
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 19 February 2021
Sec. Antigen Presenting Cell Biology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.643240
This article is part of the Research TopicNovel Platform for Antigen Delivery to Dendritic Cells for ImmunotherapyView all 10 articles
 Charlotte Castenmiller1†
Charlotte Castenmiller1† Brigitte-Carole Keumatio-Doungtsop2†
Brigitte-Carole Keumatio-Doungtsop2† Ronald van Ree1,3
Ronald van Ree1,3 Esther C. de Jong1†
Esther C. de Jong1† Yvette van Kooyk2*†
Yvette van Kooyk2*†Dendritic cells (DCs) are well-established as major players in the regulation of immune responses. They either induce inflammatory or tolerogenic responses, depending on the DC-subtype and stimuli they receive from the local environment. This dual capacity of DCs has raised therapeutic interest for their use to modify immune-activation via the generation of tolerogenic DCs (tolDCs). Several compounds such as vitamin D3, retinoic acid, dexamethasone, or IL-10 and TGF-β have shown potency in the induction of tolDCs. However, an increasing interest exists in defining tolerance inducing receptors on DCs for new targeting strategies aimed to develop tolerance inducing immunotherapies, on which we focus particular in this review. Ligation of specific cell surface molecules on DCs can result in antigen presentation to T cells in the presence of inhibitory costimulatory molecules and tolerogenic cytokines, giving rise to regulatory T cells. The combination of factors such as antigen structure and conformation, delivery method, and receptor specificity is of paramount importance. During the last decades, research provided many tools that can specifically target various receptors on DCs to induce a tolerogenic phenotype. Based on advances in the knowledge of pathogen recognition receptor expression profiles in human DC subsets, the most promising cell surface receptors that are currently being explored as possible targets for the induction of tolerance in DCs will be discussed. We also review the different strategies that are being tested to target DC receptors such as antigen-carbohydrate conjugates, antibody-antigen fusion proteins and antigen-adjuvant conjugates.
Dendritic cells (DCs) are important antigen presenting cells during the induction of immune responses and are essential in directing immune responses toward either immunity or tolerance. This decision is of great importance as undesired inflammatory responses could cause autoimmune or allergic diseases. In the periphery, DCs capture antigens and process them while migrating to the draining lymph nodes, where they present antigen-specific peptides to T lymphocytes. This migration process causes a dramatic transformation of the DC phenotype, called maturation. Maturation is associated with increased MHC-II complex levels, costimulatory molecule expression, enhanced secretion of polarizing cytokines and molecules, and alterations in chemokine receptor expression, all resulting in an optimal microenvironment to direct T cell responses (1–3). Although various DC subsets have been shown to preferentially induce specific T cell responses in non-inflammatory conditions, the induction of T cell immunity is adapted to and dictated by the encounter with pathogens (4). The unique capacity of DCs to coordinate innate and adaptive immune responses has highlighted them as potential targets for immune activating or dampening therapies to combat undesired immune responses (3).
Immunomodulatory agents such as vitamin D3, retinoic acid, rapamycin, dexamethasone, corticosteroids, ligands of the aryl hydrocarbon receptor (AhR), or specific cytokines (IL-10, TGFβ) have been key in determining the existence and function of tolerogenic DCs (tolDCs) ex vivo (Figure 1) (5–7). These tolDCs can induce tolerance through various mechanisms, including the induction of Tregs, autoreactive T cell anergy and apoptosis, and could be used in tolerizing immunotherapies (6, 8, 9). Ex vivo tolDC immunotherapies are based on re-education of patient-derived DCs to a tolerizing phenotype and the subsequent reinfusion into the body, where they suppress inflammatory immune responses (Figure 1). The first clinical study utilizing tolerogenic DCs (tolDCs) for the treatment of autoimmune diseases was performed in 2011 in adult type I diabetes (T1D) patients. Since then, phase I and II clinical trials have been conducted for T1D, rheumatoid arthritis (RA), Crohn's disease, and multiple sclerosis (MS) (5), but also for kidney and liver transplant recipients (8–10). However, due to the personalized, laborious, and expensive nature of ex vivo-generated tolDCs, new approaches for inducing tolDCs in vivo are being developed.
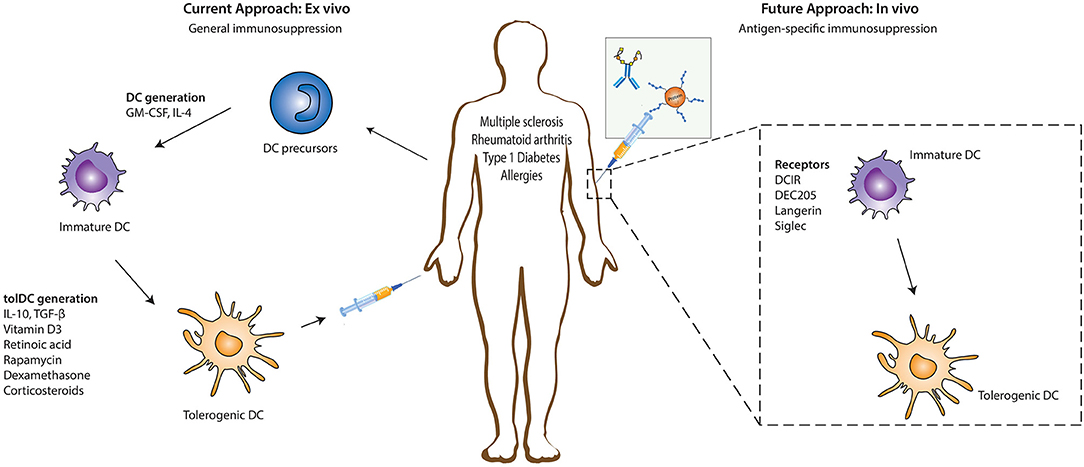
Figure 1. Ex vivo and in vivo strategies for generation of tolerogenic DCs for DC-based therapies. The current DC-based immunotherapy strategy in the treatment of immunopathologies involves the isolation of DC precursors either from PBMCs or bone marrow-derived cells which could either be allogeneic or autologous. These DC precursors are then differentiated into immature DCs in the presence of GM-CSF and recombinant IL-4 which are subsequently differentiated into tolerogenic DCs (tolDCs) by the addition of pharmacologic agents or immunomodulatory cytokines. Administration of these tolDCs leads to the generation of a suppressive immune environment which dampens inflammation. Future strategies are focusing more on in vivo targeting of DCs, where specific antigen-based vaccine formulations targeting specific receptors on DCs in their natural environment are injected into the patient. The antigen is taken up by DCs through these receptors, resulting in the induction of a tolerogenic program in DCs that leads to the generation of antigen-specific immunosuppression. DC, dendritic cells; GM-CSF, Granulocyte-macrophage colony-stimulating factor; IL-4, Interleukin 4; IL-10, Interleukin-10; TGF-β, Transforming growth factor beta.
The feasibility and potential of in vivo strategies lie in the ability of DCs to recognize and internalize antigens through surface receptors that not only route antigens to the antigen processing machinery of DCs for subsequent presentation to T cells but also transmit signals that direct anti-inflammatory immune responses. This allows direct modulation of specific DC subsets due to differential surface receptor expression profiles between them. In vivo DC-targeting has several advantages compared to ex vivo DC-targeting, including fewer hospital visits for the patient, less laborious production methods, and the possibility of large scale production, which is more cost-effective. Additionally, the induction of antigen-specific T cell responses with in vivo DC-targeting strategies reduces the risk of generalized immunosuppression, which is induced during the current ex vivo strategies using only immunosuppressive agents. The main strategies for in vivo tolDC generation take advantage of modalities binding to specific endocytic receptors on DC surfaces, ensuring the delivery of antigen of interest into the antigen-processing machinery (Figure 1) (11). Antigens could either be directly coupled to antibodies (11) or loaded on nanoparticles or in liposomes, reviewed elsewhere (12). Another strategy being explored in this regard involves chemically conjugating antigens with specific glycan structures which are ligands for DC surface receptors. In this review we discuss the different DC-subsets used for targeting, the receptors expressed on their surface that have potential to induce tolerogenic signals (but might not be inherently tolerogenic), and the current state of research in their use for the treatment of auto-immune or allergic diseases.
It is now recognized that DCs are a heterogeneous population of cells. The different subsets are defined by surface markers and transcriptome profiles, nicely reviewed by various colleagues (4, 13–16). DCs are generally classified into four major subsets, namely, CD141+ conventional DCs (cDC1s), CD1c+ conventional DCs (cDC2s), monocyte-derived DCs (moDCs), and plasmacytoid DCs (pDCs). The cDC1 subset is a relatively homogenous population that is specialized in cross-presentation of extracellular antigens and efficiently primes CD8+ T cells (16). In contrast, the cDC2 subset is a heterogeneous population and could be further subdivided in separate lineages. For example, the cDC2A and cDC2B lineage are defined by distinct developmental pathways regulated by the transcription factors T- bet and RORγt, respectively (17). Both lineages are potent stimulators of CD4+ naïve T cell, however, cDC2Bs have been shown to be more prone to secrete pro-inflammatory cytokine than cDC2As (13, 17). Additionally identified cDC2 lineages include monocyte-like DC2s, inducing Th1 responses, and DC3s, responsible for Th2, Th17 and Treg differentiation (15, 17). The moDC subset arises from monocytes and retains, like the DC3s, the monocyte marker CD14. They are recruited to inflamed tissue sites in vivo where they efficiently cross-present antigens to CD8+ T cells in peripheral tissues (18). The last subset, pDCs, differs from the other subsets as they are marked by quick secretion of pro-inflammatory type I interferons (IFN) following viral infection. pDCs are defined as CD123+ CD303+ CD304+ cells and were originally classified within the myeloid compartment. However, recent findings providing evidence for a lymphoid origin of the majority of pDCs challenges this hypothesis (4, 13, 19, 20). Finally, the tissues where DCs reside, such as lymph nodes, skin, lung, intestines and liver, offer the above mentioned DC subsets additional environmental factors to further adapt to their specific niche resulting in tissue specific DC subsets (8, 21, 22).
Although the immune system encounters many innocuous antigens, including self-antigens and allergens, the chance to develop autoimmune or allergic diseases is relatively small due to the phenomenon of “natural tolerance.” Natural tolerance is achieved through the presence of tolerance-inducing DCs located both centrally and in the periphery. Central tolerance induction is mediated by thymic epithelial cells and thymic DCs, which regulate negative selection of autoreactive T cells and induction of natural Tregs (23, 24). Even though the specific role of each thymic DC subset in peripheral immune homeostasis remains elusive, thymic pDCs and the Sirpα+ cDC subset have been proposed to contribute to the prevention of allergic or commensal-specific autoimmune diseases, as they originate from the periphery where they encounter many innocuous antigens, followed by migration to the thymus (23–25). On the other hand, peripheral tolerance is mediated by peripheral DCs, preferentially located at the border between the body and the external environment, such as lung, intestine and skin. Steady state or immature DCs were the original identified peripheral tolDCs (26–28). They exhibit low expression of co-stimulatory (CD40, CD80/86) and MHC molecules due to lack of appropriate activation signals and are able to maintain tolerance via deletion of self-reactive T cells, induction of T cell anergy or differentiation of antigen-specific Tregs (2). These immature DCs have been reported to be the primary cell types involved in maintaining tolerance in the periphery and mainly carry self-antigens. However, recent identification of partial- or semi-mature DCs with tolerizing capacities, questions the dogma that only immature DCs induce tolerance (22, 27). Similar to immunogenic DCs, tolDCs may be defined by integration of all the signals they transmit to T cells, including maturation marker expression, as well as the presence of, in this case, anti-inflammatory-related tolerizing signals consisting of surface molecule expression (PD-L1, ILT3/4, ICOSL, CTLA-4), tolerogenic cytokine profiles (IL-10, TGFβ) and the presence of other tolerance-inducing metabolites (IDO, RA) (14, 22). Furthermore, the presence or absence of pro-inflammatory cytokines seems to be decisive in inducing either immunity or tolerance, respectively. Nevertheless, no standard tolDCs profile has been established yet, and may not exist due to the great diversity between those that have been described till date.
Besides the immature and semi-mature tolDCs, several tissue-specific DCs exhibit inherent tolerogenic properties, including those in the skin and intestines. In the skin, Langerhans cells (LCs), which are characterized by the expression of Langerin (CD207), CD1a, E-Cadherin, CD39, FcεRI, and Birbeck granules, are the sole tissue-resident DC population in the epidermis (29). LCs constantly migrate from the skin to draining lymph nodes, even in steady-state conditions, and have been implicated in both immunogenic as well as tolerogenic immune reactions (30–32). In contrast, CD14+(CD141+) dermal DCs constitutively secrete the anti-inflammatory cytokine IL-10 and are prone to induce T cell anergy and Tregs that inhibit skin inflammation (33, 34). The ability to produce extensive levels of IL-10 is shared with CD14+CD16+CD141+CD163+ DCs isolated from peripheral blood, identified by Gregori and colleagues, which might correspond to the DC3 subset expressing the same surface markers (16, 35). The same group has shown that these cells express the surface receptors HLA-G, ILT2, ILT3, and ILT4 and have the potency to induce type 1 Tregs (Tr1) in vitro (36–38). In the intestines, the main subset involved in oral tolerance during steady state conditions are the CD103+ DCs in the lamina propia and mesenteric lymph nodes. They are able to prime Tregs in gut lymphoid tissues through the production of TGF-β and RA (39–42). Additionally, CD103+ DCs express high levels of RALDH2, converting vitamin A to RA which enhances Treg induction (29). These studies demonstrate that various tissues contain specific subsets of tolDCs, emphasizing the power of the immune system to adapt to specific environmental factors functioning to maintain immune homeostasis during tissue-specific circumstances.
Signals such as pathogen-associated molecular patterns (PAMPS) from pathogens, damage associated molecular patterns (DAMPS) from inflammation, and self-associated molecular patterns (SAMPS) can be recognized by pattern recognition receptors (PRRs) on the surface of DCs (21, 43). C-type lectins (CLRs) and Sialic-acid binding immunoglobulin-type lectins (Siglecs) are families of PRRs equipped with a carbohydrate recognition domain that specifically recognizes glycan moieties on host cells, pathogens, as well as innocuous antigens such as allergens (21, 43–46).
CLRs function both as adhesion molecules and endocytic receptors, but also have a function in directing immunity to various pathogens, cellular proteins and lipids (44, 47, 48). Induction of immune response through these receptors can alternate between inflammation and immune tolerance depending on several factors including the nature of the ligand (49). They recognize a large and diverse range of ligands and trigger immune responses by inducing signaling pathways via an immunoreceptor tyrosine-based activation motif (ITAM), ITAM-like motif, or immunoreceptor tyrosine-based inhibitory motif (ITIM) that signal through Syk or phosphatases (21, 44, 45, 49, 50), generating pro- or anti-inflammatory signals, respectively. Only a few CLRs, such as, DC immunoreceptor (DCIR), Clec12A and Clec12B, bear the ITIM motif (51). Moreover, an important number of CLRs do not signal through Syk or phosphatases but may bear ITAM/ITIM motifs which are important for endocytosis (45, 52). Examples include: dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), LSECtin, macrophage C-type lectin (MCL), Langerin, macrophage-galactose lectin (MGL), mannose receptor (MR) and DEC205 (11, 44, 48). These CLRs mediate antigen internalization, followed by processing and subsequent presentation via MHC-I or II molecules (53–56).
Next to CLRs, the Siglecs are also expressed on DCs and recognize self- and non-self-antigens (43, 46). Similar to CLRs, they could serve as adhesion molecules and endocytic receptors, and have been shown to be important instructors of T cell immunity (57, 58). Most members of the Siglec family signal through ITIM or ITIM-like motifs resulting in the generation of anti-inflammatory signals that modulate DC function (59). Overall, the ability of effective antigen uptake, processing, and presentation as well as the regulation of immunogenic and tolerogenic immune responses through the modulation of DC function positions DC receptors as promising candidates for novel DC-targeting immunotherapies. In the next sections, we explore current knowledge regarding the most promising DC-receptors being targeted for in vivo generation of tolDCs for the treatment of immune dysregulated pathologies.
DEC205 (CD205) is an endocytic receptor highly expressed on cDC1s and belongs to the macrophage- mannose receptor family of CLRs (54). Although the natural ligand of DEC205 remains to be elucidated, some studies suggest apoptotic and necrotic material as well as CpG motifs as consecutive ligands (60). Upon antigen encounter, DEC205 internalizes and recycles very efficiently back to the surface (61). DEC205 was one of the first receptors used for in vivo antibody targeting of DCs (Table 1). Initial experiments using model antigens, such as hen egg lysozyme or ovalbumin (OVA), coupled to anti-DEC205 antibodies, demonstrated that these antigens were taken up by DCs (82–84). When OVA-anti-DEC205 fusion antibodies were administered to mice in the presence of maturation stimuli, strong immunogenic responses were induced (85). Conversely, when anti-DEC-antigens were injected into animals without adjuvants, DCs remained non-activated as their expression levels of costimulatory molecules was comparable to those obtained in DCs from control mice (83, 85). The analysis of antigen-specific T cell populations in injected mice revealed increased numbers of antigen-specific IL-10 producing CD25+Foxp3+ Tregs that were able to suppress proliferation of CD4+ T cells in vivo (83) (Figure 2). Since then, DEC205 targeting has been tested in various autoimmune disease animal models. For instance, in experimental autoimmune encephalomyelitis (EAE), the murine model for MS (63, 66, 86), injection of the autoantigen, myelin oligodendrocyte glycoprotein (MOG) or proteolipid protein (PLP) fused to anti-DEC205-specific antibodies led to elicitation of IL-10-producing CD4+CD25+Foxp3+ Tregs, the deletion of antigen-specific CD4+ and CD8+ T cells, reduced Th17 cell activity and significantly ameliorated disease symptoms and substantially delayed the disease onset (63, 64, 67) (Figure 2). In some studies, the conversion of some autoreactive T cells into Foxp3+ pTreg cells was reported. These findings were confirmed in non-obese diabetic (NOD), a mouse model of T1D, inflammatory bowel disease (IBD) (71), proteoglycan-arthritis (68), spontaneous experimental autoimmune uveoretinitis (EAU) (72), an animal model of ocular inflammation, as well as a model of graft-vs. host disease (65, 69, 70, 73). Because the use of DEC205 antibodies has proven to be more effective than the administration of free synthetic peptides, in these models of autoimmune diabetes, it is being considered as a possible, important therapeutic tool in the treatment of various autoimmune diseases (Table 1). In summary, these data establish DC targeting via DEC205 as an effective strategy to tolerize against autoantigens to protect against autoimmunity. However, this DC targeting strategy for the induction of tolerance is yet to be tested in human settings. Moreover, the future prospects of targeting DEC205 to induce tolerance in humans may be hampered by the varied expression pattern of this receptor on the different subsets of DCs (87). Although DEC-205 is predominantly expressed in mice CD8+ DCs, dermal DCs and LCs, human DEC-205 is relatively high expressed on myeloid blood DCs and monocytes, at moderate levels on B cells, and at low levels on pDCs, T cells and natural killer cells (87, 88). This differential expression pattern of DEC205 in humans is problematic for the development of DEC205-targeted vaccines for humans due to potential offsite targeting and needs to be addressed carefully during the design of potential clinical studies.
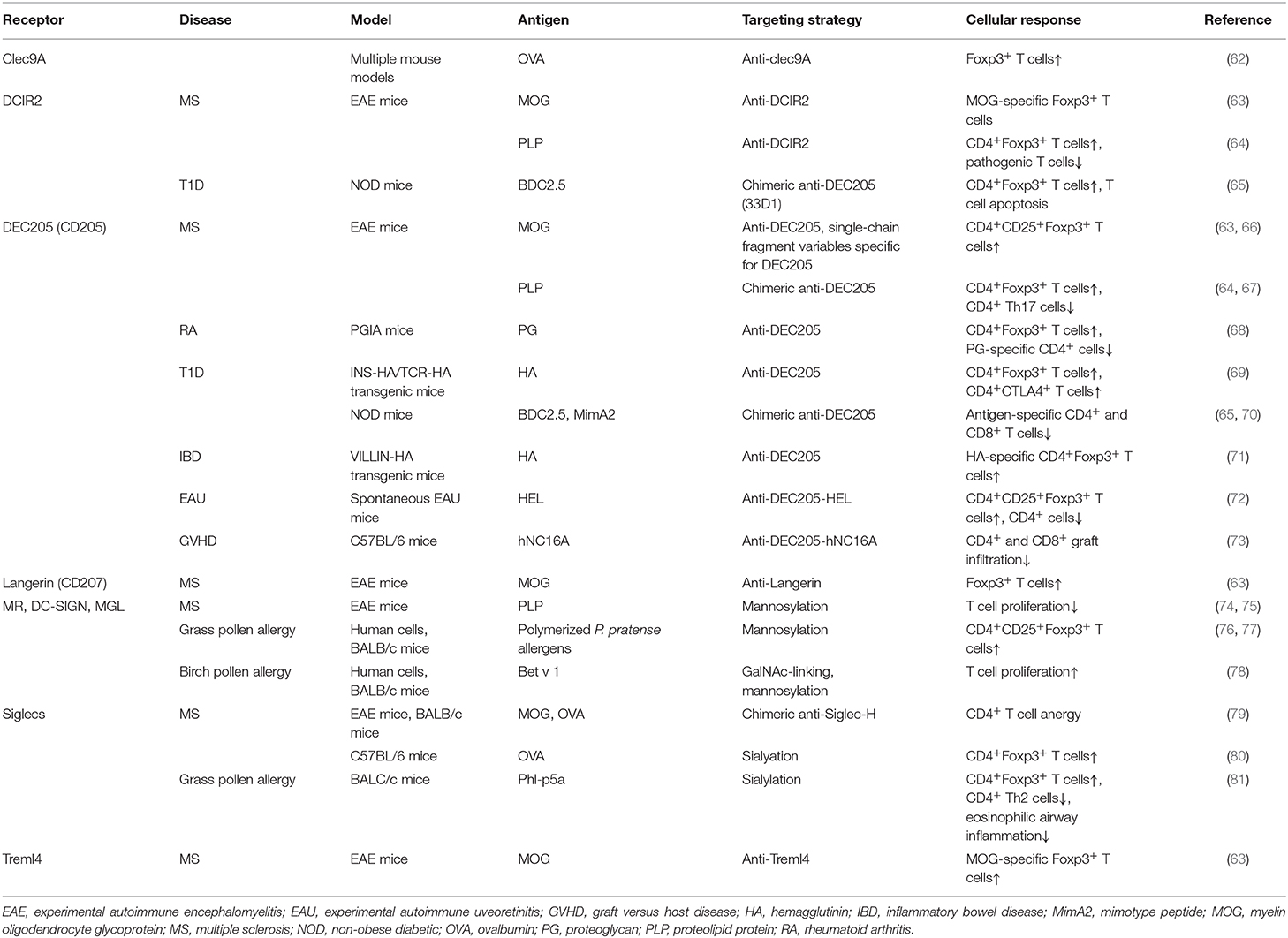
Table 1. Summary of in vivo studies to induce tolDCs using either antigen-antibody fusion compounds or carbohydrate-modified antigens.
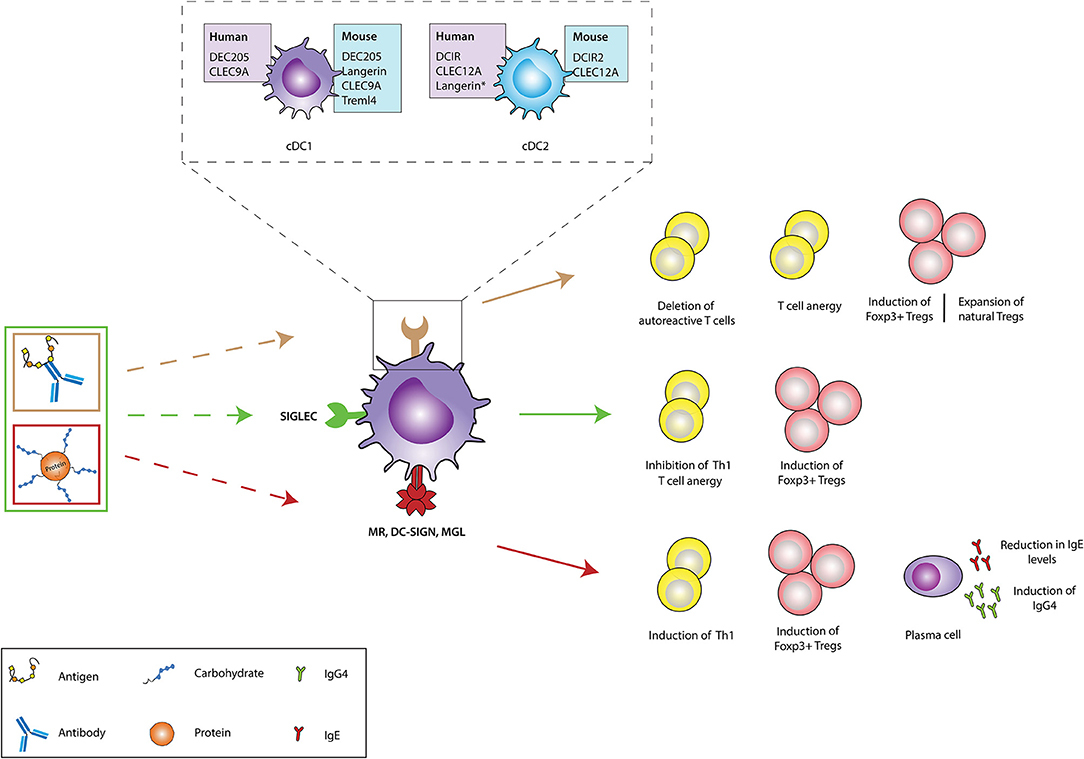
Figure 2. In vivo targeting of receptors on DCs against immunopathologies. Different receptors on dendritic cells (DCs) could be targeted using either antigen-antibody fusion compounds or carbohydrate-modified antigens. Brown arrows: the cDC1 and cDC2 subsets express a variety of receptors in both humans (purple boxes) and mice (blue boxes), which recognize and internalize fusion antibodies coupled with antigens, specific for a DC receptor and mediate the deletion of autoreactive T cells, induction of T cell anergy, generation of antigen-specific Foxp3+ Tregs and expansion of natural Tregs (These tolerogenic responses are abrogated if fusion antibodies are administered together with adjuvants like PolyI:C). The generation of either antigen-specific Foxp3+ Tregs or promotion of natural Tregs expansion depends of the receptor being targeted. Green arrows: Fusion antibodies against Siglecs, coupled with antigens or sialic acid-modified antigens bind to Siglec receptors on DCs and induce anti-inflammatory signals that result in the induction of Foxp3+ T cells, T cell anergy and the inhibition of Th1 responses. Red arrows: Targeting the MR, DC-SIGN and MGL on DCs with antigens conjugated to specific glycan moieties, result in the polarization of Th2 responses in allergy to Th1 responses and the induction of Foxp3+ T cells. Moreover, this interaction promotes the production of IgG4 blocking antibodies and mediates the reduction of IgE secretion. CLEC9A, C-type lectin domain family 9 member A; Treml4, The Triggering Receptor Expressed on Myeloid cells-like 4; DCIR, dendritic cell immunoreceptor; CLEC12A, C-type lectin domain family 12 member A; cDC1, Conventional type 1 dendritic cells; cDC2, Conventional type 2 dendritic cells; Siglec, Sialic acid-binding immunoglobulin-type lectins; MR, Mannose receptor; DC-SIGN, Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin; MGL, macrophage galactose-type C-type lectin. Langerin*: Induced expression.
DC-SIGN and MR are other members of the CLR family which recognize several mannose and fucose-containing structures, present on many antigens (49) and activate signaling pathways in CLR-expressing cells (44, 52, 60). These receptors are widely expressed on DCs and have been extensively exploited in several fields as potential targets for immunotherapy (89–91). Previously, antibody-mediated CLR targeting has been the most studied strategy for antigen delivery and activation of DCs in vivo, but in recent years glycan-based targeting approaches are gaining increasing attention (Table 1) (89, 90). Compared to antibody-mediated targeting, in glycan-based targeting, the spatial orientation of displayed carbohydrate CLR ligands can be varied more easily according to the distances between receptor binding sites thereby enhancing receptor-ligand binding and subsequent signaling (89). DC-SIGN is exclusively expressed on immature DCs and shows properties that are often, but not always, associated with Th2 polarization, suppression of inflammation and/or induction of regulatory immune response inhibiting pro-inflammatory Th1/Th17 immunity, especially when it recognizes helminth or allergen associated antigens (92–96). Interestingly, binding of the mycobacterial cell wall component Mannose-capped Lipoarabinomannan (ManLAM) to DC-SIGN inhibits DC maturation and induces IL-10 production (97). Also, the use of fucosylated ligands targeting DC-SIGN biases immune responses toward anti-Th1 responses, with an enhanced Th2 response, and has been shown to ameliorate different autoimmune conditions pre-clinically (92, 98). For instance, exposure of NOD mice to fucose-containing schistosome antigens inhibited the development of type 1 diabetes. This finding is in agreement with reports that have shown that such glycan-CLR signaling can induce a regulatory T cell phenotype having IL-10 and TGF-β production (99), which could explain the observed prevention of the development of autoimmunity in these mice (98, 100).
The MR recognizes terminal mannose, fucose and N-acetylglycosamine carbohydrates via its carbohydrate recognition domains. In humans, the MR has been identified in CD1ahigh and CD1alow dermal DCs, as well as in vitro monocyte-derived DCs and macrophages (101). In mice, the MR is mainly expressed by tissue and lymphoid-resident macrophages, but also in various endothelial cells and tracheal smooth muscle cells (101, 102). Additionally, MR expression can be detected in cultured murine moDCs, however the in vivo expression of MR on murine DCs remains unknown (61, 101). The MR has been reported to induce DC-mediated anti-inflammatory responses, including IL-10 production upon binding to some natural ligands that bind inside the MR binding sites (103). In contrast, when MR interacts with ligands that bind outside the carbohydrate recognition domains, there is no induction of IL-10 secretion, suggesting that, the efficacy of MR-targeted vaccines to induce tolerance will greatly depend on the appropriate selection of targeting vehicles and conditions (104). Notably, in a murine autoimmune model of collagen antibody-induced arthritis, treatment of mice with an epitope of Leishmania analog of the receptors for activated C kinase (LACK) from Leishmania major, inhibited joint inflammation and downregulated Th1 and Th17 cell responses through binding to the MR in CD11c+ DCs (105). Similarly, mannosylated forms of the myelin peptide PLP139−151 and MOG induced a state of tolerance in EAE mice (74, 75). Inhibition of EAE disease severity was suggested to be mediated by modulation of peripheral autoreactive T cells. This is in agreement with a study where treatment with mannosylated OVA peptides induced impaired Th1 effector functions and abrogated the activity of pre-existing effector T cells (106).
MGL is well-characterized for its specificity for terminal GalNAc (N-Acetylgalactosamine) residues, expressed by both mammalian cells and pathogens (49). In humans, MGL is expressed in vivo by human DCs of skin and lymph nodes and in vitro by macrophages and moDCs (107). In mice however, the homologs of human MGL, MGL1, and MGL2 are expressed by dermal DCs and alternatively activated macrophages. Upon ligand binding, the intracellular signaling pathways that are triggered vary extensively depending on the structure of the ligands. In this regard, it has recently been reported that, glycoconjugates from Fasciola hepatica potentiate the production of IL-10 by moDCs via engagement of MGL (94). Moreover, MGL-expressing DCs from mice infected with these glycoconjugates expanded IL-10-producing T cells and suppressed Th1 responses. Correspondingly, recent data has labeled the MGL as a negative regulator in autoimmune-induced neuroinflammation as MGL was shown to induce apoptosis of autoreactive T cells, the reduction of autoantibodies and the induction of IL-10 (108).
The use of glycan-based strategies has been substantially tested in pre-clinical and a few clinical settings for the treatment of allergies (Table 1). In these studies, carbohydrate-modified allergens were used to dampen allergic immune responses while installing antigen-specific T cell anergy both in vivo and in vitro (76, 77, 109–112). A notable mention is a study by Sirvent et al where they conjugated non-oxidized mannan from Saccharomyces cerevisae to polymerized grass pollen allergens (PM) and demonstrated that PM-treated human moDCs favor the induction of CD4+CD25highCD127−Foxp3+ Tregs over Th1 cells through PD-L1 signaling, subsequently causing an increase in the IL-10/IL-5 cytokine ratio produced by T cells (76) (Figure 2). PM was captured via the MR and DC-SIGN and proved to be hypoallergenic during in vivo skin prick tests and ex vivo basophil activation tests. The same group demonstrated that this strategy is equally effective in the treatment of canine atopic dermatitis (77). Interestingly, oxidation of mannan impaired the tolerogenic properties of PM shown in both human and mice, emphasizing the importance of the mannan structure for its functional properties (76, 109, 113). Also, Mathiesen et al. used the major birch pollen allergen, Bet v 1, coupled to defined carbohydrate structures and demonstrated that the prophylactic treatment of mice with GalNAc-coupled Bet v 1 significantly reduces IgE responses (78) (Figure 2). This finding suggests that MGL, which recognizes terminal α-and β-linked GalNAc structures, might be involved in the induction of the observed immune responses and may thus qualify as another potential candidate for specific antigen-delivery to DCs for induction of tolerance (114). Cumulatively, available data suggest that targeting DC-SIGN, MR and MGL for specific antigen delivery is a promising strategy to be further explored for the management of dysregulated immune pathologies (Figure 2).
Langerin (CD207) is a transmembrane protein that functions as an endocytic receptor by binding various sugars, including mannose, n-acetylglucosamine, fucose, and sulfated sugars, and mediates efficient antigen presentation on MHC I and II products in vivo (115). Langerin is highly expressed on surfaces of human LCs, but also at low levels on cDC2s isolated from dermal, lung, liver and lymphoid tissue (116). Yet, langerin is not expressed on circulating cDC2s isolated from blood (116). In mice, langerin is expressed on LCs and CD8α+DEC205+ cDC1s of the spleen and skin draining, however, langerin has not been identified in the homologous human cDC1 subset, indicating that langerin targeting strategies could induce distinct outcomes between mice and human experiments due to differential expression of the targeting receptor in both species (44, 52). Nevertheless, the tolerogenic role of LCs under physiological conditions as well as their accessible location at the surface of the body, marked langerin as a promising target for in vivo delivery of self-antigens to alter disease severity in autoimmune diseases (30, 31, 63). A notable mention is a report from Idoyaga et al that showed that, targeting MOG35−55 peptides to murine skin and lung langerin+ migratory DCs via conjugation with anti-langerin antibodies lessens EAE symptom severity through the induction of Foxp3+ Tregs (63) (Figure 2). The effect was comparable to the reduction of disease symptoms following administration of anti-DEC205-MOG fusion proteins. Interestingly, the langerin+ DC population is known to co-express high levels of DEC205, suggesting that the DC subset that is targeted with anti-langerin is also a target for anti-DEC205 mediated induction of Foxp3+ Tregs (117). However, in contrast to the above findings, co-administration of recombinant langerin-mAbs fused to antigen and maturation stimuli like anti-CD40 or polyI:C leads to efficient CD4+ and CD8+ T cell priming, proliferation, and differentiation (118). These results suggest that the strength of the activation signal targeted to langerin+ DC is a very important factor that must be strictly controlled in order to exploit these subsets in autoimmune therapy. Nonetheless, the ability of langerin+ DCs to induce antigen-specific Foxp3+ Tregs in lungs, suggest that anti-langerin mAbs is an attractive candidate for the treatment of respiratory dysregulated immune responses like allergies (Figure 2).
DCs are able to recognize and take up DAMPs through surface receptors such as Clec9A, a homodimeric type II transmembrane protein with a single extracellular C-type lectin-like domain expressed on cDC1s in both mice and human (119–121). The highly restricted expression of Clec9A on the human and mice cDC1 subset makes it an attractive receptor for targeting this specific subset of DCs (118). Clec9A ligation to its ligand F-actin either results in immunity or tolerance. As Clec9A promotes CD8+ T cell cross-priming, several in vitro studies have been performed to explore Clec9A targeting to induce anti-tumor immune responses (121). To determine whether Clec9A is a promising receptor for DC targeting in the context of autoimmune diseases, mice were injected with anti-Clec9A-antigen conjugates. In steady-state conditions in the absence of adjuvants, these conjugates promoted the differentiation of Foxp3+ Treg cells (62) (Figure 2). On the other hand, when anti-Clec9A was administered in combination with polyI:C, tolerance was prevented and instead promoted the development of potent antibody and Th1 or Th17 responses (62). Also, it has been reported that antigen delivery via Clec9A enhances the humoral response, even in the absence of adjuvant CpG. However, the immunoglobulin classes and resulting tolerogenic or immunogenic functions, were not explored in this study (122). Although targeting Clec9A can induce Tregs, extensive research is still needed to perfectly map out the optimal conditions that are necessary for tolerance induction.
Treml4 is another cell death receptor and binds to late apoptotic bodies necrotic cells (123). It is a member of the the triggering receptor expressed on myeloid cells (Trem)-family receptors which are primarily expressed on murine CD8+ lymphoid resident DCs and CD103+ lung DCs (63, 123). Treml4 has been investigated as a therapeutic target in a study by Idoyaga and colleagues. In this study, it was demonstrated that, intranasal inoculation of anti-Treml4-MOG peptide conjugates could induce MOG-specific Foxp3+ T cells in mice, but this did not prevent the development or promote improvement of EAE symptoms in diseased mice (63). The molecular mechanisms underlying these observations were not explored but it seems like signaling through this receptor does not produce a strong enough signal necessary for DC-mediated polarization of T cells to Tregs or the suppressive capacity of induced Tregs may be impaired in some way. Mechanistic studies addressing these issues will be very valuable in further exploring the therapeutic potential of this receptor in human settings in the context of immune pathologies. It is also important to note that the expression of Treml4 on human DC is yet to be reported. Therefore, the use of cell death sensing receptors for tolerizing therapies remains elusive until additional studies shed light on their relevance for clinical applications.
Siglec receptors are a family of receptors expressed on a wide variety of immune cells, including DCs (43, 59). They recognize sialic acid, which is the last carbohydrate structure added during the process of glycosylation, positioning sialic acid groups on the distal end of sugar-moieties (124). These sialic acid groups are present in the glycocalyx of all mammalian cells and could be considered as SAMPs (43). Siglecs are divided into two groups: (1) Siglecs that are conserved throughout different species, namely Siglec-1 (sialoadhesin), Siglec-2 (CD22), Siglec-4 myelin associated glycoprotein (MAG) and Siglec-15, and (2) the CD33-related Siglecs that have rapidly expanded and have no clear orthologs in mammalian species viz; Siglec−3 (CD33), −5, −6, −7, −8, −9, −10, −11, −14 and −16. The expression of Siglecs on myeoloid cells and their subsequent intracellular signaling pathways have been nicely reviewd by Lübbers et al. (59). In short, monocytes and monocyte-derived DCs express high levels of Siglec-3, −7 and −9, and low levels of Siglec-10. cDCs also express Siglec-3, −7, and −9, augmented with low expression levels of Siglec-2 and −15. On the other hand, pDCs only express Siglec-1, which is a non-signaling Siglec that internalizes upon ligand binding, and Siglec-5 (59). The binding affinity to sialic-acid-containing glycan varies between Siglecs. This is determined by the linkage of the sialic acid group to the underlying carbohydrate moiety (α2,3; α2,6 or α2,8 linkage). Another noteworthy feature of Siglecs is their ability to either bind their ligand via a trans interaction (on a different cell) or via a cis interaction (on the same cell). These cis interactions might contribute to sustain a tolerogenic phenotype, as surface proteins on tolerogenic DCs, immature DCs and Tregs are highly α2,6-sialylated, and could serve as a ligand for tolerogenic Siglecs (125). The conserved Siglec-2 and the CD33-related Siglec-3, and−5 till−11 contain an intracellular ITIM or ITIM-like motif that deliver negative signals via recruitment of SHP1 and SHP2 (43).
Interestingly, this immune modulating mechanism has been exploited by pathogen and cancer cells. For example, the protozoan parasite Trypanosoma cruzi enzymatically cleaves sialic acid moieties from the host and transfers them via α2,3-linkage to its own surface, subsequently downregulating pro-inflammatory IL-12 production and upregulating anti-inflammatory IL-10 production in murine DCs (126). Furthermore, various tumors upregulate α2,3; α2,6; and α2,8 sialic acid on their surface to evade anti-tumor T cell responses and to induce tumor-specific tolerance (59). Comparable to these natural Siglec-mediated immune modulation events, the tolerance inducing capacity of Siglecs could be used in therapeutic strategies to treat auto-immune and allergic diseases. So far, several in vitro and in vivo mouse studies have been performed to address this concept (Table 1). Targeting Siglec H on murine pDCs using anti-Siglec-H-antigen (OVA or MOG peptides) conjugates resulted in a decrease of CD4+ T cell expansion and Th1/Th17 differentiation, which subsequently delayed the onset and reduced disease severity in EAE when using the anti-Siglec-H-MOG conjugate (79). Similarly, direct modification of OVA and MOG peptides with α2,3 or α2,6 sialyl-lactose targeted these antigens to Siglec E on DCs and dampened pro-inflammatory responses in the same EAE mouse model upon treatment with sialylated MOG peptides (80). Siglec E targeting resulted in the induction of Foxp3+ CD4+ Tregs and inhibition of inflammatory effector cells after stimulation with LPS, both in vitro and in vivo (80) (Figure 2). Finally, the potential of sialic acid modified antigens was tested in an experimental murine model for grass pollen allergy. Subcutaneous treatment with sialic acid modified grass pollen peptides induced significant numbers of antigen specific Tregs, inhibited antigen specific effect Th2 cells, and reduced the accumulation of eosinophils (81).
Overall, these studies demonstrate that targeting DC through Siglecs could be very promising for induction of tolerogenic immune responses as a treatment for autoimmune and allergic diseases (Figure 2). However, most studies have been performed in mice and are therefore not sufficient for translation to human settings. Consequently, it is important to elucidate their potential and consequences in the human immune system.
DCIR is another member of the family of CLRs expressed on cDCs, moDCs and pDCs (44, 45, 127). The human DCIR-Fc protein has been reported to bind a variety of carbohydrate structures including Lewisb, Man3 glycans, and bisecting GlcNAc residues (127). The mouse homolog of DCIR, DCIR2 is primarily expressed on CD8+DCs. DCIR is important for the homeostasis of the immune system by, in part, regulating DC differentiation or polarization, as DCIR-deficient mice were prone to develop autoimmune encephalitis (128). As such, consistent with results obtained in mouse models (128, 129), polymorphisms of the DCIR gene are associated with the susceptibility to RA in humans (130). DCIR2+ DCs have been shown to stimulate natural Foxp3+ Tregs to mediate tolerance to self-antigens in the absence of immune stimuli (41). However, in the presence of a maturation stimulus, they induce T cell expansion and production of pro-inflammatory cytokines (41). Upon triggering with DCIR-specific mAbs, DCIR is internalized in both pDCs as well as human moDCs, resulting in efficient antigen presentation to T cells and downregulation of IFN-α production (131). Interestingly, using an anti-DCIR2-PLP139−151 and MOG fusion antibody to target DCs resulted in the amelioration of EAE symptoms (63, 64). This effect was suggested to be mediated primarily through the depletion of autoreactive T cells or induction of anergy in pathogenic T cells (Figure 2). However, DCIR2-targeting did not induce de novo generation of Ag-specific Treg from naïve CD4+ T cell precursors in the steady state due to lack of TGF-β expression by CD4+ CD11b+ DC but instead stimulated and expanded natural Tregs (64). Similarly, targeting β-Cell antigen using chimeric DCIR2 antibodies in NOD mice, elicited tolerogenic CD4+ T cell responses and induced increased T cell apoptosis while delaying diabetes induction (65). Targeting DCs with anti-DCIR2-antigen has also shown some promise in the field of transplantation where, an anti-DCIR2-MHC I monomer successfully inhibited allorecognition and the production of IgG alloantibodies leading to long-term allograft survival (132). Unexpectedly, DCIR2 targeting of mice DCs augmented spontaneous EAU development, characterized by local reduction in Tregs (133). While DCIR2 may be a promising candidate for in vivo Ag delivery in mice, this may not be the case for humans because, even though DCIR2 is highly restricted to CD11b+ DC subset in mice it is not detectable on the human CD1a/b+ cDC subset, a proposed equivalent of mouse CD11b+ DCs (130). Thus, there is need for identification of surface molecules that are specifically and similarly expressed by mouse and human DCs to allow exploration of clinical effectiveness of vaccines targeting these DC subsets.
The human myeloid inhibitory C-type lectin receptor (MICL) or Clec12A is expressed on alveolar macrophages, cDC1s, cDC2s, and pDCs, while the mouse Clec12A is expressed on myeloid cells (45, 134). Clec12A selectively binds to dead cells that have lost their plasma membrane integrity (130). The endocytic capacity of Clec12A has led to its being exploited for DC-specific antigen targeting. In this regard, antibody-mediated targeting of OVA to Clec12A in mice was able to induce potent antibody responses but no tolerogenic responses (135). Such targeting of Clec12A with anti-Clec12A antibodies seems to be sufficient for antigen internalization, processing and presentation but not for activation of DCs as reported in targeting of DEC205 with mAbs (122, 135). These mAbs may therefore simply serve to deliver Ags to DCs. The study of Clec12A in the context of immunotherapy for dysregulated immune pathologies is still in its infancy and further research is warranted given that preliminary data and the biological properties of Clec12A portrays this receptor as a promising candidate in this field.
Due to the unique capacity of DCs to coordinate innate and adaptive immune responses, they have been extensively studied and have proven to be a very promising strategy for immunotherapy. In the past decades, our knowledge of the potential of DCs in cancer and autoimmune disease/allergy therapy has expanded remarkably, advancing from the current ex vivo generated DC-based vaccines to in vivo targeting of DCs via specific receptors (Figure 1). Various compounds, such as vitamin D3, retinoic acid, dexamethasone, or IL-10, and TGFβ have shown the potency of tolDCs as immunotherapy in autoimmune diseases. However, there has been an increasing interest in moving toward in vivo targeting strategies where the induction of tolerance is achieved by targeting different receptors on DCs in their natural environment with antigen-delivering antibodies and antigen-carbohydrate conjugates. This has proven to be very effective in the amelioration of disease processes in a range of mouse models including MS, diabetes and allergies. Nevertheless, there is still a need to expand our knowledge on the potential application of such in vivo targeting strategies in human settings because, despite the many important similarities that exist between human and mouse DCs, very crucial incompatibilities between both species still limits the capacity to translate findings from one species to the other. Nonetheless, the potential for future clinical translation and therapeutic application of in vivo antigen targeting to DCs is very promising, although additional research is necessary to decipher the specific molecular mechanisms involved in the anti-disease tolerance promoted by such DCs. During the development of potential vaccines for autoimmune and allergic diseases, multiple inevitable questions need to be addressed. For instance, receptors that are not inherently tolerogenic but are capable of inducing tolerance under certain conditions, such as DEC205, DC-SIGN, and langerin, the induced tolerogenic effect is abrogated in the presence of pro-inflammatory modulators. Therefore, the appropriate optimization of vaccine formulations to target such receptors will be of utmost importance. In contrast, Siglecs have the ability to induce tolerogenic immune responses even in the presence of the pro-inflammatory modulator LPS (80, 136) and the resulting responses are not particularly affected by the presence of adjuvants. Moreover, there is still uncertainty about the right antigen-antibody/glycan dosage necessary for induction of tolerance, the duration of the resulting tolerogenic response, the effect on other immune cells expressing similar receptors as those being targeted, the use of a vehicle, and the method of administration. Finally, it may also be important to further investigate the potential positive or negative effects that receptor-specific antigen targeting may have on other myeloid cells, such as macrophages that express some of the DC receptors that can be targeted. Although further investigation is warranted, the effects might be negligible giving the lower antigen presenting capacity of macrophages. Overall, it is clear that the generation of a tolerogenic immune response via DC receptor targeting depends on the receptor, the DC subset being targeted, and the specific micro-environmental factors.
CC and B-CKD performed the literature search, wrote the manuscript, and created all figures. EdJ and YvK critically read and carefully revised all versions of the manuscript providing valuable guidance and insight. RvR critically read the manuscript and provided valuable additions. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from Health Holland (SIALLERGEN HH LSHM19073).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. (2013) 31:563–604. doi: 10.1146/annurev-immunol-020711-074950
2. Audiger C, Rahman MJ, Yun TJ, Tarbell KV, Lesage S. The importance of dendritic cells in maintaining immune tolerance. J Immunol. (2017) 198:2223–31. doi: 10.4049/jimmunol.1601629
3. Fucikova J, Palova-Jelinkova L, Bartunkova J, Spisek R. Induction of tolerance and immunity by dendritic cells: Mechanisms and clinical applications. Front Immunol. (2019) 10:2393. doi: 10.3389/fimmu.2019.02393
4. Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. (2018) 154:3–20. doi: 10.1111/imm.12888
5. Cauwels A, Tavernier J. Tolerizing strategies for the treatment of autoimmune diseases: from ex vivo to in vivo strategies. Front Immunol. (2020) 11: e674. doi: 10.3389/fimmu.2020.00674
6. Navarro-Barriuso J, Mansilla MJ, Naranjo-Gómez M, Sánchez-Pla A, Quirant-Sánchez B, Teniente-Serra A, et al. Comparative transcriptomic profile of tolerogenic dendritic cells differentiated with vitamin D3, dexamethasone and rapamycin. Sci Rep. (2018) 8:14985. doi: 10.1038/s41598-018-33248-7
7. Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. (2010) 107:20768–73. doi: 10.1073/pnas.1009201107
8. Obregon C, Kumar R, Pascual MA, Vassalli G, Golshayan D. Update on dendritic cell-induced immunological and clinical tolerance. Front Immunol. (2017) 8:1514. doi: 10.3389/fimmu.2017.01514
9. Ten Brinke A, Martinez-Llordella M, Cools N, Hilkens CMU, Van Ham SM, Sawitzki B, et al. Ways forward for tolerance-inducing cellular therapies- An afactt perspective. Front Immunol. (2019) 10:181. doi: 10.3389/fimmu.2019.00181
10. Aragão-França LS, Rocha VCJ, Cronemberger-Andrade A, Costa FHB, Vasconcelos JF, Athanazio DA, et al. Tolerogenic dendritic cells reduce airway inflammation in a model of dust mite triggered allergic inflammation. Allergy Asthma Immunol Res. (2018) 10:406–19. doi: 10.4168/aair.2018.10.4.406
11. Lehmann CHK, Heger L, Heidkamp GF, Baranska A, Lühr JJ, Hoffmann A, et al. Direct delivery of antigens to dendritic cells via antibodies specific for endocytic receptors as a promising strategy for future therapies. Vaccines. (2016) 4:8. doi: 10.3390/vaccines4020008
12. Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vaccines. (2014) 2:159–82. doi: 10.1177/2051013614541440
13. Rhodes JW, Tong O, Harman AN, Turville SG. Human dendritic cell subsets, ontogeny, and impact on HIV infection. Front Immunol. (2019) 10:1088. doi: 10.3389/fimmu.2019.01088
14. Lamendour L, Deluce-Kakwata-nkor N, Mouline C, Gouilleux-Gruart V, Velge-Roussel F. Tethering innate surface receptors on dendritic cells: a new avenue for immune tolerance induction? Int J Mol Sci. (2020) 21:1–15. doi: 10.3390/ijms21155259
15. Amon L, Lehmann CHK, Heger L, Heidkamp GF, Dudziak D. The ontogenetic path of human dendritic cells. Mol Immunol. (2020) 120:122–9. doi: 10.1016/j.molimm.2020.02.010
16. Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. (2017) 356:eaah4573. doi: 10.1126/science.aah4573
17. Brown CC, Gudjonson H, Pritykin Y, Deep D, Lavallée VP, Mendoza A, et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell. (2019) 179:846–63.e24. doi: 10.1016/j.cell.2019.09.035
18. Schlitzer A, McGovern N, Ginhoux F. Dendritic cells and monocyte-derived cells: two complementary and integrated functional systems. Semin Cell Dev Biol. (2015) 41:9–22. doi: 10.1016/j.semcdb.2015.03.011
19. Rodrigues PF, Tussiwand R. Novel concepts in plasmacytoid dendritic cell (pDC) development and differentiation. Mol Immunol. (2020) 126:25–30. doi: 10.1016/j.molimm.2020.07.006
20. Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. (2015) 15:471–85. doi: 10.1038/nri3865
21. Busold S, Nagy NA, Tas SW, van Ree R, de Jong EC, Geijtenbeek TBH. Various tastes of sugar: the potential of glycosylation in targeting and modulating human immunity via C-type lectin receptors. Front Immunol. (2020) 11:1–12. doi: 10.3389/fimmu.2020.00134
22. Iberg CA, Hawiger D. Natural and induced tolerogenic dendritic cells. J Immunol. (2020) 204:733–44. doi: 10.4049/jimmunol.1901121
23. Oh J, Shin JS. The role of dendritic cells in central tolerance. Immune Netw. (2015) 15:111–20. doi: 10.4110/in.2015.15.3.111
24. Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. (2009) 9:833–44. doi: 10.1038/nri2669
25. Proietto AI, Van Dommelen S, Zhou P, Rizzitelli A, D'Amico A, Steptoe RJ, et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci USA. (2008) 105:19869–74. doi: 10.1073/pnas.0810268105
26. Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. (2012) 30:1–22. doi: 10.1146/annurev-immunol-100311-102839
27. Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. (2002) 23:445–9. doi: 10.1016/S1471-4906(02)02281-0
28. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. (2003) 21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040
29. Adnan E, Matsumoto T, Ishizaki J, Onishi S, Suemori K, Yasukawa M, et al. Human tolerogenic dendritic cells generated with protein kinase C inhibitor are optimal for functional regulatory T cell induction—A comparative study. Clin Immunol. (2016) 173:96–108. doi: 10.1016/j.clim.2016.09.007
30. West HC, Bennett CL. Redefining the role of langerhans cells as immune regulators within the skin. Front Immunol. (2018) 8:1941. doi: 10.3389/fimmu.2017.01941
31. Azukizawa H, Döhler A, Kanazawa N, Nayak A, Lipp M, Malissen B, et al. Steady state migratory RelB+ langerin+ dermal dendritic cells mediate peripheral induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells. Eur J Immunol. (2011) 41:1420–34. doi: 10.1002/eji.201040930
32. Devi KSP, Anandasabapathy N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin Immunopathol. (2017) 39:137–52. doi: 10.1007/s00281-016-0602-0
33. Chu CC, Ali N, Karagiannis P, Di Meglio P, Skowera A, Napolitano L, et al. Resident CD141 (BDCA3) + dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp Med. (2012) 209:935–45. doi: 10.1084/jem.20112583
34. Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes LA, et al. Functional specializations of human epidermal langerhans cells and CD14+ dermal dendritic cells. Immunity. (2008) 29:497–510. doi: 10.1016/j.immuni.2008.07.013
35. Comi M, Avancini D, Santoni de Sio F, Villa M, Uyeda MJ, Floris M, et al. Coexpression of CD163 and CD141 identifies human circulating IL-10-producing dendritic cells (DC-10). Cell Mol Immunol. (2020) 17:95–107. doi: 10.1038/s41423-019-0218-0
36. Amodio G, Mugione A, Sanchez AM, Viganò P, Candiani M, Somigliana E, et al. HLA-G expressing DC-10 and CD4+ T cells accumulate in human decidua during pregnancy. Hum Immunol. (2013) 74:406–11. doi: 10.1016/j.humimm.2012.11.031
37. Comi M, Amodio G, Gregori S. Interleukin-10-producing DC-10 is a unique tool to promote tolerance via antigen-specific T regulatory type 1 cells. Front Immunol. (2018) 9:6. doi: 10.3389/fimmu.2018.00682
38. Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. (2010) 116:935–44. doi: 10.1182/blood-2009-07-234872
39. Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β -and retinoic acid-dependent mechanism. J Exp Med. (2007) 204:1757–64. doi: 10.1084/jem.20070590
40. Tordesillas L, Berin MC. Mechanisms of oral tolerance. Clin Rev Allergy Immunol. (2018) 55:107–17. doi: 10.1007/s12016-018-8680-5
41. Yamazaki S, Morita A. Dendritic cells in the periphery control antigen-specific natural and induced reulatory T cells. Front Immunol. (2013) 4:e151. doi: 10.3389/fimmu.2013.00151
42. Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. (2010) 59:595–604. doi: 10.1136/gut.2009.185108
43. Läubli H, Varki A. Sialic acid–binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell Mol Life Sci. (2020) 77:593–605. doi: 10.1007/s00018-019-03288-x
44. del Fresno C, Iborra S, Saz-Leal P, Martínez-López M, Sancho D. Flexible signaling of Myeloid C-type lectin receptors in immunity and inflammation. Front Immunol. (2018) 9:1. doi: 10.3389/fimmu.2018.00804
45. Geijtenbeek TBH, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. (2009) 9:465–79. doi: 10.1038/nri2569
46. Duan S, Paulson JC. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol. (2020) 38:365–95. doi: 10.1146/annurev-immunol-102419-035900
47. García-Vallejo JJ, Van Kooyk Y. Endogenous ligands for C-type lectin receptors: the true regulators of immune homeostasis. Immunol Rev. (2009) 230:22–37. doi: 10.1111/j.1600-065X.2009.00786.x
48. Iborra S, Sancho D. Signalling versatility following self and non-self sensing by myeloid C-type lectin receptors. Immunobiology. (2015) 220:175–84. doi: 10.1016/j.imbio.2014.09.013
49. Zizzari IG, Napoletano C, Battisti F, Rahimi H, Caponnetto S, Pierelli L, et al. MGL receptor and immunity: when the ligand can make the difference. J Immunol Res. (2015) 2015:450695. doi: 10.1155/2015/450695
50. Rapoport EM, Khaidukov SV, Gaponov AM, Pazynina GV, Tsygankova SV, Ryzhov IM, et al. Glycan recognition by human blood mononuclear cells with an emphasis on dendritic cells. Glycoconj J. (2018) 35:191–203. doi: 10.1007/s10719-017-9811-6
51. Redelinghuys P, Brown GD. Inhibitory C-type lectin receptors in myeloid cells. Immunol Lett. (2011) 136:1–12. doi: 10.1016/j.imlet.2010.10.005
52. Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. (2012) 30:491–529. doi: 10.1146/annurev-immunol-031210-101352.Signaling
53. Fehres CM, Unger WWJ, Garcia-Vallejo JJ, van Kooyk Y. Understanding the biology of antigen cross-presentation for the design of vaccines against cancer. Front Immunol. (2014) 5:149. doi: 10.3389/fimmu.2014.00149
54. Streng-Ouwehand I, Ho NI, Litjens M, Kalay H, Boks MA, Cornelissen LAM, et al. Glycan modification of antigen alters its intracellular routing in dendritic cells, promoting priming of T cells. Elife. (2016) 5:11765. doi: 10.7554/eLife.11765
55. Nair P, Amsen D, Blander JM. Co-ordination of incoming and outgoing traffic in antigen-presenting cells by pattern recognition receptors and T Cells. Traffic. (2011) 12:1669–76. doi: 10.1111/j.1600-0854.2011.01251.x
56. McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol. (2005) 17:18–24. doi: 10.1016/j.coi.2004.12.001
57. Pereira MS, Alves I, Vicente M, Campar A, Silva MC, Padrão NA, et al. Glycans as key checkpoints of T cell activity and function. Front Immunol. (2018) 9:2754. doi: 10.3389/fimmu.2018.02754
58. Crespo HJ, Lau JTY, Videira PA. Dendritic cells: a spot on sialic acid. Front Immunol. (2013) 4:491. doi: 10.3389/fimmu.2013.00491
59. Lübbers J, Rodríguez E, van Kooyk Y. Modulation of immune tolerance via siglec-sialic acid interactions. Front Immunol. (2018) 9:2807. doi: 10.3389/fimmu.2018.02807
60. Figdor CG, Van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and langerhans cells. Nat Rev Immunol. (2002) 2:77–84. doi: 10.1038/nri723
61. Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. (2000) 151:673–83. doi: 10.1083/jcb.151.3.673
62. Joffre OP, Sancho D, Zelenay S, Keller AM, Reis E, Sousa C. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur J Immunol. (2010) 40:1255–65. doi: 10.1002/eji.201040419
63. Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. (2013) 123:844–54. doi: 10.1172/JCI65260
64. Tabansky I, Keskin DB, Watts D, Petzold C, Funaro M, Sands W, et al. Targeting DEC-205-DCIR2+ dendritic cells promotes immunological tolerance in proteolipid protein-induced experimental autoimmune encephalomyelitis. Mol Med. (2018) 24:17. doi: 10.1186/s10020-018-0017-6
65. Price JD, Hotta-Iwamura C, Zhao Y, Beauchamp NM, Tarbell KV. DCIR2+ cDC2 DCs and Zbtb32 restore CD4+ T-cell tolerance and inhibit diabetes. Diabetes. (2015) 64:3521–31. doi: 10.2337/db14-1880
66. Ring S, Maas M, Nettelbeck DM, Enk AH, Mahnke K. Targeting of autoantigens to DEC205 + dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J Immunol. (2013) 191:2938–47. doi: 10.4049/jimmunol.1202592
67. Stern JNH, Keskin DB, Kato Z, Waldner H, Schallenberg S, Anderson A, et al. Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. Proc Natl Acad Sci USA. (2010) 107:17280–5. doi: 10.1073/pnas.1010263107
68. Spiering R, Margry B, Keijzer C, Petzold C, Hoek A, Wagenaar-Hilbers J, et al. DEC205 + dendritic cell–targeted tolerogenic vaccination promotes immune tolerance in experimental autoimmune arthritis. J Immunol. (2015) 194:4804–13. doi: 10.4049/jimmunol.1400986
69. Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, et al. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. (2005) 54:3395–401. doi: 10.2337/diabetes.54.12.3395
70. Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV, et al. Selective delivery of β cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc Natl Acad Sci USA. (2008) 105:6374–9. doi: 10.1073/pnas.0802644105
71. Wadwa M, Klopfleisch R, Buer J, Westendorf AM. Targeting antigens to DEC-205 on dendritic cells induces immune protection in experimental colitis in mice. Eur J Microbiol Immunol. (2016) 6:1–8. doi: 10.1556/1886.2015.00048
72. Kamoi K, Martín-Granados C, Bobu C, Wikstrom M, Degli-Esposti M, Steinman R, et al. Anti-DEC205 mediated delivery of self-antigen to dendritic cell restores tolerance in spontaneous EAU. Invest Ophthalmol Vis Sci. (2012) 53:6233.
73. Ettinger M, Gratz IK, Gruber C, Hauser-Kronberger C, Johnson TS, Mahnke K, et al. Targeting of the hNC16A collagen domain to dendritic cells induces tolerance to human type XVII collagen. Exp Dermatol. (2012) 21:395–8. doi: 10.1111/j.1600-0625.2012.01474.x
74. Kel J, Oldenampsen J, Luca M, Drijfhout JW, Koning F, Nagelkerken L. Soluble mannosylated myelin peptide inhibits the encephalitogenicity of autoreactive T cells during experimental autoimmune encephalomyelitis. Am J Pathol. (2007) 170:272–80. doi: 10.2353/ajpath.2007.060335
75. Luca ME, Kel JM, Van Rijs W, Drijfhout JW, Koning F, Nagelkerken L. Mannosylated PLP139-151 induces peptide-specific tolerance to experimental autoimmune encephalomyelitis. J Neuroimmunol. (2005) 160:178–87. doi: 10.1016/j.jneuroim.2004.11.014
76. Sirvent S, Soria I, Cirauqui C, Cases B, Manzano AI, Diez-Rivero CM, et al. Novel vaccines targeting dendritic cells by coupling allergoids to nonoxidized mannan enhance allergen uptake and induce functional regulatory T cells through programmed death ligand 1. J Allergy Clin Immunol. (2016) 138:558–67.e11. doi: 10.1016/j.jaci.2016.02.029
77. Soria I, Alvarez J, Manzano AI, López-Relaño J, Cases B, Mas-Fontao A, et al. Mite allergoids coupled to nonoxidized mannan from Saccharomyces cerevisae efficiently target canine dendritic cells for novel allergy immunotherapy in veterinary medicine. Vet Immunol Immunopathol. (2017) 190:65–72. doi: 10.1016/j.vetimm.2017.07.004
78. Mathiesen CBK, Carlsson MC, Brand S, Möller SR, Idorn M, Thor Straten P, et al. Genetically engineered cell factories produce glycoengineered vaccines that target antigen-presenting cells and reduce antigen-specific T-cell reactivity. J Allergy Clin Immunol. (2018) 142:1983–7. doi: 10.1016/j.jaci.2018.07.030
79. Loschko J, Heink S, Hackl D, Dudziak D, Reindl W, Korn T, et al. Antigen targeting to plasmacytoid dendritic cells via siglec-h inhibits Th cell-dependent autoimmunity. J Immunol. (2011) 187:6346–56. doi: 10.4049/jimmunol.1102307
80. Perdicchio M, Ilarregui JM, Verstege MI, Cornelissen LAM, Schetters STT, Engels S, et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc Natl Acad Sci USA. (2016) 113:3329–34. doi: 10.1073/pnas.1507706113
81. Hesse L, Feenstra R, Ambrosini M, de Jager WA, Petersen A, Vietor H, et al. Subcutaneous immunotherapy using modified Phl p5a-derived peptides efficiently alleviates allergic asthma in mice. Allergy Eur J Allergy Clin Immunol. (2019) 74:2495–8. doi: 10.1111/all.13918
82. Mahnke K, Ring S, Enk AH. Antibody targeting of “steady-state” dendritic cells induces tolerance mediated by regulatory T cells. Front Immunol. (2016) 7:63. doi: 10.3389/fimmu.2016.00063
83. Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. (2003) 101:4862–9. doi: 10.1182/blood-2002-10-3229
84. Maksimow M, Miiluniemi M, Marttila-Ichihara F, Jalkanen S, Hänninen A. Antigen targeting to endosomal pathway in dendritic cell vaccination activates regulatory T cells and attenuates tumor immunity. Blood. (2006) 108:1298–305. doi: 10.1182/blood-2005-11-008615
85. Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. (2002) 196:1627–38. doi: 10.1084/jem.20021598
86. Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. (2004) 20:695–705. doi: 10.1016/j.immuni.2004.05.002
87. Inaba K, Swiggard WJ, Inaba M, Meltzer J, Miryza A, Sasagawa T, et al. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell Immunol. (1995) 163:148–56. doi: 10.1006/cimm.1995.1109
88. Kato M, McDonald KJ, Khan S, Ross IL, Vuckovic S, Chen K, et al. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int Immunol. (2006) 18:857–69. doi: 10.1093/intimm/dxl022
89. Lepenies B, Lee J, Sonkaria S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv Drug Deliv Rev. (2013) 65:1271–81. doi: 10.1016/j.addr.2013.05.007
90. Li RE, van Vliet SJ, van Kooyk Y. Using the glycan toolbox for pathogenic interventions and glycan immunotherapy. Curr Opin Biotechnol. (2018) 51:24–31. doi: 10.1016/j.copbio.2017.11.003
91. Johannssen T, Lepenies B. Glycan-based cell targeting to modulate immune responses. Trends Biotechnol. (2017) 35:334–46. doi: 10.1016/j.tibtech.2016.10.002
92. Marciani DJ. Effects of immunomodulators on the response induced by vaccines against autoimmune diseases. Autoimmunity. (2017) 50:393–402. doi: 10.1080/08916934.2017.1373766
93. Favoretto BC, Casabuono AAC, Portes-Junior JA, Jacysyn JF, Couto AS, Faquim-Mauro EL. High molecular weight components containing N-linked oligosaccharides of Ascaris suum extract inhibit the dendritic cells activation through DC-SIGN and MR. Mol Immunol. (2017) 87:33–46. doi: 10.1016/j.molimm.2017.03.015
94. Rodríguez E, Carasi P, Frigerio S, da Costa V, van Vliet S, Noya V, et al. Fasciola hepatica immune regulates CD11c+ cells by interacting with the macrophage gal/GalNAc lectin. Front Immunol. (2017) 8:264. doi: 10.3389/fimmu.2017.00264
95. Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, Bouma G, et al. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int J Parasitol. (2013) 43:191–200. doi: 10.1016/j.ijpara.2012.10.021
96. Cvetkovic J, Ilic N, Gruden-Movsesijan A, Tomic S, Mitic N, Pinelli E, et al. DC-SIGN signalling induced by Trichinella spiralis products contributes to the tolerogenic signatures of human dendritic cells. Sci Rep. (2020) 10:20283. doi: 10.1038/s41598-020-77497-x
97. Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SCM, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-κB activation through Raf-1 and Syk. Nat Immunol. (2009) 10:203–13. doi: 10.1038/ni.1692
98. Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. (2009) 39:1098–107. doi: 10.1002/eji.200838871
99. Maizels RM, Smits HH, McSorley HJ. Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity. (2018) 49:801–18. doi: 10.1016/j.immuni.2018.10.016
100. Ruyssers NE, De Winter BY, De Man JG, Loukas A, Herman AG, Pelckmans PA, et al. Worms and the treatment of inflammatory bowel disease: are molecules the answer? Clin Dev Immunol. (2008) 2008:567314. doi: 10.1155/2008/567314
101. Taylor PR, Gordon S, Martinez-Pomares L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. (2005) 26:104–10. doi: 10.1016/j.it.2004.12.001
102. Linehan SA, Martínez-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. (1999) 189:1961–72. doi: 10.1084/jem.189.12.1961
103. Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. (2003) 171:4552–60. doi: 10.4049/jimmunol.171.9.4552
104. Keler T, Ramakrishna V, Fanger MW. Mannose receptor-targeted vaccines. Expert Opin Biol Ther. (2004) 4:1953–62. doi: 10.1517/14712598.4.12.1953
105. Yang F, Fan X, Huang H, Dang Q, Lei H, Li Y. A single microorganism epitope attenuates the development of murine autoimmune arthritis: regulation of dendritic cells via the mannose receptor. Front Immunol. (2018) 9:1528. doi: 10.3389/fimmu.2018.01528
106. Kel JM, De Geus ED, Van Stipdonk MJ, Drijfhout JW, Koning F, Nagelkerken L. Immunization with mannosylated peptide induces poor T cell effector functions despite enhanced antigen presentation. Int Immunol. (2008) 20:117–27. doi: 10.1093/intimm/dxm123
107. van Vliet SJ, Paessens LC, Broks-van den Berg VCM, Geijtenbeek TBH, van Kooyk Y. The C-type lectin macrophage galactose-type lectin impedes migration of immature APCs. J Immunol. (2008) 181:3148–55. doi: 10.4049/jimmunol.181.5.3148
108. Ilarregui JM, Rabinovich GA. Tolerogenic dendritic cells in the control of autoimmune neuroinflammation: an emerging role of protein-glycan interactions. Neuroimmunomodulation. (2010) 17:157–60. doi: 10.1159/000258712
109. Benito-Villalvilla C, Soria I, Subiza JL, Palomares O. Novel vaccines targeting dendritic cells by coupling allergoids to mannan. Allergo J Int. (2018) 27:256–62. doi: 10.1007/s40629-018-0069-8
110. Soria I, López-Relaño J, Viñuela M, Tudela JI, Angelina A, Benito-Villalvilla C, et al. Oral myeloid cells uptake allergoids coupled to mannan driving Th1/Treg responses upon sublingual delivery in mice. Allergy Eur J Allergy Clin Immunol. (2018) 73:875–84. doi: 10.1111/all.13396
111. Palomares F, Ramos-Soriano J, Gomez F, Mascaraque A, Bogas G, Perkins JR, et al. Pru p 3-glycodendropeptides based on mannoses promote changes in the immunological properties of dendritic and t-cells from LTP-allergic patients. Mol Nutr Food Res. (2019) 63:553. doi: 10.1002/mnfr.201900553
112. Manzano AI, Javier Cañada F, Cases B, Sirvent S, Soria I, Palomares O, et al. Structural studies of novel glycoconjugates from polymerized allergens (allergoids) and mannans as allergy vaccines. Glycoconj J. (2016) 33:93–101. doi: 10.1007/s10719-015-9640-4
113. Benito-Villalvilla C, Soria I, Pérez-Diego M, Fernández-Caldas E, Subiza JL, Palomares O. Alum impairs tolerogenic properties induced by allergoid-mannan conjugates inhibiting mTOR and metabolic reprogramming in human DCs. Allergy Eur J Allergy Clin Immunol. (2020) 75:648–59. doi: 10.1111/all.14036
114. van Vliet SJ, van Liempt E, Saeland E, Aarnoudse CA, Appelmelk B, Irimura T, et al. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol. (2005) 17:661–9. doi: 10.1093/intimm/dxh246
115. Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, et al. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. (2008) 180:3647–50. doi: 10.4049/jimmunol.180.6.3647
116. Bigley V, McGovern N, Milne P, Dickinson R, Pagan S, Cookson S, et al. Langerin-expressing dendritic cells in human tissues are related to CD1c + dendritic cells and distinct from Langerhans cells and CD141 high XCR1 + dendritic cells. J Leukoc Biol. (2015) 97:627–34. doi: 10.1189/jlb.1hi0714-351r
117. Petzold C, Schallenberg S, Stern JNH, Kretschmer K. Targeted antigen delivery to DEC-205+ dendritic cells for tolerogenic vaccination. Rev Diabet Stud. (2012) 9:305–18. doi: 10.1900/RDS.2012.9.305
118. Flacher V, Tripp CH, Mairhofer DG, Steinman RM, Stoitzner P, Idoyaga J, et al. Murine langerin + dermal dendritic cells prime CD 8 + T cells while L angerhans cells induce cross-tolerance. EMBO Mol Med. (2014) 6:1191–204. doi: 10.15252/emmm.201303283
119. Schreibelt G, Klinkenberg LJJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M, et al. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood. (2012) 119:2284–92. doi: 10.1182/blood-2011-08-373944
120. Crozat K, Tamoutounour S, Vu Manh T-P, Fossum E, Luche H, Ardouin L, et al. Cutting edge: expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8α + type. J Immunol. (2011) 187:4411–5. doi: 10.4049/jimmunol.1101717
121. Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem. (2008) 283:16693–701. doi: 10.1074/jbc.M709923200
122. Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee C-N, et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J Immunol. (2011) 187:842–50. doi: 10.4049/jimmunol.1101176
123. Hemmi H, Idoyaga J, Suda K, Suda N, Kennedy K, Noda M, et al. A new triggering receptor expressed on myeloid cells (trem) family member, trem-like 4, binds to dead cells and is a DNAX activation protein 12-linked marker for subsets of mouse macrophages and dendritic cells. J Immunol. (2009) 182:1278–86. doi: 10.4049/jimmunol.182.3.1278
124. Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. (2007) 446:1023–9. doi: 10.1038/nature05816
125. Jenner J, Kerst G, Handgretinger R, Müller I. Increased α2,6-sialylation of surface proteins on tolerogenic, immature dendritic cells and regulatory T cells. Exp Hematol. (2006) 34:1211–7. doi: 10.1016/j.exphem.2006.04.016
126. Erdmann H, Steeg C, Koch-Nolte F, Fleischer B, Jacobs T. Sialylated ligands on pathogenic Trypanosoma cruzi interact with Siglec-E (sialic acid-binding Ig-like lectin-E). Cell Microbiol. (2009) 11:1600–11. doi: 10.1111/j.1462-5822.2009.01350.x
127. García-Vallejo JJ, Bloem K, Knippels LMJ, Garssen J, van Vliet SJ, van Kooyk Y. The consequences of multiple simultaneous C-type lectin-ligand interactions: DCIR alters the endo-lysosomal routing of DC-SIGN. Front Immunol. (2015) 6:87. doi: 10.3389/fimmu.2015.00087
128. Seno A, Maruhashi T, Kaifu T, Yabe R, Fujikado N, Ma G, et al. Exacerbation of experimental autoimmune encephalomyelitis in mice deficient for DCIR, an inhibitory C-type lectin receptor. Exp Anim. (2015) 64:109–19. doi: 10.1538/expanim.14-0079
129. Fujikado N, Saijo S, Yonezawa T, Shimamori K, Ishii A, Sugai S, et al. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med. (2008) 14:176–80. doi: 10.1038/nm1697
130. Kaifu T, Iwakura Y. Dendritic cell immunoreceptor (DCIR): an ITIMharboring C-type lectin receptor. In: C-Type Lectin Receptors in Immunity. Springer Japan. (2016). p. 101–13. doi: 10.1007/978-4-431-56015-9_7
131. Meyer-Wentrup F, Cambi A, Joosten B, Looman MW, de Vries IJM, Figdor CG, et al. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J Leukoc Biol. (2009) 85:518–25. doi: 10.1189/jlb.0608352
132. Tanriver Y, Ratnasothy K, Bucy RP, Lombardi G, Lechler R. Targeting MHC class I monomers to dendritic cells inhibits the indirect pathway of allorecognition and the production of IgG alloantibodies leading to long-term allograft survival. J Immunol. (2010) 184:1757–64. doi: 10.4049/jimmunol.0902987
133. Iberg CA, Hawiger D. Advancing immunomodulation by in vivo antigen delivery to DEC-205 and other cell surface molecules using recombinant chimeric antibodies. Int Immunopharmacol. (2019) 73:575–80. doi: 10.1016/j.intimp.2019.05.037
134. Álvarez B, Nieto-Pelegrín E, Martínez de la Riva P, Toki D, Poderoso T, Revilla C, et al. Characterization of the porcine CLEC12A and analysis of its expression on blood dendritic cell subsets. Front Immunol. (2020) 11:863. doi: 10.3389/fimmu.2020.00863
135. Lahoud MH, Proietto AI, Ahmet F, Kitsoulis S, Eidsmo L, Wu L, et al. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J Immunol. (2009) 182:7587–94. doi: 10.4049/jimmunol.0900464
Keywords: dendritic cell, tolerance, immunotherapy, surface receptors, C-type lectins, Siglecs, allergy, auto immune diseases
Citation: Castenmiller C, Keumatio-Doungtsop B-C, van Ree R, de Jong EC and van Kooyk Y (2021) Tolerogenic Immunotherapy: Targeting DC Surface Receptors to Induce Antigen-Specific Tolerance. Front. Immunol. 12:643240. doi: 10.3389/fimmu.2021.643240
Received: 17 December 2020; Accepted: 02 February 2021;
Published: 19 February 2021.
Edited by:
Irina Caminschi, Monash University, AustraliaReviewed by:
Angel L. Corbi, Consejo Superior de Investigaciones Científicas (CSIC), SpainCopyright © 2021 Castenmiller, Keumatio-Doungtsop, van Ree, de Jong and van Kooyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yvette van Kooyk, eS52YW5rb295a0BhbXN0ZXJkYW11bWMubmw=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.