
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 01 April 2021
Sec. Autoimmune and Autoinflammatory Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.631055
This article is part of the Research TopicAutoimmune Vasculitis: Advances in Pathogenesis and TherapiesView all 29 articles
Biologics targeting inflammation-related molecules in the immune system have been developed to treat rheumatoid arthritis (RA), and these RA treatments have provided revolutionary advances. Biologics may also be an effective treatment for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis, particularly in patients with resistance to standard treatments. Despite the accumulation of clinical experience and the increasing understanding of the pathogenesis of vasculitis, it is becoming more difficult to cure vasculitis. The treatment of vasculitis with biologics has been examined in clinical trials, and this has also enhanced our understanding of the pathogenesis of vasculitis. A humanized anti-interleukin-5 monoclonal antibody known as mepolizumab was recently demonstrated to provide clinical benefit in the management of eosinophilic granulomatosis with polyangiitis in refractory and relapsing disease, and additional new drugs for vasculitis are being tested in clinical trials, while others are in abeyance. This review presents the new findings regarding biologics in addition to the conventional immunosuppressive therapy for ANCA-associated vasculitis.
Anti-neutrophil cytoplasmic autoantibodies (ANCAs) are the major serological markers of primary systemic necrotizing small vessel inflammation or ANCA-associated vasculitis (AAV), including granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA) (1, 2). ANCAs for proteinase 3 (PR3) are more prevalent in patients with GPA, and ANCAs for myeloperoxidase (MPO) are more prevalent in patients with MPA, but there is substantial overlap. Regarding the clinical utility of ANCAs’ specificity in the classification of the forms of AAV, ANCA specificity is more likely to be associated with the patient’s genetic predisposition (3), treatment effect(s) (4), the risk of recurrence (5, 6), and the prognosis (7) than the clinical diagnosis. Distinct cytokine profiles were identified for PR3-AAV and MPO-AAV with GPA, MPA, and EGPA (8).
These differences in circulating immuno-mediators are strongly associated with ANCA specificity, not clinical diagnosis, and the heterogeneity of AAV subtypes is associated with the clinical phenotypes identified in the traditional clinical classification of GPA and MPA (8). Clinical trial results and clinical practice data have formed the foundation of the management of AAV, which is based on the disease severity. In 2016, the European League Against Rheumatism (EULAR) updated their recommendations for the management of primary small- and medium-vessel vasculitis, including the management of AAV (9). Glucocorticoids are a central component of the management of AAV in induction and maintenance therapy and are not sufficient by themselves, especially in the context of organ invasion. For active AAV, the current treatment recommendation is to first administer high doses of glucocorticoids, followed by a gradual decrease in steroids (10).
Cyclophosphamide is also used in combination with steroids to induce remission in AAV, but its metabolites are toxic to the bladder and reproductive organs and may cause malignancy and infertility in the long term (11). In non-immediately life-threatening AAV, there was no significant difference in remission rates between treatment groups receiving daily oral versus intravenous pulsed cyclophosphamide therapy regimens, and the total dose of cyclophosphamide was reduced in the intravenous pulse group (11).
Advances in induction therapy have transformed AAV from a life-threatening disease to a chronic disease with relapse. Relapse is not uncommon, occurring in 30%–50% of patients with AAV within 5 years of onset, and often 12–18 months after treatment with immunosuppressive agents is discontinued (12). In AAV, maintenance therapy is recommended to prevent relapse after achieving remission by the induction therapy. Maintenance immunosuppressive agents such as cyclophosphamide, mycophenolate mofetil, and azathioprine are used in combination to prevent relapse after the successful induction of remission. More recently, biologic agents have begun to play an important role in the induction of clinical remission and the maintenance of remission in severe AAV. The biologic rituximab is indicated for remission induction and the management of severe and relapsed GPA and MPA, and data suggest a role for pre-emptive fixed-interval rituximab maintenance therapy in remission treatment (13, 14). Treatment with the biologic mepolizumab also provided a significantly greater number of weeks of remission and higher remission rates than a placebo when it was used as maintenance therapy for EGPA (15). Other biologics are either being tested in clinical trials or have failed because their effectiveness could not be verified, or they produced unacceptable side effects. However, it is possible that using biologics could reduce the rate of side effects caused by steroids in the treatment of AAV, by providing a new mechanism of action. This review presents new insights into novel therapeutic targets in AAV.
ANCAs are autoantibodies against cytoplasmic antigens expressed on the primary granules of neutrophils and the lysosomes of monocytes. The primary granules of neutrophils contain a series of antimicrobial proteins, including lysozyme, MPO, neutral serine proteinases (PR3, elastase, and cathepsin G), and acid hydrolases (cathepsins B and D). Autoantibodies can develop against any of these proteins, but the most clinically important antibodies are against MPO and PR3. During the active phase of AAV, ANCA is usually immunoglobulin G (IgG), but other immunoglobulin classes (IgM and IgA) have also been reported. PR3-ANCA is most frequently associated with GPA (75%), and MPO-ANCA is associated with MPA (60%) and EGPA (30%). MPO-ANCA is also associated with renal limited vasculitis (80%) (Table 1) (16, 17).
Atypical ANCAs, which do not react with either PR3 or MPO (positive by indirect immunofluorescence and negative by enzyme-linked immunosorbent assay), have been identified in a range of nonvasculitic conditions: inflammatory bowel diseases, autoimmune diseases, and malignancies.
PR3- and MPO-ANCA have also been found in chronic infections such as endocarditis, tuberculosis, human immunodeficiency virus, hepatitis C, and bartonellosis. The presence of both anti-MPO and anti-PR3 antibodies in the same patient is very rare and suggests drug-induced vasculitis (16). In a subgroup of patients (10%) whose clinical and pathological features are consistent with AAV, the test result for ANCA remains negative. Although these patients may have a similar clinical course and response to treatment, ANCA-negative patients are more likely to have renal-limited disease or less severe systemic disease (16).
Berti et al. reported that the circulating cytokine profiles were significantly different between patients with PR3-ANCA and those with MPO-ANCA (8), and they noted that compared to the PR3-ANCA group, nine biomarkers were higher in their MPO-AAV group: interleukin (IL)-6, -15, and -18, granulocyte-macrophage colony-stimulating factor, chemokine (C-X-C) ligand 8/IL-8, chemokine (C-C motif) ligand 17/THYMUS, activation-regulated chemokine, and IL-18 binding protein. In contrast, four biomarkers were higher in MPO-AAV than in PR3-AAV: soluble IL-6 receptor, soluble tumor necrosis factor (TNF) receptor II, neutrophil gelatinase-associated lipocalin, and soluble intercellular adhesion molecule-1. In active AAV, the cellular infiltration in kidney, lung, and nasal tissues is composed mainly of macrophages, T cells, and B cells (18). In a validation study of T-cell markers in 38 renal biopsies from patients with AAV, Kidder et al. observed an increased number of CD8+ T cells in the periglomerular and interstitial areas in kidneys with a crescent-shaped histology. They also reported a significant correlation between the number of CD8+ T cells and the glomerular filtration rate. Despite the low number of T lymphocytes infiltrating the glomerulus, CD8+ T cells were more predominant than CD4+ T cells (19). In contrast, T cells stimulated with MPO showed much less or no proliferative response in both patients and healthy controls (20). Thus, at least in GPA, the pathogenic role of T cells at the effector stage of AAV is not fully established, because CD4+ T cells, based on their cytokine profile and associated functions, have been shown to have two distinct types: Th1 and Th2 cells (Figure 1). In localized GPA, T cells in nasal inflammatory infiltrates were shown to express the Th1 marker CD26 (21). In addition, more interferon-alpha (IFNα)-positive cells were detected in the nasal inflammatory infiltrate in localized GPA than in generalized GPA. The tissues of eosinophilic infiltration and eosinophilia are also common in GPA and EGPA, and thus polarization to the Th2 profile would be expected in EGPA.
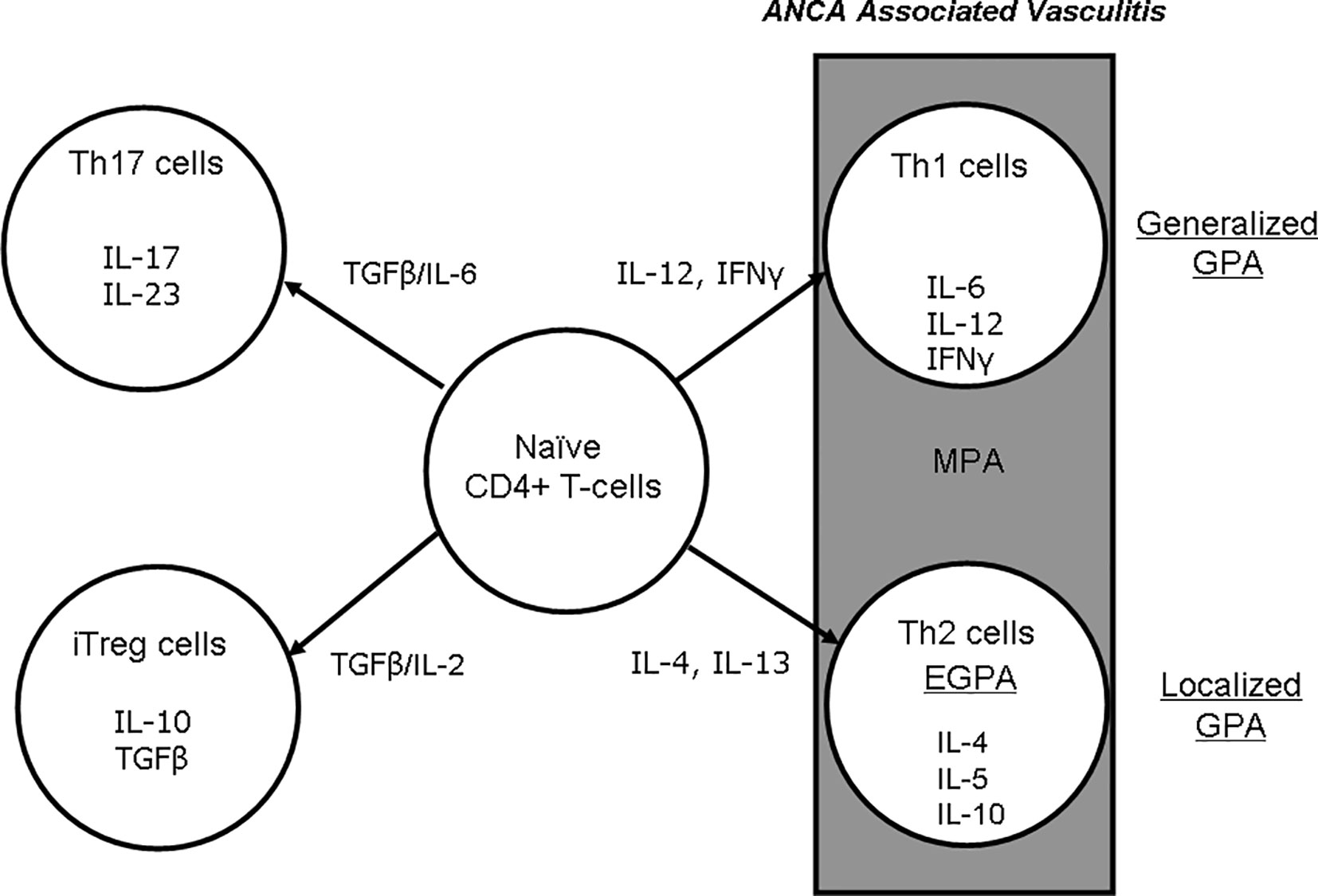
Figure 1 Differentiation of naïve CD4+ T cells to Th1, -2, -17, and iTreg cells and effects on cytokine production in AAV. AAV, ANCA-associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; EGPA, eosinophilic granulomatosis with polyangiitis; GPA, granulomatosis with polyangiitis; IFNγ, interferon gamma; IL, interleukin; iTreg, inducible regulatory T cell; MPA, microscopic polyangiitis; TGFβ, transforming growth factor beta.
Those findings were accompanied by increases in spontaneous IFNα and IL-10 produced by peripheral blood mononuclear cells in patients with localized GPA compared to generalized GPA, whereas high levels of IL-4 mRNA were detected in the nasal inflammatory infiltrates of the patients with generalized GPA. A Th2 environment in this phenomenon was confirmed in nasal granulomas of systemic GPA. In immunohistochemistry staining, IFNα was not detected in nasal biopsies of 10 patients with systemic active GPA, but IL-4 was upregulated (22). These cells are likely to be T cells, especially Th2 cells and eosinophils. These data thus support potential differences in Th responses between local and systemic GPA in nasal granulomatous lesions. Csernok et al. reported the expression of IFNα mRNA in nasal granulomas with systemic GPA, but IL-4 mRNA was only expressed in two of five patients (23). Komocsi et al. also demonstrated Th1-like cytokine production and features suggestive of CD4+ T-cell-mediated cytotoxicity. In GPA, CD4+CD28- T cells are recruited from the blood to granulomatous lesions via interaction with CD18, followed by cytokine secretion to promote monocyte accumulation and granuloma formation (24).
The Th17 T helper subset is involved in the defense against extracellular bacteria and fungi and has been implicated in autoimmune diseases (25). Th17 cells express the transcription factor RORγt (retinoic acid-related orphan receptor gamma t) and produce IL-17A–F, which are centered on IL-17A (26). IL-23 is responsible for the expansion and maturation of the Th17 subset (27). Th17 cells are also inhibited by Th1 and Th2 cytokines.
IL-17A acts on monocytes/macrophages and functions directly via ligation to the receptors expressed on monocytes (28). In peripheral blood, IL-17A induces the release of pro-inflammatory mediators from macrophages, and IL-17A induces a high percentage of Th17 cells in patients with AAV compared to healthy controls (29). Thus, IL-17A, as an important regulator and initiator of inflammation, mediates both innate and adaptive immunity. IL-17A not only defines the Th17 subset; it is also a logical therapeutic target for diseases induced by Th17-dominant autoimmune responses.
Nogueira et al. described that in a cohort of 28 patients with acute AAV and 65 patients in AAV, serum IL-17A levels were significantly higher than in healthy controls, with a similar pattern for serum IL-23 levels (30). Other researchers have found no evidence of this expansion Th17 response in AAV (31, 32). For example, Krohn et al. detected no difference in serum IL-17A levels between 70 AAV patients and the healthy controls, but interestingly, they observed a significant increase in IL-17C levels (33). Velden et al. demonstrated the presence of IL-17 in kidney tissues by immunohistochemical staining, and they reported that the percentage of Th1-like Th17 cells was higher in patients in the acute phase of the disease or in untreated remission compared to healthy controls (34). Thus, IL-17A, as an important regulator and initiator of inflammation, mediates both innate and adaptive immunity. Neutrophils can also induce Th17 cell chemotaxis, making this cell axis even more interesting as a potential target in the treatment of AAV (35).
C5a, an anaphylatoxin of complement, is a potent inflammatory mediator (36). Alternative classical and lectin pathways converge on the activation of C5, releasing C5a and C5b. C5a is a potent chemoattractant for neutrophils, and ligation of C5aR/CD88 by C5a activates neutrophils. Neutrophil priming increases the availability of ANCA antigens at the surface, where they interact with ANCA and activate neutrophils. When stimulated with proinflammatory cytokines, the terminal C5a/C5aR axis is activated, generating an automatic amplification loop that triggers an acute necrotizing vasculitis process from the primed neutrophils (resulting from their interaction with ANCA) (37). C5a acting on C5aR is a potent neutrophil chemoattractant and agonist (38), increases neutrophil adhesion, induces neutrophil degranulation, and generates reactive oxygen intermediates; C5a activates vascular endothelial cells via the C5aR, promoting cell retraction and increasing vascular permeability (39). Historically, the role of complement was thought to be limited in AAV, as renal biopsies rarely showed complement deposition and the absence of hypocomplementemia. Other clinical studies have supported these findings by showing activation of the alternative pathway in the cardiovascular system and deposition of complement components of the alternative pathway in tissues. Active AAV patients with renal involvement had higher levels of C3a and C5a in the circulation (40). These results could be the potential target in the treatment of AAV.
Cyclophosphamide is associated with reduced ovarian reserve, ovarian failure, and male infertility (41–45). A review of the prednisolone reduction regimens published in major trials has shown that on average, the cyclophosphamide doses 10 mg (after 19 weeks) and 7.5 mg (after 21 weeks) have been achieved (11, 46–51). Cyclophosphamide is usually administered orally or as pulse therapy for 3–6 months, and after remission is achieved, the cyclophosphamide is changed to a less-toxic agent. Intravenous cyclophosphamide pulse therapy may allow the reduction of the cumulative dose and consequently the toxicity. The strategy was demonstrated in the CYCLOPS study (11). Remission rates in patients with systemic but not immediately life-threatening AAV were not significantly different between those who received daily oral cyclophosphamide and those who received pulsed cyclophosphamide. With the pulsed cyclophosphamide regimen, patients could receive a cumulative dose of cyclophosphamide half that of the daily oral cyclophosphamide regimen, but the dose of corticosteroids remained the same. However, pulsed cyclophosphamide is associated with a higher risk of recurrence than daily oral cyclophosphamide (52).
Methotrexate (20-25 mg/week, orally or parenterally) can be used in place of cyclophosphamide in patients with less severe disease and normal renal function (53–62). There have been trials using either methotrexate or mycophenolate mofetil as remission-inducing agents in patients with AAV (60–62). Therefore, methotrexate should only be considered in cases of non-organ-threatening disease. So far, the induction trials using methotrexate are generally larger and have a higher grade of evidence than trials using mycophenolate mofetil. The two randomized controlled trials (RCTs) with mycophenolate mofetil to date have been conducted primarily in patients with MPA (61, 62). MPA often affects renal function, and methotrexate is not indicated in such situations. These studies did not include patients with pulmonary hemorrhage or central nervous system involvement, and mycophenolate mofetil should not be used routinely in life-threatening situations.
Representative clinical trials and the pathogenesis of AAV by the inhibition of binding of biological agents for AAV with biologics are shown in Table 2 and Figure 2.
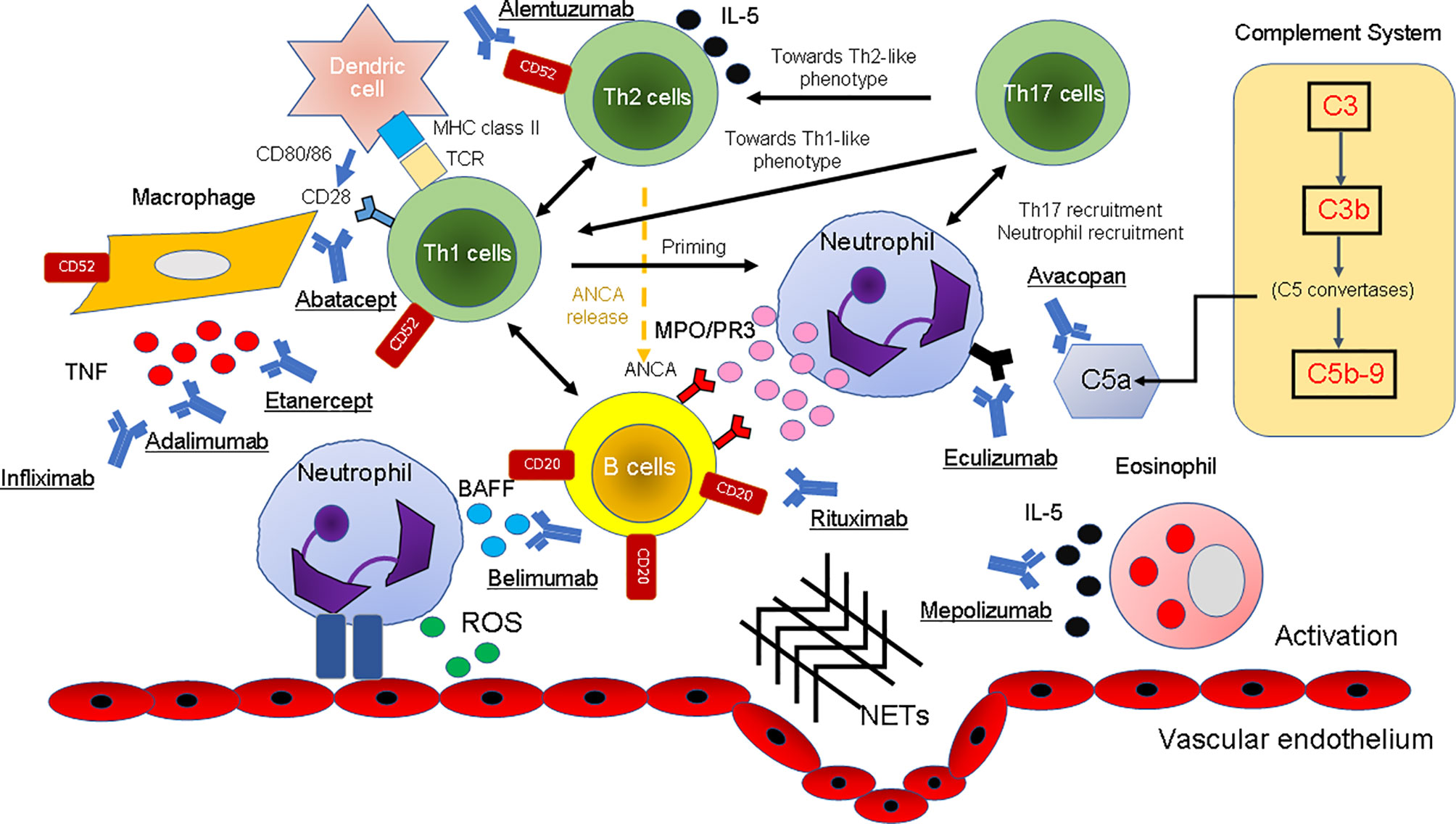
Figure 2 The pathogenesis of AAV and the inhibition of the binding of biological agents. The mechanism of the onset of AAV in the immune and complement systems in various cell types is illustrated. T cells: Etanercept, adalimumab, and infliximab as TNF-α inhibitors and alemtuzumab as an anti-CD52 inhibitor have been used for the treatment of AAV because they block the cytokine signaling pathway. By containing CTLA4, abatacept blocks the engagement of CD28 on the surface of T cells by B7.1 (CD80) or B7.2 (CD86) on the surface of APCs or B cells with its ligand, thereby inhibiting T-cell activation. B cells: B cells also act as APCs for T lymphocytes, and they produce pro-inflammatory cytokines that are useful for T-cell hyperactivity and neutrophil priming. Immunotherapies targeting B cells (depletion of B cells with rituximab and blockade of BLyS by belimumab) reduce the recruitment of these effector cells at the site of immune complex deposition, thereby reducing inflammation and tissue damage. Eosinophils: IL-5 is produced by Th2 cells and induces differentiation and maturation of human eosinophils. By neutralizing IL-5, mepolizumab inhibits the IL-5 signaling pathway and may be a therapeutic option for patients with EGPA. Complement system: Eculizumab and avacopan bind C5 and C5aR with picomolar affinity and inhibit the enzymatic activation by C5 convertases. Others: The binding of ANCA to these autoantigens activates neutrophils attached to the vascular endothelium. Degranulation of neutrophils releases ROS and NETs that damage the vascular endothelium. AAV, ANCA-associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; APCs, antigen-presenting cells; BAFF, B-cell activating factor inhibitor; BLys, B-lymphocyte stimulator; NETs, neutrophil extracellular traps; ROS, reactive oxygen species; EGPA, eosinophilic granulomatosis with polyangiitis; IL, interleukin; MPO, myeloperoxidase; PR3, proteinase 3; TNF, tumor necrosis factor.
A rationale for B-cell activation in AAV has been based on the pathogenicity of ANCA (63). B cells also acts as antigen-presenting cells for T lymphocytes (64), and they produce pro-inflammatory cytokines that are useful for T-cell hyperactivity and neutrophil priming (Figure 1). These mechanisms suggest that B-cell depletion could be a potential target for AAV therapy.
Rituximab, an anti-CD20 IgG1 chimeric mouse/human monoclonal antibody, was approved by the U.S. Food and Drug Administration in 2011 for the management of AAV. Rituximab in AAV has been used in two RCTs, the RAVE (Rituximab for the Treatment of Wegener’s Granulomatosis and Microscopic Polyangiitis) trial and the RITUXVAS trial, an international, randomized, open-label trial comparing rituximab-based regimens with standard cyclophosphamide/azathioprine regimens in the treatment of active “generalized” AAV (13, 14). There were several differences between these two trials: the RITUXVAS trial included new patients with severe renal disease, whereas the RAVE trial included new and recurrent patients with well-maintained renal function. Oral cyclophosphamide was used as a comparator in RAVE, and pulsed cyclophosphamide was used in the RITUXVAS trial. In both trials, rituximab was given as four infusions with a body surface area of 375 mg/m2, but in the RITUXVAS trial, cyclophosphamide was given in addition to rituximab for two to three cycles. Prednisolone was tapered and discontinued by 5 months in the RAVE trial, but was reduced to 5 mg by 6 months in the RITUXVAS study and continued for the remainder of the study. In the RAVE trial, after 18 months, 39% of patients in the rituximab group and 33% of those in the control group maintained complete remission (65). In that study, there was no significant difference in the total number of serious adverse events between the two groups. In the RITUXVAS trial, the rate of sustained remission was 76% in the rituximab group and 82% in the control group (14). A long-term analysis of these patients showed that relapse occurred at 24 months in 42% of the rituximab group and 36% of the cyclophosphamide group (66). Rituximab can be used in patients who are intolerant to cyclophosphamide, in patients of reproductive age, and in patients who have had substantial prior exposure to cyclophosphamide. However, the efficacy of rituximab monotherapy in severe disease has not been established and there is no consensus on the appropriate dosing regimen.
One of the hallmarks of EGPA is eosinophilic inflammation. IL-5, a major cytokine that activates eosinophils, has been postulated to be involved in the pathogenesis of EGPA. IL-5 is produced by Th2 cells and induces differentiation and maturation of human eosinophils (67). IL-5 also inhibits eosinophil apoptosis (68).
Mepolizumab is a humanized anti-IL-5 monoclonal antibody that shows clinical benefit in the management of refractory and relapsing EGPA (15). In patients with EGPA, mepolizumab resulted in a significantly greater number of weeks in remission and a higher proportion of patients in remission compared to a placebo, thus allowing for reduced glucocorticoid use. In 2015, the U.S. Food and Administration expanded the approved use of mepolizumab to treat EGPA. Limitations of the study on which the approval was based were that less than 10% of patients were ANCA positive at baseline and that no analysis of outcomes according to ANCA status was performed.
BAFF is also involved in Ig class switching and subsequent antibody production in vivo. BAFF promotes B-cell proliferation and splenic B-cell survival in vitro (69, 70). Soluble BAFF binds to three different TNF receptors: B cell maturation antigen (BCMA), transmembrane activator, calcium regulator, and cyclophilin ligand interactor (TACI), and BAFF-R (BR3). BCMA and TACI, but not BAFF-R, bind to another B receptor for a proliferation-inducing ligand that is also a cell survival ligand (71). When BAFF binds to its high affinity BAFF-R, the NF-κB pathway (both classical and non-classical pathways) and the MAPK pathway are activated, leading to the expression of genes essential for B cell survival (72).
Belimumab is a human monoclonal IgG1 antibody against B-lymphocyte-stimulating factor (B-Lys) against BAFF and is being investigated as a therapeutic option for AAV. B-lymphocyte-stimulating (B-Lys) factor is a cytokine that promotes B-cell survival, maturation, and differentiation, and B-Lys has been observed to be elevated in the serum of patients with AAV, particularly patients with GPA (73, 74). Belimumab has been used as a treatment for Lupus. The BREVAS clinical trial is a phase III multi-center, multinational, randomized and double-blind study evaluating the efficacy and safety of belimumab in combination with azathioprine for the maintenance of remission in GPA and MPA patients (75). This trial demonstrated that the addition of belimumab to a regimen of azathioprine plus low-dose glucocorticoids to maintain remission of AAV did not reduce the risk of recurrent vasculitis. However, patients who received belimumab after remission with rituximab did not experience recurrence of vasculitis. the BREVAS trial has limitations, including sample size (placebo n=52, belimumab n=54). In addition, the number of patients in remission with rituximab who received belimumab was quite low (n=14). Investigation of the maintenance of remission of AAV with belimumab as monotherapy is needed to determine the potential therapeutic benefit of this biologic. Currently, the combination of belimumab and rituximab is being investigated in patients with PR3 ANCA-positive AAV, and the primary endpoint is to compare the time to PR3 ANCA negativity with rituximab alone.
TNF-α is a multifaceted cytokine that plays a central role in inflammation and leads to the production of a wide range of other pro-inflammatory cytokines and chemokines in the kidney disease (76). In AAV, TNF-α mRNA expression is increased in leukocytes and renal tissue, indicating its involvement in the pathophysiology of the disease (77, 78). These data suggested that TNF-α induces the expression of autoantigens involved in vasculitis on the leukocyte cell membrane, preparing the cells for the effects of ANCAs.
Etanercept, a p75 Fc fusion protein against TNF-α, is a biologic agent that has been studied in AAV. A randomized controlled trial (Wegener’s Granulomatosis Etanercept Trial [WGET]) compared the ability of treatment with etanercept 25 mg 2×/week plus standard care with that of placebo plus standard care to maintain clinical remission in patients with GPA (79). Of the 174 evaluable patients, 126 (72.4%) achieved sustained remission, while only 86 (49.4%) remained in remission. There was no significant difference between the etanercept and control groups in the rates of sustained remission (69.7% vs. 75.3%) and sustained low-level disease activity (86.5% vs. 90.6%). Analysis of the relative risk of disease relapse during follow-up also showed that there was no significant difference in the incidence of disease relapse between the etanercept and control groups.
Infliximab is a chimeric mouse/human monoclonal antibody against TNF-α and has been used for the treatment of AAV (GPA and MPA). A prospective randomized controlled trial comparing infliximab with rituximab for remission induction in patients with severe refractory GPA showed that both agents were effective in inducing remission, but the general data tended to favor rituximab over infliximab; all non-responders to infliximab were thus switched to rituximab (80). Although no significant results were obtained, the trial’s analyses suggested that (i) rituximab has a higher response rate and a higher sustained remission rate, but (ii) there are some cases in which infliximab is useful.
Adalimumab is a humanized anti-TNF-α monoclonal antibody that is being studied in a phase II open-label, prospective study in patients with AAV with renal impairment. Adalimumab (40 mg every 2 weeks) plus cyclophosphamide pulsed infusion (compared to cyclophosphamide pulsed infusion alone) showed similar remission-inducing effects and adverse events, but it reduced the dose of glucocorticosteroids in the adalimumab group (81). However, that study had several limitations including a small sample size and the lack of a control group and randomization. At present, all clinical trials of anti-TNF-α antibodies as treatments for AAV have been suspended.
Concerns have been raised regarding the risk of malignancy with anti-TNF-α treatment in AAV, and the results of a subsequent analysis of the WGET trial with an extended period of follow-up (82) suggested that the increased risk of cancer was not significantly different between the intervention and placebo groups compared to the general population, and that this risk could not be attributed solely to etanercept treatment. However, all of the solid malignancies in the etanercept group occurred in patients who received cyclophosphamide, suggesting that etanercept should not be administered after cyclophosphamide because of the increased incidence of malignancy.
CTLA4-Ig, a soluble fusion protein consisting of the extracellular domain of CTLA4 and the CH2-CH3 domain of IgG modified not to bind the Fc receptor, binds to B7.1 and B7.2 with much higher affinity than CD28 and thus serves as an efficient competitive antagonist of the important B7/CD28 When CD28 on the surface of T cells binds to B7.1 (CD80) or B7.2 (CD86) on the surface of activated antigen-presenting cells (APCs) or B cells (83). It activates signaling pathways that promote T cell survival, leading to the formation of a CD40L (CD40+) receptor on T cells. CD40L (CD154) expression is induced in T cells; CD40L interacts with CD40 on APCs, resulting in further upregulation of MHC and B7 and release of cytokines and other inflammatory mediators (84). In addition, CD40L on activated T cells interacts with CD40 on antigen-specific B cells, which induces B cell proliferation and germinal center formation (85, 86). Costimulation-dependent cell-cell interactions within the germinal center lead to B cell maturation through immunoglobulin isotype switching, somatic mutations, clonal expansion of high-affinity B cells, terminal differentiation into plasma cells, and formation of memory B cells that further activate T cells by expressing B7 and acting as APCs (85, 86). which further activate T cells by expressing B7 and acting as APCs (85–87).
Abatacept is a fusion protein that fuses the Fc region of IgG1 with the extracellular domain of CTLA4 and inhibits the intracellular co-stimulation of T cells. By containing CTLA4, abatacept blocks the engagement of CD28 with its ligand, thereby inhibiting T-cell activation. Based on the rationale that the blockage of T-cell activation might impact the disease pathogenesis of GPA (88), an open-label trial was conducted to investigate the safety and efficacy of abatacept in patients with non-severe relapsing GPA. The trial’s results demonstrated that abatacept treatment induced remission in the majority of patients (80%) and was well tolerated overall (89). Eleven of the 15 patients (73%) were able to withdraw from prednisone. However, the trial had limitations including the small sample size (n=20) and uncontrolled design. The results cannot be generalized to all GPA patients, and because the study excluded patients with severe disease, no conclusions can be reached about the efficacy of abatacept at the current stage.
Alemtuzumab is a humanized anti-CD52 monoclonal antibody that selectively reduces the peripheral blood concentrations of T lymphocytes, monocytes, and macrophages. Alemtuzumab has been used to treat AAV patients who have discontinued all immunosuppressive drugs except prednisolone 10 mg/day. A small, uncontrolled study of alemtuzumab administered at doses of 4, 10, and 40 mg on consecutive days was reported, and the patients were followed for an average of 5 years (90). The majority of patients (85%) achieved clinical remission, but a significant proportion of these patients (72%) relapsed at a median period of 9 months after the treatment ended. Adverse events such as severe infections, malignancies, and Grave’s disease have been reported in patients treated with alemtuzumab (90, 91). Alemtuzumab appears to be able to bring refractory and relapsing AAV into remission. Patients treated in the above-described study had a high incidence of adverse events, particularly serious infections, and many received treatment after suffering significant morbidity with a very poor pre-treatment prognosis. Young patients with relapsing or refractory disease before suffering major vital organ damage may benefit from alemtuzumab. Careful monitoring for infections, thyroid disease (in the long term), and malignancies (over the long term) should be performed in all patients treated with alemtuzumab. In order to recommend alemtuzumab as the standard of care for refractory AAV, randomized controlled trials testing the efficacy of alemtuzumab are needed. However, clinical trials of AAV in alemtuzumab have also been suspended.
Antibodies directed against IL-17 and IL-23 (secukinumab and ustekinumab, respectively) are effectively used in other autoimmune conditions such as psoriasis (92), but no studies of these monoclonal antibodies for the treatment of vasculitis have been conducted despite the existence of a clear rationale.
Eculizumab, a commercial C5 blocking antibody, binds C5 with picomolar affinity and inhibits its enzymatic activation by C5 convertases, possibly through steric hindrance (93). Antibodies directed against C5 have shown remarkable clinical benefits for the diseases paroxysmal nocturnal hemoglobinuria (94) and atypical hemolytic uremic syndrome (95), but a study in AAV was withdrawn without a detailed description (no reference).
Avacopan (formerly known as CCX168) is a novel, orally available, highly selective human C5aR antagonist with no other known pharmacological effects (93). Avacopan does not inhibit the interaction of C5a with its associated receptor C5L2 (also called C5aR2), and avacopan is thought to have anti-inflammatory properties (96). Abacopan has also been shown to exert a protective effect in a mouse model of anti-MPO-induced glomerulonephritis (97). Abacopan, a specific C5aR antagonist, does not inhibit the formation of the terminal complement complex or the membrane attack complex C5b-9, which are required for the elimination of pathogenic endophytic bacteria such as Neisseria meningitidis.
The CLEAR and CLASSIC Phase II trials confirmed that treatment with avacopan 30 mg 2×/day is safe and can be used in place of glucocorticoids for the induction of AAV remission (98, 99). The results of the ADVOCATE trial, a large randomized trial comparing avacopan+placebo with standard glucocorticoid therapy, both in combination with cyclophosphamide or rituximab, are awaited for approval and will probably lead to a major breakthrough in the treatment of AAV (100).
Biologics play an important role in inducing and maintaining clinical remission in severe ANCA-associated vasculitis. The improved understanding of the disease process gained over the past decade has led to the identification of multiple new targets and strategies to treat this deadly disease. The development of tools to assess the pathogenesis of vasculitis and extensive experience with a series of clinical trials has established a foundation from which new agents can be evaluated.
Rituximab is indicated for the induction of remission and the management of severe recurrent GPA/MPA, although the role of preemptive, fixed-interval therapy with rituximab in maintaining remission has been suggested. Mepolizumab has shown efficacy in the management of severe refractory and relapsing EGPA. Belimumab and avacopan are undergoing clinical trials testing their efficacy and safety in AAV. Although anti-TNF-α biologics are not currently recommended as remission-inducing therapy for AAV, case reports and nonrandomized, open-label trials have provided data that justify the use of these biologics in certain relapsed and refractory cases. However, concerns remain regarding the incidence of malignancies. Abatacept has been well tolerated and a high percentage of patients have achieved disease remission and have been able to discontinue prednisone. Nevertheless, further studies are required to establish the safety and efficacy of these biologics. The clinical efficacy of alemtuzumab has been suggested, but a significant proportion of relapses and a high rate of adverse events such as severe infections were reported for this biologic. All clinical trials for AAV have been suspended at this time.
Strategies to reduce the toxicity associated with treatment without compromising efficacy are important goals for future research. Treatments need to be optimized and customized according to the severity of the patient’s disease and the risk of recurrence. This will require a better understanding of the etiology of AAV and the development of powerful biomarkers.
In conclusion, the potential benefits, adverse effects, and risks of biologic agents in the management of AAV should be considered prior to the administration of these drugs for the induction and maintenance of disease remission.
The author confirms being the sole contributor of this work and has approved it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Cornec D, Cornec-Le Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis-clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol (2016) 12:570–9. doi: 10.1038/nrrheum.2016.123
2. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol (2013) 65:1–11. doi: 10.1002/art.37715
3. Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med (2012) 367:214–23. doi: 10.1056/NEJMoa1108735
4. Unizony S, Villarreal M, Miloslavsky EM, Lu N, Merkel PA, Spiera R, et al. Clinical outcomes of treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis based on ANCA type. Ann Rheum Dis (2016) 75:1166–9. doi: 10.1136/annrheumdis-2015-208073
5. Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med (2005) 143:621–31. doi: 10.7326/0003-4819-143-9-200511010-00005
6. Fussner LA, Hummel AM, Schroeder DR, Silva F, Cartin-Ceba R, Snyder MR, et al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol (2016) 68:1700–10. doi: 10.1002/art.39637
7. Tanna A, Guarino L, Tam FW, Rodriquez-Cubillo B, Levy JB, Cairns TD, et al. Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: Evaluation of the international histological classification and other prognostic factors. Nephrol Dial Transplant (2015) 30:1185–92. doi: 10.1093/ndt/gfu237
8. Berti A, Warner R, Johnson K, Cornec D, Schroeder D, Kabat B, et al. Brief Report: Circulating cytokine profiles and antineutrophil cytoplasmic antibody specificity in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol (2018) 70:1114–21. doi: 10.1002/art.40471
9. Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis (2016) 75:1583–94. doi: 10.1136/annrheumdis-2016-209133
10. Knight A, Askling J, Granath F, Sparen P, Ekbom A. Urinary bladder cancer in Wegener’s granulomatosis: Risks and relation to cyclophosphamide. Ann Rheum Dis (2004) 63:1307–11. doi: 10.1136/ard.2003.019125
11. de Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized trial. Ann Intern Med (2009) 150:670–80. doi: 10.7326/0003-4819-150-10-200905190-00004
12. Geetha D, Jefferson JA. ANCA-associated vasculitis: Core curriculum 2020. Am J Kidney Dis (2020) 75:124–37. doi: 10.1053/j.ajkd.2019.04.031
13. Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med (2010) 363:221–32. doi: 10.1056/NEJMoa0909905
14. Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med (2010) 363:211–20. doi: 10.1056/NEJMoa0909169
15. Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med (2017) 376:1921–32. doi: 10.1056/NEJMoa1702079
16. Bossuyt X, Cohen Tervaert JW, Arimura Y, Blockmans D, Flores-Suárez LF, Guillevin L, et al. Position paper: Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol (2017) 13:683–92. doi: 10.1038/nrrheum.2017.140
17. Gioffredi A, Maritati F, Oliva E, Buzio C. Eosinophilic granulomatosis with polyangiitis: An overview. Front Immunol (2014) 5:549. doi: 10.3389/fimmu.2014.00549
18. Rasmussen N, Petersen J. Cellular immune responses and pathogenesis in c-ANCA positive vasculitides. J Autoimmun (1993) 6:227–36. doi: 10.1006/jaut.1993.1020
19. Kidder D, Bray SE, Fleming S. Differences in the frequency of macrophage and T cell markers between focal and crescentic classes of anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. J Nephropathol (2016) 6:97–102. doi: 10.15171/jnp.2017.16
20. King WJ, Brooks CJ, Holder R, Hughes P, Adu D, Savage CO. T lymphocyte responses to anti-neutrophil cytoplasmic autoantibody (ANCA) antigens are present in patients with ANCA-associated systemic vasculitis and persist during disease remission. Clin Exp Immunol (1998) 112:539–46. doi: 10.1046/j.1365-2249.1998.00615.x
21. Müller A, Trabandt A, Gloeckner-Hofmann K, Seitzer U, Csernok E, Schönermarck U, et al. Localized Wegener’s granulomatosis: Predominance of CD26 and IFN-gamma expression. J Pathol (2000) 192:113–20. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH656>3.0.CO;2-M
22. Balding CE, Howie AJ, Drake-Lee AB, Savage CO. Th2 dominance in nasal mucosa in patients with Wegener’s granulomatosis. Clin Exp Immunol (2001) 125:332–9. doi: 10.1046/j.1365-2249.2001.125002332.x
23. Csernok E, Trabandt A, Müller A, Wang GC, Moosig F, Paulsen J, et al. Cytokine profiles in Wegener’s granulomatosis: Predominance of type 1 (Th1) in the granulomatous inflammation. Arthritis Rheumatol (1999) 42:742–50. doi: 10.1002/1529-0131(199904)42:4<742::AID-ANR18>3.0.CO;2-I
24. Komocsi A, Lamprecht P, Csernok E, Mueller A, Holl-Ulrich K, Seitzer U, et al. Peripheral blood and granuloma CD4(+)CD28(–) T cells are a major source of interferon-gamma and tumor necrosis factor-alpha in Wegener’s granulomatosis. Am J Pathol (2002) 160:1717–24. doi: 10.1016/S0002-9440(10)61118-2
25. Tabarkiewicz J, Pogoda K, Karczmarczyk A, Pozarowski P, Giannopoulos K. The role of IL-17 and Th17 lymphocytes in autoimmune diseases. Arch Immunol Ther Exp (Warsz) (2015) 63:435–49. doi: 10.1007/s00005-015-0344-z
26. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell (2006) 126:1121–33. doi: 10.1016/j.cell.2006.07.035
27. Ooi JD, Phoon RK, Holdsworth SR, Kitching AR. IL-23, not IL-12, directs autoimmunity to the Goodpasture antigen. J Am Soc Nephrol (2009) 20:980–9. doi: 10.1681/ASN.2008080891
28. Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol (2009) 182:3884–91. doi: 10.4049/jimmunol.0802246
29. Abdulahad WH, Stegeman CA, Limburg PC, Kallenberg CG. Skewed distribution of Th17 lymphocytes in patients with Wegener’s granulomatosis in remission. Arthritis Rheumatol (2008) 58:2196–205. doi: 10.1002/art.23557
30. Nogueira E, Hamour S, Sawant D, Henderson S, Mansfield N, Chavele KM, et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant (2010) 25:2209–17. doi: 10.1093/ndt/gfp783
31. Szczeklik W, Jakieła B, Wawrzycka-Adamczyk K, Sanak M, Hubalewska-Mazgaj M, Padjas A, et al. Skewing toward Treg and Th2 responses is a characteristic feature of sustained remission in ANCA-positive granulomatosis with polyangiitis. Eur J Immunol (2017) 47:724–33. doi: 10.1002/eji.201646810
32. Rimbert M, Hamidou M, Braudeau C, Puéchal X, Teixeira L, Caillon H, et al. Decreased numbers of blood dendritic cells and defective function of regulatory T cells in antineutrophil cytoplasmic antibody-associated vasculitis. PloS One (2011) 6:e18734. doi: 10.1371/journal.pone.0018734
33. Krohn S, Nies JF, Kapffer S, Schmidt T, Riedel JH, Kaffke A, et al. IL-17C/IL-17 receptor E signaling in CD4+ T cells promotes TH17 cell-driven glomerular inflammation. J Am Soc Nephrol (2018) 29:1210–22. doi: 10.1681/ASN.2017090949
34. Velden J, Paust HJ, Hoxha E, Turner JE, Steinmetz OM, Wolf G, et al. Renal IL-17 expression in human ANCA-associated glomerulonephritis. Am J Physiol Renal Physiol (2012) 302:F1663–73. doi: 10.1152/ajprenal.00683.2011
35. Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood (2010) 115:335–43. doi: 10.1182/blood-2009-04-216085
36. Höchsmann B, Dohna-Schwake C, Kyrieleis HA, Pannicke U, Schrezenmeier H. Targeted therapy with eculizumab for inherited CD59 deficiency. N Engl J Med (2014) 370:90–2. doi: 10.1056/NEJMc1308104
37. Schatz-Jakobsen JA, Zhang Y, Johnson K, Neill A, Sheridan D, Andersen GR. Structural basis for eculizumab-mediated inhibition of the complement terminal pathway. J Immunol (2016) 197:337–44. doi: 10.4049/jimmunol.1600280
38. Hammerschmidt DE, Harris PD, Wayland JH, Craddock PR, Jacob HS. Complement-induced granulocyte aggregation in vivo. Am J Pathol (1981) 102:146–50.
39. Tse WY, Nash GB, Hewins P, Savage CO, Adu D. ANCA-induced neutrophil F-actin polymerization: Implications for microvascular inflammation. Kidney Int (2005) 67:130–9. doi: 10.1111/j.1523-1755.2005.00063.x
40. Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheumatol (2001) 44:912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5
41. Koyama H, Wada T, Nishizawa Y, Iwanaga T, Aoki Y. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer (1977) 39:1403–9. doi: 10.1002/1097-0142(197704)39:4<1403::AID-CNCR2820390408>3.0.CO;2-8
42. Mersereau J, Dooley MA. Gonadal failure with cyclophosphamide therapy for lupus nephritis: Advances in fertility preservation. Rheum Dis Clin North Am (2010) 36:99–108. doi: 10.1016/j.rdc.2009.12.010
43. Silva CA, Hallak J, Pasqualotto FF, Barba MF, Saito MI, Kiss MH. Gonadal function in male adolescents and young males with juvenile onset systemic lupus erythematosus. J Rheumatol (2002) 29:2000–5.
44. Schrader M, Heicappell R, Müller M, Straub B, Miller K. Impact of chemotherapy on male fertility. Onkologie (2001) 24:326–30. doi: 10.1159/000055103
45. Clowse ME, Copland SC, Hsieh TC, Chow SC, Hoffman GS, Merkel PA, et al. Ovarian reserve diminished by oral cyclophosphamide therapy for granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken) (2011) 63:1777–81. doi: 10.1002/acr.20605
46. Cohen P, Pagnoux C, Mahr A, Arène JP, Mouthon L, Le Guern V, et al. Churg-Strauss syndrome with poor-prognosis factors: a prospective multicenter trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in forty-eight patients. Arthritis Rheumatol (2007) 57:686–93. doi: 10.1002/art.22679
47. Stassen PM, Tervaert JWC, Stegeman CA. Induction of remission in active anti-neutrophil cytoplasmic antibody-associated vasculitis with mycophenolate mofetil in patients who cannot be treated with cyclophosphamide. Ann Rheum Dis (2007) 66:798–802. doi: 10.1136/ard.2006.060301
48. De Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum (2005) 52:2461–9. doi: 10.1002/art.21142
49. Mansfield N, Hamour S, Habib AM, Tarzi R, Levy J, Griffith M, et al. Prolonged disease-free remission following rituximab and low-dose cyclophosphamide therapy for renal ANCA-associated vasculitis. Nephrol Dial Transplant (2011) 26:3280–6. doi: 10.1093/ndt/gfr127
50. Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniené J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med (2003) 349:36–44. doi: 10.1056/NEJMoa020286
51. Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniené J, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol (2007) 18:2180–8. doi: 10.1681/ASN.2007010090
52. Harper L, Morgan MD, Walsh M, Hoglund P, Westman K, Flossmann O, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis (2012) 71:955–60. doi: 10.1136/annrheumdis-2011-200477
53. Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. The treatment of Wegener’s granulomatosis with glucocorticoids and methotrexate. Arthritis Rheum (1992) 35:1322–9. doi: 10.1002/art.1780351113
54. Sneller MC, Hoffman GS, Talar-Williams C, Kerr GS, Hallahan CW, Fauci AS. An analysis of forty-two Wegener’s granulomatosis patients treated with methotrexate and prednisone. Arthritis Rheum (1995) 38:608–13. doi: 10.1002/art.1780380505
55. Stone JH, Tun W, Hellman DB. Treatment of non-life threatening Wegener’s granulomatosis with methotrexate and daily prednisone as the initial therapy of choice. J Rheumatol (1999) 26:1134–9.
56. Langford CA, Talar-Williams C, Sneller MC. Use of methotrexate and glucocorticoids in the treatment of Wegener’s granulomatosis. Long-term renal outcome in patients with glomerulonephritis. Arthritis Rheum (2000) 43:1836–40. doi: 10.1002/1529-0131(200008)43:8<1836::AID-ANR20>3.0.CO;2-R
57. Stone JH. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med (2005) 352:351–61. doi: 10.1056/NEJMoa041884
58. de Groot K, Mühler M, Reinhold-Keller E, Paulsen J, Gross WL. Induction of remission in Wegener’s granulomatosis with low dose methotrexate. J Rheumatol (1998) 25:492–5.
59. Metzler C, Hellmich B, Gause A, Gross WL, de Groot K. Churg Strauss syndrome-successful induction of remission with methotrexate and unexpected high cardiac and pulmonary relapse ratio during maintenance treatment. Clin Exp Rheumatol (2004) 22:52–61.
60. Faurschou M, Westman K, Rasmussen N, de Groot K, Flossmann O, Höglund P, et al. Brief Report: Long-term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum (2012) 64:3472–7. doi: 10.1002/art.34547
61. Hu W, Liu C, Xie H, Chen H, Liu Z, Li L. Mycophenolate mofetil versus cyclophosphamide for inducing remission of ANCA vasculitis with moderate renal involvement. Nephrol Dial Transplant (2008) 23:1307–12. doi: 10.1093/ndt/gfm780
62. Han F, Liu G, Zhang X, Li X, He Q, He X, et al. Effects of mycophenolate mofetil combined with corticosteroids for induction therapy of microscopic polyangiitis. Am J Nephrol (2011) 33:185–92. doi: 10.1159/000324364
63. Wilde B, van Paassen P, Witzke O, Tervaert JWC. New pathophysiological insights and treatment of ANCA-associated vasculitis. Kidney Int (2011) 79:599–612. doi: 10.1038/ki.2010.472
64. Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: Positive feedback in systemic autoimmune disease. Nat Rev Immunol (2001) 1:147–53. doi: 10.1038/35100573
65. Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med (2013) 369:417–27. doi: 10.1056/NEJMoa1213277
66. Jones RB, Furuta S, Tervaert JW, Hauser T, Luqmani R, Morgan MD, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis (2015) 74:1178–82. doi: 10.1136/annrheumdis-2014-206404
67. Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: Comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood (1989) 73:1504–12. doi: 10.1182/blood.V73.6.1504.bloodjournal7361504
68. Yamaguchi Y, Suda T, Ohta S, Tominaga K, Miura Y, Kasahara T. Analysis of the survival of mature human eosinophils: Interleukin-5 prevents apoptosis in mature human eosinophils. Blood (1991) 78:2542–7. doi: 10.1182/blood.V78.10.2542.bloodjournal78102542
69. Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med (1999) 189:1747–56. doi: 10.1084/jem.189.11.1747
70. Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science (2001) 293:2111–4. doi: 10.1126/science.1061964
71. Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: A tutorial on B cell survival. Annu Rev Immunol (2003) 21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152
72. Rauch M, Tussiwand R, Bosco N, Rolink AG. Crucial role for BAFF-BAFF-R signaling in the survival and maintenance of mature B cells. PloS One (2009) 4:e5456. doi: 10.1371/journal.pone.0005456
73. Nagai M, Hirayama K, Ebihara I, Shimohata H, Kobayashi M, Koyama A. Serum levels of BAFF and APRIL in myeloperoxidase anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis: Association with disease activity. Nephron Clin Pract (2011) 118:c339–45. doi: 10.1159/000323393
74. Bader L, Koldingsnes W, Nossent J. B-lymphocyte activating factor levels are increased in patients with Wegener’s granulomatosis and inversely correlated with ANCA titer. Clin Rheumatol (2010) 29:1031–5. doi: 10.1007/s10067-010-1526-z
75. Jayne D, Blockmans D, Luqmani R, Moiseev S, Ji B, Green Y, et al. Efficacy and safety of belimumab and azathioprine for maintenance of remission in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized controlled study. Arthritis Rheumatol (2019) 71:952–63. doi: 10.1002/art.40802
76. Feldmann M, Pusey CD. Is there a role for TNF-alpha in anti-neutrophil cytoplasmic antibody-associated vasculitis? Lessons from other chronic inflammatory diseases. J Am Soc Nephrol (2006) 17:1243–52. doi: 10.1681/ASN.2005121359
77. Deguchi Y, Shibata N, Kishimoto S. Enhanced expression of the tumour necrosis factor/cachectin gene in peripheral blood mononuclear cells from patients with systemic vasculitis. Clin Exp Immunol (1990) 81:311–4. doi: 10.1111/j.1365-2249.1990.tb03336.x
78. Noronha IL, Krüger C, Andrassy K, Ritz E, Waldherr R. In situ production of TNF-alpha, IL-1 beta and IL-2R in ANCA-positive glomerulonephritis. Kidney Int (1993) 43:682–92. doi: 10.1038/ki.1993.98
79. Wegener’s Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med (2005) 27(352):351–61. doi: 10.1056/NEJMoa041884
80. de Menthon M, Cohen P, Pagnoux C, Buchler M, Sibilia J, Détrée F, et al. Infliximab or rituximab for refractory Wegener’s granulomatosis: Long-term followup. A prospective randomised multicentre study on 17 patients. Clin Exp Rheumatol (2011) 29:S63–71.
81. Laurino S, Chaudhry A, Booth A, Conte G, Jayne D. Prospective study of TNFalpha blockade with adalimumab in ANCA-associated systemic vasculitis with renal involvement. Nephrol Dial Transplant (2010) 25:3307–14. doi: 10.1093/ndt/gfq187
82. Silva F, Seo P, Schroeder DR, Stone JH, Merkel PA, Hoffman GS, et al. Granulomatosis with polyangiitis (Wegener) and solid malignancies among etanercept-treated patients: Long-term follow-up of a multicenter longitudinal cohort. Arthritis Rheumatol (2011) 63:2495–503. doi: 10.1002/art.30394
83. Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity (1994) 1:793–801. doi: 10.1016/S1074-7613(94)80021-9
84. Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol (1998) 16:111–35. doi: 10.1146/annurev.immunol.16.1.111
85. Lindhout E, Koopman G, Pals ST, de Groot C. Triple check for antigen specificity of B cells during germinal centre reactions. Immunol Today (1997) 18:573–7. doi: 10.1016/S0167-5699(97)01160-2
86. Tarlinton D. Germinal centers: Form and function. Curr Opin Immunol (1998) 10:245–51. doi: 10.1016/S0952-7915(98)80161-1
87. Mamula MJ. Epitope spreading: The role of self peptides and autoantigen processing by B lymphocytes. Immunol Rev (1998) 164:231–9. doi: 10.1111/j.1600-065X.1998.tb01223.x
88. Steiner K, Moosig F, Csernok E, Selleng K, Gross WL, Fleischer B, et al. Increased expression of CTLA-4 (CD152) by T and B lymphocytes in Wegener’s granulomatosis. Clin Exp Immunol (2001) 126:143–50. doi: 10.1046/j.1365-2249.2001.01575.x
89. Langford CA, Monach PA, Specks U, Seo P, Cuthbertson D, McAlear CA, et al. An open-label trial of abatacept (CTLA4-Ig) in non-severe relapsing granulomatosis with polyangiitis (Wegener’s). Ann Rheum Dis (2014) 73:1376–9. doi: 10.1136/annrheumdis-2013-204164
90. Walsh M, Chaudhry A, Jayne D. Long-term follow-up of relapsing/refractory anti-neutrophil cytoplasm antibody associated vasculitis treated with the lymphocyte depleting antibody alemtuzumab (CAMPATH-1H). Ann Rheum Dis (2008) 67:1322–7. doi: 10.1136/ard.2007.081661
91. Lockwood CM, Thiru S, Isaacs JD, Hale G, Waldmann H. Long-term remission of intractable systemic vasculitis with monoclonal antibody therapy. Lancet (1993) 341:1620–2. doi: 10.1016/0140-6736(93)90759-A
92. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis – Results of two phase 3 trials. N Engl J Med (2014) 371:326–38. doi: 10.1056/NEJMoa1314258
93. Antovic A, Mobarrez F, Manojlovic M, Soutari N, De Porta Baggemar V, Nordin A, et al. Microparticles expressing myeloperoxidase and complement C3a and C5a as markers of renal involvement in antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol (2020) 47:714–21. doi: 10.3899/jrheum.181347
94. Hillmen P, Young NS, Schubert J, Brodsky RA, Socié G, Muus P, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med (2006) 355:1233–43. doi: 10.1056/NEJMoa061648
95. Keenswijk W, Raes A, Vande Walle J. Is eculizumab efficacious in Shigatoxin-associated hemolytic uremic syndrome? A narrative review of current evidence. Eur J Pediatr (2018) 177:311–18. doi: 10.1007/s00431-017-3077-7
96. Bekker P, Dairaghi D, Seitz L, Leleti M, Wang Y, Ertl L, et al. Characterization of pharmacologic and pharmacokinetic properties of CCX168, a potent and selective orally administered complement 5a receptor inhibitor, based on preclinical evaluation and randomized Phase 1 clinical study. PloS One (2016) 11:e0164646. doi: 10.1371/journal.pone.0164646
97. Xiao H, Dairaghi DJ, Powers JP, Ertl LS, Baumgart T, Wang Y, et al. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol (2014) 25:225–31. doi: 10.1681/ASN.2013020143
98. Merkel PA, Niles J, Jimenez R, Spiera RF, Rovin BH, Bomback A, et al. Adjunctive treatment with avacopan, an oral C5a receptor inhibitor, in patients with antineutrophil cytoplasmic antibody-associated vasculitis. ACR Open Rheumatol (2020) 2:662–71. doi: 10.1002/acr2.11185
99. Jayne DRW, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, et al. Randomized trial of c5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol (2017) 28:2756–67. doi: 10.1681/ASN.2016111179
100. Merkel PA, Jayne DR, Wang C, Hillson J, Bekker P. Evaluation of the safety and efficacy of avacopan, a C5a receptor inhibitor, in patients with antineutrophil cytoplasmic antibody-associated vasculitis treated concomitantly with rituximab or cyclophosphamide/azathioprine: Protocol for a randomized, double-blind, active-controlled, Phase 3 trial. JMIR Res Protoc (2020) 9:e16664. doi: 10.2196/16664
Keywords: anti-neutrophil cytoplasmic autoantibody, anti-neutrophil cytoplasmic autoantibody-associated vasculitis, biologics, cytokine, cytokine-immunological terms
Citation: Nozaki Y (2021) New Insights Into Novel Therapeutic Targets in ANCA-Associated Vasculitis. Front. Immunol. 12:631055. doi: 10.3389/fimmu.2021.631055
Received: 19 November 2020; Accepted: 18 March 2021;
Published: 01 April 2021.
Edited by:
Joshua Daniel Ooi, Monash University, AustraliaReviewed by:
Tanya Mayadas, Brigham and Women’s Hospital and Harvard Medical School, United StatesCopyright © 2021 Nozaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuji Nozaki, eXVqaTA1MTZAbWVkLmtpbmRhaS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.