
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 25 March 2021
Sec. Microbial Immunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.638913
 Jiang Li1†
Jiang Li1† Yanfei Zhang2†
Yanfei Zhang2† Alexandria L. Jilg3
Alexandria L. Jilg3 Donna M. Wolk4
Donna M. Wolk4 Harshit S. Khara5
Harshit S. Khara5 Amy Kolinovsky6
Amy Kolinovsky6 David D. K. Rolston3
David D. K. Rolston3 Raquel Hontecillas7
Raquel Hontecillas7 Josep Bassaganya-Riera7
Josep Bassaganya-Riera7 Marc S. Williams2
Marc S. Williams2 Vida Abedi1*‡
Vida Abedi1*‡ Ming Ta Michael Lee2*‡ on behalf of Regeneron Genetics Center
Ming Ta Michael Lee2*‡ on behalf of Regeneron Genetics CenterBackground: Clostridioides difficile is a major cause of healthcare-associated and community-acquired diarrhea. Host genetic susceptibility to Clostridioides difficile infection has not been studied on a large-scale.
Methods: A total of 1,160 Clostridioides difficile infection cases and 15,304 controls were identified by applying the eMERGE Clostridioides difficile infection algorithm to electronic health record data. A genome-wide association study was performed using a linear mixed model, adjusted for significant covariates in the full dataset and the antibiotic subgroup. Colocalization and MetaXcan were performed to identify potential target genes in Clostridioides difficile infection - relevant tissue types.
Results: No significant genome-wide association was found in the meta-analyses of the full Clostridioides difficile infection dataset. One genome-wide significant variant, rs114751021, was identified (OR = 2.42; 95%CI = 1.84-3.11; p=4.50 x 10-8) at the major histocompatibility complex region associated with Clostridioides difficile infection in the antibiotic group. Colocalization and MetaXcan identified MICA, C4A/C4B, and NOTCH4 as potential target genes. Down-regulation of MICA, upregulation of C4A and NOTCH4 was associated with a higher risk for Clostridioides difficile infection.
Conclusions: Leveraging the EHR and genetic data, genome-wide association, and fine-mapping techniques, this study identified variants and genes associated with Clostridioides difficile infection, provided insights into host immune mechanisms, and described the potential for novel treatment strategies for Clostridioides difficile infection. Future replication and functional validation are needed.
Clostridioides difficile (C. difficile) is an anaerobic, Gram-positive, and spore-forming bacterium. C. difficile is a major cause of antibiotic-associated diarrhea. It is also associated with the community-acquisition of diarrhea. A surveillance study across 10 geographic areas in the United States in 2011 estimated 453,000 incident C. difficile infections (CDI) and 29,000 associated deaths per year (1). Individuals who harbor C. difficile in their gut may be carriers with no discernible symptoms, may have diarrhea of variable severity, or may progress from fulminant colitis to systemic disease and death (2). The clinical manifestations are determined by several major factors, including the strain type and associated virulence (3), the host immune response (4), disruptions in the host’s microbiome (for example, those caused by antibiotics) (5), patient medications (6), and gastric acid suppression (7).
Toxins are the major virulence factors of C. difficile (8). Toxins A (TcdA) and B (TcdB) are large secreted glucosyltransferase proteins that target intestinal epithelial cells and disrupt the epithelial barrier leading to secretory diarrhea (9). The hypervirulent BI/NAP1/027 strain is often fluoroquinolone resistant and the most common cause of CDI globally (10, 11). Host risk factors include advanced age (≥ 65 years), underlying comorbidities such as inflammatory bowel disease (IBD), organ transplantation, immunodeficiency, certain medications including antibiotics, chemotherapy, and proton pump inhibitor (PPI) (7), and prolonged hospital stay (4). Antibiotic use and altered intestinal microbiota can reduce colonization resistance against pathogens, including C. difficile, therefore, certain antimicrobial agents remain the most prominent risk factors for CDI (4, 12).
Multiple studies have investigated the genetics of the bacteria and identified genes and genetic variation that are associated with the virulence factors, deepening our understanding of the emergence and global spread of C. difficile strains (11, 13, 14). However, the identification of host genetic susceptibility to CDI has not been extensively investigated. Several candidate gene studies have mainly focused on proinflammatory cytokine genes such as interleukin-8 (IL-8) (15–17), or IBD-associated single nucleotide variants (SNVs) (18, 19). One SNV, rs4073(–251T>A), found in the promoter region of IL8, was reported to be associated with CDI (15) and recurrent CDI (16). However, this association was not validated in independent studies (17). Eight IBD-associated SNVs were found associated with CDI at a nominal significance level (p<0.05) in an ulcerative colitis population (18). Another study reported that rs2243250, an IL4-associated SNV, was associated with CDI in an IBD population (19). Hitherto, only one study with 57 CDI patients performed a genome-wide association study (GWAS) in a cohort of patients with multiple myeloma undergoing autologous stem cell transplantation. None of these associations reached genome-wide significance (20).
All the published studies suffer from small sample size (CDI cases < 60) that limit the statistical power. There is an unmet need to identify host genetic risk factors for CDI in general and in high-risk populations such as antibiotics-users. In this study, we leveraged the electronic health record (EHR) linked to genetic data and performed GWAS to identify common variants associated with CDI.
The MyCode® Community Health Initiative (MyCode) is an ongoing project with deidentified EHR linked to genomic data supporting genetic studies (21–23). The primary cohort in this study consist of approximately 92,000 consented participants from MyCode Phase I (approximately 60,000 participants) and Phase II (approximately 32,000 participants). This study was exempted as non-human subjects research by the Geisinger Institutional Review Board for using de-identified information and was approved by the MyCode Governing Board. Figure 1 illustrates the study design and pipeline for sample selection and data analysis.
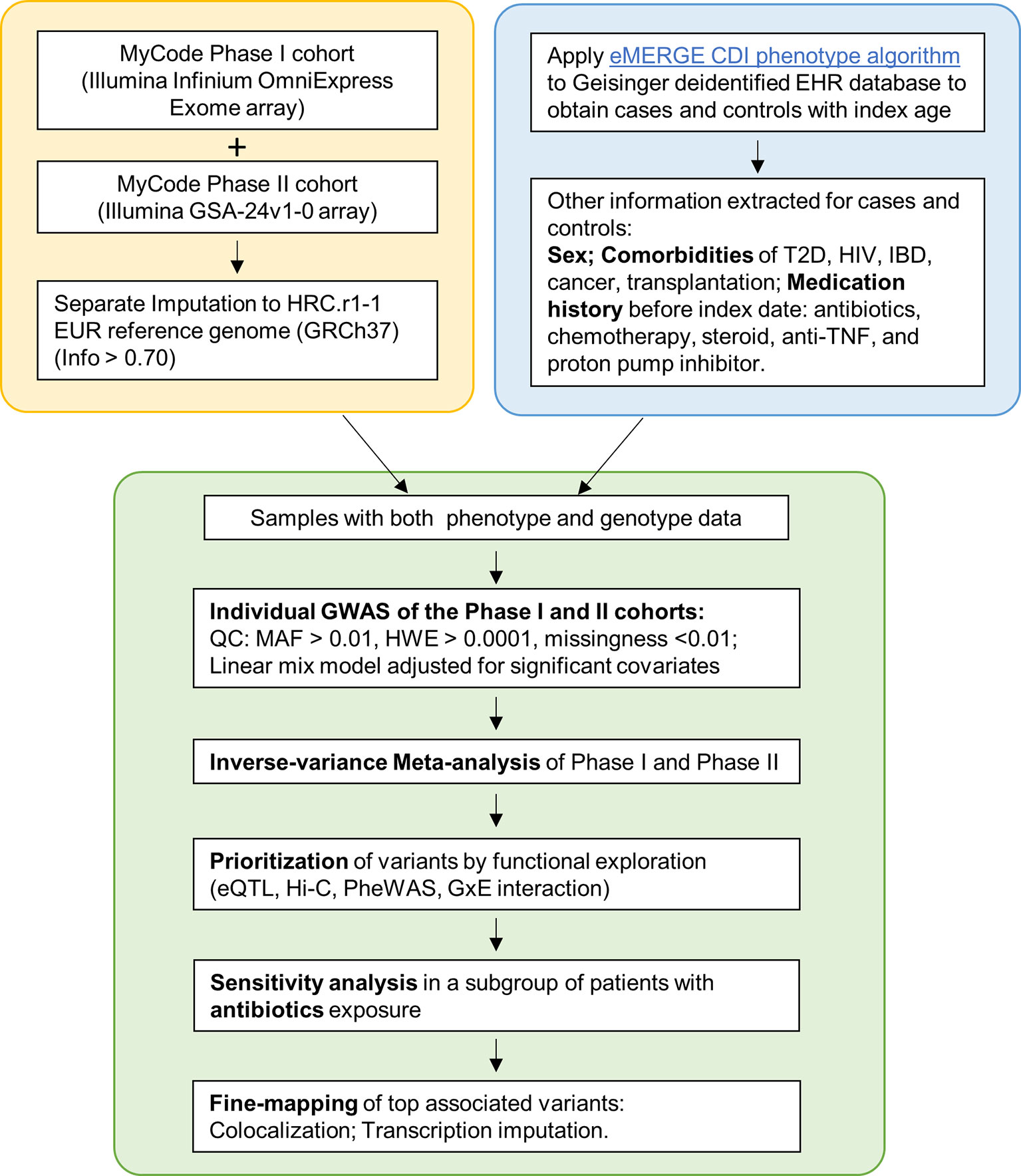
Figure 1 Flowchart of the study. Yellow box: Genotyping and imputation was conducted separately for Phase I and II. Blue box: eMERGE CDI algorithm was applied to identify cases and controls. Clinical information was extracted. Green box: individual GWAS and meta-analyses, followed by a sensitivity analysis and Fine-mapping.
Geisinger houses a continually growing deidentified EHR database for research, including information describing patients’ demographics, diagnosis, laboratory test results, prescriptions, procedures, and vital signs. A phenotyping algorithm to identify CDI cases and controls from EHR data was developed by the eMERGE Network and was implemented in the Geisinger EHR (dbGaP study accession: phs000888.v1.p1; phenotype accession: phd004976.1). Briefly, the eMERGE algorithm identified CDI cases using the reference standard, based on positive laboratory results, or the silver standard, based on diagnosis codes combined with information from the patients’ chart. Controls were defined as patients without CDI, based on negative laboratory results, but exposed to similar risk factors, such as antibiotic use, hospitalization, high-risk conditions, and medications (details in the Supplementary Note). Polymerase chain reaction (PCR) was used as the laboratory reference standard test with the BD GeneOhm™ Cdiff Assay (Becton Dickinson, Franklin Lakes, NJ) used prior to mid-April 2014 and the Xpert C. difficile/Epi assay (Cepheid, Sunnyvale CA) used after that. Only adults (age >=18) were included in this study. Risk factors and demographic information were extracted from EHR data. Using data through Dec 31, 2018, we identified 946 cases and 10,840 controls from Phase I and 214 cases and 3,304 controls from Phase II. Of these, 587 cases and 3,166 controls had antibiotics exposure prior to the index date.
The genotyping, imputation and quality control for the genetic data have been previously described (24) (Figure 1; Supplementary Methods). Pairs of individuals with first- or second-degree relatedness were identified (Supplementary Figure 1A). Principal component analyses indicated that the individuals in this study are of European ancestry (Supplementary Figure 1B). In total, 7,077,672 SNVs from Phase I, 6,683,047 SNVs from Phase II, and 6,497,3696 SNVs from the merged antibiotic-exposed group were included in the analyses.
BOLT-LMM, a linear mixed model, was adopted to test genetic associations while accounting for covariates and cryptic relatedness between individuals (25). In the sensitivity analysis of antibiotics subgroups, only the patients exposed to antibiotic risk within 7 to 62 days before the index date, were included. The significant covariates between cases and controls were adjusted. The fixed effect, inverse variance weighted meta-analyses of Phase I and II summary statistics were accomplished using METAL (26). SAIGE, which uses the saddle point approximation to calibrate the distribution of score test statistics, was also applied to account for case-control imbalance (27). GEMMA was used to test the gene x environment (GxE) interaction, which employs a linear mixed model to control for the main effects of SNV and environmental factors while testing for the interaction effect (28). The odds ratio (OR) was converted from the regression coefficient β in R (29). Open Targets Genetics was queried for the top associated SNVs to evaluate whether other significantly associated phenotypes were present.
Statistical fine-mapping using functional genomics data, such as expression quantitative trait loci (eQTL), has been used to explore the potential mechanisms of GWAS variants on diseases. Colocalization is one commonly used method that integrates GWAS and eQTL data to estimate the probability of the same variant being causal in both GWAS and eQTL studies (30). Colocalized signals provide evidence of possible causal relationships between the target gene of an eQTL and the GWAS trait. To identify causal genes and target tissues, we performed colocalization analyses of the four top associated variants at the MHC region with CDI in the antibiotic subgroup using eCAVIAR (31). The eQTL data of the whole blood and the seven gastrointestinal (GI) tissue types (colon sigmoid, colon transverse, esophageal mucosa, esophageal muscularis, gastroesophageal junction, small intestine, and stomach) from GTEx v7 data release (32) were included in the analyses. The overlapped SNVs in the GWAS and GTEx were included in the analyses. Fifty SNVs, upstream and downstream from the GWAS lead SNV in each locus, were included to calculate the colocalization posterior probability (CLPP) assuming a maximum of two causal SNVs in each locus. Only significant eGenes were examined. All SNV-gene pairs eQTL data for each eGene were then used in the analyses. CLPP represents the probability that the same variant is causal in both GWAS and QTLs. We adopted a CLPP > 0.01 as the colocalization cutoff (31). Variants that have CLPP > 0.01 and GWAS p-value <10-5 were included to identify target genes. We report the highest probability (max of CLPP value) for each target gene in the relevant tissue.
S-PrediXcan is an integrative gene-based association approach that uses summary data and pre-imputed transcriptome levels with models trained in a measured transcriptome dataset (such as GTEx) to identify genes involved in the etiology of the phenotype (33). In S-PrediXcan, the predicted expression levels are used to correlate with the phenotype in the gene association test. In our study, the GWAS summary statistics of the antibiotic subgroup were used to perform transcriptome imputation and gene-based association testing by S-PrediXcan using a pre-trained model based on GTEx v7 data (the GTEx-V7_HapMap-2017-11-29.tar.gz file in the PredictDB). The infrastructure described was used to impute gene expression at the MHC region (Chr6: 28477797~33448354, GRCh37) (33). The whole blood and the seven GI tissues were also examined.
Demographics and clinical characteristics of CDI cohorts from Phase I, Phase II, and antibiotic subgroups for CDI GWAS are listed in Table 1. CDI cases had older age, higher prevalence of antibiotic and proton pump inhibitor (PPI) use, and inflammatory bowel disease (IBD); all of which were known risk factors for CDI. Other factors such as steroid intake, anti-TNF medication, type 2 diabetes, and transplantation history were only significant in the phase I cohort, reflecting underlying heterogeneity among cohorts.
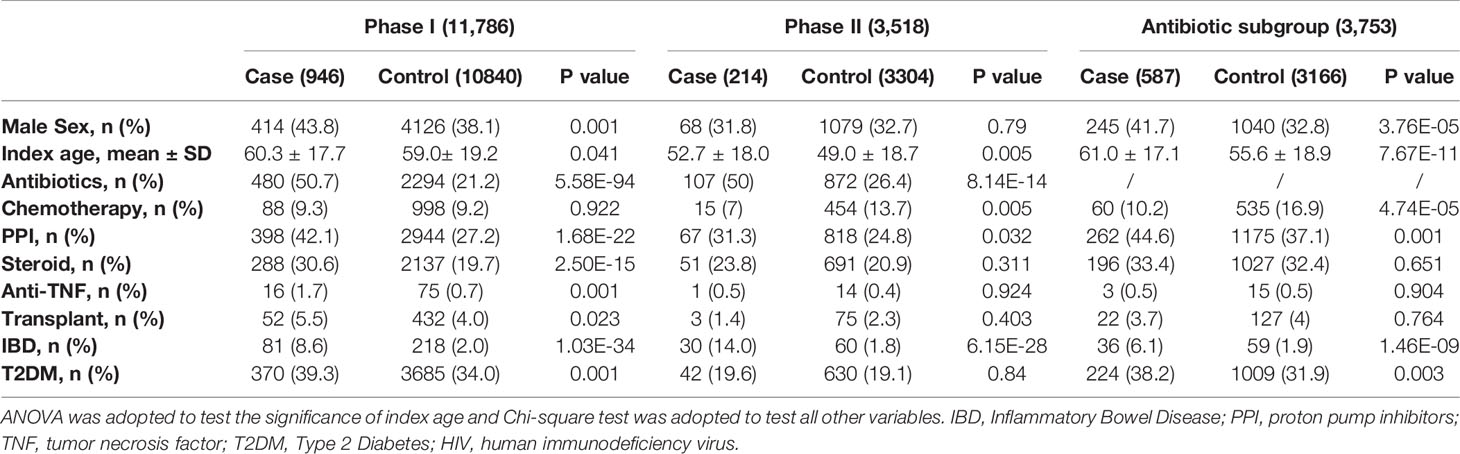
Table 1 Demographics and clinical characteristics of cohorts from phase I, phase II and antibiotics subgroup for CDI GWAS.
No variants from the meta-analyses of Phase I and II GWAS reached genome-wide significance (p<5 x10-8). The Manhattan and QQ plots for Phase I, Phase II GWAS, and the meta-analyses are displayed in Supplementary Figure 2. The top SNVs with p < 5×10-6 in the meta-analyses are listed in Supplementary Table 1. We queried the Open Targets Genetics and GTEx Portal to evaluate the potential functional impact of these top variants. The variants rs115062572 (OR = 1.74; 95%CI = 1.41-2.08; p=3.508×10-6) and rs149917912 (OR = 1.67; 95%CI =1.37-2.00; p=4.017×10-6) showed evidence of functional impact on the neighboring genes. These two variants are located in the MHC region and are in partial linkage disequilibrium (LD) in the population of European ancestry (r2 = 0.594). The rs115062572 SNV is a significant eQTL for Neurogenic Locus Notch Homolog Protein 4 (NOTCH4) in the whole blood (p=1.4x10-8) and is a significant splicing quantitative trait loci (sQTL) for MHC Class I Polypeptide-Related Sequence A (MICA) in the small intestine (p=4.2x10-7) (Supplementary Figures 3A, B). The SNV rs149917912 is an eQTL for MIR6891 (p=6.3x10-12) and a sQTL for HLA-L in whole blood (p=8.1x10-18) (Supplementary Figures 3C, D).
A sensitivity GWAS was performed in a subset of patients with antibiotic treatment to determine whether any of the top associated variants could be enriched in this high-risk cohort. The Manhattan and QQ plot for the sensitivity GWAS are shown in Figure 2. Top associated SNVs (p<1×10-5) are listed in Table 2. The rs114751021 variant in the MHC region is the only genome-wide significant variant associated with CDI (OR = 2.42; 95%CI = 1.84-3.11; p=4.50×10-8). The rs114751021 variant is also a significant eQTL for NOTCH4 in whole blood (p=5.3x10-14), and a significant sQTL for MICA in adipose tissue (p=1.6x10-6) (Supplementary Figures 3E, F). This SNV is in partial LD with rs115062572 (r2 = 0.593) and rs149917912 (r2 = 0.642), both of which demonstrated more significant associations with larger effect-size in the antibiotic subgroup than in the meta-analyses (p = 1.4×10-6 and 1.0×10-6, respectively). A regional association plot of the rs114751021 shows the top-ranked SNVs in partial LD flank a gene-rich region (Supplementary Figure 4). Three other variants showing independent suggestive association at the MHC region are identified after clumping (rs146508039, rs115062572, rs4294047; Table 2). The variants rs115062572 (OR=2.14, p=3.0×10-14) and rs149917912 (OR=1.53, p=2.0×10-13) were significantly associated with IBD or ulcerative colitis according to the UK Biobank (UKB) GWAS results (UKB Neale v2). We further examined the interaction of these SNVs and IBD but did not find significant interactions for these two SNVs in the antibiotic subgroup (p = 0.45 and 0.90, respectively).
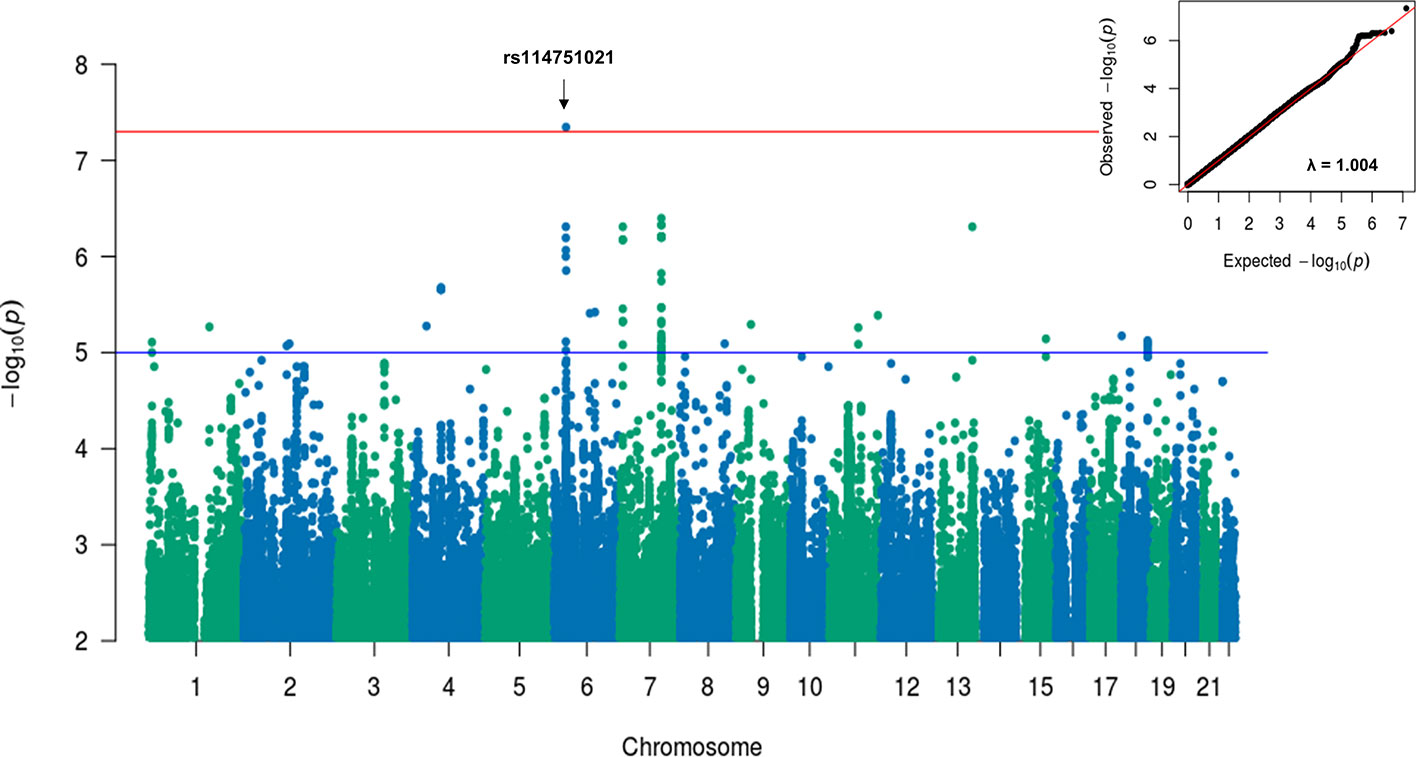
Figure 2 Manhattan and QQ plots for GWAS results associated with CDI in antibiotic treated patients with European ancestry. A linear mixed model regression adjusted for the covariates including sex, cohort, PPI, chemotherapy, T2DM, and Index Age were conducted by BOLT-LMM. Top variants with original p value < 5×10-8 were labeled. The genomic inflation factor, λGC, equals to 1.004, suggesting no evidence for systematic inflation of genome-wide test statistics.
Previously reported CDI-associated SNVS were reviewed. Associations after Bonferroni correction in our study (p<4.5x10-3, Supplementary Table 2) could not be replicated. The variants rs4073 and rs2227306 in IL8 showed a nominal significant association in the Phase I cohort (p=0.036 and 0.017), but not in the Phase II cohort (p=0.94 and 0.93 with opposite direction) or the meta-analysis, and the effect size was very small (beta < 0.01). Further, rs2227306 also showed a nominal significant association in the antibiotic subgroup (p=0.026). Another IBD-associated SNV, rs17085007, showed a nominally significant association after meta-analyses, and a small effect size (p=0.039, beta=0.008).
To identify potential causal variant and target genes, colocalization analyses of the four top loci at the MHC region with eQTL data were performed. The variants for each lead SNV that have CLPP >0.01 are listed in Supplementary Table 3. The lead SNV is the only variant in each locus that has GWAS p <1x10-5 and CLPP >0.01. The target genes and the relevant tissues for each locus are visualized in Figure 3A. The genes TCF19, MICA, ENSG00000272501, and CYP21A1P are potential target genes for multiple loci in most tissue types. The gene NOTCH4 is the target gene of two lead SNVs in whole blood, with large posterior probability (CLPP= 0.99 and 0.98). The gene C4B is the target gene of rs115062572 in two GI-tissue types. Several HLA genes are also targeted by specific SNV in specific tissues.
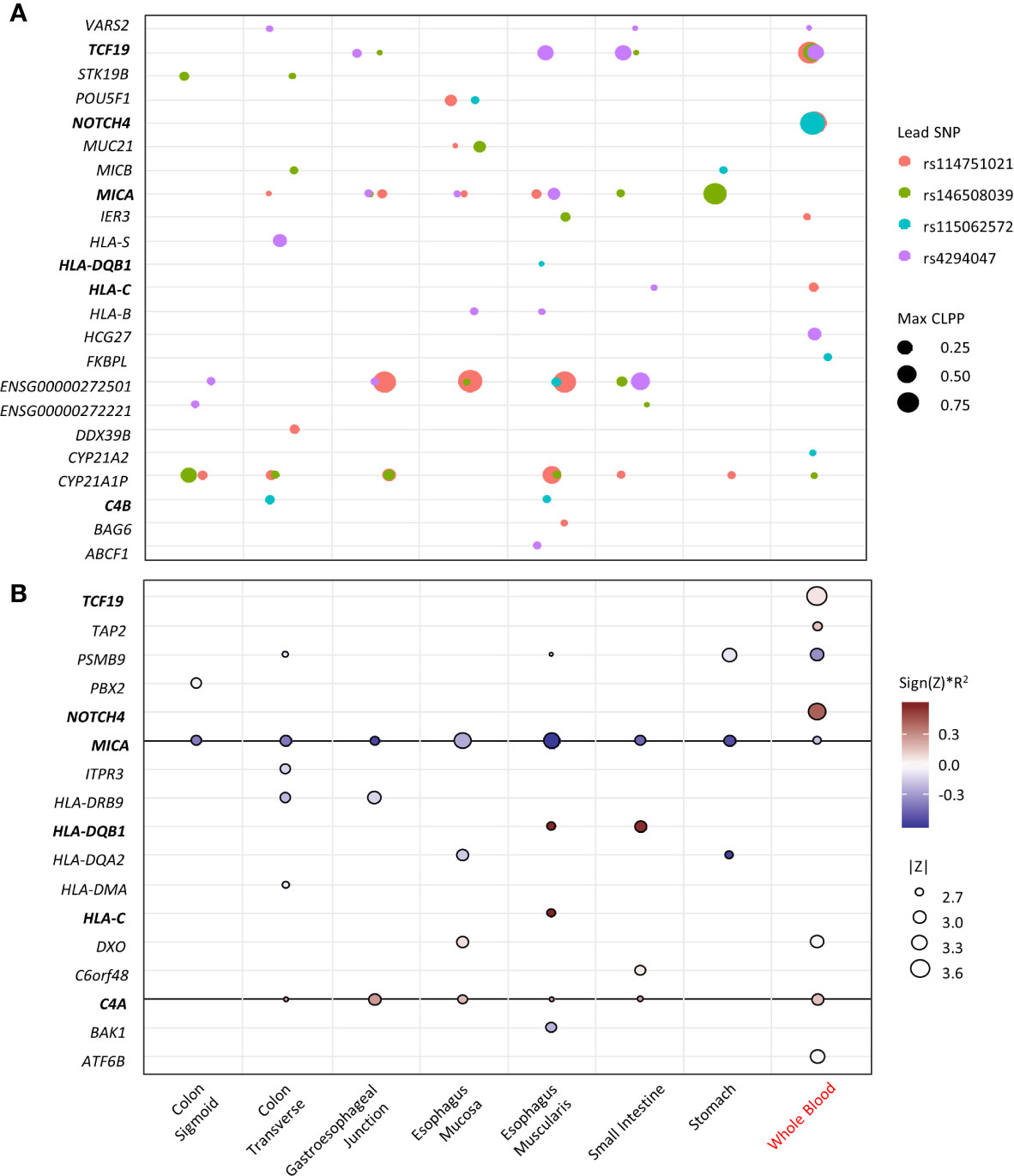
Figure 3 Fine-mapping of the top loci. X-axis represents the tissue types (black indicates GI-tissue). Y-axis represent the genes. Genes that appear in both eCAVIAR and MetaXcan are in blood. (A) Colocalization of the 4 lead SNVs at the MHC region. The dot size represents the maximum CLPP values in the corresponding locus and tissues. The color represents different locus for each lead SNV. (B) MetaXcan associations of the MHC region with CDI in antibiotic subgroup. The size of the dots represents the significance of the association between predicted expression and the CDI in patients exposed to high-risk antibiotics. Red indicates positive correlation while blue negative. Darker color indicates larger genetic component and consequently more active regulation in the tissue (R2 is a model performance measure computed as the correlation squared between observed and predicted expression, cross validated in the training set). Only associations with p<0.01 were shown.
The transcriptome imputation-based gene-level association for CDI in the antibiotic subgroup in multiple GI-tract tissues and whole blood was performed. Genes with a nominal significance level (p<0.05) are summarized in Supplementary Table 4. Genes with p<0.01 are visualized in Figure 3B. Expression in eight genes from whole blood are associated with CDI, of which NOTCH4 showed the greatest association. The expression of MICA and C4A in multiple GI-tract tissues and whole blood are significantly associated with CDI. Expression of HLA-DRB9, -DQB1, -DQA2, -DMA, and -C in some GI-tissues, but not blood is associated with CDI. MICA, HLA-DQB1, -DQA2.
In this study, we applied the eMERGE CDI algorithm to identify CDI cases and controls from a de-identified EHR database and performed GWAS followed by fine-mapping analyses. Although no genome-wide significant associations were identified after meta-analysis, the variants at the MHC region were prioritized with evidence of functional impact on neighborhood genes. The sensitivity GWAS in a subgroup analysis of high-risk patients with antibiotic use prior to CDI showed enhanced associations at the MHC region with the rs114751021 variant reaching genome-wide significance (p=4.50×10-8, OR = 2.42[1.84-3.11]). Fine-mapping of the top four lead SNVs at the MHC region by colocalization analyses and transcriptional imputation association test identified genes with potential functional significance in CDI, including MICA, C4A, C4B, and NOTCH4, in the GI-tissues and whole blood.
The symptomatic severity of CDI is the result of complex interactions among C. difficile, gut microbiota, and host factors. Host risk factors, such as older age, certain comorbidities, and exposure to antibiotics, were observed in this study (Table 1). The host immune response is another key determinant of CDI severity. The early immune response to CDI is characterized by the recruitment of neutrophils that eliminate the bacteria through phagocytosis. In addition, they secrete cytokines including IL-1β which contribute to the amplification of the inflammatory response (8). Our findings in this study may shed light on the role of the immune response during CDI. The variants at the MHC region identified in the meta-analyses showed a stronger association with larger effect size in the antibiotic subgroup. Through fine-mapping, we identified four potential target genes: MICA, C4A, C4B, and NOTCH4.
The variant MICA was identified in most of the GI-tissue types (Figure 3). It encodes the highly polymorphic major histocompatibility complex class I chain-related protein A, which serves as one of the ligands for NKG2D, an activating receptor constitutively expressed on natural killer (NK) cells, γδ T cells and other types of killer T cells (34). As with all other NKG2D ligands, MICA expression in epithelial cells is induced by stress signals caused by infection (34). For example, Escherichia coli increases MICA protein expression levels on the surface of intestinal epithelial cells by stimulating CD55 (35). In our study, a negative association of CDI risk and the MICA expression level in all the GI-tissues and blood (Figure 3B), was observed, indicating the induction of MICA expression could play a critical role in the response to C. difficile infection. Failure or altered expression of MICA could impose a higher risk for CDI.
We speculate that the recognition of C. difficile by epithelial cells induces MICA expression, leading to MICA activation of the NKG2D on NK cells and γδ T cells in the epithelium, triggering cell-mediated cytolysis in the presence of proinflammatory cytokines. Studies have found pathogens especially viruses, have evolved mechanisms to inhibit the expression of MICA on the infected host cells through masking, internalization, or retention to escape the attack from the host immune system (36). Reduction of MICA expression could be an important cause or contributing factor for CDI and restoration of MICA expression in the gut could be a treatment strategy for infection. However, whether C. difficile possesses a similar mechanism to that of viruses remained to be determined.
The genes C4A and C4B encode for complement factor C4, a protein belonging to the complement system, which plays an important role in anti-bacterial response (37). Specifically, C4 is cleaved into a small fragment C4a and a large fragment C4b, the latter is a key component together with C2a of C3 convertase (C4b2a) (37). Though the gram-positive cell wall of C. difficile, composed of a thick peptidoglycan layer that may be resistant to direct bacterial killing through the formation of a membrane attack complex (MAC), it can be opsonized to attract phagocytes and NK cells (38). The complement fragments C4b, C3b, and C1q are important and serve as opsonins. Once adhered to pathogens, they can be recognized by the complement receptor 1 which is expressed on all phagocytes to activate phagocytosis (38). Inmouse models of CDI, C4b is also important to activate C3, which participates in the elimination of translocated bacteria following C. difficile disruption of the colonic epithelium (39). These findings highlight the important role of C4 in the severity and outcome of CDI.
The NOTCH4 gene encodes for NOTCH4, a member of the Notch receptor family. Studies of NOTCH4 in infections are very limited. A study showed that Notch4-deficient mice and mice treated with Notch inhibitors were more resistant to Mycobacterium tuberculosis infection (40). Notch4 inhibited M. tuberculosis–triggered production of proinflammatory cytokines in macrophages through the inhibition of phosphorylation and ubiquitination of TAK1 (40). This is consistent with our finding that upregulated NOTCH4 is associated with a higher risk for CDI, indicating Notch inhibitors could be potential treatments for CDI.
Epithelial cells produce pro-inflammatory chemokines such as IL8, CXCL1, and CCL2, upon stimulation with C. difficile toxins (8). Previous studies have found that variants in IL8 were associated with CDI, recurrent CDI, or severe CDI using a dominant or a genotypic genetic model (15, 16, 41). However, the sample sizes in previous studies were too small (38, 23, and 18 cases) to achieve sufficient power to detect any associations with CDI. We did not replicate any of these associations in our study (Supplementary Table 2).
Although our study represents the largest GWAS for CDI to date, it has several limitations. The C. difficile strain information, which can be associated with virulence, was not available for most of the cohort, since presumptive strain type for the NAP-1 strain was only available after mid-April, 2014. Disruption of the gut microbiota was difficult to quantify, even though we included antibiotics and PPIs use–confounders that can interfere with the balance of gut microbiota. Disruption of gut microbiota was not routinely reported for most of the cohort, but as the EHR now contains information on dysbiosis, the future assessment is possible. In addition, detailed information on the antibiotics used was not available in the dataset used, so analysis to further stratify subgroups based on antibiotic exposure was not able to be performed. The use of different antibiotics could have major effects on CDI patients such as the gut microbiota and risk of recurrence. This will be included in the next phase of analysis. A demographic limitation is that the patients in our study are of predominantly European descent and from a single healthcare system. Lastly, replication in a second cohort to validate our findings was not possible as other publicly available cohorts do not have all the data elements needed for the analyses, even though a generalizable phenotyping algorithm was used.
In conclusion, while no genetic variants were identified that met genome-wide significance, several variants within the MHC region had suggestive associations coupled with mechanistic plausibility based on current knowledge of CDI pathogenesis. Fine-mapping identified the genes MICA, C4A/C4B, and NOTCH4 to be of potential interest for future studies based on associated evidence of their relevance to the host response to CDI. These results need to be validated in future studies in independent and more diverse cohorts and potential mechanisms require exploration using complementary and orthogonal investigations.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
ML, VA, JL, and YZ designed the study. YZ and JL performed all analyses and drafted the manuscript. VA and ML supervised the project. DW provided critical input on laboratory data elements and laboratory data validation. AK extracted and de-identified the clinical data from electronic health records. All authors provided critical feedback on the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Regeneron Genetic Center which provided funds for MyCode consenting, sample collection, genotyping, and genetic data processing. The internal fund to MTML from Geisinger and the external fund from the Defense Threat Reduction Agency to JB-R, RH, and VA (HDTRA1-18-1-0008) supported the data extraction and analyses. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to extend our thanks to Dr. Alison M Brodginski, for valuable discussion on clinical data. Thanks to Drs Raquel M. Martinez, Jordan Olson, as well as laboratory scientists Steve Kreis, and Barb J. Heiter who helped guide the data validation process based on historical laboratory testing. The authors would like to express their sincere gratitude to the participants and staff of MyCode.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.638913/full#supplementary-material
1. Guh AY, Mu Y, Winston LG, Johnston H, Olson D, Farley MM, et al. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N Engl J Med (2020) 382(14):1320–30. doi: 10.1056/NEJMoa1910215
2. Solomon K, Martin AJ, O’Donoghue C, Chen X, Fenelon L, Fanning S, et al. Mortality in patients with Clostridium difficile infection correlates with host pro-inflammatory and humoral immune responses. J Med Microbiol (2013) 62(Pt 9):1453–60. doi: 10.1099/jmm.0.058479-0
3. Cookson B. Hypervirulent strains of Clostridium difficile. Postgrad Med J (2007) 83(979):291–5. doi: 10.1136/pgmj.2006.056143
4. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med (2015) 372(16):1539–48. doi: 10.1056/NEJMra1403772
5. Johanesen PA, Mackin KE, Hutton ML, Awad MM, Larcombe S, Amy JM, et al. Disruption of the Gut Microbiome: Clostridium difficile Infection and the Threat of Antibiotic Resistance. Genes (Basel) (2015) 6(4):1347–60. doi: 10.3390/genes6041347
6. Jensen GL, Bross JE, Bourbeau PP, Naumovitz DW, Streater M, Gianferante LE. Risk factors for Clostridium difficile stool cytotoxin b among critically ill patients: role of sucralfate. J Infect Dis (1994) 170(1):227–30. doi: 10.1093/infdis/170.1.227
7. Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DD, Hernandez AV, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol (2015) 36(4):452–60. doi: 10.1017/ice.2014.88
8. Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol (2016) 14(10):609–20. doi: 10.1038/nrmicro.2016.108
9. Rupnik M. Heterogeneity of large clostridial toxins: importance of Clostridium difficile toxinotypes. FEMS Microbiol Rev (2008) 32(3):541–55. doi: 10.1111/j.1574-6976.2008.00110.x
10. Pepin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis (2005) 41(9):1254–60. doi: 10.1086/496986
11. He M, Miyajima F, Roberts P, Ellison L, Pickard DJ, Martin MJ, et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet (2013) 45(1):109–13. doi: 10.1038/ng.2478
12. Dial S, Kezouh A, Dascal A, Barkun A, Suissa S. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ (2008) 179(8):767–72. doi: 10.1503/cmaj.071812
13. Eyre DW, Cule ML, Wilson DJ, Griffiths D, Vaughan A, O’Connor L, et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med (2013) 369(13):1195–205. doi: 10.1056/NEJMoa1216064
14. Kumar N, Browne HP, Viciani E, Forster SC, Clare S, Harcourt K, et al. Adaptation of host transmission cycle during Clostridium difficile speciation. Nat Genet (2019) 51(9):1315–20. doi: 10.1038/s41588-019-0478-8
15. Jiang ZD, DuPont HL, Garey K, Price M, Graham G, Okhuysen P, et al. A common polymorphism in the interleukin 8 gene promoter is associated with Clostridium difficile diarrhea. Am J Gastroenterol (2006) 101(5):1112–6. doi: 10.1111/j.1572-0241.2006.00482.x
16. Garey KW, Jiang ZD, Ghantoji S, Tam VH, Arora V, Dupont HL. A common polymorphism in the interleukin-8 gene promoter is associated with an increased risk for recurrent Clostridium difficile infection. Clin Infect Dis (2010) 51(12):1406–10. doi: 10.1086/657398
17. Miyajima F, Swale A, Zhang JE, Alfirevic A, Little M, Beeching NJ, et al. Is the interleukin 8 promoter polymorphism rs4073/-251T >A associated with Clostridium difficile infection? Clin Infect Dis (2014) 58(12):e148–51. doi: 10.1093/cid/ciu152
18. Ananthakrishnan AN, Oxford EC, Nguyen DD, Sauk J, Yajnik V, Xavier RJ. Genetic risk factors for Clostridium difficile infection in ulcerative colitis. Aliment Pharmacol Ther (2013) 38(5):522–30. doi: 10.1111/apt.12425
19. Connelly TM, Koltun WA, Sangster W, Berg AS, Hegarty JP, Harris L3, et al. An interleukin-4 polymorphism is associated with susceptibility to Clostridium difficile infection in patients with inflammatory bowel disease: results of a retrospective cohort study. Surgery (2014) 156(4):769–74. doi: 10.1016/j.surg.2014.06.067
20. Apewokin S, Lee JY, Goodwin JA, McKelvey KD, Stephens OW, Zhou D, et al. Host genetic susceptibility to Clostridium difficile infections in patients undergoing autologous stem cell transplantation: a genome-wide association study. Support Care Cancer (2018) 26(9):3127–34. doi: 10.1007/s00520-018-4173-6
21. Dewey FE, Murray MF, Overton JD, Habegger L, Leader JB, Fetterolf SN, et al. Distribution and clinical impact of functional variants in 50,726 whole-exome sequences from the DiscovEHR study. Science (2016) 354(6319). doi: 10.1126/science.aaf6814
22. Carey DJ, Fetterolf SN, Davis FD, Faucett WA, Kirchner HL, Mirshahi U, et al. The Geisinger MyCode community health initiative: an electronic health record-linked biobank for precision medicine research. Genet Med (2016) 18(9):906–13. doi: 10.1038/gim.2015.187
23. Abul-Husn NS, Manickam K, Jones LK, Wright EA, Hartzel DN, Gonzaga-Jauregui C, et al. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science (2016) 354(6319). doi: 10.1126/science.aaf7000
24. Zhang Y, Poler SM, Li J, Abedi V, Pendergrass SA, Williams MS, et al. Dissecting genetic factors affecting phenylephrine infusion rates during anesthesia: a genome-wide association study employing EHR data. BMC Med (2019) 17(1):168. doi: 10.1186/s12916-019-1405-7
25. Loh PR, Tucker G, Bulik-Sullivan BK, Vilhjalmsson BJ, Finucane HK, Salem RM, et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet (2015) 47(3):284–90. doi: 10.1038/ng.3190
26. Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics (2010) 26(17):2190–1. doi: 10.1093/bioinformatics/btq340
27. Zhou W, Nielsen JB, Fritsche LG, Dey R, Gabrielsen ME, Wolford BN, et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet (2018) 50(9):1335–41. doi: 10.1038/s41588-018-0184-y
28. Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat Genet (2012) 44(7):821–4. doi: 10.1038/ng.2310
29. Linear (mixed) model effects to odds ratios. Available at: http://cnsgenomics.com/shiny/LMOR/data/shiny_lmor_func.R.
30. Spain SL, Barrett JC. Strategies for fine-mapping complex traits. Hum Mol Genet (2015) 24(R1):R111–9. doi: 10.1093/hmg/ddv260
31. Hormozdiari F, van de Bunt M, Segre AV, Li X, Joo JWJ, Bilow M, et al. Colocalization of GWAS and eQTL Signals Detects Target Genes. Am J Hum Genet (2016) 99(6):1245–60. doi: 10.1016/j.ajhg.2016.10.003
32. GTExPortal. Available at: https://gtexportal.org/home/.
33. Barbeira AN, Dickinson SP, Bonazzola R, Zheng J, Wheeler HE, Torres JM, et al. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nat Commun (2018) 9(1):1825. doi: 10.1038/s41467-018-03621-1
34. Lanier LL. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol Res (2015) 3(6):575–82. doi: 10.1158/2326-6066.CIR-15-0098
35. Tieng V, Le Bouguenec C, du Merle L, Bertheau P, Desreumaux P, Janin A, et al. Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proc Natl Acad Sci USA (2002) 99(5):2977–82. doi: 10.1073/pnas.032668099
36. Schepis D, D’Amato M, Studahl M, Bergstrom T, Karre K, Berg L. Herpes simplex virus infection downmodulates NKG2D ligand expression. Scand J Immunol (2009) 69(5):429–36. doi: 10.1111/j.1365-3083.2009.02241.x
37. Heesterbeek DAC, Angelier ML, Harrison RA, Rooijakkers SHM. Complement and Bacterial Infections: From Molecular Mechanisms to Therapeutic Applications. J Innate Immun (2018) 10(5-6):455–64. doi: 10.1159/000491439
38. Sarma JV, Ward PA. The complement system. Cell Tissue Res (2011) 343(1):227–35. doi: 10.1007/s00441-010-1034-0
39. Hasegawa M, Yada S, Liu MZ, Kamada N, Munoz-Planillo R, Do N, et al. Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity (2014) 41(4):620–32. doi: 10.1016/j.immuni.2014.09.010
40. Zheng R, Liu H, Zhou Y, Yan D, Chen J, Ma D, et al. Notch4 Negatively Regulates the Inflammatory Response to Mycobacterium tuberculosis Infection by Inhibiting TAK1 Activation. J Infect Dis (2018) 218(2):312–23. doi: 10.1093/infdis/jix636
Keywords: Clostridioides difficile, MICA, C4a, NOTCH4, GWAS
Citation: Li J, Zhang Y, Jilg AL, Wolk DM, Khara HS, Kolinovsky A, Rolston DDK, Hontecillas R, Bassaganya-Riera J, Williams MS, Abedi V and Lee MTM (2021) Variants at the MHC Region Associate With Susceptibility to Clostridioides difficile Infection: A Genome-Wide Association Study Using Comprehensive Electronic Health Records. Front. Immunol. 12:638913. doi: 10.3389/fimmu.2021.638913
Received: 07 December 2020; Accepted: 09 March 2021;
Published: 25 March 2021.
Edited by:
Gee W. Lau, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Sharvan Sehrawat, Indian Institute of Science Education and Research Mohali, IndiaCopyright © 2021 Li, Zhang, Jilg, Wolk, Khara, Kolinovsky, Rolston, Hontecillas, Bassaganya-Riera, Williams, Abedi and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Ta Michael Lee, bWxlZTJAZ2Vpc2luZ2VyLmVkdQ==;Vida Abedi, dmFiZWRpQGdlaXNpbmdlci5lZHU=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.