- 1Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, China
- 2Organ Transplantation Center, West China Hospital, Sichuan University, Chengdu, China
Background: A systematic review and meta-analysis were performed to investigate the efficacy and safety of conversion from calcineurin inhibitors (CNIs) to mammalian target of rapamycin inhibitors (mTORi) in kidney transplant recipients (KTRs).
Methods: MEDLINE, EMBASE, PubMed, and Cochrane Library were searched to identify randomized controlled trials (RCTs) that compared the continuation of CNI with conversion to mTORi therapy.
Results: Twenty-nine RCTs (5,747 KTRs) were included in our analysis. Meta-analysis of the glomerular filtration rate (SMD 0.20; 95%CI 0.10–0.31; P<0.01) and malignancy (RR 0.74; 95%CI 0.55–0.99; P=0.04) demonstrated a significant advantage of mTORi conversion over CNI continuation. However, the risk of acute rejection (RR 1.58; 95%CI 1.22–2.04; P<0.01), infection (RR 1.55; 95%CI 1.01–1.31; P=0.04), proteinuria (RR 1.87; 95%CI 1.34–2.59; P<0.01), leukopenia (RR 1.56; 95%CI 1.27–1.91; P<0.01), acne (RR 6.43; 95%CI 3.43–12.04; P<0.01), and mouth ulcer (RR 11.70; 95%CI 6.18–22.17; P<0.01) were higher in the mTORi group. More patients in the conversion group had to discontinue study medication (RR 2.52; 95%CI 1.75–3.63; P<0.01). There was no significant difference between the two groups with regard to death, graft loss, diabetes, chronic allograft nephropathy, and interstitial fibrosis/tubular atrophy.
Conclusions: Posttransplant patients have a better graft function and lower incidence of malignancy after conversion from CNI to mTORi therapy. However, this conversion strategy may be prevented by the higher drug discontinuation rate due to mTORi-associated adverse events, such as more acute rejection, infection, proteinuria, leukopenia, acne, and mouth ulcer, indicating that conversion therapy may only be a treatment option in selected patients.
Introduction
Most kidney transplant recipients (KTRs) currently receive calcineurin inhibitor (CNI) therapy, which has remarkably reduced acute rejection (AR) episodes and improved early graft survival (1). However, long-term CNI exposure may induce irreversible nephrotoxicity, resulting in progressive graft dysfunction (2, 3). The CNI can also promote cardiovascular events and malignancies, which are the leading causes of premature death with a functioning graft (4, 5). This discrepancy has prompted investigations into CNI retention strategies, which maintain adequate immunosuppressive effects without compromising safety (6).
Mammalian target of rapamycin inhibitors (mTORi) are new immunosuppressants that exhibit little or minimal nephrotoxicity (7–9). The dual immunosuppressive and antineoplastic properties of mTORi offer a distinct advantage for this class of drugs in the treatment of patients who receive kidney transplant (10, 11). Nevertheless, major concerns associated with the de novo introduction of mTORi include the risk of AR, impaired wound healing, and prolonged delayed graft function (DGF), limiting the adoption of mTORi as a first-line immunosuppressant (9, 12, 13).
Thus, increasing interest has been directed toward exploiting the synergistic actions of CNIs and mTORi by administering the CNI at the initial stage of high-risk AR after transplantation and then converting it into mTORi before the onset of CNI-induced irreversible nephrotoxicity (14). Evidence regarding the clinical benefit of this conversion strategy is conflicting. Budde et al. (15) demonstrated that mTORi combined with CNI withdrawal improved kidney function while maintaining efficacy and safety. In contrast, several studies reported that mTORi therapy not only did fail to show any overall clinical benefit but also mTORi conversion was associated with more adverse events (16) and discontinuations (17). Thus, further evidence for the validity of mTORi conversion strategy is urgently needed (18). Our study aimed to systematically identify and evaluate the currently available evidence from randomized controlled trials (RCTs) on the outcomes of conversion from the CNI to mTORi for KTRs.
Materials and Methods
Our systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (19).
Eligibility Criteria
Studies were eligible if they met the following criteria: (a) RCTs of conversion from CNIs to mTORi maintenance immunosuppressive regimen; (b) participants were the recipients of a single organ kidney transplant (first or repeat) from a living or deceased donor; (c) initial immunosuppression consisted of a CNI but not an mTORi; and (d) KTRs were randomly assigned to either continue with current CNI therapy or be converted from the CNI to mTORi. Dose modifications of CNIs, mTORi, and other concomitant immunosuppressants were permissible after randomization. Randomized trials with less than 20 cases in each group or abstracts of major transplant conferences were excluded. KTRs with a history of malignancy (other than adequately treated non-melanoma skin carcinoma) before randomization were excluded. To minimize participant overlap, when the same study was reported many times, the study with long follow-up period and complete case report was identified as the primary data source.
Search Strategy
MEDLINE, EMBASE, PubMed, and Cochrane Library were searched from the start date of each resource up to November 12, 2020 (the last literature search) using logical combinations of relevant keywords and medical subject headings that contained all spellings of the following terms: kidney transplantation, calcineurin inhibitor, CNIs, tacrolimus, cyclosporine, conversion, mTORi, sirolimus, and everolimus. No restrictions regarding the language of the publication, age of KTRs, or concomitant immunosuppressants were applied. The results were supplemented by manually searching reference lists of relevant reviews and included articles. All citations identified by this search strategy were evaluated by two independent reviewers (JZ and QZ) using titles, abstracts, and, where required, the full text to determine eligibility.
Outcome Measures
The primary endpoints were graft function, AR, graft loss, and death. The secondary outcomes were adverse events (AEs), infections, malignancies, and discontinuation of medication.
Data Extraction
Eligible RCTs were referred to throughout this paper by the first author (JZ and QZ) and the year of the earliest peer-reviewed publication. Data extraction was independently performed by two reviewers (JZ and QZ) using a predesigned form on which study design, participant characteristics, interventions, and outcome data were recorded. Missing information was requested from the trial authors or sponsors. They met to combine their findings, and the information was subsequently entered into Review Manager (RevMan) (Version 5.4, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration).
Quality Assessment and Statistical Analyses
Cochrane Collaboration’s tool was used to assess the risk of bias of randomized controlled trials (20). RevMan 5.4 was utilized for the execution of the meta-analysis. Dichotomous outcomes were expressed as risk ratios (RRs) with 95% confidence intervals (CIs), and continuous variables were expressed as mean differences (MDs) with 95% CI. Data were pooled using the standardized mean difference (SMD) as the summary effect size metric. P<0.05 was considered statistically significant. Meta-analysis was conducted using the fixed effect model in the absence of heterogeneity; otherwise, the random effect was applied. Heterogeneity was quantified via the Cochrane Q (P<0.1) and I2 statistics (I2>50%). If heterogeneity existed, a sensitivity analysis was performed to explore possible sources of heterogeneity.
Results
Search and Selection
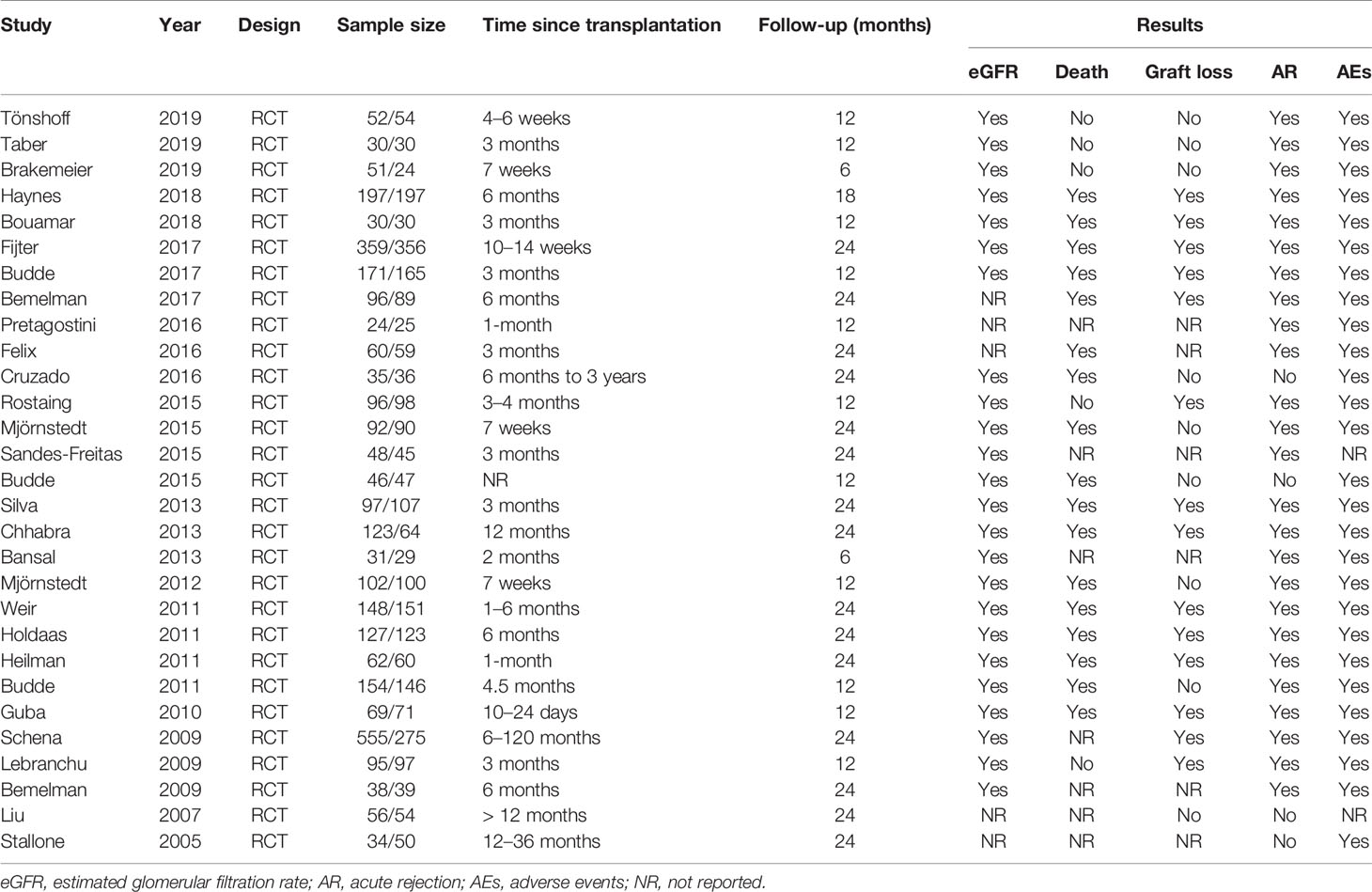
The search and selection flow chart is illustrated in Figure 1. Initial literature searches identified 1,488 citations across all databases. The titles and abstracts were reviewed, followed by full-text review of potentially eligible articles and abstracts, of which 29 RCTs met the inclusion criteria (15, 17, 21–47). The baseline overview of included studies is reported in Table 1.
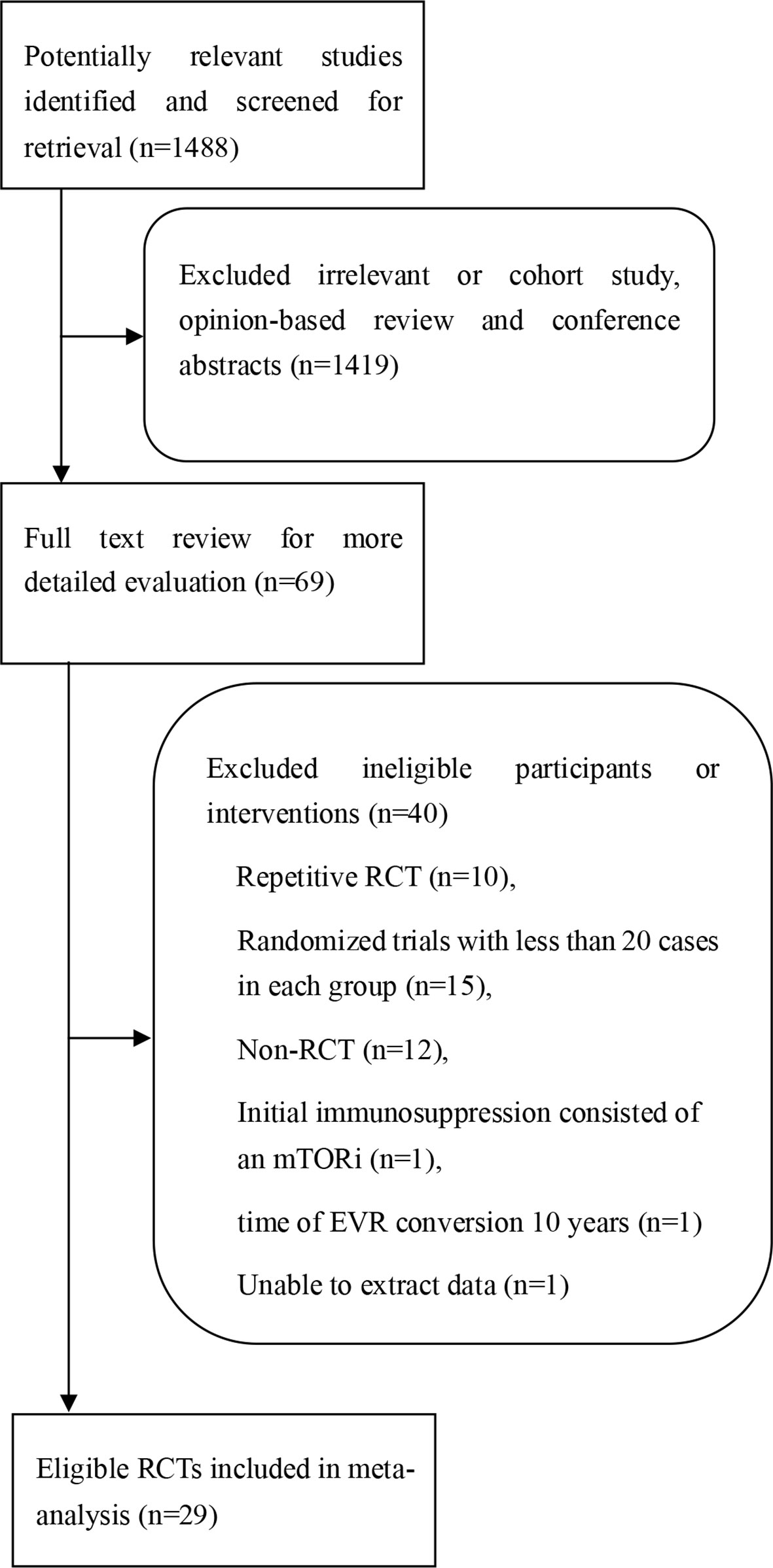
Figure 1 Procedure for the search and selection of RCTs included in the systematic review and meta-analysis.
Description of Eligible Trials
There were 29 eligible RCTs, in which a total number of 5,747 KTRs were included, whose characteristics are summarized in Table S1. Among these 29 trials, 22 trials reported the time of conversion to mTORi within 6 months after transplantation; the rest were more than 6 months. A total of 16 RCTs reported the impressive therapy of conversion from the CNI to everolimus. The patient sample size ranged from 49 to 830, and follow-up duration was from 6 to 24 months.
Quality of Included Trials
The quality of the 29 clinical trials was evaluated by Cochrane Collaboration’s tool (Figure S1). A total of 14 studies exhibited an adequate random sequence generation process, 15 trials described the methods used for allocation concealment, 2 RCTs illustrated performance bias, and 4 studies had unreported data. In short, the studies included had low to moderate risk of bias.
Primary Endpoints
The meta-analysis results of the primary endpoints are reported in Figures 2 and 3. A total of 22 trials reported renal function; the meta-analysis of the estimated glomerular filtration rate (eGFR) demonstrated a statistically significant advantage for mTORi conversion over CNI continuation (SMD 0.20; 95%CI 0.10–0.31; P<0.01) in Figure 2A. A total of 28 trials reported AR; we found that patients converted to mTORi were at a higher risk of developing AR (RR 1.58; 95%CI 1.22–2.04; P<0.01) in Figure 2B. A total of 22 trials reported graft loss; there was no difference between patients converted to mTORi and those remaining on the CNI (RR 1.14; 95%CI 0.75–1.74; P=0.53) in Figure 3A. A total of 22 trials reported mortality; we also found that there was no statistical difference in mortality between the mTORi conversion group and the CNI continuation arm (RR 0.99, 95%CI 0.64–1.55; P=0.97) in Figure 3B.
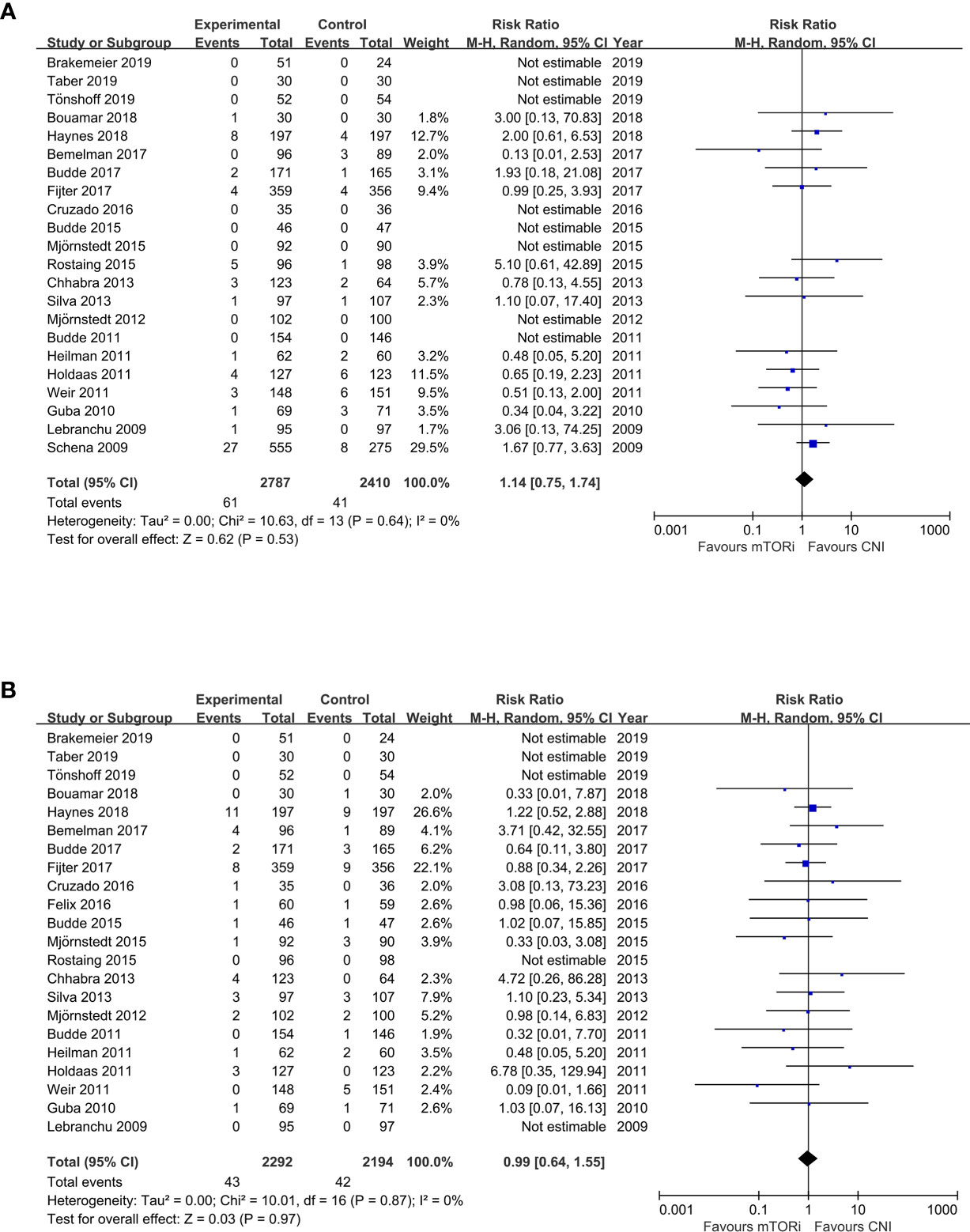
Figure 2 The forest plot of studies that compared conversion from CNIs to mTOR inhibitors versus maintenance of CNI therapy for the outcomes of (A) renal function (eGFR) and (B) acute rejection (AR).
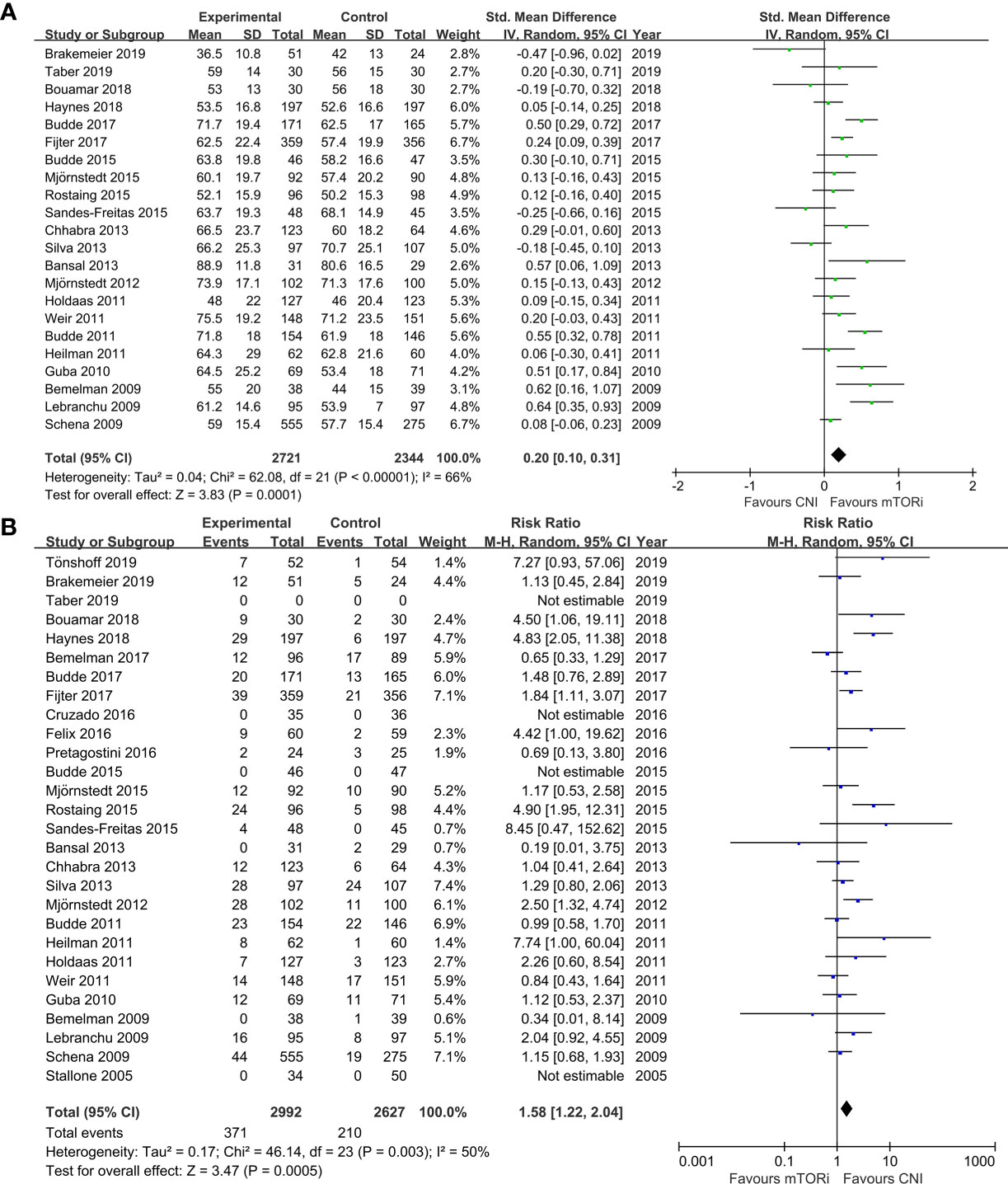
Figure 3 The forest plot of studies that compared conversion from CNIs to mTOR inhibitors versus maintenance of CNI therapy for the outcomes of (A) graft loss and (B) mortality.
Secondary Endpoints
Sixteen studies described the adverse events (AEs) and serious adverse events (sAEs). Despite the comparable rate of AEs (RR 1.03; 95%CI 0.99–1.06; P=0.11), patients converted into mTORi had a significantly higher risk to have sAEs (RR 1.23; 95%CI 1.09–1.39; P<0.01; Figure S2A). Twenty-one RCTs reported the drug discontinuation from AEs. There was a greater risk of drug discontinuation in patients who converted to mTORi compared with those on the CNI (RR 2.52; 95%CI 1.75–3.63; P<0.01; Figure S2B). Sixteen trials described infections, and eight trials described serious infections (defined as requiring hospitalization). We found that there was a higher risk of infections and serious infections in the mTORi conversion group compared with the remaining CNI group (RR 1.15; 95%CI 1.01–1.31; P=0.04; Figure S3A; RR 1.17; 95%CI 1.05–1.30; P<0.01; Figure S3B, respectively). Fifteen studies reported on cancer outcomes. Patients converted from the CNI to mTORi had lower risk of malignancy compared with those continuing on the CNI therapy (RR 0.74; 95%CI 0.55–0.99; P=0.04; Figure S4A). In addition to these advantageous results, we also found other adverse consequences brought about by the mTORi therapy, including proteinuria (RR 1.87; 95%CI 1.34–2.59; P<0.01; Figure S4B), mouth ulcer (RR 11.70; 95%CI 6.18–22.17; P<0.01; Figure S4C), anemia (RR 1.55; 95%CI 1.26–1.89; P<0.01; Figure S5A), leukopenia (RR 1.56; 95%CI 1.27–1.91; P<0.01; Figure S5B), thrombocytopenia (RR 2.45; 95%CI 1.13–5.35; P=0.02; Figure S5C), dyslipidemia (RR 1.41; 95%CI 1.27–1.58; P<0.01; Figure S6A), acne (RR 6.43; 95%CI 3.43–12.04; P<0.01; Figure S6B), diarrhea (RR 1.46; 95%CI 1.28–1.67; P<0.01, Figure S7A), and edema (RR 1.49; 95%CI 1.14–1.93; P<0.01; Figure S7B). In Figure S8, there were no differences in diabetes and interstitial fibrosis/tubular atrophy (IF/TA) between the mTORi conversion group and the CNI continuation group. On the other hand, there was no significant difference between the two groups with regard to CMV or BKV infection (Figure S9), chronic allograft nephropathy (increased serum creatine), gastroenteritis, pyrexia, posttransplant lymphoproliferative disease (PTLD), and wound-related problem (Table S3).
Discussion
The meta-analysis of the 29 eligible RCTs (involving 5,747 KTRs) demonstrated that the conversion from the CNI to mTORi after kidney transplantation was associated with an improvement in graft function and a reduced incidence of malignancy. There was no significant difference between the groups with respect to IF/TA or chronic allograft nephropathy, indicating that the improvement in graft function after conversion may primarily be due to effects other than structural improvement (48). Ten years ago, mTOR inhibitors were more commonly used, but only 1.9% of recipients were prescribed with them at transplant in 2016, increasing to 4.3% at 1-year posttransplant (49). Current studies reported that the combination of low-dose CNI (tacrolimus) and everolimus can make the difference in order to obtain the best clinic benefit for KTRs (50). Nevertheless, conversion to mTORi therapy may be prevented by the high discontinuation rate (51) due to the intolerable AEs, such as the development of serious infections, AR, mouth ulceration, proteinuria, anemia, leucopenia, thrombocytopenia, dyslipidemia, acne, diarrhea, and edema. All of these changes were statistically significant between the two groups. Thus, the application of mTORi conversion must be weighed during clinical decision-making, with the potential benefits and risks carefully assessed for each KTR.
Previous systematic review had suggested that conversion to sirolimus was associated with an improvement in short-term renal function, while none of the identified studies investigated everolimus (18, 52). Similarly, Lim et al. (53) reviewed RCTs comparing delayed conversion from CNIs to mTORi indicating that patients converted to mTORi up to 1-year posttransplant had higher GFR compared with those remaining on the CNI. Those findings were highly consistent with our meta-analysis, but we also found that there was no difference in CMV and BKV infection, and no difference in new-onset diabetes. Wolf et al. (54) concluded that the incidence of infections is lower when mTORi is combined with a CNI compared to a standard CNI therapy following renal transplantation, while we found that mTORi conversion therapy had more risk of infections.
In this systematic review, we identified all mTORi conversion RCTs involving both sirolimus and everolimus with heterogeneous treatment and varying follow-up period. We included the RCTs with more than 20 cases in each group, which made the evidence in this review stronger and more convincing. We also evaluated some outcomes (graft function, acute rejection, graft loss, death) and some secondary outcomes that are not commonly reported in other systematic reviews such as drug discontinuation and adverse events. Consequently, the current study provides critical information to guide clinical decision-making on the mTORi conversion strategy after kidney transplantation.
Nevertheless, there were potential caveats limiting the generalizability of our findings. First, besides mTORi-induced AEs, the open-label nature of the 29 RCTs may have contributed to the high discontinuation rate in the conversion group. KTRs in the conversion group underwent a major change in immunosuppression after successful transplantation, and the KTRs may have tended to revert to their original immunosuppression due to the perceived limited efficacy or newly emergent AEs. Second, the 29 RCTs differed with respect to the baseline graft function, induction therapy, conversion time after transplantation, indication for conversion, strategy for conversion (abrupt vs. stepwise), and target immunosuppressant exposure. There may be selection and measurement bias, either of which may ultimately affect the validity of the study results. Third, all KTRs received mycophenolic acid (MPA)-based immunosuppression. It has been documented that CNIs, but not mTORi, inhibit MPA enterohepatic recirculation, resulting in a 50% lower MPA exposure (55). In most of the eligible RCTs, MPA was administered at a fixed dose; after conversion, more KTRs in the conversion group may have been overexposed to MPA, ultimately contributing to the reported AEs. Fourth, most RCTs focused on KTRs with low-to-moderate immunological risk; therefore, our results cannot be extended to high-risk KTRs. Fifth, due to the difference in the number of included cases, we did not distinguish the difference between CsA and TAC conversion.
Determining the optimal timing or indication for mTORi conversion requires a balance between avoiding the high rejection risk in the initial posttransplant period and minimizing the progressive development of CNI-related nephrotoxicity. Conversion ≥1-year posttransplant may be insufficient to prevent progression to graft dysfunction, except in KTRs with normal graft function and without proteinuria (14). Conversion in the first 6 months after transplantation seems appropriate for maintenance therapy in KTRs after careful screening (14). Refining selection criteria may help both in identifying patients who will profit most from switching and in alleviating the need to reintroduce CNI therapy. Primarily based on the current consensus, the target population for mTORi conversion should be KTRs with a baseline GFR of more than 40 ml/min and normal urinary protein excretion, an absence of previous AR and subclinical rejection, and appearance of donor-specific antibody (14, 25). Periodic surveillance biopsies may also help to identify a subset of KTRs who do not develop subclinical rejection or irreversible pathological changes in the renal interstitial to guide mTORi conversion therapy (56).
The high rate of drug discontinuation in the conversion group illustrates the challenge of handling a new spectrum of AEs that differs from more familiar CNI-related AEs, including dyslipidemia, acne, mouth ulceration, proteinuria, and AR (57, 58). Most AEs occurred early after conversion and in some cases resolved without intervention within a few weeks. Some of the discontinuations in the conversion group could have been avoided with better patient selection and more refined management of mTORi-related AEs. Specific patient monitoring and the concomitant use of lipid-lowering agents, angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, and induction therapies should be considered for combating dyslipidemia, proteinuria, and AR (16, 59). The identification of the optimal timing of conversion, appropriate candidates, dosing regimens for conversion, and safe management of mTORi-related AEs will be key in minimizing the need for discontinuation, which currently limits the broad applicability of this strategy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
JZ designed the study and wrote the manuscript. XF, QZ, and LL performed the meta-analysis and did the data extraction. SF, YF, TS, and ZH checked the results. XW and TL designed the research strategy and revised the manuscript. All authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Sichuan Science and Technology Program [grant number 2019YJ0133]; Chengdu Science and Technology Program [grant number 2019-YF05-00084-SN]; and 1.3.5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University [grant number 2019-075, ZY2016104, 2021HXFH007].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.663602/full#supplementary-material
Abbreviations
AE, adverse event; AR, acute rejection; ATG, anti-thymocyte globulin; CI, confidence interval; CNI, calcineurin inhibitor; CsA, cyclosporine A; DGF, delayed graft function; eGFR, estimated glomerular filtration rate; EVL, everolimus; IF/TA, interstitial fibrosis/tubular atrophy; KTR, kidney transplant recipient; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitor; MD, mean difference; PTLD, posttransplant lymphoproliferative disease; RCT, randomized controlled trial; RR, risk ratio; SMD, standard mean difference; SRL, sirolimus; TAC, tacrolimus.
References
1. Lamb KE, Lodhi S, Meier-Kriesche HU. Long-Term Renal Allograft Survival in the United States: A Critical Reappraisal. Am J Transpl (2011) 11(3):450–62. doi: 10.1111/j.1600-6143.2010.03283.x
2. Vincenti F. Are Calcineurin Inhibitors-Free Regimens Ready for Prime Time? Kidney Int (2012) 82(10):1054–60. doi: 10.1038/ki.2012.194
3. Maluf DG, Dumur CI, Suh JL, Lee JK, Cathro HP, King AL, et al. Evaluation of Molecular Profiles in Calcineurin Inhibitor Toxicity Post-Kidney Transplant: Input to Chronic Allograft Dysfunction. Am J Transpl (2014) 14(5):1152–63. doi: 10.1111/ajt.12696
4. Mourer JS, de Koning EJ, van Zwet EW, Mallat MJ, Rabelink TJ, de Fijter JW. Impact of Late Calcineurin Inhibitor Withdrawal on Ambulatory Blood Pressure and Carotid Intima Media Thickness in Renal Transplant Recipients. Transplantation (2013) 96(1):49–57. doi: 10.1097/TP.0b013e3182958552
5. Mohan S, Hirsch J. Risk of Malignancy After Renal Transplantation. Transplantation (2013) 95(1):17–8. doi: 10.1097/TP.0b013e31827b3d70
6. Sharif A, Shabir S, Chand S, Cockwell P, Ball S, Borrows R. Meta-Analysis of Calcineurin-Inhibitor-Sparing Regimens in Kidney Transplantation. J Am Soc Nephrol (2011) 22(11):2107–18. doi: 10.1681/ASN.2010111160
7. Sutherland AI, Akhtar MZ, Zilvetti M, Brockmann J, Ruse S, Fuggle SV, et al. Alemtuzumab and Sirolimus in Renal Transplantation: Six-Year Results of a Single-Arm Prospective Pilot Study. Am J Transpl (2014) 14(3):677–84. doi: 10.1111/ajt.12572
8. Russ G, Segoloni G, Oberbauer R, Legendre C, Mota A, Eris J, et al. Superior Outcomes in Renal Transplantation After Early Cyclosporine Withdrawal and Sirolimus Maintenance Therapy, Regardless of Baseline Renal Function. Transplantation (2005) 80(9):1204–11. doi: 10.1097/01.tp.0000178393.78084.9b
9. Webster AC, Lee VW, Chapman JR, Craig JC. Target of Rapamycin Inhibitors (Sirolimus and Everolimus) for Primary Immunosuppression of Kidney Transplant Recipients: A Systematic Review and Meta-Analysis of Randomized Trials. Transplantation (2006) 81(9):1234–48. doi: 10.1097/01.tp.0000219703.39149.85
10. Peddi VR, Wiseman A, Chavin K, Slakey D. Review of Combination Therapy With mTOR Inhibitors and Tacrolimus Minimization After Transplantation. Transplant Rev (Orlando) (2013) 27(4):97–107. doi: 10.1016/j.trre.2013.06.001
11. Webster AC, Lee VW, Chapman JR, Craig JC. Target of Rapamycin Inhibitors (TOR-I; Sirolimus and Everolimus) for Primary Immunosuppression in Kidney Transplant Recipients. Cochrane Database Syst Rev (2006) (2):CD004290. doi: 10.1002/14651858.CD004290.pub2
12. Nashan B, Citterio F. Wound Healing Complications and the Use of Mammalian Target of Rapamycin Inhibitors in Kidney Transplantation: A Critical Review of the Literature. Transplantation (2012) 94(6):547–61. doi: 10.1097/TP.0b013e3182551021
13. Morath C, Arns W, Schwenger V, Mehrabi A, Fonouni H, Schmidt J, et al. Sirolimus in Renal Transplantation. Nephrol Dialysis Transpl (2007) 22(Supplement 8):viii61–5. doi: 10.1093/ndt/gfm652
14. Gatault P, Lebranchu Y. Conversion to mTOR-Inhibitor-Based Immunosuppression: Which Patients and When? Transplant Res (2013) 2(Suppl 1):S3. doi: 10.1186/2047-1440-2-S1-S3
15. Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Everolimus-Based, Calcineurin-Inhibitor-Free Regimen in Recipients of De-Novo Kidney Transplants: An Open-Label, Randomised, Controlled Trial. Lancet (2011) 377(9768):837–47. doi: 10.1016/S0140-6736(10)62318-5
16. Claes K, Meier-Kriesche HU, Schold JD, Vanrenterghem Y, Halloran PF, Ekberg H, et al. Effect of Different Immunosuppressive Regimens on the Evolution of Distinct Metabolic Parameters: Evidence From the Symphony Study. Nephrol Dial Transpl (2012) 27(2):850–7. doi: 10.1093/ndt/gfr238
17. Holdaas H, Rostaing L, Seron D, Cole E, Chapman J, Fellstrom B, et al. Conversion of Long-Term Kidney Transplant Recipients From Calcineurin Inhibitor Therapy to Everolimus: A Randomized, Multicenter, 24-Month Study. Transplantation (2011) 92(4):410–8. doi: 10.1097/TP.0b013e318224c12d
18. Mulay AV, Cockfield S, Stryker R, Fergusson D, Knoll GA. Conversion From Calcineurin Inhibitors to Sirolimus for Chronic Renal Allograft Dysfunction: A Systematic Review of the Evidence. Transplantation (2006) 82(9):1153–62. doi: 10.1097/01.tp.0000237101.58974.43
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
20. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
21. Stallone G, Infante B, Schena A, Battaglia M, Ditonno P, Loverre A, et al. Rapamycin for Treatment of Chronic Allograft Nephropathy in Renal Transplant Patients. J Am Soc Nephrol (2005) 16(12):3755–62. doi: 10.1681/ASN.2005060635
22. Liu M, Zhang W, Gu M, Yin C, Zhang WY, Lv Q, et al. Protective Effects of Sirolimus by Attenuating Connective Tissue Growth Factor Expression in Human Chronic Allograft Nephropathy. Transplant Proc (2007) 39(5):1410–5. doi: 10.1016/j.transproceed.2007.03.072
23. Bemelman FJ, de Maar EF, Press RR, van Kan HJ, ten Berge IJ. Minimization of Maintenance Immunosuppression Early After Renal Transplantation: An Interim Analysis. Transplantation (2009) 88(3):421–8. doi: 10.1097/TP.0b013e3181af1df6
24. Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, et al. Efficacy on Renal Function of Early Conversion From Cyclosporine to Sirolimus 3 Months After Renal Transplantation: Concept Study. Am J Transpl (2009) 9(5):1115–23. doi: 10.1111/j.1600-6143.2009.02615.x
25. Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, et al. Conversion From Calcineurin Inhibitors to Sirolimus Maintenance Therapy in Renal Allograft Recipients: 24-Month Efficacy and Safety Results From the CONVERT Trial. Transplantation (2009) 87(2):233–42. doi: 10.1097/TP.0b013e3181927a41
26. Guba M, Pratschke J, Hugo C, Kramer BK, Nohr-Westphal C, Brockmann J, et al. Renal Function, Efficacy, and Safety of Sirolimus and Mycophenolate Mofetil After Short-Term Calcineurin Inhibitor-Based Quadruple Therapy in De Novo Renal Transplant Patients: One-Year Analysis of a Randomized Multicenter Trial. Transplantation (2010) 90(2):175–83. doi: 10.1097/TP.0b013e3181e11798
27. Heilman RL, Younan K, Wadei HM, Mai ML, Reddy KS, Chakkera HA, et al. Results of a Prospective Randomized Trial of Sirolimus Conversion in Kidney Transplant Recipients on Early Corticosteroid Withdrawal. Transplantation (2011) 92(7):767–73. doi: 10.1097/TP.0b013e31822805d7
28. Weir MR, Mulgaonkar S, Chan L, Shidban H, Waid TH, Preston D, et al. Mycophenolate Mofetil-Based Immunosuppression With Sirolimus in Renal Transplantation: A Randomized, Controlled Spare-The-Nephron Trial. Kidney Int (2011) 79(8):897–907. doi: 10.1038/ki.2010.492
29. Mjornstedt L, Sorensen SS, von Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Improved Renal Function After Early Conversion From a Calcineurin Inhibitor to Everolimus: A Randomized Trial in Kidney Transplantation. Am J Transpl (2012) 12(10):2744–53. doi: 10.1111/j.1600-6143.2012.04162.x
30. Bansal D, Yadav AK, Kumar V, Minz M, Sakhuja V, Jha V. Deferred Pre-Emptive Switch From Calcineurin Inhibitor to Sirolimus Leads to Improvement in GFR and Expansion of T Regulatory Cell Population: A Randomized, Controlled Trial. PLoS One (2013) 8(10):e75591. doi: 10.1371/journal.pone.0075591
31. Chhabra D, Alvarado A, Dalal P, Leventhal J, Wang C, Sustento-Reodica N, et al. Impact of Calcineurin-Inhibitor Conversion to mTOR Inhibitor on Renal Allograft Function in a Prednisone-Free Regimen. Am J Transpl (2013) 13(11):2902–11. doi: 10.1111/ajt.12437
32. Silva HT Jr, Felipe CR, Garcia VD, Neto ED, Filho MA, Contieri FL, et al. Planned Randomized Conversion From Tacrolimus to Sirolimus-Based Immunosuppressive Regimen in De Novo Kidney Transplant Recipients. Am J Transpl (2013) 13(12):3155–63. doi: 10.1111/ajt.12481
33. Budde K, Rath T, Sommerer C, Haller H, Reinke P, Witzke O, et al. Renal, Efficacy and Safety Outcomes Following Late Conversion of Kidney Transplant Patients From Calcineurin Inhibitor Therapy to Everolimus: The Randomized APOLLO Study. Clin Nephrol (2015) 83(1):11–21. doi: 10.5414/CN108444
34. de Sandes-Freitas TV, Felipe CR, Campos EF, de Lima MG, Soares MF, de Franco MF, et al. Subclinical Lesions and Donor-Specific Antibodies in Kidney Transplant Recipients Receiving Tacrolimus-Based Immunosuppressive Regimen Followed by Early Conversion to Sirolimus. Transplantation (2015) 99(11):2372–81. doi: 10.1097/TP.0000000000000748
35. Mjornstedt L, Schwartz Sorensen S, von Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Renal Function Three Years After Early Conversion From a Calcineurin Inhibitor to Everolimus: Results From a Randomized Trial in Kidney Transplantation. Transpl Int (2015) 28(1):42–51. doi: 10.1111/tri.12437
36. Rostaing L, Hertig A, Albano L, Anglicheau D, Durrbach A, Vuiblet V, et al. Fibrosis Progression According to Epithelial-Mesenchymal Transition Profile: A Randomized Trial of Everolimus Versus CsA. Am J Transpl (2015) 15(5):1303–12. doi: 10.1111/ajt.13132
37. Cruzado JM, Pascual J, Sanchez-Fructuoso A, Seron D, Diaz JM, Rengel M, et al. Controlled Randomized Study Comparing the Cardiovascular Profile of Everolimus With Tacrolimus in Renal Transplantation. Transpl Int (2016) 29(12):1317–28. doi: 10.1111/tri.12862
38. Felix MJ, Felipe CR, Tedesco-Silva H, Osmar Medina-Pestana J. Time-Dependent and Immunosuppressive Drug-Associated Adverse Event Profiles in De Novo Kidney Transplant Recipients Converted From Tacrolimus to Sirolimus Regimens. Pharmacotherapy (2016) 36(2):152–65. doi: 10.1002/phar.1692
39. Pretagostini R, Poli L, Pettorini L, Lai Q, Garofalo M, Melandro F, et al. Delayed Introduction of Everolimus in De Novo Renal Transplanted Patients: A Single-Center Experience. Transplant Proc (2016) 48(2):326–8. doi: 10.1016/j.transproceed.2016.02.004
40. Bemelman FJ, de Fijter JW, Kers J, Meyer C, Peters-Sengers H, de Maar EF, et al. Early Conversion to Prednisolone/Everolimus as an Alternative Weaning Regimen Associates With Beneficial Renal Transplant Histology and Function: The Randomized-Controlled MECANO Trial. Am J Transpl (2017) 17(4):1020–30. doi: 10.1111/ajt.14048
41. Budde K, Zeier M, Witzke O, Arns W, Lehner F, Guba M, et al. Everolimus With Cyclosporine Withdrawal or Low-Exposure Cyclosporine in Kidney Transplantation From Month 3: A Multicentre, Randomized Trial. Nephrol Dial Transpl (2017) 32(6):1060–70. doi: 10.1093/ndt/gfx075
42. de Fijter JW, Holdaas H, Oyen O, Sanders JS, Sundar S, Bemelman FJ, et al. Early Conversion From Calcineurin Inhibitor- to Everolimus-Based Therapy Following Kidney Transplantation: Results of the Randomized ELEVATE Trial. Am J Transpl (2017) 17(7):1853–67. doi: 10.1111/ajt.14186
43. Bouamar R, Shuker N, Osinga JAJ, Groningen MCC-v, Wetering Jvd, Rowshani AT, et al. Conversion From Tacrolimus to Everolimus With Complete and Early Glucocorticoid Withdrawal After Kidney Transplantation: A Randomised Trial. Netherlands J Med (2018) 76(1):14–26.
44. Group CSC. Campath, Calcineurin Inhibitor Reduction, and Chronic Allograft Nephropathy (The 3C Study) - Results of a Randomized Controlled Clinical Trial. Am J Transpl (2018) 18(6):1424–34. doi: 10.1111/ajt.14619
45. Brakemeier S, Arns W, Lehner F, Witzke O, Vonend O, Sommerer C, et al. Everolimus in De Novo Kidney Transplant Recipients Participating in the Eurotransplant Senior Program: Results of a Prospective Randomized Multicenter Study (SENATOR). PLoS One (2019) 14(9):e0222730. doi: 10.1371/journal.pone.0222730
46. Taber DJ, Chokkalingam A, Su Z, Self S, Miller D, Srinivas T. Randomized Controlled Trial Assessing the Impact of Everolimus and Low-Exposure Tacrolimus on Graft Outcomes in Kidney Transplant Recipients. Clin Transpl (2019) 33(10):e13679. doi: 10.1111/ctr.13679
47. Tonshoff B, Ettenger R, Dello Strologo L, Marks SD, Pape L, Tedesco-Silva H Jr, et al. Early Conversion of Pediatric Kidney Transplant Patients to Everolimus With Reduced Tacrolimus and Steroid Elimination: Results of a Randomized Trial. Am J Transpl (2019) 19(3):811–22. doi: 10.1111/ajt.15081
48. Franz S, Regeniter A, Hopfer H, Mihatsch M, Dickenmann M. Tubular Toxicity in Sirolimus- and Cyclosporine-Based Transplant Immunosuppression Strategies: An Ancillary Study From a Randomized Controlled Trial. Am J Kidney Dis (2010) 55(2):335–43. doi: 10.1053/j.ajkd.2009.09.004
49. Har A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Robinson A, et al. OPTN/SRTR 2016 Annual Data Report: Kidney. Am J Transpl (2018) 18:18–113. doi: 10.1111/ajt.14557
50. Wolf S, Hoffmann VS, Habicht A, Kauke T, Bucher J, Schoenberg M, et al. Effects of mTOR-Is on Malignancy and Survival Following Renal Transplantation: A Systematic Review and Meta-Analysis of Randomized Trials With a Minimum Follow-Up of 24 Months. PLoS One (2018) 13(4):e0194975. doi: 10.1371/journal.pone.0194975
51. Sanchez-Escuredo A, Diekmann F, Revuelta I, Esforzado N, Ricart MJ, Cofan F, et al. An mTOR-Inhibitor-Based Protocol and Calcineurin Inhibitor (CNI)-Free Treatment in Kidney Transplant Recipients From Donors After Cardiac Death: Good Renal Function, But High Incidence of Conversion to CNI. Transpl Int (2016) 29(3):362–8. doi: 10.1111/tri.12732
52. Glover TE, Watson CJ, Gibbs P, Bradley JA, Ntzani EE, Kosmoliaptsis V. Conversion From Calcineurin to Mammalian Target of Rapamycin Inhibitors in Liver Transplantation: A Meta-Analysis of Randomized Controlled Trials. Transplantation (2016) 100(3):621–9. doi: 10.1097/TP.0000000000001006
53. Lim WH, Eris J, Kanellis J, Pussell B, Wiid Z, Witcombe D, et al. A Systematic Review of Conversion From Calcineurin Inhibitor to Mammalian Target of Rapamycin Inhibitors for Maintenance Immunosuppression in Kidney Transplant Recipients. Am J Transpl (2014) 14(9):2106–19. doi: 10.1111/ajt.12795
54. Wolf S, Lauseker M, Schiergens T, Wirth U, Drefs M, Renz B, et al. Infections After Kidney Transplantation: A Comparison of mTOR-Is and CNIs as Basic Immunosuppressants. A Systematic Review and Meta-Analysis. Transpl Infect Dis (2020) 22(3):e13267. doi: 10.1111/tid.13267
55. Chaabane A, Aouam K, Ben Fredj N, Hammouda M, Chadly Z, El May M, et al. Limited Sampling Strategy of Mycophenolic Acid in Adult Kidney Transplant Recipients: Influence of the Post-Transplant Period and the Pharmacokinetic Profile. J Clin Pharmacol (2013) 53(9):925–33. doi: 10.1002/jcph.125
56. Thierry A, Thervet E, Vuiblet V, Goujon JM, Machet MC, Noel LH, et al. Long-Term Impact of Subclinical Inflammation Diagnosed by Protocol Biopsy One Year After Renal Transplantation. Am J Transpl (2011) 11(10):2153–61. doi: 10.1111/j.1600-6143.2011.03695.x
57. Ekberg H, Bernasconi C, Noldeke J, Yussim A, Mjornstedt L, Erken U, et al. Cyclosporine, Tacrolimus and Sirolimus Retain Their Distinct Toxicity Profiles Despite Low Doses in the Symphony Study. Nephrol Dial Transpl (2010) 25(6):2004–10. doi: 10.1093/ndt/gfp778
58. Hamdy AF, Bakr MA, Ghoneim MA. Long-Term Efficacy and Safety of a Calcineurin Inhibitor-Free Regimen in Live-Donor Renal Transplant Recipients. J Am Soc Nephrol (2008) 19(6):1225–32. doi: 10.1681/ASN.2007091001
Keywords: calcineurin inhibitor, mammalian-target-of-rapamycin inhibitor, kidney transplantation, conversion, meta-analysis
Citation: Zeng J, Zhong Q, Feng X, Li L, Feng S, Fan Y, Song T, Huang Z, Wang X and Lin T (2021) Conversion From Calcineurin Inhibitors to Mammalian Target of Rapamycin Inhibitors in Kidney Transplant Recipients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Immunol. 12:663602. doi: 10.3389/fimmu.2021.663602
Received: 03 February 2021; Accepted: 16 August 2021;
Published: 03 September 2021.
Edited by:
Soldevila Gloria, National Autonomous University of Mexico, MexicoReviewed by:
Josefina M. Alberu, Tecnológico de Monterrey, MexicoJianghua Chen, Zhejiang University, China
Copyright © 2021 Zeng, Zhong, Feng, Li, Feng, Fan, Song, Huang, Wang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianding Wang, eGlhbmRpbmd3YW5nQG91dGxvb2suY29t; Tao Lin, a2lkbmV5NUAxNjMuY29t
†These authors share first authorship
 Jun Zeng
Jun Zeng Qiang Zhong
Qiang Zhong Xiaobing Feng1,2
Xiaobing Feng1,2 Linde Li
Linde Li Xianding Wang
Xianding Wang Tao Lin
Tao Lin