
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 15 April 2021
Sec. Viral Immunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.633184
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to spread worldwide as a severe pandemic. Although its seroprevalence is highly variable among territories, it has been reported at around 10%, but higher in health workers. Evidence regarding cross-neutralizing response between SARS-CoV and SARS-CoV-2 is still controversial. However, other previous coronaviruses may interfere with SARS-CoV-2 infection, since they are phylogenetically related and share the same target receptor. Further, the seroconversion of IgM and IgG occurs at around 12 days post onset of symptoms and most patients have neutralizing titers on days 14-20, with great titer variability. Neutralizing antibodies correlate positively with age, male sex, and severity of the disease. Moreover, the use of convalescent plasma has shown controversial results in terms of safety and efficacy, and due to the variable immune response among individuals, measuring antibody titers before transfusion is mostly required. Similarly, cellular immunity seems to be crucial in the resolution of the infection, as SARS-CoV-2-specific CD4+ and CD8+ T cells circulate to some extent in recovered patients. Of note, the duration of the antibody response has not been well established yet.
Time and time again, emerging and recurring pathogens have posed a threat to humanity and materialized as global challenges to public health (1). Most often, these microorganisms are effectively contained, and their emergence does not translate into a widespread disease with high morbidity or mortality rates. Nonetheless, some few noteworthy exceptions have escaped this rule, because of their own pathophysiological nature or due to insufficient efforts at containing them. The novel coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) falls into this narrow group of exceptions, as it continues to spread worldwide as a severe pandemic, with varied and often misleading estimates of its true impact (2, 3). Hence, the menace that the coronavirus disease 2019 (COVID-19) represents for global health must be met with a thorough understanding of the nature of this highly pathogenic virus, so as to focus on effective strategies that lead to its control and mitigation.
This viral pathogen has been confirmed, based on phylogenetic evidence, to be in close relationship with other highly pathogenic coronaviruses such as Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV), with which it shares common biological features, routes of transmission and the common receptor angiotensin converting enzyme 2 (ACE-2) to infect susceptible cells (4–7). The clinical course of SARS-CoV2 infection is usually asymptomatic or with mild symptoms, including fever, cough and shortness of breath; although it can course in extreme cases with respiratory failure, requiring mechanical ventilation. Moreover, this coronavirus can also lead to several extrapulmonary manifestations, such as thromboembolic complications, cardiac lesions, acute coronary syndromes, gastrointestinal symptoms, acute renal failure, liver dysfunction, hyperglycemia and diabetic ketosis, neurologic deficits, and dermatologic complications. Although these alterations can be due to direct viral infection, indirect mechanisms such as thromboinflammation, dysfunction of the immune system and dysregulation of the renin–angiotensin system have been associated with multiple organ dysfunction (8). All of these are characteristics that resemble the clinical spectrum found on diseases caused by the other aforementioned coronaviruses (9). Additionally, these coronaviruses have similar phylogenetic and clinical characteristics, and different studies have shown that the host immune response can be comparable as well, particularly regarding humoral responses (10–12).
Antibodies against SARS-CoV-2 are essential for outsmarting the virus, as a proper neutralizing response would decrease substantially the number of virions that could successfully infect ACE-2 receptor-expressing cells. Thus, research on antibody responses to SARS-CoV-2 must be a priority for the scientific community responding to the pandemic, both in terms of prophylaxis and treatment.
However, antibody response against this virus is still a subject of controversy and must be addressed carefully. Vaccine effectiveness studies, the possibility of antibody dependent enhancement (ADE) and convalescent plasma therapy, are some of the many topics of debate involving antibody responses to SARS-CoV-2, and plenty of research is yet to be done in some of these fields. However, the robust set of evidence that has surfaced provides clarity in many aspects of the humoral immune response mounted against the novel coronavirus. In this review, we provide an insight on the currently available evidence regarding the nature of antibody response to SARS-CoV-2, especially pertaining to seroprevalence, advances in convalescent plasma therapies, antibody kinetics, and antibody neutralization.
We conducted a literature search in the databases PubMed (MEDLINE), Embase, SCOPUS, and Cochrane from inception to 11 March 2021, using the following terms: “SARS-CoV-2”, “COVID-19”, “seroprevalence”, “convalescent plasma”, “neutralizing antibodies”, “antibodies”, “antibody dependent enhancement” and “kinetics”, without geographical restrictions, limited to articles published in English (Figure 1). Only articles considered relevant were included according to the authors’ criteria, including original articles, case series, experimental research, reviews, and case reports. The authors’ criteria to consider an article relevant included, among others, the article’s pertinence regarding the specific subjects of seroprevalence, convalescent plasma, kinetics and neutralization, as well as recency and sample size. Technical considerations, opinion articles and performance evaluation articles were not included. Four pre-print articles were carefully revised and included due to their relevance in the fields of study. In addition, the reference lists of each article were reviewed in order to expand the search for relevant articles. Each article underwent a double filter from two authors who schemed the databases according to each topic and deliberated on the relevance of such articles.
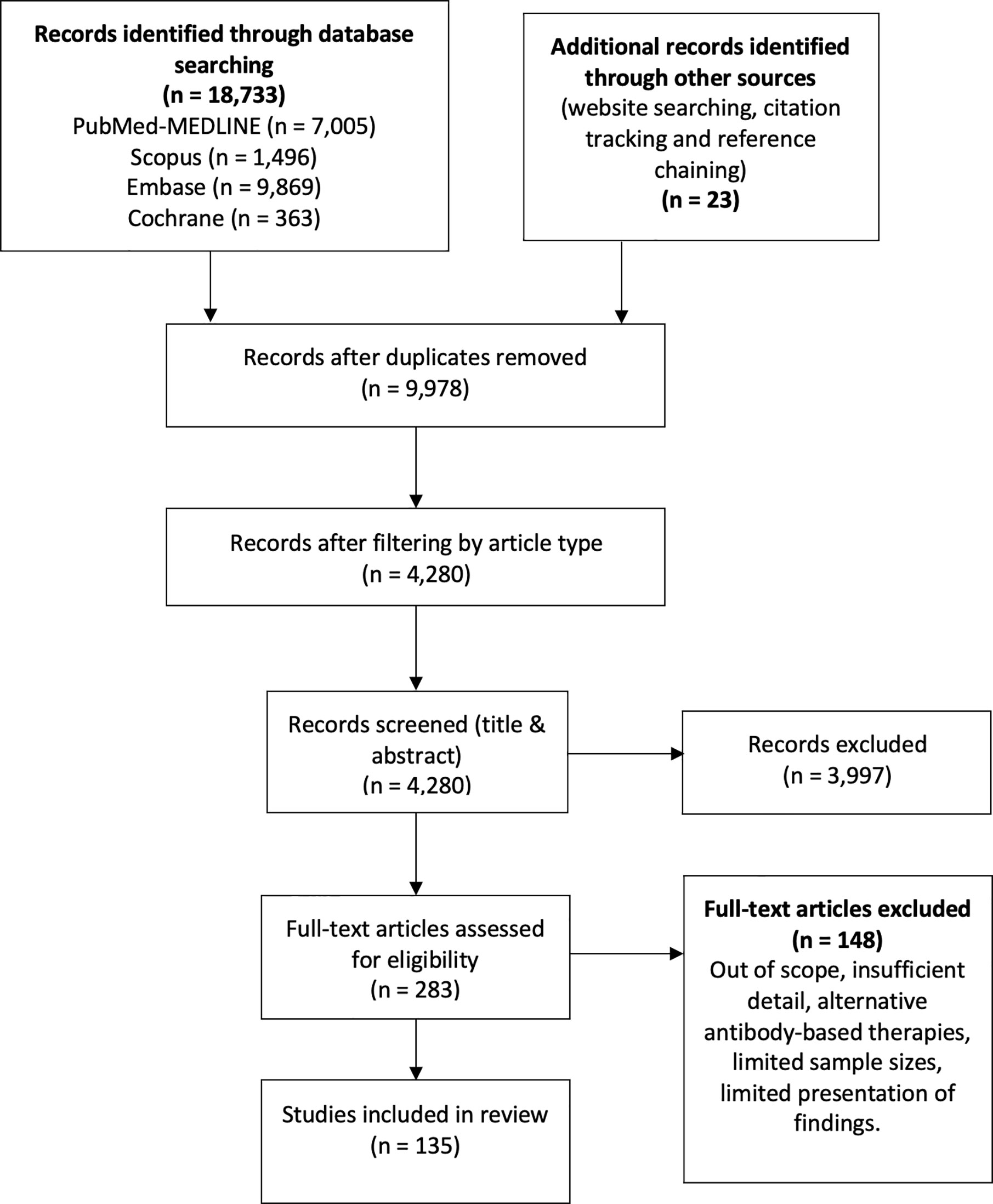
Figure 1 The flow diagram describes the process of literature review on antibody kinetics, neutralization, antibody-dependent enhancement, seroprevalence and convalescent plasma in COVID-19.
The immune response elicited against this coronavirus not only serves the evident physiological purpose of protecting against the infection, but it is also employed for evaluating the impact of this pathogen over a community. Seroprevalence has been important during this pandemic, identifying a significant number of cases that were not diagnosed by the conventional quantitative real-time polymerase chain reaction (qRT-PCR) or by antigen-based tests. As time passes, a growing body of evidence suggests that seroprevalence reflects the dynamics of the pandemic. One such resource is a systematic review and meta-analysis by Bobrovitz et al. that includes 338 studies with over 2.3 million participants (13). Seroprevalence was reported low in general population, and varied among countries and territories. Although this information is of great analytical importance, it must be carefully revised as has not yet undergone peer-review. Another powerful resource is a recent article on The Lancet, which spotlights a dashboard for tracking seroprevalence reports (14). It includes data from 73 countries, the majority of which report seroprevalence below 15%. The data shows variability among territories, with European countries such as United Kingdom, Germany and France reporting higher-range prevalences between 6.7% and 13.6%, in contrast to other countries such as China (0.8-2.46%) or Japan (0.1%). The information displayed can also be analyzed based on local or regional approaches, as nation-wide information is often difficult to gather. Although this information has a worth-mentioning availability and robustness, it is important to analyze individual reports on seroprevalence to characterize further the impact of SARS-CoV-2 over different communities and populations.
The majority of studies on seroprevalence focus on healthcare workers (HCW). Recently, a systematic review and meta-analysis was published by Galanis et al, which showed that the overall seroprevalence was estimated at 8.7% from the information of 49 studies that included 127,480 HCW (15). From this systematic review, it is important to mention that seroprevalence is higher in North American studies while it is lower in Europe, Asia and Africa, but there are also important variations in reports. This can be seen from the data of individual studies. One such example is a report by García-Basteiro et al. in which seroprevalence was reported in HCW from a reference hospital in Spain in June (16). Seropositivity either from IgG, IgM or IgA against SARS-CoV-2 was reported at 9.3%. A German-based study by Brehm et al. showed a notoriously low seroprevalence in HCW from a tertiary care center in November, with an overall seroprevalence of 1.8% (17). Similar studies have been done in China and India, with antibody positivity rates of 17.14% and 11.1% in July and November respectively, in HCW with negative swab samples (18, 19). Data from British centers reported even more dramatic scenarios, with positivity rates of 31.6% from 2,167 HCW in July (20). However, other studies conducted in North American grounds such as that of Stubblefield et al. or that of Hunter et al. display a noticeable heterogeneity, with positivity rates of 7.6% and 1.6% in July and August, respectively (21, 22). A South-American study estimated seroprevalence among HCW at a University Hospital in Colombia in December at nearly 6% (23). These compiled data connote that HCW, who have an augmented exposure to the virus and hence an increased probability of becoming infected, do have an increased seroprevalence as compared to general data from the aforementioned dashboard. Nevertheless, the values are not consistent among them, as they may also be influenced by several factors including social, demographic, professional, and others. In fact, this has been portrayed in individual studies such as that of Goldblatt et al. a cross-sectional study that determined prevalence in eight countries in January (24). Seroprevalence was significantly different in each country, ranging from 0% to 16.93%, and was linked to each national COVID-19 burden. Other individual study by Alseheri et al. showed that this marked variation occurs not only at an international scale (25). This study showed a difference in seropositivity among cities in Saudi Arabia in November, ranging from 0% to 6.31%.
While there is important data on HCW seroprevalence, a significant proportion of the studies have also been carried out in the general population, which reflect more adequately the dynamics and characteristics of the pandemic. An article investigated the seropositivity in May in Wuhan, the city that gave birth to the pandemic, and found a rate ranging between 3.2% and 3.8% (26). There is also data on other countries which have gravely suffered the repercussions of COVID-19, such as Brazil and the United States. Studies in North America by Sood et al., Bryan et al., and Havers et al. show that the prevalence varies according to the specific area, with positivity rates of 4.06%, 1.79%, and up to 6.9% respectively, all between July and August (27–29). In fact, the latter clearly demonstrates the geographical dynamics of the infection, as the authors report that the seroprevalence in the San Francisco Bay area was 1% in contrast to that of New York City, which was 6.9%. Worth noting, Spain, a nation which has suffered greatly from the pandemic as well, conducted one of the greatest epidemiological studies to establish seropositivity. Pollan et al. explored seropositivity through both point of care (POC) -testing and immunoassays in August, with 61,075 and 51,958 tests respectively (30). The seropositivity with the POC tests was 5.0%, while the immunoassays showed 4.6%. Intriguingly, one population-based study found a low seroprevalence in Brazilian territory, a nation that has been hard-struck by the pandemic. This study conducted by Silveira et al. found a seropositivity between 0.048% and 0.222% among three rounds of testing, although it is worth mentioning that this study was based on lateral flow assays (31). These findings are of particular interest because Brazil’s COVID-19 death count has been considerable throughout the pandemic, so it seems that this area of study behaves as an exception, and even in hard-hit countries it is possible to find low-transmission territories. Seroprevalence also shows a more profound impact of COVID-19 in underserved populations. A study in Mumbai from February 2021 showed that seroprevalence in non-slums was 16.1%, while it was as high as 54.1% in slums (32). Furthermore, recent individual studies from late 2020 and early 2021 have shown seroprevalence ranging from 0.09% to 22.2% (33–38). Some of these studies have used a Bayesian approach for accounting for misclassification bias, which represents more properly the data obtained in these seroprevalence studies (35, 36).
So far, the available evidence casts a shadow over several points of analysis which are shown schematically in Figure 2. First, it leads to the conclusion that seroprevalence is highly variable among territories, and that such values must be read in the light of their particular contexts. Factors such as impact on the community, social and demographic dynamics, economics and political decision-making must be indispensable for an appropriate analysis. Second, the available data on seroprevalence is so far limited, and such challenges must be met by the scientific community so as to describe broadly how this new coronavirus infection affects population on a worldwide scale. Third and last, seropositivity in HCW is evidently greater than that of the general population, which illustrates the risk for medical professionals, highlighting the need for personal protective equipment to be widely available and stresses the need for governments and institutions to strive for the safety of their professionals. For an accurate analysis of these points, it is essential to consider the quality of the antibody tests so as to diminish the incidence of confounding factors, as well as considering the inherent difficulty in estimating the impact when some COVID-19 positive individuals never develop antibodies at all. Finally, time is an important variable to consider because COVID-19 is still spreading with high peaks of infection around the world. Therefore, a dynamic seroprevalence follow-up could be set to have more precise mapping.
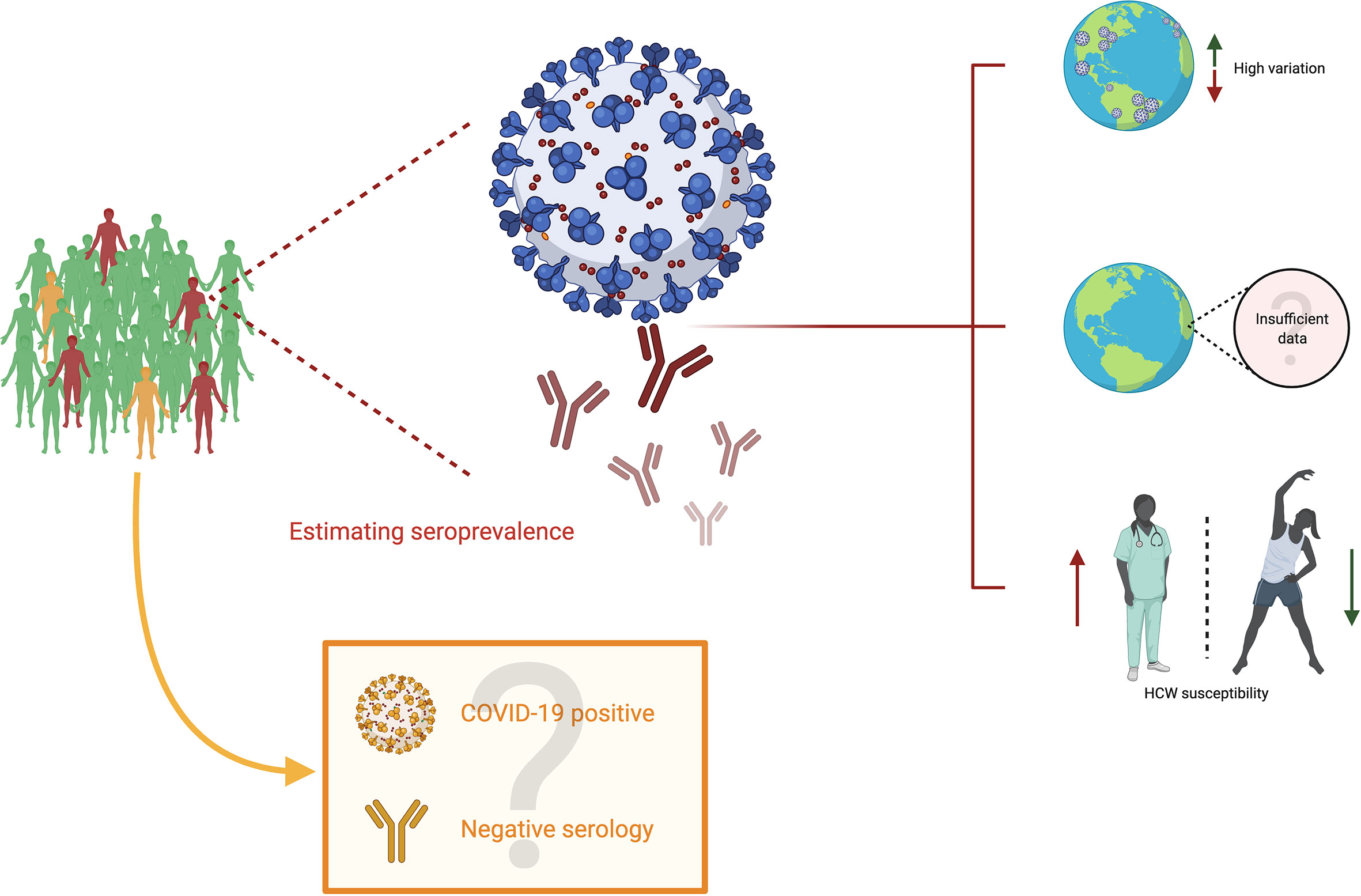
Figure 2 Seroprevalence in COVID-19. Subjects in green are uninfected people (neither current nor past) with negative serology; subjects in orange are infected people (current or past) with negative serology, and subjects in red are infected people (current or past) with positive serology. Estimating seroprevalence points to three primary conclusions. First, there appears to be a high variation among different territories worldwide. Second, although efforts to characterize the impact of SARS-CoV-2 are worth highlighting, there is still insufficient data to estimate the precise impact of this virus. Third, the studies on seroprevalence display the susceptibility of healthcare workers to SARS-CoV-2. However, the impact could be undermined due to individuals who become infected (COVID-19 positive) and have negative serological results.
The transfusion of convalescent plasma is a therapy that has been used during the infection of other human coronaviruses, such as SARS-CoV and MERS-CoV (39, 40). Moreover, it has shown to be effective and safe for human use. Convalescent plasma has antiviral characteristics, due to the presence of neutralizing and non-neutralizing antibodies, but it also has immunomodulatory properties through signaling pathways involving anti-inflammatory cytokines, complement blocking antibodies, auto-antibodies, anti-idiotype antibodies and factors involved in hemostasis, depending on the doses used (Figure 3) (41).
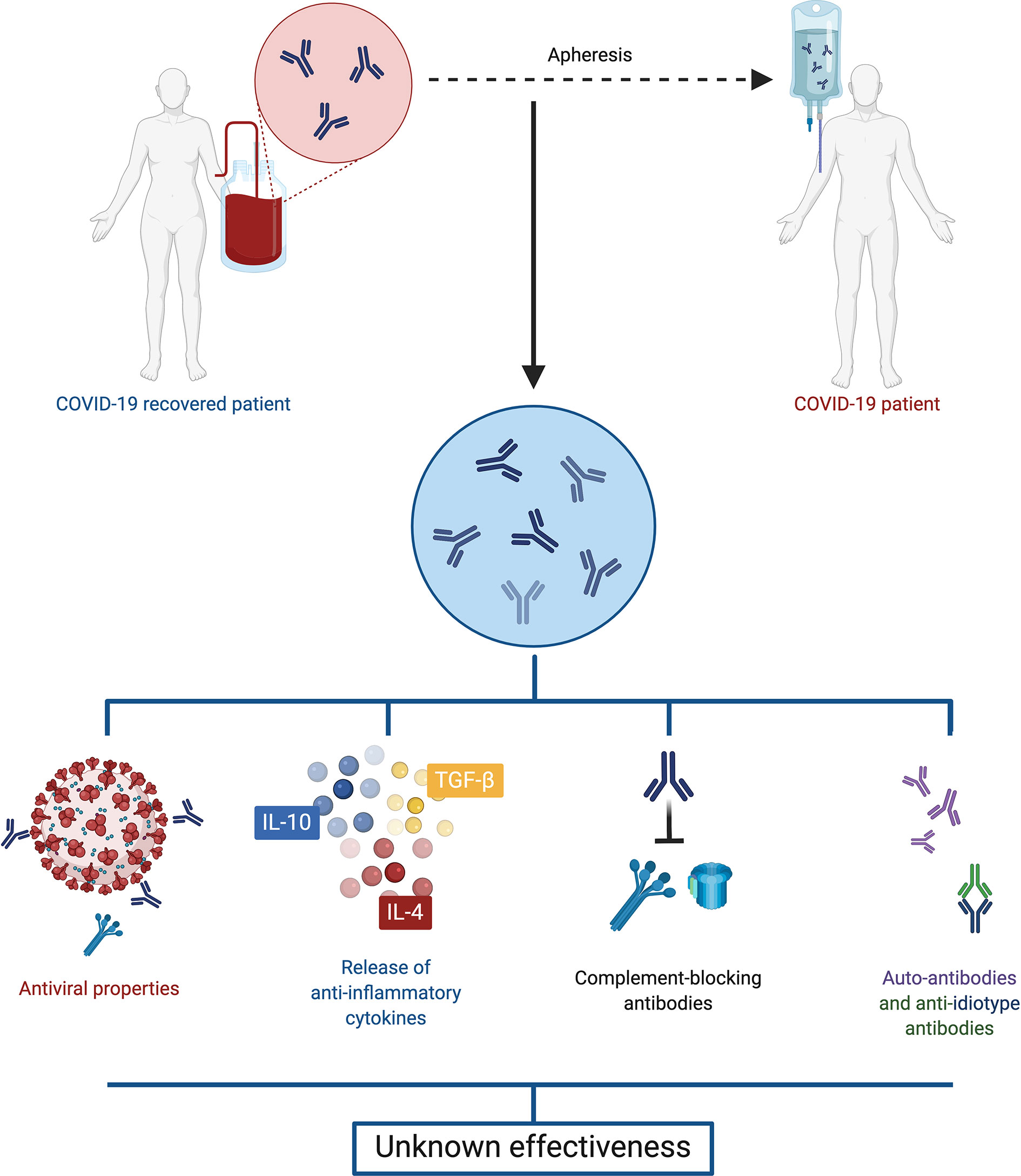
Figure 3 Convalescent plasma. Plasma retrieved from convalescent COVID-19 patients displays both antiviral and immunomodulatory properties, including anti-inflammatory cytokines, complement blocking antibodies, auto-antibodies and anti-idiotype antibodies. However, evidence is so far contradictory, and the effectiveness of this treatment is unclear.
Recent studies suggest convalescent plasma-treated patients may have increased viral clearance and improvement of clinical manifestations, in particular for fever and radiological findings (42–44), leading to a lower requirement of mechanical ventilation and shorter hospital stay (45). Worth highlighting, a study by Joyner et al. enrolled 21,987 patients who received convalescent plasma (46). Overall, the incidence of all serious adverse events was low, as transfusion reactions, thrombotic events and cardiac events were reported in less than 1% of the population sample. These findings, in accordance with those of other smaller studies, suggest that safety should not be a concern regarding treatment with it (46–49). Further, the time in which it is administered also seems to be decisive in the outcomes of transfused patients, since Joyner et al. showed a 7-day mortality rate of 8.7% (95% CI 8.3-9.2) in transfused patients within 3 days of diagnosis and 11.9% (95% CI 11.4-12.2) in transfused patients after 4 days of diagnosis (50).
However, in terms of grounded, more directly applicable data, a clinical trial could not demonstrate differences in mortality or clinical improvement within 28 days after convalescent plasma therapy (51). It is worth mentioning that this study was limited and did not obtain enough statistical power. Similarly, the PlasmAR trial enrolling 228 patients with severe COVID-19 found no differences between the group of patients treated with convalescent plasma compared to the control group at day 30 (52) and the PLACID trial that included 235 patients with moderate COVID-19, also noted no difference in mortality at 28 days in those who received convalescent plasma compared to those who received only available best standard care therapy (53).
Among the very different reported results, as listed in Table 1, uncertainty reigns. A Cochrane systematic review included 19 studies with 38,160 participants and concluded that it is so far unknown whether convalescent plasma is an effective method for reducing severity and mortality in patients with COVID-19 (54). Additionally, reports by regulatory entities have pointed to unimpressive results from these trials (55). Further randomized controlled trials must provide useful information to solve this issue.
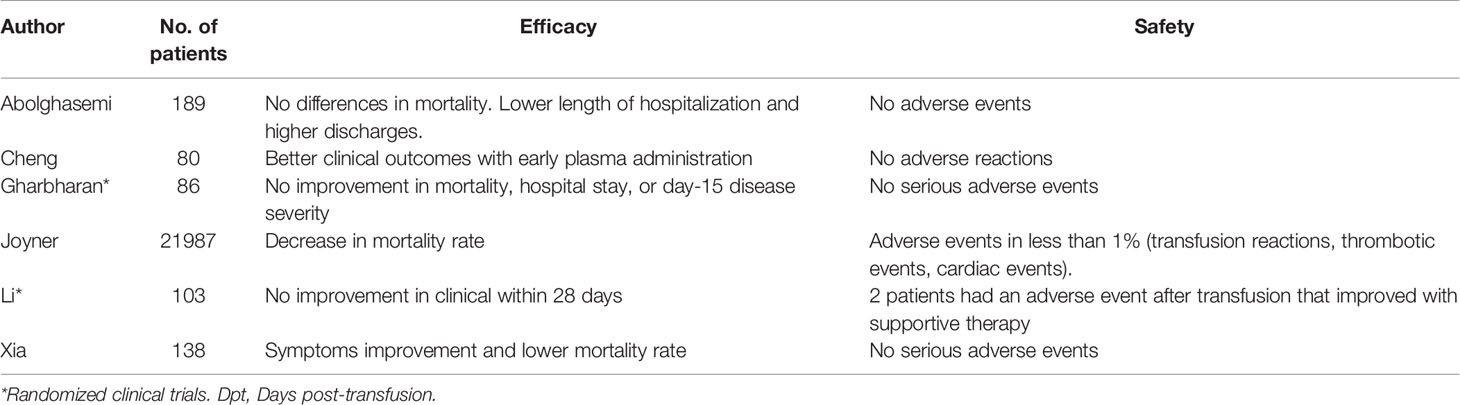
Table 1 Efficacy and safety of convalescent plasma transfusion as a treatment for COVID-19 patients.
Measuring antibody titers before transfusions is required in most cases, considering that the antibody responses in recovered patients is extremely variable. Studies have reported that around 33% of patients had titers less than 1:50 (56). Besides, it appears that mild patients may have lower neutralizing antibody titers compared to severe patients (57). In fact, only hospitalized patients who were not receiving mechanical ventilation and received plasma transfusions with higher antibody titers appear to have benefit in clinical outcomes (50). Also, in an animal model with green monkeys, it was noted that those who received higher antibody titers had lower SARS-CoV-2 levels in the respiratory compartments, less severity of virus-related lung pathology, reduction in coagulopathy and inflammatory processes (58).
Thus, considering that neutralizing antibodies could be presumed to be potentially protective during SARS-CoV-2 infection, it is necessary to know the status of neutralizing antibodies in donor plasma. However, due to the difficulty in performing neutralization assays, the nucleoprotein (NP), spike (S) 1 and S2 specific IgG could be an alternative for the indirect measurement of neutralizing antibodies in donor plasma (57). Notably, the antibody response against receptor-binding domain (RBD) has been recognized as anti-SARS-CoV-2 effective in donor plasma (56); and expansion of clones of RBD-specific memory B cells was higher in recovered patients (59). The application of these data, which resulted from more specifically focused studies and pertains to aspects of basic research, must be warranted and taken into account for designing clinical studies in a stride to solve the uncertainty mentioned before.
A fundamental, yet roughly understood matter in the humoral response to SARS-CoV-2 is the kinetics, whose importance is stressed by the need for a robust and long-lasting immunity against this coronavirus. Despite the narrow understanding on antibody kinetics in the context of COVID-19, four areas of knowledge have seen noticeable advances: differences in antibody responses between critical and non-critical COVID-19 patients; seroconversion and magnitude of antibody responses during the first weeks after infection; variability in antibody kinetics depending on their respective antigen targets; and controversy in the reliability and neutralization capacity of antibodies through time (Figure 4). It is worth mentioning that kinetics, performance, analytical and clinical validation of specific assays are also related to the topic of antibody kinetics in COVID-19, and have been broadly explored elsewhere (60–64).
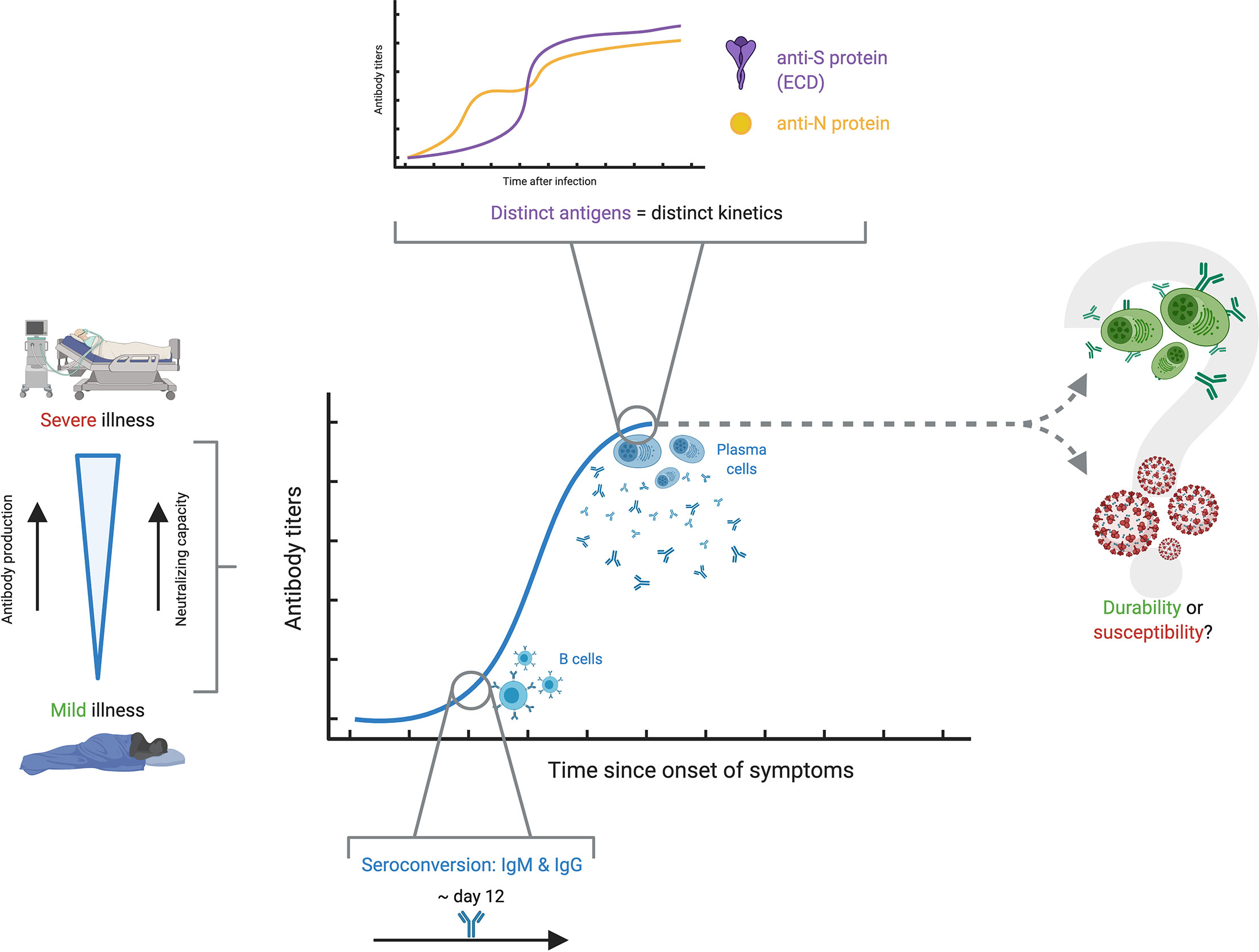
Figure 4 Antibody kinetics in COVID-19. The antibody kinetics show to be highly variable among individuals, but there seems to be a clear correspondence between severe disease, high antibody production and high neutralizing capacity, while the opposite is true with mild disease. Among the different studies, seroconversion appears at around 12 days. A characteristic finding is that there appears to be distinct kinetic profiles for the different antigens against which antibody responses are developed. The most important question which remains unsolved is whether antibody titers persist over time conferring protection, or if, on the contrary, antibody production wanes over time and renders people susceptible to reinfection.
Perhaps one of the clearest and most well-understood topics regarding antibody kinetics is the relationship with severity. The available evidence strongly suggests that severity is associated with higher levels of antibodies. Rijkers et al. showed results in a sample with 38 severe and 24 mild patients, in which the severe group developed a robust antibody response with adequate neutralizing capacity, in contrast to a mild group with only 75% seropositivity and poor neutralizing capacity (65). Several reports, including systematic reviews, opinion articles and original articles, point towards this same conclusion, strongly asserting that severity in COVID-19 patients seems to be associated with antibody production and response against this virus, although the immunological, virological or physiological bases behind this are so far unknown (66–69). Still, further research must be done on the topic, as the findings in some studies suggest differently. For example, a study by Wang et al. with 12 severe and 11 mild COVID-19 patients showed that, even though mild patients had significantly lower IgM titers compared to their severe counterparts, both groups showed comparable IgG responses at 9 days post-onset of symptoms (POS) (57). Recent evidence suggests that there is a prognostic relationship between antibody kinetics and severity of the disease in COVID-19. A report by Ren et al. has shown the correlation in severe COVID-19 patients with a delayed antibody response, in contrast to an earlier-mounted response by mild patients (70). Although the study has a limited follow-up, further data presented by Lucas et al. in a recent preprint leads to similar conclusions. These researchers showcased that not only is there a delayed antibody response in lethal COVID-19, but also that an early-mounted neutralizing response correlates with discharge (71). The authors speculate on a possible window of 14 days POS in which antibody responses must be mounted for increasing odds of survival. Altogether, these results pave the way towards an understanding of antibody kinetics and how it relates, and even dictates, COVID-19 severity.
The kinetics of seroconversion has also been evaluated in several studies. Large studies have shown seroconversion rates from 91 to 99% (72, 73). Lynch et al. point out that over 80% of patients had IgM and IgG seroconversion between 8- and 10-days POS, and highlight that patients admitted to the intensive care unit had higher peak measurements in all intervals between 6 and 20 days for IgM (74). Additionally, a different study by Orth-Höller et al. shows that positive IgG titers were found in most mild and moderate patients after two to three weeks (75). In contrast, Zhang et al. described that the production of IgM and IgG was delayed in the critical group and reached the peak at 1-month POS (76). Several studies, including an aforementioned meta-analysis, suggest that seroconversion of both IgM and IgG occurs at around 12 days POS with extensive variation, but does not shed any light towards severity (66), underlying the importance of further studies (66, 74–78). This evidence on the first phase of antibody response in COVID-19 resembles that for other coronaviruses. In other outbreak-related coronaviruses such as SARS-CoV, as well as endemic coronaviruses such as HCoV-229E, a noticeable increase in antibody response was detected 10-20 days after the onset of illness (67, 79–81).
Naturally, antibody profiles and dynamics vary depending on the isotype of interest (70, 82), but evidence has also gathered to indicate that there are dissimilar profiles of antibody kinetics depending on the antigen of interest, and consequently natural antibody levels vary significantly and cannot be characterized as a whole (83). An article by Chen et al. portrays this avidly, as serum IgM and IgG responses displayed distinct kinetic profiles against NP, RBD, S1 and the ectodomain (ECD) of the S protein (70, 84). This heterogeneity may be used in favor of the diagnostic precision, as combined detection of antigens such as NP and ECD increases test sensitivity, as well as a combination of IgM or IgG specific against N or S (84, 85). Nonetheless, attention should also be set on other, less popular antigens. There is evidence proving that targets such as ORF8 and ORF3b elicit noticeably strong antibody responses and provide very high specificity and sensitivity for evaluating antibody response, even outperforming serological assays screening for other antigens such as S or N protein (86). All of these findings further demonstrate that evidence on antibody response in terms of kinetics is worth investigating, as it could provide insight on COVID-19 diagnosis and the maintenance of durable immunity.
The main concern on antibody response kinetics to SARS-CoV-2 is the establishment of a durable protection. Based on the available evidence, it is yet to be determined if the antibody response effectively wanes over time, and if the resulting antibody titers are enough to provide protection against reinfection (87). In fact, several reports show an evident decrease in antibody response over time, which depict variable declining neutralizing antibody titers during the follow-up period of convalescent patients (68, 88). Still, it is unclear whether the quality of the remaining antibodies is enough to effectively neutralize the virus (69). These concerns are reinforced by the recently published reports on confirmed reinfections, although more research must be conducted in order to determine the nature and causes of such phenomena, and what is the role of antibody kinetics, if any (89, 90). However, there are also several reports that point to the opposite conclusion. There is compelling evidence on the persistence of robust neutralizing antibodies for months after infection, as well as favorable antibody kinetic profiles in large national studies, which range from five to eight months (72, 73, 91, 92). To assess said establishment of a durable protection, Lumley et al. studied antibody responses in healthcare workers and followed up 1265 seropositive subjects (93). Among these participants, who were followed up to 31 weeks, 2 had a positive PCR test. Other even larger studies have pointed to similar conclusions, as Abu-Raddad et al. followed 43,000 PCR-positive subjects and found an estimated incidence rate of reinfection of 0.66 per 10,000 people-weeks (94). Although this conclusion does not involve humoral response directly, it does indicate that immune protection is robust among those previously infected and that the risk of reinfection is low, and that it is mostly asymptomatic.
A parallel can be drawn over kinetics with that of other coronaviruses, as antibody responses to MERS-CoV and SARS-CoV effectively wane over time. Indeed, antibody responses can be detected in MERS patients one year after the infection, but in a notably lower concentration (95). A similar phenomenon occurs in SARS patients, as some studies have shown that antibodies can be found 2-3 years after the infection, albeit in low titers (67, 81, 96). Although data cannot be directly extrapolated from one virus to another, it is safe to assume that the data presented herein suggests the kinetics of antibodies against SARS-CoV-2 might follow a similar pattern to those of other outbreak-related coronaviruses, as antibody responses are detected in patients previously infected with SARS-CoV-2, but appear to wane as well.
Neutralizing antibodies (NAbs) are often correlated with long-term immunity in several viral infections (97). Hence, understanding the presence of NAbs in SARS-CoV-2 infection might be helpful as well. Most infected patients with SARS-CoV-2 develop variable titers of NAbs between days 14 and 20 POS (98). A better perspective on NAb production might be achieved upon a well-established comparison between age groups. In children, the onset and synthesis of NAbs seems to be similar than in adults, so most of them produce titers of neutralizing antibodies of variable level. Of note, Pengcheng Liu et al. suggest that NAbs produced during the acute phase may be insufficient for viral clearance, which could be associated with prolonged viral shedding (99).
Another important aspect surrounding antibody neutralization against SARS-CoV-2 is the influence of immunity against seasonal human coronaviruses (HCoV), and whether or not such immunity protects against SARS-CoV-2. Some published results so far suggest that there is probably no cross-neutralizing response between SARS-CoV and SARS-CoV-2 (100), which renders unlikely that previous infections with SARS-CoV provide protection against infection by SARS-CoV-2. However, other reports show the opposite. Some authors have demonstrated a partial protection for SARS-CoV-2 in patients who previously were infected by SARS-CoV (101). These studies highlight a potential of cross-reactivity from seasonal and endemic coronaviruses with SARS-CoV-2 (102). In fact, not only does it appear to exist a comparable neutralization activity between people infected by SARS-CoV-2 and those infected by different HCoVs, but evidence has surfaced as well on the interference upon entry of SARS-CoV-2 into target cells in those patients who had previous exposure to seasonal coronaviruses (103).
Besides, it has been proven that neutralizing antibodies have a positive correlation with age, male sex and severity of the disease (Figure 5) (104). Wu et al. reported that NAbs were higher among older patients compared to those middle-aged and younger, being the latter those who had lower neutralizing titers (105). Moreover, they found that titers of NAbs were positively correlated with the C-reactive protein levels and negatively with lymphocyte count at the time of admission. Of note, younger people do not necessarily develop low titers, as the same study also detected 2 patients with very high neutralizing titers (ID50:15989, 21567) in this age group, prompting other factors to be considered.
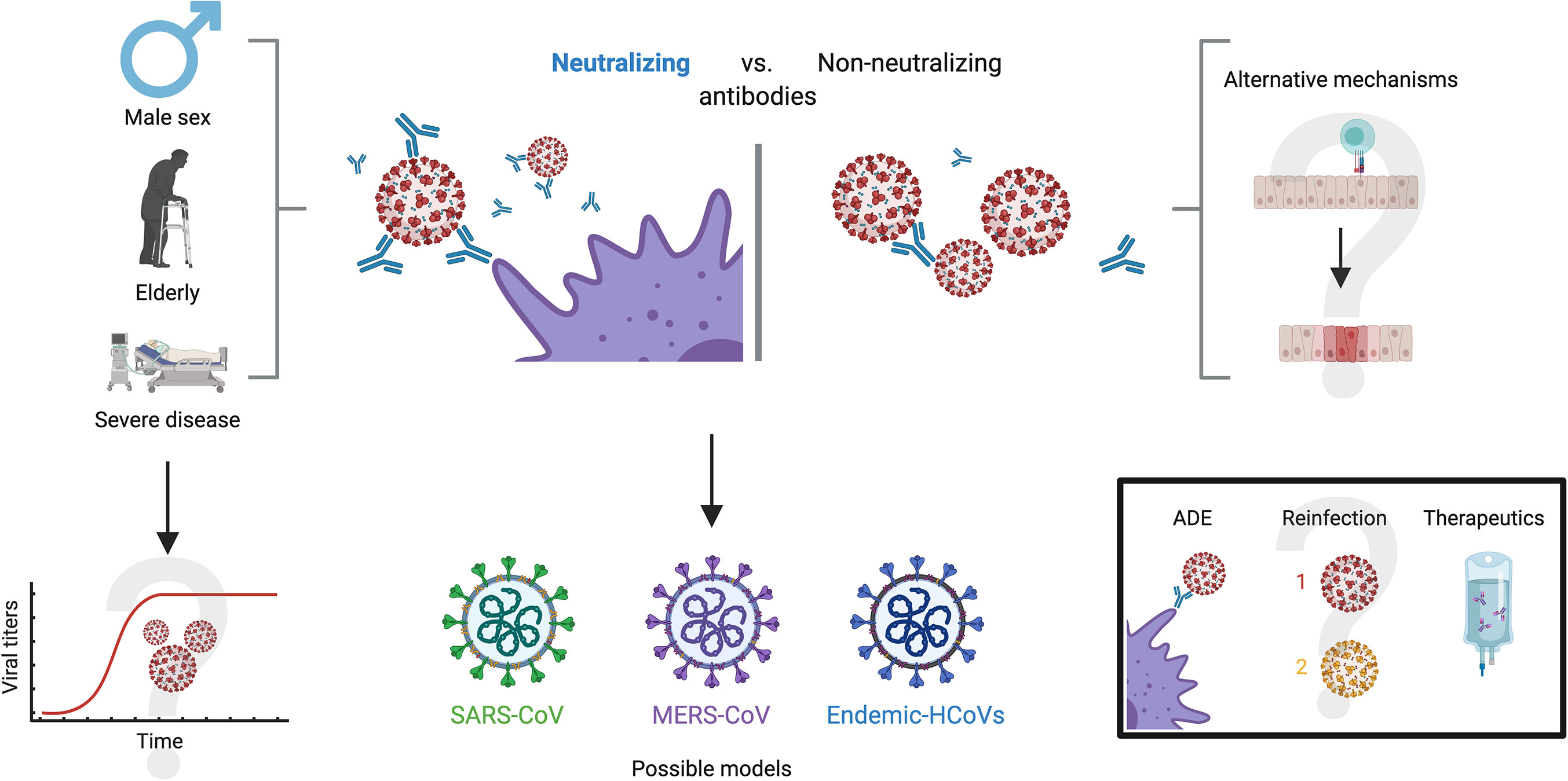
Figure 5 Antibody neutralization in COVID-19. Three characteristics appear to hold a close relationship with high antibody neutralization capacity: male sex, old age and severe disease. A possible explanation is viral persistence in patients with these characteristics, but this is yet to be confirmed. On the other side of the spectrum, alternate mechanisms have been proposed for patients that do not develop robust neutralizing antibodies, such as high cytotoxic activity and a robust innate immune response, although these remain undetermined. For investigating neutralizing dynamics against SARS-CoV-2, other models with similar coronaviruses have been explored, such as SARS-CoV, MERS-CoV and endemic CoVs. Further investigation on neutralizing capacity will present definitive solutions to the enigmas of antibody-dependent enhancement, likelihood of reinfection and the effectiveness of treatments such as convalescent plasma.
Regarding differences by gender, and despite the fact that men and women seem to have the same risk of infection by this virus (106), there are significant differences in the course and outcome of the disease between them, and worse outcomes and higher NAbs titers among the male population (98, 104, 107). Indeed, clinical outcomes show that males experience both a higher severity and fatality for COVID-19 infection than females (108, 109). Worth noting, an article by Takahashi et al. highlights the importance of specific cytokines such as IL-8 and IL-18, as well as the role of non-classical monocytes and the significantly diminished T cell responses in male patients with unfavorable outcomes (110). These findings demonstrate, altogether, that there are profound physiological differences between males and females in terms of COVID-19 progression and immunity to the virus.
Similar to the wide humoral response, the severity of COVID-19 has been correlated with titers of neutralizing antibodies against SARS-CoV-2, as these are higher among hospitalized patients or those in intensive care units (ICU) compared to patients with mild symptoms (111, 112). Although some neutralizing antibodies with strong RBD region binding affinity have been found in convalescent patients, (113), currently hospitalized patients have neutralizing titers up to 3000-fold higher than outpatients and donors of convalescent plasma (114). Despite the fact that most of the mechanisms behind these phenomena are unknown, there are several hypotheses. Some authors discuss the possibility that high titers are found in severe patients as a consequence of the strengthened and prolonged B-cell receptor stimulation (115), which might be associated with insufficient viral clearance. They also shed light on the potential role of other immune mechanisms in those with less severe outcomes. One such example might be a robust CD8+ T cell response that could confer protection against the virus more effectively, but this is yet to be proven by future investigations (115). Correlations between neutralizing capacity and disease severity have also been drawn in the context of other coronaviruses such as MERS-CoV, where peak overall antibody and neutralizing antibody levels increase in cases of severe disease, while mild and asymptomatic cases appear to have little or no neutralizing antibodies (116, 117).
The overall timing and kinetics of NAbs is a topic worthy of discussion. As mentioned in “Kinetics”, NAbs are detected earlier in non-lethal and less severe patients, and a timeframe of 14 days has been proposed to classify patients at risk of having lethal outcomes (70, 71). In fact, this might suggest a contrary immune phenotype to that of quick COVID-19 healers suggested by Chen et al. (118). These authors showed that there is a specific group of mild patients who have a shortened disease course and a sustained antibody production for nearly 100 days. Ren et al. also showed a general correspondence between the timing of NAbs detection and anti-RBD and anti-S antibody detection, thus showing that NAbs appear in a similar time frame to these other antibodies (70). The duration of NAbs is also subject to intensive research since it could provide information about protective immunity over time. However, the duration of circulating NAbs in convalescent people has not been established yet. Then, it can only be speculated based on the information on other coronaviruses, such as SARS-CoV, which has neutralizing antibodies for up to 24 months post-infection (49). Other than that, information could be extrapolated from general kinetic profiles reported by other studies, as shown above in this review.
Yet another topic which is subject of public debate regarding neutralizing antibodies is the relationship with antibody dependent enhancement (ADE) and reinfection. ADE is a phenomenon in which, contrary to the ideal condition of neutralizing antibodies, the immune system produces suboptimal antibodies that are not able to inhibit the replication cycle and, instead, facilitate viral entry to susceptible cell types (119–121) Since the beginning of the pandemic, ADE has been a possibility noted by many authors due to past experiences with other coronaviruses (122), although concrete evidence has been limited (123–127). Some authors argue that the possibility of ADE would have therapeutic implications that should be addressed urgently, in particular with vaccines, hyperimmune globulin and convalescent plasma (128, 129). Others have speculated on the possible relationship between ADE and severity in COVID-19 patients (122, 130), while some have even suggested that ADE is responsible for specific conditions such as multisystem inflammatory syndrome in children or hyperinflammation in people living with COVID-19 (131, 132). In addition, the potential role of ADE has been suggested in the Nevada reinfection case, but aside from speculation, there is no evidence or support for this hypothesis (90).
However, there is also an important number of authors who have speculated from other perspectives on ADE in COVID-19. For instance, some authors have suggested that convalescent plasma safety studies weigh against the possibility of this phenomenon in SARS-CoV-2 infection, lessening the concerns, although they do not discard this possibility (133, 134). Altogether, the multiple articles garnered about ADE display a wide spectrum of opinions on a subject that is yet to be deeply investigated and must remain within sight (Figure 5). Nevertheless, it is necessary to state that the available evidence so far on ADE and its potential role on infection by SARS-CoV2 suggests that this phenomenon is unlikely and would have limited inherence in the clinical course of COVID-19, but compelling evidence is yet to be presented on the subject, either for or against.
The nature and importance of antibodies against SARS-CoV-2 has been a subject of debate. Despite the ample areas of research on humoral response in COVID-19, neutralization and antibody kinetics have been at the center of investigation in humoral immune responses against this coronavirus. The disparities among people in these two subjects appears to be finely tuned and hold an important correspondence with the severity of the disease. As shown in this review, the antibody response, its duration and its capacity to confer protection, appears to behave more favorably in patients who had coursed with severe disease as compared to those with milder symptoms.
There is compelling evidence, indicating that some immune functions are misregulated in COVID-19 patients, such as loss of germinal centers with poor Bcl-6 activity, leading to an overall inadequate immune response (135), that may account for the few confirmed cases of reinfection (136) and, perhaps, for the persistent circulation for SARS-CoV-2. Additionally, considering the apparently low risk of ADE in the context of COVID-19 with the evidence garnered so far, the humoral immune response does not likely represent a danger in itself.
Investigating and reviewing seroprevalence in COVID-19 gives perspective on the impact of this virus over time. So far, few major studies have led conclusively to seropositivity rates that can be easily extrapolated to the general population, and that could assist analyzing the “protected population” status (14, 15). Therefore, COVID-19 response urgently needs to be enlightened with more representative data, in particular defining the neutralizing capacity of the humoral response and its duration. This information would shed light on the truly protected population compared to those which might be susceptible to infection, even if they have encountered this virus before.
Convalescent plasma is another subject that provides perspective on antibodies. Several clinical trials are currently investigating the efficacy of such a treatment at different stages of the disease, compared to different treatments and interventions (137–142). Current information has also been contradictory, as some have reported beneficial effects of convalescent plasma while others have criticized the lack of controlled groups in the studies published so far, as well as randomized clinical trials suggesting that the balance of efficacy and adverse events is still uncertain (51, 143).
Both SARS-CoV-2 specific CD4 + and CD8 + T cells, as well as B cells against SARS-CoV-2 epitopes, have been found for up to 6 months after infection in about 95% of COVID-19 patients (91). The same response that occurs with natural infection could be expected with vaccines, such as those developed by Pfizer-BioNTech, Moderna and Oxford-Astrazeneca, since they stimulate the humoral immune response directed to the SARS-CoV-2 spike protein (144–146) At the same time, it has also been seen that although the memory immune response remains for several months, it decreases over time; such is the case of the neutralizing antibody response and spike-specific CD4 + T cells, which diminish in the former 4 months post-infection. However, it seems that S-specific IgG+ memory B cells accumulate over time (147) and may be responsible for maintaining the efficacy of vaccines at long term. It should be noted that the possibility that the SARS-CoV-2 vaccines are only transiently protective in the population cannot be ruled out, which leads to the belief that the vaccines may require greater immunogenicity than natural infection or revaccinate the general population periodically, in order to maintain long-time protection.
Altogether, the evidence on antibody responses to SARS-CoV-2 is broad and ever-growing, but there are still important contradictions and uncertainties that must lead the scientific community to find better evidence that can provide a more precise scope for understanding the biological phenomena, and at the same time lead clinicians to find the best possible interventions for their patients.
MC-M and YM-C reviewed the literature and prepared drafts of the manuscript for final submission. PP, PV, and MR assisted in evaluation of the literature and revised the drafts of the manuscript. All authors contributed to the article and approved the submitted version.
Project BPIN 2020000100131 and Universidad de Antioquia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures 2–5 were created using BioRender.com.
1. Gao GF. From “A”IV to “Z”IKV: Attacks from Emerging and Re-emerging Pathogens. Cell [Internet] (2018) 172(6):1157–9. doi: 10.1016/j.cell.2018.02.025
2. Callaway BE, Cyranoski D, Mallapaty S, Stoye E, Tollefson J. Coronavirus by the numbers. Nature (2020) 579:482–3. doi: 10.1038/d41586-020-00758-2
3. World Health Organization. Estimating mortality from COVID-19. In: Scientific brief, 4 August 2020 (2020). p. 5–8. Available at: https://www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19.
4. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med (2020) 382(16):0–3. doi: 10.1101/2020.03.09.20033217
5. Somsen GA, van Rijn C, Kooij S, Bem RA, Bonn D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir Med [Internet] (2020) 8(7):658–9. doi: 10.1016/S2213-2600(20)30245-9
6. Meselson M. Droplets and Aerosols in the Transmission of SARS-CoV-2. N Engl J Med (2020) 382(21):2063. doi: 10.1056/NEJMc2009324
7. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet [Internet] (2020) 395(10224):565–74. doi: 10.1016/S0140-6736(20)30251-8
8. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med [Internet] (2020) 26(7):1017–32. doi: 10.1038/s41591-020-0968-3
9. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med (2020) 382(18):1708–20. doi: 10.1056/NEJMoa2002032
10. Jiang S, Hillyer C, Du L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol [Internet] (2020) 41(5):355–9. doi: 10.1016/j.it.2020.03.007
11. Gorse GJ, Donovan MM, Patel GB. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J Med Virol (2020) 92(5):512–7. doi: 10.1002/jmv.25715
12. Shah VK, Firmal P, Alam A, Ganguly D, Chattopadhyay S. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Front Immunol (2020) 11(August):1–17. doi: 10.3389/fimmu.2020.01949
13. Bobrovitz N, Arora RK, Cao C, Boucher E, Liu M, Rahim H, et al. Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and meta-analysis [Internet]. medRxiv. medRxiv (2020) 2020.11.17.20233460. doi: 10.1101/2020.11.17.20233460
14. Arora RK, Joseph A, Van Wyk J, Rocco S, Atmaja A, May E, et al. SeroTracker: a global SARS-CoV-2 seroprevalence dashboard. Lancet Infect Dis [Internet] (2020) 3099(20):9–10. doi: 10.1016/S1473-3099(20)30631-9
15. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect [Internet] (2021) 108(December 2019):120–34. doi: 10.1016/j.jhin.2020.11.008
16. Garcia-Basteiro AL, Moncunill G, Tortajada M, Vidal M, Guinovart C, Jiménez A, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun [Internet] (2020) 11(1):1–9. doi: 10.1038/s41467-020-17318-x
17. Brehm TT, Schwinge D, Lampalzer S, Schlicker V, Küchen J, Thompson M, et al. Seroprevalence of SARS-CoV-2 antibodies among hospital workers in a German tertiary care center: A sequential follow-up study. Int J Hyg Environ Health [Internet] (2021) 232(September 2020):113671. doi: 10.1016/j.ijheh.2020.113671
18. Chen Y, Tong X, Wang J, Huang W, Yin S, Huang R, et al. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect (2020) 81(3):420–6. doi: 10.1016/j.jinf.2020.05.067
19. Kumar N, Bhartiya S, Singh T. Duration of anti-SARS-CoV-2 antibodies much shorter in India. Vaccine [Internet] (2021) 39(6):886–8. doi: 10.1016/j.vaccine.2020.10.094
20. Grant J, Wilmore S, McCann N, Donnelly O, Lai R, Kinsella M, et al. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect Control Hosp Epidemiol (2020) 42(2):1–3. doi: 10.1017/ice.2020.402
21. Stubblefield WB, Talbot HK, Feldstein LR, Tenforde MW, Rasheed MAU, Mills L, et al. Seroprevalence of SARS-CoV-2 Among Frontline Healthcare Personnel During the First Month of Caring for Patients With COVID-19—Nashville, Tennessee. Clin Infect Dis (2020) Xx Xxxx):1–4. doi: 10.1093/cid/ciaa936
22. Hunter BR, Dbeibo L, Weaver C, Beeler C, Saysana M, Zimmerman M, et al. Seroprevalence of SARS-CoV-2 Antibodies among Healthcare Workers with Differing Levels of COVID-19 Patient Exposure. Infect Control Hosp Epidemiol (2020) 2:1–2. doi: 10.1017/ice.2020.390
23. Ariza B, Torres X, Salgado D, Cepeda M, Restrepo CG, Castellanos JC, et al. Seroprevalence and seroconversion rates to SARS-CoV-2 in interns, residents, and medical doctors in a University Hospital in Bogotá, Colombia. Infectio (2021) 25(3):145–52. doi: 10.22354/in.v25i3.938
24. Goldblatt D, Johnson M, Falup-Pecurariu O, Ivaskeviciene I, Spoulou V, Tamm E, et al. Cross-sectional prevalence of SARS-CoV-2 antibodies in healthcare workers in paediatric facilities in eight countries. J Hosp Infect (2021) 110:60–6. doi: 10.1016/j.jhin.2020.12.019
25. Alserehi HA, Alqunaibet AM, Al-Tawfiq JA, Alharbi NK, Alshukairi AN, Alanazi KH, et al. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: comparing case and control hospitals. Diagn Microbiol Infect Dis (2021) 99(3):1–5. doi: 10.1016/j.diagmicrobio.2020.115273
26. Xu X, Sun J, Nie S, Li H, Kong Y, Liang M, et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med [Internet] (2020) 26(8):1193–5. doi: 10.1038/s41591-020-0949-6
27. Sood N, Simon P, Ebner P, Eichner D, Reynolds J, Bendavid E, et al. Seroprevalence of SARS-CoV-2-Specific Antibodies among Adults in Los Angeles County, California, on April 10-11, 2020. JAMA - J Am Med Assoc (2020) 323(23):2425–7. doi: 10.1001/jama.2020.8279
28. Bryan A, Pepper G, Wener M, Fink S, Morishima C, Chaudhary A, et al. Performance Characteristics of the Abbott Architect SARS-CoV- 2 IgG Assay and Seroprevalence in Boise, Idaho. J Clin Microbiol (2020) 58(8):4–11. doi: 10.1128/JCM.00941-20
29. Havers FP, Reed C, Lim T, Montgomery JM, Klena JD, Hall AJ, et al. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med (2020) 30329:1–11. doi: 10.1101/2020.06.25.20140384
30. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet (2020) 396(10250):535–44. doi: 10.1016/S0140-6736(20)32266-2
31. Silveira MF, Barros AJD, Horta BL, Pellanda LC, Victora GD, Dellagostin OA, et al. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med [Internet] (2020) 26(8):1196–9. doi: 10.1038/s41591-020-0992-3
32. Malani A, Shah D, Kang G, Lobo GN, Shastri J, Mohanan M, et al. Seroprevalence of SARS-CoV-2 in slums versus non-slums in Mumbai, India. Lancet Glob Heal [Internet] (2021) 9(2):e110–1. doi: 10.1016/S2214-109X(20)30467-8
33. Bruckner TA, Parker DM, Bartell SM, Vieira VM, Khan S, Noymer A, et al. Estimated seroprevalence of SARS-CoV-2 antibodies among adults in Orange County, California. Sci Rep [Internet] (2021) 11(1):1–9. doi: 10.1038/s41598-021-82662-x
34. Xu R, Huang J, Duan C, Liao Q, Shan Z, Wang M, et al. Low prevalence of antibodies against SARS-CoV-2 among voluntary blood donors in Guangzhou, China. J Med Virol (2021) 93(3):1743–7. doi: 10.1002/jmv.26445
35. Yiannoutsos CT, Halverson PK, Menachemi N. Bayesian estimation of SARS-CoV-2 prevalence in Indiana by random testing. Proc Natl Acad Sci U S A (2021) 118(5):1–8. doi: 10.1073/pnas.2013906118
36. Goldstein ND, Wheeler DC, Gustafson P, Burstyn I. A Bayesian approach to improving spatial estimates of prevalence of COVID-19 after accounting for misclassification bias in surveillance data in Philadelphia, PA. Spat Spatiotemporal Epidemiol (2021) 36:1–7. doi: 10.1016/j.sste.2021.100401
37. Sam IC, Chong YM, Tan CW, Chan YF. Low postpandemic wave SARS-CoV-2 seroprevalence in Kuala Lumpur and Selangor, Malaysia. J Med Virol (2021) 93(2):647–8. doi: 10.1002/jmv.26426
38. Shakiba M, Nazemipour M, Salari A, Mehrabian F, Hashemi Nazari SS, Rezvani SM, et al. Seroprevalence of SARS-CoV-2 in Guilan Province, Iran, April 2020. Emerg Infect Dis (2021) 27(2):636–8. doi: 10.3201/eid2702.201960
39. Cheng Y, Wong R, Soo YOY, Wong WS, Lee CK, Ng MHL, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis [Internet] (2005) 24(1):44–6. doi: 10.1007/s10096-004-1271-9
40. Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw F-M, Lim WS, et al. The Effectiveness of Convalescent Plasma and Hyperimmune Immunoglobulin for the Treatment of Severe Acute Respiratory Infections of Viral Etiology: A Systematic Review and Exploratory Meta-analysis. J Infect Dis [Internet] (2015) 211(1):80–90. doi: 10.1093/infdis/jiu396
41. Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev [Internet] (2020) 19(7):102554. doi: 10.1016/j.autrev.2020.102554
42. Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of convalescent plasma therapy in two covid-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci (2020) 35(14):2–9. doi: 10.3346/jkms.2020.35.e149
43. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U.S.A. (2020) 117(17):9490–6. doi: 10.1073/pnas.2007408117
44. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 Critically Ill Patients with COVID-19 with Convalescent Plasma. JAMA - J Am Med Assoc (2020) 323(16):1582–9. doi: 10.1001/jama.2020.4783
45. Zhang L, Pang R, Xue X, Bao J, Ye S, Dai Y, et al. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging (2020) 12(8):6536–42. doi: 10.18632/aging.103102
46. Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, et al. Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients. Mayo Clin Proc (2020) 95(9):1888–97. doi: 10.1016/j.mayocp.2020.09.032
47. Abolghasemi H, Eshghi P, Cheraghali AM, Imani Fooladi AA, Bolouki Moghaddam F, Imanizadeh S, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus Apher Sci [Internet] (2020) 59(5):102875. doi: 10.1016/j.transci.2020.102875
48. Gharbharan A, Jordans CCE, GeurtsvanKessel C, Hollander JGd, Karim F, Mollema FPN, et al. Convalescent Plasma for COVID-19. A randomized clinical trial. medRxiv [Internet] (2020) 2020:7.01.20139857. doi: 10.1101/2020.07.01.20139857v1
49. Bradfute SB, Hurwitz I, Yingling AV, Ye C, Cheng Q, Noonan TP, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Neutralizing Antibody Titers in Convalescent Plasma and Recipients in New Mexico: An Open Treatment Study in Patients With Coronavirus Disease 2019. J Infect Dis (2020) 222(10):1620–8. doi: 10.1093/infdis/jiaa505
50. Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med (2021) 384(11):1–13. doi: 10.1056/NEJMoa2031893
51. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA - J Am Med Assoc (2020) 324(5):460–70. doi: 10.1001/jama.2020.12607
52. Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med (2020) 384(7):619–29. doi: 10.1056/NEJMoa2031304
53. Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ (2020) 371:1–10. doi: 10.1101/2020.09.03.20187252
54. Piechotta V, Kl C, Sj V, Doree C, Monsef I, Em W, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review (Review). Cochrane Database Syst Rev (2020) 7):1–293. doi: 10.1002/14651858.CD013600.pub2
55. Updated Evidence to Support the Emergency Use of COVID-19 Convalescent Plasma - as of 9/23/2020. (2020) 1):1–5. https://www.fda.gov/media/142386/download.
56. Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature (2020) 584(7821):437–42. doi: 10.1038/s41586-020-2456-9
57. Wang Y, Zhang L, Sang L, Ye F, Ruan S, Zhong B, et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest (2020) 130(10):5235–44. doi: 10.1172/JCI138759
58. Cross RW, Prasad AN, Borisevich V, Woolsey C, Agans KN, Deer DJ, et al. Use of convalescent serum reduces severity of COVID-19 in nonhuman primates. Cell Rep [Internet] (2021) 34(10):108837. doi: 10.1016/j.celrep.2021.108837
59. Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nat [Internet] (2020) 584(7819):115–9. doi: 10.1038/s41586-020-2380-z
60. Lau CS, Hoo SP, Yew SF, Ong SK, Lum LT, Heng PY, et al. Evaluation of an Electrochemiluminescent SARS-CoV-2 Antibody Assay. J Appl Lab Med (2020) 5(6):1313–23. doi: 10.1093/jalm/jfaa134
61. Hamilton F, Muir P, Attwood M, Vipond ANB, Hopes R, Moran E, et al. Kinetics and performance of the Abbott architect SARS-CoV-2 IgG antibody assay. J Infect (2020) xxxx):9–11. doi: 10.1101/2020.07.03.20145722
62. Tré-Hardy M, Wilmet A, Beukinga I, Favresse J, Dogné JM, Douxfils J, et al. Analytical and clinical validation of an ELISA for specific SARS-CoV-2 IgG, IgA, and IgM antibodies. J Med Virol (2020) March):1–9. doi: 10.1002/jmv.26303
63. Herroelen PH, Martens GA, De Smet D, Swaerts K, Decavele AS. Humoral Immune Response to SARS-CoV-2. Am J Clin Pathol (2020) 154(5):610–9. doi: 10.1093/ajcp/aqaa140
64. Moshe M, Daunt A, Flower B, Simmons B, Brown JC, Frise R, et al. SARS-CoV-2 lateral flow assays for possible use in national covid-19 seroprevalence surveys (React 2 ): diagnostic accuracy study. BMJ (2021) 2(React 2):1–8. doi: 10.1136/bmj.n423
65. Rijkers G, Murk JL, Wintermans B, van Looy B, van den Berge M, Veenemans J, et al. Differences in Antibody Kinetics and Functionality Between Severe and Mild Severe Acute Respiratory Syndrome Coronavirus 2 Infections. J Infect Dis (2020) 222(8):1265–9. doi: 10.1093/infdis/jiaa463
66. Borremans B, Gamble A, Prager KC, Helman SK, McClain AM, Cox C, et al. Quantifying antibody kinetics and rna detection during early-phase SARS-CoV-2 infection by time since symptom onset. Elife (2020) 9:1–27. doi: 10.7554/eLife.60122
67. Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun [Internet] (2020) 11(1):1–16. doi: 10.1038/s41467-020-18450-4
68. Luo YR, Chakraborty I, Yun C, Wu AHB, Lynch KL. Kinetics of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Avidity Maturation and Association with Disease Severity. Clin Infect Dis (2020) 2(Xx):1–3. doi: 10.1093/cid/ciaa1389
69. Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol [Internet] (2020) 5(December):1–17. doi: 10.1038/s41564-020-00813-8
70. Ren L, Zhang L, Chang D, Wang J, Hu Y, Chen H, et al. The kinetics of humoral response and its relationship with the disease severity in COVID-19. Commun Biol [Internet] (2020) 3(1):1–7. doi: 10.1038/s42003-020-01526-8
71. Lucas C, Klein J, Sundaram M, Liu F, Wong P, Silva J. Kinetics of antibody responses dictate COVID-19 outcome. medRxiv (2020). doi: 10.1101/2020.12.18.20248331
72. Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N Engl J Med (2020) 383(18):1724–34. doi: 10.1056/NEJMoa2026116
73. Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Sci (80- ) (2020) 7728(October):eabd7728. doi: 10.1126/science.abd7728
74. Lynch KL, Whitman JD, Lacanienta NP, Beckerdite EW, Kastner SA, Shy BR, et al. Magnitude and Kinetics of Anti–Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Responses and Their Relationship to Disease Severity. Clin Infect Dis (2020) Xx Xxxx):1–8. doi: 10.1093/cid/ciaa979
75. Orth-Höller D, Eigentler A, Weseslindtner L, Möst J. Antibody kinetics in primary- and secondary-care physicians with mild to moderate SARS-CoV-2 infection. Emerg Microbes Infect (2020) 9(1):1–12. doi: 10.1080/22221751.2020.1793690
76. Zhang B, Yue D, Wang Y, Wang F, Wu S, Hou H. The dynamics of immune response in COVID-19 patients with different illness severity. J Med Virol (2020) July):1–8. doi: 10.1002/jmv.26504
77. Sun J, Tang X, Bai R, Liang C, Zeng L, Lin H, et al. The kinetics of viral load and antibodiesto SARS-CoV-2. Clin Microbiol Infect [Internet] (2020) 26(12):1–16. doi: 10.1016/j.cmi.2020.08.043
78. Kowitdamrong E, Puthanakit T, Jantarabenjakul W, Prompetchara E, Suchartlikitwong P, Putcharoen O, et al. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PloS One [Internet] (2020) 15(10 October):1–11. doi: 10.1371/journal.pone.0240502
79. Callow KA, Parry HF, Sergeant M, Tyrrell DAJ. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect (1990) 105(2):435–46. doi: 10.1017/S0950268800048019
80. Hsueh PR, Huang LM, Chen PJ, Kao CL, Yang PC. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect (2004) 10(12):1062–6. doi: 10.1111/j.1469-0691.2004.01009.x
81. Mo H, Zeng G, Ren X, Li H, Ke C, Tan Y, et al. Longitudinal profile of antibodies against SARS-coronavirus in SARS patients and their clinical significance. Respirology (2006) 11(1):49–53. doi: 10.1111/j.1440-1843.2006.00783.x
82. Patil HP, Rane PS, Shrivastava S, Palkar S, Lalwani S, Mishra AC, et al. Antibody (IgA, IgG, and IgG Subtype) Responses to SARS-CoV-2 in Severe and Nonsevere COVID-19 Patients. Viral Immunol [Internet] (2021) 34(3):1–9. doi: 10.1089/vim.2020.0321
83. Georg S, Marianna Theresia T, Marianne G, Wolfgang H, Tamara S, Hasan K, et al. Assessment of S1, S2 and NCP-specific IgM, IgA, and IgG antibody kinetics in acute 1 SARS-CoV-2 infection by a microarray and twelve other immunoassays 2 3 Downloaded from. J Clin Microbiol [Internet] (2021) 1–50. doi: 10.1128/JCM.02890-20
84. Chen Y, Tong X, Li Y, Gu B, Yan J, Liu Y, et al. A comprehensive, longitudinal analysis of humoral responses specific to four recombinant antigens of SARS-CoV-2 in severe and non-severe COVID-19 patients. PloS Pathog [Internet] (2020) 16(9):1–16. doi: 10.1371/journal.ppat.1008796
85. Sun B, Feng Y, Mo X, Zheng P, Wang Q, Li P, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect (2020) 9(1):940–8. doi: 10.1080/22221751.2020.1762515
86. Hachim A, Kavian N, Cohen CA, Chin AWH, Chu DKW, Mok CKP, et al. ORF8 and ORF3b antibodies are accurate serological markers of early and late SARS-CoV-2 infection. Nat Immunol [Internet] (2020) 21(10):1293–301. doi: 10.1038/s41590-020-0773-7
87. Bahar B, Jacquot C, Mo YD, DeBiasi RL, Campos J, Delaney M. Kinetics of Viral Clearance and Antibody Production Across Age Groups in Children with Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Pediatr [Internet] (2020) 227:31–37.e1. doi: 10.1016/j.jpeds.2020.08.078
88. Marot S, Malet I, Leducq V, Zafilaza K, Sterlin D, Planas D, et al. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat Commun (2021) 12(1):1–7. doi: 10.1038/s41467-021-21111-9
89. To KK-W, Hung IF-N, Ip JD, Chu AW-H, Chan W-M, Tam AR, et al. Coronavirus Disease 2019 (COVID-19) Re-infection by a Phylogenetically Distinct Severe Acute Respiratory Syndrome Coronavirus 2 Strain Confirmed by Whole Genome Sequencing. Clin Infect Dis (2020) 2019(Xx):1–6. doi: 10.1093/cid/ciaa1275
90. Tillett RL, Sevinsky JR, Hartley PD, Kerwin H, Crawford N, Gorzalski A, et al. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis [Internet] (2020) 3099(20):1–7. doi: 10.1016/S1473-3099(20)30764-7
91. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Sci (80- ) [Internet] (2021) 371(6529):1–15. doi: 10.1126/science.abf4063
92. Zheng Y, Zhang Q, Ali A, Li K, Shao N, Zhou X, et al. Sustainability of SARS-CoV-2 Induced Humoral Immune Responses in COVID-19 Patients from Hospitalization to Convalescence Over Six Months. Virol Sin [Internet] (2021) 1–10. doi: 10.1007/s12250-021-00360-4
93. Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N Engl J Med [Internet] (2021) 384(6):533–40. doi: 10.1056/NEJMoa2034545
94. Abu-Raddad LJ, Chemaitelly H, Coyle P, Malek JA, Ahmed AA, Mohamoud YA, et al. SARS-CoV-2 reinfection in a cohort of 43,000 antibody-positive individuals followed for up to 35 weeks. medRxiv [Internet] (2021) 2021:1.15.21249731. doi: 10.1101/2021.01.15.21249731
95. van Kerkhove MD, Aswad S, Assiri A, Perera RAPM, Peiris M, El Bushra HE, et al. Transmissibility of MERS-CoV infection in closed setting, Riyadh, Saudi Arabia, 2015. Emerg Infect Dis (2019) 25(10):1802–9. doi: 10.3201/eid2510.190130
96. Cao W-C, Liu W, Zhang P-H, Zhang F, Richardus JH. Disappearance of Antibodies to SARS-Associated Coronavirus after Recovery. N Engl J Med (2007) 357(11):1162–3. doi: 10.1056/NEJMc070348
97. Zinkernagel RM, Hengartner H. Protective “immunity” by pre-existent neutralizing antibody titers and preactivated T cells but not by so-called “immunological memory.” Immunol Rev (2006) 211:310–9. doi: 10.1111/j.0105-2896.2006.00402.x
98. Wang X, Guo X, Xin Q, Pan Y, Hu Y, Li J, et al. Neutralizing Antibody Responses to Severe Acute Respiratory Syndrome Coronavirus 2 in Coronavirus Disease 2019 Inpatients and Convalescent Patients. Clin Infect Dis (2020) Xx):1–7. doi: 10.1093/cid/ciaa721
99. Liu P, Cai J, Jia R, Xia S, Wang X, Cao L, et al. Dynamic surveillance of SARS-CoV-2 shedding and neutralizing antibody in children with COVID-19. Emerg Microbes Infect (2020) 9(1):1254–8. doi: 10.1080/22221751.2020.1772677
100. Lv H, Wu NC, Tsang OTY, Yuan M, Perera RAPM, Leung WS, et al. Cross-reactive Antibody Response between SARS-CoV-2 and SARS-CoV Infections. Cell Rep [Internet] (2020) 31(9):107725. doi: 10.1016/j.celrep.2020.107725
101. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell (2020) 181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052
102. Ma Z, Li P, Ji Y, Ikram A, Pan Q. Cross-reactivity towards SARS-CoV-2: the potential role of low-pathogenic human coronaviruses. Lancet Microbe [Internet] (2020) 1(4):e151. doi: 10.1016/S2666-5247(20)30098-7
103. Ng KW, Faulkner N, Cornish GH, Rosa A, Harvey R, Hussain S, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Sci (80- ) (2020) 370(6522):eabe1107. doi: 10.1126/science.abe1107
104. Klein SL, Pekosz A, Park HS, Ursin RL, Shapiro JR, Benner SE, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest (2020) 130(11):6141–50. doi: 10.1101/2020.06.26.20139063
105. Wu F. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. Lancet Infect Dis (2020) in press:1–20. doi: 10.2139/ssrn.3566211
106. Gallian P, Pastorino B, Morel P, Chiaroni J, Ninove L, de Lamballerie X. Lower prevalence of antibodies neutralizing SARS-CoV-2 in group O French blood donors. Antiviral Res [Internet] (2020) 181:104880. doi: 10.1016/j.antiviral.2020.104880
107. Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front Public Heal (2020) 8(April):1–6. doi: 10.3389/fpubh.2020.00152
108. Mukherjee S, Pahan K. Is COVID-19 Gender-sensitive? J Neuroimmune Pharmacol Springer (2021) 16:38–47. doi: 10.1007/s11481-020-09974-z
109. Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun [Internet] (2020) 11(1):1–10. doi: 10.1038/s41467-020-19741-6
110. Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature (2020) June):1–23. doi: 10.1101/2020.06.06.20123414
111. Liu L, To KKW, Chan KH, Wong YC, Zhou R, Kwan KY, et al. High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerg Microbes Infect (2020) 9(1):1–30. doi: 10.1080/22221751.2020.1791738
112. Choe PG, Kang CK, Suh HJ, Jung J, Kang EK, Lee SY, et al. Antibody Responses to SARS-CoV-2 at 8 Weeks Postinfection in Asymptomatic Patients. Emerg Infect Dis (2020) 26(10):2484–7. doi: 10.3201/eid2610.202211
113. Cao Y, Su B, Guo X, Sun W, Deng Y, Bao L, et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell (2020) 182(1):73–84.e16. doi: 10.1016/j.cell.2020.05.025
114. Terpos E, Politou M, Sergentanis TN, Mentis A, Rosati M, Stellas D, et al. Anti–SARS-CoV-2 Antibody Responses in Convalescent Plasma Donors Are Increased in Hospitalized Patients; Subanalyses of a Phase 2 Clinical Study. Microorganisms [Internet] (2020) 8(12):1885. doi: 10.3390/microorganisms8121885
115. Chen X, Pan Z, Yue S, Yu F, Zhang J, Yang Y, et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transduct Target Ther (2020) 5(1):1–6. doi: 10.1038/s41392-020-00301-9
116. Okba NMA, Raj S, Widjaja I, de Bruin E, Farag E, Al-Hajri M, et al. Sensitive and Specific Detection of Low-Level Antibody Responses in Mild Middle East Respiratory Syndrome Coronavirus Infections. Emerg Infect Dis - (Open Access) (2019) 25(10):1868–77. doi: 10.3201/eid2510.190051
117. Ko J, Müller MA, Seok H, Eun G, Yeon J, Young S, et al. Serologic responses of 42 MERS-coronavirus-infected patients according to the disease severity. Diagn Microbiol Infect Dis (2017) 89(2):106–11. doi: 10.1016/j.diagmicrobio.2017.07.006
118. Chen Y, Zuiani A, Fischinger S, Mullur J, Atyeo C, Travers M, et al. Quick COVID-19 Healers Sustain Anti-SARS-CoV-2 Antibody Production. Cell [Internet] (2020) 183(6):1496–1507.e16. doi: 10.1016/j.cell.2020.10.051
119. Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol [Internet] (2020) 5(10):1185–91. doi: 10.1038/s41564-020-00789-5
120. Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, et al. Antibody-dependent enhancement of severe dengue disease in humans. Sci (80- ) (2017) 358(6365):929–32. doi: 10.1126/science.aan6836
121. Kulkarni R. Antibody-Dependent Enhancement of Viral Infections. In: Bramhachari P. V. (Ed.), Dynamics of Immune Activation in Viral Diseases. Singapore: Springer (2020). pp. 9–41. doi: 10.1007/978-981-15-1045-8_2
122. Tetro JA. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect [Internet] (2020) 22(2):72–3. doi: 10.1016/j.micinf.2020.02.006
123. Denner J. SARS-CoV-2 and enhancing antibodies. J Clin Virol (2020) April:1. doi: 10.1016/j.jcv.2020.104424
124. Negro F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis? Swiss Med Wkly (2020) 150(April):1. doi: 10.4414/smw.2020.20249
125. Hotez PJ, Corry DB, Bottazzi ME. COVID-19 vaccine design: the Janus face of immune enhancement. Nat Rev Immunol [Internet] (2020) 20(6):347–8. doi: 10.1038/s41577-020-0323-4
126. Fierz W, Walz B. Antibody Dependent Enhancement Due to Original Antigenic Sin and the Development of SARS. Front Immunol (2020) 11(June):1–5. doi: 10.3389/fimmu.2020.01120
127. Eroshenko N, Gill T, Keaveney K, Marianna K, Church GM, Trevejo JM, et al. Implications of antibody-dependent enhancement of infection for SARS-CoV-2 countermeasures. Nat Biotechnol (2020) 38(7):788–9. doi: 10.1038/s41587-020-0577-1
128. de Alwis R, Chen S, Gan ES, Ooi EE. Impact of immune enhancement on Covid-19 polyclonal hyperimmune globulin therapy and vaccine development. EBioMedicine (2020) 55:1–7. doi: 10.1016/j.ebiom.2020.102768
129. Fleming AB, Raabe V. Current studies of convalescent plasma therapy for COVID-19 may underestimate risk of antibody- dependent enhancement. J Clin Virol (2020) 127(January):1. doi: 10.1016/j.jcv.2020.104388
130. Tilocca B, Soggiu A, Musella V, Britti D, Sanguinetti M, Urbani A, et al. Molecular basis of COVID-19 relationships in different species: a one health perspective. Microbes Infect [Internet] (2020) 22(4–5):218–20. doi: 10.1016/j.micinf.2020.03.002
131. Rothan HA, Byrareddy SN. The potential threat of multisystem inflammatory syndrome in children during the COVID-19 pandemic. Pediatr Allergy Immunol (2020) August):1–6. doi: 10.22541/au.159224859.92720715
132. Cloutier M, Nandi M, Ullah A, Allard H. ADE and hyperinflammation in SARS-CoV2 infection- comparison with dengue hemorrhagic fever and feline infectious peritonitis. Cytokine (2020) 136:155256. doi: 10.1016/j.cyto.2020.155256
133. Im JH, Nahm CH, Baek JH, Kwon HY, Lee JS. Convalescent plasma therapy in coronavirus disease 2019: A case report and suggestions to overcome obstacles. J Korean Med Sci (2020) 35(26):1–5. doi: 10.3346/jkms.2020.35.e239
134. Yager EJ. Antibody-dependent enhancement and COVID-19: Moving toward acquittal. Clin Immunol [Internet] (2020) 217:108496. doi: 10.1016/j.clim.2020.108496
135. Kaneko N, Kuo HH, Boucau J, Farmer JR, Allard-Chamard H, Mahajan VS, et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell (2020) 183(1):143–57.e13. doi: 10.1016/j.cell.2020.08.025
136. Ledford H. Covid-19 Reinfection: Three Questions Scientists Are Asking. Nat [Internet] (2020) 585(7824):168–9. doi: 10.1038/d41586-020-02506-y
137. Convalescent Plasma - clinical trial. In: NHS Blood and Transplant [Internet]. Available at: https://www.nhsbt.nhs.uk/how-you-can-help/convalescent-plasma-clinical-trial/.
138. Convalescent Plasma for the Treatment of Severe SARS-CoV-2 (COVID-19). (2020). https://clinicaltrials.gov/ct2/show/NCT04391101.
139. Use of Convalescent Plasma Therapy for COVID-19 Patients With Hypoxia: a Prospective Randomized Trial. (2020). https://www.clinicaltrials.gov/ct2/show/NCT04356534.
140. A Clinical Trial of Convalescent Plasma Compared to Best Supportive Care for Treatment of Patients With Severe COVID-19. (2020). https://clinicaltrials.gov/ct2/show/NCT04433910.
141. Efficacy of Convalescent Plasma Therapy in the Early Care of COVID-19 Patients. (2020). https://clinicaltrials.gov/ct2/show/NCT04372979.
142. Efficacy of Convalescent Plasma Therapy in Severely Sick COVID-19 Patients. (2020). https://clinicaltrials.gov/ct2/show/NCT04425915.
143. Plebani M. Antibody responses in mild COVID-19 hospital staff. EBioMedicine (2020) 59:4–5. doi: 10.1016/j.ebiom.2020.102940
144. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med (2020) 383(27):2603–15. doi: 10.1056/NEJMoa2034577
145. Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med (2021) 384(1):80–2. doi: 10.1056/NEJMc2032195
146. Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 : a preliminary report of a phase 1 / 2, single-blind, randomised controlled trial. Lancet (2020) 467–78:476–78. doi: 10.1016/S0140-6736(20)31604-4
Keywords: therapeutics, antibodies, SARS-CoV-2, COVID-19, seroprevalence, kinetics, neutralization
Citation: Chvatal-Medina M, Mendez-Cortina Y, Patiño PJ, Velilla PA and Rugeles MT (2021) Antibody Responses in COVID-19: A Review. Front. Immunol. 12:633184. doi: 10.3389/fimmu.2021.633184
Received: 24 November 2020; Accepted: 25 March 2021;
Published: 15 April 2021.
Edited by:
Mara Biasin, University of Milan, ItalyReviewed by:
Suresh Pallikkuth, University of Miami, United StatesCopyright © 2021 Chvatal-Medina, Mendez-Cortina, Patiño, Velilla and Rugeles. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria T. Rugeles, bWFyaWEucnVnZWxlc0B1ZGVhLmVkdS5jbw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.