- 1NHC Key Laboratory of Immunodermatology, Ministry of Education Key Laboratory of Immunodermatology, National Joint Engineering Research Center for Diagnosis and Treatment of Immunologic Skin Diseases, Department of Dermatology, The First Hospital of China Medical University, Shenyang, China
- 2Department of Dermatology, The First Hospital of China Medical University, Shenyang, China
Background: Pemphigus is a rare but life-threatening autoimmune skin disease characterized by blistering on skin and/or mucous membranes. The physiological process of blister formation involves IgG antibodies against the desmogleins (Dsgs) and desmocollins (Dscs). Additional autoAbs have also been suggested to mediate the disease heterogeneity, such as anti-thyroid peroxidase (anti-TPO) and antithyroglobulin (anti-Tg) antibodies, the essential culprits of the immune system in autoimmune thyroid diseases.
Purpose: To investigate the levels and antibody positivity of anti-TPO and anti-Tg antibodies in pemphigus patients.
Methods: Antibody positivity and levels of anti-TPO and anti-Tg antibodies in pemphigus patients as compared to healthy controls were examined. A meta-analysis was conducted by reviewing six similar studies.
Results: 98 Chinese pemphigus patients and 65 healthy controls were enrolled in the study. Our meta-analysis revealed a significant correlation between increased presence of positive anti-TPO and anti-Tg antibodies and pemphigus, particularly for pemphigus vulgaris (PV). Such correlation was also observed in our own hospitalized PV patients, but not in pemphigus foliaceus (PF) patients. In addition, the status of anti-TPO and anti-Tg antibodies were also compared between females and males within PV patients, PF patients or controls, as well as compared for females or males between pemphigus patients and controls. In the analysis of T cell counts, we found abnormal low CD3 + T cell counts (< 690 n/µl) were only detected in patients whose thyroid antibody levels were less than 20 IU/ml.
Conclusion: Pemphigus patients showed higher levels and antibody positivity of anti-TPO and anti-Tg antibodies than healthy controls. Further investigations are needed to identify the pathogenic functions of these antibodies in pemphigus, as well as to identify the potential shared susceptibility genes.
Introduction
Pemphigus is a rare but life-threatening autoimmune skin disease characterized by blistering on skin and/or mucous membranes (1, 2). The pathogenesis of pemphigus is rather complicated, with genetic and environmental factors being the most well recognized contributors to this severe disease (3). The physiological process of blister formation involves IgG antibodies against the desmoglein (Dsg) 1 and 3 (4, 5), and desmocollin (Dsc) 1-3 (6–8). The most common clinical variant of pemphigus, pemphigus vulgaris (PV), is generally associated with Dsg3 for the mucosal-dominant phenotype, and both Dsg1 and Dsg3 for the mucocutaneous/cutaneous phenotype (9). Whereas autoantibodies against Dsg1 are also present in patients with pemphigus foliaceus (PF), another major clinical variant of pemphigus that displays a phenotype of blistering in the superficial epidermis (10).
Although antibodies against desmosomal proteins are clearly correlated with disease progression in most pemphigus patients, however, accumulating evidence suggested the potential participation of additional autoAbs in determining the disease heterogeneity in pemphigus patients (11–14). Several studies have focused on antithyroglobulin (anti-Tg) and anti-thyroid peroxidase (anti-TPO) autoAbs (15–20). Correlation between PV and the presence of thyroid antibodies have been documented in these studies, although the role of thyroid antibodies in mediating the cell-cell adhesion remains unclear (21). Anti-TPO and anti-Tg antibodies are essential targets of the immune system in autoimmune thyroid diseases (AITDs), and increased prevalence of AITDs such as Hashimoto’s thyroiditis and Graves’s disease has been reported in pemphigus patients in several studies (22–24).
Here we examined the levels and positivity of anti-TPO and anti-Tg antibodies in 98 Chinese pemphigus patients and 65 healthy controls, and reviewed the available literature on association between increased presence of positive anti-TPO and anti-Tg antibodies and pemphigus, particularly for PV. Such correlation was also observed in our Chinese PV patients, but not in PF patients. A gender-based comparison on the status of anti-TPO and anti-Tg antibodies was also conducted in our PV and PF patients.
Materials and Methods
Electronic Search
Databases including PubMed, EMBASE, the Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang database and Weipu database were systematically searched to identify all articles published to December of 2020. The search strategy consisted of the following index terms: (“Pemphigus Vulgaris” OR “Pemphigus Foliaceus” OR “Foliaceus, Pemphigus”) AND “Thyroid”. Additional manual search was also conducted on the references listed in identified articles.
Inclusion and Exclusion Criteria
For inclusion, the studies needed to provide sufficient information to allow the analysis of antibody positivity for anti-TPO and anti-Tg antibodies in both pemphigus patients and control individuals. Studies enrolled patients with or without history of thyroid disease were both considered eligible. No restrictions on the methodologies for detecting levels of anti-TPO and anti-Tg antibodies were made. The studies were published in English or Chinese. Articles providing insufficient data, irrelevant to our topics, review article or case reports were excluded.
Data Extraction and Quality Assessment
Two authors (H-X W, YY) independently evaluated all titles and abstracts of retrieved studies, and full texts that met the inclusion criteria were ultimately obtained. Disagreements on the eligibility of studies were resolved through discussion between the two authors, or if necessary, also with a third party (SQ). The following information was extracted from selected articles by two authors independently (H-XW, YY): name of the first author, publication year, country, sample size, type of pemphigus, thyroid function tests, gender, age and prevalence rate.
The quality of selected articles was assessed independently by two authors (H-XW, YY) by using the Newcastle-Ottawa-Scale (NOS). Each article was scored based on three dimensions: subject selection (0-3); comparability (0-4); and exposure (0-2). The total NOS score range from 0 to 8.
Study Population
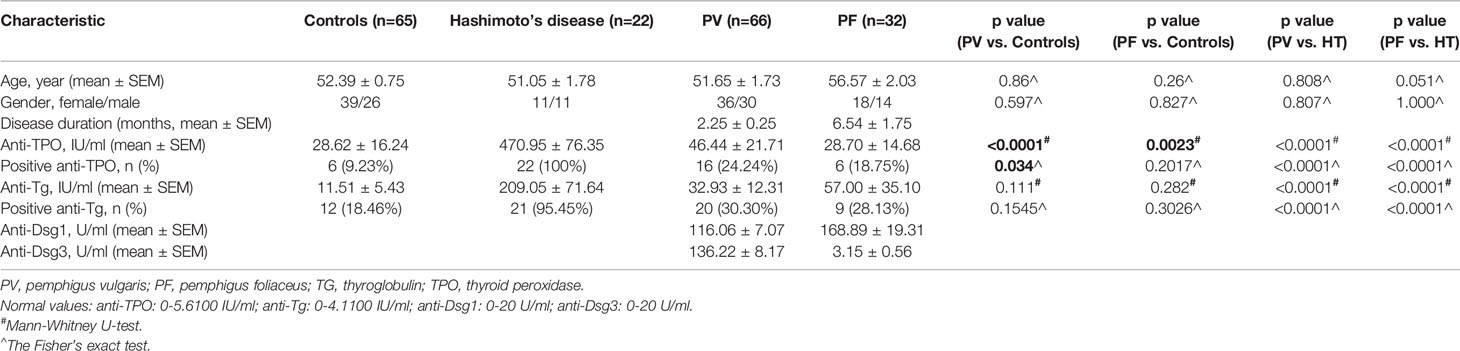
Information on 98 patients with a diagnosis of pemphigus (66 PV and 32 PF) at active phase was enrolled in our study. They were hospitalized patients of the Department of Dermatology at the First Hospital of China Medical University from 2013 to 2019. The study was approved by the ethics committee of the First Hospital of China Medical University (AF-SOP-07-1.1-01) and conducted according to the Declaration of Helsinki. Study participants gave written informed consent before they were included in the study. The diagnosis of pemphigus was confirmed based on clinical, histological, immunological (direct and indirect immunofluorescence) examinations and serological tests. Seventeen PV patients and ten PF patients were under corticosteroid therapy, immunoglobulin therapy or immunosuppressive therapy, while the other forty-nine PV patients and twenty-two PF patients were not under any treatment when the blood samples were drawn. Demographic, clinical and laboratory information of pemphigus patients were retrospectively collected from medical records. Control serum samples were collected from 65 healthy age- and sex-matched Chinese Han ethnic volunteers as described previously (25). Pemphigus patients with history of thyroid dysfunction or thyroid surgery, or malignancies were excluded.
Chemiluminescence Microparticle Immunoassay
Evaluation of serum levels of anti-TPO and anti-Tg antibodies was performed according to the manufacturer’s protocols (Abbott Park, Middletown, USA). Levels of anti-Tg > 4.1100 IU/ml and anti-TPO > 5.6100 IU/ml were defined as antibody positive.
ELISA
Serum samples were prepared at a dilution of 1:101, and each ELISA assay required 10 µl of undiluted serum sample. Elisa assay for anti-Dsg1 and anti-Dsg3 antibodies was performed using ELISA kit (MBL Intl, Japan) according to the manufacturers’ protocols. The detection range of anti-Dsg1 and anti-Dsg3 was 0-150 U/ml, and ELISA level > 20 U/ml was defined as Dsg antibody positive.
T Cell Subset Counting (Flow Cytometry)
200 µL of peripheral blood were stained for markers of CD3, CD4 and CD8, and T cell subset counting was analyzed according the manufacturer’s instructions by flow cytometry instrument (BDCantoII). Normal counts for T cell subsets were: CD3: 690-2540 n/µl; CD4:410-1590 n/µl; CD8:190-1140 n/µl.
Statistical Analysis
Statistical analysis of meta-analysis was performed by Stata software (version 15.0; Stata Corporation, Texas, USA). Pooled prevalence estimates and 95% confidence intervals (CIs) were calculated to determine the prevalence of positive thyroid antibodies in pemphigus patients. Sensitivity analysis was achieved by removing the study one by one to evaluate the reliability of the results.
Levels of anti-TPO and anti-Tg antibodies were summarized as means and were compared by the Mann-Whitney U test. Age, gender, prevalence of anti-TPO and anti-Tg antibodies between cases and controls, as well as prevalence of low T cell subsets between thyroid antibody positive group and negative group were compared using The Fisher’s exact test. Statistical analysis was performed by GraphPad Prism 7.0, and p-value < 0.05 was considered to be statistically significant.
Results
Study Selection and Characteristics
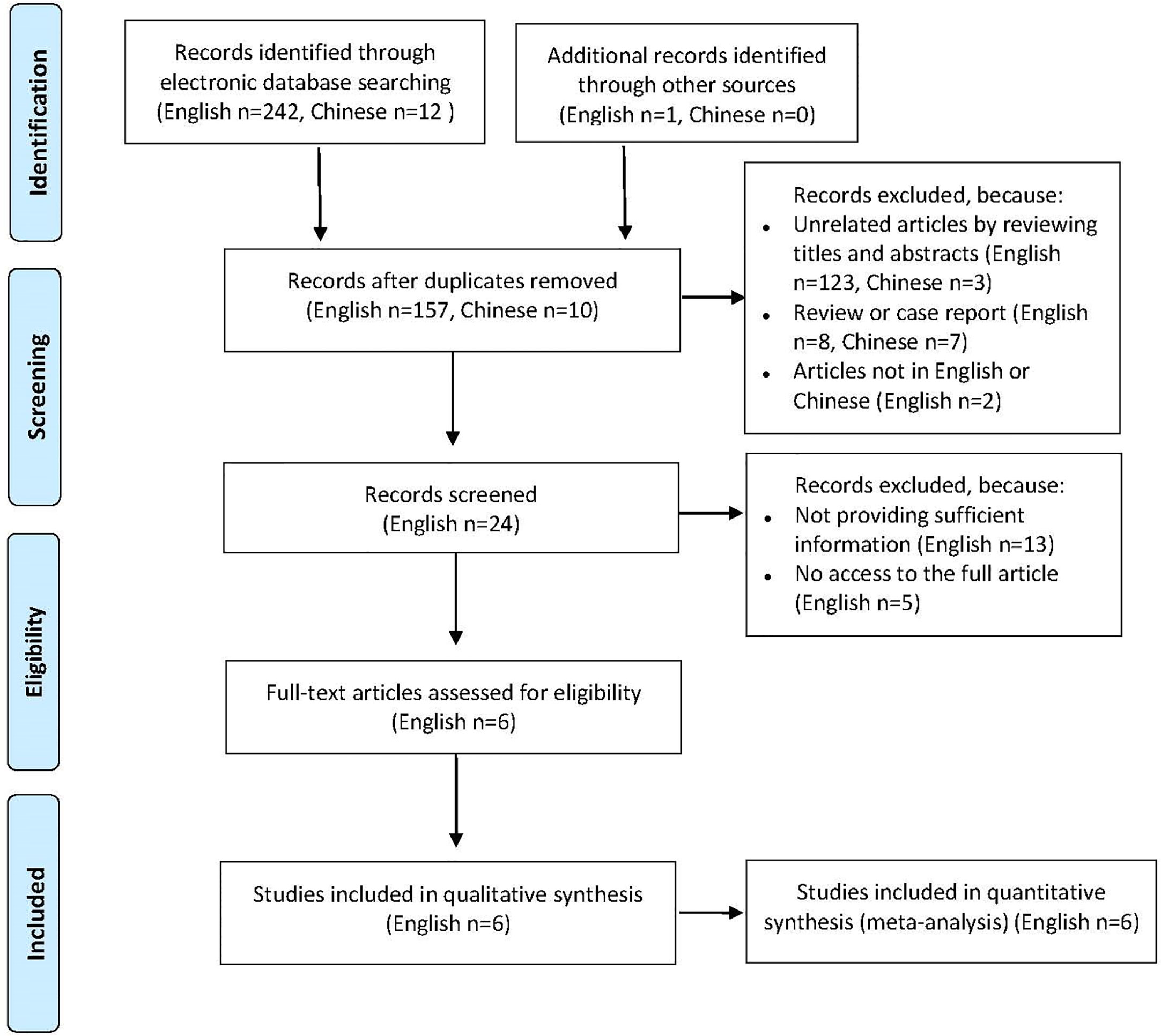
A total of 255 published articles were yielded based on the search strategy, and 6 articles were ultimately included in the meta-analysis after screening (Figure 1). Study characteristics and methodological quality were shown in Table 1 (15–20). Six selected articles were published between 2005 and 2018. All studies included a patient group and a control group, and of which four were well matched on age and sex. The sample size of patient population varied between 15 and 225, and the patients reported in 6 studies were based in USA, Italy, Turkey, Iran and Argentina, respectively. All studies enrolled patients with PV and one study additionally enrolled patients with nonparaneoplastic pemphigus.
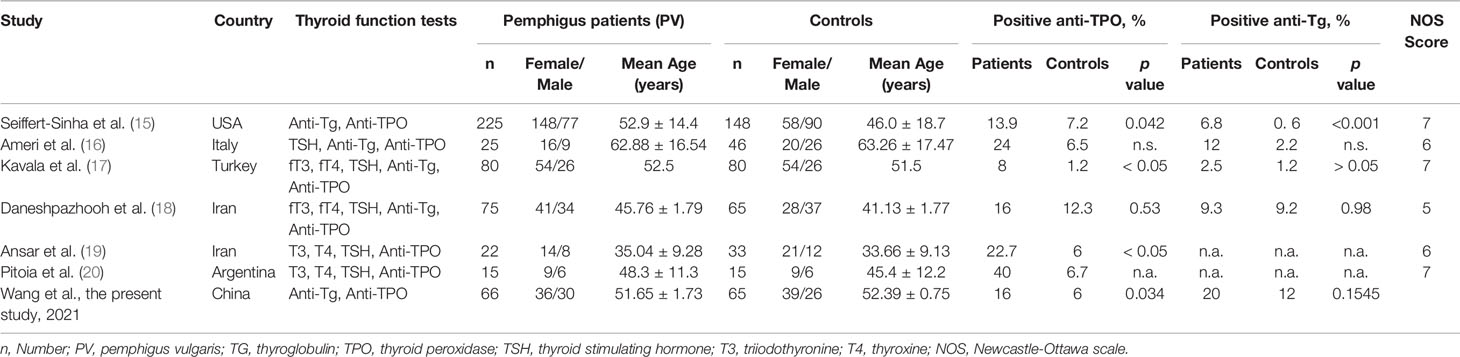
Table 1 Characteristics of studies on the prevalence of positive thyroid antibodies in patients with pemphigus.
Comparison of the Prevalence of Anti-TPO and Anti-Tg Antibodies in Pemphigus Patients and Controls
The thyroid antibody profiles were reported in all 6 studies, with both anti-TPO and anti-Tg antibodies in 4 studies, and anti-TPO antibody only in 2 studies (Table 1). The prevalence of positive anti-TPO antibody in pemphigus patients varied between 8% and 40%, and that between 2.5% and 12% for anti-Tg antibody. Meta−analysis using the fixed−effects model suggested higher prevalence of positive anti-TPO (OR=2.51, 95% CI, 1.56−4.06) and anti-Tg (OR=2.84, 95% CI, 1.30−6.23) antibodies in patients with pemphigus than healthy individuals (Figures 2A, B). In addition, sensitivity analysis proved the reliability for the result of this meta-analysis.
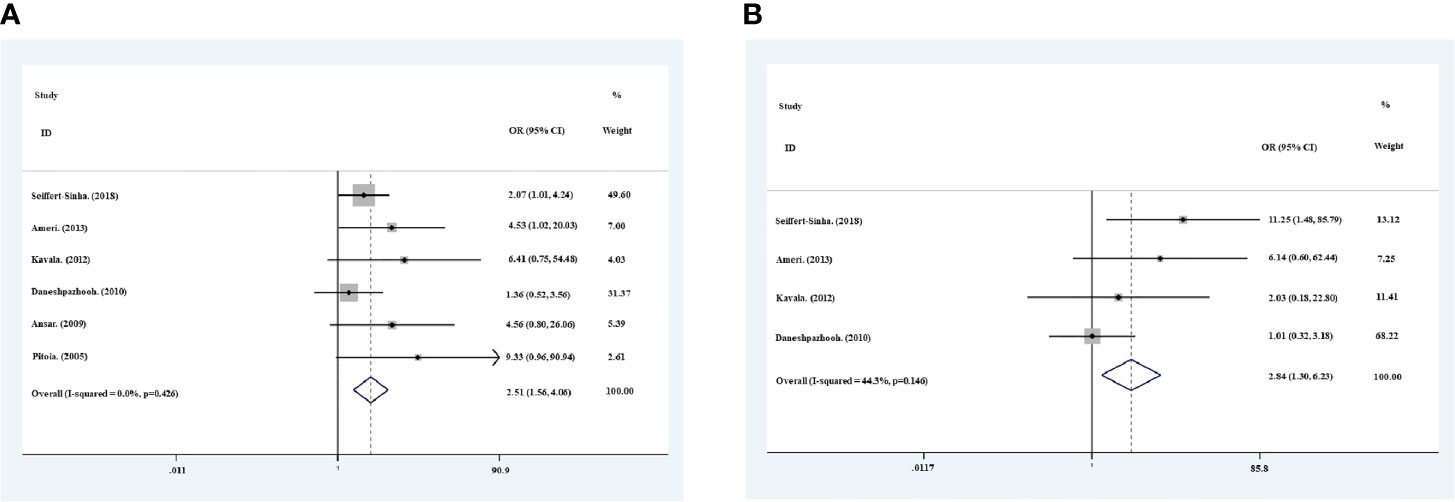
Figure 2 Meta−analysis using the fixed−effects model showed risk ratio of the prevalence of positive anti-TPO antibodies (A) and anti-Tg antibodies (B) in pemphigus patients compared with healthy controls.
Detection of Anti-TPO and Anti-Tg Antibodies in Chinese PV Patients, PF Patients, and Healthy Controls
Based on the findings of higher frequency of positive anti-TPO and anti-Tg antibodies in pemphigus patients than controls by meta-analysis, we also detected the levels of anti-TPO and anti-Tg antibodies in 98 Chinese Han ethnic pemphigus patients (66 PV patients and 32 PF patients) and 65 healthy age-, sex-matched healthy controls (Table 2). In our investigations, by using the Fisher’s exact test we found PV patients have a significantly higher positivity of anti-TPO antibody than controls (24.24% in PV patients and 9.23% in controls, p<0.0001), but such difference was not observed between PF patients and controls (18.75% in PF patients, p=0.2017). The levels of anti-TPO antibody in PV patients (p<0.0001) and PF patients (p=0.0023) were also significantly higher than controls by Mann-Whitney U test. In the detection of anti-Tg antibody, we observed increased antibody level and positivity in both PV patients and PF patients, but with no statistical significance.
Gender-Based Comparison of Anti-TPO or Anti-Tg Antibody Status in Chinese Controls, PV Patients, and PF Patients
The levels and antibody positivity of anti-TPO and anti-Tg antibodies were also compared between males and females within PV patients, PF patients or controls. As shown in Figures 3A, C, we found significantly higher levels and antibody positivity of anti-TPO antibody in PV female patients (83.34 ± 38.98 IU/ml, 38.89%) than PV male patients (2.16 ± 0.80 IU/ml, 6.67%) (plevel = 0.0081, ppositivity = 0.0032). In contrast to PV patients, the levels and antibody positivity of anti-TPO antibody in PF patients were likely to be higher in males (56.57 ± 32.13 IU/ml, 21.4%) than females (7.01 ± 4.63 IU/ml, 16.7%), although no statistical significance was reached (Figures 3B, C).
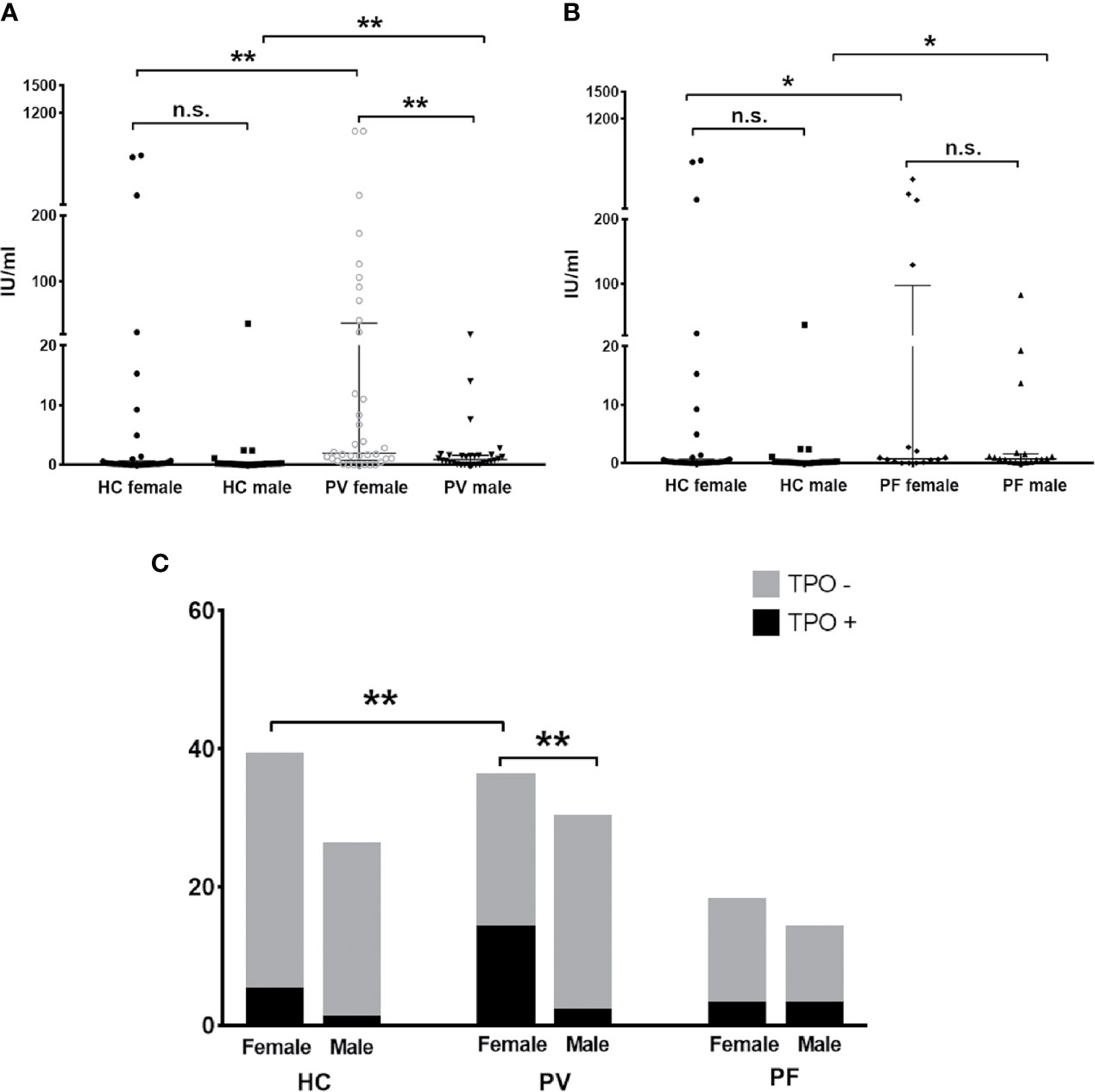
Figure 3 The status of anti-TPO antibody in PV patients, PF patients and healthy controls. (A, B) The levels of anti-TPO antibody in PV patients, PF patients and healthy controls, shown as median with interquartile range. (C) The antibody positivity of anti-TPO antibody in PV patients, PF patients and healthy controls. * indicates a significant difference (*p < 0.05, **p < 0.01, ***p < 0.001, by the Mann-Whitney U test for the comparison of antibody levels and The Fisher’s exact test for the comparison of antibody positivity).
Moreover, we found the levels of anti-TPO antibody in female PV patients and male PV patients were even higher than that of female controls (46.54 ± 26.80 IU/ml, p=0.0013) and male controls (1.75 ± 4.10 IU/ml, p=0.0069), respectively. More female PV patients were also likely to carry anti-TPO antibody than female controls (12.82%) (p=0.0157). In PF patients, the levels of anti-TPO antibody were significantly increased in males and decreased in females when compared with male controls (p=0.016) and female controls (p=0.0278), respectively.
For anti-Tg antibody detection, our results revealed significant differences in the levels and antibody positivity of anti-Tg antibodies between genders in both PV patient groups (females: 46.70 ± 6.28 IU/ml, 44.44%; males: 16.41 ± 12.63 IU/ml, 13.33%; p = 0.0286) and controls (females: 17.92 ± 8.95 IU/ml, 28.20%; males: 1.90 ± 0.61 IU/ml, 3.84%; p = 0.0003) (Figures 4A, C), but not in PF patients (females: 35.25 ± 31.01 IU/ml, 16.67%; males: 84.95 ± 70.64 IU/ml, 42.9%; p = 0.4860) (Figures 4B, C). For females or males between pemphigus patients and controls, however, differences in the levels or antibody positivity of anti-Tg antibodies were only detected in males between patients and controls (plevel, PV/C = 0.0366, plevel, PF/C = 0.0238, ppositivity, PF/C = 0.0044).
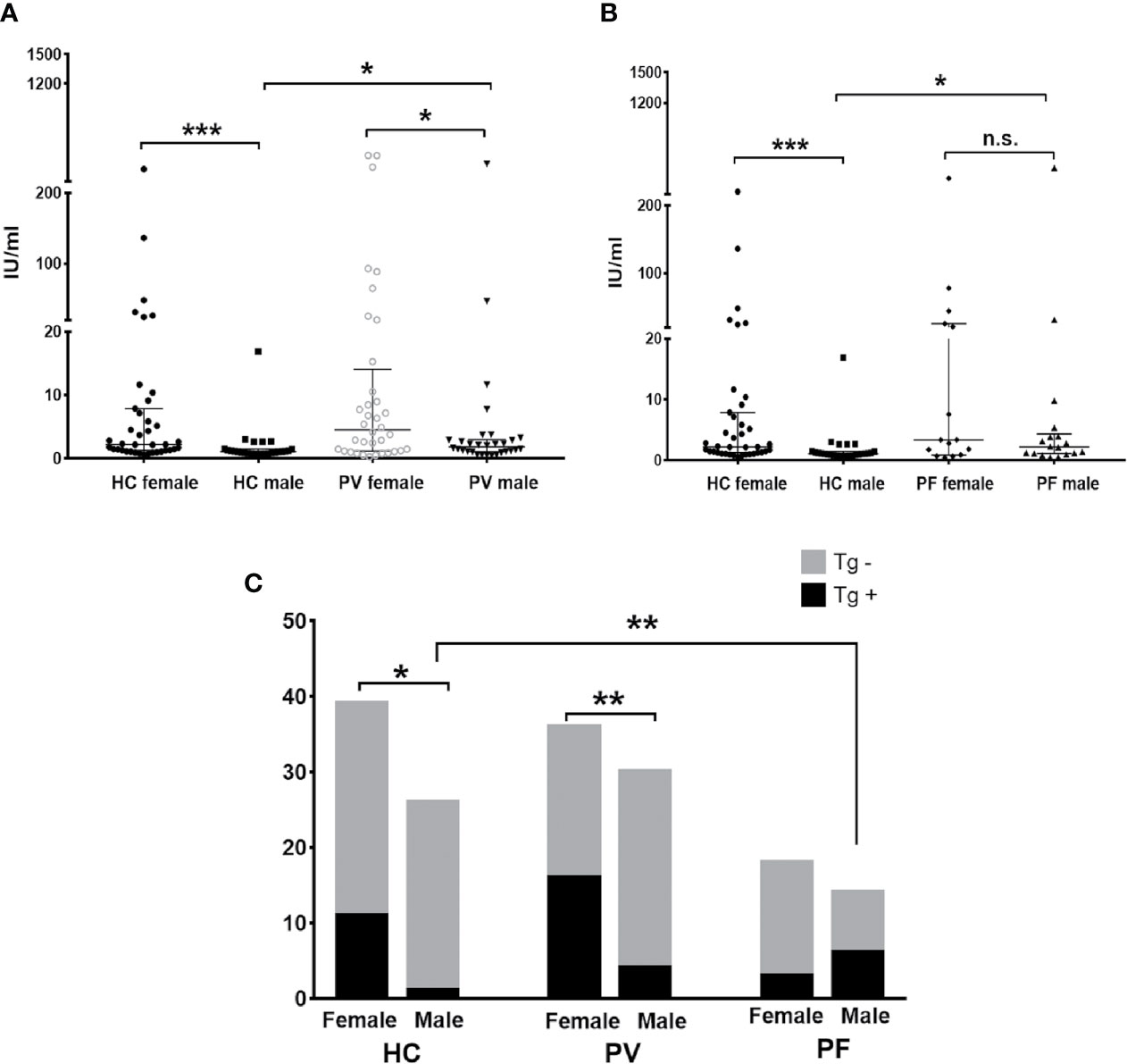
Figure 4 The status of anti-Tg antibody in PV patients, PF patients and healthy controls. (A, B) The levels of anti-Tg antibody in PV patients, PF patients and healthy controls, shown as median with interquartile range. (C) The antibody positivity of anti-Tg antibody in PV patients, PF patients and healthy controls. * indicates a significant difference (*p < 0.05, **p < 0.01, ***p < 0.001, by the Mann-Whitney U test for the comparison of antibody levels and The Fisher’s exact test for the comparison of antibody positivity).
Abnormal Low CD3 + T Cell Counts Only Present in Chinese Pemphigus Patients With Low Levels of Thyroid Antibodies
The correlation between the absolute number of T cell subsets and thyroid antibodies in pemphigus patients were also investigated in 58 PV patients and 34 PF patients for whom the T cell subset counts in peripheral blood were available. It was very interesting to find abnormal low CD3 + T cell counts (< 690 n/µl) were only present in patients whose thyroid antibody levels were less than 20 IU/ml, although with no statistical significance (Table 3). These patients also showed abnormal low numbers of CD4 + T cells (< 410 n/µl) and/or CD8 + T cells (< 190 n/µl). No correlation between other T cell subsets and thyroid antibodies were observed.
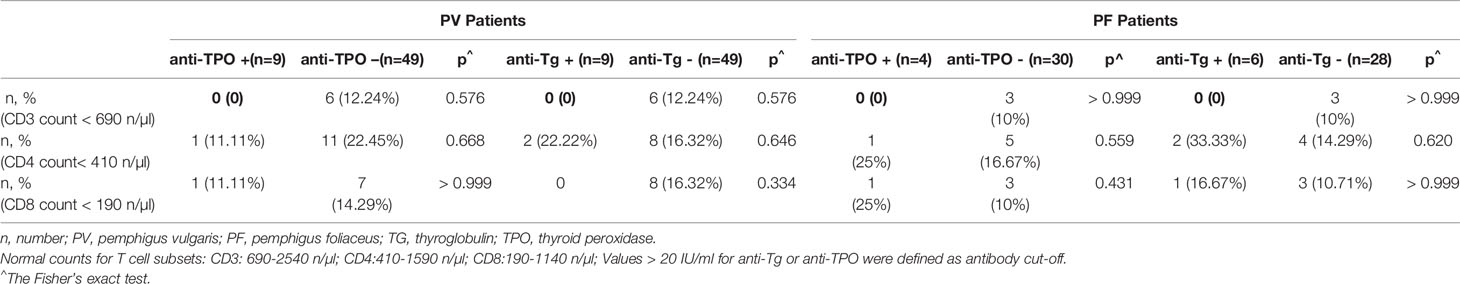
Table 3 Prevalence of abnormal low T cell subset counts in the peripheral blood of PV patients and PF patients.
Discussion
The present study has analyzed the antibody positivity and levels of anti-TPO and anti-Tg antibodies in pemphigus patients as compared to healthy controls, by performing a meta-analysis on 6 published studies, and also by examining our own hospitalized Chinese pemphigus patient population.
Additional autoimmune diseases can co-occur in individuals that are affected by an autoimmune disease, a concept known as ‘autoimmune diathesis’ (26, 27). This spurred the notion of investigating shared pathogenesis, as well as developing preventive and therapeutic strategies for these diseases (28). The most common autoimmune diseases, AITDs have been found to be co-occurred with certain autoimmune diseases, such as rheumatoid arthritis (RA), multiple sclerosis (MS), vitiligo and pemphigus (4).
Anti-TPO and anti-Tg antibodies are hallmarks of AITDs and elevated levels of these antibodies were even detectable several years before the clinical diagnosis of AITDS (29). Vitiligo patients were recommended to screen for these two antibodies due to their increased susceptibility in developing AITDS (30). Likewise, the correlations between pemphigus and anti-TPO/anti-Tg antibody positivity have been reported in several articles (15–20). We systematically searched relevant articles for a meta-analysis, and in result we identified PV patients tend to carry more anti-TPO and anti-Tg antibodies than healthy controls, implying PV patients may be at increased risk of developing AITDs than controls. In accordance with this finding, we have also confirmed higher prevalence of positive anti-TPO antibody in our own Chinese hospitalized PV as compared to healthy controls, but unfortunately not in PF patients. To our knowledge, this is the first study that investigated the thyroid antibody status in Chinese pemphigus patient population. PF patients were also rarely discussed for their thyroid antibody status, although only 32 cases of PF were included in this study due to the disease rarity. Significantly higher serum level of anti-TPO antibody was detected in our PV patients and PF patients as compared to controls, and the finding in PV patients was also consistent with two studies on Iranian and Turkish PV patients (17, 19). The levels and frequency of positive anti-Tg antibody were also likely to be higher than controls for both PV and PF patients, although with no statistical significance. These findings demonstrated generally consistent status of anti-TPO and anti-Tg antibodies between Chinese pemphigus population and previously reported other races.
It is well accepted that females are more susceptible to AITDs than males (31). Our PV female patients demonstrated higher levels and prevalence of anti-TPO and anti-Tg antibodies than males, and this finding was consistent with another study performed on American PV patients (15). In PF patients, however, both levels and antibody positivity of anti-TPO and anti-Tg antibodies were likely to be higher in males than females, although with no statistical significance, suggesting a potential higher tendency for PF male patients to develop AITDs and thyroid antibodies, and therefore, anti-TPO and anti-Tg antibodies should possibly be monitored particularly in PF male patients, and preventive strategies for AITDs may should also be performed. Whereas small sample size may also be an issue that contribute to the inconsistent results between PF patients and PV patients. Thus, validation of this finding should be performed on larger patient population and will be a feature of future studies by our group.
The impact of steroids on anti-thyroid antibodies was controversial. Ansar et al. implied autoimmune reactions could be suppressed in patients who received steroids (19), but Daneshpazhooh et al. found no significant difference in anti-thyroid antibodies between patients with and without steroid treatments (18). In accordance with Daneshpazhooh’ finding, steroids were also unlikely to influence the status of anti-thyroid antibodies in our PV and PF patients (data not shown), and this finding should be validated in the future study.
The presence of elevated anti-TPO and anti-Tg antibodies identified in pemphigus patients implied pemphigus and AITDs may potentially share susceptibility genes or alleles. Functions of influencing keratinocyte signalings by anti-TPO antibody may also be a potential explanation for the presence of elevated anti-TPO antibody in pemphigus (15).
In conclusion, our data has supported the potential correlation between thyroid antibodies and pemphigus. Particularly in Chinese population, significantly higher levels and positivity of anti-TPO antibody were observed in PV patients than controls. Further investigations are needed to be carried out to identify the pathogenic functions of anti-TPO and anti-Tg antibodies in pemphigus, as well as to identify the potential shared susceptibility genes. Given the impact on life quality and mortality of autoimmune disease, particularly for those with co-occurred conditions, quantification of anti-TPO and anti-Tg antibodies may be useful to identify pemphigus patients at risk for thyroid disease, and the necessity should also be verified in future studies on bigger sample size and more races.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of the First Hospital of China Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
H-XW analyzed and interpreted the data, and conducted the draft writing. SQ and X-HG contributed to the research concept and design, and draft revision. YY has contributed to literature searching and data extraction for the meta-analysis. J-YH and L-MZ have collected the serum samples and recorded patients’ medical information. TX and H-DC have revised the language and the content. HG has performed the serum testing. Y-FC has analyzed part of the data for meta-analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by NSFC (81903228 HG) and NSFC (81903198 QA) for data collection.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hertl M, Jedlickova H, Karpati S, Marinovic B, Uzun S, Yayli S, et al. Pemphigus. S2 Guideline for diagnosis and treatment–guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol (2015) 29(3):405–14. doi: 10.1111/jdv.12772
2. Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet (2019) 394(10201):882–94. doi: 10.1016/S0140-6736(19)31778-7
3. Kasperkiewicz M, Ellebrecht CT, Takahashi H, Yamagami J, Zillikens D, Payne AS, et al. Pemphigus. Nat Rev Dis Primers (2017) 3:17026. doi: 10.1038/nrdp.2017.26
4. Parameswaran A, Attwood K, Sato R, Seiffert-Sinha K, Sinha AA. Identification of a new disease cluster of pemphigus vulgaris with autoimmune thyroid disease, rheumatoid arthritis and type I diabetes. Br J Dermatol (2015) 172(3):729–38. doi: 10.1111/bjd.13433
5. Spindler V, Eming R, Schmidt E, Amagai M, Grando S, Jonkman MF, et al. Mechanisms Causing Loss of Keratinocyte Cohesion in Pemphigus. J Invest Dermatol (2018) 138(1):32–7. doi: 10.1016/j.jid.2017.06.022
6. Spindler V, Heupel WM, Efthymiadis A, Schmidt E, Eming R, Rankl C, et al. Desmocollin 3-mediated binding is crucial for keratinocyte cohesion and is impaired in pemphigus. J Biol Chem (2009) 284(44):30556–64. doi: 10.1074/jbc.M109.024810
7. Mindorf S, Dettmann IM, Kruger S, Fuhrmann T, Rentzsch K, Karl I, et al. Routine detection of serum antidesmocollin autoantibodies is only useful in patients with atypical pemphigus. Exp Dermatol (2017) 26(12):1267–70. doi: 10.1111/exd.13409
8. Ishii N, Teye K, Fukuda S, Uehara R, Hachiya T, Koga H, et al. Anti-desmocollin autoantibodies in nonclassical pemphigus. Br J Dermatol (2015) 173(1):59–68. doi: 10.1111/bjd.13711
9. Sinha AA, Sajda T. The Evolving Story of Autoantibodies in Pemphigus Vulgaris: Development of the “Super Compensation Hypothesis”. Front Med (Lausanne) (2018) 5:218. doi: 10.3389/fmed.2018.00218
10. Didona D, Maglie R, Eming R, Hertl M. Pemphigus: Current and Future Therapeutic Strategies. Front Immunol (2019) 10:1418. doi: 10.3389/fimmu.2019.01418
11. Sajda T, Sinha AA. Autoantibody Signaling in Pemphigus Vulgaris: Development of an Integrated Model. Front Immunol (2018) 9:692. doi: 10.3389/fimmu.2018.00692
12. Ahmed AR, Carrozzo M, Caux F, Cirillo N, Dmochowski M, Alonso AE, et al. Monopathogenic vs multipathogenic explanations of pemphigus pathophysiology. Exp Dermatol (2016) 25(11):839–46. doi: 10.1111/exd.13106
13. Nguyen VT, Ndoye A, Shultz LD, Pittelkow MR, Grando SA. Antibodies against keratinocyte antigens other than desmogleins 1 and 3 can induce pemphigus vulgaris-like lesions. J Clin Invest (2000) 106(12):1467–79. doi: 10.1172/JCI10305
14. Rafei D, Muller R, Ishii N, Llamazares M, Hashimoto T, Hertl M, et al. IgG autoantibodies against desmocollin 3 in pemphigus sera induce loss of keratinocyte adhesion. Am J Pathol (2011) 178(2):718–23. doi: 10.1016/j.ajpath.2010.10.016
15. Seiffert-Sinha K, Khan S, Attwood K, Gerlach JA, Sinha AA. Anti-Thyroid Peroxidase Reactivity Is Heightened in Pemphigus Vulgaris and Is Driven by Human Leukocyte Antigen Status and the Absence of Desmoglein Reactivity. Front Immunol (2018) 9:625. doi: 10.3389/fimmu.2018.00625
16. Ameri P, Cinotti E, Mussap M, Murialdo G, Parodi A, Cozzani E. Association of pemphigus and bullous pemphigoid with thyroid autoimmunity in Caucasian patients. J Am Acad Dermatol (2013) 68(4):687–9. doi: 10.1016/j.jaad.2012.11.022
17. Kavala M, Kural E, Kocaturk E, Zindanci I, Turkoglu Z, Can B. The evaluation of thyroid diseases in patients with pemphigus vulgaris. ScientificWorldJournal (2012) 2012:146897. doi: 10.1100/2012/146897
18. Daneshpazhooh M, Behjati J, Hashemi P, Shamohammadi S, Mortazavi H, Nazemi MJ, et al. Thyroid autoimmunity and pemphigus vulgaris: is there a significant association? J Am Acad Dermatol (2010) 62(2):349–51. doi: 10.1016/j.jaad.2009.05.024
19. Ansar A, Farshchian M, Farahnaki S, Farshchian M. Thyroid autoimmunity in Iranian patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol (2009) 23(6):719–20. doi: 10.1111/j.1468-3083.2009.03172.x
20. Pitoia F, Moncet D, Glorio R, Graciela Diaz A, Rodriguez Costa G, Carbia S, et al. Prevalence of thyroid autoimmunity in patients with pemphigus vulgaris. Med (B Aires) (2005) 65(4):307–10.
21. Cianfarani F, Baldini E, Cavalli A, Marchioni E, Lembo L, Teson M, et al. TSH receptor and thyroid-specific gene expression in human skin. J Invest Dermatol (2010) 130(1):93–101. doi: 10.1038/jid.2009.180
22. Leshem YA, Katzenelson V, Yosipovitch G, David M, Mimouni D. Autoimmune diseases in patients with pemphigus and their first-degree relatives. Int J Dermatol (2011) 50(7):827–31. doi: 10.1111/j.1365-4632.2010.04818.x
23. Kridin K, Khamaisi M, Comaneshter D, Batat E, Cohen AD. Autoimmune Thyroid Diseases and Thyroid Cancer in Pemphigus: A Big Data Analysis. Front Med (Lausanne) (2018) 5:159. doi: 10.3389/fmed.2018.00159
24. Gupta VK, Kelbel TE, Nguyen D, Melonakos KC, Murrell DF, Xie Y, et al. A globally available internet-based patient survey of pemphigus vulgaris: epidemiology and disease characteristics. Dermatol Clin (2011) 29(3):393–404vii–iii. doi: 10.1016/j.det.2011.03.016
25. Zhang L, Qi R, Yang Y, Gao X, Chen H, Xiao T. Serum miR-125a-5p and CCL17 Upregulated in Chronic Spontaneous Urticaria and Correlated with Treatment Response. Acta Derm Venereol (2019) 99(6):571–8. doi: 10.2340/00015555-3149
26. Davidson A, Diamond B. Autoimmune diseases. N Engl J Med (2001) 345(5):340–50. doi: 10.1056/NEJM200108023450506
27. Somers EC, Thomas SL, Smeeth L, Hall AJ. Autoimmune diseases co-occurring within individuals and within families: a systematic review. Epidemiology (2006) 17(2):202–17. doi: 10.1097/01.ede.0000193605.93416.df
28. Somers EC, Thomas SL, Smeeth L, Hall AJ. Are individuals with an autoimmune disease at higher risk of a second autoimmune disorder? Am J Epidemiol (2009) 169(6):749–55. doi: 10.1093/aje/kwn408
29. Vrijman C, Kroon MW, Limpens J, Leeflang MM, Luiten RM, van der Veen JP, et al. The prevalence of thyroid disease in patients with vitiligo: a systematic review. Br J Dermatol (2012) 167(6):1224–35. doi: 10.1111/j.1365-2133.2012.11198.x
30. Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, et al. Guideline for the diagnosis and management of vitiligo. Br J Dermatol (2008) 159(5):1051–76. doi: 10.1111/j.1365-2133.2008.08881.x
Keywords: anti-TPO antibody, anti-Tg antibody, pemphigus vulgaris, pemphigus foliaceus, meta-analysis, Chinese population
Citation: Wang H-X, Yang Y, Hu J-Y, Zhang L-M, Cai Y-F, Guo H, Xiao T, Chen H-D, Gao X-H and Qiao S (2021) Serum Detection of Anti-thyroid Peroxidase and Anti-thyroglobulin Antibodies in Chinese Patients With Pemphigus Vulgaris and Pemphigus Foliaceus and Literature Review. Front. Immunol. 12:653356. doi: 10.3389/fimmu.2021.653356
Received: 14 January 2021; Accepted: 25 February 2021;
Published: 16 March 2021.
Edited by:
Takashi Hashimoto, Osaka City University, JapanReviewed by:
Christoph M. Hammers, University of Lübeck, GermanyAna Maria Abreu-Velez, Georgia Dermatopathology Associates, United States
Ana Maria Roselino, University of São Paulo, Brazil
Christoph Hudemann, Philipps-University Marburg, Germany
Copyright © 2021 Wang, Yang, Hu, Zhang, Cai, Guo, Xiao, Chen, Gao and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing-Hua Gao, Z2FvYmFycnlAaG90bWFpbC5jb20=; Shuai Qiao, cXMxOTc3MDhAMTYzLmNvbQ==
 He-Xiao Wang
He-Xiao Wang Yang Yang1
Yang Yang1 Yun-Fei Cai
Yun-Fei Cai Hong-Duo Chen
Hong-Duo Chen Xing-Hua Gao
Xing-Hua Gao