
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 04 May 2021
Sec. Alloimmunity and Transplantation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.637512
This article is part of the Research TopicThe Immunotherapeutic Potential of Allogeneic Hematopoietic Stem Cell Transplantation (HSCT)View all 10 articles
 Andrea Bacigalupo1,2*
Andrea Bacigalupo1,2* Idanna Innocenti1
Idanna Innocenti1 Elena Rossi1,2
Elena Rossi1,2 Federica Sora1,2
Federica Sora1,2 Eugenio Galli1
Eugenio Galli1 Francesco Autore1
Francesco Autore1 Elisabetta Metafuni1
Elisabetta Metafuni1 Patrizia Chiusolo1,2
Patrizia Chiusolo1,2 Sabrina Giammarco1
Sabrina Giammarco1 Luca Laurenti1,2
Luca Laurenti1,2 Giulia Benintende2
Giulia Benintende2 Simona Sica1,2
Simona Sica1,2 Valerio De Stefano1,2
Valerio De Stefano1,2The aim of this review is to update the current status of allogeneic hemopoietic stem cell transplants (HSCT) for patients with myelofibrosis (MF). We have first summarized the issue of an indication for allogeneic HSCT, discussing several prognostic scoring systems, developed to predict the outcome of MF, and therefore to identify patients who will benefit of an allogeneic HSCT. Patients with low risk MF are usually not selected for a transplant, whereas patients with intermediate or high risk MF are eligible. A separate issue, is how to predict the outcome of HSCT: we will outline a clinical molecular myelofibrosis transplant scoring system (MTSS), which predicts overall survival, ranging from 90% for low risk patients, to 20% for very high risk patients. We will also discuss transfusion burden and spleen size, as predictors of transplant outcome. The choice of a transplant platform including the conditioning regimen, the stem cell source and GvHD prophylaxis, are crucial for a successful program in MF, and will be outlined. Complications such as poor graft function, graft failure, GvHD and relapse of the disease, will also be reviewed. Finally we discuss monitoring the disease after HSCT with donor chimerism, driver mutations and hematologic data. We have made an effort to make this review as comprehensive and up to date as possible, and we hope it will provide some useful data for the clinicians.
In the era of JAK inhibitors, allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative treatment for patients with Myelofibrosis (MF) (1). The American Society for Transplantation and Cellular Therapy (ASTCT) considers an allogeneic HSCT “standard of care with clinical evidence” for patients with intermediate and high risk disease (2). In order to classify patients as intermediate or high risk several models have been developed. Table 1 outlines some of the most commonly used scoring systems and the variables they are based on: IPSS (3), DIPSS (4), DIPSS-plus (5) and MIPSS70 (6). The first two are based exclusively on clinical data, the third incorporates cytogenetics and the fourth includes mutational analysis. Survival of patients with MF can be predicted using one of those models, and thus eligibility for a transplant procedure. However eligibility must also include transplant related variables, such as patients age up to 70-75 years, a good performance status, low transfusion burden, absence of a massive splenomegaly and portal hypertension and donor type. Older patients also tend to have one or more comorbidities which may increase the risk of transplant related mortality (TRM) or even preclude a transplant approach. A Panel of experts recommends considering allogeneic HSCT for patients with IPSS/DIPSS/DIPSS plus high or intermediate-2 risk (7) (Figure 1). The Panel also recommends considering an allogeneic HSCT for transplant-eligible patients with IPSS/DIPSS/DIPSS-Plus intermediate-1 risk score, who present with either refractory, transfusion-dependent anemia, a percentage of blasts in peripheral blood > 2% in at least two repeated manual measurements, adverse cytogenetics, or high-risk mutations, such as such as ASXL1, EZH2, IDH1/IDH2, SRSF2 (7)(Figure 1). In this situation, the transplant procedure should be performed in a controlled setting (registries, clinical trial) (7).
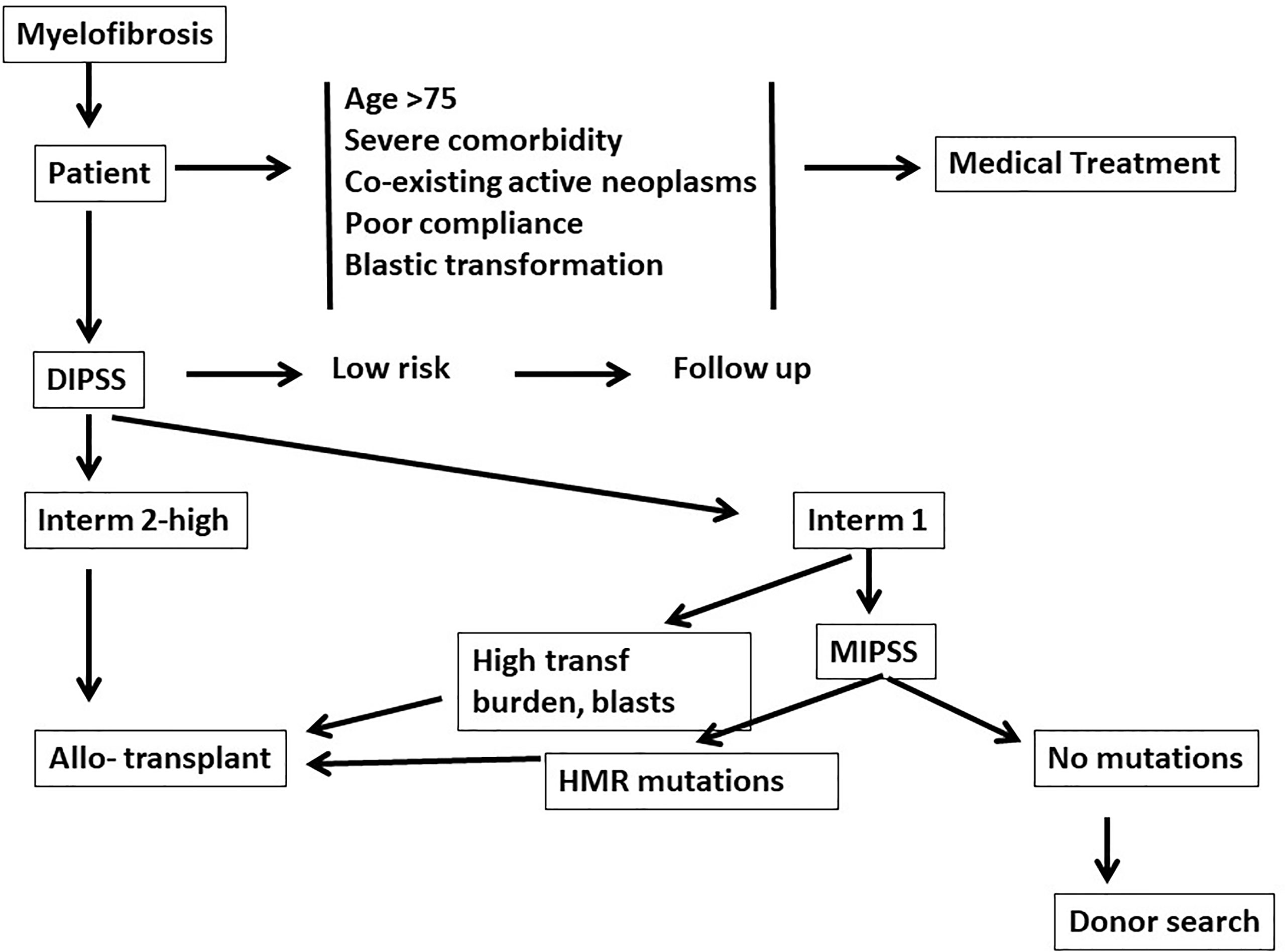
Figure 1 Eligibility for a transplant procedure in patients with myelofibrosis: medical treatment should be offered for older patients (>75 years) and/or patients with comorbidities. Dynamic international prognostic scoring system (DIPSS) will then identify patients low risk patients, who should be followed. DIPSS-intermediate 2 and high risk patients are who are strong candidates for an allogeneic transplant. DIPSS-Intermediate 1 patients with a high transfusion burden and blasts counts are also strong candidates for an allogeneic transplant. Patients may also be studied with a molecular international prognostic scoring system (MIPSS), and may be eligible for transplantation if high risk mutations (HMR) (see text) are identified.
More recently a mutation-based prognostic model has been proposed to identify candidates for HSCT among low or intermediate-1 risk DIPSS, who are expected to have similar overall survival as patients with a high risk DIPSS (8). Patients who are triple negative (JAK2/CALR/MPL) or CALR wild type and ASXL1 mutated, irrespective of DIPSS risk scores, should be considered for HSCT (8). A combination of mutation-based prognosis together with clinical data has been compiled in a recent scoring system (9).
In conclusion, we are now able to identify MF patients with a different median survival: there is consensus on the eligibility to transplant for DIPSS intermediate2/high risk patients. The presence of high risk mutations in DIPSS intermediate1/low risk patients may also suggest eligibility for a transplant procedure. The clinical conditions of the patient, the degree of HLA matching of potential donors and the patient’s choice must be considered in the final decision to transplant or not.
Splenomegaly is a common feature in patients with advanced myelofibrosis (MF) and it is a sign of extramedullary hematopoiesis (also known as myeloid metaplasia) (10). Patients may be severely symptomatic with abdominal pain, early satiety, weight loss, cytopenia, portal hypertension, and splenic infarction (10).
Splenectomy is effective in relieving symptoms, but is associated with a number of complications, as well as significant morbidity and mortality.
Peri-operative mortality is in the range of 5%-10%. The most common complications are infections, thrombosis and bleeding, occurring in up to 30% of patients (11). Patients with thrombocytopenia seemed to have an increased probability of post-splenectomy blast transformation, although this did not result in shortened survival. Leukemic transformation is more probably related to natural progression of the disease in advanced stage and to post-splenectomy redistribution of circulating blasts, not to true clonal evolution (12, 13). Hemopoietic stem cell transplantation (HSCT) offers the potential of cure for patients with intermediate or high risk myelofibrosis (14). Splenomegaly, characteristic of those patients, may lead to sequestration of transplanted stem cells and delayed hematologic recovery (3, 15) thus affecting the transplant outcome. Surgical removal of the spleen may be effective in reducing the time for neutrophil and platelet recovery (16) but its impact on relapse rate and survival is unclear (17, 18), calling for a prospective randomized trial. Pre-transplant splenectomy in MF patients was associated with a prolonged overall and event-free survival in a recently published study (19).
The advent of Janus kinase (JAK) 1/2 inhibitors, which decrease splenomegaly and alleviate MF-related symptoms, has had, as compared to old cytoreductive drugs, a major impact on the management of splenomegaly, removing some indications for splenectomy. However, in a proportion of patients, the splenic response is then lost. Many MF patients who proceed to allogeneic HSCT, are currently treated with JAK inhibitors, usually ruxolitinib: this should be tapered down over a 10- to 14-day period and should be discontinued just before the conditioning regimen (20). In one study, ruxolitinib was continued also during transplant in the attempt of preventing GvHD (21).
Splenic irradiation (SI) may also be used to reduce the spleen size and related symptoms; there are only few small studies on SI prior to transplant in MF patients (22, 23). It was demonstrated that SI alleviates splenic discomfort and reduces spleen size in a majority of MF patients, with a median duration of response of 6 months (24). Limitations of SI include prolonged pancytopenia with infectious complications. Comparable engraftment rate has been shown in patients receiving or not SI (25) as well as comparable acute and chronic GVHD incidence, post-transplant infectious complications and survival. The role of SI in leukemic transformation (LT) remains unclear and speculative. Radiotherapy may be indicated in patients who are not eligible for surgery or in patients who have lost their response to JAK2 inhibitors (26–28).
Disease based risk score. Survival of MF patients receiving medical treatment, with the exclusion of allogeneic HSCT, can be predicted by several scoring systems, reviewed in Indications for HSCT (3–7). Some studies have assessed whether these scoring systems can predict the outcome of patients after an allogeneic HSCT. DIPSS can predict post transplant survival (29), and the same has been shown for DIPSS-plus (30). In multivariate analysis, the DIPPS-plus score predicted survival, disease free survival (DFS) and TRM, together with conditioning regimen, comorbidity index (HCT-CI), patients’ age and donor type (30). In 2019, a cohort of 159 patients with secondary myelofibrosis who underwent allogeneic HSCT was analyzed retrospectively to compare the predictive value of DIPSS and MYSEC (31). The four risk groups of DIPSS did not predict survival after allogeneic HSCT, whereas MYSEC maintained its predictive role also in the post-transplant setting.
Transplant based risk score (TS). Few scoring systems have been designed exclusively for allogeneic HSCT. In 2010, a study identified spleen size, transfusion history and donor type as predictive of outcome: survival was 79% for low risk patients and 8% for high risk patients (18). In 2012 a predictive risk model including JAKV617F status, age and constitutional symptoms was proposed in the setting of 150 transplanted patients and resulted to be predictive for 5 years overall survival (OS) (32).
More recently, a scoring system has been devised, which incorporates HLA matching between donor and recipient, mutational analysis, and clinical data, at time of transplantation (MTSS), in patient with primary and secondary MF (9). This index is predictive of non-relapse mortality. In the last year we have revisited our transplant score (TS) including maximum spleen size and red blood cell transfusion burden before HSCT (18): the 5 year disease free survival (DFS) was 74% vs 36% (p=0.0001) for patients with low or high TS.
In conclusion, scoring systems designed to predict transplant outcome are available and can be used when counseling patients eligible for transplant procedures.
Donor type is an important predictor of outcome in myelofibrosis: a study from the Center for International Blood and Marrow Transplant Research (CIBMTR) on 233 transplants for myelofibrosis (33), showed that donor type was an independent risk factor for TRM, with a relative risk of death of 3.92 for matched unrelated donor (MUD) and 9.37 for mismatched unrelated donor (MMUD), when compared to matched related donor (MRD) (33). The 5 year overall survival was 56% for MRD, 48% for MUD and 34% for MMUD. The main causes of death were GvHD, infections and organ failure, in particular among MMUD grafts (33). Similar results are reported in other studies (17, 34–37). On the other hand, contrasting data exist regarding GvHD and donor type. Some studies show no significant difference among different donor types (17, 35, 38), whereas the CIBMTR shows a higher risk of GvHD for patients receiving MUD (RR 1.98) and MMUD (RR 1.52) as compared to MRD (33). Engraftment is reported to be comparable according to donor type (33, 35, 38), whereas significant differences have been described according to the stem cell source, with faster recovery with peripheral blood grafts (37, 38). Unrelated cord blood (UCB) transplants have been rarely used in myelofibrosis, and are associated with delayed engraftment and a high TRM, probably due to the significant risk for graft failure and infectious complications (39).
The addition of ATG to conventional GvHD prophylaxis, based on calcineurin inhibitor alone or combined to methotrexate or mycophenolic acid, reduces the incidence of GvHD, as one would expect (40). However, modified regimens of GvHD prophylaxis, including the use of post-transplant cyclophosphamide (PT-CY) have reduced post-transplant complications in alternative donor grafts, especially for HLA haplo-identical donor (36). A combination of calcineurin inhibitor with ATG and PTCy after reduced intensity conditioning may further reduce the risk of GvHD, improving TRM and survival, without an increased risk of relapse (41). Very recently, an interesting pilot study was conducted by Morozova and colleagues: GvHD prophylaxis with PTCY and ruxolitinib showed promising results in terms of GvHD control in a small cohort of patients with acceptable TRM (42).
In summary an HLA matched donor is the best option for myelofibrosis, in order to achieve optimal outcome: alternative donor grafts may be explored using modified regimens of GvHD prophylaxis.
Conditioning regimens in myelofibrosis were historically myeloablative (MAC), predominantly busulfan plus cyclophosphamide and total body irradiation with or without cyclophosphamide (15), but transplant related mortality (TRM) and GvHD rates were high, especially in older individuals (43).
Reduced intensity conditioning (RIC) has been increasingly used in MF, in consideration of the older age of MF patients. The first prospective EBMT multicenter phase II trial of RIC SCT consisted of busulfan (10 mg/kg) orally (or equivalent IV dose) plus fludarabine (180 mg/m2) and in vivo T-cell depletion with anti-thymocyte globulin at a dose of 3 x 10 mg/kg (for related transplantation) or 3 x 20 mg/kg (for unrelated donor transplantation): this protocol resulted in low rates of primary graft failure and rapid hematologic recovery (17). Fludarabine 90 mg/m^2, combined with melphalan 140 mg/m^2 (FLU-MEL) is an alternative RIC regimen, and has been compared in a retrospective study with the BU-FLU regimen (44). Although the FLU-MEL was associated with increased early toxicity, the long-term outcome (OS and disease-free survival) was similar in the two groups. In both regimens the use of a HLA mismatched unrelated donor was associated with worse outcome, in terms of TRM, OS and progression-free survival. A randomized study comparing fludarabine in combination with busulfan 10 mg/kg i.v. or thiotepa 12 mg/kg, failed to identify significant differences in terms of clinical outcome (45): both regimens were associated with a significant degree of mixed chimerism.
In a retrospective comparisons of RIC versus MAC regimens for myelofibrosis, the latter do not appear to protect patients from relapse (46), neither there are differences within day +100 transplant-related mortality (47). A large retrospective analysis of the EBMT in 2224 patients with myelofibrosis, compared MAC regimens (781 patients) with RIC regimens (1443 patients) (48): there was no statistically significant difference in engraftment, GvHD, TRM and overall survival; there was a trend toward a higher relapse rate with RIC.
We have recently shown that a conditioning regimen including two alkylating agents (in our case busulfan and thiotepa) with fludarabine, significantly reduced the risk of relapse when compared to regimen with one alkylating agent (either busulfan or thiotepa or melphalan) in combination with fludarabine (36). Therefore, the choice of the conditioning regimen, may play a significant role in determining the control of the disease after an allogeneic HSCT.
The efficacy of the JAK1/JAK2 inhibitor ruxolitinib in reducing spleen size and systemic symptoms, in myelofibrosis, has been established (49, 50). Currently, most patients undergoing an allogeneic HSCT have been treated with this agent with the aim of reducing splenomegaly, improving the performance status and shorten time to engraftment. A phase II trial demonstrated the feasibility of ruxolitinib therapy followed by a RIC regimen for patients with myelofibrosis (51). Appropriate tapering should be scheduled (52), although recently peri-transplant ruxolitinib has been reported (42). There is no evidence, however, that the administration of ruxolitinib pre-transplant reduces the incidence of relapse after transplant.
Patients with myelofibrosis may have one of three driver mutations (JAK2, CALR and MPL), or lack all three (triple negative patients). Ditschkowski et al. (53) showed that survival after transplantation was not significantly different for JAK2+ (75%) versus JAK2 negative (71%) patients. More recent retrospective studies have suggested a survival advantage for CALR mutation (54, 55). A large retrospective study has investigated the role of extensive mutational profiling with a targeted 16-gene panel, and has confirmed the favorable role of a CALR mutation (56). In the same study IDH2 and ASXL1 mutations confirmed their adverse prognostic role after allogenic HSCT, whereas a triple negative status (JAK2, MPL, CALR) did not appear to modify the outcome after transplant.
Minimal residual disease (MRD) should be used to identify patients achieving a complete remission after HSCT, as well as an early evidence of relapse. Alchalby et al. has shown that JAK2 negativity after allogeneic HSCT significantly reduces the risk of relapse (57). Similar results have been obtained with MPL and CALR mutations as MRD markers (58). A recent retrospective single-center study (59) has shown that that patients with detectable mutations on day +100 or at day +180 after allogeneic HSCT have a significant higher risk of clinical relapse at 5 years, as compared to molecular-negative patients (62% vs 10%, P<0.001 and 70% vs 10%, P<0.001, respectively): single different mutations have comparable predictive value on relapse.
However, 10% to 15% of patients are triple negative and cannot be followed after transplantation with a molecular marker: in these patients chimerism studies can be helpful to identify early signs of relapse. We have recently described 120 patients with chimerism data on day +30 (60), showing that early full donor chimerism is highly predictive of long-term disease control. The cumulative incidence of relapse at 5 years, was 14% vs 40% for patients with or without full donor chimerism (40). We found that a conditioning regimen including two alkylating agents (busulfan and thiotepa) induces a significantly higher rate of complete donor chimerism on day +30, as compared to patients prepared with one alkylating agent (either busulfan, melphalan or thiotepa) (87% vs 45%, p<0.0001).
MRD positive patients or patients with declining donor chimerism, who still are receiving immunosuppressive therapy, may discontinue immunosuppressive drugs and/or receive donor lymphocyte infusions (DLI), in order to achieve again full donor chimerism.
Lack of engraftment of donor stem cells is referred to as primary graft failure (PrGF), and is characterized by neutropenia, combined with mixed or no donor chimerism on bone marrow and/or peripheral blood cells (61). PrGF should be distinguished from poor graft function, or cytopenia with full donor chimerism (62). The latter suggests inappropriate function of engrafted donor stem cells and can be treated with the infusion of selected CD34+ cells from the same donor, without a preparative regimen (62). Predictive factors have not been determined, but several conditions have been associated with unsuccessful engraftment, such as the intensity of the conditioning regimen, donor type, stem cells source, number of CD34+ cells infused, GvHD prophylaxis, degree of fibrosis, degree of splenomegaly, pre-transplant thrombocytopenia (63).
The incidence of PrGF ranges from 2 to 24%. A lower rate was reported in a large prospective study from EBMT (48), with only in 2 out 103 patients with PrGF. However, 11% of patients experienced poor graft function and required an additional stem cell boost. In a subsequent pilot study, PrGF was not influenced by the intensity of conditioning regimen (64) and no other predictors were found in other studies (17, 53). Donor type appears to influence the incidence of PrGF, which is lower in patients transplanted from HLA identical donors, as compared to transplants from family mismatched and unrelated donors (65–67). Contrasting data are reported on other factors: splenectomy before HSCT, peripheral stem cell use as source of stem cells and the absence of pre-transplant thrombocytopenia have been suggested to promote engraftment in some studied (18, 66, 68), but not in other studies (65).
Patients with full donor engraftment, may still have transfusion dependent low blood counts for variable periods of time, and this is referred to as Poor graft function (PGF). In a large retrospective analysis, the proportion of patients with less than 20x10^9/l platelets between day +50 and +100 after an allogeneic HSCT, is 10% and has not changed in the time period before 2000, 2001-2010 and beyond 2010 (unpublished). A diagnosis of myelofibrosis is a negative predictor for hematologic recovery: a low platelet count is seen in 18% vs 8% of patients with or without a diagnosis of MF (unpublished). For this reason, when looking at patients receiving a top up of CD34 selected cells for PGF, the proportion of patients with MF (26%) is higher than the proportion of MF in the transplant indications (7%) (62). These patients may remain transfusion dependent for long periods of time, and may be treated either with an infusion of CD34 selected cells from the same donor, or, more recently with high dose eltrombopag. Time to trilineage recovery is however delayed with these approaches and long-lasting supportive care must be planned.
Allogeneic hematopoietic stem cell transplant remains the only curative treatment for myelofibrosis (MF). A retrospective EBMT study on 1055 patients with MF transplanted between 1995 and 2014, alive and free of their disease at two years after HSCT showed that the most common cause of death (41-61%) was relapse of MF, for all time periods (2-5years, 5-10 years) (40). There is no standardized re-treatment of relapse after allogeneic transplant. Based on limited available literature, ruxolitinib, donor leukocytes infusion (DLI), and a second allogenic HSCT are three options for relapsing MF patients; obviously, the choice depends on patients age, fitness status, molecular or hematologic relapse, and the presence of GVHD.
The use of DLI and second transplant as salvage treatment for relapsed MF after allogeneic HSCT was reported in a retrospective study some years ago (69). Out of 26 relapsed patients, 39% achieved a stable response to dose-escalated DLIs. Seventeen patients, thirteen of which non-responders to DLI, underwent a second allogeneic HSCT, achieving an ORR of 80% (9 CR and 3 PR); incidence of relapse at 1-year was 24%. The 2-year overall survival and progression-free survival were 70% and 67%, respectively.
The most consistent data derive from a recent EBMT real-life retrospective study focusing on the treatment of 251/1371 (18%) MF patients, who relapsed after an allogeneic HSCT (70). DLIs were used in 23% of patients, whereas 20% underwent DLI combined with chemotherapy and 11% had chemotherapy alone. Fifty-one patients (25%) underwent second allogeneic HSCT alone and 26 (13%) underwent DLI and a second allogeneic HSCT. The median OS from the time of relapse for patients receiving DLI alone, DLI followed by a second allogeneic HSCT or second allogeneic HSCT were 76 months, 54 months, and 27 months respectively.
Recently Chabra et al. published a small number of MF patients, mostly treated with ruxolitinb pre-transplant (71): after a median follow up of >3 years, two patients out of 37 had relapsed after HSCT (5.4%), but the study lacked a strong control group of untreated ruxolitinib patients. Indeed other recently published data in the ruxolitinib era (72), have shown no improvement in survival nor in the incidence of relapse for MF. The use of ruxolitinib after allogenic HSCT is primarily attributable to the treatment of GVHD, and only in few cases for the treatment of the relapse, mostly in combination with DLIs. One study has reported peri-transplant use of ruxolitinib (21).
In conclusion, although based on a small number of studies, the best therapeutic strategy for MF patients relapsing after an allogeneic HSCT, seems to be dose -escalated DLI, or otherwise, for non-responders, a second allogeneic HSCT. The question remains whether DLI should be infused after a lympho-depleting treatment, as currently is being done for CAR-T cells.
Patients with myelofibrosis need to be discussed to identify eligibility for transplant procedures (Figure 1). Patients over the age of 75 years, with severe comorbidities, coexisting active neoplasms, or poor compliance, should be addressed by medical treatment. Patients less than 75 years of age and fit, should be assessed for risk factors (DIPSS or other scoring systems): low risk patients should be followed regularly. DIPSS intermediate 2 or high risk patients are eligible for a transplant procedure (Figure 1). DIPSS int 1 patients should be studied with next generation sequencing (NGS): if no additional adverse mutations are found (ASXL1, EZH2, SRSF2, IDH1/2) then the search for a donor can be initiated, but the transplant may be postponed. If, on the contrary, additional adverse mutations are identified the donor search may be initiated and the transplant also programmed.
Once a transplant is programmed several facts need to be considered: in addition to patient factors such as age, comorbidities and disease phase (DIPSS), other facts need to be taken in to account, including transplant variables (donor type, stem cell source, conditioning regimen, GvHD prophylaxis), the psychological status of the patient, the presence of care givers, especially for the post-transplant discharge and logistics (transplant centers may be located at a distance from the patients’ home). The combination of all these factors will then lead to a tailored strategy in terms of optimal timing and choice of a transplant platform.
AB, SS and VS designed the study and overviewed the manuscript. Sections and authors: indications (II), splenectomy (ER), predicting outcome (FS, EG), monitoring disease (PC), Graft Failure (SG), Relapse (LL), Conditioning regimens (FA), donor type (EM), reviewed MS (GB). All authors contributed to the article and approved the submitted version.
This study was partly funded by AIRC, Associazione Italiana Ricerca contro il Cancro; grant to AB.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Tefferi A, Partain DK, Palmer JM, Slack JL, Roy V, Hogan WJ, et al. Allogeneic hematopoietic stem cell transplant overcomes the adverse survival effect of very high risk and unfavorable karyotype in myelofibrosis. Am J Hematol (2018) 93(5):649–54. doi: 10.1002/ajh.25053
2. Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, et al. Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant (2020) 26(7):1247–56. doi: 10.1016/j.bbmt.2020.03.002
3. Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood (2009) 113(13):2895–901. doi: 10.1182/blood-2008-07-170449
4. Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood (2010) 115(9):1703–8. doi: 10.1182/blood-2009-09-245837
5. Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol (2011) 29(4):392–7. doi: 10.1200/JCO.2010.32.2446
6. Guglielmelli P, Lasho TL, Rotunno G, Mudireddy M, Mannarelli C, Nicolosi M, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients With Primary Myelofibrosis. J Clin Oncol (2018) 36(4):310–8. doi: 10.1200/JCO.2017.76.4886
7. Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia (2018) 32(5):1057–69. doi: 10.1038/s41375-018-0077-1
8. Tefferi A, Nicolosi M, Mudireddy M, Szuber N, Finke CM, Lasho TL, et al. Driver mutations and prognosis in primary myelofibrosis: Mayo-Careggi MPN alliance study of 1,095 patients. Am J Hematol (2018) 93(3):348–55. doi: 10.1002/ajh.24978
9. Gagelmann N, Ditschkowski M, Bogdanov R, Bredin S, Robin M, Cassinat B, et al. Comprehensive clinical-molecular transplant scoring system for myelofibrosis undergoing stem cell transplantation. Blood (2019) 133(20):2233–42. doi: 10.1182/blood-2018-12-890889
10. Santos FP, Tam CS, Kantarjian H, Cortes J, Thomas D, Pollock R, et al. Splenectomy in patients with myeloproliferative neoplasms: efficacy, complications and impact on survival and transformation. Leuk Lymphoma (2014) 55(1):121–7. doi: 10.3109/10428194.2013.794269
11. Cervantes F. How I treat splenomegaly in myelofibrosis. Blood Cancer J (2011) 1(10):e37. doi: 10.1038/bcj.2011.36
12. Tefferi A, Mesa RA, Nagorney DM, Schroeder G, Silverstein MN. Splenectomy in myelofibrosis with myeloid metaplasia: a single-institution experience with 223 patients. Blood (2000) 95(7):2226–33. doi: 10.1182/blood.V95.7.2226.007k19_2226_2233
13. Tefferi A, Mudireddy M, Gangat N, Hanson CA, Ketterling RP, Pardanani A, et al. Risk factors and a prognostic model for postsplenectomy survival in myelofibrosis. Am J Hematol (2017) 92(11):1187–92. doi: 10.1002/ajh.24881
14. Guardiola P, Anderson JE, Bandini G, Cervantes F, Runde V, Arcese W, et al. Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European Group for Blood and Marrow Transplantation, Société Française de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center Collaborative Study. Blood (1999) 93(9):2831–8. doi: 10.1182/blood.V93.9.2831
15. Martino R, Altés A, Muñiz-Díaz E, Brunet S, Sureda A, Domingo-Albós A, et al. Reduced transfusion requirements in a splenectomized patient undergoing bone marrow transplantation. Acta Haematol (1994) 92(3):167–8. doi: 10.1159/000204213
16. von Bueltzingsloewen A, Bordigoni P, Dorvaux Y, Witz F, Schmitt C, Chastagner P, et al. Splenectomy may reverse pancytopenia occurring after allogeneic bone marrow transplantation. Bone Marrow Transpl (1994) 14(2):339–40.
17. Kröger N, Holler E, Kobbe G, Bornhäuser M, Schwerdtfeger R, Baurmann H, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood (2009) 114(26):5264–70. doi: 10.1182/blood-2009-07-234880
18. Bacigalupo A, Soraru M, Dominietto A, Pozzi S, Geroldi S, Van Lint MT, et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant (2010) 45(3):458–63. doi: 10.1038/bmt.2009.188
19. Robin M, Zine M, Chevret S, Meignin V, Munoz-Bongrand N, Moatti H, et al. The Impact of Splenectomy in Myelofibrosis Patients before Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transpl (2017) 23(6):958–64. doi: 10.1016/j.bbmt.2017.03.002
20. McLornan DP, Yakoub-Agha I, Robin M, Chalandon Y, Harrison CN, Kroger N. State-of-the-art review: allogeneic stem cell transplantation for myelofibrosis in 2019. Haematologica (2019) 104(4):659–68. doi: 10.3324/haematol.2018.206151
21. Kröger N, Shahnaz Syed Abd Kadir S, Zabelina T, Badbaran A, Christopeit M, Ayuk F, et al. Peritransplantation Ruxolitinib Prevents Acute Graft-versus-Host Disease in Patients with Myelofibrosis Undergoing Allogenic Stem Cell Transplantation. Biol Blood Marrow Transpl (2018) 24(10):2152–6. doi: 10.1016/j.bbmt.2018.05.023
22. Kalman NS, Mukhopadhyay ND, Roberts CH, Chung HM, Clark WB, McCarty JM, et al. Low-dose splenic irradiation prior to hematopoietic cell transplantation in hypersplenic patients with myelofibrosis. Leuk Lymphoma (2017) 58(12):2983–4. doi: 10.1080/10428194.2017.1321747
23. Vyas OH, Kaul E, Rosenberg AS, Gunjan L, Shah UA, Comenzo RL, et al. Splenic Irradiation and a Reduced-Intensity Conditioning Regimen Prior to Allogeneic Stem-Cell Transplantation for Myelofibrosis. Blood (2014) 124(21):3170. doi: 10.1182/blood.V124.21.3170.3170
24. Elliott MA, Chen MG, Silverstein MN, Tefferi A. Splenic irradiation for symptomatic splenomegaly associated with myelofibrosis with myeloid metaplasia. Br J Haematol (1998) 103(2):505–11. doi: 10.1046/j.1365-2141.1998.00998.x
25. Akpek G, Pasquini MC, Logan B, Agovi MA, Lazarus HM, Marks DI, et al. Effects of spleen status on early outcomes after hematopoietic cell transplantation. Bone Marrow Transpl (2013) 48(6):825–31. doi: 10.1038/bmt.2012.249
26. Bouabdallah R, Coso D, Gonzague-Casabianca L, Alzieu C, Resbeut M, Gastaut JA. Safety and efficacy of splenic irradiation in the treatment of patients with idiopathic myelofibrosis: a report on 15 patients. Leuk Res (2000) 24(6):491–5. doi: 10.1016/s0145-2126(00)00018-7
27. Wagner H, McKeough PG, Desforges J, Madoc-Jones H. Splenic irradiation in the treatment of patients with chronic myelogenous leukemia or myelofibrosis with myeloid metaplasia. Results of daily and intermittent fractionation with and without concomitant hydroxyurea. Cancer (1986) 58(6):1204–7. doi: 10.1002/1097-0142(19860915)58:6<1204::aid-cncr2820580605>3.0.co;2-g
28. Malato A, Rossi E, Tiribelli M, Mendicino F, Pugliese N. Splenectomy in Myelofibrosis: Indications, Efficacy, and Complications. Clin Lymphoma Myeloma Leuk (2020) 20(9):588–95. doi: 10.1016/j.clml.2020.04.015
29. Kröger N, Giorgino T, Scott BL, Ditschkowski M, Alchalby H, Cervantes F, et al. Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofibrosis. Blood (2015) 125(21):3347–50; quiz 3364. doi: 10.1182/blood-2014-10-608315
30. Samuelson Bannow BT, Salit RB, Storer BE, Stevens EA, Wu D, Yeung C, et al. Hematopoietic Cell Transplantation for Myelofibrosis: the Dynamic International Prognostic Scoring System Plus Risk Predicts Post-Transplant Outcomes. Biol Blood Marrow Transpl (2018) 24(2):386–92. doi: 10.1016/j.bbmt.2017.09.016
31. Gagelmann N, Eikema DJ, de Wreede LC, Koster L, Wolschke C, Arnold R, et al. CMWP of the European Society for Blood and Marrow Transplantation. Comparison of Dynamic International Prognostic Scoring System and MYelofibrosis SECondary to PV and ET Prognostic Model for Prediction of Outcome in Polycythemia Vera and Essential Thrombocythemia Myelofibrosis after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant (2019) 25(6):e204–8. doi: 10.1016/j.bbmt.2019.03.024
32. Alchalby H, Yunus DR, Zabelina T, Kobbe G, Holler E, Bornhäuser M, et al. Risk models predicting survival after reduced-intensity transplantation for myelofibrosis. Br J Haematol (2012) 157(1):75–85. doi: 10.1111/j.1365-2141.2011.09009.x
33. Gupta V, Malone AK, Hari PN, Ahn KW, Hu ZH, Gale RP, et al. Reduced-intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transpl (2014) 20(1):89–97. doi: 10.1016/j.bbmt.2013.10.018
34. Raj K, Eikema DJ, McLornan DP, Olavarria E, Blok HJ, Bregante S, et al. Family Mismatched Allogeneic Stem Cell Transplantation for Myelofibrosis: Report from the Chronic Malignancies Working Party of European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant (2019) 25(3):522–8. doi: 10.1016/j.bbmt.2018.10.017
35. Keyzner A, Shapiro S, Moshier S, Schorr E, Petersen E, Najfeld B, et al. Outcome of Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Chronic and Advanced Phase Myelofibrosis. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2016) 08:029. doi: 10.1016/j.bbmt.2016.08.029
36. Bregante S, Dominietto A, Ghiso A, Raiola AM, Gualandi F, Varaldo R, et al. Improved Outcome of Alternative Donor Transplantations in Patients with Myelofibrosis: From Unrelated to Haploidentical Family Donors. Biol Blood Marrow Transpl (2016) 22(2):324–9. doi: 10.1016/j.bbmt.2015.09.028
37. Robin M, Tabrizi R, Mohty M, Furst S, Michallet M, Bay JO, et al. Allogeneic haematopoietic stem cell transplantation for myelofibrosis: a report of the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Br J Haematol (2011) 152(3):331–9. doi: 10.1111/j.1365-2141.2010.08417.x
38. Murata M, Takenaka K, Uchida N, Ozawa Y, Ohashi K, Kim SW, et al. Comparison of Outcomes of Allogeneic Transplantation for Primary Myelofibrosis among Hematopoietic Stem Cell Source Groups. Biol Blood Marrow Transpl (2019) 25(8):1536–43. doi: 10.1016/j.bbmt.2019.02.019
39. Robin M, Giannotti F, Deconinck E, Mohty M, Michallet M, Sanz G, et al. Eurocord and Chronic Malignancies Working Party-European Group for Blood and Marrow Transplantation (CMWP-EBMT). Unrelated cord blood transplantation for patients with primary or secondary myelofibrosis. Biol Blood Marrow Transpl (2014) 20(11):1841–6. doi: 10.1016/j.bbmt.2014.06.011
40. Robin M, de Wreede LC, Wolschke C, Schetelig J, Eikema DJ, Van Lint MT, et al. Long-term outcome after allogeneic hematopoietic cell transplantation for myelofibrosis. Haematologica (2019) 104(9):1782–8. doi: 10.3324/haematol.2018.205211
41. Salas MQ, Lam W, Law AD, Kim DDH, Michelis FV, Loach D, et al. Reduced-intensity conditioning allogeneic transplant with dual T-cell depletion in myelofibrosis. Eur J Haematol (2019) 103(6):597–606. doi: 10.1111/ejh.13327
42. Morozova EV, Barabanshikova MV, Moiseev IS, Shakirova AI, Barhatov IM, Ushal IE, et al. A Prospective Pilot Study of Graft-versus-Host Disease Prophylaxis with Post-Transplantation Cyclophosphamide and Ruxolitinib in Patients with Myelofibrosis. Acta Haematol (2020) 23:1–8. doi: 10.1159/000506758
43. Kröger N, Zabelina T, Schieder H, Panse J, Ayuk F, Stute N, et al. Pilot study of reduced-intensity conditioning followed by allogeneic stem cell transplantation from related and unrelated donors in patients with myelofibrosis. Br J Haematol (2005) 128(5):690–7. doi: 10.1111/j.1365-2141.2005.05373.x
44. Robin M, Porcher R, Wolschke C, Sicre de Fontbrune F, Alchalby H, Christopeit M, et al. Outcome after Transplantation According to Reduced-Intensity Conditioning Regimen in Patients Undergoing Transplantation for Myelofibrosis. Biol Blood Marrow Transpl (2016) 22(7):1206–11. doi: 10.1016/j.bbmt.2016.02.019
45. Patriarca F, Masciulli A, Bacigalupo A, Bregante S, Pavoni C, Finazzi MC, et al. Busulfan- or Thiotepa-Based Conditioning in Myelofibrosis: A Phase II Multicenter Randomized Study from the GITMO Group. Biol Blood Marrow Transpl (2019) 25(5):932–40. doi: 10.1016/j.bbmt.2018.12.064
46. Patriarca F, Bacigalupo A, Sperotto A, Isola M, Soldano F, Bruno B, et al. Allogeneic hematopoietic stem cell transplantation in myelofibrosis: the 20-year experience of the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Haematologica (2008) 93(10):1514–22. doi: 10.3324/haematol.12828
47. Abelsson J, Merup M, Birgegård G, WeisBjerrum O, Brinch L. Brune M et al; Nordic MPD Study Group. The outcome of allo-HSCT for 92 patients with myelofibrosis in the Nordic countries. Bone Marrow Transpl (2012) 47(3):380–6. doi: 10.1038/bmt.2011.91
48. McLornan D, Szydlo R, Koster L, Chalandon Y, Robin M, Wolschke C, et al. Myeloablative and Reduced-Intensity Conditioned Allogeneic Hematopoietic Stem Cell Transplantation in Myelofibrosis: A Retrospective Study by the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant (2019) 25(11):2167–71. doi: 10.1016/j.bbmt.2019.06.034
49. Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med (2012) 366(9):787–98. doi: 10.1056/NEJMoa1110556
50. Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med (2012) 366(9):799–807. doi: 10.1056/NEJMoa1110557
51. Gupta V, Kosiorek HE, Mead A, Klisovic RB, Galvin JP, Berenzon D, et al. Ruxolitinib Therapy Followed by Reduced-Intensity Conditioning for Hematopoietic Cell Transplantation for Myelofibrosis: Myeloproliferative Disorders Research Consortium 114 Study. Biol Blood Marrow Transpl (2019) 25(2):256–64. doi: 10.1016/j.bbmt.2018.09.001
52. Robin M, Francois S, Huynh A, Cassinat B, Bay JO, Cornillon J, et al. Ruxolitinib Before Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) In Patients With myelofibrosis : a Preliminary Descriptive Report Of The JAK ALLO Study, a Phase II Trial Sponsored By Goelams-FIM In Collaboration With The Sfgmtc. Blood (2013) 122(21):306. doi: 10.1182/blood.V122.21.306.306
53. Ditschkowski M, Elmaagacli AH, Trenschel R, Steckel NK, Koldehoff M, Beelen DW. No influence of V617F mutation in JAK2 on outcome after allogeneic hematopoietic stem cell transplantation (HSCT) for myelofibrosis. Biol Blood Marrow Transpl (2006) Dec12(12):1350–1. doi: 10.1016/j.bbmt.2006.07.010
54. Alchalby H, Badbaran A, Zabelina T, Kobbe G, Hahn J, Wolff D, et al. Impact of JAK2V617F mutation status, allele burden, and clearance after allogeneic stem cell transplantation for myelofibrosis. Blood (2010) 116(18):3572–81. doi: 10.1182/blood-2009-12-260588
55. Panagiota V, Thol F, Markus B, Fehse B, Alchalby H, Badbaran A, et al. Prognostic effect of calreticulin mutations in patients with myelofibrosis after allogeneic hematopoietic stem cell transplantation. Leukemia (2014) 28(7):1552–5. doi: 10.1038/leu.2014.66
56. Kröger N, Panagiota V, Badbaran A, Zabelina T, Triviai I, Araujo Cruz MM, et al. Impact of Molecular Genetics on Outcome in Myelofibrosis Patients after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transpl (2017) 23(7):1095–101. doi: 10.1016/j.bbmt.2017.03.034
57. Alchalby H, Badbaran A, Bock O, Fehse B, Bacher U, Zander AR, et al. Screening and monitoring of MPL W515L mutation with real-time PCR in patients with myelofibrosis undergoing allogeneic-SCT. Bone Marrow Transpl (2010) 45(9):1404–7. doi: 10.1038/bmt.2009.367
58. Rumi E, Passamonti F, Arcaini L, Bernasconi P, Elena C, Pietra D, et al. Molecular remission after allo-SCT in a patient with post-essential thrombocythemia myelofibrosis carrying the MPL (W515A) mutation. Bone Marrow Transpl (2010) 45(4):798–800. doi: 10.1038/bmt.2009.231
59. Wolschke C, Badbaran A, Zabelina T, Christopeit M, Ayuk F, Triviai I, et al. Impact of molecular residual disease post allografting in myelofibrosis patients. Bone Marrow Transpl (2017) 52(11):1526–9. doi: 10.1038/bmt.2017.157
60. Chiusolo P, Bregante S, Giammarco S, Lamparelli T, Casarino L, Dominietto A, et al. Full Donor Chimerism After Allogeneic Hematopoietic Stem Cells Transplant For Myelofibrosis: The Role Of The Conditioning Regimen. Am J Hematol (2021) 96:234–40. doi: 10.1002/ajh.26042
61. Olsson RF, Logan BR, Chaudhury S, Zhu X, Akpek G, Bolwell BJ, et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia (2015) 29(8):1754–62. doi: 10.1038/leu.2015.75
62. Stasia A, Ghiso A, Galaverna F, Raiola AM, Gualandi F, Luchetti S, et al. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation. Biol Blood Marrow Transpl (2014) 20(9):1440–3. doi: 10.1016/j.bbmt.2014.05.016
63. Slot S, Smits K, van de Donk NW, Witte BI, Raymakers R, Janssen JJ, et al. Effect of conditioning regimens on graft failure in myelofibrosis: a retrospective analysis. Bone Marrow Transpl (2015) 50(11):1424–31. doi: 10.1038/bmt.2015.172
64. Kröger N, Zabelina T, Schieder H, Panse J, Ayuk F, Stute N, et al. Pilot study of reduced-intensity conditioning followed by allogeneic stem cell transplantation from related and unrelated donors in patients with myelofibrosis. Br J Haematol (2005) 128(5):690–7. doi: 10.1111/j.1365-2141.2005.05373.x
65. Gupta V, Kröger N, Aschan J, Xu W, Leber B, Dalley C, et al. A retrospective comparison of conventional intensity conditioning and reduced-intensity conditioning for allogeneic hematopoietic cell transplantation in myelofibrosis. Bone Marrow Transpl (2009) 44(5):317–20. doi: 10.1038/bmt.2009.10
66. Rondelli D, Goldberg JD, Isola L, Price LS, Shore TB, Boyer M, et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood (2014) 124(7):1183–91. doi: 10.1182/blood-2014-04-572545
67. Robin M, Tabrizi R, Mohty M, Furst S, Michallet M, Bay JO, et al. Allogeneic haematopoietic stem cell transplantation for myelofibrosis: a report of the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Br J Haematol (2011) 152(3):331–9. doi: 10.1111/j.1365-2141.2010.08417.x
68. Ballen KK, Shrestha S, Sobocinski KA, Zhang MJ, Bashey A, Bolwell BJ, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transpl (2010) 16(3):358–67. doi: 10.1016/j.bbmt.2009.10.025
69. Klyuchnikov E, Holler E, Bornhäuser M, Kobbe G, Nagler A, Shimoni A, et al. Donor lymphocyte infusions and second transplantation as salvage treatment for relapsed myelofibrosis after reduced-intensity allografting. Br J Haematol (2012) 159(2):172–81. doi: 10.1111/bjh.12013
70. McLornan DP, Szydlo R, Robin M, van Biezen A, Koster L, Blok HJP, et al. Outcome of patients with Myelofibrosis relapsing after allogeneic stem cell transplant: a retrospective study by the Chronic Malignancies Working Party of EBMT. Br J Haematol (2018) 182(3):418–22. doi: 10.1111/bjh.15407
71. Chhabra S, Narra RK, Wu R, Szabo A, George G, Michaelis LC, et al. Fludarabine/Busulfan Conditioning-Based Allogeneic Hematopoietic Cell Transplantation for Myelofibrosis: Role of Ruxolitinib in Improving Survival Outcomes. Biol Blood Marrow Transpl (2020) 26(5):893–901. doi: 10.1016/j.bbmt.2020.01.010
Keywords: myelofibrosis, allogeneic transplantation, busulfan, thiotepa, fludarabine chimerism, splenectomy
Citation: Bacigalupo A, Innocenti I, Rossi E, Sora F, Galli E, Autore F, Metafuni E, Chiusolo P, Giammarco S, Laurenti L, Benintende G, Sica S and De Stefano V (2021) Allogeneic Hemopoietic Stem Cell Transplantation for Myelofibrosis: 2021. Front. Immunol. 12:637512. doi: 10.3389/fimmu.2021.637512
Received: 03 December 2020; Accepted: 13 April 2021;
Published: 04 May 2021.
Edited by:
Hermann Einsele, Julius Maximilian University of Würzburg, GermanyReviewed by:
Federico Simonetta, Geneva University Hospitals (HUG), SwitzerlandCopyright © 2021 Bacigalupo, Innocenti, Rossi, Sora, Galli, Autore, Metafuni, Chiusolo, Giammarco, Laurenti, Benintende, Sica and De Stefano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Bacigalupo, YW5kcmVhLmJhY2lnYWx1cG9AdW5pY2F0dC5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.