- 1College of Stomatology, Chongqing Medical University, Chongqing, China
- 2Chongqing Key Laboratory of Oral Diseases and Biomedical Sciences, Chongqing, China
- 3Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education, Chongqing, China
- 4The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine Ministry of Education, School & Hospital of Stomatology, Wuhan University, Wuhan, China
- 5Department of Periodontology, School & Hospital of Stomatology, Wuhan University, Wuhan, China
Integrins refer to heterodimers consisting of subunits α and β. They serve as receptors on cell membranes and interact with extracellular ligands to mediate intracellular molecular signals. One of the least-studied members of the integrin family is integrin-α9β1, which is widely distributed in various human tissues and organs. Integrin-α9β1 regulates the physiological state of cells through a variety of complex signaling pathways to participate in the specific pathological processes of some intractable diseases. In recent years, an increasing amount of research has focused on the role of α9β1 in the molecular mechanisms of different refractory diseases and its promising potential as a therapeutic target. Accordingly, this review introduces and summarizes recent research related to integrin-α9β1, describes the synergistic functions of α9β1 and its corresponding ligands in cancer, autoimmune diseases, nerve injury and thrombosis and, more importantly, highlights the potential of α9β1 as a distinctive target for the treatment of these intractable diseases.
Introduction
Integrins are specific transmembrane proteins that function as receptors on the surface of cell membranes. The heterodimers of integrin members formed by noncovalent bonds of α-subunits and β-subunits in response to corresponding ligands in the extracellular matrix mediate intracellular mechanical and chemical signals (1). So far, 24 distinct αβ receptor complexes composed of 18 α-subunits and 8 β-subunits have been found, which play regulatory roles in different developmental and physiological processes (2, 3). However, within the integrin family, there has been relatively little research on α9β1.
Integrin-α9 (ITGA9) was found in guinea pig airway epithelial cells in 1991, when the polymerase chain reaction (PCR) technique showed a new α-subunit with a novel sequence compared to previously reported integrin subunits (4). In 1993, the human amino acid sequence and cDNA of the new subunit was determined and definitively designated as α9 (5). The α9-subunit specifically groups together with the β1-subunit, combining to form the unique heterodimer integrin-α9β1, which acts as an indispensable receptor for cellular signal responses (6).
In the last few decades, more and more research has focused on the roles of integrin-α9β1 in periods of growth, development and disease. More importantly, integrin-α9β1 has been reported as a new therapeutic target for some specific refractory diseases.
In this review, we focus on recent advances in research on integrin-α9β1. We will discuss its individual potential clinical value for the treatment of tumors, rheumatoid arthritis (RA), axon damage and thrombosis.
Structure and Function of Integrin-α9β1
The ITGA9 gene is distributed on the human chromosome 3p21.3-22.2 segment, encodes the polypeptides of 1035 amino acids, and has a size of 114.5 KD (7). The structure of α9 consists of three parts: a large N-terminal extracellular domain, a transmembrane segment and a short C-terminal cytoplasmic tail (8). Among this structure, the N-terminal portion mediates ligand binding (9), while the cytoplasmic domain specifically binds to intracellular proteins that modulate the physiological activity of cells, such as spermidine/spermine N1-acetyltransferase, which has been proven to regulate inward rectification of the inward-rectifier K+ channel to enhance cell migration with interaction of the α9 cytoplasmic domain (10).
According to their homology, α-subunits can be divided into three families. One includes subunits with a characteristic disulfide-linked cleavage site and forms heterodimers that recognize Arg-Gly-Asp (RGD)-containing ligands. Another includes subunits that contain an inserted domain close to the N-terminus but no cleavage site, which generally do not recognize RGD-containing ligands. However, α9 and α4 can form a special third subfamily that contains neither the insertion domain nor the disulfide-linked cleavage site and does not recognize the classic integrin-binding motif RGD (11). The subfamily is one of the newest and most specific integrin families from an evolutionary perspective and is only expressed in vertebrates (12). Integrin-α9 used to be known as ITGA4L (integrin-α4-like) since α9 and α4 show peptide sequence similarities (39% amino acid identity) and share several common ligands (13, 14). However, knockout of α9 and α4 results in different phenotypes in mice: α4-knockout mice die by 11-14 embryonic days due to improper chorioallantoic fusion, cardiac hemorrhage (15), and defects in all hematopoietic lineages in the fetal liver, bone marrow, and spleen (16); while α9-knockout mice die within 12 days of birth with bilateral chylothorax (17), impaired development of neutrophils (18), and defective development of lymphatic valves and venous valves (19). This indicates that α9 and α4 exert distinct as well as similar physiological functions in vivo. In addition, it is intriguing that the integrin family extensively regulates cellular directional migration in an electric field (galvanotaxis), which is necessary for precise control of wound healing, angiogenesis, immune responses and organismal development. In this potentially endogenous guidance mechanism, cells expressing α9 migrate to an anode in an electric field, whereas cells expressing α4 migrate in random directions in an electric field (20), demonstrated that integrin-α9 and α4 may differentially contribute to cell migration in vivo.
In mouse tissue, immunohistochemistry has shown that integrin-α9β1 is located in the epithelia and muscle of the trachea, digestive system, skin, veins, liver and spleen, but not in the aorta, pancreas or heart (5). Integrin-α9β1 plays an important role in cell adhesion and migration, but more and more research is showing that it has roles far beyond that. It binds to a diversity of ligands in the extracellular matrix, like the a-disintegrin and metalloprotease (ADAM) family, elastic microfibril interface-located protein1 (EMILIN1), vascular endothelial growth factor (VEGF), the extra domain A (EDA) of fibronectin, tenascin-C (TNC), osteopontin (OPN), vascular cell adhesion molecule-1 (VCAM-1) and C-motif-ligand-1 (XCL1)/lymphotactin (21–25). The interaction between these proteins and integrin-α9β1 is vital for organismal growth and development and cellular physiological activities (Table 1). Recent studies have focused on the role of α9β1 in pathological processes and its potential as a therapeutic target. And great progress has been made towards certain specific refractory diseases, including various malignant tumors, autoimmune diseases, nerve damage and thrombotic diseases. Here, we will describe in detail how integrin-α9β1 plays a pivotal role in these diseases and, more importantly, makes a promising target for clinical treatment.
Integrin-α9β1 and Corresponding Ligands Have Potential as Tumor Therapeutic Targets
Integrin-α9 is considered to be closely related to the growth, metastasis and tissue invasion of cancer. The tumor microenvironment provides a large number of ligands in the extracellular matrix, leading to complex and diverse reactions with integrins (62). The expression of α9β1 is up-regulated in many cancers and affects tumor progression through a variety of mechanisms, including regulation of cell migration and invasion, mediation of cell cycle regulatory elements, promotion of the growth of tumor-associated blood and lymphatic vessels, and alternation of the epithelial-mesenchymal transition [EMT; (23, 35, 63, 64)]. Hence, there have been successive reports on the performance of α9β1 in cooperating with specific ligands in different cancers, and on the experimental effects of ITGA9-targeted therapeutic agents (Table 2).
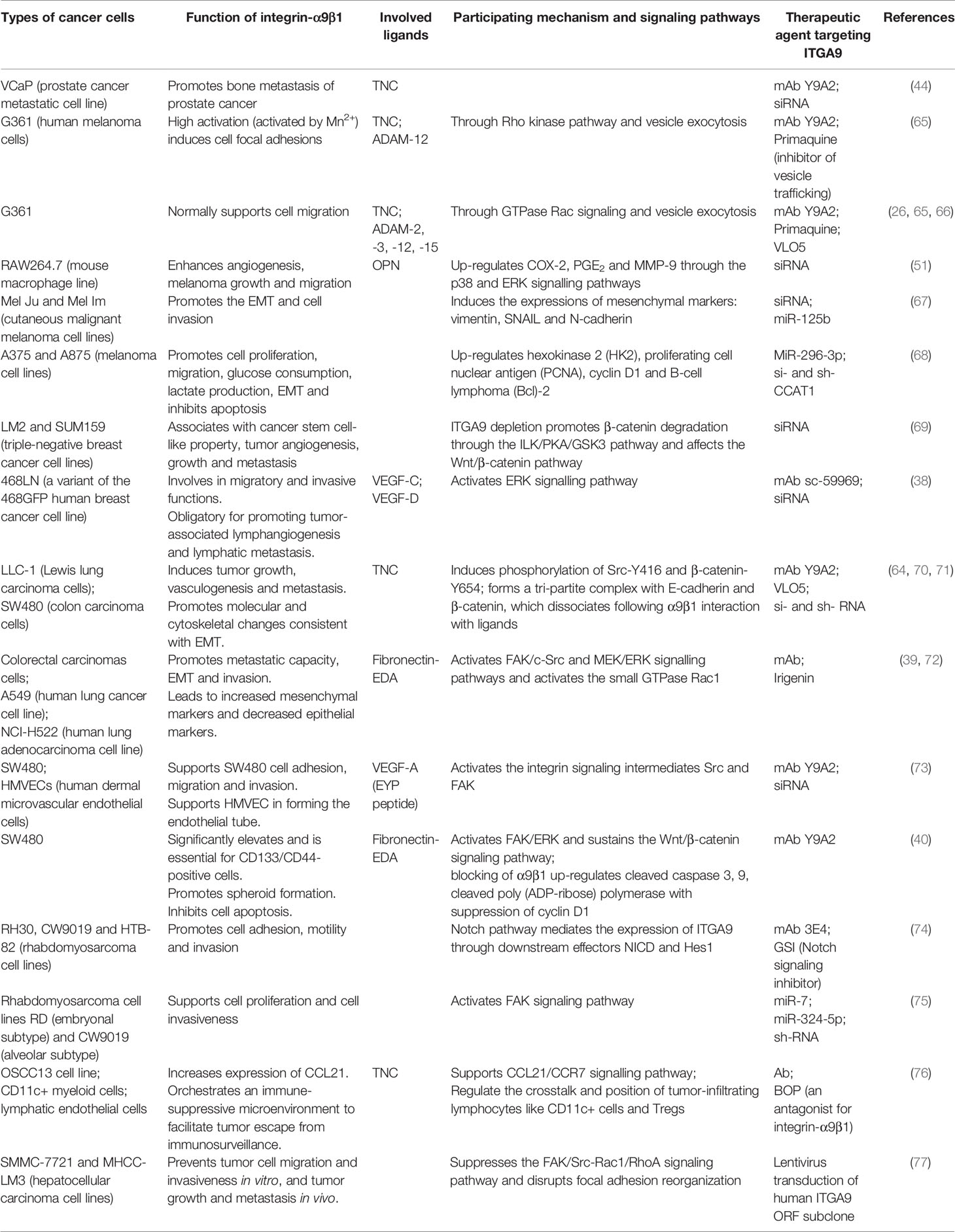
Table 2 Overview of experimental trials with therapeutic agents targeting ITGA9 in different cancers.
Prostate Cancer
The NotI-microarray analysis has identified ITGA9 as a potential biomarker for the detection and discrimination of prostate tumors with different aggressiveness and malignancy (78). In a study of prostate cancer, a human bone metastasis tissue array consisting of 63 metastasis samples was analyzed. The cells showed immunoreactivity to ITGA9 in 74% of the cancer foci associated with TNC, with the latter being an important ligand of integrin-α9β1 (44). In prostate cancer, the deposition of TNC during early cancer progression is a key marker of stromal microenvironment alternation, which is overexpressed in the endosteum when normal processes of cellular senescence and death lead to microfracture repair (79, 80). Then, bone metastatic cells interact with TNC in the endometrium through the integrin-α9β1 to accelerate the spread, whereas the activity is eliminated by small interfering RNA (siRNA) or neutralizing antibodies of α9 (44). Besides, the reaction site where human TNC binds to α9β1 is located in the isoleucine-aspartic acid-glycine motif within the third fibronectin type III repeat, which is absent in mice, which may explain why cancer rarely metastasizes to the bone in murine models, while both integrin-α9β1 and TNC are thought to have great significance in metastatic prostate cancer (81, 82).
Melanoma
Melanoma has high aggressiveness and metastasis, which has been defined as lethal melanocytic neoplasm (83). Integrin-α9β1 is up-regulated in melanoma tissue and cells, and different active states produce different effects. Under normal circumstances, the intermediate activity state of α9β1 supports cell migration through interaction with TNC and ADAM12, which are regulated by guanosine triphosphatase (GTP)-Rac signaling. However, manganese ions stimulate highly-active transformation with a protein conformation change of α9β1, which then leads to cell focal adhesion accompanied by morphological change that is dependent on the Rho kinase pathway [Figure 1A; (65, 84)]. It has been reported that focal adhesion in tumor cell is closely associated with resistance to radio- and chemotherapy. Considering that melanoma has much higher manganese levels than other cancers, this mechanism may explain the extreme radio- and chemo-resistant nature of melanoma and its low patient survival (85, 86). Another study reported that integrin-α9β1 binds to the OPN-activating p38- and ERK-signaling pathways and activator protein (AP)-1. This ultimately leads to expression of cyclooxygenase-2 (COX-2) and accompanying secretion of prostaglandin E2 (PGE2) and matrix metalloproteinase (MMP)-9 in tumor-associated macrophages (Figure 1B). These factors contribute to melanoma growth and angiogenesis (51).
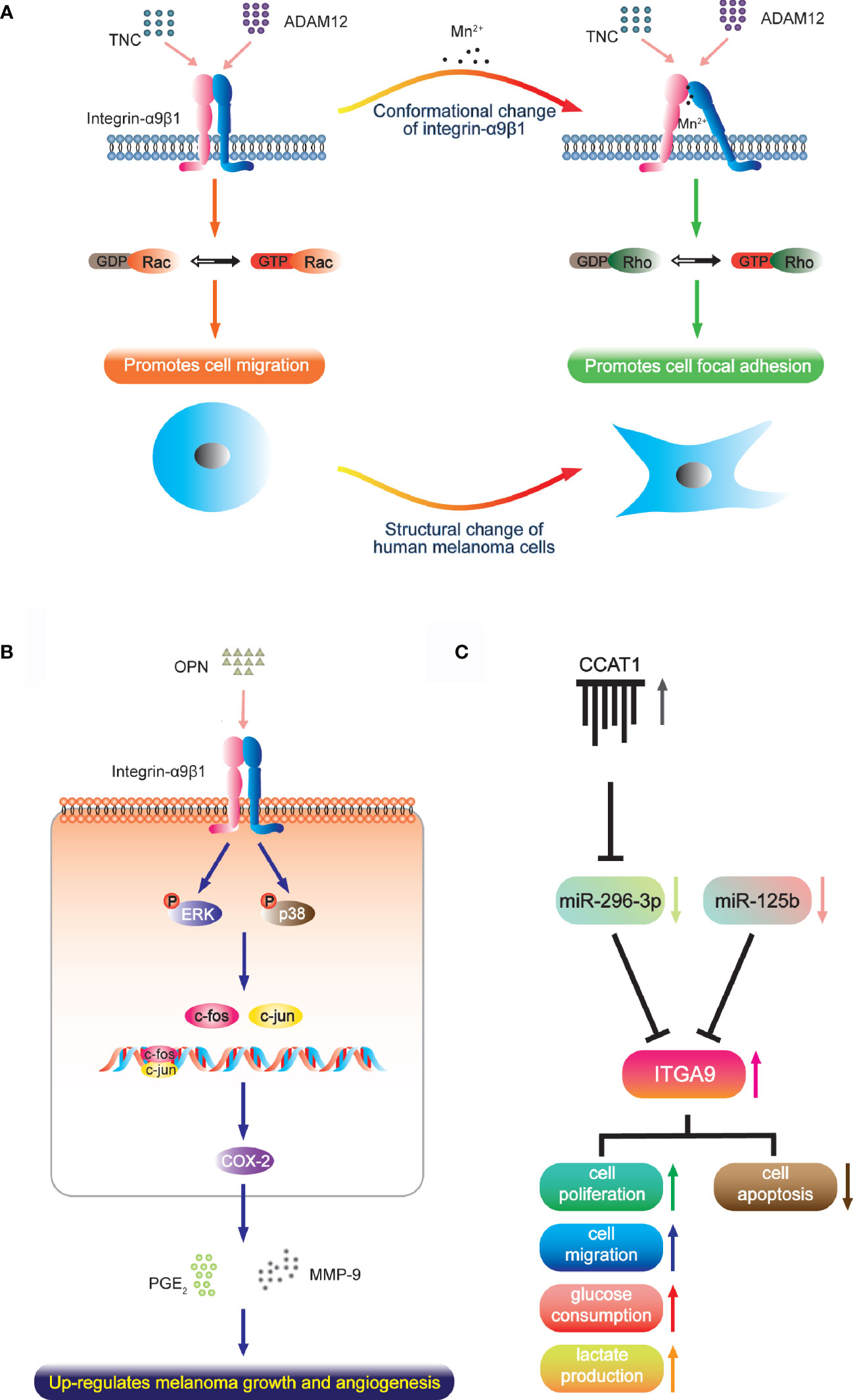
Figure 1 The function of integrin-α9β1 in melanoma. (A) Under normal conditions, the intermediate activity state of integrin-α9β1 supports cell migration via interaction with TNC and ADAM12. A high activation state (converted by manganese ions, which occur at much higher levels in melanoma than in other cancers) changes the integrin conformation and cell morphology, and induces and localizes to focal adhesions. (B) In tumour-associated macrophages, integrin-α9β1 binds to OPN, activating ERK- and p38-dependent AP-1, ultimately leading to enhanced expressions of COX-2, PGE2 and MMP-9, which contribute to melanoma growth and angiogenesis. (C) miR-296-3p (regulated by lncRNA CCAT1) and miR-125b directly target ITGA9 to mediate the cell physiology of melanoma.
The function-blocking anti-human α9 monoclonal antibody Y9A2 has the ability to inhibit adhesion of human melanoma cells. So does VLO5, a snake disintegrin that antagonizes α9β1 (26, 66). Furthermore, the regulation of endogenous integrin-α9β1 depends on cell vesicle exocytosis, and the use of primaquine (an effective inhibitor of vesicle trafficking) effectively attenuates melanoma cell attachment in a dose-dependent manner (65). These results reveal the effect of anti-α9 treatment. Moreover, ITGA9 has been reported to be the direct target of miR-125b and miR-296-3p. The expression of miR-125b is clearly decreased in primary melanoma, and even less so in the metastatic invasion phenotype, which is negatively correlated with the expression of ITGA9. Integrin-α9β1 advances cancer growth and metastasis by potentiating EMT (64) so that miR-125b shows the capacity to inhibit malignant melanoma cell invasion and metastasis by targeting ITGA9 in vitro and in vivo (67). Similarly, miR-296-3p is also downregulated in melanoma cells and tissue. It targets ITGA9 to inhibit glucose metabolism, lactic acid production, proliferation and migration of melanoma cells while inducing cell apoptosis. In addition, the long noncoding RNA (lncRNA) CCAT1 acts as a competing endogenous RNA that sponges miR-296-3p to up-regulate ITGA9 in vivo; thus, CCAT1 silencing inhibits melanoma cell growth (68). This field of research provides a new direction for the treatment of melanoma with micro-RNA and lncRNA that target ITGA9 either directly or indirectly (Figure 1C).
Breast Cancer
Integrin-α9 exists in normal human breast tissue, but the expression levels are heterogeneous in breast tumors (87). In a study of 38 human samples, the mRNA expression level was normal or increased in 45% of tumors, but decreased or absent in another 44% of samples, while 11% of samples showed that ITGA9 had a mutation or pathological alternative splicing (88). Hypermethylation of CpG-island is considered to be the critical mediation mechanism of ITGA9 inactivation of breast malignant tumors. In triple-negative breast cancer, the level of ITGA9 is drastically higher than in other subtypes of breast cancer, which is related to worse distant metastasis-free survival and recurrence-free survival rates. The nanoparticle-mediated delivery of ITGA9 siRNA into tumor cells has the capacity to sharply down-regulate angiogenesis, growth and metastasis by inducing β-catenin degradation via the integrin-linked kinase (ILK)/protein kinase A (PKA)/glycogen synthase kinase 3 (GSK3) pathway (69). The Wnt/β-catenin pathway is a crucial cascade involved in the development of cancer and is linked to decreased overall survival in breast cancer patients (89, 90). Knockdown of ITGA9 promotes β-catenin degradation, suggesting that integrin-α9 may interfere with the Wnt signaling pathway to influence the tumor microenvironment. On the other hand, integrin-α9β1 interacts with VEGF-C and -D (produced by cancer cells or by macrophages) to confer the functions of migration and invasiveness in human breast cancer cell line 468LN, which can be abrogated by antibody blocking or stable knockdown of integrin-α9. Similarly, the knockdown of ITGA9 abrogates primary tumor growth, angiogenesis, metastatic ability to the draining lymph node and intra-tumoral lymphangiogenesis in vivo in nude mice (38).
Lung Cancer
In human primary small cell lung cancer, the long-term survival rate of patients is significantly lower with higher expression of α9β1. Injection of LLC-1 lung carcinoma cells or SW480 colon carcinoma cells with over-expression of α9β1 both induce greater tumor growth and metastasis in mice (64). It is worth noting that EMT is considered to be very crucial in metastatic progression, which causes disruption to intercellular contacts and enhances cell motility to release cancer cells from the parent epithelial tissue (91). While α9β1 binding to TNC supports phenotypic and functional alterations to EMT, with increases in N-cadherin, α-SMA, vimentin and snail (mesenchymal markers), as well as a decrease in E-cadherin [epithelial marker; (64)]. The above process is accompanied by phosphorylation of β-catenin through the Src signaling pathway, which has been proved to be closely associated with EMT (70, 71). In addition, fibronectin-EDA also imparts the EMT phenotype through integrin-α9β1 (39), and irigenin (a novel lead from the Western Himalayan chemiome that can be isolated from the rhizomes of Belamcanda chinensis) has an anti-metastasis capacity by selectively blocking the α9β1 and α4β1 integrin binding sites with the C-C loop of EDA (92). In human lung cancer cell line A549 and lung adenocarcinoma cell line NCI-H522, irigenin conquers fibronectin-EDA-induced cell proliferation, migration and invasion with dose-dependent inhibition of EMT markers. Hence, irigenin may become a lead compound in the management of lung carcinoma (72).
Colon Cancer
ITGA9 antigen has been detected in the basolateral domain of colonic glandular epithelial cells at the fetal stage, but not in adults under normal circumstances. Comparatively, colon adenocarcinoma cells have the potential to express integrin-α9 with polarization features, thereby the α9 subunit may be conditional on oncofetal pattern expression in the human colonic epithelium (93). The peptide EYP of VEGF-A specifically binds to α9β1 and induces invasion of colorectal cancer cell line SW480 with phosphorylation of the integrin signaling intermediates, Src and focal adhesion kinase [FAK; (73)]. Additionally, endothelial cell-derived fibronectin-EDA binds to α9β1, which promotes colorectal cancer metastasis with EMT phenotypic conversion through activation of the FAK, ERK and Rac signaling pathways and supports endothelial tube formation (39). This research illustrates that cooperation between integrin-α9β1 and paracrine or autocrine extracellular matrix ligands is critical to the colon cancer microenvironment. Furthermore, long-term application of methotrexate, an important drug widely used in cancer therapy, carries the risk of resistance emergence (94). Simultaneously, ITGA9 is obviously up-regulated in already-developed drug-resistant colon cancer cells, whereas α9 is reported to be a promising target candidate for overcoming methotrexate resistance in colon cancer (95).
CD133+/CD44+ cancer cells, which have the properties of tumour progenitor cells, are critical in the tumorigenesis of colorectal cancer (96, 97). The expression level of integrin-α9β1 is greatly up-regulated in human colorectal cancer samples and is also increased in the CD133+/CD44+ subpopulation of SW480 cells compared with the CD133-/CD44- subpopulation. Fibronectin-EDA also increases in CD133+/CD44+ cells and activates the integrin-α9/FAK/ERK pathway to sustain Wnt/β-catenin signaling activity. The latter is critical in the development and progression of colon cancer (40). Blocking of fibronectin-EDA and α9β1 suppresses the clonogenicity and sphere-formation capacity of CD133+/CD44+ cells, and the monoclonal antibody (mAb) of integrin-α9β1 obviously promotes cell apoptosis by up-regulation of apoptotic protein markers, like caspase-3, caspase-9 and poly (ADP-ribose) polymerase, concomitant with suppressed expression of cyclin D1 (cell cycle progression-promoting protein) (40, 98). Hence, targeted methods based on integrin-α9β1 or fibronectin-EDA can serve as treatment modes for the inhibition of cancer progression and limitation of colon cancer progenitor cells.
Rhabdomyosarcoma
Rhabdomyosarcoma is an early onset malignancy, which is the most common type of soft tissue sarcoma in children. About 15% of rhabdomyosarcoma patients are diagnosed with distant metastasis (99, 100). Despite the prognosis of most patients with field cancerization being acceptable, the effectiveness of treatments for metastatic rhabdomyosarcoma is particularly poor and the long-term event-free survival rate is less than 20% (101). Integrin-α9β1 has been demonstrated as directly involved in the attachment, migration and invasiveness of rhabdomyosarcoma cells, and the specific anti-α9-blocking antibodies significantly decrease the invasive properties (74).
The Notch pathway plays an important role in the expressions of ITGA9 and N-cadherin, for which the downstream effectors Hes1 and NICD directly bind to their promoter regions. This mechanism may explain why the pharmacological Notch signaling inhibitor (GSI) impairs the adhesion of rhabdomyosarcoma cells. For the purpose of metastasis resists, Notch-inhibiting molecules serve as potential therapeutic agents (74). Moreover, after a screening process, a recent analysis selected miR-7 and miR-324-5p as the best candidates for regulating integrin-α9β1. Overexpression of both types of miRNA leads to genetic down-regulation of ITGA9 following sharp decreases in cell proliferation and invasion in vitro and in vivo, since integrin-α9β1 participates in cell proliferation and tumour growth in the two main rhabdomyosarcoma subtype cell lines RD and CW9019. In the murine model with intravenously injected tumour cells, miR-7 also induces significant impairment of rhabdomyosarcoma cell metastatic lung colonization (75). This raises the possibility of using ITGA9-mediating miRNA as a novel therapeutic tool to avoid rhabdomyosarcoma progression.
Squamous Cell Carcinoma (SCC)
In the healthy oral mucosa, integrin-α9β1 is mainly expressed at the basal and immediately suprabasal cell layers. However, in leukoplakic dysplasia, lichen planus and SCC samples, α9β1 is more diffusely expressed at the epithelial cell membranes (102), since both leucoplakia and lichen planus are considered to be potential oral malignant disorders with certain risks of malignant transformation (103). TNC is reported to be a promoter of pro-tumorigenic microenvironments as a classical ligand for integrin-α9β1, taking part in immune suppression in oral SCC (76). It works with α9β1 and TLR4 to activate the CCL21/CCR7 signaling pathway and induce the expression of CCL21, which is known to be a chemoattractant for various leukocytes and lymphoid tissue. This shifts the host’s immunogenic immune response to being a tolerogenic response and facilitates tumour progression and metastasis by evading immune surveillance (104). Either integrin-α9β1 or TNC antibodies reduce CCL21 mRNA and protein expression to improve hypoimmunity, suggesting that blocking integrin-α9β1 or TNC can alter the SCC tumour microenvironment (76).
Hepatic Fibrosis and Hepatocellular Carcinoma
Integrin-α9 chains can be detected in hepatocytes and fetal hepatoblasts, and the latter likely performing a key role in the differentiation of liver stem cells (105). Thrombin-cleaved OPN (which exhibits the α9-binding motif) interacts with α9β1 to promote activation, proliferation and migration of hepatic stellate cells via the mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) signalling pathway, which are essential for liver fibrogenesis (52). In addition, integrin-α9β1 is also important for activating the motility of hepatic stellate cells through cooperation with ligand fibronectin-EDA (106). The sustained exacerbation of liver fibrosis predisposes certain individuals to cirrhosis or even hepatocellular carcinoma (107). However, it is interesting that unlike most other types of tumors, the expression level of ITGA9 obviously declines in hepatocellular carcinoma and works as an inhibitor of the migration and invasion of hepatocellular carcinoma cells via the FAK/Src (c-Src tyrosine kinase)-Rho GTPases signalling pathway (77). ITGA9 overexpression inactivates the Rho GTPases members Rac1/RhoA and reduces FAK/Src phosphorylation, which are important in tumour angiogenesis and protease-associated metastasis (108, 109). Although the protein or mRNA of ITGA9 are roughly increased in the above-mentioned cancer types, they have previously been reported to be down-regulated in other tumors, such as human squamous cell carcinoma of the head and neck, bladder cancer and non-small-cell lung cancer, which is similar to the case for hepatocellular carcinoma (110–112). The research reviewed above reveals that ITGA9 has the potential to become a diagnostic biomarker for hepatocellular carcinoma, provides a potential treatment solution and, more importantly, shows notable tumour heterogeneity in different cancer types.
Integrin-α9β1 Serves as a New Target for the Treatment of Autoimmune Diseases
In the last decade, more and more literature has focused on the characteristics of integrin-α9β1 in autoimmune diseases. It has been identified as a novel putative therapeutic target for rheumatoid arthritis [RA; (113)]. Various extracellular matrix proteins, like TNC and OPN, have been demonstrated to up-regulate at the hyperplasia of joint synovium in RA (114, 115). They act as ligands for integrin-α9β1 to transduce intracellular mechanical signals (116) and are deeply involved in the arthritic inflammatory microenvironment (Figure 2).
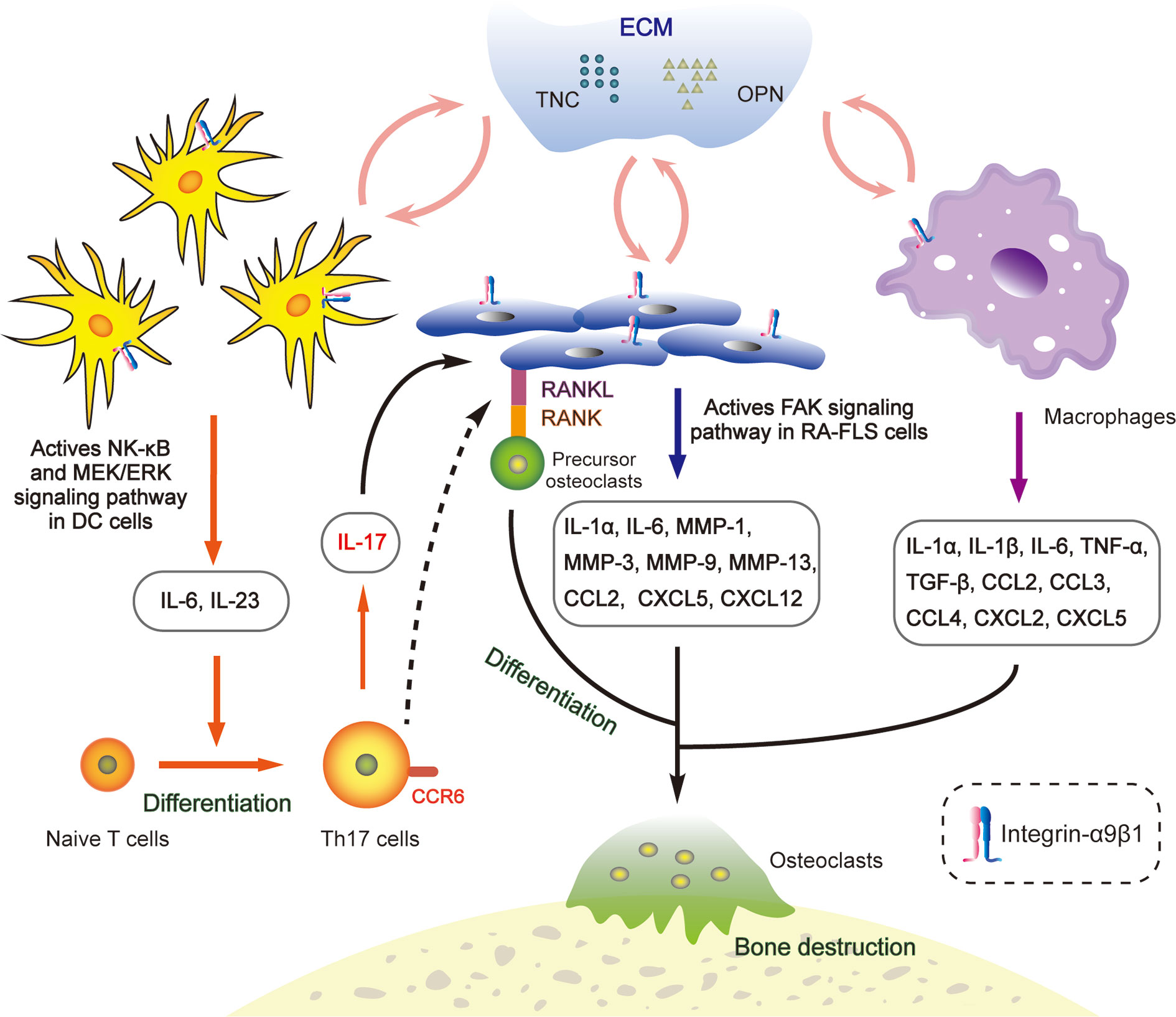
Figure 2 Interactions between integrin-α9β1 and the extracellular matrix (ECM) ligands TNC and OPN regulate the RA microenvironment. OPN and TNC bind to α9β1, promoting secretion of inflammation-related factors in conventional dendritic cells, synovial fibroblasts and macrophages, subsequently inducing osteoclast differentiation and inflammatory cell infiltration and, ultimately, leading to bone destruction.
ITGA9 is overexpressed in human RA samples and precedes the onset of arthritic symptoms in experimental models (113). The membranes of both synovial fibroblasts and synovial macrophages contain integrin-α9β1 but contribute differently to the production of various chemokines that are responsible for the recruitment and activation of inflammatory cells (117). In synovial fibroblasts, TNC and OPN interact with α9β1 to induce expressions of IL-1α, IL-6, MMP-1, -3, -9, -13, CCL2, CXCL5 and CXCL12. Meanwhile the expression levels of IL-1α, IL-6, TNF-α, IL-1β, TGF-β, CCL2, CCL3, CCL4, CXCL2 and CXCL5 increase in synovial macrophages (117, 118). Furthermore, in the conventional dendritic cells of an arthritis rodent model, integrin-α9β1 collaborates with TNC and OPN to induce secretion of IL-6 and IL-23 through both the NF-κB and MEK/ERK signalling pathways (Figure 2), which promotes the generation of pathogenic Th17 cells involved in osteoclast differentiation and bone destruction (119). Blocking of α9β1 sharply inhibits the release of these chemokines, which contribute to inflammatory cell activation and recruitment as well as angiogenesis and osteoclastogenesis (118). Therefore, integrin-α9β1 and its ligands act as key intrinsic mediators in the arthritis process (120–122).
In an arthritis animal model with inducements of anti-type II collagen antibody and lipopolysaccharide (123), injection of special anti-integrin-α9 (55A2C) antibody clearly reduced the number of arthrogenic cytokines and chemokines and ameliorated ongoing arthritis, demonstrating the therapeutic potential of the anti-α9 antibody for RA (117). Similarity, another study reported that intraperitoneal injection of blocking α9 antibody MA9-413 in collagen-induced arthritic mice also repressed the development of arthritis and inflammatory cell infiltration (113). Concurrently, it significantly decreased activated fibroblast-like synoviocyte (FLS)-derived biomarkers like MMP-3, IL-6, sRANKL and CXCL5 in plasma (124–126). MA9-413, also known as AS2535093, specifically recognizes a conserved loop region designated as amino acids 104–122 of α9, which binds to integrin-α9 but not α4 in humans and mice. In bone marrow cells of arthritic mice, MA9-413 considerably decreases RANKL and IL-6 expressions while inhibiting osteogenic differentiation (127). More important, this α9 antibody does not influence the number of immune cells in the spleen when it significantly prevents lymphocyte accumulation in the arthritic joint microenvironment, so MA9-413 plays a therapeutic role with minimal systemic immunomodulation (113).
Another study has demonstrated that the disruption of α9 with shRNA also inhibits the expression of TNC because of a positive feedback loop in which the production of TNC is dependent on α9β1-dependent FAK activation of integrin signalling (45). Compared to osteoarthritis, the FLSs of RA have activated FAK-mediated integrin-α9 signalling. Knockdown of either ITGA9 or TNC in RA-FLSs quells the phosphorylation of FAK, which is crucial for RA-FLS cell adhesion, migration and intrinsic secretion of proinflammatory mediators. Disruption of α9β1 or TNC also inhibits the spontaneous creation of MMP1, MMP3, MMP14, IL-6, cadherin-11 and TNFSF11/RANKL. Moreover, transfection of shRNA-ITGA9 in 3D cultured RA-FLSs abrogates abnormally condensed cellular accumulation structures (which reflects a pathogenic feature) and shows no proliferative reaction to stimulation of platelet-derived growth factor. Meanwhile, the production of proinflammatory regulators in response to exogenous TNF-α is nearly entirely absent compared with controls (45, 128). These results suggest that integrin-α9β1 plays an essential role in the aggressive behavior of RA-FLSs, both under autonomy conditions and exogenous inflammatory stimuli.
As mentioned above, OPN is also a key molecule in arthritis, since it binds to integrin-α9β1 via the SVVYGLR amino acid sequence rather than the full-length sequence (129). The thrombin cleaves OPN, accompanied by subsequent exposing of the SVVYGLR sequence at the N-terminal fragment in humans (130). However, in rats and mice, the amino acid sequence reacting to α9β1 is replaced by SLAYGLR (131). Intravenous injection of the specific antibody reacting to the SLAYGLR sequence inhibits the proliferation of synovium, inflammatory cell infiltration and bone erosion in murine arthritic joints (132), demonstrating that the cryptic epitope is crucial to RA pathogenesis. Deferring from thrombin, MMP-3/7 cleaves mouse OPN to expose the new sequence LRSKSRSFQVSDEQY at the C-terminal fragment, which also binds to α9β1 to participate in anti-type II collagen antibody-induced arthritis. Meanwhile, the antibody of the LRSKSRSFQVSDEQY sequence also shows an ability to diminish the pathological features of arthritis. These results indicate that MMP-3 and MMP-7 promote the development of RA via interaction between OPN and integrin-α9β1 (133).
Recently, TNC, OPN and common receptor integrin-α9β1 have been identified as promising treatment targets for more autoimmune inflammatory diseases, since both TNC- and OPN-deficient mice have shown resistance against a variety of Th1- and/or Th17-related autoimmune diseases (134). Except for RA, integrin-α9β1 is also involved in experimental autoimmune encephalomyelitis in mice via regulation of the secretion of sphingosine-1-phosphate (S1P) receptors, which affect the discharge of immune cells and are related to chronic periodontitis via the MAPK pathway (116, 135). It is remarkable that the new integrin-α9 ligand XCL1/lymphotactin has also been reported to participate in RA and autoimmune encephalomyelitis. Both α9β1-neutralizing and XCL1-neutralizing antibodies ameliorate the symptoms of autoimmune disease in murine models, and injection of α9β1-antibody relieves experimental autoimmune encephalomyelitis-related symptoms with clear reductions in spinal cord-infiltrating cells and lymphocyte egress via lymph node drainage (56, 136).
It is noteworthy that, apart from regulating lymphocyte egress via lymphatic endothelial cells, integrin-α9β1 is also essential for lymphatic valve formation and maintenance (32), which are closely related to the rejection of transplantation (137). Subconjunctival injection of α9-antibodies during orthotopic keratoplasty in mice can inhibit the formation of lymphatic valves without the intervention of lymphangiogenesis, so as to significantly improve the survival rate of grafts after transplantation (138, 139).
In conclusion, local antibody neutralization therapy using integrin-α9β1 or corresponding ligands may be prospective therapeutic directions for treating various refractory immune diseases, even for preventing immune rejection of transplanted organs. Hence, the underlying mechanism of integrin-α9β1 participation in immune-related cellular functions is worthy of further research and has valuable clinical significance.
Integrin-α9β1 Promotes Axon Regeneration
After neuronal injury of the central nervous system (CNS), it is difficult for axons to regenerate and recover function, due to the incompetent intrinsic regenerative ability of adult CNS neurons and inhibitory factors in the microenvironment (140). Axonal growth is a particular form of cell migration; meanwhile, integrins and ligands are crucial in cell adhesion and neuronal migration (141), so inhibitors of integrins that are present in the microenvironment are non-negligible factors in blocking CNS regeneration (142). For example, the integrin response suppressors, myelin-derived Nogo-A protein and chondroitin sulfate proteoglycans (CSPGs), impair integrin signalling by decreasing phosphorylated FAK and Src levels, which may be a potential factor affecting nerve self-repair (143, 144).
Furthermore, during postnatal corticospinal axon development, cortical neurons introduce integrins into their axons. However, integrins are clearly excluded from the axons when the cortical neurons mature, especially the key receptor integrin-α9β1, which is considered to be an important reason for the low regenerative competence of the CNS (145). After damage to the CNS, TNC is up-regulated, which is the main extracellular matrix glycoprotein in the CNS environment and the important ligand of integrin-α9β1; however, the α9 subunit is absent in adult neurons. Forced expression of α9 leads to neurite outgrowth in both PC12 cells and dorsal root ganglia axon (DRG) neurons of adult rats (46), suggesting that the reaction between α9β1 and TNC might play a key role in axonal regeneration. Kindlin-1 is reported to counteract the effects of CSPGs and Nogo-A to enhance integrin activation and signalling in the DRG of rats and promote axon growth with sensory axon regeneration (146). The interaction between kindlin-1-activated integrin-α9β1 and TNC overcomes the inhibitory environment of the adult axons: overexpression of kindlin-1 and ITGA9 can achieve long-distance sensory axon regeneration (> 25 mm axon length) and sensory–motor recovery in rats, which has great clinical significance, while overexpression of α9 or kindlin-1 alone is associated with substantially lower recovery and regeneration (147).
In general, it is difficult to effectively transport integrin-α9 into CNS axons, since it is restricted to being transported along axons in mature cortical neurons (148). The small GTPase Rab11 regulates the key pathway of integrin transport and participates in the transport of various neuron membrane molecules (149–151). It has been shown that α9 can be vesicle-transported through Rab11 and RCP (Rab11 effector Rab coupling protein) in differentiated PC12 cells and adult DRG neurons (152), revealing that manipulation of Rab11 and RCP may be beneficial to neuron therapy after injury. However, the transportation speed of integrins through Rab11 is not fast, and another study showed that the rapid transport of axons and cell surfaces is regulated by ARF6, which is involved in the exclusion of integrins from mature CNS axons (153). The ARF6 inactivator ACAP1 (ARF6 GTPase-activating protein) increases axonal growth, integrin-α9 externalization and anterograde transport, while the ARF6 activator EFA6 and ARNO (neuronal ARF6 guanine nucleotide exchange factors) suppress axon growth with increases in integrin retrograde transportation and internalization in the DRG of adult rats (154). Therefore, the role of ARF6 inhibitors in nerve regeneration is worthy of further exploration.
On the other hand, transplantation of human-induced pluripotent stem cell-derived neural progenitor cells (NPCs) is considered to be another potential regenerative therapy after nervous system injury, since hNPCs produce endogenous α9 and β1 subunits (155). Both wild-type and lentivirus-mediated overexpressing-α9 hNPCs induce axonal growth in the developing nervous system of rats, but their effects on spinal cord injury remain to be studied. Besides, integrin-α9β1 and TNC synergistically improve the efficiency of differentiation from mesenchymal stem cells into neuronal lineages, which has important implications for stem cell therapies (156).
In brief, integrin-α9β1 has a significant supportive effect on recovery and regeneration after nerve injury but is absent and suppressed in adult neurons. The manipulation of increased α9 expression, transport and activation, could become valuable strategies for driving integrin-dependent axonal regeneration.
Integrin-α9β1 Makes a Promising Target for Antithrombotic Therapy
Integrin-α9β1 has attracted attention as a potential new target for antithrombotic therapy in recent years (157). Numerous studies have noted the importance of neutrophils in thrombosis formation via modulation of platelet adhesion, activation and coagulation, as well as by facilitating coordinated interaction between endothelial cells and platelets (158–160). Compared to monocytes, integrin-α9β1 is highly expressed in neutrophils and is essentially for neutrophil development, since ITGA9-deficient mice have dramatic defects in neutrophil production due to a decrease in bone marrow granulocyte precursors, accompanied by a reduced capacity to differentiate bone marrow cells into granulocytes (18, 49). Besides, α9β1 is expressed on polymorphonuclear leukocytes in human blood and is up-regulated after leukocyte activation, implying its potential role in neutrophil migration through lung and synovial fibroblast barriers (161). For instance, in aspirated pneumonia patients, the expression of α9β1 in circulating neutrophils is significantly higher than that in healthy people, indicating that integrin-α9β1 may play a potential role in neutrophil extravasation (162).
It is worth noting that α9β1 regulates the physiological activity of neutrophils through a variety of different ligands. After neutrophil activation, the expression of α9β1 has been detected to increase two-to-three-fold. During neutrophil transendothelial migration through human umbilical vein endothelial (HUVE) cell-coated transwells, α9β1 was the only up-regulated β1-type integrin (163). Antibodies against either α9β1 or VCAM-1(vascular cell adhesion molecule-1) down-regulate the augmented migration across TNF-α-activated HUVE cell monolayers (49), and VCAM-1 is also a fundamental ligand for α9β1 in regulating cell adhesion [Figure 3A; (50)]. Additionally, after release from bone marrow, neutrophils undergo spontaneous apoptosis within 24 hours under normal physiological conditions (164). However, in inflammatory tissues, the survival of neutrophils is significantly prolonged, while the interaction between VCAM-1 and α9β1 involves inhibition of neutrophil apoptosis through PI3K and NF-κB activation (165). This induces an extension of lifespan upon full activation of the neutrophils, so as to promote thrombosis (166). Another study revealed that integrin-α9β1 activates the PI3K and MAPK-ERK signalling pathways in human neutrophils with NF-κB nuclear translocation, pro-apoptotic protein Bad degradation and enhanced anti-apoptotic protein Bcl-xL, resulting in spontaneous delay of cell apoptosis [Figure 3B; (167)]. The novel heterodimeric disintegrins EC3 and EC6, which have been isolated from the venom of Echis carinatus, are both effective inhibitors of adhesion mediated by reaction between integrin-α9β1 and VCAM-1. They also disrupt neutrophil migration across endothelial cells. These natural integrin inhibitors are considered to have a therapeutic potential to inhibit excessive migration of leukocytes through integrin-α9β1 (168).
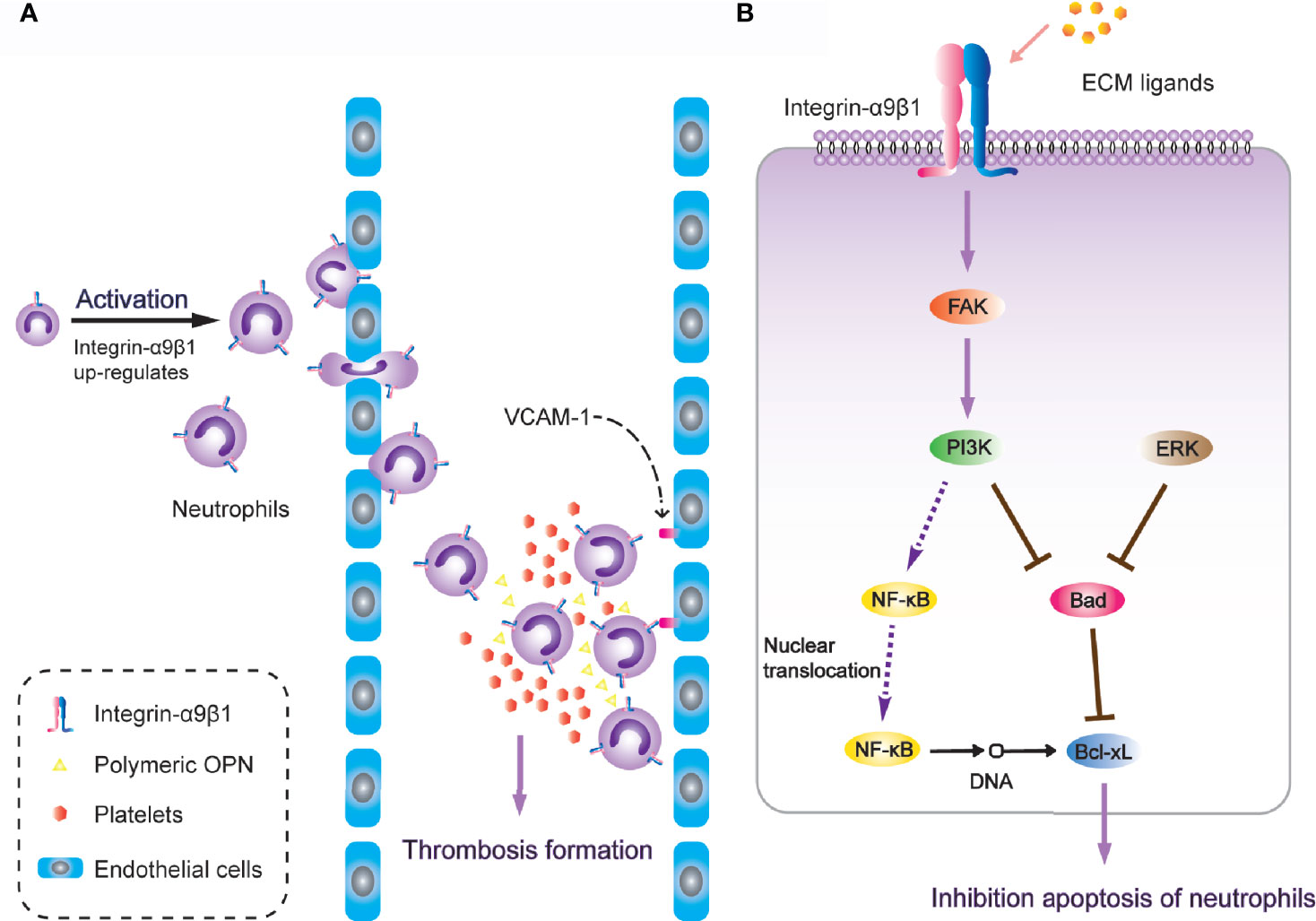
Figure 3 Integrin-α9β1 is involved in thrombosis through the regulation of chemotaxis, adhesion and apoptosis of neutrophils. (A) Integrin-α9β1 up-regulates during neutrophil activation and interacts with its ligands (VCAM-1 and Polymeric OPN), mediating neutrophil chemotactic activity and stabilizing adhesion to endothelial cells, eventually resulting in increased risk of thrombosis. (B) Integrin-α9β1 inhibits apoptosis of neutrophils through the PI3K and ERK signaling pathways.
Polymeric-OPN, which is another ligand that employs integrin-α9β1 as the receptor and is formed by transglutaminase mediation, can attract neutrophils by presenting a special binding site, while unpolymerized OPN cannot (169). Polymeric-OPN has been detected in aortic tissue and bone and induces neutrophil recruitment via α9β1 in a mouse model, in which injection of the transglutaminase inhibitor cystamine attenuates the recruitment (53). Furthermore, OPN has also been reported to interact with both integrin-α4β1 and α9β1 in neutrophils in an alcoholic liver disease rodent model, causing high hepatic neutrophil infiltration and liver injury (170). Interestingly, the reaction sites of OPN exposure reported by these studies are diverse. The latter declared that the SVVYGLR fragment of thrombin-cleaved OPN was identified by integrin-α9β1 to induce neutrophil infiltration; however, the former reported that the polymeric-OPN did not have this classical sequence, suggesting that OPN might present an undiscovered binding site after transglutaminase mediation. The same point is that α9β1 cannot recognize the complete OPN, which needs proper enzyme treatment to play the role of a ligand. Furthermore, different enzymes produce divergent binding sites, suggesting a redundant mechanism of OPN and α9β1 in neutrophil chemotaxis.
ADAM family members selectively modulate different integrin-mediated cell migrations as extracellular matrix ligands (171). ADAM9D has been proven to contribute to neutrophil activation and chemotaxis via the cooperation of integrin-α9β1 and αVβ3, concomitant with activation of the PI3K/Akt and ERK pathways, while blockade of either α9β1 or αVβ3 impairs the migration of human neutrophils toward ADAM9D (172). The PI3K/Akt pathway is involved in leukocyte function and the recruitment of both neutrophils and macrophages (173). It also leads to subsequent phosphorylation of the ERK, which supports the antiapoptotic function of integrin-α9β1 for neutrophils [Figure 3B; (167)].
It has been reported that myeloid cell-specific integrin-α9-/- mice that were less susceptible to arterial thrombosis and had unaltered hemostasis under conditions of ferric chloride and laser injury-induced thrombosis. They had reduced numbers of neutrophils, red blood cells and myeloperoxidase levels in the diminished carotid thrombi compared with normal mice. More striking was the therapy of a wild-type group with anti-α9 mAb (55A2C), which obviously suppressed ferric chloride-induced arterial thrombosis, thereby revealing the suitability of α9 as a therapeutic target for arterial thrombosis (174). It is also worth noting that deletion of the ITGA9 gene from myeloid cells can improve both short- and long-term stroke outcomes and survival rates in an ischemic stroke rodent model. This is concomitant with a reduction in the cerebral thrombo-inflammatory response, as evidenced by decreases in fibrin, platelet thrombi, neutrophils, phospho-NF-κB, TNF-α and IL-1β levels, as well as diminishment of neutrophil extracellular trap formation (web-like chromatin structures that induce activation of endothelial cells, antigen-presenting cells and platelets, as well as triggering the proinflammatory immune response, atherosclerotic plaque formation and arterial thrombosis). In addition, intravenous infusion of 55A2C antibody into hyperlipidemic mice following reperfusion significantly reduces infarct volume and improves both short-term and long-term functional outcomes (175, 176). Taken together, these studies show that the targeting of myeloid-specific integrin-α9β1 may become a new treatment direction for thrombotic diseases.
Discussion and Future Prospects
In this paper, we provide an enhanced and updated review of current research on integrin-α9β1 as a therapeutic target for different refractory diseases, focusing on the trends and changes that have occurred in the past ten years. As mentioned above, the specific antibodies, microRNA and other inhibitors that target integrin-α9β1 or corresponding ligands have shown therapeutic effects on tumors, autoimmune diseases and thrombosis. On the other hand, the overexpression, transport and activation of integrin-α9β1 hold great promise in curing axon damage.
The function of α9β1 is mainly driven by corresponding ligands in the extracellular matrix. These form a complicated signalling network and regulate the physiological and pathological behaviors of cells. However, the reactions are redundant and complex, because co-existing ligands simultaneously collaborate with integrin-α9β1 to mediate the same or different signalling pathways. Although the experiments considered in this review testify to the effectiveness of blocking these ligands to interdict the pathological process, other additional effects were not fully considered. This is because a large fraction of ligands not only interact with α9β1 but also react to other receptors and create crosstalk of molecular signalling pathways in a variety of ways (177–179). On the other hand, despite the functional alternation of α9β1 having been demonstrated to have therapeutic potential in many animal models, the possible side-effects remain to be studied. Since integrin-α9β1 also plays an indispensable role in normal physiological processes, such as the development and renewal of lymphatic and venous valves (19, 41) and proper re-epithelialization in cutaneous wound healing (180). We were pleasantly surprised to discover a very recent clinical trial using anti-α9 antibodies. ASP5094, a humanized mAb against integrin-α9, was used in a phase 2a, multicenter, randomized, double-blind study to cure refractory RA with resistance to methotrexate (181). Although intravenous ASP5094 (10 mg/kg) did not show efficacy in patients with moderate to severe refractory RA, this result could be due to insufficient exposure of ASP5094 in the target tissue. No safety signals were evident, such that ASP5094 is considered to be well-tolerated and safe overall. Because integrin-α9β1 is positioned at the cell membrane, local injection of inhibitors can act as an effective blocking factor in lesion locations (182) and play a therapeutic role through non-immunosuppressive pathways, which may benefit treatment without excessive systemic effects (45). Consequently, we look forward to further clinical trials that target α9β1 with diverse treatment modalities.
However, notwithstanding that integrin-α9β1 is distributed in so many types of organs and cells, its wider range of activity and precise mechanisms require further investigation, given that some recognized α9β1 ligands are up-regulated in many diseases. For example, TNC is remarkably increased in bronchoalveolar lavage fluid and serum in coronavirus disease 2019 (COVID-19) patients, while serum levels of VCAM-1 are also elevated in mild cases and dramatically up-regulated in patients with severe disease (183, 184). Likewise, VEGF has been reported to be involved in the brain inflammation caused by attack from severe acute respiratory syndrome coronavirus 2 (SARS-COV-2, the viral pathogen of COVID-19) (185). These extracellular matrix molecules are considered to be biomarkers or therapeutic candidates for COVID-19 and are under a recognized ligand of α9β1. For this reason, it can be inferred that α9β1 may play a regulatory role in the pathological process of COVID-19. In addition, OPN is highly associated with autoimmune diseases of the skin, such as lupus erythematosus and pemphigus vulgaris (186, 187); hence, it is likely to work with the α9β1 in skin cells to produce inflammatory reactions similar to RA, as previously described. Overall, α9β1 is a potential mediator of other diseases and further insights are urgently needed.
To summarize, treatments targeting integrin-α9β1 have been effective in many experiments and α9β1 may play important roles in more unexplored diseases. Integrin-α9β1 is of great research value as a candidate therapeutic target for clinical treatment and its future prospects are worth exploring.
Author Contributions
SX, TZ, WZ, CZ and HL collected the data. SX wrote the article. ZC and JS reviewed the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81771082, 31971282, 81800985) and the Chongqing Graduate Tutor Team (2019).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Giancotti FG, Ruoslahti E. Integrin signaling. Science (1999) 285(5430):1028–32. doi: 10.1126/science.285.5430.1028
2. Chen W, Harbeck MC, Zhang W, Jacobson JR. MicroRNA regulation of integrins. Transl Res (2013) 162(3):133–43. doi: 10.1016/j.trsl.2013.06.008
3. van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res (2001) 305(3):285–98. doi: 10.1007/s004410100417
4. Erle DJ, Sheppard D, Breuss J, Ruegg C, Pytela R. Novel integrin alpha and beta subunit cDNAs identified in airway epithelial cells and lung leukocytes using the polymerase chain reaction. Am J Respir Cell Mol Biol (1991) 5(2):170–7. doi: 10.1165/ajrcmb/5.2.170
5. Palmer EL, Ruegg C, Ferrando R, Pytela R, Sheppard D. Sequence and tissue distribution of the integrin alpha 9 subunit, a novel partner of beta 1 that is widely distributed in epithelia and muscle. J Cell Biol (1993) 123(5):1289–97. doi: 10.1083/jcb.123.5.1289
6. Takada Y, Ye X, Simon S. The integrins. Genome Biol (2007) 8(5):215. doi: 10.1186/gb-2007-8-5-215
7. Xiong W, Zeng ZY, Xia JH, Xia K, Shen SR, Li XL, et al. A susceptibility locus at chromosome 3p21 linked to familial nasopharyngeal carcinoma. Cancer Res (2004) 64(6):1972–4. doi: 10.1158/0008-5472.can-03-3253
8. Nawaz I, Hu LF, Du ZM, Moumad K, Ignatyev I, Pavlova TV, et al. Integrin alpha9 gene promoter is hypermethylated and downregulated in nasopharyngeal carcinoma. Oncotarget (2015) 6(31):31493–507. doi: 10.18632/oncotarget.5154
9. Springer TA. Folding of the N-terminal, ligand-binding region of integrin alpha-subunits into a beta-propeller domain. Proc Natl Acad Sci U S A (1997) 94(1):65–72. doi: 10.1073/pnas.94.1.65
10. Kon S, Atakilit A, Sheppard D. Short form of alpha9 promotes alpha9beta1 integrin-dependent cell adhesion by modulating the function of the full-length alpha9 subunit. Exp Cell Res (2011) 317(12):1774–84. doi: 10.1016/j.yexcr.2011.04.005
11. Yokosaki Y, Matsuura N, Higashiyama S, Murakami I, Obara M, Yamakido M, et al. Identification of the ligand binding site for the integrin alpha9 beta1 in the third fibronectin type III repeat of tenascin-C. J Biol Chem (1998) 273(19):11423–8. doi: 10.1074/jbc.273.19.11423
12. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell (2002) 110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6
13. Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell (1992) 69(1):11–25. doi: 10.1016/0092-8674(92)90115-s
14. Hoye AM, Couchman JR, Wewer UM, Fukami K, Yoneda A. The newcomer in the integrin family: integrin alpha9 in biology and cancer. Adv Biol Regul (2012) 52(2):326–39. doi: 10.1016/j.jbior.2012.03.004
15. Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development (1995) 121(2):549–60.
16. Arroyo AG, Yang JT, Rayburn H, Hynes RO. Alpha4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity (1999) 11(5):555–66. doi: 10.1016/s1074-7613(00)80131-4
17. Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV Jr., et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol (2000) 20(14):5208–15. doi: 10.1128/mcb.20.14.5208-5215.2000
18. Chen C, Huang X, Atakilit A, Zhu QS, Corey SJ, Sheppard D. The Integrin alpha9beta1 contributes to granulopoiesis by enhancing granulocyte colony-stimulating factor receptor signaling. Immunity (2006) 25(6):895–906. doi: 10.1016/j.immuni.2006.10.013
19. Bazigou E, Lyons OT, Smith A, Venn GE, Cope C, Brown NA, et al. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J Clin Invest (2011) 121(8):2984–92. doi: 10.1172/JCI58050
20. Zhu K, Takada Y, Nakajima K, Sun Y, Jiang J, Zhang Y, et al. Expression of integrins to control migration direction of electrotaxis. FASEB J (2019) 33(8):9131–41. doi: 10.1096/fj.201802657R
21. Eto K, Huet C, Tarui T, Kupriyanov S, Liu HZ, Puzon-McLaughlin W, et al. Functional classification of ADAMs based on a conserved motif for binding to integrin alpha 9beta 1: implications for sperm-egg binding and other cell interactions. J Biol Chem (2002) 277(20):17804–10. doi: 10.1074/jbc.M200086200
22. Danussi C, Petrucco A, Wassermann B, Pivetta E, Modica TM, Del Bel Belluz L, et al. EMILIN1-alpha4/alpha9 integrin interaction inhibits dermal fibroblast and keratinocyte proliferation. J Cell Biol (2011) 195(1):131–45. doi: 10.1083/jcb.201008013
23. Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem (2005) 280(6):4544–52. doi: 10.1074/jbc.M412816200
24. Murtomaki A, Uh MK, Kitajewski C, Zhao J, Nagasaki T, Shawber CJ, et al. Notch signaling functions in lymphatic valve formation. Development (2014) 141(12):2446–51. doi: 10.1242/dev.101188
25. Saika S, Sumioka T, Okada Y, Yamanaka O, Kitano A, Miyamoto T, et al. Wakayama symposium: modulation of wound healing response in the corneal stroma by osteopontin and tenascin-C. Ocul Surf (2013) 11(1):12–5. doi: 10.1016/j.jtos.2012.09.002
26. Tomczuk M, Takahashi Y, Huang J, Murase S, Mistretta M, Klaffky E, et al. Role of multiple beta1 integrins in cell adhesion to the disintegrin domains of ADAMs 2 and 3. Exp Cell Res (2003) 290(1):68–81. doi: 10.1016/s0014-4827(03)00307-0
27. Zhu X, Evans JP. Analysis of the roles of RGD-binding integrins, alpha(4)/alpha(9) integrins, alpha(6) integrins, and CD9 in the interaction of the fertilin beta (ADAM2) disintegrin domain with the mouse egg membrane. Biol Reprod (2002) 66(4):1193–202. doi: 10.1095/biolreprod66.4.1193
28. Bridges LC, Sheppard D, Bowditch RD. ADAM disintegrin-like domain recognition by the lymphocyte integrins alpha4beta1 and alpha4beta7. Biochem J (2005) 387(Pt 1):101–8. doi: 10.1042/BJ20041444
29. Rao H, Lu G, Kajiya H, Garcia-Palacios V, Kurihara N, Anderson J, et al. Alpha9beta1: a novel osteoclast integrin that regulates osteoclast formation and function. J Bone Miner Res (2006) 21(10):1657–65. doi: 10.1359/jbmr.060718
30. Lafuste P, Sonnet C, Chazaud B, Dreyfus PA, Gherardi RK, Wewer UM, et al. ADAM12 and alpha9beta1 integrin are instrumental in human myogenic cell differentiation. Mol Biol Cell (2005) 16(2):861–70. doi: 10.1091/mbc.e04-03-0226
31. Vishweswaraiah S, Veerappa AM, Mahesh PA, Jayaraju BS, Krishnarao CS, Ramachandra NB. Molecular interaction network and pathway studies of ADAM33 potentially relevant to asthma. Ann Allergy Asthma Immunol (2014) 113(4):418–24.e1. doi: 10.1016/j.anai.2014.07.009
32. Danussi C, Del Bel Belluz L, Pivetta E, Modica TM, Muro A, Wassermann B, et al. EMILIN1/alpha9beta1 integrin interaction is crucial in lymphatic valve formation and maintenance. Mol Cell Biol (2013) 33(22):4381–94. doi: 10.1128/MCB.00872-13
33. Pivetta E, Danussi C, Wassermann B, Modica TM, Del Bel Belluz L, Canzonieri V, et al. Neutrophil elastase-dependent cleavage compromises the tumor suppressor role of EMILIN1. Matrix Biol (2014) 34:22–32. doi: 10.1016/j.matbio.2014.01.018
34. Capuano A, Pivetta E, Baldissera F, Bosisio G, Wassermann B, Bucciotti F, et al. Integrin binding site within the gC1q domain orchestrates EMILIN-1-induced lymphangiogenesis. Matrix Biol (2019) 81:34–49. doi: 10.1016/j.matbio.2018.10.006
35. Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, et al. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J Biol Chem (2007) 282(20):15187–96. doi: 10.1074/jbc.M609323200
36. Lala PK, Nandi P, Majumder M. Roles of prostaglandins in tumor-associated lymphangiogenesis with special reference to breast cancer. Cancer Metastasis Rev (2018) 37(2-3):369–84. doi: 10.1007/s10555-018-9734-0
37. Mishima K, Watabe T, Saito A, Yoshimatsu Y, Imaizumi N, Masui S, et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol Cell (2007) 18(4):1421–9. doi: 10.1091/mbc.e06-09-0780
38. Majumder M, Tutunea-Fatan E, Xin X, Rodriguez-Torres M, Torres-Garcia J, Wiebe R, et al. Co-expression of alpha9beta1 integrin and VEGF-D confers lymphatic metastatic ability to a human breast cancer cell line MDA-MB-468LN. PLoS One (2012) 7(4):e35094. doi: 10.1371/journal.pone.0035094
39. Ou J, Peng Y, Deng J, Miao H, Zhou J, Zha L, et al. Endothelial cell-derived fibronectin extra domain A promotes colorectal cancer metastasis via inducing epithelial-mesenchymal transition. Carcinogenesis (2014) 35(7):1661–70. doi: 10.1093/carcin/bgu090
40. Ou J, Deng J, Wei X, Xie G, Zhou R, Yu L, et al. Fibronectin extra domain A (EDA) sustains CD133(+)/CD44(+) subpopulation of colorectal cancer cells. Stem Cell Res (2013) 11(2):820–33. doi: 10.1016/j.scr.2013.05.009
41. Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell (2009) 17(2):175–86. doi: 10.1016/j.devcel.2009.06.017
42. Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem (2002) 277(17):14467–74. doi: 10.1074/jbc.M201100200
43. Shinde AV, Bystroff C, Wang C, Vogelezang MG, Vincent PA, Hynes RO, et al. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities. J Biol Chem (2008) 283(5):2858–70. doi: 10.1074/jbc.M708306200
44. San Martin R, Pathak R, Jain A, Jung SY, Hilsenbeck SG, Pina-Barba MC, et al. Tenascin-C and Integrin alpha9 Mediate Interactions of Prostate Cancer with the Bone Microenvironment. Cancer Res (2017) 77(21):5977–88. doi: 10.1158/0008-5472.CAN-17-0064
45. Emori T, Hirose J, Ise K, Yomoda JI, Kasahara M, Shinkuma T, et al. Constitutive Activation of Integrin alpha9 Augments Self-Directed Hyperplastic and Proinflammatory Properties of Fibroblast-like Synoviocytes of Rheumatoid Arthritis. J Immunol (2017) 199(10):3427–36. doi: 10.4049/jimmunol.1700941
46. Andrews MR, Czvitkovich S, Dassie E, Vogelaar CF, Faissner A, Blits B, et al. Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci (2009) 29(17):5546–57. doi: 10.1523/JNEUROSCI.0759-09.2009
47. Nakamura-Ishizu A, Okuno Y, Omatsu Y, Okabe K, Morimoto J, Uede T, et al. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood (2012) 119(23):5429–37. doi: 10.1182/blood-2011-11-393645
48. Yoshimura E, Majima A, Sakakura Y, Sakakura T, Yoshida T. Expression of tenascin-C and the integrin alpha 9 subunit in regeneration of rat nasal mucosa after chemical injury: involvement in migration and proliferation of epithelial cells. Histochem Cell Biol (1999) 111(4):259–64. doi: 10.1007/s004180050356
49. Taooka Y, Chen J, Yednock T, Sheppard D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol (1999) 145(2):413–20. doi: 10.1083/jcb.145.2.413
50. Yang Y, Enis D, Zheng H, Chia S, Yang J, Chen M, et al. Cell Adhesion Mediated by VCAM-ITGalpha9 Interactions Enables Lymphatic Development. Arterioscler Thromb Vasc Biol (2015) 35(5):1179–89. doi: 10.1161/ATVBAHA.114.304997
51. Kale S, Raja R, Thorat D, Soundararajan G, Patil TV, Kundu GC. Osteopontin signaling upregulates cyclooxygenase-2 expression in tumor-associated macrophages leading to enhanced angiogenesis and melanoma growth via alpha9beta1 integrin. Oncogene (2014) 33(18):2295–306. doi: 10.1038/onc.2013.184
52. Cui G, Chen J, Wu Z, Huang H, Wang L, Liang Y, et al. Thrombin cleavage of osteopontin controls activation of hepatic stellate cells and is essential for liver fibrogenesis. J Cell Physiol (2019) 234(6):8988–97. doi: 10.1002/jcp.27571
53. Nishimichi N, Hayashita-Kinoh H, Chen C, Matsuda H, Sheppard D, Yokosaki Y. Osteopontin undergoes polymerization in vivo and gains chemotactic activity for neutrophils mediated by integrin alpha9beta1. J Biol Chem (2011) 286(13):11170–8. doi: 10.1074/jbc.M110.189258
54. Kourepini E, Aggelakopoulou M, Alissafi T, Paschalidis N, Simoes DC, Panoutsakopoulou V. Osteopontin expression by CD103- dendritic cells drives intestinal inflammation. Proc Natl Acad Sci U S A (2014) 111(9):E856–65. doi: 10.1073/pnas.1316447111
55. Smith LL, Cheung HK, Ling LE, Chen J, Sheppard D, Pytela R, et al. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by alpha9beta1 integrin. J Biol Chem (1996) 271(45):28485–91. doi: 10.1074/jbc.271.45.28485
56. Matsumoto N, Kon S, Nakatsuru T, Miyashita T, Inui K, Saitoh K, et al. A Novel alpha9 Integrin Ligand, XCL1/Lymphotactin, Is Involved in the Development of Murine Models of Autoimmune Diseases. J Immunol (2017) 199(1):82–90. doi: 10.4049/jimmunol.1601329
57. Staniszewska I, Zaveri S, Del Valle L, Oliva I, Rothman VL, Croul SE, et al. Interaction of alpha9beta1 integrin with thrombospondin-1 promotes angiogenesis. Circ Res (2007) 100(9):1308–16. doi: 10.1161/01.RES.0000266662.98355.66
58. Takahashi H, Isobe T, Horibe S, Takagi J, Yokosaki Y, Sheppard D, et al. Tissue transglutaminase, coagulation factor XIII, and the pro-polypeptide of von Willebrand factor are all ligands for the integrins alpha 9beta 1 and alpha 4beta 1. J Biol Chem (2000) 275(31):23589–95. doi: 10.1074/jbc.M003526200
59. Silletti S, Mei F, Sheppard D, Montgomery AM. Plasmin-sensitive dibasic sequences in the third fibronectin-like domain of L1-cell adhesion molecule (CAM) facilitate homomultimerization and concomitant integrin recruitment. J Cell Biol (2000) 149(7):1485–502. doi: 10.1083/jcb.149.7.1485
60. Staniszewska I, Sariyer IK, Lecht S, Brown MC, Walsh EM, Tuszynski GP, et al. Integrin alpha9 beta1 is a receptor for nerve growth factor and other neurotrophins. J Cell Sci (2008) 121(Pt 4):504–13. doi: 10.1242/jcs.000232
61. Majumdar M, Tarui T, Shi B, Akakura N, Ruf W, Takada Y. Plasmin-induced migration requires signaling through protease-activated receptor 1 and integrin alpha(9)beta(1). J Biol Chem (2004) 279(36):37528–34. doi: 10.1074/jbc.M401372200
62. Alphonso A, Alahari SK. Stromal cells and integrins: conforming to the needs of the tumor microenvironment. Neoplasia (2009) 11(12):1264–71. doi: 10.1593/neo.91302
63. Gupta SK, Vlahakis NE. Integrin alpha9beta1 mediates enhanced cell migration through nitric oxide synthase activity regulated by Src tyrosine kinase. J Cell Sci (2009) 122(Pt 12):2043–54. doi: 10.1242/jcs.041632
64. Gupta SK, Oommen S, Aubry MC, Williams BP, Vlahakis NE. Integrin alpha9beta1 promotes malignant tumor growth and metastasis by potentiating epithelial-mesenchymal transition. Oncogene (2013) 32(2):141–50. doi: 10.1038/onc.2012.41
65. Lydolph MC, Morgan-Fisher M, Hoye AM, Couchman JR, Wewer UM, Yoneda A. Alpha9beta1 integrin in melanoma cells can signal different adhesion states for migration and anchorage. Exp Cell Res (2009) 315(19):3312–24. doi: 10.1016/j.yexcr.2009.09.022
66. Eto K, Puzon-McLaughlin W, Sheppard D, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin alpha9beta1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem (2000) 275(45):34922–30. doi: 10.1074/jbc.M001953200
67. Zhang J, Na S, Liu C, Pan S, Cai J, Qiu J. MicroRNA-125b suppresses the epithelial-mesenchymal transition and cell invasion by targeting ITGA9 in melanoma. Tumour Biol (2016) 37(5):5941–9. doi: 10.1007/s13277-015-4409-8
68. Fan J, Kang X, Zhao L, Zheng Y, Yang J, Li D. Long Noncoding RNA CCAT1 Functions as a Competing Endogenous RNA to Upregulate ITGA9 by Sponging MiR-296-3p in Melanoma. Cancer Manag Res (2020) 12:4699–714. doi: 10.2147/CMAR.S252635
69. Wang Z, Li Y, Xiao Y, Lin HP, Yang P, Humphries B, et al. Integrin alpha9 depletion promotes beta-catenin degradation to suppress triple-negative breast cancer tumor growth and metastasis. Int J Cancer (2019) 145(10):2767–80. doi: 10.1002/ijc.32359
70. Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, et al. Integrin alpha3beta1-dependent beta-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol (2009) 184(2):309–22. doi: 10.1083/jcb.200806067
71. Park JS, Choi HI, Kim DH, Kim CS, Bae EH, Ma SK, et al. RON Receptor Tyrosine Kinase Regulates Epithelial Mesenchymal Transition and the Expression of Pro-Fibrotic Markers via Src/Smad Signaling in HK-2 and NRK49F Cells. Int J Mol Sci (2019) 20(21):5489. doi: 10.3390/ijms20215489
72. Amin A, Chikan NA, Mokhdomi TA, Bukhari S, Koul AM, Shah BA, et al. Irigenin, a novel lead from Western Himalayan chemiome inhibits Fibronectin-Extra Domain A induced metastasis in Lung cancer cells. Sci Rep (2016) 6:37151. doi: 10.1038/srep37151
73. Oommen S, Gupta SK, Vlahakis NE. Vascular endothelial growth factor A (VEGF-A) induces endothelial and cancer cell migration through direct binding to integrin {alpha}9{beta}1: identification of a specific {alpha}9{beta}1 binding site. J Biol Chem (2011) 286(2):1083–92. doi: 10.1074/jbc.M110.175158
74. Masia A, Almazan-Moga A, Velasco P, Reventos J, Toran N, Sanchez de Toledo J, et al. Notch-mediated induction of N-cadherin and alpha9-integrin confers higher invasive phenotype on rhabdomyosarcoma cells. Br J Cancer (2012) 107(8):1374–83. doi: 10.1038/bjc.2012.411
75. Molist C, Navarro N, Giralt I, Zarzosa P, Gallo-Oller G, Pons G, et al. miRNA-7 and miRNA-324-5p regulate alpha9-Integrin expression and exert anti-oncogenic effects in rhabdomyosarcoma. Cancer Lett (2020) 477:49–59. doi: 10.1016/j.canlet.2020.02.035
76. Spenle C, Loustau T, Murdamoothoo D, Erne W, Beghelli-de la Forest Divonne S, Veber R, et al. Tenascin-C Orchestrates an Immune-Suppressive Tumor Microenvironment in Oral Squamous Cell Carcinoma. Cancer Immunol Res (2020) 8(9):1122–38. doi: 10.1158/2326-6066.CIR-20-0074
77. Zhang YL, Xing X, Cai LB, Zhu L, Yang XM, Wang YH, et al. Integrin alpha9 Suppresses Hepatocellular Carcinoma Metastasis by Rho GTPase Signaling. J Immunol Res (2018) 2018:4602570. doi: 10.1155/2018/4602570
78. Dmitriev AA, Rosenberg EE, Krasnov GS, Gerashchenko GV, Gordiyuk VV, Pavlova TV, et al. Identification of Novel Epigenetic Markers of Prostate Cancer by NotI-Microarray Analysis. Dis Markers (2015) 2015:241301. doi: 10.1155/2015/241301
79. Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res (2002) 8(9):2912–23.
80. Kilian O, Dahse R, Alt V, Zardi L, Hentschel J, Schnettler R, et al. mRNA expression and protein distribution of fibronectin splice variants and high-molecular weight tenascin-C in different phases of human fracture healing. Calcif Tissue Int (2008) 83(2):101–11. doi: 10.1007/s00223-008-9156-z
81. Nemeth JA, Harb JF, Barroso U Jr., He Z, Grignon DJ, Cher ML. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res (1999) 59(8):1987–93.
82. Adams JC, Chiquet-Ehrismann R, Tucker RP. The evolution of tenascins and fibronectin. Cell Adh Migr (2015) 9(1-2):22–33. doi: 10.4161/19336918.2014.970030
84. Thodeti CK, Frohlich C, Nielsen CK, Holck P, Sundberg C, Kveiborg M, et al. Hierarchy of ADAM12 binding to integrins in tumor cells. Exp Cell Res (2005) 309(2):438–50. doi: 10.1016/j.yexcr.2005.06.020
85. Eke I, Cordes N. Focal adhesion signaling and therapy resistance in cancer. Semin Cancer Biol (2015) 31:65–75. doi: 10.1016/j.semcancer.2014.07.009
86. Doble PA, Miklos GLG. Distributions of manganese in diverse human cancers provide insights into tumour radioresistance. Metallomics (2018) 10(9):1191–210. doi: 10.1039/c8mt00110c
87. Arihiro K, Kaneko M, Fujii S, Inai K, Yokosaki Y. Significance of alpha 9 beta 1 and alpha v beta 6 integrin expression in breast carcinoma. Breast Cancer (2000) 7(1):19–26. doi: 10.1007/BF02967183
88. Mostovich LA, Prudnikova TY, Kondratov AG, Loginova D, Vavilov PV, Rykova VI, et al. Integrin alpha9 (ITGA9) expression and epigenetic silencing in human breast tumors. Cell Adh Migr (2011) 5(5):395–401. doi: 10.4161/cam.5.5.17949
89. Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene (2017) 36(11):1461–73. doi: 10.1038/onc.2016.304
90. Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, et al. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A (2000) 97(8):4262–6. doi: 10.1073/pnas.060025397
91. Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology (2007) 39(3):305–18. doi: 10.1080/00313020701329914
92. Kwon A, Chae IH, You E, Kim SH, Ahn SY, Lee OJ, et al. Extra domain A-containing fibronectin expression in Spin90-deficient fibroblasts mediates cancer-stroma interaction and promotes breast cancer progression. J Cell Physiol (2020) 235(5):4494–507. doi: 10.1002/jcp.29326
93. Basora N, Desloges N, Chang Q, Bouatrouss Y, Gosselin J, Poisson J, et al. Expression of the alpha9beta1 integrin in human colonic epithelial cells: resurgence of the fetal phenotype in a subset of colon cancers and adenocarcinoma cell lines. Int J Cancer (1998) 75(5):738–43. doi: 10.1002/(sici)1097-0215(19980302)75:5<738::aid-ijc12>3.0.co;2-2
94. Morales C, Ribas M, Aiza G, Peinado MA. Genetic determinants of methotrexate responsiveness and resistance in colon cancer cells. Oncogene (2005) 24(45):6842–7. doi: 10.1038/sj.onc.1208834
95. Kel AE, Stegmaier P, Valeev T, Koschmann J, Poroikov V, Kel-Margoulis OV, et al. Multi-omics “upstream analysis” of regulatory genomic regions helps identifying targets against methotrexate resistance of colon cancer. EuPA Open Proteom (2016) 13:1–13. doi: 10.1016/j.euprot.2016.09.002
96. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature (2007) 445(7123):111–5. doi: 10.1038/nature05384
97. O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature (2007) 445(7123):106–10. doi: 10.1038/nature05372
98. Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology (2004) 145(12):5439–47. doi: 10.1210/en.2004-0959
99. Dasgupta R, Fuchs J, Rodeberg D. Rhabdomyosarcoma. Semin Pediatr Surg (2016) 25(5):276–83. doi: 10.1053/j.sempedsurg.2016.09.011
100. Hawkins DS, Spunt SL, Skapek SX, C.O.G.S.T.S. Committee. Children’s Oncology Group’s 2013 blueprint for research: Soft tissue sarcomas. Pediatr Blood Cancer (2013) 60(6):1001–8. doi: 10.1002/pbc.24435
101. Malempati S, Weigel BJ, Chi YY, Tian J, Anderson JR, Parham DM, et al. The addition of cixutumumab or temozolomide to intensive multiagent chemotherapy is feasible but does not improve outcome for patients with metastatic rhabdomyosarcoma: A report from the Children’s Oncology Group. Cancer (2019) 125(2):290–7. doi: 10.1002/cncr.31770
102. Häkkinen L, Kainulainen T, Salo T, Grenman R, Larjava H. Expression of integrin alpha9 subunit and tenascin in oral leukoplakia, lichen planus, and squamous cell carcinoma. Oral Dis (1999) 5(3):210–7. doi: 10.1111/j.1601-0825.1999.tb00303.x
103. van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol (2009) 45(4-5):317–23. doi: 10.1016/j.oraloncology.2008.05.016
104. Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science (2010) 328(5979):749–52. doi: 10.1126/science.1185837
105. Couvelard A, Bringuier AF, Dauge MC, Nejjari M, Darai E, Benifla JL, et al. Expression of integrins during liver organogenesis in humans. Hepatology (1998) 27(3):839–47. doi: 10.1002/hep.510270328
106. Olsen AL, Sackey BK, Marcinkiewicz C, Boettiger D, Wells RG. Fibronectin extra domain-A promotes hepatic stellate cell motility but not differentiation into myofibroblasts. Gastroenterology (2012) 142(4):928–37.e3. doi: 10.1053/j.gastro.2011.12.038
107. Baglieri J, Brenner DA, Kisseleva T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int J Mol Sci (2019) 20(7):1723. doi: 10.3390/ijms20071723
108. Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol (2006) 18(5):516–23. doi: 10.1016/j.ceb.2006.08.011
109. Aspenstrom P. Activated Rho GTPases in Cancer-The Beginning of a New Paradigm. Int J Mol Sci (2018) 19(12):3949. doi: 10.3390/ijms19123949
110. Ghosh A, Ghosh S, Maiti GP, Sabbir MG, Zabarovsky ER, Roy A, et al. Frequent alterations of the candidate genes hMLH1, ITGA9 and RBSP3 in early dysplastic lesions of head and neck: clinical and prognostic significance. Cancer Sci (2010) 101(6):1511–20. doi: 10.1111/j.1349-7006.2010.01551.x
111. Anedchenko EA, Dmitriev AA, Krasnov GS, Kondrat’eva TT, Kopantsev EP, Vinogradova TV, et al. [Down-regulation of RBSP3/CTDSPL, NPRL2/G21, RASSF1A, ITGA9, HYAL1 and HYAL2 genes in non-small cell lung cancer]. Mol Biol (Mosk) (2008) 42(6):965–76. doi: 10.1134/S0026893308060058
112. Aran D, Camarda R, Odegaard J, Paik H, Oskotsky B, Krings G, et al. Comprehensive analysis of normal adjacent to tumor transcriptomes. Nat Commun (2017) 8(1):1077. doi: 10.1038/s41467-017-01027-z
113. Sugahara S, Hanaoka K, Yamamoto N. Integrin, alpha9 subunit blockade suppresses collagen-induced arthritis with minimal systemic immunomodulation. Eur J Pharmacol (2018) 833:320–7. doi: 10.1016/j.ejphar.2018.06.021
114. Ohshima S, Kobayashi H, Yamaguchi N, Nishioka K, Umeshita-Sasai M, Mima T, et al. Expression of osteopontin at sites of bone erosion in a murine experimental arthritis model of collagen-induced arthritis: possible involvement of osteopontin in bone destruction in arthritis. Arthritis Rheum (2002) 46(4):1094–101. doi: 10.1002/art.10143
115. Page TH, Charles PJ, Piccinini AM, Nicolaidou V, Taylor PC, Midwood KS. Raised circulating tenascin-C in rheumatoid arthritis. Arthritis Res Ther (2012) 14(6):R260. doi: 10.1186/ar4105
116. Kon S, Uede T. The role of alpha9beta1 integrin and its ligands in the development of autoimmune diseases. J Cell Commun Signal (2018) 12(1):333–42. doi: 10.1007/s12079-017-0413-7
117. Kanayama M, Kurotaki D, Morimoto J, Asano T, Matsui Y, Nakayama Y, et al. Alpha9 integrin and its ligands constitute critical joint microenvironments for development of autoimmune arthritis. J Immunol (2009) 182(12):8015–25. doi: 10.4049/jimmunol.0900725
118. Asano T, Iwasaki N, Kon S, Kanayama M, Morimoto J, Minami A, et al. alpha9beta1 integrin acts as a critical intrinsic regulator of human rheumatoid arthritis. Rheumatology (Oxford) (2014) 53(3):415–24. doi: 10.1093/rheumatology/ket371
119. Kanayama M, Morimoto J, Matsui Y, Ikesue M, Danzaki K, Kurotaki D, et al. alpha9beta1 integrin-mediated signaling serves as an intrinsic regulator of pathogenic Th17 cell generation. J Immunol (2011) 187(11):5851–64. doi: 10.4049/jimmunol.1101524
120. McMurray RW. Adhesion molecules in autoimmune disease. Semin Arthritis Rheum (1996) 25(4):215–33. doi: 10.1016/s0049-0172(96)80034-5
121. Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med (1998) 338(7):436–45. doi: 10.1056/NEJM199802123380706
122. Szekanecz Z, Koch AE. Vascular involvement in rheumatic diseases: ‘vascular rheumatology’. Arthritis Res Ther (2008) 10(5):224. doi: 10.1186/ar2515
123. Tanaka D, Kagari T, Doi H, Shimozato T. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis. Immunology (2006) 119(2):195–202. doi: 10.1111/j.1365-2567.2006.02424.x
124. Schurgers E, Billiau A, Matthys P. Collagen-induced arthritis as an animal model for rheumatoid arthritis: focus on interferon-gamma. J Interferon Cytokine Res (2011) 31(12):917–26. doi: 10.1089/jir.2011.0056
125. Neumann E, Lefevre S, Zimmermann B, Gay S, Muller-Ladner U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol Med (2010) 16(10):458–68. doi: 10.1016/j.molmed.2010.07.004
126. Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev (2008) 223:252–70. doi: 10.1111/j.1600-065X.2008.00648.x
127. Torikai M, Higuchi H, Yamamoto N, Ishikawa D, Fujita H, Taguchi K, et al. A novel monoclonal antibody cross-reactive with both human and mouse alpha9 integrin useful for therapy against rheumatoid arthritis. J Biochem (2020) 168(3):231–41. doi: 10.1093/jb/mvaa040
128. Shahrara S, Castro-Rueda HP, Haines GK, Koch AE. Differential expression of the FAK family kinases in rheumatoid arthritis and osteoarthritis synovial tissues. Arthritis Res Ther (2007) 9(5):R112. doi: 10.1186/ar2318
129. Kon S, Yokosaki Y, Maeda M, Segawa T, Horikoshi Y, Tsukagoshi H, et al. Mapping of functional epitopes of osteopontin by monoclonal antibodies raised against defined internal sequences. J Cell Biochem (2002) 84(2):420–32. doi: 10.1002/jcb.10039
130. Sharif SA, Du X, Myles T, Song JJ, Price E, Lee DM, et al. Thrombin-activatable carboxypeptidase B cleavage of osteopontin regulates neutrophil survival and synoviocyte binding in rheumatoid arthritis. Arthritis Rheum (2009) 60(10):2902–12. doi: 10.1002/art.24814
131. Lund SA, Wilson CL, Raines EW, Tang J, Giachelli CM, Scatena M. Osteopontin mediates macrophage chemotaxis via alpha4 and alpha9 integrins and survival via the alpha4 integrin. J Cell Biochem (2013) 114(5):1194–202. doi: 10.1002/jcb.24462
132. Yamamoto N, Sakai F, Kon S, Morimoto J, Kimura C, Yamazaki H, et al. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest (2003) 112(2):181–8. doi: 10.1172/JCI17778
133. Kon S, Nakayama Y, Matsumoto N, Ito K, Kanayama M, Kimura C, et al. A novel cryptic binding motif, LRSKSRSFQVSDEQY, in the C-terminal fragment of MMP-3/7-cleaved osteopontin as a novel ligand for alpha9beta1 integrin is involved in the anti-type II collagen antibody-induced arthritis. PLoS One (2014) 9(12):e116210. doi: 10.1371/journal.pone.0116210
134. Uede T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol Int (2011) 61(5):265–80. doi: 10.1111/j.1440-1827.2011.02649.x
135. Xu S, Jiang C, Liu H, Zhang H, Liao H, Wang X, et al. Integrin-alpha9 and Its Corresponding Ligands Play Regulatory Roles in Chronic Periodontitis. Inflammation (2020) 43(4):1488–97. doi: 10.1007/s10753-020-01226-9
136. Ito K, Morimoto J, Kihara A, Matsui Y, Kurotaki D, Kanayama M, et al. Integrin alpha9 on lymphatic endothelial cells regulates lymphocyte egress. Proc Natl Acad Sci U S A (2014) 111(8):3080–5. doi: 10.1073/pnas.1311022111
137. Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol (2010) 184(2):535–9. doi: 10.4049/jimmunol.0903180
138. Kang GJ, Truong T, Huang E, Su V, Ge S, Chen L. Integrin Alpha 9 Blockade Suppresses Lymphatic Valve Formation and Promotes Transplant Survival. Invest Ophthalmol Vis Sci (2016) 57(14):5935–9. doi: 10.1167/iovs.16-20130
139. Altiok E, Ecoiffier T, Sessa R, Yuen D, Grimaldo S, Tran C, et al. Integrin Alpha-9 Mediates Lymphatic Valve Formation in Corneal Lymphangiogenesis. Invest Ophthalmol Vis Sci (2015) 56(11):6313–9. doi: 10.1167/iovs.15-17509
140. Mahar M, Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci (2018) 19(6):323–37. doi: 10.1038/s41583-018-0001-8
141. Endo Y, Ishiwata-Endo H, Yamada KM. Cell adhesion to anosmin via alpha5beta1, alpha4beta1, and alpha9beta1 integrins. Cell Adh Migr (2018) 12(2):93–100. doi: 10.1080/19336918.2016.1221568
142. Fawcett JW. An integrin approach to axon regeneration. Eye (Lond) (2017) 31(2):206–8. doi: 10.1038/eye.2016.293
143. Hu F, Strittmatter SM. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci (2008) 28(5):1262–9. doi: 10.1523/JNEUROSCI.1068-07.2008
144. Tan CL, Kwok JC, Patani R, Ffrench-Constant C, Chandran S, Fawcett JW. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci (2011) 31(17):6289–95. doi: 10.1523/JNEUROSCI.0008-11.2011
145. Andrews MR, Soleman S, Cheah M, Tumbarello DA, Mason MR, Moloney E, et al. Axonal Localization of Integrins in the CNS Is Neuronal Type and Age Dependent. eNeuro (2016) 3(4):ENEURO.0029-16.2016. doi: 10.1523/ENEURO.0029-16.2016
146. Tan CL, Andrews MR, Kwok JC, Heintz TG, Gumy LF, Fassler R, et al. Kindlin-1 enhances axon growth on inhibitory chondroitin sulfate proteoglycans and promotes sensory axon regeneration. J Neurosci (2012) 32(21):7325–35. doi: 10.1523/JNEUROSCI.5472-11.2012
147. Cheah M, Andrews MR, Chew DJ, Moloney EB, Verhaagen J, Fassler R, et al. Expression of an Activated Integrin Promotes Long-Distance Sensory Axon Regeneration in the Spinal Cord. J Neurosci (2016) 36(27):7283–97. doi: 10.1523/JNEUROSCI.0901-16.2016
148. Cheah M, Andrews MR. Targeting cell surface receptors for axon regeneration in the central nervous system. Neural Regener Res (2016) 11(12):1884–7. doi: 10.4103/1673-5374.197079
149. Pellinen T, Ivaska J. Integrin traffic. J Cell Sci (2006) 119(Pt 18):3723–31. doi: 10.1242/jcs.03216
150. Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, et al. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci (2008) 11(4):457–66. doi: 10.1038/nn2063
151. Welz T, Wellbourne-Wood J, Kerkhoff E. Orchestration of cell surface proteins by Rab11. Trends Cell Biol (2014) 24(7):407–15. doi: 10.1016/j.tcb.2014.02.004
152. Eva R, Dassie E, Caswell PT, Dick G, ffrench-Constant C, Norman JC, et al. Rab11 and its effector Rab coupling protein contribute to the trafficking of beta 1 integrins during axon growth in adult dorsal root ganglion neurons and PC12 cells. J Neurosci (2010) 30(35):11654–69. doi: 10.1523/JNEUROSCI.2425-10.2010
153. Franssen EH, Zhao RR, Koseki H, Kanamarlapudi V, Hoogenraad CC, Eva R, et al. Exclusion of integrins from CNS axons is regulated by Arf6 activation and the AIS. J Neurosci (2015) 35(21):8359–75. doi: 10.1523/JNEUROSCI.2850-14.2015
154. Eva R, Crisp S, Marland JR, Norman JC, Kanamarlapudi V, ffrench-Constant C, et al. ARF6 directs axon transport and traffic of integrins and regulates axon growth in adult DRG neurons. J Neurosci (2012) 32(30):10352–64. doi: 10.1523/JNEUROSCI.1409-12.2012
155. Forbes LH, Andrews MR. Grafted Human iPSC-Derived Neural Progenitor Cells Express Integrins and Extend Long-Distance Axons Within the Developing Corticospinal Tract. Front Cell Neurosci (2019) 13:26. doi: 10.3389/fncel.2019.00026
156. Tucker RP, Chiquet-Ehrismann R. Tenascin-C: Its functions as an integrin ligand. Int J Biochem Cell Biol (2015) 65:165–8. doi: 10.1016/j.biocel.2015.06.003
157. Brill A. Integrin alpha9beta1: a new target to fight thrombosis. Blood (2020) 135(11):787–8. doi: 10.1182/blood.2020004999
158. Kapoor S, Opneja A, Nayak L. The role of neutrophils in thrombosis. Thromb Res (2018) 170:87–96. doi: 10.1016/j.thromres.2018.08.005
159. Noubouossie DF, Reeves BN, Strahl BD, Key NS. Neutrophils: back in the thrombosis spotlight. Blood (2019) 133(20):2186–97. doi: 10.1182/blood-2018-10-862243
160. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost (2018) 16(2):231–41. doi: 10.1111/jth.13911
161. Shang T, Yednock T, Issekutz AC. alpha9beta1 integrin is expressed on human neutrophils and contributes to neutrophil migration through human lung and synovial fibroblast barriers. J Leukoc Biol (1999) 66(5):809–16. doi: 10.1002/jlb.66.5.809
162. Taooka Y, Ohe M, Chen L, Sutani A, Higashi Y, Isobe T. Increased expression levels of integrin alpha9beta1 and CD11b on circulating neutrophils and elevated serum IL-17A in elderly aspiration pneumonia. Respiration (2013) 86(5):367–75. doi: 10.1159/000345390
163. Mambole A, Bigot S, Baruch D, Lesavre P, Halbwachs-Mecarelli L. Human neutrophil integrin alpha9beta1: up-regulation by cell activation and synergy with beta2 integrins during adhesion to endothelium under flow. J Leukoc Biol (2010) 88(2):321–7. doi: 10.1189/jlb.1009704
164. Chilvers ER, Cadwallader KA, Reed BJ, White JF, Condliffe AM. The function and fate of neutrophils at the inflamed site: prospects for therapeutic intervention. J R Coll Physicians Lond (2000) 34(1):68–74.
165. Ross EA, Douglas MR, Wong SH, Ross EJ, Curnow SJ, Nash GB, et al. Interaction between integrin alpha9beta1 and vascular cell adhesion molecule-1 (VCAM-1) inhibits neutrophil apoptosis. Blood (2006) 107(3):1178–83. doi: 10.1182/blood-2005-07-2692
166. Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H, et al. Cell Type-Specific Roles of NF-kappaB Linking Inflammation and Thrombosis. Front Immunol (2019) 10:85. doi: 10.3389/fimmu.2019.00085
167. Saldanha-Gama RF, Moraes JA, Mariano-Oliveira A, Coelho AL, Walsh EM, Marcinkiewicz C, et al. alpha(9)beta(1) integrin engagement inhibits neutrophil spontaneous apoptosis: involvement of Bcl-2 family members. Biochim Biophys Acta (2010) 1803(7):848–57. doi: 10.1016/j.bbamcr.2010.03.012
168. Marcinkiewicz C, Taooka Y, Yokosaki Y, Calvete JJ, Marcinkiewicz MM, Lobb RR, et al. Inhibitory effects of MLDG-containing heterodimeric disintegrins reveal distinct structural requirements for interaction of the integrin alpha 9beta 1 with VCAM-1, tenascin-C, and osteopontin. J Biol Chem (2000) 275(41):31930–7. doi: 10.1074/jbc.M003209200
169. Nishimichi N, Higashikawa F, Kinoh HH, Tateishi Y, Matsuda H, Yokosaki Y. Polymeric osteopontin employs integrin alpha9beta1 as a receptor and attracts neutrophils by presenting a de novo binding site. J Biol Chem (2009) 284(22):14769–76. doi: 10.1074/jbc.M901515200
170. Banerjee A, Lee JH, Ramaiah SK. Interaction of osteopontin with neutrophil alpha(4)beta(1) and alpha(9)beta(1) integrins in a rodent model of alcoholic liver disease. Toxicol Appl Pharmacol (2008) 233(2):238–46. doi: 10.1016/j.taap.2008.08.008
171. Huang J, Bridges LC, White JM. Selective modulation of integrin-mediated cell migration by distinct ADAM family members. Mol Biol Cell (2005) 16(10):4982–91. doi: 10.1091/mbc.e05-03-0258
172. Amendola RS, Martin AC, Selistre-de-Araujo HS, Paula-Neto HA, Saldanha-Gama R, Barja-Fidalgo C. ADAM9 disintegrin domain activates human neutrophils through an autocrine circuit involving integrins and CXCR2. J Leukoc Biol (2015) 97(5):951–62. doi: 10.1189/jlb.3A0914-455R
173. Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science (2000) 287(5455):1049–53. doi: 10.1126/science.287.5455.1049
174. Dhanesha N, Nayak MK, Doddapattar P, Jain M, Flora GD, Kon S, et al. Targeting myeloid-cell specific integrin alpha9beta1 inhibits arterial thrombosis in mice. Blood (2020) 135(11):857–61. doi: 10.1182/blood.2019002846
175. Dhanesha N, Jain M, Tripathi AK, Doddapattar P, Chorawala M, Bathla G, et al. Targeting Myeloid-Specific Integrin alpha9beta1 Improves Short- and Long-Term Stroke Outcomes in Murine Models With Preexisting Comorbidities by Limiting Thrombosis and Inflammation. Circ Res (2020) 126(12):1779–94. doi: 10.1161/CIRCRESAHA.120.316659
176. Doring Y, Soehnlein O, Weber C. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ Res (2017) 120(4):736–43. doi: 10.1161/CIRCRESAHA.116.309692
177. Conrad C, Benzel J, Dorzweiler K, Cook L, Schlomann U, Zarbock A, et al. ADAM8 in invasive cancers: links to tumor progression, metastasis, and chemoresistance. Clin Sci (Lond) (2019) 133(1):83–99. doi: 10.1042/CS20180906
178. Midwood KS, Chiquet M, Tucker RP, Orend G. Tenascin-C at a glance. J Cell Sci (2016) 129(23):4321–7. doi: 10.1242/jcs.190546
179. Pang X, Gong K, Zhang X, Wu S, Cui Y, Qian BZ. Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol Res (2019) 144:235–44. doi: 10.1016/j.phrs.2019.04.030
180. Singh P, Chen C, Pal-Ghosh S, Stepp MA, Sheppard D, Van De Water L. Loss of integrin alpha9beta1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. J Invest Dermatol (2009) 129(1):217–28. doi: 10.1038/jid.2008.201
181. Takeuchi T, Tanaka Y, Erdman J, Kaneko Y, Saito M, Higashitani C, et al. ASP5094, a humanized monoclonal antibody against integrin alpha-9, did not show efficacy in patients with rheumatoid arthritis refractory to methotrexate: results from a phase 2a, randomized, double-blind, placebo-controlled trial. Arthritis Res Ther (2020) 22(1):252. doi: 10.1186/s13075-020-02336-3
182. Moreno-Layseca P, Icha J, Hamidi H, Ivaska J. Integrin trafficking in cells and tissues. Nat Cell Biol (2019) 21(2):122–32. doi: 10.1038/s41556-018-0223-z
183. Zeng HL, Chen D, Yan J, Yang Q, Han QQ, Li SS, et al. Proteomic characteristics of bronchoalveolar lavage fluid in critical COVID-19 patients. FEBS J (2020). doi: 10.1111/febs.15609
184. Tong M, Jiang Y, Xia D, Xiong Y, Zheng Q, Chen F, et al. Elevated Expression of Serum Endothelial Cell Adhesion Molecules in COVID-19 Patients. J Infect Dis (2020) 222(6):894–8. doi: 10.1093/infdis/jiaa349
185. Yin XX, Zheng XR, Peng W, Wu ML, Mao XY. Vascular Endothelial Growth Factor (VEGF) as a Vital Target for Brain Inflammation during the COVID-19 Outbreak. ACS Chem Neurosci (2020) 11(12):1704–5. doi: 10.1021/acschemneuro.0c00294
186. Buback F, Renkl AC, Schulz G, Weiss M. Osteopontin and the skin: multiple emerging roles in cutaneous biology and pathology. Exp Dermatol (2009) 18(9):750–9. doi: 10.1111/j.1600-0625.2009.00926.x
Keywords: integrin-α9β1, cancer, autoimmune diseases, axon regeneration, thrombosis
Citation: Xu S, Zhang T, Cao Z, Zhong W, Zhang C, Li H and Song J (2021) Integrin-α9β1 as a Novel Therapeutic Target for Refractory Diseases: Recent Progress and Insights. Front. Immunol. 12:638400. doi: 10.3389/fimmu.2021.638400
Received: 06 December 2020; Accepted: 26 February 2021;
Published: 15 March 2021.
Edited by:
Jon Humphries, The University of Manchester, United KingdomReviewed by:
Stephen Doulgas Robinson, Quadram Institute, United KingdomVinay Shankar Swaminathan, Lund University, Sweden
Matt Jones, The University of Manchester, United Kingdom
Copyright © 2021 Xu, Zhang, Cao, Zhong, Zhang, Li and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinlin Song, c29uZ2ppbmxpbkBob3NwaXRhbC5jcW11LmVkdS5jbg==; orcid.org/0000-0002-0224-6640
 Shihan Xu1,2,3
Shihan Xu1,2,3 Zhengguo Cao
Zhengguo Cao Jinlin Song
Jinlin Song