
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 02 July 2021
Sec. Autoimmune and Autoinflammatory Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.648881
This article is part of the Research Topic Recent Advances in Antiphospholipid Syndrome View all 17 articles
 Chaojun Hu1,2†
Chaojun Hu1,2† Siting Li1,2†
Siting Li1,2† Zhijuan Xie1,2
Zhijuan Xie1,2 Hanxiao You1,2
Hanxiao You1,2 Hui Jiang1,2
Hui Jiang1,2 Yu Shi1,2
Yu Shi1,2 Wanting Qi1,2
Wanting Qi1,2 Jiuliang Zhao1,2*
Jiuliang Zhao1,2* Qian Wang1,2
Qian Wang1,2 Xinping Tian1,2
Xinping Tian1,2 Mengtao Li1,2
Mengtao Li1,2 Yan Zhao1,2
Yan Zhao1,2 Xiaofeng Zeng1,2*
Xiaofeng Zeng1,2*Background: Diagnosis of antiphospholipid syndrome (APS) is based on the positivity of laboratory criteria antiphospholipid antibodies (aPLs). Test results for aPLs could be contradictory among different detection methods as well as commercial manufacturers. This study aimed to assess and compare the diagnostic and analytic performances of four commercial assays prevalently used in China.
Methods: A total of 313 patients including 100 patients diagnosed with primary APS, 52 with APS secondary to SLE, 71 with SLE, and 90 health controls were recruited. Serum IgG, IgM, and IgA for aCL, and aβ2GPI antibodies were detected with two ELISA and two CLIA systems, and test system with the best diagnostic value was explored of its correlation with key clinical features.
Results: CLIA by YHLO Biotech Co. was considered as the system with the best predictive power, where 58.55 and 57.89% of APS patients were positive for aCL or aβ2GPI for at least one antibody (IgG or IgM or IgA). Overall, CLIA showed better performance characteristics than traditional ELISA test systems.
Conclusion: CLIA was considered as a better platform for aPL detection in APS diagnosis. A combination of other detection platforms could assist in differential diagnosis as well as in identifying high-risk patients.
The antiphospholipid syndrome (APS) is defined by the development of venous/arterial thromboses or by the occurrence of obstetrical events including recurrent fetal losses or increased perinatal morbidity, with the persistent presence of antiphospholipid antibodies (aPLs). According to the 2006 APS classification criteria, APS diagnosis is based on the positivity of at least one of the clinical criteria as well as one of laboratory criteria including lupus anticoagulant (LA), high level of anti-cardiolipin (aCL), anti-β2 glycoprotein-I (aβ2GPI) immunoglobulin isotype G (IgG) or M (IgM) (1). More recently, non-criteria aPLs including anti-aCL or anti-b2GPI IgA, anti-phosphatidylserine–prothrombin (aPS/PT) complex, anti-annexin A5 antibodies (aAnxV), etc. are receiving increasing attention (2).
APS could be associated with several severe clinical outcomes such as pulmonary embolism, acute myocardial infarction, and stroke, which demand immediate appropriate intervention. On the other hand, anticoagulant treatment commonly utilized for APS could increase bleeding risk for susceptible patients. Since aPL detection comprise a large part of APS diagnosis, a detection system with high sensitivity and specificity is required in order to timely identify APS patients as well as provide accurate clinical intervention (3). Besides, evaluation of aPLs could also contribute to prognosis and risk assessment for associated clinical manifestations (4, 5).
Numerous guidelines and studies concerning aCL and aβ2GPI tests have been published (6). However, test results for aPLs remain contradictory among different detection methods as well as commercial manufacturers, probably due to the lack of standardization for cut-off values, method of calibration and quantitation, choice of solid phase and coating, type and source of antigen, and other analytic problems (7–9). Traditionally, enzyme-linked immunosorbent assay (ELISA) was applied due to its relative time and cost-efficiency. In recent years, novel automating detection systems, such as chemiluminescent immunoassay (CLIA), addressable laser bead immunoassay (ALBIA), line immunoassay (LIA), etc. have been introduced for aPL detection, and promising results have been yielded (10–14). Automatization can improve the reproducibility and reduce interlaboratory variation, yet may show distinct performance characteristics compared to ELISA (15, 16).
More specifically, in China, home-conducted ELISA is still most widely applied at laboratories for APS diagnosis. However, an increasing number of automated analyzers have been equipped by large general hospitals with high application potentials. Regarding commercially available systems, most studies focused on measuring and comparing only one assay to laboratory-conducted ELISA (17). However, little attention has been paid to simultaneously evaluate different test systems that are commonly chosen. The aim of this study was to assess and compare the diagnostic and analytic performances of four commercial assays prevalently used in China, including two ELISA and two CLIA systems, in a Chinese prospective APS cohort. Detection of IgG, IgM, and IgA for aCL and aβ2GPI antibodies was evaluated, and a test system with the best diagnostic value was explored of its correlation with key clinical features.
This was a single-center, prospective cohort study conducted at Peking Union Medical College Hospital (PUMCH) and the National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID) from May 2017 to January 2020. A total of 313 consecutive patients were included in this study, of which 100 patients had been diagnosed with primary APS (PAPS group), 52 with APS secondary to SLE (SAPS group), 71 with SLE (SLE group), and 90 healthy controls (HC group). Diagnosis of APS was defined by clinicians according to the 2006 Sydney revised classification criteria (1). According to the criteria, IgG and IgM aCL and a2GPI were analyzed with standardized ELISA (INOVA Diagnostics) at the Key Laboratory. Lupus anticoagulant was detected and evaluated according to the ISTH recommendations. Dilute Russell viper venom time (dRVVT) testing and activated partial thromboplastin time were measured, where LAC was considered positive if the ratio of screen/confirm time ratio was >1.20. Diagnosis of SLE was based on the 1997 ACR criteria and confirmed by the 2019 EULAR/ACR criteria. Clinical manifestations were recorded for PAPS, SAPS, and SLE groups, including vascular thrombosis (arterial or venous), pregnancy morbidity, and extra-criteria manifestations, including thrombocytopenia, heart valve disease, autoimmune hemolytic anemia, and neurological disorders, etc. For the HC group, only aPL serology information was present. For each subject, 4 ml of blood was collected with the help of a BD vacutainer without anticoagulants. Blood samples were allowed to clot at room temperature for 1 h and then centrifuged at 4°C for 5 min at 3,000 rpm. Serum was collected and stored at −80°C. No sample was exposed to more than one freeze–thaw cycle before analysis. The study was approved by the ethics committee at PUMCH and fulfilled the ethical guidelines of the declaration of Helsinki. All subjects gave written informed consent.
For each study subject, IgG, IgM, and IgA isotypes of aCL and aβ2GPI were analyzed with four systems listed below: a. iFlash CLIA kits provided by YHLO Biotech Co., Shenzhen, China (Y-CLIA); b. QUANTA Flash® CLIA kits provided by INOVA Diagnostics, Inc., San Diego, CA, US, Werfen Group as sales agent (W-CLIA); c. QUANTA Lite™ ELISA kits provided by INOVA Diagnostics, Inc., San Diego, CA, US, Werfen Group as sales agent (W-ELISA); d. AESKULISA® ELISA test kits provided by Aesku.Diagnostics GmbH & Co. KG, Wendelsheim, Germany (A-ELISA). Detailed characteristics of test systems from different manufacturers were summarized in Table 2. Cut-off values were defined for each system as recommended by the manufacturer.
Statistical analysis was performed using SPSS 26.0 or R (version 3.6.2). The χ2 test or Fisher’s exact test was used for comparison of categorical variables, and Wilcoxon test was used for continuous variables after normality was explored with the Shapiro–Wilk test. Sensitivities, specificities, and accuracies in APS diagnosis were compared in the McNemar test. Youden Index, positive and negative predictive values (PPV and NPV), and odds ratio (OR) with 95% confidence interval (95% CI) were also shown. Correlation of different aPL isotype levels with clinical manifestations was calculated, and clinical events with 95% CI were displayed. Two-tailed values of p less than 0.05 were considered statistically significant.
Among 152 APS patients, there were 63 (63.0%) females for PAPS, 46 (88.5%) for SAPS, and the mean age for each was 36.3 and 32.9 years (Table 1). Mean age was 30.1+/−8.2 years in the SLE group, of which 61 (85.9%) were female, while the HC group had 41 (45.6%) female and a mean age of 43.4+/−12.2. Detailed clinical manifestations were recorded for both APS and SLE patients and were shown. Thrombosis was most commonly present, with 80 (80.0%) for PAPS and 39 (75%) for SAPS, but not in the SLE group. Patients with history of arterial or venous thrombosis were recorded for APS patients. Pregnancy morbidity, history of adverse pregnancy, microangiopathy, and LA were also observed in both PAPS and SAPS group. Of all the clinical manifestations, the prevalence of thrombocytopenia was significantly different between PAPS and SAPS group (χ2 = 4.382, p = 0.036).
As summarized in Table 2, the coating, conjugation, calibration, and cut-off values with their calculation were listed for four commercial test systems. More specifically, Y-CLIA conducted paramagnetic particle chemiluminescent immunoassay using a fully automated iFlash 3000 Chemiluminescence Immunoassay Analyzer. Recommended values with best sensitivity, specificity, and false positive results of healthy donors against APS, SLE, and other autoimmune disease patients were chosen for all antibody isotypes. For W-CLIA, antigen-specific paramagnetic bead chemiluminescent immunoassay was conducted employing the fully automated BIO-FLASH CLIA instrument. Cut-off values for all antibodies were calculated using the 99th percentile in healthy groups. W-ELISA was a semi-quantitative enzyme linked immunosorbent assay manually conducted according to the manufacturer’s instruction.
Cut-off values were set based on the evaluation of normal and positive antibody samples. For A-ELISA, assay was also manually conducted following manufacturer’s protocols, yet no information was provided for cut-off value calculation.
Antibody results obtained from four test systems were evaluated for diagnostic power with sensitivity, specificity, accuracy, Youden Index, PPV, and NPV in APS diagnosis from the HC group in Table 3. For each antibody type, sensitivity, specificity, and accuracy were compared first between the same test methods (i.e., Y-CLIA against W-CLIA, W-ELISA against A-ELISA). The better system from each method, if identified, was then compared to determine the best system, which was further evaluated for clinical manifestation prediction. As shown in Table 3, the accuracy of aCL IgG was significantly higher for Y-CLIA than W-CLIA (p < 0.001), and A-ELISA than W-ELISA (p = 0.035). The sensitivity (p < 0.001) and accuracy (p < 0.001) were both significantly higher for Y-CLIA method. For aCL IgM, sensitivity and accuracy were significantly higher for W-ELISA than A-ELISA (p < 0.001). As for aCL IgA, Y-CLIA and A-ELISA were selected respectively for comparison, and the specificity of the former was significantly higher (p = 0.031). Sensitivity and accuracy of positivity of aCL IgG, IgM, or IgA were also significantly higher for Y-CLIA than for W-CLIA (p < 0.001). Y-CLIA and W-ELISA were selected as better systems for positivity of aCL IgG or IgM, and significant difference was observed for accuracy (p = 0.022). Concerning aβ2GPI, Y-CLIA and W-CLIA were selected for comparison of IgM, whose specificity (p = 0.049) was higher that the former. Sensitivity and accuracy of positivity of aβ2GPI IgG, IgM, or IgA, as well as those of aCL IgG or IgM, were all significantly higher for Y-CLIA. All in all, Y-CLIA was considered as a system with the best predictive power.
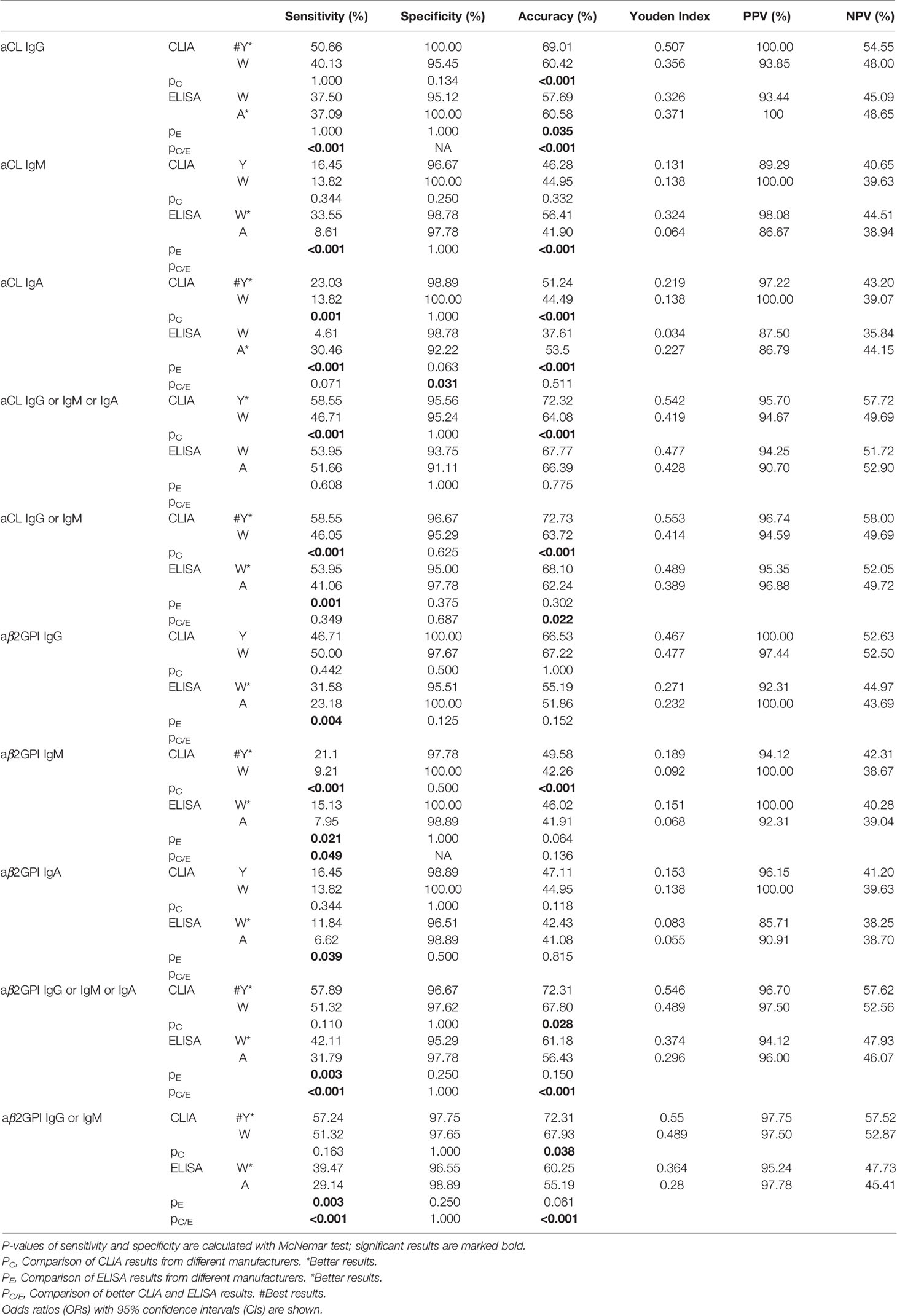
Table 3 Comparison of the predictive power of aPL tests from different test systems in APS diagnosis.
Similarly, the sensitivity, specificity, and accuracy were also compared among four systems in identifying thrombosis and pregnancy morbidity (Table 4). For thrombosis events, significant results for sensitivity and accuracy of aCL and aβ2GPI positivity were all higher for Y-CLIA than for W-CLIA and for W-ELISA than for A-ELISA. Y-CLIA still showed higher accuracy (p = 0.022 for aCL IgG or IgM and p = 0.001 for aβ2GPI IgM). As for pregnancy morbidity, significant results for specificity and accuracy of aCL and aβ2GPI positivity were significantly higher for W-CLIA than for Y-CLIA and for A-ELISA than for W-ELISA.
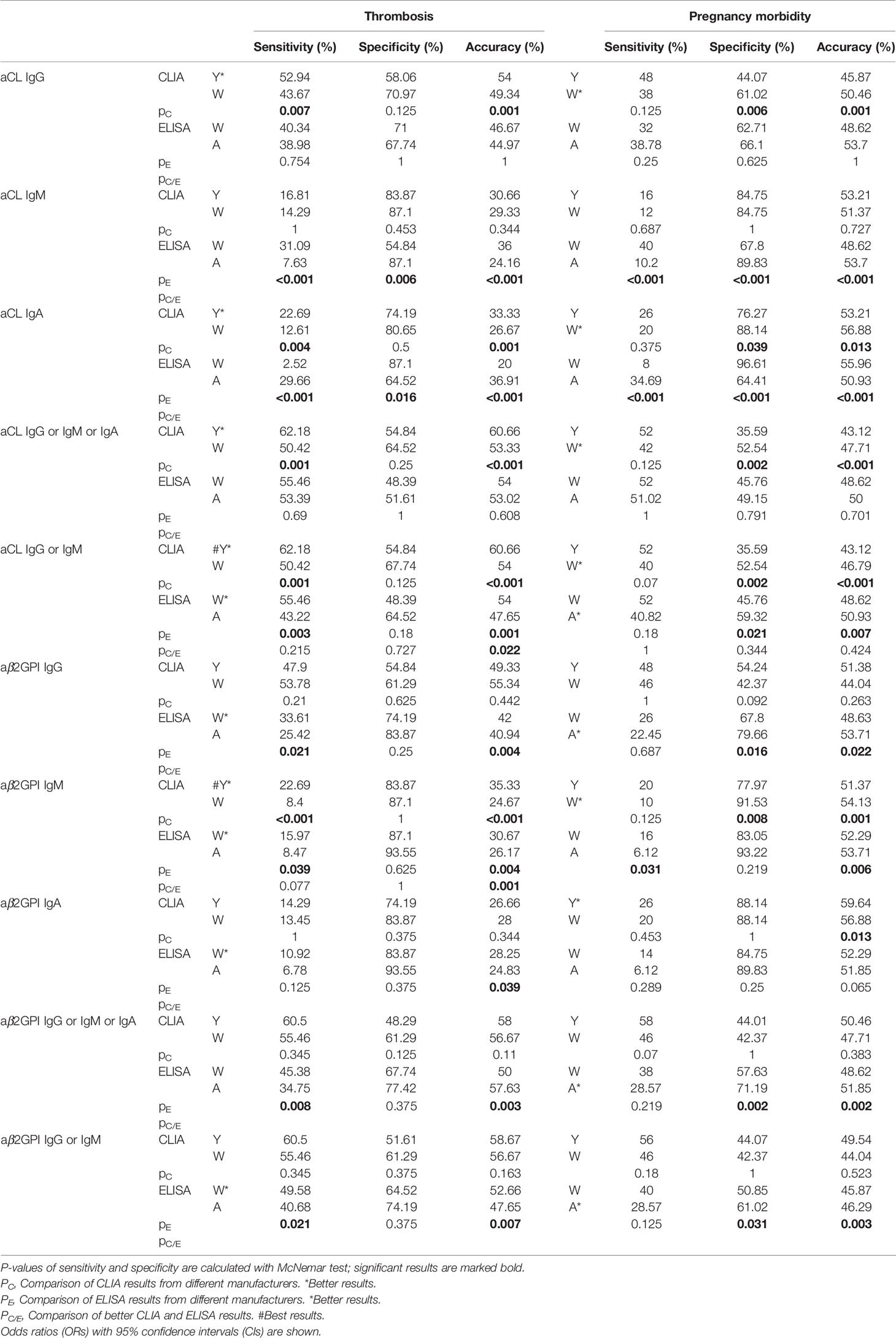
Table 4 Comparison of the predictive power of aPL tests from different test systems for criterial manifestations.
As different cut-off values were used by four test systems, the distribution of aPL test results from different manufacturers among patient groups were calculated with lg[(test result/cutoff value) +1] so that they could be visualized together as positive numbers in Figure 1. Patients positive for antibodies fell above the dotted line, and the range of distribution varied due to use of both test methods and limitation of test range for different antibodies. In general, W-CLIA had the widest range of test distribution, while W-ELISA had the narrowest. For Y-CLIA, test range limitation influenced distribution for three autoantibodies. The results of primary or secondary APS patients were compared to other groups and illustrated. Overall, most test systems could distinguish between APS patients and HC, while little significant difference was observed between PAPS and SAPS groups. For different antibodies, four test systems showed different strengths of differential diagnosis. For instance, W-CLIA was best at discrimination for aCL IgG, while A-ELISA was best at aCL IgM. Additionally, distribution of aPLs among clinical groups with the largest number of patients (i.e., thrombosis, pregnancy morbidity, and thrombocytopenia) was also illustrated in Figure 2.
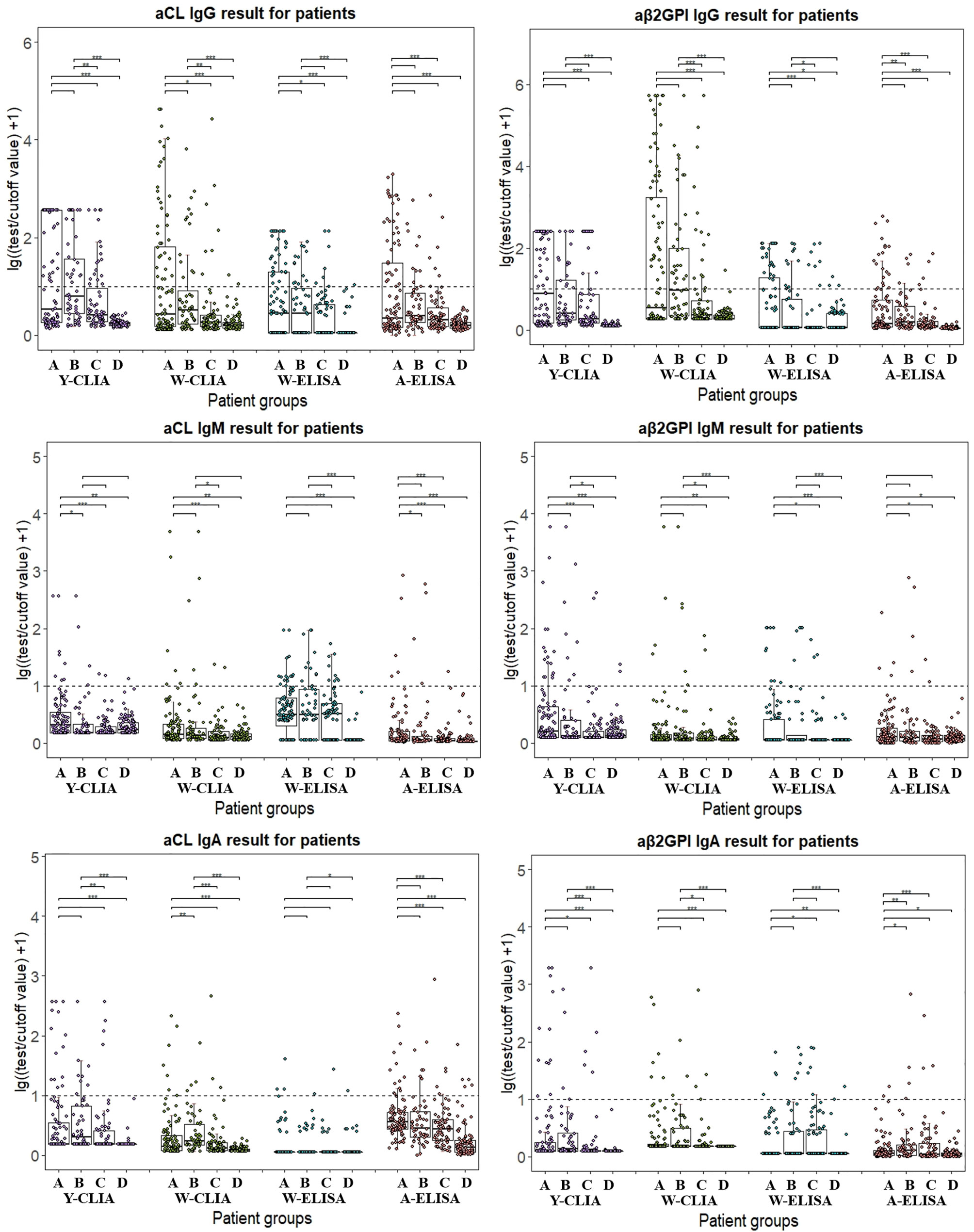
Figure 1 Distribution of aPL test results from different manufacturers among different patient groups. Test results are calculated in lg[(test result/cutoff value) +1]. A: PAPS, B: SAPS, C: SLE, D: Health control. Wilcox’s test is conducted comparing primary or secondary APS result to other patient groups. *P < 0.05, **P < 0.01, ***P < 0.001.
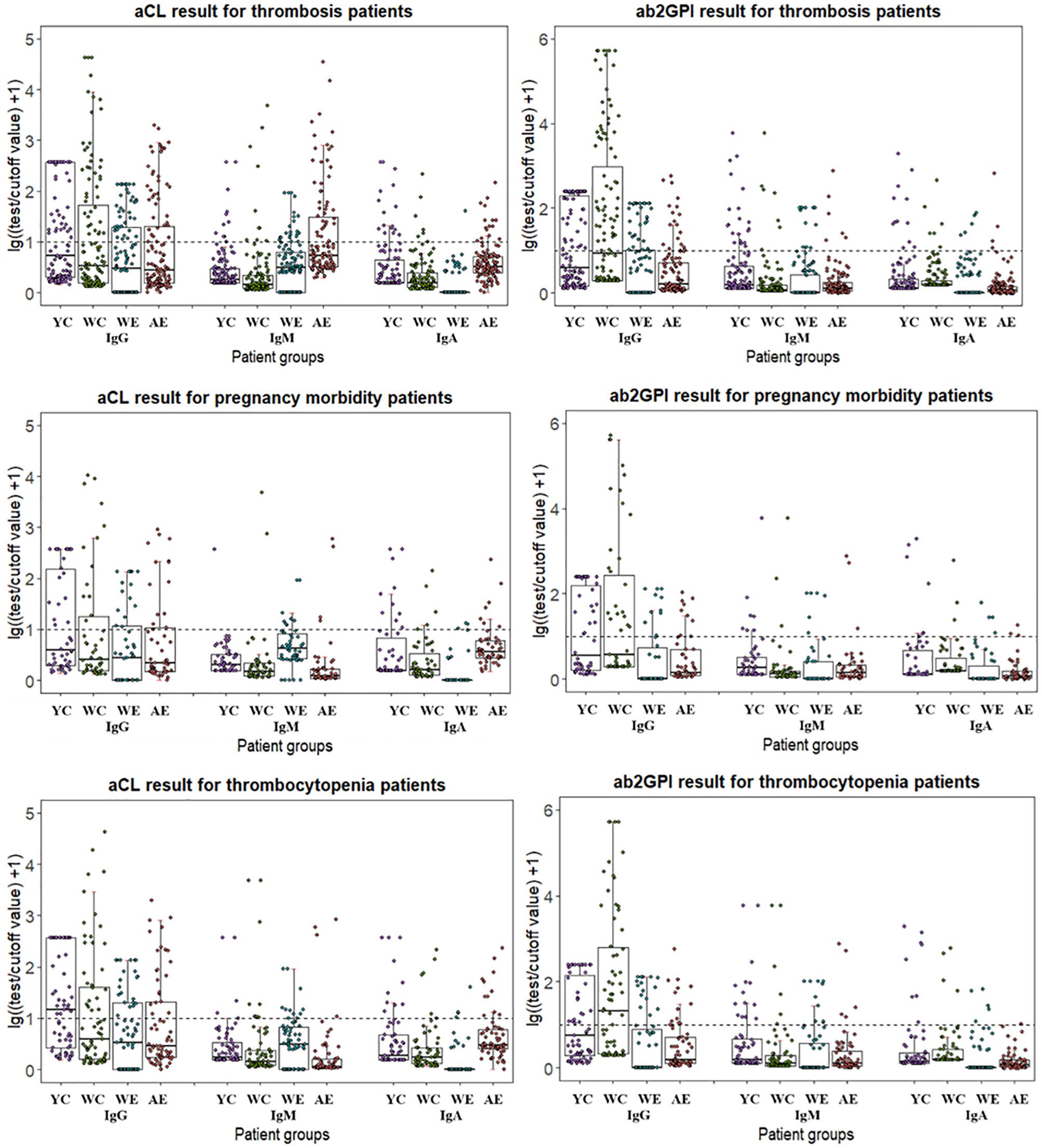
Figure 2 Distribution of aPL test results from different manufacturers for patients with different manifestations. Test results are calculated in lg[(test result/cutoff value) +1]. YC, Y-CLIA; WC, W-CLIA; WE, W-ELISA; AE, A-ELISA.
Among 152 patients, cross positivity for IgG or IgM of aCL and aβ2GpI for each of the four test systems were demonstrated with Venn diagrams (Figure 3). For aCL, 50 (32.9%) patients were tested positive for IgG or IgM by all systems. There were 12 (7.9%) patients who were tested positive only by Y-CLIA, and 13 (8.6%) were tested positive only by W-ELISA. Similarly, for aβ2GpI, 19 (12.5%) patients were test positive only by Y-CLIA, and seven (4.6%) were tested positive only by W-CLIA. When combining the positivity of aCL and aβ2GpI, Y-CLIA identified the most amount of positive patients (totally 102, 67.8%), with the highest level of patients distinguished only by the system (16, 10.5%).
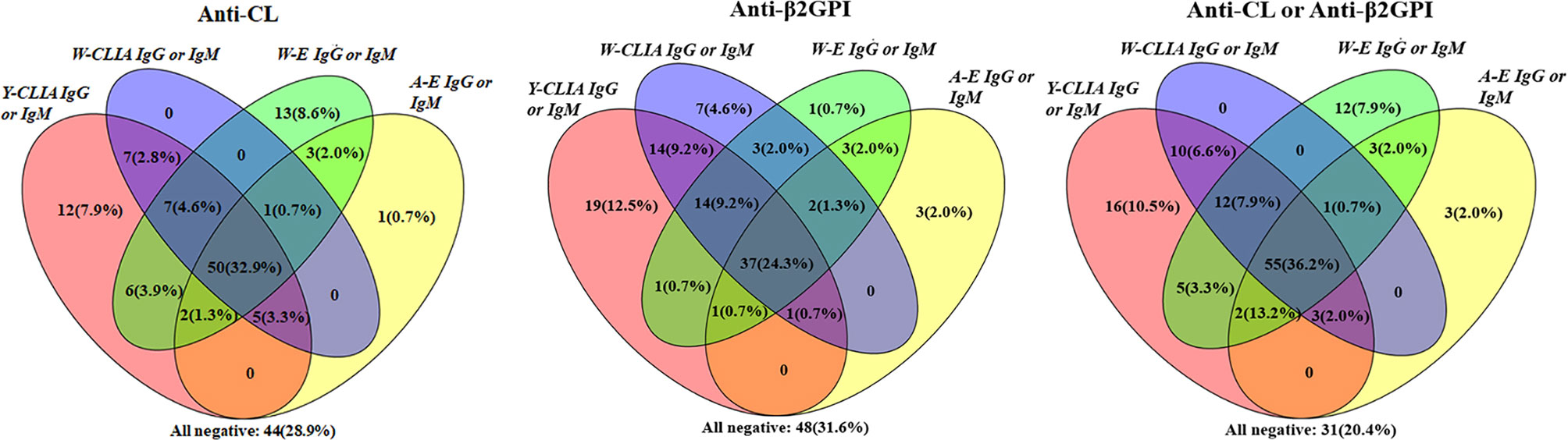
Figure 3 Cross-positivity among different tests for anti-CL, anti-β2GPI, and anti-CL or anti-β2GPI antibodies in APS patients (n = 152).
The correlation of different aPL levels by all four test systems with non-criteria clinical manifestations was further explored, with significant results presented in Table 5. Thrombocytopenia was associated with the greatest number of antibody positivity (aCL IgG by Y-CLIA, aCL IgM/aβ2GpI IgG/aβ2GpI IgM by W-CLIA, and aβ2GpI IgM by W-ELISA). Significant association was also observed for APSN, PVT, PE, DVT, and positivity of some autoantibodies by certain test systems. Little association was observed between IgA with any clinical features.
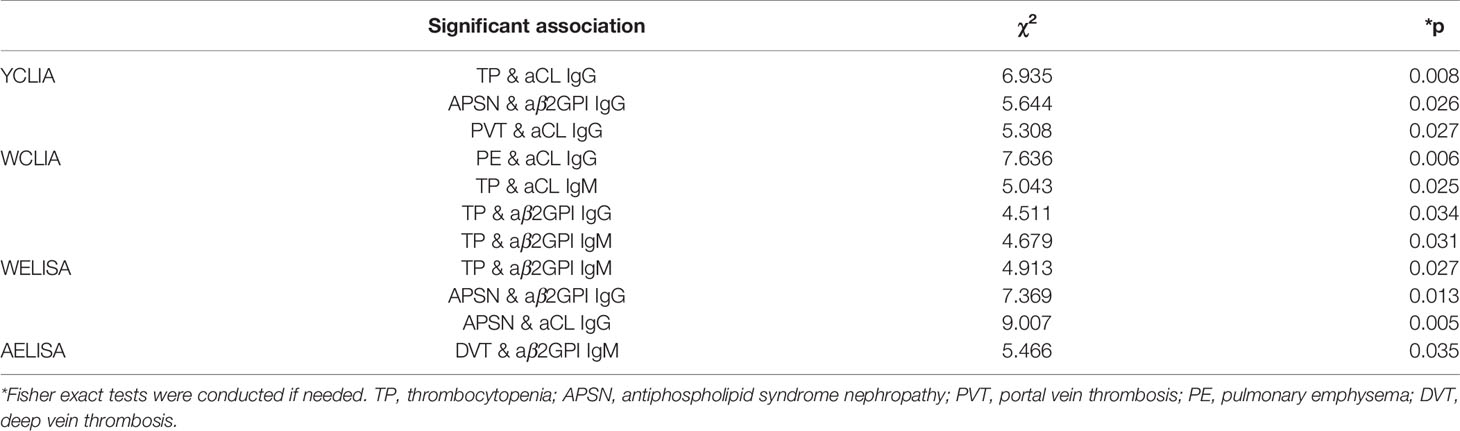
Table 5 Significant association between non-criteria clinical manifestations and aPL levels by different test systems.
APS is an autoimmune disease featuring thrombosis and/or pregnancy morbidity which may lead to severe consequences. In order to accurately identify APS patients and provide timely clinical intervention, a detection system with high sensitivity and specificity is required. In this study, the diagnostic and analytic performances of four commercial assays were compared in detecting IgG/IgM/IgA for aCL and aβ2GPI antibodies. In brief, CLIA by YHLO Biotech Co. was considered as the system with the best predictive power, where 58.55 and 57.89% of APS patients were positive for aCL or aβ2GPI for at least one antibodies (IgG or IgM or IgA). Y-CLIA also identified the greatest number of patients (67.8%) positive for aCL or aβ2GpI IgG or IgM, with the highest level of patients distinguished only by the system (16, 10.5%). Nevertheless, for Y-CLIA, little correlation of antibodies’ positivity result with thrombosis or pregnancy complication was observed. In addition, the greatest number of double/triple patients was detected by Y-CLIA. Concerning clinical manifestations, a significant association was observed between W-CLIA and TP/PE, Y-CLIA and TP, as well as combined results with TP/PE/thrombosis. Overall, CLIA showed better performance characteristics than traditional ELISA test systems.
Many previous studies have found poor agreement among different aPL assay platforms (5, 18), which may result from various factors. As shown in Table 2, depending on the coating method for solid phase, antibodies detected would either bind to cardiolipin or bind directly to β2GPI. In addition, different conjugates were applied for signal detection. A lack of universal internal standards for calibration further increased the chance of discrepancy. In addition, different cut-off values were chosen, as they stem from heterogenous reference sample groups in the original calculation. Thus, it might be better to choose the same appropriate reference population among all platforms and utilize an in-house 99th percentile cut-off value, which had been recommended by all manufacturers and confirmed by previous studies (19, 20). Nevertheless, due to the restriction of subjects, this study still chose the cut-off values provided by platform instructions respectively, which might not reflect the distribution characteristics of the disease population. Compared to ELISA, automated CLIA has the advantage of increasing reproducibility, reducing hands-on time as well as avoiding manual error, which had been proved by some previous studies.
With regard to the predictive value of aPLs detected by the four systems, Y-CLIA stood out as the best. Table 3 indicated that the sensitivity, specificity, accuracy, and Youden index were higher for Y-CLIA among each comparison whenever a significant difference was found. As for ELISAs, W-ELISA had higher predictive power for most aPLs compared to A-ELISA. However, no single detection system had stably shown better performance for all aPLs. Distribution of aPL test results in Figure 1 further reflected this inconsistency. Y-CLIA did not show better ability at distinguishing PAPS or SPAS from SLE or HC groups compared to other systems. Indeed, it had been estimated in previous studies that around 40% of patients with SLE have aPL, and APS may develop in up to 50–70% of patients with both SLE and aPLs (21). Thus, although Y-CLIA could be recommended for APS diagnosis, other systems may provide additive value for each individual aPL in differentiation, especially when SLE was involved. The predictive power of criterial manifestations indicated that besides serology diagnosis, different systems had respective strengths in predicting associate events. W-CLIA was more sensitive and accurate for thrombosis, while results from A-ELISA were more specific and accurate for pregnancy-related outcomes. Since APS diagnosis relied both on clinical and experimental criteria, inclusion of more test systems was still of great importance.
As IgG or IgM of aCL and aβ2GpI was part of the standard diagnostic criteria, cross-positivity analysis was conducted, which revealed that Y-CLIA identified the most number of patients test positive overall. However, other systems were still of great value for different aPLs, as 8.6% of aCL and 4.6% of aβ2GpI were tested positive only by W-ELISA or W-CLIA. which suggested that a combination of more test systems could increase the sensitivity of APS diagnosis. In the clinic, patients may remain persistently negative for criteria aPLs yet show typical APS clinical manifestations (defined as seronegative APS, SNAPS) (22). Alternate testing platforms could assist in final diagnosis for SNAPS patients.
According to the European League Against Rheumatism (EULAR) guidelines for APS, high-risk profiles for APS is defined as a positive LA test, the presence of double (any combination of LA, aCL or aβ2GPI antibodies) or triple (all three subtypes) aPL positivity, or the presence of persistently high aPL titers (23). It is crucial to recognize these high-risk patients in order for the early prevention of thrombotic and obstetric events (24). Thus, a cross-positivity analysis was conducted to evaluate the ability of four test systems in identifying high-risk patients concerning aCL/aβ2GPI detection (result not shown). For double-positive patients, among 94 patients (61.84%) positive for LA and aCL, eleven and nine were detected positive only by Y-CLIA and W-ELISA respectively. Among 92 patients (60.53%) positive for LA and aβ2GPI, seven and six were detected positive only by Y-CLIA and W-CLIA respectively. For 77 triple-positive patients (50.66%), nine were detected positive only by Y-CLIA and two by W-CLIA. The result suggested that a combination of more test systems could increase the sensitivity of high-risk identification for APS.
Finally, the results of different aPL isotypes tested by four systems were explored of their association with non-criteria clinical manifestations. Thrombocytopenia was associated with the greatest number of antibody positivity, and significant results were also observed for APSN, PVT, PE, and DVT. However, no other significant association was observed for other clinical features or IgA isotype. Similar results could be observed in a study conducted by us recently in a large cohort with more than 7,000 patients (25). It had been reported that the prevalence of thrombocytopenia was 20 to 46% as a manifestation of primary APS, probably because aCL may bind activated platelet membranes and cause platelet destruction (26). Although the correlation between aPLs and thrombosis or pregnancy events has been confirmed by a number of studies (27–29), conflicting results have also been observed in other reports (9, 30). In our study, venous thrombosis events (PVT, PE, and DVT) showed more correlation with aPL positivity, while little significant relationship was found with poor pregnancy outcomes. It should be noted that the number of patients with most of the recorded clinical manifestations was small (Figure 1). Consequently, the results might be strongly influenced by patient heterogeneity including age, gender, or other factors.
All in all, this study confirmed the advantage of using CLIA testing systems for aPL detection, with higher predictive power and better ability at identifying both low-titer suspected and multi-positive high-risk patients. In the future, with the reduction of test apparatus cost, fully automated CLIA could replace ELISA in the laboratory testing of aPLs for APS diagnosis and monitor. For the local population in China, Y-CLIA would be a more suitable choice concerning commercially available testing systems. Our study has some limitations. Recommended cut-off values were used and not calculated with the local population, which might decrease precision in sequential analysis. Correlation between autoantibodies and clinical manifestations, especially obstetrical related events, still needs examination. Larger sample size and inclusion of patients with a wider range of associated diseases or clinical features, as well as more high-risk patients (double/triple-positive), could further complement the study. The predictive performance of the selected test system (Y-CLIA) also needs further confirmation.
In conclusion, CLIA was considered a better platform for IgG/IgM/IgA aCL and aβ2GPI detection in APS diagnosis. Additionally, a combination of other detection platforms could assist in clinical diagnosis and differential diagnosis, increase the ability to exclude SNAPS, as well as identify high-risk patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
All authors were involved in the design of this study. CH, SL, ZX, HY, HJ, and JZ contributed to the collection of blood samples and other experimental procedures. YS and WQ were involved in data collection and pre-processing. CH and SL analyzed the data and wrote the manuscript. JZ, QW, XT, ML, and YZ contributed to the recruitment of patients and evaluation of clinical data. All authors contributed to the article and approved the submitted version.
This study was supported by the National Key Research and Development Program of China (2019YFC0840603, 2017YFC0907601, and 2017YFC0907602), the National Natural Science Foundation of China (81771780), and the CAMS Initiative for Innovative Medicine (2017-I2M-3-001 and 2019-I2M-2-008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International Consensus Statement on an Update of the Classification Criteria for Definite Antiphospholipid Syndrome (APS). J Thromb Haemost (2006) 4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Žigon P, Podovšovnik A, Ambrožič A, Tomšič M, Hočevar A, Gašperšič N, et al. Added Value of Non-Criteria Antiphospholipid Antibodies for Antiphospholipid Syndrome: Lessons Learned From Year-Long Routine Measurements. Clin Rheumatol (2019) 38(2):371–8. doi: 10.1007/s10067-018-4251-7
3. Persijn L, Decavele AS, Schouwers S, Devreese K. Evaluation of a New Set of Automated Chemiluminescense Assays for Anticardiolipin and Anti-Beta2-Glycoprotein I Antibodies in the Laboratory Diagnosis of the Antiphospholipid Syndrome. Thromb Res (2011) 128(6):565–9. doi: 10.1016/j.thromres.2011.04.004
4. Oliveira DC, Correia A, Oliveira C. The Issue of the Antiphospholipid Antibody Syndrome. J Clin Med Res (2020) 12(5):286–92. doi: 10.14740/jocmr4154
5. Chayoua W, Kelchtermans H, Moore GW, Musiał J, Wahl D, de Laat B, et al. Identification of High Thrombotic Risk Triple-Positive Antiphospholipid Syndrome Patients Is Dependent on Anti-Cardiolipin and Anti-β2glycoprotein I Antibody Detection Assays. J Thromb Haemostasis: JTH (2018) 16(10):2016–23. doi: 10.1111/jth.14261
6. Devreese KMJ, Ortel TL, Pengo V, de Laat B. Laboratory Criteria for Antiphospholipid Syndrome: Communication From the SSC of the ISTH. J Thromb Haemostasis: JTH (2018) 16(4):809–13. doi: 10.1111/jth.13976
7. Devreese KM. Antiphospholipid Antibody Testing and Standardization. Int J Lab Hematol (2014) 36(3):352–63. doi: 10.1111/ijlh.12234
8. Devreese KMJ. How to Interpret Antiphospholipid Laboratory Tests. Curr Rheumatol Rep (2020) 22(8):38. doi: 10.1007/s11926-020-00916-5
9. Chayoua W, Kelchtermans H, Moore GW, Gris JC, Musial J, Wahl D, et al. Detection of Anti-Cardiolipin and Anti-β2glycoprotein I Antibodies Differs Between Platforms Without Influence on Association With Clinical Symptoms. Thromb Haemostasis (2019) 119(5):797–806. doi: 10.1055/s-0039-1679901
10. Montaruli B, De Luna E, Erroi L, Marchese C, Mengozzi G, Napoli P, et al. Analytical and Clinical Comparison of Different Immunoassay Systems for the Detection of Antiphospholipid Antibodies. Int J Lab Hematol (2016) 38(2):172–82. doi: 10.1111/ijlh.12466
11. Grossi V, Infantino M, Benucci M, Li Gobbi F, Bandinelli F, Damiani A, et al. Two Novel Technologies for the Detection of Anti-Cardiolipin and Anti β2-Glycoprotein Antibodies in the Real Life: Chemiluminescent in Comparison to the Addressable Laser Bead Immunoassays. Immunol Invest (2020) 49(1–2):58–68. doi: 10.1080/08820139.2019.1647233
12. Thaler MA, Bietenbeck A, Steigerwald U, Büttner T, Schierack P, Lindhoff-Last E, et al. Evaluation of the Sensitivity and Specificity of a Novel Line Immunoassay for the Detection of Criteria and Non-Criteria Antiphospholipid Antibodies in Comparison to Established ELISAs. PloS One (2019) 14(7):e0220033. doi: 10.1371/journal.pone.0220033
13. Tozzoli R, Villalta D. Autoantibody Profiling of Patients With Antiphospholipid Syndrome Using an Automated Multiplexed Immunoassay System. Autoimmun Rev (2014) 13(1):59–63. doi: 10.1016/j.autrev.2013.08.007
14. Van Hoecke F, Persijn L, Decavele AS, Devreese K. Performance of Two New, Automated Chemiluminescence Assay Panels for Anticardiolipin and Anti-Beta2-Glycoprotein I Antibodies in the Laboratory Diagnosis of the Antiphospholipid Syndrome. Int J Lab Hematol (2012) 34(6):630–40. doi: 10.1111/j.1751-553X.2012.01448.x
15. Capozzi A, Lococo E, Grasso M, Longo A, Garofalo T, Misasi R, et al. Detection of Antiphospholipid Antibodies by Automated Chemiluminescence Assay. J Immunol Methods (2012) 379(1-2):48–52. doi: 10.1016/j.jim.2012.02.020
16. Mahler M, Bentow C, Serra J, Fritzler MJ. Detection of Autoantibodies Using Chemiluminescence Technologies. Immunopharmacol Immunotoxicol (2016) 38(1):14–20. doi: 10.3109/08923973.2015.1077461
17. Zhou J, Hou X, Zhang H, Wang T, Cui L. The Clinical Performance of a New Chemiluminescent Immunoassay in Measuring Anti-β2 Glycoprotein 1 and Anti-Cardiolipin Antibodies. Med Sci Monit (2018) 24:6816–22. doi: 10.12659/MSM.910369
18. Devreese KMJ. Testing for Antiphospholipid Antibodies: Advances and Best Practices. Int J Lab Hematol (2020) 42 Suppl 1:49–58. doi: 10.1111/ijlh.13195
19. Vanoverschelde L, Kelchtermans H, Musial J, de Laat B, Devreese KMJ. Influence of Anticardiolipin and Anti-β2 Glycoprotein I Antibody Cutoff Values on Antiphospholipid Syndrome Classification. Res Pract Thromb Haemostasis (2019) 3(3):515–27. doi: 10.1002/rth2.12207
20. Decavele AS, Schouwers S, Devreese KM. Evaluation of Three Commercial ELISA Kits for Anticardiolipin and Anti-Beta2-Glycoprotein I Antibodies in the Laboratory Diagnosis of the Antiphospholipid Syndrome. Int J Lab Hematol (2011) 33(1):97–108. doi: 10.1111/j.1751-553X.2010.01259.x
21. Paule R, Morel N, Le Guern V, Fredi M, Coutte L, Belhocine M, et al. Classification of Primary Antiphospholipid Syndrome as Systemic Lupus Erythematosus: Analysis of a Cohort of 214 Patients. Autoimmun Rev (2018) 17(9):866–72. doi: 10.1016/j.autrev.2018.03.011
22. Hughes GR, Khamashta MA. Seronegative Antiphospholipid Syndrome. Ann Rheum Dis (2003) 62(12):1127. doi: 10.1136/ard.2003.006163
23. Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, et al. EULAR Recommendations for the Management of Antiphospholipid Syndrome in Adults. Ann Rheum Dis (2019) 78(10):1296–304. doi: 10.1136/annrheumdis-2019-215213
24. Garcia D, Erkan D. Diagnosis and Management of the Antiphospholipid Syndrome. New Engl J Med (2018) 378(21):2010–21. doi: 10.1056/NEJMra1705454
25. Hu C, Li X, Zhao J, Wang Q, Li M, Tian X, et al. Immunoglobulin A Isotype of Antiphospholipid Antibodies Does Not Provide Added Value for the Diagnosis of Antiphospholipid Syndrome in a Chinese Population. Front Immunol (2020) 11:568503. doi: 10.3389/fimmu.2020.568503
26. Wang D, Lv W, Zhang S, Zhang J. Advances in the Research on Anticardiolipin Antibody. J Immunol Res (2019) 2019:8380214. doi: 10.1155/2019/8380214
27. Sciascia S, Cosseddu D, Montaruli B, Kuzenko A, Bertero MT. Risk Scale for the Diagnosis of Antiphospholipid Syndrome. Ann Rheum Dis (2011) 70(8):1517–8. doi: 10.1136/ard.2010.145177
28. Pengo V, Ruffatti A, Legnani C, Gresele P, Barcellona D, Erba N, et al. Clinical Course of High-Risk Patients Diagnosed With Antiphospholipid Syndrome. J Thromb Haemostasis: JTH (2010) 8(2):237–42. doi: 10.1111/j.1538-7836.2009.03674.x
29. De Carolis S, Tabacco S, Rizzo F, Giannini A, Botta A, Salvi S, et al. Antiphospholipid Syndrome: An Update on Risk Factors for Pregnancy Outcome. Autoimmun Rev (2018) 17(10):956–66. doi: 10.1016/j.autrev.2018.03.018
Keywords: antiphospholipid antibodies, antiphospholipid syndrome, chemiluminescent immunoassay, enzyme-linked immunosorbent assay, anti-β2 glycoprotein-I, anti-cardiolipin
Citation: Hu C, Li S, Xie Z, You H, Jiang H, Shi Y, Qi W, Zhao J, Wang Q, Tian X, Li M, Zhao Y and Zeng X (2021) Comparison of Different Test Systems for the Detection of Antiphospholipid Antibodies in a Chinese Cohort. Front. Immunol. 12:648881. doi: 10.3389/fimmu.2021.648881
Received: 02 January 2021; Accepted: 17 June 2021;
Published: 02 July 2021.
Edited by:
Huji Xu, Tsinghua University, ChinaReviewed by:
Cheng-De Yang, Shanghai Jiao Tong University, ChinaCopyright © 2021 Hu, Li, Xie, You, Jiang, Shi, Qi, Zhao, Wang, Tian, Li, Zhao and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiuliang Zhao, empscHVtY0BzaW5hLmNvbQ==; Xiaofeng Zeng, emVuZ3hmcHVtY0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.