians and researchers to pay close attention to factors that influence serum immunoglobulin levels in healthy adults and possibly in the context of immunosenescence. However, our study also knows some limitations. Most included studies had a moderate quality, small sample size (n <200), cross-sectional design (thus lacking longitudinal measurements of serum immunoglobulins), and were published multiple years ago (74 out of 117 included studies were published in the 20th century). We were unable to draw conclusions on certain lifestyle related, cardio-metabolic, or miscellaneous determinants due to heterogeneity in definition or a limited number of studies investigating those determinants. Therefore we could only meta-analyze results for age and sex, although a fair number of articles (n=28) were included in these meta-analyses.
The results of our systematic review and meta-analysis urge investigation of age- and sex-specific reference ranges for serum IgA and IgM. Although we did not establish associations with IgG in our main analyses, quality of included studies was generally low or moderate, total number of participants was relatively low, age of the included subjects was generally restricted to the young or middle-aged adult range, and between-study variance was high, warranting further research. When interpreting immunoglobulin levels of patients, clinicians should be aware of lower IgG levels in smokers and systemic corticosteroid users, lower IgA, IgG, and IgM levels in Caucasians, and higher IgA levels in alcohol consumers. Large population-based studies are important to confirm found associations with identified determinants (especially lifestyle and cardio-metabolic factors), while taking a wide range of potential confounders into account. Furthermore, multiple age categories should be studied in order to provide robust recommendations for age-specific reference ranges of serum immunoglobulins.
Conclusion
This systematic review and meta-analysis presents an overview of the literature highlighting determinants that influence serum immunoglobulin levels in healthy adults. In total, 117 articles published over a time span of 53 years were included. The meta-analysis indicated higher serum IgA, but lower serum IgM levels in older individuals and in males. Other identified determinants of serum IgA, IgG, and/or IgM were ethnicity, smoking, alcohol consumption, probiotics, corticosteroid use, hypertension, and acute psychological stress.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
SK: Conceptualization (equal), data curation (equal), formal analysis (lead), methodology (lead), validation (equal), visualization (lead), writing—original draft (lead), and writing—review and editing (equal). AB: Data curation (equal), validation (equal), and writing—review and editing (supporting). RP: Conceptualization (equal), supervision (equal), and writing—review and editing (supporting). MH: Conceptualization (equal), supervision (equal), and writing—review and editing (supporting). VD: Conceptualization (equal), funding acquisition (lead), supervision (equal), and writing—review and editing (equal). LC: Conceptualization (equal), supervision (equal), and writing—review and editing (equal). All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Takeda [grant number IIR-NLD-002671 to VD].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Elise Krabbendam, biomedical information specialist at the medical library of the Erasmus MC, for her contribution to the online literature search.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.664526/full#supplementary-material
References
1. Hoffman W, Lakkis FG, Chalasani G, Cells B. Antibodies, and More. Clin J Am Soc Nephrol (2016) 11:137–54. doi: 10.2215/CJN.09430915
2. Agarwal S, Busse PJ. Innate and adaptive immunosenescence. Ann Allergy Asthma Immunol (2010) 104:183–90; quiz 190-2, 210. doi: 10.1016/j.anai.2009.11.009
3. Listi F, Candore G, Modica MA, Russo M, Di Lorenzo G, Esposito-Pellitteri M, et al. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann N Y Acad Sci (2006) 1089:487–95. doi: 10.1196/annals.1386.013
4. Cassidy JT, Nordby GL, Dodge HJ. Biologic variation of human serum immunoglobulin concentrations: sex-age specific effects. J Chronic Dis (1974) 27:507–16. doi: 10.1016/0021-9681(74)90026-5
5. Obiandu C, Okerengwo AA, Dapper DV. Levels of serum immunoglobulins in apparently healthy children and adults in Port Harcourt, Nigeria. Niger J Physiol Sci (2013) 28:23–7.
6. Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol (2008) 151:42–50. doi: 10.1111/j.1365-2249.2007.03545.x
7. McMillan SA, Douglas JP, Archbold GP, McCrum EE, Evans AE. Effect of low to moderate levels of smoking and alcohol consumption on serum immunoglobulin concentrations. J Clin Pathol (1997) 50:819–22. doi: 10.1136/jcp.50.10.819
8. Maddison SE, Reimer CB. Normative values of serum immunoglobulins by single radial immunodiffusion: a review. Clin Chem (1976) 22:594–601. doi: 10.1093/clinchem/22.5.594
9. Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Settings, Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PloS One (2016) 11:e0147601. doi: 10.1371/journal.pone.0147601
10. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
11. N.H. Nih, Lung, and I. Blood. Quality assessment tool for before-after (pre-post) studies with no control group. (2014). Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (Accessed July 21, 2020).
12. Barrett DJ, Stenmark S, Wara DW, Ammann AJ. Immunoregulation in aged humans. Clin Immunol Immunopathol (1980) 17:203–11. doi: 10.1016/0090-1229(80)90088-4
13. Batory G, Jancso A, Puskas E, Redei A, Lengyel E. Antibody and immunoglobulin levels in aged humans. Arch Gerontol Geriatr (1984) 3:175–88. doi: 10.1016/0167-4943(84)90009-8
14. Beharka AA, Paiva S, Leka LS, Ribaya-Mercado JD, Russell RM, Nibkin Meydani S. Effect of age on the gastrointestinal-associated mucosal immune response of humans. J Gerontol A Biol Sci Med Sci (2001) 56:B218–23. doi: 10.1093/gerona/56.5.B218
15. Behr W, Schlimok G, Firchau V, Paul HA. Determination of reference intervals for 10 serum proteins measured by rate nephelometry, taking into consideration different sample groups and different distribution functions. J Clin Chem Clin Biochem (1985) 23:157–66. doi: 10.1515/cclm.1985.23.3.157
16. Bhat GA, Mubarik M, Bhat MY. Serum immunoglobulin profile in normal Kashmiri adults. J Postgrad Med (1995) 41:66–9.
17. Bowden M, Crawford J, Cohen HJ, Noyama O. A comparative study of monoclonal gammopathies and immunoglobulin levels in Japanese and United States elderly. J Am Geriatr Soc (1993) 41:11–4. doi: 10.1111/j.1532-5415.1993.tb05940.x
18. De Bruijn AM, Geers FC, Hylkema RS, Vermeeren R, Hofman A. Blood pressure and immunoglobulins. Clin Sci (Lond) (1983) 65:665–7. doi: 10.1042/cs0650665
19. De Bruyn AM, Klein F, Neumann H, Sandkuyl LA, Vermeeren R, Le Blansch G. The absolute quantification of human IgM and IgG: standardization and normal values. J Immunol Methods (1982) 48:339–48. doi: 10.1016/0022-1759(82)90334-9
20. Carson PJ, Nichol KL, O’Brien J, Hilo P, Janoff EN. Immune function and vaccine responses in healthy advanced elderly patients. Arch Intern Med (2000) 160:2017–24. doi: 10.1001/archinte.160.13.2017
21. Challacombe SJ, Percival RS, Marsh PD. Age-related changes in immunoglobulin isotypes in whole and parotid saliva and serum in healthy individuals. Oral Microbiol Immunol (1995) 10:202–7. doi: 10.1111/j.1399-302X.1995.tb00143.x
22. Farges MC, Minet-Quinard R, Walrand S, Thivat E, Ribalta J, Winklhofer-Roob B, et al. Immune status is more affected by age than by carotenoid depletion-repletion in healthy human subjects. Br J Nutr (2012) 108:2054–65. doi: 10.1017/S0007114512000177
23. De Greef GE, Van Tol MJ, Van Den Berg JW, Van Staalduinen GJ, Janssen CJ, Radl J, et al. Serum immunoglobulin class and IgG subclass levels and the occurrence of homogeneous immunoglobulins during the course of ageing in humans. Mech Ageing Dev (1992) 66:29–44. doi: 10.1016/0047-6374(92)90071-K
24. Grundbacher FJ. High IgM levels in women coincide with reproductive phase. Experientia (1980) 36:1360–1. doi: 10.1007/BF01960097
25. Hallgren HM, Buckley CE 3rd3rd, Gilbertsen VA, Yunis EJ. Lymphocyte phytohemagglutinin responsiveness, immunoglobulins and autoantibodies in aging humans. J Immunol (1973) 111:1101–7.
26. Hoffman PM, Robbins DS, Oldstone MB, Gibbs CJ Jr., Gajdusek DC. Humoral immunity in Guamanians with amyotrophic lateral sclerosis and parkinsonism-dementia. Ann Neurol (1981) 10:193–6. doi: 10.1002/ana.410100210
27. Hrncir Z, Nerad V, Skaunic V, Tichy M. Effect of aging on the levels of the main classes of serum immunoglobulins in active chronic hepatitis and in clinically healthy subjects. Rev Czech Med (1973) 19:215–22.
28. Ichihara K, Itoh Y, Min WK, Yap SF, Lam CW, Kong XT, et al. Diagnostic and epidemiological implications of regional differences in serum concentrations of proteins observed in six Asian cities. Clin Chem Lab Med (2004) 42:800–9. doi: 10.1515/CCLM.2004.133
29. Jayachandran M, Rani PJA, Arivazhagan P, Panneerselvam C. Neutrophil phagocytic function and humoral immune response with reference to ascorbate supplementation in aging humans. J Anti-Aging Med (2000) 3:37–42. doi: 10.1089/rej.1.2000.3.37
30. Jazayeri MH, Pourfathollah AA, Rasaee MJ, Porpak Z, Jafari ME. The concentration of total serum IgG and IgM in sera of healthy individuals varies at different age intervals. Biomed Aging Pathol (2013) 3:241–5. doi: 10.1016/j.biomag.2013.09.002
31. Kardar GA, Shams SH, Pourpak Z, Moin M. Normal value of immunoglobulins IgA, IgG, and IgM in Iranian healthy adults, measured by nephelometry. J Immunoassay Immunochem (2003) 24:359–67. doi: 10.1081/IAS-120025774
32. MacGregor RR, Shalit M. Neutrophil function in healthy elderly subjects. J Gerontol (1990) 45:M55–60. doi: 10.1093/geronj/45.2.M55
33. Maddison SE, Stewart CC, Farshy CE, Reimer CB. The relationship of race, sex, and age to concentrations of serum immunoglobulins expressed in international units in healthy adults in the USA. Bull World Health Organ (1975) 52:179–85.
34. Memeo SA, Piantanelli L, Mazzufferi G, Guerra L, Nikolitz M, Fabris N. Age related patterns of immunoglobulin serum levels in the Quechua Indians of Andean Mountains. Int J Biometeorol (1982) 26:49–52. doi: 10.1007/BF02187616
35. Modica MA, Freddi S, Caruso C. Blood IgA, IgM and IgE levels are influenced by sex and HLA phenotype. Exp Clin Immunogenet (1989) 6:251–7.
36. Paganelli R, Quinti I, Fagiolo U, Cossarizza A, Ortolani C, Guerra E, et al. Changes in circulating B cells and immunoglobulin classes and subclasses in a healthy aged population. Clin Exp Immunol (1992) 90:351–4. doi: 10.1111/j.1365-2249.1992.tb07954.x
37. Papadopoulos NG, Lianou PE, Papavassiliou Th J. Strain-dependent alterations of polymorphonuclear leukocyte phagocytosis and bactericidal function in healthy elderly subjects. Aging Immunol Infect Dis (1993) 4:223–9.
38. Phair JP, Kauffman CA, Bjornson A, Gallagher J, Adams L, Hess EV. Host defenses in the aged: evaluation of components of the inflammatory and immune responses. J Infect Dis (1978) 138:67–73. doi: 10.1093/infdis/138.1.67
39. Quintiliani L, Taggi F, Giuliani E, Buzzonetti A, D’Amico F. IgG, IgA and IgM concentration in human sera from different age groups: statistical evaluation. Boll Ist Sieroter Milan (1976) 55:241–8.
40. Radl J, Sepers JM, Skvaril F, Morell A, Hijmans W. Immunoglobulin patterns in humans over 95 years of age. Clin Exp Immunol (1975) 22:84–90.
41. Reen DJ, Murphy MB, O’Connor A, FitzGerald MX. IgG sub-class levels in healthy Irish adults–a population study. Ir J Med Sci (1981) 150:265–9. doi: 10.1007/BF02938252
42. Rowe DS, McGregor IA, Smith SJ, Hall P, Williams K. Plasma immunoglobulin concentrations in a West African (Gambian) community and in a group of healthy British adults. Clin Exp Immunol (1968) 3:63–79.
43. Stoica G, Macarie E, Michiu V, Stoica RC. Biologic variation of human immunoglobulin concentration. I. Sex-age specific effects on serum levels of IgG, IgA, IgM and IgD. Med Interne (1980) 18:323–32.
44. Toshkov A, Abrashev I, Prodanov P. Quantity of serum immunoglobulins IgG, IgA and IgM in Bulgarian blood-donors. Folia Haematol Int Mag Klin Morphol Blutforsch (1974) 101:269–71.
45. Vasson MP, Farges MC, Goncalves-Mendes N, Talvas J, Ribalta J, Winklhofer-Roob B, et al. Does aging affect the immune status? A comparative analysis in 300 healthy volunteers from France, Austria and Spain. Immun Ageing (2013) 10:38. doi: 10.1186/1742-4933-10-38
46. Veys EM, Wieme RJ. Serum IgG, IgM and IgA concentration determined by the “linear plate” immunodiffusion technique in a normal population. Clin Chim Acta (1973) 47:295–306. doi: 10.1016/0009-8981(73)90327-6
47. Yodfat Y, Keren L, Zlotnick A. Serum immunoglobulin levels in healthy adults of various ethnic groups in a rural family practice in Israel. J Fam Pract (1975) 2:419–22.
48. Kalff MW. A population study on serum immunoglobulin levels. Clin Chim Acta (1970) 28:277–89. doi: 10.1016/0009-8981(70)90092-6
49. Haferkamp O, Schlettwein-Gsell D, Schwick HG, Storiko K. Serum protein in an aging population with particular reference to evaluation of immune globulins and antibodies. Gerontologia (1966) 12:30–6. doi: 10.1159/000211530
50. Lichtman MA, Vaughan JH, Hames CG. The distribution of serum immunoglobulins, anti-gamma-G globulins (“rheumatoid factors”) and antinuclear antibodies in White and Negro subjects in Evans County, Georgia. Arthritis Rheum (1967) 10:204–15. doi: 10.1002/art.1780100306
51. Bell DY, Haseman JA, Spock A, McLennan G, Hook GE. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis (1981) 124:72–9.
52. Finch AP, Whelan CA, de Azavedo J, Greally JF. Immunoglobulin levels and auto-antibodies in normal healthy adults. Ir J Med Sci (1975) 144:463–8. doi: 10.1007/BF02939055
53. Gomez E, Ortiz V, Saint-Martin B, Boeck L, Diaz-Sanchez V, Bourges H. Hormonal regulation of the secretory IgA (sIgA) system: estradiol- and progesterone-induced changes in sIgA in parotid saliva along the menstrual cycle. Am J Reprod Immunol (1993) 29:219–23. doi: 10.1111/j.1600-0897.1993.tb00590.x
54. Lau YL, Jones BM, Yeung CY. Biphasic rise of serum immunoglobulins G and A and sex influence on serum immunoglobulin M in normal Chinese children. J Paediatr Child Health (1992) 28:240–3. doi: 10.1111/j.1440-1754.1992.tb02654.x
55. Melamed I, Kark JD, Zakuth V, Margalit G, Spirer Z. Serum immunoglobulin A levels and ethnicity in an Israeli population sample. Clin Immunol Immunopathol (1987) 42:259–64. doi: 10.1016/0090-1229(87)90013-4
56. Pongpaew P, Tungtrongchitr R, Phonrat B, Supawan V, Lertchawanakul A, Tawprasert S, et al. Serum proteins and nutritional status of free-living Thai elderly. Arch Gerontol Geriatr (1995) 20:219–27. doi: 10.1016/0167-4943(94)00616-F
57. Riches PG, Quakyi IA, Gibbs MR, Addison AE. Normal serum immunoglobulin and albumin levels in adult Ghanaians compared with levels in adults in Europe. Trop Geogr Med (1980) 32:151–7.
58. Rocker L, Kirsch KA, Stoboy H. Sex-dependent changes in plasma globulins in women and men exposed to heat stress. Isr J Med Sci (1978) 14:212–7.
59. Shiddo SA, Huldt G, Jama H, Nilsson LA, Ouchterlony O, Warsame M, et al. Reference ranges for IgG, IgM and IgA in the serum of urban and rural Somalis. Trop Geogr Med (1994) 46:27–31.
60. Sinkov D, Tolev V, Stereva T. Concentration of IgG, IgA, and IgM in terms of international units in the sera of healthy individuals. Bull World Health Organ (1973) 49:217–8.
61. White AG, Al-Riyami HA, Kuchipudi P, Daar AS. Immunoglobulins, immunoglobulin G subclasses and complement in adult Omanis. Ann Saudi Med (1997) 17:39–42. doi: 10.5144/0256-4947.1997.39
62. Zegers BJ, Stoop JW, Reerink-Brongers EE, Sander PC, Aalberse RC, Ballieux RE. Serum immunoglobulins in healthy children and adults. Levels of the five classes, expressed in international units per millilitre. Clin Chim Acta (1975) 65:319–29. doi: 10.1016/0009-8981(75)90257-0
63. Zegers BJ, Geerdink RA, Sander PC. Serum immunoglobulin levels in Trio and Wajana indians of Surinam. Vox Sang (1973) 24:457–67. doi: 10.1111/j.1423-0410.1973.tb03486.x
64. Stoop JW, Zegers BJ, Sander PC, Ballieux RE. Serum immunoglobulin levels in healthy children and adults. Clin Exp Immunol (1969) 4:101–12.
65. Amagase H, Sun B, Nance DM. Immunomodulatory effects of a standardized Lycium barbarum fruit juice in Chinese older healthy human subjects. J Med Food (2009) 12:1159–65. doi: 10.1089/jmf.2008.0300
66. Anderson R, Oosthuizen R, Maritz R, Theron A, Van Rensburg AJ. The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune functions in normal volunteers. Am J Clin Nutr (1980) 33:71–6. doi: 10.1093/ajcn/33.1.71
67. Dlouhy P, Kucera P, Kraml P, Pompachova A, Potockova J, Smejkalova V, et al. Short-term dietary intake of C18:1 trans fatty acids decreases the function of cellular immunity in healthy young men. Ann Nutr Metab (2008) 53:129–36. doi: 10.1159/000162679
68. Hartoma TR, Sotaniemi EA, Maattanen J. Effect of zinc on some biochemical indices of metabolism. Nutr Metab (1979) 23:294–300. doi: 10.1159/000176268
69. Kelley DS, Taylor PC, Nelson GJ, Mackey BE. Dietary docosahexaenoic acid and immunocompetence in young healthy men. Lipids (1998) 33:559–66. doi: 10.1007/s11745-998-0240-8
70. Kianbakht S, Ghazavi A. Immunomodulatory effects of saffron: a randomized double-blind placebo-controlled clinical trial. Phytother Res (2011) 25:1801–5. doi: 10.1002/ptr.3484
71. Kim HS, Park H, Cho IY, Paik HD, Park E. Dietary supplementation of probiotic Bacillus polyfermenticus, Bispan strain, modulates natural killer cell and T cell subset populations and immunoglobulin G levels in human subjects. J Med Food (2006) 9:321–7. doi: 10.1089/jmf.2006.9.321
72. Lomax AR, Cheung LV, Tuohy KM, Noakes PS, Miles EA, Calder PC. beta2-1 Fructans have a bifidogenic effect in healthy middle-aged human subjects but do not alter immune responses examined in the absence of an in vivo immune challenge: results from a randomised controlled trial. Br J Nutr (2012) 108:1818–28. doi: 10.1017/S0007114511007276
73. Marteau P, Vaerman JP, Dehennin JP, Bord S, Brassart D, Pochart P, et al. Effects of intrajejunal perfusion and chronic ingestion of Lactobacillus johnsonii strain La1 on serum concentrations and jejunal secretions of immunoglobulins and serum proteins in healthy humans. Gastroenterol Clin Biol (1997) 21:293–8.
74. O’Brien BC, McMurray DN. Human plasma lipid and immunologic responses to eggs and ascorbic acid. Nutr Res (1988) 8:353–66. doi: 10.1016/S0271-5317(88)80030-7
75. Park OJ, Kang NE, Chang MJ, Kim WK. Resistant starch supplementation influences blood lipid concentrations and glucose control in overweight subjects. J Nutr Sci Vitaminol (Tokyo) (2004) 50:93–9. doi: 10.3177/jnsv.50.93
76. Predy GN, Goel V, Lovlin RE, Basu TK. Immune modulating effects of daily supplementation of COLD-fX (a proprietary extract of North American ginseng) in healthy adults. J Clin Biochem Nutr (2006) 39:162–7. doi: 10.3164/jcbn.39.162
77. Prinz W, Bortz R, Bregin B, Hersch M. The effect of ascorbic acid supplementation on some parameters of the human immunological defence system. Int J Vitam Nutr Res (1977) 47:248–57.
78. Sierra S, Lara-Villoslada F, Sempere L, Olivares M, Boza J, Xaus J. Intestinal and immunological effects of daily oral administration of Lactobacillus salivarius CECT5713 to healthy adults. Anaerobe (2010) 16:195–200. doi: 10.1016/j.anaerobe.2010.02.001
79. Song HJ, Grant I, Rotondo D, Mohede I, Sattar N, Heys SD, et al. Effect of CLA supplementation on immune function in young healthy volunteers. Eur J Clin Nutr (2005) 59:508–17. doi: 10.1038/sj.ejcn.1602102
80. Zhang H, Yeh C, Jin Z, Ding L, Liu BY, Zhang L, et al. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth Syst Biotechnol (2018) 3:113–20. doi: 10.1016/j.synbio.2018.03.001
81. Kim SH, Kim HY, Kim WK, Park OJ. Nutritional status, iron-deficiency-related indices, and immunity of female athletes. Nutrition (2002) 18:86–90. doi: 10.1016/S0899-9007(01)00663-3
82. Sakem B, Nock C, Stanga Z, Medina P, Nydegger UE, Risch M, et al. Serum concentrations of 25-hydroxyvitamin D and immunoglobulins in an older Swiss cohort: results of the Senior Labor Study. BMC Med (2013) 11:176. doi: 10.1186/1741-7015-11-176
83. Bahijri SM, Ajabnoor GM, Borai A, Al-Aama JY, Chrousos GP. Effect of Ramadan fasting in Saudi Arabia on serum bone profile and immunoglobulins. Ther Adv Endocrinol Metab (2015) 6:223–32. doi: 10.1177/2042018815594527
84. Develioglu ON, Kucur M, Ipek HD, Celebi S, Can G, Kulekci M. Effects of Ramadan fasting on serum immunoglobulin G and M, and salivary immunoglobulin A concentrations. J Int Med Res (2013) 41:463–72. doi: 10.1177/0300060513476424
85. Agarwal DP, Goedde HW, Benkmann HG, Flatz G, Rahimi AG, Kaifie S, et al. Genetic polymorphism of C3 and serum levels of immunoglobulins, C3, C4 components of complement and C3-proactivator in four different populations of Afghanistan. Hum Genet (1976) 33:67–72. doi: 10.1007/BF00447288
86. Wright EP, Vlug A, Geertzen HG, Hoang TL, Nguyen DH. Serum immunoglobulins, including IgG subclasses, in Vietnamese leprosy patients. Int J Lepr Other Mycobact Dis (1985) 53:225–32.
87. Wang DY, Goodwin PR, Bulbrook RD, Hayward JL, Abe O, Utsunomiya J, et al. Possible relationship of plasma IgA, IgG and IgM to breast cancer in British and Japanese women. Eur J Cancer (1977) 13:1405–9. doi: 10.1016/0014-2964(77)90153-0
88. Grove DI, McGregor A, Forbes IJ. Impaired humoral immunity in PNG Highlanders. P N G Med J (1975) 18:1–7.
89. Tollerud DJ, Brown LM, Blattner WA, Weiss ST, Maloney EM, Kurman CC, et al. Racial differences in serum immunoglobulin levels: relationship to cigarette smoking, T-cell subsets, and soluble interleukin-2 receptors. J Clin Lab Anal (1995) 9:37–41. doi: 10.1002/jcla.1860090107
90. Rowe DS. Concentration of serum-immunoglobulins in healthy young adult males estimated by assay against the international reference preparation. Lancet (1972) 2:1232–3. doi: 10.1016/S0140-6736(72)92277-5
91. Turner MW, Voller A. Studies on immunoglobulins of Nigerians. I. The immunoglobulin levels of a Nigerian population. J Trop Med Hyg (1966) 69:99–103.
92. Wells JV. Serum immunoglobulin levels in tropical splenomegaly syndrome in New Guinea. Clin Exp Immunol (1968) 3:943–51.
93. Aral M, Ekerbicer HC, Celik M, Ciragil P, Gul M. Comparison of effects of smoking and smokeless tobacco “Maras powder” use on humoral immune system parameters. Mediators Inflammation (2006) 2006:85019. doi: 10.1155/MI/2006/85019
94. van Larebeke N, Husson B, De Coen W, Pluygers E. Immunologic and other biological parameters as a function of smoking status and of residence in areas differing in terms of air pollution. Int J Environ Health Res (2003) 13:55–69. doi: 10.1080/0960312021000063313
95. Liao M, Ye F, Zhang B, Huang L, Xiao Q, Qin M, et al. Genome-wide association study identifies common variants at TNFRSF13B associated with IgG level in a healthy Chinese male population. Genes Immun (2012) 13:509–13. doi: 10.1038/gene.2012.26
96. Yang M, Wu Y, Lu Y, Liu C, Sun J, Liao M, et al. Genome-wide scan identifies variant in TNFSF13 associated with serum IgM in a healthy Chinese male population. PloS One (2012) 7:e47990. doi: 10.1371/journal.pone.0047990
97. Mahassni SH, Ali EYI. The Effects of Firsthand and Secondhand Cigarette Smoking on Immune System Cells and Antibodies in Saudi Arabian Males. Indian J Clin Biochem (2019) 34:143–54. doi: 10.1007/s12291-018-0739-9
98. Wagner V, Skokanova K, Wagnerova M, Heribanova A, Riha M. Indicators of humoral immunity in smokers and nonsmokers working underground. J Hyg Epidemiol Microbiol Immunol (1983) 27:129–42.
99. Calapai G, Caputi AP, Mannucci C, Gregg EO, Pieratti A, Aurora Russo G, et al. A cross-sectional investigation of biomarkers of risk after a decade of smoking. Inhal Toxicol (2009) 21:1138–43. doi: 10.3109/08958370902798455
100. Ferson M, Edwards A, Lind A, Milton GW, Hersey P. Low natural killer-cell activity and immunoglobulin levels associated with smoking in human subjects. Int J Cancer (1979) 23:603–9. doi: 10.1002/ijc.2910230504
101. Gyllen P, Andersson BA, Qvarfordt I. Smokeless tobacco or nicotine replacement therapy has no effect on serum immunoglobulin levels. Respir Med (2004) 98:108–14. doi: 10.1016/j.rmed.2003.08.012
102. Kokavec A, Crowe SF. Effect of moderate white wine consumption on serum IgA and plasma insulin under fasting conditions. Ann Nutr Metab (2006) 50:407–12. doi: 10.1159/000094631
103. Romeo J, Warnberg J, Nova E, Diaz LE, Gonzalez-Gross M, Marcos A. Changes in the immune system after moderate beer consumption. Ann Nutr Metab (2007) 51:359–66. doi: 10.1159/000107679
104. De Feo P, Volpi E, Lucidi P, Cruciani G, Monacchia F, Reboldi G, et al. Ethanol impairs post-prandial hepatic protein metabolism. J Clin Invest (1995) 95:1472–9. doi: 10.1172/JCI117818
105. Butler WT, Rossen RD. Effects of corticosteroids on immunity in man. I. Decreased serum IgG concentration caused by 3 or 5 days of high doses of methylprednisolone. J Clin Invest (1973) 52:2629–40. doi: 10.1172/JCI107455
106. Goodwin JS, Clay GA. Effect of chronic ingestion of a prostaglandin E analogue on immunologic function in healthy elderly subjects. Int J Immunopharmacol (1986) 8:867–73. doi: 10.1016/0192-0561(86)90086-X
107. Khorram O, Vu L, Yen SS. Activation of immune function by dehydroepiandrosterone (DHEA) in age-advanced men. J Gerontol A Biol Sci Med Sci (1997) 52:M1–7. doi: 10.1093/gerona/52A.1.M1
108. Klinger G, Graser T, Mellinger U, Moore C, Vogelsang H, Groh A, et al. A comparative study of the effects of two oral contraceptives containing dienogest or desogestrel on the human immune system. Gynecol Endocrinol (2000) 14:15–24. doi: 10.3109/09513590009167655
109. Otolorin EQ, Adeyefa I, Konje JC, Ojengbede O, Osotimehin B, Ladipo OA. Plasma immunoglobulin, total protein and albumin levels during Norplant use by Nigerian women. Acta Obstet Gynecol Scand (1993) 72:645–7. doi: 10.3109/00016349309021158
110. Van Schoor J, Toogood JH, Pauwels RA. Differential effects of inhaled budesonide and oral prednisolone on serum immunoglobulin G and its subclasses in healthy adult volunteers. Clin Exp Allergy (1997) 27:192–5. doi: 10.1111/j.1365-2222.1997.tb00692.x
111. Wang X, Li Y, Li H, Gu Y, Song Y, Zhang Q, et al. Relationship of serum immunoglobulin levels to blood pressure and hypertension in an adult population. J Hum Hypertens (2018) 32:212–8. doi: 10.1038/s41371-018-0029-2
112. Green ML, Green RG, Santoro W. Daily relaxation modifies serum and salivary immunoglobulins and psychophysiologic symptom severity. Biofeedback Self Regul (1988) 13:187–99. doi: 10.1007/BF00999169
113. Lee M-K, Lim S, Song J-A, Kim M-E, Hur M-H. The effects of aromatherapy essential oil inhalation on stress, sleep quality and immunity in healthy adults: Randomized controlled trial. Eur J Integr Med (2017) 12:79–86. doi: 10.1016/j.eujim.2017.04.009
114. Lovas JM, Craig AR, Segal YD, Raison RL, Weston KM, Markus MR. The effects of massage therapy on the human immune response in healthy adults. J Bodywork Movement Therapies (2002) 6:143–50. doi: 10.1054/jbmt.2001.0251
115. Niu A. Effect of “Tai Chi” Exercise on Antioxidant Enzymes Activities and Immunity Function in Middle-Aged Participants. Afr J Tradit Complement Altern Med (2016) 13:87–90.
116. Park SM, Kwak YS, Ji JG. The Effects of Combined Exercise on Health-Related Fitness, Endotoxin, and Immune Function of Postmenopausal Women with Abdominal Obesity. J Immunol Res (2015) 2015:830567. doi: 10.1155/2015/830567
117. Al-Hazimi A. Effect of stress on immunity: a study among healthy blood donors at King Abdul Aziz University Hospital, Jeddah. Ann Saudi Med (2004) 24:52–4. doi: 10.5144/0256-4947.2004.52
118. Kiecolt-Glaser JK, Garner W, Speicher C, Penn GM, Holliday J, Glaser R. Psychosocial modifiers of immunocompetence in medical students. Psychosom Med (1984) 46:7–14. doi: 10.1097/00006842-198401000-00003
119. Maes M, Hendriks D, Van Gastel A, Demedts P, Wauters A, Neels H, et al. Effects of psychological stress on serum immunoglobulin, complement and acute phase protein concentrations in normal volunteers. Psychoneuroendocrinology (1997) 22:397–409. doi: 10.1016/S0306-4530(97)00042-5
120. Theorell T, Orth-Gomer K, Eneroth P. Slow-reacting immunoglobulin in relation to social support and changes in job strain: a preliminary note. Psychosom Med (1990) 52:511–6. doi: 10.1097/00006842-199009000-00003
121. Hui L, Hua F, Diandong H, Hong Y. Effects of sleep and sleep deprivation on immunoglobulins and complement in humans. Brain Behav Immun (2007) 21:308–10. doi: 10.1016/j.bbi.2006.09.005
122. Ozturk L, Pelin Z, Karadeniz D, Kaynak H, Cakar L, Gozukirmizi E. Effects of 48 hours sleep deprivation on human immune profile. Sleep Res Online (1999) 2:107–11.
123. Ruiz FS, Andersen ML, Martins RC, Zager A, Lopes JD, Tufik S. Immune alterations after selective rapid eye movement or total sleep deprivation in healthy male volunteers. Innate Immun (2012) 18:44–54. doi: 10.1177/1753425910385962
124. Dufour V, Millon L, Faucher JF, Bard E, Robinet E, Piarroux R, et al. Effects of a short-course of amoxicillin/clavulanic acid on systemic and mucosal immunity in healthy adult humans. Int Immunopharmacol (2005) 5:917–28. doi: 10.1016/j.intimp.2005.01.007
125. Roseman JM, Buckley CE. 3rd, Inverse relationship between serum IgG concentrations and measures of intelligence in elderly persons. Nature (1975) 254:55–6. doi: 10.1038/254055a0
126. Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev (2011) 10:330–5. doi: 10.1016/j.arr.2010.08.004
127. Yang Y, Chen L, Jia Y, Liu Y, Wen L, Liang Y, et al. Monoclonal gammopathy in rheumatic diseases. Clin Rheumatol (2018) 37:1751–62. doi: 10.1007/s10067-018-4064-8
128. Atkin C, Richter A, Sapey E. What is the significance of monoclonal gammopathy of undetermined significance? Clin Med (Lond) (2018) 18:391–6. doi: 10.7861/clinmedicine.18-5-391
129. Kanda N, Tsuchida T, Tamaki K. Estrogen enhancement of anti-double-stranded DNA antibody and immunoglobulin G production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum (1999) 42:328–37. doi: 10.1002/1529-0131(199902)42:2<328::AID-ANR16>3.0.CO;2-#
130. Kanda N, Tsuchida T, Tamaki K. Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin Exp Immunol (1996) 106:410–5. doi: 10.1046/j.1365-2249.1996.d01-842.x
131. Grundbacher FJ. Human X chromosome carries quantitative genes for immunoglobulin M. Science (1972) 176:311–2. doi: 10.1126/science.176.4032.311
132. Escobar V, Corey LA, Bixler D, Nance WE, Biegel A. The human X-chromosome and the levels of serum immunoglobulin M. Clin Genet (1979) 15:221–7. doi: 10.1111/j.1399-0004.1979.tb00971.x
133. Hodges-Simeon CR, Asif S, Gurven M, Blackwell AD, Gaulin SJC. Testosterone is positively and estradiol negatively associated with mucosal immunity in Amazonian adolescents. Am J Hum Biol (2019) 31:e23284. doi: 10.1002/ajhb.23284
134. Li H, Limenitakis JP, Greiff V, Yilmaz B, Scharen O, Urbaniak C, et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature (2020) 584:274–8. doi: 10.1038/s41586-020-2564-6
135. Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res (2011) 69:465–72. doi: 10.1203/PDR.0b013e318217638a
136. Grundbacher FJ. Heritability estimates and genetic and environmental correlations for the human immunoglobulins G, M, and A. Am J Hum Genet (1974) 26:1–12.
137. Norris ET, Wang L, Conley AB, Rishishwar L, Marino-Ramirez L, Valderrama-Aguirre A, et al. Genetic ancestry, admixture and health determinants in Latin America. BMC Genomics (2018) 19:861. doi: 10.1186/s12864-018-5195-7
138. He G, Adnan A, Rakha A, Yeh HY, Wang M, Zou X, et al. A comprehensive exploration of the genetic legacy and forensic features of Afghanistan and Pakistan Mongolian-descent Hazara. Forensic Sci Int Genet (2019) 42:e1–12. doi: 10.1016/j.fsigen.2019.06.018
139. McAllister-Sistilli CG, Caggiula AR, Knopf S, Rose CA, Miller AL, Donny EC. The effects of nicotine on the immune system. Psychoneuroendocrinology (1998) 23:175–87. doi: 10.1016/S0306-4530(97)00080-2
140. Kawashima K, Fujii T, Moriwaki Y, Misawa H. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci (2012) 91:1027–32. doi: 10.1016/j.lfs.2012.05.006
141. Su D, Wang X, Campbell MR, Porter DK, Pittman GS, Bennett BD, et al. Distinct Epigenetic Effects of Tobacco Smoking in Whole Blood and among Leukocyte Subtypes. PloS One (2016) 11:e0166486. doi: 10.1371/journal.pone.0166486
142. Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun (2007) 21:259–72. doi: 10.1016/j.bbi.2006.11.006
143. Silberman DM, Wald MR, Genaro AM. Acute and chronic stress exert opposing effects on antibody responses associated with changes in stress hormone regulation of T-lymphocyte reactivity. J Neuroimmunol (2003) 144:53–60. doi: 10.1016/j.jneuroim.2003.08.031
144. Phillips AC, Carroll D, Evans P, Bosch JA, Clow A, Hucklebridge F, et al. Stressful life events are associated with low secretion rates of immunoglobulin A in saliva in the middle aged and elderly. Brain Behav Immun (2006) 20:191–7. doi: 10.1016/j.bbi.2005.06.006
145. Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr (2014) 54:938–56. doi: 10.1080/10408398.2011.619671
146. Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol (2008) 42:349–61. doi: 10.1016/j.alcohol.2008.03.131
147. Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol (2010) 10:203–7. doi: 10.1016/j.coph.2010.01.006
148. Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, et al. Obligatory Role for B Cells in the Development of Angiotensin II-Dependent Hypertension. Hypertension (2015) 66:1023–33. doi: 10.1161/HYPERTENSIONAHA.115.05779
Keywords: serum immunoglobulins, adult human beings, determinants, systematic review, meta-analysis
Citation: Khan SR, van der Burgh AC, Peeters RP, van Hagen PM, Dalm VASH and Chaker L (2021) Determinants of Serum Immunoglobulin Levels: A Systematic Review and Meta-Analysis. Front. Immunol. 12:664526. doi: 10.3389/fimmu.2021.664526
Received: 05 February 2021; Accepted: 19 March 2021;
Published: 07 April 2021.
Edited by:
Rui Li, University of Pennsylvania, United StatesReviewed by:
Hanane Touil, Columbia University Irving Medical Center, United StatesHongwei Xu, Harbin Medical University, China
Copyright © 2021 Khan, van der Burgh, Peeters, van Hagen, Dalm and Chaker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Layal Chaker, bC5jaGFrZXJAZXJhc211c21jLm5s
 Samer R. Khan
Samer R. Khan Anna C. van der Burgh1,3
Anna C. van der Burgh1,3 Robin P. Peeters
Robin P. Peeters P. Martin van Hagen
P. Martin van Hagen Virgil A. S. H. Dalm
Virgil A. S. H. Dalm Layal Chaker
Layal Chaker