- Department of Surgery & Cancer, Hammersmith Hospital, Imperial College London, London, United Kingdom
Until recently, the treatment landscape for hepatocellular cancer (HCC) was dominated by tyrosine kinase inhibitors (TKIs) which offered an overall survival (OS) benefit when used both in the first-and second-line setting compared to best supportive care. However, the treatment landscape has changed with the introduction of immune checkpoint inhibitors (ICIs) for the treatment of HCC with significant improvement in OS and progression free survival reported with combination atezolizumab and bevacizumab compared to sorafenib in the first-line setting. Nonetheless, the response to ICIs is 20–30% and invariably patients will progress. What remains unclear is which therapeutics should be used following ICI exposure. Extrapolating from the evidence base in renal cell carcinoma, subsequent therapy with TKIs offers both a response and survival benefit and are recommended by European guidelines. However, there are a number of novel therapies emerging that target mechanisms of ICI resistance that hold promise both in combination with ICI or as subsequent therapy. This paper will discuss the evidence for ICIs in HCC, the position of second-line therapies following ICIs and research strategies moving forward.
Introduction
Hepatocellular cancer (HCC) is the fifth most common cause of cancer and the third leading cause of cancer related death worldwide (1). The majority of HCC develops on a background of chronic liver disease secondary to chronic hepatitis B and C, alcohol excess or non-alcoholic liver disease (2). The presence of chronic liver disease has a direct impact on liver function and often limits therapies that can be extended to patients (3). Whilst curative in the early stages, the majority of patients (>70%) will present with advanced stage cancer, and even in those receiving curative therapy with surgery or ablation, the majority will relapse within 5-years and the mainstay of treatment in this setting is that of systemic therapy (2, 4).
For over 20 years the research field has been dominated by molecular targeted agents, the majority inhibiting angiogenesis through blockade of vascular endothelial growth factor receptor (VEGFR) (2). Both in the first and second-line setting, the efficacy of these agents has been modest, with improvements in overall survival (OS) of only 2–3 months and poor objective response rates (5–9), underscoring a need for more efficacious therapeutics in this disease space. In recent years there has been an increasing appreciation of the role of the immune microenvironment in liver carcinogenesis (10). Being at the junction of the arterial and portal systemic blood flow, the liver has an important immunoregulatory role (11). The liver constitutes the largest reticulo-endothelial system (RES) in the human body, with specialized immune cells including Kupffer cells, innate T-cells, natural kills cells and liver sinusoidal endothelial cells (12). Cirrhosis results in persistent inflammation and damage to the RES leading to impaired immune surveillance and dysregulation of the immune environment, resulting in DNA damage, hepatocyte necrosis and cancer (13). A rich immune infiltrate is observed in the tumor microenvironment (TME) but this infiltrate comprises of predominantly “exhausted” pro-inflammatory T-cell (regulatory T-cells, T-regs) populations that express co-inhibitory receptors such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 and its ligand (PD-1/PDL-1), T-cell immunoglobulin, mucin-domain containing-3 (TIM-3), and myeloid derived suppressor cells (MDSCs) (14, 15). Together with the secretion of immunoregulatory cytokines, immune tolerance results which is associated with poor prognosis (16, 17). Hence, there is a strong rationale for the use of immunotherapies (ICI) in HCC. The pressing question moving forward is which agent to use in the second-line setting, with tyrosine kinase inhibitors (TKIs) currently recommended post-ICI (18, 19). The aim of this review is to summarize the evidence for ICIs in HCC with a particular focus on combination ICI-therapy and to explore the therapeutic options following ICI. To inform treatment decision-making, we will revisit the current therapeutic portfolio in HCC and discuss future treatment directions.
Immunotherapeutic Strategies in HCC
The goals of ICI can broadly be defined as either unmasking a current immune response or stimulating a new or different one (11). The majority of phase III studies have been performed using therapeutics that target molecules such as CTLA-4 and the PD-1/PDL-1 axis in an effort to unmask an immune response (10).
Single Agent Immunotherapeutic Strategies
The first ICI to be approved by the FDA for the management of HCC was nivolumab, an anti-PDL-1 antibody following the publication of CheckMate 040 (20). This was a phase I/II, uncontrolled, open labeled study that evaluated nivolumab, initially in a dose escalation, and then in a subsequent dose expansion cohort, enrolling patients with Child Pugh A and B cirrhosis who had previously received sorafenib (N = 262) (20). The study reported an overall response rate (ORR) of 20% with a 9-months survival rate of 74% (95% CI: 67–79%) which led to the phase III randomized controlled trial, Checkmate 459, in which nivolumab was tested against sorafenib in the first-line setting (21). The study failed to meet its primary endpoints of OS; median OS for nivolumab was 16.4 months (95% CI: 13.9–18.4) vs. 14.7 months (95% CI: 11.9–17.2) for sorafenib (HR 0.85, 95% CI: 0.72–1.02, p = 0.075) (21).
A similar fate awaited the much anticipated Keynote-240 study, a phase III study that randomized patients to either pembrolizumab or placebo following sorafenib therapy (22). Pembrolizumab is a highly selective humanized IgG4/κ monoclonal antibody that directly inhibits the binding of PD-1 to its ligands, PD-L1 and PD-L2. Despite an ORR of 17% in the phase II Keynote-224 study (23), the phase III study failed to meet either of its co-primary endpoints (OS or PFS). The reported median OS was numerically longer for pembrolizumab, 13.9 vs. 10.6 months for placebo, HR 0.78, 95% CI: 0.61–0.99, p = 0.024, but did not meet the pre-specified criteria for statistical significance over placebo (24). Of interest, following progression 41.7% of patients in the pembrolizumab group and 47.4% in the placebo group received subsequent anti-cancer treatment. On post-hoc analysis, the median OS was longer in the pembrolizumab group vs. placebo when survival was adjusted for subsequent anti-cancer therapies (13.9 vs. 9.3 months; HR, 0.67; 95% CI, 0.48–0.92; nominal one-sided p = 0.0066) (23). 24.8% of patients received TKIs following pembrolizumab and whilst not reported, the efficacy of individual TKIs in this sub-study would be of key interest.
Despite the absence of a clear role for single agent ICIs either in the first or second-line management of HCC, there are a number of other agents under investigation. Durvalumab, an anti-PDL1 IgG1 monoclonal, has been evaluated as part of a phase I/II study in an expansion cohort of 40 HCC patients with Child-Pugh Class A, 93% of whom were sorafenib experienced. An ORR of 10% was reported with a median OS of 13.2 months and a 56% 1-year survival rate (25). Other drugs being investigated include camrelizumab (26), cemiplimab (27) (NCT03916627), and tislelizumab, a humanized IgG4 antibody to PD-1, the efficacy of which is currently being explored in the phase III RATIONALE-301 study compared with sorafenib in the first-line setting (NCT 03412773) (28).
In addition to PD-1 and PDL-1, single agent CTLA-4 inhibitors have been investigated in HCC, although not in the context of large phase III studies. The frist CTLA-4 inhibitor to be studied in HCC was tremelimumab, a fully human IgG2 monoclonal antibody (29). The study investigated the efficacy of tremelimumab 15 mg/kg IV every 90 days in 21 patients with Hepatitis C-associated HCC and reported a response rate of 17.6% and time to tumor progression (TTP) of 6.48 months (95% CI: 3.95–9.14) (29). The reported median OS was 8.2 months and the probability of survival at 1 year was reported to be 43%. Duffy and colleagues investigated the combination of tremelimumab and ablation with the intention of inducing synergistic immunogenic cell death. Tremelimumab was administered as six infusions, 3.5 and 10 mg/kg 4-weekly followed by 3-monthly maintenance. Sub-total tumor ablation was given at day 36. Five out of 19 evaluable patients achieved a partial response, translating into a TTP of 7.4 months and OS of 12.3 months (30). Both studies demonstrated evidence of anti-viral activity with falling HCV RNA load and expansion of HCV-specific T-cell responses (29). There is a paucity of phase III data for anti-CTLA-4 monotherapy and long term efficacy data is wanting as is its efficacy across diverse etiologies of chronic liver disease.
Immunotherapy Combination Studies
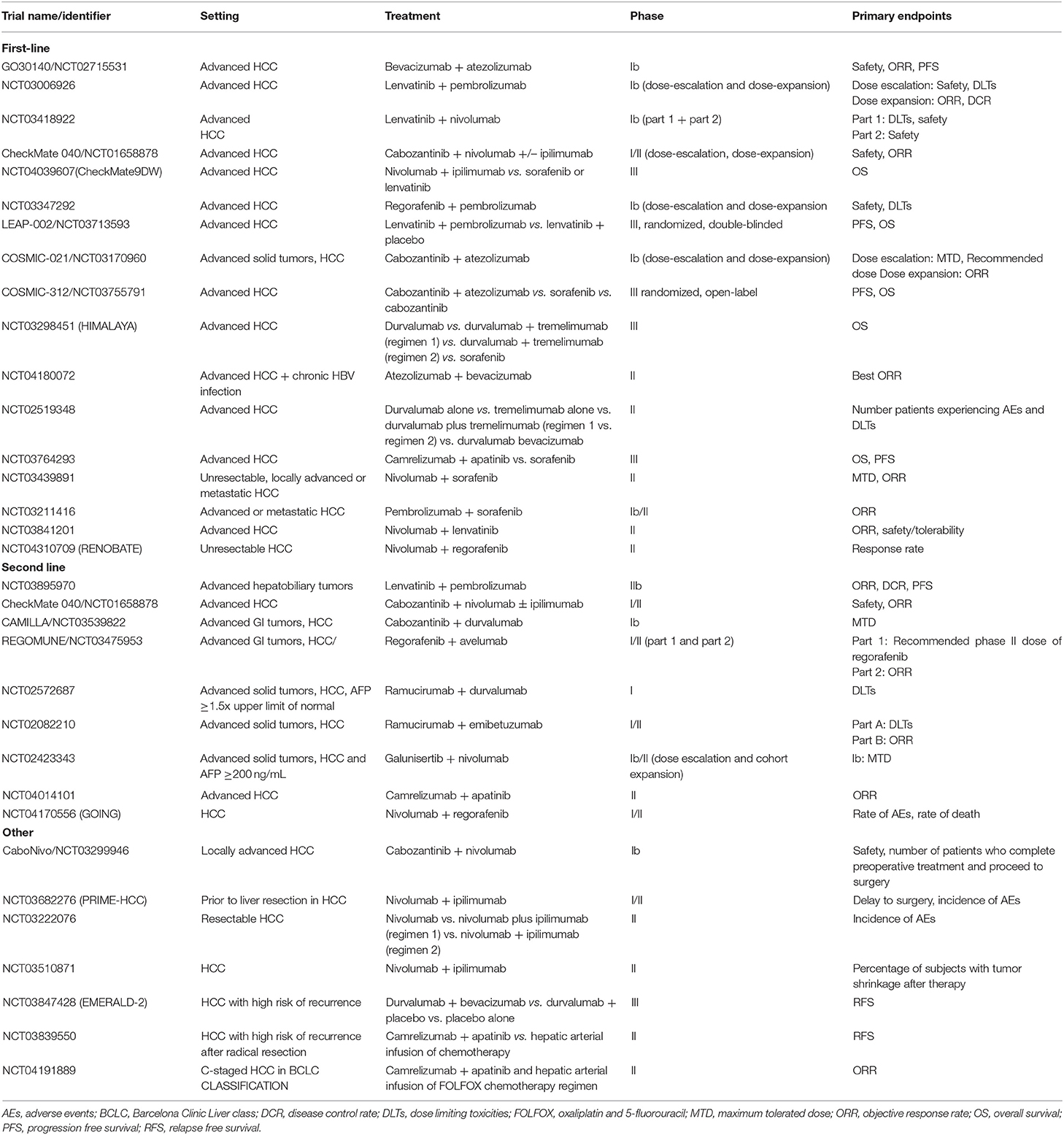
Extrapolating from the improved clinical outcomes observed in other malignancies, there are a number of clinical trials investigating the efficacy of combination therapy with both PD-1 and CTLA-4 inhibitors (Table 1). The rationale for this combination is that whilst the PD/PDL-1 pathway inhibits the effectiveness of the CD8+ T-cell response, CTLA-4 differentially suppresses the action of antigen presenting cells and T-regs. Thus, by targeting both pathways, there is the expectation of both an increase in the number of activated CD8+ cells infiltrating the tumor and an enhancement of anti-tumor activity.
Cohort 4 of the Checkmate-040 was designed to test the efficacy of varying doses of combination therapy of the CTLA-4 inhibitor, ipilimumab, and nivolumab in patients with advanced stage HCC following progression on sorafenib (arm A: nivolumab 1 mg/kg + ipilimumab 3 mg/kg, arm B: nivolumab 3 mg/kg + ipilimumab 1 mg/kg every 3 weeks for 4 doses followed by nivolumab maintenance (240 mg flat dose every 2 weeks), arm C: nivolumab 3 mg/kg + ipilimumab 1 mg/kg every 6 weeks until discontinuation due to progression or toxicity) (31). Arm A showed the greatest improvement in OS compared to arm B and C and has received accelerated approval in the United States; median OS 22.8 months (95% CI, 9.4-not reached) in arm A vs. 12.5 months (95% CI, 7.6–16.4) in arm B and 12.7 months (95% CI, 7.4–33.0) in arm C (31).
The phase III HIMALAYA study randomizes patients to receive combination therapy with tremelimumab and the PDL-1 inhibitor, durvalumab, durvalumab alone, or sorafenib in the first-line setting (NCT03298451). This trial was instigated based on promising phase I/II results that illustrated an ORR of 15% with disease control rates at 16 weeks of 57% in patients with unresectable HCC treated with durvalumab and tremelimumab with an acceptable safety profile. The authors reported that 20% of patients experienced grade ≥3 related adverse events the most common being an asymptomatic rise in AST (10%) (32).
Rationale for Combination Therapy of ICIs and Molecular Targeted Agents
The TME in HCC is hypoxic and as a consequence, is characterized by the presence of tortuous, leaky neoangiogenic vessels (33). Hypoxia has been shown to impair the function of immune effector cells and modulate the function of innate immune cells toward immunosuppression (33). Moreover, PD-1 and PD-L1 are unregulated in the hypoxic TME as a mechanism to evade anticancer immune responses, with upregulation of PD-L1 expression observed on MDSCs, dendritic and endothelial cells, as well as on tumor cells (34). Excessive production of VEGF and other pro-angiogenic factors in response to hypoxia creates a pro-tumor microenvironment by impacting on the number and function of T-regs, tumor associated macrophages, and MDSCs resulting in an immunosuppressive environment (33).
The TKI, sorafenib, targets multiple kinases including the VEGF receptor (9). Preclinical work in HCC, illustrates that the TKI, sorafenib, induces hypoxia and over-expression of PDL-1 within the tumor, resulting in accumulation of T-reg and M2-macrophages (35, 36). Moreover, in an elegant study by Shigeta and colleagues, dual blockade with anti-PD-1/VEGFR-2 therapy significantly inhibited HCC growth and improved survival in vivo (37). The authors illustrated that dual therapy resulted in an increase in cytotoxic T-cell infiltration and activation, an increase in M2 tumor-associated macrophages and a reduction in T-regs (37). Normalization of vessel architecture with dual therapy was also observed lending preclinical support for the use of combination ICI and anti-angiogenic therapy in the clinical setting.
Clinical Data for the Combination of ICIs and VEGF/VEGFR Axis Inhibitors
The first clinical trial of combination therapy to show a survival benefit in HCC was IMBrave 150 (38). In this open label, phase III study, patients with advanced stage disease were randomized to receive a combination of atezolizumab and bevacizumab or sorafenib. Patients were included if they had preserved liver function, ECOG 0-1 and an absence of main portal trunk invasion. The co-primary endpoints of OS and PFS were both achieved such that the OS at 12 months was 67.2% (95% CI, 61.3–73.1) with combination therapy compared with 54.6% for sorafenib (95% CI, 45.2–64.0) (HR 0.58, 95% CI, 0.42–0.79, p < 0.001). PFS was 6.8 months (95% CI: 5.7–8.3) for atezolizumab plus bevacizumab vs. 4.3 months (95% CI: 4.0–5.6) with sorafenib (HR0.59; 95% CI: 0.47–0.76, p < 0.0001). Of key interest, quality of life was maintained with atezolizumab plus bevacizumab compared to sorafenib in this essentially palliative population (38). Despite the promise of the trial, some outstanding questions remain. Whilst treatment related adverse events were similar in both treatment groups, discontinuation rates were higher with combination therapy, but no further details were given by the authors. Moreover, the trial does not report rates of cirrhosis which may impact on rates of drug induced adverse events in particular hepatitis, and any real-world data of the combination therapy will be of interest (38).
Numerous combination studies are currently open testing a myriad of permutations with various TKIs and ICIs (Table 1). The recently published phase Ib study of combination therapy of pembrolizumab and lenvatinib in patients with unresectable HCC reported no dose limiting toxicities in both the safety run-in (N = 6) and expansion phase (39). The authors report an ORR of 46.0% (95% CI: 36.0–56.3%), median PFS of 9.3 months (95% CI: 5.6–9.7 months) and OS of 22 months (95% CI: 20.4–not evaluable, months) (39). This combination is now being evaluated in a phase III vs. single agent lenvatinib (40). Similarly, the combination of regorafenib with pembrolizumab (NCT03347292) and cabozantinib with atezolizumab are being investigated in the first-line setting (41).
The Role of Tyrosine Kinase Inhibitors Post-ICI
Whilst IMBrave150 illustrated an OS and ORR benefit of combination therapy over sorafenib in the first-line setting, data on long-term survivorship and response to subsequent therapies is not yet available (38). Similarly, anti-PD-1 monotherapy (20, 22) and dual checkpoint inhibition with anti-CTLA-4 (31) were approved by the FDA on the basis of response rates rather than evidence of convincing OS benefit. The majority of advanced HCC patients will invariably progress and a looming question is what should be used in the second-line setting following combination ICI therapy. The recently updated European Society of Medical Oncology position regorafenib, cabozantinib, and ramucirumab as therapeutic options following failure of atezolizumab and bevacizumab, a stance that has been adopted by a number of healthcare systems (18, 19), and is supported by a recent network analysis (42). Evidence of efficacy of TKIs following ICI in HCC is limited. A post-hoc analysis of 14 patients in the CELESTIAL study who received cabozantinib third line following ICI reported a median OS of 7.9 months (95% CI 5.1–NE) which was comparable to that of patients that had received two prior regimens, median OS 8.5 months (95% CI 7.4–9.7) (43). In another small study of 30 patients with HCC who received TKIs following immunotherapy (combination nivolumab and ipilimumab (N = 2), single agent nivolumab (N = 7), pembrolizumab (N = 4) and durvalumab (N = 1), the authors report a median OS, defined from the commencement of TKI till death from any cause, of 602 days (95% CI: 124–not reached) (44). It is unclear from the published abstract if immunotherapy was administered as a single agent or combination and the full publication is awaited. Currently, there are no publications or studies considering the utility of TKIs following combination therapy.
Prior to the introduction of immunotherapy into the therapeutic armamentarium, sorafenib and lenvatinib offered a survival benefit of 2 months for patients with inoperable HCC (7, 9). For those patients who failed first-line therapy with sorafenib, three second-line options were available; regorafenib, cabozantinib and ramucirumab (5, 6, 8). None of these agents have been assessed following lenvatinib failure. Post-hoc exploratory analysis of the RESORCE study illustrated that sequential treatment with sorafenib and regorafenib resulted in a median OS of 26 months from start of sorafenib compared to 19 months in those that received sorafenib followed by placebo (45). Similar results were observed in a post-hoc analysis of the CELESTIAL trial that illustrated patients who had received prior sorafenib, cabozantinib significantly improved OS, 24.5 months compared to 18.8 months in those receiving placebo (46). In addition, post-hoc analysis of the REFLECT data that illustrates an OS benefit of second-line therapy, OS 20.8 vs. 17.0 months (HR 0.87; 95% CI 0.67–1.14) (47). Subgroup analysis illustrated that OS was greatest in those patients who had initially responded to either lenvatinib, 25.7 months (95% CI 18.5–34.6), or sorafenib 22.3 months (95% CI 14.6–not evaluable).
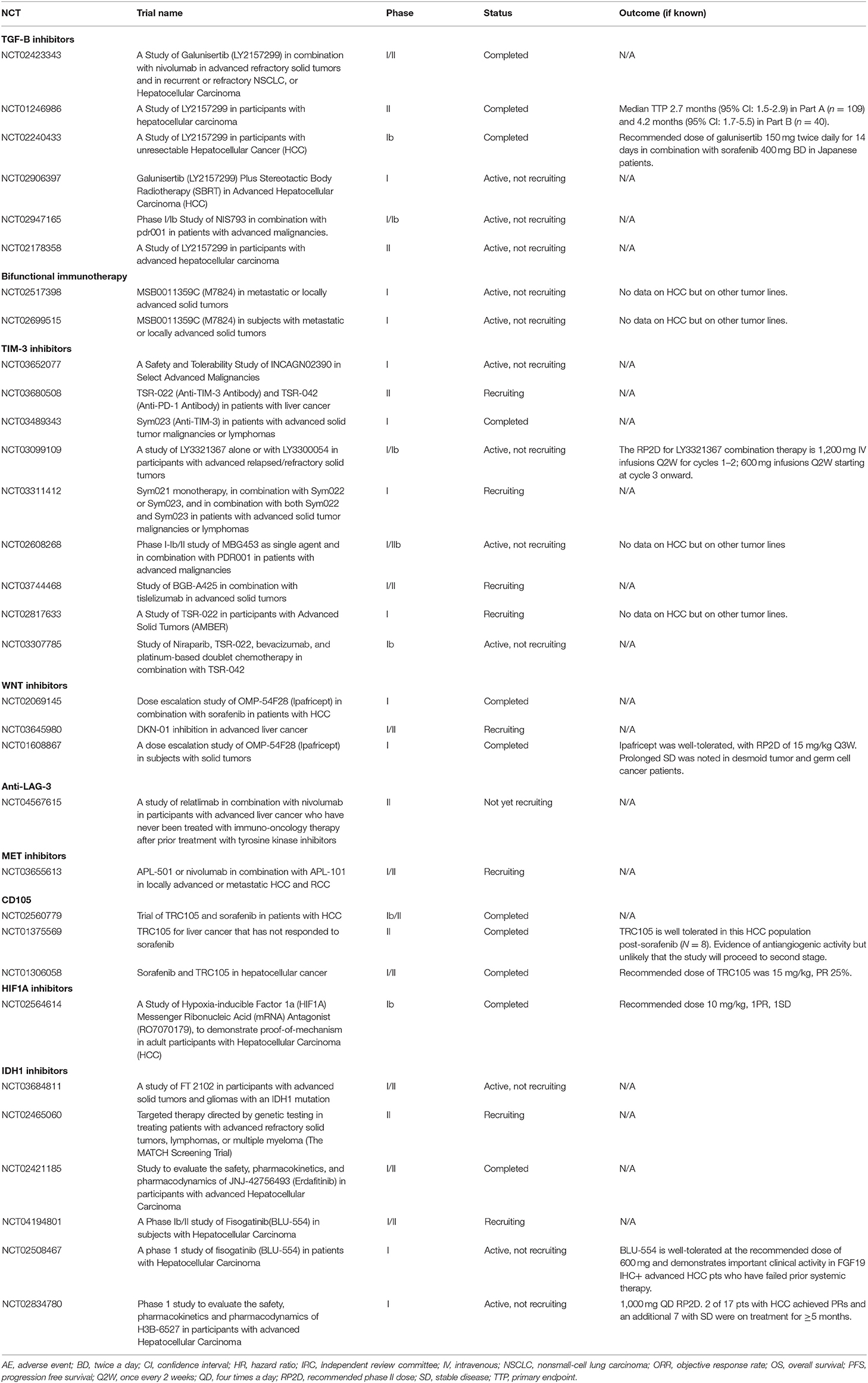
Given that all therapeutics that have previously shown activity in HCC in phase III trials target VEGFR and angiogenic signaling to some extent, it can be expected that all these agents could be successfully combined with ICI (5–9). Which TKI would be more efficacious following ICI remains to be elucidated. Extrapolating from renal cell carcinoma, another tumor driven by angiogenesis, sequential TKI use following ICI therapy is associated with incremental OS benefit, leading to international guidelines to recommend the use of any multi-targeted TKI that has not been used in the first-line setting in combination with ICI, an approach that is gaining traction in HCC (44, 48, 49). Another therapeutic approach is the evaluation of novel therapies that target ICI resistance mechanisms or alternate signaling pathways in HCC (Table 2).
Mechanisms of ICI Resistance in HCC and Treatment Strategies
Resistance to ICIs can either be primary or acquired, and the mechanisms that drive this process are an evolving field. What is clear is that “cold” tumors do not respond to ICI whilst “hot” tumors do. Cold tumors are characterized by an infiltrate of MDSCs, T-regs, low tumor mutational burden and poor antigen presentation, resulting in an inability to mount an immune response toward the tumor (50). A number of novel therapeutics are currently being developed to essentially transform a “cold” tumor microenvironment into a “hot” tumor and to enhance the endogenous T-cell response. Of these, a number are being trialed in HCC including TIM-3, and lymphocyte activation gene 3 (LAG-3) antagonists, and inhibitors of transforming growth factor β (TGFβ) receptor ligands, and tumor necrosis factor (TNF) receptor (51).
TIM-3 is a transmembrane protein expressed on exhausted CD8+ cells that is expressed with other co-inhibitory receptors such as PD-1 and CTLA-4. The combination of TSR-022, a TIM-3 antagonist, TSR-042, a novel anti-PD-1 is currently the subject of a phase II study in HCC (NCT03680508). Similarly, lymphocyte activation gene 3 (LAG-3) suppresses T-cells activation and cytokine secretion, thereby ensuring immune homeostasis and is currently the subject of clinical trials (Table 2).
The tumor growth factor-β (TGFβ) signaling pathways play a key role in cellular invasion and proliferation, driving hepatocarcinogenesis (52). In addition, TGFβ signaling in the TME has been shown to result in tumor T-cell exclusion and poor response to PD-1/PD-L1 blockade, and there is rationale to combine TGFβ with ICIs (53). Galunisertib, an oral small molecule inhibitor of the TGFβ receptor I (TGFβRI) kinase, has been evaluated in phase II study of 149 patients with HCC who had progressed following sorafenib (54). Enrollment was stratified according to AFP>1.5ULN with a median OS of 7.3 months (95% CI: 4.9–10.5) in those patients with an AFP < 1.5ULN and 16.8 months (95% CI: 10.5–24.4) with AFP > 1.5ULN (54). Galunisertib in combination with nivolumab is currently being investigated in HCC and other solid tumors (NCT02423343). OX40 is a member of the TNF receptor family that is highly expressed on activated immune cells. On ligand binding, T-cell survival, proliferation and effector function is enhanced (55). MEDI0562 is an agonistic, humanized IgG monoclonal antibody directed at OX40 that has undergone phase I evaluation with acceptable toxicity (56). It is anticipated that the combination of MEDI0562 with ICI may enhance the immunomodulatory effects.
Conclusion
Currently, for patients that receive either sorafenib or lenvatinib first-line there is a clear benefit with second-line therapy from the RESORCE, CELESTIAL, REACH 2 studies. There is no randomized evidence supporting the use of second-line ICIs following sorafenib or lenvatinib despite the prolonged survival benefit observed in the KEYNOTE-240 study. Promising results are observed with the combination of nivolumab and ipilumumab in the second-line setting which has been approved by the FDA. There is evidence that combination atezolizumab and bevacizumab improves OS in the first-line setting but there are no clear answers as to what to use second-line. What is clear is that the survival for patients with advanced HCC is improving and whilst the correct sequence and drug combination is not yet clear, the survival gains are reasons for enthusiasm. The next few years will herald an exciting time for drug development in HCC both in terms of novel therapeutics but also their accompanying biomarkers which are sorely needed.
Author Contributions
RS and LA designed and wrote the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. (2019) 144:1941–53. doi: 10.1002/ijc.31937
2. Liver EAftSot. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. (2018). 69:182–236. doi: 10.1016/j.jhep.2011.12.001
3. Pinato DJ, Sharma R, Allara E, Yen C, Arizumi T, Kubota K, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. (2017) 66:338–46. doi: 10.1016/j.jhep.2016.09.008
4. Yegin EG, Oymaci E, Karatay E, Coker A. Progress in surgical and nonsurgical approaches for hepatocellular carcinoma treatment. Hepatobiliary Pancreat Dis Int. (2016) 15:234–56. doi: 10.1016/S1499-3872(16)60097-8
5. Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Ramucirumab vs. placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2015) 16:859–70. doi: 10.1016/S1470-2045(15)00050-9
6. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2017) 389:56–66. doi: 10.1016/S0140-6736(16)32453-9
7. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib vs. sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
8. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. (2018) 379:54–63. doi: 10.1056/NEJMoa1717002
9. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
10. Pinato DJ, Guerra N, Fessas P, Murphy R, Mineo T, Mauri FA, et al. Immune-based therapies for hepatocellular carcinoma. Oncogene. (2020) 39:3620–37. doi: 10.1038/s41388-020-1249-9
11. Johnston MP, Khakoo SI. Immunotherapy for hepatocellular carcinoma: current and future. World J Gastroenter. (2019) 25:2977–89. doi: 10.3748/wjg.v25.i24.2977
12. Guillot A, Tacke F. Liver macrophages: old dogmas and new insights. Hepatol Commun. (2019) 3:730–43. doi: 10.1002/hep4.1356
13. Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. (2013) 14:996–1006. doi: 10.1038/ni.2691
14. Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. (2008) 135:234–43. doi: 10.1053/j.gastro.2008.03.020
15. Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. (2017) 153:812–26. doi: 10.1053/j.gastro.2017.06.007
16. Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. (2007) 67:10019–26. doi: 10.1158/0008-5472.CAN-07-2354
17. Baitsch L, Fuertes-Marraco SA, Legat A, Meyer C, Speiser DE. The three main stumbling blocks for anticancer T cells. Trends Immunol. (2012) 33:364–72. doi: 10.1016/j.it.2012.02.006
18. Oncology ESoM. eUpdate–Hepatocellular Carcinoma Treatment Recommendations. (2020). Available online at: https://www.esmo.org/guidelines/gastrointestinal-cancers/hepatocellular-carcinoma/eupdate-hepatocellular-carcinoma-treatment-recommendations3
19. Team NECDF. National Cancer Drugs Fund List. In: Commissioning S, editor. London: NHS (2020). p. 1–174.
20. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. (2017) 389:2492–502. doi: 10.1016/S0140-6736(17)31046-2
21. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs. sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. (2020) 30(Suppl. 5):V874–V5. doi: 10.1093/annonc/mdz394.029
22. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. (2020) 38:193–202. doi: 10.1200/JCO.19.01307
23. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. (2018) 19:940–52. doi: 10.1016/S1470-2045(18)30351-6
24. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab (Pembro) therapy vs. best supportive care (BSC) in advanced hepatocellular carcinoma (HCC): KEYNOTE-240. Ann Oncol. (2019) 30 (Suppl. 4):iv135–iv6. doi: 10.1093/annonc/mdz154.026
25. Wainberg ZA, Segal NH, Jaegar D, Lee HK, Marshall J, Antonia SJ, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J Clin Oncol. (2017) 35:4071. doi: 10.1200/JCO.2017.35.15_suppl.4071
26. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. (2020) 21:571–80. doi: 10.1016/S1470-2045(20)30011-5
27. Markham A, Duggan S. Cemiplimab: first global approval. Drugs. (2018) 78:1841–6. doi: 10.1007/s40265-018-1012-5
28. Qin S, Finn RS, Kudo M, Meyer T, Vogel A, Ducreux M, et al. RATIONALE 301 study: tislelizumab vs. sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. (2019) 15:1811–22. doi: 10.2217/fon-2019-0097
29. Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. (2013) 59:81–8. doi: 10.1016/j.jhep.2013.02.022
30. Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. (2017) 66:545–51. doi: 10.1016/j.jhep.2016.10.029
31. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol. (2020) 6:e204564. doi: 10.1001/jamaoncol.2020.4564
32. Kelley RK, Abou-Alfa GK, Bendell JC, Kim TY, Borad MJ, Yong WP, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): phase I safety and efficacy analyses. J Clin Oncol. (2017) 35:4073. doi: 10.1200/JCO.2017.35.15_suppl.4073
33. Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. (2018) 15:310–24. doi: 10.1038/nrclinonc.2018.9
34. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. (2014) 211:781–90. doi: 10.1084/jem.20131916
35. Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. (2015) 61:1591–602. doi: 10.1002/hep.27665
36. Lu LC, Lee YH, Chang CJ, Shun CT, Fang CY, Shao YY, et al. Increased expression of programmed death-ligand 1 in infiltrating immune cells in hepatocellular carcinoma tissues after sorafenib treatment. Liver Cancer. (2019) 8:110–20. doi: 10.1159/000489021
37. Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. (2020) 71:1247–61. doi: 10.1002/hep.30889
38. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
39. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
40. Llovet J, Kudo M, Cheng AL, Finn R, Galle PR, Kaneko K, et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): phase 3 LEAP-002 study. J Clin Oncol. (2019) 37:15. doi: 10.1200/JCO.2019.37.15_suppl.TPS4152
41. Kelley RK, J WO, Hazra S, Benzaghou F, Yau T, Cheng AL, et al. Cabozantinib in combination with atezolizumab vs. sorafenib in treatment-naive advanced hepatocellular carcinoma: COSMIC-312 Phase III study design. Future Oncol. (2020) 16:1525–36. doi: 10.2217/fon-2020-0283
42. Sonbol MB, Riaz IB, Naqvi SAA, Almquist DR, Mina S, Almasri J, et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. JAMA Oncol. (2020) 6:e204930. doi: 10.1001/jamaoncol.2020.4930
43. Abou-Alfa GK, Cheng AL, Saletan S, Kelley K, El-Khoueiry A, editors. PB02-04. Clinical Activity of Cabozantinib in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Anti-VEGF and Immuno-Oncology Therapy: Subgroup Analysis From the Phase 3 CELESTIAL trial. Liver Cancer Summit. Prague: EASL (2020).
44. Yau TC, Tang V, Chan J, Kwok GW, Chiu J, Leung CR, et al. Outcomes of tyrosine kinase inhibitors (TKI) after immunotherapy in unresectable or advanced hepatocellular carcinoma (HCC) patients. J Clin Oncol. (2019) 37 (Supply. 4):361. doi: 10.1200/JCO.2019.37.4_suppl.361
45. Finn RS, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. (2018) 37:361. doi: 10.1016/j.jhep.2018.04.010
46. Kelley RK, Ryoo BY, Merle P, Park JW, Bolondi L, Chan SL, et al. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: a subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open. (2020) 5:e000714. doi: 10.1136/esmoopen-2020-000714
47. Alsina A, Kudo M, Vogel A, Cheng AL, Tak WY, Ryoo BY, et al. Effects of subsequent systemic anticancer medication following first-line lenvatinib: a post hoc responder analysis from the phase 3 REFLECT study in unresectable hepatocellular carcinoma. Liver Cancer. (2020) 9:93–104. doi: 10.1159/000504624
48. Barata PC, De Liano AG, Mendiratta P, Crolley V, Szabados B, Morrison L, et al. The efficacy of VEGFR TKI therapy after progression on immune combination therapy in metastatic renal cell carcinoma. Br J Cancer. (2018) 119:160–3. doi: 10.1038/s41416-018-0104-z
49. Albiges L, Powles T, Staehler M, Bensalah K, Giles RH, Hora M, et al. Updated european association of urology guidelines on renal cell carcinoma: immune checkpoint inhibition is the new backbone in first-line treatment of metastatic clear-cell renal cell carcinoma. Eur Urol. (2019) 76:151–6. doi: 10.1016/j.eururo.2019.05.022
50. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. (2017) 168:707–23. doi: 10.1016/j.cell.2017.01.017
51. Faivre S, Rimassa L, Finn RS. Molecular therapies for HCC: Looking outside the box. J Hepatol. (2020) 72:342–52. doi: 10.1016/j.jhep.2019.09.010
52. Rawal P, Siddiqui H, Hassan M, Choudhary MC, Tripathi DM, Nain V, et al. Endothelial cell-derived TGF-beta promotes epithelial-mesenchymal transition via CD133 in HBx-Infected hepatoma cells. Front Oncol. (2019) 9:308. doi: 10.3389/fonc.2019.00308
53. Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer. (2018) 6:47. doi: 10.1186/s40425-018-0356-4
54. Faivre S, Santoro A, Kelley RK, Gane E, Costentin CE, Gueorguieva I, et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. (2019) 39:1468–77. doi: 10.1111/liv.14113
55. Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. (2016) 52:50–66. doi: 10.1016/j.ejca.2015.08.021
Keywords: HCC, second-line therapy, tyrosine kinase inhibitors, survival, immunotherapy
Citation: Sharma R and Motedayen Aval L (2021) Beyond First-Line Immune Checkpoint Inhibitor Therapy in Patients With Hepatocellular Carcinoma. Front. Immunol. 12:652007. doi: 10.3389/fimmu.2021.652007
Received: 11 January 2021; Accepted: 10 February 2021;
Published: 15 March 2021.
Edited by:
Amaia Lujambio, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Raphael Mohr, Charité—Universitätsmedizin Berlin, GermanyChristoph Roderburg, Charité—Universitätsmedizin Berlin, Germany
Copyright © 2021 Sharma and Motedayen Aval. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rohini Sharma, ci5zaGFybWFAaW1wZXJpYWwuYWMudWs=
 Rohini Sharma
Rohini Sharma Leila Motedayen Aval
Leila Motedayen Aval