- 1Department of Gastroenterology, Wenzhou People’s Hospital, Wenzhou Third Clinical Institute Affiliated to Wenzhou Medical University, Wenzhou, China
- 2Department of Pulmonary Medicine, Shanghai Respiratory Research Institute, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Department of Medical Oncology, Shanghai Pulmonary Hospital & Thoracic Cancer Institute, Tongji University School of Medicine, Shanghai, China
This study aimed to investigate the predictive value of liver metastases (LM) in patients with various advanced cancers received immune-checkpoint inhibitors (ICIs). First, clinical and survival data from a published cohort of 1,661 patients who received ICIs therapy were downloaded and analyzed. Second, a retrospective review of 182 patients with advanced non-small-cell lung cancer (NSCLC) who received PD-1/PD-L1 monotherapy was identified. Third, a meta-analysis of published trials was performed to explore the impact of LM on the efficacy of anti-PD-1/PD-L1 based therapy in advanced lung cancers. Pan-cancer analysis revealed that patients with LM had significantly shorter overall survival (OS) than those without LM (10 vs. 20 months; P < 0.0001). Subgroup analysis showed that the presence of LM was associated with markedly shorter OS than those without LM in ICI monotherapy group (P < 0.0001), but it did not reach the statistical significance in ICI-based combination therapy (P = 0.0815). In NSCLC, the presence of LM was associated with significantly inferior treatment outcomes in both pan-cancer and real-world cohort. Interestingly, ICI-based monotherapy and combination therapy could simultaneously prolong progression-free survival (PFS) and OS than chemotherapy in patients without LM. However, ICI-based monotherapy could not prolong PFS than chemotherapy in patients with LM while ICI-based combination therapy could dramatically prolong both PFS and OS. Together, these findings suggested that the presence of LM was the negative predictive factor in cancer patients received ICIs monotherapy, especially in NSCLC. ICI-based combination therapy might overcome the intrinsic resistance of LM to ICIs while the optimal combinatorial strategies remain under further investigation.
Introduction
The liver is a large and very vascular glandular organ of human beings, which secretes bile and causes important biological changes in many of the substances contained in the blood (1, 2). It is also the main sites of distant metastases in patients with advanced cancers including melanoma, gastrointestinal cancer, breast cancer, as well as lung cancer (3, 4). Approximately 15–40% of patients with advanced cancers would be diagnosed with liver metastases (LM) during his/her lifetime (5, 6). Patients with LM often have an unsatisfactory prognosis (7). To make matters worse, several previous publications revealed that the presence of LM was a negative predictive factor for molecular targeted therapy in patients with driver gene mutations (e.g. EGFR) (8), indicating that alternative treatment strategy is warranted.
Immune-checkpoint inhibitors (ICIs) targeting cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed cell death 1 (PD-1) and its ligand (PD-L1) interaction have shifted the treatment landscape of advanced cancers and significantly improved the overall survival (OS) (9–12). Currently, ICI is one of the key and standard treatment strategies for various solid tumors. Nevertheless, several recent studies reported that patients with LM cannot benefit from ICI monotherapy (13, 14). Osorio et al. analyzed 761 individual lesions from 214 patients with non-small-cell lung cancer (NSCLC) and 290 lesions from 78 patients mismatch repair deficiency (MMRD) carcinoma treated with PD-1 monotherapy and found that LM had the least responses (15). However, other studies reported that LM did not compromise the survival benefit of patients received ICIs (16, 17). These contrary findings indicated that the predictive value of LM for ICIs treatment remains further investigation.
Therefore, we performed this pan-cancer analysis to investigate the predictive value of LM in patients with various advanced cancers received ICIs. We also analyzed a real-world cohort and conducted a systematic review with meta-analysis to explore the impact of LM on the efficacy of anti-PD-1/PD-L1 based treatment in advanced lung cancers.
Methods
Data Identification and Pan-Cancer Analysis
To investigate the impact of LM on ICIs treatment outcome, we downloaded the pan-cancer clinical and survival data from a recently published cohort of 1,661 patients treated with ICIs therapy from the cBioPortal online database (https://www.cbioportal.org) (18–20). Firstly, we analyzed the predictive significance of LM in all included patients with various cancers. Then, we explored the predictive value of LM for ICIs treatment outcomes in several common types of solid tumors including melanoma, colorectal cancer and NSCLC. We also compared the tumor mutational burden (TMB) level between patients with and without LM. Similar to previous study, TMB was defined as the total number of nonsynonymous mutations including somatic, coding, base substitution, and indel mutations per megabase (mut/Mb) of genome examined.
Patients’ Selection in a Real-World Cohort
To further assess the impact of LM for ICI treatment outcome in NSCLC, we performed a retrospective review of the patients diagnosed with advanced NSCLC who received anti-PD-1/PD-L1 monotherapy from January 1, 2016 to November 1, 2020 in two medical centers. The major inclusion criteria were (i) histological or pathological confirmation of advanced NSCLC, (ii) radiological confirmation of LM including magnetic resonance imaging (MRI) and/or enhanced computed tomography (CT), and (iii) evaluable for treatment response assessment. Firstly, patients with initial diagnosis of stage IV NSCLC were identified. Then, patients with LM and sufficient clinical information were selected. Other distant metastases were detected by using whole body positron emission tomography (PET) or PET/CT, cranial and thoracic CT/MRI, abdominal ultrasound or bone scan. All of them had received anti-PD-1/PD-L1 antibodies as monotherapy, regardless of treatment lines. The dose of each type of anti-PD-1/PD-L1 antibodies was used according to the recommended dose from drug instructions or phase II/III trials. This study was conducted in accordance with the provisions of the Declaration of Helsinki and was approved by the ethics committee of each medical center.
Data Collection
The major clinicopathological parameters including age, sex, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), lung cancer histology (WHO classification) (21), sites of metastasis, therapeutic regimens and treatment lines were collected. Smoking status, ECOG PS and age were recorded at the time of initial diagnosis. A never smoker was defined as a person who had smoked less than 100 cigarettes during his/her lifetime. Which anti-PD-1/PD-L1 antibodies were selected according to clinical treatment guidelines or by the investigators’ or patients’ discretion. Response including complete response (CR), partial response (PR), stable disease (SD) and disease progression (PD) was assessed using Response Evaluation Criteria in Solid Tumors version 1.1. Progression-free survival (PFS) was assessed from the date the patient began ICI treatment to the date of PD or death of any cause. Patients who had not progressed were censored at the date of their last follow-up. OS was calculated from the beginning of immunotherapy to the date of death of any cause. Patients who were still alive or lost contact were censored at the date of last scan. The last follow-up was December 1, 2020.
Meta-Analysis of Published Trials
We performed a publication search of the PubMed/Medline, EMBASE, Google Scholar, Cochrane Library, and Web of Science databases through December 31, 2020, using “lung cancer” and “PD-L1” and “liver metastasis” and their related words. Data on the relationship between liver metastasis and OS or PFS in NSCLC patients treated with anti-PD-1/PD-L1 based treatments were collected from publications meeting the eligibility criteria. The details of our methodology are described in the Supplemental Material.
Statistical Analysis
Clinicopathologic characteristics were descriptively summarized by number and percentages. The categorical variables were compared by using chi-square test, or Fisher’s exact test when needed. The continuous variables were analyzed by ANOVA and/or Tukey’s multiple comparison tests. The difference of baseline features between different treatment groups was compared with the χ2 test. PFS was defined as the time from the date of initiation of ICIs based treatment to the date of systemic progression or death and was censored at the date of last tumor assessment (when carried out). OS was calculated from the date of ICIs based treatment start to the date of death of any cause or last follow-up. Kaplan–Meier curves with two-sided log-rank tests and Cox proportional hazards model with calculated hazard ratios (HRs) and 95% confidence intervals (CIs) were used to determine the survival difference. All P values were two-sided and considered significant at P < 0.05. All statistical analyses were performed using the SPSS statistical software, version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Pan-Cancer Analysis
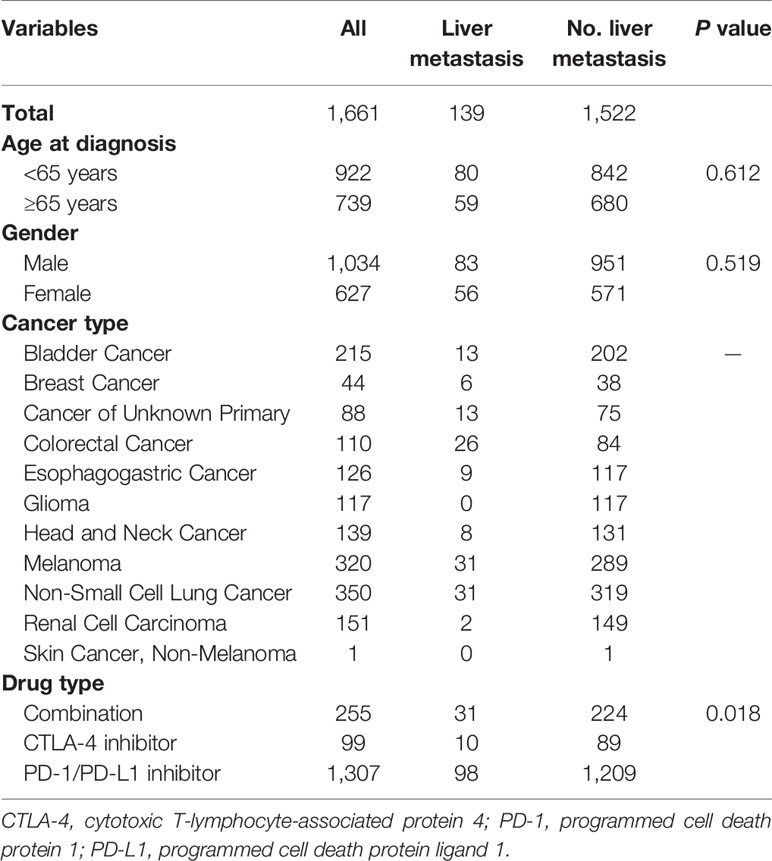
We identified a cohort of 1,661 cancer patients with 11 cancer types. Among them, 139 (8.4%) cases had LM. Baseline features of included patients were listed in Table 1. Totally, 1,034 (62.3%) male patients were included, and 739 (44.5%) cases had age ≥65 years. Most of them received PD-1/PD-L1 inhibitors treatment (78.7%). There was a significantly higher rate of patients received ICI-based combination therapy in patients with LM than those without LM (P = 0.018).
Patients with LM had significantly shorter OS than those without LM (10 vs. 20 months; HR = 1.70, P < 0.0001; Figure 1A) in all included patients. Intriguingly, TMB level was comparable between patients with and without LM (5.6 vs. 6.1, P = 0.2782; Figure 1B). Subgroup analysis showed that patients with LM also had markedly inferior OS than those without LM (9 vs. 17 months; HR = 1.79, P < 0.0001; Figure 1C) in ICI monotherapy group. However, the presence of LM was associated with inferior OS in ICI combination therapy without statistical significance (not reached vs. 41 months; HR = 1.66, P = 0.0815; Figure 1D). Interestingly, in patients treated with PD-1/PD-L1 monotherapy, the presence of LM was associated with significantly shorter OS (9 vs. 16 months; HR = 1.79, P < 0.0001; Figure 1F). Whereas the presence of LM was associated with inferior OS in CTLA-4 monotherapy but it did not reach the statistical significance (13 vs. 42 months; HR = 2.01, P = 0.0752; Figure 1E) mainly due to small sample size. We also investigated the predictive value of LM in several specific types of tumors. The presence of LM was associated with obviously worse OS in colorectal cancer (P = 0.0289; Supplemental Figure S1A) and NSCLC (P = 0.0449; Supplemental Figure S1C) group than those without LM, but it did reach the statistical significance in melanoma cohort (P = 0.0668; Supplemental Figure S1B). Multivariate analysis revealed that LM was significantly associated with worse OS (P < 0.001; Table 2). Additionally, ICIs based combination therapy and high tumor purity was significantly associated with longer OS (P < 0.001, P = 0.042, respectively; Table 2).
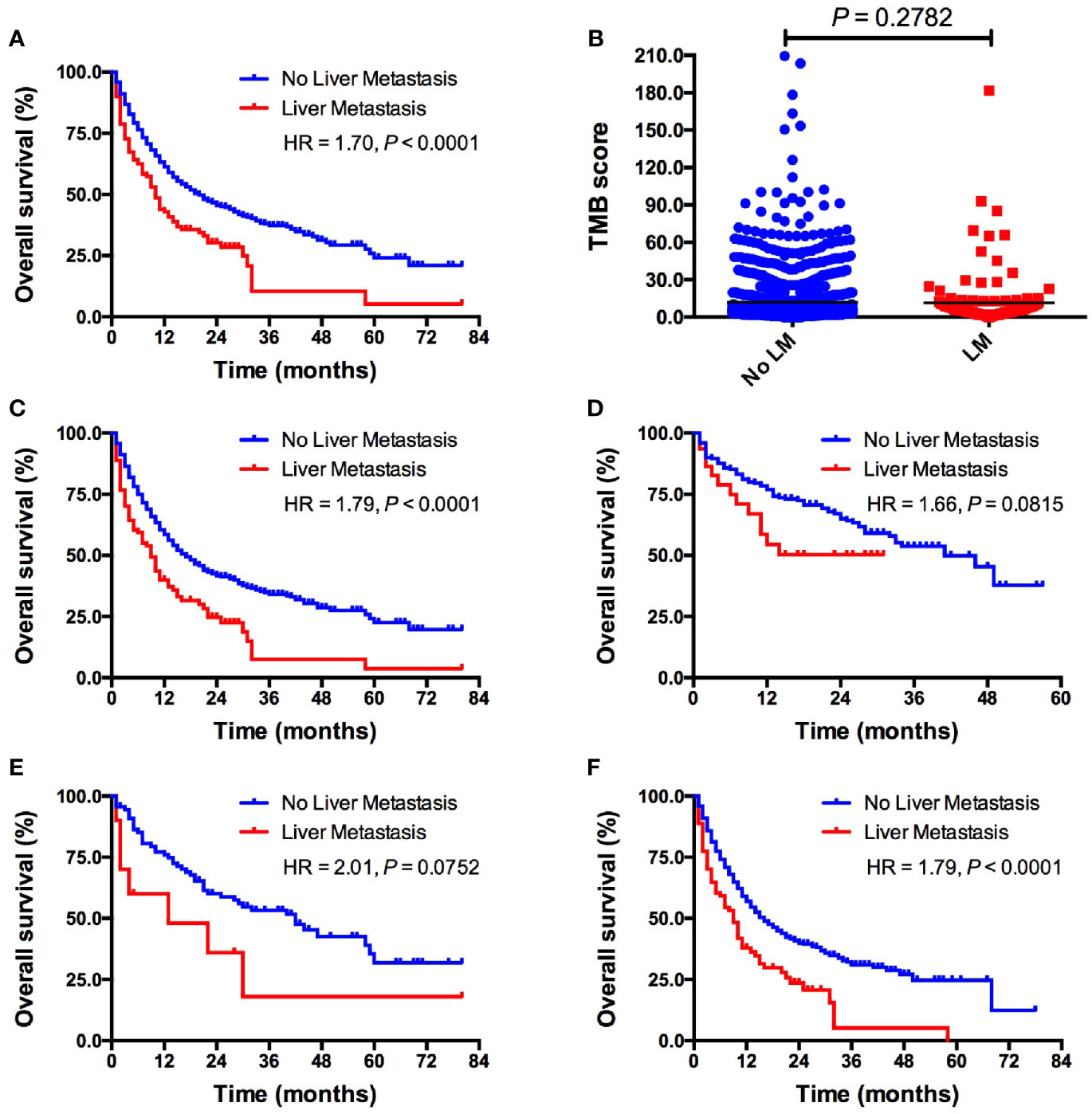
Figure 1 Pan-cancer analysis of the predictive value of LM for ICIs treatment outcomes. (A) OS comparison between patients with vs. without LM in whole cohort; (B) TMB level comparison between patients with vs. without LM in whole cohort; (C) OS comparison between patients with vs. without LM in ICIs monotherapy group; (D) OS comparison between patients with vs. without LM in ICIs based combination therapy group; (E) OS comparison between patients with vs. without LM in PD-1/PD-L1 monotherapy group; (F) OS comparison between patients with vs. without LM inCTLA-4 monotherapy group. LM, liver metastasis; TMB, tumor mutational burden; ICI, immune checkpoint inhibitor.
Baseline Features of Included Patients in Real-World Cohort
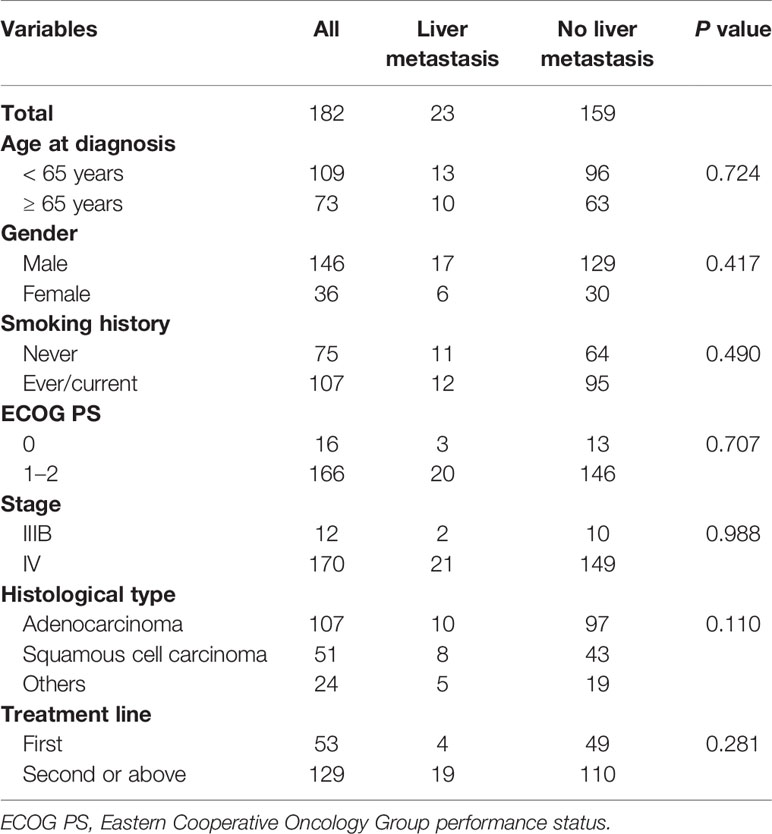
To further assess the predictive value of LM in patients with advanced NSCLC, we identified a total of 182 NSCLC patients received PD-1/PD-L1 monotherapy from January 1, 2016 to November 1, 2020 in two medical centers. Around 23 (18.0%) of them were initially diagnosed with LM. The clinical characteristics of the study population were summarized in Table 3. In total, 146 (80.2%) male patients were included, and the mean age was 61 years. Most of them were smokers (58.8%) and had performance status of ECOG 1-2 (91.2%). Adenocarcinoma is the most common histological type (58.8%). Some 53 (29.1%) patients received PD-1/PD-L1 monotherapy as first-line therapy.
The Predictive Value of LM in Real-World Cohort
Survival analyses using the Kaplan–Meier method and log-rank test showed significantly shorter PFS in patients with LM received PD-1/PD-L1 monotherapy compared to patients without LM (3.3 vs. 5.6 months; HR = 1.77, P = 0.0119; Figure 2A). Patients with LM also had significantly shorter OS than those without LM (8.2 vs. 17.6 months; HR = 1.83, P = 0.0408; Figure 2B). The objective response rate (ORR) was significantly lower in patients with LM than in patients without LM (4.3% vs. 28.9%, P = 0.0118; Figure 2C). The disease control rate (DCR) was similar between two groups (65.2% vs. 67.9%; Figure 2C). In multivariate analysis, LM was significantly associated with both shorter PFS (HR = 1.546, P = 0.039; Supplemental Table S2) and OS (HR = 1.543, P = 0.046; Supplemental Table S1). Additionally, PD-1/PD-L1 monotherapy as first-line treatment was significantly associated with longer PFS (P = 0.020; Supplemental Table S1) and OS (P = 0.027; Supplemental Table S1).
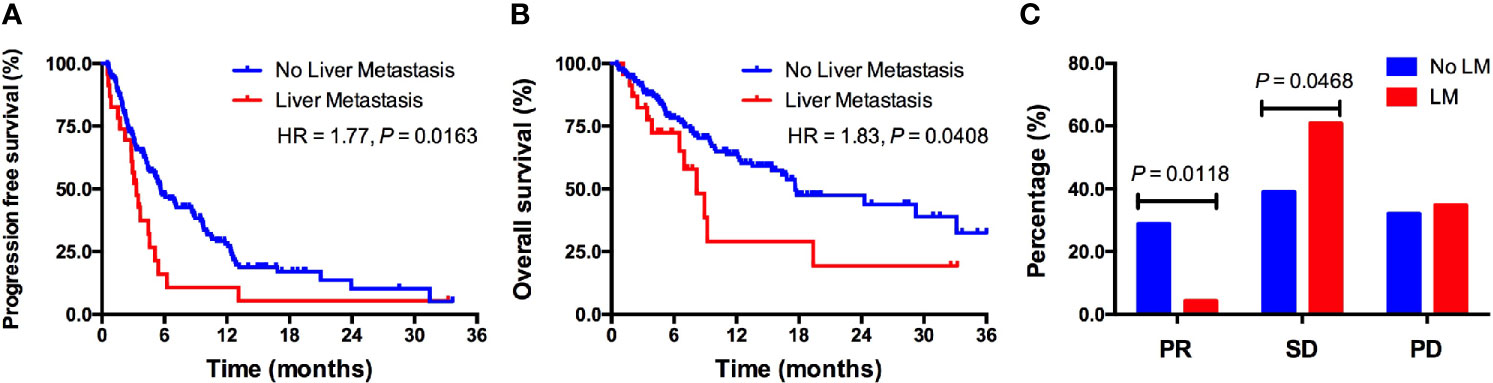
Figure 2 The predictive value of LM for ICIs treatment outcomes in a real-world cohort. (A) Kaplan–Meier curve of PFS in patients with versus without LM; (B) Kaplan–Meier curve of OS in patients with versus without LM; (C) Response rate comparison between patients with versus without LM. LM, liver metastasis; PR, partial response; SD, stable disease; PD, disease progression.
Features of Included Publication in the Meta-Analysis
Considering the negative predictive value of LM in NSCLC from both the online database and real-world cohort, we conducted a meta-analysis to compare the different treatment outcomes of anti-PD-1/PD-L1 based therapies in NSCLC with versus without LM. As shown in Supplemental Figure S2, 298 potentially relevant studies were screened. Most of the excluded publications were reviews, comments, duplications, or studies with incomplete data. The current study assessed 6,274 cases from 11 publications to investigate the distinct treatment outcomes of anti-PD-1/PD-L1 based therapies in NSCLC with versus without LM (22–32). The main features of the eligible studies are shown in Supplemental Table S2. Each included trial had the excellent methodologic quality (Supplemental Table S3).
Treatment Outcomes in NSCLC With Versus Without LM
The pooled results showed that anti-PD-1/PD-L1 based therapies was correlated with better OS (HR = 0.73, 95% CI: 0.64–0.83; P < 0.05; Figure 3A) and PFS (HR = 0.77, 95% CI: 0.60–0.94; P < 0.05; Figure 3C) when compared with standard chemotherapy in patients with LM. Similarly, the pooled results indicated that anti-PD-1/PD-L1 based therapies was associated with longer OS (HR = 0.71, 95% CI: 0.66–0.77; P < 0.05; Figure 3B) and PFS (HR = 0.66, 95% CI: 0.57–0.75; P < 0.05; Figure 3D) in patients without LM. Both results of OS showed low heterogeneity (I2 = 0.0%, P = 0.454; I2 = 0.0%; P = 0.622; respectively), but results of PFS showed high heterogeneity (I2 = 64.9%, P = 0.004; I2 = 72.9%; P < 0.001; respectively). Subgroup analysis revealed that anti-PD-1/PD-L1 monotherapy could not prolong PFS than chemotherapy in patients with LM while anti-PD-1/PD-L1 based combination therapy could significantly prolong PFS (Supplemental Figure S3). In patients without LM, both anti-PD-1/PD-L1 based monotherapy and combination therapy could simultaneously prolong PFS and OS (Supplemental Figure S3).
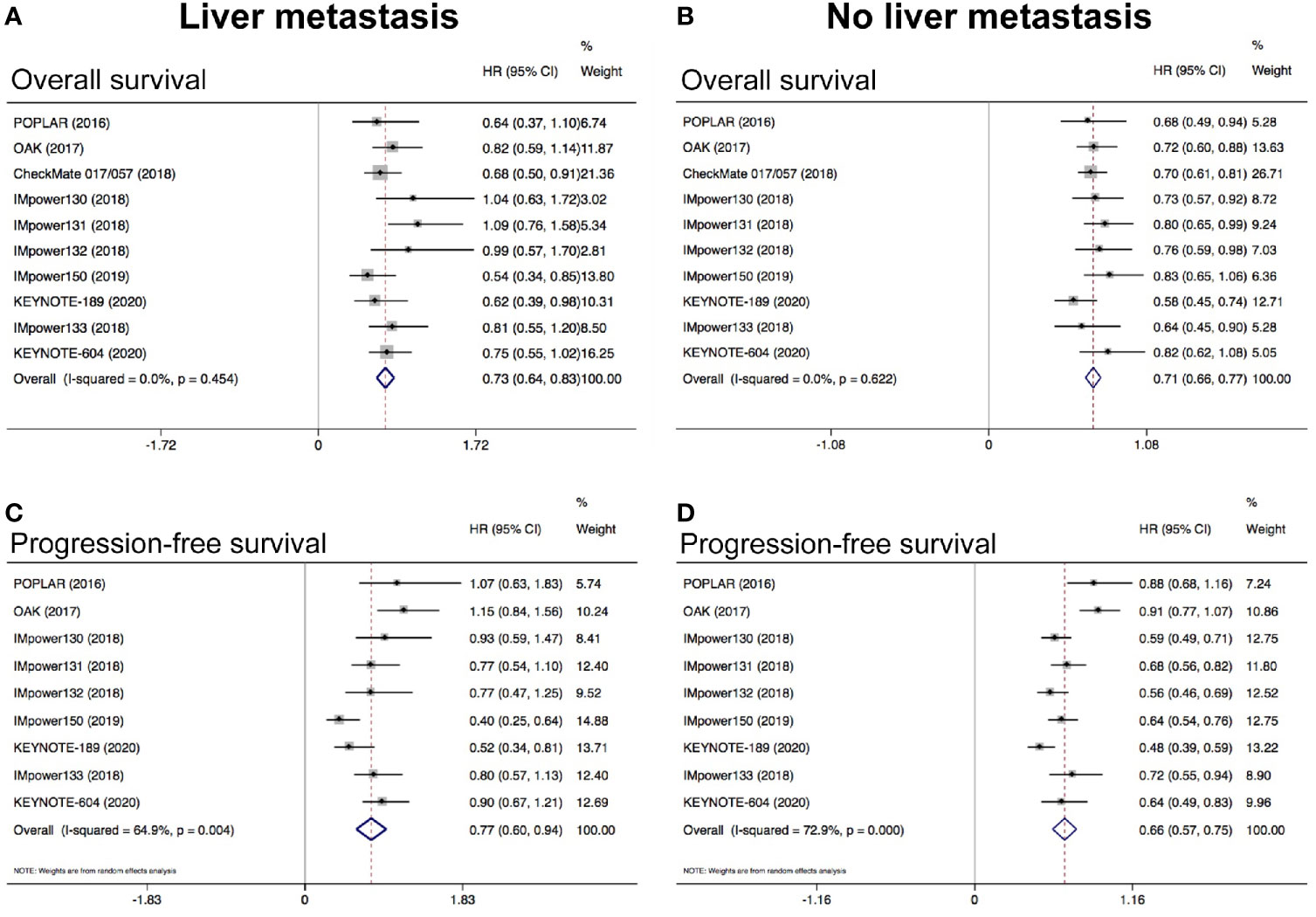
Figure 3 Meta-analysis to evaluate the predictive value of LM in NSCLC treated with ICIs. (A) Pooled analysis of OS in patients with LM; (B) Pooled analysis of OS in patients without LM; (C) Pooled analysis of PFS in patients with LM; (D) Pooled analysis of PFS in patients without LM. LM, liver metastasis.
Discussion
The present study reported that the presence of LM was correlated with significantly inferior treatment outcomes in ICI based monotherapy. However, it was not associated with significantly inferior OS in ICI based combination treatment group. In one of the most common solid tumors, the presence of LM was associated with significantly inferior treatment outcomes in patients with advanced NSCLC from both the pan-cancer and real-world cohort. Interestingly, meta-analysis revealed that anti-PD-1/PD-L1 based monotherapy and combination therapy could simultaneously prolong PFS and OS in NSCLC patients without LM. However, anti-PD-1/PD-L1 based monotherapy could not prolong PFS than chemotherapy in NSCLC patients with LM while anti-PD-1/PD-L1 based combination therapy could dramatically prolong both PFS and OS. Collectively, these findings indicate that the presence of LM was the negative predictive factor in patients with advanced cancers received ICIs monotherapy. ICI based combination therapy might overcome the intrinsic resistance of LM to ICI monotherapy while the optimal combinatorial strategies need further investigation.
As one of the most common distant metastasis in solid tumors, LM has unique the tumor immune microenvironment (3, 4). When LM-competent cells entered the liver, they would encounter a variety of cells including liver sinusoidal endothelial cells, liver-associated lymphocytes, Kupffer cells, hepatic stellate cells, dendritic cells, and portal fibroblasts (3, 4). All of them would have an impact on the biology of LM formation and progression. Previously, several elegant studies have unraveled that liver could promote the specific immune tolerance under the circumstance of viral infections, organ transplantation and autoimmune diseases via eliminating effector T cell, inducing effector T cell anergy and regulatory T cells (Tregs) (33–35). Whether LM could impair the systemic antitumoral immunity and ICI treatment outcomes remains unknown. Recently, several publications investigated the predictive value of LM for ICI efficacy. Paul et al. analyzed 336 patients with melanoma or NSCLC received pembrolizumab and reported that LM was associated with significantly reduced responses and PFS (13). Subsequently, a series of studies reported the negative predictive value of LM for ICI treatment in specific types of solid tumors (16, 36). Furthermore, our study indicated that the presence of LM was the pan-cancer negative predictive factor in patients received ICIs monotherapy. Interestingly, our data revealed that ICI based combination therapy could dramatically prolong both PFS and OS in patients with LM and the presence of LM did not significantly impair the efficacy of ICI based combination therapy. Taken together, these findings suggested that ICI monotherapy is insufficient to control the disease in patients with cancer and LM. Reasonable ICI based combination therapy need future investigation in this clinical scenario.
To unravel the mechanism of liver antitumoral immune tolerance in the context of cancer is the key to improve the clinical practice and prognosis of patients with LM. Several recent publications shed a light on this research area. Zhou et al. reported that LAG3 blockade could increase proliferation and effector cytokine production of intratumoral T-cells isolated from LM of colorectal cancer in response to both polyclonal and autologous tumor-specific stimulations, suggesting a new promising immunotherapeutic target for LM of colorectal cancer (37). James et al. observed that the presence of liver could suppress the systemic antitumor immunity in a dual-tumor immunocompetent mouse model (38). Mechanistically, coordinated activation of Tregs and modulation of intratumoral CD11b+ monocytes led to the antigen specific immune suppression. While Tregs were depleted or destabilized by using specific inhibitors, the antitumoral immune of PD-1 antibody could resuscitate within LM. More recently, Yu et al. found that LM could siphon activated CD8+ T cells from systemic circulation and induce antigen-specific Fas+CD8+ T cells undergo apoptosis following their interaction with FasL+CD11b+F4/80+ monocyte-derived macrophages (39). These immunosuppressive hepatic macrophages could be eliminated by liver-directed radiotherapy, which result in the increase of hepatic T cell survival and decrease of hepatic siphoning of T cells. These two elegant study together suggested the specific immune microenvironment of LM and ICI based combination therapy (e.g. plus CTLA-4 inhibitor, EZH2 inhibitors, radiotherapy, etc.) could rescue systemic antitumor immunity and improve the prognosis of cancer patients with LM.
These current findings had several significant limitations that should be acknowledged and treated with caution. First, relatively small number of eligible patients into the final real-world cohort analysis and the retrospective nature will inevitably have several biases such as selection bias. Meta-analysis is the archetypical observation and heterogeneous clinical trials were included without any technically correct information, making it not necessarily meaningful. Thus, the present findings must be cautiously interpreted and large-scale prospective study is eagerly warranted. Second, since PD-L1 expression results from online database was unavailable and real-world cohort did not record the PD-L1 expression, the impact of PD-L1 expression on the treatment outcomes could not be investigated. Third, details of patients with LM in published trials were not reported, making further subgroup analysis difficult. Last but not least, the mechanisms of LM conferring poor prognosis in patients treated with ICI are not well stated. Since it is much difficult to obtain the paired primary and liver metastatic lesions in clinical practice, we cannot include any specific exome and/or transcriptomic features in the multivariate analysis. Therefore, currently, we cannot make a solid conclusion on the true predictive or prognostic significance of LM. In the future, we need comprehensively study the multi-omic features including genomic, transcriptomic, proteomic, metabolic and epigenomic features, especially single-cell transcriptome analysis and TCR sequencing of both primary lesions and LM to unravel the impact of specific immune clusters (e.g. macrophages, CD8+ T cells, Tregs, etc.) on tumor progression in the liver and ICI response, and then establish the true predictive or prognostic significance of LM in patients received ICIs therapy.
In conclusion, the current study indicated that the presence of LM was the negative predictive factor in cancer patients received ICIs monotherapy. ICI based combination therapy could dramatically prolong both PFS and OS in patients with LM and the presence of LM did not significantly impair the efficacy of ICI based combination therapy, suggesting it might overcome the intrinsic resistance of LM to ICIs monotherapy. However, due to the limited clinical and survival data from this study, the optimal combinatorial strategies in patients with LM are still unknown.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Wenzhou People’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
All authors participated in the planning and execution of this study or analysis of the study data. TJ designed this study. All authors collected the data and conducted the relevant experiments. X-JC, LZ, E-DZ, and TJ collected the data and performed the statistical analyses. TJ and X-JC drafted the manuscript. LZ and E-DZ provided critical comments, suggestions and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported in part by the Shanghai Sailing Program (No. 20YF1407500).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.651086/full#supplementary-material
References
1. Trefts E, Gannon M, Wasserman DH. The Liver. Curr Biol (2017) 27:R1147–51. doi: 10.1016/j.cub.2017.09.019
2. Hoekstra LT, de Graaf W, Nibourg GA, Heger M, Bennink RJ. Stieger B and Van Gulik Tm, Physiological and Biochemical Basis of Clinical Liver Function Tests: A Review. Ann Surg (2013) 257:27–36. doi: 10.1097/SLA.0b013e31825d5d47
3. Brodt P. Role of the Microenvironment in Liver Metastasis: From Pre- to Prometastatic Niches. Clin Cancer Res (2016) 22:5971–82. doi: 10.1158/1078-0432.CCR-16-0460
4. Milette S, Sicklick JK, Lowy AM, Brodt P. Molecular Pathways: Targeting the Microenvironment of Liver Metastases. Clin Cancer Res (2017) 23:6390–9. doi: 10.1158/1078-0432.CCR-15-1636
5. Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic Patterns in Adenocarcinoma. Cancer (2006) 106:1624–33. doi: 10.1002/cncr.21778
6. Riihimaki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic Sites and Survival in Lung Cancer. Lung Cancer (2014) 86:78–84. doi: 10.1016/j.lungcan.2014.07.020
7. Ren Y, Dai C, Zheng H, Zhou F, She Y, Jiang G, et al. Prognostic Effect of Liver Metastasis in Lung Cancer Patients With Distant Metastasis. Oncotarget (2016) 7:53245–53. doi: 10.18632/oncotarget.10644
8. Jiang T, Cheng R, Zhang G, Su C, Zhao C, Li X, et al. Characterization of Liver Metastasis and Its Effect on Targeted Therapy in EGFR-mutant Nsclc: A Multicenter Study. Clin Lung Cancer (2017) 18 631–9.e2. doi: 10.1016/j.cllc.2017.04.015
9. Patel SA, Minn AJ. Combination Cancer Therapy With Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity (2018) 48:417–33. doi: 10.1016/j.immuni.2018.03.007
10. Ribas A, Wolchok JD. Cancer Immunotherapy Using Checkpoint Blockade. Science (2018) 359:1350–5. doi: 10.1126/science.aar4060
11. Galon J, Bruni D. Approaches to Treat Immune Hot, Altered and Cold Tumours With Combination Immunotherapies. Nat Rev Drug Discov (2019) 18:197–218. doi: 10.1038/s41573-018-0007-y
12. Hegde PS, Chen DS. Top 10 Challenges in Cancer Immunotherapy. Immunity (2020) 52:17–35. doi: 10.1016/j.immuni.2019.12.011
13. Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver Metastasis and Treatment Outcome With Anti-PD-1 Monoclonal Antibody in Patients With Melanoma and NSCLC. Cancer Immunol Res (2017) 5:417–24. doi: 10.1158/2326-6066.CIR-16-0325
14. Wang X, Ji Q, Yan X, Lian B, Si L, Chi Z, et al. The Impact of Liver Metastasis on Anti-PD-1 Monoclonal Antibody Monotherapy in Advanced Melanoma: Analysis of Five Clinical Studies. Front Oncol (2020) 10:546604. doi: 10.3389/fonc.2020.546604
15. Osorio JC, Arbour KC, Le DT, Durham JN, Plodkowski AJ, Halpenny DF, et al. Lesion-Level Response Dynamics to Programmed Cell Death Protein (Pd-1) Blockade. J Clin Oncol (2019)37:3546–55. doi: 10.1200/JCO.19.00709
16. Qin BD, Jiao XD, Liu J, Liu K, He X, Wu Y, et al. The Effect of Liver Metastasis on Efficacy of Immunotherapy Plus Chemotherapy in Advanced Lung Cancer. Crit Rev Oncol Hematol (2020) 147:102893. doi: 10.1016/j.critrevonc.2020.102893
17. Vokes EE, Ready N, Felip E, Horn L, Burgio MA, Antonia SJ, et al. Nivolumab Versus Docetaxel in Previously Treated Advanced non-Small-Cell Lung Cancer (CheckMate 017 and CheckMate 057): 3-Year Update and Outcomes in Patients With Liver Metastases. Ann Oncol (2018) 29:959–65. doi: 10.1093/annonc/mdy041
18. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor Mutational Load Predicts Survival After Immunotherapy Across Multiple Cancer Types. Nat Genet (2019) 51:202–6. doi: 10.1038/s41588-018-0312-8
19. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the Cbioportal. Sci Signal (2013) 6:pl1. doi: 10.1126/scisignal.2004088
20. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov (2012) 2:401–4. doi: 10.1158/2159-8290.CD-12-0095
21. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thoracic Oncol Off Publ Int Assoc Study Lung Cancer (2011) 6:244–85. doi: 10.1097/JTO.0b013e318206a221
22. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Graf Finckenstein F and Brahmer Jr, Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
23. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
24. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab Versus Docetaxel for Patients With Previously Treated non-Small-Cell Lung Cancer (POPLAR): A Multicentre, Open-Label, Phase 2 Randomised Controlled Trial. Lancet (2016) 387:1837–46. doi: 10.1016/S0140-6736(16)00587-0
25. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab Versus Docetaxel in Patients With Previously Treated non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
26. Gadgeel S, Rodriguez-Abreu D, Speranza G, Esteban E, Felip E, Domine M, et al. Pietanza MC and Garassino Mc, Updated Analysis From Keynote-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol (2020) 38:1505–17. doi: 10.1200/JCO.19.03136
27. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol (2020) 38:2369–79. doi: 10.1200/JCO.20.00793
28. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in Combination With Carboplatin Plus Nab-Paclitaxel Chemotherapy Compared With Chemotherapy Alone as First-Line Treatment for Metastatic Non-Squamous Non-Small-Cell Lung Cancer (Impower130): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2019) 20:924–37. doi: 10.1016/S1470-2045(19)30167-6
29. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous Non-Small-Cell Lung Cancer (Impower131): Results From a Randomized Phase III Trial. J Thorac Oncol (2020)15:1351–60. doi: 10.1016/j.jtho.2020.03.028
30. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab Plus Chemotherapy for First-Line Treatment of Non-Squamous Non-Small Cell Lung Cancer: Results From the Randomized Phase III Impower132 Trial. J Thorac Oncol (2020)16:653–64. doi: 10.1016/j.jtho.2020.11.025
31. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
32. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab Plus Bevacizumab and Chemotherapy in Non-Small-Cell Lung Cancer (Impower150): Key Subgroup Analyses of Patients With EGFR Mutations or Baseline Liver Metastases in a Randomised, Open-Label Phase 3 Trial. Lancet Respir Med (2019) 7:387–401. doi: 10.1016/S2213-2600(19)30084-0
33. Crispe IN. Hepatic T Cells and Liver Tolerance. Nat Rev Immunol (2003) 3:51–62. doi: 10.1038/nri981
34. Li F, Tian Z. The Liver Works as a School to Educate Regulatory Immune Cells. Cell Mol Immunol (2013) 10:292–302. doi: 10.1038/cmi.2013.7
35. Doherty DG. Immunity, Tolerance and Autoimmunity in the Liver: A Comprehensive Review. J Autoimmun (2016) 66:60–75. doi: 10.1016/j.jaut.2015.08.020
36. Lin Z, Liu Q, Wei Q, Lin L, Chen X, Xue D. Hyperprogressive Disease in Advanced Cancer Patients With Liver Metastasis Treated With PD-1 Inhibitors: Two Case Reports. Ann Transl Med (2020) 8:1100. doi: 10.21037/atm-20-3928
37. Zhou G, Noordam L, Sprengers D, Doukas M, Boor PPC, van Beek AA, et al. Blockade of LAG3 Enhances Responses of Tumor-Infiltrating T Cells in Mismatch Repair-Proficient Liver Metastases of Colorectal Cancer. Oncoimmunology (2018) 7:e1448332. doi: 10.1080/2162402X.2018.1448332
38. Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, et al. Regulatory T Cell Control of Systemic Immunity and Immunotherapy Response in Liver Metastasis. Sci Immunol (2020)5:eaba0759. doi: 10.1126/sciimmunol.aba0759
Keywords: pan-cancer, liver metastases, immune checkpoint inhibitor, prognosis, treatment outcome
Citation: Chen X-J, Ren A, Zheng L, Zheng E-D and Jiang T (2021) Pan-Cancer Analysis Identifies Liver Metastases as Negative Predictive Factor for Immune Checkpoint Inhibitors Treatment Outcome. Front. Immunol. 12:651086. doi: 10.3389/fimmu.2021.651086
Received: 08 January 2021; Accepted: 04 June 2021;
Published: 24 June 2021.
Edited by:
Jian Zhang, Southern Medical University, ChinaReviewed by:
Caicun Zhou, Shanghai Pulmonary Hospital, ChinaRafael Rosell, Catalan Institute of Oncology, Spain
Copyright © 2021 Chen, Ren, Zheng, Zheng and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Juan Chen, MzY2NTM1MjU1QHFxLmNvbQ==; Tao Jiang, dG9ueWppYW5nZHJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiao-Juan Chen
Xiao-Juan Chen Aiqun Ren1†
Aiqun Ren1†