
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 22 March 2021
Sec. Nutritional Immunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.643420
Lactobacillus (L.) plantarum strains, belong to lactic acid bacteria group, are considered indispensable probiotics. Here, we performed meta-analysis to evaluate the regulatory effects of L. plantarum on the immunity during clinical trials. This meta-analysis was conducted by searching across four most common literature databases, namely, Cochrane Central Register of Controlled Trials, Web of Science, Embase, and PubMed. Clinical trial articles that met the inclusion and exclusion criteria were analyzed by Review Manager (version 5.3). p-value < 0.05 of the total effect was considered statistically significant. Finally, total of 677 references were retrieved, among which six references and 18 randomized controlled trials were included in the meta-analysis. The mean differences observed at 95% confidence interval: interleukin (IL)-4, −0.48 pg/mL (−0.79 to −0.17; p < 0.05); IL-10, 9.88 pg/mL (6.52 to 13.2; p < 0.05); tumor necrosis factor (TNF)-α, −2.34 pg/mL (−3.5 to −1.19; p < 0.05); interferon (IFN)-γ, −0.99 pg/mL (−1.56 to −0.41; p < 0.05). Therefore, meta-analysis results suggested that L. plantarum could promote host immunity by regulating pro-inflammatory and anti-inflammatory cytokines.
Probiotics have been studied extensively by researchers since their discovery in early twentieth century by Elie Metchnikoff. Their probiotic effects are well-acclaimed by researchers and consumers. Probiotics regulate the gut microbiome, maintain intestinal homeostasis, and modulate other physiological conditions (1). Probiotic products are used to treat/alleviate clinical conditions like constipation, cardiovascular diseases, hyperlipidemia, hyperglycemia, and hypertension (2, 3). Lactobacilli are commonly found in fermented food products and are frequently used in industrial food fermentation (4), and many members of this genera are generally recognized as safe (GRAS). Lactobacilli are Gram-positive bacteria which are found in various natural environments. Modern food and health industries have been investigating and developing a broad range of probiotic strains which have been isolated from nutrient rich habitats such foods, feeds, plants, animals, and humans. It has been substantially reported that probiotics confer beneficial effects to human health (5). Recent works have also shown that the administration of lactobacilli have abated antibiotic-induced side effects, such as gut dysbiosis, diarrhea, and immune dysregulation (6–8). Some of the most used probiotic Lactobacillus spp. are Lactobacillus acidophilus, Lactobacillus casei, and Lactobacillus plantarum (L. plantarum) among others.
Recently, L. plantarum has been applied in health-promoting products as some strains have shown promising clinical results, such as regulating gastrointestinal function, lowering serum cholesterol, and enhancing immunity (9–11). One of the most obvious beneficial health effects of consuming probiotic products is immune regulation. With the advent in cell biology and molecular biology, a deeper understanding of how probiotics modulate the immune system has been gained (12, 13). Robust evidence has come from clinical and experimental works on the L. plantarum species. This species has been found to regulate the innate and adapted cellular and humoral immunity (14). For example, Prakoeswa and his coworkers (15) had shown that taking L. plantarum IS-10506 could boost the levels of interleukin (IL)-4 and IL-17 of adults with atopic dermatitis. Moreover, L. plantarum could alleviate oxidative stress after a triathlon by increasing the levels of anti-inflammation (IL-10) while decreasing the level of pro-inflammation [tumor necrosis factor (TNF)-α, IL-6, and IL-8] (16). Besides, L. plantarum IS-10506 could regulate the immune system by decreasing IL-17, IL-4, and interferon (IFN)-γ levels in serum of children with atopic dermatitis (17).
Meta-analysis was first christened by Glass, a British psychologist (18). In 2001, Egger and colleagues (19) made point that meta-analysis is a valid method to evaluate the effects of the interventions on subjects by combining the results of multiple research data for statistical analysis. Some researchers have used meta-analysis to explore the therapeutic effects of probiotics on allergies and the modulation of intestinal flora (20, 21). L. plantarum has many physiological functions, among which immunity is one of the most important (11). However, to the best of our knowledge, there is no specific data indicating the effects of L. plantarum in immunity regulation. Therefore, we designed this study to perform meta-analysis on effects of L. plantarum administration, mainly focusing on the immunoregulatory studies conducted in the last decades.
Four databases, namely Cochrane Central Register of Controlled Trials (https://www-cochranelibrary-com.ezproxy.cul.columbia.edu/), Web of Science (http://apps.webofknowledge.com/), Embase (https://www-embase-com.ezproxy.cul.columbia.edu/#search), and PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), were selected for reference retrieval. All the studies on the effect of intervention of L. plantarum on host immune published in and before October 2019 were included. The “subject” field (including title, keyword and abstract) was used to retrieve literature. The combination of keywords was based on Boolean operation, and the use of keywords was as follows: (Lactobacillus plantarum or L. plantarum) and (immunological/immunity/immune/allergy/eczema/rhinitis/asthma/cytokines/atopic dermatitis).
Exclusion criteria: (1) meta-analysis, letter, review, report, meeting summary, etc.; (2) animal test, in vitro test, and other non-clinical trials; (3) studies without original data; (4) studies applying prebiotics or other additives; (5) non-randomized controlled trials.
Inclusion criteria: (1) clinical studies of L. plantarum taken by human subjects; (2) studies employing immunity-related outcome indicators; (3) randomized controlled trials.
The data were extracted from the articles that met the inclusion and exclusion criteria. More than two people independently extracted the data, made decisions, and compared the conclusions of each article. In case of any disagreement, a third individual member was introduced to solve the problem through discussion and consultation. When extracting the required data from the original research, the authenticity and reliability of the data were considered. Bias caused by the subjective judgment of the evaluator was avoided as much as possible. If the discussion still failed to solve the existing problems, the details of problems occurring in the data extraction process were to be included. To ensure the reliability of the extracted data and minimize bias and errors, systematic training was given to the members who participated in the analysis and data extraction.
The information extracted in this study included: type of trial, probiotic strains, number of participants, type of intervention, and intervention results [tumor necrosis factor (TNF)-α, IL-4, IL-10, and IFN-γ].
Before the meta-analysis, the units of indicators were standardized. Review Manager 5.3 (Cochrane Collaboration Network, London, UK) was used to conduct this meta-analysis. Forest plots were used to detect the heterogeneity of the articles. Funnel maps were used to detect the possible publication bias (22). The sample size, mean difference, and 95% confidence interval (CI) were calculated to analyze the results of continuous variables. The average difference was calculated by subtracting the baseline data from the data after intervention, and the difference was directly used if given in the original article. If 95% CI includes zero, there is no statistical significance. If the data after intervention and the baseline data were given, statistical analysis was done after calculation. p-value < 0.05 of the total effect quantity was considered statistically significant in the confidence interval method. I2 was an indicator that measured the degree of heterogeneity in multiple studies. If I2 was <50%, the heterogeneity between studies was considered acceptable. When there was no heterogeneity between studies, the fixed effect model method should be used; otherwise, the random effect model should be applied (23).
In total, 677 references were retrieved from the four databases (after removing duplicated studies) (Figure 1). Based on the title and abstract, 641 irrelevant articles were excluded since those were animal trials and in vitro studies. The remaining 36 articles were relevant after reading the full text. Thirty of these articles were excluded according to the inclusion and exclusion criteria (two articles were not related to this meta-analysis, one article was not placebo-controlled trial, 17 articles were insufficient, and ten articles were based on non-blind trials). Eventually, only six articles were considered in this current meta-analysis. Among the included articles: Costabile et al. (24) measured two immune-related indicators, so this study was considered as two independent trials; Hirose et al. (25) measured two different immune-related indicators; Huang et al. (26) measured four different immune-related indicators; Lew et al. (27) measured four different immune-related indicators; Prakoeswa et al. (17) measured three different immune-related indicators; and Yang et al. (28) measured three different immune-related indicators. Therefore, this meta-analysis included 18 randomized controlled trials.
Data were extracted from the selected clinical trials. Extracted data, including probiotic strains, interventions, experiment types, intervention doses, sample size, and outcome index information, are presented in Table 1. The total number of participants was 1,047, including 514 in the probiotic group and 533 in the placebo group. Moreover, the immune parameters selected for this meta-analysis were TNF-α, IL-4, IFN-γ, and IL-10.
Review Manager 5.3 (Cochrane Collaboration Network, London, UK) was used to perform statistical analysis on four indicators: TNF-α (four studies), IL-4 (five studies), IFN-γ (four studies), and IL-10 (five studies). The mean difference and 95% confidence interval of these indicators are shown in Figures 2–5.
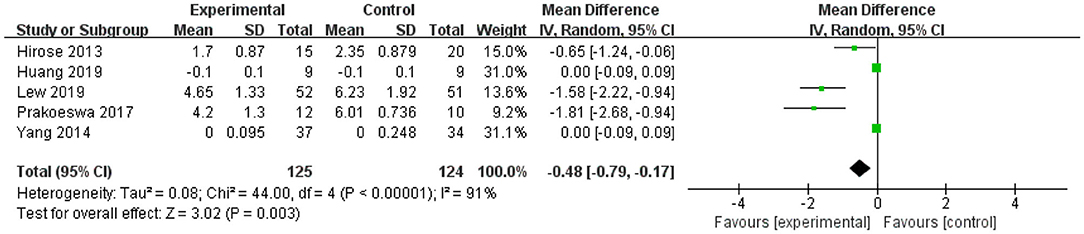
Figure 2. Forest plot of effect of Lactobacillus plantarum on IL-4. SD, standard deviation; Total, sample size of each group; Mean Difference, the mean value of the experimental group minus that of the control; 95% CI, 95% confidence intervals.
After intervention, the 95% CI of two of the five studies of IL-4 were 0 (the lower limit was <0 and the upper limit was >0) (Figure 2). The upper and lower limits of 95% CI of the other three studies were <0, indicating that these studies were statistically significant, and L. plantarum treatment was effective for IL-4. The mean difference of the total effect was −0.48 pg/mL (p < 0.05), its 95% CI was −0.79 to −0.17 pg/mL, indicating that L. plantarum could effectively decrease the level of IL-4 and modulate the host immunity (Figure 2).
For IL-10, the forest plot showed that the 95% CI of the first and last studies among the five included studies were 0 (the lower limit was <0 and the upper limit was >0), meaning that there was no statistical significance in this study and L. plantarum treatment was not effective in modulating the level of IL-10. The upper and lower limits of 95% CI of the other three studies were >0, indicating that these studies were statistically significant and that L. plantarum was effective in regulating the level of IL-10. The mean difference of the total effect was 9.88 pg/mL (p < 0.05), and the 95% CI was 6.52 to 13.2 pg/mL, suggesting the effectiveness of L. plantarum in enhancing the level of IL-10 and modulating the host immunity (Figure 3).
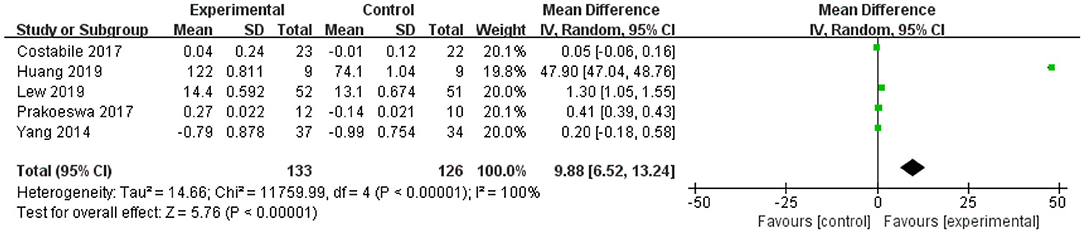
Figure 3. Forest plot of effect of Lactobacillus plantarum on IL-10. SD, standard deviation; Total, sample size of each group; Mean Difference, the mean value of the experimental group minus that of the control; 95% CI, 95% confidence intervals.
The forest plot of TNF-α (Figure 4) showed that the 95% CI in one of the four studies was 0 (the lower limit was <0 and the upper limit was >0), representing this study was not statistically significant for TFN-α. The lower and upper limits of the other three studies were all lower than 0, indicating that these studies were statistically significant, and L. plantarum treatment was effective in modulating the level of TNF-α. The mean difference of the total effect was −2.34 pg/mL (p < 0.05), and the 95% CI was −3.5 to −1.19 pg/mL, indicating that L. plantarum could effectively reduce the content of TNF-α and modulate the immune system (Figure 4).
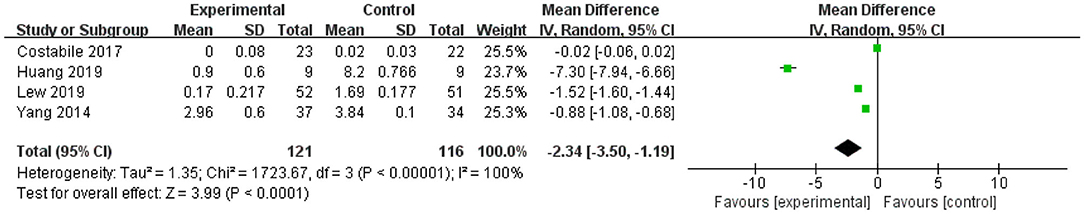
Figure 4. Forest plot of effect of Lactobacillus plantarum on TNF-α. SD, standard deviation; Total, sample size of each group; Mean Difference, the mean value of the experimental group minus that of the control; 95% CI, 95% confidence intervals.
The forest plot of IFN-γ revealed that the upper and lower limits of the 95% CI were lower than 0 in all four studies, indicating significant effects for IFN-γ. The L. plantarum treatment was effective for modulating the level of IFN-γ. The mean difference of the total effect was −0.99 pg/mL (p < 0.05), and the 95% CI was −1.56 to −0.41 pg/mL, suggesting that L. plantarum treatment could effectively reduce the content of IFN-γ to modulate the immune system (Figure 5).
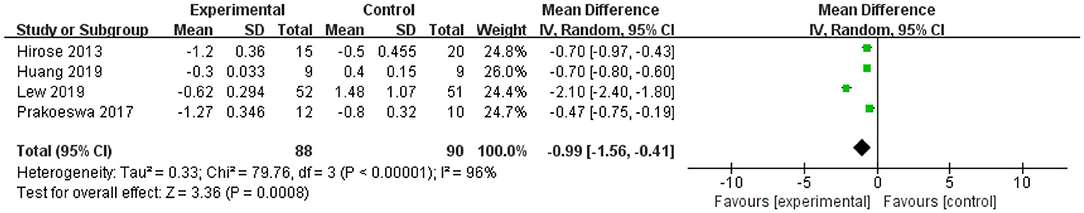
Figure 5. Forest plot of effect of Lactobacillus plantarum on IFN-γ. SD, standard deviation; Total, sample size of each group; Mean Difference, the mean value of the experimental group minus that of the control; 95% CI, 95% confidence intervals.
In addition, we explored publication bias between included studies using funnel plots for all four immune parameters, and the results showed that slight publication bias existed across the 18 randomized controlled trials (Supplementary Figures 1–4).
This study performed a meta-analysis to evaluate the effect of L. plantarum in immune regulation. The results showed that L. plantarum significantly increased the level of IL-10 while significantly reduced the levels of IL-4, IFN-γ, and TNF-α (p < 0.05).
IL-4 is a T cell-derived growth factor for B cells. Over the last few decades, immunology studies have substantially expanded the knowledge about the cellular sources and functions of IL-4 (29). IL-4, as an anti-inflammatory cytokine, has been used in clinical biomaterials to promote wound healing after implantation surgery and reduce immune response (30). IL-4 mainly promotes the proliferation of CD8 + T cells, and endogenous IL-4 inhibits the secretion of IL-10 and promotes the release of TNF-α and GM-CSF (31). In a previous trial, L. plantarum IM76 significantly decreased IL-4 level in allergic mice, induced by house dust allergens (32). Similarly, the probiotic function of L. plantarum IS-10506, a probiotic strain isolated from the Indonesian fermented milk product dadih, could downregulate IL-4 in children with mild atopic dermatitis (17). Besides, the lysates of L. plantarum could inhibit IL-4 level in Nc/Nga mice and regulate inflammatory diseases like atopic dermatitis (33). The results of these studies are consistent with the current findings that the IL-4 level was significantly decreased by the consumption of L. plantarum. Interleukin-4 is a T cell-derived growth factor for B cells. In the last few decades, immunology studies have significantly expanded the knowledge about the cellular sources and functions of IL-4. Apart from T cells, IL-4 is also produced by myeloid cells, such as mast cells and basophils, and innate lymphocytes, such as NTK cells. It is a representative cytokine of the type 2 immune response, and it plays a direct role in modulating the host immunity (29).
The meta-analysis found that consuming L. plantarum enhanced IL-10 production. One study showed that L. plantarum 299 could regulate the ratio of Th1 and Th2 cells by modulating the production of IL-10 (34). Another study showed L. plantarum LS/07 could promote the IL-10 production in rats with colitis; and thus, L. plantarum LS/07 could be applicable in treating inflammation disease (35). Besides, administrating L. plantarum CIRM653 upregulated the IL-10 level in rats infected with Klebsiella pneumonia (36). As an anti-inflammatory factor, IL-10 is vital in protecting the host from tissue damage during acute phases of the immune response toward a pathogenic infection. Such regulatory mechanism helps maintain T cells homeostasis. In addition, IL-10 can be produced by almost all immune cells to autoregulate the functioning of these cells (37). IL-10 exerts immunosuppression through antigen presenting cells (APCs). IL-10 can significantly inhibit APCs, especially macrophages and dendritic cells (DCs). IL-10 can reduce the expression of major histocompatibility complex (MHC) class II molecules and co-stimulatory molecules (CD) 80 and CD86. Moreover, it can promote the expression of B7-H1 molecules and reduce the APC antigen presentation ability (38). Another important role of IL-10 is to inhibit the secretion of pro-inflammatory factors such as IL-1, IL-6, IL-12 and tumor necrosis factors by DCs and macrophages (39). In conclusion, IL-10 can act on many immune cell subsets and exert immunosuppression in various ways, which plays an important role in anti-inflammatory ailments.
The results showed that intake of L. plantarum significantly reduced the TNF-α level, which is in conformity with a previous study showing the supplementation of L. plantarum lowered the TNF-α concentration in male Wistar rats having metabolic syndrome (40). In addition, ingesting L. plantarum C4 could inhibit TNF-α production in male BALB/c mice after moderate physical exercise (41). Furthermore, one L. plantarum strain attenuated the TNF-α level in rats with non-alcoholic steatohepatitis (NASH) (42). TNF-α is a cytokine secreted mainly by macrophages, and it functions to modulate systemic inflammation by regulating immune cells. On the other hand, as an endogenous pyrogen, it can cause fever, apoptosis meanwhile prevent tumorigenesis and virus replication (43). In the inflammatory state, TNF-α can promote the expression of other inflammatory cytokines and aggravate inflammation. Moreover, it can also lead to the movement of neutrophils, interfere with the function of gastric mucosal endothelial cells and cause gastric mucosal damage (44). TNF receptor can transmit survival and death signals to cells, which plays an important role in cell proliferation, differentiation, apoptosis, regulation of immune response, and induction of inflammation (45).
The current meta-analysis found that consuming L. plantarum significantly reduced IFN-γ production in human subjects. Similarly, one published article found that administering L. plantarum KLDS1.038 downregulated IFN-γ secretion and promoted the recovery of mice by immunosuppression (46). The study of Lew and coworkers showed that L. plantarum P-8 could reduce the level of proinflammatory cytokines such as IFN-γ in stressed adults (27). Another study indicated that L. plantarum C4 could reduce IFN-γ production and avoid intestinal infections in mice through immunosuppression (47). Interferon-γ is a pro-inflammatory cytokine, which is mainly produced by activated NK cells and Th cells. The major biological effect of IFN-γ is immune-stimulation, inducing multiple antigen-presenting cells to express MHC-I/II molecules, activating macrophages and monocytes, enhancing lytic activity of immune cells, as well as enhancing the secretion of other cytokines such as IL-1, IL-6, IL-8, and TNF-α. Although IFN-γ serves as a pro-inflammatory cytokine and enhances the host immunity, which is desirable in many scenarios; however, its overshooting in some occasions might cause harmful tissue damages to the host. Thus, it would be useful to take advantage of the immunosuppression property of L. plantarum to protect from excessively strong immune responses in some cases (48).
Recently, several in depth studies have been conducted on the mechanisms of probiotics in immunoregulation (49, 50). However, specific underlying mechanisms of how L. plantarum interacts with the host are obscure.
Some studies have shown that L. plantarum strains exerted its immune regulatory function by competing with the pathogenic bacteria for limiting trophins, inhibiting the growth of pathogenic bacteria, regulating the intestinal microecology, and forming a biological barrier (51). Besides, L. plantarum produces lactic acid, diacetyl, and bacteriocin through metabolism, which could modulate the colonic environment and microbiome to improve its competitive advantages among other coexisting microflora (51, 52). Moreover, L. plantarum might interact with the host immunity via several mechanisms. Firstly, the immunomodulatory effect of L. plantarum could be attained via affecting the non-specific immune response and enhancing the viability of various types of immune cells, such as polymorphonuclear cells (mainly neutrophils) and cell involved in mononuclear phagocytosis (e.g., macrophages). These cells together with natural killer cells stimulate the secretion of mononuclear factors, such as lysosomal enzymes, and reactive oxygen species (53). The second mechanism is to affect the bacterial-specific immune responses, such as the increasing in the levels of IgA, IgG, and IgM in serum and the mucosal surface. Thus, augmenting the humoral immune function, promoting maturation and proliferation of B lymphocytes, T lymphocytes, and enhancing cellular immune response are some of the mechanisms of immunomodulation by L. plantarum (54). On the other hand, L. plantarum might reduce the production of pro-inflammation cytokines (such as TNF-α and IFN-γ) and induce peripheral tolerance via IL-10-induced antigen-specific T cell disability.
The meta-analysis showed that L. plantarum could significantly increase the level of IL-10 while decrease the levels of IL-4, TNF-α, and IFN-γ (p < 0.05). Both TNF-α and IFN-γ are pro-inflammatory cytokines. Many previous studies have drawn their conclusions on the effect of L. plantarum on the immune system by showing the up-regulation or down-regulation of a specific pro-inflammatory or anti-inflammatory factor, while the results found that L. plantarum could regulate the immunity via down-regulating multiple pro-inflammatory factors and up-regulating anti-inflammatory factors through a summary analysis of a large number of studies.
To minimize publication bias, this meta-analysis was performed objectively by two independent researchers. However, this study still has some unavoidable limitations. First, the heterogeneity of some indicators was significant, suggesting variations between studies. The discrepancies might be due to different types and doses of probiotics intervened in each study, as well as other factors such as diet, physical conditions, scale of study and so on. Second, each randomized controlled trials included in the current meta-analysis was performed in a different region, and the genetic background of subjects and their intrinsic microbiome might played a role in influencing the trial outcomes even if the same probiotics would have been applied. Third, the small sample size of some included studies might be affected the reliability and validity of the conclusions.
We performed a meta-analysis on the effects of L. plantarum administration, mainly focusing on the immunoregulatory studies conducted in the last decade. The obtained results showed that L. plantarum significantly increased the level of IL-10, whereas, significantly reduced the level of IL-4, IFN-γ, and TNF-α (p < 0.05). Therefore, meta-analysis results suggested that L. plantarum could promote host immunity by co-regulating pro-inflammatory and anti-inflammatory cytokines.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
WZhan designed the study. WZhao and CP retrieved references and extracted data of included references. WZhan and WZhao analyzed the data and discussed. WZhao and L-YK wrote the manuscript. HS edited and revised the final manuscript. WZhao, CP, HS, L-YK, and WZhan revised drafts and approved the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China under Grant (no. 31922071) and the Program for Grassland Elite of Inner Mongolia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.643420/full#supplementary-material
1. Hou Q, Zhao F, Liu W, Lv R, Khine WWT, Han J, et al. Probiotic-directed modulation of gut microbiota is basal microbiome dependent. Gut Microbes. (2020) 12:1–20. doi: 10.1080/19490976.2020.1736974
2. Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A. Probiotics, gut microbiota and their influence on host health and disease. Mol Nutr Food Res. (2016) 61. doi: 10.1002/mnfr.201600240
3. Toscano M, De Grandi R, Pastorelli L, Vecchi M, Drago L. A consumer's guide for probiotics: 10 golden rules for a correct use. Digest Liver Dis. (2017) 49:1177–84. doi: 10.1016/j.dld.2017.07.011
4. Cao C, Wang J, Liu Y, Kwok L-Y, Zhang H, Zhang W. Adaptation of Lactobacillus plantarum to ampicillin involves mechanisms that maintain protein homeostasis. Msystems. (2020) 5. doi: 10.1128/mSystems.00853-19
5. Ding RX, Goh WR, Wu RN, Yue XQ, Luo X, Khine WWT, et al. Revisit gut microbiota and its impact on human health and disease. J Food Drug Anal. (2019) 27:623–31. doi: 10.1016/j.jfda.2018.12.012
6. Gueimonde M, Sánchez B, de los Reyes-Gavilán CG, Margolles A. Antibiotic resistance in probiotic bacteria. Front Microbiol. (2013) 4:202. doi: 10.3389/fmicb.2013.00202
7. Mantegazza C, Molinari P, D'Auria E, Sonnino M, Morelli L, Zuccotti GV. Probiotics and antibiotic-associated diarrhea in children: a review and new evidence on Lactobacillus Rhamnosus GG during and after antibiotic treatment. Pharmacol Res. (2018) 128:63–72. doi: 10.1016/j.phrs.2017.08.001
8. Wang L, Liu C, Chen M, Ya T, Huang W, Gao P, et al. A novel Lactobacillus plantarum strain P-8 activates beneficial immune response of broiler chickens. Int Immunopharmacol. (2015) 29:901–7. doi: 10.1016/j.intimp.2015.07.024
9. Lee SJ, Bose S, Seo JG, Chung WS, Lim CY, Kim H. The effects of co-administration of probiotics with herbal medicine on obesity, metabolic endotoxemia and dysbiosis: a randomized double-blind controlled clinical trial. Clin Nutr. (2014) 33:973–81. doi: 10.1016/j.clnu.2013.12.006
10. Fuentes MC, Lajo T, Carrión JM, Cuñé J. A randomized clinical trial evaluating a proprietary mixture of Lactobacillus plantarum strains for lowering cholesterol. Mediterr J Nutr Metab. (2016) 9:125–35. doi: 10.3233/MNM-160065
11. Hakansson A, Aronsson CA, Brundin C, Oscarsson E, Molin G, Agardh D. Effects of Lactobacillus plantarum and Lactobacillus paracasei on the peripheral immune response in children with celiac disease autoimmunity: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. (2019) 11:1925–36. doi: 10.3390/nu11081925
12. Liu CF, Tseng KC, Chiang SS, Lee BH, Hsu WH, Pan TM. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J Sci Food Agr. (2011) 91:2284–91. doi: 10.1002/jsfa.4456
13. Wang K, Li W, Rui X, Chen X, Jiang M, Dong M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int J Biol Macromol. (2014) 63:133–9. doi: 10.1016/j.ijbiomac.2013.10.036
14. Wang J, Zhao X, Yang Y, Zhao A, Yang Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int J Biol Macromol. (2015) 74:119–26. doi: 10.1016/j.ijbiomac.2014.12.006
15. Prakoeswa CRS, Bonita L, Karim A, Herwanto N, Surono IS. Beneficial effect of Lactobacillus plantarum IS-10506 supplementation in adults with atopic dermatitis: a randomized controlled trial. J Dermatol Treat. (2020) 1:1–8. doi: 10.1080/09546634.2020.1836310
16. Huang HY, Tsao SP. 2020. Long term supplementation of Lactobacillus plantarum PS128 attenuated high-intensity exercise induced acute-phase inflammation in triathletes. Curr Dev Nutr. (2020) 4(Suppl. 2):1564. doi: 10.1093/cdn/nzaa062_021
17. Prakoeswa CRS, Herwanto N, Prameswari R, Astari L, Sawitri S, Hidayati AN, et al. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef Microbes. (2017) 8:833–40. doi: 10.3920/BM2017.0011
18. Glass GV. Primary, secondary, and meta-analysis of research. Educ Res. (1976) 5:3–8. doi: 10.3102/0013189X005010003
19. Egger M, Smith GD, Schneider M. Systematic reviews of observational studies. Systematic Reviews in Health Care: Meta-Analysis in Context. London: BMJ Publishing Group (2015).
20. Zajac AE, Adams AS, Turner JH. A systematic review and meta-analysis of probiotics for the treatment of allergic rhinitis. Int Forum of Allergy Rh. (2015) 5:524–32. doi: 10.1002/alr.21492
21. Tench ML, Hansen TL, Warren LK. Meta-analysis of probiotic effectiveness for reducing diarrhea and altering the gut microbiome in horses. J Equine Vet Sci. (2019) 76:40. doi: 10.1016/j.jevs.2019.03.020
22. Miller LE, Ouwehand AC, Ibarra A. Effects of probiotic-containing products on stool frequency and intestinal transit in constipated adults: systematic review and meta-analysis of randomized controlled trials. Ann Gastroenterology. (2017) 30:629–39. doi: 10.20524/aog.2017.0192
23. Yang Y, Guo Y, Kan Q, Zhou XG, Zhou XY, Li Y. A meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Braz J Med and Biol Res. (2014) 47:804–10. doi: 10.1590/1414-431X20143857
24. Costabile A, Buttarazzi I, Kolida S, Quercia S, Gibson GR. An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. Plos ONE. (2017) 12:e0187964. doi: 10.1371/journal.pone.0187964
25. Hirose Y, Yamamoto Y, Yoshikai Y, Murosaki S. Oral intake of heat-killed Lactobacillus plantarum L-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. J Nutr Sci. (2013) 2:39–46. doi: 10.1017/jns.2013.35
26. Huang WC, Wei CC, Huang CC, Chen WL, Huang HY. The beneficial effects of Lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients. (2019) 11:353–40. doi: 10.3390/nu11020353
27. Lew LC, Hor YY, Yusoff NAA, Choi SB, Yusoff MSB, Roslan NS, et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study. Clin Nutr. (2019) 38:2053–64. doi: 10.1016/j.clnu.2018.09.010
28. Yang HJ, Min TK, Lee HW, Pyun BY. Efficacy of probiotic therapy on atopic dermatitis in children: a randomized, double-blind, placebo-controlled trial. Allergy Asthma Immun. (2014) 6:208–15. doi: 10.4168/aair.2014.6.3.208
29. Ho I, Miaw SC. Regulation of IL-4 expression in immunity and diseases. Adv Exp Med Biol. (2016) 941:31–77. doi: 10.1007/978-94-024-0921-5_3
30. Sato T, Pajarinen J, Behn A, Jiang X, Lin TH, Loi F, et al. The effect of local IL-4 delivery or CCL2 blockade on implant fixation and bone structural properties in a mouse model of wear particle induced osteolysis. J Biomed Mater Res. (2016) 104:2255–62. doi: 10.1002/jbm.a.35759
31. Peng J, Xiao Y, Wan X, Chen Q, Wang H, Li J, et al. Enhancement of immune response and anti-infection of mice by porcine antimicrobial peptides and interleukin-4/6 fusion gene encapsulated in chitosan nanoparticles. Vaccines. (2020) 8:552–66. doi: 10.3390/vaccines8030552
32. Kim JK, Kim JY, Kim HI, Han MJ, Kim DH. Bifidobacterium longum and Lactobacillus plantarum alleviate house dust mite allergen-induced allergic rhinitis by regulating IL-4, IL-5, and IL-10 expression. Food Agr Immunol. (2019) 30:581–93. doi: 10.1080/09540105.2019.1608161
33. Kim H, Kim HR, Kim NR, Jeong BJ, Lee JS, Jang S, et al. Oral administration of Lactobacillus plantarum lysates attenuates the development of atopic dermatitis lesions in mouse models. J Microbiol. (2015) 53:47–52. doi: 10.1007/s12275-015-4483-z
34. Le B, Yang SH. Efficacy of Lactobacillus plantarum in prevention of inflammatory bowel disease. Toxicol Rep. (2018) 5:314–7. doi: 10.1016/j.toxrep.2018.02.007
35. Stofilova J, Langerholc T, Botta C, Treven P, Gradisnik L, Salaj R, et al. Cytokine production in vitro and in rat model of colitis in response to Lactobacillus plantarum LS/07. Biomed Pharmacother. (2017) 94:1176–85. doi: 10.1016/j.biopha.2017.07.138
36. Marjolaine V-D, Sylvie M, Sophie G, Thomas B, Balestrino D, Bertrand E, et al. Immunomodulatory effects of Lactobacillus plantarum on inflammatory response induced by Klebsiella pneumoniae. Infect Immun. (2019) 87:e00570–00519. doi: 10.1128/IAI.00570-19
37. Rojas JM, Avia M, Martin V, Sevilla N. IL-10: a multifunctional cytokine in viral infections. J Immunol Res. (2017) 2017:6104054. doi: 10.1155/2017/6104054
38. Wilke CM, Wei S, Wang L, Kryczek I, Kao J, Zou W. Dual biological effects of the cytokines interleukin-10 and interferon-γ. Cancer Immunol Immun. (2011) 60:1529–41. doi: 10.1007/s00262-011-1104-5
39. Pestka S, Krause CD, Sarkar D, Walter MR, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. (2004) 22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622
40. Korkmaz OA, Sumlu E, Koca HB, Pektas MB, Kocabas A, Sadi G, et al. Effects of Lactobacillus plantarum and Lactobacillus helveticus on renal insulin signaling, inflammatory markers, and glucose transporters in high-fructose-fed rats. Medicina-Lithuania. (2019) 55:207. doi: 10.3390/medicina55050207
41. Appukutty M, Ramasamy K, Rajan S, Vellasamy S, Ramasamy R, Radhakrishnan AK. Effect of orally administered soy milk fermented with Lactobacillus plantarum LAB12 and physical exercise on murine immune responses. Benef Microbes. (2015) 6:491–6. doi: 10.3920/BM2014.0129
42. Werawatganon D, Somanawat K, Tumwasorn S, Klaikeaw N, Siriviriyakul P. Lactobacillus plantarum attenuates oxidative stress and liver injury in rats with nonalcoholic steatohepatitis. Pharmacogn Mag. (2018) 14:471–6. doi: 10.4103/pm.pm_279_18
43. Mendonça A, Cardoso M, Cardoso F. Cloning and expression of a recombinant tumor necrosis factor-α (TNF-α) protein. Ann Med. (2019) 51:36. doi: 10.1080/07853890.2018.1561799
44. Khotib J, Utami NW, Gani MA, Ardianto C. The change of proinflammatory cytokine tumor necrosis factor α level in the use of meloxicam in rat model of osteoarthritis. J Basic Clin Physiol Pharmacol. (2019) 30:185–93. doi: 10.1515/jbcpp-2019-0331
45. Rodrigues FF, Morais MI, Melo ISF, Augusto PSA, Machado RR. Clindamycin inhibits nociceptive response by reducing tumor necrosis factor-α and CXCL-1 production and activating opioidergic mechanisms. Inflammopharmacology. (2020) 28(Suppl. 1):1–13. doi: 10.1007/s10787-019-00670-w
46. Meng Y, Li B, Jin D, Zhan M, Lu J, Huo G. Immunomodulatory activity of Lactobacillus plantarum KLDS1.0318 in cyclophosphamide-treated mice. Food Nutr Res. (2018) 62:1–9. doi: 10.29219/fnr.v62.1296
47. De Montijo-Prieto S, Moreno E, Bergillos-Meca T, Lasserrot A, Ruiz-Lopez M.-D, Ruiz-Bravo A, et al. A Lactobacillus plantarum strain isolated from kefir protects against intestinal infection with Yersinia enterocolitica O9 and modulates immunity in mice. Res Microbiol. (2015) 166:626–32. doi: 10.1016/j.resmic.2015.07.010
48. Zheng X, Xie T, Lin Y, Yang J, Huang L. Immune response and mechanisms of IFN-γ in administration for keratomycosis. Ocul Immunol Inflamm. (2018) 27:958–67. doi: 10.1080/09273948.2018.1491604
49. Plaza-Diaz J, Javier Ruiz-Ojeda F, Gil-Campos M, Gil A. Immune-mediated mechanisms of action of probiotics and synbiotics in treating pediatric intestinal diseases. Nutrients. (2018) 10:42. doi: 10.3390/nu10010042
50. Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A, Sharif SK, Asghari-Jafarabadi M, Zavvari S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition. (2014) 30:430–5. doi: 10.1016/j.nut.2013.09.007
51. de Vos PZ, Mujagic BJ, de Haan RJ, Siezen PA, Bron M, Meijerink JM, et al. Lactobacillus plantarum strains can enhance human mucosal and systemic immunity and prevent non-steroidal anti-inflammatory drug induced reduction in T regulatory cells. Front Immunol. (2017) 8:1000–17. doi: 10.3389/fimmu.2017.01000
52. Dash G, Raman R.P, Prasad KP, Makesh M, Pradeep MA, Sen S. Evaluation of paraprobiotic applicability of Lactobacillus plantarum in improving the immune response and disease protection in giant freshwater prawn, macrobrachium rosenbergii (de Man, 1879). Fish Shellfish Immun. (2015) 43:167–74. doi: 10.1016/j.fsi.2014.12.007
53. Ozdemir O. Various effects of different probiotic strains in allergic disorders: an update from laboratory and clinical data. Clin Exp Immunol. (2010) 160:295–304. doi: 10.1111/j.1365-2249.2010.04109.x
Keywords: Lactobacillus plantarum, immunity, meta-analysis, IL-4, IL-10, TNF-α, IFN-γ
Citation: Zhao W, Peng C, Sakandar HA, Kwok L-Y and Zhang W (2021) Meta-Analysis: Randomized Trials of Lactobacillus plantarum on Immune Regulation Over the Last Decades. Front. Immunol. 12:643420. doi: 10.3389/fimmu.2021.643420
Received: 12 January 2021; Accepted: 24 February 2021;
Published: 22 March 2021.
Edited by:
Haruki Kitazawa, Tohoku University, JapanCopyright © 2021 Zhao, Peng, Sakandar, Kwok and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenyi Zhang, emhhbmd3ZW55aXppQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.